Abstract
Schistosomiasis affects nearly 240 million people in predominately low and middle-income countries and ranks second in the number of cases and socioeconomic burden among all parasitic diseases. Despite the enormous burden posed by schistosomes, our understanding of how schistosomiasis impacts infected human tissues remains limited. Intestinal schistosomiasis in animal models leads to goblet cell hyperplasia, likely increasing mucus production and reflecting an intestinal type 2 immune response. However, it is unknown whether these same changes occur in schistosome-infected humans. Using immunofluorescence and light microscopy, we compared the abundance and morphology of goblet cells in patients diagnosed with schistosomiasis to uninfected controls. The mucin-containing vesicles in goblet cells from schistosome-infected patients were significantly larger (hypertrophic) than uninfected individuals, although goblet cell hyperplasia was absent in chronic human schistosomiasis. Additionally, we examined tuft cells in the large intestinal epithelium of control and schistosome-infected patients. Tuft cell numbers expand during helminth infection in mice, but these cells have not been characterized in human parasite infections. We found no evidence of tuft cell hyperplasia during human schistosome infection. Thus, our study provides novel insight into schistosome-associated changes to the intestinal epithelium in humans, suggesting an increase in mucus production by large intestinal goblet cells but relatively minor effects on tuft cell numbers.
Keywords: Schistosomiasis, schistosome, parasitic worm, goblet cell, tuft cell, neglected tropical disease
INTRODUCTION
Schistosomiasis is a debilitating infectious disease caused by parasitic flatworms of the genus Schistosoma. An estimated 240 million people required preventative treatment for schistosomiasis in 2019, making it one of the most common parasitic infections worldwide (1). Typical symptoms include abdominal pain, diarrhea, gastrointestinal bleeding, hematuria, and portal hypertension (1,2). Schistosomiasis predominantly affects impoverished rural communities without safe drinking water and adequate sanitation infrastructure. Cercariae, the infectious larval form of schistosomes, infect human hosts by penetrating skin exposed to contaminated fresh water. Once inside the host, schistosome larvae migrate through the circulatory system to the hepatic portal where they mature into adult worms and form mating pairs. These paired adult worms then travel to their permanent residence in the mesenteric (S. mansoni, S. japonicum, S. mekongi, S. intercalatum, and S. guineesis) or pelvic (S. haematobium) veins. Adult worms persist inside their human host for years, but do not induce local inflammation or directly cause symptoms. Rather, the immunopathology associated with chronic schistosome infection is caused by localized inflammatory responses against schistosome eggs (2–4).
Female schistosomes release approximately 300 to 3500 eggs per day (5,6), with approximately half of the eggs traversing the endothelium and across the intestinal or urinary epithelium before excretion in stool or urine. The remaining eggs are caught in the bloodstream and are ultimately trapped in distal tissues, principally the liver. When embedded in host tissues, the immunogenic schistosome eggs induce localized granulomatous inflammation, causing fibrosis and tissue-specific lesions that damage host organs (7–10). Studies in mice and humans indicate that schistosome eggs induce a type 2 immune inflammatory response (11–15).
Type 2 immunity is commonly associated with intestinal parasite infections and includes physiological changes such as increased gut motility and mucus secretion by the epithelium. Schistosome eggs elicit many cardinal features of type 2 immunity in mice, including an expansion of CD4+ T helper 2 (Th2) cells, eosinophils, basophils, and M2 polarized macrophages, as well as increased production of IgE and the cytokines IL-4, IL-5, and IL-13 (4,8,13–15). In addition, the number of mucin-secreting epithelial cells called goblet cells significantly increased in both the small and large intestines of mice and non-human primates infected with S. mansoni (16–18). Because adult schistosomes reside in the mesenteric venules, they are unlikely to be directly affected by goblet cell hyperplasia. However, increased mucus production fortifies the intestinal barrier, which may alter the susceptibility of individuals with schistosomiasis to co-infections with pathogenic enteric bacteria and other parasites.
Like schistosomes, parasitic worms such as Nippostrongylus brasiliensis and Heligmosomoides polygyrus induce type 2 immunity (19,20) and goblet cell hyperplasia in the small intestine of mice (23–25). These roundworms also increase the abundance of chemosensory epithelial cells called tuft cells. These cells detect luminal dwelling parasites and initiate type 2 immunity (25–30), but despite the central role of tuft cells in type 2 immunity and epithelial remodeling, their abundance and distribution during schistosome infection remains unknown.
Animal models are frequently used to study the pathogenesis of schistosomiasis in host tissue, including schistosome-associated type 2 immunity and epithelial remodeling. These models allow synchronization of infections and access to tissues that can be difficult to acquire in patients. Schistosoma endemic regions are often medically underserved, and patients are less likely to provide biopsies or undergo surgical procedures that would yield schistome-infected tissue. Consequently, the intestinal tissue response in human schistosomiasis or whether observations in animal models translate to human disease is poorly understood. This study utilizes human tissue biopsies from patients with schistosomiasis to evaluate goblet and tuft cell remodeling during chronic infection. Here, we report hypertrophy of mucin-containing vesicles in the goblet cells of patients with schistosomiasis. However, unlike previous studies performed in animal models, we find no difference in goblet cell abundance between control and infected groups. Furthermore, we enumerated tuft cells in the human large intestine and found the frequency of these cells unchanged regardless of infection status. In summary, this work characterizes how schistosomiasis shapes the landscape of tuft and goblet cells in human large intestines and identifies clear differences and similarities in schistosome-induced epithelial remodeling between humans and other mammalian species.
MATERIALS & METHODS
Ethics Statement
All tubular gastrointestinal tract biopsies with a diagnosis including the word “schistosomiasis” were received through the Department of Pathology at Stanford Hospital and evaluated following the approved Institutional Review Board (IRB) protocol. The slides were reviewed by board-certified fellowship-trained gastrointestinal pathologists (GWC and EF). This study utilized archived excess human tissues that were removed for routine clinical purposes, therefore specific written consent was not required under the approved IRB. Detailed information regarding patient age, sex, presentation of symptoms, and other relevant clinical information are included in Table 1. Animal studies and experiments were approved and performed in accordance with Stanford’s Institutional Animal Care and Use Committee (IACUC #32914), and the National Institutes of Health guidelines for animal use and care.
