Abstract
The review provides a comprehensive update (previous report: Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2008;119(3):504–32) on clinical diagnostic utility of transcranial magnetic stimulation (TMS) in neurological diseases. Most TMS measures rely on stimulation of motor cortex and recording of motor evoked potentials. Paired-pulse TMS techniques, incorporating conventional amplitude-based and threshold tracking, have established clinical utility in neurodegenerative, movement, episodic (epilepsy, migraines), chronic pain and functional diseases. Cortical hyperexcitability has emerged as a diagnostic aid in amyotrophic lateral sclerosis. Single-pulse TMS measures are of utility in stroke, and myelopathy even in the absence of radiological changes. Short-latency afferent inhibition, related to central cholinergic transmission, is reduced in Alzheimer’s disease. The triple stimulation technique (TST) may enhance diagnostic utility of conventional TMS measures to detect upper motor neuron involvement. The recording of motor evoked potentials can be used to perform functional mapping of the motor cortex or in preoperative assessment of eloquent brain regions before surgical resection of brain tumors. TMS exhibits utility in assessing lumbosacral/cervical nerve root function, especially in demyelinating neuropathies, and may be of utility in localizing the site of facial nerve palsies. TMS measures also have high sensitivity in detecting subclinical corticospinal lesions in multiple sclerosis. Abnormalities in central motor conduction time or TST correlate with motor imspairment and disability in MS. Cerebellar stimulation may detect lesions in the cerebellum or cerebello-dentatothalamo-motor cortical pathways. Combining TMS with electroencephalography, provides a novel method to measure parameters altered in neurological disorders, including cortical excitability, effective connectivity, and response complexity.
Keywords: Motor evoked potential, neurological disorders, short interval intracortical inhibition, transcranial magnetic stimulation
1. Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive technique for stimulating the human brain, first described in the 1980s (Barker et al., 1985). The TMS stimulator passes a large, brief current through a coil, generating a strong time-varying electromagnetic field perpendicular to the transducing coil positioned over the scalp (Siebner et al., 2022). The magnetic field, which penetrates the scalp and skull, and is not attenuated by tissues surrounding the brain, induces an electric field in the underlying brain tissue. When stimulating the primary motor cortex (M1), the induced electric field transsynaptically activates cortical output cells (pyramidal neurons) resulting in descending corticospinal volleys, which are reflected in a motor evoked potential (MEP) (Rossini et al., 2015). Cortical TMS effects are dependent on whether a focal (figure of eight) or non-focal (circular) coils are used, pulse waveform (monophasic vs biphasic), number of pulses (e.g. paired-pulse), strength of stimulation (subthreshold vs threshold) and direction of induced cortical currents, which result in activation of distinct neuronal elements (Corp et al., 2021, Di Lazzaro et al., 2002b, Di Lazzaro et al., 1999b, Di Lazzaro et al., 2012, Di Lazzaro and Rothwell, 2014, Rossini et al., 1994, Rossini et al., 2015, Rossini et al., 2019, Siebner et al., 2022, Sommer et al., 2018).
The clinical diagnostic utility of TMS techniques have been reported across and expanding range of neurological diseases, including neurodegenerative, inflammatory, or lesional brain or spinal disorders, as well as clinical utility in investigating central pathophysiology in chronic pain, movement, episodic (epilepsy), and structural brain (stroke) disorders (Chen et al., 2008, Di Lazzaro et al., 2021, Rossi et al., 2021, Smith and Stinear, 2016, Vucic et al., 2013b). Since the last IFCN committee report (Chen et al., 2008), there have been significant advances in clinical applications of TMS in neurological diseases, leading to greater understanding of pathophysiology and development of novel diagnostic approaches. Threshold tracking TMS has emerged as a potential diagnostic technique for amyotrophic lateral sclerosis (Menon et al., 2015), while single and paired-pulse TMS (constant stimulus) techniques, as well as TMS-EEG, have yielded novel diagnostic and prognostic cortical biomarkers (Corp et al., 2021, de Goede et al., 2016, Di Lazzaro et al., 2021, Keser et al., 2022). TMS mapping of motor cortex representation based on image-guided navigated procedure of MEP recording is now an essential technique in the preoperative evaluation of brain tumor surgery to improve postoperative functional outcome. Consequently, the review will discuss advances in clinical utility of different TMS techniques, including single, paired, and triple pulse TMS, as well as TMS-EEG. The utility of repetitive TMS and other plasticity inducing techniques (such as paired-associative stimulation) will not be discussed and the reader is directed to dedicated reviews on the topic (Antal et al., 2022, Di Lazzaro et al., 2021, Gogulski et al., 2022, Harmelech et al., 2023, Lefaucheur et al., 2014, Motolese et al., 2022, Somaa et al., 2022, van den Bos et al., 2022). The first section will provide an update on specific TMS techniques, including threshold tracking TMS and TMS-EEG. The second section will discuss the application of the TMS techniques in neurological disease with an emphasis on clinical diagnostic utility.
2. TMS techniques and outcome measures
2.1. Measures of corticospinal projection
Motor threshold (MT) has been traditionally defined as the lowest TMS stimulation intensity capable of eliciting a small motor evoked potential (MEP). Rest MT (RMT) typically refers to the lowest intensity required to elicit an MEP amplitude ≥50μV with target muscle at rest in at least 5 of 10 trials (Rossini et al., 1994, Rossini et al., 2015, Rothwell et al., 1999), while active MT (AMT) is defined as lowest intensity required to elicit an MEP amplitude ≥200 μV during slight isometric tonic muscle contraction. RMT is always higher than AMT. With the threshold tracking method, RMT is defined as stimulus intensity required to generate and maintain an MEP amplitude of 0.2 mV (±20%), a target that lies in the middle of the steepest portion of the TMS input-output (IO) curve (Fisher et al., 2002, Vucic et al., 2006). Adaptive methodology, which uses a S-shaped metric function to model the probabilistic nature of MT and the relationship between TMS intensity and MEP amplitude (Awiszus, 2003, Rossini et al., 2015), is an alternative method of measuring MT. The mean difference between the adaptive and “constant stimulus” traditional methods was ~ 2.3% of maximal stimulator output using AMT, being higher in the former (Silbert et al., 2013).
MT indicates the excitability of a central core of neurons that represent the target muscle in the primary motor cortex (M1) and excitability of brainstem or spinal cord motor neurons. MT is lower in intrinsic hand muscles compared to proximal arm, truncal or lower limb muscles (Chen et al., 1998), reflecting difference in the strength of corticospinal projections. Voltage gated sodium channel blockers, such as phenytoin and carbamazepine, which reduce membrane excitability, increase MT (Chen R. et al., 1997, Ziemann et al., 1996a). Motor thresholds are decreased after administration of ketamine, an NMDA receptor antagonist, that simultaneously activates glutamatergic neurotransmission at AMPA and kainite non-NMDA receptors (Di Lazzaro et al., 2003). These pharmacological findings suggest that MT reflects membrane excitability of corticospinal neurons and short-lasting glutamatergic AMPA transmission. The lowest threshold for the hand motor hotspot with a figure-of-eight coil is obtained by placing the handle about 45 degrees to the sagittal line to induce posterior-anterior directed currents in the brain. For the leg motor area, the coil is placed with the handle at 90 degrees to the sagittal line with the center of coil close to Cz (Groppa et al., 2012). Motor thresholds are higher in older adults (Bashir et al., 2014), but comparable between male and females and between dominant and non-dominant limbs (Livingston et al., 2010).
2.1.2. Input-output curve, MEP amplitude, and MEP mapping
The magnetic input-output (IO) curve and MEP amplitude assess neurons that are less excitable or spatially distant from the center of target muscle representation in the M1 (Chen, 2000, Hallett et al., 1999). The gradient of the sigmoidal IO curve is determined by the degree of activation of corticospinal neurons as well as the strength of corticospinal projections onto the target muscle. Muscles with lower MT, such as intrinsic hand muscles, exhibit steeper IO curves (Chen et al., 1998), as do younger adults with no gender effects (Pitcher et al., 2003). IO curve gradients are steeper in the non-dominant compared to the dominant hemisphere (Daligadu et al., 2013), suggesting that the non-dominant hemisphere may have a higher level of excitation or a lower level of inhibition. The slope of the IO curve is increased by drugs that increase adrenergic transmission and decreased by sodium and calcium channel blockers as well as agents that enhance GABAergic effects (Ziemann et al., 2015). It should be stressed that the MEP amplitude is significantly smaller than the maximal compound muscle action potential amplitude (Rosler et al., 2002). This is related to desynchronization of descending corticospinal volleys resulting in phase cancellation and asynchronous recruitment of spinal or bulbar motor neurons.
Marked trial-to-trial variability of MEP amplitude with constant TMS intensity is a well-known phenomenon (Kiers et al., 1993). The physiological mechanisms underlying MEP variability include: (i) fluctuation of neuronal excitability at cortical and spinal cord levels (Rossini et al., 2015), (ii) timing of TMS stimulus application in relation to the peaks or troughs of specific cortical oscillatory states (Metsomaa et al., 2021), and (iii) activation of target muscle (Darling et al., 2006). Specifically, TMS delivered during the trough and rising phase of the rhythm generates larger MEPs, while TMS at peak and the falling phase of μ rhythm elicits smaller MEPs (Wischnewski et al., 2022, Zrenner et al., 2018). MEP variability may be reduced, and amplitude increased when TMS is triggered at the optimal phase of individualized oscillation (Torrecillos et al., 2020). The MEP variability prompted β the development of the threshold tracking technique which relies on TMS intensity rather than MEP amplitude as an outcome measure (Vucic et al., 2006).
TMS mapping can be used for probing cortical motor representation and enabling delineation of somatotopy of different muscle groups. Although different mapping protocols have been utilized, these are often used to locate the center of gravity (COG) (Wassermann et al., 1992). The most basic method includes applying a 1 cm grid on the scalp and stimulating each point on the grid with TMS intensity at 110~120% RMT [landmark-guided mapping] (Sondergaard et al., 2021a). The mapping procedure starts from the selected muscle motor hotspot and moves in either anterior-posterior or medial-lateral direction at each marking on the grid until no MEP can be obtained, indicating the edge of the map. The coil is subsequently moved to identify borders of the map. Usually, 10–20 trials per site are recorded (Classen et al., 1998). Although more sampling trials per site provide more precise COG measurement, it is more time consuming (Classen et al., 1998). The COG is calculated from the amplitude weighted average of the MEP amplitude at each stimulation site, or the MEP amplitude at a site can be presented as the ratio to the averaged MEP size of the whole mapping area (Ngomo et al., 2012).
At present, conventional cortical mapping methods based on anatomical landmarks are outdated, due to the development of image-guided navigation tools. Navigation is based on a frameless stereotaxic system dedicated to TMS, co-registration of the coil positioning on the scalp and individual brain imaging (MRI or fMRI) of the subject (Lefaucheur, 2010) This is the best way to ensure the accuracy of coil placement and the reliability and repeatability of cortical mapping with reduced variability between sessions (Gugino et al., 2001). Navigation systems have the advantage of providing real-time feedback and demonstrate the location of the sites of cortical stimulation producing MEPs relative to classical anatomical structures, such as the motor hand knob, central sulcus or other gyral features (Jonker et al., 2019). Presurgical navigated TMS mapping procedures are now largely used in clinical practice to delineate eloquent cortical regions and preserve motor or language functions from deleterious lesions secondary to brain tumor resection or epilepsy surgery (Lefaucheur and Picht, 2016). Robotic-assisted TMS may lead to further improve coil handling and mapping procedure accuracy in combination with navigation (Ginhoux et al., 2013, Harquel et al., 2016).
2.1.3. Central motor conduction time
Central motor conduction time (CMCT) includes the excitation time of motor cortical neurons, conduction via the corticospinal tract and time to excite spinal motor neurons to threshold. CMCT is measured as the difference between MEP latency and spinal motor neuron latency to the target muscle, known as the peripheral motor conduction time (PMCT). The PMCT can be estimated using the F-wave method as reflected by the following formula (F+M-1)/2, where F represents the shortest F-wave latency, M is the distal motor latency and 1 ms represents the turnaround time for spinal motor neurons activated antidromically (Mills, 1999). It has also been suggested that the longest F-wave latency may be used (Olivier et al., 2002). Alternatively, PMCT may be estimated by subtracting the MEP or compounding muscle action potential onset latencies, induced by magnetic or electrical stimulation respectively over the vertebral columns, from the cortical MEP latency (Mills and Murray, 1986). The latter method excites the spinal nerves at the spinal foramen and has the advantage of being recordable form most muscle. As for the latter method, CMCT may be overestimated, especially when recoding from lower limb muscles, since the conduction time in proximal nerve root segment between spinal cord and exit foramen is included. To overcome this overestimation, we should use cortico-conus conduction time (CCCT) for leg muscles (Matsumoto et al., 2010a).
To obtain the shortest CMCT, the target muscle should be activated at ~ 10% to 20% of maximum background force (Mills, 1999). The active MEP latency is 1.5-to-2.5 ms shorter than rest MEP latency (Mano et al., 1992), termed “latency jump”, and is more prominent in children (Caramia et al., 1993). It is recommended to superimpose at least five responses and measure the shortest latency. Contraction of homologous contralateral muscles is an option for patients unable to produce adequate target muscle contraction (Mariorenzi et al., 1991).
Age is weakly correlated with CMCT in adults (Claus, 1990, Mano et al., 1992, Matsumoto et al., 2012, Mills and Nithi, 1997b). Immaturity of the corticospinal system, as in preterm and term babies, results in longer CMCT (Eyre, 2007). When measured from the lower limbs, CMCT correlates with height, although this correlation is not evident in upper limb CMCT (Matsumoto et al., 2012, Rossini et al., 1987, Wochnik-Dyjas et al., 1997). Additionally, upper limb CMCT is not influenced by gender or hand dominance, and there are no significant side-to-side differences (Livingston et al., 2010, Toleikis et al., 1991). In contrast, lower limb CMCT is marginally shorter in women, even allowing for differences in height (Toleikis et al., 1991).
