Abstract
Purpose
Evaluating procedure-related complications and perinatal outcomes after intrauterine transfusion (IUT) before or after 20+0 weeks of gestation in fetuses with severe anemia due to intrauterine human parvovirus B19 infection.
Methods
A retrospective study investigating fetuses requiring IUT for fetal Parvo B19 infection in two tertiary referral centers between December 2002 and December 2021. Procedure-related complications, intrauterine fetal death (IUFD), and perinatal outcome were correlated to gestational age (GA) at first IUT, the presence of hydrops and fetal blood sampling results.
Results
A total of 186 IUTs were performed in 103 fetuses. The median GA at first IUT was 19+3 (13+0–31+4) weeks of gestation. IUFD occurred in 16/103 fetuses (15.5%). Overall survival was 84.5% (87/103). Hydrops (p = 0.001), lower mean hemoglobin at first IUT (p = 0.001) and low platelets (p = 0.002) were strongly associated with IUFD. There was no difference observed in fetuses transfused before or after 20+0 weeks of gestation.
Conclusion
IUT is a successful treatment option in fetuses affected by severe anemia due to parvovirus B19 infection in specialized centers. In experienced hands, IUT before 20 weeks is not related to worse perinatal outcome.
Keywords: Fetal therapy, Intrauterine transfusion, Fetal infection by parvovirus B19, Fetal anemia, Fetal hydrops
What does this study add to the clinical work
| Anemia due to parvovirus B19 infection can be successfully treated in experienced centers from the early second trimester with similar complications rates < 20+0 and ≥ 20+0 weeks of gestation. Overall, survival to delivery is high but factors associated with non-survival are fetal hydrops, the severity of anemia and thrombocytopenia. |
Introduction
Parvovirus B19, a single-stranded round DNA virus is responsible for erythema infectiosum, a common illness observed in children. In most cases the infection will be asymptomatic, sometimes patients experience flu-like symptoms, the above-mentioned erythema infectiosum or arthropathies. In cases of intrauterine infections, parvovirus B19 attacks hematopoietic system cells, endothelial cells, placental cells, fetal liver and heart cells by binding to the P antigen. Erythroid progenitors in the bone marrow are targeted by the virus, inducing a shorter half-life of fetal red blood cells. This mainly affects hematopoiesis during the hepatic stage, consecutively leading to hemolysis and red blood cell aplasia [1–5]. Due to suppression of platelet precursors and direct cytotoxic effects on megakaryocytes, fetal thrombocytopenia is also commonly seen in fetuses affected by parvovirus B19 infection [6, 7]. The transplacental transmission rate is between 17 and 33%. Fetal anemia and hydrops are usually observed between 3 and 12 weeks after maternal infection. Fetal loss rate is reported at around 9% [8, 9]. According to a large observational study, most intrauterine fetal deaths (IUFD) are observed up to 4 weeks after maternal infection. Fetal losses occurred only in those infected before 20 weeks. The highest risk of IUFD was observed in infections between 9 and 16 weeks, and the highest risk for hydrops in infections between 13 and 20 weeks [8]. A recent systematic review and meta-analysis exploring the outcome of fetuses affected by parvovirus B19 infection included 35 observational studies and found a spontaneous resolution of the infection in 58.2% of the cases in non-hydropic fetuses [10]. Spontaneous resolution, however, was rare in hydropic fetuses (5%). Hydrops was associated with an increased risk for intrauterine or perinatal death. In this systematic review, IUT was performed in 78.7% of hydropic and 29.6% of non-hydropic fetuses. Fetal loss after IUT was higher in hydropic fetuses [10]. Fetal anemia is diagnosed by Doppler ultrasound measurement of the peak systolic velocity in the middle cerebral artery (MCA-PSV). This measurement is possible from the late first and early second trimester, in combination with other sonographic signs such as nuchal/generalized skin edema, echogenic bowel, cardiomegaly, placentomegaly and polyhydramnios [11]. Most fetuses do not develop relevant anemia or hydrops within the first 12 weeks after seroconversion and therefore do not need intrauterine transfusion. However, for severely anemic fetuses an IUT represents an important and in most cases life-saving treatment [12].
Data on intrauterine treatment of parvovirus B19-related anemia are limited [10, 13–17]. Parvovirus B19 often affects fetuses in the early second trimester but published data is mainly from IUTs performed after 20 weeks of gestation. We have previously published our experience of IUTs before 20 weeks of gestation [17]. Overall survival was 80.0% (44/55). Hydrops was present in 38.2% (21/55) and was strongly associated with IUFD (p = 0.001). There was no difference with regard to IUFD, hydrops, or hemoglobin concentration at first IUT in fetuses with transfusion before or after 16+0 weeks [17]. The aim of this study was to compare outcomes in pregnancies with severe anemia caused by parvovirus B19 infection and treated by IUT before or after 20+0 weeks of gestation.
Materials and methods
This retrospective study included in total 103 fetuses that underwent an IUT due to parvovirus B19 infection at any time during pregnancy in two tertiary centers between December 2002 and December 2021. Maternal characteristics including gestational age (GA) at first IUT, fetal sonographic manifestations, fetal blood sampling results, details of IUT procedures, procedure-related complications, and perinatal outcome data were analyzed.
