Abstract
Introduction:
Pulmonary function tests (PFT) are a frequent component of heart transplant evaluation. In cardiac surgery abnormal PFTs, especially reduced DLCO, have been associated with poor outcomes. We sought to evaluate the impact of pre-transplant PFTs on post-transplant pulmonary outcomes and patient survival.
Methods:
Among the 652 adult heart transplant recipients between 1/1/2010-7/31/2021, 462 had PFTs and constituted the patient cohort. Obstructive ventilatory defects (OVD), restrictive ventilatory defects (RVD), and reduced DLCO were defined according to established criteria. The primary outcome was the combined endpoint of a post-transplant pulmonary complication defined as reintubation, post-operative pneumonia, prolonged intubation, or tracheostomy. Secondary outcomes included 90-day all-cause mortality, length of stay, and the odds of individual pulmonary complications. Kaplan-Meier survival analysis, multivariable Cox proportional-hazards regression, and multivariable logistic regression were performed to compare outcomes between the groups.
Results:
Patients with severe OVD (OR 1.48, 95% CI 1.18-5.23, p=0.02) or severely reduced DLCO (OR 1.95, 95% CI 1.19-3.20, p=0.008) had increased odds of post-transplant pulmonary complications. Following multivariable adjustment, severe OVD (aOR 2.67, 95% CI 1.15-6.19, p=0.02) and severely reduced DLCO (aOR 1.79, 95% CI 1.05-3.04) remained strongly associated with post-transplant pulmonary complications. Patients with any degree of extrinsic RVD, moderate or less OVD, or moderately reduced DLCO or less did not have an increased odds of post-transplant pulmonary complications. Ninety-day post-transplant survival was significantly reduced for both severe OVD (97.2% vs. 86.5%, p=0.04) and severely reduced DLCO (97.3% vs. 90.4%, p=0.004). Post-transplant ICU and hospital length of stay was nominally longer for both groups as well.
Conclusion:
Severe OVD or severely reduced DLCO on pre-heart transplant PFTs were associated with increased odds of post-transplant pulmonary complications and early mortality.
Keywords: Heart transplant, pulmonary function test, pulmonary complications, survival, length of stay
Introduction
Heart transplantation (HT) continues to be the gold standard therapy for patients with advanced heart failure, with a current one-year survival of over 90% and a median survival of over 13 years.1 Unfortunately, this life-saving therapy remains a limited resource restricted by the availability of suitable donors for all patients who would benefit. Appropriate patient selection remains an important component of organ stewardship to maximize the appropriate utilization of a donated heart. Pulmonary function tests (PFTs) are a noninvasive measure of lung function that are a common component of HT evaluation, however guidance on specific thresholds are not provided by the ISHLT guidelines for patient selection.2,3 These tests include a variety of measurements including forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, and diffusion capacity for carbon monoxide (DLCO), which may be used to characterize restrictive and/or obstructive ventilatory defects as well as diffusion abnormalities in gas exchange. PFTs in patients with severe and chronic heart failure are frequently abnormal, with decreased diffusing capacity and a restrictive ventilatory defect being the most common abnormalities, which may reflect prior thoracic surgeries and impaired gas exchange due to heart failure.4-6
Despite the ubiquity of PFTs in the pre-transplant evaluation, there are limited data on the impact of abnormal PFTs on post-transplant outcomes. Prior expert guidance on the selection of heart transplant candidates suggested that significant obstructive pulmonary disease, defined as a FEV1 <1 L/minute, should be an absolute contraindication for heart transplant. Additionally, severe pulmonary dysfunction (FEV1<40% normal) was suggested to serve as a relative contraindication, though there were no associated clinical outcome data. A recent study analyzed a subset of patients from the International Society for Heart and Lung Transplantation registry with pre-transplant PFTs and identified patients with a FEV1 or FVC < 50% predicted had an increased risk of mortality and longer length of stay. However there were no data on pulmonary outcomes.7 The objective of this study was to evaluate the impact of pre-transplant PFTs on post-transplant pulmonary outcomes and patient survival to help further refine appropriate patient selection heart transplantation.
Methods
Study Population
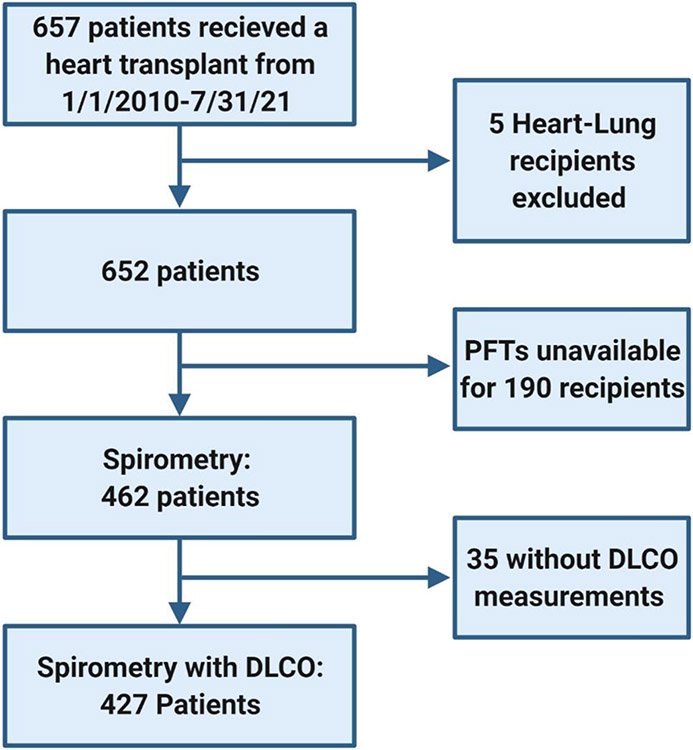
All adult patients who underwent heart transplantation at Columbia University Irving Medical Center from January 1st, 2010 to July 31st, 2021 were included. Among the 657 patients transplanted during the study period (5 heart-lung transplant recipients were excluded) spirometry data were available for 462 patients and 427 patients had both spirometry & DLCO data available (Figure 1). The 190 patients without spirometry were excluded, and the data in this study is germane only to patients who were able to undergo PFTs prior to transplantation. Demographic and clinical data were collected from the electronic medical record.
