Abstract
Aims
Heart failure (HF) is one of the leading causes of cardiovascular morbidity and mortality. HF with preserved ejection fraction (HFpEF), or diastolic failure, accounts for half of all HF cases and differs from HF with reduced ejection fraction (HFrEF). Patients with HFpEF are typically older, female, and commonly seen with chronic kidney disease (CKD), one of the leading independent risk factors for mortality in these patients. Unfortunately, drugs that had shown significant improvements in mortality in HFrEF have not shown similar benefits in HFpEF. Recently, sodium glucose transporter 2 inhibitors (SGLT2i) have been shown to reduce cardiovascular morbidity and mortality in HFrEF patients and slow down CKD progression. This study aimed to elucidate the impact of this drug class on mortality and risk of end stage renal disease in patients with HFpEF, which is currently unclear.
Methods and results
We retrospectively analysed the Research Data Warehouse containing electronic health records from de‐identified patients (n = 1 266 290) from the University of Mississippi Medical Center from 2013 to 2022. HFpEF patients had an average follow‐up of 4 ± 2 years. Factors associated with increased all‐cause mortality during HFpEF included age, male sex, and CKD. Interestingly, the only treatments associated with significant improvements in survival were angiotensin converting enzyme inhibitors/angiotensin receptor blockers and SGLT2i, regardless of CKD or diabetes status. Additionally, SGLT2i use was also associated with significant decrease in the risk of end stage renal disease.
Conclusions
Our results support the use of SGLT2i in an HFpEF population with relatively high rates of hypertension, CKD, and black race and suggests that improvements in mortality may be through preserving kidney function.
Keywords: Heart failure with preserved ejection fraction, Heart failure, SGLT2 inhibitors, Antihypertensive therapy, Chronic kidney disease, End stage renal disease
Introduction
Heart failure (HF) is growing in the United States and is estimated to have a 5 year mortality of 40%. 1 HF can present with preserved ejection fraction (HFpEF) or diastolic failure, which differs from HF with reduced ejection fraction (HFrEF) epidemiologically and mechanistically. HFpEF accounts for half of all HF cases with similar mortality as HFrEF. HFpEF patients usually present with increased myocardial stiffness, hypertension, left ventricular concentric hypertrophy, and impaired diastolic function. Further, approximately 50–60% of HFpEF patients have chronic kidney disease (CKD), 2 which is a significant independent predictor of mortality in these patients. 3
Hypertension is the most important attributable risk factor for diastolic dysfunction, with most of the current treatments being antihypertensive therapies. Treatments that have shown significant efficacy in HFrEF include beta‐blockers (BB), angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), diuretics, mineralocorticoid antagonists (MRA), vasodilators, and angiotensin receptor blocker‐neprilysin inhibitors (ARNI). However, these therapies have not convincingly shown a reduction in cardiovascular morbidity or mortality in HFpEF patients.
Sodium glucose transporter 2 inhibitors (SGLT2i) have been classically used as an antidiabetic drug due to its ability to increase glucose excretion and other favourable effects on body weight, blood pressure, and kidney disease progression. 4 Indeed, SGLT2i have been shown to significantly slow renal disease and reduce the risk of end stage renal disease (ESRD) in patients with type 2 diabetes (T2D) and in HFrEF patients independent of diabetes. 5 , 6 , 7 In fact, in 2021, the FDA approved the SGLT2i dapagliflozin in CKD patients, including non‐diabetics, to reduce CKD progression and death. 8 , 9 In the more recent EMPEROR‐Preserved trial, approximately 2 years of SGLT2i treatment was shown to significantly slow down the decline in renal function and reduce HF hospitalizations in HFpEF patients. 10 However, SGLT2i has not yet been shown to significantly improve mortality or risk of ESRD in patients with HFpEF, leaving the utility of this treatment and its impact on renal disease progression in this population unclear. 10 Further, SGLT2i use in the black population has not been well characterized and requires further investigation. Therefore, we used a large Research Data Warehouse (RDW) containing over 1 million patients and 30 million unique encounters to investigate the impact of common HF drugs on the all‐cause mortality in patients diagnosed with HFpEF. We hypothesized that SGLT2i would reduce mortality in these patients through renal protective mechanisms and that this effect persists regardless of race.
