Abstract
Aims
Since the withdrawal of HeartWare (HVAD) from the global market, there is an ongoing discussion if and which patients require prophylactically exchange for a HeartMate 3 (HM3). Therefore, it is important to study outcome differences between HVAD and HM3 patients. Because centres differ in patient selection and standard of care, we performed a propensity score (PS)‐based study including centres that implanted both devices and aimed to identify which HVAD patients are at highest risk.
Methods and results
We performed an international multi‐centre study (n = 1021) including centres that implanted HVAD and HM3. PS‐matching was performed using clinical variables and the implanting centre. Survival and complications were compared. As a sensitivity analysis, PS‐adjusted Cox regression was performed. Landmark analysis with conditional survival >2 years was conducted to evaluate long‐term survival differences. To identify which HVAD patients may benefit from a HM3 upgrade, Cox regression using pre‐operative variables and their interaction with device type was performed. Survival was significantly better for HM3 patients (P < 0.01) in 458 matched patients, with a median follow‐up of 23 months. Within the matched cohort, HM3 patients had a median age of 58 years, and 83% were male, 80% of the HVAD patients were male, with a median age of 59 years. PS‐adjusted Cox regression confirmed a significantly better survival for HM3 patients when compared with HVAD, with a HR of 1.46 (95% confidence interval 1.14–1.85, P < 0.01). Pump thrombosis (P < 0.01) and ischaemic stroke (P < 0.01) occurred less in HM3 patients. No difference was found for haemorrhagic stroke, right heart failure, driveline infection, and major bleeding. Landmark‐analysis confirmed a significant difference in conditional survival >2 years after implantation (P = 0.03). None of the pre‐operative variable interactions in the Cox regression were significant.
Conclusions
HM3 patients have a significantly better survival and a lower incidence of ischaemic strokes and pump thrombosis than HVAD patients. This survival difference persisted after 2 years of implantation. Additional research using post‐operative variables is warranted to identify which HVAD patients need an upgrade to HM3 or expedited transplantation.
Keywords: Left ventricular assist device, LVAD, End‐stage heart failure, Mechanical circulatory support, Centrifugal continuous flow pump
Introduction
Since Medtronic's announcement in June 2021 on withdrawing HeartWare (HVAD, Medtronic, Minneapolis, MN, USA) from the global market, HVADs are not implanted anymore. 1 Approximately 4000 patients worldwide are still on HVAD support, of which many patients are on destination therapy or on a long waiting list for heart transplantation. It remains unclear which HVAD patients are at highest risk and elective device exchange is considered by some.
It is therefore important to study differences in outcomes between patients on HM3 and HVAD support. No randomized controlled trial has been performed comparing long‐term outcomes of HM3 and HVAD. Both third generation devices were only compared with its predecessor the axial flow pump HMII. 4 , 5 , 6 Instead, retrospective comparisons were conducted. Several single‐centre studies were performed comparing HM3 and HVAD. 7 , 8 , 9 , 10 , 11 Different outcomes were considered, but all studies showed a worse complication profile for HVAD. None of those studies showed a significant difference in survival for HM3 and HVAD. This could be caused by the low number of patients included, for which registry‐based studies with larger patient numbers could be the solution. Three registry‐based studies were performed, which showed a higher incidence of pump thrombosis and device malfunction in HVAD patients 12 and fewer neurological events for patients on HM3 support. 12 , 13 Potapov et al. found a non‐significant difference in survival, whereas Pagani et al. showed a significant better 1 year survival for patients on HM3 support. 12 , 14
Those general registry‐based studies included a higher number of patients, but were limited by several factors such as missing data. More importantly, registry‐based studies usually include all centres, including centres that only implant one of the two devices. These centres may have a different standard‐of‐care and patient selection. Therefore, we propose a multi‐centre propensity score based approach to compare survival in patients on HVAD and HM3 support. Moreover, we aimed to identify which HVAD patients are at greatest device‐specific risk and HM3 upgrade or priority status on the waiting list for heart transplantation may be desired.
Methods
This investigator‐initiated study was designed as a multi‐centre retrospective study to compare outcome of patients on HVAD and HM3 support. The study was approved by the local ethics committee of the University Medical Centre Utrecht (UMCU), the Netherlands (METC: 20‐195). The study was conducted in accordance with Good Clinical Practice and the 2002 Declaration of Helsinki and in compliance with the ISHLT Ethics Statement. The need for informed consent was waived.
Inclusion criteria
All adult patients (n = 1021) that were primarily implanted with a (sintered) HVAD or HM3 LVAD in one of the participating centres up until December 2019 were included for analysis. Participating centres were as follows: Heart and Diabetes Centre North Rhine‐Westphalia (HDZ NRW, Bad Oeynhausen, Germany), Medical University of Vienna (MedUni Vienna, Vienna, Austria), University Medical Centre Utrecht (UMCU, Utrecht, the Netherlands) and Rabin Medical Centre (RMC, Petah Tikva, Israel). Only centres that implanted both devices were asked to participate in the current multi‐centre study, to eliminate the effect of the performance of the centre on the outcome. Patients that received an HVAD with non‐sintered inflow cannula were excluded from analysis, as this
was an important design change preventing tissue ingrowth encircling the external surface of the inflow cannula, which might result in emboli. 15 Baseline characteristics and outcome data were collected by each centre using the electronic health record and their databases.
