Abstract
The integration of external information with the internal state of the body is central to the survival of virtually every multicellular organism. Yet, a complete picture of the mechanisms that govern this process is lacking. In this article, we synthesize evidence demonstrating that astrocytes sense the momentary arousal state – through neuromodulator release – as well as the sensory inputs – through local synaptic activity – and respond to them with changes in Ca2+ signaling. We hypothesize that astrocytes integrate sensory signals with the internal state, and that this process is necessary for securing optimal behavior. Finally, we argue that dysfunctional astrocytic Ca2+ signaling could be an underlying factor in disorders characterized by disrupted sensory processing.
Keywords: Glial cells, calcium signaling, internal state, sensory processing, neuromodulation
How are external signals and internal state information merged in the brain?
The brain is constantly integrating signals conveying information about the inner states of the body with external sensory inputs from the outside world to produce appropriate, context-dependent behaviors [1–4]. This process ensures both efficient behavioral shifts when conditions change and selecting relevant information for memory consolidation to guide future behavior.
The current model for this integration process implicates that sensory information is encoded by fast and spatially constricted neuronal synaptic transmission, while internal state information is relayed brain-wide by slowly acting neuromodulators. In this Opinion article, we argue that astrocytes, an often-overlooked type of brain cell in systems neuroscience, also contribute to the integration of these two information sources, adding a new dimension to our understanding of how external and internal information is merged in the brain.
We first provide a short overview of the nature and characteristics of astrocytic calcium (Ca2+) signals. Second, we present evidence, mainly from rodent models demonstrating that astrocytes respond to both arousal and sensory inputs with changes in their Ca2+ signaling. Next, we formulate a general hypothesis about the integration of arousal and sensory information in astrocytes. Finally, we discuss this hypothesis in light of recent data and outline outstanding questions and future experiments which can extend our knowledge about the role of astrocytes in cognitive functions.
Astrocytic Ca2+ signals
Astrocytic Ca2+ signaling has been characterized in a wide range of species and preparations, including human tissue [5], ferrets [6], rodents [7–15], zebrafish [16], and flies [17,18]. Surges in intracellular Ca2+ concentration are predominantly caused by Ca2+ release from internal stores, triggered by G-protein-coupled receptor-mediated activation of the inositol triphosphate (IP3) pathway. However, other IP3-independent mechanisms such as Ca2+-permeable ion channels, exchangers, and transporters are likely also involved (reviewed in [19,20]). The spatiotemporal organization of Ca2+ signals in astrocytes is diverse (reviewed in [20–22]): Spatially, events range from local responses in fine astrocytic processes to responses extending throughout the cell, with the latter often occurring in many astrocytes in parallel [8–10,23] or spreading from one astrocyte to another [12,24]. Similarly, the time course of responses varies notably, from hundreds of milliseconds to minutes. Unfortunately, understanding of the underlying mechanisms and functional role of this diversity is still very limited. A growing body of literature suggests that astrocytic Ca2+ signaling regulates neuronal activity and behavior, and the phylogenetic conservation of Ca2+ signaling in astrocytes suggests that this signaling has important functions. However, the question of when and why astrocytic Ca2+ signals are evoked remains to be answered. Interestingly, astrocytes express receptors for both neuromodulators and neurotransmitters [25], indicating they are able to sense changes in arousal state as well as sensory input.
Astrocytes signal arousal state
The ability of animals to interact with external events highly depends on their body’s internal state, including their momentary arousal level [1–4] (Box 1).
Box 1: The internal state of arousal.
In psychology and related fields, arousal is defined as a state of physiological activation associated with sensory stimulation or a state of excitement linked to an emotion. While arousal is sometimes conceptualized as a distinct state, in reality, arousal comprises a spectrum ranging from states of lower to higher arousal. Arousal level is indexed by biological measures such as pupil diameter, heart and breathing rate, and shifts in brain activity patterns. Essential regulators of arousal in the nervous system are neuromodulators, such as noradrenaline (NA) and acetylcholine (ACh), which are increased in the aroused brain [1–4]. An inherent challenge when evaluating sensory input-related astrocytic Ca2+ responses in awake-behaving animals is, therefore, the potential influence of concomitant arousal. In this article, as a simplification, we differentiate between non-arousing sensory stimulation, such as the display of visual gratings and subtle whisker stimulation, and arousal-inducing salient sensory stimulation, such as startle-related air-puff stimulation and forced locomotion. In future experiments, monitoring arousal fluctuations during sensory experiences are essential and will allow researchers to disentangle the contribution of arousal and sensation and provide important new insight into the interaction between the two.
