Abstract
The continuous expansion of immunocompromised patient populations at-risk for developing life-threatening opportunistic fungal infections in recent decades has helped develop a deeper understanding of antifungal host defenses, which has provided the foundation for eventually devising immune-based targeted interventions in the clinic. This review outlines how genetic variation in certain immune pathway-related genes may contribute to the observed clinical variability in the risk of acquisition and/or severity of fungal infections and how immunogenetic-based patient stratification may enable the eventual development of personalized strategies for antifungal prophylaxis and/or vaccination. Moreover, this review synthesizes the emerging cytokine-based, cell-based, and other immunotherapeutic strategies that have shown promise as adjunctive therapies for boosting or modulating tissue-specific antifungal immune responses in the context of opportunistic fungal infections.
Keywords: fungal infections, immunogenetic risk, antifungal immunity, immunotherapy
Introduction
Since the 1980s, the global burden of opportunistic fungal infections has dramatically increased in humans. Life-threatening fungal infections such as Pneumocystis jirovecii pneumonia (PJP), cryptococcal meningoencephalitis, and disseminated histoplasmosis are AIDS-defining illnesses that continue to cause significant morbidity and mortality in resource-poor settings with limited access to antiretroviral and antifungal therapies [1]. The introduction of myeloablative chemotherapy and targeted immunosuppressive therapies for patients with neoplastic and autoimmune conditions has expanded the burden of opportunistic fungal infections such as invasive aspergillosis (IA) [2, 3]. Modern advances in the clinical management of critically ill patients in the intensive care unit (ICU) has resulted in an increased frequency of invasive candidiasis in recent decades [4–6]. The advent of allogeneic hematopoietic stem cell transplantation (HSCT) and solid organ transplantation (SOT) has transformed the management of patients with hematological malignancies and end-organ failure but has resulted in complex iatrogenic immunosuppressive states that significantly heighten host susceptibility to opportunistic fungal infections [7].
Despite improvements over the past two decades, the clinical outcome of immunocompromised patients who develop opportunistic fungal infections remains poor despite the administration of antifungal therapy. Several factors contribute to this clinical observation. Firstly, prompt diagnosis of fungal infections remains problematic due to the suboptimal sensitivity and specificity of available culture- and biomarker-based fungal diagnostic tests [8]. Delayed diagnosis in turn results in delayed initiation of appropriate antifungal therapy, which has been shown to increase patient mortality in the setting of invasive candidiasis, IA, and mucormycosis [9–11]. Secondly, despite the discovery and introduction in the clinic of several new antifungal agents [12, 13], the in vivo efficacy of antifungal therapy in immunosuppressed patients remains hindered by drug-drug interactions, pharmacokinetic and pharmacodynamic challenges, and drug toxicities. The emergence of antifungal resistance such as with multidrug-resistant Candida auris, echinocandin-resistant Candida glabrata, azole-resistant Cryptococcus neoformans, and azole-resistant Aspergillus fumigatus is particularly concerning as it can underlie suboptimal treatment responses and poor patient outcomes [14–17]. Thirdly, the profound net state of immunosuppression of at-risk patients is a major driver of susceptibility to acquisition of, and poor outcomes after, fungal infection, and immune restoration is often required to control opportunistic fungal infections in spite of the administration of antifungal therapy [18–20].
Thus, alongside improving fungal diagnostic tests, developing novel, broad-spectrum, and non-toxic antifungal agents, and investigating fungal virulence traits, enhancing our understanding of antifungal host defenses is essential for improving patient outcomes. This review first outlines how the recent surge in the characterization of genetic variants (single nucleotide polymorphisms [SNPs]) in certain immune pathway-related genes (Table 1) can be exploited in the clinic with the goal to assess the individualized risk of fungal infection development and prognosis in at-risk patients and to develop personalized strategies for antifungal prophylaxis, immunotherapy, and/or vaccination that could improve patient outcomes (Figure 1). Moreover, this review briefly discusses certain cytokine-based, cell-based, and other immunotherapeutic interventions that have shown promise in mouse models of fungal disease and/or human patients for boosting or dampening antifungal immune responses to benefit the infected mammalian host.
Table 1.
Key genetic variants and their reported association with the development of fungal infections in vulnerable patients.
| Genetic variants associated with the development of aspergillosis | ||||||||
| TLR1 | 4p14 | rs5743611 (0.02) | R80T | HSCT recipients | Caucasian | Development of IA | Unknown | [61] |
| TLR1 together with TLR6 | 4p14 | rs4833095 (0.43) and rs5743810 (0.12) | N248S and S249P | HSCT recipients | Caucasian | Development of IA | Unknown | [61] |
| TLR3 | 4q35 | rs3775296 (0.18) | +95C/A | HSCT donors | Caucasian | Development of IA | Impaired TLR3 expression and responsiveness in CD141+ DCs, impaired DC-induced CD8+ T cell proliferation | [57] |
| TLR4 | 9q33 | rs4986790 (0.06) rs4986791 (0.04) |
D299G T399I |
Unrelated (but not related) HSCT donors | Caucasian | Development of IA, nonrelapse death post-HSCT (yes) | Unknown | [53, 59, 62] |
| TLR4 | 9q33 | rs4986790 (0.06) | D299G | Patients with CCPA | ND | Development of CCPA | Unknown | [58] |
| TLR5 | 1q41 | rs5744168 (0.05) | R392* | HSCT recipients | Caucasian | Development of IA | Unknown | [60] |
| TLR6 | 4p14 | rs5743810 (0.12) | S249P | HSCT donors | Caucasian | Development of IA | Unknown | [46] |
| CLEC1A (Mel-Lec) | 12p13 | rs2306894 (0.33) | G26A | HSCT donors | Caucasian | Development of IA | Impaired production of IL-1β and IL-8 by macrophages | [50] |
| CLEC7A (DECTIN-1) | 12p13 | rs16910526 (0.04) | Y238* | HSCT donors and recipients | Caucasian | Development of IA (yes) | Impaired β-glucan binding; impaired production of pro-inflammatory cytokines by PBMCs | [41, 42, 46] |
| CLEC7A (DECTIN-1) | 12p13 | rs7309123 (0.28) | c.375-1404C/G | HSCT donors or patients with hematological malignancy | Caucasian | Development of IA (yes) | Decreased CLEC7A mRNA expression in whole blood | [46, 49] |
| CD209 (DC-SIGN) | 19p13 | rs7248637 (0.23) | *898T/C | HSCT donors or patients with hematological malignancy | Caucasian | Development of IA (yes) | Unknown | [46, 49] |
| CARD9 | 9q34 | rs4077515 (0.37) | S12N | Patients with ABPA | ND | Development of ABPA | Increased Aspergillus-induced RelB nuclear translocation and IL-5 production by PBMCs carrying the SNP in homozygosity | [39] |
| NOD2 | 16q12 | rs2066842 (0.10) | P268S | HSCT donors | Caucasian | Development of IA | Decreased production of IL-1β by Aspergillus-stimulated PBMCs | [74] |
| APCS (PTX2) | 1q23 | rs2808661 (0.10) rs3753869 (0.41) |
V144V | HSCT donors | Caucasian | Development of IA | Decreased SAP levels | [81] |
| PTX3 | 3q25 | rs2305619 (0.44) rs3816527 (0.29) |
281GG 734AA |
HSCT donors | Caucasian | Development of IA (yes) | Decreased PTX3 expression; decreased phagocytosis and fungicidal activity of neutrophils | [46, 76] |
| PLG | 6q26 | rs4252125 (0.14) | D472N | HSCT recipients | Caucasian | Development of IA (yes) | Unknown | [117] |
| AGER (RAGE) | 6p21 | rs2070600 (0.07) | −374T/A | HSCT donors or recipients (T cell-depleted transplant s) | Caucasian | Development of IA | Unknown | [114] |
| S100B | 21q22 | rs9722 (0.27) | +427C/T | HSCT donors | Caucasian | Development of IA (yes) | Unknown | [46, 114] |
| IFNG | 12q15 | rs2069705 (0.48) | −1616 C/T | HSCT recipients | Caucasian | Development of IA (yes) | Unknown | [46, 82] |
| CXCL10 | 4q21 | rs1554013 (0.30) rs3921 (0.31) rs4257674 (0.31) |
+11101 C/T +1642 C/G −1101 A/G |
HSCT recipients | Caucasian | Development of IA (yes) | Decreased CXCL10 mRNA expression in DCs | [46, 82] |
| CX3CR1 | 3p22 | rs7631529 (0.05) rs9823718 (0.14) |
A allele G allele |
HSCT donors and patients with hematological malignancy | Caucasian | Development of IA | Unknown | [100] |
| TNFR1 | 12p13 | rs4149570 (0.30) | −609G/T | HSCT donors and patients with hematological malignancy | Caucasian | Development of IA (yes) | Decreased TNFR1 mRNA expression in whole blood | [46, 85] |
| TNFR2 | 1p36 | VNTR | −322 | Patients with hematological malignancy | Caucasian | Development of IA | Unknown | [84] |
| ILIB | 2q14 | rs16944 (0.49) | −511C/T | SOT recipients | Caucasian | Development of mold colonization and IMI | Decreased production of pro-inflammatory cytokines by Aspergillus-stimulated PBMC | [89] |
| ILIB | 2q14 | rs3917354 | T/- | Patients with CCPA | Caucasian | Development of CCPA | Unknown | [88] |
| ILIRN | 2q14 | rs4252041 (0.02) | C/T | Patients with CCPA | Caucasian | Development of CCPA | Unknown | [88] |
| ILIA together with ILIB and ILIRN | 2q14 | rs1800587 (0.28) and rs1143627 (0.47) and 86-bp VNTR | −889C and −511T and VNTR2 | Patients with hematological malignancy | Caucasian | Development of IA | Unknown | [87] |
| IL10 | 1q32 | rs1800896 (0.27) | −1082GG | Patients with hematological malignancy | Caucasian | Development of IA | Unknown | [93] |
| IL10 | 1q32 | rs1800896 (0.27) and rs1800871 (0.43) and rs1800872 (0.43) | −1082G and −819C and −592A | HSCT recipients | Asian | Development of IA | Unknown | [94] |
| IL15 | 4q31 | rs1519551 (0.38) rs6842735 (0.08) rs12508866 (0.15) |
A/G G/T T/C |
Patients with CCPA | Caucasian | Development of CCPA | Unknown | [88] |
| DEFB1 | 8p23 | rs1800972 (0.14) | −44C/G | SOT recipients | Caucasian | Development of mold colonization and IMI | Unknown | [89] |
| CALM1 | 14q32 | rs12885713 (0.30) | CC genotype | HSCT recipients | Caucasian | Development of IA | Decreased CALM1 transcription | [116] |
| FLOT1 | 6p21 | rs3094127 (0.27) | CC genotype | HSCT donors | Caucasian | Development of IA | Decreased production of IL-1β and IL-6 by Aspergillus-stimulated macrophages carrying the GG genotype | [279] |
| Genetic variants associated with the development of invasive candidiasis | ||||||||
| TLR1 | 4pl4 | rs5743611 (0.02) rs4833095 (0.43) rs5743618 (0.20) |
R80T N248S I602S |
Patients with candidemia | Caucasian (no increase d risk in African Americans) | Development of candidemia | Impaired production of pro-inflammatory cytokines by PBMC | [55] |
| TLR4 | 9q33 | rs4986790 (0.06) rs4986791 (0.04) |
D299G T399I |
Patients with candidemia | Caucasian | Development of candidemia | Unknown | [56] |
| IFIH1 (MDA5) | 2q24 | rs1990760 (0.36) rs3747517 (0.41) |
A946T H843R |
Patients with candidemia | Caucasian | Development of candidemia | Impaired production of pro-inflammatory cytokines by Candida-stimulated PBMC | [280] |
| VAV3 | 1p13 | rs4914950 (0.37) | CC carriage | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [51] |
| IL10 | 1q32 | rs1800896 (0.27) | AA carriage | Patients with candidemia | Caucasian | Development of persistent fungemiain candidemic patients | Unknown | [95] |
| IL12B | 5q33 | rs41292470 | INS/INS | Patients with candidemia | Caucasian | Development of persistent fungemia in candidemic patients | Unknown | [95] |
| TNFA | 6p21 | rs1800629 (0.09) | AA/GA carriage | Surgical ICU patients at-risk for invasive candidiasis | Caucasian | Development of intra-abdominal candidiasis | Unknown | [83] |
| DEFB1 | 8p23 | rs1800972 (0.14) | GG/CG carriage | Surgical ICU patients at-risk for invasive candidiasis | Caucasian | Development of intra-abdominal candidiasis | Unknown | [83] |
| CCL8 | 17q12 | 1kg_17_29697448 | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [109] |
| CXCR1 | 2q35 | rs2234671 (0.14) | S276T (CG+GG carriage) | Patients with candidemia | Caucasian | Development of disseminated candidiasis in candidemic patients | Impaired neutrophil degranulation and fungal killing | [96] |
| CX3CR1 | 3p22 | rs3732378 (0.09) | T280M (CC+CT carriage) | Patients with candidemia | Caucasian | Development of candidemia and disseminated candidiasis in candidemic patients (yes) | Impaired monocyte survival | [97, 98] |
| STAT1 | 2q32 | rs16833172 (0.09) | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [109] |
| PSMB8 | 6p21 | rs3198005 (0.01) | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [109] |
| SP110 | 2q37 | rs3769845 (0.48) | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [109] |
| TAGAP | 6q25 | rs3127214 (0.24) | N/A | Patients with candidemia | Caucasian | Development of candidemia and disseminated candidiasis in candidemic patients | Unknown | [108] |
| CD58 | 1p13 | rs17035850 (0.24) | N/A | Patients with candidemia | Caucasian | Development of candidemia and persistent fungemia in candidemic patients | Unknown | [108] |
| LCE4 - C1orf68 | 1q21 | rs4845320 (0.10) | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [108] |
| CD82 | 11p11 | rs7932712 (0.36) | N/A | Patients with candidemia | Caucasian | Development of candidemia | Unknown | [281] |
| Genetic variants associated with the development of VVC | ||||||||
| TLR2 | 4q31 | rs5743704 (0.01) | P631H | Women with RVVC | Caucasian | Development of RVVC | Decreased production of IL-17A by Candida-stimulated PBMC | [47] |
| CLEC7 A (DECT IN-1) | 12p13 | rs16910526 (0.04) | Y238* | Women with RVVC | Caucasian | Development of RVVC (no) | Impaired β-glucan binding; impaired production of pro-inflammatory cytokines by PBMC | [47, 48, 102] |
| NLRP3 | 1q44 | rs74163773 (42-bp VNTR) | 12/9 genotype | Women with RVVC | Caucasian | Development of RVVC | Increased vaginal levels of IL-1 β and decreased vaginal levels of IL-1Ra | [71] |
| IL4 | 5q31 | rs2243250 (0.47) | −589C/T | Women with RVVC | Caucasian | Development of RVVC | Increased vaginal levels of IL-4 and decreased vaginal level of NO | [282] |
| IL22 | 12q15 | rs2227485 (0.48) | CC+CT carriage | Women with RVVC | Caucasian | Development of RVVC | Increased vaginal levels of IL-22 and decreased vaginal level of IL-17A, TNF-α, and calprotectin in women with the protective TT genotype | [102] |
| IDOl | 8p11 | rs3808606 (0.46) | CC+CT carriage | Women with RVVC | Caucasian | Development of RVVC | Increased vaginal levels of IL-22 and decreased vaginal level of IL-17A and TNF-α in women with the protective TT genotype | [102] |
| SIGLE Cl5 | 18q12 | rs2919643 (0.40) | CC+CT carriage | Women with RVVC | Caucasian | Development of RVVC | Increased production of IL-17A, IL-22, and IFN-γ by Candida-stimulated PBMC of women carrying the disease-associated C allele | [73] |
| DSG1 | 18q21 | rs200520431 (0.01) | D/I (D the high-risk allele) | Women with VVC | Caucasian | Development of VVC | Unknown | [110] |
| PRKCH | 14q23 | rs2251260 (0.49) | T/C (T the high-risk allele) | Women with VVC | Caucasian | Development of VVC | Unknown | [110] |
| Genetic variants associated with the development of cryptococcosis | ||||||||
| TLR1 | 4p14 | rs5743563 (0.18) | T/T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis | Unknown | [54] |
| TLR1 | 4p14 | rs5743604 (0.47) | T/T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis and more severe disease | Unknown | [54] |
| TLR2 | 4q31 | rs3804099 (0.41) | T/T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis and more severe disease | Unknown | [54] |
| TLR6 | 4p14 | rs3796508 (0.03) | G/A | HIV-infected and HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis in both HIV-infected and HIV-negative patients (yes) | Unknown | [54] |
| TLR9 | 3p21 | rs164637 (0.03) | C/T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis | Unknown | [54] |
| TLR9 | 3p21 | rs352140 (0.42) | T/T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis | Unknown | [54] |
| CLEC6 A (DECT IN-2) | 12p13 | rs11045418 (0.35) | CC+CT carriage | HIV-negative patients with cryptococcosis | Asian (Chinese Han) | Development of pulmonary (but not meningeal) cryptococcosis | Unknown | [283] |
| PTX3 | 3q25 | rs2305619 (0.44) | 281AA | HIV-negative patients with cryptococcosis | Asian (Chinese Han) | Development of cryptococcosis | Unknown (increased serum levels of PTX3 in individuals carrying the AA genotype) | [80] |
| FCGR2A | 1q23 | rs1801274 (0.44) | H131R | HIV-infected and HIV-negative patients with cryptococcal meningitis | Multiple | Development of cryptococcosis in HIV-negative (but not HIV-infected) patients | Unknown | [105-107] |
| FCGR2B | 1q23 | rs1050501 (0.19) | I232T | HIV-negative patients with cryptococcal meningitis | Asian (Chinese Han) | Development of cryptococcal meningitis in HIV-negative patients | Unknown | [105] |
| FCGR3 A | 1q23 | rs396991 (0.42) | F158V | HIV-infected and HIV-negative patients with cryptococcosis | Multiple | Development of cryptococcosis in both HIV-infected and HIV-negative patients (variable validation) | Impaired antibody-dependent NK cell-mediated ADCC | [105-107] |
| FCGR3 B | 1q23 | N/A | NA1/NA2 alleles | HIV-negative patients with cryptococcosis | Multiple | Development of cryptococcosis | Unknown | [105, 106] |
| CSF1 (M-CSF) | 1p13 | rs1999713 (0.47) rs1999714 (0.46) rs1999715 (0.48) |
N/A | HIV-infected patients | African descent | Development of HIV-associated cryptococcosis (yes) | Unknown | [103] |
| Genetic variants associated with the development of PJP | ||||||||
| IL4 | 5q31 | rs2243250 (0.47) | −589C/T (CT+TT carriage) | HIV-infected patients | Caucasian | Development of PJP | Unknown | [91] |
| Genetic variants associated with the development of blastomycosis | ||||||||
| IL6 locus | 7p15 | rs1800796 (0.31) rs1524107 (0.31) rs2066992 (0.31) |
N/A | Individual s of Hmong ancestry | Asian | Development of severe blastomycosis | Decreased production of IL-6 by immortalized B cells and decreased production of IL-17A by CD4+ T cells | [90] |
| Genetic variants associated with the development of mycetoma | ||||||||
| IL8 | 4q13 | rs4073 (0.48) | −251T/A | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [92] |
| IL10 | 1q32 | rs1800872 (0.43) | −592A/C | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [284] |
| CCL5 | 17q12 | rs2280788 (0.03) rs2280789 (0.19) |
−28C/G −In1/1 T/C |
Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [284] |
| CXCR2 | 2q35 | rs2230054 (0.49) | +785C/T | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [92] |
| TSP4 | 5q14 | N/A | A389P | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [92] |
| NOS2 | 17q11 | rs1800482 (0.02) | G-954C (Lambarene) | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma and more severe mycetomalesions | Unknown (decreased NOS activity) | [92] |
| CR1 | 1q32 | N/A | SI2 and McCa alleles | Patients with mycetoma | African descent (Sudanese) | Development of mycetoma | Unknown | [92] |
| CHIT1 | 1q32 | rs3831317 | Presence of the 24-bp insertion | Patients with mycetoma | African descent (Sudanese) | Development of invasive mycetoma by M. mycetomatis | Unknown (decreased chitotriosidase activity) | [118] |
Abbreviations: ABPA, allergic bronchopulmonary aspergillosis; ADCC, antibody-dependent cell-mediated cytotoxicity; AGER, Advanced glycosylation end-product specific receptor; CALM1, calmodulin 1; CCPA, Chronic cavitary pulmonary aspergillosis; CCL, CC chemokine ligand; CXCL, CXC chemokine ligand; DC, dendritic cell; DC-SIGN, Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; DEFB1, defensin β1; DSG1, desmoglein 1; FLOT1, flotillin 1; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; IA, invasive aspergillosis; ICU, intensive care unit; IFIH1, interferon-induced with helicase C domain 1; IFN, interferon; IL, interleukin; IMI, invasive mold infection; LCE4A, late cornified envelope 4A; MAF, minor allelic frequency (frequency of the second most frequent allele in 1000 Genomes combined population); MDA5, melanoma differentiation-associated protein 5; N/A, not available; ND, not defined; NO, nitric oxide; NOS, nitric oxide synthase; PJP, Pneumocystis jirovecii pneumonia; PLG, plasminogen; PRKCH; protein kinase C; PSMB8, Proteasome subunit beta type-8; PTX3, pentraxin 3; RAGE, receptor for advanced glycation end-products; RVVC, recurrent vulvovaginal candidiasis; SAP, serum amyloid P component; S100B, S100 calcium binding protein beta; SOT, solid organ transplantation; STAT1, Signal transducer and activator of transcription 1; TAGAP, T cell activation RhoGTPase activating protein; TLR, Toll-like receptor; TNF, tumor necrosis factor; VNTR, variable number of tandem repeats; VVC, vulvovaginal candidiasis.