Endpoints
The follow‐up of the study was until January 2021. The primary endpoint was death or urgent heart transplantation (HTx) during follow‐up. Urgent HTx was defined as a transplantation that was performed after a patient received a priority status on the waiting list. Patients were censored if receiving HTx after normal listing or for ongoing support at follow‐up. Secondary endpoints were complications that were registered locally according to the definitions of Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS): major bleeding, ischaemic stroke, haemorrhagic stroke, pump thrombosis, right heart failure, or driveline infections.
Statistical analysis
Baseline characteristics of all unmatched patients were presented as median and interquartile range (IQR) for continuous variables, and categorical variables were presented as number or percentage. The Mann–Whitney U test was used for continuous variables, and the Fisher's exact test was used for categorical variables. The percentage of missing data was displayed for all variables per centre.
The across method was used to create two comparable groups, combining multiple imputation and propensity score (PS) matching. At first, missing data of all variables necessary for PS‐matching were imputed using multiple imputation (R‐package: mice), resulting in five imputed datasets. 16 The PS was calculated for every patient within each dataset. Subsequently, the average PS was calculated for each patient, which was then used to perform 1:1 matching using the nearest neighbour method, with a calliper of 0.1 without replacement. The covariates that were used for PS‐matching are reported in Table 1 . Patients with pre‐operative temporary support were considered as being a separate group from INTERMACS 1 and were classified as ‘pre‐operative temporary support’. In addition to those clinical covariates, the implanting centre was used for PS‐matching as well. Because HVADs were available prior to the introduction of HM3, the number of months after the start of the first implantation (February 2012) in the study cohort was considered for PS‐matching, to ensure comparing survival of patients implanted within the same time period. Within the PS‐matched groups, differences between both groups were tested using the Wilcoxon signed‐rank test or McNemar test. The standardized mean difference (SMD) was calculated to assess the balance after matching. We assumed having two comparable groups if the SMD was <10% for all covariates.
Table 1.
Baseline characteristics of all patients (before PS‐matching) categorized by device type and centre
| Covariate | HDZ NRW (n = 560) | MedUni Vienna (n = 218) | UMCU (n = 165) | RMC (n = 78) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| HM3 (n = 194) | HVAD (n = 366) | HM3 (n = 103) | HVAD (n = 115) | HM3 (n = 80) | HVAD (n = 85) | HM3 (n = 56) | HVAD (n = 22) | ||
| Sex (% male) | 88.1 | 82.2 | 89.3 | 81.7 | 65.0 | 64.7 | 85.7 | 81.8 | <0.01 |
| Age (years) | 60 [53–66] | 58 [50–63] | 64 [56–68] | 60 [52–66] | 56 [47–61] | 58.0 [49–62] | 61.0 [55–66] | 63 [58–69] | <0.01 |
| Ischaemic CMP (%) | 50.5 | 48.8 | 66.0 | 60.0 | 23.8 | 38.8 | 64.3 | 63.6 | <0.01 |
| BMI (kg/m2) | 25.7 [24–30] | 25.3 [23–28] | 27.2 [24–31] | 25.4 [23–29] | 24.7 [22–28] | 24.0 [22–27] | 26.1 [23–29] | 23.5 [21–26] | <0.01 |
| BSA (m2) | 2.0 [1.9–2.2] | 2.0 [1.8–2.1] | 2.0 [1.9–2.2] | 1.9 [1.8–2.1] | 2.0 [1.8–2.1] | 1.9 [1.8–2.1] | 1.9 [1.8–2.0] | 1.8 [1.6–1.9] | <0.01 |
| eGFR (ml/min/1.73 m2) | 53 [36–78] | 56 [35–84] | 51 [35–90] | 57 [38–90] | 61 [43–83] | 63 [47–90] | 65 [46–86] | 59 [39–90] | 0.07 |
| Bilirubin (μmol/L) | 22 [13–33] | 24 [15–38] | 13 [9–21] | 22 [12–39] | 19 [12–37] | 19 [12–30] | 15 [10–20] | 23 [12–30] | <0.01 |
| RV‐function (1 = poor, 2 = moderate, 3 = reasonable, 4 = good) | 3 [2–4] | 3 [2–4] | 4 [2–4] | 4 [2–4] | 3 [2–3] | 2 [2–3] | 3 [3–4] | 3 [2–3] | <0.01 |
| Diabetes (%) | 35.0 | 30.1 | 33.0 | 26.1 | 15.0 | 11.8 | 55.4 | 50.0 | <0.01 |
| Stroke (%) | 11.7 | 14.4 | 7.8 | 9.6 | 3.8 | 8.2 | 8.9 | 13.6 | 0.14 |
| Concomitant surgery (%) | 34.0 | 30.6 | 23.3 | 16.5 | 23.8 | 29.4 | 5.4 | 9.1 | <0.01 |
| Pre‐operative support (%) | 18.0 | 36.9 | 9.7 | 31.3 | 12.5 | 27.1 | 5.4 | 9.1 | <0.01 |
| INTERMACS 1 (%) | 4.6 | 9.3 | 10.7 | 7.8 | 6.2 | 4.7 | 3.6 | 0.0 | 0.24 |
| INTERMACS 2 (%) | 36.6 | 27.6 | 21.4 | 15.7 | 32.5 | 30.6 | 10.1 | 22.7 | <0.01 |
| INTERMACS 3–7 (%) | 40.7 | 26.2 | 58.3 | 45.2 | 48.8 | 37.6 | 80.4 | 68.2 | <0.01 |
| Number of months | 75 [63–78] | 42 [29–58] | 69 [51–83] | 38 [18–61] | 75 [61–85] | 62 [43–79] | 73 [61–82] | 32 [21–46] | <0.01 |
Abbreviations: BMI, body mass index; BSA, body surface area; CMP, cardiomyopathy; eGFR, estimated glomerular filtration rate; HDZ NRW, Heart and Diabetes Centre North Rhine‐Westphalia; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; MedUni Vienna, Medical University of Vienna; PS, propensity score; RMC, Rabin Medical Centre; RV, right ventricle; UMCU, University Medical Centre Utrecht.
