Abstract
Introduction
Previous studies have identified lesions commonly found in placentas associated with stillbirth but have not distinguished across a range of gestational ages (GAs). The objective of this study was to identify lesions associated with stillbirths at different GAs by adapting methods from the chemical machine learning field to assign lesion importance based on correlation with GA.
Methods
Placentas from the Stillbirth Collaborative Research Network were examined according to standard protocols. GAs at stillbirth were categorized as: <28 weeks (extreme preterm stillbirth [PTSB]), 28–33’6 weeks (early PTSB), 34–36’6 weeks (late PTSB), ≥37 weeks (term stillbirth). We identified and ranked the most discriminating placental features, as well as those that were similar across GA ranges, using Kernel Principal Covariates Regression (KPCovR).
Results
These analyses included 210 (47.2%) extreme PTSB, 85 (19.1%) early PTSB, 62 (13.9%) late PTSB, and 88 (19.8%) term stillbirths. When we compute the KPCovR, the first principal covariate indicates that there are four lesions (acute funisitis & nucleated fetal red blood cells found in extreme PTSB; multifocal reactive amniocytes & multifocal meconium found in term stillbirth) that distinguish GA ranges among all stillbirths.
Discussion
There are distinct placental lesions present across GA ranges in stillbirths; these lesions are identifiable using sophisticated feature selection. Further investigation may identify histologic changes across gestations that relate to fetal mortality.
Keywords: Placental Lesions, Stillbirth, Intrauterine Fetal Demise, Feature Selection, Kernel Principal Covariates Regression
Introduction
Understanding placental pathology in cases of intrauterine fetal demise (IUFD/stillbirth) has significant implications for uncovering the etiologies of stillbirth and, ultimately, helping reduce the high rate of preventable stillbirths in the United States [1–3]. Placental examination has proven of great utility in identifying risk factors and causes for stillbirth since its inception, even recently uncovering patterns of vascular malperfusion in stillbirths associated with COVID-19 infection [3,4]. Therefore, understanding which placental lesions are present in stillbirths of all etiologies and gestational ages is vitally important.
Previous studies identified placental lesions present in stillbirths across a range of gestational ages (GAs) [5]. Developmental, inflammatory, and circulatory lesions were compared across placentas associated with stillbirths delivered at the following GAs: less than 24 weeks, 24 weeks to 31 weeks 6 days, 32 weeks to 36 weeks 6 days, and greater than or equal to 37 weeks. While this study comprehensively described these lesions (see supplemental table 1), analyses were limited to comparisons across groups, which does not identify lesions unique to each GA range. Tiwari et al. (2022) studied placental findings of preterm stillbirths versus livebirths and term stillbirths versus livebirths but did not compare lesions between preterm and term stillbirths [6]. Others have sought to identify lesions common within different trimesters of stillbirths and the prevalence of placental causes of death across GAs, but none have identified which lesions are unique to specific GA ranges [7,8].
Therefore, we sought to use feature importance techniques to identify placental lesions associated with stillbirths at different GAs. Feature importance refers to techniques that calculate how useful certain measures are in explaining the variance of a sample or a target variable; such methods include Exploratory Factor Analysis and Principal Components Analysis. We utilized a method borrowed from chemistry and engineering fields, Kernel Principal Covariates Regression (KPCovR), first created by Helfrecht, Cersonsky, et al. in 2020 [9,10]. This method constructs a non-linear latent-space projection simultaneously minimizing regression error and maximizing projection variance. This is done by considering a mixing of two optimization functions; the losses used in kernel ridge regression and kernel principal components analysis. The first dimension of the resulting projection (first kernel principal covariate) contains the constructed feature for each sample resulting from this minimization. We hypothesized that, with this method, we would be able to classify which lesions best explained the variance in placental findings in stillbirths across GA ranges.
Methods
Data
Data were derived from the Stillbirth Collaborative Research Network (SCRN), a comprehensive study of stillbirths and livebirths in the United States. Data were collected at 59 hospitals across five geographic regions from 2006 to 2009 [11]. Each center’s Institutional Review Board approved study procedures, as well as the Data Coordinating and Analysis Center. Participants gave written informed consent for each portion of the study, including postmortem and placental examinations. SCRN procedures, including inclusion/exclusion criteria, have been described previously [11].
For these analyses, participants were included if they delivered a singleton stillbirth of GA > 20 weeks and consented to complete placental examination and were excluded if they delivered a live birth or a singleton stillbirth of GA <20 weeks or did not have complete placental examination. Protocols for placental and postmortem examination have been described previously [12,13]. Study pathologists received centralized training in placental examinations, resulting in standardized assessments. Participants also completed surveys regarding demographics (including self-reported minority race [Black, Asian, American Indian/Alaskan Native, Pacific Islander, multiple races, other]), maternal health history, and current pregnancy history.
Placental features
Features included in these analyses encompassed those previously identified by Pinar et al. (2014), as well as a number of related lesions and gross findings [5]. Developmental, inflammatory, circulatory, findings of the placental disc, and multifocal findings (across membranes, chorionic plate, umbilical cord, etc.) were included. Specific lesions were classified as focal (present in one area on a single side), multifocal/patchy (present in more than one area on multiple sides), or diffuse (full thickness of placental disc, all sections involved). Lesions included and their definitions are described in supplemental table 2.
