Abstract
Thoracoabdominal normothermic regional perfusion (TA-NRP) has recently begun being utilized in the United States for recovery of cardiothoracic allografts from some donors after circulatory death (DCD), but data on lungs recovered in this method is limited to case reports. We conducted a national retrospective review of lung transplants from DCD donors recovered using TA-NRP. Of the 434 total DCD lung transplants performed between January 2020 and March 2022, 17 were recovered using TA-NRP. Compared to direct recovery DCD transplants, recipients of TA-NRP DCD transplants had lower likelihood of ventilation >48 hours (23.5% vs 51.3%, p = 0.027) and similar likelihood of predischarge acute rejection, requirement for extracorporeal membrane oxygenation at 72 hours, hospital lengths of stay, and survival at 30, 60, and 90 days post-transplant. These early data suggest that DCD lung recovery using TA-NRP might be a safe way to further expand the donor pool and warrant further study.
Keywords: lung transplantation, outcomes, donation after circulatory death, normothermic regional perfusion
Donation after circulatory death (DCD) has been increasingly used to address the organ shortage and has demonstrated excellent outcomes in lung transplants.1,2 For cardiac transplantation, however, DCD has not been as widely adopted given the ischemic insult to myocardial tissue.3 A novel strategy involving thoracoabdominal normothermic regional perfusion (TA-NRP) for recovery of cardiothoracic allografts has been increasingly utilized in the United States, primarily driven by cardiac recovery teams to increase the availability of cardiac allografts from DCD donors. TA-NRP involves in situ reperfusion of thoracic and abdominal organs via extracorporeal membrane oxygenation prior to organ retrieval. Following declaration of death and a 2 to 5 min standoff time, chest entry is performed, the right atrium and ascending aorta are cannulated, and extracorporeal flow is established with a reperfusion time of up to 90 min, after which cross clamp and recovery are performed in identical fashion as a brain-dead donor recovery.1,4
With increased interest in utilizing TA-NRP for cardiac transplant,3,5 we sought to evaluate the early outcomes of lung transplants from TA-NRP DCD donors in the United States. We retrospectively reviewed adult (≥18 years) lung-only transplants from DCD donors between January 1, 2020 and March 31, 2022 in the United Network for Organ Sharing (UNOS) database. To determine organ recovery during which TA-NRP was likely utilized, we considered a transplant to have used TA-NRP if the interval between asystole and aortic cross-clamp time was ≥50 min. This interval was chosen based on the 2020 ISHLT consensus statement, which suggests a TA-NRP interval of 45 to 90 min.1,3,5 The time between asystole and cross-clamp includes stand-off time after declaration of death (2-5 min), chest entry (2 min), and, in the cases of TA-NRP, the reperfusion interval prior to cross-clamp. All other DCD transplants were considered direct recovery transplants. Our threshold of 50 min captures nearly all TA-NRP donors described by Hoffman et al3 and Smith et al,5 while minimizing the number of direct recovery donors captured. Baseline characteristics and outcomes were assessed using Wilcoxon rank sum and chi-square testing for continuous and categorical variables, respectively. Post-transplant survival at 30, 60, and 90 days was assessed using time-to-event analysis and log-rank tests. This study was deemed exempt for the need for institutional review board approval by the Johns Hopkins Institutional Review Board.