The primary endpoint in both unmatched and matched patient groups was assessed using Kaplan–Meier analysis with 95% confidence intervals (CI), censoring for ongoing support at the end of the follow‐up, left ventricular assist device (LVAD) explantation and non‐urgent heart transplantation. Log‐rank testing was performed to assess difference in survival between HVAD and HM3 patients. In addition, competing risk analysis was conducted to evaluate differences in the cumulative incidences of all secondary outcomes: major bleeding, ischaemic and haemorrhagic stroke, pump thrombosis, right heart failure, and driveline infection.
In addition to the primary analysis, two sensitivity analyses were performed. PS‐adjusted Cox regression was performed using the average PS in all imputed datasets. The second sensitivity analysis that was performed was similar to the primary analysis. However, now patients were also censored for urgent heart transplantation, since this was usually performed in previous literature.
To identify in which HVAD patients an HM3 upgrade should be considered, we first performed a landmark analysis to check if the survival difference remains after 2 years after implantation. We included only patients that survived for more than 2 years for PS‐matching (with a similar approach as the primary analysis). Lastly, to assess which HVAD patients are more at risk for the primary outcome (death and urgent HTx) when compared with HM3 patients, we performed a Cox regression (complete case analysis) with the pre‐operative variables age at primary implantation, sex, body surface area (BSA), body mass index (BMI), diabetes, and stroke, in addition to device type and the interaction with the variables and device type. Because the proportional hazard assumption for sex was not met, we used a step function for the regression coefficient of sex, that is, a time‐dependent coefficient, using three segments (<1 year, >1 and <2 years, and >2 years after primary implantation).
A P value <0.05 was considered statistically significant. All statistical analyses were performed using R software version 4.0.3.
Results
Between 2011 and December 2019, 1021 adult patients were primarily implanted with HM3 (n = 433) or sintered inflow cannula HVAD (n = 588) in all four participating centres. Baseline characteristics of all patients are presented in Table 1 , categorized per centre and device type. Within all centres, fewer females were implanted with HM3, and patients on HVAD support had a lower BSA and BMI. In addition, age at implantation, the number of patients with ischaemic cardiomyopathy, concomitant surgery, bilirubin, diabetes, and the number of months after the first implantation differed significantly within each centre. The percentage of patients on temporary support before LVAD implantation differed across centres, but overall temporal support was more common in HVAD patients. Furthermore, patients on HVAD support more often had a stroke in their medical history. Table S2 shows the percentage of missing data for each covariate per centre. A minimum of 14 out of 16 covariates per patient were available.
Matching substantially reduced imbalance, as the SMD was smaller than 10% for all covariates (Figure 1 ). After PS‐matching, 458 matched patients were included for analysis (Table 2 ). Patients were followed‐up for a median of 23 months (IQR: 30 months). Both before and after PS‐matching, survival was significantly better for HM3, with P < 0.01 (Figure 2 ). The cumulative incidences of important complications were compared in PS‐matched patients (Figure 3 ). Ischaemic stroke (P < 0.01) and pump thrombosis (P < 0.01) more frequently occurred in patients on HVAD support. The incidences for major bleeding (P = 0.49), haemorrhagic stroke (P = 0.11), right heart failure (P = 0.92), and driveline infections (P = 0.96) did not differ significantly between HM3 and HVAD.
Figure 1.
