Abstract
This article updates and extends an earlier meta-analysis (Westerhof et al., 2014) on the longitudinal effects of subjective aging on health outcomes. A systematic search in different databases (PsycInfo, PubMed, Web of Science, and Scopus) resulted in 99 articles, reporting on 107 studies. Participants: Studies had a median sample size of 1,863 adults with a median age of 66 years. A randomized effect meta-analysis showed a significant, small effect (Likelihood Ratio (LR) = 1.347; 95% CI = 1.300 – 1.396; p < .001), similar in magnitude to the previous meta-analysis of 19 studies. Although the results showed high heterogeneity in the longitudinal link between subjective aging and health outcomes, there were no differences in effects according to chronological age of participants, welfare state status (more or less developed social security system), length of follow-up, type of health-related outcome, or quality of the study. Effects were stronger for multi-item measures of self-perceptions of aging than for the frequently used single-item measures assessing subjective age, especially for indicators of physical health. Based on this meta-analysis, building on five times more studies than the 2014 review, we consider the associations of measures of subjective aging with health and longevity across time as robust, albeit small in size. Future research should concentrate on the clarification of pathways mediating the relation between subjective aging and health outcomes, as well as potential bidirectional effects.
Keywords: subjective age, self-perceptions of aging, physical health, mental health, longevity, meta-analysis
The concept of subjective aging (SA) addresses how people reflect on their own development and aging as they move through adulthood and old age (Brandtstädter & Rothermund, 2002; Diehl et al., 2021; Wurm et al., 2017). That is, aside from using their chronological age as a marker of their position in the life course, individuals interpret their behavioral experiences with their own aging process to establish a sense of SA (Settersten & Hagestad, 2015). As has been argued, this sense of SA becomes an important part of aging individuals’ self and identity (Diehl et al., 2015, 2021; Levy, 2022). Conceptually, SA is an individual-level variable indicating how a person perceives, interprets, and evaluates their own aging against existing cultural representations of what aging and old age may mean. The latter is frequently described as age stereotyping operating at the societal level, with potential differences between societies, countries, or cultures when seen in a more global context (e.g., Löckenhoff et al., 2009; Pinquart & Wahl, 2021). SA and age stereotypes are certainly interconnected and SA has been found to mediate the connection among general age stereotypes and health at the individual level (Brothers et al., 2021).
Following an earlier meta-analysis on SA and health outcomes (Westerhof et al., 2014), the current meta-analysis focuses exclusively on consequences of SA for individuals’ development and aging, with a particular emphasis on health-related outcomes (see also Wurm et al., 2017). Given the large increase of research interest in this topic in the past decade, the current study provides an update and extension of the earlier meta-analysis on the longitudinal effects of SA on health outcomes and longevity by Westerhof et al. (2014).
Previous Research
In the previous meta-analysis, Westerhof et al. (2014) synthesized the available evidence on associations between various indicators of SA with health-related outcomes up to June 2013. Solely relying on longitudinal studies (N = 19), the authors found an overall significant effect of measures of SA on a range of health markers, including functional health, health-related quality of life, physical illnesses, and longevity (Likelihood Ratio (LR) = 1.429; 95% confidence interval = 1.273–1.604; p < .001). These findings were robust as observed effects did neither vary across different conceptualizations of SA (comparing measures of SA to measures of self-perceptions of aging—SPA), nor by study quality. Furthermore, most studies controlled for a range of confounding variables, such as gender, level of education, baseline health, depressive symptoms, and loneliness. However, the analyses also revealed pronounced heterogeneity among the included studies. Studies with a shorter period of follow-up and focusing on health (versus longevity) had a stronger effect, suggesting that more proximal effects were stronger than more distal effects. Stronger effects were found in studies with younger participants than older participants (age at baseline in the studies varied between 57 and 85 years, with a median age of 63 years), suggesting that the effects of SA are stronger when age-related health problems have not yet emerged and might still be more easily influenced. Lastly, effects were stronger in countries where state provisions of welfare were minimal. Tying in with sociological descriptions of different welfare state regimes (Bambra, 2007; Deeming, 2017; Esping-Andersen, 1990), some welfare states (e.g., Scandinavian countries) give more state support to older citizens, for example, in ensuring basic health and retirement provisions. Other states, such as the United States, rely more on the responsibilities of individual citizens to care for themselves. In the first kind of welfare states provisions are often tied to chronological age, making it more relevant to incorporate chronological age in one’s SA. In the latter kind of welfare state regimes, SA might matter more for health-related outcomes as provisions are less equally distributed across individuals. In sum, measures of SA had a small but consistent and significant effect on health-related outcomes.
Because the meta-analysis of Westerhof et al. (2014) had only 19 available studies at the time, its statistical power was limited, in particular with regard to the moderation analyses. Furthermore, it was not possible to distinguish between different measures of SA beyond subjective age versus SPA or between different health outcomes beyond health and longevity. An additional limitation was that the variables used in the moderation analyses were often confounded. For example, studies focusing on health had younger samples than studies on longevity, so it was unclear whether the outcome or the sample characteristics produced the results (Westerhof et al., 2014).
A recent flurry of systematic and meta-analytical reviews has further investigated the relation of SA with health-related outcomes (Chang et al., 2020; Debreczeni & Bailey, 2021; Diehl et al., 2021; Kotter-Grühn et al., 2016; Sabatini et al., 2020; Tully-Wilson et al., 2021; Westerhof & Wurm, 2018; Wurm et al., 2017). The systematic review by Chang et al. (2020) largely focused on ageism, but also included more than 50 studies on self-perceptions of aging and health. Debreczeni and Bailey (2021) focused exclusively on subjective age and included data from 24 independent cross-sectional and longitudinal studies. Tully-Wilson et al. (2021) included longitudinal evidence from 21 independent datasets but considered only the unidimensional measure of attitudes toward one’s own aging (Lawton, 1975) as an indicator of SA. Finally, Sabatini et al. (2020) analyzed data from 6 studies that used a multidimensional measure of SA based on the concept of awareness of age-related change (Diehl et al., 2021; Diehl & Wahl, 2010). These recently published reviews (Chang et al., 2020; Tully-Wilson et al., 2020) and meta-analyses (Debreczeni & Bailey, 2021; Sabatini et al., 2020) provide further evidence for the link between SA and health outcomes.
Even though the number of studies has increased over the past decade, the picture emerging from these recent reviews and meta-analyses on the effects of SA on health-related outcomes has remained incomplete for three reasons. First, several studies focused on a single specific SA construct, and thus did not compare different, competing SA constructs. Second, several of these more recent reviews and meta-analyses (e.g., Debreczeni & Bailey, 2021; Sabatini et al., 2020) included cross-sectional studies in their study pool potentially overestimating the associations between SA and outcomes and ignoring the fact that only data from longitudinal studies permit directional conclusions. Thus, we argue that a more comprehensive and integrative analysis of SA and health outcomes is in order with the intention of updating and extending the meta-analysis of Westerhof et al. (2014) in a comprehensive way.
Update of Previous Meta-Analytical Findings
The first goal of the current meta-analysis was therefore to update the meta-analysis of Westerhof et al. (2014) across a larger number of longitudinal studies. In line with the earlier systematic reviews and meta-analyses, we hypothesized that SA is a significant predictor of health-related outcomes over time (Hypothesis 1.1). The update also included the analysis of potential moderators, expecting to confirm the earlier results. We hypothesized that effects would be similar (a) for measures of subjective age versus measures of SPA and (b) regardless of study quality (Hypothesis 1.2). Additionally, we hypothesized that effects would be larger for (a) markers of health as compared to longevity, (b) a shorter period of follow-up, (c) a younger sample, and (d) states with a less supportive welfare regime (Hypothesis 1.3).
Extension 1: Comparing different measures of SA
A major limitation of recent systematic reviews and meta-analyses is that they were selective in terms of which measures of SA were included. For example, Debreczeni and Bailey (2021) only considered subjective age, Tully-Wilson et al. (2021) only included attitudes toward own aging as SA indicators. Westerhof et al. (2014) could not make comparisons between subjective age and self-perceptions of aging and were forced to collapse unidimensional and multidimensional measures of SPA due to statistical power problems. However, no meta-analysis to date was able to make more fine-grained comparisons of measures of SA.
Authors have distinguished between several different constructs of SA (Diehl et al., 2014; Wurm et al., 2017), including subjective age (sometimes called age identity) and different conceptualizations regarding SPA. Subjective age refers to how old (or young) a person feels irrespective of their chronological age, whereas the term SPA refers to how a person interprets their own aging process (Diehl et al., 2014; Faudzi et al., 2019; Kastenbaum et al., 1972; Pinquart & Wahl, 2021). SPA may be further conceptualized as a unidimensional construct, placing adults’ perceptions on a single continuum from positive to negative (e.g., attitudes toward one’s own aging; Lawton, 1975; Miche et al., 2014), or as a multidimensional construct, capturing distinct dimensions of adults’ aging experiences, such as perceived gains and losses in particular life domains due to growing older (Brothers et al., 2018; Laidlaw et al., 2007; Marquet et al., 2016; Steverink et al., 2001).
The importance of utilizing a multidimensional approach and distinguishing between the perception of age-related gains and losses was raised already by Keller et al. (1989) Furthermore, it dates back to the fundamental insight by Baumeister et al. (2001) and an extensive body of research that negative experiences and evaluations tend to have more impact on behavior than positive ones. Although a recent study found stronger support for adults’ perceptions of age-related gains as predictors of longevity (Wurm & Schäfer, 2022), other studies have shown stronger associations between age-related losses and markers of health (Brothers et al., 2017, 2018; Dutt et al., 2018a).
Hence, we assessed the differential impact of SA measures as follows: (a) subjective age, (b) unidimensional measures like attitudes towards one’s own aging, (c) perceived age-related gains, and (d) perceived age-related losses as assessed by multidimensional measures. We expected that multidimensional measures of losses would show the strongest effects on health and longevity (Hypothesis 2.1).
Extension 2: Comparing different health outcomes
Another important limitation of the existing systematic reviews and meta-analyses is that the analyses so far have been limited in their comparisons of the effects of SA across different potential health outcomes. Health outcomes that have been studied, but could not be compared in detail are as diverse as well-being, health behaviors, biomarkers, mental health, subjective physical health, objective physical health, and longevity.
Theoretical frameworks, like the stereotype embodiment theory, have also been proposed that help to explain why SA is related to health outcomes across time (Diehl & Wahl, 2010; Levy, 2009; Weiss & Kornadt, 2018; Wurm et al., 2017). A first distinction can be made between pathways, health states, and longevity: pathways may contribute to important individual differences in health states across time (e.g., Boehmer, 2006; Levy et al., 2002; Moser et al., 2011; Wurm et al., 2007). In the long run, these pathways may contribute to premature mortality or longevity, respectively (e.g., Kotter-Grühn et al., 2009; Levy et al., 2002; Maier & Smith, 1999; Markides & Pappas, 1982; Wurm & Schäfer, 2022). Regarding health states, it has been argued that it is important to distinguish between mental health (e.g., depressive symptoms), subjective physical health (e.g., self-rated health), and objective physical health (e.g., physician-reported health; Diehl & Wahl, 2010; Wurm et al., 2017). Various pathways that have been distinguished are psychological, behavioral, and physiological pathways (Kuypers & Bengtson, 1973; Levy, 2009; Wurm et al., 2013).
Psychological pathways
Psychological pathways linking measures of SA to health indicators include, for instance, maintaining a positive perception of one’s own aging process. This is generally considered an adaptive strategy in later life because it helps to maintain a consistent and positive self-concept in a culture that generally devalues old age and older adults (Levy, 2022; Westerhof & Barrett, 2005). A more consistent and positive self-concept contributes to well-being (Mock & Eibach, 2011; Wurm et al., 2008), which, in turn, has been found to be related to health and longevity over time (Chida & Steptoe, 2008; Lamers et al., 2012).
Behavioral pathways
Behavioral pathways linking SA with health include preventive health behaviors and coping efforts. For example, individuals with younger and more positive SPA are more inclined to engage in preventive health behaviors, such as greater physical activity (e.g., Levy & Myers, 2004; Wurm et al., 2010). They are also more likely to engage in task-oriented as opposed to avoidance-oriented coping strategies (Boehmer, 2007), which, in turn, contribute to health and longevity across time.
Physiological pathways
Physiological pathways through which SA is linked to health indicators are manifold. For example, more negative SA may lead to physiological responses, such as greater cardiovascular stress (Levy et al., 2000). In addition, more negative SA has been found to be associated with higher plasma concentrations of inflammatory biomarkers, such as C-reactive protein (CRP; Stephan et al., 2015). Overall, physiological pathways seem so far the least researched and the various biomarkers are diverse and associated with health measures and longevity in complex ways (Schönstein et al., 2022).
The current meta-analysis assessed the impact of SA on a variety of health outcomes: pathways, health states, and longevity. Even though theoretical reasoning suggests that pathways might mediate between SA on the one hand and health states on the other, and that health states might mediate between pathways and longevity, few studies have actually tested these mediating effects (e.g., for an exception, see Levy & Bashivi, 2018). Hence, we can only treat pathways, health states, and longevity as separate outcomes and not their interrelations in a mediating model. Specifically, we examined the associations of SA with several health-related outcomes including: (a) psychological pathways, (b) behavioral pathways, and (c) physiological pathways, (d) mental health states, (e) subjective states of physical health, (f) objective states of physical health, and (g) longevity, or chance of survival after a number of years. We expected that the effects would be stronger for pathways (a-c) than for the mental and physical health states (d-f) as pathways theoretically contribute to the latter in the long run. We also expected that the effects would be stronger for health states (d-f) than for longevity (g), as the former contribute to longevity over time (Hypothesis 2.2).
Extension 3: Comparing different measures of SA on different health outcomes
A last limitation of existing systematic reviews and meta-analyses is that there might have been some confounding among variables used in subgroup analyses and meta-regression analyses. For example, the earlier finding that effects of SA are stronger for markers of health as compared to longevity could have also been the result of the fact that studies on markers of health had younger samples than the studies on longevity (Westerhof et al., 2014). Given the large increase in available studies in the past ten years, the present study addressed these limitations as much as possible. Specifically, we aimed to determine the differential impact of subjective age versus self-perceptions of aging on pathways, health states, and longevity. In particular, we expected that Hypothesis 2.2 would be confirmed for both measures of subjective age and measures of self-perceptions of aging. That is, we expected to see no interaction between measures of SA and measures of pathways, health states, and longevity (Hypothesis 2.3).