Figure 1.
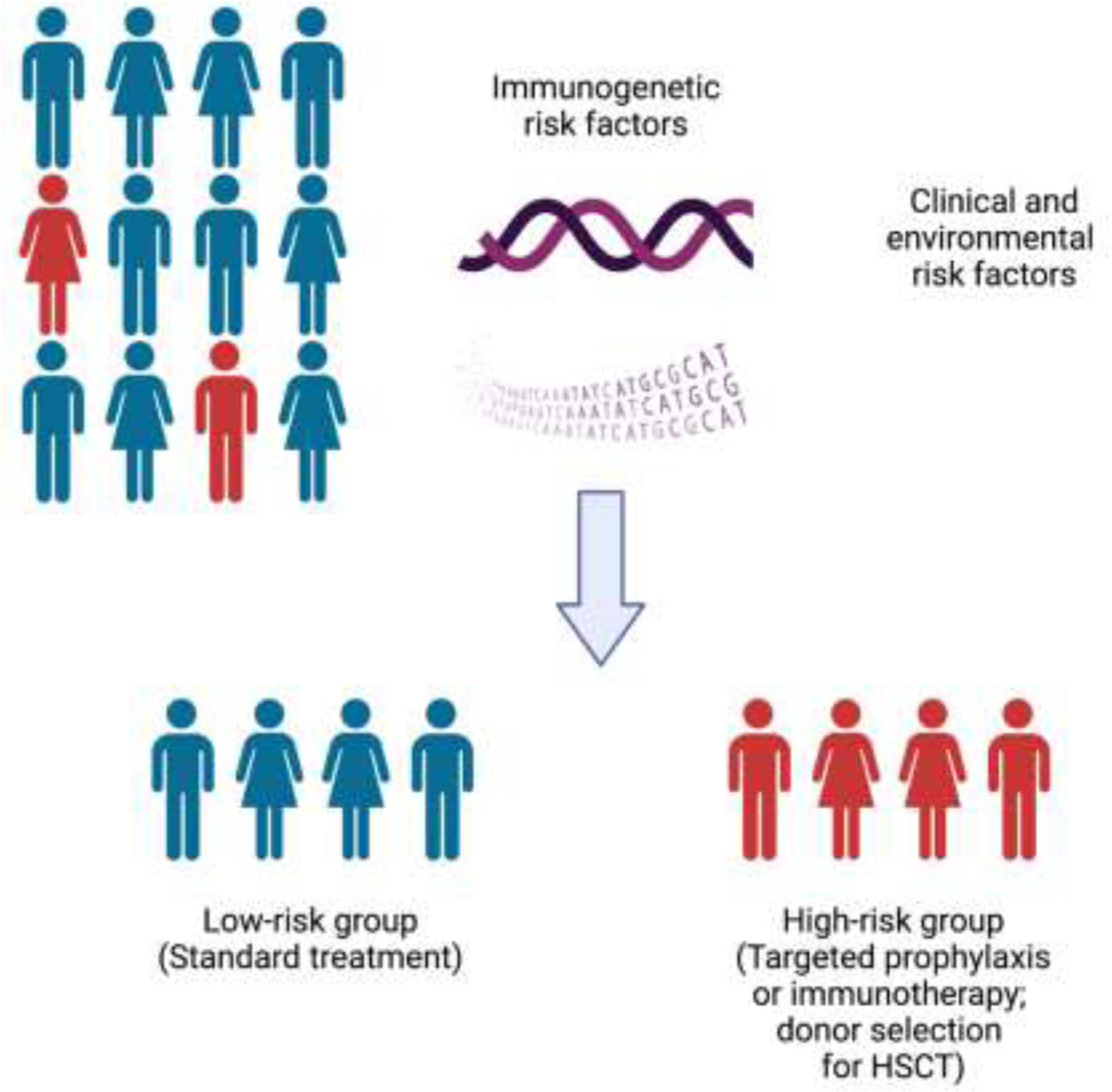
Personalized immunogenetic risk assessment in patients at risk for fungal infections.
Created with BioRender.com.
1. Immunogenetic-based risk assessment for fungal disease
An important clinical observation in patients infected with pathogenic fungi (and other non-fungal pathogens) is that there is significant heterogeneity in the clinical severity and outcome of infections and that clinical and environmental risk factors alone are not sufficient to fully explain the variable patient-specific risk for acquiring these infections. For example, although the majority of ICU patients share similar clinical risk factors for invasive candidiasis (e.g., broad-spectrum antibiotic use, central venous catheters, total parenteral nutrition), the majority of these patients do not develop the infection and when candidemia develops in a subset of them, the clinical severity varies significantly among patients who share similar clinical and microbiological risk factors for developing poor outcome [4, 18]. In addition, despite the ubiquitous environmental exposure to Aspergillus via inhalation and similar clinical risk factors (e.g., neutropenia, corticosteroid use) among allogeneic HSCT recipients, only ~10% of them develop IA. Moreover, <10% of HIV-infected patients with similarly decreased CD4+ T cell counts develop cryptococcal meningoencephalitis despite widespread environmental Cryptococcus exposure [21]. Taken together, these observations indicate that individual genetic variations in immune pathway genes (either alone or in combination) may confer increased susceptibility to or protection from fungal infection.
Indeed, several studies have now demonstrated the contribution of selected immune-related gene SNPs in increasing susceptibility to opportunistic fungal infections in humans (Table 1). These studies provide genetic associations that may help us develop immunogenetic-based risk assessment in patients at-risk for opportunistic fungal infections, which could lead to individualization of antifungal prophylaxis, immunotherapy, or vaccination, and/or optimization of donor selection for recipients of allogeneic HSCT. However, these studies also have limitations. First, biases may be inadvertently introduced by imbalanced population stratification, by small patient sample sizes, by variable clinical practices between different hospitals (e.g., different antifungal prophylaxis or immunosuppressive drug regimens), by the identified SNPs being in linkage disequilibrium with SNPs in other genes that are responsible for the observed phenotype, and/or by the identified SNPs influencing the risk of fungal disease indirectly by affecting other clinical risk factors for acquisition of the fungal infection (e.g., graft-versus-host disease in the setting of allogeneic HSCT). Secondly, validation studies in independent patient cohorts are lacking for most of the reported genetic associations, which are often examined in patients from only one ethnic group (typically Caucasian). Thirdly, in most reported genetic associations, whether the SNPs are dysfunctional thereby conferring impairment in antifungal host defenses is not experimentally examined. Therefore, additional studies in large patient cohorts from multiple ethnic backgrounds with rigorous multivariate statistical analyses and corroborating functional evaluations are warranted to determine which SNPs may be ideal candidates for proceeding with formal clinical trial testing of SNP-based individualized risk stratification and antifungal prophylaxis in at-risk patients. Despite these limitations, these genetic association studies provide critical information about the role of certain immune genes and pathways in host protection against pathogenic fungi, often corroborating mouse studies that reveal the mechanisms by which these genes promote effective antifungal host defense. Although an exhaustive discussion on the role of all reported SNPs is beyond the scope of this review (see Table 1 for detailed summary), the contribution of certain key immune-related SNPs genes in antifungal immune responses is briefly presented below.
1.1. Genetic variation in fungal-sensing pattern recognition receptor (PRR) genes
A breakthrough in the field of fungal immunology over the past two decades has been the discovery and characterization of the role of C-type lectin receptors (CLRs) as the critical fungal-sensing molecules that drive protective antifungal immune responses [22–26]. DECTIN-1 (CLEC7A) is the prototypic CLR recognizing fungal β-glucan [27] but a growing number of CLRs are being discovered and studied whose roles in antifungal host defense is less defined (reviewed in detail elsewhere [23]). In brief, engagement of CLRs by fungal pathogen-associated molecular patterns (PAMPs) promotes the sequential activation of Syk, protein kinase C-δ and the Vav proteins, and the CARD9/BCL10/MALT1 signalosome that in turn leads to the activation of the canonical NF-κB pathway [23]. DECTIN-1 engagement also promotes H-Ras/RASGRF1-mediated, CARD9-dependent ERK activation and Raf-1-mediated, CARD9-independent non-canonical NF-κB pathway activation [28, 29]. Collectively, CLR/Syk/CARD9 signaling drives the production of pro-inflammatory cytokines and chemokines, inflammasome activation, recruitment and effector function of myeloid phagocytes, and Th17 cell differentiation [23, 30–32]. Importantly, human CARD9 deficiency is an autosomal recessive primary immunodeficiency disorder (PID) that causes fungal infection-specific susceptibility with a particular predilection for a) chronic mucocutaneous candidiasis (CMC), associated with decreased circulating Th17 cells in some patients; b) central nervous system (CNS)-targeted candidiasis, associated with impaired neutrophil recruitment and effector function in the fungus-infected CNS; c) IA that exhibits a unique tropism for extrapulmonary tissues; d) cutaneous and CNS phaeohyphomycosis; and c) deep-seated dermatophytosis [30–37].
Not surprisingly, several studies have examined the potential role of SNPs in CLR signaling pathway genes in contributing to fungal infection susceptibility in vulnerable patients. Although CARD9 SNPs have not been associated with candidemia in hospitalized patients [38], the CARD9 S12N SNP, which promotes enhanced type-2 immune responses via macrophage-dependent IL-5 release, was associated with the development of allergic bronchopulmonary aspergillosis (ABPA) in humans, corroborated by investigations in a CARD9S12N knock-in mouse [39]. Among the CLRs, the best studied SNP has been CLEC7A Y238*, which alters the carbohydrate-recognition domain of DECTIN-1 and impairs β-glucan-dependent pro-inflammatory cytokine production by human peripheral blood mononuclear cells (PBMCs) when present in heterozygosity or homozygosity [40–45]. In several studies, the presence of Y238* in either donors or recipients of allogeneic HSCT has been associated with a greater risk of developing IA, indicative of a role of DECTIN-1 signaling in both myeloid phagocytes and epithelial cells for anti-Aspergillus protection, as also shown in mice [41, 42, 46]. The CLEC7A Y238* SNP has also been associated with a greater risk of mucosal Candida colonization in allogeneic HSCT recipients, of recurrent vulvovaginal candidiasis (RVCC), but not candidemia, and of severe phaeohyphomycosis and disseminated coccidioidomycosis [38, 43–45, 47, 48]. Other CLRs in which SNPs have been implicated in the risk of IA following HSCT include CLEC1A (which encodes MelLec that recognizes fungal DHN melanin) and CD209 (which encodes DC-SIGN) whereas a VAV3 SNP has been associated with the risk of candidemia in hospitalized patients [46, 49–51].
Although absent Toll-like receptor (TLR) (and IL-1 receptor) signaling in humans who have inherited MYD88 deficiency does not increase the risk of developing spontaneous fungal infections [52], genetic variation in several TLRs (i.e., TLR1, TLR3, TLR4, TLR5, TLR6, TLR9) has been associated with a greater risk of developing IA in HSCT recipients, of candidemia in hospitalized patients, and/or of cryptococcosis in HIV-infected or HIV-negative individuals [46, 53–62]. Several TLR SNPs have been reported (Table 1), with the best studied being the dysfunctional TLR4 SNPs D299G and T399I, which have been shown to cause hyporesponsiveness to inhaled LPS challenge in humans [63]. These TLR4 SNPs have been associated with an increased risk of developing IA following HSCT, as well as developing chronic cavitary pulmonary aspergillosis and candidemia [53, 56, 58, 59, 62]. Although TLR4 has been shown to recognize PAMPs and secreted virulence factors from Candida, Cryptococcus, and Scedosporium species [64–66], the exact mechanisms by which genetic variation in TLR4 heightens the risk of these infections in humans remain elusive.
Among other classes of PRRs, the NLRP3, NLRC4, and NLRP10 inflammasomes have been implicated in protective host defense against mucosal and invasive fungal infections (IFIs) in mice [26, 67–70]. A variable number tandem repeat in the NLRP3 gene was associated with increased vaginal IL-1β levels and enhanced risk of recurrent vulvovaginal candidiasis (RVVC) in women, a condition characterized by maladaptive, neutrophil-driven, NLRP3/IL-1β-associated vaginal inflammation [71, 72]. Similarly, a SNP in the sialic acid-binding lectin SIGLEC15 was associated with increased IL1B and NLRP3 expression and enhanced the risk of RVVC in women [73]. Nod2-deficient mice are resistant to IA and the presence of the NOD2 SNP P268S affected the production of IL-1β by human PBMCs and was associated with the development of IA following HSCT [74].
The soluble long pentraxin 3 (PTX3) binds to bacteria, viruses, and fungi and facilitates their opsonization, uptake, and killing by immune cells. Ptx3-deficient mice are highly susceptible to IA [75] and dysfunctional PTX3 SNPs in donors of HSCT recipients have been identified as a major risk factor for IA [46, 76]. Neutrophils from individuals carrying the PTX3 SNPs exhibited impaired phagocytosis and intracellular fungal killing, a defect that could be restored in vitro by administration of recombinant PTX3 [76]. Genetic variation in PTX3 has also been associated with the development of IA in patients with SOT and chronic obstructive pulmonary disease and with the development of cryptococcosis in HIV-negative patients [77–80]. PTX2, also known as serum amyloid P component (SAP), is another PRR of the pentraxin family that binds to Aspergillus conidia and facilitates phagocytosis by neutrophils [81]. Apcs-deficient mice are susceptible to IA and SNPs in APCS (which encodes PTX2) were associated with decreased SAP levels and an increased risk of IA following HSCT [81].
1.2. Genetic variation in cytokine, chemokine, and their receptor genes
Following fungal invasion, the production of pro-inflammatory cytokines and chemokines in infected tissues orchestrates the recruitment and activation of immune cells that promote fungal clearance and sterilizing immunity. Several studies have examined the potential role of SNPs in cytokine, chemokine, and their receptor genes in contributing to fungal infection susceptibility in at-risk patients. SNPs in IFNG and the IFN-γ-inducible chemokine CXCL10 have been associated with an increased risk of developing IA in HSCT recipients in independent patient cohorts [46, 82]. A TNFA SNP was associated with the development of intra-abdominal candidiasis in surgical ICU patients [83] and genetic variation in the TNF receptors, TNFR1 and TNFR2, has been associated with an increased risk of IA in patients with HSCT or hematological malignancies [46, 84, 85], in agreement with reports of invasive candidiasis and IA in individuals receiving TNF-α inhibitors [18, 86].
SNPs in IL1A, IL1B, and/or IL1RN have been associated with an increased risk of airway colonization and invasive pulmonary infection by inhaled mold fungi in SOT recipients, chronic cavitary pulmonary aspergillosis, and/or IA in patients with hematological malignancies (Table 1) [87–89]. Genetic variation in IL4 and IL6 have been associated with a greater risk of developing PJP in HIV-infected patients and blastomycosis in individuals of Hmong ancestry, respectively [90, 91]. SNPs in the chemokine IL8 and its receptor CXCR2 in Sudanese individuals have been associated with an increased risk of developing the World Health Organization (WHO)-designated neglected tropical disease mycetoma, a chronic, progressive, and debilitating granulomatous infection that is endemic in tropical and subtropical areas and causes significant morbidity in affected individuals [92]. Genetic variation in IL10 has been associated with the development of IA in HSCT recipients and patients with hematological malignancy [93, 94], whereas SNPs in both IL10 and IL12B were shown to correlate with an increased risk of persistent candidemia in hospitalized patients [95].
Mouse neutrophils rely on the chemokine receptor CXCR1 for degranulation and non-oxidative Candida killing and Cxcr1-deficient mice are susceptible to invasive candidiasis [96]. The dysfunctional CXCR1 SNP S276T was associated with impaired neutrophil degranulation and fungal killing and an increased risk of disseminated candidiasis in candidemic hospitalized patients [96]. The monocyte/macrophage-targeted chemokine receptor CX3CR1 is critical for fungal clearance and host survival in a mouse model of invasive candidiasis by mediating macrophage accumulation in the infected kidney via inhibition of caspase 3-dependent apoptosis [97]. In humans, the dysfunctional CX3CR1 SNP T280M was associated with impaired ERK- and AKT-mediated monocyte survival and increased susceptibility to developing candidemia and disseminated candidiasis among candidemic hospitalized patients in independent Caucasian patient cohorts [97, 98]. In contrast, the CX3CR1 SNP T280M did not increase the risk of RVVC in women [99]. Moreover, genetic variation at the CX3CR1 locus has been associated with the development of IA in patients with HSCT and hematological malignancies [100], although the mechanistic basis of CX3CR1-dependent anti-Aspergillus host defense has not been examined to date.
