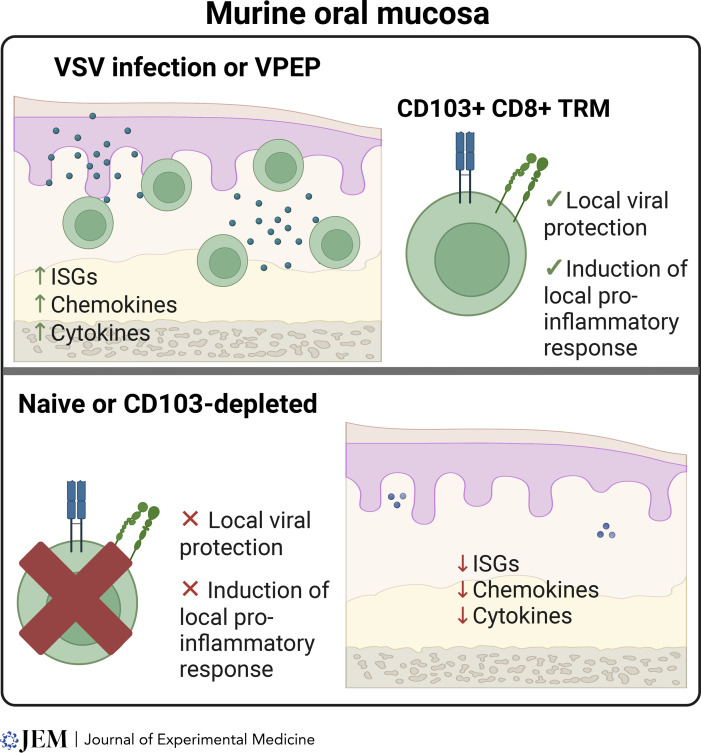
Stolley et al. discovered that the oral mucosa houses CD8+ CD103+ resident memory T cells, which are important for protecting against local viral infections in mice.
Abstract
The oral mucosa serves as a frontline defense against many infections. Stolley et al. (2023. J. Exp. Med. https://doi.org/10.1084/jem.20221853) discovered that the oral mucosa houses CD8+ CD103+ resident memory T cells, which are important for protecting against local viral infections in mice.
The oral barrier serves as a critical gateway for various microbes, acting as the first line of defense for our respiratory and digestive systems. With a diverse array of microbial communities, the oral mucosa is prone to a wide range of viral infections capable of initiating immune responses and reappearing during stress or immunosuppression. The unique physiology and anatomy of the mouth leave it susceptible also to inflammatory diseases and ongoing immune stimulation. As such, a better understanding of the complex interplay between microbes and immune responses is vital for maintaining overall well-being.

Insights from Marcus Buggert.
Memory CD8+ T cells play a crucial role in detecting and clearing pathogens. Distinct memory CD8+ T cell subsets occupy specific niches, patrolling secondary lymphoid and non-lymphoid tissues through continuous recirculation of the blood through constant surveillance by cytotoxic effector memory subsets (Buggert et al., 2020; Lange et al., 2022; Szabo et al., 2019). In contrast, resident memory T cells (TRMs) remain stationed in tissues for rapid pathogen sensing. TRMs are typically characterized by their lack of recirculation in parabiosis experiments and increased expression of canonical markers such as CD69 and CD103 (Masopust et al., 2010; Masopust et al., 2006). Their differentiation is strongly influenced by various environmental factors, resulting in a diverse group of non-recirculating cells that adapt to their environment (Poon et al., 2023). While mucosal immunology and TRM biology have been investigated in certain tissues, the formation and function of TRMs in the oral mucosa and periodontium remain largely uncharted. A more profound understanding of CD8+ TRMs in the mouth could illuminate their roles in oral health and disease and possibly lead to novel therapeutic targets.