Statistical analyses
For the purposes of these analyses, we chose to separate stillbirths into gestational age ranges according to the American College of Obstetricians and Gynecologists: extreme preterm stillbirth (<28 weeks), early preterm stillbirth (28 – 336/7 weeks), late preterm stillbirth (34 – 366/7 weeks), and term stillbirth (≥37 weeks) [14,15]. Sample characteristics were compared across groups using Chi-Square tests (categorical) or Kruskall-Wallis tests (continuous) in RStudio (version 2022.07.1, R version 4.1.3).
KPCovR (scikit-matter v0.1) was utilized to determine those features that best correlated and explained most variance observed in placentas associated with stillbirth at different GAs. Placental features (n = 54) were preprocessed using one-hot encoding; features were first linearly correlated to GA, then encoded into a non-linear similarity matrix (radial basis function [RBF] kernel, γ=0.1) to determine our subject similarities. We cross-validated our kernel hyperparameters using leave-one-out cross-validation on a grid search (GridSearchCV and KernelRidge, scikit-learn v1.0). Using this RBF kernel, we computed the correlation of the lesions with the latent space projection over 1000 random train / test draws in a 90/10 proportion.
First principal covariate was reported, which represents the feature of this data that explains the most variance (similarly to Principal Components Analysis). Once this first dimension was constructed, we then completed post-hoc analyses (in RStudio) to understand which features were more common within each gestational age range. Chi-Square tests were used to compare across and between GA ranges; p-values <0.05 were considered significant.
Results
Sample characteristics
We identified 445 placentas that met study inclusion criteria (Figure 1): 210 extreme preterm stillbirth (GA <28 weeks), 85 early preterm stillbirth (GA 28 – 336/7 weeks), 62 late preterm stillbirth (GA 34 – 366/7 weeks), and 88 term stillbirth (GA ≥37 weeks). The distributions of minority race and use of assisted reproductive technologies significantly differed across all GA ranges; there were no other significant differences in demographic or maternal history measures (Table 1). Fetuses delivered at earlier gestational ages were, as expected, of smaller weight.
Figure 1:
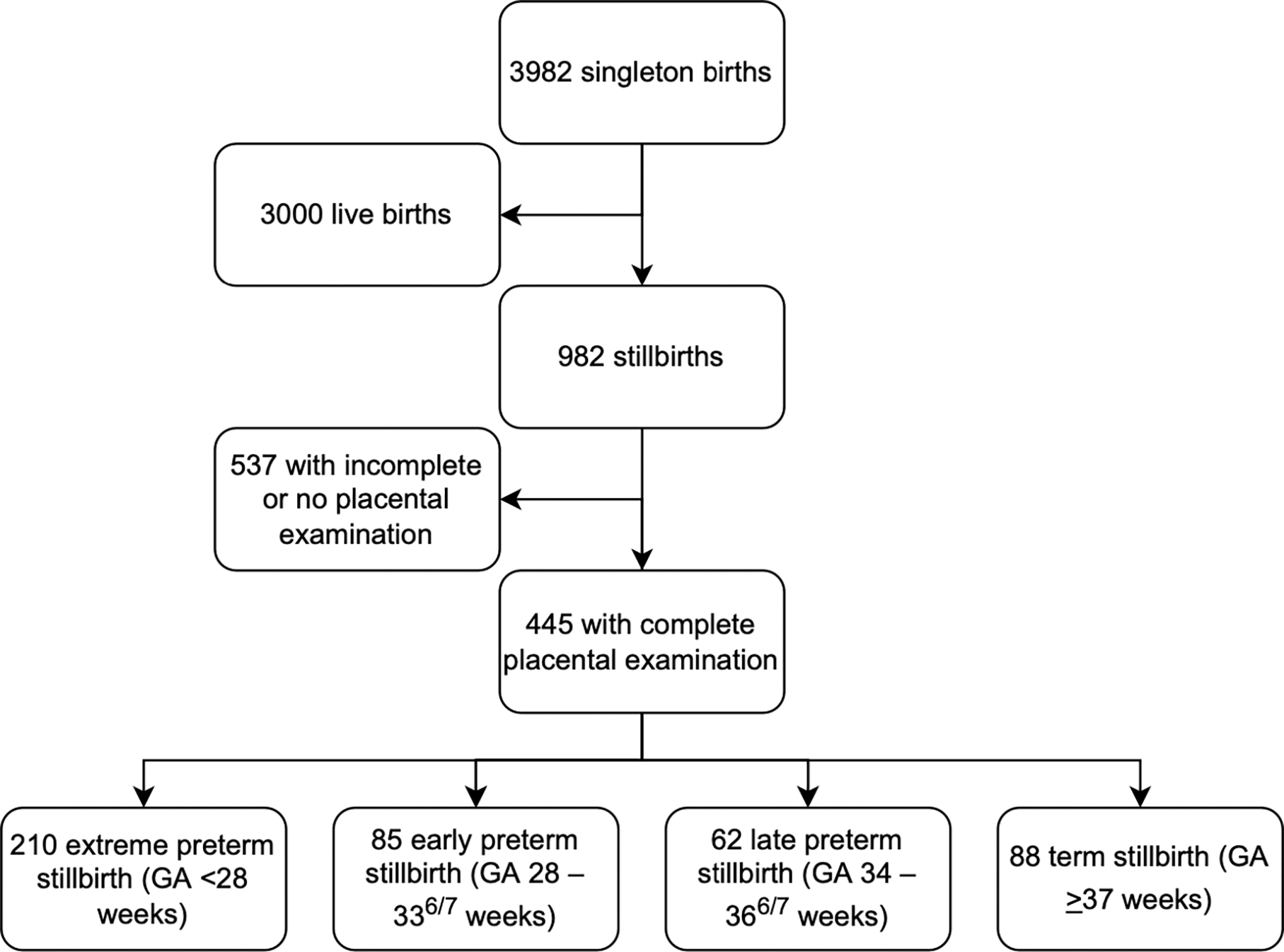
Sample derivation. A flowchart of included and excluded participants in this study. 3982 participants were considered; 445 were ultimately included.