Table 1.
Clinical information about the patient cohort used in this study.
| Control Patients | ||||||
|---|---|---|---|---|---|---|
| Age | Sex | Travel History | Region Biopsied | Symptoms | Additional Relevant | Serology |
| 34 | Male | N/A | Unidentified colonic region | Chronic diarrhea | None | Not tested |
| 40 | Male | 2017: Australia and New Zealand | Transverse colon | None, Crohn’s surveillance | Ileocolonic Crohn’s disease | Not tested |
| 48 | Female | N/A | Unidentified colonic region | Chronic diarrhea and pain | None | Not tested |
| 55 | Female | N/A | Descending colon | Worsening ulcerative colitis symptoms over past 6 months | Left-sided ulcerative colitis | Not tested |
| 56 | Female | N/A | Descending colon | Intermittent diarrhea | None | Not tested |
| 57 | Male | N/A | Sigmoid colon | Sigmoid colitis | None | Not tested |
| 61 | Female | N/A | Rectum | Nausea, vomiting, diarrhea | Aplastic anemia, status post hematopoietic stem cell transplant | Not tested |
| 80 | Female | N/A | Unidentified colonic region | Diarrhea | None | Not tested |
| Schistosomiasis Patients | ||||||
| Age | Sex | Travel History | Region Biopsied | Symptoms | Additional Relevant | Serology |
| 35 | Female | 2009: Yemen | Rectum | Chronic left flank pain, nausea and passage of tissue per rectum | Present: Chronic active gastritis 2016: H. pylori infection |
Positive |
| 51 | Female | Recent travel to Philippines | Rectosigmoid colonic junction | Chronic non-bloody diarrhea | Tubular colonic adenoma and hyperplastic polyp present in same biopsy | Negative |
| 61 | Female | N/A | Ascending colon | None, presents for high-risk colon cancer surveillance | None | Not tested |
| 66 | Male | N/A | Cecum | None, colonoscopy screening | Tubulovillous adenoma present in same biopsy | Negative |
| 70 | Male | Immigrant from China | Rectum | Rectal bleeding and pain | Prior schistosomiasis infection during childhood, treated twice | Not tested |
| 71 | Female | Recent immigrant from China | Rectum | None, follow-up for prior rectal adenocarcinoma | 2018: Rectal adenocarcinoma | Negative |
| 86 | Female | N/A | Rectum | None | 2005 and 2010: Prior schistosomiasis infection in hepatic flexure | Not tested |
Immunofluorescence microscopy
Human tissue biopsies were fixed in formalin, embedded in paraffin (FFPE), and cut into 5μm thick sections. FFPE tissue sections were deparaffinized and hydrated in decreasing concentrations of ethanol. Heat-mediated antigen retrieval was performed in 10mM sodium citrate buffer + 0.05% Tween-20, pH 6.0 for 20 minutes at 98°C. Afterward, slides were washed and blocked in PBS containing 3% BSA, 2.5% donkey serum, 0.1% saponin for 1 hour at room temperature. Primary antibodies were incubated overnight at 4°C. Secondary antibodies and DAPI were applied for 1 hour at room temperature. Primary antibodies included: Rabbit anti-pEGFR (1:200 dilution, ab182618, Abcam), Mouse anti-E-Cadherin (1:200 dilution, 610181, BD Biosciences), Goat anti-ChAT (1:200 dilution, AB144P, MilliporeSigma), Mouse anti-COX1 (1:200 dilution, sc-19998, Santa Cruz Biotechnology, Inc.). DNA was labeled with DAPI (0.5 μg/ml, Roche). Mouse colonic tissue was fixed in 4% paraformaldehyde overnight at 4°C and embedded in paraffin. 5μm thick sections were processed as described above, but murine tuft cells were stained with Rabbit anti-DCAMLK1 (1:200, ab31704 Abcam). Images were captured with a Zeiss LSM 700 confocal microscope and processed/analyzed with FIJI.
Histology
FFPE tissue sections were deparaffinized and hydrated as described above. Alcian blue/nuclear red staining or hematoxylin and eosin (H&E) staining were performed using standard procedures. Images were captured with a Nikon Eclipse Ni light microscope and processed/analyzed with FIJI.
Tuft cell enumeration
Human tuft cells were identified by immunofluorescence microscopy as described above. Image analysis was conducted in FIJI. Cells were manually enumerated using the Cell Counter plugin. First, individual epithelial cells were counted using the DAPI and E-Cadherin channels as reference. Only epithelial cells with a visible lumen-facing border were counted. Next, the pEGFR and ChAT channels were used as reference to enumerate tuft cells among the previously established population of epithelial cells. Tuft cell enumeration was represented as a percent of total epithelial cells. Between 650 and 2500 epithelial cells were analyzed per patient. Tuft cell frequencies in control and schistosome-infected groups were compared using Mann-Whitney U tests. All statistical analyses and graphs were generated using GraphPad Prism 8.
Mouse tuft cells were identified and enumerated by immunofluorescence microscopy as previously described (27–29).
Goblet cell enumeration and area analysis
Goblet cells were identified by alcian blue/nuclear red staining as described above. Consecutive serial sections were used for goblet cell microscopy and analysis. This was done to maximize the similarity in tissue structure between sections, allowing for direct comparison of cell counts. The same regions of tissue used to enumerate tuft cells were also used for goblet cell analysis. Cell counter files marking the epithelium, which were generated during tuft cell enumeration, were superimposed onto the images of corresponding alcian blue staining and used as a guide for goblet cell enumeration. Goblet cells were manually enumerated using the Cell Counter plugin. Image analysis was conducted in FIJI. Goblet cell abundance was represented as a percent of total epithelial cells. For goblet cell mucin aggregate area analysis, images were de-identified and independently analyzed by two different individuals. Goblet cell area was measured manually using the polygon selection tool. Only goblet cells with a visible lumen-facing border and associated nucleus were quantified. 100 goblet cells per patient were randomly selected for goblet cell area analysis. Goblet cell frequency and mucin aggregate area in control and schistosome-infected groups were compared using Mann-Whitney U tests. All statistical analyses and graphs were generated using GraphPad Prism.
