Abstract
Cardiovascular physiology and pathophysiology display pronounced circadian rhythms. The study is designed to examine whether the time of day of physical activity is associated with cardiovascular mortality. We analyzed 94,489 UK Biobank adults with objectively measured physical activity, including 53,328 morning-type participants and 30,962 evening-type participants based on self-reported chronotypes. The time of day of peak physical activity was categorized using a machine learning algorithm: early morning (n=18,477), late morning (n=25,700), midday (reference) (n=27,803), and night (n=22,509). Hazard ratios of cardiovascular mortality were examined using the Cox proportional hazards model. During a median follow-up of 6.9 years (interquartile range, 6.3-7.4 years), we identified 629 cardiovascular deaths. The hazard of cardiovascular mortality was elevated in the early morning group (hazard ratio=1.56, 95% Confidence Interval [1.23-1.98]) and night group (1.49, [1.18-1.88]) but not in the late morning group (1.21, [0.98-1.47]) compared to the referent midday group. In the chronotype-stratified analysis, the increased cardiovascular mortality in the morning group was only observed in the evening-type participants, while the increased cardiovascular mortality in the night group was only observed in the morning-type participants. In conclusion, optimizing the timing of peak physical activity according to cardiovascular circadian rhythms and individual chronotypes could be a potential therapeutic target that brings additional health benefits.
Keywords: cohort study, timing of physical activity, chronotype, circadian rhythm, cardiovascular physiology, machine learning, accelerometry
Introduction
Regular physical activity is inversely associated with cardiovascular disease (CVD) mortality, especially in patients with pre-existing CVD (Jeong et al. 2019). Considering that cardiovascular physiology and pathophysiology display pronounced circadian rhythms (Crnko et al. 2019), whether the time of day of physical activity is associated with CVD mortality is worth investigating.
Timing is critical in cardiovascular health. Compelling evidence reveals that sudden cardiac death and non-fatal cardiovascular events are subject to circadian variations, with the risk peaking in the early morning (Muller et al. 1987; Cohen et al. 1997). A recent study found that the likelihood of plaque rupture, a potential underlying mechanism of exertion-related sudden death in patients with CVD (Burke et al. 1999), has a two-fold increase in the morning (Araki et al. 2021), suggesting that the cardiovascular system is more vulnerable to triggers of cardiovascular events (e.g., physical exertion) in the morning (Atkinson et al. 2010). The evidence raises a clinically relevant question: Is morning a less favorable time of day to engage in physical activity in terms of cardiovascular health?
Early morning is accompanied by a rapid increase in blood pressure, which is potentially contributing to the increased CVD incidence within the few hours after awaking (Bilo et al. 2018). Additionally, accumulating evidence suggests that blood pressure reactivity to physical exertion is also increased in the early morning. In a study of intermittent exercise among trained athletes, the end-exercise systolic and diastolic blood pressures in the early morning were higher than that in the evening (systolic 196.8 vs 185.5 mmHg; diastolic 96.1 vs 89.5 mmHg) (Faisal et al. 2010). In another study among hypertensive and non-hypertensive participants, the authors observed a noticeably higher reactivity of systolic blood pressure in the early morning than other times of day (Jones et al. 2006). For example, the reactivity index was 4 mmHg/logarithm of activity change between 10:00h and 12:00h and was approximately 2 mmHg/logarithm of activity change between 18:00h and 20:00h, indicating a two-fold greater increase in systolic blood pressure for a given change in physical activity level when performed in the morning than in the evening. Additionally, a greater increase in rate-pressure product (a surrogate measure of myocardial oxygen demand) in the early morning was observed in patients recruited from a hypertension clinic (Atkinson et al. 2009). The increase in rate-pressure product per logged activity counts was approximately 670 beat·min−1·mmHg between 08:00h and 10:00h, and was 500 beat·min−1·mmHg between 12:00h and 14:00h (34% higher in the early morning).
It has been well recognized that the benefits of physical activity outweigh the associated risks. However, the risks could be augmented if physical activities are performed during non-optimal timing. With the evidence described above, it is reasonable to speculate that performing physical activity in the early morning may lead to disproportionately higher cardiovascular stress due to the concurrence of the physical activity-induced blood pressure rise and the diurnal variation-associated blood pressure surge. Given that the blood pressure exerted on the arterial plaque is the main mechanical stress that leads to plaque rupture (Li et al. 2009), the increased cardiovascular reactivity to physical activity in the early morning may increase the chance of plaque rupture during physical exertion compared to the same level of physical activity at other times of day.
Hence, we hypothesize that the timing of physical activity plays a vital role in cardiovascular health. However, few studies have investigated whether long-term survival is influenced by the time of day of physical activity. Therefore, this study was designed to examine, after adjusting for the volume of physical activity (i.e., overall acceleration average), whether the time of day of physical activity is independently associated with CVD mortality.
Methods
Participants
The UK Biobank project is a prospective longitudinal cohort with approximately 500,000 participants aged 40 to 69 years recruited from England, Wales, and Scotland lasting 2006 through 2010. In this current study, we analyzed approximately 100,000 participants with objectively measured physical activity data collected between February 2013 and December 2015. The diagram of the study sample is presented in Figure 1. We examined the association of time-of-day physical activity with CVD mortality. The analysis of the de-identified data in this current study was approved by the UK Biobank Team (Approval ID: 85118), and the entire Biobank project is authorized by the North West Multi-centre Research Ethics Committee (MREC) (UK Biobank 2022).
Figure 1.
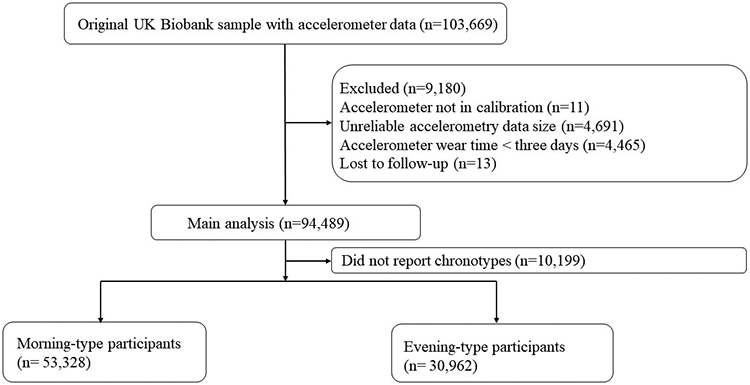
Diagram of participants selection.
