Abstract
Aims
Transthyretin amyloid cardiomyopathy (ATTR CM) is a progressive and severe heart disease with physical and psychological implications. The Nordic PROACT study was conducted to investigate the health‐related quality of life (HRQoL) in ATTR CM patients.
Methods and results
The Nordic PROACT study was a cross‐sectional non‐interventional study conducted in 12 cardiology hospital clinics across Norway, Sweden, Finland and Denmark. Men and women aged ≥18 years diagnosed with symptomatic ATTR CM were included. The investigator provided information on medical history, biomarkers, current treatment, co‐morbidities and disease severity according to the New York Heart Association (NYHA) class and the National Amyloidosis Centre (NAC) staging. Patients completed the HRQoL questionnaires in the form of the Kansas City Cardiomyopathy Questionnaire (KCCQ), the EQ‐5D‐5L index with Visual Analog Scale (VAS), and the Major Depression Inventory (MDI). A total of 169 patients (mean ± SD age 77.7 ± 6.2 years) were included. Ninety‐two per cent were men. Seventy‐six per cent had wildtype ATTR CM (ATTRwt CM) and 15% had a hereditary form of ATTR CM (ATTRv CM) while 9% were genetically unclassified. Most patients were in NYHA class II (54%) and NAC stage 1 (53%). Participation in randomized clinical trials (RCT) was noted in 58% of the patients. The 169 ATTR CM patients had a mean ± SD KCCQ score of 64.3 ± 23.1 for total symptom score, 64.8 ± 20.9 for overall summary score (OSS) and 65.1 ± 21.5 for clinical summary score. The EQ‐5D‐5L total utility score was 0.8 ± 0.2 and the EQ‐5D‐5L VAS score was 62.9 ± 20.6. The vast majority (89%) did not report any signs of depression. Patients with ATTRv CM had a higher KCCQ OSS as compared with ATTRwt CM, while EQ‐5D‐5L utility score, EQ‐5D‐5L VAS and MDI were similar. Non‐RCT participants had a poorer HRQoL as compared with RCT participants as reflected in lower KCCQ OSS and EQ‐5D‐5L VAS scores and a higher MDI score. Patients with higher NYHA classes and NAC disease stages had a poorer HRQoL as demonstrated by lower KCCQ and EQ‐5D‐5L scores and higher MDI scores. Correlation between KCCQ, EQ‐5D‐5L and MDI and the covariate NYHA class remained significant (P < 0.05) after adjusting for multiple testing.
Conclusions
KCCQ scores were lower than previously reported for patients with other heart diseases of non‐ATTR CM origin. The HRQoL measures correlated well to NYHA class and NAC disease stage. The prevalence of depression appeared to be low.
Keywords: Amyloidosis, Cardiomyopathy, Transthyretin, ATTR CM, Observational study, Patient‐reported outcome measures, Quality of life
Introduction
Transthyretin amyloid cardiomyopathy (ATTR CM) is a progressive, debilitating and life‐threatening disease characterized by the accumulation of amyloid fibrils of misfolded transthyretin (TTR) protein in the heart. 1 , 2 , 3 The progressive accumulation leads to development of restrictive cardiomyopathy with clinical heart failure and arrhythmia. 4 , 5 ATTR CM is a systemic disease with carpal tunnel syndrome and spinal stenosis as typical non‐cardiac manifestations. 4 , 6 , 7
ATTR CM might develop due to a pathogenic mutation in the transthyretin gene, leading to the inherited form of ATTR CM (ATTRv CM), or associated with aging in the wild‐type form (ATTRwt CM) in which the specific disease cause is not known. ATTRwt CM, which is the most prevalent form and predominantly affects men above 60 years, was previously considered as a rare condition but is currently recognized as a prevalent cause of heart failure and arrythmia in the elderly population. 8 , 9 The prevalence of ATTRv CM varies across regions and many different mutations have been identified in the TTR gene. Within the Nordic countries, the Leu111Met is seen in Denmark whereas the Val30Met is prevalent in certain regions of Sweden. 10 , 11
ATTR CM severity can be evaluated according to New York Heart Association (NYHA) classes 12 and National Amyloidosis Centre (NAC) disease stages which both provide prognostic information. 13 The treatment of the disease includes symptomatic medical treatment, specific disease modifying medication, and—for those with ATTRv CM—possibly liver transplantation. 1 , 4
The physical symptoms of ATTR CM can have a significant impact on activities of daily life and affect the quality of life (QoL). The disease progressively reduces physical capacity, limits patients' ability to take part in social activities and has been associated with significant financial burdens. 2 , 14 Data for patient‐reported outcome measures (PROMs) are limited for patients with ATTR CM and data from the Nordic countries have not been previously reported. Available non‐Nordic reports have demonstrated impaired QoL as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), EQ‐5D/EQ‐5D‐3L, the 12‐Item Short Form Health Survey (SF‐12), and the Hospital Anxiety and Depression Scale (HADS). 2 , 14 , 15 , 16 , 17 , 18
Nordic PROACT (patient‐reported outcomes in transthyretin amyloid cardiomyopathy trial) explored for the first time the health‐related QoL (HRQoL) of contemporary patients from Norway, Sweden, Finland, and Denmark living with symptomatic ATTR CM. Furthermore, correlates were investigated between HRQoL as measured by KCCQ, EQ‐5D‐5L, and Major Depression Inventory (MDI), and factors like disease severity, diagnostic delay, and co‐morbidities.
Methods
Study design
The Nordic PROACT study was a one‐time cross‐sectional non‐interventional study conducted in 12 hospital cardiology outpatient clinics across Norway, Sweden, Finland, and Denmark between January 2021 and January 2022. The study consisted of two electronic questionnaires, one designed for the investigator, referred to as the eCaseReportForm (eCRF), and one designed for the patient, referred to as the ePatientReportedOutcomes (ePRO).
Inclusion and exclusion criteria
Eligible patients were men and women aged ≥18 years and diagnosed with symptomatic ATTR CM three or more months prior to study participation. Carriers of TTR mutations without symptoms of ATTR CM were not included in the study. Written informed consent was obtained from every participant prior to study inclusion.
Medical history
The patients' medical history was completed by the investigator by use of the eCRF and included time of diagnosis, age, gender, time of onset of symptoms, co‐morbidities, ATTR CM phenotype and genotype, current medical treatment, biomarkers, data for left ventricular ejection fraction (LVEF), and disease stage (NYHA class and NAC stage). Co‐morbidities were defined as those medically treated at time of inclusion independent of ATTR CM or non‐ATTR CM origin. Time since diagnosis reflects the period between final ATTR CM diagnosis and latest control visit/completion of the QoL assessment questionnaires of the Nordic PROACT study. Diagnostic delay was defined as time from first cardiological examination to the diagnostic date of biopsy and/or 99mTc‐DPD scintigraphy.