Mice
Specific-pathogen-free C57BL/6 mice were bred and housed in microisolator cages at Stanford University. Five female mice between 6 and 10 weeks of age were used in experiments. No animals were excluded for analysis. Large intestine were excised from specific-pathogen-free (SPF) mice without parasite infections. The luminal contents were flushed and the large intestines were swiss-rolled for immunofluorescence microscopy. Animal studies and experiments were approved and carried out following Stanford’s Institutional Animal Care and Use Committee, and the National Institutes of Health guidelines for animal use and care.
RESULTS
To determine the influence of schistosomiasis on large intestinal goblet and tuft cell remodeling, we obtained schistosome-infected large intestinal tissue from patients at Stanford University Medical Center. These infected patients ranged in age from 35 to 86 years old and presented with various symptoms related to gastrointestinal diseases (Table 1). Schistosome infections were diagnosed histologically by the presence of visible schistosome eggs surrounded by granulomatous inflammation (Fig. 1). Uninfected control biopsies were age and sex-matched with the schistosome-infected groups. These uninfected patients also presented with various gastrointestinal symptoms but displayed no evidence of schistosomiasis or other gastrointestinal infections (Table 1).
Fig. 1. Schistosome egg surrounded by granulomatous inflammation.
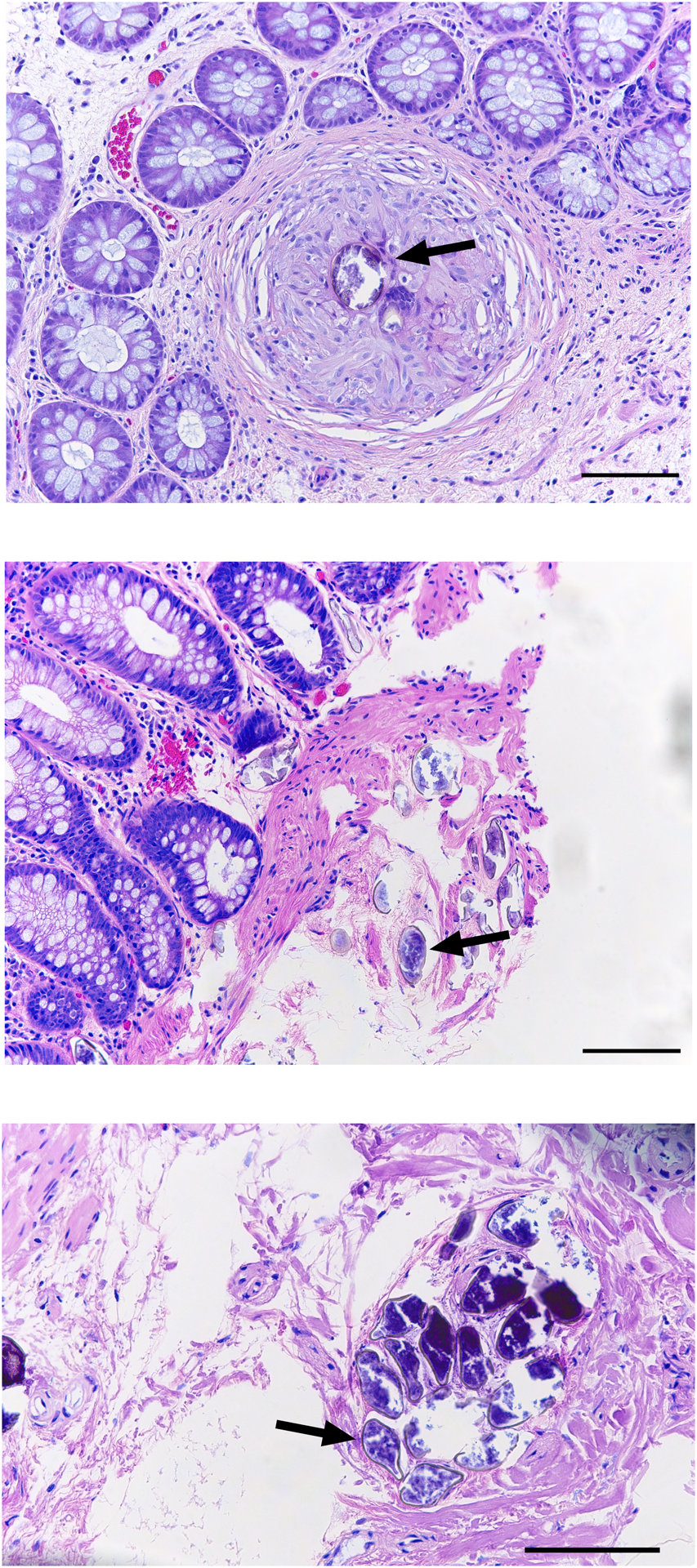
Hematoxylin and eosin stained colonic sections from three patients with intestinal schistosomiasis. Black arrow indicates a schistosome egg surrounded by a granuloma in the colonic lamina propria. Scale bar, 100 μm.
Analysis of goblet cell size and abundance in schistosome-infected patients
Hyperplasia of mucus-producing goblet cells is a well-characterized feature of anti-parasite immunity in various mammalian models, including in the large intestines of schistosome-infected mice and baboons (16–18). However, whether human goblet cells undergo similar changes following parasite infection remains unclear. To determine the effects of schistosome infection on the abundance and morphology of goblet cells, we compared biopsies of schistosome-infected human large intestines to uninfected controls using quantitative microscopy (Fig. 2A). Contrary to experiments using animal models, we found no difference in goblet cell abundance between schistosome-infected and control groups (p = 0.8665) (Fig. 2B). However, we observed that mucin-containing vesicles in goblet cells appeared larger (hypertrophy) in schistosome-infected samples than control samples.
Fig. 2. Chronic intestinal schistosomiasis induces goblet cell hypertrophy in the human colon.