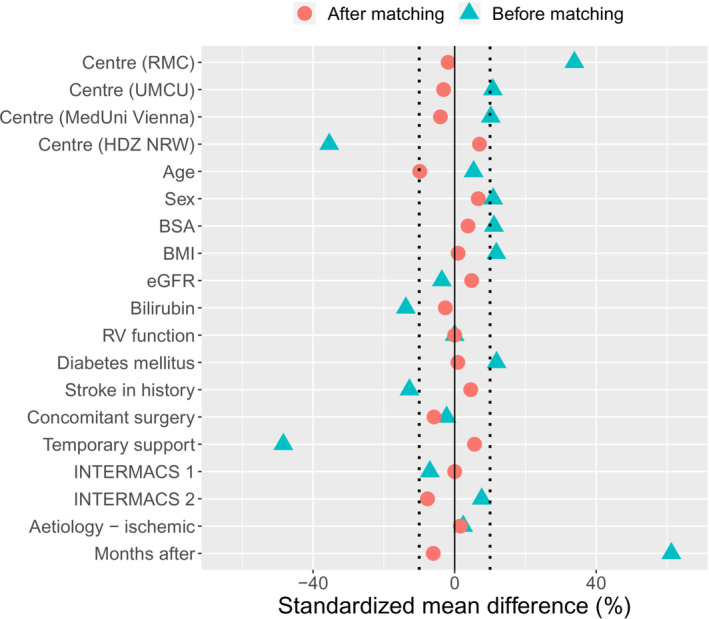
Love plot showing the standardized mean difference (SMD) before and after PS‐matching. BMI, body mass index; BSA, body surface area; eGFR, estimated glomerular filtration rate; HDZ NRW, Heart and Diabetes Centre North Rhine‐Westphalia; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; MedUni Vienna, Medical University of Vienna; PS, propensity score; RMC, Rabin Medical Centre; RV, right ventricular; UMCU, University Medical Centre Utrecht.
Table 2.
Baseline characteristics before and after PS‐matching
| Covariate | Unmatched patients | P value | PS‐matched patients | P value | |||
|---|---|---|---|---|---|---|---|
| HM3 (n = 433) | HVAD (n = 588) | HM3 (n = 229) | HVAD (n = 229) | ||||
| Centre (%) | HDZ NRW | 44.8 | 62.2 | <0.01 | 51.1 | 47.6 | 0.90 |
| MedUni Vienna | 23.8 | 19.6 | 22.3 | 24.0 | |||
| UMCU | 18.5 | 14.5 | 21.8 | 23.1 | |||
| RMC | 12.9 | 3.7 | 22.3 | 24.0 | |||
| Sex (% male) | 83.8 | 79.9 | 0.10 | 82.5 | 79.9 | 0.55 | |
| Age (years) | 60 [53–66] | 58 [50–64] | <0.01 | 58 [51–65] | 59 [52–64] | 0.28 | |
| Ischaemic CMP (%) | 51.0 | 49.8 | 0.75 | 51.5 | 50.7 | 0.93 | |
| BMI (kg/m2) | 25.7 [23–29] | 25.0 [23–28] | <0.01 | 25.5 [23–29] | 25.6 [23–29] | 0.91 | |
| BSA (m2) | 2.0 [1.8–2.2] | 2.0 [1.8–2.1] | <0.01 | 2.0 [1.8–2.1] | 2.0 [1.8–2.1] | 0.36 | |
| eGFR (mL/min/1.73 m2) | 56 [39–83] | 57 [37–90] | 0.71 | 59 [39–88] | 58 [39–87] | 0.66 | |
| Bilirubin (μmol/L) | 18 [11–30] | 23 [14–37] | <0.01 | 20 [12–34] | 21 [12–32] | 0.74 | |
| RV function (1 = poor, 2 = moderate, 3 = reasonable, 4 = good) | 3 [2–4] | 3 [2–4] | 0.03 | 3 [2–4] | 3 [2–4] | 0.32 | |
| Diabetes (%) | 33.4 | 27.4 | 0.05 | 29.6 | 28.9 | 0.95 | |
| Stroke (%) | 8.8 | 12.5 | 0.08 | 11.6 | 10.2 | 0.73 | |
| Concomitant surgery (%) | 25.9 | 26.9 | 0.77 | 26.2 | 28.8 | 0.60 | |
| Pre‐operative support (%) | 13.4 | 33.3 | <0.01 | 20.1 | 17.9 | 0.63 | |
| INTERMACS 1 (%) | 6.2 | 8.2 | 0.30 | 7.9 | 7.9 | 1.00 | |
| INTERMACS 2 (%) | 28.9 | 25.5 | 0.26 | 27.9 | 31.4 | 0.47 | |
| INTERMACS 3–7 (%) | 51.5 | 33.2 | <0.01 | 44.1 | 42.8 | 0.85 | |
| Number of months | 74 [60–85] | 43 [29–62] | <0.01 | 64 [53–79] | 67 [51–80] | 0.73 | |
Abbreviations: BMI, body mass index; BSA, body surface area; CMP, cardiomyopathy; eGFR, estimated glomerular filtration rate; HDZ NRW, Heart and Diabetes Centre North Rhine‐Westphalia; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; MedUni Vienna, Medical University of Vienna; PS, propensity score; RMC, Rabin Medical Centre; RV, right ventricle; UMCU, University Medical Centre Utrecht.