During murine vulvovaginal candidiasis (VVC), IL-22 is protective by dampening excessive NLRP3 activation and IL-1β release and ameliorating neutrophil-induced immunopathology [101]. SNPs in IL22 and IDO1 correlated with resistance to RVVC in women, associated with increased vaginal levels of IL-22 and decreased vaginal levels of pro-inflammatory cytokines [102]. A recent genome-wide association study (GWAS) in HIV-infected patients of African descent revealed an association between genetic variation in CSF1 (which encodes M-CSF) and the development of cryptococcosis, which was validated in an independent patient cohort [103]. M-CSF has been shown to promote the survival and activation of resident microglia, which are thought of as critical mediators of anti-cryptococcal host defense in the infected CNS tissue [104].
1.3. Genetic variation in other immune-related genes
Additional genetic association studies have highlighted the importance of other immune-related genes in antifungal host defenses, although more studies are required to discern the mechanistic basis of these findings. For example, SNPs in the Fcγ receptors FCGR2A, FCGR2B, FCGR3A, and FCGR3B have been associated with an increased risk of cryptococcosis in HIV-infected and HIV-negative patients (Table 1) [105–107]. A GWAS in hospitalized patients uncovered an association between genetic variation in TAGAP, CD58, and LCE4A-C1orf68 with developing invasive candidiasis [108]. SNPs in STAT1 and other type I interferon-regulated genes also correlate with the risk of invasive candidiasis in hospitalized patients [109]. Another study performed 23 GWAS in >200,000 individuals of European descent and found an association between the risk of VVC and genetic variation in both DSG1 (which encodes desmoglein 1) and PRKCH (which encodes protein kinase C eta) [110]. DSG1 contributes to maintaining barrier function and epidermal integrity [111] and PRKCH regulates keratinocyte differentiation [112], thus pointing to a potential contribution of these molecules to mucocutaneous host defense against Candida that merits further investigation.
The role of danger-associated molecular pattern (DAMP)-associated signaling in antifungal immunity has been less studied, with a reported contribution for the receptor for advanced glycation end products (RAGE) and its ligand S100B in restraining immunopathology during murine IA [113]. In addition, a SNP in AGER (which encodes RAGE) in both donors and recipients of HSCT and a SNP in S100B in donors of HSCT have been associated with a greater risk of developing IA following HSCT [46, 114]. In addition, LC3-associated phagocytosis (LAP) has been shown to promote Aspergillus clearance within macrophages in a DECTIN-1/Syk-dependent, calcium/calmodulin-regulated manner and can be counteracted by Aspergillus conidial melanin [115, 116]. A SNP in CALM1 (which encodes calmodulin 1) that decreases CALM1 mRNA levels was recently shown to correlate with increased risk of IA following HSCT [116]. More studies will be needed to understand the mechanisms by which genetic variation in the PLG gene (which encodes plasminogen) is associated with the risk of IA in HSCT recipients [117] and by which genetic variation in the CHIT1 gene (which encodes the chitin-degrading enzyme chitotriosidase) is associated with the risk of mycetoma caused by the fungus Madurella mycetomatis [118].
1.4. Targeted antifungal prophylaxis based on increased immunological risk
Since the 1990s, the introduction of fluconazole prophylaxis has significantly decreased the incidence of invasive candidiasis in high-risk patients with allogeneic HSCT [119, 120]. More recently, prophylaxis with the mold-active triazole, posaconazole, was shown to decrease the incidence of IA and other IFIs in high-risk patients with allogeneic HSCT and graft-versus-host disease or hematological malignancy and prolonged neutropenia [121, 122]. However, universal administration of antifungal prophylaxis, particularly beyond the high-risk setting of allogeneic HSCT, poses significant challenges. Specifically, the number of patients needed to administer prophylaxis to prevent one fungal infection is often quite high even when using clinical risk factors to enrich for higher-risk individuals. Moreover, in most studies there is no observed survival benefit, antifungal agents are costly and exhibit toxicities and drug-drug interactions, and drug-resistant fungal strains can emerge during antifungal prophylaxis [123–126]. Therefore, immunogenetic-based risk assessment could help individualize antifungal prophylaxis by selecting patients with a greater risk for developing IFIs, thereby decreasing the numbers needed to treat to prevent disease and minimizing the cost, toxicities, and drug resistance risk associated with widespread use of antifungal prophylaxis.
Proof-of-concept for the beneficial use of targeted antifungal prophylaxis has been demonstrated in two high-risk groups of patients with certain immunological conditions that dramatically predispose them to IA. Firstly, patients with chronic granulomatous disease (CGD), caused by mutations in any of the subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex that impair phagocyte-dependent oxidative burst, have a ~40% lifetime risk of developing IA, which is the leading cause of infection-associated mortality in this PID [127]. The use of itraconazole prophylaxis has dramatically decreased the incidence of IFIs in CGD patients, thereby improving their outcomes [128]. Secondly, patients with lymphoma who receive the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib are at increased risk for developing IA, underscoring the role of phagocyte BTK in anti-Aspergillus host defense [129, 130]. The incidence of IA appears to be ~2–4% in patients receiving ibrutinib monotherapy and increases to ~5–10% when ibrutinib is co-administered with corticosteroids [131–136]. When ibrutinib was co-administered with corticosteroids and chemotherapeutic agents in patients with refractory primary CNS lymphoma (TEDDI-R), 39% of them developed IA [137]. To enable the observed high rates of durable lymphoma remissions with TEDDI-R treatment in these patients without the high rate of IA, isavuconazole prophylaxis has now been added to the TEDDI-R regimen and has thus far prevented the development of IA in an ongoing clinical trial [138].
1.5. Targeted vaccination based on increased immunological risk
Besides guiding targeted antifungal prophylaxis, immunogenetic-based risk assessment could also help individualize fungal vaccination strategies by selecting patients with a greater risk for developing invasive candidiasis in the ICU. While several fungal vaccine candidates have been investigated in murine models of various opportunistic fungal infections (reviewed elsewhere [139–141]), an immunogenetic risk-based, targeted vaccination strategy could now become achievable with the recent development of the NDV-3A vaccine, the first-in-human fungal vaccine to exhibit immunogenicity, tolerability, and clinical efficacy in patients [142, 143]. NDV-3A, which is based on the recombinant N terminus of the Candida albicans adhesin Als3 (rAls3p-N) [144] combined with an aluminum hydroxide adjuvant, improved survival of mice systemically infected with C. albicans by eliciting potent antibody and cell-mediated immune responses [145]. Mechanistically, vaccination induced the generation of IFN-γ- and IL-17-producing CD4+ T cells, which promoted the production of phagocyte-recruiting chemokines such as CXCL1, mediated phagocyte trafficking and activation at the site of fungal invasion, and decreased tissue fungal burden [145]. Although the eventual goal of the NDV-3A vaccination platform is to determine whether it can prevent the development of invasive candidiasis in high-risk acutely ill patients in the ICU, it was initially evaluated in the context of VVC. Thus, in a murine model of VVC, administration of the NDV-3A vaccine protected animals from vaginal fungal proliferation in a manner dependent on both B and T cells [146]. In a Phase I clinical trial, NDV-3A vaccination was safe and immunogenic in healthy adults resulting in IgA and IgG antibody responses as well as IFN-γ- and IL-17-producing CD4+ T cells [143]. In a Phase II randomized, double-blind, placebo-controlled clinical trial, NDV-3A vaccination of women with RVVC was safe, immunogenic, and exhibited clinical efficacy by decreasing the frequency of symptomatic episodes of VVC [142]. Future clinical trials will be needed to determine whether NDV-3A protects against invasive candidiasis in high-risk ICU patients. To decrease the number of patients needed to treat to prevent fungal disease, patient selection in such a clinical trial could be based on both clinical and immunogenetic risk factors of invasive candidiasis development.
1.6. Outlook
Moving forward, the challenge in the field will be to determine which of the reported SNPs in several immune-related genes are ideal candidates for formal testing in clinical trials of targeted antifungal prophylaxis or vaccination, whether present alone or in combination. In that regard, the combined presence of TLR4 and IFNG SNPs was shown to promote additive susceptibility to IA compared to carriage of each SNP alone [59]. Similarly, the combined presence of two or more SNPs within the CD58, TAGAP, and LCE4A-C1orf68 loci markedly increased the risk of invasive candidiasis (~20-fold) compared to carriage of each SNP alone [108]. As mentioned earlier, ideal candidates are dysfunctional SNPs that their genetic association with increased risk of fungal disease has been validated in independent patient cohorts and across ethnic backgrounds with corroborating immunological mechanistic data in mouse models of fungal disease and primary human cells.
2. Immunotherapeutic modalities to boost antifungal immune responses
The suboptimal clinical outcomes and severe net state of immunosuppression of patients who suffer from opportunistic fungal disease has sparked a growing interest in the role of certain adjunctive immunotherapeutic modalities that could help augment antifungal immune responses and complement conventional antifungal treatment. These interventions can be categorized into cytokine- and cell-based and are briefly highlighted below.
2.1. Cytokine-based and other non-cellular interventions
2.1.1. IFN-γ-based interventions
IFN-γ was discovered in 1965 as a viral inhibitory factor in phytohemagglutinin-stimulated human leukocyte cultures [147]. Since then, IFN-γ has been shown to exert pleiotropic effects during immune homeostasis, inflammation, autoimmunity, and host defense against intracellular pathogens including Cryptococcus and endemic dimorphic fungi such as Coccidioides [148–150].
In 1991, a randomized, double-blind, placebo-controlled clinical trial showed that recombinant IFN-γ reduced the frequency of serious (including fungal) infections in patients with CGD resulting in its FDA approval in this patient population [151]. Although a few studies have since reported a potential beneficial role for adjunctive IFN-γ in the management of chronic progressive pulmonary aspergillosis or of invasive candidiasis or IA in patients with critical illness, hematological malignancies, or HSCT, definite evidence for the widespread utility of this intervention in these patient populations is lacking [152–155]. Recent reports have suggested a potential role for the combination of recombinant IFN-γ with the immune checkpoint inhibitor nivolimab in the management of mucormycosis [156, 157], highlighting the need for additional preclinical and clinical studies that will precisely define the contribution of immune checkpoint blockade in the treatment of opportunistic (including fungal) infections [158].
The contribution of IFN-γ in host defense against infections by intra-macrophagic pathogens is well-recognized. Thus, the role of IFN-γ in the management of patients infected by intra-macrophagic fungi has been examined in the setting of cryptococcosis and coccidioidomycosis. Firstly, the administration of recombinant IFN-γ was shown to accelerate Cryptococcus clearance from the cerebrospinal fluid (CSF) in HIV-infected patients with cryptococcal meningoencephalitis [159, 160], consistent with several studies demonstrating that impaired type-1 immune responses and decreased IFN-γ levels are predictive of poor outcomes in this disease [161–163]. IFN-γ may also boost immunity and help remit cryptococcal meningoencephalitis in patients with idiopathic CD4 lymphopenia (ICL) [164]. Secondly, the administration of recombinant IFN-γ has been reported to remit severe, treatment-refractory coccidioidomycosis in some patients [165]. More recently, the co-administration of recombinant IFN-γ with the IL-4/IL-13 receptor inhibitor, dupilumab, augmented IFN-γ and decreased IL-4 production and resulted in rapid clinical remission in another patient with disseminated, treatment-refractory coccidioidomycosis [166]. In some patients with autosomal recessive partial IFN-γR1 deficiency who are at-risk for infections by intra-macrophagic pathogens, IFN-γ (or IFN-α) immunotherapy may boost immune responses and promote clinical remission [167].
Besides monogenic disorders in the IFN-γ signaling pathway [168], cryptococcosis can also develop in patients with neutralizing autoantibodies against IFN-γ [169]. Rituximab (which targets CD20) and daratumumab (which targets CD38) deplete B cells and plasma cells, respectively, and have shown promise as adjunctive therapies in patients with treatment-refractory non-tuberculous mycobacterial disease associated with a reduction in the titers of anti-IFN-γ neutralizing autoantibodies [170, 171]. Future studies are warranted to define the role of these biologics in the management of infections by intra-macrophagic fungi in patients with anti-IFN-γ autoantibodies.
2.1.2. Colony stimulating factor-based interventions
G-CSF and GM-CSF are FDA-approved for accelerating neutrophil reconstitution in patients with hematological malignancies and HSCT recipients and have been shown to decrease the frequency and/or duration of episodes of febrile neutropenia and associated infections, albeit without an observed survival benefit [172, 173]. In mice, both G-CSF and GM-CSF improved the survival of immunosuppressed animals with invasive candidiasis or IA [174–177]. Moreover, treatment of Aspergillus-infected, transplanted mice with M-CSF instructed myeloid commitment in hematopoietic stem cells via direct activation of the transcription factor PU.1, thereby decreasing tissue fungal proliferation and improving survival [178]. Mechanistically, GM-CSF primes oxidative burst and the fungicidal activity of neutrophils in the Aspergillus-infected mouse lung [174], whereas dendritic cell-derived IL-23 release promotes the release of GM-CSF by NK cells, which in turn primes the candidacidal activity of neutrophils in the infected mouse kidney [179, 180]. In a mouse model of antibiotic pre-exposure and subsequent invasive candidiasis, impaired lymphocyte-mediated GM-CSF (and IL-17) responses in the intestine led to systemic bacterial translocation and increased mortality, which could be partially rescued by recombinant GM-CSF immunotherapy [181]. In a Phase IV randomized clinical trial that compared the role of G-CSF and GM-CSF in prevention and treatment of IFIs in allogeneic HSCT recipients, GM-CSF demonstrated superior efficacy and significantly decreased the incidence of invasive candidiasis and fungal infection-associated mortality relative to G-CSF [182]. Another study recently reported favorable clinical responses in some patients with invasive candidiasis, IA, mucormycosis and other invasive fungal infections when adjuvant GM-CSF treatment was used together with conventional antifungal therapy [183].
Decreased GM-CSF associated with defective H-RAS/RASGRF-1/ERK responses have been documented in some CARD9-deficient patients with CNS candidiasis and adjunctive GM-CSF immunotherapy was associated with clinical remission in these patients [184, 185]. However, in another CARD9-deficient patient with CNS candidiasis caused by different missense CARD9 mutations and intact H-RAS/RASGRF-1/ERK responses, GM-CSF immunotherapy appeared to drive eosinophil-driven CNS immunopathology and disease worsening, pointing to differential effects of various CARD9 mutations on the H-RAS/RASGRF-1/ERK signaling axis and differing clinical responses to GM-CSF [186]. Other CARD9-deficient patients with invasive candidiasis were reported to achieve clinical remission with G-CSF immunotherapy [187, 188]. More studies are needed to define the optimal immunotherapeutic intervention(s) in patients with CARD9 deficiency, a subset of whom require HSCT to control treatment-refractory invasive fungal disease [189].
2.1.3. IL-7
IL-7 was discovered in 1988 as a growth factor that stimulated the proliferation of lymphoid progenitors [190]. In a Phase II, randomized, double-blind clinical trial of IL-7 in septic patients, IL-7 was well-tolerated, it inhibited lymphocyte apoptosis, reversed sepsis-associated lymphopenia, and induced lymphocyte proliferation, activation, and release of IFN-γ and IL-17 [191]. In CD4-depleted, Pneumocystis-infected mice, IL-7 prevented T cell apoptosis, increased lymphocyte recruitment, activation, and IFN-γ release, and decreased tissue fungal proliferation [192]. In a two-hit experimental murine model of bacterial peritonitis caused by cecal ligation and puncture followed by Candida sepsis, IL-7 immunotherapy promoted lymphocyte proliferation, activation, IFN-γ release, and expression of adhesion molecules leading to improved host survival [193, 194]. In an immunocompetent individual who developed a mixed would infection by Trichosporon asahii, Fusarium, and Saksenaea species and had failed antifungal and surgical treatment, receipt of adjunctive IL-17 immunotherapy led to improved lymphocyte counts and function, fungal clearance, and clinical remission [195]. Another setting where IL-7 immunotherapy has shown promising clinical results is ICL, a condition that heightens the risk for cryptococcosis and other opportunistic infections such as progressive multifocal leukoencephalopathy (PML) [196]. Indeed, IL-7 immunotherapy increased the numbers of circulating and tissue-resident T cells and enhanced their function [197], and exhibited promising clinical effects in an ICL patient with PML [198]. Thus, the potential role of IL-7 immunotherapy in prevention and/or treatment of opportunistic (including fungal) infections in ICL patients warrants further study.
2.1.4. TLR and CLR cooperative activity against chromoblastomycosis
Chromoblastomycosis is a WHO-designated neglected tropical disease most often caused by the melanin-bearing yeast fungus Fonsecaea pedrosoi, which upon traumatic skin inoculation infects the subcutaneous tissues leading to progressive, disfiguring, and often treatment-refractory disease in tropical and subtropical areas [199]. F. pedrosoi activates the Syk/CARD9 signaling pathway via the CLR Mincle (Clec4e) but it fails to activate TLR-mediated immune responses, thereby leading to impaired pro-inflammatory cytokine responses in the infected mouse skin [200]. Notably, exogenous application of the TLR7 agonist imiquimod in F. pedrosoi-infected mice reinstated effective pro-inflammatory immune responses and facilitated infection clearance [200]. Concordantly, topical application of imiquimod combined with antifungal therapy leads to clinical remission in patients with treatment-refractory chromoblastomycosis [201–203], highlighting the key role of fungal-sensing PRR cooperation in mounting effective antifungal responses in mice and humans [18, 65, 204].
2.2. Cell-based interventions
2.2.1. Granulocyte transfusions
Granulocyte transfusions were introduced in the clinic in the 1960s and early controlled studies showed remarkable clinical and survival benefits in neutropenic patients with invasive infections [205]. Although the advent of corticosteroid and G-CSF use has led to improved donor neutrophil mobilization, granulocyte transfusions remain challenging due to the high cost, technical difficulties in harvesting large numbers of granulocytes that are required for efficacy, short shelf-life (~24 hours), transfusion-induced pulmonary reactions, and HLA alloimmunization, which is particularly problematic in individuals anticipating HSCT [206–209]. Although granulocyte transfusions decreased lung fungal burden and improved survival in Aspergillus-infected neutropenic mice [210], most human studies have reported conflicting results regarding their clinical efficacy and marked variability in granulocyte transfusion practices, which collectively preclude reliable conclusions about their potential role as adjunctive therapies in immunosuppressed patients [211]. In a recent multicenter, randomized, controlled clinical trial termed RING (Resolving Infection in Neutropenia with Granulocytes) that was not completed as planned due to suboptimal patient enrollment, adjunctive transfusion of G-CSF/dexamethasone-mobilized granulocytes did not demonstrate a clinical benefit over standard antimicrobial treatment alone in neutropenic patients with invasive infections [212]. However, in a post hoc analysis, individuals who received a high granulocyte dose (i.e., ≥0.6 × 109 cells/Kg per transfusion) tended to have better clinical outcomes compared to those who received lower granulocyte doses [212]. The NIH experience with granulocyte transfusions indicates that certain patient groups may exhibit a clinical benefit such as those with hematological malignancies and refractory fusariosis or CGD patients with refractory bacterial or fungal infections [213, 214].