Method
Transparency and Openness
The study was preregistered in Prospero (CRD42020197690), an international database for prospective registration of systematic reviews. We applied guidelines from the PRISMA statement on transparent reporting on meta-analyses, in particular regarding search strategy, eligibility criteria, quality assessment, and publication bias (Moher et al., 2009). The general analytical approach for the present study was adapted from Westerhof et al. (2014). The source data were available in the articles, although some authors provided additional aggregated data upon request. Upon review, the Ethics Committee of the Faculty Behavioural, Management, and Social Sciences at the University of Twente, the Netherlands, exempted the study from ethical assessment following Dutch standards for ethical conduct of scientific research with humans (Study title: Assessing the Impact of Subjective Aging on Health-Related Outcomes: An Updated Meta-Analysis of the Evidence from Longitudinal Studies; protocol number: BFD-BMS/EC-5-2022). The complete data, PRISMA checklist, and Ethics statement are available from osf.io/62rdf (Westerhof, 2022).
Search Strategy and Selection of Studies
A systematic search was performed in four electronic databases: PsycInfo, Pubmed, Scopus, and Web of Science, up to January 26, 2022. The main search strategy was based on two key concepts: SA and longitudinal studies. The databases were searched for articles with these components in either title, abstract, or keywords. Terms referring to SA included the following while allowing for plurals and spelling differences (e.g., aging vs. ageing): age identity, aging-related cognitions, aging satisfaction, age views, aging views, attitude toward own aging, awareness of age-related change, cognitive age, felt age, images of aging, perceived age, psychological age, self-perceptions of aging, subjective age, subjective aging, and views on aging. Terms referring to longitudinal studies included the following: longitudinal, panel, prospective, or over time. In order not to limit any health outcomes beforehand, we did not use terms referring to specific health outcomes in the search strategy but rather checked whether the study was health-related during the inclusion process. Studies in peer-reviewed journals were searched, applying no limitations on publication year or language. Furthermore, the reference lists of other recent meta-analyses were cross-checked, and the authors’ expert base provided further insight on additional eligible studies, including recently published papers.
Three authors (ANB, JSS, and HYT) independently rated potentially eligible studies based on the full-text papers. All articles that were identified by the database searches were rated by two authors. Disagreements between raters were resolved by having the third rater review the paper and with all raters reaching a consensus.
Studies were included if they reported the effects of SA on health-related outcomes, such as well-being, health behaviors, coping, biomarkers, functional measures, mental health, physical health, or longevity over time. Studies were excluded when any of the following conditions were met: (a) The study addressed other facets of ageism but not SA (e.g., perceived age discrimination, perceived age by others, stereotypes about older persons, societal age views); (b) the study did not address health-related outcomes (e.g., job performance, cognitive performance, personality); (c) the study design was not longitudinal (e.g., experimental study, cross-sectional study, intervention study); (d) the study did not focus on trait-like characterizations of SA based on ecological momentary assessment designs (e.g., state-like measurements, such as daily assessment studies as they focus on short-term variations in health) because these studies offer micro-longitudinal observational intervals that could not directly be compared with traditional longitudinal studies; (e) the study did not examine the impact of SA on health-related outcomes (e.g., studies addressing change in SA), or (f) the article did not report enough data to be included in the meta-analysis (even after consulting the authors when not enough data were obtained).
The flow diagram of the study selection is shown in Figure 1. Searching databases resulted in 1,088 unique records. After removing 629 duplicate records, 459 articles remained. Cross-checking the expert base and reference lists of other meta-analyses resulted in 78 additional articles. Of these 537 articles, 438 were excluded based on full-text reviews (see Figure 1 for reasons). This process resulted in a total of 99 articles included in the updated meta-analysis: five times more than the nineteen studies included in the meta-analysis from about a decade ago (Westerhof et al., 2014).
Figure 1.
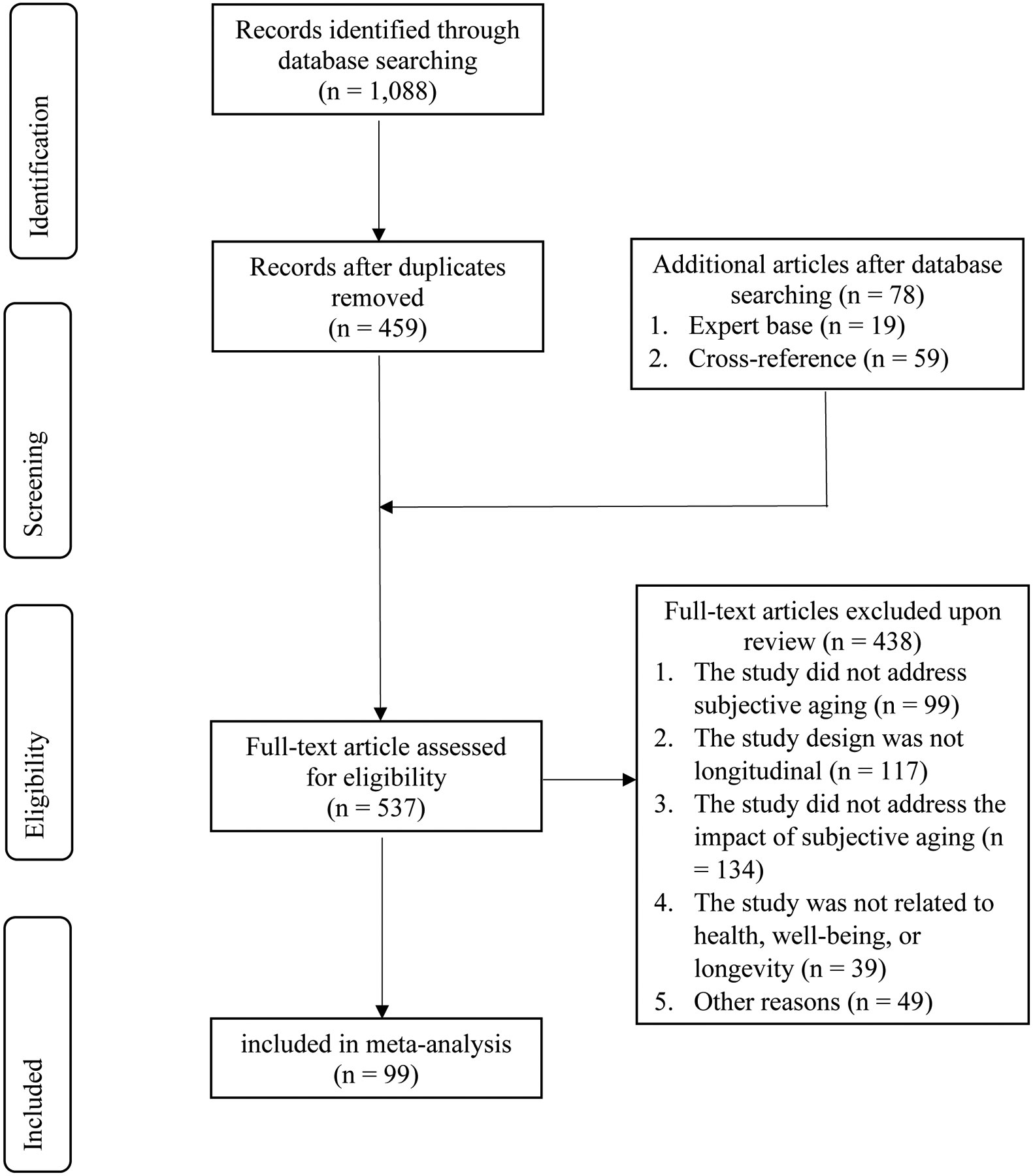
Flow Diagram of Study Selection
Data Extraction and Meta-Analytic Strategy
We used the software Comprehensive Meta-Analysis (CMA; Borenstein et al., n.d.) to meta-analytically combine study findings. The articles reported hazard ratios, risk ratios, odds ratios, likelihood ratios, regression coefficients, standardized effects in structural equation models, effects in multilevel models, correlations, or means. When both bivariate relations of SA to health-related outcomes and more advanced analyses were reported (e.g., controlling for confounders like demographic variables, psychosocial functioning, and baseline health variables), the results of the latter were used for the meta-analysis. We used findings of unidirectional models (e.g., the effect of SA on health) even when bidirectional models (e.g., including the effects of both SA on health and health on SA) were available to make the best possible comparison between studies. Similarly, we used the baseline measure of SA and the follow-up health-related outcome rather than change across time as not all studies assessed change across time. In cases where sufficient data were not available in the published article, we contacted authors of published studies to request standardized estimates.
Two articles included two studies and three articles included three studies with independent sampling of participants. These were considered separate studies, so the 99 articles reported findings from a total of 107 independent studies. We refer to a total of 107 studies (rather than 99 articles) in the remainder of the article. When studies presented findings at multiple time points, the findings for the longest follow-up were used. In total, this resulted in findings from 252 analyses. Several studies reported more than one health-related outcome, so a meta-analysis for the particular study was done to include only the average effect size per study. However, when a study reported results of SA measures that fell into different categories (e.g., both subjective age and SPA), the study results were analyzed separately in the subgroup analyses. The same logic was applied when health-related outcomes were reported across categories (e.g., both mental and physical health).
Hazard, risk, or odds ratios with their 95% confidence intervals (CI) were extracted. When regression coefficients or correlations were reported, these were converted to odds ratios in CMA. Likelihood ratios (LR; Lamers et al., 2012) were used to refer to different ratios (hazard, risk, and odds ratios). All ratios were computed so that LRs above 1 indicate a positive association of SA to health-related outcomes. All LRs were weighted by the inverse of their standard errors. A LR was considered statistically significant if the 95% CI did not include the value of 1. As we expected heterogeneity across studies, a random-effects meta-analysis was performed. This assumes that the studies are estimating different but related effects rather than being replicas of each other. It also adjusts the study weights according to the level of heterogeneity in order to compute the 95% CI around the pooled effect estimate (Deeks et al., 2008).
Study quality was assessed with a protocol that provides a total score for each included study. The protocol was based on the quality checklists outlined by Wong et al. (2008) and by Lamers et al. (2012), and reflects the protocol used in the Westerhof et al. (2014) meta-analysis. To note, the retention rate criterion utilized in the Westerhof et al. (2014) meta-analysis was replaced with the attrition information criterion to account for more information in the current meta-analysis. A total of six quality criteria were applied and coded as 0 (not applicable) or 1 (applicable): probability sampling (n = 70), response rate 60% or above (n = 37), attrition information (n = 68), multi-item scale with Cronbach’s alpha .70 or higher (n = 39), control for any confounding variables, such as gender, chronological age, level of education, and loneliness (n = 97), and control for baseline values of outcome variables (n = 61; not coded for studies on longevity). The overall quality of the study was assessed by counting the number of applicable items and dividing them either by five (i.e., for longevity studies) or six (i.e., for all other studies), which resulted in scores between 0 and 1. Based on the scoring categorization applied in Westerhof et al. (2014), studies were classified in three groups: scores ≤ .33 (n = 10), between .34 and .66 (n = 39), and ≥ .67 (n = 58). The information to rate study quality was extracted by ANB, AB, and HYT, and was double-checked by GW. Discrepancies were resolved by reassessing the criteria for the article and, if necessary, generating a new study quality score.
The analysis proceeded in several steps. To assess Hypothesis 1.1, an analysis was performed to estimate the overall effect across all studies. We also examined heterogeneity or the variation in effect sizes between studies. The Q-test indicates the probability of heterogeneity, and the I2 index indicates its magnitude (0–30% is low; 30–75% is moderate; 75–100% is high; Deeks et al., 2008). Publication bias towards an overreporting of positive findings was assessed with three indices (Ferguson & Brannick, 2012): the funnel plot, skewness, and Egger’s test of intercept. The funnel plot examines effect size (LR) against standard error. Skewed distributions towards the left or right indicate a possible publication bias. The Egger’s test of intercept is the correlation between the precision of the study (i.e., the inverse of the standard error) and the standardized effect (i.e., the effect size divided by its standard error). Duval and Tweedie’s (2000) trim and fill analysis estimates effect sizes after correcting for publication bias.
To assess Hypothesis 1.2 and 1.3, four subgroups and two meta-regression analyses were performed similar to those reported in Westerhof et al. (2014). SA measures were categorized into measures of subjective age versus measures of SPA, whereas outcomes were categorized into health-related outcomes versus longevity. We also extracted the length of follow-up in years (e.g., always the longest follow-up reported in a study), the average age of the sample (e.g., at the first, baseline measurement of the study), and the welfare support system in the country where the study was done (e.g., USA, Canada, Australia, United Kingdom with less state support and Germany, Switzerland, Finland with more state support; Esping-Andersen, 1990; Bambra, 2007). Subgroup analyses compared the effect sizes according to SA measures (i.e., subjective age versus SPA), outcome measures (i.e., health-related outcomes versus longevity), welfare regimes (i.e., less or more state support), and quality scores (i.e., ranging from 1 to 3). Unrestricted maximum likelihood mixed effects meta-regression regressed the effect size per study on the time of follow-up and the average sample age.
To assess Hypothesis 2.1, SA measures were grouped into four categories: (a) subjective age, (b) unidimensional measures of self-perceptions of aging, (c) multidimensional gain-oriented, and (d) multidimensional loss-oriented assessments of self-perceptions of aging. The categorization was based on the actual use of the instrument. For example, some studies divided the attitudes towards aging instrument into subscales of gains and losses (e.g., Mejia et al., 2020), even though it is originally a unidimensional instrument (Lawton, 1975).
To assess Hypothesis 2.2, health-related outcomes were divided into seven categories: (a) psychological pathways (measures of well-being, like life satisfaction, positive affect, negative affect, psychological well-being), (b) behavioral pathways (measures like physical activity, preventive health behavior, coping behavior), (c) physiological measures (e.g., biomarkers), (d) mental health states (e.g., depressive symptoms, anxiety symptoms), (e) subjective physical health states (e.g., self-reported health conditions, self-reported hospitalizations, self-rated health), (f) objective physical health states (e.g., frailty or diagnosed diseases, dementia-related disorders), and (g) longevity.
