Abstract
Background:
Obesity is associated with higher mortality following trauma, though the pathogenesis is unclear. Both obesity and trauma are associated with syndecan-1 shedding and metalloproteinase-9 (MMP-9) activation, which can adversely affect endothelial cell function. We recently demonstrated that fibrinogen stabilizes endothelial cell surface syndecan-1 to reduce shedding and maintain endothelial barrier integrity. We thus hypothesized that MMP-9 activation and syndecan-1 shedding would be exacerbated by obesity after trauma but attenuated by fibrinogen-based resuscitation.
Materials and methods:
ApoE null (−/−) mice were fed a Western diet to induce obesity. Mice were subjected to hemorrhage shock and laparotomy then resuscitated with Lactated Ranger’s (LR) or LR containing fibrinogen and compared to null and lean sham wild type mice. Mean arterial pressure (MAP) was monitored. Bronchial alveolar lavage protein as an indicator of permeability and lung histopathologic injury were assessed. Syndecan-1 protein and active MMP-9 protein were measured.
Results:
MAP was similar between lean sham and ApoE−/− sham mice. However, following hemorrhage, ApoE−/− mice resuscitated with fibrinogen had significantly higher MAP than LR mice. Lung histopathologic injury and permeability were increased in LR compared to fibrinogen resuscitated animals. Compared with lean sham mice, both active MMP-9 and cleaved syndecan-1 level were significantly higher in ApoE−/− sham mice. Resuscitation with fibrinogen. but not lactated Ringers, largely reduced these changes.
Conclusion:
Fibrinogen as a resuscitative adjunct in ApoE−/− mice after hemorrhage shock augmented MAP and reduced histopathologic injury and lung permeability, suggesting fibrinogen protects the endothelium by inhibiting MMP-9-mediated syndecan-1 cleavage in obese mice.
Keywords: obesity, hemorrhage shock, MMP-9, syndecan-1, fibrinogen, lung permeability
Introduction
Obesity is associated with a higher risk of mortality following blunt trauma. The severely injured trauma patients with obesity (BMI ≥ 30) have a mortality rate from 24% to 32% compared to nonobese patients (BMI < 25) (Neville et al, 2004; Choban et al, 1991; Nelson et al, 2012). The pathogenesis underlying the high mortality associated with obesity is not well understood. Nonetheless, it has been reported that obese individuals were very likely to have dysfunction of the endothelium, or endotheliopathy (Cooke et al., 2020), we postulated that the high mortality may be due to the endotheliopathy.
The endothelial glycocalyx is a complex network of molecules that projects from the surface of endothelial cells into the vessel lumen (Reitsma et al, 2007). It is formed by membrane-bound sulfated proteoglycans, consisting of a core protein (syndecan-1 in endothelial cells) with glycosaminoglycan side chains and membrane glycoproteins attached with sialoproteins (Reitsma et al, 2007). Syndecan-1 functions as a soluble modulator when it is released from the cell surface by ectodomain shedding (Rangarajan et al, 2020). Shedding is mediated by “sheddases”, among which is metalloproteinase-9 (MMP-9), in disease states including hemorrhagic shock (Altshuler et al., 2012; Dong et al., 2009; Turner et al., 2016; Zhang et al, 2021).
We and others have reported that the syndecan-1 ectodomain is shed following hemorrhagic shock in vivo and in humans (Gonzalez et al, 2017; Haywood-Watson et al., 2011; Jonansson et al, 2017; Kozar et al, 2011). This shedding results in loss of the endothelial glycocalyx and is also associated with endothelial hyperpermeability, inflammation and shock, and is an independent predictor of mortality following trauma (Gonzalez et al, 2017; Haywood-Watson et al., 2011; Jonansson et al, 2017; Kozar et al, 2011; Johansson et al., 2001). Interestingly, obesity is also associated with glycocalyx shedding (Diebel et al, 2021). ApoE null mice fed with high-fat-diet develop obesity and atherosclerosis, accompanied by the development of a metabolic syndrome phenotype (King et al, 2010). Similar to human metabolic syndrome, ApoE null obese mice have impaired fasting glucose, impaired glucose tolerance, modest dyslipidemia and increased levels of the inflammation (King et al, 2010). We thus hypothesize that syndecan-1 shedding (representative of glycocalyx shedding) is mediated by MMP-9 and the consequence of syndecan-1 shedding in ApoE null mice after hemorrhage shock. Based on our prior work demonstrating that fibrinogen stabilized cell surface syndecan-1 to maintain endothelial barrier (Chipman et al, 2021; Wu et al, 2019), we further hypothesize that fibrinogen inhibits MMP-9 to prevent sydecan-1 shedding in obese mice after hemorrhage shock.