2.2.2. Infusion of Aspergillus-specific T cells or fungus-targeted chimeric antigen receptor (CAR) T cells
Lymphocytes are dispensable for host defense against IA as Rag2Il2rg−/− mice that lack innate and adaptive lymphoid cells control inhaled Aspergillus conidia without developing invasive infection, similarly to humans with quantitative or qualitative defects in lymphoid cells [18, 215]. However, because T lymphocytes can augment the anti-Aspergillus effector function of myeloid phagocytes via the production of IFN-γ, IL-17, and/or other soluble factors [216, 217], the adoptive transfer of Aspergillus-specific T cells was investigated in a mouse model of IA following HSCT and was found to confer resistance to the infection [218]. In a clinical trial, Perrucio and colleagues generated Aspergillus-specific T cells by limiting dilution, which requires >20 days, and infused them in 10 patients with IA following HSCT, whereas 13 additional HSCT recipients with IA did not receive this adoptive immunotherapy [219]. All 10 recipients of Aspergillus-specific T cells had normalization of serum galactomannan levels within 6 weeks of cell infusion, whereas serum galactomannan levels remained elevated in all 13 control patients who did not receive Aspergillus-specific T cells. Moreover, 9/10 patients who received Aspergillus-specific T cells cleared the infection and only one succumbed to IA, whereas 7/13 of control individuals who did not receive Aspergillus-specific T cells died from IA [219]. No infusion toxicities or graft-versus-host disease were noted in that study. The advent of more rapid methods for the clinical scale generation of Aspergillus-specific T cells according to good manufacturing practice conditions holds promise for the potential further clinical development of this immunotherapeutic intervention [220].
Furthermore, Kumaresan et al. bioengineered fungus-targeting cytotoxic T cells by enforcing expression of a CAR that recapitulates the specificity of the β-glucan-sensing DECTIN-1 fused to the CD28 and CD3-ζ cytoplasmic signaling domain [221]. The genetically modified DECTIN-1-CAR T cells bound specifically to β-glucan, expressed perforin and granzyme, exhibited a central memory phenotype, produced IFN-γ, and were able to recognize and lyse Aspergillus conidia and hyphae in vitro and in vivo in the lung and skin of immunosuppressed mice [221]. Moreover, a recent study described the development of CAR T cells that recognize conserved epitopes on the surface of A. fumigatus hyphae, but not of other Aspergilli or mold species. These cells were shown to a) secrete pro-inflammatory cytokines upon exposure to A. fumigatus, b) prime the antifungal effector function of macrophages, and c) secrete perforin and granzyme B for direct antifungal activity. Adoptive transfer of these cells into Aspergillus-infected mice led to their accumulation in the lung and resulted in decreased tissue fungal burden and a survival benefit in neutropenic mice [222]. Although the cost of such a CAR T cell-based immunotherapeutic approach would be currently prohibitory in the clinic, this study provides proof-of-concept that bioengineering fungus-directed cytotoxic T cells with specificity to carbohydrates and/or other fungal epitopes can be harnessed to target life-threatening fungal infections in vulnerable patients.
3. Immunotherapeutic modalities to ameliorate immunopathology
Although susceptibility to fungal infections is most often driven by impaired host resistance, in certain settings, the development of opportunistic fungal disease is characterized by maladaptive immune responses that can drive local detrimental immunopathology thereby impairing host tolerance to the infection. In such conditions, treatment with anti-inflammatory agents that dampen exuberant immune responses can help control the infection. Three such examples are briefly highlighted below.
3.1. Corticosteroids for neutrophil-mediated immunopathology during disseminated candidiasis
Although neutrophils are critical for promoting sterilizing immunity in the setting of invasive candidiasis (and IA) in mice and humans [223], their aberrant accumulation and activation at the site of fungal infection may also come at the cost of tissue immunopathology and damage [224]. In the mouse model of invasive candidiasis, excessive, CCR1-mediated neutrophil recruitment in the infected kidney at the late phase of the infection underlies immunopathology and renal injury [225–227]. Besides the chemokine receptor CCR1, leukotriene B4, the CLR dendritic cell natural killer lectin group receptor-1 (DNGR-1), the lectin galectin-3, the tyrosine kinase Tec, the suppressor of TCR signaling (Sts) phosphatases, the endoribonuclease MCPIP1, and IL-17C have also been implicated in neutrophil-driven immunopathology in the mouse model of invasive candidiasis [228–233].
In humans, neutrophil-mediated immunopathology can be seen in a subset of neutropenic patients with hepatosplenic candidiasis upon recovery of their neutrophil counts and manifests clinically with worsening of symptoms and persistent fever [234]. Corticosteroids are often used to ameliorate excessive inflammatory responses and improve clinical symptoms in these patients [234]. A recent study examined peripheral blood immune responses in patients with chronic disseminated candidiasis upon neutrophil recovery and found neutrophilia, increased numbers of IFN-γ-producing T cells, enhanced T cell activation, and elevated plasma levels of pro-inflammatory molecules such as IL-1β, TNF-α, IL-6, and soluble CD25 [235]. Collectively, a better understanding of the molecular drivers of inflammation in this clinical condition may help develop targeted pharmacological strategies to inhibit excessive neutrophil-driven immunopathology, as corticosteroids significantly heighten susceptibility to opportunistic (including fungal) infections [236].
3.2. Treatment of cryptococcosis-associated immune reconstitution inflammatory syndrome (IRIS)
Although IFN-γ-producing T cells are crucial for facilitating sterilizing immunity during cryptococcosis, their aberrant accumulation and activation at the site of infection may also promote immunopathogenic effects. Clinically, these effects can be observed in HIV-negative patients with cryptococcosis [237] and in a subset of HIV-infected patients with cryptococcosis who develop IRIS after initiation of antiretroviral therapy that promotes immune reconstitution [238, 239]. The risk of HIV-associated IRIS is greater in patients with greater HIV viremia, more severe CD4+ T cell lymphopenia, and active infection at the time of initiation of antiretroviral therapy [238, 239]. In these patients, the use of corticosteroids may ameliorate excessive inflammation and improve neurological symptoms [240, 241].
In mice infected with Cryptococcus neoformans without CD4+ T cell depletion, which models non-HIV-associated human infection, excessive accumulation of Th1 cells is mediated by the release of the chemokine CXCL10 by activated resident glial cells and promotes immunopathology in the infected CNS [242, 243]. These effects were ameliorated by inhibiting the CXCL10-targeted chemokine receptor CXCR3, which improved mouse survival [242]. In non-HIV-infected patients with cryptococcal meningoencephalitis, robust accumulation of CXCR3-expressing Th1 cells in the CNS-infected tissue and increased CSF levels of CXCL10 are also observed, yet the immune response is ineffective and is associated with neuronal damage [244].
In mouse models of C. neoformans-associated IRIS, increased production of IFN-γ, TNF-α, and IL-6 are seen in inflamed tissue, and in one of the studies the accumulation of Th1 cells was sufficient to drive CNS immunopathology associated with induction of the expression of aquaporin-4, a molecule that regulates water influx in the brain parenchyma [245, 246]. Moreover, HIV-infected patients with IRIS have increased serum levels of IL-6, IL-7, and IFN-γ increased CSF levels of CXCL10, enhanced frequencies of activated HLA-DR+ CD14+ monocytes, and enriched frequencies of effector memory IFN-γ- and IL-17-producing CD4+ T cells compared to HIV-infected patients without IRIS [239, 247–249]. A better understanding of the molecular determinants of cryptococcosis-associated IRIS could enable more targeted treatments relative to corticosteroids. For example, inhibition of exaggerated TNF-α responses with adalimumab or thalidomide has been reported to successfully treat a few HIV-infected patients with cryptococcosis-associated IRIS [250, 251].
3.3. Janus kinase (JAK) inhibitors for CMC in autoimmune regulator (AIRE) deficiency
Another important breakthrough in the field of fungal immunology over the past decade has been the discovery that the IL-17R signaling pathway promotes protective host defense against mucosal candidiasis in mice and humans [252]. Indeed, patients with complete genetic deficiencies in the IL-17 receptors IL-17RA and IL-17RC or their adaptor molecule ACT1/TRAF3IP2 develop fully penetrant CMC [252–255] and several other PIDs that underlie CMC susceptibility feature varying degrees of decreased circulating Th17 cells and/or impaired IL-17 cellular responses [18, 256]. Mild, treatment-responsive oral candidiasis, but not CMC, develops in a small proportion of patients treated with IL-17 pathway-targeting biologics (mean frequency, ~1–10%) [257].
CMC develops in ~85% of patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), caused by loss-of-function mutations in the AIRE gene that impair central immune tolerance [258]. Many APECED patients carry neutralizing autoantibodies against IL-17A (frequency, ~35%), IL-17F (~20–80% depending on the patient cohort), and IL-22 (frequency, ~85%), yet the association between CMC and neutralizing IL-17 autoantibodies is incompletely penetrant [257, 259–262], indicating that other immunological factors also contribute to CMC susceptibility. Indeed, we recently showed that exuberant IFN-γ production by mucosal CD4+ and CD8+ T cells drives oral candidiasis susceptibility in Aire-deficient mice by driving epithelial barrier disruption, which can be ameliorated by genetic deletion of IFN-γ or pharmacological JAK-STAT inhibition with ruxolitinib [263]. Similarly, excessive IFN-γ/JAK/STAT responses were observed in the oral mucosa of APECED patients [263], suggesting that JAK inhibition may be an effective immunomodulatory strategy for CMC in this patient population, a hypothesis that is currently been tested in an ongoing clinical trial. This finding provides a conceptual framework for classifying mucosal fungal susceptibility across a spectrum of impaired type-17 mucosal host resistance and/or immunopathology-causing type-1 mucosal inflammation.
Furthermore, patients with STAT1 gain-of-function mutations who are universally susceptible to severe CMC, exhibit enhanced IFN-γ cellular responses, and a subset, but not all, of them have decreased numbers of circulating Th17 cells [264–266]. Strikingly, JAK-STAT inhibition with ruxolitinib or itacitinib leads to clinical remission of CMC in patients with STAT1 gain-of-function mutations, in many of whom, CMC remission is seen without an increase in the frequency of circulating Th17 cells, pointing to IL-17-independent ameliorating mechanisms [267–275]. Thus, JAK inhibitor-induced dampening of excessive IFN-γ mucosal responses may contribute to the beneficial immunotherapeutic effects of JAK inhibitors in this CMC-manifesting patient population. Importantly, the expanding number of PIDs that feature CMC in the context of autoinflammation or autoimmunity and exhibit intact or even enhanced IL-17 responses (e.g., Down syndrome, mutations in ELF4 or IKZF2) may reveal additional clinical conditions in which CMC is promoted by mucosal type-1 inflammation and, therefore, could perhaps also be therapeutically targeted with benefit with JAK-STAT inhibition [276–278].
Conclusions
Opportunistic fungal infections represent significant causes of morbidity and mortality in vulnerable patients with critical illness and various inherited and acquired immunodeficiency states. Herein, an overview was presented of how our improved understanding of the cellular and molecular determinants of fungus-, tissue-, cell type-, and context-specific antifungal immune responses could be exploited in the clinical context to benefit fungus-infected patients. Taken together, immunogenetic-based risk assessment, individualization of antifungal prophylaxis and vaccination, and targeted immunotherapies that boost inadequate immune responses or ameliorate maladaptive immunopathogenic responses hold promise for improving the clinical management and prognosis of susceptible patients who suffer from life-threatening fungal infections.
Highlights.
Fungal infections affect immunocompromised patients, and their outcomes remain poor despite treatment.
Immunogenetic profiling may enable personalized risk assessment, and targeted antifungal prophylaxis and vaccination strategies.
A Candida albicans Als3-based vaccine is the first-in-human efficacious fungal vaccine.
Targeted immunotherapy holds promise for modulating antifungal immune responses in vulnerable patients with opportunistic fungal infections.
Acknowledgements
This work was supported by the Division of Intramural Research of the NIAID, NIH (ZIA AI001175 to MSL).
Abbreviations:
- PJP
Pneumocystis jirovecii pneumonia
- IA
invasive aspergillosis
- ICU
intensive care unit
- HSCT
hematopoietic stem cell transplantation
- SOT
solid organ transplantation
- SNP
single nucleotide polymorphism
- PRR
pattern recognition receptor
- CLR
C-type lectin receptor
- PAMP
pathogen-associated molecular pattern
- PID
primary immunodeficiency disorder
- CMC
chronic mucocutaneous candidiasis
- CNS
central nervous system
- ABPA
allergic bronchopulmonary aspergillosis
- PBMCs
peripheral blood mononuclear cells
- IFIs
invasive fungal infections
- RVVC
recurrent vulvovaginal candidiasis
- VVC
vulvovaginal candidiasis
- TLR
Toll-like receptor
- PTX3
pentraxin 3
- SAP
serum amyloid P component
- WHO
World Health Organization
- GWAS
genome-wide association study
- DAMP
danger-associated molecular pattern
- RAGE
receptor for advanced glycation end products
- LAP
LC3-associated phagocytosis
- CGD
chronic granulomatous disease
- NADPH
nicotinamide adenine dinucleotide phosphate
- BTK
Bruton’s tyrosine kinase
- CSF
cerebrospinal fluid
- ICL
idiopathic CD4 lymphopenia
- PML
progressive multifocal leukoencephalopathy
- CAR
chimeric antigen receptor
- DNGR-1
dendritic cell natural killer lectin group receptor-1
- STS
suppressor of TCR signaling
- IRIS
immune reconstitution inflammatory syndrome
- JAK
Janus kinase
- AIRE
autoimmune regulator
- APECED
autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR, Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis, Lancet Infect Dis (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Segal BH, Aspergillosis N Engl J Med 360(18) (2009) 1870–84. [DOI] [PubMed] [Google Scholar]
- [3].Lionakis MS, Hohl TM, Call to Action: How to Tackle Emerging Nosocomial Fungal Infections, Cell Host Microbe 27(6) (2020) 859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kullberg BJ, Arendrup MC, Invasive Candidiasis N Engl J Med 373(15) (2015) 1445–56. [DOI] [PubMed] [Google Scholar]
- [5].Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ, Invasive candidiasis, Nat Rev Dis Primers 4 (2018) 18026. [DOI] [PubMed] [Google Scholar]
- [6].Strollo S, Lionakis MS, Adjemian J, Steiner CA, Prevots DR, Epidemiology of Hospitalizations Associated with Invasive Candidiasis, United States, 2002–2012(1), Emerg Infect Dis 23(1) (2016) 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lionakis MS, Iliev ID, Hohl TM, Immunity against fungi, JCI Insight 2(11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E, Molecular and nonmolecular diagnostic methods for invasive fungal infections, Clin Microbiol Rev 27(3) (2014) 490–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chamilos G, Lewis RE, Kontoyiannis DP, Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis, Clin Infect Dis 47(4) (2008) 503–9. [DOI] [PubMed] [Google Scholar]
- [10].Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH, Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign, Clin Infect Dis 44(3) (2007) 373–9. [DOI] [PubMed] [Google Scholar]
- [11].Kollef M, Micek S, Hampton N, Doherty JA, Kumar A, Septic shock attributed to Candida infection: importance of empiric therapy and source control, Clin Infect Dis 54(12) (2012) 1739–46. [DOI] [PubMed] [Google Scholar]
- [12].Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Lass-Florl C, Prattes J, Spec A, Thompson GR 3rd, Wiederhold N, Jenks JD, The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin, Drugs 81(15) (2021) 1703–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lamoth F, Lewis RE, Kontoyiannis DP, Investigational antifungal agents for invasive mycoses: a clinical perspective, Clin Infect Dis (2022). [DOI] [PubMed] [Google Scholar]
- [14].Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA, Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations, Clin Infect Dis 56(12) (2013) 1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chow NA, Gade L, Tsay SV, Forsberg K, Greenko JA, Southwick KL, Barrett PM, Kerins JL, Lockhart SR, Chiller TM, Litvintseva AP, U.S.C.a.I. Team, Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey, Lancet Infect Dis 18(12) (2018) 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Verweij PE, Chowdhary A, Melchers WJ, Meis JF, Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles?, Clin Infect Dis 62(3) (2016) 362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stone NR, Rhodes J, Fisher MC, Mfinanga S, Kivuyo S, Rugemalila J, Segal ES, Needleman L, Molloy SF, Kwon-Chung J, Harrison TS, Hope W, Berman J, Bicanic T, Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis, J Clin Invest 129(3) (2019) 999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lionakis MS, Levitz SM, Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts, Annu Rev Immunol 36 (2018) 157–191. [DOI] [PubMed] [Google Scholar]
- [19].Kontoyiannis DP, Bodey GP, Hanna H, Hachem R, Boktour M, Girgaway E, Mardani M, Raad II, Outcome determinants of fusariosis in a tertiary care cancer center: the impact of neutrophil recovery, Leuk Lymphoma 45(1) (2004) 139–41. [DOI] [PubMed] [Google Scholar]