2.1.4. Cortical Silent period
The cortical silent period (CSP) refers to electrical silence of background electromyography (EMG) activity in a contracting muscle following suprathreshold TMS of M1 and varies from 50-to-300 ms (Cantello et al., 1992). The CSP duration increases with stimulation intensity, but is not related to size of the preceding MEP response (Triggs et al., 1992) or strength of target muscle contraction (Inghilleri et al., 1993, Kimiskidis et al., 2005). Low levels of muscle contraction are suggested to avoid muscle fatigue that may inadvertently prolong the CSP duration (Hunter et al., 2006). The CSP duration is longer with anterior-to-posterior compared to posterior-to-anterior directed currents (Orth and Rothwell, 2004). Moreover, the CSP can be elicited with subthreshold TMS intensity without a preceding MEP (Trompetto et al., 2001), suggesting that CSP is not directly related to MEP generation.
The CSP can be recorded in different muscles such as lower limb (Ziemann et al., 1993), facial (Werhahn et al., 1995), diaphragm (Lefaucheur and Lofaso, 2002) and sphincter muscles (Lefaucheur, 2005), although the duration is longest when recorded from intrinsic hand muscles. The first 50ms of CSP involves spinal inhibitory circuits (Fuhr et al., 1991, Pierrot-Deseilligny and Burke, 2012, Rossini et al., 2015), while the later parts of the CSP are of cortical origin mediated by GABAergic neurotransmission acting via GABAB receptors (Classen and Benecke, 1995, Stetkarova and Kofler, 2013). The non-dominant hand exhibits longer CSP duration than the dominant hand, suggesting that circuits underlying CSP generation are less excitable in the dominant hemisphere (Priori et al., 1999). Although most of studies revealed reduced CSP in older adults (Davidson and Tremblay, 2013, Oliviero et al., 2006, Sale and Semmler, 2005), some studies reported a comparable CSP duration between young or older adults (Fujiyama et al., 2012, Hunter et al., 2008). CSP duration is not affected by gender (Shibuya et al., 2016a).
Ipsilateral inhibition (ipsilateral silent period, iSP) induced by motor cortex stimulation can be measured by interruption of ongoing voluntary EMG activity in muscles ipsilateral to cortical TMS (Chen et al., 2008). The iSP reflects transcallosal inhibition (Meyer et al., 1995), although non-callosal pathways caudal to the corpus callosum may also contribute (Compta et al., 2006). The iSP usually begins 30~40 ms after a TMS and lasts for 20-to-25 ms (Meyer et al., 1995). Although iSP duration could be a simple measure of the iSP response, the recommended measurement is to normalize the area of the rectified trace between onset and offset of the iSP to the pre-stimulus mean baseline EMG level (Kuo et al., 2017). To attain the largest iSP response, at least 60% of maximal TMS output may be required (Meyer et al., 1995). To avoid muscle fatigue, its recommend that participants either sustain a low-level contraction (15–20% maximum voluntary contraction) for the entire duration of the trial, or perform short, near-maximal contraction bursts with standard inter-trial rest intervals between each subsequent stimuli (Hupfeld et al., 2020). The latter option may function better in older populations who are more susceptible to muscle fatigue. The iSP onset, end latency and transcallosal time, as well as area, is increased in older adults (Davidson and Tremblay, 2013, Petitjean and Ko, 2013), suggesting that transcallosal inhibition declines with age. In contrast, the extent of muscle contraction, direction of TMS induced current, or limb dominance do not appreciably affect the iSP (Chen et al., 2003, Davidson and Tremblay, 2013, Hunter et al., 2006, Kuo et al., 2017).
2.2. Measures of cortical inhibition and facilitation
2.2.1. Short interval intracortical inhibition
SICI was first described in 1993 and is the most frequently used paired-pulse TMS paradigm to evaluate motor cortex excitability (Kujirai et al., 1993). Primary motor cortex (M1) stimulation with a subthreshold conditioning stimulus (CS) followed by suprathreshold test stimulus (TS) at inter-stimulus intervals (ISI) of 1-to-6 ms decreases MEP amplitude compared to TS alone, termed the “constant stimulus” method (Kujirai et al., 1993).
Subsequently, a threshold tracking paired-pulse TMS technique was developed, whereby a fixed MEP amplitude (0.2 mV±20%) was tracked by a test stimulus (TS), with ISIs increased in a sequential ascending order (Vucic et al., 2006). SICI is heralded by a greater conditioned-test stimulus intensity required to generate and maintain a target MEP response, developing between ISIs of 1-to-7 ms [Figure 1] (Awiszus et al., 1999, Fisher et al., 2002, Vucic et al., 2006). Two maximum phases of inhibition have been described, occurring at ISIs of about 1 and 2.5-to-3 ms (Fisher et al., 2002, Hanajima et al., 2003, Roshan et al., 2003, Vucic et al., 2006). The inter-session reliability and reproducibility of mean SICI (between ISIs 1-to-7 ms), as reflected by a low intraclass correlation coefficient (ICC), was established (Matamala et al., 2018), suggesting a potential clinical diagnostic utility. Recently, a threshold tracking TMS paradigm was developed with ISIs delivered in a pseudo-random fashion, with 10 stimuli at each ISI level (Tankisi et al., 2021), and was shown to exhibit comparable reliability and reproducibility as the “constant stimulus” method (Nielsen et al., 2021).
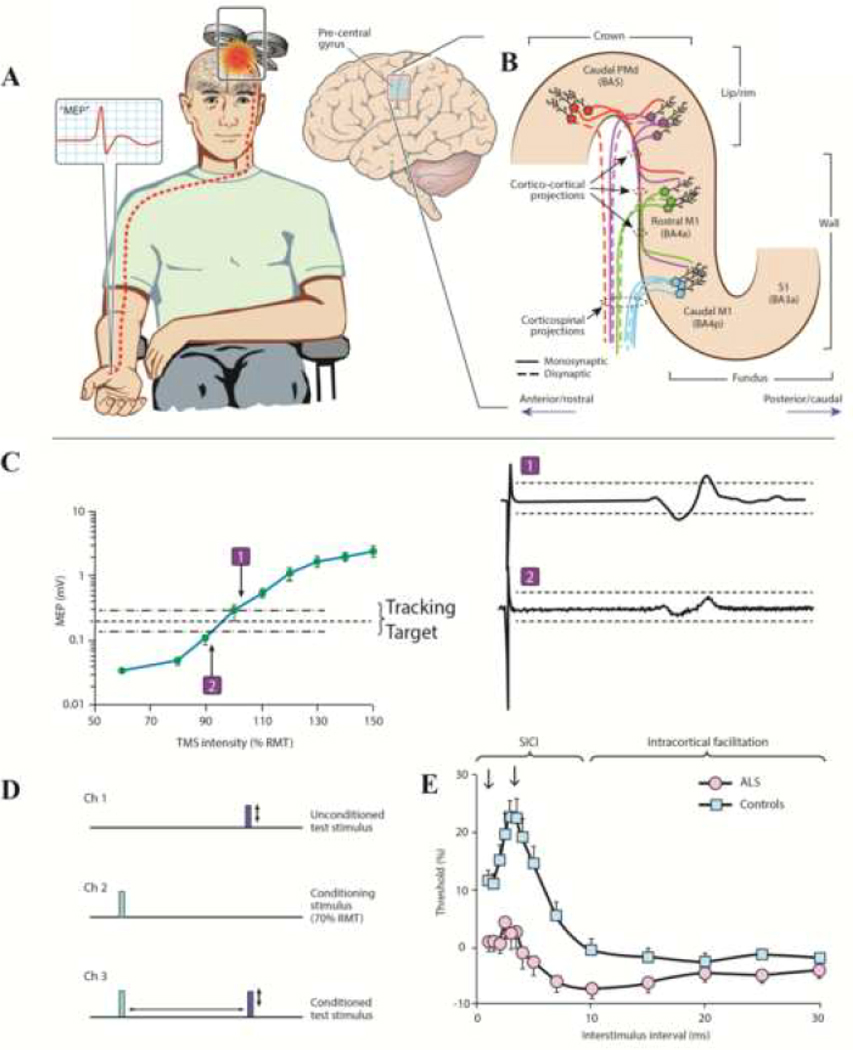
Figure 1: Principles of single and paired-pulse TMS.
(A) Transcranial magnetic stimulation using a figure of eight coil and applied over the primary motor cortex (M1), elicits a motor evoked potential (MEP, red potential in inset) from a target muscle. (B) Candidate descending corticomotoneuronal pathways from the precentral gyrus that contribute to the MEP response. Direct neuronal activation most likely occurs in the lip/rim regions of the motor hand knob. Activation spreads to the rostral and caudal parts of the M1, via cortico-cortical synaptic transmission, potentially contributing to indirect waves (I-waves). There is a greater preponderance of fast-conducting monosynaptic cortico-motoneuronal neurons in the caudal M1 (BA4p) compared to the rostral M1 (BA4a) is highlighted. The exact transition between rostral M1 and caudal dorsal premotor cortex (PMd) in the lip/rim region of the gyrus is gradual and varies across subjects. Additional corticospinal pathways may be activated by TMS via excitation of postcentral primary somatosensory cortex (S1) and its cortico-cortical projections to rostral/caudal M1. (C) For threshold tracking TMS, a target of 0.2 mV (±20%) is selected which lies in the steepest portion of the stimulus response curve. As such, if the MEP response is larger than the tracking target (potential-1) the subsequent stimulus intensity is reduced, while if the MEP response is smaller than the tracking target (potential-2), the subsequent stimulus intensity is reduced. (D) The paired pulse paradigm is illustrated. Channel 1 records an unconditioned test stimulus, defined as TMS intensity required to generate and maintain the tracking target, which signifies the resting motor threshold (RMT) when using the threshold tracking technique. Channel 2 monitors the subthreshold conditioning stimulus (does not generate MEP) and channel 3 records the conditioned-test stimulus at interstimulus intervals of 1–30 ms. (E) When utilizing the threshold tracking TMS technique, short interval intracortical inhibition (SICI) is represented as increased conditioned-test stimulus intensity required to generate and maintain the tracking target, developed between 1–7 ms. Intracortical facilitation is represented as reduced conditioned-test stimulus intensity. In amyotrophic lateral sclerosis (ALS) patients SICI is reduced and ICF increased, signifying cortical hyperexcitability.
A cortical origin of SICI was suggested by epidural recordings, whereby the subthreshold CS suppressed recruitment of late I waves (especially I3 waves) elicited by the TS (Di Lazzaro and Rothwell, 2014). Pharmacological studies have suggested that inhibitory interneuronal circuits acting, via GABAA receptors, mediate the second phase of SICI at ISIs 2.5-to-3 ms (Di Lazzaro et al., 2007a, Ziemann et al., 1996a, Ziemann et al., 2015). Increased axonal refractoriness or synaptic mechanisms have been proposed as underlying physiological mechanisms mediating SICI at ISI 1ms (Chen, 2004, Fisher et al., 2002, Hanajima et al., 2003, Vucic et al., 2011a, Vucic et al., 2009), as well as shunting inhibition by opening channels in proximal dendrites targeted by incoming afferents (Paulus and Rothwell, 2016).
SICI is a general inhibitory effect which is evident in different muscles, including proximal arm (Abbruzzese et al., 1999), facial (Paradiso et al., 2005), lower limb (Chen et al., 1998, Menon et al., 2018) muscles, as well as the trapezius (Menon et al., 2018), diaphragm (Demoule et al., 2003) and sphincter muscles (Lefaucheur, 2005). While SICI appears not be to be influenced by handedness or hemispheric laterality (Cahn et al., 2003, Dharmadasa et al., 2019, Menon et al., 2019), some have reported a reduction of SICI in the dominant hemisphere in right handed subjects (Hammond et al., 2004, Ilic et al., 2004). Given that a recent study suggested that brain derived neurotrophic factor (BDNF) polymorphism might influence interhemispheric balance of SICI, the discrepancies across different studies may be explained by variations of BDNF polymorphism in the studied populations (Dubbioso et al., 2022b). Additionally, male and female subjects exhibit comparable SICI values (Cahn et al., 2003, Hermsen et al., 2016).
SICI critically depends on the CS and TS intensities, being absent when the TS intensity is < 110% RMT (Garry and Thomson, 2009) and increases with higher TS intensities (Daskalakis et al., 2004, Roshan et al., 2003, Sanger et al., 2001). At low CS intensities, SICI is reduced or absent and increases as the CS intensity is increased, but then diminishes at even higher CS intensities and becoming facilitatory when CS is close to RMT, resulting in a U-shaped curve response (Chen et al., 1998, Peurala et al., 2008, Vucic et al., 2009). SICI may be reduced immediately after contraction of target muscle and is influenced by coil type (Dharmadasa et al., 2019, Menon et al., 2018, Van den Bos et al., 2018, Vucic et al., 2011a). Preferential recruitment of interneuronal circuits generating I3 waves was correlated with higher SICI values (Hanajima et al., 1998, Higashihara et al., 2020). At a physiological level, SICI may serve to focus output from motor cortex to enable selective activation of specific muscles and prevent unwanted activation of other muscles (Rosenkranz and Rothwell, 2004, Stinear and Byblow, 2003, Zoghi et al., 2003).
2.2.2. Intracortical facilitation
Intracortical facilitation (ICF) is elicited with a similar paradigm as SICI, except the ISI is between 8–30 ms, with the most prominent facilitation evident from 10–15 ms (Kujirai et al., 1993, Vucic et al., 2006). ICF is not a rebound disinhibition of SICI as its threshold is slightly higher (Chen et al., 1998, Ziemann et al., 1996b). Since ICF can be produced by subthreshold CS that does not evoke descending corticospinal volleys, ICF likely occurs at a cortical level. Epidural spinal recordings did not show changes in amplitude or number of D-wave or I-waves with ICF (Di Lazzaro et al., 2006b), suggesting that ICF may be either mediated by cortical circuits other than those generating I-waves, or yet to be discovered physiological mechanisms operating at a spinal level (Chen et al., 2008, Di Lazzaro and Rothwell, 2014). Administration of NMDA receptor antagonist dextromethorphan reduced ICF (Ziemann et al., 1998a), while chronic administration of the serotonin re-uptake inhibitor paroxetine enhanced ICF (Gerdelat-Mas et al., 2005), suggesting the involvement of glutamatergic and serotonergic neurotransmission in generating ICF. Sodium channel blockers, age and gender did not appreciably impact ICF (Bhandari et al., 2016, Chen R. et al., 1997, Shibuya et al., 2016a). While one study reported greater ICF in the dominant hemisphere (Civardi et al., 2000), others have not reported hemispheric asymmetry (Lefaucheur et al., 2008).