Table 1:
Sample characteristics
| Extreme Preterm Stillbirth | Early Preterm Stillbirth | Late Preterm Stillbirth | Term Preterm Stillbirth | p-value* | |
|---|---|---|---|---|---|
| (n = 210) | (n = 85) | (n = 62) | (n = 88) | ||
|
| |||||
| Maternal age (years) | 27.0 ± 6.7 | 28.3 ± 7.2 | 27.1 ± 6.2 | 28.2 ± 7.0 | 0.4 |
|
| |||||
| Race (minority) | 94 (44.8) | 35 (41.2) | 20 (32.3) | 25 (28.4) | 0.038 |
|
| |||||
| Ethnicity (Hispanic) | 58 (27.8) | 30 (35.3) | 21 (33.9) | 32 (36.4) | 0.4 |
|
| |||||
| Education (years) | 12.8 ± 2.9 | 12.8 ± 3.2 | 12.6 ± 3.6 | 12.9 ± 2.9 | 0.9 |
|
| |||||
| Any chronic illness | 144 (68.6) | 52 (61.2) | 43 (69.4) | 65 (73.9) | 0.3 |
| Diabetes | 7 (3.6) | 3 (3.8) | 3 (5.1) | 9 (11.1) | 0.1 |
| Hypertension | 23 (12.0) | 14 (17.7) | 10 (16.9) | 15 (18.5) | 0.4 |
| Cardiovascular disease | 121 (57.6) | 47 (55.3) | 35 (56.5) | 53 (60.2) | 0.9 |
| Gastrointestinal disease | 100 (47.6) | 37 (43.5) | 31 (50.0) | 38 (43.2) | 0.8 |
| Mental health condition | 24 (11.4) | 9 (10.6) | 9 (14.5) | 17 (19.3) | 0.3 |
| Obesity | 58 (27.6) | 23 (27.1) | 17 (27.4) | 20 (22.7) | 0.8 |
|
| |||||
| Obstetric history | |||||
| Past IUFD | 38 (18.1) | 9 (10.6) | 10 (16.1) | 14 (15.9) | 0.5 |
| Number of pregnancies | 2.6 ± 1.6 | 2.6 ± 1.6 | 2.4 ± 1.7 | 2.8 ± 1.9 | 0.7 |
| Assisted reproductive technology | 49 (23.3) | 29 (34.1) | 13 (21.0) | 10 (11.4) | 0.005 |
|
| |||||
| Fetal characteristics | |||||
| Sex (female) | 78 (37.1) | 31 (36.5) | 19 (30.6) | 39 (44.3) | 0.4 |
| Weight (g) | 425 ± 363 | 1181 ± 530 | 2246 ± 671 | 3120 ± 680 | <0.001 |
All values listed as number (percent) or mean ± standard deviation.
Chi-Square difference test (categorical) or Kruskall-Wallis test (continuous); p<0.05 significant.
Kernel Principal Covariates Regression
As has been suggested by literature, we chose to equally minimize the regression and projection loss, and our resulting first principal covariate maps onto gestational age with Pearson correlation coefficient of 0.89 ± 0.01. The correlation of the original parameters with this first principal covariate encodes how much their similarity across subjects delineates the different gestational age thresholds. We found that acute funisitis, multifocal meconium, and multifocal reactive amniocytes had the strongest correlations with the first principal covariate (Table 2, Figures 2&3).