Figure 1.
Study Flow chart
Study Definitions
Spirometry was defined based on the Global Initiative for Chronic Obstructive Lung Disease definitions.8 An obstructive ventilatory defect (OVD) was defined by a FEV1/FVC ratio below 0.70 with an FEV1 below 80% predicted, a restrictive ventilatory defect (RVD) was defined as a FEV1/FVC ratio above 0.70 and FVC below 80% predicted, and normal spirometry was FEV1/FVC ratio above 0.7 with FEV1 and FVC above 80% predicted. Notably, RVD can be only suspected since total lung capacity was not part of the pre-transplant protocol and was lacking. The severity of spirometric abnormalities (for both OVD and RVD) were graded according to the American Thoracic Society guidelines: mild (FEV1≥70% predicted), moderate (50%<FEV1<69%), and severe (FEV1<50%).9 Abnormalities in DLCO were defined as: Mildly decreased (60-74% predicted), moderately decreased (40-60% predicted), and severely decreased (<40% predicted).
Clinical post-transplant pulmonary complications included reintubation (unplanned, nonprocedural reintubation), post-operative pneumonia (clinical assessment of the physicians taking care of the patient with administration of antibiotics during the index hospitalization), prolonged intubation (intubation greater than 5 days [90th percentile]), and tracheostomy. Comparisons were made to two established surgical risk scores for post-operative pulmonary complications. The ARISCAT Risk Index10, which aims to predict a composite including respiratory failure, respiratory infection, pleural effusion, atelectasis, pneumothorax, bronchospasm, aspiration pneumonitis incorporates advanced age, low preoperative oxygen saturation, a respiratory infection within the past month, preoperative anemia, upper abdominal or thoracic surgery, surgery lasting more than two hours, and emergency surgery. The Gupta score11 intends to predict the risk of mechanical ventilation for longer than 48 hours or reintubation within 30 days, and includes functional status, American Society of Anesthesiologists class, sepsis, emergency case status, and type of surgery.
Study Outcomes
The primary outcome was the combined endpoint of a post-transplant pulmonary complication defined as reintubation, post-operative pneumonia, prolonged intubation, or tracheostomy. Secondary outcomes included 90-day all-cause mortality and the odds of individual pulmonary complications.
Statistical Analysis
Demographic and clinical variables were expressed as mean (± standard deviation) or median with interquartile range (IQR) for continuous variables depending on normality and count (with percentage) for categorical variables. Group comparisons were made with X2 test Kruskal–Wallis one-way analysis of variance where appropriate. Kaplan-Meier survival analysis, Cox proportional-hazards regression, and logistic regression were performed to compare outcomes between the groups. The proportional hazards assumption was tested using Schoenfeld residuals and was not violated. Multicollinearity was assessed through the variance inflation factor, tolerance, and an eigensystem analysis of covariance. A multivariable logistic regression model was generated to assess for significant predictors and confounders of post-transplant pulmonary complications. Variables included were those in Table 3. Additional exploratory analyses of the individual components of primary endpoint were performed and were not adjusted for multiple comparisons. The adjusted model included those that had a p<0.20 on univariable analysis. A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). The study was approved by the Columbia University Irving Medical Center Institutional Review Board.
Table 3.
Univariate and multivariable predictors of post-transplant pulmonary complications
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Severe OVD | 2.48 (1.18-5.23) | 0.01 | 2.67 (1.15-6.19) | 0.02 |
| Severe RVD | 1.20 (0.56-2.57) | 0.63 | ||
| Severely Reduced DLCO | 1.95 (1.19-3.20) | 0.008 | 1.79 (1.05-3.04) | 0.03 |
| Female | 0.85 (0.53-1.34) | 0.47 | ||
| Age (Per ten years) | 1.002 (0.85-1.18) | 0.98 | ||
| BMI | 1.06 (1.01-1.10) | 0.01 | 1.03 (0.98-1.08) | 0.29 |
| Non-white | 0.97 (0.55-1.72) | 0.99 | ||
| Ischemic Cardiomyopathy | 1.56 (1.002-2.42) | 0.05 | 1.25 (0.76-2.05) | 0.13 |
| Intra-aortic Balloon Pump | 0.79 (0.42-1.47) | 0.45 | ||
| VA-ECMO | 3.60 (0.99-12.96) | 0.05 | 3.22 (0.82-12.64) | 0.09 |
| LVAD | 1.70 (1.14-2.54) | 0.01 | 1.46 (0.78-2.72) | 0.24 |
| Prior Cardiac Surgery | 1.50 (1.00-2.25) | 0.05 | 1.20 (0.78-2.72) | 0.49 |
| Pre-Transplant Cigarette Use | 1.56 (0.84-1.87) | 0.26 | ||
| Diabetes Mellitus | 1.55 (1.005-2.40) | 0.05 | 1.20 (9.72-1.99) | 0.11 |
| Prior Stroke | 1.34 (0.67-2.65) | 0.41 | ||
| Stage 3 CKD (30<GFR<60 ml/min/1.73 m2) | 1.24 (90.82-1.88) | 0.32 | ||
| Stage 3 CKD+ (GFR <30 ml/min/1.73 m2) | 0.88 (0.34-2.16) | 0.60 | ||
| Location at the time of Transplant | ||||
| CCU vs. Home | 0.87 (0.56-1.34) | 0.45 | ||
| Hospitalized, not in CCU vs. Home | 0.54 (0.29-0.998) | 0.07 | 0.69 (0.33-1.41) | 0.18 |
Results
Baseline characteristics
One-hundred and two patients had normal spirometry, 252 had an RVD (13.5% severe), and 108 had an OVD (27.8% severe). Patients with an OVD more commonly had an ischemic etiology, had a history of cigarette use, were black, and had an LVAD (Table 1). Those with an RVD were younger, more commonly black, less frequently smoked pre-transplant, and were less likely to have an ICD. Patients with a reduced DLCO were more likely to have an ischemic cardiomyopathy or be undergoing retransplant, were more commonly former smokers, diabetic, were less likely to have an IABP at time of transplant, more commonly had an LVAD at time of transplant, and more commonly had Stage III CKD or worse at time of transplant (Table 2).