Fetal anemia was assessed by pulsed wave Doppler ultrasound evaluation of the peak systolic velocity in the middle cerebral artery (MCA-PSV) [18, 19]. In all cases a detailed anatomic scan was performed and presence or absence of polyhydramnios, placentomegaly, tricuspid regurgitation, cardiomegaly, ascites, echogenic bowel and skin edema were evaluated. The diagnosis of fetal infection was confirmed either by the quantitative viral load in fetal blood by polymerase chain reaction (PCR) in 68/103 fetuses (66.0%) or by fetal sonographic suspicion of severe anemia in combination with maternal IgM antibodies. In cases with an elevated MCA-PSV above 1.5 multiples of the median (MoM), an IUT was planned [19]. For fetuses between 12+0 and 14+6 weeks of gestation reference values are described in the publication by Hellmund et al. [17].
Fetal blood sampling was possible in 169/186 IUTs and a complete blood count was performed. Severe thrombocytopenia was considered if < 50 platelets/nl. If fetal blood sampling was not successful (n = 17), the decision on the amount of blood transfused was solely based on the estimated fetal weight (without hydrops). A fetal weight adapted volume of packed red blood cells (PRBCs) (0 rhesus-negative, cross-matched, cytomegalovirus-negative, irradiated, mean hemoglobin concentration 26.1 g/dl) was administered during the same intervention.
All the procedures were conducted by experienced operators of the two centers. Spinal needles of 20–25 G were used. To not cause volume overload, 30–50 ml PRBCs/kg estimated fetal weight were transfused. Antenatal corticosteroids to reduce the incidence and severity of respiratory distress syndrome were not administered prior to IUTs. The necessity for additional IUTs depended on the hemoglobin concentration after IUT and echocardiographic and Doppler sonographic results.
The next day, an ultrasound examination with Doppler was performed. Depending on the results, further follow-up was planned. The total number of transfusions per fetus and the interval between IUTs were evaluated. The perinatal outcome of survivors and non-survivors were compared.
Bleeding from the puncture site, dislocation of the needle, unsuccessful puncture, fetal bradycardia or IUFD within 24 h after IUT were categorized as procedure-related complications.
To investigate the hypothesis that earlier transfusions cause more complications, the study group was divided into fetuses transfused < 20+0 weeks of gestation (57 fetuses) and fetuses treated at ≥ 20+0 weeks of gestation (46 fetuses). Survival rate was also compared in IUTs < 16+0 and ≥ 20+0 weeks of gestation.
Part of the IUTs < 20+0 weeks of gestation have been previously published [15].
Data analysis was performed with IBM SPSS software version 27 (SPSS Inc., Chicago, IL, USA). Differences between subgroups were calculated with the Fisher exact test, Mann- Whitney-U-Test or Pearson correlation coefficient wherever appropriate. A p value < 0.05 was considered significant.
Patient data, technical aspects of IUT, complications, fetal blood sampling results and perinatal outcomes were collected from the Viewpoint Database Version 5 and Orbis Version 08.04.37.21, the electronic hospital information system.
Ethical review and approval were waived in view of the retrospective nature of the study.
Results
103 patients underwent intrauterine transfusion for fetal anemia due to parvo B19 infection during the study period. In all cases, an increased MCA-PSV above 1.5 MoM was detected. 44/103 (42.7%) of fetuses were hydropic. Additional sonographic findings were tricuspid regurgitation (n = 53; 51.5%), ascites (n = 51; 49.5%), cardiomegaly (n = 42; 40.8%), pericardial effusion (n = 38; 36.9%), echogenic bowel (n = 32; 31.1%), skin edema (n = 31; 30.1%), placentomegaly (n = 33; 32.0%) and polyhydramnios (n = 25; 24.3%) whereas pleural effusion (n = 11; 10.7%) was less common. Reasons for referral were maternal seroconversion regarding human parvovirus B19 (n = 41; 39.8%), ascites/pericardial effusion or presence of fetal hydrops (n = 37; 35.9%), suspected maternal parvovirus exposure or maternal fever (n = 11; 10.7%), suspected fetal anemia (n = 9; 8.7%) and fetal echogenic bowel (n = 5; 4.9%) (Table 1).
Table 1.