Disease severity assessments
Disease severity was assessed by the medical staff at the latest visit according to NYHA and NAC. NYHA categorizes patients in one of four classes based on their heart disease associated physical activity limitations, with higher classes reflecting more limitations. 12 NAC staging is based on N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) above or below 3000 ng/L and estimated glomerular filtration rate (eGFR) above or below 45 mL/min and is divided into different groups as follows: Stage 1: NT‐proBNP ≤3000 ng/L and eGFR ≥45 mL/min/1.73 m2; stage 3: NT‐proBNP >3000 ng/L and eGFR <45 mL/min/1.73 m2; and stage 2: all other level combinations). 13
Quality of life assessments
QoL assessment tools in Nordic PROACT included the three validated questionnaires: KCCQ, EQ‐5D‐5L (index and Visual Analog Scale (VAS), and MDI. All QoL assessments were completed by the patient by use of the ePRO tool.
KCCQ is a 23‐item patient‐completed questionnaire that assesses health status and HRQoL in patients with heart failure and is also validated for patients with heart failure with preserved ejection fraction (HFpEF). Items assess the ability to perform activities of daily living, frequency and severity of symptoms, the impact of these symptoms, and HRQoL. Scoring yields scores for 6 domains (physical limitation, symptom stability, symptoms, self‐efficacy, social limitation, and QoL), two summary scores (Functional Summary and Clinical Summary), as well as an Overall Summary score (OSS). Domain scores are transformed to a 0 to 100 range, which is often divided into 25‐point ranges as follows: 0 to 24: very poor to poor; 25 to 49: poor to fair; 50 to 74: fair to good; and 75 to 100: good to excellent. 19
EQ‐5D‐5L is a brief, self‐administered generic health status instrument consisting of two parts. In the first part respondents are asked to indicate their current health state on 5 modalities (mobility, self‐care, usual activities, pain or discomfort, and anxiety or depression) with each modality having 5 levels of function, ranging from no problem to extreme problem/unable to do the activity. This results in a 5‐digit number that combined with existing population weights for all EQ‐5D‐5L health states enables the calculation of a single index value (the utility score) between 0 and 1 for every patient, where 1 is equal to a situation of perfect health and 0 is equal to being dead. The second part is a subject's self‐rating of current health state on a VAS ranging from ‘best imaginable health state’ (score of 100) to ‘worst imaginable health state’ (score of 0). 20
MDI is a brief, 13‐item scale constructed to enable both an ICD‐10 and DSM‐IV diagnosis of clinical depression in addition to an estimate of symptom severity. For this study, rating scales were used to categorize patients as having no depression, mild depression, moderate depression, or severe depression. 21 , 22
Statistical analyses
Variables were summarized using descriptive statistics. Statistical tests and confidence intervals were performed and assessed from a 5%‐significance level (two‐sided). Subgroup analyses were conducted for gender (male/female), ATTR CM type (wildtype/hereditary) and randomized clinical trial (RCT) participation (+/− RCT participation). Multiple linear regression was used to analyse KCCQ, EQ‐5D‐5L and MDI according to gender, age, type of disease, years since diagnosis, disease severity, treatment, diagnostic delay and co‐morbidities. Wilcoxon and Kruskal–Wallis tests were used when the covariate had, respectively, two and more than two categories. Bonferroni's adjustment was used to take multiple testing into account. Pearson correlation coefficients analysis was conducted to assess any correlations between the various HRQoL measures included in the study. All data were analysed using SAS software (SAS statistical programme version 9.04).
Results
Patient disposition
A total of 169 patients were enrolled in the study, 70 patients from Denmark, 61 from Sweden, 19 from Norway, and 19 from Finland.
Patient characteristics
Of the 169 patients, 92% were men and 8% were women. Mean age was 78 years (77 for men and 83 for women). Three of four patients (76%) had ATTRwt CM, 15% had ATTRv CM and 9% were not genetically classified. In terms of disease severity assessment, 54% of patients were in NYHA class II and 35% were in NYHA class III, while 53% of patients were in NAC stage 1. Mean time since diagnosis was 3.1 years (Table 1 ).
TABLE 1.
Patient characteristics
| All patients (N = 169) | |
|---|---|
| Age (years) | 77.7 ± 6.2 |
| Male gender | 155 (91.7) |
| Type of ATTR CM | |
| Hereditary | 25 (14.8) |
| Wildtype | 129 (76.3) |
| ATTR CM type not determined | 15 (8.9) |
| Co‐morbidities a | |
| Any | 163 (96.4) |
| Atrial fibrillation | 116 (68.6) |
| Hypertension | 80 (47.3) |
| Ischaemic heart disease | 34 (20.1) |
| Gastrointestinal disease | 20 (11.8) |
| Spinal stenosis | 19 (11.2) |
| Diabetes | 17 (10.1) |
| Diagnostic delay b | 1.7 ± 3.3 |
| Time since ATTR CM diagnosis (years) | 3.1 ± 1.8 |
| <2 | 71 (42.0) |
| >2–4 | 72 (42.6) |
| >4–6 | 17 (10.1) |
| >6 | 8 (4.8) |
| Missing or not applicable/available | 1 (0.6) |
| NYHA stage | |
| I | 13 (7.7) |
| II | 92 (54.4) |
| III | 59 (34.9) |
| IV | 3 (1.8) |
| Missing or not applicable/available | 2 (1.2) |
| NAC stage | |
| 1 | 90 (53.3) |
| 2 | 43 (25.4) |
| 3 | 31 (18.3) |
| Missing or not applicable/available | 5 (3.0) |
| Current medical treatment for ATTR CM | |
| ATTR CM modifying treatment | 14 (8.3) |
| Symptomatic treatment c | 55 (32.5) |
| No ATTR CM treatment | 1 (0.6) |
| Participation in RCT | 98 (58.0) |
| Missing or not applicable/available | 1 (0.6) |
Data are presented as n (%) or mean ± SD.
RCT, randomized clinical trial; NAC, National Amyloidosis Centre; NYHA, New York Heart Association; 99mTc‐DPD, 99mTechnetium labelled 3,3‐diphosphono‐1,2‐propanodicarboxylic acid.
Co‐morbidities were defined as those medically treated at time of inclusion independent of ATTR CM or non‐ATTR CM origin.
Diagnostic delay is defined as time from first cardiological examination to the diagnostic date of biopsy and/or 99mTc‐DPD scintigraphy.
Patients were defined as receiving symptomatic treatment if they only received symptomatic treatment.
The 25 patients with ATTRv CM had the following mutations: Val30Met (n = 10), Val122Ile (n = 2), Thr60Ala (n = 1) and Leu111Met (n = 1). Mutation details were not provided for the remaining 9 ATTRv CM patients.