Accelerometer Data Processing
Physical activity was objectively measured for up to seven days using a dominant wrist-worn accelerometer (Model AX3, Axivity Ltd, Newcastle, UK). The self-reported physical activity data were not used due to the nature of self-reporting having considerable bias potential and because the time of day of physical activity cannot be determined using existing Biobank questionnaires. The processing of raw accelerometer data was done by the UK Biobank expert group (Doherty et al. 2017). Non-wear time is defined as consecutive little to no movement (all three axes <13 milli-gravity) over at least a 60-minute time period; this was removed from the final data. The UK Biobank expert group performed imputation of non-wear data through similar time-of-day acceleration and intensity distribution to reduce the wear-time bias. Details of the imputation process and coding can be found at https://github.com/activityMonitoring/biobankAccelerometerAnalysis. In this current study, we excluded individuals whose accelerometer was not in calibration, whose accelerations data size was unusually low or high, and those with less than three days of valid accelerometer wear. It was reported that at least three days of wear was required to make the error within 10% compared to the seven-day perfect wear (Doherty et al. 2017).
Main Exposure
The time of day of peak physical activity was categorized using an unsupervised machine learning algorithm (kmeans in R Stats Package) (Hartigan and Wong 1979). Kmeans clustering is a type of unsupervised algorithm as there are no predetermined labels that guide the clustering analysis. Instead, the clustering is inferred entirely from the unlabeled dataset; therefore, the categories being identified are representative of the patterns within the data. Supplemental Figure S1 illustrates a visualized example of kmeans clustering based on a dataset that has two variables (two-dimensional). The algorithm starts with choosing k numbers of initial centers and assigning data points to those centers (k=2 in the example). Then, the algorithm refines the location of the centers and reassigns data points by reducing the distance of the data points to its center. When the distance cannot be further reduced, the algorithm stops. In our dataset of hourly physical activity, there were 24 variables (24-dimensional) in the clustering analysis. Although our analysis cannot be visualized due to its high dimensions, the algorithm functions in a similar process as explained in the two-dimensional dataset.
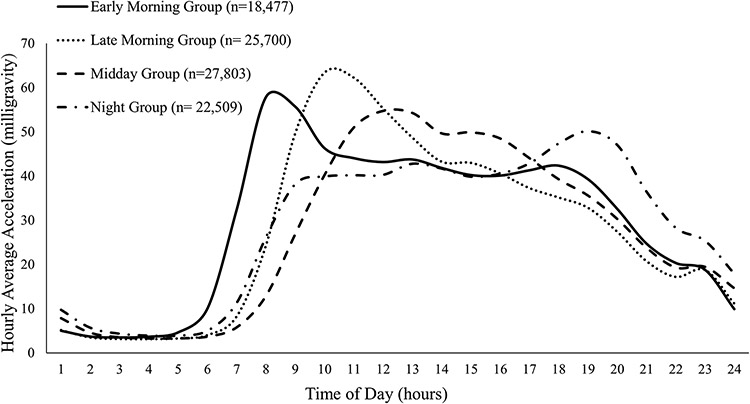
In data preparation, the hourly proportions of the daily total were calculated as hourly magnitude divided by daily total magnitude (Data-Field 90027-90050 and 90012, in accordance with the Data Identifier within the UK Biobank repository). This preparation process allows the machine learning algorithm to focus on the diurnal variation of physical activity rather than the volume of physical activity. The most important step in applying kmeans is to determine the value of k (i.e., the number of clusters). We found that four clusters (k=4) were the most reasonable selection for testing our hypothesis. When the number of clusters was less than four, morning and night individuals were assigned to the same cluster. When the number of clusters was greater than four, participants who were active in the morning were assigned to multiple sub-clusters (e.g., early morning, mid-morning, and late morning), suggesting a risk of overfitting. Based on the processed 24 variables, the machine learning algorithm identified four time-of-day patterns of physical activity (Figure 2). It has been reported that CVD risk increases in the morning with a secondary increase in the evening (Scheer et al. 2010), making midday a theoretically low-risk time window. Therefore, the midday group was treated as the referent group in statistical analysis.
Figure 2.
Diurnal patterns of physical activity by machine learning-based clustering.
Primary Outcome
The primary outcome is CVD mortality, which is defined as deaths due to cardiovascular disease, including I00-I79 coded by the International Classification of Diseases, Tenth Revision (ICD-10). Death records were provided by the National Health Service Digital in England and Wales and by the National Health Service Central Register in Scotland. Both primary and contributory causes of death were provided. However, only data from the primary causes of death (Data-Field 40001) as well as date of death (Data-Field 40000) were used for the mortality analysis. The study participants were censored based on date of death or end of follow-up, which was September 30, 2021, in England and Wales; and October 31, 2021, in Scotland. Follow-up duration was calculated from the start of accelerometer wear to the date of death or end of follow-up, whichever came first.
Covariates
The covariates of our survival analysis were the potential confounding variables of the physical activity-CVD mortality association based on previously reported UK biobank studies (Tikkanen et al. 2018). Covariates included age at recruitment (years, as a continuous variable), sex (male and female), ethnicity (White, Black, Asian, mixed, and others), Townsend Deprivation Index (quartiles), body-mass index (body weight in kg divided by square of height in meters; <18.5 [underweight], 18.6-24.9 [healthy weight], 25-29.9 [overweight], >30 [obese] kg/m2), smoking status (never, former, and current), alcohol consumption (never, former, and current), healthy eating score (zero [least healthy] to five [healthiest]; one point for meeting each of the following: fruit and vegetable intake ≥ 5 portions/week; oily fish ≥ 1 serving/week, non-oily fish ≥ 2 servings/week; processed meat ≤ 1 times/week; red meat intake ≤ 3 time/week) (Public Health England 2022), a family history of heart disease and stroke (yes and no), hypertension (hypertensive if systolic blood pressure ≥ 130, diastolic blood pressure ≥ 80 mmHg, physician-diagnosed hypertension or use of anti-hypertensive medications), high cholesterol (total cholesterol > 6.2 mmol/L or use of cholesterol medication), diabetes (physician diagnosed diabetes, HbA1c ≥ 48 mmol/mol, or use of anti-diabetic medication), long-standing illness (yes and no), a personal history of heart attack, angina, and stroke (yes and no), and general health status (excellent, good, fair, and poor).