In mammals, periods of heightened arousal correlate with increased noradrenaline (NA) and acetylcholine (ACh) signaling [26–28]. Recent in vivo experiments in awake mice have reignited research into NA-mediated astrocytic Ca2+ signaling [8,9], and there is strong evidence now supporting the notion that astrocytes reflect arousal changes by NA-driven global Ca2+ signaling [19–22,29], confirming early ex vivo data [30–32] and further evidenced by the cellular expression pattern of noradrenergic receptors [33,34]. Indeed, electrical or optogenetic stimulation of NA-producing locus coeruleus neurons leads to a sharp increase in astrocytic Ca2+ in the somatosensory cortex of awake and anesthetized mice [23,35].
Astrocytic Ca2+ signaling has been investigated in both externally triggered (e.g., air-puff whisker stimulation, tail stimulation, or forced locomotion) and internally generated (e.g., spontaneous locomotion bouts) arousal-increasing contexts. Concretely, salient sensory stimulation [7–9,36–38] and self-initiated locomotion bouts [10,11,39,40] elicit NA-mediated Ca2+ signaling in large cortical astrocytic networks, through activation of the alpha1 noradrenergic receptor. Although most studies on NA and astrocytic signaling have been done in rodents, there is evidence from zebrafish and drosophila to support that this type of signaling is evolutionarily conserved [16,18].
The close association between arousal and astrocytic signaling is corroborated by experiments showing that astrocytic Ca2+ signaling is notably reduced during natural sleep and anesthesia compared to wakefulness [41–45]. Interestingly, large synchronized astrocytic Ca2+ events, extending across the soma and processes, are consistently associated with awakening from sleep [41–43], and these Ca2+ events are most likely induced by NA release [46]. While most research on astrocytes and arousal has focused on NA, another important regulator of arousal, ACh, can also elicit astrocytic Ca2+ signaling [47,48]. Collectively, the literature strongly supports the idea that astrocytes respond to state-related neuromodulators and that astrocytic Ca2+ signaling is a general correlate of arousal.
Astrocytes respond to sensory input
Mounting evidence indicates that astrocytes can respond to sensory inputs in vivo [6,9,12–15,24,39,48–51] and local synaptic transmission ex vivo [13–15,20,50,52–55]. Curiously, astrocytic responses to sensory stimulation in vivo appear to depend on the arousal state of animals. For instance, astrocytes in the visual cortex of mice exhibit robust visual-evoked Ca2+ signals during locomotion (i.e., increased arousal) [9,37,39]. Interestingly, these Ca2+ signals are larger than signals triggered by arousal alone, suggesting an interaction between sensory inputs and arousal in astrocytes [9, 34]. By contrast, during periods of quiescence, visual-evoked Ca2+ is weak or non-existent [9,37,39]. These findings suggest that high arousal is necessary for sensory-activated astrocytes, yet sensory inputs still trigger astrocytic Ca2+ signals during anesthesia, a state characterized by low arousal [6,12–14,24,45–47]. Furthermore, after pharmacologically blocking arousal-related astrocytic Ca2+ signals in awake mice, visual input-driven Ca2+ transients in the soma [51] and whisker stimulation-induced Ca2+ events in the processes [15] are still observed. This aligns with the idea that local sensory-evoked synaptic activity is adequate to drive astrocytic Ca2+ signaling independent of the arousal state. Together, these results suggest that astrocytic signaling reflects both sensory signals and arousal levels.
Astrocytes may integrate arousal state and sensory information
We hypothesize that astrocytes integrate external sensory signals (carried as synaptic activity in neuronal circuits) and internal arousal state information (relayed by neuromodulators such as NA and ACh) through differential Ca2+ signaling (Figure 1). We postulate that arousal-related signaling shifts astrocytes into a distinct activity mode, which increases the probability of exhibiting Ca2+ signals in response to sensory inputs. Hence, if the sensory input-driven synaptic activity is insufficient to elicit measurable Ca2+ signaling alone, neuromodulators such as NA and ACh can push astrocytes above a theorized activation threshold. Conversely, if sensory input is strong enough, it can trigger astrocytic Ca2+ signaling without notable arousal. Intriguingly, our proposal is in congruence with recent preliminary work showing that the probability that astrocytic Ca2+ signals originating in the processes will propagate to the soma increases during states of higher arousal [56], suggesting that arousal primes astrocytes to be more responsive to sensory input.
Figure 1. Astrocytic Ca2+ signaling as an integrator of external and internal information.