The patients' medical history was characterized by previous diagnoses given before the ATTR CM diagnosis that are typical cardiac and non‐cardiac red flags for ATTR CM. These included atrial fibrillation (53%), carpal tunnel syndrome (45%), HFpEF (25%) not identified as ATTR CM, and spinal stenosis (20%). Fifty‐four per cent also had a history of hypertension and 21% of ischaemic heart disease.
At time of inclusion, the vast majority (96%) had one or more co‐morbidities for which they received medical treatment, the most frequent being atrial fibrillation (69%). Eight per cent of all patients received ATTR CM modifying treatment and 58% of all patients were enrolled in an RCT while participating in the present non‐interventional study (Table 1 ). As no information was provided on the type of RCT or the specific medications involved, specific treatment data was not available for RCT participants. Among patients not enrolled in an RCT, 20% received disease‐modifying treatment.
Cardiovascular profiles as described by NT‐proBNP levels, eGFR and LVEF at the latest control visit are listed in Table 2 along with the patients' self‐reported current symptoms. Nearly 80% had a NT‐proBNP above 1000 ng/L and 55% had an eGFR below 60. Patients were distributed evenly between those with an LVEF above 50% and those with an LVEF below 50% while 24% had an LVEF below 40% (Table 2 ).
TABLE 2.
Cardiovascular measures and symptoms
| All patients (N = 169) | |
|---|---|
| NT‐proBNP (ng/L) a | |
| <1000 | 32 (20.4) |
| 1000–2999 | 61 (38.9) |
| 3000–5999 | 41 (26.1) |
| >6000 | 23 (14.6) |
| eGFR (mL/min/1.73 m2) b | |
| <45 | 45 (27.9) |
| 45–59 | 44 (27.3) |
| 60–89 | 69 (42.9) |
| > 90 | 3 (1.9) |
| LVEF (%) | |
| <40 | 40 (23.7) |
| 40–49 | 37 (21.9) |
| 50–60 | 50 (29.6) |
| >60 | 18 (10.7) |
| Missing or not applicable/available | 24 (14.2) |
| Current symptoms c | |
| Shortness of breath at physical activity/exercise | 125 (74.0) |
| Shortness of breath at daily physical activity | 91 (53.8) |
| Problems with balance | 74 (43.8) |
| Pronounced fatigue | 72 (42.6) |
| Swelling of lower legs and ankles (oedema) | 64 (37.9) |
| Physical weakness | 61 (36.1) |
| Feeling disorders in the hand and/or feet | 56 (33.1) |
| Dizziness/fainting | 45 (26.6) |
Data are presented as n (%).
eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐Terminal pro B‐type Natriuretic Peptide.
Based on the 157 patients for whom NT‐proBNP was available.
Based on the 161 patients for whom eGFR was available.
Symptoms were recorded by the patients. Only the most prevalent symptoms are listed, and one patient may have reported more than one symptom.
Patients with ATTRv CM (n = 25) and ATTRwt CM (n = 129) were comparable regarding age (mean age was 77 years for ATTRv CM and 78 years for ATTRwt CM) and disease severity as assessed by NYHA and NAC. In contrast, the proportion of women was higher (20% vs. 6%), the diagnostic delay was shorter (0.6 vs. 2.1 years), and time since diagnosis was longer (4.4 vs. 2.9 years) among ATTRv CM patients as compared with those with ATTRwt CM. The proportion of patients enrolled in an RCT was high both among patients with ATTRv CM and patients with ATTRwt CM (52% vs. 65%, respectively), while considerably more patients with ATTRv CM received disease‐modifying treatment (28% vs. 5%). At time of inclusion, more patients with ATTRv CM received treatment for co‐morbidities like neuropathy (20% vs. 1.6%) and gastrointestinal disease (16% vs. 12%) while fewer received treatment for spinal stenosis (4% vs. 11%) and diabetes (0% vs. 13%) as compared with ATTRwt CM patients.
Patients not enrolled in an RCT (n = 70) and those enrolled in an RCT (n = 98) were comparable in terms of age, gender distribution, and diagnostic delay. Time since diagnosis was also comparable, though more non‐RCT participants as compared with RCT participants (20% vs. 8%) were diagnosed within the last year. Slightly more non‐RCT participants as compared with RCT participants had ATTRv CM (17% vs. 13%). Distribution across NYHA class was similar, whereas NAC stage allocation differed somewhat (the percentage distribution across NAC stage 1/2/3 was 49/21/26 for non‐RCT participants vs. 57/29/13 for RCT participants). At time of inclusion, more non‐RCT participants than RCT participants received treatment for co‐morbidities like ischaemic heart disease (27% vs. 15%), gastrointestinal disease (20% vs. 6%) and neuropathy (10% vs. 1%).
Patient‐reported outcome measures (PROMs)
Patients reported the lowest mean KCCQ domain score in symptom stability (47) and the highest mean score in self‐efficacy (75). Means for the other domain scores ranged from 61 to 68 and were similar to the mean summary scores, which were 64 for total symptom score, 65 for OSS and 65 for clinical summary score (Table 3 ).
TABLE 3.
Quality of life measures
| All patients (N = 169) | |
|---|---|
| KCCQ: domain scores | |
| Physical limitation a | 66.2 ± 24.1 |
| Symptom stability b | 46.9 ± 15.0 |
| Symptom frequency b | 60.5 ± 26.0 |
| Symptom burden b | 68.0 ± 22.0 |
| Self‐efficacy b | 74.6 ± 23.2 |
| Quality of life b | 62.2 ± 24.6 |
| Social limitation c | 67.1 ± 24.9 |
| KCCQ: summary scores | |
| Total symptom score b | 64.3 ± 23.1 |
| <50 | 47 (27.8) |
| 50–75 | 63 (37.3) |
| >75 | 58 (34.3) |
| Overall summary score | 64.8 ± 20.9 |
| <50 | 43 (25.4) |
| 50–75 | 67 (39.6) |
| >75 | 59 (34.9) |
| Clinical summary score | 65.1 ± 21.5 |
| <50 | 42 (24.9) |
| 50–75 | 65 (38.5) |
| >75 | 62 (36.7) |
| EQ‐5D‐5L utility scores | |
| Mobility | 1.2 ± 1.1 |
| Self‐care | 0.4 ± 0.7 |
| Usual activities | 1.2 ± 1.1 |
| Pain/discomfort | 1.0 ± 1.0 |
| Anxiety/depression | 0.6 ± 0.8 |
| Total score | 0.8 ± 0.2 |
| EQ‐5D‐5L VAS | |
| Your health today | 62.9 ± 20.6 |
| <50 | 36 (21.3) |
| 50–75 | 77 (45.6) |
| >75 | 54 (32.0) |
| MDI rating scale | |
| No depression | 151 (89.3) |
| Mild depression | 5 (3.0) |
| Moderate depression | 6 (3.6) |
| Severe depression | 7 (4.1) |
Data are presented as mean ± SD or n (%).