2.2.3. Short interval intracortical facilitation
Short interval intracortical facilitation (SICF) is recorded by using a paired-pulse paradigm whereby a suprathreshold first stimulus (S1) and subthreshold or threshold second stimulus (S2) is delivered at short ISIs leading to an increase in the conditioning-test MEP amplitude (Chen and Garg, 2000, Ziemann et al., 1998b). Alternatively, both S1 and S2 stimuli may be set to motor thresholds also resulting in conditioning-test MEP facilitation (Tokimura et al., 1996). Using this constant stimulus method, three SICF facilitation peaks have been identified at discrete ISIs: 1.1–1.5, 2.3–3.0, and 4.1–4.5 ms (labeled as SICF-1, SICF-2 and SICF-3). Recently, a threshold tracking paradigm was developed whereby S1 and S2 were set to threshold, and SICF was reflected by reduction in test stimulus intensity required to generate and maintain a target MEP response of 0.2 mV (±20%) (Van den Bos et al., 2018). As with the constant stimulus method, SICF developed between ISIs of 1–5 ms, with two peaks evident at ISI 1.5 and 3 ms. Voluntary target muscle contraction, handedness and age do not affect SICF (Bäumer et al., 2007, Chen et al., 2008, Ilic et al., 2004, Van den Bos et al., 2018), although assessment with a figure-of-eight coil compared to circular coil and lower tracking targets (0.2 vs 1.0 mV) increase SICF (Van den Bos et al., 2018).
The precise physiological mechanisms mediating SICF remain to be fully elucidated.
It has been proposed that facilitatory interactions of I-waves at a motor cortical level form the basis of SICF (Ziemann et al., 2015, Ziemann et al., 1998c). TMS modelling studies of induced I-waves suggested that the suprathreshold S1 stimulus leads to subliminal depolarization of a subpopulation of cortical neurons (Rusu et al., 2014). A subsequent subthreshold stimulus (S2) applied at short ISIs causes the subliminally depolarized neurons to reach threshold, thereby generating an MEP potential and resulting in facilitation (Hanajima et al., 2002). Support for a cortical origin was suggested by the observed periodicity of SICF peaks, which occur at 1.5 ms (~660 Hz), being consistent with I-wave frequency (Amassian et al., 1987). Pharmaco-TMS studies have provided additional support for a cortical origin, documenting a modulating effect on SICF by a variety of neurotransmitter systems (Ilic et al., 2003, Ilic et al., 2002, Korchounov and Ziemann, 2011, Ziemann et al., 2015), all of which are involved in the neuronal circuitry underlying I-wave generation (Di Lazzaro and Ziemann, 2013). The facilitating effects of SICI on SICF (Wagle-Shukla et al., 2009) provided additional evidence for importance of cortical neuronal circuitry in SICF via disynaptic inhibition. TMS intensities and paired-pulse intervals for SICF overlap with SICI, and recruitment of SICF may explain the reduction of SICI at high CS intensities (Ni et al., 2013, Peurala et al., 2008). Therefore, it has been suggested the CS intensity for SICI be kept below AMT and the ISI occur at the trough of SICF to minimize the influence of SICF on SICI (Rossini et al., 2015).
2.2.4. Long interval intracortical inhibition
Long-interval intracortical inhibition (LICI) is typically elicited by a suprathreshold CS followed by a suprathreshold TS at ISI from 50 to 200 ms (Valls-Sole et al., 1992, Vucic et al., 2006, Wassermann et al., 1996). Evidence that LICI occurs at a cortical level includes; (i) finding of no change in spinal excitability at more than 50 ms after suprathreshold TMS (Fuhr et al., 1991), (ii) absence of LICI with paired transcranial electrical stimulation (Inghilleri et al., 1993), and (iii) epidural recordings disclosing marked reduction of descending corticospinal test volleys (Chen et al., 1999b, Di Lazzaro et al., 2002a, Nakamura et al., 1997). With reduction of CS intensity to subthreshold levels, facilitation may be observed (Chen et al., 1998, Vallence et al., 2014). LICI appears to be mediated by GABAB post-synaptic receptors (McDonnell et al., 2006) and may be enhanced by GABAB receptor agonists (baclofen), GABA analogs (vigabatrin) and GABA uptake inhibitor (tiagabine) (McDonnell et al., 2006, Pierantozzi et al., 2004a, Ziemann et al., 2015). There is evidence that LICI at ISI 100 ms is more prominent in the dominant hemisphere in younger adults (Hammond and Garvey, 2006) and this asymmetry decreases with age (Vallence et al., 2017). LICI is reduced with increasing TS intensity (Sanger et al., 2001) and is not substantially affected by target muscle contraction (Chen R. et al., 1997). Late cortical disinhibition following LICI has been described, which represent a period of late facilitation after LICI (Cash et al., 2010, Caux-Dedeystère et al., 2014). Using a triple pulse stimulation paradigm, LICI inhibits SICI, likely through the pre-synaptic GABAB receptor mediated inhibition (Ni et al., 2011).
2.2.5. Interhemispheric inhibition and interhemispheric facilitation
Interhemispheric inhibition (IHI) is typically recorded by delivering a suprathreshold CS to M1 in one hemisphere followed by a suprathreshold TS to the opposite M1 (Ferbert et al., 1992, van den Bos et al., 2021). Two types of IHI have been described: short-latency IHI (SIHI) between ISI 6-to-11 ms (maximum at ISI ~9.6 ms), and long-latency IHI (LIHI) between ISIs 20-to-50 ms (Chen et al., 2003, Ni et al., 2020). SIHI and LIHI are more prominent in distal than proximal muscles (Perez and Cohen, 2009, Rossini et al., 2015). Higher CS intensities elicit IHIs at longer ISIs [> 50 ms] (Ferbert et al., 1992). Cervical epidural recordings showed that IHI occurs at a cortical level since it was associated with reduction of later I-waves (particularly I3) (Di Lazzaro et al., 1999c). At a physiological level, CS exerts an inhibitory effect via activation of excitatory transcallosal fibers that activate GABAergic inhibitory circuits in the opposite motor cortex and thereby lead to inhibition of the MEP response evoked by a TS (Irlbacher et al., 2007, Reis et al., 2008).
SIHI can be elicited from the premotor cortex with subthreshold test stimuli that are medially directed (Mochizuki et al., 2004) or suprathreshold test stimuli with anteriorly directed currents (Bäumer et al., 2007). LIHI can be elicited by conditioning stimulation of the contralateral somatosensory cortex (Iwata et al., 2016, Ni et al., 2009). Handedness or hemispherical dominance may affect IHI, with stronger inhibition when the conditioning stimulation is applied over the dominant hemisphere (Bäumer et al., 2007, Netz et al., 1995). This, however, is not a universal finding (De Gennaro et al., 2004), and could be related to use of different stimulation coils and induced current directions.
Interhemispheric facilitation (IHF) may be elicited by applying the CS over the M1 (at ISIs 3-to-6 ms) or premotor cortex (ISIs 6-to-8 ms) ipsilateral to the target muscle, followed by a TS delivered to the contralateral M1 (Bäumer et al., 2006, Hanajima et al., 2001). IHF can be elicited either during active muscle contraction or at rest, with CS set to subthreshold (target muscle at rest) or suprathreshold (target muscle is active) (Bäumer et al., 2006, Hanajima et al., 2001) intensities. The magnitude and latency distribution of IHF correlates with IHI (Ni et al., 2020). Magnetic (lateral-medial direction) and anodal electrical stimulation may generate IHF, suggesting that activation of corticospinal neurons and subsequent transmission through the corpus callosum is a likely mechanism (Hanajima et al., 2001). The facilitatory effects are also related to I3 wave recruitment, suggesting a role for interneuronal circuits (Hanajima et al., 2001). Long-latency IHF (at ISIs >80 ms) has also been reported with suprathreshold CS delivered to M1 or subthreshold CS to dorsal premotor cortex or supplementary motor area (Fiori et al., 2017).
2.2.6. Short latency afferent inhibition
Afferent input from cutaneous or mixed nerves innervating the hand may decrease cortical excitability if delivered prior to TMS applied over the contralateral motor cortex, termed short-latency afferent inhibition (SAI). MEP amplitude is reduced when electrical stimulation of the median nerve at the wrist is delivered 18-to-28 ms before a TMS stimulus (Ni et al., 2011, Tokimura et al., 2000). The ISI is slightly longer for digital cutaneous nerve stimulation to account for conduction time from the digit to wrist. For example, MEP amplitude of intrinsic hand muscles is reduced when preceded by digital nerve stimulation of the index finger 20–50 ms before TMS (Tamburin et al., 2005). SAI in the abductor digit minimi muscle can be elicited when stimulation from the 5th digit preceded TMS by 20 to 45 ms (Tamburin et al., 2001). Mixed nerve stimulation activates muscle afferents, joint and cutaneous mechanoreceptors, whereas digital stimulation only activated cutaneous fibers (Turco et al., 2018b).
Maximum SAI occurs at ISIs of ~N20 latency plus 2 ms, or ~20–22ms for median nerve stimulation at wrist and ~25ms for digit stimulation (Bikmullina et al., 2009, Rossini et al., 2015). SAI reaches maximal level at a stimulating intensity that recruits all the sensory afferents as reflected by the sensory nerve action potential amplitude (3 times the sensory perception threshold for digital nerve stimulation, or 1.2 times motor threshold for mixed nerve stimulation) (Bailey et al., 2016, Turco et al., 2018b). SAI is mediated by cortical mechanisms since epidural recordings disclosed suppression of I2 and I3 waves (Tokimura et al., 1996), and appears unrelated to alterations in spinal cord excitability (Delwaide and Olivier, 1990). Importantly, anterior-posterior directed currents leads to less SAI than posterior-anterior directed TS (Ni et al., 2010), suggesting that specific interneuronal circuits generating later I-waves exhibit different sensitivity to SAI. Mixed nerve SAI is reduced during movement or just before movement begins (Asmussen et al., 2013), indicating a modulating effect of motor cortex on afferent inputs. Additionally, SAI is enhanced by cholinergic transmission (Di Lazzaro et al., 2005, Di Lazzaro et al., 2000, Fujiki et al., 2006) and reduced by GABAergic transmission (Di Lazzaro et al., 2007a, Teo et al., 2009). Higher test MEP amplitude is associated with lower SAI (Ni et al., 2011), although this is not a universal finding (Toepp et al., 2021). Intersession test-retest reliability is high, and SAI is not affected by age, gender, or time of day. However, some normal subjects still showed fluctuation between inhibitory and facilitatory responses (Toepp et al., 2021).
2.2.7. Long latency afferent inhibition
When a peripheral nerve afferent stimulation is applied ~200 ms before a contralateral TMS to the motor cortex, the MEP amplitude is reduced and is termed long-latency afferent inhibition (LAI) (Chen et al., 1999a). Peripheral nerve stimulation may be from a mixed or pure sensory nerve. The response is typically recorded form an intrinsic hand muscle. With cutaneous nerve stimulation (typically digit 3), the ISI range of LAI is ~200 to 600 ms when recording from the abductor pollicis brevis (Chen et al., 1999a). Mixed and cutaneous nerve stimulations lead to LAIs of similar magnitude (Abbruzzese et al., 2001, Turco et al., 2017). The stimulation intensity required to achieve maximal LAI magnitude is ~ 50% of maximum sensory nerve action potential amplitude, representing an intensity of two times sensory perception threshold for digital nerves or motor threshold for mixed nerves. Given the long interval between peripheral stimulation and a subsequent TMS pulse, the afferent input may be relayed through the basal ganglia-thalamocortical loop to the contralateral primary somatosensory cortex, posterior parietal cortex and secondary somatosensory cortex before arriving at the motor cortex (Allison et al., 1989, Kawamura et al., 1996, Sailer et al., 2003, Turco et al., 2017). There is evidence that LAI is modulated by GABAA receptor mediated circuits (Turco et al., 2018a). Similar to SAI, age and gender does not affect LAI (Toepp et al., 2021).
2.2.8. Cerebellar inhibition
The modulatory effects of cerebellar stimulation on the contralateral motor, termed cerebellum-to-motor cortex inhibition (CBI), was first studied by high-voltage electrical stimulation (Groiss and Ugawa, 2013, Ugawa et al., 1991a) and later by double cone magnetic stimulation (Fernandez et al., 2018a, Mooney et al., 2022, Rurak et al., 2022, Spampinato et al., 2020, Ugawa et al., 1995b). Magnetic cerebellar stimulation is most reliably elicited by using a double cone coil, positioned over the midpoint on a line between inion and external auditory meatus (Ugawa et al., 1995b, Werhahn et al., 1996) or 3–5 cm lateral and 0 or 2 cm above the inion (Fernandez et al., 2018a), with upward induced current in the cerebellar cortex (Reis et al., 2008). The cerebellar conditioning stimulation intensity is set at 5–10% below AMT for foramen magnum double cone stimulation (Ugawa et al., 1995b, Werhahn et al., 1996). To activate cerebellar Purkinje cells, the intensity of cerebellar CS is usually high and may cause discomfort. The double cone coil provides the most consistent CBI results, with no further increase in inhibition when CS intensity exceeds 60% of maximum stimulator output [MSO] (Fernandez et al., 2018b). Figure-of-eight coils do not elicit an adequate and consistent CBI response, and therefore are not recommended (Fernandez et al., 2018b, Ugawa et al., 1995b, Werhahn et al., 1996). Suppression is not observed with electrical test stimulation applied to the primary motor cortex, suggesting that interactions occur at the cortical level.