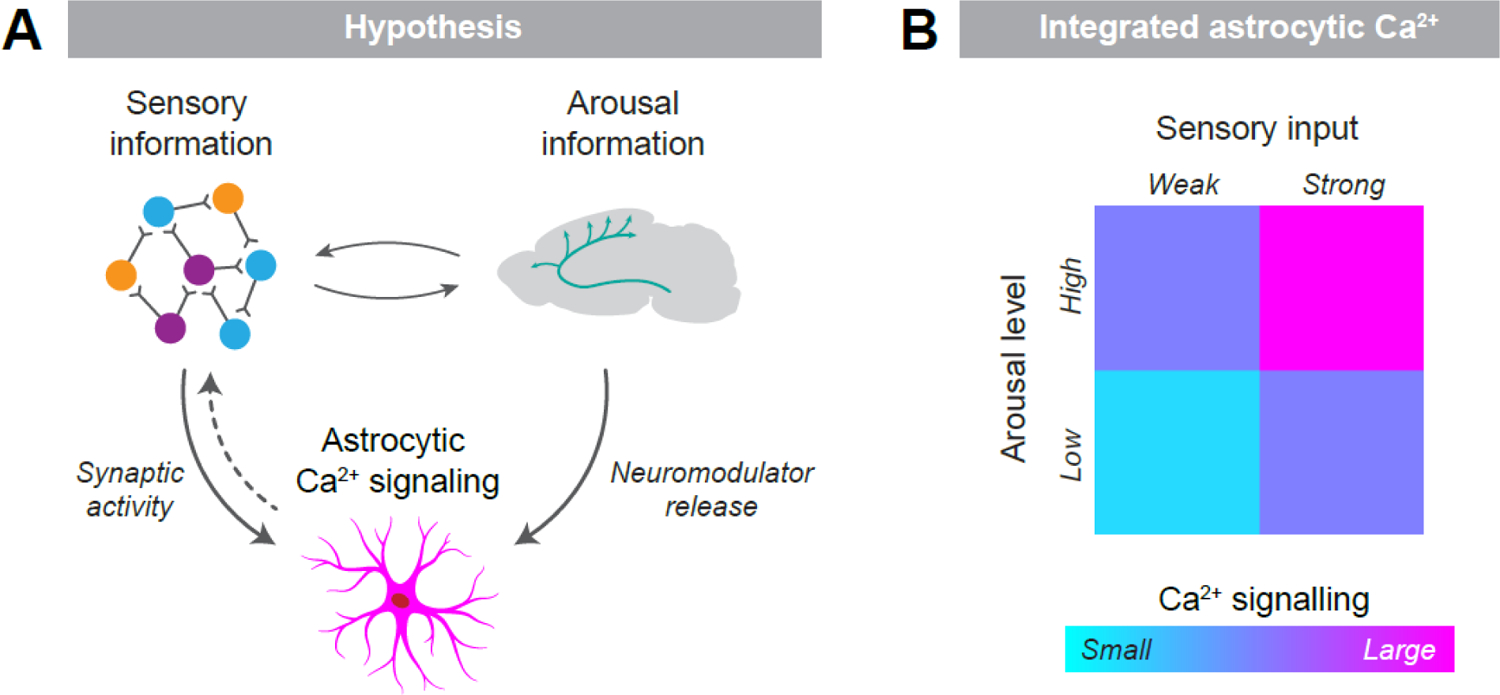
(A) Visualization of the proposed concept: astrocytes integrate sensory information, conveyed by synaptic activity in neuronal circuits, and arousal information, conveyed by neuromodulator release, by means of differential Ca2+ signaling. Salient sensory stimulation can increase arousal levels, and in turn, arousal-related neuromodulators affect sensory processing in neuronal circuits. While not discussed in detail here, the signaling events downstream of astrocytic Ca2+ that facilitate interactions with neuronal activity and modulate sensory experiences remain elusive (dotted arrow; see Outstanding questions). (B) Heat map illustrating the anticipated magnitude of astrocytic Ca2+ signaling due to the integration of arousal level and sensory input.
While the precise mechanism of the proposed integration is currently unresolved, it is tempting to speculate that global arousal signaling is primarily mediated by Ca2+ release from internal stores through the IP3 pathway, and local sensory signaling is initially mediated by IP3-independent Ca2+ transients, such as via activation of Ca2+-permeable ion channels. It is plausible that these two different signaling pathways converge; arousal-related intracellular Ca2+ released from internal stores can increase the conductance of Ca2+-permeable ion channels and thereby amplify sensory-related signaling, while local Ca2+ transients mediated by ion channels can stimulate Ca2+ release from internal stores through Ca2+-induced Ca2+ release [57]. Although this mechanistic explanation is likely overly simplified, it captures the core elements of our proposed hypothesis and thus seems a reasonable starting point for guiding future experiments aiming to decipher the subcellular mechanisms of the integrated astrocytic Ca2+ signal.
Finally, we propose that downstream of the integrated Ca2+ signal, astrocytes can feed back to neural circuits to modulate sensory processing and, ultimately, behavior. Astrocyte-to-neuron signaling remains largely enigmatic, but we propose that it can occur as a result of direct gliotransmitter release (such as ATP [53]) and/or through indirect regulation of glutamate and GABA uptake [58,59] or extracellular potassium ion levels [60]. Through these mechanisms, astrocytes can both enhance and dampen synaptic activity, and future experiments are needed to determine if and when this occurs (see Outstanding Questions).