Methods
Study population
The RDW contains >45 million electronic health records (EHR) from >1 million unique patients from the University of Mississippi Medical Center (UMMC), a large academic health science centre. Patient data are de‐identified are extracted from the EHR system, Epic, and are exempt from Institutional Review Board approval. 11 Data from 1 January 2013 to 31 December 2021 were retrieved from patients ≥18 years old. There were 35 803 patients with an HF diagnosis with 6 016 566 unique encounters. Of these, 5569 patients had a positive diagnosis for HFpEF or diastolic dysfunction without any systolic HF or HFrEF diagnoses or record of ejection fraction <40% at baseline or throughout follow‐up. Of these HFpEF patients, 2368 patients had at least 20 visits over the course of a year or longer at UMMC clinics to select patients that were dependent on UMMC for regular medical care. Clinical diagnoses for hypertension, CKD, ESRD, T2D, myocardial infarction (MI), proteinuria, and pulmonary hypertension were also queried.
HFpEF therapies of interest included BBs (carvedilol 3–6 mg; metoprolol 25–200 mg; labetalol 100–300 mg; nadolol 20–40 mg; nebivolol 2.5–20 mg; propranolol 10–60 mg; sotalol 80 mg), ARNI (sacubitril/valsartan 24/26 mg to 97/103 mg), MRA (eplerenone 25–50 mg; spironolactone 25–100 mg), vasodilators (amlodipine 2.5–10 mg; verapamil 120–240 mg; diltiazem 30–300 mg; hydralazine 10–100 mg; minoxidil 2.5–10 mg; nifedipine 20–90 mg), diuretics (chlorthalidone 25–50 mg; chlorothiazide 250–500 mg; hydrochlorothiazide 12.5–25 mg; indapamide 2.5 mg; metolazone 2.5–10 mg), and ACEi/ARB (benazepril 10–40 mg; valsartan 40–320 mg; candesartan 4–16 mg; captopril 6–25 mg; enalapril 2.5–20 mg; irbesartan 150–300 mg; lisinopril 2.5–40 mg; losartan 25–100 mg; olmesartan 20–40 mg; quinapril 20–40 mg; ramipril 2.5–10 mg; telmisartan 40–80 mg; valsartan 40–320 mg), and SGLT2i (canagliflozin 100–300 mg; dapagliflozin 5–10 mg; empagliflozin 5–25 mg; ertugliflozin 5 mg). Patients taking a combination of the above‐mentioned formulations were flagged as taking both classes. Only patients that were prescribed a drug for at least 90 days during the follow‐up were considered for a given drug class.
Statistical analysis
Baseline characteristics were summarized as means ± standard deviations and compared with t‐tests for continuous factors. Categorical factors are summarized as percentages and compared using Fisher's exact test. Survival and ESRD event curves were plotted and stratified by each drug using the Kaplan–Meier method and compared using a Cox proportional hazards regression model. Models measuring survival time were adjusted for sex, age, and race along with the presence of hypertension, T2D, and CKD. Models measuring time to first ESRD diagnosis included these same adjusters except for presence of CKD due to perfect correlation with the outcome. The proportional hazards assumption was assessed for each model fit, and results were reported in the form of hazard ratios (HRs) with their respective 95% confidence intervals (CIs) and P‐values. Similarly, survival curves were plotted with bands representing 95% CIs. We considered P‐values <0.05 to indicate a statistically significant difference. All statistical analyses were performed with R version 4.2.1.
Results
Of the 1 266 290 total patients in the RDW, 2368 patients had a positive diagnosis for HFpEF or diastolic HF without any systolic HF or HFrEF diagnoses or record of ejection fraction <40%. These patients were mostly female (63%), obese (35 ± 10 kg/m2 body mass index) had normal ejection fraction (65%) and an average of 177 ± 196 total UMMC visits over an average follow‐up of 4 ± 2 years (Table 1 ). This population was also associated with a relatively high prevalence of hypertension (87%), CKD (58%), and T2D (56%). Overall mortality of the entire cohort was 16%.
Table 1.
Baseline characteristics of heart failure with preserved ejection fraction patients stratified by race and chronic kidney disease.