Methods and Materials
Mouse model of hemorrhagic shock
The Animal Care and Use Committee of Institutes of the University of Maryland School of Medicine approved the animal use protocol (IACUC#0818008) and The Institutional Review Board approved the use of human blood components in mice (IBC#00006292). All procedures were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. ApoE null mice (male, 8–9 weeks old) that were fed Western diet ± ad libitum for 12 weeks were subjected to our validated coagulopathic model of trauma-hemorrhagic shock (HS) (Peng et al, 2013). We chose male mice because that female sex protects from organ failure after major trauma hemorrhage (Trentzsch et al., 2014). Under isoflurane anesthesia, a midline laparotomy incision was made, the intestines were inspected and the incision closed. The right femoral artery was cannulated for continuous hemodynamic monitoring and blood withdrawal or resuscitation. After a 10-min period of equilibration, mice were bled to a mean arterial pressure (MAP) of 30 ± 5 mmHg for 90 min. Mice were then resuscitated with lactated Ringer’s (LR) solution (3 × bled volume), or fibrinogen (fib) (0.5 mg fibrinogen/g body weight) in LR (3 × bled volume) within 15 min. After 3 hours, mice were euthanized by exsanguination under isoflurane anesthesia and the lungs were harvested for assays as described below. The right lung root was ligated, dissected and frozen on dry ice for RNA extraction and Western blot analysis. Next, a tracheal catheter (IV catheter, 22G) was inserted, and the left lung washed with 0.4 ml PBS three times through the catheter. The retained bronchoalveolar lavages (BALs) were pooled in a 1.5 ml tube. The left lung was further filled with 0.4 ml OCT:PBS (1:1), saved in OCT mold and frozen for cryostat sectioning. ApoE null sham mice and C57BL/6 lean sham mice (sex and age matched) underwent anesthesia and placement of catheters but were not subjected to hemorrhagic shock and laparotomy. After feeding with fat-diet, ApoE null mice weigh 41.5 ± 1.4 g (means ± SE, n=15) in comparison to lean mice 27 ± 0.5 g (n=5). There were four mouse groups in the present study as shown below:
| Group# | Treatment | Mouse type | Western diet | Cannulation | Laparotomy | Hemorrhage shock | LR | Fibrinogen |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Group 1 | WT SHAM | C57BL6 | No | Yes | No | No | No | No |
| Group 2 | SHAM | ApoE null |
Yes | Yes | No | No | No | No |
| Group 3 | HS+LR | APOE null |
Yes | Yes | Yes | Yes | Yes | No |
| Group 4 | HS+fib | ApoE null |
Yes | Yes | Yes | Yes | No | Yes |
Lung Permeability
Total protein in the BAL fluid was measured with the BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA) as an indicator of permeability. It has been demonstrated that BAL protein level reflects the lung permeability extent (Fu et al., 2008).
Lung histopathologic injury
Lung tissue sections were stained with hematoxylin and eosin (H&E) and scored 0–3 each for alveolar wall thickness, capillary congestion, and leukocyte infiltration as initially described by Hart et al. and as we have reported (Peng et al, 2013; Hart et al, 2005). The overall lung injury score was evaluated by two individuals in a blinded fashion by averaging the three parameters.
Western blotting
Western blotting was performed according to the instructions of antibodies’ manufactures. The Western blots of lung homogenates were probed with anti-syndecan-1 ectodomain antibody (sc-12765, Santa Cruz Biotechnology), anti-(matrix metallopeptidase) MMP-9 antibody (MA5–15886, Invitrogen) and anti-GAPDH antibody (PA1–987, Thermo Fisher Scientific).
Lung syndecan-1 mRNA
Fresh frozen lung tissue was harvested for syndecan-1 mRNA measurements by RT-qPCR. The primer sequences used for detecting syndecan-1 were: forward: 5’- GAAGAAGAAGGACGAAGGCAG-3’; and reverse: 5’-CCTCCTGTTTGGTGGGC-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control for syndecan-1. Relative RNA amount was calculated using the 2^−ΔΔCt method as described previously (Livak et al., 2001).
Statistical analysis
Prism 6 was used for statistical analysis. MAP data are reported as means ± standard deviation (SD) and were analyzed using two-way-repeated-measures ANOVA followed by Bonferroni multiple comparisons. For non-MAP data, the values are reported as medians and interquartile range (IQR) and were analyzed using Kruskal-Wallis test followed by Dunn’s post-hoc test for multiple comparisons. P<0.05 was considered statistically significant.
Results
Mean arterial pressure was improved by fibrinogen in ApoE null mice after hemorrhage shock.
Baseline MAP did not differ between groups and there was no difference in MAP between WT lean sham mice and ApoE null sham mice (Fig. 1). However, in ApoE null mice undergoing hemorrhage, mice resuscitated with fibrinogen had a significant increase in MAP at 0.5 h, 1 h, 2 h, and 3 h post resuscitation compared to ApoE null mice undergoing HS + LR (Fig. 1).
Fig. 1.