Table 1.
Baseline characteristics based on spirometry pattern
| Normal Spirometry |
Restrictive Ventilatory Defect |
Obstructive Ventilatory Defect |
p-value | |
|---|---|---|---|---|
| n | 102 | 252 | 108 | |
| PFT Severity (%) | ||||
| Mild | 81 (32.1) | 18 (16.7) | ||
| Moderate | 137 (54.3) | 60 (55.6) | ||
| Severe | 34 (13.5) | 30 (27.8) | ||
| Moderate or greater reduced DLCO (%) | 53 (52.0) | 163 (64.7) | 73 (67.6) | 0.04 |
| Severely Reduced DLCO | 7 (6.9) | 50 (21.8) | 27 (27.8) | |
| Male (%) | 78 (76.5) | 173 (68.7) | 91 (84.3) | 0.007 |
| Age at Transplant | 59 (50-66) | 54 (43-62) | 61 (53-65) | <0.0001 |
| BMI | 26.3 (23.0-29.6) | 25.8 (23.3-29.3) | 26.1 (22.8-30.6) | 0.54 |
| Ethnicity (%) | 0.002 | |||
| White | 64 (62.7) | 112 (44.4) | 65 (60.2) | |
| Black | 15 (14.7) | 76 (30.2) | 31 (28.7) | |
| Hispanic | 18 (17.7) | 47 (18.7) | 9 (8.3) | |
| Other | 5 (4.9) | 17 (6.8) | 3 (2.8) | |
| HF Etiology (%) | 0.04 | |||
| Ischemic | 25 (24.5) | 57 (22.6) | 36 (33.3) | |
| Non-ischemic | 58 (56.9) | 150 (59.5) | 58 (53.7) | |
| Restrictive/Infiltrative | 8 (7.8) | 11 (4.4) | 10 (9.2) | |
| Retransplant | 7 (6.9) | 19 (7.5) | 0 (0) | |
| Congenital | 4 (3.9) | 15 (6.0) | 4 (3.7) | |
| Pre-Transplant Smoking (%) | 49 (48.0) | 96 (38.1) | 66 (61.1) | 0.0003 |
| Prior Cardiac Surgery (%) | 42 (41.2) | 86 (34.1) | 50 (46.3) | 0.08 |
| Obstructive Sleep Apnea (%) | 13 (13.8) | 15 (6.0) | 7 (6.5) | 0.08 |
| Implantable Cardioverter Defibrillator (%) | 82 (80.4) | 180 (71.4) | 92 (85.2) | 0.01 |
| Diabetes Mellitus (%) | 30 (29.4) | 69 (27.4) | 25 (23.2) | 0.57 |
| Prior Stroke (%) | 6 (5.9) | 23 (9.1) | 10 (9.3) | 0.57 |
| IABP (%) | 19 (18.6) | 29 (11.5) | 10 (9.3) | 0.09 |
| ECMO (%) | 0 (0) | 8 (3.2) | 2 (1.9) | 0.17 |
| LVAD (%) | 40 (39.2) | 119 (47.2) | 61 (56.5) | 0.04 |
| Prior Cardiac Surgery (%) | 42 (41.2) | 86 (34.1) | 50 (46.3) | 0.08 |
| CKD (%) | 0.20 | |||
| GFR>60 mL/min/1.73 m2 | 59 (57.9) | 155 (61.5) | 49 (45.4) | |
| GFR 30-60 mL/min/1.73 m2 | 40 (39.2) | 79 (31.4) | 53 (49.1) | |
| GFR<30 mL/min/1.73 m2 | 3 (2.9) | 18 (7.1) | 6 (5.5) | |
| Location at the Time of Transplant | ||||
| CCU | 41 (40.2) | 92 (36.5) | 36 (33.3) | 0.47 |
| Hospitalized, not in CCU | 20 (19.6) | 41 (16.3) | 15 (13.9) | |
| Home | 41 (40.2) | 119 (47.2) | 57 (52.8) | |
| Functional Status at the Time of Transplant |
0.22 | |||
| Performs ADLs without assistance | 9 (8.8) | 20 (7.9) | 11 (10.2) | |
| Performs ADLs with some assistance | 45 (44.1) | 51 (38.9) | 51 (47.2) | |
| Requires considerable ADL assistance | 25 (24.5) | 86 (34.1) | 22 (20.4) | |
| Hospitalization necessary | 23 (22.6) | 48 (19.1) | 24 (22.2) | |
| Right Heart Catheterization | ||||
| PA Systolic (mmHg) | 38 (28-50) | 42 (32-53) | 46 (35-56) | 0.01 |
| PA Diastolic (mmHg) | 17 (11-24) | 19 (14-25) | 20 (15-26) | 0.03 |
| PCWP (mmHg) | 16 (10-23) | 19 (12-25) | 20 (14-25) | 0.06 |
| PVR (Woods Unit) | 2.13 (1.38-2.92) | 2.25 (1.41-3.06) | 2.46 (1.73-3.61) | 0.15 |
| Cardiac Index (L/min/m2) | 2.01 (1.73-2.46) | 2.04 (1.74-2.55) | 1.94 (1.65-2.31) | 0.14 |
Table 2.