Cerebellar stimulation suppresses the MEP response at ISIs of 5–8 ms mediated by cerebellar activation and spinal inhibitory processes (ISI 7–8 ms) (Fernandez et al., 2018a, Werhahn et al., 1996). Underscoring the importance of cerebellar activation are findings that CBI was absent in patients with cerebellar degeneration (Ugawa et al., 1997). Activation of Purkinje cells, located in the cerebellar posterior lobules V-VIII and ~30 mm below the scalp, mediate development of CBI (Hardwick et al., 2014). Purkinje cell activation reduces the tonic facilitatory drive from dentate nucleus to contralateral M1 through the dentate-thalamo-cortical pathways (Pinto A. D. and Chen R., 2001, Ugawa et al., 1997). Cerebellar inhibition is more prominent with smaller (~0.5 mV) than larger test MEP amplitudes (~2 mV), a finding related to either predominant I1 wave modulation, or projection of dentate-thalamo-cortical fibers to the core of cortical hand muscle representation area (Pinto Andrew D and Chen Robert, 2001, Reis et al., 2008). Cerebellar inhibition may modulate premotor cortex excitability with maximal inhibition at ISI of 7 ms when the TS induced currents are directed anterior-posteriorly in the motor cortex (Spampinato et al., 2020).
It should be stressed that two independent cerebello-M1 pathways may contribute to CBI (Spampinato et al., 2020). Specifically, one cerebello-M1 pathway (assessed by posterior-anterior directed currents) targets excitability of M1 layer 5 pyramidal neurons in the rostral lip, while the other pathway (activated by anterior-posterior directed currents) targets excitability of neurons in the premotor cortex that project onto M1. Assessment of these pathways should be considered in pathophysiological studies. Of relevance, CBI may reduce SICI and increase ICF, suggesting an effect on inhibitory and facilitatory cortical circuits (Daskalakis et al., 2004). Factors such as age influence CBI, with the magnitude of cerebellar inhibition being smaller in older adults, a finding potentially mediated by an age-related loss of Purkinje cells (Rurak et al., 2022). Others have reported the converse and argued that the increase in CBI in older adults was a compensatory mechanism to support age-related motor function decline (Mooney et al., 2022). The discordant findings may be related to use of different coil types with figure-of-eight coil used by the former (Rurak et al., 2022) and double-cone coil in latter study (Mooney et al., 2022). At a physiological level, the pathways assessed by CBI seem to be important for gait performance, whereby greater CBI is associated with a faster 10-meter walking time (Rurak et al., 2022). This finding was attributed to importance of rhythmic upper limb movement in gait control (Ortega et al., 2008).
Inadvertent stimulation of CSTs by the CS may confound CBI by three potential mechanisms: (i) collision of antidromic CST with descending M1 volleys; (ii) activation of CST collaterals which activate cortical inhibitory neurons; and (iii) depolarization of spinal motor neurons by descending CST volleys (Fisher et al., 2009, Ugawa et al., 1994a). The intensity of cerebellar stimulation should always be adjusted relative to CST activation with foramen magnum level stimulation.
2.3. Triple stimulation technique
2.3.1. TST methodology:
The triple stimulation technique (TST) is a collision method, first designed to measure conduction blocks in peripheral nerves and was subsequently adapted to study the corticospinal conduction (Magistris et al., 1999). The TST circumvents problems encountered with TMS. Namely, MEP response are variable in size and smaller than CMAP responses, a phenomenon related to central desynchronization, and thereby precluding a reliable quantitative evaluation of central motor conduction based on MEP amplitude. The desynchronization of descending volleys leads to phase cancellation of motor unit potentials, accounting for the MEP variability and smaller amplitude, even when facilitated with background muscle contractions, when compared to CMAP amplitudes (Magistris et al., 1999, Magistris et al., 1998b, Rosler et al., 2002).
The triple stimulation technique (TST) corrects the desynchronization of descending corticospinal volleys and quantify central motor conduction (Magistris et al., 1999, Magistris et al., 1998b, Z’Graggen et al., 2005). The proof of concept comes from the resultant MEP amplitude, which corresponds more closely to CMAP amplitudes, with the excitation of entire motor neuron pool innervating the target muscle in healthy subjects (Buhler et al., 2001, Magistris et al., 1998b). Triple stimulation technique has become an established tool in clinical practice and research, contributing to a better understanding of motor cortex physiology. When combined with paired pulse stimulation protocols, TST may exclude a role for desynchronization in mediating intracortical inhibition and facilitation (Caranzano et al., 2017).
The principle of TST is explained in Figure 2. TST consists of three successive stimuli with pre-defined delays. The TMS pulse (1st stimulus) is delivered over the motor cortex, followed by two supra-maximal electrical stimuli delivered to the nerve innervating a target muscle. The first electrical stimulus is delivered distally (2nd stimulus) while the second electrical stimulus is applied proximally (3rd stimulus) with at Erb’s point or sciatic nerve at the gluteal fold. In healthy subjects, the descending discharges from TMS collides with antidromic discharges elicited by the 2nd stimulus leading to cancellation. The 3rd stimulus elicits synchronous discharges resulting in a supramaximal CMAP. In central motor dysfunction, the TMS induced descending discharges fail to reach the peripheral axon, either totally or partly, resulting in a paucity of collision with the 2nd stimulus. Consequently, descending discharges from the 3rd stimulus collide with antidromic discharges from the 2nd stimulus resulting in smaller CMAP responses. Commercially available software is available which sets the interstimulus intervals between the three stimuli, ensuring optimal collision, and thereby facilitation the translation of TST into clinical practice.
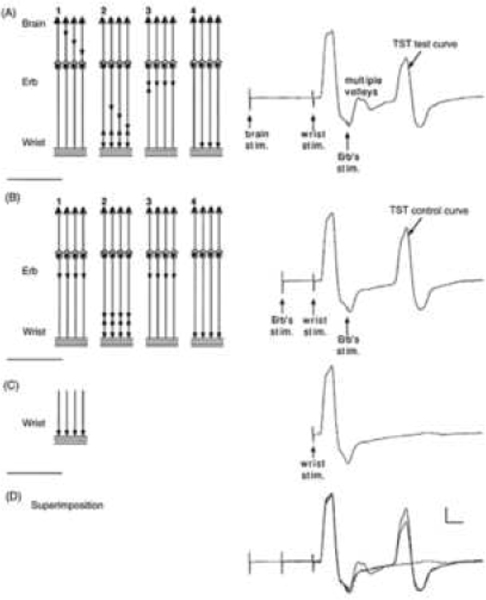
Figure 2: The triple stimulation test (TST) principle.
On the left, a schematic diagram of the motor tract is simplified to four corticospinal axons with monosynaptic connections to four peripheral axons (a simplification which does not account for the complexity of corticospinal connections); horizontal lines represent the muscle fibres of the four motor units. Recordings are shown on the right: (A) TST test, (B) TST control, (C) response to a single stimulus at wrist and (D) superimposition of recordings A, B and C. In this example a submaximal transcranial stimulus excites 75% of the axons (three axons out of four). Desynchronization of the three action potentials is assumed to occur within the corticospinal tract (or possibly at the spinal cell level). (A, 1) Transcranial stimulation excites three out of four axons. (A, 2) After a delay, a maximal stimulus applied to the wrist evokes the first negative (upward) deflection in the TST test trace; this response is followed by that of the multiple-discharge volleys (not figured on the left scheme). (A, 3) After a delay, a maximal stimulus is applied to Erb’s point; (A, 4) a synchronized response from the three axons excited initially by the transcranial stimulus is recorded as the second large deflection of TST test trace. (B, 1) A maximal stimulus is applied to Erb’s point; (B, 2) after a delay, a maximal stimulus applied to the wrist evokes the first deflection of TST control trace; (B, 3) after a delay, a maximal stimulus is applied to Erb’s point; (B, 4) a synchronized response from the four axons is recorded as the second deflection of TST control trace. (C) The response evoked by stimulating the wrist serves as a baseline for measurement of the amplitude and area of the second deflection of the TST curves. (D) On the superimposed traces, the smaller size of the second deflection of the TST test trace, compared with that of the TST control trace, demonstrates that not all spinal axons of the target muscle were excited by transcranial stimulation (in this example both amplitude and area ratios should be 75% if the four individual MUPs have identical sizes). Calibrations: 2 mV and 5 ms. (Figure from Magistris, M. R., K. M. Rosler, A. Truffert and J. P. Myers (1998). “Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials.” Brain 121: 437–450 (with kind permission of the authors and Oxford University Press (Magistris et al., 1998a).)
The TST response is compared to that of a control curve obtained by triple stimulation performed on the peripheral nerve [Erb’s point (1st stimulus)-to-wrist (2nd stimulus)-to Erb’s point (3rd stimulus)] paradigm. The proportion of spinal motor neuron pool of the target muscle discharged by TMS is quantified by the amplitude ratio of the TST test to the TST control curves. A TST amplitude ratio >93% can always be obtained in healthy subjects and TST exhibits good test-to-test reliability (Buhler et al., 2001, Humm et al., 2004b, Magistris and Rösler, 2003, Magistris et al., 1999, Magistris et al., 1998b). Modified TST protocols correcting for volume conduction of adjacent hand muscles (Ziemann et al., 2004) and an extended TST protocol including a fourth (quadruple) and a fifth (quintuple) stimulus have also been described, enabling a more precise estimate of the number of repetitive spinal motor neuron discharges (Z’Graggen et al., 2005), although these techniques are yet to be applied in clinical practice.
2.3.2. Clinical utility of TST
TST enables precise quantification of central and peripheral conduction deficits that result from reduced excitability, loss of cortical motor neurons, conduction block in the corticospinal tracts or proximal peripheral motor nerve segments. TST is two to three times more sensitive than standard TMS and may detect even minor deficits (Buhler et al., 2001, Magistris et al., 1999, Magistris et al., 1998b). In central demyelinating diseases, TST may quantify temperature-dependent conduction blocks underlying the Uhthoff phenomenon (Humm et al., 2004b). Additionally, TST appears to be reliable in monitoring disease course and effects of therapeutic interventions in multiple sclerosis (Hofstadt-van Oy et al., 2015). Compared to conventional MEP, the TST correlates better with the clinical performance and global disability in patients with multiple sclerosis (Giffroy et al., 2019).
Separately, TST has been proven sensitive in detecting loss of corticomotor neurons in amyotrophic lateral sclerosis, even at a subclinical stage, and in distinguishing central from lower motor neuron degeneration. TST complements the standard neurophysiological evaluation and improves diagnostic accuracy in ALS (Grapperon et al., 2021, Kleine et al., 2010, Komissarow et al., 2004, Rösler et al., 2000, Wang et al., 2019).
In disorders of the peripheral nervous system, particularly multifocal motor neuropathy with proximal conduction block (MMN), TST may differentiate conduction block from temporal dispersion in proximal nerve segments and increases the sensitivity for detecting proximal conduction block from 60% to 90% compared to standard neurophysiological studies (Attarian et al., 2005, Deroide et al., 2007). When combined with neuroimaging (MRI), TST increases the sensitivity for detecting brachial plexus pathology, thereby providing further support for diagnosis of MMN (Caranzano et al., 2021). Of relevance, TST may also detect proximal conduction blocks in other peripheral nerve pathologies such as Guillain–Barre syndrome (Taieb et al., 2015) and chronic inflammatory demyelinating polyradiculoneuropathy (Attarian et al., 2015).
TST has also been applied in a number of central nervous system disorders such as Parkinson’s disease (Xu et al., 2020), multiple system atrophy (Eusebio et al., 2007) and spinocerebellar ataxia type 6 (Sakuma et al., 2005), although the clinical relevance remains to be determined.
At a clinical level, the use of TST has some caveats. Notably, TST is limited to the study of central conduction to distal hand and foot muscles (Buhler et al., 2001, Magistris et al., 1999, Magistris et al., 1998b, Rosler et al., 2002). Absence or marked reduction of CMAP responses preclude TST studies. Additionally, TST cannot differentiate central conduction deficit from proximal peripheral conduction block, and clinical correlation is required. Confounding effects of sub-maximal peripheral stimulation also need consideration (Caranzano et al., 2021). Potential risk of injury with needle stimulation, when used for proximal sciatic nerve stimulation also needs consideration, although this is a rare adverse event. Another major limitation of TST is the fact that the technique is rather painful, limiting its use for monitoring patients’ follow-up.
2.4. Other TMS techniques
2.4.1. Foramen magnum stimulation
Activation of the corticospinal tracts (CST) at the foramen magnum was first described with a high voltage electrical stimulation (Ugawa et al., 1991b), and later with TMS using a double-cone coil (Ugawa et al., 1994c). The site of foramen magnum stimulation (FMS) seems to be at either the foramen magnum or CST decussation [cervicomedullary junction] (Ugawa et al., 1992, Ugawa et al., 1991b, Ugawa et al., 1994c), preferentially activated by TMS currents induced in a parallel direction to the decussation (Taylor, 2006). Upward induced currents at foramen magnum are more effective than downward currents (Ugawa et al., 1989), with optimal TMS coil position being mid-way between the inion and mastoid process ipsilateral to the target muscle (Shirota et al., 2011). The MEP latency with FMS is 2–3 ms shorter than D-wave latency and was not impacted by voluntary contraction, contrasting with motor cortical stimulation (Ugawa et al., 1991b, Ugawa et al., 1994c). The physiological differences could relate to the fact that FMS evokes a single descending volley (Taylor et al., 2002, Ugawa et al., 1991b, Ugawa et al., 1994c), in contrast to cortical stimulation that elicits multiple descending volleys by a single pulse stimulation (Day et al., 1987).