Table 2:
Correlations and post-hoc analyses of placental lesions
| Correlation Coefficient1 | Extreme Preterm Stillbirth | Early Preterm Stillbirth | Late Preterm Stillbirth | Term Stillbirth | p-value2 | |
|---|---|---|---|---|---|---|
|
| ||||||
| Acute funisitis | −0.30 ± 0.01 | 28 (13.3)ˠ | 2 (2.4) | 1 (1.6) | 2 (2.3) | <0.001 |
|
| ||||||
| Acute umbilical arteritis | −0.21 ± 0.01 | 10 (4.8)ˠˠ | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0.030 |
|
| ||||||
| Acute chorionic vasculitis | −0.19 ± 0.02 | 22 (10.5) | 1 (1.2) | 2 (3.2) | 4 (4.5) | 0.012 |
|
| ||||||
| Diffuse terminal villous immaturity | 0.12 ± 0.02 | 6 (2.9) | 3 (3.5) | 8 (12.9)ˠˠ | 5 (5.7) | 0.022 |
|
| ||||||
| Parenchymal infarct | 0.09 ± 0.02 | 44 (21.0) | 37 (43.5)ˠ | 16 (25.8) | 20 (22.7) | <0.001 |
|
| ||||||
| Retroplacental hematoma | −0.26 ± 0.02 | 52 (24.8)ˠˠ | 17 (20.0) | 9 (14.5) | 5 (5.7) | 0.001 |
|
| ||||||
| Accelerated villous maturity | −0.04 ± 0.02 | 40 (19.0)ˠ | 28 (32.9) | 23 (37.1)ˠˠ | 0 (0.0) | <0.001 |
|
| ||||||
| Nucleated fetal red blood cells | 0.26 ± 0.02 | 45 (21.4)ˠ | 29 (34.1) | 28 (45.2) | 31 (35.2) | 0.001 |
|
| ||||||
| Placental disc | ||||||
| Hemorrhage | −0.21 ± 0.02 | 83 (39.5)ˠˠ | 26 (30.6) | 15 (24.2) | 19 (21.6) | 0.009 |
| Syncytial knots | 0.25 ± 0.02 | 22 (10.5) | 29 (34.1)ˠˠ | 8 (12.9) | 25 (28.4) | <0.001 |
| Trophoblast proliferation | −0.17 ± 0.02 | 45 (21.4) | 16 (18.8) | 4 (6.5)ˠˠ | 9 (10.2) | 0.012 |
|
| ||||||
| Decidual vasculopathy | −0.14 ± 0.02 | 41 (19.5) | 19 (22.4) | 5 (8.1)ˠˠ | 7 (8.0)ˠˠ | 0.009 |
|
| ||||||
| Multifocal | ||||||
| Reactive amniocytes | 0.38 ± 0.02 | 16 (7.6) | 6 (7.1) | 14 (22.6)ˠˠ | 32 (36.4)ˠˠ | <0.001 |
| Calcifications | 0.17 ± 0.02 | 1 (0.5) | 3 (3.5) | 0 (0.0) | 7 (8.0)ˠˠ | <0.001 |
| Meconium | 0.29 ± 0.02 | 41 (19.5) | 29 (34.1) | 16 (25.8) | 43 (48.9)ˠˠ | <0.001 |
Pearson correlation with first principal covariate.
Chi-Square difference test; p<0.05 significant.
Post-hoc analysis indicates significantly higher/lower relative to all other ranges.
Post-hoc analysis indicates significantly higher/lower relative to 1–2 other ranges.
Lesions not significantly different across ranges (did not contribute to variance explained in sample): thrombi of or disrupted fetal vessels, chorionic vascular degeneration, chorioamnionitis, chorangioma, chorangiomatosis, chorangiosis, chorionic or disc karyorrhexis, acute umbilical phlebitis, umbilical vascular lesion, punctate or gross umbilical hemorrhages, true knot, single umbilical artery, umbilical degeneration, irregular disc lobulations, disc inclusion bodies, deciduitis, amniotic bands, disc fibrin deposition, avascular villi, terminal villous hypoplasia, intravillous thrombus, diffuse villous fibrin deposition, intervillitis, acute or chronic villitis, abnormal membrane insertion, abundant blood clots, or multifocal edema, hemosiderin, amnion nodosum, or necrosis.
Figure 2:
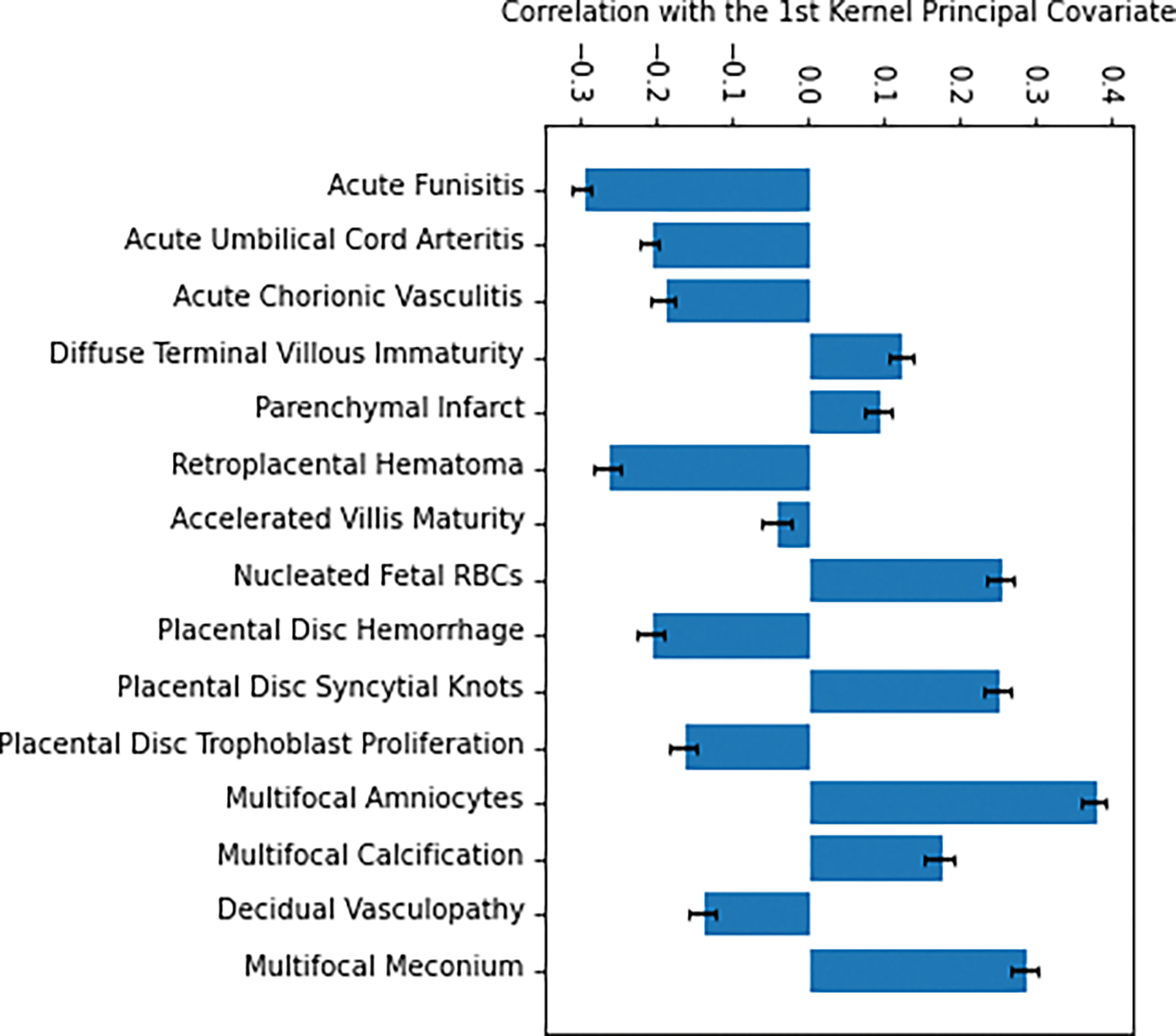
Feature correlation with first principal covariate. Correlation coefficient with 1st Kernel principal covariate for various lesions shown with standard error bars.
