Abstract
Bacterial flagella are powered by a motor that converts a transmembrane electrochemical potential of either H+ or Na+ into mechanical work. In Escherichia coli, the MotA and MotB proteins form the stator and function in proton translocation, whereas the FliG protein is located on the rotor and is involved in flagellar assembly and torque generation. The sodium-driven polar flagella of Vibrio species contain homologs of MotA and MotB, called PomA and PomB, and also contain two other membrane proteins called MotX and MotY, which are essential for motor rotation and that might also function in ion conduction. Deletions in pomA, pomB, motX, or motY in Vibrio cholerae resulted in a nonmotile phenotype, whereas deletion of fliG gave a nonflagellate phenotype. fliG genes on plasmids complemented fliG-null strains of the parent species but not fliG-null strains of the other species. FliG-null strains were complemented by chimeric FliG proteins in which the C-terminal domain came from the other species, however, implying that the C-terminal part of FliG can function in conjunction with the ion-translocating components of either species. A V. cholerae strain deleted of pomA, pomB, motX, and motY became weakly motile when the E. coli motA and motB genes were introduced on a plasmid. Like E. coli, but unlike wild-type V. cholerae, motility of some V. cholerae strains containing the hybrid motor was inhibited by the protonophore carbonyl cyanide m-chlorophenylhydrazone under neutral as well as alkaline conditions but not by the sodium motor-specific inhibitor phenamil. We conclude that the E. coli proton motor components MotA and MotB can function in place of the motor proteins of V. cholerae and that the hybrid motors are driven by the proton motive force.
Many bacteria swim by rotating their flagella, the filamentous organelles that function as a propeller. Flagellar rotation is carried out by a rotary motor in the cell membrane at the base of the flagellar filament. The motor complex generating torque converts ion flux to motor rotation. The source of energy for motor rotation is the electrochemical gradient of protons or, in some species, sodium ions across the cytoplasmic membrane. Extensive studies on the proton-driven motors of Escherichia coli and Salmonella enterica serovar Typhimurium showed that the rotor part of the motor is composed of the FliG, FliM, and FliN proteins, whereas the stator complex consists of the MotA and MotB proteins (for reviews, see references 5, 6, 9, and 24). MotA and MotB interact via their transmembrane regions and function as the proton-conducting channel (8, 31, 34). Although the mechanism of the conversion of electrochemical energy into mechanical work is not completely understood at the molecular level, torque generation is believed to occur at an interface between cytoplasmic domains of the MotA-MotB complexes and the C-terminal domain of FliG (15, 20, 32).
The architecture of the sodium-type motor is much less well defined. In the sodium-driven polar flagella of Vibrio alginolyticus and Vibrio parahaemolyticus, four proteins, PomA, PomB, MotX, and MotY, have been shown to be essential for rotation and may comprise the stator (2, 26, 27, 29). PomA and PomB have sequence similarities to MotA and MotB, respectively. The rotation of sodium-driven flagella is specifically inhibited by phenamil, an amiloride analog, and mutations conferring resistance to phenamil mapped to the pomA and pomB genes, implicating both proteins in sodium transfer (16, 18). More indirectly, MotX was also implicated in Na+ channel function (26). Recently, the V. parahaemolyticus FliG, FliM, and FliN proteins were demonstrated to be important in flagellum assembly (7).
Vibrio cholerae, the causative agent of the severe diarrheal disease cholera, is motile via a single polar sheathed flagellum. The life cycle of V. cholerae consists of a free-swimming phase outside the host and a virulent phase when colonizing the human small intestine. While motility is thought to contribute to the pathogenicity of V. cholerae, the relationship between motility and virulence is not yet understood (30). Interestingly, alterations in motility phenotypes were found to correlate with changes in expression of the major virulence genes (12). Recently, induced changes in the membrane sodium flux were found to affect virulence gene regulation perhaps by affecting motility, suggesting an intriguing interplay of sodium energetics, motility, and virulence in this organism (14).
The polar flagellum of V. cholerae was recently demonstrated to be sodium driven (14, 19), and gene homologs of pomA, pomB, motX, motY, and fliG are present in the genome. In the present study, we analyzed the involvement of the four putative stator proteins, PomA, PomB, MotX, and MotY, and the torque-generating FliG protein in flagellum function and assembly in V. cholerae and tested specific mutations in these genes for functional complementation by their E. coli counterparts.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
V. cholerae strain O395N1 was used for mutagenesis of the pomAB, motX, motY, and fliG genes (Table 1). E. coli strain DH5αλpir was used to maintain the suicide plasmids during cloning steps, whereas E. coli strain β2155 (supplemented with 0.002% diaminopimelic acid) was used as the host of the suicide plasmids for conjugation with V. cholerae cells. The E. coli fliG deletion strain DFB225 (named EcΔG in this study) was kindly provided by D. Blair (21). The plasmid vector pBAD-24 (13) was used for the cloning and expression of the various fliG genes, with 0.02% l-arabinose used for induction. Plasmid pJZ19 (34) (named pMotAB in this study) carrying the E. coli motAB genes in pACYC184 was generously provided by D. Blair. MotAB is expressed from the trp promoter. Plasmid pLS25 (from D. Blair) was used as the template in a PCR to clone the E. coli fliG gene into pBAD-24 (pBAD-EcG). All strains were grown in Luria broth (LB) containing the appropriate antibiotics at the following concentrations: streptomycin, 100 μg/ml; ampicillin, 50 μg/ml; and chloramphenicol, 10 μg/ml. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) and phenamil were purchased from Sigma.