| Variable | All (n = 2368) | White | Black | ||
|---|---|---|---|---|---|
| HFpEF (n = 488) | HFpEF + CKD (n = 490) | HFpEF (n = 505) | HFpEF + CKD (n = 850) | ||
| Follow‐up (year) | 4.0 ± 2 | 3.7 ± 2 | 3.6 ± 2 | 4.1 ± 2 # | 4.4 ± 2*, # |
| Visits (#) | 177 ± 196 | 125 ± 127 | 186 ± 187* | 130 ± 119 | 229 ± 249*, # |
| Mortality (%) | 16 | 12 | 23* | 11 | 18*, # |
| Hospitalization (%) | 75 | 65 | 82* | 63 | 84* |
| Age (year) | 64 ± 14 | 69 ± 13 | 70 ± 12 | 60 ± 14 # | 60 ± 14 # |
| Female (%) | 63 | 62 | 53* | 74 # | 63*, # |
| SBP (mmHg) | 140 ± 24 | 133 ± 21 | 134 ± 22 | 144 ± 23 # | 148 ± 25 # |
| DBP (mmHg) | 76 ± 14 | 72 ± 13 | 72 ± 12 | 81 ± 14 # | 79 ± 15 # |
| HR (b.p.m.) | 77 ± 15 | 77 ± 16 | 75 ± 16 | 80 ± 15 | 78 ± 13 |
| BMI (kg/m2) | 35 ± 10 | 32 ± 9 | 32 ± 9 | 38 ± 9 # | 37 ± 9 # |
| EF (%) | 65 ± 10 | 59 ± 13 | 65 ± 9 | 63 ± 11 | 69 ± 7 |
| HTN drugs (#) | 2.1 ± 1.5 | 1.6 ± 1.4 | 1.9 ± 1.4* | 2.2 ± 1.5 # | 2.5 ± 1.4*, # |
| Creatinine (mg/dL) | 1.8 ± 2.2 | 0.9 ± 0.3 | 1.6 ± 1.5* | 0.9 ± 2.2 | 3.0 ± 3.1*, # |
| Co‐morbidities | |||||
| HTN (%) | 87 | 74 | 88* | 86 # | 94*, # |
| T2D (%) | 56 | 41 | 53* | 50 # | 71*, # |
| MI (%) | 22 | 21 | 30* | 15 # | 22*, # |
| ESRD (%) | 18 | 0 | 14* | 0 | 42*, # |
| Proteinuria (%) | 14 | 1 | 11* | 3 # | 29*, # |
| Pulmonary HTN (%) | 19 | 17 | 19 | 16 | 23* |
Note: HFpEF indicates heart failure with preserved ejection fraction. Mortality and hospitalization represent unadjusted rates for any cause. Mean ± SD are shown.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease before or during follow‐up; DBP, diastolic blood pressure; EF, ejection fraction; ESRD, end stage renal disease before or during follow‐up; HR, heart rate; HTN, hypertension; MI, myocardial infarction previously or during follow‐up; SBP, systolic blood pressure; T2D, type 2 diabetes.
P < 0.05 vs. HFpEF.
P < 0.05 vs. White.
As compared with White patients, Black patients with or without CKD were associated with significantly lower age, were more female, and had higher blood pressure, increased body mass index, and increased number of prescribed drugs. Black patients were also associated with higher prevalence of hypertension, T2D, and proteinuria, but lower MI prevalence. In both White and Black populations, CKD was associated with more clinical visits, higher age, and higher plasma creatinine (Table 1 ). This CKD population was also more likely to be hypertensive, diabetic, proteinuric, and have a MI. Table 2 reports the adjusted Cox models for variables that significantly increased the risk of all‐cause mortality. These factors included age (HR 1.30 for each decade), male sex (HR 1.47), and CKD status (HR 1.68). Conversely, race, hypertension status, and diabetic status did not significantly increase the risk of death (Table 2 ). However, age, sex, race, hypertension status, and diabetes status all increased the risk of ESRD.
Table 2.
Adjusted Cox models for all‐cause mortality and end stage renal disease.
| Variable | Death from any cause | End stage renal disease | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age | 1.03 (1.02–1.04) | <0.001 | 0.97 (0.97–0.98) | <0.001 |
| Sex | ||||
| Female | ‐ | ‐ | ‐ | ‐ |
| Male | 1.47 (1.19–1.80) | <0.001 | 1.57 (1.22–2.02) | <0.001 |
| Race | ||||
| Black | ‐ | ‐ | ‐ | ‐ |
| White | 1.20 (0.96–1.49) | 0.11 | 0.37 (0.27–0.52) | <0.001 |
| HTN | 0.92 (0.65–1.30) | 0.65 | 3.46 (1.63–7.37) | 0.001 |
| T2D | 0.81 (0.66–1.01) | 0.06 | 2.85 (2.09–3.89) | <0.001 |
| CKD | 1.68 (1.34–2.11) | <0.001 | ‐ | ‐ |
Note: HTN indicates hypertension diagnosis. CKD was removed from the end stage renal disease analysis.
Abbreviations: CKD, chronic kidney disease; T2D, type 2 diabetes.
HFpEF treatments and their associated risk of ESRD development are shown in Figure 1 . BB, vasodilator, and diuretic use was associated with increased risk of ESRD after adjusting for age, sex, race, hypertension status, and diabetes status (HR 1.79, 2.35, and 1.32, respectively). MRA reduced the risk of ESRD (HR 0.69 [95% CI: 0.49–0.98], P = 0.036) (Figure 1 ). ARNI and ACEi/ARB were not associated with significant changes in ESRD risk.