Characteristics of cases with parvovirus B19 infection and intrauterine transfusion (IUT)
| Total number of fetusus | 103 |
| Number of IUTs (total, n = 186) | |
| 1 | 46 (44.7%) |
| 2 | 39 (37.9%) |
| 3 | 12 (11.6%) |
| 4 | 5 (4.8%) |
| 5 | 0 |
| 6 | 1 (1%) |
| Route of transfusion | |
| Intravascular | 167 (89.8%) |
| Intraperitoneal | 13 (7.0%) |
| Intracardiac | 6 (3.2%) |
| Weeks of gestation at first IUT (median) | 19+3 (13+0- 31+4) |
| IUTs < 20 + 0 weeks | 100 (53.8%) |
| IUTs ≥ 20 + 0 weeks | 86 (46.2%) |
| Mean interval between 1st and 2nd IUT, days | 6.6 |
| Mean interval between 2nd and 3rd IUT, days | 13.1 |
| Mean interval between 1st and last IUT, days | 12.3 |
| Hydrops | 44 (42.7%) |
| Placental site | |
| Anterior wall | 57 (55.3%) |
| Posterior wall | 46 (44.7%) |
| Fetal Death | 16/103 (15.5%) |
| Overall Survival | 87/103 (84.5%) |
| Fetal Loss Rate at 1st IUT | 12/103 (11.7%) |
| Fetal Loss Rate at follow-up IUTs | 4/57 (7.0%) |
A total of 186 IUTs were performed in 103 fetuses between 13+0 and 31+4 weeks of gestation. Up to six subsequent IUTs were required in 57/103 fetuses (55.3%). Median gestational age at first IUT was 19+3 weeks. 57 fetuses (55.3%) were transfused before and 46 (44.7%) after 20+0 weeks.
The most common access was intravascular (in the umbilical vein) in 89.8% (n = 167) and the most common site of transfusion was the placental cord insertion (81.7%); an intraperitoneal approach was necessary for 7% of IUTs. Intracardiac IUT was performed in six cases (3.2%) as a rescue procedure and in one case primarily due to difficult access. Peritoneal access was significantly more common in transfusions before 20+0 weeks of gestation (p = 0.006). Spinal needles of 20–25 G were used. In 174/186 IUTs this information was available. The 20G needle was used in 55 (31.6%), the 22G needle in 108 (62.1%) and the 25G needle in 11 (6.3%) IUTs.
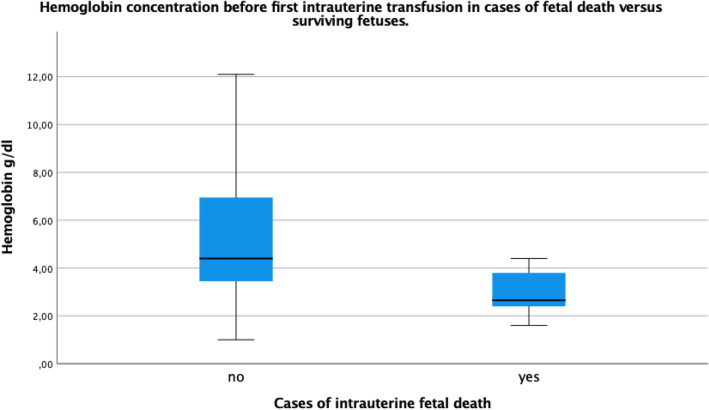
The hemoglobin concentration at the first IUT was available in 90/103 fetuses (87.4%) and in 90.3% of all procedures (168/186), information on platelet count was available in 75 fetuses (72.8%). The mean Hb concentration before the first IUT was 5.0 g/dl (0.3–12.1 g/dl, SD 2.58). Mean hemoglobin concentration before the first IUT was significantly lower in non-survivors (2.63 g/dl, SD 1.16) than in survivors (5.3 g/dl, SD 2.55) (p = 0.001) (Fig. 1).
Fig. 1.
Hemoglobin concentration in g/dl before first intrauterine transfusion in cases of surviving versus non-surviving fetuses
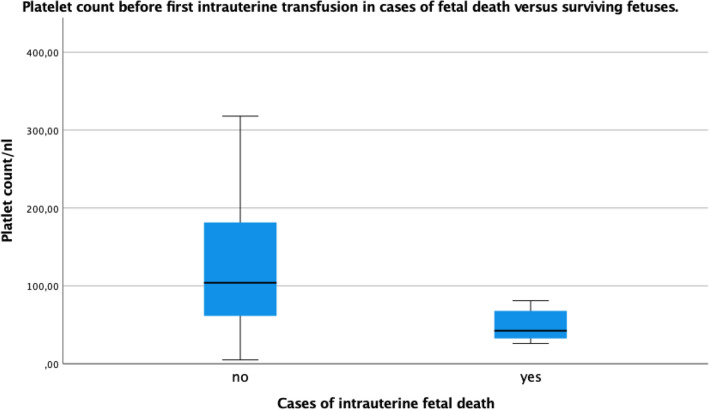
Thrombocytopenia below 100 platelets/nl was present in 37/75 fetuses (49.3%). The platelet count was significantly lower in non-survivors (p = 0.02) (Fig. 2). Severe thrombocytopenia below 50 platelets/nl was much rarer in the survivor group (12/67;17.9%) than in the non-survivor group (5/8; 62.5%) (p = 0.013). Platelets were given in 10 of the 186 (5.4%) transfusions in case of thrombocytopenia according to the operators’ choice and were not administered routinely. We also observed a correlation between the hemoglobin and thrombocyte concentration before the first IUT (p = 0.001).
Fig. 2.