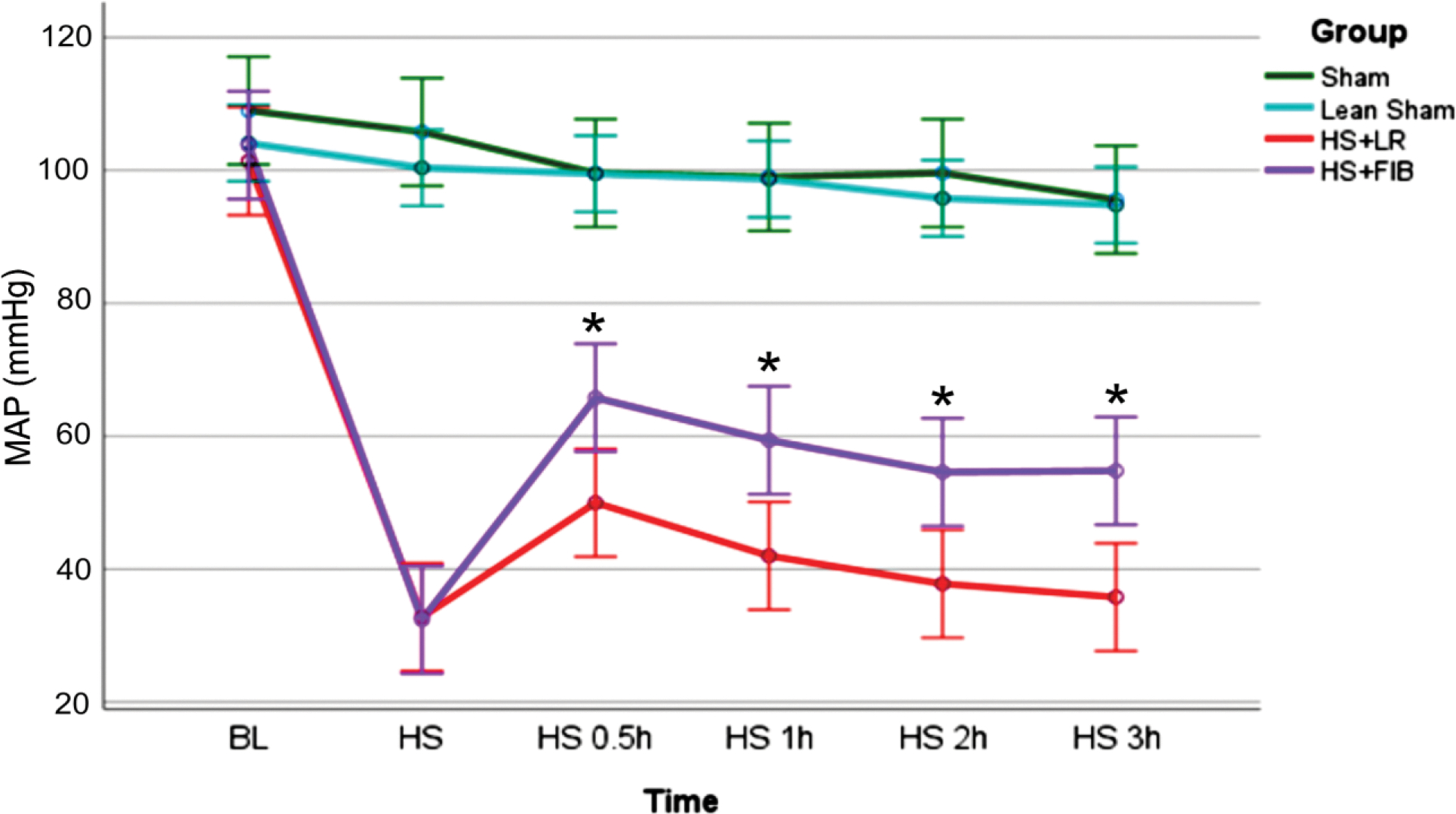
Mean arterial pressure (MAP) over time. ApoE null mice after feeding with 12 weeks Western diet underwent 90 min of hemorrhagic shock followed by resuscitation with either fibrinogen (fib) or LR and were compared to ApoE null sham mice (SHAM) or C57BL/6 wild type lean sham mice (WT-SHAM). Data is reported as mean ± SD with n=5 per group and analyzed by two-way-repeated-measures ANOVA followed by Bonferroni multiple comparisons. Asterisk indicates MAP in HS+fib was significantly higher than that in HS+LR at the respective time points. fib: fibrinogen, LR: lactated ringer’s, HS: hemorrhagic shock.
Fibrinogen reduced lung histopathologic injury and decreased lung permeability in ApoE null mice after hemorrhage shock
Lung structure in terms of alveolar wall thickness, capillary congestion, and leukocyte infiltration, did not show marked damage in ApoE null sham mice compared with wild type lean sham mice (Fig. 2A and 2B). However, hemorrhage shock plus resuscitation with LR (HS+LR) significantly increased lung histopathologic injury (Fig. 2A and 2B). Notably, resuscitation with fibrinogen (HS+fibrinogen) significantly reduced lung injury compared with resuscitation with LR (Fig. 2A and 2B).