- [20].Nucci M, Anaissie EJ, Queiroz-Telles F, Martins CA, Trabasso P, Solza C, Mangini C, Simoes BP, Colombo AL, Vaz J, Levy CE, Costa S, Moreira VA, Oliveira JS, Paraguay N, Duboc G, Voltarelli JC, Maiolino A, Pasquini R, Souza CA, Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection, Cancer 98(2) (2003) 315–9. [DOI] [PubMed] [Google Scholar]
- [21].Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, Denning DW, Loyse A, Boulware DR, Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis, Lancet Infect Dis 17(8) (2017) 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brown GD, Innate antifungal immunity: the key role of phagocytes, Annu Rev Immunol 29 (2011) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown GD, Willment JA, Whitehead L, C-type lectins in immunity and homeostasis, Nat Rev Immunol 18(6) (2018) 374–389. [DOI] [PubMed] [Google Scholar]
- [24].Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B, A homozygous CARD9 mutation in a family with susceptibility to fungal infections, N Engl J Med 361(18) (2009) 1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J, Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity, Nature 442(7103) (2006) 651–6. [DOI] [PubMed] [Google Scholar]
- [26].Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J, Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence, Nature 459(7245) (2009) 433–6. [DOI] [PubMed] [Google Scholar]
- [27].Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S, Dectin-1 is a major beta-glucan receptor on macrophages, J Exp Med 196(3) (2002) 407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, Hsu YM, Yang L, Guan JH, Xu GT, Lin X, CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity, J Exp Med 211(11) (2014) 2307–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB, Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk, Nat Immunol 10(2) (2009) 203–13. [DOI] [PubMed] [Google Scholar]
- [30].Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, Kuijpers TW, Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency, Blood 121(13) (2013) 2385–92. [DOI] [PubMed] [Google Scholar]
- [31].Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EM, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS, CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System, PLoS Pathog 11(12) (2015) e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Drummond RA, Swamydas M, Oikonomou V, Zhai B, Dambuza IM, Schaefer BC, Bohrer AC, Mayer-Barber KD, Lira SA, Iwakura Y, Filler SG, Brown GD, Hube B, Naglik JR, Hohl TM, Lionakis MS, CARD9(+) microglia promote antifungal immunity via IL1-beta- and CXCL1-mediated neutrophil recruitment, Nat Immunol 20(5) (2019) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corvilain E, Casanova JL, Puel A, Inherited CARD9 Deficiency: Invasive Disease Caused by Ascomycete Fungi in Previously Healthy Children and Adults, J Clin Immunol 38(6) (2018) 656–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lanternier F, Mahdaviani SA, Barbati E, Chaussade H, Koumar Y, Levy R, Denis B, Brunel AS, Martin S, Loop M, Peeters J, de Selys A, Vanclaire J, Vermylen C, Nassogne MC, Chatzis O, Liu L, Migaud M, Pedergnana V, Desoubeaux G, Jouvion G, Chretien F, Darazam IA, Schaffer AA, Netea MG, De Bruycker JJ, Bernard L, Reynes J, Amazrine N, Abel L, Van der Linden D, Harrison T, Picard C, Lortholary O, Mansouri D, Casanova JL, Puel A, Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species-induced meningoencephalitis, colitis, or both, J Allergy Clin Immunol 135(6) (2015) 1558–68 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Boudghene Stambouli O, Guellil B, Jacobs F, Goffard JC, Schepers K, del Marmol V, Boussofara L, Denguezli M, Larif M, Bachelez H, Michel L, Lefranc G, Hay R, Jouvion G, Chretien F, Fraitag S, Bougnoux ME, Boudia M, Abel L, Lortholary O, Casanova JL, Picard C, Grimbacher B, Puel A, Deep dermatophytosis and inherited CARD9 deficiency, N Engl J Med 369(18) (2013) 1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rieber N, Gazendam RP, Freeman AF, Hsu AP, Collar AL, Sugui JA, Drummond RA, Rongkavilit C, Hoffman K, Henderson C, Clark L, Mezger M, Swamydas M, Engeholm M, Schule R, Neumayer B, Ebel F, Mikelis CM, Pittaluga S, Prasad VK, Singh A, Milner JD, Williams KW, Lim JK, Kwon-Chung KJ, Holland SM, Hartl D, Kuijpers TW, Lionakis MS, Extrapulmonary Aspergillus infection in patients with CARD9 deficiency, JCI Insight 1(17) (2016) e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Drummond RA, Franco LM, Lionakis MS, Human CARD9: A Critical Molecule of Fungal Immune Surveillance, Front Immunol 9 (2018) 1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rosentul DC, Plantinga TS, Oosting M, Scott WK, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Laird GM, Joosten LA, van der Meer JW, Perfect JR, Kullberg BJ, Netea MG, Johnson MD, Genetic variation in the dectin-1/CARD9 recognition pathway and susceptibility to candidemia, J Infect Dis 204(7) (2011) 1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu X, Xu JF, Zheng G, Lu HW, Duan JL, Rui W, Guan JH, Cheng LQ, Yang DD, Wang MC, Lv QZ, Li JX, Zhao X, Chen CX, Shi P, Jia XM, Lin X, CARD9(S12N) facilitates the production of IL-5 by alveolar macrophages for the induction of type 2 immune responses, Nat Immunol 19(6) (2018) 547–560. [DOI] [PubMed] [Google Scholar]
- [40].Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG, Human dectin-1 deficiency and mucocutaneous fungal infections, N Engl J Med 361(18) (2009) 1760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chai LY, de Boer MG, van der Velden WJ, Plantinga TS, van Spriel AB, Jacobs C, Halkes CJ, Vonk AG, Blijlevens NM, van Dissel JT, Donnelly PJ, Kullberg BJ, Maertens J, Netea MG, The Y238X stop codon polymorphism in the human beta-glucan receptor dectin-1 and susceptibility to invasive aspergillosis, J Infect Dis 203(5) (2011) 736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D’Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A, Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity, Blood 116(24) (2010) 5394–402. [DOI] [PubMed] [Google Scholar]
- [43].Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG, Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients, Clin Infect Dis 49(5) (2009) 724–32. [DOI] [PubMed] [Google Scholar]
- [44].Drummond RA, Desai JV, Hsu AP, Oikonomou V, Vinh DC, Acklin JA, Abers MS, Walkiewicz MA, Anzick SL, Swamydas M, Vautier S, Natarajan M, Oler AJ, Yamanaka D, Mayer-Barber KD, Iwakura Y, Bianchi D, Driscoll B, Hauck K, Kline A, Viall NS, Zerbe CS, Ferre EM, Schmitt MM, DiMaggio T, Pittaluga S, Butman JA, Zelazny AM, Shea YR, Arias CA, Ashbaugh C, Mahmood M, Temesgen Z, Theofiles AG, Nigo M, Moudgal V, Bloch KC, Kelly SG, Whitworth MS, Rao G, Whitener CJ, Mafi N, Gea-Banacloche J, Kenyon LC, Miller WR, Boggian K, Gilbert A, Sincock M, Freeman AF, Bennett JE, Hasbun R, Mikelis CM, Kwon-Chung KJ, Belkaid Y, Brown GD, Lim JK, Kuhns DB, Holland SM, Lionakis MS, Human Dectin-1 deficiency impairs macrophage-mediated defense against phaeohyphomycosis, J Clin Invest 132(22) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hsu AP, Korzeniowska A, Aguilar CC, Gu J, Karlins E, Oler AJ, Chen G, Reynoso GV, Davis J, Chaput A, Peng T, Sun L, Lack JB, Bays DJ, Stewart ER, Waldman SE, Powell DA, Donovan FM, Desai JV, Pouladi N, Long Priel DA, Yamanaka D, Rosenzweig SD, Niemela JE, Stoddard J, Freeman AF, Zerbe CS, Kuhns DB, Lussier YA, Olivier KN, Boucher RC, Hickman HD, Frelinger J, Fierer J, Shubitz LF, Leto TL, Thompson Iii GR, Galgiani JN, Lionakis MS, Holland SM, Immunogenetics associated with severe coccidioidomycosis, JCI Insight (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fisher CE, Hohl TM, Fan W, Storer BE, Levine DM, Zhao LP, Martin PJ, Warren EH, Boeckh M, Hansen JA, Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation, Blood 129(19) (2017) 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rosentul DC, Delsing CE, Jaeger M, Plantinga TS, Oosting M, Costantini I, Venselaar H, Joosten LA, van der Meer JW, Dupont B, Kullberg BJ, Sobel JD, Netea MG, Gene polymorphisms in pattern recognition receptors and susceptibility to idiopathic recurrent vulvovaginal candidiasis, Front Microbiol 5 (2014) 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Usluogullari B, Gumus I, Gunduz E, Kaygusuz I, Simavli S, Acar M, Oznur M, Gunduz M, Kafali H, The role of Human Dectin-1 Y238X Gene Polymorphism in recurrent vulvovaginal candidiasis infections, Mol Biol Rep 41(10) (2014) 6763–8. [DOI] [PubMed] [Google Scholar]
- [49].Sainz J, Lupianez CB, Segura-Catena J, Vazquez L, Rios R, Oyonarte S, Hemminki K, Forsti A, Jurado M, Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection, PLoS One 7(2) (2012) e32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, Hardison SE, Dambuza IM, Valsecchi I, Kerscher B, Plato A, Wallace CA, Yuecel R, Hebecker B, da Gloria Teixeira Sousa M, Cunha C, Liu Y, Feizi T, Brakhage AA, Kwon-Chung KJ, Gow NAR, Zanda M, Piras M, Zanato C, Jaeger M, Netea MG, van de Veerdonk FL, Lacerda JF, Campos A, Carvalho A, Willment JA, Latge JP, Brown GD, Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus, Nature 555(7696) (2018) 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Roth S, Bergmann H, Jaeger M, Yeroslaviz A, Neumann K, Koenig PA, Prazeres da Costa C, Vanes L, Kumar V, Johnson M, Menacho-Marquez M, Habermann B, Tybulewicz VL, Netea M, Bustelo XR, Ruland J , Vav Proteins Are Key Regulators of Card9 Signaling for Innate Antifungal Immunity, Cell Rep 17(10) (2016) 2572–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Picard C, Casanova JL, Puel A, Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency, Clin Microbiol Rev 24(3) (2011) 490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, Rodrigues SD, Li S, Hansen JA, Zhao LP, Aderem A, Boeckh M, Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation, N Engl J Med 359(17) (2008) 1766–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jiang YK, Wu JQ, Zhao HZ, Wang X, Wang RY, Zhou LH, Yip CW, Huang LP, Cheng JH, Chen YH, Li H, Zhu LP, Weng XH, Genetic influence of Toll-like receptors on non-HIV cryptococcal meningitis: An observational cohort study, EBioMedicine 37 (2018) 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Netea MG, Toll-like receptor 1 polymorphisms increase susceptibility to candidemia, J Infect Dis 205(6) (2012) 934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Van der Graaf CA, Netea MG, Morre SA, Den Heijer M, Verweij PE, Van der Meer JW, Kullberg BJ, Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection, Eur Cytokine Netw 17(1) (2006) 29–34. [PubMed] [Google Scholar]
- [57].Carvalho A, De Luca A, Bozza S, Cunha C, D’Angelo C, Moretti S, Perruccio K, Iannitti RG, Fallarino F, Pierini A, Latge JP, Velardi A, Aversa F, Romani L, TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients, Blood 119(4) (2012) 967–77. [DOI] [PubMed] [Google Scholar]
- [58].Carvalho A, Pasqualotto AC, Pitzurra L, Romani L, Denning DW, Rodrigues F, Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis, J Infect Dis 197(4) (2008) 618–21. [DOI] [PubMed] [Google Scholar]
- [59].de Boer MG, Jolink H, Halkes CJ, van der Heiden PL, Kremer D, Falkenburg JH, van de Vosse E, van Dissel JT, Influence of polymorphisms in innate immunity genes on susceptibility to invasive aspergillosis after stem cell transplantation, PLoS One 6(4) (2011) e18403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grube M, Loeffler J, Mezger M, Kruger B, Echtenacher B, Hoffmann P, Edinger M, Einsele H, Andreesen R, Holler E, TLR5 stop codon polymorphism is associated with invasive aspergillosis after allogeneic stem cell transplantation, Med Mycol 51(8) (2013) 818–25. [DOI] [PubMed] [Google Scholar]
- [61].Kesh S, Mensah NY, Peterlongo P, Jaffe D, Hsu K, M VDB, O’Reilly R, Pamer E, Satagopan J, Papanicolaou GA, TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation, Ann N Y Acad Sci 1062 (2005) 95–103. [DOI] [PubMed] [Google Scholar]
- [62].Koldehoff M, Beelen DW, Elmaagacli AH, Increased susceptibility for aspergillosis and post-transplant immune deficiency in patients with gene variants of TLR4 after stem cell transplantation, Transpl Infect Dis 15(5) (2013) 533–9. [DOI] [PubMed] [Google Scholar]
- [63].Schroder NW, Schumann RR, Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease, Lancet Infect Dis 5(3) (2005) 156–64. [DOI] [PubMed] [Google Scholar]
- [64].Figueiredo RT, Fernandez PL, Dutra FF, Gonzalez Y, Lopes LC, Bittencourt VC, Sassaki GL, Barreto-Bergter E, Bozza MT, TLR4 recognizes Pseudallescheria boydii conidia and purified rhamnomannans, J Biol Chem 285(52) (2010) 40714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ, Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors, J Clin Invest 116(6) (2006) 1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dang EV, Lei S, Radkov A, Volk RF, Zaro BW, Madhani HD, Secreted fungal virulence effector triggers allergic inflammation via TLR4, Nature (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Joly S, Eisenbarth SC, Olivier AK, Williams A, Kaplan DH, Cassel SL, Flavell RA, Sutterwala FS, Cutting edge: Nlrp10 is essential for protective antifungal adaptive immunity against Candida albicans, J Immunol 189(10) (2012) 4713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA, An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans, Cell Host Microbe 5(5) (2009) 487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD, Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection, Cell Host Microbe 17(3) (2015) 357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG, A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans, PLoS Pathog 7(12) (2011) e1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jaeger M, Carvalho A, Cunha C, Plantinga TS, van de Veerdonk F, Puccetti M, Galosi C, Joosten LA, Dupont B, Kullberg BJ, Sobel JD, Romani L, Netea MG, Association of a variable number tandem repeat in the NLRP3 gene in women with susceptibility to RVVC, Eur J Clin Microbiol Infect Dis 35(5) (2016) 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yano J, Sobel JD, Nyirjesy P, Sobel R, Williams VL, Yu Q, Noverr MC, Fidel PL Jr., Current patient perspectives of vulvovaginal candidiasis: incidence, symptoms, management and post-treatment outcomes, BMC Womens Health 19(1) (2019) 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Jaeger M, Pinelli M, Borghi M, Constantini C, Dindo M, van Emst L, Puccetti M, Pariano M, Ricano-Ponce I, Bull C, Gresnigt MS, Wang X, Gutierrez Achury J, Jacobs CWM, Xu N, Oosting M, Arts P, Joosten LAB, van de Veerdonk FL, Veltman JA, Ten Oever J, Kullberg BJ, Feng M, Adema GJ, Wijmenga C, Kumar V, Sobel J, Gilissen C, Romani L, Netea MG, A systems genomics approach identifies SIGLEC15 as a susceptibility factor in recurrent vulvovaginal candidiasis, Sci Transl Med 11(496) (2019). [DOI] [PubMed] [Google Scholar]
- [74].Gresnigt MS, Cunha C, Jaeger M, Goncalves SM, Malireddi RKS, Ammerdorffer A, Lubbers R, Oosting M, Rasid O, Jouvion G, Fitting C, Jong DJ, Lacerda JF, Campos A Jr., Melchers WJG, Lagrou K, Maertens J, Kanneganti TD, Carvalho A, Ibrahim-Granet O, van de Veerdonk FL, Genetic deficiency of NOD2 confers resistance to invasive aspergillosis, Nat Commun 9(1) (2018) 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A, Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response, Nature 420(6912) (2002) 182–6. [DOI] [PubMed] [Google Scholar]
- [76].Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loffler J, Maertens JA, Bell AS, Inforzato A, Barbati E, Almeida B, Santos e Sousa P, Barbui A, Potenza L, Caira M, Rodrigues F, Salvatori G, Pagano L, Luppi M, Mantovani A, Velardi A, Romani L, Carvalho A, Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation, N Engl J Med 370(5) (2014) 421–32. [DOI] [PubMed] [Google Scholar]
- [77].Cunha C, Monteiro AA, Oliveira-Coelho A, Kuhne J, Rodrigues F, Sasaki SD, Schio SM, Camargo JJ, Mantovani A, Carvalho A, Pasqualotto AC, PTX3-Based Genetic Testing for Risk of Aspergillosis After Lung Transplant, Clin Infect Dis 61(12) (2015) 1893–4. [DOI] [PubMed] [Google Scholar]
- [78].He Q, Li H, Rui Y, Liu L, He B, Shi Y, Su X, Pentraxin 3 Gene Polymorphisms and Pulmonary Aspergillosis in Chronic Obstructive Pulmonary Disease Patients, Clin Infect Dis 66(2) (2018) 261–267. [DOI] [PubMed] [Google Scholar]
- [79].Wojtowicz A, Lecompte TD, Bibert S, Manuel O, Rueger S, Berger C, Boggian K, Cusini A, Garzoni C, Hirsch H, Khanna N, Mueller NJ, Meylan PR, Pascual M, van Delden C, Bochud PY, Swiss Transplant Cohort S., PTX3 Polymorphisms and Invasive Mold Infections After Solid Organ Transplant, Clin Infect Dis 61(4) (2015) 619–22. [DOI] [PubMed] [Google Scholar]
- [80].Zhang W, Liao Q, Liu Y, Wu S, Deng J, Xiao Y, Ma Y, Xie Y, Kang M, PTX3 gene polymorphism associated with cryptococcosis in HIV-uninfected Chinese patients, Mycoses 64(4) (2021) 405–411. [DOI] [PubMed] [Google Scholar]
- [81].Doni A, Parente R, Laface I, Magrini E, Cunha C, Colombo FS, Lacerda JF, Campos A Jr., Mapelli SN, Petroni F, Porte R, Schorn T, Inforzato A, Mercier T, Lagrou K, Maertens J, Lambris JD, Bottazzi B, Garlanda C, Botto M, Carvalho A, Mantovani A, Serum amyloid P component is an essential element of resistance against Aspergillus fumigatus, Nat Commun 12(1) (2021) 3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mezger M, Steffens M, Beyer M, Manger C, Eberle J, Toliat MR, Wienker TF, Ljungman P, Hebart H, Dornbusch HJ, Einsele H, Loeffler J, Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells, Blood 111(2) (2008) 534–6. [DOI] [PubMed] [Google Scholar]
- [83].Wojtowicz A, Tissot F, Lamoth F, Orasch C, Eggimann P, Siegemund M, Zimmerli S, Flueckiger UM, Bille J, Calandra T, Marchetti O, Bochud PY, Fungal S Infection Network of, Polymorphisms in tumor necrosis factor-alpha increase susceptibility to intra-abdominal Candida infection in high-risk surgical ICU patients*, Crit Care Med 42(4) (2014) e304–8. [DOI] [PubMed] [Google Scholar]
- [84].Sainz J, Perez E, Hassan L, Moratalla A, Romero A, Collado MD, Jurado M, Variable number of tandem repeats of TNF receptor type 2 promoter as genetic biomarker of susceptibility to develop invasive pulmonary aspergillosis, Hum Immunol 68(1) (2007) 41–50. [DOI] [PubMed] [Google Scholar]