Statistical Analysis
Descriptive data were analyzed using the Analysis of Variance for continuous variables and the Chi-square test for categorical variables. We fitted a Cox proportional hazards model to examine the association between time-of-day physical activity and CVD mortality. The time of day of peak physical activity was treated as a categorical variable with the midday group as the referent group. The volume of physical activity was treated as a continuous variable using a restricted cubic spline with three knots placed at the 25th, 50th, and 75th percentiles (Desquilbet and Mariotti 2010). To explore whether performing the same volume of physical activity at different times of day influences mortality outcomes, we examined the association between time of day and CVD mortality with adjustment for physical activity volume and all covariates mentioned previously.
We also conducted chronotype-stratified analysis on CVD mortality to explore whether the outcome is driven by a circadian misalignment (e.g., evening-type individuals perform physical activity in the early morning or vice versa). The chronotypes of Biobank participants were assessed using a single touch-screen question with six choices (Question: Do you consider yourself to be? Choices: definitely morning, more morning than evening, more evening than morning, definitely evening, don’t know, and prefer not to answer). In our analysis, we stratified the study participants into two general types: morning type (definitely morning + more morning than evening), and evening type (more evening than morning + definitely evening).
To explore whether the association between time of day and CVD mortality is influenced by a personal history of CVD, we stratified the sample into 6,672 participants with pre-existing CVD and 87,817 participants without CVD. In the Cox model with pre-existing CVD, we analyzed the association between time of day and CVD mortality in 6,672 participants with a personal history of heart disease and stroke at baseline (the date of accelerometer wear). In the Cox model without CVD, we analyzed the association between time of day and CVD mortality after excluding 6,672 participants with heart disease and stroke at baseline.
In sensitivity analyses, we repeated the analysis after excluding participants whose job involves night shifts (n = 3,697). We also excluded participants who died within two years of follow-up (n = 531) to account for reverse causation bias. Finally, we further adjusted for employment (employed, retired, and unemployed) and total sleep duration (<=6 hours, 7-8 hours, and >=9 hours). Employment status could be an important confounding variable by affecting daily schedule and the time available for physical activity. Also, the diurnal patterns of physical activity in our study (Figure 2) indicated differences in sleep duration across the time-of-day groups. We conducted additional analysis on non-CVD mortality and all-cause mortality. We also conducted subdistribution analysis of competing risk (non-CVD morality as a competing event) according to an established method (Fine and Gray 1999; Scrucca et al. 2010).
The proportional hazards assumption was justified by visual inspections of the log (-log (survival function)) vs log (time) across the time-of-day groups. Missing data of covariates were imputed using multivariate imputation by chained equations (R mice Package, m=5). A similar approach to the missingness of Biobank data has been reported previously (Tikkanen et al. 2018). Less than 5% of values were missing for any covariates except for high cholesterol (5.5% missing). We did not impute physical activity data and mortality data. The pooled estimates of mortality outcomes were combined based on Rubin’s rules (Rubin 1996). Statistical significance was determined with a 2-sided alpha level of 0.05. Data were analyzed in May 2022 using the R software 4.1.0.
Results
During a median of 6.9 years (interquartile range, 6.3-7.4 years) of follow-up, we identified 629 CVD deaths out of the 94,489 participants. The descriptive statistics of study participants are summarized in Table 1. In general, participants in the early morning and night groups were five years younger compared to the midday group, and six years younger compared to the late morning group. The percentage of females was not substantially different across the four groups. There was a significant association between the time of day of physical activity and employment status. In the early morning and night groups, more than 75% of participants were employed, whereas only 54.1% of participants in the midday group were employed. The late morning group had the lowest employment rate (46.5%) and the highest retirement rate (47.1%). Violin plots were used to visualize the distribution of individual physical activity volume across time-of-day groups stratified by sex and age (Supplemental Figure S2)
Table 1.