Figure 3:
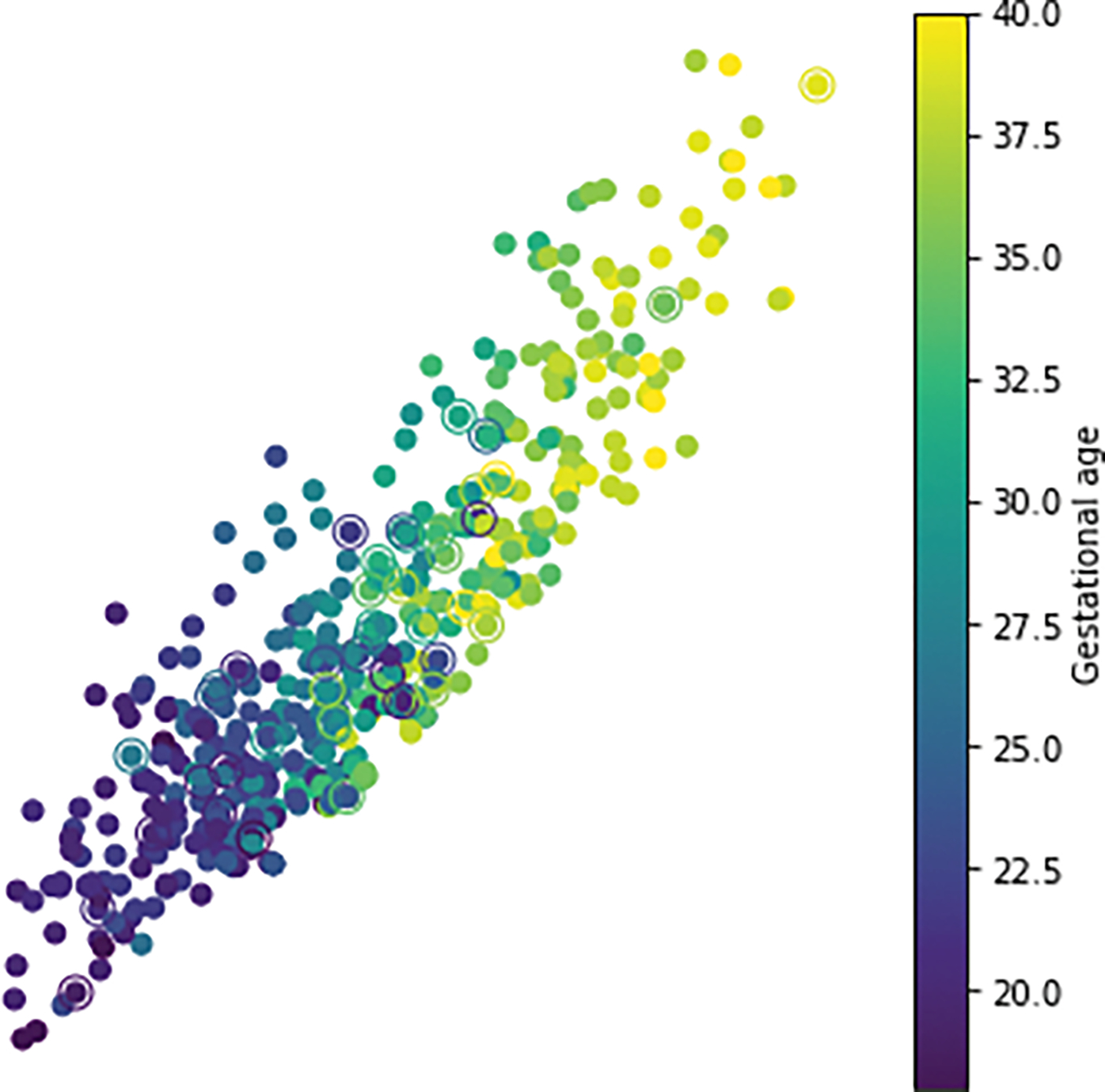
Feature mapping across gestational ages. Each dot represents a lesion, color coded according to approximate gestational age to which it is mapped.
Post-hoc analyses
Several lesions had significantly different distributions across all ranges. Numerous other lesions did not differ in their distributions; these lesions did not contribute to the variance observed in the sample (Table 2).