Fig. 2.
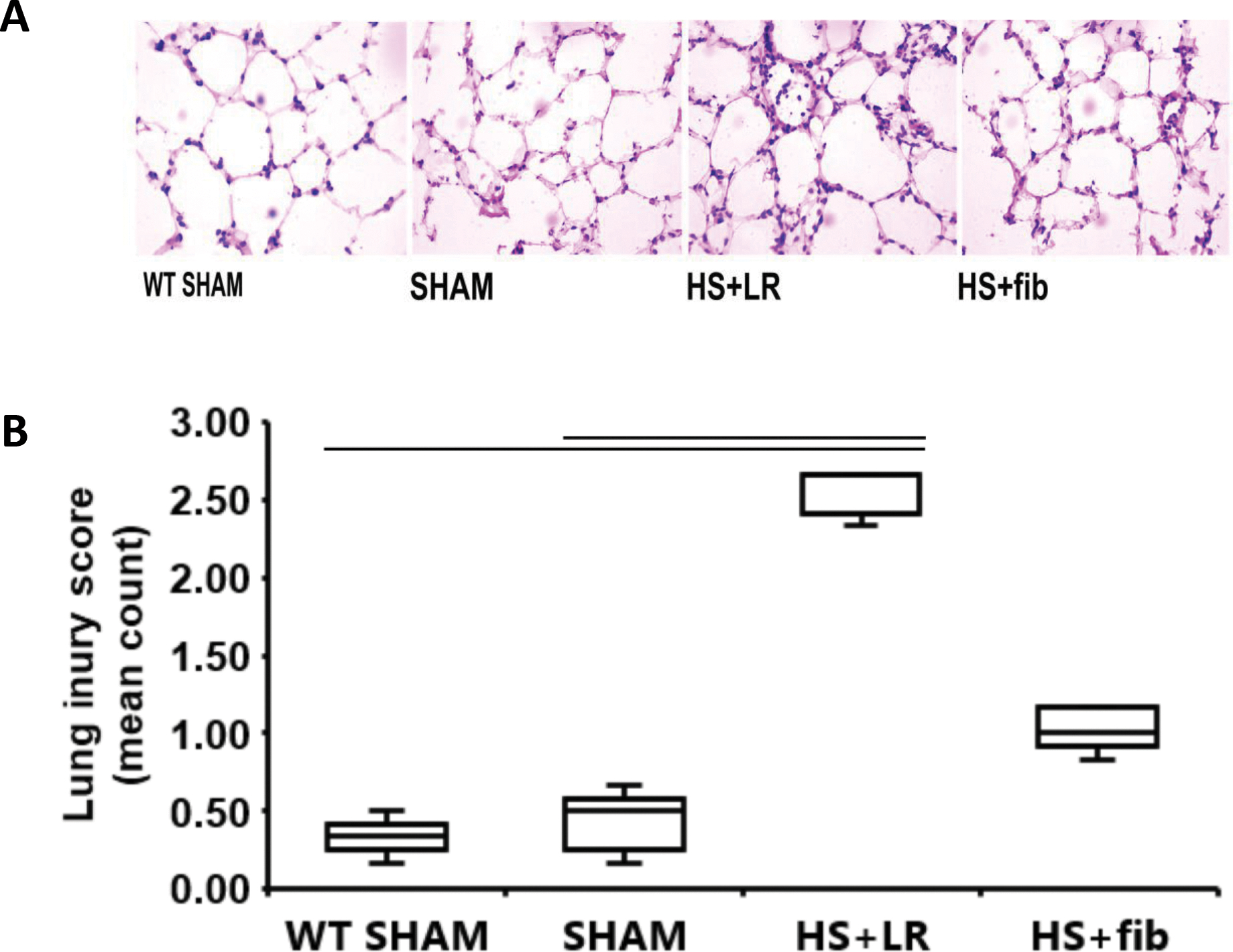
Lung histopathologic injury. Mice were treated as described in Fig. 1. Shown are representative H&E images (A) and the corresponding lung injury score counts (B). The values are presented as medians with IQR. Bars indicate relationships with p<0.05; n=5 per group.
BAL protein concentration was measured to determine lung vascular integrity. We first detected a relative high level of protein content in BAL obtained from ApoE null sham mice when compared with BAL from wild type lean mice, suggesting baseline lung vascular leakage in ApoE null sham mice (Fig. 3). HS+LR further elevated BAL protein level but HS+fibrinogen reduced BAL protein compared with HS+LR (Fig. 3), demonstrating that fibrinogen prevents pulmonary vascular leakage.
Fig. 3.
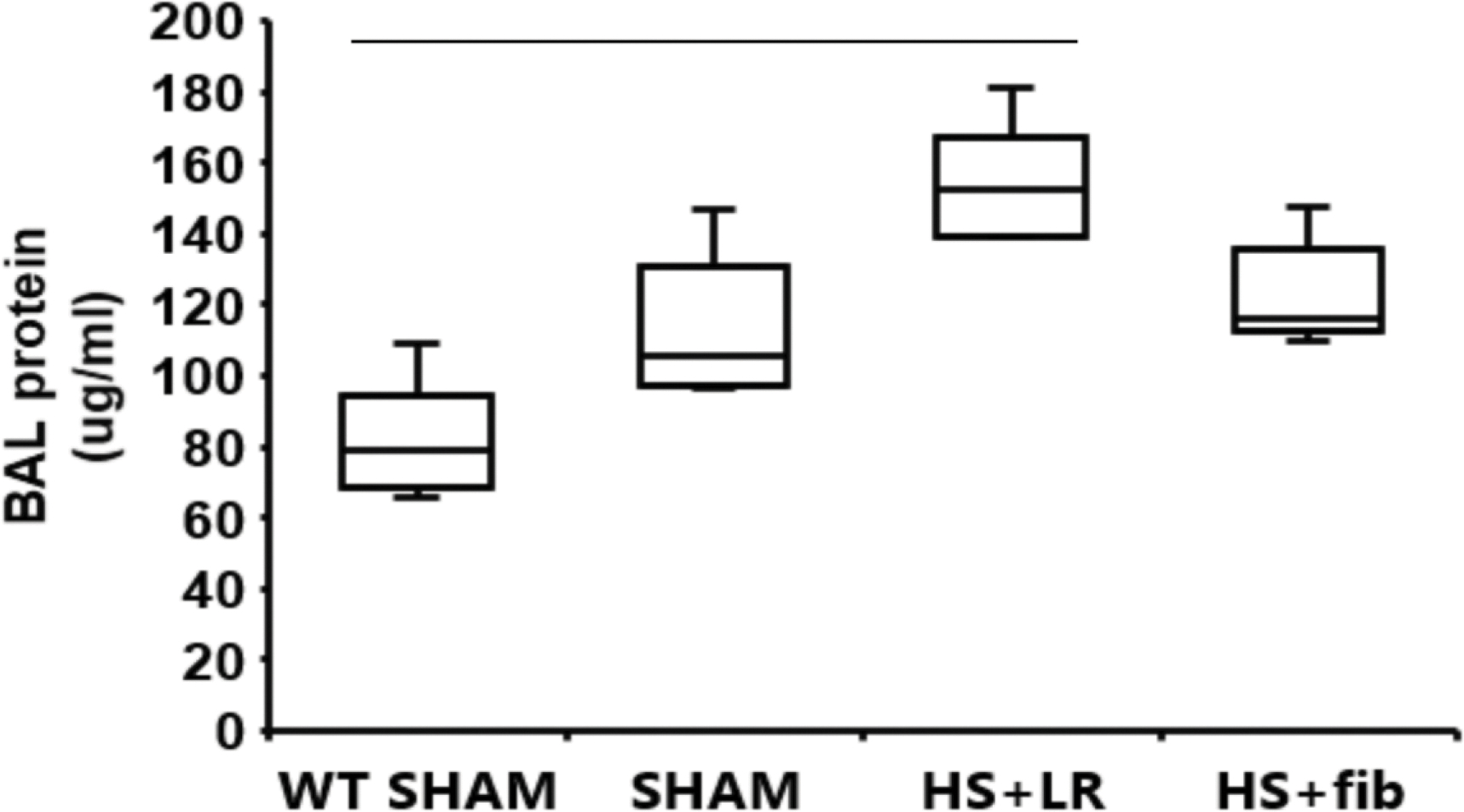
Lung permeability. Mice were treated as described in Fig. 1. BAL protein concentration was measured as an indicator of lung permeability. The values are presented as medians with IQR. Bars indicate relationships with p<0.05; n=5 per group.