To this end, Stolley et al. (2023) developed a set of models to study oral CD8+ TRMs and assess if systemic viral infections produce memory CD8+ T cells in the oral mucosa. They first compared lymphocytic choriomeningitis virus and vesicular stomatitis virus expressing OVA (VSV-ova), showing that particularly VSV-ova generated strong TRM responses in the oral cavity. Notably, they demonstrated that mice previously exposed to VSV-ova were protected from vaccinia virus expressing OVA. The protection was unaffected by FTY720 treatment, suggesting that local TRMs protect against viral infections in the oral mucosa. The authors further explored if CD8+ T cells with a TRM phenotype in the oral mucosa were truly resident and how this related to surface phenotypes. Through transfer and parabiosis experiments (Masopust et al., 2010), they could demonstrate that CD8+ T cells in the oral mucosa showed a host bias, indicating residency, with CD103 expression being a core marker to identify oral TRMs. The “dirty” mouse model developed by the lab (Beura et al., 2016) confirmed CD103 as a reliable marker of oral residency among CD8+ T cells in non–specific pathogen–free mice. Cohousing VSV-ova immune mice with pet shop mice had little effect on oral CD8+ T cell abundance and phenotype but increased total TRMs. Altogether these data indicated that the number of memory CD8+ T cells in the oral mucosa is flexible and influenced by microbial experience.
The same group has previously shown that isolation of T cells, particularly TRMs, is very inefficient from many tissues (Steinert et al., 2015). As such, they developed a viral-prime, epitope-pull (VPEP) strategy by priming antigen-specific CD8+ T cells with intravenous VSV-ova infection followed by an oral peptide mixture application. This led to more oral mucosa memory CD8+ T cells compared to systemic VSV-ova infection alone. Parabiosis confirmed that VPEP-generated oral CD8+ T cells were mostly TRMs. TGFβ signaling was crucial for TRM differentiation in the mouth, consistent with its role as a critical cytokine for CD103+ TRM formation (Qiu et al., 2021). VPEP produced high densities of CD103+ TRMs with unique phenotypic signatures in the oral mucosa, reflecting their distinct tissue microenvironment. More detailed analysis revealed CD8+ T cells near taste buds in the tongue’s soft palate and surrounding circumvallate papillae. As such, TRMs seem broadly distributed throughout the oral mucosa and periodontium, where flow cytometry–based cell isolation significantly underestimates their true number following VPEP.
Other studies have shown that TRMs can be locally reactivated and fortify immunity and residency in other sites, such as draining LNs (Beura et al., 2018). The data supported a similar scenario in the oral cavity, with antigen swabbing increasing total oral CD8+ T cells through the expansion of recirculating CD8+ T cells and increasing dendritic cells and macrophages in mouth-draining LNs. Bulk RNA sequencing revealed upregulated antimicrobial gene signatures and chemokine genes in antigen-treated mice, with many identified genes potentially involved in oral physiology and disease. They finally developed a proof-of-principle approach to selectively eliminate CD103+ TRMs in the oral mucosa by transferring wild-type antigen-specific CD8+ T cells into CD103-deficient hosts and treating them with anti-CD103 antibodies conjugated to Saporin-toxin. This treatment depleted >90% of CD103+ TRMs in the oral mucosa, leaving CD103− CD8+ T cells unaffected. CD103+ CD8+ T cell depletion altered TRM-driven gene expression. When depleted prior to oral peptide swabbing, transcriptional changes and accumulation of CD8+ T cells and other leukocytes in the oral cavity were significantly reduced. Collectively, this set of data highlights the critical role of CD103+ TRM in the transcriptional response to secondary antigen exposure in the oral mucosa. The paper presents several interesting implications. First, it supports previous human observations that most CD103+ T cells in the oral cavity are likely TRMs (Niessl et al., 2021; Sobkowiak et al., 2019). Notably, mice were protected from a heterologous challenge with the vaccinia virus in FTY720-treated mice, suggesting that local TRMs can provide protection against viral infections in the oral mucosa without recruiting other cell types. While the study did not explore the functional mechanisms of TRM-mediated protection, the data indicate that TRMs may protect from overt disease by fortifying oral barriers.