Hypothesis 2.3 stated that the effects of different measures of SA would be similar for different measures of health-related outcomes. To have enough studies within each category, SA measures were divided into two categories (subjective age and SPA) and health-related outcomes in four categories (pathways: a-c, mental health: d, physical health: e-f, and longevity: g). In this way, similarities and differences in health-related outcomes could be assessed separately for subjective age and for SPA and then compared to each other.
Results
Descriptive Findings
Table 1 provides an overview of the 107 studies included. The median year of publication was 2018 (ranging from 1982 to 2022). The median sample size of the studies was 1,863 participants, varying from 58 to 18,373 participants. The median of the average participant age was 66 years (ranging from 40 to 90 years). The median follow-up time was 4.5 years, varying between 2 weeks and 23 years.
Table 1.
Overview of Included Studies
| Author(s) (year) | Study | Country | N | Follow-up (yr) | Study Quality | Mean age (yr) | Predictors | Health Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub age | SPA | Path | Ment hlth | Phys hlth | Long | ||||||||
| 1 | Avidor, Levi-Beltz, & Solomon (2021) | IL | 125 | 7 | 50 | 58 | X | X | |||||
| 2.1 | Avidor, Palgi, & Solomon (2021) | Study 1 | IL | 226 | 6 | 50 | 65 | X | X | ||||
| 2.2 | Avidor, Palgi, & Solomon (2021) | Study 2 | IL | 132 | 1 | 67 | 66 | X | X | ||||
| 3 | Ayalon (2016) | HRS | USA | 4,121 | 4 | 100 | 74 | U | Sub | ||||
| 4 | Barnes-Farrell & Petery (2018) | HRS | USA | 2,156 | 4 | 83 | 69 | X | X | Sub | |||
| 5 | Barrett & Toothman (2016) | MIDUS | USA | 872 | 9 | 83 | 50 | X | Psy | ||||
| 6 | Benyamini & Burns (2019) | RAH | USA | 851 | 10 | 40 | 73 | X | X | ||||
| 7 | Beyer et al. (2015) | DEAS | FRG | 277 | 2.5 | 83 | 73 | U | Beh | Sub | |||
| 8 | Beyer et al. (2019) | DEAS | FRG | 2,367 | 3 | 83 | 73 | G,L | Beh | ||||
| 9 | Boeder & Tse (2020) | DEAS | FRG | 3,745 | 6 | 67 | 61 | U,L,G | Psy | X | Sub | ||
| 10 | Boehmer (2006) | BLS | FRG | 159 | 0.42 | 0 | 63 | X | Sub | ||||
| 11 | Boehmer (2007) | BLS | FRG | 159 | 0.42 | 0 | 63 | X | Psy, Beh | Sub | |||
| 12 | Brothers et al. (2016) | FRG, USA | 537 | 2.5 | 33 | 64 | G,L | Psy | |||||
| 13 | Cheng et al. (2012) | HKG | 83 | 0.25 | 33 | 58 | U | Ob | |||||
| 14 | Choi & Dinitto (2014) | NHATS | USA | 5,371 | 1 | 50 | 74 | X | X | ||||
| 15 | Cohn-Schwartz et al. (2020) | HRS | USA | 1,823 | 8 | 100 | 68 | U | X | Sub | |||
| 16 | Dutt, Gabrian, & Wahl (2018) | FRG | 356 | 2.5 | 67 | 63 | G,L | X | |||||
| 17 | Dutt & Wahl (2019) | FRG | 299 | 4.5 | 67 | 63 | L | X | |||||
| 18 | Dutt, Wahl, & Rupprecht (2018) | FRG | 356 | 5 | 50 | 63 | G,L | X | |||||
| 19 | Fass et al. (2020) | DEAS | FRG | 1,027 | 12 | 83 | 64 | L | Sub | ||||
| 20 | Freeman et al. (2016) | TILDA | IE | 6,065 | 2 | 50 | 63 | L | X | ||||
| 21 | Fundenberger et al. (2020) | NHATS | USA | 1,679 | 7 | 67 | 75 | X | Sub | ||||
| 22 | Gale et al. (2018) | LBC1936 | GB | 271 | 7 | 50 | 79 | G,L | Beh | ||||
| 23 | Gale & Cooper (2018) | ELSA | GB | 3,505 | 6 | 83 | 70 | L | Ob | ||||
| 24 | Gum & Ayalon (2018) | HRS | USA | 4,606 | 4 | 100 | 65 | U | X | ||||
| 25 | Hajek & König (2020) | DEAS | FRG | 18,373 | 15 | 67 | 65 | X | Psy | ||||
| 26 | Han (2018) | HRS | USA | 3,382 | 4 | 100 | 74 | U | X | Sub | |||
| 27 | Han & Richardson (2015) | HRS | USA | 3,921 | 4 | 100 | 65 | U | X | ||||
| 28 | Kaspar et al. (2021) | FRG | 1,863 | 3.5 | 40 | 87 | G,L | X | |||||
| 29 | Kim et al. (2014) | HRS | USA | 6,177 | 4 | 83 | 71 | U | Beh | ||||
| 30 | Klusmann et al.(2019) | KLS | FRG | 557 | 1 | 50 | 44 | G | Beh | ||||
| 31 | Kotter-Grühn et al. (2009) | BASE | FRG | 496 | 16 | 80 | 85 | X | U | X | |||
| 32 | Kwak et al. (2014) | HRS | USA | 5,938 | 4 | 100 | 65 | U | X | ||||
| 33 | Lahav et al. (2020) | IL | 88 | 15 | 33 | 64 | X | Phy | |||||
| 34 | Levy et al. (2018) | HRS | USA | 4,765 | 4 | 50 | 72 | U | Ob | ||||
| 35 | Levy & Bavishi (2018) | HRS | USA | 4,149 | 6 | 80 | 68 | U | X | ||||
| 36 | Levy & Myers (2004) | OLSAR | USA | 241 | 20 | 50 | 57 | U | Beh | ||||
| 37 | Levy & Myers (2005) | OLSAR | USA | 620 | 23 | 60 | 63 | U | X | ||||
| 38 | Levy & Slade (2019) | HRS | USA | 5,702 | 6 | 50 | 65 | U | Beh | ||||
| 39 | Levy, Slade, & Kasl (2002) | OLSAR | USA | 433 | 20 | 50 | 62 | U | Sub | ||||
| 40 | Levy, Slade, Kunkel, & Kasl (2002) | OLSAR | USA | 660 | 23 | 60 | 63 | U | X | ||||
| 41 | Li et al. (2021) | NHATS | USA | 2,592 | 4 | 50 | 75 | X | Ob | ||||
| 42 | Liang (2018) | SSAPURs | CN | 5,702 | 4 | 83 | 70 | X | Sub | ||||
| 43 | Liang (2020) | SSAPURs | CN | 5,612 | 4 | 83 | 70 | X | X | ||||
| 44 | Lim et al. (2013) | ACPC | USA | 290 | 1 | 20 | 63 | X | X | ||||
| 45 | Losada-Baltar, et al. (2021) | ES | 1,549 | .04 | 50 | 43 | U | X | |||||
| 46 | Luo & Li (2020) | HRS | USA | 10,212 | 6 | 83 | 66 | U | Sub | ||||
| 47 | Maier & Smith (1999) | BASE | FRG | 513 | 4.5 | 60 | 85 | U | X | ||||
| 48 | Markides & Pappas (1982) | SATX | USA | 460 | 4 | 60 | 65 | X | X | ||||
| 49 | McLachlan et al. (2020) | LBC 1936 | GB | 758 | 8 | 40 | 73 | G,L | Phy | ||||
| 50 | Mejía et al. (2017) | HRS | USA | 1,231 | 6 | 83 | 66 | U | Sub | ||||
| 51 | Mejía et al. (2020) | HRS | USA | 2,717 | 4 | 83 | 66 | G,L | Phy | Ob | |||
| 52 | Mock & Eibach (2011) | MIDUS | USA | 1,170 | 10 | 50 | 53 | X | Psy | ||||
| 53 | Moser et al. (2011) | LC65+ | CH | 883 | 1 | 33 | 69 | U | Sub | ||||
| 54 | Nieves-Lugo, et al. (2021) | USA | 549 | 4.5 | 33 | 61 | X | U | Ob | ||||
| 55 | Palgi et al. (2018) | HRS | USA | 4,938 | 6 | 50 | 69 | X | X | Sub | |||
| 56 | Palgi et al. (2019) | IL | 132 | 2 | 50 | 65 | X | X | |||||
| 57 | Petashnick, et al. (2022) | IL | 164 | 3 | 67 | 81 | X | Phy | X | Sub | |||
| 58 | Qiao et al. (2021) | ELSA | GB | 6,475 | 11 | 50 | 65 | X | Ob | ||||
| 59 | Rippon & Steptoe (2015) | ELSA | GB | 6,489 | 9 | 40 | 66 | X | X | ||||
| 60 | Rippon & Steptoe (2018) | ELSA | GB | 7,546 | 4 | 67 | 66 | X | X | Sub | |||
| 61 | Sargent-Cox et al. (2012) | ALSA | AUS | 1,212 | 16 | 67 | 77 | U | Ob | ||||
| 62 | Sargent-Cox et al. (2014) | ALSA | AUS | 1,507 | 16 | 40 | 77 | U | X | ||||
| 63 | Schroyen et al. (2017) | BE | 58 | 1 | 67 | 74 | U | Psy | Sub | ||||
| 64 | Schroyen et al. (2020) | BE | 140 | 6 | 40 | 73 | U | X | |||||
| 65 | Segel-Karpas et al. (2021) | MIDUS | USA | 3,591 | 8 | 100 | 69 | U | X | ||||
| 66 | Siebert et al. (2018) | ILSE | FRG | 260 | 12 | 67 | 63 | U | Ob | ||||
| 67 | Spuling et al. (2013) | DEAS | FRG | 3,038 | 6 | 50 | 61 | X | X | Sub | |||
| 68.1 | Stephan et al. (2015) | HRS | USA | 2,023 | 4 | 67 | 74 | X | Phy | ||||
| 68.2 | Stephan et al. (2015) | NHATS | USA | 3,279 | 2 | 67 | 76 | X | Phy | ||||
| 69.1 | Stephan et al. (2016) | MIDUS | USA | 3,209 | 9 | 50 | 47 | X | Sub | ||||
| 69.2 | Stephan et al. (2016) | HRS | USA | 3,779 | 4 | 50 | 68 | X | Sub | ||||
| 69.3 | Stephan et al. (2016) | NHATS | USA | 3,418 | 2 | 50 | 76 | X | Sub | ||||
| 70 | Stephan et al. (2019) | HRS | USA | 3,339 | 4 | 83 | 69 | X | Phy | ||||
| 71.1 | Stephan, Sutin, Bayard, & Terracciano (2017) | MIDUS | USA | 2,350 | 9 | 67 | 56 | X | Beh | ||||
| 71.2 | Stephan, Sutin, Bayard, & Terracciano (2017) | HRS | USA | 4,066 | 6 | 67 | 68 | X | Beh | ||||
| 71.3 | Stephan, Sutin, Bayard, & Terracciano (2017) | NHATS | USA | 3,541 | 3 | 67 | 76 | X | Beh | ||||
| 72 | Stephan, Sutin, Luchetti, & Terracciano (2017) | HRS | USA | 5,748 | 4 | 83 | 74 | X | Ob | ||||
| 73 | Stephan, Sutin, Luchetti, & Terracciano (2018) | NHATS | USA | 4,262 | 4 | 83 | 76 | X | Ob | ||||
| 74 | Stephan, Sutin, Luchetti, & Terracciano (2021) | HRS | USA | 2,253 | 7 | 83 | 67 | X | Phy | ||||
| 75.1 | Stephan, Sutin, & Terracciano (2018) | MIDUS | USA | 4,898 | 19 | 60 | 48 | X | X | ||||
| 75.2 | Stephan, Sutin, & Terracciano (2018) | HRS | USA | 6,220 | 6 | 60 | 70 | X | X | ||||
| 75.3 | Stephan, Sutin, & Terracciano (2018) | NHATS | USA | 6,494 | 3 | 60 | 77 | X | X | ||||
| 76 | Stephan, Sutin, Wurm & Terracciona (2021) | HRS | USA | 10,695 | 9 | 33 | 69 | X | U | Sub | |||
| 77 | Sun et al. (2017) | HRS | USA | 4,735 | 4 | 100 | 69 | U | Sub | ||||
| 78 | Tovel et al. (2019) | IL | 892 | 2 | 83 | 81 | U | Sub | |||||
| 79 | Uotinen et al. (2005) | EP | FIN | 1,165 | 13 | 20 | 73 | X | X | ||||
| 80 | Veenstra et al. (2020) | NorLAG | NO | 4,502 | 15 | 83 | 58 | X | Psy | Sub | |||
| 81 | Veenstra et al. (2021) | NorLAG | NO | 1,432 | 10 | 80 | 73 | X | X | ||||
| 82 | Warmoth et al. (2018) | ELSA | GB | 2,418 | 6 | 100 | 70 | U | Ob | ||||
| 83 | Wettstein, Spuling, Cengia, & Nowossadeck (2021) | DEAS | FRG | 5,039 | 3 | 50 | 64 | X | Sub | ||||
| 84 | Wettstein, Wahl, & Kornadt (2021) | DEAS | FRG | 4,588 | 3 | 67 | 64 | X | U,G,L | X | |||
| 85 | Wettstein, Wahl, & Siebert (2020) | ILSE | FRG | 894 | 20 | 67 | 54 | U | Ob | ||||
| 86 | Wettstein, Wahl & Spuling (2021) | DEAS | FRG | 2,499 | 9 | 83 | 71 | U,G,L | Sub | ||||
| 87 | Whitehead (2019) | USA | 89 | .67 | 50 | 77 | U | Phy | Sub | ||||
| 88 | Wienert et al. (2015) | FRG | 541 | .08 | 67 | 40 | X | Beh | |||||
| 89 | Wienert et al. (2017) | FRG | 571 | .08 | 50 | 41 | X | Beh | |||||
| 90 | Wolff et al. (2017) | DEAS | FRG | 252 | 2.5 | 100 | 73 | L | Psy | Sub | |||
| 91 | Wurm et al. (2007) | DEAS | FRG | 1,286 | 6 | 83 | 57 | G,L | Sub | ||||
| 92 | Wurm et al. (2008) | DEAS | FRG | 1,286 | 6 | 67 | 57 | G | Psy | Sub | |||
| 93 | Wurm et al. (2010) | DEAS | FRG | 1,286 | 6 | 83 | 57 | G | Beh | ||||
| 94 | Wurm et al. (2013) | DEAS | FRG | 678 | 0.5 | 100 | 73 | L | Psy | Sub | |||
| 95 | Wurm & Benyamini (2014) | DEAS | FRG | 1,286 | 3 | 83 | 62 | L | X | Sub | |||
| 96 | Wurm & Schäfer (2022) | DEAS | FRG | 2,400 | 23 | 80 | 59 | X | G,L | X | |||
| 97 | Zee & Weiss (2019) | MIDUS | USA | 5,762 | 14 | 67 | 59 | X | Phy | ||||
| 98 | Zhang et al. (2020) | CLHLS | CN | 10,051 | 8 | 60 | 90 | L | X | ||||
| 99 | Zhao et al. (2017) | CLHLS | CN | 10,051 | 9 | 60 | 85 | U | X | ||||
Notes: Study Abbreviations: ALSA=Australian Longitudinal Study BASE=Berlin Aging Study BLS=Berlin Longitudinal Study on Quality of Life after Tumor Surgery CLHLS= Chinese Longitudinal Healthy Longevity Survey EP=Evergreen Project DEAS=German Aging Survey ELSA=English Longitudinal Study of Ageing HRS=Health and Retirement Study ILSE=Interdisciplinary Longitudinal Study of Adult Development KLS=Konstanz Life Study (not currently abbreviated but we could - Klusmann) LBC1936=Lothian Birth Cohort 1936 LC65+=Lausanne Cohort Study MIDUS=Survey on Midlife in the United States NHATS=National Health and Aging Trends Study NorLAG=Norwegian Life Course, Ageing and Generation Study OLSAR= Ohio Longitudinal Study of Aging and Retirement RAH =Rutgers Aging and Health Study SSAPURs=Sample Survey of the Aged Population in Urban/Rural China SATX=Survey in San Antonio, Texas SSP=Study on chronically institutionalized Schizophrenia Patients TILDA= The Irish Longitudinal Study on Ageing
Country Abbreviations: AUS=Australia BE=Belgium CH=Switzerland CN=China ES=Spain FIN=Finland DE=Germany GB=United Kingdom HKG=Hong Kong IE=Ireland IL= Israel NO=Norway RU=Russia USA=United States
- Sub Age = Subjective Age
- SPA = Self-Perceptions of Aging
- U = Unidimensional
- L = Multidimensional, loss-oriented
- G = Multidimensional, gains-oriented
Health Outcome Categorization:
- Psy = Psychological Pathways (e.g, life satisfaction, positive or negative affect, psychological well-being)
- Beh = Behavioral Pathways (e.g, physical activity, preventive health services, coping)
- Phy = Physiological Pathways (e.g, biomarkers)
Ment. Hlth = Mental Health States (e.g, depressive symptoms, anxiety symptoms)
- Sub = Subjective indicators of physical health (e.g, self-reported health conditions, self-reported hospitalizations, self-rated health
- Ob = Objective indicators of physical health (e.g, frailty or diagnosed diseases)
Long = Longevity
First, a total of 52 studies assessed subjective age, with the majority using a single-item felt-age question (n = 44 studies); 60 studies focused on SPA, with 27 studies using the Attitude Toward Own Aging (ATOA) subscale of the Philadelphia Geriatric Center Morale Scale (Lawton, 1975), 9 studies used the Aging-Related Cognition-Scales (AgeCog; Steverink et al., 2001; Wurm et al., 2007), 5 used the Awareness of Age-Related Change questionnaire (AARC; Brothers et al., 2018; Kaspar et al., 2019), 4 used the Attitudes to Aging Questionnaire (AAQ; Laidlaw et al., 2007; Marquet et al., 2016), and 1 used the Images of Aging Scale as adapted for self-ratings (IAS; Levy et al., 2004). Studies using instruments to assess SPA were further divided into unidimensional measures (e.g., ATOA) and multidimensional measures (e.g., AgeCog, AARC, AAQ, IAS).