The clinical utility of FMS is in localizing the site of CST lesions rostral or caudal to the pyramidal decussation (Ugawa et al., 1996), and the clinical utility was demonstrated in the following settings; (i) detecting subclinical lesions, (ii) identifying multiple CST lesions, (iii) unmasking CST dysfunction that could be clinically masked by presence of peripheral neuropathy, and (iv) establishing the presence of distinct disease-conduction delay rostral to foramen magnum was shown to distinguish cervical myeloradiculopathy from amyotrophic lateral sclerosis (ALS) (Ugawa et al., 1996). Separately, prolonged cortical to brainstem (CTX-BST) conduction times were reported in ALS, although is less sensitive at detecting CST dysfunction than prolonged CMCT (Tokimura et al., 2020). Despite potential clinical utility, FMS has not been used widely due to pain associated with stimulation. Foramen magnum stimulation may fail to elicit an MEP response which could be overcome by a paired-pulse FMS paradigm (Matsumoto et al., 2008), although there is limited clinical experience in using this technique. Separately, FMS may be used to assess the excitability of spinal motor neurons (Taylor, 2006) and spinal cord synaptic efficacy (Cortes et al., 2011, Fitzpatrick et al., 2016, Yamashita et al., 2021), which may be of clinical utility in spinal cord injuries (Vastano and Perez, 2020), although further research is required.
2.4.2. Conus stimulation
Stimulation of the conus enables assessment of the cortico-conus motor conduction time (CCCT) and cauda equina conduction time (CECT), which reflect conduction in proximal peripheral nerve segments innervating the lower limbs (Figure 3). Conus stimulation is achieved by using a large 20-cm-diameter Magnetic Augmented Translumbosacral Stimulation (MATS) coil (Matsumoto et al., 2009b). The MATS coil is positioned lateral to midline and contralateral to recording muscle site. The edge of the MATS coil is placed over the L1, L3 or L5/S1 spinous processes with stimulus intensity set to maximal stimulator output (Matsumoto et al., 2009b). The magnetic stimulation induced currents flowing in either upward or downward direction in the body, and three MEP responses are evoked at different directions of the induced currents. The optimal induced current direction is defined as the direction in which the largest response was elicited with a stable latency (Matsumoto et al., 2010a, Matsumoto et al., 2013a). When recording from the tibialis anterior (TA) muscle (Figure 2C and D), proximal segments of the cauda equina are activated by positioning the magnetic augmented translumbosacral stimulation (MATS) coil over the L1 spinous process and inducing current flow upward, while neuro-foraminal stimulation is achieved by placing the coil over the L5 spinous process for inducing current flow 45° downward from the horizontal direction (Matsumoto et al., 2009a, Matsumoto et al., 2009b). A similar stimulation set-up was used when recording from the abductor hallucis (AH) muscle (L1 or L3 spinous process and upward induced current, Figure 2A and B), with the MATS coil placed over the S1 spinous process and induced current flowing 60° downward from the horizontal direction for optimal neuroforaminal activation (Matsumoto et al., 2009a).
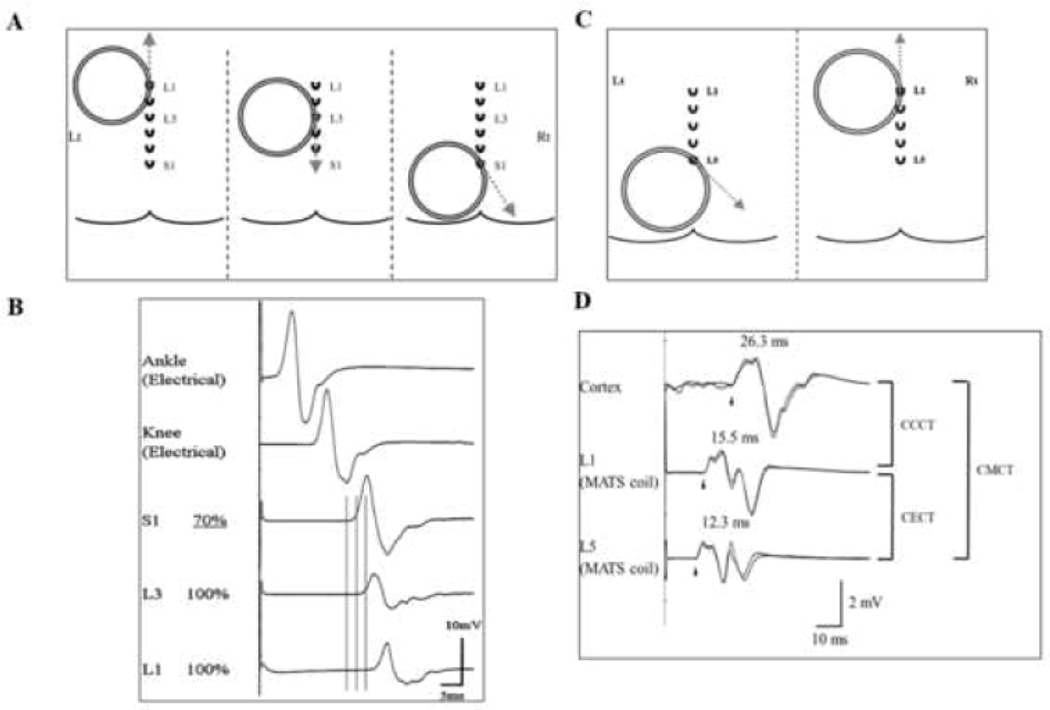
Figure 3: Stimulation of the lumbosacral region.
(A) Position of the magnetic augmented translumbosacral stimulation (MATS) coil in magnetic stimulation of cauda equina with motor evoked potentials (MEP) recorded over the adductor hallucis (AH). The coil edge was positioned over the L1, L3, and S1 spinous processes. The induced current directions are illustrated by grey dashed lines and are tangential to the direction of coil winding over the activation sites. (B) At L1 and L3 levels, MATS coil stimulations failed to elicit a supramaximal MEP response. At the S1 level, stimulating nerves within the neuro-foramina, the MEP responses are supramaximal elicited at a TMS intensity of 70% maximal stimulator output. The MEP onset latency differences between the L1 and L3 stimulation levels suggest that cauda equina in the spinal canal at L1 and L3 levels were activated separately. Cauda equina conduction time (CECT) is calculated by subtracting the S1 from L1 elicited MEP onset latencies when recoding from AH. Tibial nerve compound muscle action potential (CMAP) responses were illustrated with ankle and knee stimulation. (C) Conus stimulation method when recoding over the right tibialis anterior muscle. For proximal cauda equina stimulation, the edge of MATS coil is positioned over the L1 spinous process for inducing currents in an upward direction (dashed grey arrow), while for neuroforaminal activation the edge of the MATS coil is positioned over L5 with induced current direction being 45° downward from a horizontal direction. (D) The MEP responses elicited with cortical, L1 and L5 stimulation are illustrated. The cortico-conus motor conduction time (CCCT) is calculated by subtracting the MEP onset latency elicited by L1 from cortical stimulation. Additionally, CECT is measured by subtracting MEP onset latency elicited by L1 form L5 stimulation. Central motor conduction time (CMCT) is represented by addition of CCCT and CECT.
The CECT is calculated by subtracting the L5/S1 evoked MEP latency from that evoked at the L1 level. The CCCT is calculated by subtracting the MEP latency evoked with L1 from motor cortical stimulation, while central motor conduction time can be calculated by subtracting MEP latency at L5/S1 stimulation from cortical MEP latencies (Matsumoto et al., 2010a, Matsumoto et al., 2013a). A limitation of conus stimulation relates to submaximal activation of neural elements, thereby limiting the possibility of establishing conduction block at the cauda equina.
At a clinical level, assessment of CCCT may identify CST dysfunction in the setting of peripheral nerve disease or when upper motor neuron signs are absent (Murakami et al., 2019, Tokimura et al., 2020, Tokushige et al., 2013). Separately, CECT may be prolonged in demyelinating neuropathies, such as chronic inflammatory demyelinating polyradiculoneuropathy, demyelinating Guillain-Barré Syndrome phenotypes, anti-myelin-associated glycoprotein (MAG) polyneuropathy, POEMS syndrome and Charcot-Marie-Tooth disease type 1 (Maccabee et al., 2011, Matsumoto et al., 2015, Matsumoto et al., 2010b), where it may be of diagnostic utility. Additionally, prolonged CECT was also reported in primary malignant lymphoma of the cauda equina (Matsumoto et al., 2009a) and in lumbar spinal canal stenosis (Senocak et al., 2009). Larger studies are required to determine the diagnostic utility of conus stimulation in peripheral nervous system disorders, particularly developing optimal diagnostic cut-off criteria.
2.4.3. Facial nerve stimulation
The facial nerve can be directly stimulated by TMS with a 90 mm circular coil positioned over the ipsilateral parieto-occipital region, with the base of the coil over the mastoid process (canalicular stimulation) (Chen et al., 2008, Rimpiläinen et al., 1993, Rösler et al., 1989, Schmid et al., 1992, Schriefer et al., 1988, Wolf et al., 1995). The site of facial nerve stimulation remains controversial, although appears to be within the internal acoustic meatus where the nerve transitions from low-resistance cerebrospinal fluid to high-resistance petrous bone (Schmid et al., 1992). Cortical MEPs are elicited by stimulation of the facial area in the contralateral motor cortex, with the optimal position being ~2 cm lateral and ~1 cm anterior to the position that evokes the strongest contraction in hand muscles (Paradiso et al., 2005). Facilitation of the target muscle is often required to record an MEP response (Rösler et al., 1989). The TMS elicited responses are compared to facial nerve CMAP responses evoked by electrical stimulation at the stylomastoid fossa or further along the facial nerve. The three stimulation sites allow assessment of three segments (cortico-proximal, transosseal, and distal) of the cortico-facial projection.
The MEP and CMAP responses may be recorded from any facial muscle, including orbicularis oculi, oris, nasalis, mentalis, and buccinator, and should be recorded bilaterally to enable a side-to-side comparison. The facial MEP responses are smaller than CMAP responses, and may be contaminated by a number of artefacts, including volume conduction from uncrossed ipsilateral MEPs, blink and other facial reflexes, peripheral stimulation of the ipsilateral facial nerve, and possibly by activation of muscles innervated by the trigeminal nerve (Paradiso et al., 2005, Türk et al., 1994, Urban et al., 1997). Normative values have been previously reported and should be established with each laboratory prior to undertaking studies in neurological diseases (Rimpiläinen et al., 1992, Rösler et al., 1989, Rösler et al., 1995, Urban et al., 1997). The clinical utility of magnetic stimulation in facial nerve disorders is discussed below.
2.4.4. Spinal nerve stimulation in peripheral neuropathy
The spinal nerve stimulation is sometimes used to evaluate the proximal parts of peripheral nerves. Focal lesions between Erb’s point and neuroforamina, i.e., brachial plexus or spinal nerves just distal to neuroforamina, can be detected in demyelinating neuropathies (Matsumoto et al., 2013a, Matsumoto L. et al., 2010), and focal lesions between knee and neuroforamina, i.e., the sacral nerves, sacral plexus, or spinal nerves just distal to neuroforamina, can also be detected (Matsumoto et al., 2013b).
2.5. TMS-EEG
TMS in combination with EEG (TMS-EEG) enables direct assessment of cortical circuits, by-passing sensory and motor pathways, as TMS-EEG is not reliant on the integrity of these systems. Moreover, TMS-EEG can activate cortical neurons with a wide range of stimulation intensities, thereby providing full excitability profiles, from threshold to saturation (Casali et al., 2013, Kähkönen et al., 2005, Komssi et al., 2004, Rosanova et al., 2009). Consequently, input-output properties of cortical neurons and circuits can be better assessed, which has pathological implications. Additionally, TMS-EEG offers an unambiguous measure of connectivity, namely causal interactions within the thalamocortical system (Massimini et al., 2005, Morishima et al., 2009, Paus, 2005).
The flexibility of TMS-EEG affords unprecedented opportunities for exploring and modulating cortical excitability but also represents a challenge, especially when probing cortical areas outside M1. Indeed, in the absence of a motor read-out, when a TMS coil is positioned over the region of interest, the actual impact of the induced electric field on cortical neurons is difficult to predict, even when utilizing individual head models and TMS navigation systems (Lioumis and Rosanova, 2022). Key factors such as microscale axon orientation, cytoarchitectonics and local neuronal excitability remain unaccounted for and may dramatically affect the interaction between the induced electric field and brain activity. Differences in the strength of direct cortical activation have been highlighted as a major problem affecting the reproducibility of TMS-EEG studies in assessing cortical excitability and connectivity (Belardinelli et al., 2019). Maximizing the direct impact of stimulation on cortical neurons, while minimizing collateral effects such as cranio-facial muscle, auditory or somatosensory activation, is a key prerequisite for improving the reproducibility, signal-to-noise ratio (SNR), as well as clinical utility of TMS-EEG.
Although off-line software tools are available for reducing artifacts (Mutanen et al., 2022), controlling for quality of EEG signals in real-time is the most effective strategy for recording reliable TMS evoked potentials (TEP). Utilizing a software that enables setting of stimulation parameters based on real time visualization EEG signals, may be critical for recording good quality TEPs, and has been successfully implemented in the study of brain-injury patients (Casali et al., 2013, Casarotto et al., 2016, Rosanova et al., 2018, Sinitsyn et al., 2020). A free-release MATLAB-based tool, called rt-TEPs (real-time TEP), is available to assist in the implementation of this approach (Casarotto et al., 2022).
Together with tools that enable on-line assessment of TEP quality, other tools have been developed to control for confounding factors such as the auditory evoked potentials (AEP) produced by TMS “clicks”. The AEPs can be abolished by continuously playing a white noise through inserted earplugs during acquisition of TEPs (Paus et al., 2001), administering continuous masking noise that reproduces the time-varying spectral content of the coil “click” (Massimini et al., 2005), or by interposing a foam layer between the TMS coil and scalp (ter Braack et al., 2015). More recently, a highly flexible and freely available tool that can generate effective and safe masking noises, customized for each TMS device and tailored on subject’s perception, has been released (Russo et al., 2022).
Developing such tools is critical as researchers and clinicians would like to avoid situations such as that illustrated in Figure 4. Here, it is shown how despite reasonable a priori assumptions based on anatomical and biophysical data, TMS can have little impact on the underlying cortex. Under these circumstances, lack of real-time control on cortical impact, artifacts and confounding factors may result in a ‘false TEP’ with little initial activation and late symmetrical, central topography typical of an AEP. The figure also shows how this fundamental drawback can be readily controlled for and prevented during the experiment if the operator’s actions during the measurement are informed by tools such as rt-TEP. In this case, responses consistent with the effect of direct cortical stimulation can be easily obtained, and are characterized by a strong initial activation under the TMS coil followed by asymmetric topography, which is specific to the stimulated site, and high SNR.