Figure 1.
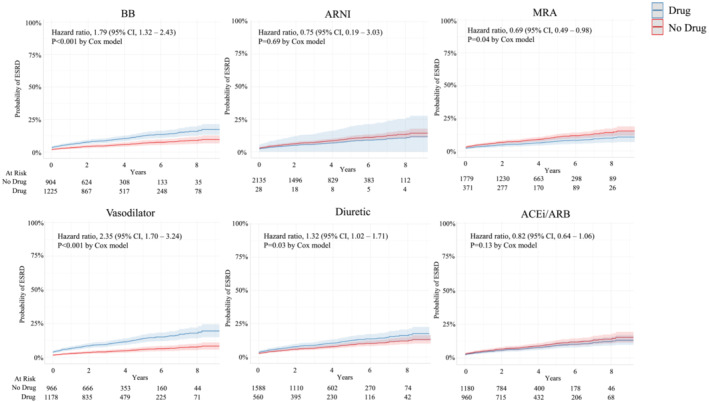
Risk of end stage renal disease in heart failure with preserved ejection fraction stratified by drug class after adjusting for age, sex, race, hypertension status, and diabetes status.
Figure 2 shows the probability of survival in patients prescribed the different treatments after adjusting for age, sex, race, and status of hypertension, diabetes, and CKD. The inclusion criteria for patients being seen at UMMC for at least 1 year are reflected in the 100% survival at 1 year (Figure 2 ). ACEi/ARB was associated with a 25% lower risk of death (HR 0.75 [95% CI: 0.60–0.92], P = 0.008). BB, ARNI, MRA, vasodilator, or diuretic use was not associated with any significant effects on all‐cause mortality, although there was a trend for ARNI to reduce the risk of death (HR 0.37 [95% CI: 0.09–1.48]), but this did not reach statistical significance (P = 0.16) (Figure 2 ). After adjusting for sex, age, race, hypertension, and T2D there was an 87% lower risk of ESRD for those being treated with SGLT2i compared with those that were not (HR 0.13 [95% CI: 0.04–0.42], P = 0.007) (Figure 3 ). After adjusting for sex, age, race, hypertension, CKD, and T2D there was a 93% lower risk of death for those treated with SGLT2i (HR 0.07 [95% CI: 0.01–0.47], P = 0.007) (Figure 3 ).
Figure 2.
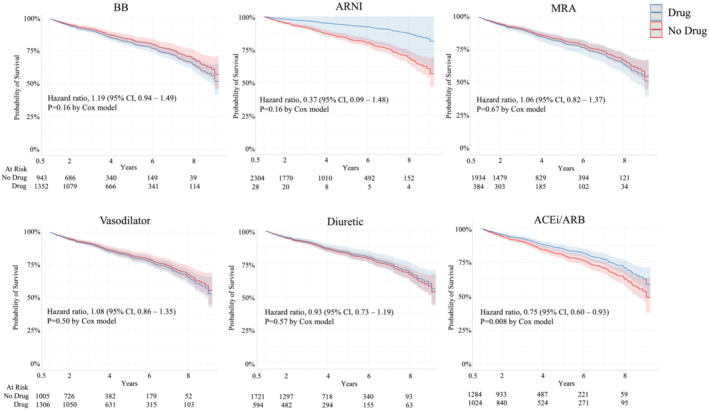
Risk of all‐cause mortality in patients with heart failure with preserved ejection fraction stratified by drug class after adjusting for age, sex, race, hypertension status, diabetes status, and chronic kidney disease status.
Figure 3.
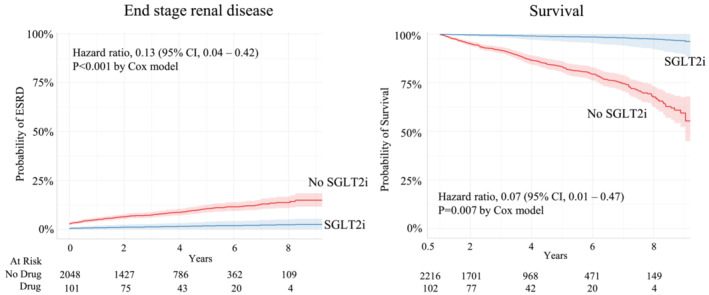
Risk of end stage renal disease and all‐cause mortality in heart failure with preserved ejection fraction patients prescribed SGLT2i after adjusting for age, sex, race, hypertension status, and diabetes status. Kaplan–Meier curves for mortality were also adjusted for chronic kidney disease status.