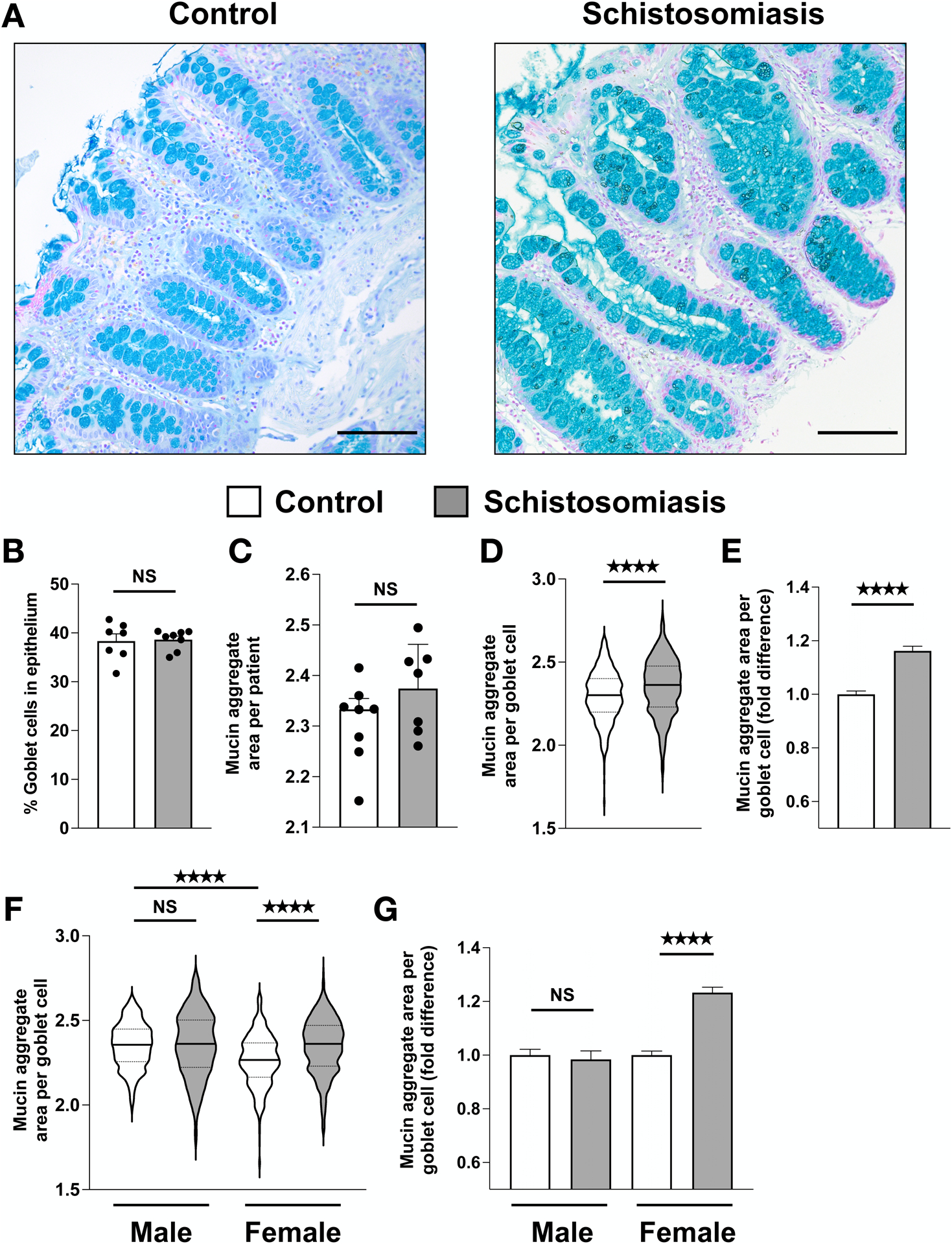
(A) Representative images of goblet cells in colonic sections stained with Alcian blue and nuclear red in uninfected and schistosome-infected patients (scale bar, 100 μm). (B) Colonic goblet cell abundance. Points represent individual patients. (C) Average goblet cell mucin aggregate area in μm2 plotted on a log scale. Points represent individual patients. (D) Mucin aggregate area per goblet cell. Violin plots show the median (solid black line) and quartiles (dashed lines) mucin area for individual goblet cells on a log scale. 100 goblet cells were analyzed for each individual patient. (E) Fold difference of mucin aggregate area per goblet cell. (F) Mucin aggregate area per goblet cell separated by sex. (G) Fold difference of mucin aggregate area per goblet cell separated by sex. Data are plotted as means with SEM. **** p < 0.0001; NS, not significant; using Mann-Whitney tests.
To test whether schistosome infection induces goblet cell hypertrophy, we measured the area of apical mucin-containing vesicles in 100 goblet cells per patient sample. Quantification of goblet cell size was analyzed independently by two individuals blinded to the identity of the samples to account for variation in individual researcher analysis. The results from both individual’s analyses are highly consistent (Fig. 2 and Fig. S1). Schistosome-infected patients have significantly enlarged goblet cell mucin-aggregates compared to controls on a per-cell basis (p <0.0001) (Fig. 2D). Although individual goblet cell mucin-aggregates were highly variable in size within each patient sample, goblet cells from schistosome-infected patients were 16.2% larger than controls on average (Fig. 2E). When analyzed on a per patient basis, these differences were not significant (p = 0.7789), which may reflect the high degree of intra-patient goblet cell variability (Fig. 2C). When analyzed by sex, goblet cells of male controls were significantly larger (29.0%) compared to female controls (p-value < 0.0001), but not infected males (p = 0.4382). Goblet cells of infected females were significantly larger (22%) compared to female controls (p < 0.0001), but not infected males (p = 0.8210) (Fig. 2F–G). These data suggest that chronic intestinal schistosome infection stimulates human large intestinal goblet cell hypertrophy. Female patients in this study drove this response; however, larger patient cohorts are required to robustly determine whether there are sex-specific differences in goblet cell hypertrophy during human schistosomiasis.
Analysis of tuft cell abundance in schistosome-infected patients
Tuft cells recently emerged as key players in anti-parasite immunity in the gastrointestinal tract. Studies conducted in mice revealed that tuft cells sense intestinal parasites and release IL-25 and other type 2 immune effector molecules in response. These effector molecules stimulate group 2 innate lymphoid cells (ILC2) to produce IL-13 and initiate type 2 immunity in the gut (25–29). Under homeostatic conditions, tuft cells sparsely populate the intestinal epithelium. However, during enteric parasite infection of mice, tuft cell abundance increases in the small intestine in response to IL-13 produced by ILC2s but not in the large intestine (25–27,31). Whether human tuft cells display a similar pattern during parasite infection is unknown.