Baseline characteristics based on DLCO abnormality
| Normal DLCO | Abnormal DLCO | p-value | |
|---|---|---|---|
| n | 54 | 373 | |
| DLCO Reduction Severity (%) | |||
| Mild | 84 (22.5) | ||
| Moderate | 205 (55.0) | ||
| Severe | 84 (22.5) | ||
| Male (%) | 37 (68.5) | 280 (75.1) | 0.30 |
| Age at Transplant | 54.5 (47.0-62.0) | 58.0 (48.0-64.0) | 0.23 |
| BMI | 24.9 (22.4-28.0) | 26.2 (23.3-29.7) | 0.13 |
| Ethnicity (%) | 0.28 | ||
| White | 30 (55.5) | 193 (51.8) | |
| Black | 9 (16.7) | 103 (27.6) | |
| Hispanic | 12 (22.2) | 56 (15.0) | |
| Other | 3 (5.6) | 21 (5.6) | |
| HF Etiology (%) | 0.02 | ||
| Ischemic | 6 (11.1) | 98 (26.3) | |
| Non-ischemic | 43 (79.6) | 207 (55.5) | |
| Restrictive/Infiltrative | 2 (3.7) | 26 (7.0) | |
| Retransplant | 1 (1.9) | 22 (5.9) | |
| Congenital | 2 (3.7) | 20 (5.3) | |
| Pre-Transplant Smoking (%) | 15 (27.8) | 178 (47.7) | 0.006 |
| Prior Cardiac Surgery (%) | 20 (37.0) | 143 (38.3) | 0.85 |
| Obstructive Sleep Apnea (%) | 9 (16.7) | 24 (6.4) | 0.009 |
| Implantable Cardioverter Defibrillator (%) | 39 (72.2) | 289 (77.5) | 0.39 |
| Diabetes Mellitus (%) | 8 (14.8) | 103 (27.6) | 0.05 |
| Prior Stroke (%) | 3 (5.6) | 31 (8.3) | 0.48 |
| IABP (%) | 21 (41.1) | 25 (6.7) | <0.0001 |
| ECMO (%) | 2 (3.7) | 8 (2.1) | 0.48 |
| LVAD (%) | 19 (35.2) | 181 (48.5) | 0.06 |
| Prior Cardiac Surgery (%) | 20 (37.0) | 143 (38.3) | 0.85 |
| CKD (%) | 0.02 | ||
| GFR>60 mL/min/1.73 m2 | 40 (74.1) | 203 (54.4) | |
| GFR 30-60 mL/min/1.73 m2 | 13 (24.1) | 146 (39.2) | |
| GFR<30 mL/min/1.73 m2 | 1 (1.8) | 24 (6.4) | |
| Location at the time of Transplant | 0.11 | ||
| CCU | 27 (50.0) | 132 (35.4) | |
| Hospitalized, not in CCU | 8 (14.8) | 62 (16.6) | |
| Home | 19 (35.2) | 179 (48.0) | |
| Functional Status at the Time of Transplant | 0.04 | ||
| Performs ADLs without assistance | 8 (14.8) | 29 (7.8) | |
| Performs ADLs with some assistance | 18 (33.3) | 158 (42.3) | |
| Requires considerable ADL assistance | 11 (20.4) | 113 (30.3) | |
| Hospitalization necessary | 17 (31.5) | 73 (19.6) | |
| Right Heart Catheterization | |||
| PA Systolic (mmHg) | 42 (33-55) | 42 (31-53) | 0.79 |
| PA Diastolic (mmHg) | 20 (14-25) | 18 (14-25) | 0.5 |
| PCWP (mmHg) | 20 (14-25) | 19 (12-24) | 0.41 |
| PVR (Woods Unit) | 2.6 (1.47-3.51) | 2.23 (1.46-3.06) | 0.23 |
| Cardiac Index (L/min/m2) | 1.93 (1.79-2.28) | 2.04 (1.70-2.54) | 0.25 |
Pulmonary outcomes
Patients with a severe OVD (n=30) had a 148% increase in the odds of post-transplant pulmonary complications (OR 2.48, 95% CI 1.18-5.23, P=0.02) compared with patients with a moderate or less OVD. The increased likelihood of pulmonary complications was limited to those with severe OVD, as those with a mild to moderate OVD did not have an increased likelihood of pulmonary complications compared to patients with no OVD (OR 1.13, 95% CI 0.66-1.93, P=0.66). RVD of any severity (Severe RVD vs. moderate or less: OR 1.20, 95% CI 0.56-2.57, p=0.63; mild-moderate RVD vs. no RVD: OR 1.16, 95% CI 0.77-1.76, p=0.48) was not associated with post-transplant pulmonary complications. Notably, none of the patients with severe RVD had radiographic evidence of parenchymal lung disease. Comparable to OVD, patients with a severely reduced DLCO had a 95% increase in the odds of post-transplant pulmonary complications (OR 1.95, 95% CI 1.19-3.20, p=0.008), while patients with mild to moderate reductions in DLCO did not demonstrate a markedly increased risk (OR 1.06, 95% CI 0.55-2.05, p=0.87). A combined group of those with severe OVD and/or severely reduced DLCO (n=102) had an increased risk (OR 1.90, 95% CI 1.20-3.00, p=0.006) and both remained significantly associated with pulmonary complications in a multivariable model with both parameters (Severe OVD: OR 2.56, 95% CI 1.13-5.81; Severely reduced DLCO: OR 1.78, HR 1.07-2.95). Additionally, individually pre-transplant PaO2/FiO2 ratio, peak inspiratory flow, and peak expiratory flow were not predictive of adverse events post-transplant.