KCCQ, Kansas City Cardiomyopathy Questionnaire; MDI, Major Depression Inventory; VAS, Visual Analog Scale.
Based on 167 patients.
Based on 168 patients.
Based on 165 patients.
For the generic tool EQ‐5D‐5L, mean utility scores within the five domains ranged from 0.4 for self‐care to 1.2 for mobility and usual activities. Mean total EQ‐5D‐5L score was 0.8 and mean VAS score was 63 (Table 3 ).
Finally, categorization of depression state according to MDI showed that the vast majority (89%) had no depression (Table 3 ).
Patients with ATTRv CM (n = 25) had generally higher KCCQ scores as compared with those with ATTRwt CM (n = 129): Mean total symptom score was 73 vs. 66, mean OSS was 70 vs. 64 and mean clinical summary score was 70 vs. 65. EQ‐5D‐5L utility score, EQ‐5D‐5L VAS and MDI were similar in the two groups.
Non‐RCT participants tended to have lower KCCQ scores as compared with those participating in an RCT: Mean total symptom score was 60 vs. 67, mean OSS was 61 vs. 68, and mean clinical summary score was 61 vs. 68. Likewise, mean EQ‐5D‐5L VAS was lower among non‐RCT participants (59 vs. 66), and mean MDI was higher (12 vs. 8). Mean EQ‐5D‐5L utility score did not differ between the groups.
PROMs according to disease severity and other covariates
Patients with higher NYHA classes had a poorer quality of life as reflected in lower KCCQ and EQ‐5D‐5L scores and higher MDI scores (Figure 1 , A‐C). The same was the case when disease severity was assessed by use of NAC stages (Figure 2 , A‐C).
Figure 1.
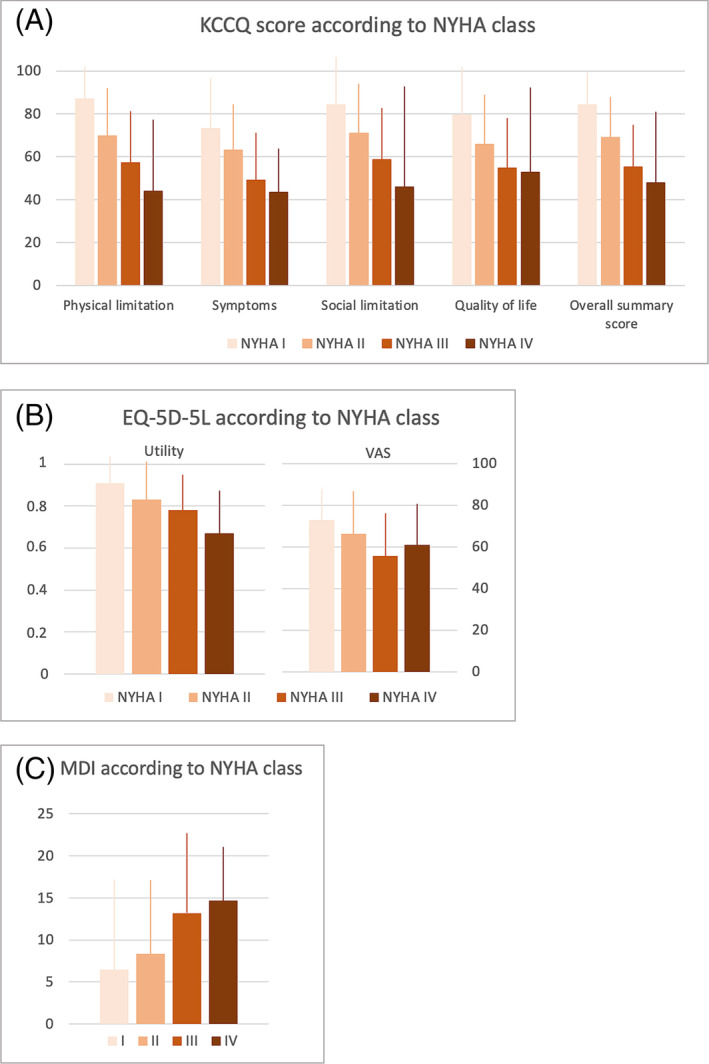
Disease severity as assessed by NYHA according to KCCQ (A), EQ‐5D‐5L (B), and MDI (C).
Figure 2.
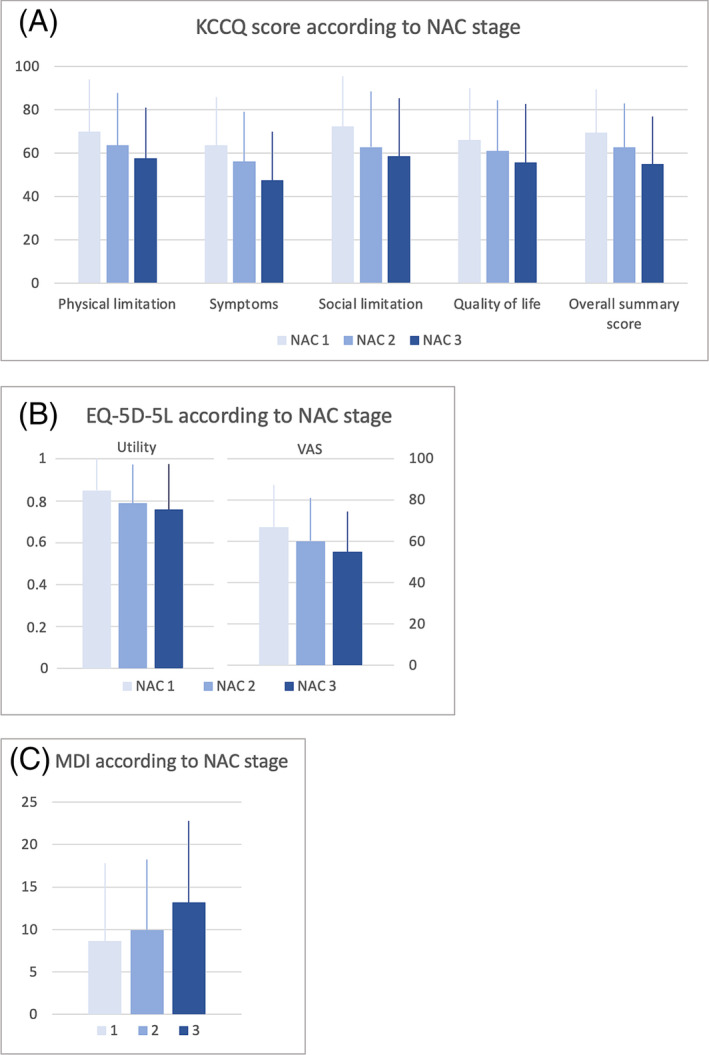
Disease severity as assessed by NAC according to KCCQ (A), EQ‐5D‐5L (B), and MDI (C).