TABLE 1.
Bacterial strains and plasmids used for complementation analyses
| Strain or plasmid | Description | Source, parent, and/or reference |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| O395N1 | Wild type | |
| VcΔX | ΔmotX | O395N1, this study |
| VcΔY | ΔmotY | O395N1 (20) |
| VcΔAB | ΔpomAB | O395N1, this study |
| VcΔG | ΔfliG | O395N1 (20) |
| VcΔXYAB | ΔmotX ΔmotY ΔpomAB | O395N1, this study |
| VcΔXYABG | ΔmotX ΔmotY ΔpomAB ΔfliG | O395N1, this study |
| HM-1 | Spontaneously hypermotile | VcΔXYABG, pMotAB, pBAD-VcG |
| HM-2 | Spontaneously hypermotile | HM-1 |
| HM-3 | Spontaneously hypermotile | HM-2 |
| E. coli EcΔG | (DFB225) ΔfliG | D. Blair (22) |
| Plasmids | ||
| pACYC184 | Camr, cloning vector | |
| pMotAB | (pJZ19), E. coli fliG in pACYC184 | D. Blair (6) |
| pBAD-24 | Ampr, cloning vector | |
| pBAD-VcG | V. cholerae fliG in pBAD-24 | This study |
| pBAD-EcG | E. coli fliG in pBAD-24 | This study |
| pBAD-FP1 | V. cholerae-E. coli chimeric fliG in pBAD-24 | This study |
| pBAD-FP2 | E. coli-V. cholerae chimeric fliG in pBAD-24 | This study |
Genetic manipulations.
Mutants of V. cholerae were generated by homologous recombination. Preliminary sequence data for V. cholerae were obtained from The Institute for Genomic Research website (http://www.tigr.org). The genes and surrounding sequences were amplified by PCR using specific primers and cloned into the plasmid vector pCR2.1 (Invitrogen) or pUC19. Internal deletions were generated by using convenient restriction sites present in the genes, and the DNA was then subcloned into pWM91 (28) (generously provided by B. Wanner). An in-frame deletion in fliG was constructed using internal SalI (filled in with T4 polymerase) and SnaBI sites; this results in deletion of amino acids 94 to 258. The pomAB deletion was constructed by using internal BamHI and BglII sites; this results in an out-of-frame deletion of amino acids 63 to 243 encoded by pomA and amino acids 1 to 187 encoded by pomB. The motX deletion was constructed using HincII and PmlI sites, deleting amino acids 1 to 79. AccI (filled in by T4 polymerase) and HincII sites were used to delete amino acids 34 to 295 encoded by motY. The mutated alleles were introduced into the chromosome of strain O395N1 following sucrose selection as described elsewhere (11). Plasmid DNA was prepared by using a Qiagen (Chatsworth, Calif.) Miniprep extraction kit and introduced into bacteria by electroporation as described by the supplier. The FP-1 chimeric construct was generated by replacement of a SalI-EcoRI fragment of pBAD-VcG containing the C-terminal 268 bp of the fliG gene with a SalI-EcoRI fragment containing the 272-bp C-terminal fragment of E. coli fliG. pBAD-FP2 was constructed by replacement of the SalI-HindIII fragment of pBAD-EcG containing the C-terminal 272 bp of the E. coli fliG gene with a SalI-HindIII fragment containing the 268 bp of the V. cholerae fliG.
Motility assays.
Motility phenotypes were assessed for swarm diameter following inoculation into 0.3% soft agar. Swarm plates were inoculated with cells toothpicked from colonies. Bacterial cells were also assayed for swimming ability under a dark-field microscope after the addition of various compounds. CCCP was added at 30 μM, and phenamil was added at 50 to 100 μM. A score of +++ indicates that more than 70% of the bacteria were swimming. Swimming of the V. cholerae parental strain was scored ++++ to indicate the increased speed compared to E. coli or the hybrid Vibrio strain. A score of − indicates that less than 10% of the bacteria were swimming.
RESULTS
Involvement of the V. cholerae PomAB, MotX, MotY, and FliG proteins in flagellar function and assembly.
