Abstract
In patients with heart failure with preserved ejection fraction (HFpEF), sudden cardiac death (SCD) accounts for approximately 25–30% of all‐cause mortality and 40% of cardiovascular mortality in properly adjudicated large clinical trials. The mechanism of SCD in HFpEF remains unknown but thought to be driven by arrhythmic events. Apart from atrial fibrillation, which is prevalent in approximately 45% of HFpEF patients, the true burden of other cardiac arrhythmias in HFpEF remains undetermined. The incidence and risk of clinically significant advanced cardiac conduction disease with bradyarrhythmias and ventricular arrhythmias remain less known. Recommendations have been made for long‐term cardiac rhythm monitoring to determine the incidence of arrhythmias and clarify mechanisms and mode of death in HFpEF patients. In animal studies, spontaneous ventricular arrhythmias and SCD are significantly elevated in HFpEF animals compared with controls without heart failure. In humans, these studies are scant, with a few published small‐size studies suggesting an increased incidence of ventricular arrhythmias in HFpEF. Higher rates of clinically significant conduction disease and cardiac pacing are seen in HFpEF compared with the general population. Excepting atrial fibrillation, the predictive effect of other arrhythmias on heart failure hospitalization, all‐cause mortality, and precisely SCD remains unknown. Given the high occurrence of SCD in the HFpEF population, it could potentially become a target for therapeutic interventions if driven by arrhythmias. Studies to address these knowledge gaps are urgently warranted. In this review, we have summarized data on arrhythmias and SCD in HFpEF while highlighting avenues for future research in this area.
Keywords: Arrhythmias, Heart failure with preserved ejection fraction, Ventricular arrhythmia, Atrial arrhythmia, Bradyarrhythmia
Introduction
Heart failure resulting from several aetiologies is now considered a global pandemic, affecting more than 64 million people worldwide. 1 In the Western world where ascertainment of cases is fairly feasible, the incidence and prevalence of heart failure is increasing, resulting in enormous and rising cost implications. 2 , 3 In the USA, for example, the annual median costs for heart failure care are estimated at ~$25 000 per patient. 4 Heart failure is currently clinically classified based on a single measure of cardiac contractility, the left ventricular ejection fraction (LVEF). Heart failure with preserved ejection fraction (HFpEF) is defined with LVEF ≥50%, contrasted with heart failure with borderline or mid‐range ejection fraction (HFmrEF) where LVEF is 41–49%, and heart failure with reduced ejection fraction (HFrEF) where LVEF is ≤40%. 5 , 6 HFpEF currently represents approximately 50% of all heart failure patients; the proportion of HFpEF is increasing and will soon become the predominant form of heart failure. 6 , 7 , 8
Apart from atrial fibrillation (AF), 9 , 10 the true burden of arrhythmias in HFpEF remains unknown but is likely high and possibly contributing to increased morbidity and reduced survival in this patient group. The predictive effect of arrhythmias on heart failure hospitalization, all‐cause mortality, and sudden cardiac death (SCD) in HFpEF also remains unknown. Identifying the specific HFpEF phenotypes at higher risk of life‐threatening arrhythmias and SCD could lead to evidence‐based interventions to reduce arrhythmia‐induced morbidity and mortality in HFpEF. About a quarter or more of HFpEF patients suffer SCD as mode of death. Unlike HFrEF where most cases of SCD are due to ventricular arrhythmias, the proportion of patients dying from malignant arrhythmias among SCD in HFpEF patients remains unknown. 11 , 12 The mechanisms (arrhythmic vs. other causes) driving fatal events in HFpEF especially SCD are not well known. Mapping the natural history of HFpEF with respect to arrhythmias using longitudinal data appears to be urgently required. Death and hospitalizations resulting from arrhythmias would be a promising target for therapeutic intervention once we know their burdens and predictive effects on these hard outcomes. The objective of this review is to summarize the available data on arrhythmia in HFpEF especially with reference to SCD.
High morbidity and mortality in HFpEF including SCD
Although declining, the 30‐day re‐hospitalization rates following admission for heart failure are still approximately 20% in developed countries. 6 , 13 , 14 , 15 Despite improvements in survival, and irrespective of advances in therapy, the prognosis of heart failure remains poor with median survival of 2 years, and high 5‐year absolute mortality rates of 50–75%, and significantly greater compared with heart failure‐free individuals. 2 , 7 , 16 , 17 The prognosis of HFpEF subgroup is equally poor with a median survival of 2 years and 5‐year mortality of ~75%. 7 The significant morbidity with loss of economic productivity, the high mortality, and the astronomical cost implications associated with the management of heart failure have rendered it a high‐priority area for policymakers, clinicians, and researchers. 2 , 5 , 6 Therefore, novel clinical research perspectives are still warranted. All‐cause and cardiovascular mortality are significantly elevated in HFpEF patients compared with matched controls without heart failure. 18 , 19 , 20 The risk of all‐cause mortality in HFpEF patients is two to four times that of controls without heart failure and normal left ventricular systolic function. 21 , 22 , 23
In randomized controlled trials (RTCs), majority of deaths in HFpEF patients were cardiovascular (~60–70% of total deaths), whereas 20–30% were due to non‐cardiac causes. Observational epidemiological studies, however, report lower proportions of cardiovascular deaths (∼50–60%) in HFpEF patients when compared with RCTs. 12 Case definition of mode of death in HFpEF patients is hampered by semantics as some studies use sudden death (SD), whereas others use SCD, but closer review revealed that the criteria used to defined SD and SCD in various clinical trials are similar. 12 Therefore, SD and SCD are considered synonymous with respect to HFpEF, based on data from various studies. In this review, we will be using the term SCD. In HFpEF patients, SCD accounted for approximately 25–30% of all mortality and 40% of cardiovascular mortality, whereas worsening heart failure accounted for 20–30% of cardiovascular deaths, and MI and stroke accounted for a minority of cardiovascular deaths (each ~5–15%) 12 , 18 , 20 (Figure 1 ). Conversely, in HFrEF patients, 80–85% of deaths are due to cardiovascular causes, with sudden death accounting for about ~45% of cardiovascular deaths and worsening heart failure ~25%. 12 , 20 , 24 Cardiac dysrhythmias are responsible for majority of SCD in patients with HFrEF. 25 , 26 Ventricular arrhythmias are present at the time of death in 76% of SCD in patients implanted with an implantable cardioverter defibrillator (ICD). 27 Unlike HFrEF where most of the cases of SCD are due to malignant ventricular arrhythmias [ventricular tachycardia (VT) and ventricular fibrillation (VF)], the proportion of HFpEF patients with SCD due to malignant arrhythmias remains unknown but is probably high. 11 , 12 The mechanisms driving fatal events in HFpEF especially SCD remain elusive. Mapping the natural history of HFpEF with respect to arrhythmias using longitudinal data is strongly required. Malignant tachy (or brady) arrhythmias may be important causes of SCD, but we just do not know. This is important as a high‐risk group could exist where an ICD or a permanent pacemaker may be of benefit.
Figure 1.
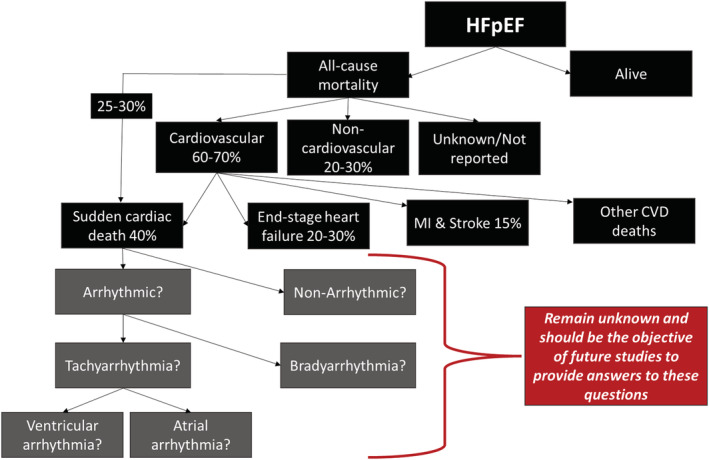
Causes of mortality in heart failure with preserved ejection fraction (HFpEF). 12 , 18 , 20
Treatment options in HFpEF are limited compared with HFrEF
Clinical trials have shown morbidity and mortality benefits of several pharmacotherapies and cardiac implantable electronic devices (CIEDs) like ICD and cardiac resynchronization therapy (CRT) implantations in HFrEF patients, leading to established guidelines for HFrEF management. 5 , 6 , 28 Unfortunately, as shown in Table 1 , major randomized controlled clinical trials have failed to show significant mortality beneficial effects of pharmacological therapy in HFpEF patients. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 Though observational studies seem to suggest some mortality benefit of renin–angiotensin blockade in HFpEF, RCTs did not show significant all‐cause mortality benefit or reduction in heart failure hospitalization. 40 Although beta‐blockers are an established part of the pharmacological armamentarium in HFrEF, randomized clinical trials in HFpEF patients have not shown any significant reduction in all‐cause mortality or heart failure hospitalization, 36 , 47 despite meta‐analysis of observational studies suggesting some reduction in all‐cause mortality associated with beta‐blocker use in HFpEF. 41 , 48 , 49 Mineralocorticoid receptor antagonists use did not significantly reduce the occurrence of the primary endpoint at intention‐to‐treat analysis of the TOPCAT trial or Aldo‐DHF in HFpEF patients. 38 , 39 The DIG ancillary trial did not show any benefit of digoxin on HFpEF with respect to total mortality and all‐cause mortality or cardiovascular hospitalizations when compared with placebo. 32 In the PARAGON‐HF trial, angiotensin receptor‐neprilysin inhibitor (ARNI) did not significantly reduce hospitalizations for heart failure and death from cardiovascular causes in HFpEF. 42
Table 1.