We next performed multivariable adjustment, generating a model with risk factors that were associated with an increased risk of pulmonary complications including BMI, ischemic etiology, VA-ECMO, LVAD, prior cardiac surgery, diabetes, and patient location at the time of transplant. After adjustment, severe OVD (adjusted OR 2.67, 95% CI 1.15-6.19 p=0.02) and a severely reduced DLCO (adjusted OR 1.79, 95% CI 1.05-3.04, p=0.03) remained associated with an increased odds of post-transplant pulmonary complications (Table 3). Notably, other risk factors were no longer significantly associated with post-transplant pulmonary complications. It is worth mentioning that VA-ECMO had the largest effect size, though did not meet significance due to a low number of patients.
Subgroup Analyses
Previously suggested individual PFT cutoffs including FEV1< 1 L/min (OR 1.55, 95% CI 0.26-9.42) and FVC <50% predicted (OR 1.36, 95% CI 0.65-2.85) were not significant predictors of pulmonary outcomes, whereas FEV1<50% was independently associated with post-transplant pulmonary complications (OR 1.86, 95% CI 1.08-3.21, p=0.02), similar to severe OVD and severely reduced DLCO.
Individual Pulmonary Complications
Pulmonary complications occurred in 127 individual patients (Table 2). Analyzing individual pulmonary complications, those with a severe OVD had an increased odds of all individual complications, though the most significant was the 380% increased odds of reintubation (95% CI 1.52-9.54, p=0.004) and 221% increased odds of post-operative pneumonia (95% CI 1.03-4.75, p=0.04). The odds of each individual pulmonary complication were significantly increased among those with a severely reduced DLCO (Table 4).
Table 4.
Individual post-transplant pulmonary complications expressed as count (%) or Odds Ratio (95% CI)
| Reintubation | Tracheostomy | Post- Operative Pneumonia |
Prolonged Intubation |
Any Pulmonary Complication |
|
|---|---|---|---|---|---|
| Total Patients | 39 | 20 | 112 | 50 | 139 |
| Normal Spirometry (n=102) | 9 (8.8) | 3 (2.9) | 17 (16.7) | 6 (5.9) | 20 (19.6) |
| OVD (n=108) | 11 (10.2) | 4 (3.7) | 32 (29.6) | 13 (12.0) | 39 (36.1) |
| Mild-Moderate OVD (n=78) | 4 (5.1) | 2 (2.6) | 20 (25.6) | 8 (10.3) | 24 (30.8) |
| Severe OVD (n=30) | 7 (23.3) | 2 (6.7) | 12 (40.0) | 5 (16.7) | 15 (50.0) |
| RVD (n=252) | 19 (7.5) | 13 (5.2) | 63 (25.0) | 31 (12.3) | 80 (31.8) |
| Mild-Moderate RVD (n=218) | 15 (6.9) | 10 (4.6) | 53 (24.3) | 25 (11.5) | 68 (31.2) |
| Severe RVD (n=34) | 4 (11.8) | 3 (8.8) | 10 (29.4) | 6 (17.7) | 12 (35.3) |
| Reduced DLCO (n=373) | 33 (8.9) | 18 (4.8) | 92 (24.7) | 41 (9.6) | 113 (30.3) |
| Mildly-Moderately Reduced DLCO (n=289) | 18 (6.2) | 10 (3.5) | 64 (22.2) | 27 (9.3) | 78 (27.0) |