Fibrinogen inhibited pulmonary MMP-9 activation in ApoE null mice after hemorrhage shock
As shown in Fig. 4A and 4B, compared with lean wild type sham mice, ApoE null sham mice had significantly increased levels of active MMP-9. Though HS+LR reduced active MMP-9, it was further lessened by HS+fibrinogen resuscitation.
Fig. 4.
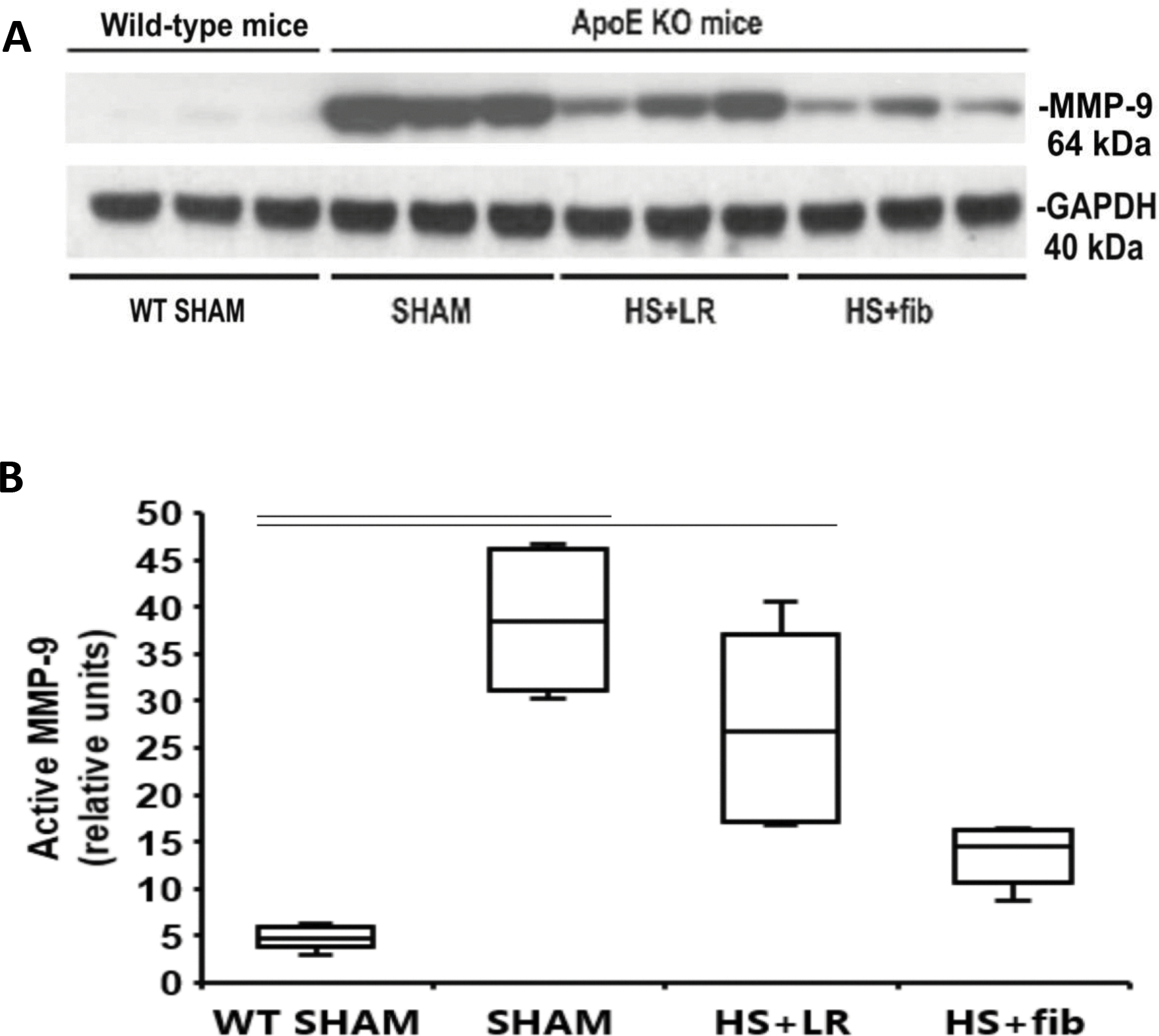
Western blot analysis of active MMP-9 in the lung tissues. Mice were treated as described in Fig. 1. Lung tissues were homogenized for electrophoresis and Western blot analysis. A: Representative immunoblotting for active MMP-9 and loading control GAPDH. B: Summaries of active MMP-9 band intensities (normalized to GAPDH). The values are presented as medians with IQR. Bars indicate relationships with p<0.05; n=5 per group.