Platelet count before first intrauterine transfusion in cases of surviving versus non-surviving fetuses
The overall survival rate was 84.5% (87/103). Sixteen (15.5%) IUFD occurred in 103 transfused fetuses. The IUFD rate after the first transfusion was 11.7% (12/103). In follow-up IUTs, fetal loss was rare (7.0%; 4/57). In 14 fetuses, death was noted during transfusion or within 24 h; in the other 2, death was detected on follow-up. Hydrops (p = 0.001) and low hemoglobin at first IUT (p = 0.001) were significantly associated with IUFD. Furthermore, posterior placental localization was associated with fetal loss (68.8%, 11/16 fetuses; p = 0.05). Prior to IUFD in these fetuses, dislocation of the needle (n = 2), bleeding from the puncture site (n = 3) and bradycardia during IUT (n = 1) were observed. Maternal body mass index on the other hand did not show any influence on procedure-related fetal loss (p = 0.97) (Table 2).
Table 2.
Procedure-related complications < 20 + 0 and ≥ 20 + 0 weeks of gestation
| Procedure-related complications | < 20 + 0 weeks | ≥ 20 + 0 weeks |
|---|---|---|
| Overall (n, %) | 15 (15%) | 9 (10.5%) |
| Bradycardia (n) | 1 | 1 |
| Bleeding from puncture site (n) | 2 | 1 |
| Unsucessfull puncture (n) | 1 | 1 |
| Dislocation of needle (n) | 2 | 1 |
| Fetal death within 24 h (n) | 9 | 5 |
Complications such as IUFD within 24 h, bradycardia, bleeding from the puncture site, contractions, dislocation of the needle or unsuccessful puncture occurred in 24/186 IUTs (12.9%). The median year of IUTs performed was 2009. To investigate if a learning curve exists, complication rates before and after 2009 were compared but did not differ significantly (p = 0.801). All operators were experienced and had performed at least 50 fetal blood samplings of the umbilical vein prior.
There was a lower survival rate in fetuses undergoing their first IUT < 20+0 weeks of gestation (80.7%) compared to ≥ 20+0 weeks of gestation (89.1%), but this did not reach statistical significance (p = 0.284). No differences in survival rate could be detected if IUTs < 16+0 (73.3% survival) and ≥ 20+0 (89.1% survival) weeks of gestation were compared (p = 0.204). Factors associated with IUFD after IUT are hydrops, mean hemoglobin before first IUT, mean platelet count, severe thrombocytopenia and posterior placental position (details Tables 3, 4).
Table 3.
Characteristics of survivors and non-survivors in transfused fetuses
| Characteristics | Survivor | Non-survivor | P value |
|---|---|---|---|
| Hydrops (%, n) | 35.6% (31) | 81.3% (13) | 0.001 |
| Median gestational age at 1st IUT | 19+2 | 18+4 | 0.18 |
| Mean hemoglobin before 1st IUT, g/dl | 5.3 (1–12.1) | 2.63 (0.3–4.4) | 0.001 |
| Mean platelet count/nl | 126.6 (5–318) | 49.1 (26–81) | 0.002 |
| Severe thrombocytopenia (< 50 platelets/nl) (%, n) | 17.9% (12) | 62.5% (5) | 0.013 |
| Posterior placental localization (%, n) | 40.2% (35) | 68.8% (11) | 0.05 |
| Mean maternal BMI | 24.6 (17.6–37.1) | 24.8 (19–33.2) | 0.97 |
p value < 0.05 is considered significant
Table 4.
Characteristics of fetuses with parvovirus B19 infection transfused < 20 + 0 and ≥ 20 + 0 weeks of gestation
| Characteristics | < 20 + 0 weeks | ≥ 20 + 0 weeks | p value |
|---|---|---|---|
| Hydrops (%, n) | 38.6% (22) | 47.8% (22) | 0.42 |
| Mean hemoglobin before 1st IUT, g/dl | 5.0 (0.3–10.7) | 4.94 (1.1–12.1) | 0.66 |
| Survivor (%, n) | 80.7% (46) | 89.1% (41) | 0.28 |
| Non-survivor (%, n) | 19.3% (11) | 10.9% (5) | 0.28 |
| Number of IUTs (%, n) | 0.39 | ||
| 1 | 50.9% (29) | 36.9% (17) | |
| 2 | 29.8% (17) | 47.8% (22) | |
| 3 | 12.3% (7) | 10.9% (5) | |
| 4 | 7% (4) | 2.2% (1) | |
| 5 | 0 | 0 | |
| 6 | 0 | 2.2% (1) |
p value < 0.05 is considered significant
Discussion
Our study investigated 103 fetuses affected by severe parvovirus B19 infection requiring transfusion. We found an overall survival rate of 84.5% (87/103 fetuses).
Survival rates in alloimmune fetal anemia vary between 77 and 97%, depending on GA at transfusion [20–22]. Survival rates for fetuses transfused at < 22 weeks due to alloimmunization are reported between 76 and 80% [23–25]. A large retrospective study analyzing 1678 IUTs in alloimmune fetal anemia observed fetal demise more often < 20+0 (17.0%, survival 83%) than ≥ 20+0 weeks of gestation (1.9%, survival 98.1%) [22]. The necessity for transfusion in fetal anemia due to parvovirus B19 infection usually occurs earlier in pregnancy compared to red blood cell alloimmunization. Additionally, at the time of diagnosis and IUT more fetuses are hydropic and severely anemic compared to fetuses affected by red blood cell alloimmunization. In a review, perinatal survival after IUT was 66.7–72.2% in parvovirus B19-infected fetuses versus 80.0–93.5% in cases of red blood cell alloimmunization [26]. Survival rates in cases of parvovirus infection have been reported between 33 and 100% in the literature [6, 8, 13–16, 27–39]. GA varied between studies, but cases before 20 weeks of gestation were less commonly included. Part of our data already published investigating IUTs performed before pregnancy week 20+0, found a survival of 80.0% (44/55) [17]. In our study, we also observed a trend towards lower survival < 20+0 weeks of gestation (80.7%) compared to ≥ 20+0 weeks of gestation (89.1%), but this did not reach statistical significance (p = 0.284).