Figure 2.
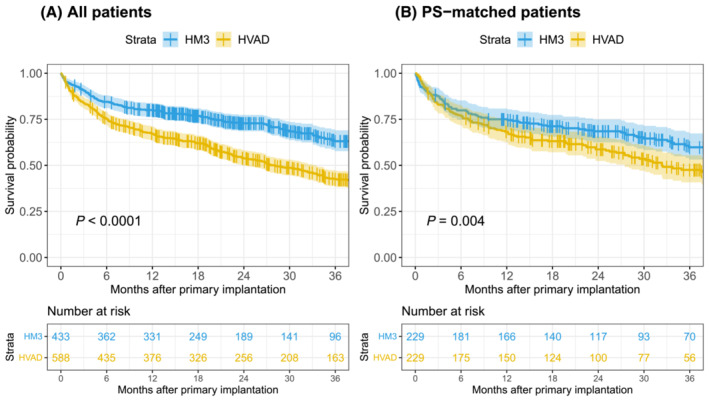
Kaplan–Meier survival of (A) All patients and (B) propensity score matched patients, censoring for non‐urgent transplantation and ongoing support at follow‐up.
Figure 3.
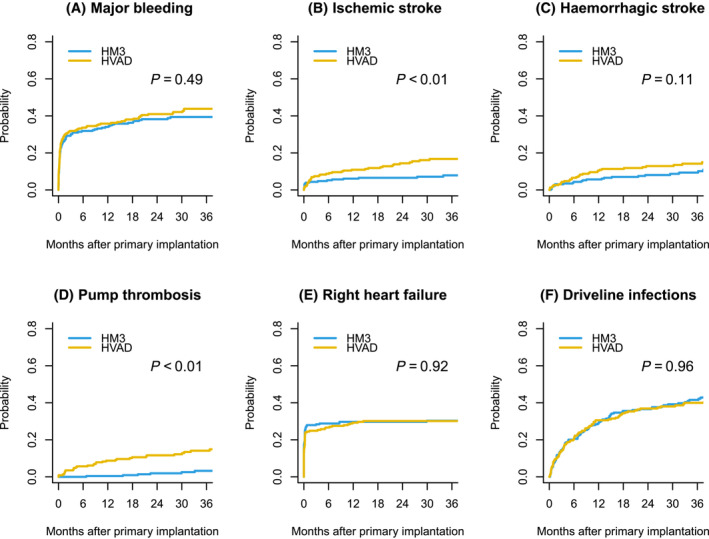
Cumulative incidence of propensity‐score matched patients of complications: (A) Major Bleeding. (B) Ischaemic stroke. (C) Haemorrhagic stroke. (D) Pump thrombosis. (E) Right heart failure. (F) Driveline infections. Death, heart transplantation and ongoing support were considered as a competing risk.
In total, in two patients, the LVAD was explanted after a median of 6 months, and 69 patients received a heart transplantation after a median of 18 months (IQR: 18 months). Table 3 shows the number of patients with urgent and non‐urgent HTx, the number of deaths and the number of LVAD explants. Patients on HVAD support more often received an urgent HTx, and a higher percentage of HVAD patients died during follow‐up.
Table 3.
Number of patients that were explanted, (non‐urgent) HTx and causes of death in PS‐matched groups
| PS‐matched patients (n = 458) | |||
|---|---|---|---|
| HVAD (n = 229) | HM3 (n = 229) | ||
| Explant n (%) | 1 (0.4) | 1 (0.4) | |
| HTx n (%) | Non‐urgent | 18 (7.9) | 20 (8.7) |
| Urgent | 18 (7.9) | 13 (5.7) | |
| Death n (%) | 111 (48.5) | 82 (35.8) | |
| Cause of death n (%) | Device malfunction | 0 (0.0) | 1 (0.4) |
| Infection | 9 (3.9) | 6 (2.6) | |
| Stroke | 21 (9.2) | 13 (5.7) | |
| Multi‐organ failure | 49 (19.3) | 43 (18.8) | |
| RV failure | 2 (1.7) | 1 (0.9) | |
| Other | 17 (7.4) | 14 (6.1) | |
| Unknown | 10 (4.3) | 3 (1.3) | |
Abbreviations: HTx; heart transplantation, RV; right ventricle, PS; propensity score.
The PS‐adjusted Cox regression also showed a significantly better survival for HM3 when compared with HVAD, with a hazard ratio (HR) of 1.46 (95% CI 1.14–1.85) with P < 0.01. The sensitivity analysis where patients were censored for urgent heart transplantation confirmed a significant worse survival for patients on HVAD support, with P < 0.01.
The landmark analysis confirmed a significant better conditional survival in patients that were on HM3 support for more than 2 years (P = 0.03). Lastly, we analysed which variables are predictive for a worse survival for HVAD patients compared with patients on HM3 support for a subset of variables. Table S2 reports the HRs and P values of all used variables and interactions. None of the interactions were significant.