Second, for health-related outcome variables, 11 studies used measures of psychological pathways, 14 studies assessed behavioral pathways, 10 studies focused on physiological pathways, 25 studies used indicators of mental health, 35 used subjective indicators of physical health, 13 measured objective indicators of physical health, and longevity was included as an outcome variable in 20 studies.
Studies were conducted in the United States (n = 49), Europe (n = 45), Australia (n = 2), Israel (n = 7), Hong Kong (n = 1), and China (n = 4). Welfare regime for the country in which the study took place was also accounted for in the current meta-analysis. Israel and the European countries of Germany, Switzerland, Spain, Belgium, Finland, and Norway were categorized as having more state support, and the United States, Hong Kong, Ireland, Great Britain, China, and Australia as having less state support.
Update of Earlier Results
To assess Hypothesis 1.1, the overall effects of SA on health-related outcomes were analyzed. Figure 2 provides an overview of the results of the meta-analysis. Likelihood Ratios (LR) of 1 indicate no relation, LR higher than 1 show that more youthful and positive SA were related to more positive health-related outcomes, and those LR lower than 1 show that more youthful and positive SA were related to less positive health-related outcomes. The overall effect was significant (LR = 1.346; 95% CI = 1.299 – 1.396; p < .001). It corresponds to a correlation of .12 and can be interpreted as small in size (Chen et al., 2010). The size of the effect was similar to the result from the previous meta-analysis as the confidence intervals overlapped (LR = 1.429; 95% CI = 1.273 – 1.604; Westerhof et al., 2014). Hence, as expected, more youthful and positive SA were related to more positive health-related outcomes.
Figure 2a.
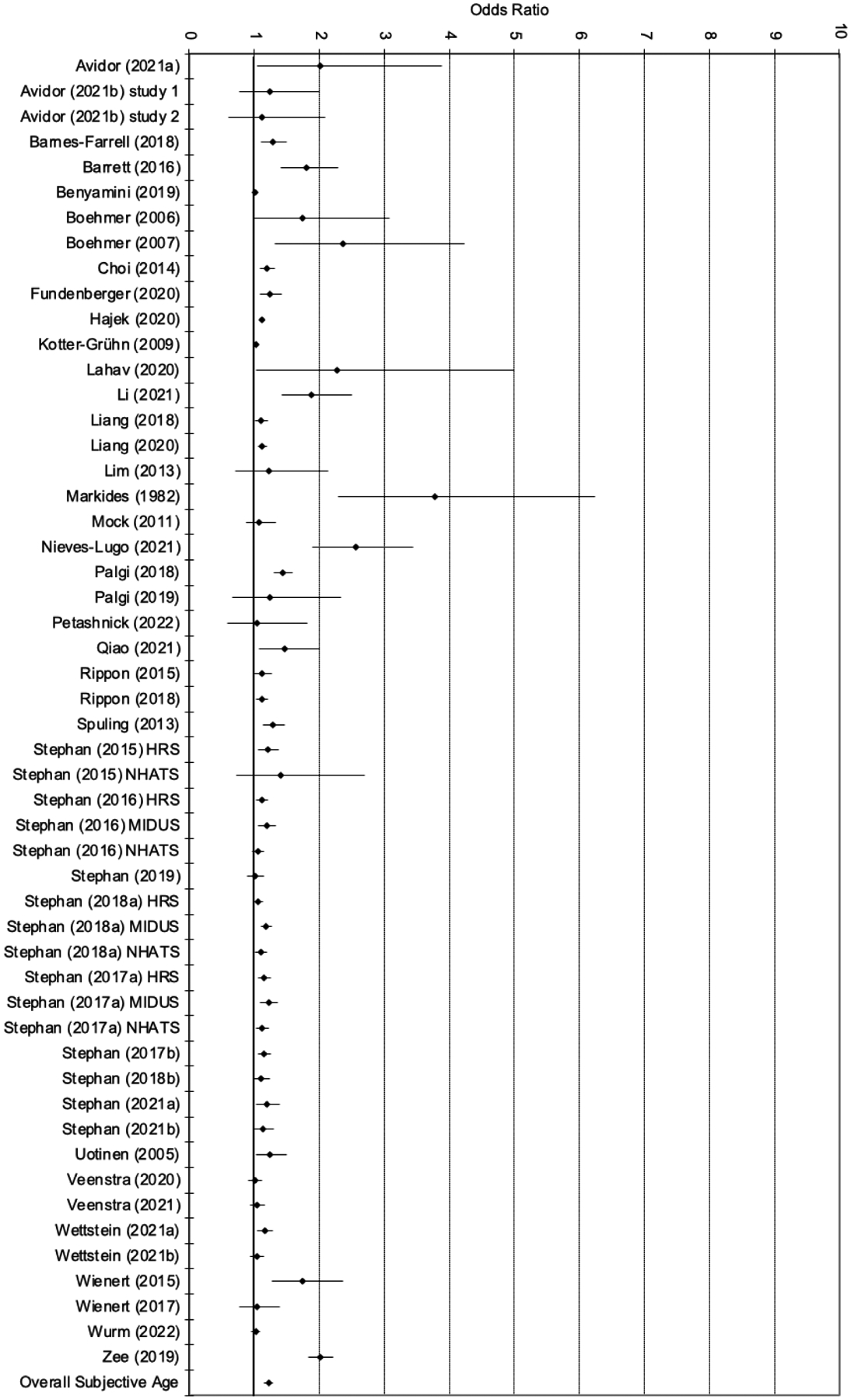
Forest plot of effect sizes (95% confidence interval; Subjective Age)
The effect sizes differed across studies between LR = 1.004 (Veenstra et al., 2021b) and LR = 3.772 (Markides & Pappas, 1982). Significant effects in the expected direction (e.g., positive SPA or younger subjective age predicted health/longevity) were reported in 79 studies (74%), whereas 28 studies (26%) reported no significant effects. None of the studies reported any significant reverse effect (e.g., younger subjective age or positive SPA associated with poorer health outcomes). Hence, not all studies supported Hypothesis 1.1, but when significant effects were found, they supported the hypothesis, such that more positive SA was associated with better health-related outcomes. The variability of the effect sizes was significantly larger than would be expected from sampling error alone (Q106 = 2932.3; p < .001; I2 = 96.4). Some studies had LRs above 3.0 and could be considered possible outliers (Cheng et al., 2012; Markides & Pappas, 1982; Schroyen et al., 2017, 2020). As these studies were among the smaller ones in terms of number of participants, the overall effect was still significant and small after their exclusion (LR = 1.335; 95% CI = 1.288 – 1.384; p < .001). Similarly, the variability was still significant and high (Q102 = 2891.6; p < .001; I2 = 96.5).
With regard to publication bias, the funnel plot shows a concentration of studies on the right. The Egger’s test of intercept was significant (t105 = 4.726; p < .001), which indicates a significant positive correlation between study precision and standardized effect. The trim and fill analysis showed that looking for missing studies to the left of the mean resulted in an adjusted value with 9 trimmed studies (LR = 1.305; 95% CI = 1.258 – 1.353), which is not significantly different from the value in the current meta-analysis (LR = 1.346; 95% CI = 1.299 – 1.396; p < .001). Keeping heterogeneity and possible publication bias in mind, these findings provided longitudinal support for Hypothesis 1.1 and SA as a predictor of subsequent health-related outcomes.
Subgroup analyses and meta-regressions updating the results from the previous meta-analysis might explain some of the heterogeneity of effects. First, the predictor variables were categorized as measures of subjective age on the one hand and measures of SPA on the other hand. As can be seen in Table 2, the predictive effects were stronger for SPA measures than for subjective age measures. Second, the study quality (e.g., categorized as low, intermediate, or high) moderated the effect sizes. Specifically, the effect sizes were higher for studies with lower quality. Third, when the health-related outcomes were grouped into two classes (health versus longevity), no significant differences were found. Fourth, a meta-regression was carried out for the number of years between the baseline measurement and the follow-up (e.g., range between 2 weeks and 23 years). The length of the follow-up period was not significantly related to outcomes (slope = 0.0002; 95% CI = −0.010 – 0.010; p = .975). Fifth, the average age of the sample at baseline was used in a meta-regression analysis with the average age ranging between 40 and 90 years. No significant relation was found with effect sizes (slope = −0.002; 95% CI = −0.008 – 0.003; p = .399); studies with older participants showed less strong effects. Lastly, a country’s welfare regime was assessed as a possible moderator of the effects; however, there were no differences between countries with more or less state support (Table 2).
Table 2.
Predictive Effects of SA Measures on Health Outcomes: Update of the Subgroup Analyses of Westerhof et al. (2014) based on N = 107 studies
| N | LR | 95% CI | Q | df | p | |
|---|---|---|---|---|---|---|
| Measures of subjective aging1 | 23.7 | 1 | <.001 | |||
| Subjective age | 52 | 1.217 | 1.155–1.283 | |||
| Self-perceptions of aging | 61 | 1.455 | 1.385–1.529 | |||
| Study quality | 9.6 | 2 | .008 | |||
| Lower | 10 | 1.673 | 1.419–1.972 | |||
| Intermediate | 39 | 1.277 | 1.195–1.365 | |||
| Higher | 58 | 1.371 | 1.302–1.443 | |||
| Outcome measure | 0.8 | 1 | .383 | |||
| Health | 87 | 1.362 | 1.305–1.422 | |||
| Longevity | 20 | 1.306 | 1.199–1.422 | |||
| Welfare state regime2 | 0.3 | 1 | .555 | |||
| Less state support | 63 | 1.334 | 1.274–1.396 | |||
| More state support | 43 | 1.366 | 1.281–1.457 |
N = Number of studies; LR = Likelihood Ration; CI = Confidence Interval; df = degrees of freedom
Four studies measured both subjective age and self-perceptions of aging
One study was carried out in both USA and Germany
To control for possible confounding relations between the different subgroups and moderators, a multivariate meta-regression was conducted, including measure of SA (subjective age versus SPA), study quality (lower, intermediate, higher), outcome type (health versus longevity), length of follow-up, average sample age, and welfare state (more or less support). As in the bivariate analyses, only the type of SA measure and the study quality were significant: Measures of SPA and studies with lower quality had a stronger effect on health-related outcomes.
These findings were only partly in line with the earlier meta-analysis and our corresponding hypotheses 1.2 and 1.3. In particular, studies using measures of SPA had a stronger effect than studies focusing on subjective age for both health and longevity, and studies with a lower quality had stronger effects. In contrast, no such differences had been found in the 2014 meta-analysis. Furthermore, the updated meta-analysis no longer supported significant differences in effect sizes according to length of follow-up, average sample age, or welfare regime.