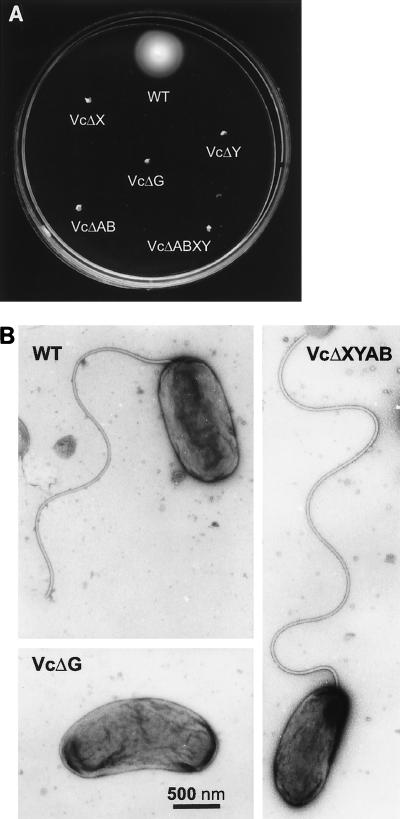
To study the roles of the V. cholerae pomAB, motX, motY, and fliG homologous genes in flagellar function and assembly, we created strains with specific deletion mutations in these genes, a strain deleted in the four putative stator genes, as well as a strain lacking all of these genes. Analyses of the resulting strains in soft agar plates and under light microscopy showed that all mutant strains displayed nonmotile phenotypes (Fig. 1A). Electron microscopy (EM) studies of these strains revealed that all strains with deletions in the putative stator genes, including the quadruple mutant (Fig. 1B), produced apparently normal flagella. In contrast, strains with a deletion in the fliG gene did not produce any flagella as analyzed by EM (Fig. 1B).
FIG. 1.
Analyses of mutants for motility and flagellum production. Swarms in soft agar plates incubated for 8 h at 37°C (A) and electron micrographs (B) of the V. cholerae strain O395N1 (WT [wild-type]) and the motX (VcΔX), motY (VcΔY), pomAB (VcΔAB), and fliG (VcΔG) mutant derivatives as well as the motX motY pomAB (VcΔXYAB) quadruple mutant strain are shown.
Complementation of V. cholerae and E. coli ΔfliG strains with plasmids carrying fliG.
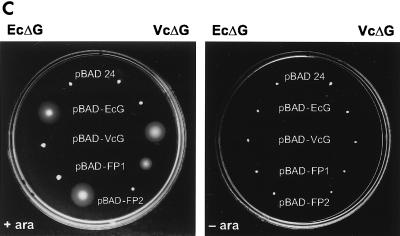
The V. cholerae predicted FliG protein has 39.5% amino acid sequence identity with the E. coli FliG protein (Fig. 2A). To address whether the V. cholerae and E. coli FliG proteins can functionally complement each other, we introduced plasmids with different fliG genes under an arabinose-inducible promoter into V. cholerae and E. coli fliG deletion strains. No restoration of the motility phenotypes as assayed in soft agar plates was observed with either the V. cholerae ΔfliG strain harboring the E. coli fliG gene on a plasmid or the E. coli ΔfliG strain carrying the V. cholerae fliG gene on a plasmid (Fig. 2C). However, the fliG genes did complement their parental mutations (Fig. 2C). A fusion protein consisting of the N-terminal portion of V. cholerae FliG fused to the C-terminal domain of E. coli FliG (FP-1 [Fig. 2B]) was capable of complementing the V. cholerae, but not the E. coli, fliG deletion strain (Fig. 2C). Similarly, the inverse E. coli-V. cholerae fusion protein (FP-2 [Fig. 2B]) complemented the E. coli but not V. cholerae ΔfliG strain (Fig. 2C). EM studies of the V. cholerae ΔfliG strain carrying different plasmids showed that none or only very few bacteria produced flagella when the E. coli wild-type FliG or FP-2 was expressed. In contrast, normal flagella were observed when the V. cholerae wild-type or chimeric FP-1 protein was expressed (data not shown).
FIG. 2.
Complementation of fliG mutants by plasmids carrying various fliG genes. (A) Amino acid sequence alignment of the E. coli and V. cholerae FliG proteins. The arrow indicates the position of the junction between the two domains in the fusion proteins. (B) Diagram of the chimeric FliG proteins. Hatched boxes indicate V. cholerae sequence, and open boxes indicate E. coli sequence. Numbers correspond to amino acid residues. (C) Swarming abilities in the presence or absence of arabinose (ara) of the E. coli (EcΔG) or V. cholerae (VcΔG) fliG deletion strains complemented by plasmids carrying the E. coli (pBAD-EcG), V. cholerae (pBAD-VcG), or chimeric (pBAD-FP1, pBAD-FP2) fliG genes. pBAD-24 is the parent vector and contains no flagellar genes. Plates were incubated for 8 h at 37°C.
Complementation of the V. cholerae pomAB, motX, and motY genes by the E. coli motAB genes.