Multiple linear regression analyses confirmed this pattern and further demonstrated significant correlations between co‐morbidity and, respectively, KCCQ and EQ‐5D‐5L, as well as between years since diagnosis and EQ‐5D‐5L (Table 4 ). However, after adjusting for multiple testing, it was primarily the correlation between KCCQ, EQ‐5D‐5L and MDI and the covariate NYHA class that remained significant. No consistent correlations were found between the individual PROMs and, respectively, gender, age, type of disease, treatment category, and diagnostic delay (Table 4 ).
TABLE 4.
Correlations between individual proms and covariates
| EQ‐5D‐5L | KCCQ scores | MDI | ||||||
|---|---|---|---|---|---|---|---|---|
| Utility | VAS | Physical limitation | Symptom frequency | Social limitation | Quality of life | Overall summary | Depression score | |
| Gender | 0.8822 | 0.5257 | 0.4600 | 0.5415 | 0.8084 | 0.1093 | 0.8353 | 0.8306 |
| Age category | 0.0458 | 0.0893 | 0.0213 | 0.1089 | 0.1704 | 0.1933 | 0.1157 | 0.2126 |
| Type of disease | 0.0871 | 0.8129 | 0.7566 | 0.1431 | 0.4015 | 0.4218 | 0.3577 | 0.3561 |
| Years since diagnosis | 0.0439 | 0.0299 | 0.1012 | 0.1453 | 0.1539 | 0.0385 | 0.0711 | 0.3116 |
| NYHA class | 0.0010/0.0114 | 0.0032/0.0383 | <.0001/0.0003 | <.0001/<.0001 | 0.0012/0.0139 | 0.0011/0.0134 | <.0001/<.0001 | 0.0003/0.0036 |
| NAC stage | 0.0164 | 0.0067 | 0.0284 | 0.0008/0.0096 | 0.0240 | 0.1917 | 0.0073 | 0.0210 |
| Treatment category | 0.2927 | 0.8990 | 0.0208 | 0.3541 | 0.1499 | 0.8514 | 0.1499 | 0.5854 |
| Diagnostic delay | 0.7844 | 0.0229 | 0.6645 | 0.0132 | 0.1275 | 0.0952 | 0.0668 | 0.1396 |
| Co‐morbidities | 0.0212 |
0.0010 /0.0094 |
0.0211 | 0.0185 | 0.0434 | 0.0174 | 0.0075 | 0.0822 |
P‐values for regression analyses for EQ‐5D‐5L, KCCQ and MDI according to covariates. Significant P‐values are indicated in bold, and P‐values that were still significant after Bonferroni correction are indicated in bold italics.
Covariate categories were as follows: Gender: Male/Female, Age category: <70 years/71–80 years/>81 years, Type of disease: hereditary ATTR CM/wildtype ATTR CM, Years since diagnosis: 0–5/over 5, NYHA class: NYHA I/NYHA II/NYHA III/NYHA IV, NAC stage: Stage 1/Stage 2/Stage 3, Treatment category: Symptomatic treatment/ATTR CM disease modifying treatment/The patient is participating in a randomized clinical trial, Diagnostic Delay: ≤1 year/>1 year, Co‐morbidities: Yes/No.
NYHA, New York Heart Association; NAC, National Amyloidosis Centre; KCCQ, Kansas City Cardiomyopathy Questionnaire; MDI, Major depression inventory; VAS, visual analogue scale.
Significant correlations were found between all the individual PROMs. Correlations were strong (correlation coefficient ≥0.8 or ≤ − 0.8) between KCCQ OSS and, respectively, KCCQ (Physical limitation), KCCQ (Social limitation), and KCCQ (Quality of life), with all having p‐values < 0.001. Correlations were weak (correlation coefficient <0.4 or > − 0.4) between KCCQ (Symptoms) and, respectively, other KCCQ scores, EQ‐5D‐5L and MDI, all p‐values <0.05. All remaining correlations were moderate (correlation coefficient 0.4 to 0.8 or −0.4 to −0.8) with p‐values < 0.001.
Discussion
As the first of its kind, the Nordic PROACT study provides a thorough description of HRQoL in 169 contemporary patients living with symptomatic ATTR CM in Norway, Sweden, Finland and Denmark. The study population consisted of both ATTRwt and ATTRv CM patients with a predominance of wildtype disease. Even though most patients were in NYHA class II/NAC stage 1 and the mean diagnostic delay of 1.7 years was relatively short as compared with previous reports, the HRQoL was impaired. 14 , 23 , 24 , 25 , 26 , 27
KCCQ
The HRQoL assessed by the KCCQ OSS in the present ATTR‐CM patients showed a mean value of 65. This is in line with the mean values of 65–67 for KCCQ OSS at baseline reported for ATTR CM patients enrolled in CTs 28 , 29 and somewhat higher than for ATTR CM patients characterized by profound diagnostic delay and advanced disease progression, for whom KCCQ OSS mean values below 60 have been reported. 2 , 14 Data from observational studies and clinical trial baseline data from patients with heart diseases like HFpEF, heart failure with reduced ejection fraction (HFrEF) and obstructive hypertrophic cardiomyopathy report mean KCCQ OSS values of at least 70. 30 , 31 , 32 , 33 Bearing in mind the pitfalls of cross‐study comparisons, this might suggest a clinically relevant difference in HRQoL between patients with ATTR CM and patients with other heart failure diseases, with ATTR CM patients being more affected. Possible explanations may involve the systemic nature of ATTR CM, the limited effect of heart failure medication for the treatment of ATTR CM, and the later diagnosis of ATTR CM as compared with other heart failure aetiologies.