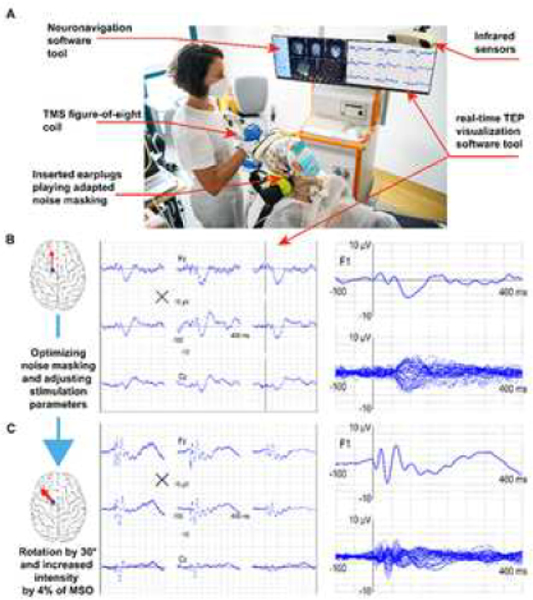
Figure 4: TMS-EEG principles.
(A) Key elements (pointed by red arrows) of a TMS-EEG set-up employed in a clinical setting. (B and C) These panels directly compare the final average TMS evoked potentials [(TEP] (150 trials) collected during two sessions. Although both responses have been obtained by setting stimulation parameters based on reasonable a priori anatomical (position and orientation with respect to the cortical gyrus) and physiological (maximum stimulator output [MSO, %] at or above resting motor threshold [RMT]) information, they differ in fundamental ways. The responses in B show small early activations and are characterized by larger, late symmetric components which are maximal over midline channels, like those reported previously (Conde et al., 2019; Chung et al., 2018). These waveforms are hardly consistent with the effects of direct cortical stimulation, which is expected to trigger responses that are large immediately after the pulse and specific for the stimulation site (Keller et al., 2014; Kundu et al., 2020). Conversely, the TEP reported in C fulfills these basic criteria and is similar to those described in previous studies (Rosanova et al., 2009; Casarotto et al., 2016; Sinitsyn et al., 2020). In this case, a strong initial activation is followed by an overall asymmetric wave shape with high signal-to-noise ratio (SNR). Obtaining this kind of responses only required maximizing the immediate impact of TMS on early (8–50 ms) components through slight adjustments of the intensity (by 4% MSO) and orientation of stimulation (30° counterclockwise), while at the same time optimizing noise masking. Making such adjustments is relatively straightforward but would be impossible based on a priori information alone and can only be done if the operator is guided in real-time by informative visual feedback (rt-TEP) about the immediate effects of TMS.
To obtain high-quality TEPs, the adjustment of TMS parameters may involve noise masking optimization, intensity changes and small coil rotations (as illustrated in Figure 4). Although a few manual rotations of the TMS coil are generally effective in increasing the SNR, a systematic search of the optimal electric field orientation is practically unfeasible. Such fine tuning requires more sophisticated strategies and hardware, such as an EEG-based adaptive search algorithms coupled with electronically controlled two-coil transducers (Souza et al., 2022, Tervo et al., 2020, Tervo et al., 2022). Combining rt-TEP with advanced closed-loop systems represents a promising strategy whereby fundamental stimulation parameters are first set by the operator, based on visual feed-back, and then automatically optimized in a closed-loop fashion.
Beside the appropriate experimental procedures, the reliability of TMS-EEG measurements critically depends on the hardware. Active amplifiers tend to induce long-lasting decay artifacts that are more prominent and difficult to eliminate, often masking early TEPs components. The accuracy of the TMS-navigation unit is also a key factor. Specifically, the settings (coil position coordinates and rotation) identified during the initial parameter search must be precisely retrieved and held steady throughout the experiment and across repeated measurements (Lioumis and Rosanova, 2022). TMS hardware, TMS coils and pulse waveshapes can differ in their focality, efficacy on stimulating cortical circuits and collateral effects (magnetic artifacts, sensory and auditory stimulation) (Koponen et al., 2020). With theoretical and technical improvements, the quality and informativeness of TEPs is likely to improve.
2.5.1. Clinical measurements: principles and examples:
Experimental procedures and tools to record reproducible TEPs are being improved, standardized and shared within the TMS-EEG community (Belardinelli et al., 2019, Siebner et al., 2019) with the aim of establishing TMS-EEG as a reliable clinical tool (Julkunen et al., 2022). Recent preclinical studies have suggested that several biomarkers can be already extracted from TEPs that may serve as potential pathophysiological, diagnostic, and prognostic biomarkers in neurological patients (Tremblay et al., 2019).
2.5.1.1. Time-domain, early and late components
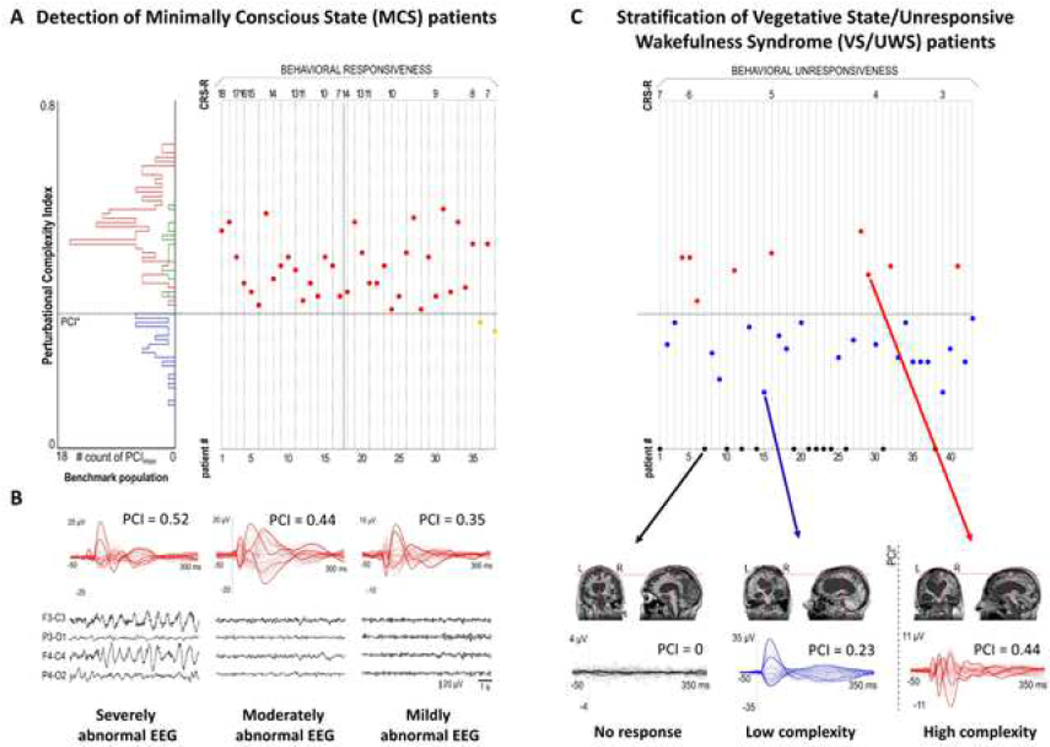
Early TEP components (0–50 ms) have been considered as markers of cortical excitability, possibly reflecting the immediate reactivity of local cortical neuronal populations (Moliadze et al., 2003, Mueller et al., 2014, Romero et al., 2019). Among different time-domain measurements, the peak-to-peak amplitude and slope of early TEPs at the individual channel level, as well as multi-channel measurements such as local and global mean field power in early time windows, have been used to detect changes in cortical excitability over time or after neuromodulatory interventions (Esser et al., 2006, Huber et al., 2013, Ly et al., 2016, Romero Lauro et al., 2014). Amplitude changes of early TEPs and regional cortical hyperexcitability have been demonstrated in Alzheimer’s disease at specific cortical locations, suggesting potential clinical utility (Casarotto et al., 2011, Casula et al., 2022, Julkunen et al., 2011, Julkunen et al., 2008), although the variation in cortical atrophy across studies may limit interpretability given the inherent variability of stimulation parameters. Early TEPs have a high individual test-retest reproducibility (Casarotto et al., 2010), and may be of utility in assessing disease progression or treatment effects in neurological diseases. Of relevance, early TEPs have been effectively used to measure the increase in frontal cortex excitability induced by electroconvulsive treatment (Casarotto et al., 2013) and local modulations of cortical excitability by dopaminergic agents in Parkinson’s disease (Casarotto et al., 2019, Leodori et al., 2022, Turco et al., 2018c).
Selective alterations of late TEPs components (>50 ms) have been linked to pharmacological modulation of cortical inhibition (Premoli et al., 2014), or pathological cortical adaptation dynamics in severe brain injury (Rosanova et al., 2018) and stroke (Sarasso et al., 2020, Tscherpel et al., 2020). Abnormalities of later TEP components have been observed in Parkinson’s disease (Maidan et al., 2021), whereas deep brain stimulation of the subthalamic nucleus and L-Dopa intake increases late TEPs (Casula et al., 2017). Finally, alterations of late TEP components may be of utility as a biomarker of epileptogenic cortical foci and a measure of anti-epileptic drug effects (Kimiskidis et al., 2017, Valentin et al., 2008).
2.5.1.2. Spectral features:
Alterations in membrane properties of cortical and thalamic neurons, as well as alterations in their patterns of connectivity, underlie most neurological conditions, leading to distinctive changes in oscillatory dynamics (Hughes and Crunelli, 2005, Jeong, 2004, Llinás et al., 1999, Soininen et al., 1992). Such alterations can be studied with EEG recordings, although spontaneous rhythms are variable and their topography can change radically in response to eye opening, planning of simple movements or cognitive activity. A complementary way of probing frequency tuning of brain circuits is to apply direct perturbations to detect the main rate of ensuing oscillations, the so-called natural frequency. Following an early (0–20 ms) stereotypical sharp component, TMS consistently evokes (i) alpha-band oscillations after stimulation of the occipital cortex, (ii) beta-band oscillations after stimulation of the parietal cortex, and (iii) fast beta/gamma- band oscillations after stimulation of the frontal cortex (Rosanova et al., 2009). Dampening of beta-band responses have been reported in Parkinson’s disease after unilateral surgical lesioning of the ventrolateral thalamic nucleus (Van Der Werf et al., 2006). A marked reduction of gamma-band TMS-evoked oscillation was reported in the frontal cortex of schizophrenia patients, possibly related to thalamic dysfunction (Ferrarelli et al., 2012, Guller et al., 2012). Slowing of the natural frequency was reported in cortical areas overlying subcortical strokes (Pellicciari et al., 2018, Sarasso et al., 2020, Tscherpel et al., 2020). As such, TMS-evoked EEG oscillations may provide valuable clinical information about the state of cortico-subcortical (especially thalamic) loops.
2.5.1.3. Connectivity:
Long-range interactions of neuronal populations represent a key aspect of brain function. Such interactions are typically inferred based on measures of functional connectivity that rely on correlation-based analyses of spontaneous activity. A limitation of these measures of temporal correlation among time series, such as cross-correlation, coherence, phase-locking value, is the possible biasing by common drivers, correlated inputs, and noise. A more informative and clinically relevant measure is effective connectivity, which refers to the ability of a specific neuronal population causally influencing the activity of connected neuronal groups within a system (Friston, 2011, Lee et al., 2003). TMS-EEG offers a straightforward way to measure effective connectivity in the human brain.
In studies of patients with severe brain injury, resulting in an Unresponsive Wakefulness Syndrome (UWS), a dramatic reduction in spread of TMS-evoked activity has been reported both at the source and sensory level (Casarotto et al., 2016, Ragazzoni et al., 2013, Rosanova et al., 2012). Notably, recovery of effective connectivity paralleled and often heralded recovery of consciousness. Changes in effective connectivity have also been demonstrated in stroke (Borich et al., 2016, Casula et al., 2021) and genetic generalized epilepsy patients (Vlachos et al., 2022), whereas no major variations were found in early-stage patients with multiple-sclerosis (Zipser et al., 2018). Clinical results show that perturbational measures of connectivity with TMS-EEG are sensitive and potentially prognostic, especially after diffuse, multifocal and focal brain injury. A potential role for TMS-EEG assessing and prognosticating mild traumatic brain injury has been recently proposed (Coyle et al., 2018).
2.5.1.4. Complexity:
The TEPs are characterized by high differentiation (i.e., different areas have different natural frequencies) and high integration (i.e., causal interactions among distant areas). Inspired by theoretical principles, TMS-EEG-based measures of complexity have been developed to simultaneously quantify differentiation and integration in corticothalamic networks. These measures have been clinically utilized by different centers in large patient cohorts, representing a novel approach to stratifying Disorders of Consciousness (DoC) (Casarotto et al., 2016, Sinitsyn et al., 2020, Wang et al., 2022) and will be discussed below in section 3.12.
2.6. Peristimulus time histogram (PSTH)
The PSTH TMS technique assesses corticomotoneuronal system integrity by evaluating a small number of corticomotoneurons that converge onto a specific population of spinal motor neurons (Weber and Eisen, 2000). The primary peak (PP) reflects the firing probability of a single voluntarily recruited motor unit induced by a sub-threshold TMS stimulus. In healthy controls, PP occurs ~20–25 ms after the stimulus, is well synchronized and of short duration, in keeping with activation of fast-conducting monosynaptic pathways. The PP reflects the rising edge of the underlying excitatory post-synaptic potential (EPSP) evoked at the anterior horn cell. Additionally, excitatory, and inhibitory effects on motor neurons may be assessed, as can the strength of synaptic inputs. Latency, amplitude, and dispersion (calculated by bins excess, duration, and synchronicity) of the PP is typically evaluated.