Doublecortin-like Kinase 1 (DCLK1) is commonly used to identify tuft cells in mice but does not mark tuft cells in humans (32–34). Instead, choline acetyltransferase (ChAT), phosphorylated epidermal growth factor receptor (pEGFR; Y1068), and cyclooxygenase 1 (COX1) have been used to identify human tuft cells (29,32,35,36). Therefore, we stained large intestinal samples with anti-pEGFR, anti-ChAT, and anti-COX1 and observed co-localization of all three markers in slender epithelial cells that morphologically resemble tuft cells (Fig. 3A). Because of the variable quality of COX1 staining in tuft cells, we evaluated tuft cell abundance using only anti-pEGFR and anti-ChAT.
Fig. 3. Colonic tuft cell hyperplasia is absent in patients with chronic intestinal schistosomiasis.
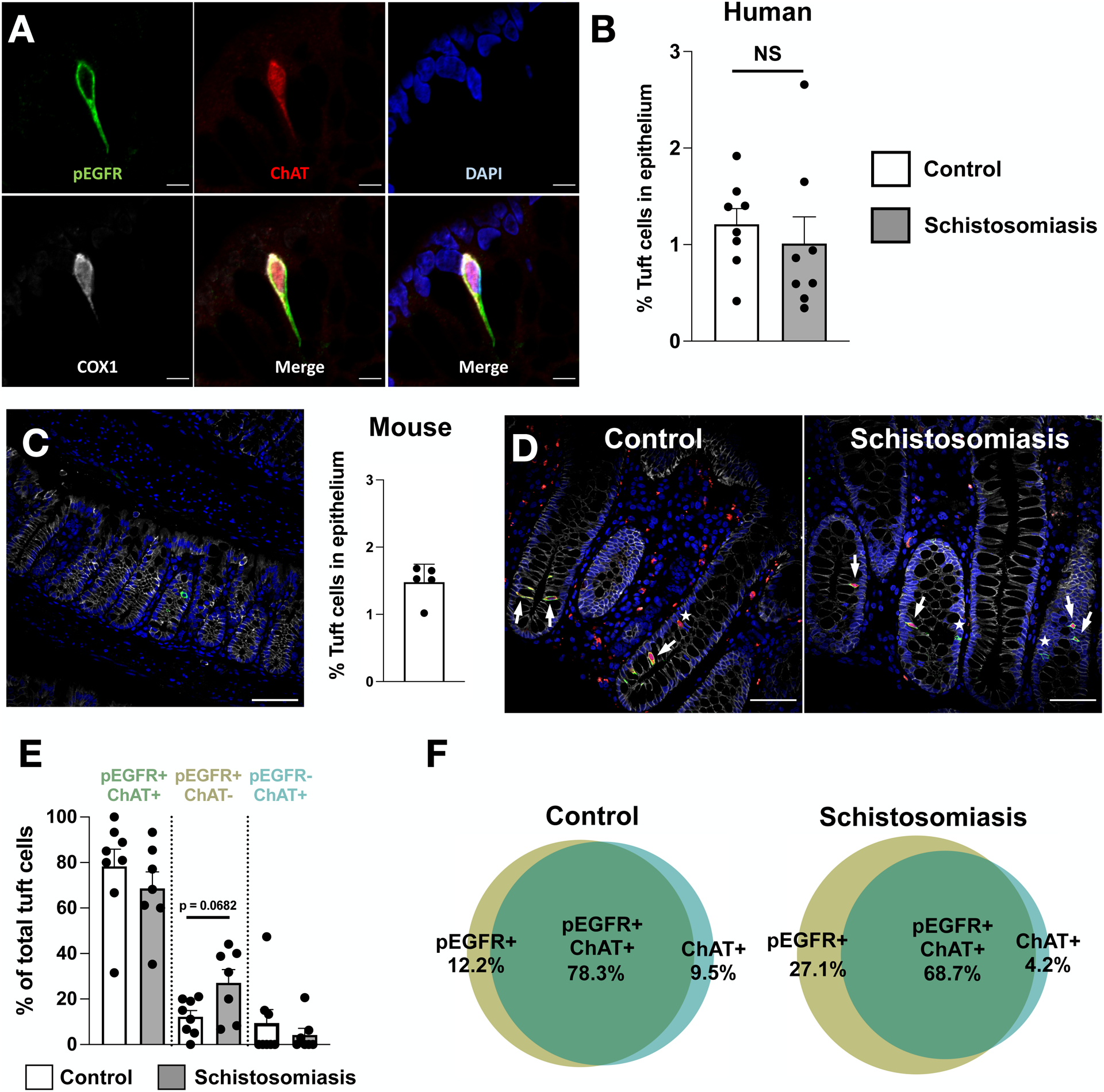
(A) Representative image of a colonic tuft cell in an uninfected patient. pEGFR is shown in green, ChAT in red, DAPI in blue, and COX1 in white (scale bar, 5 μm). (B) Abundance of pEGFR/ChAT double-positive colonic tuft cells in uninfected and schistosome-infected patients. Points represent individual patients. (C) Representative image of tuft cells in the colon of specific-pathogen free (SPF) mice without parasites. Tuft cells are stained with DCLK1 in green, E-Cadherin in red, and DAPI in blue (scale bar, 50 μm). Tuft cell abundance in colonic epithelium of SPF mice determined by quantitative microscopy. Points represent individual mice. (D) Representative images of colonic tuft cells in uninfected and schistosome-infected patients. pEGFR is shown in green, ChAT in red, DAPI in blue, and E-Cadherin in white. White arrows depict pEGFR/ChAT double-positive tuft cells. White stars depict pEGFR or ChAT single-positive tuft cells (scale bar, 50 μm). (E-F) Proportion of pEGFR/ChAT double-positive, pEGFR single-positive, and ChAT single-positive colonic tuft cells in uninfected and schistosome-infected patients. Data are plotted as means with SEM in (E) or as venn-diagrams in (F). Points represent individual patients. NS, not significant; using Mann-Whitney tests.