The reported pattern with lowest domain scores for symptom stability and highest scores for self‐efficacy is in line with previous reports. 2 , 14 Self‐efficacy measures the patient's knowledge of, and confidence in, their medical care, including what they should do if their heart failure gets worse. 19
EQ‐5D‐5L
The overall HRQoL as measured by EQ‐5D‐5L was comparable to previous reports of ATTR CM patients and patients diagnosed with heart failure, but slightly lower—at least in terms of VAS scores—than for patients with cardiac diagnoses like ischaemic heart disease, arrhythmia and congenital heart disease. 2 , 16 , 18 , 34 When compared with the general population in the Scandinavian countries, the mean EQ‐5D‐5L VAS score of 63 in the Nordic PROACT population was lower than a VAS score of 75 in Swedish men from the general population with an age of 75–79 years. 35 The EQ‐5D‐5L mean utility score of 0.8 demonstrated in the present ATTR CM population was also slightly lower as compared with a mean EQ‐5D‐5L utility score of 0.9 demonstrated in the general Danish population aged above 70 years of age. 36 In Norway, however, overall HRQoL in the general population aged 70–79 years was similar to that of the ATTR CM study population with a mean EQ‐5D‐5L utility score of 0.8. 37
RCT participants vs. non‐RCT participants
The majority (58%) of the study group participated in an RCT. Even though this is a well‐known term when including patients with a rare disease like ATTR CM for which many new treatment strategies are under investigation, the large proportion of patients participating in an RCT may blur the transparency of the treatment category and slightly affect how the patient questionnaires are answered. A split analysis showed that this subgroup, though of comparable age, diagnostic delay and NYHA class, had a better HRQoL, both as measured by KCCQ and EQ‐5D‐5L VAS, than those not participating in a trial. The difference, or at least some of it, may be explained by the markedly higher proportion of co‐morbidities such as ischaemic heart disease, gastrointestinal disease and neuropathy in non‐RCT participants along with the lower proportion of patients in NAC stage 3 among trial participants. Though details on the specific RCTs are not available, it is possible that some RCT participants receive disease‐modifying treatment, which can diminish the progression of the disease and thereby improve HRQoL, as shown for tafamidis and patisiran. 16 , 38 Moreover, participating in an RCT might improve QoL per se, due to the increased attention and support received by trial participants. Indeed, the difference between a mean KCCQ OSS of 58 observed among ATTR CM patients in a large observational study 14 and of approximately 67 at baseline in two large RCTs 16 , 29 corresponds to the differences found between the subgroups of RCT and non‐RCT participants in our study (61 vs. 68).
ATTRv CM patients vs. ATTRwt CM patients
The relatively small subgroup (n = 25) of patients with ATTRv CM were characterized by having a better HRQoL as assessed by KCCQ as compared with patients with ATTRwt CM, while EQ‐5D‐5L and MDI were similar. A very short diagnostic delay and a high proportion of patients receiving disease‐modifying treatment may explain some of this difference.
MDI
To the best of our knowledge, this is the first report of depression as evaluated by MDI in patients with ATTR CM. Heart failure is associated with an increased risk of depression, and large meta‐analyses of heart failure patients have reported an estimated overall prevalence of depression of 20–30%, with similar prevalence in patients with HFpEF and HFrEF and across aetiologies. 39 This is markedly higher than the observed 11% of ATTR CM patients reported to have any degree of depression according to MDI in the present study. Different assessment tools may explain some of the differences, with MDI being referred to as a rather conservative instrument with few false positives. 40 The clinical relevance of this difference should be interpreted with caution, given both the different assessment tools used and the limited size of the Nordic PROACT study population. Likewise, no conclusions can be drawn from the slight difference in mean MDI found between RCT participants and non‐RCT participants. The association between depression and NYHA class is in accordance with previous reports for patients with heart failure. 41 , 42
Correlates between PROMs, disease severity, and co‐morbidities
Significant correlations were found between the HRQoL measures of KCCQ and EQ‐5D‐5L, and the severity of ATTR CM as defined by increasing NYHA class or NAC stage. HRQoL, whether measured by the heart specific KCCQ questionnaire or by the generic EQ‐5D‐5L tool, worsened with the severity of disease. This was the case both for the tools' domain/utility scores and for the total scores. Likewise, patients with profound disease severity scored higher on the MDI scale, indicative of more depression‐like symptoms, although most patients stayed in the ‘no depression’ category. Previous reports in heart failure patients have also documented association between NYHA and KCCQ as well as between NYHA and EQ‐5D‐5L. 33 , 43 , 44 , 45 To our knowledge, the Nordic PROACT study is the first to investigate correlations between NAC staging and different HRQoL measures.
Though both methods of classifying disease severity correlated well to the analysed PROMs, NYHA classes showed a stronger correlation than NAC stages, in that the correlation between NYHA classes and the individual PROMs stayed significant after Bonferroni correction. This difference may relate to the fact that NYHA classification is based on the patients' physical limitations and symptoms. 12 Even though the tool reflects the physician's perspective and is subject to interobserver variability, it mirrors the patient's disease perception and therefore may relate more closely to QoL. In contrast, NAC staging, though specific for ATTR CM, relates to expected survival and not functional capacity or symptoms perceived by the patient. 13
Many of the reported co‐morbidities are part of the pathological picture for ATTR CM. The significant correlation between the presence of co‐morbidity and measures of HRQoL is as expected, and likely reflects a greater disease burden from the patient's perspective with the presence of symptoms requiring medical treatment. The significant correlation between years since diagnosis and EQ‐5D‐5L likely mirrors the progressive nature of ATTR CM, though the pattern was not consistent for KCCQ scores and MDI.
Study limitations
No control group was included in the Nordic PROACT study. Further, due to the limited size of the study population, no comparisons were conducted between the four Nordic countries. That said, few, if any, differences would be expected between Norway, Sweden, Finland and Denmark, as the Nordic countries are quite homogenous in terms of public health and health services.
Whereas the cardiac biomarker NT‐proBNP was measured in most patients and used in the NAC staging, data on troponin levels was only available for a small part of the enrolled patients. For this reason, troponin levels are not reported here. Being a validated marker of disease severity and cardiac involvement, the inclusion of troponin would have been preferable.
For ATTR CM, the nature and degree of co‐morbidities as well as the treatment of these is essential for the patients' prognoses, as exemplified by atrial fibrillation, the most prevalent co‐morbidity in the present study, and the associated increased risk of potentially fatal thromboembolic events. The Nordic PROACT study did not collect data on the specific medications prescribed for the co‐morbidities recorded as being treated. However, in the case of atrial fibrillation, a recent real‐world study showed that while 64% of atrial fibrillation patients in the Scandinavian countries are treated with non‐vitamin K antagonist oral anticoagulants, the rest receives vitamin K antagonists. 46 It is assumed that the ATTR CM patients who received treatment for atrial fibrillation in the present study showed a similar treatment distribution.
A significant proportion of the patients were enrolled in an RCT while participating in the Nordic PROACT study. This may blur the transparency of the received treatments and affect the results slightly. Nonetheless, the distribution of non‐RCT and RCT participants in the present study reflects the current clinical situation with a patient population of a limited size combined with multiple ongoing RCTs, and excluding patients enrolled in an RCT would have resulted in a non‐representative patient population.
The Nordic PROACT study was conducted during the second year of the COVID‐19 pandemic. This may have influenced the recruitment of patients as well as the questionnaire responses.