Intravascular transfusion is considered the standard treatment for severe fetal anemia, regardless of its different etiologies. In our study, an intravascular access was possible in the majority (89.8%) of all IUTs. There was a significantly higher rate of peritoneal transfusions before gestational week 20+0 as described in the literature [40]. In a small study investigating an intracardiac approach between 17 and 23 weeks of gestation in fetuses with hydrops due to red blood cell alloimmunization (3 cases) or parvovirus B19 infection (5 cases), a survival rate of 75% was reported [34]. In our case series, however, in 6/186 IUTs (3.2%) an intracardiac transfusion was necessary leading to IUFD in all cases. 5/6 cases were as a rescue procedure in hydropic fetuses.
Thrombocytopenia is related to fetal hemoglobin count in anemic and hydropic fetuses as described by Segata et al. [6]. They described 11 hydropic fetuses of whom 75% were thrombocytopenic [6]. In our series, we observed thrombocytopenia in 68% of fetuses and 22.7% had severe thrombocytopenia. The platelet count was significantly lower in fetuses with hydrops compared to non-hydropic fetuses (p = 0.0001) and thrombocytopenia was also more commonly seen in non-survivors (p = 0.002). There was no difference observed between fetuses transfused in < 20+0 and ≥ 20+0 weeks of gestation (p = 0.126). We also observed a correlation between the hemoglobin and thrombocyte concentration before the first IUT as described in the literature [35] (Table 5).
Table 5.
| Study (first author) | Transfused case | GA at first transfusion (weeks of gestation) | Hydrops | Cases with severe thrombocytopenia | Survival rate (%; n) | |
|---|---|---|---|---|---|---|
| < 20 + 0 | ≥ 20 + 0 | |||||
| Smoleniec, 1994 [15] | 5 | 1 | 4 | 5 | 1 | 60 (3/5) |
| Fairley, 1995 [27] | 12 | n.a | n.a | 7 | n.a | 75 (9/12) |
| Cameron, 1997 [28] | 3 | 0 | 3 | 3 | n.a | 33 (1/3) |
| Odibo, 1998 [29] | 3 | 1 | 2 | 3 | n.a | 100 (3/3) |
| Rodis, 1998 [30]a | 164 | n.a. (18–32) | n.a | 137 | n.a | 83.5 (137/164) |
| Forestier, 1999 [31] | 7 | 1 | 6 | 7 | 2 | 85.7 (6/7) |
| Schild, 1999 [14] | 30 | 3 | 27 | 30 | 11 | 80 (24/30) |
| Enders, 2004 [8] | 16 | n.a | n.a | 16 | n.a | 87.5 (14/16) |
| Segata, 2007 [6] | 11 | 3 | 8 | 8 | 7 | 72.7 (8/11) |
| De Haan, 2008 [37] | 30 | 2 | 28 | 30 | 14 | 77 (23/30) |
| Simms, 2009 [13] | 8 | n.a. (18–27) | n.a | 8 | 2 | 62.5 (5/8) |
| Chauvet, 2011 [32] | 19 | n.a | n.a | 19 | n.a | 47 (11/19) |
| Garabedian, 2014 [33] | 13 | 0 | 13 | 5 | n.a | 76.9 (10/13) |
| Macé, 2014 [16] | 17 | 0 | 17 | 17 | 9 | 76 (13/17) |
| Melamed, 2015 [35] | 29 | 4 | 25 | 25 | 11 | 75.9 (22/29) |
| Mackie, 2015 [34] | 5 | 2 | 3 | 5 | 2 | 60 (3/5) |
| Zavattoni, 2016 [36] | 9 | 0 | 9 | 9 | n.a | 66.7 (6/9) |
| Sanchéz-Durán, 2020 [39] | 10 | n.a | n.a | 8 | n.a | 80 (8/10) |
| Current Studyb | 103 | 57 | 46 | 44 | 17 | 84.5 (87/103) |
| Total | 494 | 74/265c | 191/265c | 386/494 | 76 | 79.5 (393/494) |
GA gestational age, n.a. not available
aMulticenter survey
b55 cases were previously published [17]
cThe timing of the first IUT could be classified as < 20 and ≥ 20 weeks of gestation in 265 of the 494 cases
Data on neurodevelopmental outcome after fetal parvovirus B19 infection are heterogeneous. Dembinski et al. reported normal neurodevelopment after hydrops and IUT in 20 survivors with follow-up ranging from 13 months to 9 years of age [41] whereas Nagel et al. observed a delayed psychomotor development in 5 of 16 (32%) surviving infants at 1 to 42 months of age. At the median age of five years (range 1.5–13 years), De Jong et al. from the same center reported neurodevelopmental impairment in only 11% (2/28) of hydropic cases treated with IUT [42]. In our study, no follow-up data on postnatal neurodevelopment or cardiac function was available. Another limitation of our study was its retrospective design and the lack of blood sample values in some cases of IUT, especially in those transfused before 20 weeks of gestation with a 25-G needle. Proof of fetal parvovirus B19 infection was not possible in these cases but was very probable due to maternal seroconversion, sonographic signs—all these fetuses were hydropic—and severely elevated MCA-PSV. IUTs were performed by very experienced operators/centers and therefore the high rate of survival especially in fetuses transfused before 20 weeks of gestation of pregnancy might not be transferrable to centers with less expertise in this field.