Outstanding Questions.
What are the specific molecular and cellular events downstream of astrocytic Ca2+ surges that facilitate interaction with neural circuits and regulation of sensory processing? While some clues to the mechanisms exist, a full and definitive picture is currently lacking, and would require a multitude of carefully planned in vitro, ex vivo and in vivo studies.
How does arousal affect astrocytic Ca2+ signaling in subcortical sensory areas (e.g., the lateral geniculate nucleus or superior colliculus)? How do other internal states (e.g., hunger, anxiety, aggression) induce astrocytic Ca2+ signaling to affect sensory input integration? What neuromodulators and hormones might mediate such effects? Carefully designed experiments in freely moving animals will allow addressing these questions. Advances in deep-tissue imaging, genetically encoded biosensors, and miniaturized microscopy are bound to offer insights into these processes.
What is the relation between the temporal dynamics of the astrocytic Ca2+ signal and the temporal window of sensory information integration? Systematic testing of the temporal organization of sensory inputs and the transitions between internal states is needed to tackle this question.
How does attenuation, or facilitation, of astrocytic Ca2+ signaling affect sensory discrimination performance in the healthy and diseased brain? How do changes in arousal regulate this? Leveraging psychophysical experiments with careful titration of sensory information salience and real-time monitoring of neuromodulation can offer insights into these unknowns.
Predictions and evidence for the behavioral role of astrocytic Ca2+ signaling
The most direct support for our hypothesis that astrocyte Ca2+ serves as a molecular substrate for integrating arousal and sensory information comes from studies manipulating astrocytic Ca2+ signaling in behaving animals. Here, we interpret the results of such studies through the lens of our hypothesis by discussing how they align with specific predictions.
Prediction 1:
Attenuating astrocytic Ca2+ signaling will result in impaired integration of information conveyed by sensory-evoked neuronal and arousal states (i.e., necessity).
A novel tool that allows astrocytic Ca2+ signaling manipulation is iβARK, an inhibitory peptide that attenuates Gq G-protein-coupled receptor-dependent Ca2+ elevations [36]. Interestingly, mice with brain-wide expression of iβARK in astrocytes exhibit deficient sensory adaptation to repeated obnoxious stimuli [36]. In this experimental context, sensory input remains unchanged, while the arousal level of animals likely diminishes as they habituate to repeated stimulation [61,62]. Thus, the deficient behavioral adaptation of iβARK-expressing mice suggests that astrocytic Ca2+ signaling secures a behavioral response that matches the subjects’ arousal state with incoming sensory information. Furthermore, attenuation of Ca2+ signaling led to impaired memory performance in tasks that relies on consolidating sensory information [36]. Specifically, this deficit was obvious in a subtle object exploration task (i.e., object-location recognition), while performance in a task with salient novelty (i.e., object recognition) remained unaffected. Similarly, although using different tests, memory processes are affected in another mouse model with reduced Ca2+ signals in astrocytes: namely mice lacking the IP3 type 2 receptor (IP3R2 KO) [7,63]. These mice exhibit subtle memory impairments for newly acquired sensory information and deficient long-term plasticity in the hippocampus and somatosensory cortex [64–69].
Notably, the findings from iβARK and IP3R2 KO manipulations are in congruence with results from mice expressing an artificial Ca2+ pump that dampens Ca2+ signaling in both the soma and processes of astrocytes [59,70,71]. Such manipulation in the striatum leads to reduced neuronal excitability and excessive self-grooming [59]. Interestingly, NA-deficient mice also exhibit increased self-grooming [72]. A parsimonious interpretation of these results is that astrocytic Ca2+ signaling in response to arousal, signaled by NA release, and accumulation of sensory information from the periphery (e.g., orofacial area) is important for the termination of self-grooming [73]. Similar evidence comes from zebrafish, where astrocytic Ca2+ signaling increases after repeated bouts of futile swimming to control when an animal ultimately gives up [16]. These studies suggest that normal astrocytic Ca2+ signaling is necessary to ensure context-dependent sensory-guided behaviors.
It is important to note that none of the currently available astrocytic Ca2+ loss-of-function tools permit specific attenuation of either arousal-related or synaptic activity-induced astrocytic Ca2+ transients. Therefore, there are no straightforward means to currently dissociate between the two sources of Ca2+ signaling in astrocytes and establish a causal description of their roles.
Prediction 2:
Artificial induction of astrocytic Ca2+ signaling that resembles naturally occurring dynamics will facilitate sensory information accumulation, leading to improved behavioral performance (i.e., sufficiency).