Baseline characteristics of study participants. *
| Midday Group (Reference) (n=27,803) |
Early Morning Group (n=18,477) |
Late Morning Group (n= 25,700) |
Night Group (n= 22,509) |
P-value | |
|---|---|---|---|---|---|
| Sociodemographic Variables | |||||
| Age at Recruitment (years) | 57.9 (7.0) | 53.6 (7.9) | 59.1 (6.9) | 52.7 (7.8) | <0.001 |
| Female | 15,506 (55.8) | 10,096 (54.6) | 14,877 (57.9) | 12,721 (56.5) | <0.001 |
| Race/Ethnicity | |||||
| White | 25,831 (93.2) | 16,837 (91.4) | 24,201 (94.5) | 20,067 (89.5) | <0.001 |
| Black | 82 (0.3) | 105 (0.6) | 48 (0.2) | 115 (0.5) | |
| Asian | 921 (3.3) | 805 (4.4) | 719 (2.8) | 1,239 (5.5) | |
| Mixed | 757 (2.7) | 564 (3.1) | 552 (2.2) | 807 (3.6) | |
| Other | 115 (0.4) | 101 (0.5) | 96 (0.4) | 197 (0.9) | |
| Employment | |||||
| Employed | 14,924 (54.1) | 14,106 (76.8) | 11,855 (46.5) | 17,381 (77.7) | <0.001 |
| Retired | 10,670 (38.7) | 3,358 (18.3) | 12,004 (47.1) | 3,471 (15.5) | |
| Unemployed | 2,004 (7.3) | 904 (4.9) | 1,646 (6.5) | 1,525 (6.8) | |
| Townsend Deprivation Index | |||||
| 1st quartile, least deprived | 7,190 (25.9) | 4,165 (22.6) | 7,102 (27.7) | 5,084 (22.6) | <0.001 |
| 2nd quartile | 7,088 (25.5) | 4,389 (23.8) | 6,887 (26.8) | 5,213 (23.2) | |
| 3rd quartile | 6,922 (24.9) | 4,750 (25.8) | 6,395 (24.9) | 5,527 (24.6) | |
| 4th quartile | 6,557 (23.6) | 5,133 (27.8) | 5,270 (20.5) | 6,640 (29.6) | |
| Lifestyle Factors | |||||
| Body-Mass Index (kg/m2) | |||||
| < 18.5 | 130 (0.5) | 126 (0.7) | 146 (0.6) | 134 (0.6) | <0.001 |
| 18.5-24.9 | 10,072 (36.3) | 7,252 (39.3) | 9,974 (38.9) | 9,279 (41.4) | |
| 25-29.9 | 11,868 (42.8) | 7,396 (40.1) | 10,944 (42.7) | 8,612 (38.4) | |
| ≥ 30 | 5,672 (20.4) | 3,663 (19.9) | 4,590 (17.9) | 4,414 (19.7) | |
| Healthy Eating Score | 3.3 (0.9) | 3.3 (1.0) | 3.4 (0.9) | 3.2 (0.9) | <0.001 |
| Tobacco Use | |||||
| Never | 14,982 (54.0) | 10,967 (59.5) | 14,503 (56.6) | 13,388 (59.6) | <0.001 |
| Previous | 10,553 (38.1) | 6,279 (34.1) | 9,816 (38.3) | 7,243 (32.3) | |
| Current | 2,195 (7.9) | 1,185 (6.4) | 1,302 (5.1) | 1,822 (8.1) | |
| Alcohol Intake | |||||
| Never | 658 (2.4) | 622 (3.4) | 758 (3.0) | 717 (3.2) | <0.001 |
| Previous | 695 (2.5) | 594 (3.2) | 668 (2.6) | 626 (2.8) | |
| Current | 26,430 (95.1) | 17,244 (93.4) | 24,248 (94.4) | 21,142 (94.0) | |
| Sleep Duration (hours) | |||||
| ≤ 6 | 5,473 (19.7) | 4,823 (26.2) | 5,172 (20.2) | 5,231 (23.3) | <0.001 |
| 7-8 | 19,937 (71.9) | 12,901 (70.0) | 18,603 (72.6) | 16,127 (71.9) | |
| ≥9 | 2,313 (8.3) | 711 (3.9) | 1,839 (7.2) | 1,083 (4.8) | |
| Other Risk Factors and Chronic Conditions | |||||
| Family History of CVD | 16,683 (60.0) | 9,956 (53.9) | 15,452 (60.1) | 11,992 (53.3) | <0.001 |
| Hypertension | 21,572 (77.7) | 13,252 (71.8) | 20,249 (78.9) | 15,536 (69.1) | <0.001 |
| High Cholesterol | 10,521 (40.0) | 5,504 (31.5) | 9,944 (40.9) | 6,901 (32.5) | |
| Heart Attack | 694 (2.5) | 292 (1.6) | 692 (2.7) | 316 (1.4) | <0.001 |
| Angina | 1,207 (4.3) | 542 (2.9) | 1,154 (4.5) | 614 (2.7) | <0.001 |
| Stroke | 449 (1.6) | 210 (1.1) | 426 (1.7) | 248 (1.1) | <0.001 |
| Diabetes | 1,187 (4.3) | 702 (3.8) | 969 (3.8) | 844 (3.8) | <0.001 |
| Long-Standing Illness | 8,602 (31.6) | 4,650 (25.6) | 7,310 (29.0) | 5,933 (26.9) | <0.001 |
| General Health Status | |||||
| Excellent | 5,492 (19.8) | 4,242 (23.0) | 5,592 (21.8) | 5,181 (23.1) | <0.001 |
| Good | 16,693 (60.1) | 11,017 (59.8) | 15,763 (61.5) | 13,056 (58.2) | |
| Fair | 4,719 (17.0) | 2,809 (15.2) | 3,730 (14.6) | 3,536 (15.8) | |
| Poor | 8,49 (3.1) | 364 (2.0) | 548 (2.1) | 669 (3.0) | |
| Accelerometer Parameters | |||||
| Mean Acceleration | 26.1 (21.6 - 31.3) | 28.3 (23.6 - 33.8) | 26.5 (22.0 - 31.9) | 27.8 (23.0 - 33.3) | <0.001 |
Data are mean (SD) for normally distributed variables, median (interquartile range) for non-normally distributed variables, and number (percent) for categorical variables.
In the full-sample analysis, the volume of physical activity was inversely associated with CVD mortality in a curvilinear manner (P for non-linearity < 0.001) (Supplemental Figure S3). After adjusting for the volume of physical activity, the time of day of physical activity was significantly associated with CVD mortality. The hazard of CVD mortality was 56% higher in the early morning group (95% Confidence Interval [CI]: 23 to 98%, P < 0.001) and 49% higher in the night group (95% CI: 18 to 88%, P < 0.001) than in the referent group (midday), while the hazard of CVD mortality was not significantly different between the late morning and midday groups (Table 2).
Table 2.
Cox proportional hazards regression for the association between time of day and CVD mortality. *
| Midday Group (Reference) |
Early Morning Group |
Late Morning Group |
Night Group | |
|---|---|---|---|---|
| Full Sample (n=94,489) | ||||
|
|
||||
| No. of Deaths | 175 | 117 | 208 | 129 |
| No. of Participants | 27,803 | 18,477 | 25,700 | 22,509 |
| Hazard Ratio | 1 (Reference) | 1.56 (1.23 – 1.98) | 1.21 (0.98 – 1.47) | 1.49 (1.18 – 1.88) |
| With Pre-Existing CVD (n=6,672) | ||||
|
|
||||
| No. of Deaths | 46 | 34 | 69 | 41 |
| No. of Participants | 2,267 | 1,035 | 2,204 | 1,166 |
| Hazard Ratio | 1 (Reference) | 1.87 (1.20 – 2.92) | 1.49 (1.02 – 2.17) | 1.90 (1.24 – 2.92) |
| Without CVD (n=87,817) | ||||
|
|
||||
| No. of Deaths | 129 | 83 | 139 | 88 |
| No. of Participants | 25,536 | 17,442 | 23,496 | 21,343 |
| Hazard Ratio | 1 (Reference) | 1.44 (1.09 – 1.91) | 1.11 (0.87 – 1.41) | 1.33 (1.01 – 1.75) |
The full-sample analysis adjusted for physical activity volume, age, sex, ethnicity, Townsend Deprivation Index, body-mass index, smoking, alcohol intake, healthy eating score, a family history of heart disease and stroke, hypertension, high cholesterol, diabetes, long-standing illness, a personal history of heart attack, angina, and stroke, and general health status.