In summary, anemia due to parvovirus B19 infection can be successfully treated in experienced centers from the early second trimester with similar complication rates < 20+0 and ≥ 20+0 weeks of gestation. In the vast majority of fetuses (> 80%), one or two IUTs are sufficient to bridge the time until intrauterine recovery. Overall, survival to delivery is high. Factors associated with non-survival are fetal hydrops, the severity of anemia and thrombocytopenia. An intravascular approach is possible in most cases.
Author contributions
Conceptualization: UG, AG and RB; Methodology: AH, PK and UG; formal analysis: PK and AH; investigation: PK and AH; data curation: PK, CB, BS, MD, CB, RB, AG and OMG; writing original draft preparation: PK; writing, review and editing: UG, BS, AG, PB and JJC; visualization: PK, PB and JJC; supervision: UG and AG; All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived in view of the retrospective nature of the study.
Informed consent statement
Patient consent was waived due to retrospective analysis. Patients were not contacted, and data collection was within the scope of routine patient care.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Philipp Kosian, Email: philipp.kosian@ukbonn.de.
Astrid Hellmund, Email: astrid.hellmund@praenatalmedizin-bonn.de.
Otilia-Maria Geist, Email: praenatalmedizin@klinikum-lev.de.
Ulrich Gembruch, Email: ulrich.gembruch@ukbonn.de.
References
- 1.Schwarz TF, Roggendorf M, Hottentrager B, Deinhardt F, Enders G, Gloning KP, Schramm T, Hansmann M. Human parvovirus B19 infection in pregnancy. Lancet. 1988;2(8610):566–567. doi: 10.1016/s0140-6736(88)92684-0. [DOI] [PubMed] [Google Scholar]
- 2.Levy R, Weissman A, Blomberg G, Hagay ZJ. Infection by parvovirus B 19 during pregnancy: a review. Obstet Gynecol Surv. 1997;52(4):254–259. doi: 10.1097/00006254-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Alger LS. Toxoplasmosis and parvovirus B19. Infect Dis Clin North Am. 1997;11(1):55–75. doi: 10.1016/s0891-5520(05)70341-x. [DOI] [PubMed] [Google Scholar]
- 4.Markenson GR, Yancey MK. Parvovirus B19 infections in pregnancy. Semin Perinatol. 1998;22(4):309–317. doi: 10.1016/s0146-0005(98)80019-0. [DOI] [PubMed] [Google Scholar]
- 5.Rodis JF (1999) Parvovirus infection. Clin Obstet Gynecol 42(1):107–120. 10.1097/00003081-199903000-00016(quiz 174–105) [DOI] [PubMed]
- 6.Segata M, Chaoui R, Khalek N, Bahado-Singh R, Paidas MJ, Mari G. Fetal thrombocytopenia secondary to parvovirus infection. Am J Obstet Gynecol. 2007;196(1):61. doi: 10.1016/j.ajog.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Bruno E, Briddell R, Cooper R, Srivastava C, van Besien K, Hoffman R. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood. 1990;76(10):1997–2004. doi: 10.1182/blood.V76.10.1997.1997. [DOI] [PubMed] [Google Scholar]
- 8.Enders M, Weidner A, Zoellner I, Searle K, Enders G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24(7):513–518. doi: 10.1002/pd.940. [DOI] [PubMed] [Google Scholar]
- 9.Dijkmans AC, de Jong EP, Dijkmans BA, Lopriore E, Vossen A, Walther FJ, Oepkes D. Parvovirus B19 in pregnancy: prenatal diagnosis and management of fetal complications. Curr Opin Obstet Gynecol. 2012;24(2):95–101. doi: 10.1097/GCO.0b013e3283505a9d. [DOI] [PubMed] [Google Scholar]
- 10.Bascietto F, Liberati M, Murgano D, Buca D, Iacovelli A, Flacco ME, Manzoli L, Familiari A, Scambia G, D'Antonio F. Outcome of fetuses with congenital parvovirus B19 infection: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52(5):569–576. doi: 10.1002/uog.19092. [DOI] [PubMed] [Google Scholar]
- 11.Kempe A, Rosing B, Berg C, Kamil D, Heep A, Gembruch U, Geipel A. First-trimester treatment of fetal anemia secondary to parvovirus B19 infection. Ultrasound Obstet Gynecol. 2007;29(2):226–228. doi: 10.1002/uog.3925. [DOI] [PubMed] [Google Scholar]
- 12.de Jong EP, Walther FJ, Kroes AC, Oepkes D. Parvovirus B19 infection in pregnancy: new insights and management. Prenat Diagn. 2011;31(5):419–425. doi: 10.1002/pd.2714. [DOI] [PubMed] [Google Scholar]