Trials of pharmacotherapies in HFpEF
| Study | Year, size, and population | Randomization (study arms) | Median follow‐up | Primary outcomes | Results a |
|---|---|---|---|---|---|
|
CHARM‐Preserved Yusuf et al. 29 |
2003 N = 3023, HFpEF |
Candesartan vs. placebo | 36.6 months | CVD death or HF hospitalization | HR 0·86 (0·74–1·00) P = 0.051 |
|
SENIORS Flather et al. 30 |
2005 N = 2128, HFpEF |
Nebivolol vs. placebo | 21 months |
‐ All‐cause mortality or CVD hospitalization ‐ All‐cause mortality |
‐ HR 0.86 (0.74–0.99) P = 0.039 ‐ HR 0.88 (0.71–1.08) P = 0.21 |
|
PEP‐CHF Cleland et al. 31 |
2006 N = 850, HFpEF |
Perindopril vs. placebo | 2.1 years | All‐cause mortality and HF hospitalization | HR 0.919 (0.700–1.208) P = 0.545 |
|
DIG ancillary trial Ahmed et al. 32 |
2006 N = 988, HFpEF |
Digoxin vs. placebo | 37 months | HF hospitalization or HF mortality | HR 0.82 (0.63–1.07) P = 0.136 |
|
I‐PRESERVE Massie et al. 33 |
2008 N = 4128, HFpEF |
Irbesartan vs. placebo | 49.5 months | All‐cause mortality or CVD hospitalization | HR 0.95 (0.86–1.050) P = 0.35 |
|
HK‐DHF Yip et al. 34 |
2008 N = 150, HFpEF |
Diuretics alone vs. diuretics plus irbesartan vs. diuretics plus ramipril | 1 year | Recurrent hospitalization | Rates were similar in all groups (12.2%, 11.1%, 11.4%) |
|
ALL‐HAT Grimm et al. 35 |
2009 N = 1479 with heart failure (LVEF >35%) |
‐ Lisinopril vs. chlorthalidone ‐ Amlodipine vs. chlorthalidone ‐ Doxazosin vs. chlorthalidone |
1 year | HF hospitalization |
‐ OR 1.33 (0.65–2.74) P =0.44 ‐ OR 1.08 (0.53–2.21) P =0.83 ‐ OR 1.33 (0.65–2.74) P =0.44 |
|
J‐DHF Yamamoto et al. 36 |
2013 N = 245, HFpEF |
Carvedilol vs. no carvedilol | 3.2 years | CVD death and HF hospitalization | HR 0.902 (0.546–1.48) P = 0.6854 |
|
RELAX Redfield et al. 37 |
2013 N = 216, HFpEF |
Sildenafil vs. placebo | 24 weeks |
‐ Changes in VO2 max ‐ Changes in 6MWT distance |
‐ Changes in VO2 max P =0.90 ‐ Changes 6MWT P = 0.92 |
|
Aldo‐DHF Edelmann et al. 38 |
2013 N = 422, HFpEF |
Spironolactone vs. placebo | 12 months |
‐ Diastolic function (E/e′) ‐ Peak VO2 (mL/min/kg) |
‐ −1.5 (−2.0 to −0.9) P = 0.001 ‐ +0.1 (−0.6 to +0.8) P =0.8. |
|
TOPCAT Pitt et al. 39 |
2014 N = 3445, HFpEF |
Spironolactone vs. placebo | 3.3 years | CVD death, aborted SCA, or HF hospitalization | HR 0.89 (0.77–1.04) P = 0.14 |
|
EMPA‐REG OUTCOME Zinman et al. 50 |
2015 N = 7020 (T2DM + CVD risk) |
Empagliflozin vs. placebo | 3.1 years | CVD death, non‐fatal MI or non‐fatal stroke | HR 0.86 (0.74–0.99) P = 0.04 for superiority |
|
Meta‐analysis Bavishi et al. 48 |
2015 N = 27 099 (17 studies), HFpEF |
Beta‐blocker vs. placebo/no beta‐blocker | At least 1 year |
Observational studies: ‐ All‐cause mortality ‐ HF hospitalization |
‐ RR 0.81 (0.72–0.90) P < 0.001 ‐ RR 0.79 (0.57–1.10) P > 0.05 |
|
RCTs only: ‐ All‐cause mortality ‐ HF hospitalization |
‐ RR 0.94 (0.67–1.32) P = 0.72 ‐ RR 0.90 (0.54–1.49) P = 0.68 |
||||
|
Meta‐analysis Khan et al. 40 |
2017 N = 17 284 (13 studies), HFpEF |
ACEI or ARB vs. placebo or standard therapy | 24.8 months |
All‐cause mortality ‐RCTs only ‐Observational studies |
‐ RR 1.02 (0.93–1.11) P = 0.68 ‐ RR 0.91 (0.87–0.95) P = 0.005 |
|
Meta‐analysis Zheng et al. 41 |
2017 N = 18 101 (25 RCTs), HFpEF |
‐ Beta‐blocker vs. placebo ‐ ACE‐Is vs. placebo ‐ ARBs vs. placebo ‐ MRA vs. placebo |
‐ | All‐cause mortality |
‐ RR: 0.78 (0.65–0.94) P = 0.008 ‐ RR: 1.10 (0.85–1.43) P = 0.46 ‐ RR: 1.02 (0.93–1.12) P = 0.71 ‐ RR: 0.92 (0.79–1.08) P = 0.32 |
|
CANVAS trials Neal et al. 51 |
2017 N = 10 142 (T2DM + CVD risk) |
Canagliflozin vs. placebo | 188.2 weeks | CVD death, non‐fatal MI or non‐fatal stroke | HR 0.86 (0.75–0.97) P < 0.001 (non‐inferiority); P = 0.02 (superiority) |
|
PARAGON‐HF Solomon et al. 42 |
2019 N = 4822, HFpEF |
Sacubitril–valsartan vs. valsartan | 35 months | HF hospitalization and CVD death | Rate ratio 0.87 (0.75–1.01) P = 0.06 |
|
DECLARE‐TIMI 58 Wiviott et al. 52 |
2019 N = 17 160, T2DM + CVD risk (HF N = 1724) |
Dapagliflozin or placebo | 4.2 years |
‐ MACE ‐ CVD death or HF hospitalization |
‐ Dapagliflozin non‐inferior to placebo (P < 0.001) ‐ HR 0.83 (0.73–0.95) P = 0.005 |
|
VITALITY‐HFpEF Armstrong et al. 64 |
2020 N = 789, HFpEF |
Vericiguat 15 and 10 mg/d vs. placebo | 24 weeks |
‐ KCCQ PLS least‐squares mean difference ‐ 6‐MW distance mean scores |
‐ Vericiguat 15 and 10 mg/d vs. placebo (P = 0.47 and P = 0.80) ‐ P = 0.45 (15 mg/d) and P = 0.81 (10 mg/d) |
|
SOLOIST‐WHF Bhatt DL et al. 59 |
2021 N = 1222 (T2DM + HF) (LVEF ≥50%, N = 256 patients) |
Sotagliflozin vs. placebo | 9.0 months | CVD death, HF hospitalization or urgent visit |
‐ All patients: HR 0.67 (0.52–0.85) P < 0.001 ‐ LVEF ≥50%: HR 0.48 (0.27–0.86) |
|
SCORED Bhatt et al. 60 |
2021 N = 10 584 (T2DM + CKD + CVD risk); (HF N = 3283, LVEF ≥50%, N = 1667) |
Sotagliflozin vs. placebo | 16 months | CVD death, HF hospitalization or urgent visit |
‐ All patients: HR 0.74 (0.63–0.88) P < 0.001 ‐ LVEF ≥50%: HR 0.72 (0.52–0.99) |
|
Pooled data SOLOIST‐WHF and SCORED, presented at ESC 2021 |
2021 N = 11 784 |
Sotagliflozin vs. placebo | 24 months | CVD death, HF hospitalization or urgent visit |
HFpEF: HR 0.73 (0.45–0.89) P = 0.009 All patients: HR 0.72 (0.63–0.82) P = 0.000002 |
|
EMPEROR‐Preserved Anker et al. 57 |
2021 N = 5988, HFpEF |
Empagliflozin (10 mg once daily) vs. placebo | 26.2 months |
‐ CVD death or HF hospitalization ‐ CVD death ‐ HF hospitalization |
‐ HR 0.79 (0.69–0.90) P < 0.001 ‐ HR 0.91 (0.76–.09) ‐ HR 0.73 (0.61–0.88) P < 0.001 |
|
Nassif et al. 65 |
2021 N = 324, HFpEF |
Dapagliflozin or placebo | 12 weeks |
‐ Improvement in KCCQ‐CS ‐ Improvement 6MWT |
‐ Improved KCCQ‐CS (P = 0.001) ‐ Improved 6MWT (P = 0.007) |
|
DELIVER Solomon et al. 58 |
2022 N = 6263, HFpEF |
Dapagliflozin vs. placebo | 2.3 years |
‐ Worsening HF or CVD death ‐ Worsening HF ‐ CVD death |
‐ HR 0.82 (0.73–0.92) P < 0.001 ‐ HR 0.79 (0.69–0.91) ‐ HR 0.88 (0.74–1.05) |
6MMT, 6‐min walk test; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; KCCQ‐CS, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid antagonist; OR, odds ratio; RR, relative risk; SCA, sudden cardiac arrest; T2DM, type 2 diabetes mellitus.
