Abstract
Context:
There is ongoing discourse about the impact of advance care planning (ACP) on end-of-life (EOL) care. No meta-analysis exists to clarify ACP’s impact on patients with cancer.
Objective:
To investigate the association between, and moderators of, ACP and aggressive versus comfort-focused EOL care outcomes among patients with cancer.
Methods:
Five databases were searched for peer-reviewed observational/experimental ACP-specific studies that were published between 1990-2022 that focused on samples of patients with cancer. Odds ratios were pooled to estimate overall effects using inverse variance weighting.
Results:
Of 8,673 articles, 21 met criteria, representing 33,541 participants and 68 effect sizes (54 aggressive, 14 comfort-focused). ACP was associated with significantly lower odds of chemotherapy, intensive care, hospital admissions, hospice use fewer than seven days, hospital death, and aggressive care composite measures. ACP was associated with 1.51 times greater odds of do-not-resuscitate orders. Other outcomes—cardiopulmonary resuscitation, emergency department admissions, mechanical ventilation, and hospice use—were not impacted. Tests of moderation revealed that the communication components of ACP produced greater reductions in the odds of hospital admissions compared to other components of ACP (e.g., documents); and, observational studies, not experimental, produced greater odds of hospice use.
Conclusion:
This meta-analysis demonstrated mixed evidence of the association between ACP and EOL cancer care, where tests of moderation suggested that the communication components of ACP carry more weight in influencing outcomes. Further disease-specific efforts to clarify models and components of ACP that work and matter to patients and caregivers will advance the field.
Keywords: advance care planning, communication, cancer, aggressive care, comfort-focused end-of-life care, meta-analysis
Introduction
Despite an accumulated body of literature on advance care planning (ACP) over the past 30 years, the recent discourse has focused attention on the ambiguous impact of ACP on end-of-life (EOL) care.(1,2) ACP represents a process that “enables individuals to define goals and preferences for future medical treatment and care, to discuss these goals and preferences with family and health-care providers, and to record and review these preferences if appropriate.”(3) A central goal of this process is to ensure persons with serious illnesses receive care at the EOL that is concordant with their preferences. Although EOL preferences are highly individualized, evidence suggests that persons with serious illnesses generally prefer approaches to EOL care that are comfort-focused and desire to avoid aggressive interventions, such as mechanical ventilation.(4-6) Yet, the evidence base to support the role of ACP in achieving such outcomes is mixed, and largely relies on individual studies or narrative reviews.(7-12)
We conducted a meta-analysis to investigate the association between ACP and various aggressive and comfort-focused EOL care outcomes across an extant body of literature among persons with cancer. Of note, no meta-analysis exists to clarify the overall association between ACP and various aggressive and comfort-focused EOL care outcomes among persons with cancer, nor have key moderators that could explain variation in the overall association been explored. While a few meta-analyses exist that indicate support for the role of ACP in achieving more short-term outcomes, such as improved ACP knowledge,(13) increased advance directive documentation,(14-16) or enhanced patient outcomes (e.g., quality of life, satisfaction with care),(17-19) these studies represent general populations of seriously ill patients.(13-17,19) Condition-specific inquiry, as well as meta-analyses examining long-term outcomes of ACP at the EOL, remain limited.(18,19) Investigating the association between ACP and EOL care outcomes may be particularly advantageous among studies of patients with cancer due to cancer’s often protracted illness trajectory and relatively predictable decline in the terminal illness phase,(20,21) allowing the opportunity for ACP to be implemented early in the illness trajectory and to function as it is intended—a preparatory tool for later “in-the-moment” decision making.(22) Patients with cancer are also more likely to be offered(23) and engage(24) in ACP compared to other serious illness conditions, further augmenting the potential to understand the overall impact of ACP on EOL care outcomes.
Estimating the overall associations between ACP and EOL care outcomes requires contextualization using moderator analysis. Key variables that could alter the association between ACP and EOL care outcomes include the type of ACP under study as well as study design. Conceptualizations of ACP differ across studies and tend to focus on different ACP “components.” Some studies conceptualize ACP as primarily a documentation process, whereas others conceptualize ACP as primarily a communication process, and still others conceptualize ACP more holistically to include both documentation and communication components.(11,25) Further, both observational and experimental studies contribute to the accumulated body of literature on ACP, where observational studies typically explore the impacts of routinized ACP and experimental studies, enhanced approaches to ACP. Thus, clarifying the association between ACP and various EOL care outcomes requires moderator analysis to account for sources of variation in effect sizes based on the type of ACP under study (i.e., how ACP was conceptualized) and study design. Therefore, using PRISMA guidelines(25) this meta-analysis aimed to: 1) examine the associations between ACP and the use of aggressive- and comfort-focused EOL care outcomes among patients with cancer, and 2) to test moderators (ACP type, study design) of these associations.
Methods
Eligibility Criteria
Studies were included if they contained: (1) an examination of the association between at least one component of ACP (documents, communication, or both) and one EOL care outcome, (2) included an exclusive sample of adult patients with cancer or a mixed sample of adults with serious illnesses where outcomes were reported at the level of the disease groups, (3) included a comparison group, and (4) included sufficient information to compute effect sizes. Studies were excluded if they: (1) lacked either ACP variables or EOL care outcomes, (2) had a non-adult or non-patient (e.g., healthcare providers) sample, (3) did not stratify for cancer-specific outcomes, (4) did not include sufficient information to compute effect sizes, or (5) used do-not-resuscitate orders as a proxy for ACP.
Search Strategy
ACP-related search terms were combined with cancer-related search terms across five databases: CINAHL, Cochrane Library, PubMed, Scopus, and Web of Science (Appendix). Specifically, the search phrase utilized in each database included the following MeSH terms: advance care planning, advance directives, and neoplasm. Additional keywords (and variations thereof) were also included in the search phrase: healthcare surrogate, healthcare proxy, health proxy, healthcare agent, power of attorney, cancer, oncology, tumor, and malignancy. All ACP-related search terms were separated from the cancer-related search terms parenthetically in the search phrase. Within the two parenthetical groupings, search terms were combined using the “OR” Boolean operator. Between parenthetical groupings, the “AND” Boolean operator was used in the search phrase. Outcome-specific search terms were not utilized (e.g., “mechanical ventilation,” “hospice”) as to avoid narrowing the initial catchment of articles. The living will search term was not utilized as it is subsumed under the MeSH term advance care planning. Search limiters utilized in each database included: peer-reviewed articles published in English since 1990 (coinciding with the Patient Self Determination Act).
Study Selection
The first author (KL) conducted the initial title and abstract screening. A second author (RM) independently conducted title and abstract screening for 20% of the articles to verify proper adjudication of articles at this level of the search. To ensure consistent application of eligibility criteria at the level of full-text review, three authors (KL, RM, SS) independently reviewed a subset of the full-text articles. The authors met to compare these assessments, discuss discrepancies, and achieve consensus. Using this consensus-driven application of the eligibility criteria, the remaining full-text articles were divided and a 40% overlap in full-text reviewed articles was maintained.
Data Abstraction
To ensure consistent data abstraction procedures, three authors (KL, RM, SS) conducted joint data abstraction for a subset of the included studies using the study codebook (Table A, Appendix). The authors met to compare these abstractions, discuss discrepancies, and achieve consensus. Using this consensus-driven application of the codebook, the remaining full-text articles were divided, and a 60% overlap maintained for the data abstraction. Beyond study and sample characteristics, ACP characteristics, and effect size data for the EOL care outcomes were abstracted and coded for each study into uniform coding sheets. Once completed, the authors met to compare the uniform coding sheets, discuss discrepancies, and achieve consensus.
ACP Type.
ACP was categorized into one of three “types” based on the ACP components toward which the study was oriented: 1) documentation components only (living will only, healthcare surrogate designation only, or both—synonymous with an advance directive); 2) communication components only (i.e., inclusive of communication that ranged from an exploration of EOL preferences to more targeted communication about prognosis and goals of care);(26) or 3) full ACP (i.e., embracing both documentation and communication components).
EOL Care Outcomes.
EOL care outcomes encompassed two global EOL care outcome groupings: 1) aggressive; and 2) comfort-focused. Each of these groupings contained various healthcare utilization outcomes that have been previously defined as important outcomes of successful ACP as well as indicators of the quality of EOL care delivery among patients with cancer.(27-29) Aggressive EOL care outcomes included: chemotherapy administration within the last 14 days of life; an emergency department, hospital, or intensive care unit admission in the last 30 days of life; the receipt of aggressive interventions, such as, mechanical ventilation, cardiopulmonary resuscitation, or dialysis; a hospice admission fewer than seven days; or a hospital death.(27) Comfort-focused EOL care outcomes included: documentation of a do-not-resuscitate order, withdrawal of life-sustaining treatments, hospice use, and home death.(30,31) Raw outcome data (counts of patients with and without the outcome according to ACP groups) were abstracted. When raw data were not reported, the logarithm of the reported odds ratio was computed, and the corresponding confidence interval was abstracted and utilized to compute variance of the log odds ratio (Appendix). When both unadjusted and adjusted odds ratios were reported, the unadjusted estimates were utilized.