3.0. TMS abnormalities in neurological diseases (Table 1)
Table 1:
Clinical diagnostic utility of TMS in neurological diseases: Consensus opinion
| Disease | Characteristic TMS findings | Potential Clinical Utility | Clinical aspects that may affect interpretation |
|---|---|---|---|
| Amyotrophic lateral sclerosis (ALS) | SICI ↓ SICF ↑ ICF ↑ ⟷ RMT⟷ ↓ ↑ MEP/CMAP amplitude↑ or ↓* CSP duration ↓ CMCT ↑ |
-SICI is a potential diagnostic biomarker in differentiating ALS from mimicking disorders -Useful in differentiating ALS from other neuromuscular mimicking disorders. |
-Riluzole therapy may transiently increase SICI -Changes in cortical excitability according to disease progression |
| Parkinson’s disease (PD) | SICI ↓ SICF ↑ ICF ⟷ ↓ RMT ⟷ ↓ (PD subtype) I/O curve steeper at resting state SAI ↓ (disease progression) CSP duration ⟷ ↓ ISP duration ⟷ ↓ (PD subtype) Normal SICI on I3 waves |
-SICI and SICF might be used as biomarkers of disease progression -SAI might be used to predict PD dementia and falls -Limited diagnostic utility |
- ON or OFF-drug condition -Tremor-dominant or akinetic-rigid subtype --Disease duration or severity of symptoms |
| Parkinsonism (MSA, PSP) | CMCT ↑ (MSA, PSP) SICF⟷ |
-Prolonged CMCT might be used to differentiate MSA/PSP from PD --Limited diagnostic utility |
-Parkinsonism or cerebellar ataxia predominant MSA subtypes |
| Lewy Body Disease | SICI ↓ ICF ↓ SAI ↓ |
-SAI may help differentiate LBD (SAI ↓) from Parkinsonian syndrome and FTD (SAI⟷) --Limited diagnostic utility |
|
| Huntington’s disease (HD) | SAI ↓ SICI ⟷ ↓ CSP duration ↑ (early stage) |
-Limited diagnostic utility | -Different charges according to disease stage -HD patients are not able to be fully relaxed |
| Dystonia | SICI ↓ LICI ↓ CSP ↓ IHI ↓ (with mirror movements) Surround inhibition ↓ |
-Limited diagnostic utility | -Test on affected side or unaffected side -Homogeneity of dystonia presentation -No single parameter can be used to prove organic dystonia |
| Tics and Tourette’s syndrome | SICI ↓ CSP duration ↓ | -Limited diagnostic utility | -Timing of assessment (before tics occur or when tics are suppressed) |
| Cervical spondylitic myelopathy | CMCT ↑ MEP amplitude ↓ |
-Prolonged CMCT is a major and objective criterion for the diagnosis of pyramidal tract lesion in the context of myelopathy | MEP of APB muscle is most sensitive |
| Spinal cord injury | RMT ↑ CMCT ↑ MEP amplitude ↓ |
-Prolonged CMCT is a major and objective criterion for the diagnosis of pyramidal tract lesion in the context of myelopathy | -No single measurement can predict gait and balance outcome after SCI |
| Alzheimer’s disease (AD) | RMT ↓ AMT ↓ CSP duration ⟷ SICI ⟷ ICF ⟷ LICI ⟷ SAI ↓ |
-Potential diagnostic utility -SICI-ICF/SAI ratio may help differentiate AD from FTD -SICI-ICF may help differentiate AD from LBD |
-SAI may be increased by acetylcholinesterase inhibitors |
| Mild cognitive impairment due to Alzheimer’s disease | RMT ↓ SICI ⟷ ICF ⟷ LICI ⟷ SAI ↓ |
-Potential diagnostic utility -SICI-ICF/SAI ratio may help differentiate MCI-AD from MCI-FTD -SICI-ICF may help differentiate MCI-AD from MCI-LBD |
-Interpretation may be hampered by the heterogeneity of MCI and the paucity of studies performed in patients with a biomarker supported diagnosis of MCI |
| Frontotemporal dementia (FTD) | RMT ⟷ CMCT ↑ SICI ↓ ICF ↓ SAI ⟷ |
-Potential diagnostic utility -SICI-ICF/SAI ratio may help differentiate FTD from AD -SAI may help differentiate FTD from LBD |
|
| Epilepsy | RMT ⟷ CSP duration ↑ SICI ↓ ICF ⟷ LICI ↓ |
↓ SICI and LICI may be useful in discriminating seizure from syncope Follow up clinical condition |
-Antiepileptic medications cause ↑RMT, SICI & LICI |
| Myoclonus epilepsy | SICI ↓ SICI ↓ even on I3 waves RMT ↓ |
-Limited diagnostic utility | Anti-epileptic drugs affect the results |
| Migraine without aura | SICI ↓ (at ISI 4ms) SICF ⟷ SICF ↑ (suprathreshold conditioning stimulus; weak test stimulus) SICF ↓ (preictal phase) RMT⟷ ↓ ↑ LICI ⟷ (up to 120 ms ISI) LICI ↓ (150% test stimulus) LICI ↓ (250 ms ISI) SAI ⟷ SAI ↓ (preictal phase) CSP ⟷ (interictal) CSP duration ↓ (interictal, women) |
-TMS changes vary according to the phase of migraine cycle -Limited diagnostic utility - |
-RMT/PT, and SAI change with proximity of migraine attack -ICF changes with conditioning/test stimulus intensity and proximity of migraine attack -CSP duration decreases with focused sustained attention and sleep restriction |
| Migraine with aura | SICI ↓ SICF ↑ RMT⟷ Steeper I/O curve at rest CSP duration ↓ LICI 250ms ↑ CBI↓ SAI ↓ (when disease progressed) |
- PT might be used to discriminate between transient ischemic attacks and aura without headache | - 1Hz rTMS reduces PT - deficits of cortical inhibition are related more to aura rather than headache mechanisms - CSP shortens also in facial muscles - topiramate modulates occipital cortex excitability |
| Chronic migraines | RMT↓ SICI absent |
-Limited diagnostic utility -Potential biomarker of treatment effects |
Botulinum toxin therapy partially normalizes SICI after 12-months treatment |
| Episodic cluster headaches | SICI ↓ (ictal) SICI ↓ (allodynia) SICF ↑ (preictal and ictal) SICF ↑ (allodynia) RMT ⟷ CSP duration ⟷ |
-Limited utility | Changes in paired-pulse TMS variables are ipsilateral to the pain side; inhibitory changes are ictal; facilitatory changes are both interictal and ictal. |
| Medication-overuse headache | CSP duration ⟷ (NSAIDs alone or in combination) CSP duration ↓ (triptans) |
-Limited utility | - CSP changes reveal medication-induced neural adaptation in motor cortex |
| Multiple Sclerosis | CMCT ↑ TST ↓ MEP amplitude ↓ (or desynchronized) TST-MEP amplitude ↓ |
-CMCT increase or MEP amplitude decrease after fatiguing exercise -SICI ↓ -SICF ↓ -SAI ↓ -CSP duration ↑ -ISP ↑ -Limited diagnostic utility -Potential prognostic utility |
-TMS measures may be affected by multiple sclerosis type (RRMS vs. SPMS/PPMS), and treatment (corticosteroids and immunomodulatory drugs) and the presence of fatigue |
| Neuropathic pain | SICI ↓ (contralateral to pain side) |
-SICI might be a biomarker to select candidates for analgesic cortical neuromodulation -Limited diagnostic utility |
-Defective SICI can be restored by therapeutic intervention producing analgesic effects |
| Stroke | RMT ↑ MEP latency ↑ MEP amplitude ↓ Shallower I/O curves SICI ↓ |
-Potential prognostic utility: Absent upper limb MEPs predicts worse motor recovery and outcomes | -Depending on post-stroke phase (acute, sub-acute, or chronic) |
| Cerebellar disease | CBI ↓ | -Differentiate cerebellar ataxia due to cerebellar or cerebellar efferent pathways dysfunction from that due to cerebellar afferent pathways dysfunction, or from non-cerebellar ataxia | -CBI changes may be seen for compensation of basal ganglia dysfunction (movement disorders) |
| Facial nerve disorders | MEP ↓ or absent Prolonged transosseal conduction time |
-May localize facial nerve dysfunction -Prognostication (if MEP present better prognosis) -Limited diagnostic utility |
|
| Brain Tumors | RMT ↑ or ↓ (tumor hemisphere compared to contralateral hemisphere) MEP latency ↑ MEP amplitude ↓ |
-Preoperative brain mapping -Seed regions for function-based tractography -Preoperative risk stratification -Postoperative transcallosal disinhibition -Limited diagnostic utility |
-Edema -Patient cooperation |
| Functional neurological disorders (paretic disorders) | RMT, SICI, ICF ⟷ RMT, SICI ↑ MEP duration with voluntary contraction ⟷ MEP amplitude with movement imagination ↓ |
-Change in MEP amplitude with movement imagination -Elemental measures in functional dystonia are similar to other types of dystonia - Limited clinical utility |
|
| Dystonic functional neurological disorders | SICI ↓ LICI ↓ CSP duration ↓ Forearm reciprocal inhibition ↓ Cutaneous silent period ↑ |
- Limited clinical utility in differentiating functional from organic dystonia |
Neurophysiological measures in functional dystonia are similar to other types of dystonia. |
APB: Abductor pollicis brevis, CBI: Cerebellar inhibition of the motor cortex, CMCT: Central motor conduction time, CSP: Cortical silent period, GABA: Gamma-aminobutyric acid, LBD: Lewy body disease, IHI: Interhemispheric inhibition, I/O curve: Input-output curve, ISP: Ipsilateral silent period, LICI: Long-interval intracortical inhibition, MEP: Motor evoked potential, MSA: Multiple system atrophy, PPMS: Primary progressive multiple sclerosis, PSP: Progressive supranuclear palsy, RMT: Resting motor threshold, PT phosphene threshold; NSAID (nonsteroidal anti-inflammatory drugs): RRMS: Relapsing-remitting multiple sclerosis, SAI: Short latency afferent inhibition, SCI: Spinal cord injury, SICF: Short-interval intracortical facilitation, SICI: Short-interval intracortical inhibition, SPMS: Secondary progressive multiple sclerosis, TST: Triple-stimulation technique.
no change or normal
reduced
increased.
The MEP amplitude, expressed as a percentage of the compound muscle action potential response (MEP/CMAP), is increased in strong limbs without marked UMN signs, and also in the early stages of ALS. In most ALS patients, the MEP amplitude patients is decreased. It should be stressed that a Delphi consensus process was not possible.
3.1. Neurodegenerative disorders
3.1.1. Amyotrophic lateral sclerosis/motor neuron disease
Amyotrophic lateral sclerosis (ALS) is a relentlessly progressive neurodegenerative disorder of the human motor system characterized by upper [UMN] and lower motor neuron [LMN] dysfunction (Kiernan et al., 2011, Kiernan et al., 2020). Fundamental to understanding of ALS pathogenesis pertains to the relationship between upper and lower motor neuron dysfunction. An UMN origin for ALS has been proposed, whereby corticomotoneuronal hyperexcitability mediated neurodegeneration via an anterograde glutaminergic mechanism, the dying forward hypothesis (Eisen et al., 1992). In contrast, LMN dysfunction has been proposed as the primary event in ALS pathogenesis with UMN dysfunction occurring as a secondary phenomenon [dying-back hypothesis] (Fischer et al., 2004, Williamson and Cleveland, 1999), while others have suggested that upper and lower motor neuron degeneration occur independently and in a stochastic manner [independent degeneration hypothesis] (Ravits et al., 2007). Additionally, identification of upper and lower motor neuron dysfunction is critical for ALS diagnosis (Shefner et al., 2020). Transcranial magnetic stimulation has provided vital insights in the understanding of ALS pathogenesis and has emerged as an important diagnostic technique. Early reports described hyperexcitability of motor cortex with lower-than-normal motor threshold, in particular recruiting the same motor units contributing to spontaneous fasciculations (Caramia et al., 1991).
Paired-pulse TMS studies have consistently identified cortical hyperexcitability as a pathogenic mechanism in ALS (Figure 1), mediated by a combination of reduced cortical inhibition and increased cortical facilitation (Vucic et al., 2018). Reduction or absence of SICI has been identified as an early and intrinsic feature in ALS (Blair et al., 2010, Hanajima et al., 1996, Sommer et al., 1999, Stefan et al., 2001, Tankisi et al., 2022, Vucic and Kiernan, 2006, Vucic and Kiernan, 2008, Yokota et al., 1996, Zanette et al., 2002b, Ziemann et al., 1997b), correlating with peripheral neurodegeneration (Vucic and Kiernan, 2006) and preceding the development of LMN dysfunction (Menon et al., 2015). Reduction of SICI is an adverse prognostic biomarker in ALS (Shibuya et al., 2016b), is associated with disease evolution (Dharmadasa et al., 2020, Menon et al., 2017, Shibuya et al., 2017) and development of clinical features such as the split hand-phenomenon (Bae et al., 2014, Menon et al., 2014). Additionally, SICI reduction is evident in clinically pure lower motor neuron ALS phenotypes, including flail arm and leg variants of ALS (Menon et al., 2016, Vucic and Kiernan, 2007), where it is an adverse prognostic biomarker. It has been argued that the reduction in SICI may represent a compensatory mechanisms in response to peripheral neurodegeneration (Zanette et al., 2002b), although the findings of normal cortical excitability in ALS mimicking disorders (Menon et al., 2015, Vucic et al., 2011b, Vucic and Kiernan, 2008, Vucic et al., 2010), along with partial and transient normalization of SICI with the anti-glutaminergic agent riluzole (Geevasinga N. et al., 2016a, Vucic et al., 2013a), argues against a compensatory mechanism in ALS. Dysfunction or degeneration of GABAergic interneuronal circuits, acting via GABAA, was postulated to mediate the reduction of SICI in ALS (Clark et al., 2021, Nihei et al., 1993, Zhang et al., 2016).
Increased activity of cortical facilitatory circuits also contribute to development of cortical hyperexcitability and ALS pathogenesis. Short interval intracortical facilitation, a biomarker of cortical excitatory circuit function (Di Lazzaro et al., 1999c, Rusu et al., 2014, Van den Bos et al., 2018), is increased in ALS and accompanied by reduction in SICI (van den Bos et al., 2018). Overactivity of facilitatory circuits correlated with a greater degree of functional disability and development of UMN signs.