Results are effect size and 95% confidence intervals (in brackets).
Encouragingly, recent clinical trials of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors 50 , 51 , 52 , 53 , 54 have demonstrated significant reductions in cardiovascular mortality and heart failure hospitalization in type 2 diabetic patients and in the general heart failure population irrespective of diabetes, though the strongest benefits are seen in those with heart failure compared with those without heart failure 53 and in HFrEF patients compared with HFpEF. 54 Strong benefits associated with SGLT2 use are seen in HFrEF (DAPA‐HF and EMPEROR‐Reduced), depicting significant reduction in heart failure hospitalization or parenteral therapy for heart failure or cardiovascular death in HFrEF patients, irrespective of diabetes status. 55 , 56 Although the EMPEROR‐Preserved and DELIVER trials of SGLT2 inhibitor use in HFpEF patients depicted a significant reduction in risk of composite outcome of cardiovascular death or hospitalization for heart failure, this was mainly driven by reduction in heart failure hospitalization in the SGTLT2 inhibitor group and not by mortality. 57 , 58 The SOLOIST‐WHF and SCORED trials of combined SGLT2/SGLT1 inhibition in diabetic patients have shown some encouraging results (significant reduction in a composite of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure) in acutely decompensated heart failure patients, including a subgroup of patients with LVEF >50%. 59 , 60 There have also been reported favourable cardiovascular outcomes associated with the use glucagon‐like peptide‐1 (GLP‐1) receptor agonists in type 2 diabetic patients, but no specific clinical trials have been done on the HFpEF subgroup. 61 , 62 , 63 The above rubric indicates that beyond contemporary diuretics use for fluid retention and SGLT2 inhibitor use for morbidity benefit, specific therapeutic interventions with noteworthy prognostic significance for the management of HFpEF have remained elusive, and the search continues. Could tracking and treatment of arrhythmias be beneficial in HFpEF?
Possible impact of LVEF on treatment efficacy in HFpEF
HFpEF is a heterogeneous multisystem disease with a myriad of risks factors, potential aetiologies, pathophysiologic pathways, and multiple sub‐phenotypes. 66 There have been suggestions that LVEF is an effect modifier in treatment outcomes in HFpEF patients with declining benefit observed with increasing LVEF and specifically more benefit observed in patient with LVEF <60% compared with LVEF ≥60%. 57 Earlier studies of pharmacological therapies in HFpEF patients that were negative for the overall effect of the intervention to the primary outcomes suggested some benefit in the patients with lower LVEF categories. 42 , 67 , 68 For example, in the overall CHARM study, the risk of the primary outcome of cardiovascular death or HF hospitalization was significantly reduced in the candesartan compared with placebo in the HFrEF group (P < 0.001) and HMmrEF group (P = 0.02, but not HFpEF) (P = 0.57). 67 In the TOPCAT study, stronger estimated benefits of spironolactone were observed at the lower end of the ejection fraction spectrum with respect to the primary endpoint. 68 In the PARAGON‐HF trial, there was significant benefit of treatment with sacubitril–valsartan compared with valsartan only in patients with LVEF ≤57% (median value), but in not those with LVEF >57% in subgroup analysis. 42 More recently, in the EMPEROR‐Preserved trial, empagliflozin compared with placebo significantly reduced the risk of the primary outcome in HFpEF patients with LVEF <60%, but not in patients with LVEF ≥60%, with the LVEF effect appearing to be a continuum with up‐trending benefit observed with down‐trending LVEF. 57 However, in the DELIVER trial, it was observed that benefits of SGLT2 inhibitor dapagliflozin compared with placebo with respect to hospitalization for heart failure exacerbation or cardiovascular death were similar among patients with a LVEF ≥60% and those with LVEF <60%. 58 Based on the above findings, it will seem rational in clinical settings to suggest dapagliflozin in HFpEF patients with an LVEF ≥60% and not the others.
AF burden in HFpEF
AF is present in ~45% of HFpEF patients, and the presence of AF in these patients is associated with an increased risk of all‐cause mortality, heart failure hospitalization, and stroke. 9 , 10 , 69 , 70 , 71 , 72 The prevalence and incidence of AF is higher in the HFpEF population compared with HFrEF and appears to increase proportionally with increasing LVEF in the setting of heart failure. 9 , 70 , 71 , 73 Possible explanations of high concurrent occurrence of HFpEF and AF is the fact they share similar risk factors like hypertension, diabetes, and obesity, as well as the fact that elevated ventricular filling pressure seen in HFpEF might lead to left atrial dilatation, fibrosis, and AF. 73 , 74 It is also possible that HFpEF and AF are parallel manifestations of some myocardial or systemic disease. 74
Rhythm control with antiarrhythmic drugs and/or catheter ablation for AF in HFpEF
In HFpEF, left ventricular filling and preload are significantly dependent on atrial contraction to compensate for the poor ventricular compliance. The onset of AF results in loss of atrial systolic kick, increased left ventricular filling pressure, and thus setting the stage for possible decompensation of HFpEF. 75 Antiarrhythmic drugs (AAD) are effective in treatment of AF in HFpEF patients for medium‐term to long‐term maintenance of sinus rhythm and mortality, with class III AAD more frequently used in majority of studies followed by class IC and then class IA drugs. 75 , 76 , 77 , 78 Catheter ablation for AF is safe and appears to be effective in maintaining sinus rhythm in HFpEF patients similarly to what is seen in HFrEF patients, 79 , 80 and of equivalent efficacy to patients without heart failure. 81 There is accruing evidence from observational studies that compared with medical therapy, catheter ablation in HFpEF is associated with significant reduction is AF recurrence, 81 , 82 , 83 , 84 as well as heart failure hospitalization, the composite of heart failure hospitalization and mortality, and all‐cause mortality alone. 81 , 82 , 83 However, the efficacy of catheter ablation in HFpEF with respect to heart failure hospitalization and mortality is not universally observed. 84 A subgroup analysis of heart failure patients (55% were HFpEF) enrolled into the EAST‐AFNET4 trial showed that systematic early rhythm control therapy using AAD or catheter ablation compared with usual care was associated with significant reduction in the composite primary outcome of cardiovascular death, stroke, or hospitalization for heart failure or for acute coronary syndrome after median follow of 5 years. 85 In RAFAS trial where median LVEF was 63% and 62% in the early rhythm arm and usual care arm respectively, early rhythm control was associated with significant reduction in recurrence of AF and occurrence of ischaemic stroke at 12 months follow‐up, although no all‐cause mortality or any‐cause hospitalization benefit was observed. 86 However, it is uncertain how many patients had underlying heart failure. More RCTs of AF ablation in HFpEF‐specific patients with respect to survival outcome are warranted. Catheter ablation for AF in HFrEF patients has been shown to reduce mortality and heart failure hospitalization. 87 , 88
Malignant arrhythmias in HFpEF
Given that SCD properly adjudicated in large clinical trials accounted for 25–30% of total deaths in HFpEF patients, 12 , 18 , 20 SCD could potentially become a target for therapeutic interventions if specific underlying arrhythmias are identified. The incidence of SCD due to ventricular tachyarrhythmias or significant bradyarrhythmias can be reduced with ICD or pacemaker implantation or antiarrhythmic medication or combination of these. Knowing the actual risk of ventricular arrhythmias in specific HFpEF phenotype(s) will be a prerequisite to consideration of trial of VT ablation for example and other therapies. Recommendations have been made for long‐term cardiac rhythm monitoring to clarify mechanisms and mode of death in HFpEF patients. 12 , 18 , 20 In animal studies, spontaneous ventricular arrhythmias and SCD have been observed to be significantly higher in HFpEF animals compared with controls without heart failure. 11 In humans, these studies are scant, with a few published small‐size studies yielding somewhat conflicting results. 89 , 90 , 91
One recently published small sample size study of 113 patients (VIP‐HF study) consisting of combined HFmrEF and HFpEF patients implanted with implantable loop recorders (ILR) to capture incident tachyarrhythmias and bradyarrhythmias showed 0.6, 11.5, and 3.2 per 100 person‐years incidence of sustained VT, non‐sustained VT, and bradyarrhythmia, respectively, during a median follow‐up of 1.8 years. 89 The incidence of sustained ventricular tachyarrhythmias in this HFmrEF/HFpEF population was low, whereas the number of clinically relevant bradycardias was more than expected, though the findings were limited by small sample size as the study recruited less than half of the intended sample size. Another small study of 125 patients that monitored arrhythmias through a 14‐day ambulatory cardiac monitor or permanent pacemaker demonstrated that HFpEF patients might have a relatively high, and possibly under‐appreciated burden of non‐sustained ventricular tachycardia (NSVT), which conferred a higher risk of mortality. 90 In a small retrospective study of patients who underwent ambulatory cardiac monitoring, VT was more prevalent in HFpEF patients compared with patients without heart failure (37% vs. 16%, P = 0.001) and increased QTc interval was associated with risk of VT. 91 The findings of these two later small studies suggest that the HFpEF ventricular myocardium might be a substrate for elevated malignant arrhythmogenicity. QRS duration has been shown to be predictive of a composite of cardiovascular death, aborted cardiac arrest, or heart failure hospitalization in HFpEF patients. 92
There are no large sample size human studies of incident ventricular arrhythmias or conduction disease or clear heart rhythm at time of death in pure HFpEF patients with LVEF ≥50%, and it remains uncertain how many of these patients die from preventable arrhythmias. Clinical studies in this area are urgently needed to fill to huge knowledge gap and guide future management of HFpEF patients. We are unaware of any current ongoing large study designed to determine the prevalence, incidence, and risks of arrhythmias and their predictive effect on mortality including SCD in HFpEF patients or the mechanisms of SCD in these patients. Without understanding the above, it might not be possible to design intervention clinical trials aimed at reducing SCD in HFpEF patients.