Risk of Bias
Risk of bias was assessed using an adapted tool for systematic reviews and meta-analyses that are inclusive of non-randomized study designs.(32) Two authors (KL, SS) independently assessed the risk of bias for 40% of the studies and compared ratings. When discrepancies were identified, definitions outlined in the risk of bias tool(32) were revisited and discussed until consensus was achieved. The remaining studies were assessed by the first author (KL). Studies were adjudicated as: low risk of bias with eight or more “low risk” indicators, moderate risk of bias with four to seven “low risk” indicators, or high risk of bias with zero to three “low risk” indicators.
Publication Bias
Publication bias(33,34) was assessed in two ways. Begg and Mazumdar’s rank correlation test is a regression analysis of the rank correlation (Kendall’s tau) between the effects sizes and their associated standard errors.(35) Egger’s regression test is a regression of the standard normal deviate of effect size from zero for each study onto precision (1/SE) for each study.(35) Both are tests of funnel plot asymmetry and indicate the possibility of publication bias when significant.
Synthesis of Results
Effect Size.
The effect size was the odds ratio (OR). The analysis was computed using the logarithm of the odds ratio (LOR) and its associated variance (VOR).(35) Model parameters were then back-transformed using an exponent function for more interpretable results.
Statistical Analysis.
This meta-analysis was conducted using the metafor package in R statistical software(36) and was based on the methods set forth by Hedges and Olkin(37) and further described by Cooper, Hedges, and Valentine (Appendix).35 A series of subset meta-analyses were conducted for each individual EOL care outcome. To determine whether a fixed or random effects model was indicated, a test of homogeneity was first conducted using the Q statistic (Qtotal). A significant Q statistic indicated a random effects model, a non-significant Q statistic indicated a fixed effects model. To compute the overall effect, each effect size was weighted according to inverse variance. Under a fixed effects model, variance constituted sampling error, or within study variation. Under a random effects model, variance constituted both within study and between study (Tau2) variation. Between study variation was estimated using DerSimonian and Laird methods,(38) and its magnitude was interpreted using the I2 value, where 25%, 50%, 75% indicated a low, medium, and high amount of between study variation, respectively.(39) The overall effect size was estimated by taking the sum of the weighted individual effects and dividing them by the sum of the individual weights (Appendix). Significance testing for the overall effect size was conducted on a z-distribution under the null hypothesis that the overall effect size was zero. A priori significance was set at p < .05.
Moderator analysis.
Both ACP type (documentation components only, communication components only, full ACP) and study design (observational, experimental) were tested as moderators of the association between ACP and each EOL care outcome. To determine whether a weighted fixed effects model with a moderator or a mixed effects model was indicated, the QM (test of moderator) and QE (test of residual between study variation) statistics were utilized.(35,37) A significant QM indicated moderation, and the QE guided the selection of the fixed (i.e., non-significant QE) or mixed (i.e., a significant QE) effects model. Due to the categorical moderators, a weighted statistical model was utilized to test moderation, where group effect sizes within each study, according to the categories of the moderator, were weighted using inverse variance. The sum of the weighted effect sizes was then divided by the sum of the weights for each group. Hypothesis testing determined if the effect of the moderator (QM) was significant (i.e., the group effects were significantly different from each other based on the value of the moderator). If significant, group effect sizes were interpreted.
Results
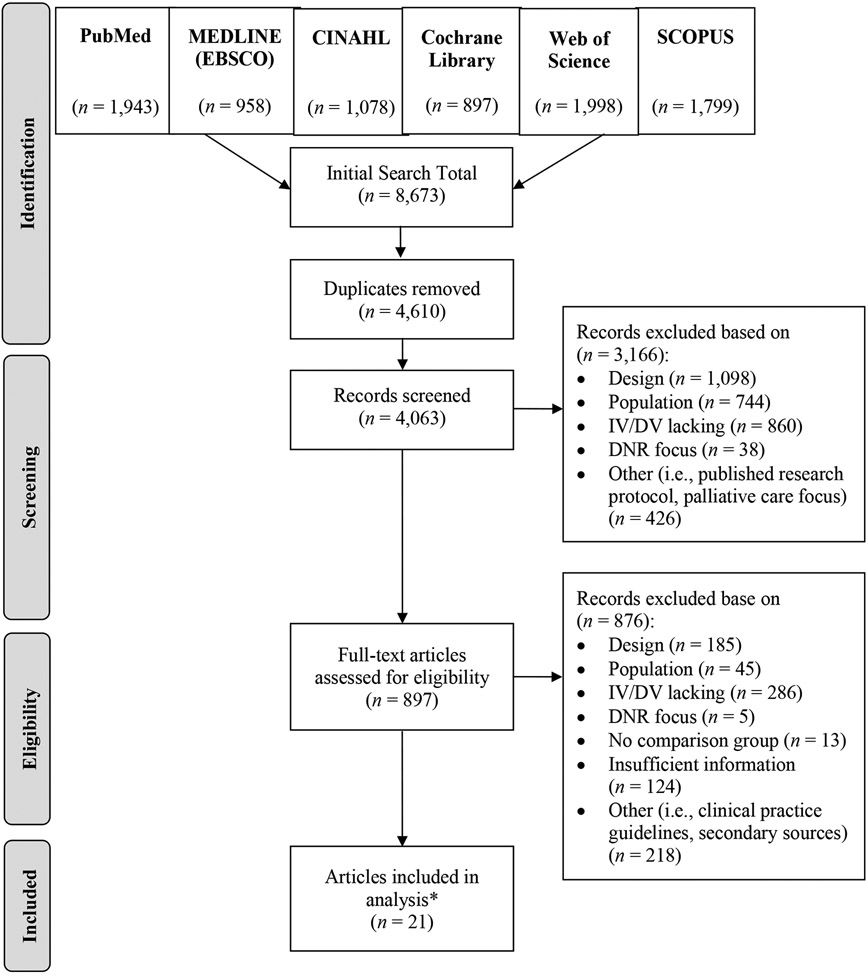
The combined searches yielded 8,673 articles, with 4,063 unique articles for review after removing duplicates, and 21 articles (representing 20 unique studies) that met criteria (Figure 1).
Figure 1. PRISMA Flow Diagram.
*21 articles, representing 20 unique studies
Study Characteristics
Among the 21 included articles, there were 68 effect sizes (54 aggressive and 14 comfort-focused EOL care outcomes) (Table 1). Effect sizes for the aggressive EOL care outcomes included chemotherapy (k = 7), intensive care unit admission (k = 7), hospital admission (k = 6), hospice use fewer than seven days (k = 5), cardiopulmonary resuscitation (k = 5), hospital death (k = 4), mechanical ventilation (k = 4), emergency department admission (k = 4), composite measures of aggressive interventions (k = 4), and other miscellaneous outcomes (e.g., artificial nutrition, dialysis, surgery) (k = 8). Effect sizes for the comfort-focused EOL care outcomes included hospice use (k = 5), do-not-resuscitate orders (k = 5), and other miscellaneous outcomes (e.g., home death, no escalation of care) (k = 4). The type of ACP under study varied across the 20 unique studies (documentation components only, n = 9, 45%; communication components only, n = 8, 40%; full ACP, n = 3, 15%). Study designs were largely observational (n = 16, 80%), and a minority were experimental (n = 4, 20%). Most were conducted in the United States (n = 16, 80%), with the remaining across Australia, Japan, Spain, and Taiwan. Half were published within the past five years, and publication dates ranged between 2001-2022.
Table 1.