- [85].Sainz J, Salas-Alvarado I, Lopez-Fernandez E, Olmedo C, Comino A, Garcia F, Blanco A, Gomez-Lopera S, Oyonarte S, Bueno P, Jurado M, TNFR1 mRNA expression level and TNFR1 gene polymorphisms are predictive markers for susceptibility to develop invasive pulmonary aspergillosis, Int J Immunopathol Pharmacol 23(2) (2010) 423–36. [DOI] [PubMed] [Google Scholar]
- [86].Marty FM, Lee SJ, Fahey MM, Alyea EP, Soiffer RJ, Antin JH, Baden LR, Infliximab use in patients with severe graft-versus-host disease and other emerging risk factors of non-Candida invasive fungal infections in allogeneic hematopoietic stem cell transplant recipients: a cohort study, Blood 102(8) (2003) 2768–76. [DOI] [PubMed] [Google Scholar]
- [87].Sainz J, Perez E, Gomez-Lopera S, Jurado M, IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level, J Clin Immunol 28(5) (2008) 473–85. [DOI] [PubMed] [Google Scholar]
- [88].Smith NL, Hankinson J, Simpson A, Bowyer P, Denning DW, A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis, Clin Microbiol Infect 20(8) (2014) O480–8. [DOI] [PubMed] [Google Scholar]
- [89].Wojtowicz A, Gresnigt MS, Lecompte T, Bibert S, Manuel O, Joosten LA, Rueger S, Berger C, Boggian K, Cusini A, Garzoni C, Hirsch HH, Weisser M, Mueller NJ, Meylan PR, Steiger J, Kutalik Z, Pascual M, van Delden C, van de Veerdonk FL, Bochud PY, Swiss Transplant Cohort S., Swiss S Transplant Cohort Study, IL1B and DEFB1 Polymorphisms Increase Susceptibility to Invasive Mold Infection After Solid-Organ Transplantation, J Infect Dis 211(10) (2015) 1646–57. [DOI] [PubMed] [Google Scholar]
- [90].Merkhofer RM Jr., O’Neill MB, Xiong D, Hernandez-Santos N, Dobson H, Fites JS, Shockey AC, Wuethrich M, Pepperell CS, Klein BS, Investigation of Genetic Susceptibility to Blastomycosis Reveals Interleukin-6 as a Potential Susceptibility Locus, MBio 10(3) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wojtowicz A, Bibert S, Taffe P, Bernasconi E, Furrer H, Gunthard HF, Hoffmann M, Osthoff M, Cavassini M, Bochud PY, Swiss HIVCS, IL-4 polymorphism influences susceptibility to Pneumocystis jirovecii pneumonia in HIV-positive patients, AIDS 33(11) (2019) 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].van de Sande WW, Fahal A, Verbrugh H, van Belkum A, Polymorphisms in genes involved in innate immunity predispose toward mycetoma susceptibility, J Immunol 179(5) (2007) 3065–74. [DOI] [PubMed] [Google Scholar]
- [93].Sainz J, Hassan L, Perez E, Romero A, Moratalla A, Lopez-Fernandez E, Oyonarte S, Jurado M, Interleukin-10 promoter polymorphism as risk factor to develop invasive pulmonary aspergillosis, Immunol Lett 109(1) (2007) 76–82. [DOI] [PubMed] [Google Scholar]
- [94].Seo KW, Kim DH, Sohn SK, Lee NY, Chang HH, Kim SW, Jeon SB, Baek JH, Kim JG, Suh JS, Lee KB, Protective role of interleukin-10 promoter gene polymorphism in the pathogenesis of invasive pulmonary aspergillosis after allogeneic stem cell transplantation, Bone Marrow Transplant 36(12) (2005) 1089–95. [DOI] [PubMed] [Google Scholar]
- [95].Johnson MD, Plantinga TS, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Scott WK, Netea MG, Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study, Clin Infect Dis 54(4) (2012) 502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Swamydas M, Gao JL, Break TJ, Johnson MD, Jaeger M, Rodriguez CA, Lim JK, Green NM, Collar AL, Fischer BG, Lee CC, Perfect JR, Alexander BD, Kullberg BJ, Netea MG, Murphy PM, Lionakis MS, CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival, Sci Transl Med 8(322) (2016) 322ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM, CX3CR1-dependent renal macrophage survival promotes Candida control and host survival, J Clin Invest 123(12) (2013) 5035–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Collar AL, Swamydas M, O’Hayre M, Sajib MS, Hoffman KW, Singh SP, Mourad A, Johnson MD, Ferre EM, Farber JM, Lim JK, Mikelis CM, Gutkind JS, Lionakis MS, The homozygous CX3CR1-M280 mutation impairs human monocyte survival, JCI Insight 3(3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Break TJ, Jaeger M, Solis NV, Filler SG, Rodriguez CA, Lim JK, Lee CC, Sobel JD, Netea MG, Lionakis MS, CX3CR1 is dispensable for control of mucosal Candida albicans infections in mice and humans, Infect Immun 83(3) (2015) 958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lupianez CB, Martinez-Bueno M, Sanchez-Maldonado JM, Badiola J, Cunha C, Springer J, Lackner M, Segura-Catena J, Canet LM, Alcazar-Fuoli L, Lopez-Nevot MA, Fianchi L, Aguado JM, Pagano L, Lopez-Fernandez E, Alarcon-Riquelme M, Potenza L, Goncalves SM, Luppi M, Moratalla L, Solano C, Sampedro A, Gonzalez-Sierra P, Cuenca-Estrella M, Lagrou K, Maertens JA, Lass-Florl C, Einsele H, Vazquez L, Group P.S., Loeffler J, Rios-Tamayo R, Carvalho A, Jurado M, Sainz J, Polymorphisms within the ARNT2 and CX3CR1 Genes Are Associated with the Risk of Developing Invasive Aspergillosis, Infect Immun 88(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Borghi M, De Luca A, Puccetti M, Jaeger M, Mencacci A, Oikonomou V, Pariano M, Garlanda C, Moretti S, Bartoli A, Sobel J, van de Veerdonk FL, Dinarello CA, Netea MG, Romani L, Pathogenic NLRP3 Inflammasome Activity during Candida Infection Is Negatively Regulated by IL-22 via Activation of NLRC4 and IL-1Ra, Cell Host Microbe 18(2) (2015) 198–209. [DOI] [PubMed] [Google Scholar]
- [102].De Luca A, Carvalho A, Cunha C, Iannitti RG, Pitzurra L, Giovannini G, Mencacci A, Bartolommei L, Moretti S, Massi-Benedetti C, Fuchs D, De Bernardis F, Puccetti P, Romani L, IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis, PLoS Pathog 9(7) (2013) e1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kannambath S, Jarvis JN, Wake RM, Longley N, Loyse A, Matzaraki V, Aguirre-Gamboa R, Wijmenga C, Doyle R, Paximadis M, Tiemessen CT, Kumar V, Pittman A, Meintjes G, Harrison TS, Netea MG, Bicanic T, Genome-Wide Association Study Identifies Novel Colony Stimulating Factor 1 Locus Conferring Susceptibility to Cryptococcosis in Human Immunodeficiency Virus-Infected South Africans, Open Forum Infect Dis 7(11) (2020) ofaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN, Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain, Neuron 82(2) (2014) 380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hu XP, Wu JQ, Zhu LP, Wang X, Xu B, Wang RY, Ou XT, Weng XH, Association of Fcgamma receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients, PLoS One 7(8) (2012) e42439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Meletiadis J, Walsh TJ, Choi EH, Pappas PG, Ennis D, Douglas J, Pankey GA, Larsen RA, Hamill RJ, Chanock S, Study of common functional genetic polymorphisms of FCGR2A, 3A and 3B genes and the risk for cryptococcosis in HIV-uninfected patients, Med Mycol 45(6) (2007) 513–8. [DOI] [PubMed] [Google Scholar]
- [107].Rohatgi S, Gohil S, Kuniholm MH, Schultz H, Dufaud C, Armour KL, Badri S, Mailliard RB, Pirofski LA, Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease, mBio 4(5) (2013) e00573–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kumar V, Cheng SC, Johnson MD, Smeekens SP, Wojtowicz A, Giamarellos-Bourboulis E, Karjalainen J, Franke L, Withoff S, Plantinga TS, van de Veerdonk FL, van der Meer JW, Joosten LA, Sokol H, Bauer H, Herrmann BG, Bochud PY, Marchetti O, Perfect JR, Xavier RJ, Kullberg BJ, Wijmenga C, Netea MG, Immunochip SNP array identifies novel genetic variants conferring susceptibility to candidaemia, Nat Commun 5 (2014) 4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Smeekens SP, Ng A, Kumar V, Johnson MD, Plantinga TS, van Diemen C, Arts P, Verwiel ET, Gresnigt MS, Fransen K, van Sommeren S, Oosting M, Cheng SC, Joosten LA, Hoischen A, Kullberg BJ, Scott WK, Perfect JR, van der Meer JW, Wijmenga C, Netea MG, Xavier RJ, Functional genomics identifies type I interferon pathway as central for host defense against Candida albicans, Nat Commun 4 (2013) 1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tian C, Hromatka BS, Kiefer AK, Eriksson N, Noble SM, Tung JY, Hinds DA, Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections, Nat Commun 8(1) (2017) 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, Spiegel R, Eytan O, Geller S, Peleg S, Shomron N, Goh CSM, Wilson NJ, Smith FJD, Pohler E, Simpson MA, McLean WHI, Irvine AD, Horowitz M, McGrath JA, Green KJ, Sprecher E, Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting, Nat Genet 45(10) (2013) 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kashiwagi M, Ohba M, Chida K, Kuroki T, Protein kinase C eta (PKC eta): its involvement in keratinocyte differentiation, J Biochem 132(6) (2002) 853–7. [DOI] [PubMed] [Google Scholar]
- [113].Sorci G, Giovannini G, Riuzzi F, Bonifazi P, Zelante T, Zagarella S, Bistoni F, Donato R, Romani L, The danger signal S100B integrates pathogen- and danger-sensing pathways to restrain inflammation, PLoS Pathog 7(3) (2011) e1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Cunha C, Giovannini G, Pierini A, Bell AS, Sorci G, Riuzzi F, Donato R, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A, Genetically-determined hyperfunction of the S100B/RAGE axis is a risk factor for aspergillosis in stem cell transplant recipients, PLoS One 6(11) (2011) e27962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, Chavakis T, Netea MG, van de Veerdonk FL, Brakhage AA, El-Benna J, Beauvais A, Latge JP, Chamilos G, Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity, Cell Host Microbe 19(1) (2016) 79–90. [DOI] [PubMed] [Google Scholar]
- [116].Kyrmizi I, Ferreira H, Carvalho A, Figueroa JAL, Zarmpas P, Cunha C, Akoumianaki T, Stylianou K, Deepe GS Jr., Samonis G, Lacerda JF, Campos A Jr., Kontoyiannis DP, Mihalopoulos N, Kwon-Chung KJ, El-Benna J, Valsecchi I, Beauvais A, Brakhage AA, Neves NM, Latge JP, Chamilos G, Calcium sequestration by fungal melanin inhibits calcium-calmodulin signalling to prevent LC3-associated phagocytosis, Nat Microbiol 3(7) (2018) 791–803. [DOI] [PubMed] [Google Scholar]
- [117].Zaas AK, Liao G, Chien JW, Weinberg C, Shore D, Giles SS, Marr KA, Usuka J, Burch LH, Perera L, Perfect JR, Peltz G, Schwartz DA, Plasminogen alleles influence susceptibility to invasive aspergillosis, PLoS Genet 4(6) (2008) e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Verwer PE, Notenboom CC, Eadie K, Fahal AH, Verbrugh HA, van de Sande WW, A Polymorphism in the Chitotriosidase Gene Associated with Risk of Mycetoma Due to Madurella mycetomatis Mycetoma--A Retrospective Study, PLoS Negl Trop Dis 9(9) (2015) e0004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, Shadduck RK, Shea TC, Stiff P, Friedman DJ, et al. , A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation, N Engl J Med 326(13) (1992) 845–51. [DOI] [PubMed] [Google Scholar]
- [120].Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, Meyers JD, Bowden RA, Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study, J Infect Dis 171(6) (1995) 1545–52. [DOI] [PubMed] [Google Scholar]
- [121].Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, AnguloGonzalez D, Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia, N Engl J Med 356(4) (2007) 348–59. [DOI] [PubMed] [Google Scholar]
- [122].Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S, Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease, N Engl J Med 356(4) (2007) 335–47. [DOI] [PubMed] [Google Scholar]
- [123].Benjamin DK Jr., Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, Natarajan G, Burchfield DJ, White RD, Shattuck KE, Neu N, Bendel CM, Kim MR, Finer NN, Stewart DL, Arrieta AC, Wade KC, Kaufman DA, Manzoni P, Prather KO, Testoni D, Berezny KY, Smith PB, Fluconazole Prophylaxis Study T., Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial, JAMA 311(17) (2014) 1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Safran DB, Dawson E, The effect of empiric and prophylactic treatment with fluconazole on yeast isolates in a surgical trauma intensive care unit, Arch Surg 132(11) (1997) 1184–8; discussion 1188–9. [DOI] [PubMed] [Google Scholar]
- [125].Schuster MG, Edwards JE Jr., Sobel JD, Darouiche RO, Karchmer AW, Hadley S, Slotman G, Panzer H, Biswas P, Rex JH, Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial, Ann Intern Med 149(2) (2008) 83–90. [DOI] [PubMed] [Google Scholar]
- [126].Timsit JF, Azoulay E, Schwebel C, Charles PE, Cornet M, Souweine B, Klouche K, Jaber S, Trouillet JL, Bruneel F, Argaud L, Cousson J, Meziani F, Gruson D, Paris A, Darmon M, Garrouste-Orgeas M, Navellou JC, Foucrier A, Allaouchiche B, Das V, Gangneux JP, Ruckly S, Maubon D, Jullien V, Wolff M, Group E.T., Empirical Micafungin Treatment and Survival Without Invasive Fungal Infection in Adults With ICU-Acquired Sepsis, Candida Colonization, and Multiple Organ Failure: The EMPIRICUS Randomized Clinical Trial, JAMA 316(15) (2016) 1555–1564. [DOI] [PubMed] [Google Scholar]
- [127].Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, Yockey L, Darnell DN, Barnhart L, Daub J, Boris L, Rump AP, Anderson VL, Haney C, Kuhns DB, Rosenzweig SD, Kelly C, Zelazny A, Mason T, DeRavin SS, Kang E, Gallin JI, Malech HL, Olivier KN, Uzel G, Freeman AF, Heller T, Zerbe CS, Holland SM, Common severe infections in chronic granulomatous disease, Clin Infect Dis 60(8) (2015) 1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, Marciano B, Eisenstein EM, Turner ML, DeCarlo ES, Starling JM, Holland SM, Itraconazole to prevent fungal infections in chronic granulomatous disease, N Engl J Med 348(24) (2003) 2416–22. [DOI] [PubMed] [Google Scholar]
- [129].Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, Armstrong-James D, Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus, EMBO molecular medicine 7(3) (2015) 240–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D, Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis, Blood 132(18) (2018) 1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Chamilos G, Lionakis MS, Kontoyiannis DP, Call for Action: Invasive Fungal Infections Associated With Ibrutinib and Other Small Molecule Kinase Inhibitors - Targeting Immune Signaling Pathways, Clin Infect Dis 66(1) (2018) 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, Dupont M, Dreyfus B, Ferrant E, Herbaux C, Laribi K, Le Calloch R, Malphettes M, Paul F, Souchet L, Truchan-Graczyk M, Delavigne K, Dartigeas C, Ysebaert L, C.L.L.g. French Innovative Leukemia Organization, Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib, Blood 131(17) (2018) 1955–1959. [DOI] [PubMed] [Google Scholar]
- [133].Gold JAW, Tolu SS, Chiller T, Benedict K, Jackson BR, Incidence of Invasive Fungal Infections in Patients Initiating Ibrutinib and other Small Molecule Kinase Inhibitors - United States, July 2016-June 2019, Clin Infect Dis (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ruchlemer R, Ben-Ami R, Bar-Meir M, Brown JR, Malphettes M, Mous R, Tonino SH, Soussain C, Barzic N, Messina JA, Jain P, Cohen R, Hill B, Mulligan SP, Nijland M, Herishanu Y, Benjamini O, Tadmor T, Okamoto K, Arthurs B, Gottesman B, Kater AP, Talha M, Eichhorst B, Korem M, Bogot N, De Boer F, Rowe JM, Lachish T, Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: An observational study, Mycoses 62(12) (2019) 1140–1147. [DOI] [PubMed] [Google Scholar]
- [135].Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, Redelman-Sidi G, Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer, Clin Infect Dis 67(5) (2018) 687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zarakas MA, Desai JV, Chamilos G, Lionakis MS, Fungal Infections with Ibrutinib and Other Small-Molecule Kinase Inhibitors, Curr Fungal Infect Rep 13(3) (2019) 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, Cole DE, Melani C, Higham CS, Desai JV, Ceribelli M, Chen L, Thomas CJ, Little RF, Gea-Banacloche J, Bhaumik S, Stetler-Stevenson M, Pittaluga S, Jaffe ES, Heiss J, Lucas N, Steinberg SM, Staudt LM, Wilson WH, Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma, Cancer Cell (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Simard JM, C.; Lakhotia R; Lionakis MS; Pittaluga S; Phelan JD; Muppidi JR; Chou LL; Holdhoff M; Giantz M; Butman JA; Lucas AN; Steinberg SM; Jaffe ES; Staudt LM; Wilson WH; Roschewski M, Preliminary Results of a Response-Adapted Study of Ibrutinib and Isavuconazole with Temozolomide, Etoposide, Liposomal Doxorubicin, Dexamethasone, Rituximab (TEDDI-R) for Secondary CNS Lymphoma, Blood 136 (2020) 24–25.32430494 [Google Scholar]
- [139].Levitz SM, Huang H, Ostroff GR, Specht CA, Exploiting fungal cell wall components in vaccines, Semin Immunopathol 37(2) (2015) 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Nanjappa SG, Klein BS, Vaccine immunity against fungal infections, Curr Opin Immunol 28 (2014) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Cassone A, Casadevall A, Recent progress in vaccines against fungal diseases, Curr Opin Microbiol 15(4) (2012) 427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Edwards JE Jr., Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, Schodel F, Marchus E, Lizakowski M, DeMontigny EA, Hoeg J, Holmberg T, Cooke MT, Hoover K, Edwards L, Jacobs M, Sussman S, Augenbraun M, Drusano M, Yeaman MR, Ibrahim AS, Filler SG, Hennessey JP Jr., A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial, Clin Infect Dis 66(12) (2018) 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, Edwards JE Jr., Hennessey JP Jr., NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults, Vaccine 30(52) (2012) 7594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE Jr., Filler SG, Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells, PLoS Biol 5(3) (2007) e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, Fu Y, French SW, Edwards JE Jr., Spellberg B, Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice, PLoS Pathog 5(12) (2009) e1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Ibrahim AS, Luo G, Gebremariam T, Lee H, Schmidt CS, Hennessey JP Jr., French SW, Yeaman MR, Filler SG, Edwards JE Jr., NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response, Vaccine 31(47) (2013) 5549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wheelock EF, Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin, Science 149(3681) (1965) 310–1. [DOI] [PubMed] [Google Scholar]