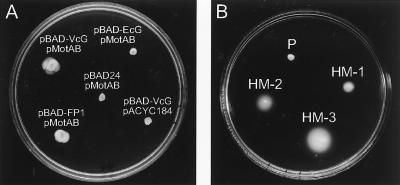
The N-terminal domain of the E. coli FliG protein is believed to interact with the flagellar basal body, whereas the C-terminal domain interacts with the MotA-MotB complex (20). Thus, for functional complementation of the ion-translocating subunits of the flagella, we anticipated the need for a chimeric FliG protein. A fusion protein consisting of the N-terminal portion of V. cholerae FliG fused to the C-terminal domain of E. coli FliG was constructed (Fig. 2B). The V. cholerae strain carrying chromosomal deletions in the pomAB, motX, motY, and fliG genes (VcΔABXYG) was transformed with the plasmids encoding either the wild-type V. cholerae or E. coli fliG gene or the V. cholerae-E. coli fliG chimeric construct (FP-1) from an arabinose-inducible promoter. A second plasmid containing the E. coli motAB genes was then introduced. Some, although very small, swarm circles were observed in soft agar plates only in the strain carrying the V. cholerae wild-type or chimeric fliG (FP-1) gene only in the presence of arabinose (Fig. 3A). This motility was dependent on the presence of the E. coli motAB genes, as the control strain harboring pACYC184 produced no appreciable motility (Fig. 3A). Interestingly, spontaneous hypermotile mutants that when isolated produced larger motility circles in soft agar plates than the parental strain were readily observed (Fig. 3B). Moreover, these hypermotile strains also produced further hypermotile variants, and several such hypermotile strains when isolated demonstrated increasingly larger motility circles (Fig. 3B). Similar increasingly motile variants were also isolated from the strain with the chimeric fliG gene (data not shown). Normal flagellum production by the motile strains was demonstrated by EM (data not shown).
FIG. 3.
Complementation of V. cholerae VcΔXYABG by plasmids carrying the E. coli motAB and different fliG genes. (A) Swarm circles of the quintuple deletion strain carrying the pMotAB or pACYC184 control plasmid as well as either pBAD-24, pBAD-EcG, pBAD-VcG, or pBAD-FP1. (B) Swarming behavior of the parental strain (P) and of spontaneous hypermotile derivatives (HM-1, HM-2, and HM-3) of strain VcΔXYABG carrying pMotAB and pBAD-VcG. Both soft agar plates contain arabinose. Plates were incubated overnight at 37°C.
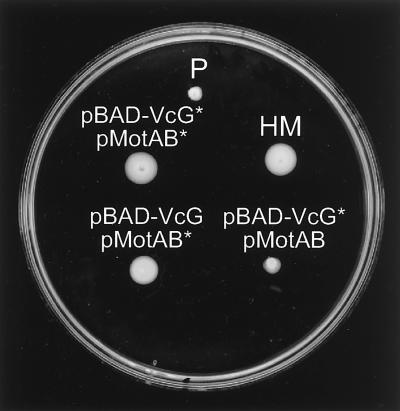
To test whether the mutations are linked to the E. coli motAB or V. cholerae fliG gene, plasmid DNA isolated from some hypermotile strains was transformed into strain VcΔABXYG, selecting for both plasmid markers. The majority of the resulting strains did not display greater motility than the original strain (data not shown). However, we were able to isolate some pMotAB plasmids that, when transformed with the original pBAD-FliG construct, resulted in increased swarming circles (Fig. 4). Three such plasmids that were independently isolated had a mutation in Tyr-61 of MotB.
FIG. 4.
Linking of the hypermotile phenotype to the pMotAB plasmid. Swarm circles in an arabinose-containing soft agar plate of V. cholerae strain VcΔXYABG carrying plasmids pMotAB and pBAD-VcG from different origins. Shown are the parental strain (P) and one of the spontaneous hypermotile derivatives (HM). Both plasmids, pMotAB and pBAD-VcG, isolated from the HM strain were transformed back into the host strain either together or with the original nonmutated plasmids. An asterisk indicates that the plasmid was derived from the hypermotile strain. Plates were incubated overnight at 37°C.
Analysis of the coupling ion used for motility of V. cholerae strain VcΔABXYG, carrying the V. cholerae fliG and E. coli motAB genes on plasmids.
The E. coli flagellar motor is known to use the translocation of protons as the energy source for rotation, whereas the polar flagella of V. cholerae were found to be sodium driven. By replacing the V. cholerae pomAB, motX, and motY genes by the E. coli motAB genes, we generated a functional hybrid flagellar motor and wished to investigate which coupling ion, H+ or Na+, was used for the observed motility. We investigated the energy requirement of several isolated hypermotile variants, both chromosomal (HM-1 and HM-2) and motAB dependent (HM-3) (data not shown), as the original hybrid motor strain produced these mutants so readily that a pure population of cells could not be analyzed in this strain. The protonophore CCCP, a compound known to collapse proton motive force (PMF), inhibited motility of the E. coli control strain and the V. cholerae hybrid motor strains under neutral as well as alkaline conditions (Table 2). In contrast, the V. cholerae control strain was inhibited at neutral pH but was insensitive to CCCP at an alkaline pH (Table 2). Interestingly, the addition of even very small amounts of CCCP (2 to 5 μM) at either pH completely inhibited motility of the V. cholerae hybrid motor strain, whereas much larger concentrations of CCCP (25 to 50 μM) were required to block motility of the E. coli strain (data not shown).
TABLE 2.