In CVD-stratified analysis, the association between the time of day of physical activity and CVD mortality was strengthened in patients with pre-existing CVD and attenuated in individuals without CVD. The hazard of CVD mortality was 87% higher in the early morning group (95% CI: 20 to 192%, P = 0.007) and 90% higher in the night group (95% CI: 24 to 192%, P = 0.004) than in the referent group (midday) among patients with pre-existing CVD. Among participants without pre-existing CVD, the hazard of CVD mortality was 44% higher in the early morning group (95% CI: 9 to 91%, P = 0.01) and 33% higher in the night group (95% CI: 1 to 75%, P = 0.04) than in the referent group (midday).
In chronotype-stratified analysis, CVD mortality was 52% (95% CI: 9 to 111%, P = 0.01) higher in the night group than in the referent group among the morning-type individuals. On the other hand, CVD mortality was 117% (95% CI: 36 to 248%, P = 0.001) higher in the early morning group than in the referent group among the evening-type individuals. (Table 3)
Table 3.
Cox proportional hazards regression for the association between time of day and CVD mortality stratified by chronotypes. *
| Midday Group (Reference) |
Early Morning Group |
Late Morning Group |
Night Group | |
|---|---|---|---|---|
| Morning-Type Individuals (n=53,328) | ||||
|
|
||||
| No. of Deaths | 87 | 79 | 143 | 61 |
| No. of Participants | 13,074 | 13,257 | 17,043 | 9,954 |
| Hazard Ratio | 1 (Reference) | 1.30 (0.95 – 1.77) | 1.13 (0.87 – 1.48) | 1.52 (1.09 – 2.11) |
| Evening-Type Individuals (n=30,962) | ||||
|
|
||||
| No. of Deaths | 70 | 25 | 45 | 52 |
| No. of Participants | 11,542 | 3,554 | 5,887 | 9,979 |
| Hazard Ratio | 1 (Reference) | 2.17 (1.36 – 3.48) | 1.23 (0.85 – 1.80) | 1.37 (0.95 – 1.98) |
Adjusted for physical activity volume, age, sex, ethnicity, Townsend Deprivation Index, body-mass index, smoking, alcohol intake, healthy eating score, a family history of heart disease and stroke, hypertension, high cholesterol, diabetes, long-standing illness, a personal history of heart attack, angina, and stroke, and general health status.
In sensitivity analyses, CVD mortality outcomes were unchanged when excluding individuals who reported nightshift work (Supplemental Table S1). Also, results were similar after excluding participants who died within two years of follow-up (Supplemental Table S2). Further adjustment for employment and total sleep duration did not affect the association between time of day and CVD mortality (Supplemental Table S3). Also, we found that the time-of-day categories were not significantly associated with non-CVD mortality or all-cause mortality (Supplemental Table S4). The outcomes based on competing risk analysis were consistent with our main analysis (Supplemental Table S5).
Discussion
In this prospective cohort study of the UK Biobank dataset, we investigated whether performing peak physical activity at different times of day is associated with CVD mortality. In addition to re-confirming the beneficial inverse association between physical activity volume and CVD mortality, we found that the time of day of peak physical activity was a significant predictor of CVD mortality independent of the volume of physical activity, especially in patients with pre-existing CVD. Specifically, the risk of CVD mortality was higher in those who accumulated their greatest volume of physical activity in the early morning (early morning group) and those accumulating most of their physical activity at night (night group), as compared with the referent midday group. In patients with pre-existing CVD, the risk of dying from CVD had a nearly two-fold increase in the early morning and night groups compared to the midday group. Our findings suggest that diurnal pattern is a novel and important dimension of health-benefiting physical activity that is not defined by the traditional dimensions (i.e., frequency, duration, and intensity). Interestingly, we found that the time-of-day categories were not significantly associated with non-CVD mortality or all-cause mortality, which supports our hypothesis that the time of day of physical activity mainly influences cardiovascular health.
Notably, the chronotype-stratified analysis revealed that the increased CVD mortality in the morning group was only observed in the evening-type participants, while the increased CVD mortality in the night group was only observed in the morning-type participants. These findings suggest that the association between physical activity timing and CVD mortality is likely to be driven by a circadian misalignment between chronotypes and physical activity behavior. Our finding is consistent with previous chronotype research. Both time of day and chronotype can influence the cardiovascular responses to physical activity (Dunn and Taylor 2017). Depending on the chronotype, the highest cardiovascular reactivity to physical activity may not always occur in the early morning. It has been revealed that morning-type individuals experienced the highest increase in heart rate and rate-pressure product when physical activity was performed in the afternoon (Dunn and Taylor 2017).
The association between time-of-day physical activity and cardiovascular health has also been observed in a cross-sectional study based on the Look AHEAD dataset. Qian and colleagues (2021) revealed that men who were physically active in the early morning had a higher risk of developing coronary heart disease compared to those who were active at midday, after adjusting for the duration and intensity of physical activity. On the other hand, a study among patients with coronary artery disease who underwent supervised cardiac rehabilitation found no time-of-day difference in exercise-associated cardiovascular risk (Murray et al. 1993). The authors revealed that supervised exercise training performed in the morning and afternoon was safe and had no significant difference in cardiac events between the two groups (3.0±1.3 [07:30h] versus 2.4±1.5 [15:00h] events per 100 000 patient-hours). The low incidence of cardiac events in that study is in agreement with the established safety of cardiac rehabilitation in clinical settings. Nonetheless, our study was based on free-living accelerometry data. Due to the lack of proper medical supervision and emergency care, the cardiovascular risk could be much higher during free-living physical exertion than during supervised training (Thompson et al. 2003). We speculated that the different setting of physical activity was a possible explanation for the discrepancy between our findings and evidence from cardiac rehabilitation.