Conclusions
The Nordic PROACT study demonstrated that HRQoL as assessed by KCCQ was lower in patients with ATTR CM as compared with previous reports from patients with other cardiac diseases. The overall HRQoL as measured by EQ‐5D‐5L was comparable to previous reports of ATTR CM patients and patients diagnosed with heart failure, but slightly lower than for patients with other cardiac diagnoses. The prevalence of depression was low. Clear correlations were found between measures of HRQoL and disease severity, as assessed by NYHA and NAC, underlining the importance of early diagnosis and treatment.
Conflict of interest
J.L., E.G., and S.H.P. were paid by Pfizer for their work as members of the study steering committee and were paid by Pfizer for their work as investigators in the study. E.B., M.O., G.S., J.K., T.R., and E.C. were paid by Pfizer for their work as investigators in the study. F.G. and P.E. are consultants for Pfizer. D.W.W. was a paid contractor to Pfizer for her work as the CRO project leader role of the study. A.B.B., M.V., T.P., K.J., M.K., and L.L.N. are full‐time employees of Pfizer. The authors report no other competing interests in this work.
Funding
The Nordic PROACT study was sponsored by Pfizer.
Acknowledgements
We thank Justyna Modrzynska and Andreas Habicht (Signifikans, Vedbæk, Denmark) for support with the statistical analyses, Katrine Bay (Bay Writing, Copenhagen, Denmark) for writing up the manuscript and Peter Bo Poulsen (Pfizer Denmark, Ballerup, Denmark) for support through the entire process.
Eldhagen, P. , Lehtonen, J. , Gude, E. , Gustafsson, F. , Bagger‐Bahnsen, A. , Vakevainen, M. , Pilgaard, T. , Wedell‐Wedellsborg, D. , Poulsen, S. H. , and Nordic PROACT study group (2023) Health‐related quality of life among transthyretin amyloid cardiomyopathy patients. ESC Heart Failure, 10: 1871–1882. 10.1002/ehf2.14350.
References
- 1. Garcia‐Pavia P, Bengel F, Brito D, Damy T, Duca F, Dorbala S, Nativi‐Nicolau J, Obici L, Rapezzi C, Sekijima Y, Elliott PM. Expert consensus on the monitoring of transthyretin amyloid cardiomyopathy. Eur J Heart Fail. 2021; 23: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart M, Shaffer S, Murphy B, Loftus J, Alvir J, Cicchetti M, Lenderking WR. Characterizing the high disease burden of transthyretin amyloidosis for patients and caregivers. Neurol Ther. 2018; 7: 349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rozenbaum MH, Large S, Bhambri R, Stewart M, Whelan J, van Doornewaard A, Dasgupta N, Masri A, Nativi‐Nicolau J. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR‐CM): a targeted literature review. Cardiol Ther. 2021; 10: 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yilmaz A, Bauersachs J, Bengel F, Büchel R, Kindermann I, Klingel K, Knebel F, Meder B, Morbach C, Nagel E, Schulze‐Bahr E, aus dem Siepen F, Frey N. Diagnosis and treatment of cardiac amyloidosis: position statement of the German cardiac society (DGK). Clin Res Cardiol. 2021; 110: 479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012; 126: 1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, Ciliberti P, Biagini E, Salvi F, Branzi A. Transthyretin‐related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010; 7: 398–408. [DOI] [PubMed] [Google Scholar]
- 7. Bay K, Gustafsson F, Maiborg M, Bagger‐Bahnsen A, Strand AM, Pilgaard T, Poulsen SH. Suspicion, screening, and diagnosis of wild‐type transthyretin amyloid cardiomyopathy: a systematic literature review. ESC Heart Fail. 2022; 9: 1524–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 9. Rubin J, Maurer MS. Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu Rev Med. 2020; 71: 203–219. [DOI] [PubMed] [Google Scholar]
- 10. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019; 73: 2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Damy T, Kristen AV, Suhr OB, Maurer MS, Planté‐Bordeneuve V, Yu CR, Ong ML, Coelho T, Rapezzi C, THAOS Investigators . Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the transthyretin amyloidosis outcomes survey (THAOS). Eur Heart J. 2019; 43: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Criteria Committee of the New York Heart Association . Functional capacity and objective assessment. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, 9th ed. Boston MA: Little, Brown & Co.; 1994. p 253–256. [Google Scholar]
- 13. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 14. Lane T, Fontana M, Martinez‐Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG, Caringal‐Galima J, Manwani R, Sharpley FA, Wechalekar AD, Lachmann HJ, Mahmood S, Sachchithanantham S, Drage EPS, Jenner HD, McDonald R, Bertolli O, Calleja A, Hawkins PN, Gillmore JD. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019; 140: 16–26. [DOI] [PubMed] [Google Scholar]
- 15. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR‐ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 16. Hanna M, Damy T, Grogan M, Stewart M, Gundapaneni B, Patterson TA, Schwartz JH, Sultan MB, Maurer MS. Impact of Tafamidis on health‐related quality of life in patients with transthyretin amyloid cardiomyopathy (from the Tafamidis in transthyretin cardiomyopathy clinical trial). Am J Cardiol. 2021; 141: 98–105. [DOI] [PubMed] [Google Scholar]
- 17. Coelho T, Maurer MS, Suhr OB. THAOS ‐ the transthyretin amyloidosis outcomes survey: initial report on clinical manifestations in patients with hereditary and wild‐type transthyretin amyloidosis. Curr Med Res Opin. 2013; 29: 63–76. [DOI] [PubMed] [Google Scholar]
- 18. Damy T, Adams D, Bridoux F, Grateau G, Planté‐Bordeneuve V, Ghiron Y, Farrugia A, Pelcot F, Taieb C, Labeyrie C, Jaccard A. Amyloidosis from the patient perspective: the French daily impact of amyloidosis study. Amyloid. 2022: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 20. Group, E . EuroQoL: a new facility for the measurement of health‐related quality of life. Health Policy. 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 21. Olsen LR, Jensen DV, Noerholm V, Martiny K, Bech P. The internal and external validity of the major depression inventory in measuring severity of depressive states. Psychol Med. 2003; 33: 351–356. [DOI] [PubMed] [Google Scholar]
- 22. Bech PER, Wermuth L. Applicability and validity of the major depression inventory in patients with Parkinson's disease. Nord J Psychiatry. 1998; 52: 305–310. [Google Scholar]
- 23. Ladefoged B, Dybro A, Povlsen JA, Vase H, Clemmensen TS, Poulsen SH. Diagnostic delay in wild type transthyretin cardiac amyloidosis ‐ a clinical challenge. Int J Cardiol. 2020; 304: 138–143. [DOI] [PubMed] [Google Scholar]