As compared with White HFpEF patients, there was a significantly higher portion of Black patients prescribed SGLT2i, BB, vasodilators, and ACEi/ARB (Table 3 ). CKD patients were more likely to be prescribed BB and vasodilators. There were no race–drug interactions aside from a 43% reduced risk of death for Black patients that were not prescribed a BB as compared with Black patients that were prescribed a BB (Figure S1 ). This effect was not seen in White patients.
Table 3.
Drug prescriptions among White and Black heart failure with preserved ejection fraction patients with or without chronic kidney disease.
| Variable | All (n = 2368) | White | Black | ||
|---|---|---|---|---|---|
| HFpEF (n = 488) | HFpEF + CKD (n = 490) | HFpEF (n = 505) | HFpEF + CKD (n = 850) | ||
| Drugs | |||||
| SGLT2i (%) | 5 | 3 | 3 | 6 | 6 # |
| ARNI (%) | 1 | 2 | 2 | 1 | 1 # |
| BB (%) | 60 | 46 | 58* | 56 # | 70*, # |
| Vasodilator (%) | 57 | 40 | 52* | 53 # | 72*, # |
| Diuretic (%) | 26 | 15 | 19 | 34 # | 32 # |
| ACEi/ARB (%) | 45 | 35 | 40 | 51 # | 50 # |
| MRA (%) | 17 | 19 | 18 | 16 | 16 |
Note: HFpEF indicates heart failure with preserved ejection fraction. Only drugs that were prescribed for at least 90 days were considered.
Abbreviations: ACEi/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta‐blocker; CKD, chronic kidney disease before or during follow‐up; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium glucose transport inhibitor.
P < 0.05 vs. HFpEF.
P < 0.05 vs. White.
Discussion
Although survival for HFrEF has improved over the past 20 years, there has been no improvement in HFpEF survival. 12 Indeed, therapies that have shown a significant impact in HFrEF have not translated well in HFpEF. In a primarily Black hypertensive population, the current study demonstrated that HFpEF was associated with significantly high prevalence of hypertension, diabetes, CKD, and mortality. However, patients prescribed either ACEi/ARB or SGLT2i were associated with a significantly decreased risk of all‐cause mortality (25% and 93%, respectively). This effect of SGLT2i was significantly associated with an 87% reduced risk of ESRD. These data warrant further investigation into the use of these drugs in non‐diabetic HFpEF, especially in Black patients.
SGLT2i have been shown to reduce morbidity and mortality in HFrEF. 13 However, few clinical trials have investigated this drug in HFpEF. Some believe SGLT2i should be considered for all patients with HF based on its efficacy in patients with or without CKD or T2D. 14 For example, the EMPULSE trial showed in‐hospital initiation of SGLT2i in patients with acute HF resulted in significant clinical benefit across all levels of ejection fraction. 14 In the DELIVER trial, HFpEF patients had no significant benefit in all‐cause mortality with SGLT2i use and had a 3 year mortality of around 20%, with or without the drug. In the current analysis, the all‐cause mortality was slightly lower, 16% with an average follow‐up of 4 years (Table 1 ). This may have been due to an older population (72 vs. 64 years) or inclusion of patients with previous HFrEF, which the current analysis excluded. In the EMPEROR‐Preserved trial, SGLT2i reduced the risk of HF hospitalization in HFpEF patients with or without diabetes, but no direct effect was seen on mortality. 10 Clearly, SGLT2i mechanism of action to increase the excretion of glucose cannot fully explain the benefits on clinical events across a broad phenotype of HF, CKD, and glucose control. Some believe that SGLT2i may improve outcomes due to direct cardiac effects in addition to renal‐mediated effects, 15 but clinical evidence is lacking.
In the current study, SGLT2i significantly reduced the risk of ESRD which supports this treatment as an effective therapy to slow renal disease progression in HFpEF (Figure 3 ). SGLT2i have been shown to delay ESRD even in patients with advanced CKD. Indeed, recent trials suggest, these benefits persist even in patients with Stage 4 CKD (estimated GFR 15–30 mL/min/1.73 m2). 16 Aside from renin‐angiotensin system (RAS) inhibitors, SGLT2i has been the only drug has been shown to consistently slow CKD progression in the last 20 years. 8 And this effect is additive in CKD patients already taking RAS inhibitors. 9
The heart and kidney interact in a complex and tight relationship in HF. Around 60% of HF patients have CKD, 17 one of the strongest independent risk factors and predictors of mortality in patients with HF, both HFpEF and HFrEF. 18 , 19 , 20 Treatments that block the RAS (e.g. ACEi/ARB) are the cornerstone of effectively delaying the progression of CKD. Activation of the RAS occurs in many patients with HFpEF and most likely relates to the severity HF or renal disease. 21 The renoprotective effects of RAS inhibitors are well established in several different populations, including T2D and CKD. RAS inhibitors have also been shown to reduce mortality in HFpEF (although this was unadjusted for confounders). 22 While the current analysis did not demonstrate significant effects on incidence of ESRD with ACEi/ARB, there was a significant reduction in ESRD risk with MRA. These effects persisted after adjusting for age, sex, race, hypertension, and T2D. Studies commonly report the attenuated efficacy of RAS inhibitors in Black patients, especially during the treatment of hypertension. However, some clinical studies show beneficial effects of these drugs in Black CKD patients. 23 Similarly, our results support the use of these drugs during HFpEF, regardless of race.