SGLT2 inhibitor and arrhythmias
In clinical trials of SGLT2 inhibitors vs. placebo, both atrial arrhythmias and SCD were significantly reduced in the SGLT2 inhibitor‐treated group compared with placebo treatment group. In most of these trials, arrhythmias and SCD were reported as adverse clinical events in the safety profile section. 93 , 94 In a meta‐analysis of 34 randomized trials with 63 166 patients in patients with type 2 diabetes or heart failure, SGLT2 inhibitors were associated with a significant reduction in the risk of incident atrial arrhythmias and the ‘SCD’ component of the SCD outcome compared with control, but not ventricular arrhythmias. 93 Although majority of the studies included in this meta‐analysis were HFrEF, it is possible that the morbidity benefits observed in the HFpEF SGLT2i trials could be linked to reduction in incident arrhythmias. Future studies with specific arrhythmia outcomes are required. However, the molecular mechanisms of the SGLT2 inhibitors in association with arrhythmia reduction remain uncertain. 94
Predictors of SCD in HFpEF
Management of HFpEF is challenging given the heterogeneity of the disease and its multiple phenotypes. It will not be absurd to hypothesize that SCD is driven by malignant tachyarrhythmias or bradyarrhythmias. Determining the phenotype of HFpEF patients at highest risk of SCD and the mechanism(s) of SCD remain paramount prerequisites before the design and conduct of clinical intervention trials. Although prognostic scores and specific risk models for SCD have been identified in the HFpEF population, 95 , 96 , 97 , 98 , 99 specific predictors that could be directly modified to reduce SCD have remained elusive. To compound the situation, these risk scores also included non‐HFpEF patients, which raises the potential for non‐generalizability to pure HFpEF patients. A multivariable risk prediction model for SCD consisting of age, gender, history of myocardial infarction, history of diabetes mellitus, presence of left bundle branch block on electrocardiogram, and NT‐proBNP level in HFpEF patients was developed in a post hoc analysis of the I‐PRESERVE trial in 2014 99 and recently validated in the TOPCAT trial. 100 Another model with similar findings was still developed in the I‐PRESERVE trial and validated in the CHARM‐Preserved and TOPCAT trials. 98 Although these models made progress by identifying patients with HFpEF who have a greater risk of SCD over time, none of these predictors can be intervened upon acutely to prevent SCD, and the mechanism of SCD (arrhythmic vs. other causes) in HFpEF still remains unknown. The I‐PRESERVE, CHARM‐Preserve, and TOPCAT also included patients with mid‐range ejection fraction (HFmrEF), 98 , 99 which could slightly dilute the true HFpEF population defined as congestive heart failure with LVEF ≥50%. We hypothesize that the incidence of arrhythmias in HFpEF patients is significantly high and predictive of SCD and that arrhythmic mechanisms drive SCD. We also hypothesize that there is an identifiable risk model for specific HFpEF phenotypes at higher risk of SCD. Identified HFpEF patients at higher risk could be considered for clinical trials of electrophysiological (EP) study +/− cardiac implantable electronic device like an ICD or pacemaker and/or antiarrhythmic medications vs. conservative management as part of the search for efficacious evidence‐based therapeutic interventions. Pulmonary hypertension is common and present in majority of HFpEF patients and predictor of mortality in these patients. 101 Although isolated post‐capillary pulmonary hypertension is likely a complication HFpEF, it possible that the combined post‐capillary type could be part of the spectrum of the heterogeneous HFpEF disease. Pulmonary hypertension should be tested in future SCD risk prediction models in HFpEF. For a start, funding of future large studies to undertake long‐term cardiac monitoring in HFpEF patients at high risk of SCD will be key. This high‐risk group could be identified using the aforementioned multivariable risk prediction models for SCD,98, 99, 100 with the caveat that these models were developed in a study that also included HFmrEF patients and was not homogeneously classified as HFpEF. Therefore, there is the need for risk prediction models of SCD in pure HFpEF patients with LVEF of ≥50%, with possible consideration of testing parameters from cardiac MRI in future models.
Device therapies in HFpEF?
Prior studies have shown that HFpEF is significantly associated with chronotropic incompetence, which portends impaired aerobic capacity. 102 , 103 , 104 , 105 , 106 For example, chronotropic incompetence was significantly more common in patients with HFpEF compared with matched healthy controls as measured by the percentage of the heart rate reserve used during maximal exercise (63% vs. 2%, P = 0.001) and percentage of predicted maximal HR (34% vs. 2%, P = 0.001). 102 Based on these observations, it was felt that cardiac pacing may improve chronotropic function and thus overall functional capacity in HFpEF. 102 , 103 , 104 However, there are no published randomized control trials of pacing compared with no pacing in HFpEF patients with observed chronotropic incompetence. The myPACE study is the first randomized controlled study currently enrolling to test the hypothesis that atrial pacing at moderately higher heart rates compared with the standard backup setting of 60 bpm might provide important benefits for pacemaker patients with HFpEF. 107
The prevalence of permanent pacemaker (PPM) implantation in HFpEF varies between 8 and 18%. 104 , 108 , 109 In one large registry of ~14 000 HFpEF patients (LVEF >40%), the prevalence of PPM was 18%. The incidence of new pacemaker implantation within 1 year after index hospitalization for HFpEF exacerbation was approximately 7%, and PPM use was more common among older patients, men, patients with AF, and patients with wider QRS duration (≥140 ms). 104 These rates of pacemaker implantation in HFpEF are significantly higher than what is seen in the general adult population during routine clinical care, 108 , 110 suggesting a greater burden of conduction disease in these patients. Even though this might be true, there is also a chance that we observe a significant bias here as patients in need for pacemaker implantation are more likely to receive the diagnosis of HFpEF in contrast with the general population where the disease is largely underdiagnosed (especially elderly women). 66 However, it has been observed that the presence of PPM in HFpEF is associated with adverse clinical outcomes driven mainly by HF hospitalization, but not mortality, raising the concern that right ventricular (RV) pacing leading to ventricular dyssynchrony might be detrimental in HFpEF. 111 Albeit the non‐availability of details about RV pacing percentage in the above study, as well as data on the indication and mode of pacing, suggestions have been made that in HFpEF patients with atrioventricular block who have a pacing indication, a CRT might be preferable. Nonetheless, there are no prospective randomized control trials that have tested this hypothesis and further studies in this area are warranted. In patients with ventricular pacing indication and/or anticipated high‐percentage ventricular pacing and LVEF >35–50%, the BLOCK‐HF trial showed that implanting a biventricular pacemaker compared with RV pacing significantly improved the primary endpoint, which was a composite of death, urgent care visit for heart failure, or a 15% increase in LV end‐systolic volume index. 112 Meanwhile, the BIOPACE study revealed no superiority of biventricular pacing compared with RV pacing in patients with atrioventricular block and any LVEF (mean LVEF was 55.4%). 113
Currently, ICD implantation in HFpEF is only indicated for secondary prevention or high‐risk infiltrative or hypertrophic cardiomyopathies, whereas in HFrEF there are clear established guidelines for routine clinical use. 28 Also, in HFrEF, catheter ablation for VT is associated with a significant reduction in the odds of appropriate ICD therapies, appropriate ICD shocks, ventricular arrhythmia storms, and cardiac hospitalizations in patients with ischaemic cardiomyopathy. 114 Similarly, in non‐ischaemic cardiomyopathy, VT ablation for ventricular arrhythmias is associated with significant reduction in long‐term VT recurrences and cardiac death. 115 Data on the risk of ventricular arrhythmias in human HFpEF patients and their treatment are lacking. It is important therefore to determine the risk of ventricular arrhythmias in HFpEF as well as identifying a higher risk subgroup that could benefit from antiarrhythmic medications and possible device therapy. Studies in this area are highly encouraged. The effectiveness of catheter ablation for AF in HFpEF for morbidity benefit is now well known, 85 , 116 and a few observational studies suggest some mortality. 81 , 82 , 83
Conclusion and future directions
The true burden of lethal and non‐lethal (apart from AF) arrhythmias and their impact on morbidity and survival in HFpEF remain elusive. 9 , 10 , 69 , 70 , 71 , 72 It remains unknown whether SCD that accounts for 25–30% of all deaths in HFpEF patients is driven by malignant arrhythmias, and thus potentially preventable. 12 , 18 , 20 Studies aimed at determining the incidence and risk of arrhythmias as well as their predictive effect on mortality and heart failure hospitalization are urgently needed. The mechanisms of SCD are presently unexplored and unresolved, and development of novel therapies or re‐adoption of existing treatment strategies to prevent SCD in HFpEF patients will most likely be predicated on understanding these mechanisms. This renders full comprehension of the mechanisms of SCD a prerequisite before design of clinical interventions studies aimed at reducing SCD in HFpEF. Studies designed with objective of determining the mechanism of SCD (arrhythmic or non‐arrhythmic) are required. Given the prohibitive cost implications, if arrhythmia burden is found to significantly elevated and driving SCD, then a subgroup of HFpEF patients at elevated risk for SCD will need to be further identified for targeted treatment. Therefore, derivation of a risk prediction model for the phenotype of HFpEF at highest risk of malignant arrhythmias and SCD using the modern definition of HFpEF (heart failure with LVEF ≥50%), is also required. Exploration of new frontiers via randomized control trials like the utility of device therapy in HFpEF should be considered.