Fibrinogen did not modulate syndecan-1 mRNA after hemorrhagic shock and resuscitation in ApoE mice.
Syndecan-1 mRNA was reduced in ApoE null sham mice (0.54 ± 0.2) compared to WT sham (1.0 ± 0.3) and the levels were not affected by hemorrhagic shock and resuscitation with either LR or fibrinogen (ApoE HS+LR 0.50 ± 0.2 vs ApoE HS+fib 0.54 ± 0.1; p> 0.05). Data is reported as mean ± SD with n=5 per group.
Fibrinogen inhibits syndecan-1 cleavage in ApoE null mice after hemorrhage shock
As MMP-9 is a syndecan-1 sheddase, we determined if syndecan-1 was cleaved at the tissue level. Compared with WT lean sham mice, ApoE null sham mice had significantly increased levels of cleaved syndecan-1 fragments (molecular weight: 37 kDa), suggesting that the baseline high MMP-9 activity caused syndecan-1 cleavage in the lung tissues (Fig. 5A and 5B). Although HS+LR had minimal effect on the high levels of cleaved syndecan-1, HS+fibrinogen reduced the levels, suggesting fibrinogen is capable of inhibiting syndecan-1 cleavage (Fig. 5A and 5B).
Fig. 5.
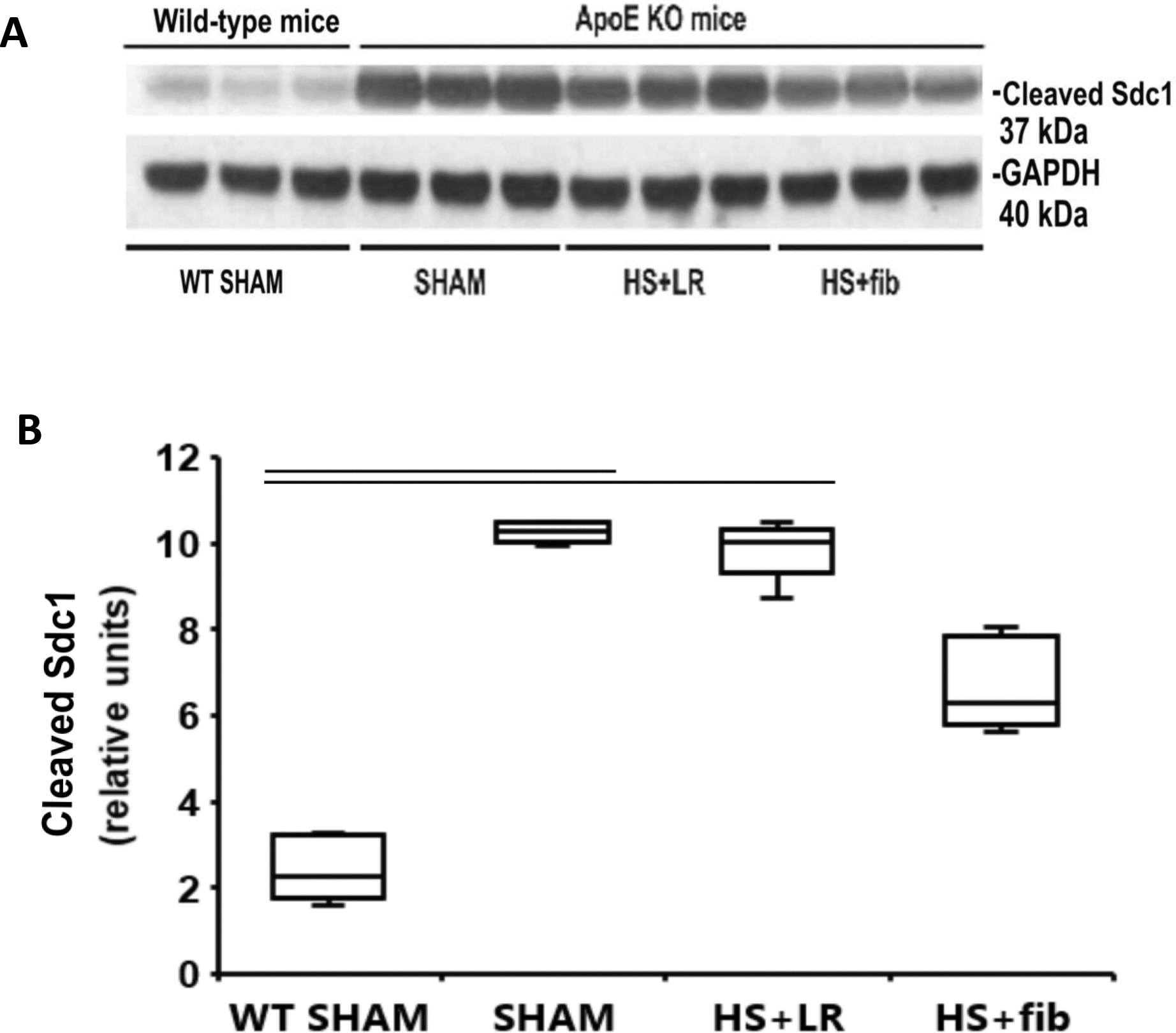
Western blot analysis of cleaved syndecan-1 fragments in the lung tissues. Mice were treated as described in Fig. 1. Lung tissues were homogenized for electrophoresis and Western blot analysis. A: Representative immunoblotting for cleaved syndecan-1 fragment and loading control GAPDH. B: Summaries of active MMP-9 band intensities (normalized to GAPDH). The values are presented as medians with IQR. Bars indicate relationships with p<0.05; n=5 per group.