| Severely Reduced DLCO (n=84) | 15 (17.9) | 8 (9.5) | 28 (33.3) | 14 (16.7) | 35 (41.7) |
| Odds Ratio | |||||
| OVD (n=108) | |||||
| Mild-Moderate OVD (n=78) | 0.63 (0.21-1.85), p=0.40 | 0.56 (0.13-2.47), p=0.44 | 1.18 (0.67-2.08), p=0.56 | 0.97 (0.44-2.19), p=0.96 | 1.13 (0.66-1.93), p=0.66 |
| Severe OVD (n=30) | 3.80 (1.52-9.54), p=0.004 | 1.64 (0.36-7.44), p=0.52 | 2.21 (1.03-4.75), 0.04 | 1.72 (0.63-4.72), p=0.29 | 1.48 (1.18-5.23), p=0.02 |
| RVD (n=252) | |||||
| Mild-Moderate RVD (n=218) | 0.70 (0.35-1.41), p=0.32 | 1.39 (0.52-3.73), p=0.51 | 1.06 (0.68-1.65), p=0.81 | 1.30 (0.69-2.44), p=0.41 | 1.16 (0.77-1.76), p=0.48 |
| Severe RVD (n=34) | 1.80 (0.56-5.80), p=0.32 | 2.01 (0.53-7.7), p=0.31 | 1.30 (0.58-2.89), p=0.52 | 1.65 (0.62-4.39), p=0.31 | 1.20 (0.56-2.57), p=0.63 |
| Reduced DLCO (n=373) | |||||
| Mildly-Moderately Reduced DLCO (n=289) | 1.73 (0.39-7.67), p=0.47 | 1.90 (0.24-15.15), p=0.54 | 1.00 (0.50-2.00), p=0.99 | 1.29 (0.43-3.84), p=0.65 | 1.06 (0.55-2.05), p=0.87 |
| Severely Reduced DLCO (n=84) | 3.51 (1.71-7.20), p=0.0006 | 3.18 (1.23-8.17), p=0.02 | 1.76 (1.04-2.96), p=0.03 | 2.01 (1.01-3.98), p=0.04 | 1.95 (1.19-3.20), p=0.008 |
Secondary Outcomes
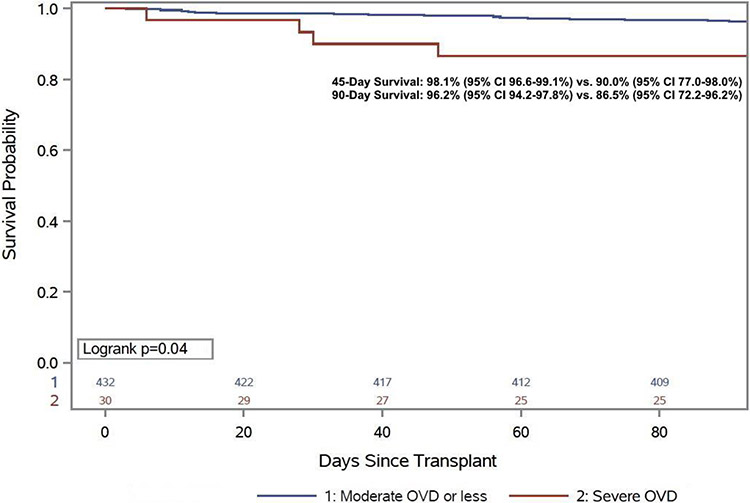
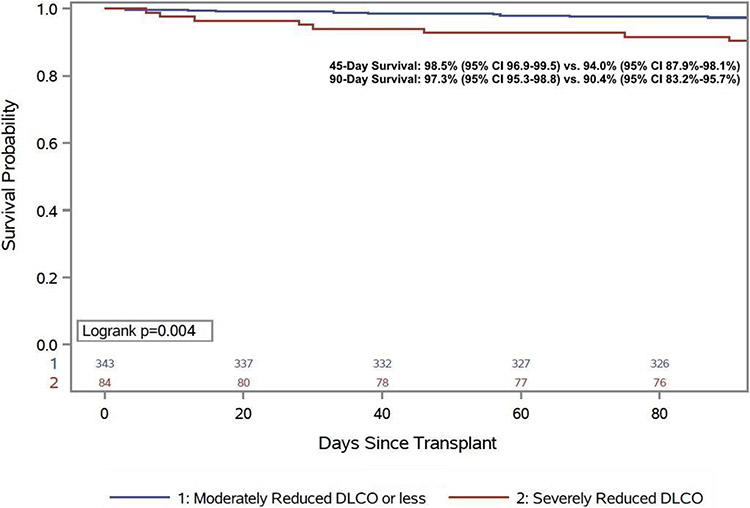
Analogous to pulmonary outcomes, 90-day mortality was greater among patients with a severe OVD or a severely reduced DLCO. Patients with a severely reduced OVD experienced more than a 10% absolute increase in 90-day mortality (13.5% vs. 2.8%, HR 2.05, 95% CI 1.03-4.08, p=0.04, Figure 2) and patients with a severely reduced DLCO (9.6% vs. 2.7%, HR 1.96, 95% CI 1.23-3.12, p=0.005, Figure 3) had nearly a 7% increase in 90-day mortality. Among patients with severe OVD and/or a severely reduced DLCO, no individual risk factor was significantly more frequent, though there was the suggestion that the individuals who died had an overall greater number of comorbid conditions (Supplemental Table 1) and had a trend towards a higher Charlson Comorbidity Index (4 [IQR 3-5] vs. 3 [IQR 2-4], p=0.08). Early mortality was not impacted by RVD (Severe RVD: 4.6% vs. 5.9%, p=0.69). Median time spent in the ICU and the hospital post-transplant was numerically longer for individuals with a severe OVD or severely reduced DLCO, but the difference was of marginal clinical significance. For those with a severe OVD the post-transplant length of stay (LOS) was a median four days longer (24 days [IQR 15-39 days] vs. 20 days [IQR 15-29 days] p=0.31) and ICU LOS was one day longer. Similarly, for those with a severely reduced DLCO post-transplant LOS was two days longer (22 days [IQR 18-31] vs. 20 days [IQR 15-29], p=0.005) and ICU LOS was also two days longer (LOS 8 days [IQR 5-12] vs. 6 [IQR 5-10], p=0.04).