Extension 1: Comparing Different Measures of SA
The second goal was to extend the analyses of the previous meta-analysis (Westerhof et al., 2014). The effects of different operationalizations of SA and the effects on different health-related outcomes could be analyzed with more precision due to the larger number of studies and broader variation in instruments. The SA measures were categorized into four classes: subjective age, attitudes towards own aging, multidimensional measures of age-related gains, and multidimensional measures of age-related losses. Table 3 shows that effects of the instruments focusing on perceptions of age-related gains were the only ones of the measures of SPA that did not significantly differ from the effects of subjective age measures. However, there were no significant differences among the three types of measures used to assess SPA. Hence, Hypothesis 2.1, which proposed that the effect of multidimensional measures of losses would show the strongest health effects was not supported by the data.
Table 3.
Subgroup Analyses for Predictor and Outcome Measures
| N | LR | 95% CI | Q | df | p | |
|---|---|---|---|---|---|---|
| SA measures | 25.5 | 3 | <.001 | |||
| Subjective age | 52 | 1.233 | 1.154–1.316 | |||
| Self-perceptions of aging | ||||||
| Unidimensional | 39 | 1.579 | 1.464–1.703 | |||
| Losses | 21 | 1.488 | 1.343–1.650 | |||
| Gains | 16 | 1.373 | 1.220–1.546 | |||
| Health measures | 7.4 | 6 | .283 | |||
| Psychological pathways | 9 | 1.351 | 1.128–1.617 | |||
| Behavioral pathways | 14 | 1.256 | 1.093–1.442 | |||
| Physiological pathways | 11 | 1.441 | 1.227–1.693 | |||
| Mental health | 25 | 1.529 | 1.381–1.693 | |||
| Subjective physical health | 35 | 1.391 | 1.279–1.513 | |||
| Objective physical health | 14 | 1.499 | 1.307–1.719 | |||
| Longevity | 20 | 1.327 | 1.190–1.481 | |||
| Health measures by SA measures | 19.0 | 7 | <.001 | |||
| Subjective age | ||||||
| All three pathways | 16 | 1.282 | 1.130–1.454 | |||
| Mental health | 12 | 1.362 | 1.169–1.587 | |||
| Physical health | 21 | 1.290 | 1.160–1.436 | |||
| Longevity | 11 | 1.154 | 0.999–1.332 | |||
| Self-perceptions of aging | ||||||
| All three pathways | 15 | 1.348 | 1.178–1.542 | |||
| Mental health | 14 | 1.608 | 1.414–1.828 | |||
| Physical health | 30 | 1.520 | 1.386–1.542 | |||
| Longevity | 11 | 1.447 | 1.251–1.674 |
Extension 2: Comparing Different Health Outcomes
The health-related outcome measures were also categorized into more fine-grained groups, comparing (a) psychological measures of well-being, (b) measures of health-related behaviors and coping behaviors, (c) physiological measures of health, (d) mental health states, (e) subjective physical health states, (f) objective physical health states, and (g) longevity. Overall, there were no significant differences between the outcomes (see Table 3). Hence, Hypothesis 2.2, which stated that the effect of SA would be strongest for the pathways, followed by indicators of health states and least strong for longevity was not supported by the data.
Extension 3: Comparing Different Measures of SA on Different Health Outcomes
Lastly, it was assessed whether different SA measures had similar effects on different health-related outcomes. To have sufficient numbers of studies across the categories, the SA measures were classified as subjective age measures versus measures of SPA. The effects were compared across four categories of health-related outcomes: pathways, mental health, physical health, and longevity. Overall, the effect across these two by four categories was significant (Table 3). When comparing the effects between subjective age and SPA, the differences were smallest for the pathways as compared to the differences for mental health, physical health, and longevity. However, the individual coefficients did not differ from each other. Hence, Hypothesis 2.3 that the effects on different measures of health would be similar for different measures of SA was supported.
Discussion
This article provides an update and extension of a meta-analysis of longitudinal studies on the relation between different measures of SA and health outcomes (Westerhof et al., 2014). The fact that we were able to identify 80 new articles since the previous meta-analysis, with five-times more studies in total, shows this is a vibrant research field. The increase might be related to (1) the availability of longitudinal data that examine the impact of SA on health outcomes, (2) a growing awareness of the potential impact of SA on health, and (3) the possibilities for interdisciplinarity that the topic of SA holds (i.e., it brings together behavioral sciences with social sciences and health sciences). Overall, these articles provide an interesting empirical piece of the puzzle that shows that aging is not a universal, biologically programmed process of decline, but also depends a good deal on individual and societal constructions.
The first goal of the article was to update the earlier meta-analysis with the findings from studies that have been published since 2014. The increase in the number and diversity of studies allowed for a more comprehensive, representative, and valid picture of the longitudinal associations of SA with health-related outcomes. The main findings showed that both measures of subjective age and SPA have an effect on health-related outcomes over time (Hypothesis 1.1). This confirmed findings from the earlier meta-analysis (Westerhof et al., 2014) and other systematic reviews and meta-analyses (Chang et al., 2020; Debreczeni & Bailey, 2021; Diehl et al., 2021; Kotter-Grühn et al., 2016; Sabatini et al., 2020; Tully-Wilson et al., 2021; Westerhof & Wurm, 2018; Wurm et al., 2017) and thereby showed the consistency of the effects. Similar to the previous meta-analysis, the overall effect size was small, though the observed effects were statistically significant. There were some indications of publication bias, but effect size did not seem to be overestimated in the trim and fill analysis. Furthermore, studies of lower quality found stronger effects. As the effect was still significant in the trim-and-fill analysis and in studies of higher quality, consistent with the conclusions of other systematic reviews and meta-analyses in the field (Chang et al., 2020; Debreczeni & Bailey, 2021; Diehl et al., 2021; Kotter-Grühn et al., 2016; Sabatini et al., 2020; Tully-Wilson et al., 2021; Westerhof & Wurm, 2018; Wurm et al., 2017), and comparable to other psychosocial variables related to health and longevity (e.g., social isolation, and loneliness, Holt-Lunstad et al., 2015; or well-being, Chida & Steptoe, 2008), it can be concluded that the findings showed a meaningful pattern of longitudinal associations with health and longevity.
Whereas the current meta-analysis focused on individual perspectives on SA, it would be interesting to take a more societal perspective as well. The longitudinal relation of subjective aging to health outcomes might have important consequences for health care, for example. This would ask for studies from a public health perspective to relate the effects of SA to health economics, like healthcare consumption and costs, similar to those that have been computed for age stereotypes (Levy et al., 2020). Similar to interpretations of health gains related to well-being, more positive SA at the individual level might result in important health gains at the population level (Huppert, 2009).
Yet, there was considerable heterogeneity among studies. In contrast to Hypotheses 1.2 and 1.3 that were derived from the earlier meta-analysis (Westerhof et al., 2014), we found that measures of SPA had a stronger impact on health-related outcomes than measures of subjective age. As there were more studies that were better balanced across the different analyses in the current meta-analysis, this suggests that there might have been a confounding between the moderators that were assessed in the earlier meta-analysis. The finding that the type of SA measure was the only significant moderator could also be interpreted as an indicator of the robustness of the association of SA with health-related outcomes. That is, the effect sizes did not vary by participants’ age, welfare state regime, or length of follow-up. This suggests that the effects of SA were not very much influenced by contextual factors and may reflect more of an intrinsic psychological process. Yet, further studies need to clarify how the existing heterogeneity can be best explained, beyond the two types of measures of SA.
The finding that measures of SPA had stronger effects on health outcomes than subjective age can be related to methodological factors: the former measures are more reliable as they consist of multiple items that cover a broader range of experiences across time, whereas subjective age is commonly measured with a single item, assessing a generalized feeling at a particular moment. Additionally, subjective age is differently operationalized, with some studies using a continuous variable (e.g., felt age) and others use a categorical variable (e.g., feeling younger, about the same, or older than one’s chronological age). As subjective age still showed a significant effect, a single item can be a parsimonious solution, for example, in large-scale epidemiological studies that need to plan the number of items carefully. Measures of SPA should, however, be the method of choice when there is more room for in-depth assessment of SA. As relations with health-related outcomes tend to be stronger for SPA, studies could rely on less participants in achieving appropriate statistical power.
The second goal of this article was to contribute to this area of inquiry by extending the previous meta-analysis (Westerhof et al., 2014). Hypothesis 2.1 extended the comparison between subjective age and SPA to different measures of the latter concept. Contrary to the a priori study hypothesis, no significant differences were found between unidimensional and multidimensional measures of SPA. Nevertheless, it should be noted that relatively new and more diverse instruments were used to assess adults’ perceived gains and losses in particular life domains (Brothers et al., 2018; Laidlaw et al., 2007; Marquet et al., 2016; Steverink et al., 2001), resulting in a more limited number of studies. In contrast, unidimensional measures of SPA included the ATOA scale, which is itself already some 50 years old (Lawton, 1975; Miche et al., 2014) and has been used in many more studies. A recent study (Wurm & Schäfer, 2022) compared the impact of gain-related SPA (i.e., perceptions of ongoing personal development) with two loss-related measures (i.e., perceptions of physical and social losses) for longevity. This study showed a greater importance of gain-related SPA for longevity (Wurm & Schäfer, 2022). In contrast, another study which examined the impact of gain- and loss-related SPA on depressive symptoms pointed to a larger role of loss-related SPA for this health outcome (Dutt et al., 2018b). This suggests a need to further investigate the interaction of various SA measures with a variety of health outcomes. Hence, the advantage of going multidimensional in SA assessment as compared to unidimensional in health prediction remains an open issue. This is especially true as some multidimensional instruments assess gains and losses in different domains in life (e.g., psychological gains and social and physical losses; Steverink et al., 2001). One strategy to deal with this currently open issue would be to include several measures of both subjective age and different dimensions of SPA in a single study to control for their interrelations and thereby better assess their relative impact on different health-related outcomes. Overall, it is important to further examine the validity of different SA measures (e.g., Spuling et al., 2020), compare differences in scales’ reliabilities, and make choices about instruments more explicit in relation to the purpose and conceptual background of studies.
Hypothesis 2.2 extended the comparison across seven different classes of health-related outcomes but was not supported by the findings. That is, no significant differences were found between the different health outcomes. Interestingly, these findings also open up the possibility to further explore mediating effects, for example, those of the pathways on health states and/or those of health states on longevity. Although some studies have assessed mediation (e.g., Levy & Bashivi, 2018), the number of studies that have done so was too small to carry out a meta-analysis on these mediating effects.
Finally, Hypothesis 2.3 was supported by the results. Although the differences between subjective age and SPA were smallest for the pathways in comparison to mental and physical health and longevity, SPA had stronger effects on health outcomes than subjective age. This finding further supports the results of Hypothesis 2.1.
Limitations
To our knowledge, this study provides the most up-to-date and most comprehensive meta-analysis on the longitudinal effects of measures of SA on health-related outcomes. Nevertheless, we made some choices to restrict the inclusion of studies in the current meta-analysis. For example, we did not include dissertations, chapters, or grey literature. This might have helped to counteract the publication bias. Furthermore, we did not consider cognitive decline as an outcome in this meta-analysis. The primary reason for doing so is that two recently published meta-analyses already considered cognitive performance, in general, as an outcome in relation to two SA indicators that continue to represent the bulk of existing SA research, namely subjective age (Debreczeni & Bailey, 2020) and attitudes toward own aging (Tully-Wilson et al., 2021). Both found a weak but significant effect in the expected direction; that is, an older subjective age and less positive attitudes towards one’s own aging predicted, on average, more cognitive decline over time. Another limitation concerns the attribution of causality. Although most studies did control for other variables known to be related to health-related outcomes, it cannot be ruled out that other variables might still confound or even explain the effects of SA found in this article. Similarly, there might also be a reverse causality in that health effects have an impact on SA. Available studies seem to be more in support that the direction of effects goes from SA to health-related outcomes rather than from health-related outcomes to SA (e.g., Spuling et al., 2013) and the current meta-analysis only used longitudinal studies where SA preceded the health outcomes in time.
To summarize, in assessing the effects of measures of SA on health-related outcomes, the current meta-analysis provides evidence that SPA show stronger associations with health outcomes across time than subjective age, but both have a significant impact on a large variety of measures of health and longevity across many studies and countries.
Figure 2b.