Studies have shown that artificial enhancement of astrocytic Ca2+ signaling during sensory-guided learning promotes memory formation and retention [74–76]. These findings support the prediction that elevating Ca2+ signaling will prime astrocytes to sense local synaptic activity and enhance sensory information accumulation, causing improved behavioral performance. However, an important limitation of these studies is the inherently artificial nature of the evoked Ca2+ signal. The highly complex spatiotemporal dynamics of astrocytic Ca2+ signals cannot currently be replicated with optogenetic or chemogenetic manipulations [77]. Finally, while currently available data appears to confirm specific predictions derived from our hypothesis, the multifaceted role of astrocytic Ca2+ signaling is undoubtedly more complex than outlined here, and many open questions remain to be addressed (see Outstanding Questions).
Astrocytic Ca2+ signaling and pathophysiological conditions
Abnormal astrocytic Ca2+ signaling appears to be a pathophysiology component of various conditions, including disorders associated with disrupted sensory processing and arousal [78,79]. Sensory hypersensitivity and hyperarousal are hallmarks of autism spectrum disorders, Fragile X, and Rett syndromes [80–82]. Conversely, sensory hyposensitivity and hypovigilance are associated with major depression and dementia [83–86]. Interestingly, astrocytes derived from individuals with autism spectrum disorders, Rett or Fragile X syndrome exhibit exacerbated Ca2+ signaling ex vivo [87–89], and mice deficient in astrocytic Ca2+ signaling exhibit long-range functional connectivity changes in vivo consistent with those seen in patients with major depressive disorder [90]. Moreover, recent reports suggest that astrocytic Ca2+ signaling is less responsive to NA and sensory inputs in two different models of Alzheimer’s disease [91,92]. Interestingly, some antidepressant medications, such as ketamine, could act through astrocytes [93]. It is, therefore, tempting to speculate that distorted astrocytic Ca2+ signaling is a key component of the cellular pathophysiology underlying sensory and arousal-related deficits in various brain disorders, and astrocytes could be a valid treatment target.
Concluding remarks and future perspectives
Considering astrocytes as integral players in cognitive function adds a new layer to the mechanistic understanding of information processing in the brain. While neurons can deliver fast and precise information, and neuromodulators provide long-lasting and long-ranging signals to tune brain activity, astrocytic Ca2+ signaling is somewhere in the middle: slow yet sufficiently fast for regulating behavior, diffused yet defined, and gradual yet punctual. It is these properties that enable astrocytes to partake in the integration of external sensory information, carried as synaptic activity in sensory neuronal circuits, with momentary internal arousal state relayed by neuromodulators. According to our hypothesis, astrocytic Ca2+ signaling is central in fine-tuning the dynamic range of sensory processing: permitting the integration of subtle sensory inputs during aroused states while also responding to strong sensory inputs in the absence of notable arousal. This optimum sensory dynamic range facilitates appropriate behavioral responses and drives memory formation.
Astrocytes are increasingly appreciated as a vastly heterogeneous cell-type population between and within brain areas [94–97]. Astrocytic Ca2+ signaling likely depends on the specific astrocyte subtype and is adapted to respond to local neuronal activity patterns and neuromodulation. While most studies in this context have focused on astrocytic Ca2+ signaling in the neocortex, technological progress now allows for the investigation of astrocyte activity in subcortical areas, such as the hippocampus [98,99] and the striatum [59]. Furthermore, microglia, the resident immune cells of the central nervous system, interact with astrocytes and neurons [100] and can regulate neuronal activity in an arousal-dependent manner [101,102]. Future work will undoubtedly widen current knowledge of the diversity and function of state-dependent glial cell signaling.
There is growing recognition that tackling some of the fundamental questions neuroscience is grappling with requires large datasets collected using advanced tools in a streamlined manner. In view of this perspective, there has been a move towards centralized brain observatories and large collaborations (e.g., the Allen Brain Observatoryi, the International Brain Laboratoryii, and the BRAIN Initiativeiii) [103]. While glial cells are yet to be part of the focus of such large-scale initiatives, placing astrocytes and other glial cells within their remit, would allow uncovering these cells’ roles in brain function and behavior. Crucially, well-defined hypotheses – for instance the proposed roles of astrocytic Ca2+ dynamics in controlling behavior, as presented here – are essential to make the most out of the new age of neuroscience research.
Much remains to be learned about how astrocytic Ca2+ signaling is regulated by, and in turn influences, neuronal activity. However, two things are certain: one, communication between neurons and astrocytes is vastly more complicated than previously thought, and two, continuing technological progress is allowing detailed dissection of this communication. It is our hope that future work, guided by specific hypotheses, will significantly expand current understanding of how astrocytes regulate internal state-dependent sensory processing.