Discussion
In this multi‐centre retrospective study, we compared survival and incidence of major complications in patients on HM3 and HVAD support (graphical abstract). After PS‐matching, patients on HM3 support showed a significantly better survival, with a lower incidence of ischaemic strokes, and pump thrombosis, whereas major bleeding, haemorrhagic stroke, right heart failure, and driveline infections did not significantly differ. Also, in patients who survived for more than 2 years, survival was significantly better in HM3 patients. We were not able to identify which HVAD patients are more at risk compared with HM3 patients using a subset of pre‐operative variables.
So far, four single‐centre retrospective studies and three registry‐based studies compared the outcome of patients on HM3 and HVAD support. 7 , 8 , 9 , 10 , 12 , 13 Different methods were used, with PS‐matching being the most used method. In addition to survival, different complication types were compared in HM3 and HVAD patients. Potapov et al. studied the combined incidence of pump thrombosis and outflow graft twisting, which did not differ significantly between both groups. 12 In line with the current study, two studies found a significantly higher incidence of pump thrombosis for patients on HVAD support, whereas Schramm et al. showed no significant difference between both groups. 7 , 9 , 10 Two out of five studies reported a significant higher incidence of stroke for patients on HVAD support. 7 , 8 , 10 , 12 , 13 Two single‐centre studies and a registry‐based study observed a significantly higher incidence of haemorrhagic stroke in HVAD patients, 8 , 9 , 12 but not of ischaemic stroke in contrast to our study. 8 , 9 Furthermore, we found fewer thrombotic events in HM3 patients, potentially due to the different pump designs. Both devices are third generation devices with a continuous and centrifugal blood flow pattern, but the HM3 has a fully magnetically levitated rotor, a relatively wide rotor housing, and an artificial pulse. 17
We demonstrated no difference in right heart failure and major bleeding incidences, which is in line with previous research. 9 , 12 Driveline infections were diagnosed significantly more often in patients on HVAD support in the study of Schramm et al., whereas Potapov et al. found a trend towards a higher incidence of infections for HM3 patients. 7 , 8 These variations across single‐centres emphasize the importance of considering the implanting centre as covariate when combining patient outcomes because these differences may be caused by centre specific effects in addition to a dissimilar patient population. Although differences in complications existed, all retrospective studies confirmed a less favourable complication profile for patients on HVAD support compared with HM3. 7 , 8 , 9 , 10 , 12 , 13
Itzhaki Ben Zadok et al. observed a better survival free from stroke or device change in HM3 supported patients. 10 In addition, Pagani et al. observed a significant better 1 year survival in patients on HM3 support when compared with patients on HVAD support. 14 Although in several studies insignificant, the Kaplan–Meier survival curves of HVAD were less favourable for HVAD patients in all studies. An important difference in the current and previous studies is that next to death, we also considered urgent heart transplantation as the primary endpoint. Without the urgent heart transplantation, the patient would probably not have survived and we therefore consider this as an adverse outcome in addition to death. Because in literature patients that receive heart transplantation, including patients that were listed as highly urgent, are usually censored, we performed a sensitivity analysis. This confirmed our primary results.
In comparison with previous studies, this study is unique in also considering the implanting centre as a covariate for PS‐matching. We showed that patients on HM3 and HVAD support differed significantly on several covariates across the four included centres. Besides different patient characteristics, probably due to a different patient selection, it is likely that there are centre specific effects that affect the results, as all centres have a different standard‐of‐care. For example, anti‐thrombotic and anti‐coagulation protocols may differ among centres. Moreover, the hospital and surgeon volume may differ. Cowger et al. demonstrated that higher volume LVAD centres have better survival rates. 18 In addition, the number of implantations per surgeon was correlated with reduced mortality. 19 In addition to measurable and known differences between centres, it is likely that there are other unknown covariates related to the implanting centre. To reduce this centre bias as much as possible, we used centre as a covariate to match on.
Clinicians now face the question if HVAD patients may benefit from a HM3 exchange. Cogswell et al. studied patients undergoing HVAD to HM3 exchange and HVAD to HVAD exchange. 3 The results need to be interpreted carefully, as the subgroups undergoing HVAD to HM3 exchange for a specific cause were generally sicker than patients who would be undergoing an elective device exchange. Even though device exchange may lead to better clinical outcomes on a population level, the replacement surgery entails peri‐operative risks such as bleeding, right heart failure, or stroke. 2 The benefit of the device exchange should outweigh those initial post‐operative risks. Both the current study and previous research showed a worse patient profile for patients before HVAD implantation. To determine which HVAD patients are more at risk, we performed a Cox regression with a subset of pre‐operative variables and its interaction with device type. None of the interaction terms were significant. Therefore, we conclude that the selection of patients who will benefit from a HM3 upgrade, cannot be made based on solely pre‐operative variables. Post‐operative information should be included to identify HVAD patients at high but reducible risk.