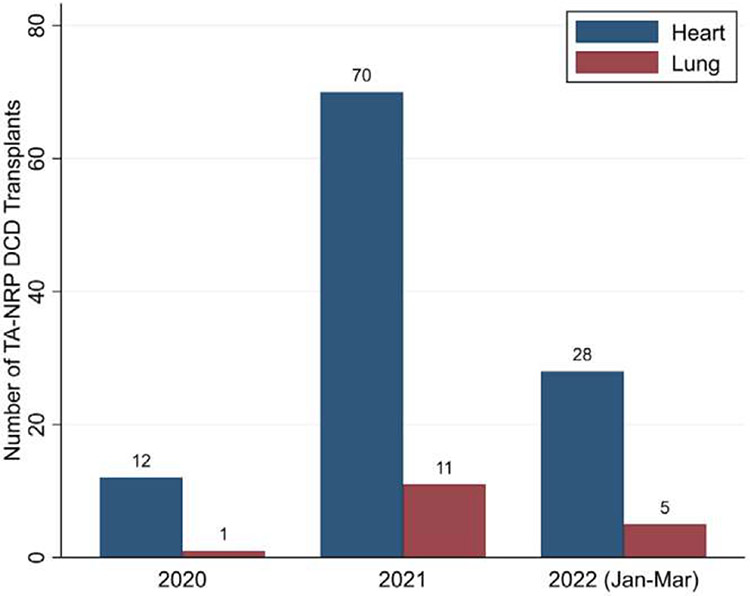
Of the 434 total DCD lung transplants, 17 (3.9%) were recovered using TA-NRP by 12 lung transplant centers (Figure 1). TA-NRP donors had a lower median age than direct recovery donors (28 [21-36] vs 40 [29-49] years, p = 0.003; Table 1), similar time from withdrawal of life support to asystole (23.5 [16-31] vs 20 [15-26] min, p = 0.2), and, by definition, longer asystole to aortic cross-clamp time (100 [72-117] vs 7 [4-9] min, p < 0.001). Ex vivo lung perfusion (EVLP) was utilized in 1 (5.9%) TA-NRP and 86 (20.6%) direct recovery transplants (p = 0.2).
Figure 1.
Number of heart and lung transplants performed from donation after circulatory death (DCD) donors after thoracoabdominal normothermic regional perfusion (TA-NRP) in the United States.
Table 1.
Baseline Donor, Recipient, and Lung Transplant Characteristics between Direct Recovery vs Thoracoabdominal Normothermic Regional Perfusion (TA-NRP) Donation After Circulatory Death (DCD) Transplants in the United States. Significant Values (p < 0.05) are Bolded
| Variable, n (%) | Direct recovery (n = 417) |
TA-NRP (n = 17) |
p-value |
|---|---|---|---|
| Donor characteristics | |||
| Age (years), median (IQR) | 40 (29-49) | 28 (21-36) | 0.003 |
| Male sex | 247 (59.2%) | 16 (94.1%) | 0.004 |
| Race/Ethnicity | 0.24 | ||
| White | 298 (71.5%) | 12 (70.6%) | |
| Black | 47 (11.3%) | 0 (0.0%) | |
| Hispanic | 61 (14.6%) | 5 (29.4%) | |
| Other | 11 (2.6%) | 0 (0.0%) | |
| Cause of death | 0.44 | ||
| Anoxia | 163 (39.1%) | 9 (52.9%) | |
| Cerebrovascular/Stroke | 119 (28.5%) | 2 (11.8%) | |
| Head trauma | 126 (30.2%) | 6 (35.3%) | |
| Other | 9 (2.2%) | 0 (0.0%) | |
| Abnormal chest x-ray | 289 (69.3%) | 13 (76.5%) | 0.53 |
| ≥20 pack year smoking history | 29 (7.0%) | 0 (0.0%) | 0.62 |
| Pa02/Fi02 ratio <300 | 40 (9.6%) | 0 (0.0%) | 0.39 |
| Ex vivo lung perfusion | 86 (20.6%) | 1 (5.9%) | 0.21 |
| Recipient characteristics | |||
| Age (years), median (IQR) | 62 (54-67) | 62 (58-64) | 0.86 |
| Male sex | 248 (59.5%) | 14 (82.4%) | 0.059 |
| Ethnicity | 0.88 | ||
| White | 323 (77.5%) | 14 (82.4%) | |
| Black | 46 (11.0%) | 1 (5.9%) | |
| Hispanic | 37 (8.9%) | 2 (11.8%) | |
| Other | 11 (2.6%) | 0 (0.0%) | |
| Diagnosis | 0.32 | ||
| Obstructive disease | 120 (28.8%) | 6 (35.3%) | |
| Pulmonary vascular disease | 12 (2.9%) | 0 (0.0%) | |
| Cystic fibrosis | 8 (1.9%) | 0 (0.0%) | |
| Restrictive disease | 264 (63.3%) | 9 (52.9%) | |
| Other | 13 (3.1%) | 2 (11.8%) | |
| Lung allocation score, median (IQR) | 38.7 (34.6-50.4) | 37.9 (34.2-39.3) | 0.27 |
| Total days on waitlist, median (IQR) | 31 (10-119) | 42 (12-76) | 0.90 |
| Transplant characteristics | |||
| Ischemic time (hours), median (IQR) | 7.1 (5.7-9.7) | 5.1 (4.4-6.0) | <0.001 |
| Donor to recipient hospital distance (miles), median (IQR) | 179 (76-359) | 142 (9-331) | 0.40 |
| Withdrawal of life support to asystole time (min), median (IQR) | 20 (15-26) | 23.5 (16-31) | 0.20 |
| Agonal time (min), median (IQR) | 16 (11-22) | 17.5 (14-25) | 0.35 |
| Asystole to clamp time (min), median (IQR) | 7 (4-9) | 100 (72-117) | <0.001 |
Abbreviations: TA-NRP, thoracoabdominal normothermic regional perfusion; IQR, interquartile range. Agonal time defined as time between systolic blood pressure <80 mm Hg or 02 saturation <80% and asystole.