Previous physiologic studies have provided insights into the mechanisms of increased CVD mortality in the early morning group. Early morning exercise leads to exaggerated blood pressure and heart rate elevation during exercise (Jones et al. 2006; Atkinson et al. 2009) and greater intravascular shear stress following exercise (Jones et al. 2009). The increased hemodynamic forces and extra workload on the heart during morning physical activity is a particular concern for patients with pre-existing CVD. Additionally, the acute post-exercise hypotension following one session of exercise and the chronic blood pressure reduction following weeks of exercise training were both attenuated when exercise was performed in the early morning (Jones et al. 2008; Brito et al. 2018). The beneficial effect of physical activity on blood pressure could be diminished by the early morning timing. Collectively, compared to physical activity at other times of day, morning physical activity is associated with an increase in physical activity-induced stress and a decrease in physical activity-elicited benefits.
Our findings also indicated increased CVD mortality in the night group. Those participants displayed increased movement a few hours prior to presumed bedtime (Figure 2), which may be due to nighttime exercise. Given that sleep is essential for cardiovascular rest and recovery (Redeker and Hedges 2002), we speculate that exercise before bedtime can potentially undermine the quality of rest and recovery. This is supported by findings that show exercise performed within two hours before bedtime significantly increased sleep heart rate (Myllymäki et al. 2011), a recognized cardiovascular risk factor in patients with hypertension and the general population (Cuspidi et al. 2018; Eguchi et al. 2009). Our speculation is also supported by the effect of nighttime exercise on the circadian rhythm of hormones. For example, the nocturnal increase of melatonin, an important antioxidant and anti-inflammatory hormone, is suppressed and delayed by exercising at night (Monteleone et al. 1990; Van Reeth et al. 1994). Nonetheless, evidence from healthy and fit individuals reported that exercising prior to bedtime did not negatively influence sleep duration and stages (e.g., rapid eye movements sleep) (Stutz et al. 2019). Future studies should target susceptible populations with or at risk for CVD and should assess outcomes that directly concern cardiovascular health to elucidate the effects of nighttime exercise on cardiovascular health.
Understanding the association between time-of-day physical activity and cardiovascular health is important as CVD continues to be the leading cause of death globally. Thirty-two percent of all deaths worldwide in 2019 (17.9 million) were attributed to CVD (World Health Organization 2022). Previously, reconsidering the circadian rhythm of blood pressure and optimizing the timing of antihypertensive medication administration improved cardiovascular prognosis (Hermida 2011). Our findings provide an innovative perspective on the prevention and management of CVD through synchronizing health-benefiting behaviors with cardiovascular diurnal variations on an individualized basis. This synchronization could be a particularly promising strategy in patients with pre-existing CVD as evidenced by the nearly two-fold differences in CVD mortality risk between the time-of-day groups.
Another implication of our findings is the relatively lower CVD mortality in the late morning and midday groups. Participants in these two groups are mainly active between 09:00h and 16:00h, which is a common time for work (i.e., occupation). This finding aligns well with the current public health effort of promoting worksite physical activity to improve health and fitness (Reed et al. 2017). For example, interrupting prolonged sitting by performing short bouts of physical activity has shown promising acute effects on cardiometabolic health (Saunders et al. 2018).
We employed a machine learning algorithm to classify the diurnal patterns of accelerometer-measured physical activity in a large sample of free-living adults. The main rationale for using kmeans includes its objectivity and its advantage in pattern identification. Firstly, the simple categorization method based on a percentage of physical activity within a time window is subject to the selection of percentage cut-points and the selection of time window. For example, defining the early morning category as “>50% of physical activity accumulated between 0700h and 0900h” is likely to produce different outcomes as opposed to “>50% of physical activity accumulated between 0800h and 1000h”. When using kmeans, this important decision was essentially left to the dataset itself. It is possible that the four time-of-day groups in our study are representative of the major diurnal patterns of physical activity within the UK Biobank population.
Secondly, the kmeans algorithm runs automated recognition of patterns, which is a great way to explore what patterns of physical activity are within the dataset. For example, the existence of individuals whose physical activity peaks at two different times of day is a potential threat to the validity of our study. However, even with a higher number of clusters in machine learning (e.g., 5-10 clusters), we failed to identify a category of participants that displayed two or more marked peaks of physical activity at different times of day (Supplemental Figure S4), suggesting that the multiple-peak patterns, if exist, would only account for a small portion of the study population. On the other hand, we did identify the pattern that peaked in the afternoon/early evening when the number of clusters was set at eight or higher. Also, we identified participants that displayed a relatively stable pattern with no noticeable peaks when the number of clusters was eight or higher.
We conducted additional sensitivity analysis using the time-of-day categorization based on eight clusters to test the robustness of our outcome and to examine the CVD mortality in the afternoon group and the stable group. To be consistent with the main analysis, the midday group was treated as the reference group. The two stable patterns were combined into one group in analysis due to their similarity (Supplemental Figure S5). The outcome of sensitivity analysis (Supplemental Table S6) agreed well with our main analysis that the early morning and night are less favorable times of day to perform physical activity, whereas the late morning, midday, and afternoon are favorable time windows for physical activity. These findings indicate that our main outcome still holds true when the number of clusters is changed.
There are some limitations to this study. Although the UK Biobank has a very large sample size, the study participants are not nationally representative. In our analysis, more than 90% of participants are White and reside in healthier and socioeconomically advantaged areas. Future studies are warranted to test the generalizability of our findings in other socioeconomic and ethnic groups. Another limitation is the lack of differentiation between recreational and occupational physical activity in accelerometry data. A recent meta-analysis revealed that occupational physical activity is not beneficially associated with CVD mortality (Cillekens et al. 2022). However, our outcomes are unlikely to be driven by occupational physical activity because the early morning and night groups were physically active during non-working hours, and our outcomes were unchanged with further adjustment for employment. It is noteworthy that our epidemiologic design cannot demonstrate causality. Despite the adjustment for well-recognized confounding variables of the physical activity-mortality association, our analysis cannot eliminate residual unmeasured confounders. It is possible that participants who are physically active in the morning or at night have other underlying behavioral, environmental, physiological, or even genetic factors that serve as the true cause of the observed increase in CVD mortality. In this case, randomized clinical trials that promote unsupervised physical activity are needed to establish the causal relationship between the time of day of physical activity and CVD outcomes.