Conflict of interest
None.
Yuyun, M. F. , Kinlay, S. , Singh, J. P. , and Joseph, J. (2023) Are arrhythmias the drivers of sudden cardiac death in heart failure with preserved ejection fraction? A review. ESC Heart Failure, 10: 1555–1569. 10.1002/ehf2.14248.
References
- 1. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd‐Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe Z, Abera SF, Abil OZ, Abraha HN, Abu‐Raddad LJ, Abu‐Rmeileh NME, Accrombessi MMK, Acharya D, Acharya P, Ackerman IN, Adamu AA, Adebayo OM, Adekanmbi V, Adetokunboh OO, Adib MG, Adsuar JC, Afanvi KA, Afarideh M, Afshin A, Agarwal G, Agesa KM, Aggarwal R, Aghayan SA, Agrawal S, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akinyemiju T, Akseer N, al‐Aly Z, al‐Eyadhy A, al‐Mekhlafi HM, al‐Raddadi RM, Alahdab F, Alam K, Alam T, Alashi A, Alavian SM, Alene KA, Alijanzadeh M, Alizadeh‐Navaei R, Aljunid SM, Alkerwi A', Alla F, Allebeck P, Alouani MML, Altirkawi K, Alvis‐Guzman N, Amare AT, Aminde LN, Ammar W, Amoako YA, Anber NH, Andrei CL, Androudi S, Animut MD, Anjomshoa M, Ansha MG, Antonio CAT, Anwari P, Arabloo J, Arauz A, Aremu O, Ariani F, Armoon B, Ärnlöv J, Arora A, Artaman A, Aryal KK, Asayesh H, Asghar RJ, Ataro Z, Atre SR, Ausloos M, Avila‐Burgos L, Avokpaho EFGA, Awasthi A, Ayala Quintanilla BP, Ayer R, Azzopardi PS, Babazadeh A, Badali H, Badawi A, Bali AG, Ballesteros KE, Ballew SH, Banach M, Banoub JAM, Banstola A, Barac A, Barboza MA, Barker‐Collo SL, Bärnighausen TW, Barrero LH, Baune BT, Bazargan‐Hejazi S, Bedi N, Beghi E, Behzadifar M, Behzadifar M, Béjot Y, Belachew AB, Belay YA, Bell ML, Bello AK, Bensenor IM, Bernabe E, Bernstein RS, Beuran M, Beyranvand T, Bhala N, Bhattarai S, Bhaumik S, Bhutta ZA, Biadgo B, Bijani A, Bikbov B, Bilano V, Bililign N, Bin Sayeed MS, Bisanzio D, Blacker BF, Blyth FM, Bou‐Orm IR, Boufous S, Bourne R, Brady OJ, Brainin M, Brant LC, Brazinova A, Breitborde NJK, Brenner H, Briant PS, Briggs AM, Briko AN, Britton G, Brugha T, Buchbinder R, Busse R, Butt ZA, Cahuana‐Hurtado L, Cano J, Cárdenas R, Carrero JJ, Carter A, Carvalho F, Castañeda‐Orjuela CA, Castillo Rivas J, Castro F, Catalá‐López F, Cercy KM, Cerin E, Chaiah Y, Chang AR, Chang HY, Chang JC, Charlson FJ, Chattopadhyay A, Chattu VK, Chaturvedi P, Chiang PPC, Chin KL, Chitheer A, Choi JYJ, Chowdhury R, Christensen H, Christopher DJ, Cicuttini FM, Ciobanu LG, Cirillo M, Claro RM, Collado‐Mateo D, Cooper C, Coresh J, Cortesi PA, Cortinovis M, Costa M, Cousin E, Criqui MH, Cromwell EA, Cross M, Crump JA, Dadi AF, Dandona L, Dandona R, Dargan PI, Daryani A, das Gupta R, das Neves J, Dasa TT, Davey G, Davis AC, Davitoiu DV, de Courten B, de la Hoz FP, de Leo D, de Neve JW, Degefa MG, Degenhardt L, Deiparine S, Dellavalle RP, Demoz GT, Deribe K, Dervenis N, Des Jarlais DC, Dessie GA, Dey S, Dharmaratne SD, Dinberu MT, Dirac MA, Djalalinia S, Doan L, Dokova K, Doku DT, Dorsey ER, Doyle KE, Driscoll TR, Dubey M, Dubljanin E, Duken EE, Duncan BB, Duraes AR, Ebrahimi H, Ebrahimpour S, Echko MM, Edvardsson D, Effiong A, Ehrlich JR, El Bcheraoui C, El Sayed Zaki M, el‐Khatib Z, Elkout H, Elyazar IRF, Enayati A, Endries AY, Er B, Erskine HE, Eshrati B, Eskandarieh S, Esteghamati A, Esteghamati S, Fakhim H, Fallah Omrani V, Faramarzi M, Fareed M, Farhadi F, Farid TA, Farinha CS, Farioli A, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fentahun N, Fereshtehnejad SM, Fernandes E, Fernandes JC, Ferrari AJ, Feyissa GT, Filip I, Fischer F, Fitzmaurice C, Foigt NA, Foreman KJ, Fox J, Frank TD, Fukumoto T, Fullman N, Fürst T, Furtado JM, Futran ND, Gall S, Ganji M, Gankpe FG, Garcia‐Basteiro AL, Gardner WM, Gebre AK, Gebremedhin AT, Gebremichael TG, Gelano TF, Geleijnse JM, Genova‐Maleras R, Geramo YCD, Gething PW, Gezae KE, Ghadiri K, Ghasemi Falavarjani K, Ghasemi‐Kasman M, Ghimire M, Ghosh R, Ghoshal AG, Giampaoli S, Gill PS, Gill TK, Ginawi IA, Giussani G, Gnedovskaya EV, Goldberg EM, Goli S, Gómez‐Dantés H, Gona PN, Gopalani SV, Gorman TM, Goulart AC, Goulart BNG, Grada A, Grams ME, Grosso G, Gugnani HC, Guo Y, Gupta PC, Gupta R, Gupta R, Gupta T, Gyawali B, Haagsma JA, Hachinski V, Hafezi‐Nejad N, Haghparast Bidgoli H, Hagos TB, Hailu GB, Haj‐Mirzaian A, Haj‐Mirzaian A, Hamadeh RR, Hamidi S, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Hasan M, Hassankhani H, Hassen HY, Havmoeller R, Hawley CN, Hay RJ, Hay SI, Hedayatizadeh‐Omran A, Heibati B, Hendrie D, Henok A, Herteliu C, Heydarpour S, Hibstu DT, Hoang HT, Hoek HW, Hoffman HJ, Hole MK, Homaie Rad E, Hoogar P, Hosgood HD, Hosseini SM, Hosseinzadeh M, Hostiuc M, Hostiuc S, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Hu G, Huang JJ, Huynh CK, Iburg KM, Ikeda CT, Ileanu B, Ilesanmi OS, Iqbal U, Irvani SSN, Irvine CMS, Islam SMS, Islami F, Jacobsen KH, Jahangiry L, Jahanmehr N, Jain SK, Jakovljevic M, Javanbakht M, Jayatilleke AU, Jeemon P, Jha RP, Jha V, Ji JS, Johnson CO, Jonas JB, Jozwiak JJ, Jungari SB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kalani R, Kanchan T, Karami M, Karami Matin B, Karch A, Karema C, Karimi N, Karimi SM, Kasaeian A, Kassa DH, Kassa GM, Kassa TD, Kassebaum NJ, Katikireddi SV, Kawakami N, Karyani AK, Keighobadi MM, Keiyoro PN, Kemmer L, Kemp GR, Kengne AP, Keren A, Khader YS, Khafaei B, Khafaie MA, Khajavi A, Khalil IA, Khan EA, Khan MS, Khan MA, Khang YH, Khazaei M, Khoja AT, Khosravi A, Khosravi MH, Kiadaliri AA, Kiirithio DN, Kim CI, Kim D, Kim P, Kim YE, Kim YJ, Kimokoti RW, Kinfu Y, Kisa A, Kissimova‐Skarbek K, Kivimäki M, Knudsen AKS, Kocarnik JM, Kochhar S, Kokubo Y, Kolola T, Kopec JA, Kosen S, Kotsakis GA, Koul PA, Koyanagi A, Kravchenko MA, Krishan K, Krohn KJ, Kuate Defo B, Kucuk Bicer B, Kumar GA, Kumar M, Kyu HH, Lad DP, Lad SD, Lafranconi A, Lalloo R, Lallukka T, Lami FH, Lansingh VC, Latifi A, Lau KMM, Lazarus JV, Leasher JL, Ledesma JR, Lee PH, Leigh J, Leung J, Levi M, Lewycka S, Li S, Li Y, Liao Y, Liben ML, Lim LL, Lim SS, Liu S, Lodha R, Looker KJ, Lopez AD, Lorkowski S, Lotufo PA, Low N, Lozano R, Lucas TCD, Lucchesi LR, Lunevicius R, Lyons RA, Ma S, Macarayan ERK, Mackay MT, Madotto F, Magdy Abd el Razek H, Magdy Abd el Razek M, Maghavani DP, Mahotra NB, Mai HT, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Manda AL, Manguerra H, Manhertz T, Mansournia MA, Mantovani LG, Mapoma CC, Maravilla JC, Marcenes W, Marks A, Martins‐Melo FR, Martopullo I, März W, Marzan MB, Mashamba‐Thompson TP, Massenburg BB, Mathur MR, Matsushita K, Maulik PK, Mazidi M, McAlinden C, McGrath JJ, McKee M, Mehndiratta MM, Mehrotra R, Mehta KM, Mehta V, Mejia‐Rodriguez F, Mekonen T, Melese A, Melku M, Meltzer M, Memiah PTN, Memish ZA, Mendoza W, Mengistu DT, Mengistu G, Mensah GA, Mereta ST, Meretoja A, Meretoja TJ, Mestrovic T, Mezerji NMG, Miazgowski B, Miazgowski T, Millear AI, Miller TR, Miltz B, Mini GK, Mirarefin M, Mirrakhimov EM, Misganaw AT, Mitchell PB, Mitiku H, Moazen B, Mohajer B, Mohammad KA, Mohammadifard N, Mohammadnia‐Afrouzi M, Mohammed MA, Mohammed S, Mohebi F, Moitra M, Mokdad AH, Molokhia M, Monasta L, Moodley Y, Moosazadeh M, Moradi G, Moradi‐Lakeh M, Moradinazar M, Moraga P, Morawska L, Moreno Velásquez I, Morgado‐da‐Costa J, Morrison SD, Moschos MM, Mountjoy‐Venning WC, Mousavi SM, Mruts KB, Muche AA, Muchie KF, Mueller UO, Muhammed OS, Mukhopadhyay S, Muller K, Mumford JE, Murhekar M, Musa J, Musa KI, Mustafa G, Nabhan AF, Nagata C, Naghavi M, Naheed A, Nahvijou A, Naik G, Naik N, Najafi F, Naldi L, Nam HS, Nangia V, Nansseu JR, Nascimento BR, Natarajan G, Neamati N, Negoi I, Negoi RI, Neupane S, Newton CRJ, Ngunjiri JW, Nguyen AQ, Nguyen HT, Nguyen HLT, Nguyen HT, Nguyen LH, Nguyen M, Nguyen NB, Nguyen SH, Nichols E, Ningrum DNA, Nixon MR, Nolutshungu N, Nomura S, Norheim OF, Noroozi M, Norrving B, Noubiap JJ, Nouri HR, Nourollahpour Shiadeh M, Nowroozi MR, Nsoesie EO, Nyasulu PS, Odell CM, Ofori‐Asenso R, Ogbo FA, Oh IH, Oladimeji O, Olagunju AT, Olagunju TO, Olivares PR, Olsen HE, Olusanya BO, Ong KL, Ong SK, Oren E, Ortiz A, Ota E, Otstavnov SS, Øverland S, Owolabi MO, P A M , Pacella R, Pakpour AH, Pana A, Panda‐Jonas S, Parisi A, Park EK, Parry CDH, Patel S, Pati S, Patil ST, Patle A, Patton GC, Paturi VR, Paulson KR, Pearce N, Pereira DM, Perico N, Pesudovs K, Pham HQ, Phillips MR, Pigott DM, Pillay JD, Piradov MA, Pirsaheb M, Pishgar F, Plana‐Ripoll O, Plass D, Polinder S, Popova S, Postma MJ, Pourshams A, Poustchi H, Prabhakaran D, Prakash S, Prakash V, Purcell CA, Purwar MB, Qorbani M, Quistberg DA, Radfar A, Rafay A, Rafiei A, Rahim F, Rahimi K, Rahimi‐Movaghar A, Rahimi‐Movaghar