- [148].Ivashkiv LB, IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy, Nat Rev Immunol 18(9) (2018) 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lionakis MS, Netea MG, Holland SM, Mendelian genetics of human susceptibility to fungal infection, Cold Spring Harb Perspect Med 4(6) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL, Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity, Semin Immunol 26(6) (2014) 454–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group, N Engl J Med 324(8) (1991) 509–16. [DOI] [PubMed] [Google Scholar]
- [152].Buddingh EP, Leentjens J, van der Lugt J, Dik WA, Gresnigt MS, Netea MG, Pickkers P, Driessen GJ, Interferon-gamma Immunotherapy in a Patient With Refractory Disseminated Candidiasis, Pediatr Infect Dis J 34(12) (2015) 1391–4. [DOI] [PubMed] [Google Scholar]
- [153].Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, Monneret G, Venet F, Bleeker-Rovers CP, van de Veerdonk FL, Pickkers P, Pachot A, Kullberg BJ, Netea MG, Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series, BMC Infect Dis 14 (2014) 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Dignani MC, Rex JH, Chan KW, Dow G, deMagalhaes-Silverman M, Maddox A, Walsh T, Anaissie E, Immunomodulation with interferon-gamma and colony-stimulating factors for refractory fungal infections in patients with leukemia, Cancer 104(1) (2005) 199–204. [DOI] [PubMed] [Google Scholar]
- [155].Kelleher P, Goodsall A, Mulgirigama A, Kunst H, Henderson DC, Wilson R, Newman-Taylor A, Levin M, Interferon-gamma therapy in two patients with progressive chronic pulmonary aspergillosis, Eur Respir J 27(6) (2006) 1307–10. [DOI] [PubMed] [Google Scholar]
- [156].Grimaldi D, Pradier O, Hotchkiss RS, Vincent JL, Nivolumab plus interferon-gamma in the treatment of intractable mucormycosis, Lancet Infect Dis 17(1) (2017) 18. [DOI] [PubMed] [Google Scholar]
- [157].Serris A, Ouedrani A, Uhel F, Gazzano M, Bedarida V, Rouzaud C, Bougnoux ME, Raphalen JH, Poiree S, Lambotte O, Martin-Blondel G, Lanternier F, Case Report: Immune Checkpoint Blockade Plus Interferon-Gamma Add-On Antifungal Therapy in the Treatment of Refractory Covid-Associated Pulmonary Aspergillosis and Cerebral Mucormycosis, Front Immunol 13 (2022) 900522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Abers MS, Lionakis MS, Kontoyiannis DP, Checkpoint Inhibition and Infectious Diseases: A Good Thing?, Trends Mol Med 25(12) (2019) 1080–1093. [DOI] [PubMed] [Google Scholar]
- [159].Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker LG, Wood R, Harrison TS, Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial, AIDS 26(9) (2012) 1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, Reboli A, Aberg J, Hasbun R, Hsu HH, Recombinant interferon- gamma 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis, J Infect Dis 189(12) (2004) 2185–91. [DOI] [PubMed] [Google Scholar]
- [161].Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, Levitz SM, Harrison TS, Koup RA, The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis, J Infect Dis 207(12) (2013) 1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, Tomlinson G, Kropf P, Noursadeghi M, Harrison TS, Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis, PLoS Pathog 11(4) (2015) e1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, Sheldon J, Chierakul W, Peacock S, Day N, White NJ, Harrison TS, IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis, J Immunol 174(3) (2005) 1746–50. [DOI] [PubMed] [Google Scholar]
- [164].Netea MG, Brouwer AE, Hoogendoorn EH, Van der Meer JW, Koolen M, Verweij PE, Kullberg BJ, Two patients with cryptococcal meningitis and idiopathic CD4 lymphopenia: defective cytokine production and reversal by recombinant interferon- gamma therapy, Clin Infect Dis 39(9) (2004) e83–7. [DOI] [PubMed] [Google Scholar]
- [165].Duplessis CA, Tilley D, Bavaro M, Hale B, Holland SM, Two cases illustrating successful adjunctive interferon-gamma immunotherapy in refractory disseminated coccidioidomycosis, J Infect 63(3) (2011) 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Tsai M, Thauland TJ, Huang AY, Bun C, Fitzwater S, Krogstad P, Douine ED, Nelson SF, Lee H, Garcia-Lloret MI, Butte MJ, Disseminated Coccidioidomycosis Treated with Interferon-gamma and Dupilumab, N Engl J Med 382(24) (2020) 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sologuren I, Boisson-Dupuis S, Pestano J, Vincent QB, Fernandez-Perez L, Chapgier A, Cardenes M, Feinberg J, Garcia-Laorden MI, Picard C, Santiago E, Kong X, Janniere L, Colino E, Herrera-Ramos E, Frances A, Navarrete C, Blanche S, Faria E, Remiszewski P, Cordeiro A, Freeman A, Holland S, Abarca K, Valeron-Lemaur M, Goncalo-Marques J, Silveira L, Garcia-Castellano JM, Caminero J, Perez-Arellano JL, Bustamante J, Abel L, Casanova JL, Rodriguez-Gallego C, Partial recessive IFN-gammaR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds, Hum Mol Genet 20(8) (2011) 1509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, Okada S, Boisson-Dupuis S, Casanova JL, Bustamante J, Mendelian susceptibility to mycobacterial disease: 2014–2018 update, Immunol Cell Biol 97(4) (2019) 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM, Adult-onset immunodeficiency in Thailand and Taiwan, N Engl J Med 367(8) (2012) 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, Pancholi MJ, Yang LM, Priel DL, Uzel G, Freeman AF, Hayes CE, Baxter R, Cohen SH, Holland SM, Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection, Blood 119(17) (2012) 3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ochoa S, Ding L, Kreuzburg S, Treat J, Holland SM, Zerbe CS, Daratumumab (Anti-CD38) for Treatment of Disseminated Nontuberculous Mycobacteria in a Patient With Anti-Interferon-gamma Autoantibodies, Clin Infect Dis 72(12) (2021) 2206–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Nemunaitis J, Rabinowe SN, Singer JW, Bierman PJ, Vose JM, Freedman AS, Onetto N, Gillis S, Oette D, Gold M, et al. , Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer, N Engl J Med 324(25) (1991) 1773–8. [DOI] [PubMed] [Google Scholar]
- [173].Ohno R, Tomonaga M, Kobayashi T, Kanamaru A, Shirakawa S, Masaoka T, Omine M, Oh H, Nomura T, Sakai Y, et al. , Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia, N Engl J Med 323(13) (1990) 871–7. [DOI] [PubMed] [Google Scholar]
- [174].Kasahara S, Jhingran A, Dhingra S, Salem A, Cramer RA, Hohl TM, Role of Granulocyte-Macrophage Colony-Stimulating Factor Signaling in Regulating Neutrophil Antifungal Activity and the Oxidative Burst During Respiratory Fungal Challenge, J Infect Dis 213(8) (2016) 1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kullberg BJ, Netea MG, Curfs JH, Keuter M, Meis JF, van der Meer JW, Recombinant murine granulocyte colony-stimulating factor protects against acute disseminated Candida albicans infection in nonneutropenic mice, J Infect Dis 177(1) (1998) 175–81. [DOI] [PubMed] [Google Scholar]
- [176].Mayer P, Schutze E, Lam C, Kricek F, Liehl E, Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice, J Infect Dis 163(3) (1991) 584–90. [DOI] [PubMed] [Google Scholar]
- [177].Polak-Wyss A, Protective effect of human granulocyte colony-stimulating factor (hG-CSF) on Cryptococcus and Aspergillus infections in normal and immunosuppressed mice, Mycoses 34(5–6) (1991) 205–15. [DOI] [PubMed] [Google Scholar]
- [178].Kandalla PK, Sarrazin S, Molawi K, Berruyer C, Redelberger D, Favel A, Bordi C, de Bentzmann S, Sieweke MH, M-CSF improves protection against bacterial and fungal infections after hematopoietic stem/progenitor cell transplantation, J Exp Med 213(11) (2016) 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S, IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells, Immunity 40(1) (2014) 117–27. [DOI] [PubMed] [Google Scholar]
- [180].Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C, Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection, PLoS Pathog 10(7) (2014) e1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Drummond RA, Desai JV, Ricotta EE, Swamydas M, Deming C, Conlan S, Quinones M, Matei-Rascu V, Sherif L, Lecky D, Lee CR, Green NM, Collins N, Zelazny AM, Prevots DR, Bending D, Withers D, Belkaid Y, Segre JA, Lionakis MS, Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria, Cell Host Microbe (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Wan L, Zhang Y, Lai Y, Jiang M, Song Y, Zhou J, Zhang Z, Duan X, Fu Y, Liao L, Wang C, Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention and Treatment of Invasive Fungal Disease in Recipients of Allogeneic Stem-Cell Transplantation: A Prospective Multicenter Randomized Phase IV Trial, J Clin Oncol 33(34) (2015) 3999–4006. [DOI] [PubMed] [Google Scholar]
- [183].Chen TK, Batra JS, Michalik DE, Casillas J, Patel R, Ruiz ME, Hara H, Patel B, Kadapakkam M, Ch’Ng J, Small CB, Zagaliotis P, Ragsdale CE, Leal LO, Roilides E, Walsh TJ, Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor (rhu GM-CSF) as Adjuvant Therapy for Invasive Fungal Diseases, Open Forum Infect Dis 9(11) (2022) ofac535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, Alirezaie N, Majewski J, Sheppard DC, Behr MA, Foulkes WD, Vinh DC, CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy, Clin Infect Dis 59(1) (2014) 81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Gavino C, Hamel N, Zeng JB, Legault C, Guiot MC, Chankowsky J, Lejtenyi D, Lemire M, Alarie I, Dufresne S, Boursiquot JN, McIntosh F, Langelier M, Behr MA, Sheppard DC, Foulkes WD, Vinh DC, Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in French-Canadians, J Allergy Clin Immunol 137(4) (2016) 1178–88 e1-7. [DOI] [PubMed] [Google Scholar]
- [186].Drummond RA, Zahra FT, Natarajan M, Swamydas M, Hsu AP, Wheat LJ, Gavino C, Vinh DC, Holland SM, Mikelis CM, Lionakis MS, GM-CSF therapy in human caspase recruitment domain-containing protein 9 deficiency, J Allergy Clin Immunol 142(4) (2018) 1334–1338 e5. [DOI] [PubMed] [Google Scholar]
- [187].Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C, Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E, Immunity 35(4) (2011) 611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Du B, Shen N, Hu J, Tao Y, Mo X, Cao Q, Complete clinical remission of invasive Candida infection with CARD9 deficiency after G-CSF treatment, Comp Immunol Microbiol Infect Dis 70 (2020) 101417. [DOI] [PubMed] [Google Scholar]
- [189].Queiroz-Telles F, Mercier T, Maertens J, Sola CBS, Bonfim C, Lortholary O, Constantino-Silva RMN, Schrijvers R, Hagen F, Meis JF, Herkert PF, Breda GL, Franca JB, Filho NAR, Lanternier F, Casanova JL, Puel A, Grumach AS, Successful Allogenic Stem Cell Transplantation in Patients with Inherited CARD9 Deficiency, J Clin Immunol (2019). [DOI] [PubMed] [Google Scholar]
- [190].Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S, Stimulation of B-cell progenitors by cloned murine interleukin-7, Nature 333(6173) (1988) 571–3. [DOI] [PubMed] [Google Scholar]
- [191].Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS, Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial, JCI Insight 3(5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Ruan S, Samuelson DR, Assouline B, Morre M, Shellito JE, Treatment with Interleukin-7 Restores Host Defense against Pneumocystis in CD4+ T-Lymphocyte-Depleted Mice, Infect Immun 84(1) (2016) 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Unsinger J, Burnham CA, McDonough J, Morre M, Prakash PS, Caldwell CC, Dunne WM Jr., Hotchkiss RS, Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis, J Infect Dis 206(4) (2012) 606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Shindo Y, Unsinger J, Burnham CA, Green JM, Hotchkiss RS, Interleukin-7 and anti-programmed cell death 1 antibody have differing effects to reverse sepsis-induced immunosuppression, Shock 43(4) (2015) 334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Turnbull IR, Mazer MB, Hoofnagle MH, Kirby JP, Leonard JM, Mejia-Chew C, Spec A, Blood J, Miles SM, Ransom EM, Potter RF, Gaut JP, Remy KE, Hotchkiss RS, IL-7 Immunotherapy in a Nonimmunocompromised Patient With Intractable Fungal Wound Sepsis, Open Forum Infect Dis 8(6) (2021) ofab256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Zonios DI, Falloon J, Bennett JE, Shaw PA, Chaitt D, Baseler MW, Adelsberger JW, Metcalf JA, Polis MA, Kovacs SB, Kovacs JA, Davey RT, Lane HC, Masur H, Sereti I, Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors, Blood 112(2) (2008) 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Sheikh V, Porter BO, DerSimonian R, Kovacs SB, Thompson WL, Perez-Diez A, Freeman AF, Roby G, Mican J, Pau A, Rupert A, Adelsberger J, Higgins J, Bourgeois JS Jr., Jensen SM, Morcock DR, Burbelo PD, Osnos L, Maric I, Natarajan V, Croughs T, Yao MD, Estes JD, Sereti I, Administration of interleukin-7 increases CD4 T cells in idiopathic CD4 lymphocytopenia, Blood 127(8) (2016) 977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Miskin DP, Chalkias SG, Dang X, Bord E, Batson S, Koralnik IJ, Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia, Neurol Neuroimmunol Neuroinflamm 3(2) (2016) e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Santos D, de Azevedo C, Vicente VA, Queiroz-Telles F, Rodrigues AM, de Hoog GS, Denning DW, Colombo AL, The global burden of chromoblastomycosis, PLoS Negl Trop Dis 15(8) (2021) e0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Sousa Mda G, Reid DM, Schweighoffer E, Tybulewicz V, Ruland J, Langhorne J, Yamasaki S, Taylor PR, Almeida SR, Brown GD, Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin, Cell Host Microbe 9(5) (2011) 436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].de Sousa Mda G, Belda W Jr., Spina R, Lota PR, Valente NS, Brown GD, Criado PR, Benard G, Topical application of imiquimod as a treatment for chromoblastomycosis, Clin Infect Dis 58(12) (2014) 1734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Belda W Jr., Criado PR, Passero LFD, Successful treatment of chromoblastomycosis caused by Fonsecaea pedrosoi using imiquimod, J Dermatol 47(4) (2020) 409–412. [DOI] [PubMed] [Google Scholar]
- [203].Huang XW, Xu MN, Dai SQ, Zeng K, Li L, Case Report: Short-Term Application of Topical Imiquimod Is Practical for Chromoblastomycosis, Am J Trop Med Hyg 105(6) (2021) 1696–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL, Immune defence against Candida fungal infections, Nat Rev Immunol 15(10) (2015) 630–42. [DOI] [PubMed] [Google Scholar]
- [205].Higby DJ, Yates JW, Henderson ES, Holland JF, Filtration leukapheresis for granulocyte transfusion therapy. Clinical and laboratory studies, N Engl J Med 292(15) (1975) 761–6. [DOI] [PubMed] [Google Scholar]
- [206].Quillen K, Wong E, Scheinberg P, Young NS, Walsh TJ, Wu CO, Leitman SF, Granulocyte transfusions in severe aplastic anemia: an eleven-year experience, Haematologica 94(12) (2009) 1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Bow EJ, Schroeder ML, Louie TJ, Pulmonary complications in patients receiving granulocyte transfusions and amphotericin B, Can Med Assoc J 130(5) (1984) 593–7. [PMC free article] [PubMed] [Google Scholar]
- [208].Heuft HG, Goudeva L, Sel S, Blasczyk R, Equivalent mobilization and collection of granulocytes for transfusion after administration of glycosylated G-CSF (3 microg/kg) plus dexamethasone versus glycosylated G-CSF (12 microg/kg) alone, Transfusion 42(7) (2002) 928–34. [DOI] [PubMed] [Google Scholar]
- [209].O’Donghaile D, Childs RW, Leitman SF, Blood consult: granulocyte transfusions to treat invasive aspergillosis in a patient with severe aplastic anemia awaiting mismatched hematopoietic progenitor cell transplantation, Blood 119(6) (2012) 1353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Martinez M, Chen V, Tong AJ, Hamilton K, Clemons KV, Stevens DA, Experimental evidence that granulocyte transfusions are efficacious in treatment of neutropenic hosts with pulmonary aspergillosis, Antimicrob Agents Chemother 57(4) (2013) 1882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].West KA, Gea-Banacloche J, Stroncek D, Kadri SS, Granulocyte transfusions in the management of invasive fungal infections, Br J Haematol 177(3) (2017) 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Price TH, Boeckh M, Harrison RW, McCullough J, Ness PM, Strauss RG, Nichols WG, Hamza TH, Cushing MM, King KE, Young JA, Williams E, McFarland J, Holter Chakrabarty J, Sloan SR, Friedman D, Parekh S, Sachais BS, Kiss JE, Assmann SF, Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection, Blood 126(18) (2015) 2153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kadri SS, Remy KE, Strich JR, Gea-Banacloche J, Leitman SF, Role of granulocyte transfusions in invasive fusariosis: systematic review and single-center experience, Transfusion 55(9) (2015) 2076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Marciano BE, Allen ES, Conry-Cantilena C, Kristosturyan E, Klein HG, Fleisher TA, Holland SM, Malech HL, Rosenzweig SD, Granulocyte transfusions in patients with chronic granulomatous disease and refractory infections: The NIH experience, J Allergy Clin Immunol 140(2) (2017) 622–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A, Inflammatory monocytes orchestrate innate antifungal immunity in the lung, PLoS Pathog 10(2) (2014) e1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, Lehrnbecher T, Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus, Blood 107(6) (2006) 2562–9. [DOI] [PubMed] [Google Scholar]
- [217].Taylor PR, Roy S, Leal SM Jr., Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E, Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2, Nat Immunol 15(2) (2014) 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, Burchielli E, Nkwanyuo G, Pitzurra L, Velardi A, Romani L, A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation, Blood 102(10) (2003) 3807–14. [DOI] [PubMed] [Google Scholar]
- [219].Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, Capanni M, Urbani E, Mancusi A, Aversa F, Martelli MF, Romani L, Velardi A, Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation, Blood 106(13) (2005) 4397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Tramsen L, Koehl U, Tonn T, Latge JP, Schuster FR, Borkhardt A, Uharek L, Quaritsch R, Beck O, Seifried E, Klingebiel T, Lehrnbecher T, Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy, Bone Marrow Transplant 43(1) (2009) 13–9. [DOI] [PubMed] [Google Scholar]