- 24. Bishop E, Brown EE, Fajardo J, Barouch LA, Judge DP, Halushka MK. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018; 25: 174–179. [DOI] [PubMed] [Google Scholar]
- 25. Lauppe RE, Liseth Hansen J, Gerdesköld C, Rozenbaum MH, Strand AM, Vakevainen M, Kuusisto J, Gude E, Gustafsson F, Smith JG. Nationwide prevalence and characteristics of transthyretin amyloid cardiomyopathy in Sweden. Open Heart. 2021; 8: e001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lauppe R, Liseth Hansen J, Fornwall A, Johansson K, Rozenbaum MH, Strand AM, Väkeväinen M, Kuusisto J, Gude E, Smith JG, Gustafsson F. Prevalence, characteristics, and mortality of patients with transthyretin amyloid cardiomyopathy in the Nordic countries. ESC Heart Fail. 2022; 9: 2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AWJM, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 28. Hanna M, Damy T, Grogan M, Stewart M, Gundapaneni B, Sultan MB, Maurer MS. Tafamidis and quality of life in people with transthyretin amyloid cardiomyopathy in the study ATTR‐ACT: a plain language summary. Future Cardiol. 2022; 18: 165–172. [DOI] [PubMed] [Google Scholar]
- 29. Judge DP, Kristen AV, Grogan M, Maurer MS, Falk RH, Hanna M, Gillmore J, Garg P, Vaishnaw AK, Harrop J, Powell C, Karsten V, Zhang X, Sweetser MT, Vest J, Hawkins PN. Phase 3 multicenter study of Revusiran in patients with hereditary transthyretin‐mediated (hATTR) amyloidosis with cardiomyopathy (ENDEAVOUR). Cardiovasc Drugs Ther. 2020; 34: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sepehrvand N, Savu A, Spertus JA, Dyck JRB, Anderson T, Howlett J, Paterson I, Oudit GY, Kaul P, McAlister FA, Ezekowitz JA, the Alberta HEART Investigators* . Change of health‐related quality of life over time and its association with patient outcomes in patients with heart failure. J Am Heart Assoc. 2020; 9: e017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis EF, Claggett BL, McMurray JJV, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Zile MR, Desai AS, Solomon SD, Swedberg K. Health‐related quality of life outcomes in PARADIGM‐HF. Circ Heart Fail. 2017; 10. [DOI] [PubMed] [Google Scholar]
- 32. Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021; 78: 2505–2517. [DOI] [PubMed] [Google Scholar]
- 33. Chandra A, Vaduganathan M, Lewis EF, Claggett BL, Rizkala AR, Wang W, Lefkowitz MP, Shi VC, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV, Solomon SD. Health‐related quality of life in heart failure with preserved ejection fraction: the PARAGON‐HF trial. JACC Heart Fail. 2019; 7: 862–874. [DOI] [PubMed] [Google Scholar]
- 34. Berg SK, Rasmussen TB, Thrysoee L, Lauberg A, Borregaard B, Christensen AV, Ekholm O, Juel K, Svanholm JR. DenHeart: differences in physical and mental health across cardiac diagnoses at hospital discharge. J Psychosom Res. 2017; 94: 1–9. [DOI] [PubMed] [Google Scholar]
- 35. Teni FS, Gerdtham UG, Leidl R, Henriksson M, Åström M, Sun S, Burström K. Inequality and heterogeneity in health‐related quality of life: findings based on a large sample of cross‐sectional EQ‐5D‐5L data from the Swedish general population. Qual Life Res. 2022; 31: 697–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen MB, Jensen CE, Gudex C, Pedersen KM, Sørensen SS, Ehlers LH. Danish population health measured by the EQ‐5D‐5L. Scand J Public Health. 2021; 51: 14034948211058060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garratt AM, Hansen TM, Augestad LA, Rand K, Stavem K. Norwegian population norms for the EQ‐5D‐5L: results from a general population survey. Qual Life Res. 2022; 31: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Obici L, Berk JL, González‐Duarte A, Coelho T, Gillmore J, Schmidt HHJ, Schilling M, Yamashita T, Labeyrie C, Brannagan TH III, Ajroud‐Driss S, Gorevic P, Kristen AV, Franklin J, Chen J, Sweetser MT, Wang JJ, Adams D. Quality of life outcomes in APOLLO, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin‐mediated amyloidosis. Amyloid. 2020; 27: 153–162. [DOI] [PubMed] [Google Scholar]
- 39. Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. 2020; 22: 2007–2017. [DOI] [PubMed] [Google Scholar]
- 40. Nielsen MG, Ørnbøl E, Bech P, Vestergaard M, Christensen KS. The criterion validity of the web‐based major depression inventory when used on clinical suspicion of depression in primary care. Clin Epidemiol. 2017; 9: 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Celik E, Cay S, Sensoy B, Murat S, Oksuz F, Cankurt T, Ali Mendi M. Heart failure functional class associated with depression severity but not anxiety severity. Acta Cardiol Sin. 2016; 32: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gottlieb SS, Kop WJ, Ellis SJ, Binkley P, Howlett J, O'Connor C, Blumenthal JA, Fletcher G, Swank AM, Cooper L, HF‐ACTION Investigators . Relation of depression to severity of illness in heart failure (from heart failure and a controlled trial investigating outcomes of exercise training [HF‐ACTION]). Am J Cardiol. 2009; 103: 1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rubio R, Palacios B, Varela L, Fernández R, Camargo Correa S, Estupiñan MF, Calvo E, José N, Ruiz Muñoz M, Yun S, Jiménez‐Marrero S, Alcoberro L, Garay A, Moliner P, Sánchez‐Fernández L, Soria Gómez MT, Hidalgo E, Enjuanes C, Calero‐Molina E, Rueda Y, San Saturnino M, Garcimartín P, López‐Ibor JV, Segovia‐Cubero J, Comin‐Colet J. Quality of life and disease experience in patients with heart failure with reduced ejection fraction in Spain: a mixed‐methods study. BMJ Open. 2021; 11: e053216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP. Assessing health‐related quality‐of‐life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ‐5D‐based utilities in the EXPLORER‐HCM trial. J Med Econ. 2022; 25: 51–58. [DOI] [PubMed] [Google Scholar]
- 45. Capota R, Militaru S, Ionescu AA, Rosca M, Baicus C, Popescu BA, Jurcut R. Quality of life status determinants in hypertrophic cardiomyopathy as evaluated by the Kansas City cardiomyopathy questionnaire. Health Qual Life Outcomes. 2020; 18: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halvorsen S, Johnsen SP, Madsen M, Linder M, Sulo G, Ghanima W, Gislason G, Hohnloser SH, Jenkins A, al‐Khalili F, Tell GS, Ehrenstein V. Effectiveness and safety of non‐vitamin K antagonist oral anticoagulants and warfarin in atrial fibrillation: a Scandinavian population‐based cohort study. Eur Heart J Qual Care Clin Outcomes. 2022; 8: 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]