Continuous variables were compared using Wilcoxon rank sum testing and expressed as median (interquartile range). Categorial variables were compared using Chi-square testing and expressed as number (percent).
Recipients of TA-NRP grafts had lower likelihood of ventilation >48 hours (23.5% vs 51.3%, p = 0.027) and trended towards shorter hospital lengths of stay (15 [10-28.5] vs 23 [15-39] days, p = 0.060; Table 2). Recipients of TA-NRP grafts had similar rates of intubation (30.8% vs 46.7%, p = 0.4) and extracorporeal membrane oxygenation at 72 hours (7.7% vs 17.3%, p = 0.7), as well as predischarge acute rejection (11.8% vs 7.0%, p = 0.4). On Kaplan–Meier analysis, TA-NRP versus direct recovery recipients had similar 30-day (100% vs 96.4%, p = 0.4), 60-day (100% vs 95.4%, p = 0.4), and 90-day (92.9% vs 93.6%, p > 0.9) survival.
Table 2.
Outcomes Following Transplantation with Lung Donors after Circulatory Death (DCD) Recovered Using Direct Recovery vs Thoracoabdominal Normothermic Regional Perfusion (TA-NRP) in the United States. Significant Values (p < 0.05) are Bolded
| Variable, n (%) | Direct recovery (n = 417) |
TA-NRP (n = 17) |
p-value |
|---|---|---|---|
| Ventilatory support >48 h | 214 (51.3%) | 4 (23.5%) | 0.027 |
| Intubation at 72 h | 184 (46.7%) | 4 (30.8%) | 0.40 |
| ECMO at 72 h | 68 (17.3%) | 1 (7.7%) | 0.71 |
| Predischarge acute rejection | 29 (7.0%) | 2 (11.8%) | 0.35 |
| Dialysis | 50 (12.0%) | 1 (5.9%) | 0.71 |
| Hospital length of stay (days), median (IQR) | 23 (15-39) | 15 (10-28.5) | 0.060 |
| Survival at 30-d post-transplant | 15 (100%) | 390 (96.4%) | 0.43 |
| Survival at 60-d post-transplant | 14 (100%) | 380 (95.4%) | 0.38 |
| Survival at 90-d post-transplant | 13 (92.9%) | 372 (93.6%) | 0.99 |
Abbreviations: TA-NRP, thoracoabdominal normothermic regional perfusion; IQR, interquartile range; ECMO, extracorporeal membrane oxygenation.
Continuous variables were compared using Wilcoxon rank sum testing and expressed as median (interquartile range). Categorial variables were compared using Chi-squared testing and expressed as number (percent). Post-transplant survival was assessed using Kaplan Meier time-to-event analysis and log-rank tests.
This is the first national study of lung transplant outcomes using TA-NRP DCD allografts. Currently, the effects of TA-NRP on lung allograft function are largely unknown; particular concerns include the limited lung perfusion prior to return of cardiac activity, the potential for reperfusion with byproducts from the abdominal compartment6, and the ethics surrounding this technique.7 TA-NRP in lung transplantation has only been described in case reports by Urban et al6,8 and Vandendriessche et al9 Our results on the early national experience with TA-NRP lungs demonstrated satisfactory outcomes, with no differences in perioperative outcomes and short-term post-transplant survival, though with younger and more nonsmoking donors. These results support the further study of lung grafts recovered using TA-NRP.