Effects of inhibitors on motilitya
| Strain | Motility
|
|||||
|---|---|---|---|---|---|---|
| CCCP
|
Phenamil, pH 6.5
|
|||||
| pH 6.5
|
pH 8.5
|
|||||
| 0 | 30 μM | 0 | 30 μM | 0 | 100 μM | |
| EcΔG, pBAD-EcG | +++ | − | +++ | − | +++ | +++ |
| VcΔG, pBAD-VcG | ++++ | − | ++++ | +++ | ++++ | − |
| HM-1 | +++ | − | +++ | − | +++ | +++ |
| HM-2 | +++ | − | +++ | − | +++ | +++ |
Motilities of the V. cholerae (VcΔG, pBAD-VcG) and E. coli (EcΔG, pBAD-EcG) control strains as well as two hypermotile derivatives of the V. cholerae hybrid motor strains (HM-1 and HM-2) were assayed under the microscope. Motility was scored as described in Materials and Methods.
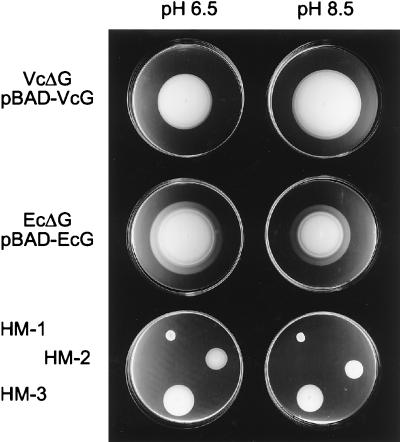
The sodium channel blocker phenamil, an amiloride analog known to specifically block the sodium-translocating portion of flagella (4), inhibited motility of the V. cholerae parental strain but not of the V. cholerae hybrid motor strains or the E. coli control strain (Table 2). The addition of increasing concentrations of NaCl to LB increased the swimming speed of the V. cholerae parental strain but not of the E. coli or V. cholerae hybrid motor strain (data not shown). Similarly, increased pH resulted in increased swarm circles by the V. cholerae parental strain but not by the E. coli or V. cholerae hybrid motor strain (Fig. 5). Together, these results strongly suggest that this hybrid flagellar motor, like the E. coli but unlike the V. cholerae flagellar motor, uses protons as the coupling ion for rotation.
FIG. 5.
Effects of different medium pHs on swarm circles. Motility of the V. cholerae (VcΔG, pBAD-VcG) and E. coli (EcΔG, pBAD-EcG) control strains as well as several spontaneous hypermotile derivatives of the V. cholerae hybrid motor strain (HM-1, HM-2, and HM-3) were assayed in arabinose-containing soft agar plates with a pH of 6.5 or 8.5.
DISCUSSION
It was recently reported that the single polar flagellum of V. cholerae is energized by the translocation of sodium ions (14, 19). The structures and functions of many proteins in the proton-driven flagella of E. coli and S. enterica serovar Typhimurium have been extensively studied (9, 24), whereas less is known about the architecture of sodium-driven flagella. The now completed sequence of the V. cholerae genome presents the first opportunity for extensive sequence comparisons of various proteins constituting the two types of flagellar motors. The single polar flagella of V. alginolyticus and V. parahaemolyticus, like those of V. cholerae, utilize an electrochemical gradient of sodium ions (sodium motive force [SMF]) as energy stock for flagellar rotation (3, 17). In these two species, four proteins, PomA, PomB, MotX, and MotY, are believed to form the sodium ion-conducting channel and the stator of the motor (2, 26, 27, 29). PomA and PomB have some sequence homology to the E. coli MotA and MotB proteins, which form the proton-conducting complex and stator of the E. coli flagella. In this study, we identified the V. cholerae gene homologs for pomA, pomB, motX, and motY from the genomic database and created V. cholerae strains with specific deletions in these genes as well as a strain deleted in all four genes. These strains showed nonmotile phenotypes but produced apparently normal flagella, indicating that these proteins, like the E. coli and V. parahaemolyticus stator proteins, are required for flagellar function but not assembly.
In E. coli, only three proteins, MotA, MotB, and FliG, participate closely in torque generation. Torque generation is believed to occur at the interface between cytoplasmic domains of the MotA-MotB complexes and the C-terminal domain of FliG (20, 32). Recently, the structure of the C-terminal domain of the Thermatoga maritima FliG protein was determined (22). A fliG deletion strain of V. cholerae did not produce flagella, suggesting that like in E. coli and V. parahaemolyticus, the V. cholerae FliG protein is required for flagellum assembly. Whereas the E. coli and V. cholerae fliG mutant strains were readily complemented by their homologous genes, expression of the heterologous fliG genes did not restore motility. Motility required chimeric FliG proteins where the N termini determined species specificity, showing that the C-terminal regions of the two FliG proteins are functionally interchangeable. Although the C-terminal domains of the two FliG proteins have high amino acid sequence homology, it is still remarkable that the E. coli FliG C-terminal domain can functionally interact with the sodium-translocating components (presumably PomA) of the Vibrio flagella and vice versa. This suggests very similar mechanisms of torque generation in the two types of motors. In contrast, the N-terminal domains of the FliG proteins apparently cannot interact properly with other flagellar proteins of the heterologous species. Similarly, the E. coli and V. parahaemolyticus FliG proteins did not functionally complement each other (7). Furthermore, a chimeric E. coli-T. maritima FliG protein, but not the full-length T. maritima FliG, restored flagellum production and motility of an E. coli fliG mutant strain (22).