In our analysis, reverse causation cannot be entirely ruled out. It is possible that either the underlying CVD risk could cause the difference in diurnal patterns, or the diurnal patterns of physical activity could cause the difference in CVD mortality outcomes. If the former is true (reverse causation is true), we would observe a higher level of CVD risk in the morning group at baseline. However, the descriptive analyses of baseline characteristics suggest that the reverse causation is unlikely because the prevalence of CVD risk factors (e.g., hypertension, high cholesterol, and diabetes) and the prevalence of diagnosed CVD (e.g., heart attack, angina, and stroke) were both higher in the referent midday group than in the early morning group. Additionally, excluding deaths within two years of follow-up did not appreciably attenuate the association between time of day and CVD mortality, which also reduced the bias associated with reverse causality.
In conclusion, early morning and night appeared less favorable times of day to engage in physical activity compared to midday, controlling for the total volume of physical activity. While acknowledging the substantial health benefits of regular physical activity, optimizing the timing of physical activity according to cardiovascular circadian rhythms and individual chronotype could be a potential therapeutic target that brings additional health benefits.
Supplementary Material
Sources of Funding:
This work was supported by the NH-INBRE (New Hampshire – Institutional Development Award (IDeA) Networks of Biomedical Research Excellence), P20GM103506, from the National Institute of General Medical Sciences of the NIH. https://www.nigms.nih.gov/research/drcb/IDeA/Pages/INBRE.aspx
Footnotes
Disclosures: The authors report no conflict of interest.
Data Availability Statements:
The data underlying this article were provided by the UK Biobank Access Management Team by permission. Data are available for approved researchers with permission of the UK Biobank Access Management Team.
References
- 1.Araki M, Yonetsu T, Kurihara O, Nakajima A, Lee H, Soeda T, Minami Y, Higuma T, Kimura S, Takano M, et al. 2021. Circadian variations in pathogenesis of ST-segment elevation myocardial infarction: An optical coherence tomography study. J Thromb Thrombolysis. 51:379–87. doi: 10.1007/s11239-020-02220-6 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson G, Leary A, George KP, Murphy MB, Jones H. 2009. 24-Hour variation in the reactivity of rate-pressure-product to everyday physical activity in patients attending a hypertension clinic. Chronobiol Int. 26:958–73. doi: 10.1080/07420520903044455 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson G, Jones H, Ainslie PN. 2010. Circadian variation in the circulatory responses to exercise: relevance to the morning peaks in strokes and cardiac events. Eur J Appl Physiol. 108:15–29. doi: 10.1007/s00421-009-1243-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilo G, Grillo A, Guida V, Parati G. 2018. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr Blood Press Control. 11:47–56. doi: 10.2147/IBPC.S130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brito LE, Pecanha T, Fecchio RA, Rezende R, Sousa P, Silva-Júnior N, Abreu A, Silva G, Mion-Junior D, Halliwill J, et al. 2018. Morning vs evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. 51:653–62. doi: 10.1249/MSS.0000000000001852 [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. 1999. Plaque Rupture and Sudden Death Related to Exertion in Men With Coronary Artery Disease. JAMA. 281:921–926. doi: 10.1001/jama.281.10.921 [DOI] [PubMed] [Google Scholar]
- 7.Cillekens B, Huysmans MA, Holtermann A, van Mechelen W, Straker L, Krause N, van der Beek AJ, Coenen P. 2022. Physical activity at work may not be health enhancing. A systematic review with meta-analysis on the association between occupational physical activity and cardiovascular disease mortality covering 23 studies with 655 892 participants. Scand J Work Environ Health. 48:86–98. doi: 10.5271/sjweh.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. 1997. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 79: 1512–6. doi: 10.1016/S0002-9149(97)00181-1 [DOI] [PubMed] [Google Scholar]
- 9.Crnko S, Du Pré BC, Sluijter JP, Van Laake LW. 2019. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 16:437–47. doi: 10.1038/s41569-019-0167-4 [DOI] [PubMed] [Google Scholar]
- 10.Cuspidi C, Facchetti R, Bombelli M, Sala C, Tadic M, Grassi G, Mancia G. 2018. Night-time heart rate nondipping: clinical and prognostic significance in the general population. J Hypertens. 36:1311–7. doi: 10.1097/HJH.0000000000001703 [DOI] [PubMed] [Google Scholar]
- 11.Desquilbet L, Mariotti F. 2010. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 29:1037–57. doi: 10.1002/sim.3841 [DOI] [PubMed] [Google Scholar]
- 12.Doherty A, Jackson D, Hammerla N, Plötz T, Olivier P, Granat MH, White T, Van Hees VT, Trenell MI, Owen CG, et al. 2017. Large scale population assessment of physical activity using wrist worn accelerometers: the UK biobank study. PloS one. 12:e0169649. doi: 10.1371/journal.pone.0169649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JS, Taylor CE. 2014. Cardiovascular reactivity to stressors: effect of time of day? Chronobiol Int. 31:166–174. doi: 10.3109/07420528.2013.833517 [DOI] [PubMed] [Google Scholar]
- 14.Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, Kario K. 2009. Nocturnal non-dipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens. 27:2265. doi: 10.1097/HJH.0b013e328330a938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faisal A, Beavers KR, Hughson RL. 2010. O2 uptake and blood pressure regulation at the onset of exercise: interaction of circadian rhythm and priming exercise. Am J Physiol Heart Circ Physiol. 299:H1832–42. doi: 10.1152/ajpheart.00762.2010 [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 17.Hartigan JA, Wong MA. 1979. A K-means clustering algorithm. Applied Statistics. 28:100–8. doi: 10.2307/2346830. [DOI] [Google Scholar]