While recovery using TA-NRP has increased over the last 2 years, uptake of this practice for lung transplantation lags behind other organs. Of the total 146 TA-NRP donors, only 17 (11.6%) lungs were transplanted. Lungs from an additional 8 (5.5%) TA-NRP donors were recovered for transplant but discarded, resulting in a discard rate of 32%, compared to a discard rate of 24.4% for DCD donors previously reported.10 Meanwhile, hearts were transplanted from 110 TA-NRP donors (75.3%), kidneys from 134 (91.8%), and livers from 82 (56.2%). Hearts were transplanted from 23 of the 25 donors (92%) in which lungs were recovered. Although standard recovery may be preferred for DCD lungs, our study suggests that the lungs from DCD donors with TA-NRP performed for cardiac transplant might currently be underutilized and may offer a safe way to further expand the donor pool, particularly as interest in TA-NRP for cardiac transplant continues to grow.4 Additionally, TA-NRP may act as an alternative to EVLP, allowing for assessment of marginal DCD donor lungs without the added costs associated with EVLP and increasing accessibility of marginal DCD lungs to programs without a dedicated EVLP team.
This study has several limitations. Given the novelty of TA-NRP, it is not yet available as a variable in the UNOS database and therefore our identification of patients using asystole and cross-clamp time might result in misclassification. The registry database also does not have more granular information on pulmonary function during the TA-NRP phase or reasoning for discarding the TA-NRP lungs. Lastly, the recent uptake of this procedure limits the available follow-up time.
In conclusion, we report on the outcomes of the first 17 lung transplants performed following TA-NRP recovery in the United States. Our analysis demonstrates satisfactory perioperative outcomes and short-term survival. Future studies should continue to assess the safety and organ utilization rate of this technique.
Abbreviations:
- DCD
donation after circulatory death
- ECMO
extracorporeal membrane oxygenation
- EVLP
ex vivo lung perfusion
- NRP
normothermic regional perfusion
- TA-NRP
thoracoabdominal normothermic regional perfusion
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure statement
Errol Bush discloses participation in an Innovation Advisory Board for Ethicon, Inc.
References
- 1.Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant 2020;39:501–17. 10.1016/j.healun.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Siddique A, Urban M, Strah H, et al. Controlled DCD lung transplantation: circumventing imagined and real barriers: time for an international taskforce? J Heart Lung Transplant 2022;41:1198–203. 10.1016/j.healun.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman JRH, McMaster WG, Rali AS, et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant 2021;40:1408–18. 10.1016/j.healun.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Pasrija C, Tipograf Y, Shah AS, Trahanas JM. Normothermic regional perfusion for donation after circulatory death donors. Curr Opin Organ Transplant, 10.1097/MOT.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 5.Smith DE, Kon ZN, Carillo JA, et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg 2022;164:557–68. 10.1016/j.jtcvs.2021.07.059. e1. [DOI] [PubMed] [Google Scholar]
- 6.Urban M, Castleberry AW, Markin NW, et al. Successful lung transplantation with graft recovered after thoracoabdominal normothermic perfusion from donor after circulatory death. Am J Transplant 2022;22:294–8. 10.1111/ajt.16806. [DOI] [PubMed] [Google Scholar]
- 7.DeCamp M, Snyder Sulmasy L, Fins JJ. Point: does normothermic regional perfusion violate the ethical principles underlying organ procurement? Yes. Chest 2022;162:288–90. 10.1016/j.chest.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Urban M, Bishawi M, Castleberry AW, et al. Novel use of mobile ex-vivo lung perfusion in donation after circulatory death lung transplantation. Prog Transpl 2022;32:190–1. 10.1177/15269248221087437. [DOI] [PubMed] [Google Scholar]
- 9.Vandendriessche K, Tchana-Sato V, Ledoux D, et al. Transplantation of donor hearts after circulatory death using normothermic regional perfusion and cold storage preservation. Eur J Cardiothorac Surg 2021;60:813–9. 10.1093/ejcts/ezab139. [DOI] [PubMed] [Google Scholar]
- 10.Choi AY, Jawitz OK, Raman V, et al. Predictors of nonuse of donation after circulatory death lung allografts. J Thorac Cardiovasc Surg 2021;161:458–66. 10.1016/j.jtcvs.2020.04.111.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]