- [221].Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, Roszik J, Rabinovich B, Olivares S, Krishnamurthy J, Zhang L, Najjar AM, Huls MH, Lee DA, Champlin RE, Kontoyiannis DP, Cooper LJ, Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection, Proc Natl Acad Sci U S A 111(29) (2014) 10660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Seif M, Kakoschke TK, Ebel F, Bellet MM, Trinks N, Renga G, Pariano M, Romani L, Tappe B, Espie D, Donnadieu E, Hunniger K, Hader A, Sauer M, Damotte D, Alifano M, White PL, Backx M, Nerreter T, Machwirth M, Kurzai O, Prommersberger S, Einsele H, Hudecek M, Loffler J, CAR T cells targeting Aspergillus fumigatus are effective at treating invasive pulmonary aspergillosis in preclinical models, Sci Transl Med 14(664) (2022) eabh1209. [DOI] [PubMed] [Google Scholar]
- [223].Desai JV, Lionakis MS, The role of neutrophils in host defense against invasive fungal infections, Curr Clin Microbiol Rep 5(3) (2018) 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D, Neutrophils: Between host defence, immune modulation, and tissue injury, PLoS Pathog 11(3) (2015) e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Lionakis MS, Albert ND, Swamydas M, Lee CR, Loetscher P, Kontoyiannis DP, Pharmacological Blockade of the Chemokine Receptor CCR1 Protects Mice from Systemic Candidiasis of Hematogenous Origin, Antimicrob Agents Chemother 61(3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lionakis MS, Fischer BG, Lim JK, Swamydas M, Wan W, Richard Lee CC, Cohen JI, Scheinberg P, Gao JL, Murphy PM, Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis, PLoS Pathog 8(8) (2012) e1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P, An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis, J Immunol 158(5) (1997) 2356–62. [PubMed] [Google Scholar]
- [228].del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavin C, Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans, Immunity 38(6) (2013) 1176–86. [DOI] [PubMed] [Google Scholar]
- [229].Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, Conti HR, Hernandez Mir G, Sirakova T, Childs EC, Smithgall TE, Biswas PS, Kolls JK, McGeachy MJ, Kolattukudy PE, Gaffen SL, MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation, Immunity 43(3) (2015) 475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Zwolanek F, Riedelberger M, Stolz V, Jenull S, Istel F, Koprulu AD, Ellmeier W, Kuchler K, The non-receptor tyrosine kinase Tec controls assembly and activity of the noncanonical caspase-8 inflammasome, PLoS Pathog 10(12) (2014) e1004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Lee EKS, Gillrie MR, Li L, Arnason JW, Kim JH, Babes L, Lou Y, Sanati-Nezhad A, Kyei SK, Kelly MM, Mody CH, Ho M, Yipp BG, Leukotriene B4-Mediated Neutrophil Recruitment Causes Pulmonary Capillaritis during Lethal Fungal Sepsis, Cell Host Microbe 23(1) (2018) 121–133 e4. [DOI] [PubMed] [Google Scholar]
- [232].Huang J, Meng S, Hong S, Lin X, Jin W, Dong C, IL-17C is required for lethal inflammation during systemic fungal infection, Cell Mol Immunol 13(4) (2016) 474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Naseem S, Frank D, Konopka JB, Carpino N, Protection from systemic Candida albicans infection by inactivation of the Sts phosphatases, Infect Immun 83(2) (2015) 637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Legrand F, Lecuit M, Dupont B, Bellaton E, Huerre M, Rohrlich PS, Lortholary O, Adjuvant corticosteroid therapy for chronic disseminated candidiasis, Clin Infect Dis 46(5) (2008) 696–702. [DOI] [PubMed] [Google Scholar]
- [235].Candon S, Rammaert B, Foray AP, Moreira B, Gallego Hernanz MP, Chatenoud L, Lortholary O, Chronic Disseminated Candidiasis During Hematological Malignancies: An Immune Reconstitution Inflammatory Syndrome With Expansion of Pathogen-Specific T Helper Type 1 Cells, J Infect Dis 221(11) (2020) 1907–1916. [DOI] [PubMed] [Google Scholar]
- [236].Lionakis MS, Kontoyiannis DP, Glucocorticoids and invasive fungal infections, Lancet 362(9398) (2003) 1828–38. [DOI] [PubMed] [Google Scholar]
- [237].Panackal AA, Wuest SC, Lin YC, Wu T, Zhang N, Kosa P, Komori M, Blake A, Browne SK, Rosen LB, Hagen F, Meis J, Levitz SM, Quezado M, Hammoud D, Bennett JE, Bielekova B, Williamson PR, Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis, PLoS Pathog 11(5) (2015) e1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Barber DL, Andrade BB, Sereti I, Sher A, Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none, Nat Rev Microbiol 10(2) (2012) 150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, Greenwald JH, Roby G, Mican J, Sher A, Roederer M, Sereti I, Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome, Blood 116(19) (2010) 3818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Anjum S, Dean O, Kosa P, Magone MT, King KA, Fitzgibbon E, Kim HJ, Zalewski C, Murphy E, Billioux BJ, Chisholm J, Brewer CC, Krieger C, Elsegeiny W, Scott TL, Wang J, Hunsberger S, Bennett JE, Nath A, Marr KA, Bielekova B, Wendler D, Hammoud DA, Williamson P, Outcomes in Previously Healthy Cryptococcal Meningoencephalitis Patients Treated With Pulse Taper Corticosteroids for Post-infectious Inflammatory Syndrome, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 73(9) (2021) e2789–e2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Meintjes G, Scriven J, Marais S, Management of the immune reconstitution inflammatory syndrome, Curr HIV/AIDS Rep 9(3) (2012) 238–50. [DOI] [PubMed] [Google Scholar]
- [242].Xu J, Neal LM, Ganguly A, Kolbe JL, Hargarten JC, Elsegeiny W, Hollingsworth C, He X, Ivey M, Lopez R, Zhao J, Segal B, Williamson PR, Olszewski MA, Chemokine receptor CXCR3 is required for lethal brain pathology but not pathogen clearance during cryptococcal meningoencephalitis, Sci Adv 6(25) (2020) eaba2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Neal LM, Xing E, Xu J, Kolbe JL, Osterholzer JJ, Segal BM, Williamson PR, Olszewski MA, CD4(+) T Cells Orchestrate Lethal Immune Pathology despite Fungal Clearance during Cryptococcus neoformans Meningoencephalitis, mBio 8(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Panackal AA, Wuest SC, Lin Y-C, Wu T, Zhang N, Kosa P, Komori M, Blake A, Browne SK, Rosen LB, Hagen F, Meis J, Levitz SM, Quezado M, Hammoud D, Bennett JE, Bielekova B, Williamson PR, Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis, PLOS Pathog 11(5) (2015) e1004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Khaw YM, Aggarwal N, Barclay WE, Kang E, Inoue M, Shinohara ML, Th1-Dependent Cryptococcus-Associated Immune Reconstitution Inflammatory Syndrome Model With Brain Damage, Frontiers in Immunology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Eschke M, Piehler D, Schulze B, Richter T, Grahnert A, Protschka M, Muller U, Kohler G, Hofling C, Rossner S, Alber G, A novel experimental model of Cryptococcus neoformans-related immune reconstitution inflammatory syndrome (IRIS) provides insights into pathogenesis, Eur J Immunol 45(12) (2015) 3339–50. [DOI] [PubMed] [Google Scholar]
- [247].Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Lee SJ, Kambugu A, Janoff EN, Bohjanen PR, Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study, PLoS Med 7(12) (2010) e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Chang CC, Omarjee S, Lim A, Spelman T, Gosnell BI, Carr WH, Elliott JH, Moosa MY, Ndung’u T, French MA, Lewin SR, Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome, J Infect Dis 208(10) (2013) 1604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Akilimali NA, Muema DM, Specht C, Chang CC, Moosa MS, Levitz SM, Lewin SR, French MA, Ndung’u T, Cryptococcosis-Associated Immune Reconstitution Inflammatory Syndrome Is Associated With Dysregulation of IL-7/IL-7 Receptor Signaling Pathway in T Cells and Monocyte Activation, J Acquir Immune Defic Syndr 80(5) (2019) 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Gaube G, De Castro N, Gueguen A, Lascoux C, Zagdanski AM, Alanio A, Molina JM, Treatment with adalimumab for severe immune reconstitution inflammatory syndrome in an HIV-infected patient presenting with cryptococcal meningitis, Med Mal Infect 46(3) (2016) 154–6. [DOI] [PubMed] [Google Scholar]
- [251].Kwon HY, Han YJ, Im JH, Baek JH, Lee JS, Two cases of immune reconstitution inflammatory syndrome in HIV patients treated with thalidomide, Int J STD AIDS 30(11) (2019) 1131–1135. [DOI] [PubMed] [Google Scholar]
- [252].Gaffen SL, Moutsopoulos NM, Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity, Sci Immunol 5(43) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL, Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity, Science 332(6025) (2011) 65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, Puel A, Li X, Casanova JL, An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis, Immunity 39(4) (2013) 676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, Migaud M, Boisson B, Bolze A, Itan Y, Goudin N, Cottineau J, Picard C, Abel L, Bustamante J, Casanova JL, Puel A, Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis, J Exp Med 212(5) (2015) 619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL, Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis, Curr Opin Allergy Clin Immunol 12(6) (2012) 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Oikonomou V, Break TJ, Gaffen SL, Moutsopoulos NM, Lionakis MS, Infections in the monogenic autoimmune syndrome APECED, Curr Opin Immunol 72 (2021) 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Constantine GM, Lionakis MS, Lessons from primary immunodeficiencies: Autoimmune regulator and autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, Immunol Rev 287(1) (2019) 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL, Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I, J Exp Med 207(2) (2010) 291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Ferre EM, Rose SR, Rosenzweig SD, Burbelo PD, Romito KR, Niemela JE, Rosen LB, Break TJ, Gu W, Hunsberger S, Browne SK, Hsu AP, Rampertaap S, Swamydas M, Collar AL, Kong HH, Lee CR, Chascsa D, Simcox T, Pham A, Bondici A, Natarajan M, Monsale J, Kleiner DE, Quezado M, Alevizos I, Moutsopoulos NM, Yockey L, Frein C, Soldatos A, Calvo KR, Adjemian J, Similuk MN, Lang DM, Stone KD, Uzel G, Kopp JB, Bishop RJ, Holland SM, Olivier KN, Fleisher TA, Heller T, Winer KK, Lionakis MS, Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, JCI Insight 1(13) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A, Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines, J Exp Med 207(2) (2010) 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Orlova EM, Sozaeva LS, Kareva MA, Oftedal BE, Wolff ASB, Breivik L, Zakharova EY, Ivanova ON, Kampe O, Dedov II, Knappskog PM, Peterkova VA, Husebye ES, Expanding the Phenotypic and Genotypic Landscape of Autoimmune Polyendocrine Syndrome Type 1, J Clin Endocrinol Metab 102(9) (2017) 3546–3556. [DOI] [PubMed] [Google Scholar]
- [263].Break TJ, Oikonomou V, Dutzan N, Desai JV, Swidergall M, Freiwald T, Chauss D, Harrison OJ, Alejo J, Williams DW, Pittaluga S, Lee CR, Bouladoux N, Swamydas M, Hoffman KW, Greenwell-Wild T, Bruno VM, Rosen LB, Lwin W, Renteria A, Pontejo SM, Shannon JP, Myles IA, Olbrich P, Ferre EMN, Schmitt M, Martin D, Genomics C Computational Biology, Barber DL, Solis NV, Notarangelo LD, Serreze DV, Matsumoto M, Hickman HD, Murphy PM, Anderson MS, Lim JK, Holland SM, Filler SG, Afzali B, Belkaid Y, Moutsopoulos NM, Lionakis MS, Aberrant type 1 immunity drives susceptibility to mucosal fungal infections, Science 371(6526) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, Ouachee-Chardin M, Fouyssac F, Girisha KM, Etzioni A, Van Montfrans J, Camcioglu Y, Kerns LA, Belohradsky B, Blanche S, Bousfiha A, Rodriguez-Gallego C, Meyts I, Kisand K, Reichenbach J, Renner ED, Rosenzweig S, Grimbacher B, van de Veerdonk FL, Traidl-Hoffmann C, Picard C, Marodi L, Morio T, Kobayashi M, Lilic D, Milner JD, Holland S, Casanova JL, Puel A, S.G.-o.-F.S.G. International, Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype, Blood 127(25) (2016) 3154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL, Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis, J Exp Med 208(8) (2011) 1635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, Kullberg BJ, van der Meer JW, Lilic D, Veltman JA, Netea MG, STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis, N Engl J Med 365(1) (2011) 54–61. [DOI] [PubMed] [Google Scholar]
- [267].Bloomfield M, Kanderova V, Parackova Z, Vrabcova P, Svaton M, Fronkova E, Fejtkova M, Zachova R, Rataj M, Zentsova I, Milota T, Klocperk A, Kalina T, Sediva A, Utility of Ruxolitinib in a Child with Chronic Mucocutaneous Candidiasis Caused by a Novel STAT1 Gain-of-Function Mutation, J Clin Immunol 38(5) (2018) 589–601. [DOI] [PubMed] [Google Scholar]
- [268].Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, Irvine AD, Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation, J Allergy Clin Immunol 135(2) (2015) 551–3. [DOI] [PubMed] [Google Scholar]
- [269].Kayaoglu B, Kasap N, Yilmaz NS, Charbonnier LM, Geckin B, Akcay A, Eltan SB, Ozturk G, Ozen A, Karakoc-Aydiner E, Chatila TA, Gursel M, Baris S, Stepwise Reversal of Immune Dysregulation Due to STAT1 Gain-of-Function Mutation Following Ruxolitinib Bridge Therapy and Transplantation, J Clin Immunol 41(4) (2021) 769–779. [DOI] [PubMed] [Google Scholar]
- [270].Moriya K, Suzuki T, Uchida N, Nakano T, Katayama S, Irie M, Rikiishi T, Niizuma H, Okada S, Imai K, Sasahara Y, Kure S, Ruxolitinib treatment of a patient with steroid-dependent severe autoimmunity due to STAT1 gain-of-function mutation, Int J Hematol 112(2) (2020) 258–262. [DOI] [PubMed] [Google Scholar]
- [271].Mossner R, Diering N, Bader O, Forkel S, Overbeck T, Gross U, Grimbacher B, Schon MP, Buhl T, Ruxolitinib Induces Interleukin 17 and Ameliorates Chronic Mucocutaneous Candidiasis Caused by STAT1 Gain-of-Function Mutation, Clin Infect Dis 62(7) (2016) 951–3. [DOI] [PubMed] [Google Scholar]
- [272].Olivier N, Boralevi F, Fricain JC, Doutre MS, Utility of ruxolitinib in a patient with chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation, J Eur Acad Dermatol Venereol (2022). [DOI] [PubMed] [Google Scholar]
- [273].Rosenberg JM, Peters JM, Hughes T, Lareau CA, Ludwig LS, Massoth LR, Austin-Tse C, Rehm HL, Bryson B, Chen YB, Regev A, Shalek AK, Fortune SM, Sykes DB, JAK inhibition in a patient with a STAT1 gain-of-function variant reveals STAT1 dysregulation as a common feature of aplastic anemia, Med (N Y) 3(1) (2022) 42–57 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, Ma C, Torgerson TR, Rosenzweig SD, Fleisher TA, Notarangelo LD, Hanson IC, Forbes LR, Chatila TA, Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation, J Allergy Clin Immunol 139(5) (2017) 1629–1640 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Baris S, Alroqi F, Kiykim A, Karakoc-Aydiner E, Ogulur I, Ozen A, Charbonnier LM, Bakir M, Boztug K, Chatila TA, Barlan IB, Severe Early-Onset Combined Immunodeficiency due to Heterozygous Gain-of-Function Mutations in STAT1, J Clin Immunol 36(7) (2016) 641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Hetemaki I, Kaustio M, Kinnunen M, Heikkila N, Keskitalo S, Nowlan K, Miettinen S, Sarkkinen J, Glumoff V, Andersson N, Kettunen K, Vanhanen R, Nurmi K, Eklund KK, Dunkel J, Mayranpaa MI, Schlums H, Arstila TP, Kisand K, Bryceson YT, Peterson P, Otava U, Syrjanen J, Saarela J, Varjosalo M, Kekalainen E, Loss-of-function mutation in IKZF2 leads to immunodeficiency with dysregulated germinal center reactions and reduction of MAIT cells, Sci Immunol 6(65) (2021) eabe3454. [DOI] [PubMed] [Google Scholar]
- [277].Kong XF, Worley L, Rinchai D, Bondet V, Jithesh PV, Goulet M, Nonnotte E, Rebillat AS, Conte M, Mircher C, Gurtler N, Liu L, Migaud M, Elanbari M, Habib T, Ma CS, Bustamante J, Abel L, Ravel A, Lyonnet S, Munnich A, Duffy D, Chaussabel D, Casanova JL, Tangye SG, Boisson-Dupuis S, Puel A, Three Copies of Four Interferon Receptor Genes Underlie a Mild Type I Interferonopathy in Down Syndrome, J Clin Immunol 40(6) (2020) 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Tyler PM, Bucklin ML, Zhao M, Maher TJ, Rice AJ, Ji W, Warner N, Pan J, Morotti R, McCarthy P, Griffiths A, van Rossum AMC, Hollink I, Dalm V, Catanzaro J, Lakhani SA, Muise AM, Lucas CL, Human autoinflammatory disease reveals ELF4 as a transcriptional regulator of inflammation, Nat Immunol 22(9) (2021) 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Schmidt F, Thywissen A, Goldmann M, Cunha C, Cseresnyes Z, Schmidt H, Rafiq M, Galiani S, Graler MH, Chamilos G, Lacerda JF, Campos A Jr., Eggeling C, Figge MT, Heinekamp T, Filler SG, Carvalho A, Brakhage AA, Flotillin-Dependent Membrane Microdomains Are Required for Functional Phagolysosomes against Fungal Infections, Cell Rep 32(7) (2020) 108017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Jaeger M, van der Lee R, Cheng SC, Johnson MD, Kumar V, Ng A, Plantinga TS, Smeekens SP, Oosting M, Wang X, Barchet W, Fitzgerald K, Joosten LA, Perfect JR, Wijmenga C, van de Veerdonk FL, Huynen MA, Xavier RJ, Kullberg BJ, Netea MG, The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections, Eur J Clin Microbiol Infect Dis 34(5) (2015) 963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Tam JM, Reedy JL, Lukason DP, Kuna SG, Acharya M, Khan NS, Negoro PE, Xu S, Ward RA, Feldman MB, Dutko RA, Jeffery JB, Sokolovska A, Wivagg CN, Lassen KG, Le Naour F, Matzaraki V, Garner EC, Xavier RJ, Kumar V, van de Veerdonk FL, Netea MG, Miranti CK, Mansour MK, Vyas JM, Tetraspanin CD82 Organizes Dectin-1 into Signaling Domains to Mediate Cellular Responses to Candida albicans, J Immunol 202(11) (2019) 3256–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Babula O, Lazdane G, Kroica J, Linhares IM, Ledger WJ, Witkin SS, Frequency of interleukin-4 (IL-4) −589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis, Clin Infect Dis 40(9) (2005) 1258–62. [DOI] [PubMed] [Google Scholar]
- [283].Hu XP, Wang RY, Wang X, Cao YH, Chen YQ, Zhao HZ, Wu JQ, Weng XH, Gao XH, Sun RH, Zhu LP, Dectin-2 polymorphism associated with pulmonary cryptococcosis in HIV-uninfected Chinese patients, Med Mycol 53(8) (2015) 810–6. [DOI] [PubMed] [Google Scholar]
- [284].Mhmoud NA, Fahal AH, van de Sande WW, The association between the interleukin-10 cytokine and CC chemokine ligand 5 polymorphisms and mycetoma granuloma formation, Med Mycol 51(5) (2013) 527–33. [DOI] [PubMed] [Google Scholar]