Study and Sample Characteristics
| Author (Year) |
Country | Design | ACP Typea | Outcomes | Analyzed Sample (n) |
Mean Age (years) |
Males (%) | Racial/ Ethnic Minority (%) |
Cancer Inclusion Criteria |
|---|---|---|---|---|---|---|---|---|---|
| Ahluwalia(61) (2015) | United States | Observational: retrospective chart review | Communication: prognosis and end-of-life preferences | Use of Aggressive Care:
|
665 | 66.4 | 97.1 | 25.3 | Terminal cancer indicators |
| Cagle(51) (2020) | United States | Observational: secondary analysis of a national dataset of a prospective cohort | Documents: advance directive | Comfort-focused Care:
|
271 | 76.2 | 59.4 | 25b | Cancer-related death cases |
| Chen(62) (2019)/Wen(63) (2020) | Taiwan | Experimental: RCT of an ACP intervention | Communication: prognosis and end-of-life preferences | Use of Aggressive Care:
|
430 | missc | 70.4 | miss | Terminal cancer indicators |
| Dalmau-Bueno(64) (2021)d | Spain | Observational: case control study | Documents: advance directive | Use of Aggressive Care:
|
2,338 | missc | 48.7 | miss | Cancer-related death cases |
| Diamond(65) (2016) | United States | Observational: retrospective cohort study | Documents: healthcare surrogate designation | Use of Aggressive Care:
|
160 | 63.4 | 58 | 38 | Diagnosis-specific |
| Halpern(66) (2011) | United States | Observational: retrospective chart review | Documents: living will or healthcare surrogate designation | Use of Aggressive Care:
|
1,121 | 61.5 | 60 | 21.2 | Diagnosis-specific |
| Ishikawa(67) (2018) | Japan | Observational: retrospective chart review | Communication: prognosis | Use of Aggressive Care:
|
107 | 77 | 46.7 | miss | Terminal cancer indicators |
| Jeurkar(68) (2012)d | United States | Observational: retrospective chart review | Documents: advance directive | Comfort-focused Care:
|
3,561 | 72.6 | 50.9 | 10.9 | Terminal cancer indicators |
| Johnson(8) (2018)d | Australia | Experimental: RCT of an ACP intervention | Full ACP | Use of Aggressive Care:
|
150 | 65.5 | 53.4 | miss | Terminal cancer indicators |
| Mack(50) (2012) | United States | Observational: secondary analysis of a cancer registry database | Communication: end-of-life preferences | Use of Aggressive Care:
|
1,231 | missc | 62 | 24 | Terminal cancer indicators |
| McDermott(69) (2020) | United States | Observational: retrospective cohort study | Documents:e healthcare surrogate designation and/or living will | Use of Aggressive Care:
|
15,092 | 64.5 | 56.7 | 14.6 | Terminal cancer indicators |
| Narang(45) (2015) | United States | Observational: secondary analysis of a national dataset of a prospective cohort | Documents:f living will | Use of Aggressive Care:
|
1,985 | missc | 54 | 13.7b | Cancer-related death cases |
| Peltier(47) (2017) | United States | Experimental: evaluation of an ACP-centered program | Full ACP | Use of Aggressive Care:
|
69 | miss | 52.2 | 28 | Cancer-related death cases |
| Prater(70) (2019) | United States | Observational: retrospective chart review | Communication: end-of-life preferences | Use of Aggressive Care:
|
1,185 | 63.7 | 51.6 | 12.9 | Terminal cancer indicators |
| Prater(71) (2022) | United States | Observational: retrospective medical claims analysis | Communication: ACP encounter (via billing code) | Use of Aggressive Care:
|
3,705 | 56.2 | 40.4 | miss | Terminal cancer indicators |
| Rocque(46) (2017) | United States | Experimental: evaluation of an ACP-centered program | Full ACP | Use of Aggressive Care:
|
608 | 76.4 | 53.8 | 19 | Terminal cancer indicators |
| Salazar(72) (2022) | United States | Observational: retrospective chart review | Documents: advance directive | Use of Aggressive Care:
|
113 | missc | 58 | 20 | Cancer-related death cases |
| Sedhom(73) (2021) | United States | Observational: retrospective chart review | Communication: prognosis and goals-of-care | Use of Aggressive Care:
|
147 | missc | 51.7 | 19.7b | Diagnosis-specific |
| Wallace(74) (2001) | United States | Observational: retrospective chart review | Documents: advance directive | Use of Aggressive Care:
|
270 | 55 | 56.7 | miss | Diagnosis-specific |
| Wright(49) (2008) | United States | Observational: retrospective cohort study | Communication: end-of-life preferences | Use of Aggressive Care:
|
332 | 57.9 | 55.1 | 36 | Terminal cancer indicators |
Documents (living will only, healthcare surrogate only, or both—synonymous with an advance directive), Communication (end-of-life preferences, prognosis, or goals of care), 3) Full ACP (all three components), or 4) unspecified ACP
Hispanic ethnicity reported as a separate variable, and thus, was not reflected in the proportion of minorities
Mean age data not reported, instead median or age ranges described
Studies where cancer death cases comprised a subset of the full sample, but demographics were only reported for the full sample
Study conceptualization of ACP included a range of ACP behaviors, inclusive of physician orders for life-sustaining treatment (POLST), however, the proportion of ACP that was POLST was not specified
Outcomes associated with healthcare surrogate designation and communication were also reported; however, there was participant crossover between ACP types, so only one type was utilized for the analysis
Abbreviations: ACP is advance care planning, AML is acute myeloid leukemia, CPR is cardiopulmonary resuscitation, DNR is do-not-resuscitate, ED is emergency department, ICU is intensive care unit, miss is missing,
Note: Terminal cancer indicators included patients with advanced stage and/or metastatic disease, progressive disease, disease unresponsive to treatment, or patients who were hospice eligible
Sample Characteristics
There were 33,541 participants across the analyzed samples, with sample sizes ranging from 69 to 15,092 and a median sample size of 519 (Table 1). Among the 13 studies reporting age-related means, the sample mean age was 65.9 years (range: 55-77 years; median: 64.5 years). The average sample contained 57% males (range: 40-97%; median: 55%) and 22% persons from underrepresented racial and/or ethnic backgrounds (range: 11-38%; median: 21%), however characteristics of participants from underrepresented backgrounds were missing in six studies. Over half (n = 11, 55%) of the studies included participants with terminal cancer indicators (i.e., advanced stage and/or metastatic disease, progressive disease, disease unresponsive to treatment, or patients who were hospice eligible), and the remaining included cancer-related death cases (typical of retrospective chart reviews) (n = 5, 25%) or diagnoses-specific criteria (e.g., which commonly included colorectal, lung, and/or pancreatic cancers) (n = 4, 20%).
Risk of Bias
A majority of studies were adjudicated with a moderate risk of bias (n = 14, 70%) assessment, and the remainder high (n = 5, 25%) or low (n = 1, 5%) risk of bias (Table B, Appendix).
Publication Bias
All Begg and Mazumdar’s rank correlation tests and Egger’s regression tests were non-significant (Table 2), with two exceptions: Egger’s regression test was significant in the subset meta-analysis performed on the chemotherapy (p = .018) and hospice use fewer than seven days (p = .022) outcomes. However, in both instances, Begg and Mazumdar’s rank correlation tests were non-significant. Taken together, this generally indicates that there was not sufficient evidence of publication bias, but there remains some potential for publication bias among the meta-analyses conducted on the chemotherapy and hospice use fewer than seven days outcomes.
Table 2.
Subset Meta-analyses by Study Outcomes
| Outcome Subset (Model) |
(n) I2 |
Publication Bias Tests |
Effect Sizes (k) |
LOR | SE | z | 95% CI | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | p | ||||||||
| Aggressive End-of-life Care Outcomes | ||||||||||||
| Chemotherapy (FE) | (3,169) | EG: p = .018 BM: p = .136 |
7 | −0.32 | 0.12 | −2.71 | −0.55 | −0.09 | 0.72 | 0.57 | 0.92 | .007 |
| ICU Admission (FE) | (17,875) | EG: p = .976 BM: p = .562 |
7 | −0.34 | 0.05 | −6.38 | −0.44 | −0.23 | 0.71 | 0.64 | 0.79 | <.001 |
| Hospital Admission (RE) | (20,772) I2 = 61.5% |
EG: p = 1.00 BM: p = .787 |
6 | −0.60 | 0.13 | −4.70 | −0.85 | −0.35 | 0.55 | 0.43 | 0.70 | <.001 |
| Hospice Use < 7 days (RE) | (2,811) I2 = 68.1% |
EG: p = .022 BM: p = .083 |
5 | −0.51 | 0.24 | −2.15 | −0.97 | −0.04 | 0.60 | 0.38 | 0.96 | .032 |
| CPR (FE) | (4,491) | EG: p = .107 BM: p = .483 |
5 | −0.27 | 0.20 | −1.39 | −0.66 | 0.11 | 0.76 | 0.52 | 1.12 | .164 |
| Hospital Death (RE) | (17,335) I2 = 98.4% |
EG: p = .057 BM: p = .750 |
4 | −0.74 | 0.32 | −2.30 | −1.38 | −0.11 | 0.48 | 0.25 | 0.89 | .021 |
| Mechanical Ventilation (RE) | (3,370) I2 = 76.6% |
EG: p = .163 BM: p = 1.00 |
4 | −0.27 | 0.31 | −0.89 | −0.88 | 0.33 | 0.76 | 0.42 | 1.39 | .375 |
| ED Admission (FE) | (18,107) | EG: p = .662 BM: p = .750 |
4 | −0.20 | 0.24 | −0.82 | −0.66 | 0.27 | 0.82 | 0.52 | 1.31 | .410 |
| Aggressive Interventionsa (FE) | (3,994) | EG: p = .762 BM: p = .750 |
4 | −0.53 | 0.13 | −4.14 | −0.77 | −0.28 | 0.59 | 0.46 | 0.76 | <.001 |
| Comfort-focused End-of-life Care Outcomes | ||||||||||||
| Hospice Use (RE) | (2,511) I2 = 63.1% |
EG: p = .786 BM: p = .483 |
5 | 0.27 | 0.20 | 1.33 | −0.13 | 0.67 | 1.31 | 0.88 | 1.95 | .185 |
| DNR Order (RE) | (3,837) I2 = 62.7% |
EG: p = .497 BM: p = .817 |
5 | 0.41 | 0.21 | 1.98 | 0.003 | 0.83 | 1.51 | 1.00 | 2.28 | .048 |
Indicates varying composite measures of aggressive end-of-life care indicators across studies, including combinations of chemotherapy in the last 14 days of life, acute care in the last 30 days of life, ICU care in the last 30 days of life, and/or intensive interventions (e.g., mechanical ventilation, CPR, hemodialysis, gastric tube placement) within the last 30 days of life.