V, Rahman M, Rahman MH, Rahman MA, Rahman SU, Rai RK, Rajati F, Ram U, Ranjan P, Ranta A, Rao PC, Rawaf DL, Rawaf S, Reddy KS, Reiner RC, Reinig N, Reitsma MB, Remuzzi G, Renzaho AMN, Resnikoff S, Rezaei S, Rezai MS, Ribeiro ALP, Roberts NLS, Robinson SR, Roever L, Ronfani L, Roshandel G, Rostami A, Roth GA, Roy A, Rubagotti E, Sachdev PS, Sadat N, Saddik B, Sadeghi E, Saeedi Moghaddam S, Safari H, Safari Y, Safari‐Faramani R, Safdarian M, Safi S, Safiri S, Sagar R, Sahebkar A, Sahraian MA, Sajadi HS, Salam N, Salama JS, Salamati P, Saleem K, Saleem Z, Salimi Y, Salomon JA, Salvi SS, Salz I, Samy AM, Sanabria J, Sang Y, Santomauro DF, Santos IS, Santos JV, Santric Milicevic MM, Sao Jose BP, Sardana M, Sarker AR, Sarrafzadegan N, Sartorius B, Sarvi S, Sathian B, Satpathy M, Sawant AR, Sawhney M, Saxena S, Saylan M, Schaeffner E, Schmidt MI, Schneider IJC, Schöttker B, Schwebel DC, Schwendicke F, Scott JG, Sekerija M, Sepanlou SG, Serván‐Mori E, Seyedmousavi S, Shabaninejad H, Shafieesabet A, Shahbazi M, Shaheen AA, Shaikh MA, Shams‐Beyranvand M, Shamsi M, Shamsizadeh M, Sharafi H, Sharafi K, Sharif M, Sharif‐Alhoseini M, Sharma M, Sharma R, She J, Sheikh A, Shi P, Shibuya K, Shigematsu M, Shiri R, Shirkoohi R, Shishani K, Shiue I, Shokraneh F, Shoman H, Shrime MG, Si S, Siabani S, Siddiqi TJ, Sigfusdottir ID, Sigurvinsdottir R, Silva JP, Silveira DGA, Singam NSV, Singh JA, Singh NP, Singh V, Sinha DN, Skiadaresi E, Slepak ELN, Sliwa K, Smith DL, Smith M, Soares Filho AM, Sobaih BH, Sobhani S, Sobngwi E, Soneji SS, Soofi M, Soosaraei M, Sorensen RJD, Soriano JB, Soyiri IN, Sposato LA, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stein DJ, Steiner C, Steiner TJ, Stokes MA, Stovner LJ, Subart ML, Sudaryanto A, Sufiyan M'B, Sunguya BF, Sur PJ, Sutradhar I, Sykes BL, Sylte DO, Tabarés‐Seisdedos R, Tadakamadla SK, Tadesse BT, Tandon N, Tassew SG, Tavakkoli M, Taveira N, Taylor HR, Tehrani‐Banihashemi A, Tekalign TG, Tekelemedhin SW, Tekle MG, Temesgen H, Temsah MH, Temsah O, Terkawi AS, Teweldemedhin M, Thankappan KR, Thomas N, Tilahun B, To QG, Tonelli M, Topor‐Madry R, Topouzis F, Torre AE, Tortajada‐Girbés M, Touvier M, Tovani‐Palone MR, Towbin JA, Tran BX, Tran KB, Troeger CE, Truelsen TC, Tsilimbaris MK, Tsoi D, Tudor Car L, Tuzcu EM, Ukwaja KN, Ullah I, Undurraga EA, Unutzer J, Updike RL, Usman MS, Uthman OA, Vaduganathan M, Vaezi A, Valdez PR, Varughese S, Vasankari TJ, Venketasubramanian N, Villafaina S, Violante FS, Vladimirov SK, Vlassov V, Vollset SE, Vosoughi K, Vujcic IS, Wagnew FS, Waheed Y, Waller SG, Wang Y, Wang YP, Weiderpass E, Weintraub RG, Weiss DJ, Weldegebreal F, Weldegwergs KG, Werdecker A, West TE, Whiteford HA, Widecka J, Wijeratne T, Wilner LB, Wilson S, Winkler AS, Wiyeh AB, Wiysonge CS, Wolfe CDA, Woolf AD, Wu S, Wu YC, Wyper GMA, Xavier D, Xu G, Yadgir S, Yadollahpour A, Yahyazadeh Jabbari SH, Yamada T, Yan LL, Yano Y, Yaseri M, Yasin YJ, Yeshaneh A, Yimer EM, Yip P, Yisma E, Yonemoto N, Yoon SJ, Yotebieng M, Younis MZ, Yousefifard M, Yu C, Zadnik V, Zaidi Z, Zaman SB, Zamani M, Zare Z, Zeleke AJ, Zenebe ZM, Zhang K, Zhao Z, Zhou M, Zodpey S, Zucker I, Vos T, Murray CJL. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories: a systematic analysis for the global burden of disease study 2017. The Lancet. 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner L, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG, American Heart Association Advocacy Coordinating Committee , Council on Arteriosclerosis, Thrombosis and Vascular Biology , Council on Cardiovascular Radiology and Intervention , Council on Clinical Cardiology , Council on Epidemiology and Prevention , Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013; 6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, di Tanna GL. A systematic review of medical costs associated with heart failure in the USA (2014‐2020). Pharmacoeconomics. 2020; 38: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, Butler J, de Jr C, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride P, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation , American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]
- 7. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol. 2017; 70: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 8. Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW, Wong SC, Kim LK. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med. 2016; 129: 635.e15–635.e26. [DOI] [PubMed] [Google Scholar]
- 9. Son MK, Park JJ, Lim NK, Kim WH, Choi DJ. Impact of atrial fibrillation in patients with heart failure and reduced, mid‐range or preserved ejection fraction. Heart. 2020; 106: 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goyal P, Almarzooq ZI, Cheung J, Kamel H, Krishnan U, Feldman DN, Horn EM, Kim LK. Atrial fibrillation and heart failure with preserved ejection fraction: insights on a unique clinical phenotype from a nationally‐representative United States cohort. Int J Cardiol. 2018; 266: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho JH, Zhang R, Aynaszyan S, Holm K, Goldhaber JI, Marbán E, Cingolani E. Ventricular arrhythmias underlie sudden death in rats with heart failure and preserved ejection fraction. Circ Arrhythm Electrophysiol. 2018; 11: e006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017; 69: 556–569. [DOI] [PubMed] [Google Scholar]
- 13. Anand V, Garg SK, Koene R, Thenappan T. Abstract 17286: national trends in hospital readmission rates in congestive heart failure patients. Circulation. 2016; 134: A17286‐A. [Google Scholar]
- 14. Khan MS, Sreenivasan J, Lateef N, Abougergi MS, Greene SJ, Ahmad T, Anker SD, Fonarow GC, Butler J. Trends in 30‐ and 90‐day readmission rates for heart failure. Circ Heart Fail. 2021; 14: e008335. [DOI] [PubMed] [Google Scholar]
- 15. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, Fonarow GC. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018; 3: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med. 2002; 347: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 17. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA. 2004; 292: 344–350. [DOI] [PubMed] [Google Scholar]
- 18. Manolis AS, Manolis AA, Manolis TA, Melita H. Sudden death in heart failure with preserved ejection fraction and beyond: an elusive target. Heart Fail Rev. 2019; 24: 847–866. [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019; 124: 1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaduganathan M, Michel A, Hall K, Mulligan C, Nodari S, Shah SJ, Senni M, Triggiani M, Butler J, Gheorghiade M. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail. 2016; 18: 54–65. [DOI] [PubMed] [Google Scholar]
- 21. Kupari M, Lindroos M, Iivanainen AM, Heikkilä J, Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4‐year prognosis in the Helsinki ageing study. J Intern Med. 1997; 241: 387–394. [DOI] [PubMed] [Google Scholar]
- 22. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. J Am Coll Cardiol. 1999; 33: 1948–1955. [DOI] [PubMed] [Google Scholar]
- 23. Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The cardiovascular health study. Ann Intern Med. 2002; 137: 631–639. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 25. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004; 95: 754–763. [DOI] [PubMed] [Google Scholar]
- 26. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996; 335: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 27. Nikolaidou T, Johnson MJ, Ghosh JM, Marincowitz C, Shah S, Lammiman MJ, Schilling RJ, Clark AL. Postmortem ICD interrogation in mode of death classification. J Cardiovasc Electrophysiol. 2018; 29: 573–583. [DOI] [PubMed] [Google Scholar]
- 28. al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018; 138: e272–e391. [DOI] [PubMed] [Google Scholar]
- 29. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved trial. Lancet. 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 30. Flather MD, Shibata MC, Coats AJS, van Veldhuisen D, Parkhomenko A, Borbola J, Cohen‐Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler‐Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole‐Wilson PA, SENIORS Investigators . Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005; 26: 215–225. [DOI] [PubMed] [Google Scholar]
- 31. Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J. 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 32. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure. Circulation. 2006; 114: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 34. Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008; 94: 573–580. [DOI] [PubMed] [Google Scholar]