Discussion
Our study demonstrated that fibrinogen-supplemented resuscitation improved hemodynamics, reduced lung permeability and lessened lung injury in ApoE null mice after hemorrhage shock. These protective effects of fibrinogen were associated with reductions in MMP-9 activity and syndecan-1 cleavage.
Certain MMPs under pathological conditions cause endothelial glycocalyx shedding, resulting in endothelial barrier dysfunction (Junger et al., 2012; Peng et al., 2021). It has been reported that MMP-2, MMP-7, MMP-9 and ADAM 17 all can cleave endothelial glycocalyx (Jin et al, 2021). Among these MMPs, MMP-9 and ADAM 17 have been reported to target syndecan-1 specifically and to increase in the plasma in hemorrhage shock (Junger et al., 2012; Peng et al., 2021). ADAM17 is also known as TNFα-converting enzyme (TACE) and cleaves pro-TNFα to release active TNFα (Pruessmeyer et al., 2009). ADAM17 cleavage activity also mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells (Pruessmeyer et al., 2009). However, ADAM17 has also been shown beneficial effect by inhibiting inflammation (Rovida et al., 2001), our present study thus focuses on MMP-9.
MMP-9 play an important role in obesity-mediated adipose tissue remodeling and obesity-mediated vascular inflammation. For examples, it has been reported that MMP-9 expression was upregulated in adipose tissue biopsies in individuals with obesity and insulin resistance (Unal et al, 2010). Further, most of the MMP-9 expression was found in the stromal vascular fraction of the biopsies (Unal et al, 2010). We also detected a large amount of activated MMP-9 in the lung tissues of ApoE null mice fed with high fat diet. Importantly, even under basal conditions, ApoE null mouse lung vessels were leaky as evidenced by the increased BAL protein concentration when compared to wild type lean mice. Since MMP-9 has been reported to mediate vascular permeability in sepsis and traumatic brain injury (Cui et al., 2015; Muradahvili et al., 2015), our data suggests that MMP-9 is likely involved in the mechanism underlying lung permeability as well.
Fibrinogen is a 340 kDa dimeric glycoprotein comprised of two sets of three polypeptide chains (Aα, Bβ and γ) that are interconnected by disulfide bridges (Kattula et al, 2017). We have shown that fibrinogen stabilizes cell surface syndecan-1 on endothelial cells (Wu et al, 2019), suggesting that the protective effects demonstrated by fibrinogen in the current study were at the cell surface level. The fact that syndecan-1 mRNA was not affected by resuscitation with fibrinogen in ApoE mice also supports a role for fibrinogen at the cell surface rather than transcriptional level. Syndecan-1 can function as a receptor for heparan sulfate-binding molecules such as fibrinogen by binding to their heparin binding domains (Martino et al, 2017; Ramani et al, 2013). Interestingly MMP-9 also contains heparan sulfate binding domains and thus may directly target cell surface syndecan-1 via a similar mechanism (Martino et al, 2017; Ramani et al, 2013). We propose that the binding of fibrinogen to syndecan-1 may shield syndecan-1’s cleavage site from MMP-9. On the other hand, it is also possible that fibrinogen, a substrate of MMP-9 (Chelladurai et al, 2012), can saturate the heparan binding sites on MMP-9, preventing MMP-9 from binding to and cleaving syndecan-1. In ApoE null mice not exposed to supplemental fibrinogen, MMP-9 was unopposed, thus explaining the enhanced cleavage of syndecan-1 detected in the present study.
A limitation of present study was not measuring the baseline fibrinogen levels in the mice. It has been reported that fibrinogen level was increased in obese individuals (Bo et al., 2004) and in the livers from ApoE-deficient mice after feeding 10 weeks of Western diet compared with that in wild type mice without feeding Western diet (Grainger et al., 2004). We postulated that fibrinogen levels are higher in the ApoE null mice in the present study and hemorrhage results in a decrease in the circulation. A second limitation is that the present study only investigated one member of ADAM family that were identified as syndecan-1 sheddases. As mentioned above, although MMP-9 is known to be upregulated and plays a role in hemorrhagic shock (Altshuler et al., 2012; Dong et al., 2009; Turner et al., 2016; Zhang et al, 2021), there are other ADAM family members may also have contributed to our findings.
Conclusion
The present study demonstrated that increased active MMP-9 is correlated with increased syndecan-1 cleavage in the lung tissues of ApoE null mice fed with high-fat-diet. Additionally, fibrinogen as a resuscitative adjunct after hemorrhage shock, augmented mean blood pressure, reduced lung permeability and histopathologic injury in ApoE null mice, suggesting that fibrinogen inhibits syndecan-1 cleavage by MMP-9 in these mice. These data support fibrinogen may be a putative therapeutic avenue for mitigating post hemorrhage shock lung injury in obese individuals.