Within extreme preterm stillbirth, we identified higher rates of acute funisitis and lower rates of accelerated villous maturity and nucleated fetal red blood cells relative to all other ranges. Acute umbilical arteritis was more common in this group than in early preterm or term stillbirth. Hemorrhage of the placental disc and retroplacental hematoma were more common in this group than in late preterm or term stillbirth.
Within early preterm stillbirth, we identified higher rates of parenchymal infarct compared to all other ranges. Increased syncytial knots of the chorionic villi were more common in this group compared to extreme or late preterm stillbirth.
There were no lesions more common in late preterm stillbirth group compared to all other groups. Diffuse terminal villous immaturity and multifocal reactive amniocytes were more common in this group than in extreme or early preterm stillbirth; decidual vasculopathy and trophoblast proliferation of the placental disc were less common comparatively. Accelerated villous maturity was more common in the late preterm stillbirth group compared to extreme preterm and term stillbirth.
Finally, there were no lesions more common within term stillbirths compared to all other groups. Decidual vasculopathy was less common in this group compared to extreme and early preterm stillbirth, whereas multifocal reactive amniocytes were present more frequently, comparatively. Multifocal calcifications and meconium were present more commonly in this group compared to extreme and late preterm stillbirth.
Discussion
Using Kernel Principal Covariates Regression, we were able to identify distinct placental lesions present across different GAs in a sample of singleton stillbirths. This method, which has not before been used in medicine, was able to identify lesions with a principal component strongly correlated with GA, with a coefficient of 0.89. These findings point to unique patterns of placental dysfunction across different GA ranges, which may offer valuable insight into stillbirth etiology.
Acute funisitis was present more commonly in extreme preterm stillbirths (13.3% of this group compared to 1.6–2.4% in other groups) without more acute chorioamnionitis (compared to other ranges), suggesting that the fetal inflammatory response is more significant in stillbirths of this GA. Funisitis has been associated with impending onset of preterm labor, neonatal morbidity, and multiorgan fetal involvement [16]. Funisitis has been frequently associated with stillbirth, particularly acute and subacute necrotizing funisitis; in many (if not all) of these cases, chorioamnionitis was coexistent with funisitis [17–19]. Funisitis, unlike histological chorioamnionitis, is also frequently associated with positive post-mortem microbiology cultures [20]. Our findings suggest that, while chorioamnionitis occurs at all gestational ages, funisitis is a unique feature of late second-trimester gestation that might contribute more commonly to stillbirths in these cases. This is consistent with the literature, which has identified infection-related stillbirth at these earlier gestational ages in high-resource settings [21–23].
Lower rates of accelerated villous maturity and nucleated fetal red blood cells were also observed in the extreme preterm stillbirth group compared to other ranges. As both findings are known to be associated with fetal hypoxia or placental malperfusion, this result is suggestive of a lower rate of hypoxic causes of death among earlier stillbirths [24,25]. Accelerated villous maturity is higher in late preterm stillbirth relative to extreme preterm and term stillbirth, implying the converse: hypoxic causes of fetal death may be more common among late preterm stillbirths compared to extreme preterm and term stillbirths. While nucleated fetal red blood cells were observed more often in association with extreme preterm stillbirth, we did not have data for the percent of the total fetal red blood cells that were nucleated, which can vary across gestational ages. Further analyses of the identified cause of death in these stillbirths may further elucidate this difference.
Parenchymal infarcts were found more commonly in early preterm stillbirths compared to other groups. Such infarcts are known to be associated with hypertensive disorders of pregnancy, particularly in more severe disease [26]. However, in our cohort, there were no differences in hypertensive disease across GA ranges. This may point to the presence of more severe disease in early preterm stillbirths, or, alternatively, more preterm deliveries in fetuses of GA ≥34 weeks. Such deliveries may be expected in, for example, preeclampsia with severe features, given current ACOG guidance [27].
While those lesions identified above were significant in differentiating between stillbirths of different GA ranges, there are a number of previously identified lesions that did not significantly differ in prevalence among GA ranges [5]. Among these were single umbilical artery, velamentous or furcate cord insertion, chorioamnionitis, acute umbilical cord phlebitis, chorionic vascular degeneration, acute or chronic villitis, intervillous thrombus, avascular villi, and edema. We can determine from the absence of differences in these lesions that, possibly, structural differences in the umbilical cord (both single umbilical artery and abnormal insertion) might contribute to stillbirths at many GA ranges; as suggested previously, this might indicate a need for closer surveillance of pregnancies with these findings. Other lesions listed here might be more significant in differentiating stillbirth versus livebirth, thus related to the etiologies of many stillbirths, but may also not be specific to GA ranges.
Our study has several strengths, including our novel methodology. By using a method of feature importance that allows for correlation with a continuous variable such as GA, we were able to identify features that vary along this range or delineate different GA ranges. Additionally, we utilized this method for the first time in medical analyses, therefore introducing this technique, with source code available on GitHub, as a viable option for other clinical researchers, particularly when the relationship between measurements and observables requires a non-linear approach to mapping and regression.