Figure 2:
90-day post-transplant survival stratified by obstructive ventilatory defect severity
Figure 3:
90-day post-transplant survival stratified by diffusion capacity for carbon monoxide severity
A sensitivity analysis comparing patients who didn’t have PFTs to those who had PFTs but did not have a severe OVD or severely reduced DLCO and to those with PFTs and a severe OVD and/or severely reduced DLCO. Outcomes were similar for patients in the first two groups, but those with severe abnormalities had a 163% increased risk of 90-day mortality (95% CI 1.06-6.55, p=0.03, Supplemental Figure 1).
Accuracy of Established Surgical Pulmonary Risk Scores
The ARISCAT Score placed all patients in the high-risk category based on the type and length of surgery, and consequently overestimated the risk of events (42.1% predicted vs. 30.1% in this study). However, it should be noted that pulmonary complications in this score include minor complications that we did not assess including pleural effusion, atelectasis, pneumothorax, and bronchospasm. The Gupta score overestimated the risk for patients with a lower risk (7% predicted vs. actual 3.9% in the study cohort) but underestimated the risk for higher risk patients (14% predicted vs. 45.6% in the study cohort and 23.6% predicted vs. 50.6% in the study cohort).
Discussion
The field of heart transplantation has evolved over the past decade with changes in bridging strategies with durable and temporary mechanical circulatory support, improved patient outcomes, and a new United States allocation system in 2018. During this period patient selection has also evolved with changes in ISHLT guidance on recipient age, BMI, and prior malignancy.3 Pre-transplant PFTs have been recommended, but there is a dearth of data on what constitutes a relative or absolute contraindication to heart transplantation.2 This study evaluated 462 heart transplant recipients with pre-transplant PFTs and demonstrated: 1) Patients with a severe OVD had a 267% increase in the adjusted odds of post-transplant pulmonary complications, but those with mild or moderate OVD did not; 2) Patients with a severely reduced DLCO had almost twice the adjusted odds of post-transplant pulmonary complications, while those with a mild or moderately reduced DLCO did not; 3) 90-day mortality was greater among patients with a severe OVD or a severely reduced DLCO; and 4) Severe RVD without evidence of parenchymal pulmonary disease was not associated with an increased odds of pulmonary complications or early mortality.
Pulmonary complications following cardiac surgery are relatively infrequent, with prior studies reporting an incidence of pneumonia ranging from 2.6-20%.12,13 The minority of these surgeries were heart transplant or LVAD implantation, however the risk of pneumonia was nearly 3-fold higher in that population.12 This is consistent with previously published data suggesting nearly one-third of heart transplant recipients will experience a pulmonary complication.14,15 In our study, 30% of patients experienced a post-transplant pulmonary complication, though certain groups were disproportionately impacted. Patients with a severe OVD had nearly four times the risk of reintubation and more than twice the risk of post-operative pneumonia. Patients with a severely reduced DLCO also represent a high-pulmonary risk group. However, we do not feel that a severe OVD or a severely reduced DLCO should be considered an absolute contraindication to transplant listing, as half of the patients with these PFT findings did not experience a post-transplant pulmonary complication. Rather, these objective pulmonary data should assist selection committees to make the best decision for each individual patient based on the presence or absence of additional comorbidities. For those patients, pre-operative pulmonary evaluation and optimization, early extubation, incentive spirometry, and early mobilization may be of value.
The Society of Thoracic Surgeons contribution to the American Board of Internal Medicine Choosing Wisely campaign noted that PFTs should not be routinely recommended prior to cardiac surgery for patients without pulmonary symptoms, though comment that PFTs can be helpful in determining the risk of cardiac surgery. There is a well-established association between clinically defined pre-existing pulmonary disease and worse outcomes after cardiac surgery, but few studies incorporating PFT data. Two retrospective studies identified severe OVD as a significant predictor of early mortality following CABG.16,17 This parallels our data where patients with a severe OVD had a 10.7% absolute increase in 90-day mortality and patients with a severely reduced DLCO experienced 6.9% absolute increase in 90-day mortality. While post-transplant pulmonary complications surely contributed to the increased risk of early death among these patients, the severe PFT abnormalities are also likely a surrogate for other comorbid conditions (measured or unmeasured) that play a part as well. Reassuringly, patients with moderate or less OVD, moderate or less reductions in DLCO, and any RVD had early post-transplant outcomes akin to those with normal PFTs.
The presence of an RVD did not portend worse pulmonary or overall outcomes following heart transplantation in this study. When assessing the etiology of an RVD, clinicians often consider intrinsic lung diseases (e.g., interstitial lung disease), extrinsic disorders (i.e., chest wall or pleural disease), or neuromuscular disorders. Advanced heart failure patients frequently have an extrinsic disorder resulting from prior cardiac surgery, marked cardiomegaly, or an LVAD that predisposes a patient to an RVD. It is important to emphasize that while a severe RVD was not associated with poor outcomes, none of the patients with a severe RVD had a history of a neuromuscular disease or radiographic evidence of a pulmonary parenchymal abnormality. Patients with an RVD attributable to those conditions require further study.