Funding
This work was supported by the National Institutes of Health RO1GM129533 (RK).
Footnotes
Disclosure: The authors reported no proprietary or commercial interest in any product mentioned on concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altshuler AE, Penn AH, Yang JA, et al. Protease activity increases in plasma, peritoneal fluid, and vital organs after hemorrhagic shock in rats. PLoS One. 2012;7:e32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo M, et al. Body fat is the main predictor of fibrinogen levels in healthy non-obese men. Metabolism. 2004; 53: 984–988. [DOI] [PubMed] [Google Scholar]
- Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J. 2012;40:766–782. [DOI] [PubMed] [Google Scholar]
- Chipman AM, Wu F, Kozar RA. Fibrinogen inhibits microRNA-19b, a novel mechanism for repair of haemorrhagic shock-induced endothelial cell dysfunction. Blood Transfus. 2021;19:420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choban PS, Weireter LJ Jr, Maynes C: Obesity and increased mortality in blunt trauma. J Trauma 1991;31:1253–1257. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Endotheliopathy of Obesity. Circulation. 2020;142:380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N, Wang H, Long Y, et al. Dexamethasone Suppressed LPS-Induced Matrix Metalloproteinase and Its Effect on Endothelial Glycocalyx Shedding. Mediators Inflamm. 2015;2015:912726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Song YN, Liu WG, et al. Mmp-9, a potential target for cerebral ischemic treatment. Curr Neuropharmacol. 2009;7:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebel LN, Marinica AL, Edelman D, et al. The effect of perturbations of the glycocalyx on microvascular perfusion in the obese trauma population: an in vitro study. Trauma Surg Acute Care Open. 2021;6:e000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Birukova AA, Xing J, et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009; 33:612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J Am Coll Surg. 2017; 225:419–427. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004; 173:6366–6375. [DOI] [PubMed] [Google Scholar]
- Hart ML, Ceonzo KA, Shaffer LA, et al. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q1. J Immunol. 2005;174:6373–6380. [DOI] [PubMed] [Google Scholar]
- Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6:e23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Fang F, Gao W, et al. The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Front Cell Neurosci. 2021; 15:739699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PI, Henriksen HH, Stensballe J, et al. Traumatic Endotheliopathy: A Prospective Observational Study of 424 Severely Injured Patients. Ann Surg. 2017; 265:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J, Rasmussen LS, et al. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011; 254:194–200. [DOI] [PubMed] [Google Scholar]
- Junger WG, Rhind SG, Rizoli SB, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012; 38:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Pruett PS, Thompson CA, et al. Heparan sulfate chains of syndecan-1 regulate ectodomain shedding. J Biol Chem. 2012;287:9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida E, Paccagnini A, Del Rosso M, et al. TNF-alpha-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol. 2001;166:1583–1589. [DOI] [PubMed] [Google Scholar]
- Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler Thromb Vasc Biol. 2017;37:e13–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Hatch NW, Chan HW, et al. A murine model of obesity with accelerated atherosclerosis. Obesity (Silver Spring). 2010;18:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Naruo H, Yoshitomi Y, et al. Matrix metalloproteinase-9 associated with heparan sulphate chains of GPI-anchored cell surface proteoglycans mediates motility of murine colon adenocarcinoma cells. J Biochem. 2008;143:581–592. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Ranga A, et al. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muradashvili N, Benton RL, Saatman KE, et al. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab Brain Dis. 2015;30:411–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville AL, Brown CV, Weng J, et al. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg 2004;139:983–987. [DOI] [PubMed] [Google Scholar]
- Nelson J, Billeter AT, Seifert B, et al. Obese trauma patients are at increased risk of early hypovolemic shock: a retrospective cohort analysis of 1,084 severely injured patients. Crit Care. 2012;16:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JT, Li H, Dillon L, et al. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000; 46:307–315. [DOI] [PubMed] [Google Scholar]
- Peng Z, Ban K, LeBlanc A, et al. Intraluminal tranexamic acid inhibits intestinal sheddases and mitigates gut and lung injury and inflammation in a rodent model of hemorrhagic shock. J Trauma Acute Care Surg. 2016;81:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Pati S, Potter D, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmeyer J, Martin C, Hess FM, et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced Shedding of Syndecan-1 and Its Role in Driving Disease Pathogenesis and Progression. J Histochem Cytochem. 2020; 68:823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic AM, Burns-Kurtis CL, Gout B, et al. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68:799–814. [DOI] [PubMed] [Google Scholar]
- Trentzsch H, et al. Female sex protects from organ failure and sepsis after major trauma hemorrhage. Injury. 2014; 45 Suppl 3:S20–8 [DOI] [PubMed] [Google Scholar]
- Turner RJ, Sharp FR. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke . Front Cell Neurosci. 2016;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal R, Yao-Borengasser A, Varma V, et al. Matrix metalloproteinase-9 is increased in obese subjects and decreases in response to pioglitazone. J Clin Endocrinol Metab. 2010;95:2993–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Kozar RA. Fibrinogen Protects Against Barrier Dysfunction Through Maintaining Cell Surface Syndecan-1 In Vitro. Shock. 2019;51:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabluchanskiy A, Ma Y, Iyer RP, et al. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang JT, Pan Y, et al. Syndecan-1 Shedding by Matrix Metalloproteinase-9 Signaling Regulates Alveolar Epithelial Tight Junction in Lipopolysaccharide-Induced Early Acute Lung Injury. J Inflamm Res. 2021;14:5801–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]