- 18.Hermida RC, Ayala DE, Mojón A, Fernández JR. 2011. Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 58:1165–73. doi: 10.1016/j.jacc.2011.04.043 [DOI] [PubMed] [Google Scholar]
- 19.Jeong SW, Kim SH, Kang SH, Kim HJ, Yoon CH, Youn TJ, Chae IH. 2019. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 40:3547–55. doi: 10.1093/eurheartj/ehz564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones H, Atkinson G, Leary A, George K, Murphy M, Waterhouse J. 2006. Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension. 47:778–84. doi: 10.1161/01.HYP.0000206421.09642.b5 [DOI] [PubMed] [Google Scholar]
- 21.Jones H, Green DJ, George KP, Black MA, Atkinson G. 2009. Evidence for a greater elevation in vascular shear stress after morning exercise. Med Sci Sports Exerc. 41:1188–93. doi: 10.1249/MSS.0b013e318195109c [DOI] [PubMed] [Google Scholar]
- 22.Jones H, Pritchard C, George K, Edwards B, Atkinson G. 2008. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 104:481–9. doi: 10.1007/s00421-008-0797-4 [DOI] [PubMed] [Google Scholar]
- 23.Li ZY, Taviani V, Tang T, Sadat U, Young V, Patterson A, Graves M, Gillard JH. 2009. The mechanical triggers of plaque rupture: shear stress vs pressure gradient. Br J Radiol. 82:S39–45. doi: 10.1259/bjr/15036781 [DOI] [PubMed] [Google Scholar]
- 24.Monteleone P, Maj M, Fusco M, Orazzo C, Kemali D. 1990. Physical exercise at night blunts the nocturnal increase of plasma melatonin levels in healthy humans. Life Sci. 47:1989–95. doi: 10.1016/0024-3205(90)90432-Q [DOI] [PubMed] [Google Scholar]
- 25.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. 1987. Circadian variation in the frequency of sudden cardiac death. Circulation. 75:131–8. doi: 10.1161/01.CIR.75.1.131 [DOI] [PubMed] [Google Scholar]
- 26.Murray PM, Herrington DM, Pettus CW, Miller HS, Cantwell JD, Little WC. 1993. Should patients with heart disease exercise in the morning or afternoon? Arch Intern Med. 153:833–6. doi: 10.1001/archinte.1993.00410070031004 [DOI] [PubMed] [Google Scholar]
- 27.Myllymäki T, Kyröläinen H, Savolainen K, Hokka L, Jakonen R, Juuti T, Martinmäki K, Kaartinen J, Kinnunen ML, Rusko H. 2011. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. J Sleep Res. 20:146–53. doi: 10.1111/j.1365-2869.2010.00874.x [DOI] [PubMed] [Google Scholar]
- 28.Public Health England. A quick guide to the government's healthy eating recommendations. London. PHE publications. [Accessed 2022 May 20] https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/742746/A_quick_guide_to_govt_healthy_eating_update.pdf [Google Scholar]
- 29.Qian J, Walkup MP, Chen SH, Brubaker PH, Bond DS, Richey PA, Jakicic JM, Hu K, Scheer FA, Middelbeek RJ, et al. 2021. Association of objectively measured timing of physical activity bouts with cardiovascular health in type 2 diabetes. Diabetes Care. 44:1046–54. doi: 10.2337/dc20-2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redeker NS, Hedges C. 2002. Sleep during hospitalization and recovery after cardiac surgery. J Cardiovasc Nurs. 17:56–68. doi: 10.1097/00005082-200210000-00006 [DOI] [PubMed] [Google Scholar]
- 31.Reed JL, Prince SA, Elliott CG, Mullen KA, Tulloch HE, Hiremath S, Cotie LM, Pipe AL, Reid RD. 2017. Impact of workplace physical activity interventions on physical activity and cardiometabolic health among working-age women: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 10:e003516. doi: 10.1161/circoutcomes.116.003516 [DOI] [PubMed] [Google Scholar]
- 32.Rubin DB. 1996. Multiple imputation after 18+ years. J Am Stat Assoc. 91:473–489. doi: 10.1080/01621459.1996.10476908 [DOI] [Google Scholar]
- 33.Saunders TJ, Atkinson HF, Burr J, MacEwen B, Skeaff CM, Peddie MC. 2018. The acute metabolic and vascular impact of interrupting prolonged sitting: a systematic review and meta-analysis. Sports Med. 48:2347–66. doi: 10.1007/s40279-018-0963-8 [DOI] [PubMed] [Google Scholar]
- 34.Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. 2010. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. PNAS. 107:20541–6. doi: 10.1073/pnas.100674910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scrucca L, Santucci A, Aversa F. 2010. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 45:1388–95. doi: 10.1038/bmt.2009.359 [DOI] [PubMed] [Google Scholar]
- 36.Stutz J, Eiholzer R, Spengler CM. 2019. Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis. Sports Med. 49:269–87. doi: 10.1007/s40279-018-1015-0 [DOI] [PubMed] [Google Scholar]
- 37.Thompson PD, Buchner D, Piña IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, et al. 2003. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 107:3109–16. doi: 10.1161/01.CIR.0000075572.40158.77 [DOI] [PubMed] [Google Scholar]
- 38.Tikkanen E, Gustafsson S, Ingelsson E. 2018. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank Study. Circulation. 137:2583–91. doi: 10.1161/circulationaha.117.032432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biobank UK. UK Biobank ethics and governance framework. [Accessed 2022 May 10] https://www.ukbiobank.ac.uk/media/0xsbmfmw/egf.pdf [Google Scholar]
- 40.Van Reeth O, Sturis J, Byrne MM, Blackman JD, L'Hermite-Baleriaux M, LeProult R, Oliner C, Refetoff S, Turek FW, Van Cauter E. 1994. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol Endocrinol Metab. 266:E964–74. doi: 10.1152/ajpendo.1994.266.6.E964 [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. [Accessed 2022 July 20] https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the UK Biobank Access Management Team by permission. Data are available for approved researchers with permission of the UK Biobank Access Management Team.