Highlights.
A growing body of literature suggests that astrocytic Ca2+ signaling regulates various aspects of neuronal activity and behavior.
We propose that astrocytes are in a unique position to integrate internal and external signals via astrocytic Ca2+ signaling.
Astrocytes respond to internal arousal signals by detecting neuromodulator release such as noradrenaline and acetylcholine and elevating their intracellular Ca2+ concentration.
Astrocytes also respond to external sensory inputs by sensing local synaptic activity that leads to Ca2+ transients.
Manipulation of astrocytic Ca2+ signaling results in altered acquisition of sensory information.
We hypothesize that arousal can amplify weak sensory input at the level of astrocytes.
Acknowledgments
We want to thank members of the Center for Translational Neuromedicine and the Del Monte Institute for Neuroscience for discussions on the ideas presented in this manuscript. This work was supported by the National Institutes of Health Grant K01NS110981 to N.A.S., the National Science Foundation NSF1926781 to N.A.S., European Union under Marie Skłodowska-Curie Fellowship (ANCoDy, #101064009) to A.A., an ONO Rising Star Fellowship to A.A., a Lundbeck Foundation, Experiment Grant to R.N.R. (R370-2021-764), and the Novo Nordisk Foundation (NNF20OC0066419) to C.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The author declares no competing interests.
References
- 1.Flavell SW et al. (2022) The emergence and influence of internal states. Neuron 110,2545–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGinley MJ et al. (2015) Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 87, 1143–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris KD and Thiele A (2011) Cortical state and attention. Nat Rev Neurosci 12, 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH and Dan Y (2012) Neuromodulation of Brain States. Neuron 76, 109–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarrete M et al. (2013) Astrocyte Calcium Signal and Gliotransmission in Human Brain Tissue. Cereb Cortex 23, 1240–1246 [DOI] [PubMed] [Google Scholar]
- 6.Schummers J et al. (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan R et al. (2015) Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 18, 708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding F et al. (2013) α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paukert M et al. (2014) Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82, 1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn A et al. (2009) Motor Behavior Activates Bergmann Glial Networks. Neuron 62, 400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dombeck DA et al. (2007) Imaging Large-Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice. Neuron 56, 43–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X et al. (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neurosci 9, 816–823 [DOI] [PubMed] [Google Scholar]
- 13.Lines J et al. (2020) Astrocytes modulate sensory-evoked neuronal network activity. Nat Commun 11, 3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsu Y et al. (2015) Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 18, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stobart JL et al. (2018) Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 98, 726–735 [DOI] [PubMed] [Google Scholar]
- 16.Mu Y et al. (2019) Glia Accumulate Evidence that Actions Are Futile and Suppress Unsuccessful Behavior. Cell 178, 27–43 [DOI] [PubMed] [Google Scholar]
- 17.Blum ID et al. (2020) Astroglial Calcium Signaling Encodes Sleep Need in Drosophila. Curr Biol 31, 150–162.e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Z et al. (2016) Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkhratsky A and Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98,239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semyanov A et al. (2020) Making sense of astrocytic calcium signals — from acquisition to interpretation. Nat Rev Neurosci 21, 551–564 [DOI] [PubMed] [Google Scholar]
- 21.Bazargani N and Attwell D (2016) Astrocyte calcium signaling: the third wave. Nature Neurosci 2016 19:2 19, 182–189 [DOI] [PubMed] [Google Scholar]
- 22.Shigetomi E et al. (2016) Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26, 300–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekar LK et al. (2008) Locus Coeruleus α-Adrenergic–Mediated Activation of Cortical Astrocytes In Vivo. Cerebral Cortex 18, 2789–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gee JM et al. (2014) Imaging Activity in Neurons and Glia with a Polr2a-Based and Cre-Dependent GCaMP5G-IRES-tdTomato Reporter Mouse. Neuron 83, 1058–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y et al. (2014) An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci 34, 11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reimer J et al. (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 7, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohani S et al. (2022) Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nature Neurosci 25, 1706–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinck M et al. (2015) Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron 86, 740–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kjaerby C et al. (2017) Does Global Astrocytic Calcium Signaling Participate in Awake Brain State Transitions and Neuronal Circuit Function? Neurochem Res 42, 1810–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy S and MacVicar BA (1995) Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 15, 5535–5550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Y and McCarthy KD (1997) Responses of Bergmann glia and granule neurons in situ to N-methyl-D-aspartate, norepinephrine, and high potassium. J Neurochem 68, 2405–2411 [DOI] [PubMed] [Google Scholar]
- 32.Salm AK and McCarthy KD (1990) Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia 3, 529–538 [DOI] [PubMed] [Google Scholar]