- 35. Grimm RH, Davis BR, Piller LB, Cutler JA, Margolis KL, Barzilay J, Dart RA, Graumlich JF, Murden RA, Randall OS, for the ALLHAT Collaborative Research Group . Heart failure in ALLHAT: did blood pressure medication at study entry influence outcome? J Clin Hypertens (Greenwich). 2009; 11: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese diastolic heart failure study (J‐DHF). Eur J Heart Fail. 2013; 15: 110–118. [DOI] [PubMed] [Google Scholar]
- 37. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, RELAX Trial. Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013; 309: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo‐DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013; 309: 781–791. [DOI] [PubMed] [Google Scholar]
- 39. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 40. Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J. Renin‐angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Fail. 2017; 4: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, Ayis S. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Heart. 2018; 104: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen D, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 43. Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther. 2003; 17: 133–139. [DOI] [PubMed] [Google Scholar]
- 44. Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC. A randomized double‐blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010; 3: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S, ALLHAT Collaborative Research Group . Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Circulation. 2008; 118: 2259–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parthasarathy HK, Pieske B, Weisskopf M, Andrews CD, Brunel P, Struthers AD, MacDonald TM. A randomized, double‐blind, placebo‐controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail. 2009; 11: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Veldhuisen DJ, Cohen‐Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole‐Wilson PA, Flather MD, SENIORS Investigators . Beta‐blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure). J Am Coll Cardiol. 2009; 53: 2150–2158. [DOI] [PubMed] [Google Scholar]
- 48. Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta‐blockers in heart failure with preserved ejection fraction: a meta‐analysis. Heart Fail Rev. 2015; 20: 193–201. [DOI] [PubMed] [Google Scholar]
- 49. Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J. Effects of beta‐blockers on heart failure with preserved ejection fraction: a meta‐analysis. PLoS ONE. 2014; 9: e90555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 51. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 52. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire D, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 53. Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018; 138: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 55. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 56. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 57. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–la Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 58. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin‐Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer‐Gamba MA, al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022; 387: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 59. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 60. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG, SCORED Investigators . Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2020; 384: 129–139. [DOI] [PubMed] [Google Scholar]
- 61. Khan MS, Fonarow GC, McGuire DK, Hernandez AF, Vaduganathan M, Rosenstock J, Handelsman Y, Verma S, Anker SD, McMurray JJV, Kosiborod MN, Butler J. Glucagon‐like peptide 1 receptor agonists and heart failure. Circulation. 2020; 142: 1205–1218. [DOI] [PubMed] [Google Scholar]
- 62. Heuvelman VD, Van Raalte DH, Smits MM. Cardiovascular effects of glucagon‐like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovasc Res. 2020; 116: 916–930. [DOI] [PubMed] [Google Scholar]
- 63. Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP‐1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. 2018; 17: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J, VITALITY‐HFpEF Study Group . Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY‐HFpEF randomized clinical trial. JAMA. 2020; 324: 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M, Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021; 27: 1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah SJ, Borlaug BA, Kitzman DW, McCulloch AD, Blaxall BC, Agarwal R, Chirinos JA, Collins S, Deo RC, Gladwin MT, Granzier H, Hummel SL, Kass DA, Redfield MM, Sam F, Wang TJ, Desvigne‐Nickens P, Adhikari BB. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation. 2020; 141: 1001–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail. 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 68. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Satish M, Guddeti R, Wenzl F, Walters R, Alla VM. Abstract 12813: atrial fibrillation and heart failure with preserved ejection fraction: A systematic review and meta‐analysis. Circulation. 2020; 142: A12813‐A. [Google Scholar]
- 70. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. JACC Heart Fail. 2017; 5: 565–574. [DOI] [PubMed] [Google Scholar]
- 71. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ, Anand IS, O'Meara E, Rouleau JL, Sweitzer NK, Fang JC, Saksena S, Pitt B, Pfeffer MA, Solomon SD. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail. 2018; 6: 689–697. [DOI] [PubMed] [Google Scholar]
- 73. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 74. Packer M, Lam CSP, Lund LH, Redfield MM. Interdependence of atrial fibrillation and heart failure with a preserved ejection fraction reflects a common underlying atrial and ventricular myopathy. Circulation. 2020; 141: 4–6. [DOI] [PubMed] [Google Scholar]
- 75. Prasitlumkum N, Chokesuwattanaskul R, Cheungpasitporn W, Kewcharoen J, Thongprayoon C, Bathini T, Vallabhajosyula S, Jongnarangsin K. Rhythm control in patients with heart failure with preserved ejection fraction: a meta‐analysis. J Clin Med. 2021; 10: 4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kelly JP, DeVore AD, Wu J, Hammill BG, Sharma A, Cooper LB, Felker GM, Piccini JP, Allen LA, Heidenreich PA, Peterson ED, Yancy CW, Fonarow GC, Hernandez AF. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines‐heart failure. J Am Heart Assoc. 2019; 8: e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. al‐Jazairi MIH, Nguyen BO, de With RR, Smit MD, Weijs B, Hobbelt AH, Alings M, Tijssen JGP, Geelhoed B, Hillege HL, Tieleman RG, van Veldhuisen DJ, Crijns HJGM, van Gelder IC, Blaauw Y, Rienstra M, for the RACE 3 Investigators . Antiarrhythmic drugs in patients with early persistent atrial fibrillation and heart failure: results of the RACE 3 study. Europace. 2021; 23: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Al‐Sadawi M, Aleem S, Aslam F, Jacobs R, Stevens G, Almasry I, Singh A, Fan R, Rashba E. Rhythm versus rate control for atrial fibrillation in heart failure with preserved ejection fraction. Heart Rhythm O2. 2022; 3: 520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, Hoffmayer KS, Krummen D, Ho G, Raissi F, Birgersdotter‐Green U, Feld GK, Hsu JC. Meta‐analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021; 142: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Black‐Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, al‐Khatib SM, Atwater BD, Daubert JP, Frazier‐Mills C, Grant AO, Hegland DD, Jackson KP, Jackson LR, Koontz JI, Lewis RK, Sun AY, Thomas KL, Bahnson TD, Piccini JP. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018; 15: 651–657. [DOI] [PubMed] [Google Scholar]
- 81. Gu G, Wu J, Gao X, Liu M, Jin C, Xu Y. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction: a meta‐analysis. Clin Cardiol. 2022; 45: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rattka M, Kühberger A, Pott A, Stephan T, Weinmann K, Baumhardt M, Aktolga D, Teumer Y, Bothner C, Scharnbeck D, Rottbauer W, Dahme T. Catheter ablation for atrial fibrillation in HFpEF patients‐a propensity‐score‐matched analysis. J Cardiovasc Electrophysiol. 2021; 32: 2357–2367. [DOI] [PubMed] [Google Scholar]