Abbreviations: BM is Begg and Mazumdar’s rank correlation test of funnel plot asymmetry; CPR is cardiopulmonary resuscitation, DNR is do-not-resuscitate, ED is emergency department, EG is Eggers regression test of funnel plot asymmetry; FE is fixed effects model, ICU is intensive care unit, RE is random effects model
Note: I2 values indicate the estimated amount of between study variation (i.e., heterogeneity) under the random effects RE models
Synthesis of Results
An overview of the results from the series of subset meta-analyses and tests of moderation is presented in Table 2 and Table 3, respectively (See Figures A-K in the Appendix for forest plots). Abstracted effect size data can be referenced in Table 4.
Table 3.
Moderator Analyses
| Moderator (Model) |
Outcome QM(df), p |
Moderator Levels |
LOR | SE | z | 95% CI | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | p | |||||||
| ACP Type (ME) | Hospital Admission QM(2) = 12.29, p = .002 |
Documentation components only | −0.40 | 0.05 | −8.21 | −0.50 | −0.31 | 0.67 | 0.61 | 0.74 | <.001 |
| Communication components only | −0.91 | 0.14 | −6.64 | −1.18 | −0.64 | 0.40 | 0.31 | 0.53 | <.001 | ||
| Full ACP | −0.40 | 0.17 | −2.34 | −0.74 | −0.07 | 0.67 | 0.48 | 0.94 | .019 | ||
| Study Design (FE with moderators) | Hospice Use QM(1) = 4.94, p = .026 |
Observational Study | 0.42 | 0.15 | 2.86 | 0.13 | 0.71 | 1.52 | 1.14 | 2.03 | .004 |
| Experimental Study | −0.11 | 0.18 | −0.58 | −0.48 | 0.26 | 0.90 | 0.62 | 1.29 | .559 | ||
Abbreviations: ACP is advance care planning; FE is fixed effects; ME is mixed effects
Table 4.
Effect Size Data
| Author (Year): Outcome | Treatment Group (n) |
Control Group (n) |
Treatment Positivea (n) |
Treatment Negativedb (n) |
Control Positivec (n) |
Control Negatived (n) |
LOR | VOR |
|---|---|---|---|---|---|---|---|---|
| Aggressive End-of-life Care Outcomes | ||||||||
| Ahluwalia (2015): Acute care | 311 | 354 | 85 | 226 | 120 | 234 | −0.310 | 0.029 |
| Ahluwalia (2015): Chemotherapy | 311 | 354 | 33 | 278 | 43 | 311 | −0.153 | 0.064 |
| Ahluwalia (2015): Hospice use fewer than 7 days | 311 | 354 | 117 | 194 | 152 | 202 | −0.221 | 0.025 |
| Ahluwalia (2015): Aggressive care | 311 | 354 | 26 | 285 | 37 | 317 | −0.246 | 0.072 |
| Chen (2019): CPR | 215 | 215 | 10 | 205 | 11 | 204 | −0.100 | 0.201 |
| Chen (2019): ICU care | 215 | 215 | 19 | 196 | 16 | 199 | 0.187 | 0.125 |
| Chen (2019): Mechanical ventilation | 215 | 215 | 23 | 192 | 16 | 199 | 0.399 | 0.116 |
| Dalmau-Bueno (2021): Artificial nutrition | 778 | 1560 | 18 | 760 | 63 | 1497 | −0.575 | 0.073 |
| Dalmau-Bueno (2021): CPR | 778 | 1560 | 0 | 778 | 2 | 1558 | −0.915 | 2.402 |
| Dalmau-Bueno (2021): Dialysis | 778 | 1560 | 6 | 772 | 16 | 1544 | −0.288 | 0.231 |
| Dalmau-Bueno (2021): ED admission | 778 | 1560 | 586 | 192 | 1321 | 239 | −0.594 | 0.012 |
| Dalmau-Bueno (2021): Mechanical ventilation | 778 | 1560 | 66 | 712 | 201 | 1359 | −0.467 | 0.022 |
| Dalmau-Bueno (2021): Surgery | 778 | 1560 | 114 | 664 | 287 | 1273 | −0.272 | 0.015 |
| Diamond (2016): Hospice use fewer than 7 days | 102 | 58 | 16 | 86 | 20 | 38 | −1.040 | 0.150 |
| Halpern (2011): CPR | 710 | 411 | 38 | 672 | 24 | 387 | −0.092 | 0.072 |
| Ishikawa (2018): Hospital death | 54 | 53 | 9 | 45 | 44 | 9 | −3.196 | 0.267 |
| Johnson (2018): Chemotherapy | 62 | 80 | 13 | 49 | 11 | 69 | 0.509 | 0.203 |
| Johnson (2018): Hospital death | 68 | 83 | 12 | 56 | 16 | 67 | −0.108 | 0.179 |
| Mack (2012): Acute Care | 1082 | 149 | 421 | 661 | 72 | 77 | −0.384 | 0.031 |
| Mack (2012): Aggressive care | 1082 | 149 | 494 | 588 | 88 | 61 | −0.541 | 0.032 |
| Mack (2012): Chemotherapy | 1082 | 149 | 157 | 925 | 39 | 110 | −0.737 | 0.042 |
| Mack (2012): Hospice use fewer than 7 days | 1082 | 149 | 185 | 897 | 25 | 124 | 0.023 | 0.055 |
| Mack (2012): ICU care | 1082 | 149 | 62 | 1020 | 8 | 141 | 0.069 | 0.149 |
| McDermott (2020): ED admission | 5145 | 9947 | --- | --- | --- | --- | 0.174 | 0.012 |
| McDermott (2020): Hospital admission | 5145 | 9947 | --- | --- | --- | --- | −0.400 | 0.002 |
| McDermott (2020): Hospital death | 5145 | 9947 | --- | --- | --- | --- | −0.431 | 0.002 |
| McDermott (2020): ICU care | 5145 | 9947 | --- | --- | --- | --- | −0.342 | 0.003 |
| Narang (2015): Aggressive care | 1601 | 384 | --- | --- | --- | --- | −0.713 | 0.076 |
| Narang (2015): Hospital death | 1601 | 384 | --- | --- | --- | --- | −0.073 | 0.023 |
| Peltier (2017): Chemotherapy | 24 | 45 | 6 | 18 | 9 | 36 | 0.288 | 0.361 |
| Peltier (2017): ED admission | 24 | 45 | 14 | 10 | 25 | 20 | 0.113 | 0.261 |
| Peltier (2017): Hospital admission | 24 | 45 | 15 | 9 | 31 | 14 | −0.284 | 0.282 |
| Peltier (2017): ICU admission | 24 | 45 | 3 | 21 | 8 | 37 | −0.414 | 0.534 |
| Prater (2019): Hospital admission | 519 | 666 | --- | --- | --- | --- | −0.211 | 0.014 |
| Prater (2019): Hospital admission | 60 | 1125 | --- | --- | --- | --- | −1.050 | 0.053 |
| Prater (2019): Hospital admission | 152 | 1033 | --- | --- | --- | --- | −0.342 | 0.019 |
| Prater (2022): Hospital admission | 158 | 3547 | 57 | 101 | 2007 | 1540 | −0.837 | 0.029 |
| Rocque (2017): Chemotherapy | 437 | 171 | 62 | 375 | 32 | 139 | −0.331 | 0.057 |
| Rocque (2017): Hospice use fewer than 3 days | 437 | 171 | 20 | 417 | 11 | 160 | −0.360 | 0.150 |
| Rocque (2017): Hospital admission | 437 | 171 | 200 | 237 | 96 | 75 | −0.417 | 0.033 |
| Rocque (2017): ED admission | 437 | 171 | 199 | 238 | 91 | 80 | −0.308 | 0.033 |
| Rocque (2017): ICU admission | 437 | 171 | 77 | 360 | 41 | 130 | −0.388 | 0.048 |
| Salazar (2022): Aggressive care (a) | --- | --- | --- | --- | --- | --- | −0.799 | 0.345 |
| Salazar (2022): Aggressive care (b) | --- | --- | --- | --- | --- | --- | 0.247 | 0.275 |
| Salazar (2022): Chemotherapy | --- | --- | --- | --- | --- | --- | 0.658 | 0.449 |
| Salazar (2022): Hospital admission | --- | --- | --- | --- | --- | --- | −0.545 | 0.202 |
| Salazar (2022): ICU care | --- | --- | --- | --- | --- | --- | −0.545 | 0.202 |
| Sedhom (2021): Hospice use fewer than 3 days | 94 | 53 | 7 | 87 | 15 | 38 | −1.59 | 0.247 |
| Wallace (2001): CPR | 135 | 135 | 10 | 125 | 16 | 119 | −0.519 | 0.179 |
| Wallace (2001): Mechanical ventilation | 135 | 135 | 59 | 76 | 57 | 78 | 0.061 | 0.061 |
| Wright (2008): CPR | 123 | 209 | 1 | 122 | 14 | 195 | −2.170 | 1.085 |
| Wright (2008): Chemotherapy | 123 | 209 | 5 | 118 | 14 | 195 | −0.527 | 0.285 |
| Wright (2008): ICU care | 123 | 209 | 5 | 118 | 26 | 183 | −1.210 | 0.252 |
| Wright (2008): Mechanical ventilation | 123 | 209 | 2 | 121 | 23 | 186 | −0.527 | 0.285 |
| Comfort-focused End-of-life Care Outcomes | ||||||||