Our study has several limitations. First, we have some missing data, but a minimum of 14 out of 16 covariates were complete. Multiple imputation was used to be able to include all patients for matching. Moreover, almost half of the patients were implanted in one of the four centres, which may affect generalizability. However, we do not expect this to heavily impact generalizability of the study results, as the difference in primary outcome between HM3 and HVAD was consistent across the four centres and the centre effect was minimized by PS‐matching.
Another limitation of the study is the observational nature of the study. Even though imbalance was drastically reduced due to PS‐matching, the risk of unknown or unmeasured covariates that are more imbalanced after matching cannot be excluded. 20 We minimized centre‐specific effects by including the centre as a covariate for PS‐matching. Sensitivity analysis was performed using PS‐adjusted Cox regression, which showed a significant better survival for HM3, confirming our primary analysis. The current study was not designed to advise about which patients on HVAD support need elective device exchange, but can inform HVAD patients and their treating physicians in their shared‐decision making. Ongoing research is still warranted to identify those at highest risk for complications or death within the HVAD group. Although randomized controlled trials are needed to confirm retrospective studies, registries have shown to be valuable resources for outcome research. With further enriched registries, a continuous learning healthcare system can be developed, allowing early detection of a possible difference in device performance. The current proposed method provides guidance for the design of future registries and research (graphical abstract), to enable comparison of outcome in short cycles or even real time.
We demonstrated that in a multi‐centre PS‐matched cohort, patients on HM3 support have a significantly better survival than patients on HVAD support. In addition, a significantly higher incidence of ischaemic strokes and pump thrombosis was found in patients on HVAD support. Future research is warranted to pinpoint which HVAD patients are at highest risk. The current proposed method provides guidance for the design of future registries and research, to enable comparison of outcome in short cycles or even real time.
Conflict of interest
L. N., O. I. B. Z, E. A., M. M, S. P. W. G., J. R., F. R., A.‐M. O., T. B.‐G., B. B.‐A., F. W. A., and R. S. have no conflict of interest. The UMCU, which employs L. W. L. received consultancy fees from Medtronic, Abbott, Vifor, and Novartis, outside the submitted work. MedUni Vienna, which employs D. Z. reports grants, personal fees, and non‐financial support from Medtronic and Abbott, and D. W. reports personal fees from Abbott and Xenios/Fresenius, outside the submitted work.
Funding
The collaboration project is co‐funded by the PPP Allowance made available by Health‐Holland, Top Sector Life Sciences & Health, to stimulate public–private partnerships (LVAD–LVAD, LSHM19035).
Supporting information
Table S1. Percentage of missing data categorized by device type.
Table S2. Hazard ratios of the static pre‐operative variables and its interaction with device type, with a time dependent coefficient for sex to fulfil the PH‐assumption.
Numan, L. , Zimpfer, D. , Zadok, O. I. B. , Aarts, E. , Morshuis, M. , Guenther, S. P. W. , Riebandt, J. , Wiedemann, D. , Ramjankhan, F. Z. , Oppelaar, A.‐M. , Ben‐Gal, T. , Ben‐Avraham, B. , Asselbergs, F. W. , Schramm, R. , and Van Laake, L. W. (2023) Identifying patients at risk: multi‐centre comparison of HeartMate 3 and HeartWare left ventricular assist devices. ESC Heart Failure, 10: 1656–1665. 10.1002/ehf2.14308.
Rene Schramm and Linda W. Van Laake, shared last authorship. University Medical Centre Utrecht, Utrecht University.
References
- 1. Medtronic . Left ventricular assist device (LVAD) for advanced heart failure still walking beside you [Internet]. 2021. [cited 2021 Nov 2]. Available from: https://www.medtronic.com/us‐en/patients/treatments‐therapies/ventricular‐assist‐device.html [Accessed on: 10th October 2021]
- 2. Austin MA, Maynes EJ, Gadda MN, O'malley TJ, Morris RJ, Mahek KS, Pirlamarla PR, Alvarez RJ, Entwistle JW, Massey HT, Tchantchaleishvilli A. Continuous‐flow LVAD exchange to a different pump model: systematic review and meta‐analysis of the outcomes. Artif Organs 2021; 45: 696–705. [DOI] [PubMed] [Google Scholar]
- 3. Cogswell R, Cantor RS, Vorovich E, Kilic A, Stehlik J, Cowger JA, Habib RH, Kirklin JK, Pagani FD, Atluri P. HVAD to HeartMate 3 device exchange: a Society of Thoracic Surgeons Intermacs analysis. Ann Thorac Surg 2021; 1–7: 1672–1678. [DOI] [PubMed] [Google Scholar]