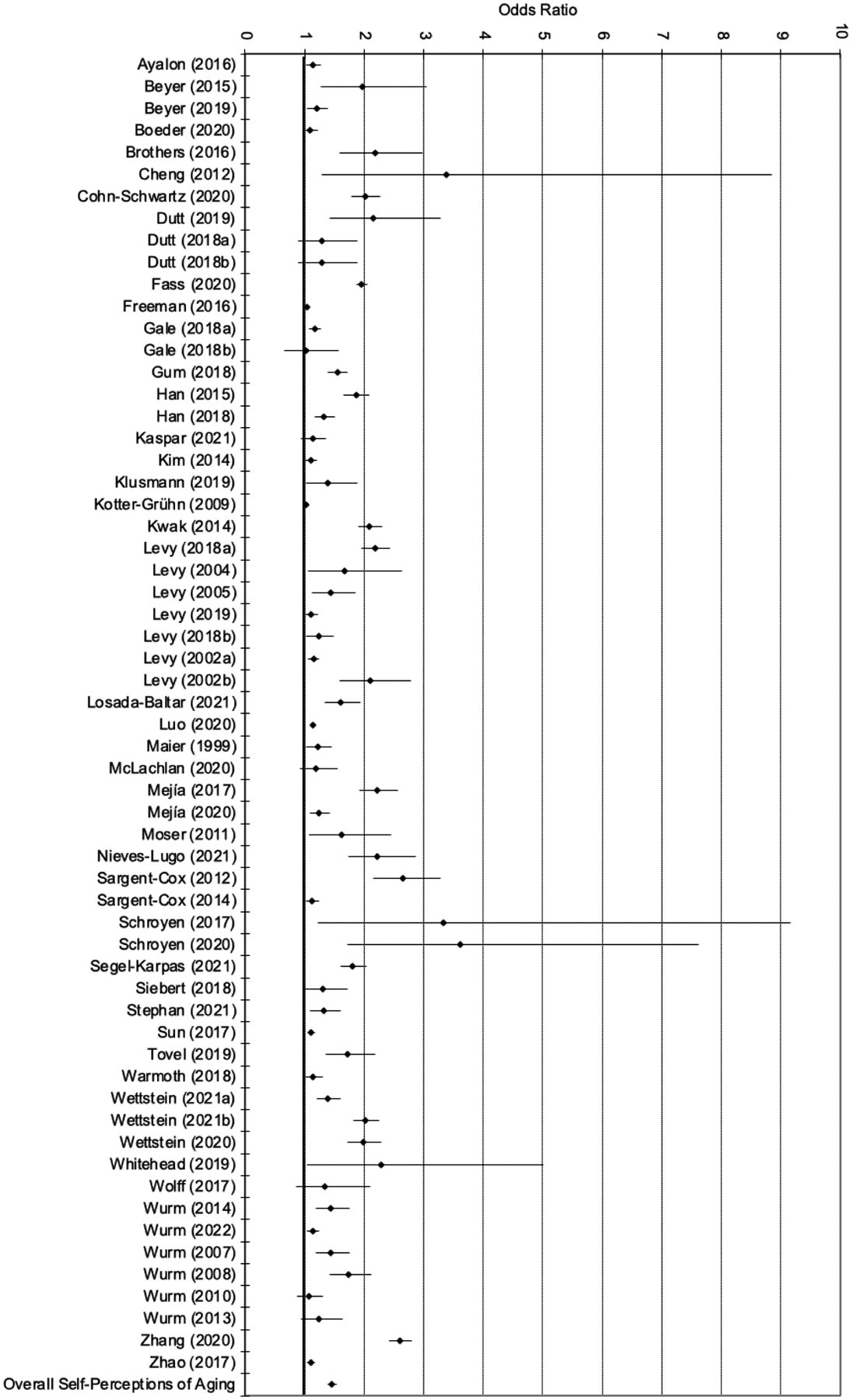
Forest plot of effect sizes (95% confidence interval; Self-Perceptions of Aging)
Public Significance.
This article focuses on the effects that measures of subjective aging (i.e., how a person perceives, interprets, and evaluates their own aging) have on health outcomes later in life. Based on a systematic search of available literature, the results of over 100 studies were analyzed. Across all studies it was found that measures of subjective aging indeed have an effect on health outcomes later in life. Promoting positive views on subjective aging in public health might therefore result in important health gains.
Acknowledgments
The work of Abigail M. Nehrkorn-Bailey, Han-Yun Tseng, and Manfred Diehl was supported by a grant from the National Institute on Aging, National Institutes of Health (Principal Investigator: Manfred Diehl, R01 AG051723).
Footnotes
We have no conflicts of interest to disclose.
A previous version of this manuscript was presented at the 2022 Bi-Annual Scientific Meeting of the German Society of Gerontology and Geriatric Medicine, Frankfurt am Main, Germany.
The complete data, PRISMA checklist, and Ethics statement for this study are available on osf.io/62rdf
References
- *.Avidor S, Palgi Y, & Solomon Z (2021). The moderating role of views of aging in the longitudinal relationship between physical health and mental distress. The Journals of Gerontology: Series B, 76(5), 871–880. 10.1093/geronb/gbaa212 [DOI] [PubMed] [Google Scholar]
- *.Avidor S, Levi-Belz Y, & Solomon Z (2021). What predicts unremitting suicidal ideation? A prospective examination of the role of subjective age in suicidal ideation among ex-prisoners of war. Psychological Trauma, 13(3), 338. 10.1037/tra0000958 [DOI] [PubMed] [Google Scholar]
- *.Ayalon L (2016). Satisfaction with aging results in reduced risk for falling. International Psychogeriatrics, 28(5), 741–747. 10.1017/S1041610215001969 [DOI] [PubMed] [Google Scholar]
- Bambra C (2007). Going beyond the three worlds of welfare capitalism: Regime theory and public health research. Journal of Epidemiological Community Health, 61(12), 1098–1102. doi: 10.1136/jech.2007.064295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Barnes-Farrell JL, & Petery GA (2018). The moderating role of employment status and gender on relationships between psychological age and health: A two-wave cross-lagged panel analysis of data from the Health and Retirement Study. Work, Aging and Retirement, 4(1), 79–95. 10.1093/workar/wax019 [DOI] [Google Scholar]
- *.Barrett AE, & Toothman EL (2016). Explaining age differences in women’s emotional well-being: The role of subjective experiences of aging. Journal of Women & Aging, 28(4), 285–296. 10.1080/08952841.2015.1017426 [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, & Vohs KD (2001). Bad is stronger than good. Review of General Psychology, 5(4), 323–370. 10.1037/1089-2680.5.4.323 [DOI] [Google Scholar]
- *.Benyamini Y, & Burns E (2020). Views on aging: older adults’ self-perceptions of age and of health. European Journal of Ageing, 17(4), 477–487. 10.1007/s10433-019-00528-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Beyer AK, Wiest M, & Wurm S (2019). There is still time to be active: Self-perceptions of aging, physical activity, and the role of perceived residual lifetime among older adults. Journal of Aging and Physical Activity, 27(6), 807–815. 10.1123/japa.2018-0380 [DOI] [PubMed] [Google Scholar]
- *.Beyer AK, Wolff JK, Warner LM, Schüz B, & Wurm S (2015). The role of physical activity in the relationship between self-perceptions of ageing and self-rated health in older adults. Psychology & Health, 30(6), 671–685. 10.1080/08870446.2015.1014370 [DOI] [PubMed] [Google Scholar]
- *.Boeder J, & Tse DC (2021). Measuring self-perceptions of aging: Differences between measures when predicting health outcomes. The Journals of Gerontology: Series B, 76(5), 825–835. 10.1093/geronb/gbaa064 [DOI] [PubMed] [Google Scholar]
- *.Boehmer S (2006). Does felt age reflect health-related quality of life in cancer patients? Psycho-Oncology, 15(8), 726–738. 10.1002/pon.1011 [DOI] [PubMed] [Google Scholar]
- *.Boehmer S (2007). Relationships between felt age and perceived disability, satisfaction with recovery, self-efficacy beliefs and coping strategies. Journal of Health Psychology, 12(6), 895–906. 10.1177/1359105307082453 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, & Rothstein H (n.d.). Comprehensive meta-analysis: Version 2.0. Retrieved from http://www.metaanalysis.com/pages/downloads/Meta-Analysis-Manual.pdf
- Brandtstädter J, & Rothermund K (2002). The life-course dynamics of goal pursuit and goal adjustment: A two-process framework. Developmental Review, 22(1), 117–150. 10.1006/drev.2001.0539 [DOI] [Google Scholar]
- *.Brothers A, Gabrian M, Wahl HW, & Diehl M (2016). Future time perspective and awareness of age-related change: Examining their role in predicting psychological well-being. Psychology and Aging, 31(6), 605. 10.1037/pag0000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers AF, Gabrian M, Wahl H-W, & Diehl M (2018). A new multi-dimensional questionnaire to assess Awareness of Age-Related Change (AARC). The Gerontologist, 59(3), e141–e151. 10.1093/geront/gny006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers A, Kornadt AE, Nehrkorn-Bailey A, Wahl HW, & Diehl M (2021). The effects of age stereotypes on physical and mental health are mediated by self-perceptions of aging. The Journals of Gerontology: Series B, 76(5), 845–857. 10.1093/geronb/gbaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers A, Miche M, Diehl M, & Wahl H-W (2017). Examination of associations among three distinct subjective aging constructs and their relevance for predicting developmental correlates. The Journals of Gerontology: Series B, 72(4), 547–560. 10.1093/geronb/gbv085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C, Bei B, Gilson K-M, Komiti A, Jackson H, & Judd F (2016). Antecedents of Attitudes to Aging: A Study of the Roles of Personality and Well-being. The Gerontologist, 56(2), 256–265. 10.1093/geront/gnu041 [DOI] [PubMed] [Google Scholar]
- Chang E-S, Kannoth S, Levy S, Wang S-Y, Lee JE, & Levy BR (2020). Globalreach of ageism on older person’s health: A systematic review. PloS ONE, 15(1), e0220857. 10.1371/journal.pone.0220857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, & Chen S (2010). How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Communications in Statistics—Simulation and Computation®, 39(4), 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- *.Cheng ST, Yip LC, Jim OT, & Hui AN (2012). Self-perception of aging and acute medical events in chronically institutionalized middle-aged and older persons with schizophrenia. International Journal of Geriatric Psychiatry, 27(9), 907–913. 10.1002/gps.2798 [DOI] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2008). Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine, 70(7), 741–756. 10.1097/PSY.0b013e31818105ba [DOI] [PubMed] [Google Scholar]
- *.Choi NG, & DiNitto DM (2014). Felt age and cognitive-affective depressive symptoms in late life. Aging & Mental Health, 18(7), 833–837. 10.1080/13607863.2014.886669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cohn-Schwartz E, Segel-Karpas D, & Ayalon L (2021). Longitudinal dyadic effects of aging self-perceptions on health. The Journals of Gerontology: Series B, 76(5), 900–909. 10.1093/geronb/gbaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debreczeni FA, & Bailey PE (2021). A systematic review and meta-analysis of subjective age and the association with cognition, subjective well-being, and depression. The Journals of Gerontology: Series B, 76(3), 471–482. 10.1093/geronb/gbaa069 [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, & Altman DG (2008). Analysing data and undertaking meta-analysis. In Higgins JPT & Green S (Eds.), Cochrane handbook for systematic reviews of interventions (pp. 243–296). Wiley. 10.1002/9780470712184.ch9 [DOI] [Google Scholar]
- Deeming C (2017). The Lost and the New ‘Liberal World’ of Welfare Capitalism: A Critical Assessment of Gøsta Esping-Andersen’s The Three Worlds of Welfare Capitalism a Quarter Century Later. Social Policy and Society, 16(3), 405–422. 10.1017/S1474746415000676 [DOI] [Google Scholar]
- Diehl M, Brothers A., & Wahl H-W (2021). Self-perceptions and awareness of aging: Past, present, and future. In Schaie KW & Willis SL (Eds.), Handbook of the psychology of aging (9th ed., pp. 155–179). Academic Press. 10.1016/B978-0-12-816094-7.00001-5 [DOI] [Google Scholar]
- Diehl MK, & Wahl HW (2010). Awareness of age-related change: Examination of a (mostly) unexplored concept. The Journals of Gerontology: Series B, 65(3), 340–350. 10.1093/geronb/gbp110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Wahl HW, Barrett AE, Brothers AF, Miche M, Montepare JM, … Wurm S (2014). Awareness of aging: Theoretical considerations on an emergingconcept. Developmental Review, 34(2), 93–113. 10.1016/j.dr.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Wahl H-W, Brothers A, & Miche M (2015). Subjective aging and awareness of aging: Toward a new understanding of the aging self. In Diehl M & Wahl H-W (Eds.), Annual review of gerontology and geriatrics, Vol. 35, 2015: Subjective aging: New developments and future directions (pp. 1–28). Springer Publishing. [Google Scholar]
- *.Dutt AJ, Gabrian M, & Wahl HW (2018a). Awareness of age-related change and depressive symptoms in middle and late adulthood: Longitudinal associations and the role of self-regulation and calendar age. The Journals of Gerontology: Series B, 73(6), 944–953. 10.1093/geronb/gbw095 [DOI] [PubMed] [Google Scholar]
- *.Dutt AJ, & Wahl HW (2019). Future time perspective and general self-efficacy mediate the association between awareness of age-related losses and depressive symptoms. European Journal of Ageing, 16(2), 227–236. 10.1007/s10433-018-0482-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dutt AJ, Wahl HW, & Rupprecht FS (2018b). Mindful vs. mind full: Processing strategies moderate the association between subjective aging experiences and depressive symptoms. Psychology and Aging, 33(4), 630–642. 10.1037/pag0000245 [DOI] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration (2010). C-reactive protein concentration and risk ofcoronary heart disease, stroke, and mortality: An individual participant meta-analysis. The Lancet, 375(9709), 132–140. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esping-Andersen G (1990). The three worlds of welfare capitalism. Princeton University Press. [Google Scholar]
- *.Faß E, Pyun H, & Schlesinger T (2020). Perception of aging in the relation between sport activity and self-rated health in middle and older age: A longitudinal analysis. SSM-Population Health, 11, 100610. 10.1016/j.ssmph.2020.100610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faudzi FNM, Armitage CJ, Bryant C, & Brown LJE (2019). A systematic review of the psychometric properties of self-report measures of attitudes to aging. Research on Aging, 41(6), 549–574. 10.1177/0164027518825117 [DOI] [PubMed] [Google Scholar]
- Ferguson CJ, & Brannick MT (2012). Publication bias in psychological science: Prevalence, methods for identifying and controlling, and implications for the use of meta-analyses. Psychological Methods, 17(1), 120. 10.1037/a0024445 [DOI] [PubMed] [Google Scholar]
- *.Freeman AT, Santini ZI, Tyrovolas S, Rummel-Kluge C, Haro JM, & Koyanagi A (2016). Negative perceptions of ageing predict the onset and persistence of depression and anxiety: Findings from a prospective analysis of the Irish Longitudinal Study on Ageing (TILDA). Journal of Affective Disorders, 199, 132–138. 10.1016/j.jad.2016.03.042 [DOI] [PubMed] [Google Scholar]
- *.Fundenberger H, Stephan Y, Hupin D, Barth N, Terracciano A, & Canada B (2022). Prospective associations between subjective age and fear of falling in older adults. Aging & Mental Health, 26(1), 86–91. 10.1080/13607863.2020.1856775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gale CR, Čukić I, Chastin SF, Dall PM, Dontje ML, Skelton DA, … & Seniors USP Team. (2018). Attitudes to ageing and objectively-measured sedentary and walking behaviour in older people: The Lothian Birth Cohort 1936. Plos One, 13(5), e0197357. 10.1371/journal.pone.0197357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gale CR, & Cooper C (2018). Attitudes to ageing and change in frailty status: the English Longitudinal Study of Ageing. Gerontology, 64(1), 58–66. 10.1159/000477169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gum AM, & Ayalon L (2018). Self-perceptions of aging mediate the longitudinal relationship of hopelessness and depressive symptoms. International Journal of Geriatric Psychiatry, 33(4), 591–597. 10.1002/gps.4826 [DOI] [PubMed] [Google Scholar]
- *.Hajek A, & König HH (2020). Feeling too old? Consequences for subjective well-being. Longitudinal findings from the German Ageing Survey. Archives of Gerontology and Geriatrics, 90, 104–127. 10.1016/j.archger.2020.104127 [DOI] [PubMed] [Google Scholar]
- *.Han J (2018). Chronic illnesses and depressive symptoms among older people: functional limitations as a mediator and self-perceptions of aging as a moderator. Journal of Aging and Health, 30(8), 1188–1204. 10.1177/0898264317711609 [DOI] [PubMed] [Google Scholar]