To assess the effects of schistosome infection on large intestinal tuft cell abundance, we compared the number of large intestinal tuft cells in schistosome-infected and control patients using anti-pEGFR and anti-ChAT (Fig. 3D). Double-positive tuft cells comprised 1.21% of the large intestinal epithelium in controls and 1.01% of the epithelium of schistosome-infected samples (p = 0.3282) (Fig. 3B). These results suggest that large intestinal tuft cell hyperplasia is absent during intestinal schistosomiasis in humans and that large intestinal tuft cell abundance is comparable to tuft cells in mouse large intestines at homeostasis (Fig. 3C) and with previous publications (31,37). Although the tuft cell markers anti-pEGFR and anti-ChAT largely co-localized, both control and infected patients contained distinct populations of epithelial cells that expressed only one of these markers. Among control patients, 78.3% of tuft cells were double positive for anti-pEGFR and anti-ChAT compared to 68.7% of tuft cells in schistosome-infected patients (p = 0.2946). In the control group, 12.2% of tuft cells were single positive for pEGFR compared to 27.1% in the infected group, a difference which trended toward significance (p = 0.0682). Tuft cells that were single positive for ChAT comprised 9.5% of tuft cells among control patients compared to 4.2% among infected patients (p = 0.9413) (Fig. 3E–F). The non-uniform expression of the conventional tuft cell markers ChAT and pEGFR may reflect heterogeneity in human tuft cells.
DISCUSSION
The response to schistosomiasis in humans has often focused on factors circulating in peripheral blood, such as antibodies, cytokines, and immune cells, while far less is understood about human tissue responses to these infections. Blood samples are much easier to collect than tissue samples from schistosome-infected patients, who are located primarily in regions with underdeveloped medical and research infrastructure. Furthermore, it can be difficult to accurately determine the duration of infection among individuals who live in highly endemic areas and are subject to frequent reinfection. Accordingly, animal models are often used to study host tissue responses to schistosome infection, but how well these findings reflect human biology is unclear. Our study begins to address this shortcoming by analyzing the large intestinal epithelial tissue response to schistosome infection using biopsies from patients diagnosed with intestinal schistosomiasis. We observed that mucus-producing goblet cells undergo hypertrophy, while the numbers of parasite-sensing tuft cells appear largely unaltered during chronic schistosome infection in humans. These findings are an important comparator to animal studies and offer insights into how schistosomiasis influences intestinal epithelial composition and morphology in humans.
In this study, we observed that large intestinal goblet cells in patients with intestinal schistosomiasis have, on average, larger mucin-containing vesicles compared to uninfected controls. This phenomenon, referred to as goblet cell hypertrophy, likely reflects an increase in mucus production, which is a canonical feature of anti-parasite immunity in the gut. The mucus barrier protects the epithelium from injury and may dislodge luminal parasites from the gut. (38). Whether schistosome-induced goblet cell hypertrophy is coupled to increased mucus secretion was not possible to examine in our study, as the patient biopsies did not contain an intact mucus layer. Furthermore, it remains unclear whether the various intestinal schistosome species (S. mansoni, S. japonicum, S. mekongi, S. guineesis, S. intercalatum) elicit different tissue responses in the host. Future studies would benefit from determining whether specific schistosome species influence goblet cells and other intestinal tissue responses.
We found no correlation between age and magnitude of hypertrophic goblet cell response (data not shown). However, segregating data by sex revealed that female patients in this study drove the hypertrophic response seen in the schistosome-infected group. Whether this is due to undersampling males or reflects biological differences between the sexes remains unclear. Interestingly, previous findings indicate that human and non-human females typically have elevated immune responses to parasite infection compared to conspecific males (39–41). Additionally, the prevalence and intensity of schistosome infections in endemic areas are lower among females than males (42,43). While differences in exposure to contaminated waters may account for discrepancies in parasite burden and disease intensity between the sexes, females produce more schistosome-specific serum IgA and exhibit enhanced regulatory T-cell responses during schistosome infection, which are processes known to mitigate tissue-damaging inflammation and protect against reinfection (44). Further investigation with a larger patient cohort will be necessary to elucidate sex-specific differences in mucus production and other tissue responses to schistosomiasis in humans.
In animal models of helminth infection, goblet cell hypertrophy and hyperplasia are consistent features of anti-parasite immunity that presumably serve to increase mucus production in the intestine (29,45). We observed goblet cell hypertrophy in schistosome-infected patients; however, in contrast to animal models of schistosomiasis, there was no goblet cell hyperplasia. This discrepancy could be influenced by the duration of infection, as the host immune response is known to change throughout the various stages of schistosomiasis. The first five weeks following schistosome infection are characterized by a potent Th1 response, during which goblet cell hyperplasia is absent in animal models (18). Approximately six weeks post-infection, mature parasites begin to release antigenic eggs, which begins the acute stage characterized by a dominant Th2 response. Goblet cell hyperplasia occurs in animal models during the acute stage of infection (16–18). During the chronic stage, which begins 12 weeks post-infection, the magnitude of the Th2 response decreases significantly over time as immune hyporesponsiveness to egg antigens progresses (8). Like many schistosome-infected patients, the exact timing and duration of infection among our cohort are not clear. However, they were likely chronically infected for years prior to this study, as no recent travel history to schistosome-endemic areas was reported at the time of the biopsy collection. Because the cytokine environments differ substantially between the acute and chronic stages of infection, the duration of infection may account for the difference in goblet cell abundance observed in prior animal studies and our study on humans. It remains unclear whether humans undergo a hyperplastic goblet cell response during acute schistosomiasis that diminishes over time. Surprisingly, schistosome-induced large intestinal goblet cell hyperplasia has been reported as independent of type 2 immunity in mice and instead relies on IL-22 (19) . Acute schistosomiasis increases IL-22 expression in mouse large intestines, and human peripheral blood mononuclear cells obtained from chronically infected individuals produce IL-22 in response to schistosome egg stimulation (46). IL-22 also has been implicated in small intestinal goblet cell hyperplasia (24), reflecting a common response between small and large intestinal goblet cells to this cytokine. Accordingly, the large intestinal goblet cell hypertrophy observed in schistosome-infected patients may indicate a similar response in the small intestine. However, small intestinal tuft cell hyperplasia requires type 2 cytokines and would not likely respond to IL-22 associated with Schistosomiasis. Future investigation is needed to further characterize the cytokine environments within human tissues across various stages of schistosome infection and determine if type 2 cytokines or IL-22 drives goblet cell hyperplasia during acute human schistosomiasis.