To investigate whether the sodium-translocating components of the V. cholerae flagella can functionally be complemented by the E. coli proton-translocating proteins, a V. cholerae strain deleted in the pomAB, motX, and motY genes was transformed with the E. coli motAB genes. Some, although very small, swarm circles in soft agar plates were observed in this strain, with spontaneous hypermotile variants appearing readily (data not shown). As the C-terminal domain of the FliG protein is involved in torque generation by interacting with the ion channel components, we expected that the V. cholerae-E. coli chimeric FliG fusion protein may better interact with the MotAB proteins. A V. cholerae strain deleted in the four putative stator genes and the fliG gene carrying the E. coli motAB genes and expressing either the full-length V. cholerae or chimeric FliG proteins displayed equally small motility zones. This indicates that the C terminus of the V. cholerae FliG protein can interact efficiently with the E. coli MotAB proteins. Both strains produced spontaneous hypermotile variants, and in most strains the hypermotile phenotype was not linked to the plasmids, i.e., the motAB or fliG gene. It is hard to speculate where these non-plasmid-linked mutations might map. It is possible that these mutations result in better recognition and installation of the foreign MotAB protein by chaperone-like proteins. Alternatively, the mutations might be in systems involved in the generation of electrochemical gradients of protons or sodium ions across the membrane, thus increasing the available energy source for flagellar rotation. However, the increased swarm circles might be a result of improved chemotaxis behavior. As the FliG protein is part of the switch complex that regulates direction of flagellar rotation in response to interaction with CheY (24), perhaps the hybrid motor cannot properly interact with the chemotaxis machinery. Further experiments are required to understand the basis for the increased swarming phenotype in these mutant strains. In summary, we have created a V. cholerae strain that is motile by using a hybrid flagellar motor composed of the V. cholerae flagellar machinery interacting with the E. coli MotAB proteins. This hybrid motor strain may provide a useful tool to help us better understand the processes involved in flagellar assembly and protein interactions required for flagellar function.
To investigate which coupling ion, H+ or Na+, was used for flagellar rotation by the hybrid motor, we used inhibitors such as CCCP and phenamil, an amiloride homolog known to specifically block the sodium-translocating portion of the flagella (4). Together with data from assays using different H+ or Na+ concentrations, we concluded that the hybrid motor strain, like E. coli but unlike V. cholerae, uses PMF as the driving energy source. Thus, the mechanisms of converting electrochemical energy into rotational energy in proton- or sodium-driven flagella seem to be similar and are functionally interchangeable. Furthermore, this suggests that the FliG protein does not directly interact with the ion flux across the membrane or that the sites of interaction can interact with either Na+ or H+. It would be interesting to create a similar but reverse hybrid flagellar motor by introducing the V. cholerae PomAB and MotXY proteins into a motAB deletion E. coli strain to assess their ability to function perhaps after induction of an artificial SMF. It was recently reported that the V. parahaemolyticus motAB genes alone did not restore motility of an E. coli motAB mutant strain (7).
Little is known about the residues involved in the ion selectivity of the sodium-translocating flagellar channel molecules. Recently, the Rhodobacter spheroides MotA protein was found to functionally complement a pomA mutant of V. alginolyticus, thus creating a hybrid motor (1). However, this motor was still using the coupling of sodium ion flux, indicating that the MotA and PomA proteins alone do not dictate the ion specificity. The ion specificity of our hybrid motor has been switched from sodium ions to protons. Apparently, the E. coli MotAB proteins are specialized to translocate protons but not sodium ions even in a V. cholerae host environment that supposedly provides a strong SMF. Even a V. cholerae strain deleted for only the pomAB genes complemented by the E. coli MotAB proteins seemed to use protons rather than sodium ions for flagellum rotation (data not shown), indicating that the presence of the MotXY proteins does not influence the coupling ion used by the MotAB proteins. By introducing the E. coli motA or motB gene alone into our various stator deletion V. cholerae strains, we should be able to address which proteins are involved in ion selectivity.
The hybrid motor strain might allow us to understand the underlying mechanism for the significantly increased speed of sodium-driven flagella compared to proton-driven flagella. The E. coli flagellum is known to rotate at about 15,000 rpm (23), whereas Vibrio flagella have been reported to achieve as high as 100,000 rpm (25). Perhaps the function of the MotXY proteins is to further stabilize the motor in the membrane to allow faster rotation. Alternatively, these two proteins might form an ion channel independent of PomAB, adding to the available energy conversion. However, a V. cholerae pomAB deletion strain complemented with the E. coli motAB genes showed no significant increase in swarm circles or swimming speed compared to the similar strain that has all four stator genes deleted (data not shown). This indicates that the MotXY proteins either do not functionally interact with the E. coli MotAB proteins or are not involved in swimming speed. Further studies on these strains might reveal the underlying mechanism for the difference in speed between the different types of motors.