- *.Han J, & Richardson VE (2015). The relationships among perceived discrimination, self-perceptions of aging, and depressive symptoms: A longitudinal examination of age discrimination. Aging & Mental Health, 19(8), 747–755. 10.1080/13607863.2014.962007 [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, & Stephenson D (2015). Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspectives on Psychological Science, 10(2), 227–237. 10.1177/1745691614568352 [DOI] [PubMed] [Google Scholar]
- Huppert FA (2009). A new approach to reducing disorder and improving well-being. Perspectives on Psychological Science, 4(1), 108–111. 10.1111/j.1745-6924.2009.01100.x [DOI] [PubMed] [Google Scholar]
- Kaspar R, Gabrian M, Brothers A, Wahl HW, & Diehl M (2019). Measuring awareness of age-related change: Development of a 10-item short form for use in large-scale surveys. The Gerontologist, 59(3), e130–e140. 10.1093/geront/gnx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kaspar R, Wahl HW, & Diehl M (2021). Awareness of age-related change as a behavioral determinant of survival time in very old age. Frontiers in Psychology, 12, Article 727560. 10.3389/fpsyg.2021.727560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenbaum R, Derbin V, Sabatini P, & Artt S (1972). “The ages of me”: Toward personal and interpersonal definitions of functional aging. Aging and Human Development, 3(2), 197–211. 10.2190/TUJR-WTXK-866Q-8QU7 [DOI] [Google Scholar]
- *.Kim ES, Moored KD, Giasson HL, & Smith J (2014). Satisfaction with aging and use of preventive health services. Preventive Medicine, 69, 176–180. 10.1016/j.ypmed.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Klusmann V, Sproesser G, Wolff JK, & Renner B (2019). Positive self-perceptions of aging promote healthy eating behavior across the life span via social-cognitive processes. The Journals of Gerontology: Series B, 74(5), 735–744. 10.1093/geronb/gbx139 [DOI] [PubMed] [Google Scholar]
- Kornadt AE, Siebert JS, & Wahl H-W (2019). The interplay of personality and attitudes toward own aging across two decades of later life. PLoSONE, 14(10), e0223622. https://doi.org/10.1371/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kotter-Grühn D, Kleinspehn-Ammerlahn A, Gerstdorf D, & Smith J (2009). Self-perceptions of aging predict mortality and change with approaching death: 16-year longitudinal results from the Berlin Aging Study. Psychology and Aging, 24(3), 654–667. 10.1037/a0016510 [DOI] [PubMed] [Google Scholar]
- Kotter-Grühn D, Kornadt AE, & Stephan Y (2016). Looking beyond chronological age: Current knowledge and future directions in the study of subjective age. Gerontology, 62(1), 86–93. 10.1159/000438671 [DOI] [PubMed] [Google Scholar]
- Kuypers JA, & Bengtson VL (1973). Social breakdown and competence. Human Development, 16(3), 181–201. 10.1159/000271275 [DOI] [PubMed] [Google Scholar]
- *.Kwak M, Ingersoll-Dayton B, & Burgard S (2014). Receipt of care and depressive symptoms in later life: The importance of self-perceptions of aging. The Journals of Gerontology: Series B, 69(2), 325–335. 10.1093/geronb/gbt128 [DOI] [PubMed] [Google Scholar]
- *.Lahav Y, Avidor S, Stein JY, Zhou X, & Solomon Z (2020). Telomere length and depression among ex-prisoners of war: the role of subjective age. The Journals of Gerontology: Series B, 75(1), 21–29. 10.1093/geronb/gby006 [DOI] [PubMed] [Google Scholar]
- Laidlaw K, Power MJ, & Schmidt S (2007). The attitudes to ageing questionnaire (AAQ): development and psychometric properties. International Journal of Geriatric Psychiatry, 22(4), 367–379. 10.1002/gps.1683 [DOI] [PubMed] [Google Scholar]
- Lamers S, Bolier L, Westerhof GJ, Smit F, & Bohlmeijer ET (2012). The impact of emotional well-being on long-term recovery and survival in physical illness: A meta-analysis. Journal of Behavioral Medicine, 35(5), 538–547. 10.1007/s10865-011-9379-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP (1975). The Philadelphia geriatric center morale scale: A revision. Journal of Gerontology, 30(1), 85–89. 10.1093/geronj/30.1.85 [DOI] [PubMed] [Google Scholar]
- Levy BR (2009). Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science, 18(6), 332–336. 10.1111/j.1467-8721.2009.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR (2022). The role of structural ageism in age beliefs and health of older persons. JAMA Network Open, 5(2), e2147802. 10.1001/jamanetworkopen.2021.47802 [DOI] [PubMed] [Google Scholar]
- *.Levy BR, & Bavishi A (2018). Survival advantage mechanism: Inflammation as a mediator of positive self-perceptions of aging on longevity. The Journals of Gerontology: Series B, 73(3), 409–412. 10.1093/geronb/gbw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Hausdorff JM, Hencke R, & Wei JY (2000). Reducing cardiovascular stress with positive self-stereotypes of aging. The Journals of Gerontology: Series B, 55(4), P205–P213. 10.1093/geronb/55.4.P205 [DOI] [PubMed] [Google Scholar]
- Levy BR, Kasl SV, & Gill TM (2004). Image of aging scale. Perceptual and Motor Skills, 99(1), 208–210. [DOI] [PubMed] [Google Scholar]
- *.Levy BR, & Myers LM (2004). Preventive health behaviors influenced by self-perceptions of aging. Preventive Medicine, 39(3), 625–629. 10.1016/j.ypmed.2004.02.029 [DOI] [PubMed] [Google Scholar]
- *.Levy BR, & Myers LM (2005). Relationship between respiratory mortality and self-perceptions of aging. Psychology & Health, 20(5), 553–564. 10.1080/14768320500066381 [DOI] [Google Scholar]
- *.Levy BR, & Slade MD (2019). Positive views of aging reduce risk of developing later-life obesity. Preventive Medicine Reports, 13, 196–198. 10.1016/j.pmedr.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Chang ES, Kannoth S, & Wang SY (2020). Ageism amplifies cost and prevalence of health conditions. The Gerontologist, 60(1), 174–181. 10.1093/geront/gny131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Levy BR, Slade MD, & Kasl SV (2002). Longitudinal benefit of positive self-perceptions of aging on functional health. The Journals of Gerontology: Series B, 57(5), P409–P417. 10.1093/geronb/57.5.P409 [DOI] [PubMed] [Google Scholar]
- *.Levy BR, Slade MD, Kunkel SR, & Kasl SV (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261. 10.1037/0022-3514.83.2.261 [DOI] [PubMed] [Google Scholar]
- *.Levy BR, Slade MD, Pietrzak RH, & Ferrucci L (2018). Positive age beliefs protect against dementia even among elders with high-risk gene. PloS One, 13(2), e0191004. 10.1371/journal.pone.0191004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Li Y, Liu M, Miyawaki CE, Sun X, Hou T, Tang S, & Szanton SL (2021). Bidirectional relationship between subjective age and frailty: a prospective cohort study. BMC Geriatrics, 21(1), 1–9. 10.1186/s12877-021-02344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Liang K (2018). The longitudinal association between age identity and physical functioning among urban Chinese older adults. Journal of Aging and Physical Activity, 26(3), 486–491. 10.1123/japa.2017-0023 [DOI] [PubMed] [Google Scholar]
- *.Liang K (2020). Differential associations between subjective age and depressive symptoms among urban and rural Chinese older adults. Aging & Mental Health, 24(8), 1271–1277. 10.1080/13607863.2019.1663489 [DOI] [PubMed] [Google Scholar]
- *.Lim MY, Stephens EK, Novotny P, Price K, Salayi M, Roeker L, … & Jatoi A (2013). Self-perceptions of age among 292 chemotherapy-treated cancer patients: Exploring associations with symptoms and survival. Journal of Geriatric Oncology, 4(3), 249–254. 10.1016/j.jgo.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löckenhoff CE, De Fruyt F, Terracciano A, McCrae RR, De Bolle M, Costa PT, … & Yik M (2009). Perceptions of aging across 26 cultures and their culture-level associates. Psychology and Aging, 24(4), 941–954. 10.1037/a0016901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Losada-Baltar A, Martínez-Huertas JÁ, Jiménez-Gonzalo L, Pedroso-Chaparro MDS, Gallego-Alberto L, Fernandes-Pires J, & Márquez-González M (2022). Longitudinal correlates of loneliness and psychological distress during the lockdown situation due to COVID-19. Effects of age and self-perceptions of aging. The Journals of Gerontology: Series B, 77(4), 652–660. 10.1093/geronb/gbab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Luo MS, & Li LW (2020). Are self-perceptions of aging associated with health trajectories among middle-aged and older adults? The Gerontologist, 60(5), 841–850. 10.1093/geront/gnz092 [DOI] [PubMed] [Google Scholar]
- *.Maier H, & Smith J (1999). Psychological predictors of mortality in old age. The Journals of Gerontology: Series B, 54B(1), P44–P54. 10.1093/geronb/54B.1.P44 [DOI] [PubMed] [Google Scholar]
- Maluf CB, Barreto SM, Giatti L, Ribeiro AL, Vidigal PG, Azevedo DR, … & Miller MA (2020). Association between C reactive protein and all-cause mortality in the ELSA-Brasil cohort. Journal of Epidemiology and Community Health, 74(5), 421–427. 10.1136/jech-2019-213289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Markides KS, & Pappas C (1982). Subjective age, health, and survivorship in old age. Research on Aging, 4(1), 87–96. 10.1177/016402758241004 [DOI] [Google Scholar]
- Marquet M, Missotten P, Schroyen S, van Sambeek I, van den Akker M, Van Den Broeke C, … & Adam S (2016). A validation of the French version of the Attitudes to Aging Questionnaire (AAQ): Factor structure, reliability and validity. Psychologica Belgica, 56(2), 80–100. 10.5334/pb.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.McLachlan KJ, Cole JH, Harris SE, Marioni RE, Deary IJ, & Gale CR (2020). Attitudes to ageing, biomarkers of ageing and mortality: the Lothian Birth Cohort 1936. Journal of Epidemiology and Community Health, 74(4), 377–383. 10.1136/jech-2019-213462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mejía ST, Giasson HL, Smith J, & Gonzalez R (2020). Concurrent and enduring associations between married partners’ shared beliefs and markers of aging. Psychology and Aging, 35(7), 925–936. 10.1037/pag0000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mejía ST, & Gonzalez R (2017). Couples’ shared beliefs about aging and implications for future functional limitations. The Gerontologist, 57(Suppl_2), S149–S159. 10.1093/geront/gnx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miche M, Elsässer VC, Schilling OK, & Wahl HW (2014). Attitude toward own aging in midlife and early old age over a 12-year period: Examination of measurement equivalence and developmental trajectories. Psychology and Aging, 29(3), 588–600. 10.1037/a0037259 [DOI] [PubMed] [Google Scholar]
- *.Mock SE, & Eibach RP (2011). Aging attitudes moderate the effect of subjective age on psychological well-being: evidence from a 10-year longitudinal study. Psychology and Aging, 26(4), 979–986. 10.1037/a0023877 [DOI] [PubMed] [Google Scholar]
- *.Moher D, Liberati A, Tetzlaff J, Altman DG, & PRISMA Grou (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151(4), 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- *.Moser C, Spagnoli J, & Santos-Eggimann B (2011). Self-perception of aging and vulnerability to adverse outcomes at the age of 65–70 years. The Journals of Gerontology: Series B, 66(6), 675–680. 10.1093/geronb/gbr052 [DOI] [PubMed] [Google Scholar]
- *.Nieves-Lugo K, Ware D, Althoff K, Brennan-Ing M, Meanley S, Brown AL, … & Plankey M (2021). Negative perception of aging is associated with frailty transitions within a cohort of sexual minority men. Innovation in Aging, 5(4), igab035. 10.1093/geroni/igab035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Palgi Y, Ayalon L, Avidor S, Segel-Karpas D, & Bodner E (2018). On the edge: The association between extreme values of proportional felt-age and functioning. Psychiatry Research, 270, 538–543. 10.1016/j.psychres.2018.10.035 [DOI] [PubMed] [Google Scholar]
- *.Palgi Y, Shrira A, Avidor S, Hoffman Y, Bodner E, & Ben-Ezra M (2019). Understanding the long-term connections between posttraumatic stress, subjective age, and successful aging among midlife and older adults. European Journal of Psychotraumatology, 10(1), 1583523. 10.1080/20008198.2019.1583523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Petashnick JR, Shrira A, Hoffman Y, Palgi Y, Kavé G, & Shmotkin D (2022). Subjective Age and Late-Life Functional Status: Mediating and Moderating Effects. The Journals of Gerontology: Series B, 77(1), 61–70. 10.1093/geronb/gbab181 [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Wahl HW (2021). Subjective age from childhood to advanced old age: A meta-analysis. Psychology and Aging, 36(3), 394–406. 10.1037/pag0000600 [DOI] [PubMed] [Google Scholar]
- *.Qiao H, Du X, Li S, Sun Y, Feng W, & Wu Y (2021). Does older subjective age predict poorer cognitive function and higher risk of dementia in middle-aged and older adults? Psychiatry Research, 298, Article 113807. 10.1016/j.psychres.2021.113807 [DOI] [PubMed] [Google Scholar]
- *.Rippon I, & Steptoe A (2015). Feeling old vs being old: Associations between self-perceived age and mortality. JAMA Internal Medicine, 175(2), 307–309. 10.1001/jamainternmed.2014.6580 [DOI] [PubMed] [Google Scholar]
- *.Rippon I, & Steptoe A (2018). Is the relationship between subjective age, depressive symptoms and activities of daily living bidirectional?. Social Science & Medicine, 214, 41–48. 10.1016/j.socscimed.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Silarova B, Martyr A, Collins R, Ballard C, Anstey KJ, Kim S, & Clare L (2020). Associations of awareness of age-related change with emotional and physical well-being: A systematic review and meta-analysis. The Gerontologist, 60(6), e477–e490. 10.1093/geront/gnz101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sargent-Cox KA, Anstey KJ, & Luszcz MA (2012). The relationship between change in self-perceptions of aging and physical functioning in older adults. Psychology and Aging, 27(3), 750–760. 10.1037/a0027578 [DOI] [PubMed] [Google Scholar]