- 13.Simms RA, Liebling RE, Patel RR, Denbow ML, Abdel-Fattah SA, Soothill PW, Overton TG. Management and outcome of pregnancies with parvovirus B19 infection over seven years in a tertiary fetal medicine unit. Fetal Diagn Ther. 2009;25(4):373–378. doi: 10.1159/000236149. [DOI] [PubMed] [Google Scholar]
- 14.Schild RL, Bald R, Plath H, Eis-Hubinger AM, Enders G, Hansmann M. Intrauterine management of fetal parvovirus B19 infection. Ultrasound Obstet Gynecol. 1999;13(3):161–166. doi: 10.1046/j.1469-0705.1999.13030161.x. [DOI] [PubMed] [Google Scholar]
- 15.Smoleniec JS, Pillai M. Management of fetal hydrops associated with parvovirus B19 infection. Br J Obstet Gynaecol. 1994;101(12):1079–1081. doi: 10.1111/j.1471-0528.1994.tb13586.x. [DOI] [PubMed] [Google Scholar]
- 16.Mace G, Sauvan M, Castaigne V, Moutard ML, Cortey A, Maisonneuve E, Garel C, Dhombres F, Boujenah J, Mailloux A, Carbonne B. Clinical presentation and outcome of 20 fetuses with parvovirus B19 infection complicated by severe anemia and/or fetal hydrops. Prenat Diagn. 2014;34(11):1023–1030. doi: 10.1002/pd.4413. [DOI] [PubMed] [Google Scholar]
- 17.Hellmund A, Geipel A, Berg C, Bald R, Gembruch U. Early intrauterine transfusion in fetuses with severe anemia caused by parvovirus B19 infection. Fetal Diagn Ther. 2018;43(2):129–137. doi: 10.1159/000477208. [DOI] [PubMed] [Google Scholar]
- 18.Cosmi E, Mari G, Delle Chiaie L, Detti L, Akiyama M, Murphy J, Stefos T, Ferguson JE, Hunter D, Hsu CD, Abuhamad A, Bahado-Singh R. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia resulting from parvovirus infection. Am J Obstet Gynecol. 2002;187(5):1290–1293. doi: 10.1067/mob.2002.128024. [DOI] [PubMed] [Google Scholar]
- 19.Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ, Dorman KF, Ludomirsky A, Gonzalez R, Gomez R, Oz U, Detti L, Copel JA, Bahado-Singh R, Berry S, Martinez-Poyer J, Blackwell SC. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000;342(1):9–14. doi: 10.1056/NEJM200001063420102. [DOI] [PubMed] [Google Scholar]
- 20.Canlorbe G, Mace G, Cortey A, Cynober E, Castaigne V, Larsen M, Mailloux A, Carbonne B. Management of very early fetal anemia resulting from red-cell alloimmunization before 20 weeks of gestation. Obstet Gynecol. 2011;118(6):1323–1329. doi: 10.1097/AOG.0b013e318235e3bb. [DOI] [PubMed] [Google Scholar]
- 21.Van Kamp IL, Klumper FJ, Oepkes D, Meerman RH, Scherjon SA, Vandenbussche FP, Kanhai HH. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. Am J Obstet Gynecol. 2005;192(1):171–177. doi: 10.1016/j.ajog.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 22.Zwiers C, Lindenburg ITM, Klumper FJ, de Haas M, Oepkes D, Van Kamp IL. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound Obstet Gynecol. 2017;50(2):180–186. doi: 10.1002/uog.17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yinon Y, Visser J, Kelly EN, Windrim R, Amsalem H, Seaward PG, Ryan G. Early intrauterine transfusion in severe red blood cell alloimmunization. Ultrasound Obstet Gynecol. 2010;36(5):601–606. doi: 10.1002/uog.7696. [DOI] [PubMed] [Google Scholar]
- 24.Poissonnier MH, Picone O, Brossard Y, Lepercq J. Intravenous fetal exchange transfusion before 22 weeks of gestation in early and severe red-cell fetomaternal alloimmunization. Fetal Diagn Ther. 2003;18(6):467–471. doi: 10.1159/000073145. [DOI] [PubMed] [Google Scholar]
- 25.Lindenburg IT, van Kamp IL, van Zwet EW, Middeldorp JM, Klumper FJ, Oepkes D. Increased perinatal loss after intrauterine transfusion for alloimmune anaemia before 20 weeks of gestation. BJOG. 2013;120(7):847–852. doi: 10.1111/1471-0528.12063. [DOI] [PubMed] [Google Scholar]
- 26.Lindenburg IT, van Kamp IL, Oepkes D. Intrauterine blood transfusion: current indications and associated risks. Fetal Diagn Ther. 2014;36(4):263–271. doi: 10.1159/000362812. [DOI] [PubMed] [Google Scholar]
- 27.Fairley CK, Smoleniec JS, Caul OE, Miller E. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet. 1995;346(8986):1335–1337. doi: 10.1016/s0140-6736(95)92346-2. [DOI] [PubMed] [Google Scholar]