In E. coli, a strong PMF is generated by respiration under neutral conditions, whereas an alkaline environment results in an opposite ΔpH and a PMF is harder to maintain. Some bacteria, including several Vibrio species, can switch to a sodium cycle of energy, thus enabling the cells to maintain a neutral cytoplasmic pH under alkaline conditions. At neutral pH, an H+/Na+ antiporter converts the PMF generated by respiration into SMF, whereas at alkaline pH an enzyme complex, called NQR (NADH-quinone oxidoreductase), can generate an SMF directly linked to respiration (for reviews, see references 10 and 33). Therefore, motility of V. cholerae is sensitive to the ionophore CCCP, an agent widely used to collapse PMF, at neutral but not alkaline pH. Interestingly, we noticed that the sensitivity of the motility to CCCP was markedly different between the Vibrio hybrid motor strain and the E. coli control strain. Much less CCCP was required to completely prevent motility of the V. cholerae hybrid motor strain compared to the E. coli control under neutral as well as alkaline conditions. One possible explanation for this is that V. cholerae cells may be inherently more sensitive to CCCP, perhaps due to their membrane composition or lack of efflux systems. Alternatively, this difference in CCCP sensitivity might reflect differences in the strength of PMF production between these organisms. Perhaps at neutral pH V. cholerae, but not E. coli, converts a substantial portion of the PMF into SMF. At alkaline pH, E. coli might have a specific mechanism, such as induction of an electrogenic antiporter, for maintaining a PMF that is lacking in V. cholerae, as Vibrio cells usually switch to the sodium cycle of energy under these conditions. Creating hybrid motor strains might provide useful tools to investigate the differences in membrane bioenergetics between organisms.
Motility is an important virulence factor in a variety of pathogenic bacteria and, in some cases, is inversely regulated with other virulence factors (30). Motility in V. cholerae is known to be negatively regulated by the ToxR regulon; conversely, some motility mutants, including a pomB insertion mutant, showed altered expression levels of the main virulence factors (12). Inhibition of the V. cholerae SMF-generating NQR enzyme complex, either by mutation or addition of a specific inhibitor, resulted in increased virulence gene expression by affecting expression of the regulatory protein ToxT (14). It was proposed that the effect of loss of NQR activity on toxT transcription may be indirectly mediated by affecting motility. The sodium influx through the flagellum may somehow be transduced into altered transcription of toxT, possibly by affecting the regulatory proteins TcpP and TcpH (14). The hybrid motor strain presented in this study will help elucidate how changes in membrane sodium energetics and motility affect virulence gene expression in V. cholerae. We can now investigate whether the sodium influx through the flagella is sensed by an as yet uncharacterized mechanism or if the motion of the bacteria or perhaps flagellar rotation speed are signals resulting in changes of gene expression.
ACKNOWLEDGMENTS
We thank David F. Blair for providing plasmids pJZ19 and pSL25 and the E. coli ΔfliG strain DFB225 as well as for many helpful suggestions. We especially thank Eric J. Rubin and John J. Mekalanos for encouragement and many discussions. We thank Ikuro Kawagishi, Igor I. Brown, and A. Brooun for comments on the manuscript and Gillian Chesney for technical assistance. We are grateful to G. Murti for the EM analyses.
This study was supported by Cancer Center Support Grant CA 21765 and ALSAC (American Lebanese Syrian Associated Charities).