- 4. Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel C, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanadam V, Sayer G, Cotts WG, Tatooles AJ, Babu A, Chromsky D, Katz JZ, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 5. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017; 376: 451–460. [DOI] [PubMed] [Google Scholar]
- 6. Carmelo AM, Rogers JG, Tatooles AJ, Bhat G, Slaugther MS, Birks EJ, Mokadam NA, Mahr C, Miller JS, Markham DW, Jeevanandam V, Uriel N, Aaronson KD, Vassiliades TA, Pagani FD. HVAD: the ENDURANCE supplemental trial. JACC Hear Fail 2018; 6: 792–802. [DOI] [PubMed] [Google Scholar]
- 7. Schramm R, Zittermann A, Morshuis M, Schoenbrodt M, Von Roessing E, Dossow V, Koster A, Fox H, Hakim‐Meibodi K, Gummert JF. Comparing short‐term outcome after implantation of the HeartWare® HVAD® and the Abbott® HeartMate 3®. ESC Hear Fail 2020; 7: 908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mueller M, Hoermandinger C, Richter G, Mulzer J, Tsyganenko D, Krabatsch T, Starck C, Stein J, Schoenrath F, Falk V, Potapov E. Retrospective 1‐year outcome follow‐up in 200 patients supported with HeartMate 3 and HeartWare left ventricular assist devices in a single centre. Eur J Cardiothorac Surg 2020; 57: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 9. Numan L, Ramjankhan FZ, Oberski DL, Oerlemans MIFJ, Aarts E, Gianoli M, Van Der Heijden JJ, De Jonge N, Van Der Kaaij NP, Meuwese CL, Mokhles MM, Oppelaar A, De Waal EEC, Asselbergs FW, Van Laake LW. Propensity score‐based analysis of long‐term outcome of patients on HeartWare and HeartMate 3 left ventricular assist device support. ESC Hear Fail 2021; 8: 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben Zadok OI, Ben‐Avraham B, Shaul A, Hammer Y, Rubachevski V, Aravot D, Kornowski R, Ben‐Gal T. An 18‐month comparison of clinical outcomes between continuous‐flow left ventricular assist devices. Eur J Cardiothorac Surg 2019; 56: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 11. Mihalj M, Heinisch PP, Schober P, Wieser M, Martinelli M, de By TM, Schefold JC, Luedi MM, Kadner A, Carrell T, Mohacsi P, Hunziker L, Reineke D. Third‐generation continuous‐flow left ventricular assist devices: a comparative outcome analysis by device type. ESC Hear Fail 2022; 9: 3469–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potapov EV, Nersesian G, Lewin D, Özbaran M, de By TMMH, Stein J, Pya Y, Gummert J, Ramjankhan F, Zembala MO, Damman K, Carrel T, Meyns B, Zimpfer D, Netuka I. Propensity score‐based analysis of long‐term follow‐up in patients supported with durable centrifugal left ventricular assist devices: the EUROMACS analysis. Eur J Cardio‐Thoracic Surg 2021; 00: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Cho S‐M, Mehaffey JH, Myers SL, Cantor RS, Starling RC, Kirklin JK, Jacobs JP, Kern J, Uchino K, Yarboro L. Cerebrovascular events in patients with centrifugal‐flow left ventricular assist devices: a propensity score matched analysis from the Intermacs registry. Circulation 2021; 144: 763–772. [DOI] [PubMed] [Google Scholar]
- 14. Pagani FD, Cantor R, Cowger J, Goldstein DJ, Teuteberg JJ, Mahr CW, Atluri P, Kilic A, Maozami N, Habib RH, Naftel D, Kirklin JK. Concordance of treatment effect: an analysis of the Society of Thoracic Surgeons Intermacs database. Ann Thorac Surg 2021; 4: 1–11. [DOI] [PubMed] [Google Scholar]
- 15. Soltani S, Kaufmann F, Vierecke J, Kretzschmar A, Hennig E, Stein J, Hetzer R, Krabatsch T, Potapov EV. Design changes in continuous‐flow left ventricular assist devices and life‐threatening pump malfunctions. Eur J Cardiothorac Surg 2014; 47: 984–989. [DOI] [PubMed] [Google Scholar]
- 16. van Buuren S, Groothuis‐Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 17. Bourque K, Cotter C, Dague C, Harjes D, Dur O, Duhamel J, Spink K, Walsh K, Burke E. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J 2016; 62: 375–383. [DOI] [PubMed] [Google Scholar]
- 18. Cowger JA, Stulak JM, Shah P, Dardas TF, Pagani FD, Dunlay SM, Maltais S, Aaronson KD, Singh R, Mokadam NA, Kirklin JK, Salerno CT. Impact of center left ventricular assist device volume on outcomes after implantation: an INTERMACS analysis. JACC Hear Fail 2017; 5: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis KF, Hohmann SF, Doukky R, Levine D, Johnson T. The impact of hospital and surgeon volume on in‐hospital mortality of ventricular assist device recipients. J Card Fail 2016; 22: 226–231. [DOI] [PubMed] [Google Scholar]
- 20. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, Nichols M, Stone GW, Pocock SJ. Comparison of propensity score methods and covariate adjustment. J Am Coll Cardiol 2017; 69: 345–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of missing data categorized by device type.
Table S2. Hazard ratios of the static pre‐operative variables and its interaction with device type, with a time dependent coefficient for sex to fulfil the PH‐assumption.