Limitations:
There are several limitations that need to be acknowledged. First is that this was a retrospective cohort study from a single transplant center. While we attempted to promote generalizability using standard PFT criteria, some of the findings may be impacted by institutional practices and may limit external validity. Next, lung volumes and total lung capacity were uncommonly measured pre-operatively and therefore RVD was based on a reduced FVC rather than a reduction in TLC. As a result, mixed restrictive and obstructive ventilatory patterns may be misclassified. Additionally, the transplant selection process may have already filtered many with severe PFT abnormalities, introducing the possibility of collider bias and creating modestly sized groups with severe PFT abnormalities (Supplemental Figure 2). Further, since individual predictors of adverse events were unable to be identified, a more comprehensive pulmonary assessment may better clinically phenotype candidates at the greatest risk. More, in 2020 the Respiratory Physiology lab our institution transitioned from the NHANES equation to the Global Lung Function Initiative equation to calculate the normality. This change likely resulted in minor changes to calculations of the percent predicted, but this happened consistently across the cohort at the same time and did not. We conducted a sensitivity analysis excluding data after 2020 (using only NHANES) and found the results similar (data not shown). Lastly, patients without available PFT data were excluded from the analysis and introduces the possibility of selection bias. The missing PFT data were due to patients who were admitted to the CCU and underwent an urgent inpatient transplant evaluation. At our institution, patients in the CCU are not able to be transported to our institution’s PFT laboratory (which is an avenue and two blocks away). Due to the volume of transfers from outside institutions and acute decompensations of patients from our own institution, many patients who underwent an inpatient evaluation and listing did not have PFT data. We elected to perform only complete-case analysis for two reasons. First, the missing data was secondary to patients who had an acute inpatient transplant evaluation due to a system-based issue that precluded performance of PFTs. Second, the focus of the study was to assess if pre-transplant PFTs had utility in the transplant evaluation and we felt the best way to answer the study question was with actual patient PFT data and not imputed data. As a result, these data are only applicable to patients who have had PFTs prior to hospitalization or those capable of undergoing PFTs prior to transplantation.
Conclusion:
Severe OVD or severely reduced DLCO on pre-transplant PFTs were associated with an increased risk of post-transplant pulmonary complications and early mortality. All other patients with PFT abnormalities, including those with a severe RVD, were able to be safely transplanted without an increased risk of post-transplant pulmonary complications or early mortality.
Supplementary Material
Funding:
KJC has been supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant K23 HL148528.
Disclosures:
VKT has been supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant K08 HL146964. PCC is recipient of a research grant from Abbott; he also serves as a consultant for the same company. GTS serves as a consultant for Abbott. N.U. serves on advisory boards for Leviticus and Livemetric/Cormetric; he also serves as a consultant for Abbott and Medtronic.
Non-standard abbreviations:
- DLCO
Diffusion capacity for carbon monoxide
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- OVD
Obstructive ventilatory defects
- PFT
Pulmonary function test
- RVD
Restrictive ventilatory defect
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Khush KK, Hsich E, Potena L, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult heart transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant 2021;40(10):1035–1049. (In eng). DOI: 10.1016/j.healun.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J Heart Lung Transplant 2006;25(9):1024–42. (In eng). DOI: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016;35(1):1–23. (In eng). DOI: 10.1016/j.healun.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agustí A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. Am J Respir Crit Care Med 2016;194(11):1319–1336. (In eng). DOI: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 5.Melenovsky V, Andersen MJ, Andress K, Reddy YN, Borlaug BA. Lung congestion in chronic heart failure: haemodynamic, clinical, and prognostic implications. Eur J Heart Fail 2015;17(11):1161–71. (In eng). DOI: 10.1002/ejhf.417. [DOI] [PubMed] [Google Scholar]
- 6.Güder G, Brenner S, Störk S, Hoes A, Rutten FH. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail 2014;16(12):1273–82. (In eng). DOI: 10.1002/ejhf.183. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren SW, Lowes BD, Lyden E, et al. Pulmonary Function Testing Pre-heart Transplant Predicts Posttransplant Survival. Transplant Direct 2021;7(10):e752. (In eng). DOI: 10.1097/txd.0000000000001177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD Guidelines: A Review of the 2018 GOLD Report. Mayo Clinic Proceedings 2018;93(10):1488–1502. DOI: 10.1016/j.mayocp.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–68. (In eng). DOI: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 10.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113(6):1338–50. (In eng). DOI: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 11.Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest 2011;140(5):1207–1215. (In eng). DOI: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- 12.Ailawadi G, Chang HL, O'Gara PT, et al. Pneumonia after cardiac surgery: Experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. The Journal of Thoracic and Cardiovascular Surgery 2017;153(6):1384–1391.e3. DOI: 10.1016/j.jtcvs.2016.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kollef MH, Sharpless L, Vlasnik J, Pasque C, Murphy D, Fraser VJ. The Impact of Nosocomial Infections on Patient Outcomes Following Cardiac Surgery. Chest 1997;112(3):666–675. DOI: 10.1378/chest.112.3.666. [DOI] [PubMed] [Google Scholar]
- 14.Lenner R, Padilla ML, Teirstein AS, Gass A, Schilero GJ. Pulmonary complications in cardiac transplant recipients. Chest 2001;120(2):508–13. (In eng). DOI: 10.1378/chest.120.2.508. [DOI] [PubMed] [Google Scholar]
- 15.Camkiran Firat A, Komurcu O, Zeyneloglu P, Turker M, Sezgin A, Pirat A. Early postoperative pulmonary complications after heart transplantation. Transplant Proc 2015;47(4):1214–6. (In eng). DOI: 10.1016/j.transproceed.2014.11.058. [DOI] [PubMed] [Google Scholar]
- 16.Saleh HZ, Mohan K, Shaw M, et al. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardiothorac Surg 2012;42(1):108–13; discussion 113. (In eng). DOI: 10.1093/ejcts/ezr271. [DOI] [PubMed] [Google Scholar]
- 17.O'Boyle F, Mediratta N, Chalmers J, et al. Long-term survival of patients with pulmonary disease undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 2013;43(4):697–703. (In eng). DOI: 10.1093/ejcts/ezs454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.