REFERENCES
- 1.Asai Y, Kawagishi I, Sockett R E, Homma M. Hybrid motor with H+- and Na+-driven components can rotate Vibrio polar flagella by using sodium ions. J Bacteriol. 1999;181:6332–6338. doi: 10.1128/jb.181.20.6332-6338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai Y, Kojima S, Kato H, Nishioka N, Kawagishi I, Homma M. Putative channel components for the fast-rotating sodium-driven flagellar motor of a marine bacterium. J Bacteriol. 1997;179:5104–5110. doi: 10.1128/jb.179.16.5104-5110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atsumi T, McCarter L, Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature. 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- 4.Atsumi T, Sugiyama S, Cragoe E J, Jr, Imae Y. Specific inhibition of the Na+-driven flagellar motors of alkalophilic Bacillus strains by the amiloride analog phenamil. J Bacteriol. 1990;172:1634–1639. doi: 10.1128/jb.172.3.1634-1639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg H C. Torque generation by the flagellar rotary motor. Biophys J. 1995;68:163–167. [PMC free article] [PubMed] [Google Scholar]
- 6.Blair D F. How bacteria sense and swim. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 7.Boles B R, McCarter L L. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J Bacteriol. 2000;182:1035–1045. doi: 10.1128/jb.182.4.1035-1045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun T F, Poulson S, Gully J B, Empey J C, Van Way S, Putnam A, Blair D F. Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli. J Bacteriol. 1999;181:3542–3551. doi: 10.1128/jb.181.11.3542-3551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeRosier D J. The turn of the screw: the bacterial flagellar motor. Cell. 1998;93:17–20. doi: 10.1016/s0092-8674(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 10.Dimroth P. Primary sodium ion translocating enzymes. Biochim Biophys Acta. 1997;1318:11–51. doi: 10.1016/s0005-2728(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 11.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardel C, Mekalanos J J. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Häse C C, Mekalanos J J. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 1999;96:3183–3187. doi: 10.1073/pnas.96.6.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irikura V M, Kihara M, Yamaguchi S, Sockett H, Macnab R M. Salmonella typhimurium fliG and fliN mutations causing defects in assembly, rotation, and switching of the flagellar motor. J Bacteriol. 1993;175:802–810. doi: 10.1128/jb.175.3.802-810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaques S, Kim Y-K, McCarter L L. Mutations conferring resistance to phenamil and amiloride, inhibitors of sodium-driven motility of Vibrio parahaemolyticus. Proc Natl Acad Sci USA. 1999;96:5740–5745. doi: 10.1073/pnas.96.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawagishi I, Maekawa Y, Atsumi T, Homma M, Imae Y. Isolation of the polar and lateral flagellum-defective mutants in Vibrio alginolyticus and identification of their flagellar driving energy source. J Bacteriol. 1995;177:5158–5160. doi: 10.1128/jb.177.17.5158-5160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima S, Asai Y, Atsumi T, Kawagishi I, Homma M. Na+-driven flagellar motor resistant to phenamil, an amiloride homolog, caused by mutations in putative channel components. J Mol Biol. 1999;285:1537–1547. doi: 10.1006/jmbi.1998.2377. [DOI] [PubMed] [Google Scholar]
- 19.Kojima S, Yamamoto K, Kawagishi I, Homma M. The polar flagellar motor of Vibrio cholerae is driven by a Na+ motive force. J Bacteriol. 1999;181:1927–1930. doi: 10.1128/jb.181.6.1927-1930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd S A, Blair D F. Charged residues of the rotor protein FliG essential for torque generation in the flagellar motor of Escherichia coli. J Mol Biol. 1997;266:733–744. doi: 10.1006/jmbi.1996.0836. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd S A, Tang H, Wang X, Billings S, Blair D F. Torque generation in the flagellar motor of Escherichia coli: evidence of a direct role for FliG but not for FliM or FliN. J Bacteriol. 1996;178:223–231. doi: 10.1128/jb.178.1.223-231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd S A, Whitby F G, Blair D F, Hill C P. Structure of the C-terminal domain of FliG, a component of the rotor in the bacterial flagellar motor. Nature. 1999;400:472–475. doi: 10.1038/22794. [DOI] [PubMed] [Google Scholar]
- 23.Lowe G, Meister M, Berg H C. Rapid rotation of flagellar bundles in swimming bacteria. Nature. 1987;325:637–640. [Google Scholar]
- 24.Macnab R. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–146. [Google Scholar]
- 25.Magariyama Y, Sugiyama S, Muramoto K, Maekawa Y, Kawagishi I, Imae Y. Very fast flagellar rotation. Nature. 1994;371:752. doi: 10.1038/371752b0. [DOI] [PubMed] [Google Scholar]
- 26.McCarter L L. MotX, the channel component of the sodium-type flagellar motor. J Bacteriol. 1994;176:5988–5998. doi: 10.1128/jb.176.19.5988-5998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarter L L. MotY, a component of the sodium-type flagellar motor. J Bacteriol. 1994;176:4219–4225. doi: 10.1128/jb.176.14.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf W W, Jiang W, Daniels L L, Kim S K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allel replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 29.Okunishi I, Kawagishi I, Homma M. Cloning and characterization of motY, a gene coding for a component of the sodium-driven flagellar motor in Vibrio alginolyticus. J Bacteriol. 1996;178:2409–2415. doi: 10.1128/jb.178.8.2409-2415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottemann K M, Miller J F. Roles for motility in bacterial-host interactions. Mol Microbiol. 1997;24:1109–1117. doi: 10.1046/j.1365-2958.1997.4281787.x. [DOI] [PubMed] [Google Scholar]
- 31.Tang H, Braun T F, Blair D F. Motility protein complexes in the bacterial flagellar motor. J Mol Biol. 1996;261:209–221. doi: 10.1006/jmbi.1996.0453. [DOI] [PubMed] [Google Scholar]
- 32.Thomas D R, Morgan D G, DeRosier D J. Rotational symmetry of the C ring and a mechanism for the flagellar rotary motor. Proc Natl Acad Sci USA. 1999;96:10134–10139. doi: 10.1073/pnas.96.18.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unemoto T, Hayashi M. Na+-translocating NADH-quinone reductase of marine and halophilic bacteria. J Bioenerg Biomembr. 1993;25:385–391. doi: 10.1007/BF00762464. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J, Lloyd S A, Blair D F. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc Natl Acad Sci USA. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]