| Cagle (2020): Hospice use | 110 | 139 | 83 | 27 | 91 | 48 | 0.483 | 0.081 |
| Halpern (2011): DNR order | 710 | 411 | 174 | 536 | 98 | 313 | 0.036 | 0.021 |
| Halpern (2011): No escalation of care | 710 | 411 | 37 | 673 | 20 | 391 | 0.072 | 0.081 |
| Halpern (2011): Withdrawal of treatments | 710 | 411 | 61 | 649 | 28 | 383 | 0.251 | 0.056 |
| Jeurkar (2012): Home death | --- | --- | --- | --- | --- | --- | 0.599 | 0.003 |
| Mack (2012): Hospice use | 1082 | 149 | 686 | 396 | 30 | 119 | 1.927 | 0.046 |
| Narang (2015): Treatments limited or withheld | 1601 | 384 | --- | --- | --- | --- | 0.920 | 0.063 |
| Peltier (2017): DNR order | 24 | 45 | 21 | 3 | 36 | 9 | 0.560 | 0.520 |
| Peltier (2017): Hospice use | 24 | 45 | 19 | 5 | 32 | 11 | 0.267 | 0.375 |
| Rocque (2017): Hospice use | 437 | 171 | 296 | 141 | 121 | 50 | −0.142 | 0.039 |
| Wen (2020): DNR order | 196 | 196 | 168 | 28 | 164 | 32 | 0.158 | 0.079 |
| Wallace (2001): DNR order | 135 | 135 | 26 | 109 | 15 | 120 | 0.646 | 0.123 |
| Wright (2008): Hospice use | 123 | 209 | 93 | 30 | 120 | 89 | 0.833 | 0.064 |
| Wright (2008): Hospice greater than 7 days | 123 | 209 | 80 | 43 | 93 | 116 | 0.842 | 0.055 |
Aggressive EOL Care Outcomes
Subset Meta-analyses by Study Outcomes.
The overall association between ACP and chemotherapy (OR = 0.72, p = .007, k = 7), ICU admissions (OR = 0.71, p < .001, k = 7), hospital admissions (OR = 0.55, p < .001, k = 6), hospice use fewer than 7 days (OR = 0.60, p = .032, k = 5), hospital death (OR = 0.48, p = .021, k = 4), and composite aggressive intervention outcomes (OR = 0.59, p < .001, k = 4) were each significant. These significant associations represented reduced odds (ranging from a 28% to a 52% reduction) of these aggressive EOL care outcomes when patients with cancer had engaged in ACP, compared to those who had not (Table 2), and suggests a protective effect of ACP against these outcomes. The remaining associations between ACP and other aggressive EOL care outcomes were non-significant—cardiopulmonary resuscitation (p = .164), mechanical ventilation (p = .375), and emergency department admissions (p = .410).
Recognizing that cultural variation exists in approaches to ACP and EOL care (e.g., approaches that emphasize deference to the healthcare provider’s authority and/or promote collective, familial decision-making),10 a series of subset sensitivity meta-analyses was conducted removing effect sizes from studies conducted outside of the United States. This sensitivity analysis revealed that all estimates of the association between ACP and aggressive EOL care outcomes (chemotherapy, ICU admission, cardiopulmonary resuscitation, hospital death, mechanical ventilation, ED admission) remained consistent, except for hospital death, which became non-significant (p = .112) (Table C, Appendix). However, this sensitivity analysis was reduced to two effect sizes, introducing the possibility of Type II error. Sensitivity analyses were not conducted for hospital admission, hospice use fewer than seven days, or composite measures of aggressive care, as these subsets lacked effect sizes from studies outside of the United States.
Moderation Effects.
ACP type was a significant moderator of the association between ACP and hospital admissions at the EOL (Table 3). Although all categories of ACP type were significantly associated with reduced odds of hospital admissions, studies with the communication components only ACP type were associated with greater reduced odds of hospital admissions at the EOL (OR = 0.40, p < .001, representing a 60% reduced odds), compared to studies oriented toward the documentation components (OR = 0.67, p < .001, representing a 33% reduced odds) or full ACP (OR = 0.67, p = .019, representing a 33% reduced odds). ACP type was not a significant moderator among the ICU admission (p = .696), cardiopulmonary resuscitation (p = .679), mechanical ventilation (p = .359), ED admission (p = .808) outcomes (Table D, Appendix). Study design was not a significant moderator among the ICU admission (p = .570), hospital admission (p = .748), or ED admission (p = .808) outcomes. Otherwise, ACP type and study design were not tested as moderators due to the limited number of effect sizes at each category of the moderator within the outcome subsets.
Comfort-focused EOL Care Outcomes
Subset Meta-analyses by Study Outcome.
The overall association between ACP and hospice use was non-significant (p = .185). However, the overall association between ACP and do not resuscitate orders was significant (OR = 1.51, 95%, p = .048, k = 5), indicating patients with cancer who had engaged in ACP experienced 1.51 times increase in the odds of completing a do not resuscitate order, compared to those patients who had not engaged in ACP (Table 2). After studies conducted outside of the United States were removed for the sensitivity analysis, the association between ACP and do-not-resuscitate orders became non-significant (p = .070). Sensitivity analyses were not conducted for the hospice use outcome, as these subsets lacked effect sizes from studies outside of the United States.
Moderation Effects.
Although the association between ACP and hospice use was not significant in the overall analysis, moderation analysis revealed study design was a significant moderator of this association (p = .026) (Table 3), indicating that there was a significant association between ACP and hospice use among the studies with observational designs (OR = 1.52, 95% CI: 1.14; 2.03, p = .004), and a non-significant association among studies with experimental designs (p = .559). However, the study design was not a significant moderator of the association between ACP and do-not-resuscitate orders (p = .672) (Table D, Appendix). ACP type was not tested as a moderator among the hospice use and do-not-resuscitate order outcomes due to the limited number of effect sizes at each category of the moderator.
Discussion
This is the first meta-analysis to synthesize accumulated evidence of the associations between ACP and various aggressive and comfort-focused EOL care outcomes among patients with cancer as well as to identify key moderators of these associations. While the findings demonstrated mixed impacts of ACP at the EOL, there was a general trend toward less aggressive and more comfort-focused EOL care among patients who had engaged in ACP, compared to those who had not engaged. Moderation analysis revealed a greater reduction in the odds of hospital admissions at the EOL when ACP took a more communication-focused approach and a greater likelihood of hospice use in observational studies.