Another intriguing aspect is the strategies employed to enhance local immunity, such as the prime-pull strategy VPEP. The COVID-19 pandemic has focused attention on upper respiratory tract vaccines, particularly nasal vaccines (Topol and Iwasaki, 2022). However, SARS-CoV-2 can also spread through the oral cavity, where mouth cells are viral targets (Huang et al., 2021). The strategy by Stolley et al. (2023) may not be directly applicable to humans; however, it demonstrates that innovative strategies could be used to expand local TRMs in humans. The authors speculate that VPEP strategies might augment TRM responses, which is an appealing idea when combined with mRNA vaccines to induce systemic responses. Mucosal vaccines have typically worked well in murine models but not humans, highlighting the need to improve stable antigen delivery to the oral mucosa to enhance local cellular immunity in oral barriers.
The paper also has implications for research on cancer and autoimmune pathogenesis. The study reveals that oral TRM reactivation leads to regional upregulation of antitumor genes, suggesting that deliberate human oral TRM reactivation might offer a mechanism for directing neoantigen-specific or chimeric antigen receptor T cells into oral cancers. Given the ease of oral mucosa swabbing, this method could be a promising alternative to more invasive therapies that may cause severe disfigurement and negatively impact essential life functions. Furthermore, the study uncovers intriguing connections between oral TRM reactivation, saliva production, and taste. The influence of reactivation on these aspects of oral health could have significant clinical implications for disorders affecting saliva production, such as Sjogren’s syndrome, and may potentially reshape our understanding of taste perception.
Induction of oral CD103+ TRMs promotes a localized pro-inflammatory response and provides protection against viral disease. ISG, interferon-stimulated gene.
In summary, research on the immunology of the oral cavity remains limited and underexplored for ambiguous reasons, despite its notable significance. Only a few studies, including the work of Stolley et al. (2023), have investigated the role of memory T cells in this context. To gain a comprehensive understanding of how T cells may contribute to maintaining normal homeostasis and their potential implications in various diseases, such as oral cancer, Sjogren’s syndrome, and SARS-CoV-2 infection, additional studies in both mice and humans are essential. The stage has been set through the identification of TRMs as the “guardians of the oral barriers,” but further research is needed to elucidate the full extent of their significance in health and disease.
References
- Beura, L.K., et al. 2016. Nature. 10.1038/nature17655 [DOI] [Google Scholar]
- Beura, L.K., et al. 2018. Immunity. 10.1016/j.immuni.2018.01.015 [DOI] [Google Scholar]
- Buggert, M., et al. 2020. Cell. 10.1016/j.cell.2020.11.019 [DOI] [Google Scholar]
- Huang, N., et al. 2021. Nat. Med. 10.1038/s41591-021-01296-8 [DOI] [Google Scholar]
- Lange, J., et al. 2022. Mucosal Immunol. 10.1038/s41385-021-00467-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust, D., et al. 2010. J. Exp. Med. 10.1084/jem.20090858 [DOI] [Google Scholar]
- Masopust, D., et al. 2006. J. Immunol. 10.4049/jimmunol.176.4.2079 [DOI] [Google Scholar]
- Niessl, J., et al. 2021. Sci. Immunol. 10.1126/sciimmunol.abk0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, M.M.L., et al. 2023. Nat. Immunol. 10.1038/s41590-022-01395-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Z., et al. 2021. Cells. 10.3390/cells10050989 [DOI] [Google Scholar]
- Sobkowiak, M.J., et al. 2019. Eur. J. Immunol. 10.1002/eji.201847759 [DOI] [Google Scholar]
- Steinert, E.M., et al. 2015. Cell. 10.1016/j.cell.2015.03.031 [DOI] [Google Scholar]
- Stolley, J.M., et al. 2023. J. Exp. Med. 10.1084/jem.20221853 [DOI] [Google Scholar]
- Szabo, P.A., et al. 2019. Sci. Immunol. 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol, E.J., and Iwasaki A.. 2022. Sci. Immunol. 10.1126/sciimmunol.add9947 [DOI] [PubMed] [Google Scholar]