- *.Sargent-Cox KA, Anstey KJ, & Luszcz MA (2014). Longitudinal change of self-perceptions of aging and mortality. The Journals of Gerontology: Series B, 69(2), 168–173. 10.1093/geronb/gbt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, & Visser M (2006). Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American Journal of Medicine, 119(6), 526.e9–526.e17. 10.1016/j.amjmed.2005.10.049 [DOI] [PubMed] [Google Scholar]
- Schönstein A, Dallmeier D, Denkinger M, Rothenbacher D, Klenk J, Bahrmann A, & Wahl H-W (2021). Health and subjective views on aging: Longitudinal findings from the ActiFE Ulm Study. The Journals of Gerontology: Series B, 76(7), 1349–1359. 10.1093/geronb/gbab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönstein A, Trares K, & Wahl H-W (2022). Subjective views on aging and objective aging biomarkers: Achievements and questions in an emerging research area. In Palgi Y, Shrira A, & Diehl M (Eds.), Subjective views of aging: Theory, research, and practice (pp. 153–168). Springer Nature. [Google Scholar]
- *.Schroyen S, Letenneur L, Missotten P, Jérusalem G, & Adam S (2020). Impact of self-perception of aging on mortality of older patients in oncology. Cancer Medicine, 9(7), 2283–2289. 10.1002/cam4.2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schroyen S, Missotten P, Jerusalem G, Van den Akker M, Buntinx F, & Adam S (2017). Association between self-perception of aging, view of cancer and health of older patients in oncology: A one-year longitudinal study. BMC Cancer, 17(1), 1–8. 10.1186/s12885-017-3607-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Segel-Karpas D, Cohn-Schwartz E, & Ayalon L (2022). Self-perceptions of aging and depressive symptoms: the mediating role of loneliness. Aging & Mental Health, 26(7), 1495–1501. 10.1080/13607863.2021.1991275 [DOI] [PubMed] [Google Scholar]
- Seidler AL, & Wolff JK (2017). Bidirectional associations between self-perceptions of aging and processing speed across 3 years. GeroPsych, 30(2), 49–59. 10.1024/1662-9647/a000165. [DOI] [Google Scholar]
- Settersten RA, & Hagestad GO (2015). Subjective aging and new complexities of the life course. Annual Review of Gerontology and Geriatrics, 35(1), 29–53. 10.1891/0198-8794.35.29 [DOI] [Google Scholar]
- *.Siebert JS, Wahl HW, Degen C, & Schröder J (2018). Attitude toward own aging as a risk factor for cognitive disorder in old age: 12-year evidence from the ILSE study. Psychology and Aging, 33(3), 461–472. 10.1037/pag0000252 [DOI] [PubMed] [Google Scholar]
- *.Spuling SM, Miche M, Wurm S, & Wahl H-W (2013). Exploring the causal interplay of subjective age and health dimensions in the second half of life: A cross-lagged panel analysis. Zeitschrift für Gesundheitspsychologie, 21(1), 5–15. 10.1026/0943-8149/a000084 [DOI] [Google Scholar]
- Stephan Y, Sutin AR, & Terracciano A (2015). Younger subjective age is associated with lower C-reactive protein among older adults. Brain, Behavior, and Immunity, 43, 33–36. https://doi-org.ezproxy2.utwente.nl/10.1016/j.bbi.2014.07.019 [DOI] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, Bayard S, & Terracciano A (2017). Subjective age and sleep in middle-aged and older adults. Psychology & Health, 32(9), 1140–1151. 10.1080/08870446.2017.1324971 [DOI] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2017). Feeling older and the development of cognitive impairment and dementia. The Journals of Gerontology: Series B, 72(6), 966–973. 10.1093/geronb/gbw085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2018). Subjective age and risk of incident dementia: Evidence from the National Health and Aging Trends survey. Journal of Psychiatric Research, 100, 1–4. 10.1016/j.jpsychires.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, Luchetti M, & Terracciano A (2021). An older subjective age is related to accelerated epigenetic aging. Psychology and Aging, 36(6), 767–772. 10.1037/pag0000607 [DOI] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, & Terracciano A (2015). “Feeling younger, walking faster”: subjective age and walking speed in older adults. Age, 37(5), 1–12. 10.1007/s11357-015-9830-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, & Terracciano A (2016). Feeling older and risk of hospitalization: Evidence from three longitudinal cohorts. Health Psychology, 35(6), 634–637. 10.1037/hea0000335 [DOI] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, & Terracciano A (2018). Subjective age and mortality in three longitudinal samples. Psychosomatic Medicine, 80(7), 659–664. 10.1097/PSY.0000000000000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, & Terracciano A (2019). Subjective age and cystatin C among older adults. The Journals of Gerontology: Series B, 74(3), 382–388. 10.1093/geronb/gbx124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Stephan Y, Sutin AR, Wurm S, & Terracciano A (2021). The Journals of Gerontology: Series B, 76(5), 910–919. 10.1093/geronb/gbaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steverink N, Westerhof GJ, Bode C, & Dittmann-Kohli F (2001). The personal experience of aging, individual resources, and subjective well-being. The Journals of Gerontology: Series B, 56B, P364–P373. 10.1093/geronb/56.6.P364 [DOI] [PubMed] [Google Scholar]
- *.Sun JK, Kim ES, & Smith J (2017). Positive self-perceptions of aging and lower rate of overnight hospitalization in the US population over age 50. Psychosomatic Medicine, 79(1), 81–90. 10.1097/PSY.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tovel H, Carmel S, & Raveis VH (2019). Relationships among self-perception of aging, physical functioning, and self-efficacy in late life. The Journals of Gerontology: Series B, 74(2), 212–221. 10.1093/geronb/gbx056 [DOI] [PubMed] [Google Scholar]
- Tully-Wilson C, Bojack R, Millear PM, Stallman HM, Allen A, & Mason J (2021). Self-perceptions of aging: A systematic review of longitudinal studies. Psychology and Aging, 36(7), 773–789. 10.1037/pag0000638 [DOI] [PubMed] [Google Scholar]
- *.Uotinen V, Rantanen T, & Suutama T (2005). Perceived age as a predictor of old age mortality: A 13-year prospective study. Age and Ageing, 34(4), 368–372. 10.1093/ageing/afi091 [DOI] [PubMed] [Google Scholar]
- *.Veenstra M, Daatland SO, & Aartsen M (2021). The role of subjective age in sustaining wellbeing and health in the second half of life. Ageing & Society, 41(11), 2446–2466. 10.1017/S0144686X2000032X [DOI] [Google Scholar]
- *.Veenstra M, Løset GK, & Daatland SO (2021). Socioeconomic inequalities in mortality after age 67: The contribution of psychological factors. Frontiers in Psychology, 12, Article 717959. 10.3389/fpsyg.2021.717959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, … & Harris TB (2002). Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The Health ABC Study. The Journals of Gerontology: Series A, 57(5), M326–M332. 10.1093/gerona/57.5.M326 [DOI] [PubMed] [Google Scholar]
- *.Warmoth K, Tarrant M, Abraham C, & Lang IA (2018). Relationship between perceptions of ageing and frailty in English older adults. Psychology, Health & Medicine, 23(4), 465–474. 10.1080/13548506.2017.1349325 [DOI] [PubMed] [Google Scholar]
- Weiss D, & Kornadt AE (2018). Age-stereotype internalization and dissociation: Contradictory processes or two sides of the same coin? Current Directions in Psychological Science, 27(6), 477–483. 10.1177/0963721418777743 [DOI] [Google Scholar]
- Westerhof GJ (2022). Assessing the Impact of Subjective Aging on Health-Related Outcomes: An Updated Meta-Analysis of the Evidence from Longitudinal Studies. [Data, Prisma checklist, and ethical approval]. 10.17605/OSF.IO/62RDF [DOI] [Google Scholar]
- Westerhof GJ, & Barrett AE (2005). Age identity and subjective well-being: A comparison of the United States and Germany. The Journals of Gerontology: Series B, 60(3), S129–S136. 10.1093/geronb/60.3.S129 [DOI] [PubMed] [Google Scholar]
- Westerhof GJ, Miche M, Brothers AF, Barrett AE, Diehl M, Montepare JM, Wahl H-W, & Wurm S (2014). The influence of subjective aging on health and longevity: A meta-analysis of longitudinal data. Psychology and Aging, 29(4), 793–802. 10.1037/a0038016 [DOI] [PubMed] [Google Scholar]
- Westerhof GJ, & Wurm S (2018). Subjective aging and health. In Knight BG & Wahl H-W (Eds.), Oxford Research Encyclopedia of Psychology and Aging (pp. 1–30). Oxford University Press. 10.1093/acrefore/9780190236557.013.4 [DOI] [Google Scholar]
- *.Wettstein M, Spuling SM, Cengia A, & Nowossadeck S (2021). Feeling younger as a stress buffer: Subjective age moderates the effect of perceived stress on change in functional health. Psychology and Aging, 36(3), 322–337. 10.1037/pag0000608 [DOI] [PubMed] [Google Scholar]
- *.Wettstein M, Wahl HW, & Kornadt AE (2021). Longitudinal associations between perceived stress and views on aging: Evidence for reciprocal relations. Psychology and Aging, 36(6), 752–766. 10.1037/pag0000632 [DOI] [PubMed] [Google Scholar]
- *.Wettstein M, Wahl HW, & Siebert JS (2020). 20-year trajectories of health in midlife and old age: Contrasting the impact of personality and attitudes toward own aging. Psychology and Aging, 35(6), 910–924. 10.1037/pag0000464 [DOI] [PubMed] [Google Scholar]
- *.Wettstein M, Werner-Wahl H, & Spuling SM (2021). Nine-year changes in self-reported problems with vision and hearing among older adults: do subjective age views matter? Aging & Mental Health, 25(12), 2200–2212. 10.1080/13607863.2020.1822290 [DOI] [PubMed] [Google Scholar]
- *.Whitehead BR (2019). Investigating the function spiral in later life: Aging attitudes, physical activity, and gait. Journal of Health Psychology, 24(14), 1955–1964. 10.1177/1359105317710814 [DOI] [PubMed] [Google Scholar]
- *.Wienert J, Gellert P, & Lippke S (2017). Physical activity across the life-span: Does feeling physically younger help you to plan physical activities? Journal of Health Psychology, 22(3), 324–335. 10.1177/13591053156034 [DOI] [PubMed] [Google Scholar]
- *.Wienert J, Kuhlmann T, & Lippke S (2015). Direct effects of a domain-specific subjective age measure on self-reported physical activity: Is it more important how old you are or how old you feel? Health Psychology Report, 3(2), 131–139. 10.5114/hpr.2015.51450 [DOI] [Google Scholar]
- *.Wolff JK, Schüz B, Ziegelmann JP, Warner LM, & Wurm S (2017). Short-term buffers, but long-term suffers? Differential effects of negative self-perceptions of aging following serious health events. The Journals of Gerontology: Series B, 72(3), 408–414. 10.1093/geronb/gbv058 [DOI] [PubMed] [Google Scholar]
- Wong WC, Cheung CS, & Hart GJ (2008). Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerging Themes in Epidemiology, 5(1), 1–4. 10.1186/1742-7622-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wurm S, & Benyamini Y (2014). Optimism buffers the detrimental effect of negative self-perceptions of ageing on physical and mental health. Psychology & Health, 29(7), 832–848. 10.1080/08870446.2014.891737 [DOI] [PubMed] [Google Scholar]
- Wurm S, Diehl M, Kornadt AE, Westerhof GJ, & Wahl H-W (2017). How do views on aging affect health outcomes in adulthood and late life? Explanations for an established connection. Developmental Review, 46, 27–43. 10.1016/j.dr.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wurm S, & Schäfer SK (2022). Gain- but not loss-related self-perceptions of aging predict mortality over a period of 23 years: A multidimensional approach. Journal of Personality and Social Psychology, 123(3), 636–653. 10.1037/pspp0000412 [DOI] [PubMed] [Google Scholar]
- *.Wurm S, Tesch-Römer C, & Tomasik MJ (2007). Longitudinal findings on aging related cognitions, control beliefs, and health in later life. The Journals of Gerontology: Series B, 62B(3), P156–P164. 10.1093/geronb/62.3.P156 [DOI] [PubMed] [Google Scholar]
- *.Wurm S, Tomasik MJ, & Tesch-Römer C (2008). Serious health events and their impact on changes in subjective health and life satisfaction: The role of age and a positive view on aging. European Journal of Ageing, 5(2), 117–127. 10.1007/s10433-008-0077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wurm S, Tomasik MJ, & Tesch-Römer C (2010). On the importance of a positive view on ageing for physical exercise among middle-aged and older adults: Cross-sectional and longitudinal findings. Psychology and Health, 25(1), 25–42. 10.1080/08870440802311314 [DOI] [PubMed] [Google Scholar]
- *.Wurm S, Warner LM, Ziegelmann JP, Wolff JK, & Schüz B (2013). How do negative self-perceptions of aging become a self-fulfilling prophecy? Psychology and Aging, 28(4), 1088–1097. 10.1037/a0032845 [DOI] [PubMed] [Google Scholar]
- *.Zee KS, & Weiss D (2019). High-quality relationships strengthen the benefits of a younger subjective age across adulthood. Psychology and Aging, 34(3), 374–388. 10.1037/pag0000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Zhang X, Kamin ST, Liu S, Fung HH, & Lang FR (2020). Negative self-perception of aging and mortality in very old Chinese adults: the mediation role of healthy lifestyle. The Journals of Gerontology: Series B, 75(5), 1001–1009. 10.1093/geronb/gby136 [DOI] [PubMed] [Google Scholar]
- *.Zhao Y, Dupre ME, Qiu L, & Gu D (2017). Changes in perceived uselessness and risks for mortality: evidence from a national sample of older adults in China. BMC PublicHealth, 17(1), 1–9. 10.1186/s12889-017-4479-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