Aggressive EOL Care Outcomes
ACP was associated with significantly reduced odds of five of eight aggressive EOL care outcomes—chemotherapy, hospital admissions, ICU admissions, hospital deaths, and delayed hospice use. However, no significant associations with emergency department admissions, cardiopulmonary resuscitation, or mechanical ventilation were detected. Although beneficial, our estimates were significantly lower in magnitude than prior research.(19, 40) A previous metaanalysis estimated a 50% reduction in the odds of chemotherapy (versus a 28% reduction herein), an 87% reduction in the odds of cardiopulmonary resuscitation (versus a non-significant association herein), and a 56% reduction in the odds of ICU admissions (versus 29% reduction herein). Another review also documented a larger influence of ACP on ICU admissions (37% risk reduction) among patients at high risk for death.(40) However, this review did not examine ACP in isolation: synthesized effects were inclusive of studies with either ACP or palliative care intervention approaches. Our estimates are likely more precise than these prior studies given the larger number of effect sizes in this analysis (i.e., four to seven effect sizes per outcome) compared to prior (i.e., two to three effect sizes per outcome). Further, by keeping the focus on ACP-specific studies with cancer-directed inquiry, this meta-analysis offers a “cleaner” examination of the impacts of ACP within a distinct context. Thus, this analysis overcomes the limitations of prior reviews, which attempt to synthesize the evidence for ACP among studies with heterogenous populations, disease states, interventions, and outcomes.(10,12)
Comfort-focused EOL Care Outcomes
ACP was significantly associated with increased odds of completing do-not-resuscitate orders, but not hospice use. The 1.51 times increase in the odds of do-not-resuscitate order completion when patients with cancer had engaged in ACP is intuitive, in that, this association likely reflects the natural “life course” of ACP—a progression from the prospective articulation of preferences across the chronic illness course to actualizing those preferences “in-the-moment” during the terminal illness course through medical orders, such as do-not-resuscitate orders.(22,26) The non-significant overall association with hospice use is less intuitive. This finding could be a function of the timing of ACP in relation to hospice enrollment, in that, some patients may not have engaged in ACP until the moment of hospice enrollment. It may be that engagement in ACP follows the actual decision to enroll in hospice to document the agreed-upon plan, making the direction of the effects between ACP communication, documentation, and hospice use less clear. Alternatively, this finding may also be explained by prior studies which indicate that although patients generally state they desire quality of life at the EOL (i.e., comfort-focused care) over length of life, a significant minority may shift their inclination toward care that increases the potential for length of life (i.e., aggressive care) when faced with serious illnesses.(41)
Tests of Moderation
Although optimal approaches to ACP have been difficult to discern in prior reviews,(10,12,42) the tests of moderation empirically support that communication-focused approaches to ACP may produce a more robust influence over certain EOL care outcomes than other approaches. This substantiates viewpoints that the real power of ACP lies in its communication components, where the process of communication serves the critical function of informing, tending to emotion, and providing counsel.(43) Although all ACP types (i.e., documents only, communication only, full ACP) produced a significant reduction in the odds of hospital admissions in the last 30 days of life, this is the first meta-analysis to document the enhanced magnitude of this effect with a specific ACP approach. Still prominent viewpoints also note the critical importance of the documentation components of ACP, specifically the need to document a healthcare surrogate in advance to facilitate a shared understanding of treatment preferences and goals.(2, 43) This gives credence to prior conclusions that ACP is an “interconnected set of elements relying on each other,”(10) where preference-based communication likely functions iteratively across the illness trajectory to bring the other components of ACP to life.(11,44, 45)
Study design was also a significant moderator among the five studies that reported hospice use effects sizes. Patients with cancer were more likely to experience hospice use among the observational studies of ACP, not the experimental studies. There are several possible explanations for this finding. It could indicate that ACP may have been particularly well-executed in routine clinical practice in the observational studies—suggesting modifications to the ACP intervention approach in the experimental studies (both employed full ACP through Respecting Choices) (46,47) were needed to optimize outcomes beyond what was possible through routine care. Thus, the non-significant association between ACP and hospice use among the experimental studies echo prior conclusions that the Respecting Choices model of ACP may not influence long-term outcomes, such as those at the EOL, and indicate that more work remains to understand its impact.(48) At the same time, two(49,50) of the three observational studies(49-51) employed the communication components of ACP close to the time of the patients’ deaths (e.g., within 1-4 months), suggesting that such conclusions may again be confounded by ACP timing.
Implications
In contrast to recent questions regarding the value of ACP,(1,2) this meta-analysis empirically supports the significant influence of ACP on certain aggressive and comfort-focused EOL care outcomes among patients with cancer, as well as circumstances where the value of ACP is enhanced when it is delivered in certain forms (i.e., communication components only). This suggests that ACP does hold value for patients with cancer at the EOL, and that continued efforts to support ACP engagement among patients with cancer are warranted, given their engagement has historically remained modest (40-69%).(45,52,53) Further, the cancer illness trajectory may offer the most “lab-like” environment to understand the association between ACP and EOL care outcomes, given its protracted illness trajectory and relatively predictable decline prior to and during the terminal illness phase. In this context, ACP functions as a preparatory process across the illness trajectory to later inform “in-the-moment” decisions.(22) Thus, the estimates herein may indicate a best-case scenario of the impacts of ACP at the EOL. Other serious illness trajectories, such as heart failure, have less predictable courses resulting in a lack of “key moments” to initiate ACP,(23) challenges that may reduce the impacts of ACP on EOL care in these disease-specific contexts. Further inquiry into the differential effects of ACP at the EOL among patients with various serious illness diagnoses is needed to inform disease-specific considerations for ACP.(11)
Despite an accumulated body of evidence on ACP over the last 30 years,(1) this metaanalysis also reveals that the evidence on which to inform the next steps in ACP science is not as robust as it seems, once the inquiry is narrowed to a distinct context (i.e., studies uniquely focused on ACP in the cancer population). While this meta-analysis included more effect sizes than prior ACP meta-analyses, the narrowed inclusion context still resulted in a limited number of effect sizes for each subset meta-analysis. Further, these effect sizes largely represented observational studies of existing ACP practices/programs (80% of studies) and were much less representative of experimental efforts to enhance ACP approaches. This suggests that questions regarding the utility of ACP may be premature and based on limited, less rigorous evidence. This also presents the possibility that the detected associations reflect the impact of confounders, rather than the unique impact of ACP on outcomes. For example, patients who possess an underlying preference for comfort-focused end-of-life care may be more likely to complete ACP; thus, it is possible that underlying preferences could be driving the detected associations, not the ACP process.(54) As the discipline of palliative care expands, our ability to discern the unique impacts of ACP may be challenged as ACP is often embedded as a critical component of palliative care delivery or other multifaceted interventions, rather than in isolation.
This meta-analysis focused on the long-term impacts of ACP at the EOL, leaving questions about the extent of its impact on outcomes at other time points along the cancer illness trajectory. Although the EOL care outcomes offered the advantage of synthesizing the evidence on more readily and objectively measured outcomes of ACP, evidence suggests that patients with cancer may utilize ACP more for its social and relational aspects than for directing care.(55) In this way, this meta-analysis may speak more to the benefits of ACP for healthcare systems rather than patients and caregivers.(56,57) This reinforces the need to shift attention to more patient- and caregiver-centered outcomes in future meta-analyses using the Organizing Framework of ACP Outcomes.(29) While the pursuit of understanding the association between ACP and the “holy grail” outcome of goal-concordant care remains elusive and may never be precisely measured,(29) meta-analytic inquiry into the ACP outcomes that patients and caregivers deem more salient (e.g., perceived value of the communication and documentation components of ACP, satisfaction with the amount of information given, sense of control the ACP process provided) offers a critical path forward in furthering ACP science.(11, 29, 43) Achieving this goal will require increased efforts at standardization of ACP outcomes across studies.(12,29)
Limitations
These findings should be considered in light of several limitations. As ACP is understood to be a process that unfolds across the illness trajectory,(3) the detected associations may be underestimated as ACP was typically treated as a dichotomous (“yes/no”) variable, leaving little insight into the nature in which ACP was implemented or timed. This prevented a more dynamic understanding of the impacts of ACP at the EOL when operationalized as an iterative process over the disease course (i.e., “dose” of ACP received, timing of ACP).(58) Relatedly, it was infeasible to determine whether ACP documents were available at the time of treatment decisions or contained relevant information, both possible contributors to a lack of effect on certain EOL outcomes. It may be that documents that are available and contain relevant information such as POLST may be more useful in changing EOL outcomes.(59) Thus, modifications to survey measures, such as The Bereaved Family Survey – Inpatient, may be warranted to capture whether ACP documents were available at the EOL and the extent to which the documents were utilized to inform EOL care decisions.(60) Given the inclusion of both observational and experimental studies, this meta-analysis is not necessarily confirmatory and limits our ability to make causal inferences. However, it does provide valuable insights into the strength of the association between ACP and a multitude of EOL care outcomes among patients with cancer. Finally, the limited ability to conduct tests of moderation leaves questions about other sources of variation among the effect sizes. Among those tests of moderation that were possible, there were often only two effect sizes per level of the moderator, leaving the chance that any negative tests of moderation (Table C, Appendix) represent a Type II error.