- 33.Hertz L et al. (2010) Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int 57, 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao Y and Sutin J (1992) Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia 6, 108–117 [DOI] [PubMed] [Google Scholar]
- 35.Oe Y et al. (2020) Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nat Commun 11, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagai J et al. (2021) Specific and behaviorally consequential astrocyte Gq GPCR signaling attenuation in vivo with iβARK. Neuron 109, 2256–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray SR et al. (2021) Noradrenergic terminal short-term potentiation enables modality-selective integration of sensory input and vigilance state. Sci Adv 7, eabk1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L et al. (2020) Ethanol abolishes vigilance-dependent astroglia network activation in mice by inhibiting norepinephrine release. Nat Commun 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slezak M et al. (2019) Distinct Mechanisms for Visual and Motor-Related Astrocyte Responses in Mouse Visual Cortex. Curr Biol 29, 3120–3127.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin H et al. (2020) Monitoring Astrocytic Ca2+ Activity in Freely Behaving Mice. Front Cell Neurosci 14, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingiosi AM et al. (2020) A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Current Biology DOI: 10.1016/j.cub.2020.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bojarskaite L et al. (2020) Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat Commun 11, 3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunematsu T et al. (2021) Region-specific and state-dependent astrocyte Ca2+ dynamics during the sleep-wake cycle in mice. Journal Neurosci 41, 5440–5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidyanathan T. v et al. (2021) Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. Elife 10, e63329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thrane AS et al. (2012) General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. PNAS 109, 18974–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kjaerby C et al. (2022) Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nature Neurosci 25, 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takata N et al. (2011) Astrocyte Calcium Signaling Transforms Cholinergic Modulation to Cortical Plasticity In Vivo. Journal of Neuroscience 31, 18155–18165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen N et al. (2012) Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. PNAS 109, E2832–E2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winship IR et al. (2007) Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J Neurosci 27, 6268–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanemaru K et al. (2014) In Vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca2+ indicator. Cell Rep 8, 311–318 [DOI] [PubMed] [Google Scholar]
- 51.Sonoda K et al. (2018) Astrocytes in the mouse visual cortex reliably respond to visual stimulation. Biochem Biophys Res Commun 505, 1216–1222 [DOI] [PubMed] [Google Scholar]
- 52.Deemyad T et al. (2018) Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat Commun 9, 4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsen EMM et al. (2021) Spinal astroglial cannabinoid receptors control pathological tremor. Nat Neurosci 24, 658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariotti L et al. (2018) Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat Commun 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panatier A et al. (2011) Astrocytes Are Endogenous Regulators of Basal Transmission at Central Synapses. Cell 146, 785–798 [DOI] [PubMed] [Google Scholar]
- 56.Rupprecht P et al. (2022) Centripetal integration of past events by hippocampal astrocytes. bioRxiv DOI: 10.1101/2022.08.16.504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge MJ et al. (2000) The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 58.Armbruster M et al. (2022) Neuronal activity drives pathway-specific depolarization of peripheral astrocyte processes. Nat Neurosci 25, 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X et al. (2018) Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang F et al. (2012) Astrocytes Modulate Neural Network Activity by Ca2+-Dependent Uptake of Extracellular K+. Sci Signal 5, ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terrazzino S et al. (2002) Effect of Development of Habituation to Restraint Stress on Hypothalamic Noradrenaline Release and Adrenocorticotropin Secretion. J Neurochem 65, 263–267 [DOI] [PubMed] [Google Scholar]
- 62.Breton-Provencher V et al. (2022) Spatiotemporal dynamics of noradrenaline during learned behaviour. Nature 606, 732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petravicz J et al. (2008) Loss of IP3 Receptor-Dependent Ca2+ Increases in Hippocampal Astrocytes Does Not Affect Baseline CA1 Pyramidal Neuron Synaptic Activity. J Neurosci 28, 4967–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherwood MW et al. (2017) Astrocytic IP 3 Rs: Contribution to Ca 2+ signalling and hippocampal LTP. Glia 65, 502–513 [DOI] [PubMed] [Google Scholar]
- 65.Liu J-H et al. (2022) Distinct roles of astroglia and neurons in synaptic plasticity and memory. Mol Psychiatry 27, 873–885 [DOI] [PubMed] [Google Scholar]
- 66.Pinto-Duarte A et al. (2019) Impairments in remote memory caused by the lack of Type 2 IP 3 receptors. Glia 67. 1976–198 [DOI] [PubMed] [Google Scholar]
- 67.Butcher JB et al. (2022) A requirement for astrocyte IP3R2 signaling for whisker experience-dependent depression and homeostatic upregulation in the mouse barrel cortex. Front Cell Neurosci 16, 905285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agulhon C et al. (2010) Hippocampal Short- and Long-Term Plasticity Are Not Modulated by Astrocyte Ca 2+ Signaling. Science 327, 1250–1254 [DOI] [PubMed] [Google Scholar]