National disparities in HF care and outcomes continue. Black patients are disproportionately affected by factors that are significant risk factors for HF, including hypertension, obesity, and diabetes. Black patients have the higher age‐adjusted rate of incident HFrEF and HFpEF. 24 Interestingly, Black HFpEF patients have greater co‐morbidities and increased risk of HF hospitalizations but statistically similar mortality as Whites with HFpEF. 25 In the current study, we found similar results in Black HFpEF patients, with similar all‐cause mortality, but significantly increased risk of developing ESRD (Table 2 ). Blacks have also been shown to be more often affected by social determinants of health including lower income. Out of pocket expenses for SGLT2i can cost around $900/year for Medicare patients, 26 and 16% of HF patients report delaying or refusing care due to financial reasons. 27 In the current study, Black patients with and without CKD were more often on Medicaid as compared with White patients (Table S1 ), which may incentivize physicians to not include SGLT2 inhibitors in the treatment plans of HFpEF. However, in the current study, Black HF patients in the current study were more commonly prescribed SGLT2i, with or without CKD, as compared with Whites (Table 3 ). The effects of SGLT2i among minority populations, although likely, require further investigation. Unfortunately, Black patients have been poorly represented in most SGLT2i trial populations (~5%), 7 , 9 , 10 , 28 warranting further investigation into the impact of this treatment in these patients. Again, our data suggest that SGLT2i improves all‐cause mortality and risk of ESRD in HFpEF, regardless of race.
In the current study, most patients were prescribed BB (60%), especially in Blacks and in CKD patients (Table 3 ). These patients were associated with increased risk of developing ESRD, (Figure 1 ). Interestingly, there was a 43% reduced risk of death for black patients that were not prescribed a BB as compared with Black patients that were (Figure S1 ). The mechanisms for this are unknown. BB reduce all‐cause mortality and are currently recommended in HFrEF; however, BB use has not been shown to improve mortality more than placebo in HFpEF patients >50%. 29 Similarly, a retrospective analysis of TOPCAT demonstrated, for HFpEF patients treated with MRA, BB use increased the risk of HF hospitalizations. 30 Surprisingly, in HFpEF patients without a previous MI, BB use actually increased the risk of all‐cause mortality by 50%. 31 These data demonstrate a lack of understanding of BB in HFpEF and highlight the need for empirical data supporting the use of common HFrEF medications in this population. In the current study, we included all BB drug classes and did not take into consideration the differences between beta selective and beta non‐selective, such as carvedilol, which has been shown to significantly reduce HFrEF mortality, but again, data demonstrating beneficial effects in HFpEF are lacking. 32
In HFrEF patients, ARNI, such as sacubitril/valsartan, have demonstrated reduced HF hospitalization, cardiovascular mortality, and all‐cause mortality compared with ACEi when initiated in the outpatient setting, 33 and have rapid and significant clinical benefits among patients already hospitalized for HF. 34 , 35 In HFpEF, ARNI have not shown any additional benefit in HF hospitalizations and cardiovascular mortality as compared with ARB alone but may be especially effective in CKD. For example, ARNI improved composite outcome of HF hospitalizations and cardiovascular death in HFpEF patients with an estimated GFR 30–60 mL/min/1.73 m2. 36 Indeed, ARNI have been shown to slow the progression of CKD in HFpEF patients as compared with ARB alone. 37 However, the effect of ARNI in patients with advanced CKD (<30 mL/min/1.73 m2) is unknown. Unfortunately, the number of CKD patients taking ARNI in the current analysis was low (n = 14), making the analysis and interpretation of ARNI effects on ESRD and mortality risk difficult. Regardless, CKD patients prescribed ARNI were associated with a lower unadjusted mortality rate (7% vs. 20%) as compared with CKD patients not taking ARNI (not shown). This evidence coupled with the current data warrants further long‐term studies on the impact of ARNI on the cardiovascular morbidity and mortality in HFpEF patients.