Conclusion
Reconciling a large body of ACP literature, and employing nuanced analyses of key moderators, this meta-analysis demonstrated mixed evidence of the associations between ACP and aggressive and comfort-focused outcomes at the EOL among seriously ill patients with cancer. This mixed evidence suggests that the impact of ACP on EOL care outcomes is not a foregone conclusion and that work remains to enhance ACP science. By limiting our analysis to ACP-centered studies with exclusive cancer samples, this meta-analysis overcomes limitations of prior reviews that have attempted to synthesize the evidence for ACP among heterogenous populations, disease states, interventions, and/or outcomes. Therefore, the findings may offer the best-case scenario of the ultimate influence of ACP on EOL care outcomes when effects are distilled down within a specific context. Yet many other outcomes of ACP remain, warranting further inquiry into the impacts of ACP on a broader set of outcomes that are more salient to patients and caregivers. Tests of moderation were also suggestive that the communication components of the ACP process may carry more weight in influencing these EOL care outcomes. Thus, efforts to directly engage patients and caregivers in the communication components of ACP is a crucial aspect of augmenting ACP outcomes alongside other ACP components. Further, the largely observational studies with moderate risk for bias herein, suggest that any questions about the continued utility of ACP may be premature, based on limited, less rigorous evidence, and make the mistake of throwing the ACP baby out with the bathwater. Thus, this meta-analysis suggests that certain aspects of ACP continue to be an important tool in the preparatory toolbox as patients with cancer and their providers prepare for future EOL. Efforts to further unpack the impact of ACP study design elements, as well as ACP model components in specific disease populations on a broader set of patient-and caregiver-centered outcomes, are critical to advancing the field.
Supplementary Material
Key Message:
This meta-analysis documents mixed evidence of the associations between ACP and EOL care outcomes among patients with cancer, and the enhanced impact of ACP’s communication components, suggesting that questions about the value of ACP are premature and may result in mistakenly throwing the ACP baby out with the bathwater.
Disclosures:
During the conduct of this study, Dr. Levoy was supported, in part, by a Future of Nursing Scholars Award from the Robert Wood Johnson Foundation, a Doctoral Degree Scholarship in Cancer Nursing (131753-DSCN-18-072-SCN) from the American Cancer Society, and a NIH/NINR Ruth L. Kirschstein National Research Service Award program (T32 NR009356). Dr. Sullivan was also supported, in part, by a NIH/NINR Ruth L. Kirschstein Predoctoral Individual National Research Service Award (F31 NR016394), the National Library of Medicine National Institute of Aging Institutional Grants for Research Training in Biomedical Informatics (T15 LM012495), and an NIH/NIA award R03 AG067159. Dr. Hickman is currently supported by NIH/NIA awards R33 AG057353, R01 AG057733, and R01 AG056618. Dr. Meghani is currently supported by NIH/NINR award R01 NR017853 and NIH/NCI award R01 CA270483.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morrison SR. Advance directives/care planning: Clear, simple, and wrong. J Palliat Med. 2020;23(7):878–879. doi: 10.1089/JPM.2020.0272 [DOI] [PubMed] [Google Scholar]
- 2.Morrison SR, Meier DE, Arnold RM. What’s wrong with advance care planning? JAMA. 2021;326(16):1575–1576. doi: 10.1001/jama.2021.16430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: An international consensus supported by the European Association for Palliative Care. Lancet Oncol. 2017;18(9):e543–e551. doi: 10.1016/S1470-2045(17)30582-X [DOI] [PubMed] [Google Scholar]
- 4.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–214. doi: 10.1111/JGS.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyland DK, Heyland R, Dodek P, et al. Discordance between patients’ stated values and treatment preferences for end-of-life care: results of a multicentre survey. BMJ Support Palliat Care. 2017;7(3):292–299. doi: 10.1136/BMJSPCARE-2015-001056 [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Gomes B, Higginson IJ. End-of-life care--what do cancer patients want? Nat Rev Clin Oncol. 2014;11(2):100–108. doi: 10.1038/NRCLINONC.2013.217 [DOI] [PubMed] [Google Scholar]
- 7.Ashana DC, Chen X, Agiro A, et al. Advance care planning claims and health care utilization among seriously ill patients near the end of life. JAMA Netw Open. 2019;2(11):e1914471–e1914471. doi: 10.1001/JAMANETWORKOPEN.2019.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SB, Butow PN, Bell ML, et al. A randomised controlled trial of an advance care planning intervention for patients with incurable cancer. Br J Cancer. 2018;119(10):1182–1190. doi: 10.1038/S41416-018-0303-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: A systematic review. Palliat Med. 2014;28(8):1000–1025. doi: 10.1177/0269216314526272 [DOI] [PubMed] [Google Scholar]
- 10.Jimenez G, Tan WS, Virk AK, et al. Overview of systematic reviews of advance care planning: Summary of evidence and global lessons. J Pain Symptom Manage. 2018;56(3):436–459.e25. doi: 10.1016/J.JPAINSYMMAN.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 11.Rosa WE, Izumi S, Sullivan D, et al. Advance care planning in serious illness: A narrative review. J Pain Symptom Manage. 2022;0(0). doi: 10.1016/J.JPAINSYMMAN.2022.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahan RD, Tellez I, Sudore RL. Deconstructing the complexities of advance care planning outcomes: What do we know and where do we go? A scoping review. J Am Geriatr Soc. 2021;69(1):234–244. doi: 10.1111/jgs.16801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain A, Corriveau S, Quinn K, et al. Video decision aids to assist with advance care planning: a systematic review and meta-analysis. BMJ Open. 2015;5(6):e007491. doi: 10.1136/BMJOPEN-2014-007491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oczkowski SJ, Chung HO, Hanvey L, Mbuagbaw L, You JJ. Communication tools for end-of-life decision-making in ambulatory care settings: A systematic review and meta-Analysis. PLoS One. 2016;11(4). doi: 10.1371/JOURNAL.PONE.0150671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–489. doi: 10.1016/J.JAMDA.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Ng AYM, Takemura N, Xu X, et al. The effects of advance care planning intervention on nursing home residents: A systematic review and meta-analysis of randomised controlled trials. Int J Nurs Stud. 2022;132:104276. doi: 10.1016/J.IJNURSTU.2022.104276 [DOI] [PubMed] [Google Scholar]
- 17.Pimsen A, Kao CY, Hsu ST, Shu BC. The effect of advance care planning intervention on hospitalization among nursing home residents: A systematic review and meta-Analysis. J Am Med Dir Assoc. 2022;23(9):1448–1460.e1. doi: 10.1016/J.JAMDA.2022.07.017 [DOI] [PubMed] [Google Scholar]
- 18.Schichtel M, Wee B, Perera R, Onakpoya I. The effect of advance care planning on heart failure: A systematic review and meta-analysis. J Gen Intern Med. 2020;35(3):874–884. doi: 10.1007/S11606-019-05482-W/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baidoobonso S Patient care planning discussions for patients at the end of life: An evidence-based analysis. Ont Health Technol Assess Ser. 2014;14(19):1. Accessed September 1, 2022. [PMC free article] [PubMed] [Google Scholar]
- 20.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289(18):2387–2392. doi: 10.1001/JAMA.289.18.2387 [DOI] [PubMed] [Google Scholar]
- 21.Lunney JR, Lynn J, Hogan C. Profiles of older Medicare decedents. J Am Geriatr Soc. 2002;50(6):1108–1112. doi: 10.1046/J.1532-5415.2002.50268.X [DOI] [PubMed] [Google Scholar]
- 22.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–261. doi: 10.7326/0003-4819-153-4-201008170-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vleminck A, Pardon K, Beernaert K, et al. Barriers to advance care planning in cancer, heart failure and dementia patients: A focus group study on general practitioners’ views and experiences. PLoS One. 2014;9(1):e84905. doi: 10.1371/journal.pone.0084905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovell A, Yates P. Advance care planning in palliative care: a systematic literature review of the contextual factors influencing its uptake 2008-2012. Palliat Med. 2014;28(8):1026–1035. doi: 10.1177/0269216314531313 [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levoy K, Tarbi EC, de Santis JP. End-of-life decision making in the context of chronic life-limiting disease: A concept analysis and conceptual model. Nurs Outlook. 2020;68(6). doi: 10.1016/j.outlook.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26(23):3860–3866. doi: 10.1200/JCO.2007.15.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284–292. doi: 10.1001/JAMA.2015.18604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sudore RL, Heyland DK, Lum HD, et al. Outcomes that define successful advance care planning: A delphi panel consensus. J Pain Symptom Manage. 2018;55(2):245–255.e8. doi: 10.1016/J.JPAINSYMMAN.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenker Y, Tiver GA, Hong SY, White DB. Association between physicians’ beliefs and the option of comfort care for critically ill patients. Intensive Care Med. 2012;38(10):1607–1615. doi: 10.1007/S00134-012-2671-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bern-Klug M A framework for categorizing social interactions related to end-of-life care in nursing homes. Gerontologist. 2009;49(4):495–507. doi: 10.1093/GERONT/GNP098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arditi C, Burnand B, Peytremann-Bridevaux I. Adding non-randomised studies to a Cochrane review brings complementary information for healthcare stakeholders: An augmented systematic review and meta-analysis. BMC Health Serv Res. 2016;16(1):1–19. doi: 10.1186/S12913-016-1816-5/FIGURES/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis: prevention, assessment and adjustments. Hoboken, NJ: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 34.Rosenthal R The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. doi: 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- 35.Cooper H, Hedges LV, Valentine JC, eds.: The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009. [Google Scholar]