- 69.Petravicz J et al. (2014) Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X et al. (2021) Local and CNS-Wide Astrocyte Intracellular Calcium Signaling Attenuation In Vivo with CalEx flox Mice. J Neurosci 41, 4556–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohmbach K et al. (2022) An astrocytic signaling loop for frequency-dependent control of dendritic integration and spatial learning. Nat Commun 13, 7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lustberg DJ et al. (2022) Norepinephrine and dopamine contribute to distinct repetitive behaviors induced by novel odorant stress in male and female mice. Horm Behav 144, 105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Z et al. (2022) A brain-to-spinal sensorimotor loop for repetitive self-grooming. Neuron 110, 874–890.e7 [DOI] [PubMed] [Google Scholar]
- 74.Adamsky A et al. (2018) Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71.e14 [DOI] [PubMed] [Google Scholar]
- 75.Kol A et al. (2020) Astrocytes contribute to remote memory formation by modulating hippocampal–cortical communication during learning. Nat Neurosci 23, 1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwai Y et al. (2021) Transient Astrocytic Gq Signaling Underlies Remote Memory Enhancement. Front Neural Circuits 15, 658343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu X et al. (2020) Improved tools to study astrocytes. Nature Reviews Neurosci 21, 121–138 [DOI] [PubMed] [Google Scholar]
- 78.Lee H-G et al. (2022) Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov 21, 339–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brandebura AN et al. (2022) Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat Rev Neurosci 24, 23–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drobnyk W et al. (2019) Sensory Integration and Functional Reaching in Children With Rett Syndrome/Rett-Related Disorders. Clin Med Insights Pediatr 13, 117955651987195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Balasco L et al. (2020) Sensory Abnormalities in Autism Spectrum Disorders: A Focus on the Tactile Domain, From Genetic Mouse Models to the Clinic. Front Psychiatry 10, 1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sinclair D et al. (2017) Sensory processing in autism spectrum disorders and Fragile X syndrome—From the clinic to animal models. Neurosci Biobehav Rev 76, 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schiller CE et al. (2013) Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J Affect Disord 151, 756–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Litwińska-Bołtuć M et al. (2021) Clinical effectiveness of the electrodermal orienting reactivity test for evaluating relapse and recurrence risk in patients hospitalized for depression. BMC Psychiatry 21, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berardi AM et al. (2005) Sustained Attention in Mild Alzheimer’s Disease. Dev Neuropsychol 28, 507–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Politoff AL et al. (1995) Severity of Dementia Correlates with Loss of Broad-Band Visual Cortical Responses. Dement Geriatr Cogn Disord 6, 169–173 [DOI] [PubMed] [Google Scholar]
- 87.Allen M et al. (2022) Astrocytes derived from ASD individuals alter behavior and destabilize neuronal activity through aberrant Ca2+ signaling. Mol Psychiatry 27, 2470–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong Q et al. (2018) Mechanism and consequence of abnormal calcium homeostasis in Rett syndrome astrocytes. Elife 7, e33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren B et al. (2023) Dysregulated cholesterol metabolism, aberrant excitability and altered cell cycle of astrocytes in fragile X syndrome. Glia DOI: 10.1002/glia.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J et al. (2022) Astrocyte dysfunction drives abnormal resting-state functional connectivity in depression. Sci Adv 8, eabo2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lines J et al. (2022) Astrocyte-neuronal network interplay is disrupted in Alzheimer’s disease mice. Glia 70, 368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Åbjørsbråten KS et al. (2022) Impaired astrocytic Ca2+ signaling in awake-behaving Alzheimer’s disease transgenic mice. Elife 11, e75055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marc D et al. (2022) Astroglial mediation of fast-acting antidepressant effect in zebrafish. bioRxiv DOI: 10.1101/2022.12.29.522099v1 [DOI] [Google Scholar]
- 94.Khakh BS and Deneen B (2019) The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42, 187–207 [DOI] [PubMed] [Google Scholar]
- 95.ben Haim L and Rowitch DH (2016) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18, 31–41 [DOI] [PubMed] [Google Scholar]
- 96.Chai H et al. (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95, 531–549.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.John Lin CC et al. (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curreli S et al. (2022) Complementary encoding of spatial information in hippocampal astrocytes. PLoS Biol 20, e3001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doron A et al. (2022) Hippocampal astrocytes encode reward location. Nature 609, 772–778 [DOI] [PubMed] [Google Scholar]
- 100.Schafer DP et al. (2013) The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia 61, 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stowell RD et al. (2019) Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci 22, 1782–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu YU et al. (2019) Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci 22, 1771–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koch C et al. (2022) Next-generation brain observatories. Neuron 110, 3661–3666 [DOI] [PubMed] [Google Scholar]