The current retrospective analysis of EHR using UMMC's RDW is a very powerful and clinically relevant tool. However, this study comes with limitations. For example, the current analysis is a single center study in Mississippi with social determinants of health that are dissimilar to most of the United States (e.g. high proportion of poverty and black race). While this suggests that these observations may not be broadly representative, they appear consistent with other published studies. Much of the cohort had missing data on HF severity (New York Heart Association class) and specific parameters describing heart size and function (e.g. pro brain natriuretic peptide levels, mitral E/A ratio, ventricular wall thickness, and atrial diameter). While UMMC clinics have standardized echocardiography procedures for diagnosing diastolic dysfunction and HFpEF, the retrospective design does not assure that these were rigorously adhered to in routine daily practice. Further, the term ‘HFpEF’ was not always used for diagnosis in the RDW, and therefore, the analysis included ‘diastolic HF’ in addition to the diagnostic criteria. Many lifestyle factors such as diet and physical activity as well as social determinants of health like income were not recorded in our institution's data warehouse. Indeed, these factors have shown to play an important role in HFpEF. For example, in HFpEF patients with CKD, low salt diet improved diastolic function in just 3 weeks. 38 In the current study, treatments such as BB, vasodilators, and diuretics were associated with increased risk of ESRD but not mortality. This does not definitively exclude the beneficial effects of these drugs in HFpEF treatment but, rather, may relate to physician bias to prescribe these drugs to HF patients with more advanced HF or CKD.
Conclusions
Many treatments have shown significant benefit in HFrEF but have failed to similarly treat HFpEF. There is much clinical evidence suggesting the significant role of SGLT2i in HFrEF, with less known about HFpEF. Here, we show significant improvements in survival with SGLT2i, regardless of CKD or diabetes status. Additionally, SGLT2i use was associated with significant decrease in the risk of ESRD. In view of the empirical data demonstrating the ability of SGLT2i to preserve kidney function paired with the common occurrence of CKD in HF, more effort is needed for exploring the patient populations that may benefit with this treatment.
Conflict of interest
The authors declare that there are no competing interests.
Funding
This work was supported by grants from the National Institute on Minority Health and Health Disparities (R00 MD014738), National Institute of General Medical Sciences (U54 GM115428), and the Joe and Dorothy Dorsett Brown Foundation to JSC; National Institute of General Medical Sciences (U54 GM115428 and P20 GM121334) to STL and (P20 GM104357) to the UMMC Department of Physiology.
Supporting information
Figure S1. Survival in black and white heart failure with preserved ejection fraction patients treated with and without beta blocker therapy after adjusting for age, sex, hypertension, chronic kidney disease, and type 2 diabetes.
Table S1. Types of insurance among white and black heart failure with preserved ejection fraction patients with or without chronic kidney disease.
Clemmer, J. S. , Ward, T. J. , and Lirette, S. T. (2023) Retrospective analysis of SGLT2 inhibitors in heart failure with preserved ejection fraction. ESC Heart Failure, 10: 2010–2018. 10.1002/ehf2.14347.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner L, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke Statistics‐2020 update: a report from the American Heart Association. Circulation. 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, van Heerebeek L, Hillege HL, Lam CS, Navis G, Voors AA. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016; 18: 588–598. [DOI] [PubMed] [Google Scholar]
- 3. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, Wehrens XHT, Deswal A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012; 59: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsia DS, Grove O, Cefalu WT. An update on sodium‐glucose co‐transporter‐2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017; 24: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019; 393: 31–39. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 8. Jafar TH. FDA approval of dapagliflozin for chronic kidney disease: a remarkable achievement? Lancet. 2021; 398: 283–284. [DOI] [PubMed] [Google Scholar]
- 9. Heerspink HJL, Stefansson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC, DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR‐Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 11. UMMC . University of Mississippi Medical Center, Center for Informatics and Analytics. Patient cohort. Explorer. 2020. [Google Scholar]
- 12. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 14. Adekunle AO, Adzika GK, Mprah R, Ndzie Noah ML, Adu‐Amankwaah J, Rizvi R, Akhter N, Sun H. Predominance of heart failure with preserved ejection fraction in postmenopausal women: intra‐ and extra‐cardiomyocyte maladaptive alterations Scaffolded by estrogen deficiency. Front Cell Dev Biol. 2021; 9: 685996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan A, Abidi E, El‐Yazbi A, Eid A, Booz GW, Zouein FA. Direct cardiovascular impact of SGLT2 inhibitors: mechanisms and effects. Heart Fail Rev. 2018; 23: 419–437. [DOI] [PubMed] [Google Scholar]