- 83. Androulakis E, Sohrabi C, Briasoulis A, Bakogiannis C, Saberwal B, Siasos G, Tousoulis D, Ahsan S, Papageorgiou N. Catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta‐analysis. J Clin Med. 2022; 11: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arora S, Jaswaney R, Jani C, Zuzek Z, Thakkar S, Patel HP, Patel M, Patel N, Tripathi B, Lahewala S, Arora N, Josephson R, Osman MN, Hoit BD, Kowlgi G, Mulpuru SK, DeSimone CV, Viles‐Gonzalez J, Deshmukh A. Catheter ablation for atrial fibrillation in patients with concurrent heart failure. Am J Cardiol. 2020; 137: 45–54. [DOI] [PubMed] [Google Scholar]
- 85. Rillig A, Magnussen C, Ozga A‐K, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Gulizia M, Haegeli L, Heidbuchel H, Kuck KH, Ng A, Szumowski L, van Gelder I, Wegscheider K, Kirchhof P. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021; 144: 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Park J, Shim J, Lee JM, Park JK, Heo J, Chang Y, Song TJ, Kim DH, Lee HA, Yu HT, Kim TH, Uhm JS, Kim YD, Nam HS, Joung B, Lee MH, Heo JH, Pak HN, for the RAFAS Investigators* . Risks and benefits of early rhythm control in patients with acute strokes and atrial fibrillation: a multicenter, prospective, randomized study (the RAFAS trial). J Am Heart Assoc. 2022; 11: e023391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 88. AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019; 19: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Veldhuisen DJ, van Woerden G, Gorter TM, van Empel VPM, Manintveld OC, Tieleman RG, Maass AH, Vernooy K, Westenbrink BD, Gelder IC, Rienstra M. Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: the VIP‐HF study. Eur J Heart Fail. 2020; 22: 1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gutierrez A, Ash J, Akdemir B, Alexy T, Cogswell R, Chen J, Adabag S. Nonsustained ventricular tachycardia in heart failure with preserved ejection fraction. Pacing Clin Electrophysiol. 2020; 43: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 91. Cho JH, Leong D, Ebinger J, Yoon S‐H, Bresee C, Ehdaie A, Shehata M, Wang X, Chugh SS, Marban E, Cingolani E. Rhythm disturbance in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2020; 75: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Joseph J, Claggett BC, Anand IS, Fleg JL, Huynh T, Desai AS, Solomon SD, O'Meara E, Mckinlay S, Pitt B, Pfeffer MA, Lewis EF. QRS duration is a predictor of adverse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2016; 4: 477–486. [DOI] [PubMed] [Google Scholar]
- 93. Fernandes GC, Fernandes A, Cardoso R, Penalver J, Knijnik L, Mitrani RD, Myerburg RJ, Goldberger JJ. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta‐analysis of 34 randomized controlled trials. Heart Rhythm. 2021; 18: 1098–1105. [DOI] [PubMed] [Google Scholar]
- 94. Kolesnik E, Scherr D, Rohrer U, Benedikt M, Manninger M, Sourij H, von Lewinski D. SGLT2 inhibitors and their antiarrhythmic properties. Int J Mol Sci. 2022; 23: 1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ferrero P, Iacovoni A, D'Elia E, Vaduganathan M, Gavazzi A, Senni M. Prognostic scores in heart failure ‐ critical appraisal and practical use. Int J Cardiol. 2015; 188: 1–9. [DOI] [PubMed] [Google Scholar]
- 96. Sharma K, Mok Y, Kwak L, Agarwal SK, Chang PP, Deswal A, Shah AM, Kitzman DW, Wruck LM, Loehr LR, Heiss G, Coresh J, Rosamond WD, Solomon SD, Matsushita K, Russell SD. Predictors of mortality by sex and race in heart failure with preserved ejection fraction: ARIC community surveillance study. J Am Heart Assoc. 2020; 9: e014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jones RC, Francis GS, Lauer MS. Predictors of mortality in patients with heart failure and preserved systolic function in the Digitalis investigation group trial. J Am Coll Cardiol. 2004; 44: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 98. Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, Granger CB, Køber L, Komajda M, McKelvie RS, Pfeffer MA, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Developing and validating models to predict sudden death and pump failure death in patients with heart failure and preserved ejection fraction. Clin Res Cardiol. 2021; 110: 1234–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014; 16: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 100. Adabag S, Langsetmo L. Sudden cardiac death risk prediction in heart failure with preserved ejection fraction. Heart Rhythm. 2020; 17: 358–364. [DOI] [PubMed] [Google Scholar]
- 101. Hoeper MM, Lam CSP, Vachiery JL, Bauersachs J, Gerges C, Lang IM, Bonderman D, Olsson KM, Gibbs JSR, Dorfmuller P, Guazzi M, Galiè N, Manes A, Handoko ML, Vonk‐Noordegraaf A, Lankeit M, Konstantinides S, Wachter R, Opitz C, Rosenkranz S. Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further research. Eur Heart J. 2017; 38: 2869–2873. [DOI] [PubMed] [Google Scholar]
- 102. Phan TT, Shivu GN, Abozguia K, Davies C, Nassimizadeh M, Jimenez D, Weaver R, Ahmed I, Frenneaux M. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2010; 3: 29–34. [DOI] [PubMed] [Google Scholar]
- 103. Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006; 114: 2138–2147. [DOI] [PubMed] [Google Scholar]
- 104. Khazanie P, Hellkamp AS, Fonarow GC, Curtis LH, Al‐Khatib SM, Hernandez AF. Permanent pacemaker use among patients with heart failure and preserved ejection fraction: findings from the acute decompensated heart failure national registry (ADHERE) national registry. Am Heart J. 2018; 198: 123–128. [DOI] [PubMed] [Google Scholar]
- 105. Sarma S, Stoller D, Hendrix J, Howden E, Lawley J, Livingston S, Adams‐Huet B, Holmes C, Goldstein DS, Levine BD. Mechanisms of chronotropic incompetence in heart failure with preserved ejection fraction. Circ Heart Fail. 2020; 13: e006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Palau P, Domínguez E, Seller J, Sastre C, Bayés‐Genís A, Núñez J. Chronotropic incompetence predicts distance walked in six‐minute walk test in heart failure with preserved ejection fraction. J Card Fail. 2020; 26: 1024–1025. [DOI] [PubMed] [Google Scholar]
- 107. Infeld M, Wahlberg K, Cicero J, Meagher S, Habel N, Muthu Krishnan A, Silverman DN, Lustgarten DL, Meyer M. Personalized pacing for diastolic dysfunction and heart failure with preserved ejection fraction: design and rationale for the myPACE randomized controlled trial. Heart Rhythm O2. 2022; 3: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT, Pavri BB, Kurtz SM. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012; 60: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 109. Lund LH, Benson L, Dahlström U, Edner M, Friberg L. Association between use of β‐blockers and outcomes in patients with heart failure and preserved ejection fraction. JAMA. 2014; 312: 2008–2018. [DOI] [PubMed] [Google Scholar]
- 110. Uslan DZ, Tleyjeh IM, Baddour LM, Friedman PA, Jenkins SM, St Sauver JL, Hayes DL. Temporal trends in permanent pacemaker implantation: a population‐based study. Am Heart J. 2008; 155: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shen L, Jhund PS, Docherty KF, Petrie MC, Anand IS, Carson PE, Desai AS, Granger CB, Komajda M, McKelvie RS, Pfeffer MA, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Prior pacemaker implantation and clinical outcomes in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2019; 7: 418–427. [DOI] [PubMed] [Google Scholar]
- 112. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, Shinn T, St. John Sutton M. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013; 368: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 113. Coordinators Btia . BioPace (Biventricular pacing for atrio‐ventricular BlOck to Prevent cArdiaC dEsynchronization) trial preliminaary results. Presented at: European Society of Cardiology Congress, Barcelona, Spain, 1 September 2014. 2014.
- 114. Martinez BK, Baker WL, Konopka A, Giannelli D, Coleman CI, Kluger J, Cronin EM. Systematic review and meta‐analysis of catheter ablation of ventricular tachycardia in ischemic heart disease. Heart Rhythm. 2020; 17: e206–e219. [DOI] [PubMed] [Google Scholar]
- 115. Okubo K, Gigli L, Trevisi N, Foppoli L, Radinovic A, Bisceglia C, Frontera A, D'Angelo G, Cireddu M, Paglino G, Mazzone P, Della Bella P. Long‐term outcome after ventricular tachycardia ablation in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2020; 13: e008307. [DOI] [PubMed] [Google Scholar]
- 116. Aldaas OM, Lupercio F, Malladi CL, Mylavarapu PS, Darden D, Han F, Hoffmayer KS, Krummen D, Ho G, Raissi F, Feld GK, Hsu JC. Catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur Heart J. 2020; 41. [Google Scholar]