Until now, human tuft cells have not been examined in the context of parasite infection, including in response to schistosome eggs. Nor have tuft cells been analyzed in animal models of schistosomiasis. The results of this study suggest that tuft cells have a similar abundance in the large intestinal epithelium of mice and humans (31), and that chronic schistosomiasis does not induce tuft cell hyperplasia in the human large intestine. Mouse models of type 2 immunity also do not induce tuft cell hyperplasia in the large intestine as they do in the ileum, suggesting that the tissue-specific response of tuft cells to parasite infection is consistent between mice and humans (25–27). We could not acquire small intestinal biopsies from parasite-infected patients to include in this study and whether large intestinal tuft cells respond to schistosome infection in ways that do not induce hyperplasia remains unclear.
Tuft and goblet cells are specialized epithelial cells that play critical roles in maintaining a healthy relationship with the gut microbiome and defending the host against pathogens. These cells work in concert to monitor the microbial environment, initiate anti-parasite immunity, and fortify the mucus barrier. Understanding how schistosomiasis shapes the epithelial and immune landscapes may yield valuable insights into how schistosome infection alters intestinal function, vulnerability to co-infection, and the severity of concurrent illness. By examining the behavior of tuft and goblet cells in patients with schistosomiasis, our study lays the groundwork for understanding the important relationship between schistosome infection and host epithelial responses in humans.
Supplementary Material
Fig. S1. Goblet cell mucin aggregate area separated by individual patients. 100 goblet cells were analyzed per patient and plotted as μm2 on a log scale. Violin plots show the median (solid black line) and quartiles (dashed lines) aggregate mucin area.
Fig. S2. Second independent analysis of human colonic goblet cell hyperplasia associated with chronic schistosomiasis (A) Average colonic goblet cell mucin aggregate area per patient in uninfected and schistosome-infected patients. Points represent individual patients. (B) Mucin aggregate area per goblet cell plotted as μm2 on a log scale. Violin plots show the aggregate mucin area; median (solid black line) and quartiles (dashed lines). (C) Fold difference of mucin aggregate area per goblet cell. (D) Mucin aggregate area per goblet cell separated by sex. (E) Fold difference of mucin aggregate area per goblet cell separated by sex. (F) Goblet cell mucin aggregate area separated by individual patients. Data are plotted as means with SEM.
**** p < 0.0001; NS, not significant; using Mann-Whitney tests.
ACKNOWLEDGEMENTS
We thank members of the Howitt lab for discussion of this work and Connie Fung, Miles Tyner, and Leila DeSchepper for their comments on this manuscript. This research was supported by National Institutes of Health (NIH) R01DK128292 and R21AI171222 (to M.R. Howitt).
Footnotes
Disclosures: None
DECLARATION OF INTERESTS
The authors declare no conflicting interests.
REFERENCES
- 1.Schistosomiasis [Internet]. [cited 2022 Jan 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 2.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018. Dec;4(1):13. [DOI] [PubMed] [Google Scholar]
- 3.Colley DG, Secor WE. Immunology of human schistosomiasis. Parasite Immunol. 2014. Aug;36(8):347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002. Jul;2(7):499–511. [DOI] [PubMed] [Google Scholar]
- 5.Moore DV, Sandground JH. The Relative Egg Producing Capacity of Schistosoma Mansoni and Schistosoma Japonicum. The American Journal of Tropical Medicine and Hygiene. 1956. Sep 1;5(5):831–40. [DOI] [PubMed] [Google Scholar]
- 6.Cheever AW, Macedonia JG, Mosimann JE, Cheever EA. Kinetics of egg production and egg excretion by Schistosoma mansoni and S. japonicum in mice infected with a single pair of worms. Am J Trop Med Hyg. 1994. Mar;50(3):281–95. [DOI] [PubMed] [Google Scholar]
- 7.Fu CL, Odegaard JI, Herbert DR, Hsieh MH. A Novel Mouse Model of Schistosoma haematobium Egg-Induced Immunopathology. PLOS Pathogens. 2012. Mar 29;8(3):e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz C, Fallon PG. Schistosoma “Eggs-Iting” the Host: Granuloma Formation and Egg Excretion. Frontiers in Immunology. 2018;9:2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hams E, Aviello G, Fallon PG. The Schistosoma Granuloma: Friend or Foe? Front Immunol. 2013. Apr 15;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costain AH, MacDonald AS, Smits HH. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front Immunol. 2018. Dec 20;9:3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araújo MI, de Jesus AR, Bacellar O, Sabin E, Pearce E, Carvalho EM. Evidence of a T helper type 2 activation in human schistosomiasis. Eur J Immunol. 1996. Jun;26(6):1399–403. [DOI] [PubMed] [Google Scholar]
- 12.Williams ME, Montenegro S, Domingues AL, Wynn TA, Teixeira K, Mahanty S, et al. Leukocytes of patients with Schistosoma mansoni respond with a Th2 pattern of cytokine production to mitogen or egg antigens but with a Th0 pattern to worm antigens. J Infect Dis. 1994. Oct;170(4):946–54. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz C, Oeser K, Costa CP da, Layland LE, Voehringer D. T Cell–Derived IL-4/IL-13 Protects Mice against Fatal Schistosoma mansoni Infection Independently of Basophils. The Journal of Immunology. 2014. Oct 1;193(7):3590–9. [DOI] [PubMed] [Google Scholar]
- 14.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie ANJ. Schistosome Infection of Transgenic Mice Defines Distinct and Contrasting Pathogenic Roles for IL-4 and IL-13: IL-13 Is a Profibrotic Agent. The Journal of Immunology. 2000. Mar 1;164(5):2585–91. [DOI] [PubMed] [Google Scholar]
- 15.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999. Jan 15;162(2):920–30. [PubMed] [Google Scholar]
- 16.Fallon PG, Smith P, Richardson EJ, Jones FJ, Faulkner HC, Van Snick J, et al. Expression of Interleukin-9 Leads to Th2 Cytokine-Dominated Responses and Fatal Enteropathy in Mice with Chronic Schistosoma mansoni Infections. Infect Immun. 2000. Oct;68(10):6005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farah IO, Nyindo M. Acute schistosomiasis mansoni in the baboon Papio anubis gives rise to goblet-cell hyperplasia and villus atrophy that are modulated by an irradiated cercarial vaccine. Parasitology Research. 1997. Mar 5;83(3):281–4. [DOI] [PubMed] [Google Scholar]