- 28.Cameron AD, Swain S, Patrick WJ. Human parvovirus B19 infection associated with hydrops fetalis. Aust N Z J Obstet Gynaecol. 1997;37(3):316–319. doi: 10.1111/j.1479-828x.1997.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 29.Odibo AO, Campbell WA, Feldman D, Ling PY, Leo MV, Borgida AF, Rodis JF. Resolution of human parvovirus B19-induced nonimmune hydrops after intrauterine transfusion. J Ultrasound Med. 1998;17(9):547–550. doi: 10.7863/jum.1998.17.9.547. [DOI] [PubMed] [Google Scholar]
- 30.Rodis JF, Borgida AF, Wilson M, Egan JF, Leo MV, Odibo AO, Campbell WA. Management of parvovirus infection in pregnancy and outcomes of hydrops: a survey of members of the Society of Perinatal Obstetricians. Am J Obstet Gynecol. 1998;179(4):985–988. doi: 10.1016/s0002-9378(98)70203-0. [DOI] [PubMed] [Google Scholar]
- 31.Forestier F, Tissot JD, Vial Y, Daffos F, Hohlfeld P. Haematological parameters of parvovirus B19 infection in 13 fetuses with hydrops foetalis. Br J Haematol. 1999;104(4):925–927. doi: 10.1046/j.1365-2141.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 32.Chauvet A, Dewilde A, Thomas D, Joriot S, Vaast P, Houfflin-Debarge V, Subtil D. Ultrasound diagnosis, management and prognosis in a consecutive series of 27 cases of fetal hydrops following maternal parvovirus B19 infection. Fetal Diagn Ther. 2011;30(1):41–47. doi: 10.1159/000323821. [DOI] [PubMed] [Google Scholar]
- 33.Garabedian C, Philippe M, Vaast P, Wibaut B, Salleron J, Delsalle A, Rakza T, Subtil D, Houfflin-Debarge V. Is intrauterine exchange transfusion a safe procedure for management of fetal anaemia? Eur J Obstet Gynecol Reprod Biol. 2014;179:83–87. doi: 10.1016/j.ejogrb.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Mackie FL, Pretlove SJ, Martin WL, Donovan V, Kilby MD. Fetal intracardiac transfusions in hydropic fetuses with severe anemia. Fetal Diagn Ther. 2015;38(1):61–64. doi: 10.1159/000369798. [DOI] [PubMed] [Google Scholar]
- 35.Melamed N, Whittle W, Kelly EN, Windrim R, Seaward PG, Keunen J, Keating S, Ryan G. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212(6):793. doi: 10.1016/j.ajog.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 36.Zavattoni M, Paolucci S, Sarasini A, Tassis B, Rustico M, Quarenghi A, Piralla A, Baldanti F. Diagnostic and prognostic value of molecular and serological investigation of human parvovirus B19 infection during pregnancy. New Microbiol. 2016;39(3):181–185. [PubMed] [Google Scholar]
- 37.de Haan TR, van den Akker ES, Porcelijn L, Oepkes D, Kroes AC, Walther FJ. Thrombocytopenia in hydropic fetuses with parvovirus B19 infection: incidence, treatment and correlation with fetal B19 viral load. BJOG. 2008;115(1):76–81. doi: 10.1111/j.1471-0528.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- 38.Nagel HT, de Haan TR, Vandenbussche FP, Oepkes D, Walther FJ. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109(1):42–47. doi: 10.1097/01.AOG.0000249611.67873.94. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez-Duran MA, Higueras MT, Aviles-Garcia M, Maiz N, Rodriguez-Aliberas M, Arevalo S, Vazquez E, Ruiz Campillo CW, Carreras E. Perinatal outcomes and central nervous system abnormalities following intrauterine fetal transfusion: 17 years' experience in a tertiary center. Transfusion. 2020;60(11):2557–2564. doi: 10.1111/trf.16087. [DOI] [PubMed] [Google Scholar]
- 40.Howe DT, Michailidis GD. Intraperitoneal transfusion in severe, early-onset Rh isoimmunization. Obstet Gynecol. 2007;110(4):880–884. doi: 10.1097/01.AOG.0000284623.15170.0d. [DOI] [PubMed] [Google Scholar]
- 41.Dembinski J, Haverkamp F, Maara H, Hansmann M, Eis-Hubinger AM, Bartmann P. Neurodevelopmental outcome after intrauterine red cell transfusion for parvovirus B19-induced fetal hydrops. BJOG. 2002;109(11):1232–1234. doi: 10.1046/j.1471-0528.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- 42.De Jong EP, Lindenburg IT, van Klink JM, Oepkes D, van Kamp IL, Walther FJ, Lopriore E. Intrauterine transfusion for parvovirus B19 infection: long-term neurodevelopmental outcome. Am J Obstet Gynecol. 2012;206(3):204. doi: 10.1016/j.ajog.2011.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.