Our study should also be interpreted in the context of several limitations. Our data were collected from 2006–2009; therefore, more recent patterns in pregnancy outcomes are not accounted for in our analyses, such as findings related to the ongoing COVID-19 pandemic [28]. The SCRN data was also collected prior to the release of the Amsterdam consensus, thus excluding several important lesions that were not commonly reported in our sample (i.e., villous chorangiosis, villous agglutination, meconium-associated vascular necrosis). Furthermore, though placental examination was standardized across SCRN hospitals, it is possible that inter-examiner differences may introduce bias into our placental data, especially given that not all pathologists were subspecialty-trained. However, all placental sampling and examinations were overseen by the primary SCRN pathologists, who maintained quality with the help of the SCRN steering committee.
Using a novel analytic method, we identified unique placental features associated with stillbirth at differing gestational ages. Further studies may link these features to specific etiologies and therefore validate these associated conditions. Specifically, we intend to investigate the relationships between lesions identified by our analyses and Initial Causes of Fetal Death (INCODE) assessment [29]. Understanding of the conditions leading to stillbirth at different gestational ages may be of benefit for obstetricians in seeking to reduce preventable stillbirths.
Supplementary Material
Highlights:
SCRN contains comprehensive placental examination data for stillbirths.
-
Lesions are found in association with stillbirth at different gestational ages.
Funisitis and nucleated fetal red cells are found in extreme preterm stillbirth.
Multifocal reactive amniocytes and meconium are found in term stillbirth.
Kernel Principal Covariates Regression can delineate placental features.
Funding details
This work was supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10-HD045953 Brown University, Rhode Island; U10-HD045925 Emory University, Georgia; U10-HD045952 University of Texas Medical Branch at Galveston, Texas; U10-HDO45955 University of Texas Health Sciences Center at San Antonio, Texas; U10-HD045944 University of Utah Health Sciences Center, Utah; and U01-HD045954 RTI International, RTP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was previously presented at the Society for Pediatric Pathology Fall 2022 meeting, for which authors were presented with the Harry B. Neustein Award for Technological Advancement in the Study of Pediatric Pathology.
Abbreviations:
- GA
gestational age
- IUFD
intrauterine fetal demise
- KPCovR
Kernel Principal Covariates Regression
- RBF
radial basis function
- SCRN
Stillbirth Collaborative Research Network
Footnotes
Conflict of interest
The authors have no financial or other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dongarwar D, Aggarwal A, Barning K, Salihu HM, Trends in Stillbirths and Stillbirth Phenotypes in the United States: An Analysis of 131.5 Million Births, International Journal of Maternal and Child Health and AIDS. 9 (2020) 146. 10.21106/IJMA.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Page JM, Thorsten V, Reddy UM, Dudley DJ, Hogue CJR, Saade GR, Pinar H, Parker CB, Conway D, Stoll BJ, Coustan D, Bukowski R, Varner MW, Goldenberg RL, Gibbins K, Silver RM, Potentially Preventable Stillbirth in a Diverse U.S. Cohort, Obstetrics and Gynecology. 131 (2018) 336. 10.1097/AOG.0000000000002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kulkarni AD, Palaniappan N, Evans MJ, Placental Pathology and Stillbirth: A Review of the Literature and Guidelines for the Less Experienced, Journal of Fetal Medicine 2017 4:4. 4 (2017) 177–185. 10.1007/S40556-017-0133-3. [DOI] [Google Scholar]
- [4].Bunnell ME, Koenigs KJ, Roberts DJ, Quade BJ, Hornick JL, Goldfarb IT, Third trimester stillbirth during the first wave of the SARS-CoV-2 pandemic: Similar rates with increase in placental vasculopathic pathology, Placenta. 109 (2021) 72–74. 10.1016/J.PLACENTA.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pinar H, Goldenberg RL, Koch MA, Heim-Hall J, Hawkins HK, Shehata B, Abramowsky C, Parker CB, Dudley DJ, Silver RM, Stoll B, Carpenter M, Saade G, Moore J, Conway D, Varner MW, Hogue CJR, Coustan DR, Sbrana E, Thorsten V, Willinger M, Reddy UM, Placental Findings in Singleton Stillbirths, Obstetrics and Gynecology. 123 (2014) 325. 10.1097/AOG.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tiwari P, Gupta MM, Jain SL, Placental findings in singleton stillbirths: a case-control study from a tertiary-care center in India, J Perinat Med. 50 (2022) 753–762. 10.1515/JPM-2021-0179/MACHINEREADABLECITATION/RIS. [DOI] [PubMed] [Google Scholar]
- [7].Stanek J, Biesiada J, Relation of placental diagnosis in stillbirth to fetal maceration and gestational age at delivery, J Perinat Med. 42 (2014) 457–471. 10.1515/JPM-2013-0219/MACHINEREADABLECITATION/RIS. [DOI] [PubMed] [Google Scholar]
- [8].Korteweg FJ, Erwich JJHM, Holm JP, Ravisé JM, van der Meer J, Veeger NJGM, Timmer A, Diverse placental pathologies as the main causes of fetal death, Obstetrics and Gynecology. 114 (2009) 809–817. 10.1097/AOG.0B013E3181B72EBE. [DOI] [PubMed] [Google Scholar]
- [9].Helfrecht BA, Cersonsky RK, Fraux G, Ceriotti M, Structure-Property Maps with Kernel Principal Covariates Regression, Mach Learn Sci Technol. 1 (2020). 10.48550/arxiv.2002.05076. [DOI] [Google Scholar]
- [10].Goscinski A, Principe V, Fraux G, Kliavinek S, Helfrecht BA, Ceriotti M, Cersonsky RK, lab-cosmo/scikit-cosmo: Version 0.1.2 (v0.1.2), Zenodo. (2022). 10.5291/zenodo.6793953. [DOI] [Google Scholar]
- [11].Parker CB, Hogue CJR, Koch MA, Willinger M, Reddy UM, Thorsten VR, Dudley DJ, Silver RM, Coustan D, Saade GR, Conway D, Varner MW, Stoll B, Pinar H, Bukowski R, Carpenter M, Goldenberg R, Stillbirth Collaborative Research Network: design, methods and recruitment experience, Paediatr Perinat Epidemiol. 25 (2011) 425–435. 10.1111/J.1365-3016.2011.01218.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinar H, Koch MA, Hawkins H, Heim-Hall J, Shehata B, Thorsten VR, Carpenter M, Lowichik A, Reddy UM, The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination Protocol, Am J Perinatol. 28 (2011) 781. 10.1055/S-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pinar H, Koch M, Hawkins H, Heim-Hall J, Abramowsky C, Thorsten V, Carpenter M, Zhou H, Reddy U, The stillbirth collaborative research network postmortem examination protocol, Am J Perinatol. 29 (2012) 187–202. 10.1055/S-0031-1284228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].American College of Obstetricians and Gynecologists, Extremely Preterm Birth, (2022). https://www.acog.org/womens-health/faqs/extremely-preterm-birth (accessed October 29, 2022).