- 16. Chertow GM, Vart P, Jongs N, Toto RD, Gorriz JL, Hou FF, McMurray JJV, Correa‐Rotter R, Rossing P, Sjöström CD, Stefánsson BV, Langkilde AM, Wheeler DC, Heerspink HJL, DAPA‐CKD Trial Committees and Investigators . Effects of dapagliflozin in stage 4 chronic kidney disease. J Am Soc Nephrol. 2021; 32: 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lofman I, Szummer K, Dahlstrom U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail. 2017; 19: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 18. Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003; 138: 917–924. [DOI] [PubMed] [Google Scholar]
- 19. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen D, Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006; 113: 671–678. [DOI] [PubMed] [Google Scholar]
- 20. van de Wouw J, Broekhuizen M, Sorop O, Joles JA, Verhaar MC, Duncker DJ, Danser AHJ, Merkus D. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: a focus on microcirculatory factors and therapeutic targets. Front Physiol. 2019; 10: 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vergaro G, Aimo A, Prontera C, Ghionzoli N, Arzilli C, Zyw L, Taddei C, Gabutti A, Poletti R, Giannoni A, Mammini C, Spini V, Passino C, Emdin M. Sympathetic and renin‐angiotensin‐aldosterone system activation in heart failure with preserved, mid‐range and reduced ejection fraction. Int J Cardiol. 2019; 296: 91–97. [DOI] [PubMed] [Google Scholar]
- 22. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin‐angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012; 308: 2108–2117. [DOI] [PubMed] [Google Scholar]
- 23. Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER 3rd, Norris K, O'Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley‐Brown D, Tisher CC, Toto RD, Wright JT Jr, Xu S, African American Study of Kidney Disease and Hypertension (AASK) Study Group . Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001; 285: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 24. Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, Wruck LM, Rosamond WD. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the atherosclerosis risk in communities study). Am J Cardiol. 2014; 113: 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis EF, Claggett B, Shah AM, Liu J, Shah SJ, Anand I, O’Meara E, Sweitzer NK, Rouleau JL, Fang JC, Desai AS, Retta TM, Solomon SD, Heitner JF, Stamos TD, Boineau R, Pitt B, Pfeffer MA. Racial differences in characteristics and outcomes of patients with heart failure and preserved ejection fraction in the treatment of preserved cardiac function heart failure trial. Circ Heart Fail. 2018; 11: e004457. [DOI] [PubMed] [Google Scholar]
- 26. Faridi KF, Dayoub EJ, Ross JS, Dhruva SS, Ahmad T, Desai NR. Medicare coverage and out‐of‐pocket costs of quadruple drug therapy for heart failure. J Am Coll Cardiol. 2022; 79: 2516–2525. [DOI] [PubMed] [Google Scholar]
- 27. Thomas A, Valero‐Elizondo J, Khera R, Warraich HJ, Reinhardt SW, Ali HJ, Nasir K, Desai NR. Forgone medical care associated with increased health care costs among the U.S. heart failure population. JACC Heart Fail. 2021; 9: 710–719. [DOI] [PubMed] [Google Scholar]
- 28. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 29. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson Å, Wikstrand J, Kotecha D, Beta‐blockers in Heart Failure Collaborative Group . Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J. 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silverman DN, Plante TB, Infeld M, Callas PW, Juraschek SP, Dougherty GB, Meyer M. Association of beta‐blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019; 2: e1916598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsujimoto T, Kajio H. Beta‐blocker use and cardiovascular event risk in patients with heart failure with preserved ejection fraction. Sci Rep. 2018; 8: 9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. carvedilol heart failure study group. N Engl J Med. 1996; 334: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 33. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 34. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019; 380: 539–548. [DOI] [PubMed] [Google Scholar]
- 35. Morrow DA, Velazquez EJ, DeVore AD, Desai AS, Duffy CI, Ambrosy AP, Gurmu Y, McCague K, Rocha R, Braunwald E. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER‐HF trial. Circulation. 2019; 139: 2285–2288. [DOI] [PubMed] [Google Scholar]
- 36. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen D, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 37. Mc Causland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, van Veldhuisen DJ, Zannad F, Comin‐Colet J, Pfeffer MA, McMurray JJV, Solomon SD. Angiotensin‐neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020; 142: 1236–1245. [DOI] [PubMed] [Google Scholar]
- 38. Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovács SJ, Kolias TJ. Low‐sodium DASH diet improves diastolic function and ventricular‐arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013; 6: 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Survival in black and white heart failure with preserved ejection fraction patients treated with and without beta blocker therapy after adjusting for age, sex, hypertension, chronic kidney disease, and type 2 diabetes.
Table S1. Types of insurance among white and black heart failure with preserved ejection fraction patients with or without chronic kidney disease.