- 36.R: The R Project for Statistical Computing. Accessed September 27, 2022. https://www.r-project.org/
- 37.Hedges LV and Olkin I.: Statistical methods for meta-analysis. 1st ed. Orlando, FL: Academic Press; 1985 [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/BMJ.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: A systematic review. Crit Care Med. 2015;43(5):1102. doi: 10.1097/CCM.0000000000000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhotra C, Xiang L, Ozdemir S, et al. A comparison of attitudes toward length and quality of life between community-dwelling older adults and patients with advanced cancer. Psychooncology. 2017;26(10):1611–1617. doi: 10.1002/PON.4344 [DOI] [PubMed] [Google Scholar]
- 42.Chan B, Sim HW, Zimmermann C, Krzyzanowska MK. Systematic review of interventions to facilitate advance care planning (ACP) in cancer patients. J Clin Oncol. 2016;34(26_suppl):21–21. doi: 10.1200/JCO.2016.34.26_SUPPL.21 [DOI] [Google Scholar]
- 43.Pottash M The limits of advance care planning. Pallimed. November 22, 2021. Accessed December 29, 2022. https://www.pallimed.org/2021/11/the-limits-of-advance-care-planning.html [Google Scholar]
- 44.Ranganathan A, Gunnarsson O, Casaret D. Palliative care and advance care planning for patients with advanced malignancies. Ann Palliat Med. 2014;3(3):144–149. doi: 10.3978/J.ISSN.2224-5820.2014.07.04 [DOI] [PubMed] [Google Scholar]
- 45.Narang AK, Wright AA, Nicholas LH. Trends in advance care planning in cancer patients: Results from a national, longitudinal survey. JAMA Oncol. 2015;1(5):601. doi: 10.1001/JAMAONCOL.2015.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocque GB, Dionne-Odom JN, Sylvia Huang CH, et al. Implementation and impact of patient lay navigator-led advance care planning conversations. J Pain Symptom Manage. 2017;53(4):682–692. doi: 10.1016/J.JPAINSYMMAN.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peltier WL, Gani F, Blissitt J, et al. Initial Eexperience with “Honoring Choices Wisconsin”: Implementation of an advance care planning pilot in a tertiary care setting. J Palliat Med. 2017;20(9):998–1003. doi: 10.1089/JPM.2016.0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacKenzie MA, Smith-Howell E, Bomba PA, Meghani SH. Respecting Choices and related models of advance care planning: A systematic review of published evidence. Am J Hosp Palliat Care. 2018;35(6):897–907. doi: 10.1177/1049909117745789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673. doi: 10.1001/JAMA.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30(35):4387–4395. doi: 10.1200/JCO.2012.43.6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagle JG, Lee J, Ornstein KA, Guralnik JM. Hospice utilization in the United States: A prospective cohort study comparing cancer and noncancer deaths. J Am Geriatr Soc. 2020;68(4):783–793. doi: 10.1111/JGS.16294 [DOI] [PubMed] [Google Scholar]
- 52.Levoy K, Buck H, Behar-Zusman V. The impact of varying levels of advance care planning engagement on perceptions of the end-of-life experience among caregivers of deceased patients with cancer. Am J Hosp Palliat Care. 2020;37(12):1045–1052. doi: 10.1177/1049909120917899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three us adults completes any type of advance directive for end-of-life care. Health Aff. 2017;36(7):1244–1251. doi: 10.1377/HLTHAFF.2017.0175 [DOI] [PubMed] [Google Scholar]
- 54.Yoo SH, Lee J, Kang JH, et al. Association of illness understanding with advance care planning and end-of-life care preferences for advanced cancer patients and their family members. Support Care Cancer. 2020;28:2959–2967. doi: 10.1007/s00520-019-05174-5 [DOI] [PubMed] [Google Scholar]
- 55.Johnson S, Kerridge I, Butow PN, Tattersall MHN. Advance care planning: Is quality end of life care really that simple? Intern Med J. 2017;47(4):390–394. doi: 10.1111/IMJ.13389 [DOI] [PubMed] [Google Scholar]
- 56.Henson LA, Gomes B, Koffman J, et al. Factors associated with aggressive end of life cancer care. Support Care Cancer. 2016;24(3):1079–1089. doi: 10.1007/S00520-015-2885-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pfaff K, Markaki A. Compassionate collaborative care: An integrative review of quality indicators in end-of-life care. BMC Palliat Care. 2017;16(1). doi: 10.1186/S12904-017-0246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bestvina CM, Polite BN. Implementation of advance care planning in oncology: A review of the literature. J Oncol Pract. 2017;13(10):657–662. doi: 10.1200/JOP.2017.021246 [DOI] [PubMed] [Google Scholar]
- 59.Hickman SE, Keevern E, Hammes BJ. Use of the physician orders for life-sustaining treatment program in the clinical setting: A systematic review of the literature. J Am Geriatr Soc. 2015;63(2):341–350. doi: 10.1111/JGS.13248 [DOI] [PubMed] [Google Scholar]
- 60.Center for Health Equity Research and Promotion. The Bereaved Family Survey-Inpatient. US Department of Veterans Affairs. Updated April 13, 2018. Accessed December 29, 2022. https://www.cherp.research.va.gov/CHERP/PROMISE/The_PROMISE_Survey.asp. [Google Scholar]
- 61.Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834–841. doi: 10.1089/jpm.2014.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CH, Chen JS, Wen FH, et al. An individualized, interactive intervention promotes terminally ill cancer patients' prognostic awareness and reduces cardiopulmonary resuscitation received in the last month of life: Secondary analysis of a randomized clinical trial. J Pain Symptom Manage. 2019;57(4):705–714.e7. doi: 10.1016/j.jpainsymman.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 63.Wen FH, Chen CH, Chou WC, et al. Evaluating if an advance care planning intervention promotes do-not-resuscitate orders by facilitating accurate prognostic awareness. J Natl Compr Canc Netw. 2020;18(12):1658–1666. doi: 10.6004/jnccn.2020.7601 [DOI] [PubMed] [Google Scholar]
- 64.Dalmau-Bueno A, Saura-Lazaro A, Busquets JM, Bullich-Marín I, García-Altés A. Advance directives and real-world end-of-life clinical practice: A case-control study. BMJ Support Palliat Care. 2021;12(e3):e337–e344. doi: 10.1136/bmjspcare-2020-002851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond EL, Russell D, Kryza-Lacombe M, et al. Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol. 2016;18(1):78–86. doi: 10.1093/neuonc/nov156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halpern NA, Pastores SM, Chou JF, Chawla S, Thaler HT. Advance directives in an oncologic intensive care unit: A contemporary analysis of their frequency, type, and impact. J Palliat Med. 2011;14(4):483–489. doi: 10.1089/jpm.2010.0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishikawa T, Fukui S, Okamoto Y. Advance care planning and home death in patients with advanced cancer: A structured interview analysis. Int J Palliat Nurs. 2018;24(9):418–426. doi: 10.12968/ijpn.2018.24.9.418 [DOI] [PubMed] [Google Scholar]
- 68.Jeurkar N, Farrington S, Craig TR, et al. Which hospice patients with cancer are able to die in the setting of their choice? Results of a retrospective cohort study. J Clin Oncol. 2012;30(22):2783–2787. doi: 10.1200/JCO.2011.41.5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDermott CL, Engelberg RA, Sibley J, Sorror ML, Curtis JR. The association between chronic conditions, end-of-life health care use, and documentation of advance care planning among patients with cancer. J Palliat Med. 2020;23(10):1335–1341. doi: 10.1089/jpm.2019.0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prater LC, Wickizer T, Bower JK, Bose-Brill S. The impact of advance care planning on end-of-life care: Do the type and timing make a difference for patients with advanced cancer referred to hospice?. Am J Hosp Palliat Care. 2019;36(12):1089–1095. doi: 10.1177/1049909119848987 [DOI] [PubMed] [Google Scholar]
- 71.Prater LC, O'Rourke B, Schnell P, et al. Examining the association of billed advance care planning with end-of-life hospital admissions among advanced cancer patients in hospice. Am J Hosp Palliat Care. 2022;39(5):504–510. doi: 10.1177/10499091211039449 [DOI] [PubMed] [Google Scholar]
- 72.Salazar MM, DeCook LJ, Butterfield RJ, et al. End-of-life care in patients undergoing allogeneic hematopoietic cell transplantation. J Palliat Med. 2022;25(1):97–105. doi: 10.1089/jpm.2021.0093 [DOI] [PubMed] [Google Scholar]
- 73.Sedhom R, Blackford AL, Gupta A, et al. End-of-life characteristics associated with short hospice length of stay for patients with solid tumors enrolled in phase I clinical trials. J Natl Compr Canc Netw. 2021;19(6):686–692. doi: 10.6004/jnccn.2020.7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace SK, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294–2298. doi: 10.1097/00003246-200112000-00010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.