- [15].Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF, DeFranco EA, Late Preterm Birth, Rev Obstet Gynecol. 3 (2010) 10. 10.1097/aog.0b013e31816499f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM, Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance, Am J Obstet Gynecol. 213 (2015) S29–S52. 10.1016/J.AJOG.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jacques SM, Qureshi F, Necrotizing funisitis: A study of 45 cases, Hum Pathol. 23 (1992) 1278–1283. 10.1016/0046-8177(92)90296-F. [DOI] [PubMed] [Google Scholar]
- [18].Navarro C, Blanc WA, Subacute necrotizing funisitis: A variant of cord inflammation with a high rate of perinatal infection, J Pediatr. 85 (1974) 689–697. 10.1016/S0022-3476(74)80521-4. [DOI] [PubMed] [Google Scholar]
- [19].Seong JS, Park CW, Moon KC, Park JS, Jun JK, Necrotizing funisitis is an indicator that intra-amniotic inflammatory response is more severe and amnionitis is more frequent in the context of the extension of inflammation into Wharton’s jelly, Taiwan J Obstet Gynecol. 60 (2021) 840–850. 10.1016/J.TJOG.2021.07.011. [DOI] [PubMed] [Google Scholar]
- [20].Monari F, Gabrielli L, Gargano G, Annessi E, Ferrari F, Rivasi F, Facchinetti F, Fetal bacterial infections in antepartum stillbirth: A case series, Early Hum Dev. 89 (2013) 1049–1054. 10.1016/J.EARLHUMDEV.2013.08.010. [DOI] [PubMed] [Google Scholar]
- [21].McClure EM, Goldenberg RL, Infection and stillbirth, Semin Fetal Neonatal Med. 14 (2009) 182–189. 10.1016/J.SINY.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McClure EM, Dudley DJ, Reddy UM, Goldenberg RL, Infectious Causes of Stillbirth: A Clinical Perspective, Clin Obstet Gynecol. 53 (2010) 635. 10.1097/GRF.0B013E3181EB6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McClure EM, Saleem S, Goudar SS, Garces A, Whitworth R, Esamai F, Patel AB, Tikmani SS, Mwenechanya M, Chomba E, Lokangaka A, Bose CL, Bucher S, Liechty EA, Krebs NF, Yogesh Kumar S, Derman RJ, Hibberd PL, Carlo WA, Moore JL, Nolen TL, Koso-Thomas M, Goldenberg RL, Stillbirth 2010–2018: a prospective, population-based, multi-country study from the Global Network, Reprod Health. 17 (2020) 1–9. 10.1186/S12978-020-00991-Y/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kingdom JCP, Kaufmann P, Oxygen and placental villous development: Origins of fetal hypoxia, Placenta. 18 (1997) 613–621. 10.1016/S0143-4004(97)90000-X. [DOI] [PubMed] [Google Scholar]
- [25].Hermansen MC, Nucleated red blood cells in the fetus and newborn, Arch Dis Child Fetal Neonatal Ed. 84 (2001) F211–F215. 10.1136/FN.84.3.F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gibbins KJ, Silver RM, Pinar H, Reddy UM, Parker CB, Thorsten V, Willinger M, Dudley DJ, Bukowski R, Saade GR, Koch MA, Conway D, Hogue CJ, Stoll BJ, Goldenberg RL, Stillbirth, hypertensive disorders of pregnancy, and placental pathology, Placenta. 43 (2016) 61–68. 10.1016/J.PLACENTA.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].American College of Obstetricians and Gynecologists, Gestational Hypertension and Preeclampsia (PB 222), Obstetrics & Gynecology. 133 (2020) 168–186. [Google Scholar]
- [28].Schwartz DA, Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy, Viruses 2022, Vol. 14, Page 458. 14 (2022) 458. 10.3390/V14030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dudley DJ, Goldenberg R, Conway D, Silver RM, Saade GR, Varner MW, Pinar H, Coustan D, Bukowski R, Stoll B, Koch MA, Parker CB, Reddy UM, A new system for determining the causes of stillbirth, Obstetrics and Gynecology. 116 (2010) 254–260. 10.1097/AOG.0B013E3181E7D975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