- 18.Marillier RG, Michels C, Smith EM, Fick LC, Leeto M, Dewals B, et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svetić A, Madden KB, Zhou XD, Lu P, Katona IM, Finkelman FD, et al. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. The Journal of Immunology. 1993. Apr 15;150(8):3434–41. [PubMed] [Google Scholar]
- 20.Urban JF, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci U S A. 1991. Jul 1;88(13):5513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller HRP, Nawa Y. Nippostrongylus brasiliensis: Intestinal goblet-cell response in adoptively immunized rats. Experimental Parasitology. 1979. Feb 1;47(1):81–90. [DOI] [PubMed] [Google Scholar]
- 22.Cywińska A, Czumińska K, Schollenberger A. Granulomatous inflammation during Heligmosomoides polygyrus primary infections in FVB mice. J Helminthol. 2004. Mar;78(1):17–24. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa N, Horii Y, Oinuma T, Suganuma T, Nawa Y. Goblet cell mucins as the selective barrier for the intestinal helminths: T-cell-independent alteration of goblet cell mucins by immunologically “damaged” Nippostrongylus brasiliensis worms and its significance on the challenge infection with homologous and heterologous parasites. Immunology. 1994. Mar;81(3):480–6. [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JE, Stockinger B, Helmby H. IL-22 Mediates Goblet Cell Hyperplasia and Worm Expulsion in Intestinal Helminth Infection. PLoS Pathog. 2013. Oct 10;9(10):e1003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016. Mar 18;351(6279):1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature. 2016. Jan 14;529(7585):221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerbe F, Jay P. Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol. 2016. Nov;9(6):1353–9. [DOI] [PubMed] [Google Scholar]
- 28.Schneider C, O’Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol. 2019. Sep;19(9):584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGinty JW, Ting HA, Billipp TE, Nadjsombati MS, Khan DM, Barrett NA, et al. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity. Immunity. 2020. Mar;52(3):528–541.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyesola OO, Shanahan MT, Kanke M, Mooney BM, Webb LM, Smita S, et al. PGD2 and CRTH2 counteract Type 2 cytokine–elicited intestinal epithelial responses during helminth infection. J Exp Med. 2021. Jul 20;218(9):e20202178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL, et al. Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science. 2018. Apr 13;360(6385):204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schütz B, Ruppert AL, Strobel O, Lazarus M, Urade Y, Büchler MW, et al. Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep. 2019. Dec;9(1):17466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Song W, Wang J, Wang T, Xiong X, Qi Z, et al. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. Journal of Experimental Medicine. 2019. Nov 21;217(2):e20191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee A, Herring CA, Chen B, Kim H, Simmons AJ, Southard-Smith AN, et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology. 2020. Dec;159(6):2101–2115.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinley ET, Sui Y, Al-Kofahi Y, Millis BA, Tyska MJ, Roland JT, et al. Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight. 2(11):e93487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen TSR, Mahmood B, Damm MB, Backe MB, Dahllöf MS, Poulsen SS, et al. Combined activity of COX-1 and COX-2 is increased in non-neoplastic colonic mucosa from colorectal neoplasia patients. BMC Gastroenterol. 2018. Feb 27;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howitt MR, Cao YG, Gologorsky MB, Li JA, Haber AL, Biton M, et al. The Taste Receptor TAS1R3 Regulates Small Intestinal Tuft Cell Homeostasis. IH. 2020. Jan 1;4(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018. Aug;16(8):457–70. [DOI] [PubMed] [Google Scholar]
- 39.Zuk M, McKean KA. Sex differences in parasite infections: Patterns and processes. International Journal for Parasitology. 1996. Oct 1;26(10):1009–24. [PubMed] [Google Scholar]
- 40.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience & Biobehavioral Reviews. 2000. Aug 1;24(6):627–38. [DOI] [PubMed] [Google Scholar]
- 41.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology. 2004;26(6–7):247–64. [DOI] [PubMed] [Google Scholar]
- 42.Degu G, Mengistu G, Jones J. Some factors affecting prevalence of and immune responses to Schistosoma mansoni in schoolchildren in Gorgora, northwest Ethiopia. Ethiop Med J. 2002. Oct;40(4):345–52. [PubMed] [Google Scholar]
- 43.Marguerite M, Gallissot MC, Diagne M, Moreau C, Diakkhate MM, Roberts M, et al. Cellular immune responses of a Senegalese community recently exposed to Schistosoma mansoni: correlations of infection level with age and inflammatory cytokine production by soluble egg antigen-specific cells. Tropical Medicine & International Health. 1999;4(8):530–43. [DOI] [PubMed] [Google Scholar]
- 44.Remoué F, To Van D, Schacht AM, Picquet M, Garraud O, Vercruysse J, et al. Gender-dependent specific immune response during chronic human Schistosomiasis haematobia. Clin Exp Immunol. 2001. Apr;124(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity. 2018. Jul;49(1):33–41.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sertorio M, Hou X, Carmo RF, Dessein H, Cabantous S, Abdelwahed M, et al. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61(4):1321–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Goblet cell mucin aggregate area separated by individual patients. 100 goblet cells were analyzed per patient and plotted as μm2 on a log scale. Violin plots show the median (solid black line) and quartiles (dashed lines) aggregate mucin area.
Fig. S2. Second independent analysis of human colonic goblet cell hyperplasia associated with chronic schistosomiasis (A) Average colonic goblet cell mucin aggregate area per patient in uninfected and schistosome-infected patients. Points represent individual patients. (B) Mucin aggregate area per goblet cell plotted as μm2 on a log scale. Violin plots show the aggregate mucin area; median (solid black line) and quartiles (dashed lines). (C) Fold difference of mucin aggregate area per goblet cell. (D) Mucin aggregate area per goblet cell separated by sex. (E) Fold difference of mucin aggregate area per goblet cell separated by sex. (F) Goblet cell mucin aggregate area separated by individual patients. Data are plotted as means with SEM.
**** p < 0.0001; NS, not significant; using Mann-Whitney tests.