A comparable increase in cortical hyperexcitability has also been reported in familial ALS cohorts, including phenotypes linked to mutations in superoxide dismutase-1 (Vucic et al., 2008), fused in sarcoma (Williams et al., 2013) and c9orf72 genes (Geevasinga Nimeshan et al., 2015). Significant correlations between cortical hyperexcitability and LMN dysfunction has been established (Geevasinga Nimeshan et al., 2015, Vucic and Kiernan, 2010), with asymptomatic mutation carriers exhibiting normal cortical function (Geevasinga Nimeshan et al., 2015, Vucic et al., 2008). As with sporadic ALS cohorts, cortical hyperexcitability precedes the clinical development of familial ALS by months (Vucic et al., 2008). The findings from familial ALS cohorts have supported the notion that ALS is a multistep process (Al-Chalabi et al., 2014, Vucic et al., 2020, Vucic et al., 2019), with cortical hyperexcitability being an important pathogenic step.
Reduction of LICI has been previously reported in ALS (Zanette et al., 2002a, 2002b). The reduction in LICI was accompanied by SICI reduction and correlated with greater disease severity and UMN dysfunction. Similarly, reduction of transcallosal inhibition (SIHI and LIHI) has been reported in ALS and correlated with a faster rate of disease progression and greater degree of muscle weakness (van den Bos et al., 2021). Degeneration of long-latency inhibitory circuits, acting via GBABB receptors (Ziemann et al., 2015), was postulated to underlie these abnormalities in ALS.
Single-pulse TMS has provided additional evidence for the pathogenic importance of cortical hyperexcitability in ALS (van den Bos et al., 2019, Vucic et al., 2018). The cortical silent period was shown to be consistently reduced in ALS (Geevasinga Nimeshan et al., 2015, Geevasinga N. et al., 2015, Menon et al., 2016, Mills, 2003, Triggs et al., 1999, Vucic and Kiernan, 2006, Vucic and Kiernan, 2007, 2008, Zanette et al., 2002b). The reduction in CSP duration appears more prominent in early disease stages and is specific for ALS compared to other neuromuscular diseases (Menon et al., 2015, Vucic et al., 2011b, Vucic and Kiernan, 2008, Vucic et al., 2010). Moreover, reduction of RMTs has been reported as an early feature of ALS (Eisen and Weber, 2001, Mills and Nithi, 1997a) and associated with profuse fasciculations, preserved muscle bulk and hyperreflexia. Resting motor threshold increases with disease progression, potentially reflecting underlying UMN degeneration (Mills and Nithi, 1997a). The reduction in RMT is not an invariable finding, with some studies reporting a normal (Menon et al., 2017, Mills and Nithi, 1997a, Vucic and Kiernan, 2006, Zanette et al., 2002b), or even increased RMT (Berardelli et al., 1991, Eisen et al., 1990, Miscio et al., 1999, Triggs et al., 1999, Urban et al., 2001), reflecting clinical heterogeneity of ALS. Of relevance, TMS studies using neuronavigation have reported an increase in RMT, with a reduction in the mean motor cortical map area which could be used as a biomarker of upper motor neuron dysfunction (Chervyakov et al., 2015).
An increase in MEP amplitude has also been reported as an early feature of ALS and correlating with LMN dysfunction (Menon et al., 2015, Menon et al., 2014, Vucic and Kiernan, 2006, Vucic and Kiernan, 2007). The increase in MEP amplitude is likely to reflect enhanced corticomotoneuronal glutamatergic activity and provides further support for the pathogenic importance of cortical hyperexcitability in ALS.
The diagnosis of ALS relies on identifying concomitant upper and lower motor neuron signs in one or more body regions, with evidence of rapid disease progression (Shefner et al., 2020). Given the absence of a pathognomonic test, clinically based and consensus driven diagnostic criteria have been developed for ALS (Brooks, 1994, Brooks et al., 2000, de Carvalho et al., 2008, Shefner et al., 2020). Limitations in eliciting UMN signs in ALS, in part due to superimposed LMN dysfunction, has been well documented (Swash, 2012), and impacts on the sensitivity of the ALS diagnostic criteria (Costa et al., 2012, Geevasinga Nimeshan et al., 2016, Geevasinga N. et al., 2016b, Higashihara et al., 2012, Turner et al., 2009).
Threshold tracking TMS has proven to be a robust and objective biomarker of UMN dysfunction in ALS (Menon et al., 2015, Vucic et al., 2011b). Specifically, the presence of cortical dysfunction, as heralded by reduction of SICI or motor cortex inexcitability, reliably differentiates ALS from neuromuscular mimicking disorders, hastening the diagnosis of ALS by ~ 8 months when compared to clinical criteria (Vucic et al., 2011b). Importantly, identification of cortical dysfunction enhances the diagnostic utility of the Awaji criteria by 34% irrespective of site of onset or disease stage (Menon et al., 2015). Additionally, sub-clinical identification of UMN dysfunction has further aided the diagnosis of ALS (Menon et al., 2016, Vucic and Kiernan, 2007). Separately, prolonged CMCT has also been reported as a potential diagnostic biomarker of UMN dysfunction (Eisen et al., 1990, Mills, 2003, Tokimura et al., 2020), although the sensitivity appears to be poor (Menon et al., 2015). While the main limitation of threshold tracking TMS was broader availability, the recent commercialization of the technique will likely lead to translation of threshold tracking TMS into clinical practice and therapeutic trial setting.
The PSTH technique has also disclosed abnormalities in ALS, characterised by increased dispersion and/or desynchronization of the PP (Kohara et al., 1996b, Mills, 1995, Nakajima et al., 1997). Specifically, small amplitude, delayed and desynchronized PPs, along with longer excitatory postsynaptic potential (EPSP) rise times of reduced amplitude, have been reported in ALS (Awiszus and Feistner, 1993, Eisen et al., 1996, Mills, 1995).
Degeneration of fast-conducting with relative preservation of slow conducting motor pathways probably account for these findings (Eisen et al., 1996). An increase in EPSP amplitude was reported in a proportion of ALS patients (Eisen et al., 1996), potentially reflecting glutamate excitotoxicity. A limited number of longitudinal studies in ALS have suggested progression of PP abnormalities, characterised by increasing desynchronization (double peaks) and delay of PP (Weber et al., 2000). The second component of the PP probably reflect activation of higher threshold slow-conducting pathways, that could be explained by development of cortical hyperexcitability.
Abnormalities of PSTH have also been reported in multiple sclerosis and stroke (Boniface et al., 1991, Kohara et al., 1996a), although the presence of double PPs are typically evident in ALS (Weber and Eisen, 2000, Weber et al., 2009), suggesting potential diagnostic utility. While PSTH is a sensitive method for detecting UMN dysfunction (Weber and Eisen, 1999, 2000), the technique is complex, not readily applicable in clinical practice and sensitivity to detect subclinical UMN dysfunction remains to be determined.
3.1.2. Dementia
3.1.2.1. Alzheimer’s disease
(AD) is the leading cause of dementia (Gustavsson et al., 2022, Scheltens et al., 2021), clinically, characterized by amnestic cognitive impairment and dysfunction in other cognitive domains that interfere with activities of daily living (Knopman et al., 2021). Pathologically, AD is characterized by the accumulation of amyloid-β plaques and tau neurofibrillary tangles, and macroscopically by atrophy beginning in the entorhinal cortex, which spreads to the limbic and paralimbic regions, and ultimately neocortical associative areas (Frisoni et al., 2010). Although the neocortex becomes affected in more advanced stages of the disease, deficits in functional connectivity have been observed in early disease stages (Brier et al., 2012; Dennis and Thompson, 2014; Ferreri et al., 2003). Thus, TMS may represent a useful tool for in vivo functional evaluation of cortical networks in AD [see review (Di Lazzaro et al., 2021)].
Motor cortex excitability is increased in AD as revealed by reduction of RMT (Alagona et al., 2004, Brem et al., 2013, de Carvalho et al., 1997, Di Lazzaro et al., 2004, Di Lazzaro et al., 2008, Di Lorenzo et al., 2013, Ferreri et al., 2011, Hoeppner et al., 2012, Inghilleri et al., 2006, Issac et al., 2013, Khedr et al., 2011, Martorana et al., 2009, Martorana et al., 2008, Motta et al., 2018, Schirinzi et al., 2018, Terranova et al., 2013, Trebbastoni et al., 2012, Wang et al., 2016) and AMT (Di Lazzaro et al., 2007b, Khedr et al., 2011, Pepin et al., 1999, Wegrzyn et al., 2013).
The increase in motor cortex excitability reflects functional changes in cortical neurotransmission involving the intricate relationships between GABAergic, glutamatergic, and cholinergic neurotransmission in M1 and resulting in an imbalance between excitatory and inhibitory activities (Di Lazzaro et al., 2004). Given that TMS produces high frequency repetitive discharge of pyramidal neurons and non-NMDA receptors are more involved in high frequency discharge, it has been suggested that enhanced excitability in AD represents enhanced neurotransmission via the non-NMDA receptors (Brem et al., 2013, Di Lazzaro et al., 2004).
Synaptic GABAA activity, as reflected by SICI, was reported to be unchanged in AD by some (Alberici et al., 2008, Benussi et al., 2018a, Benussi et al., 2018b, Benussi et al., 2017, Di Lazzaro et al., 2004, Di Lazzaro et al., 2002c, Di Lazzaro et al., 2008, Di Lazzaro et al., 2007b, Di Lorenzo et al., 2013, Martorana et al., 2013, Motta et al., 2018, Nardone et al., 2008, Olazarán et al., 2013, Pepin et al., 1999), although a reduction in SICI has also been reported (Hoeppner et al., 2012, Liepert et al., 2001, Martorana et al., 2008, Nardone et al., 2006, Olazarán et al., 2010, Pierantozzi et al., 2004b). A recent meta-analysis reported that SICI is reduced only in AD patients with longer symptom duration (Mimura et al., 2021), and it can be speculated that SICI impairment manifests only in advanced stages of AD, and that the discordant findings may be related to the varied patient characteristics across different studies.
Additionally, GABAB inhibitory neurotransmission as evaluated by CSP duration, was reported to be unchanged in most studies (Alagona et al., 2004, Di Lazzaro et al., 2002c, Inghilleri et al., 2006, Issac et al., 2013, Liepert et al., 2001, Trebbastoni et al., 2012). In contrast, reduction of LICI, another measure of GABAB neurotransmission, has been reported in a few studies (Benussi et al., 2017, Benussi et al., 2020c, Brem et al., 2013). Further studies are needed to confirm this finding. Separately, intracortical excitability as probed by ICF, tends to be reduced in AD (Alberici et al., 2008, Benussi et al., 2018b, Benussi et al., 2017, Benussi et al., 2020c, Di Lorenzo et al., 2013, Liepert et al., 2001, Martorana et al., 2013, Motta et al., 2018, Nardone et al., 2008, Nardone et al., 2006, Olazarán et al., 2010). Cholinergic-mediated inhibition evaluated with SAI was significantly decreased in AD (Bella et al., 2016, Benussi et al., 2021a, Benussi et al., 2022, Benussi et al., 2018b, Benussi et al., 2017, Benussi et al., 2020c, Di Lorenzo et al., 2013, Di Lorenzo et al., 2019, Hwang et al., 2018, Koch et al., 2016, Motta et al., 2018, Nardone et al., 2014, Schirinzi et al., 2018, Yildiz et al., 2018).
Treatment of AD patients with acetylcholinesterase inhibitors (Di Lazzaro et al., 2004), L-dopa (Martorana et al., 2009, Nardone et al., 2014) and dopamine agonists (Koch et al., 2014a, Martorana et al., 2013), was reported to normalize SAI. Interhemispheric connectivity, as assessed by interhemispheric silent period (iSP), was abnormal in AD patients as disclosed by prolonged latencies (Hoeppner et al., 2012, Khedr et al., 2011, Wegrzyn et al., 2013). Parietal-to-motor (PPC-M1) connectivity was also shown to be impaired in AD (Bonnì et al., 2013).
TMS related techniques have been successfully applied in the differential diagnosis of AD from other neurodegenerative disorders [see review (Di Lazzaro et al., 2021)]. Considering that the abnormality of single TMS measures, such as motor threshold, is not specific to AD as it has been observed in different neurodegenerative disorders such as ALS (Vucic et al., 2013b), researchers have investigated whether combined measures evaluating multiple parameters and thus multiple neurotransmitter circuits may be of greater diagnostic utility. In an early multicenter study [175 participants], using a complex parameter combining SICI, ICF and SAI measures, it was shown that TMS can differentiate AD from frontotemporal dementia (FTD) and healthy controls with a high sensitivity and specificity (Benussi et al., 2017). These initial findings were confirmed in larger study (N=694) implementing a machine learning algorithm approach based on TMS measures (SICI, ICF, SAI and LICI), that accurately distinguished AD form other neurodegenerative disorders with a high diagnostic accuracy ranging from 89 to 92% (Benussi et al., 2020c).
3.1.2.2. Frontotemporal dementia
(FTD) is one of the most frequent neurodegenerative dementing disorders after AD and is characterized by behavioral abnormalities, language impairment, and deficits of executive functions (The Lund and Manchester Groups, 1994). Three different variants have been proposed according to the clinical presentation: (i) behavioral variant of FTD (bvFTD) (Rascovsky et al., 2011), (ii) agrammatic variant of primary progressive aphasia (avPPA) and (iii) semantic variant of PPA (svPPA) (Gorno-Tempini et al., 2011). In approximately 10–30% cases, a genetic mutation may be found in the microtubule associated protein tau (MAPT) or granulin (GRN) gene, or expansion on chromosome 9 open reading frame 72 (C9orf72) (Benussi et al., 2021c).
Several motor circuit abnormalities have been reported in FTD, including reduction in M1 excitability or absent MEPs, increase in MEP latencies and CMCT (Bae et al., 2016, Burrell et al., 2011, Chandra et al., 2016, Di Lazzaro et al., 2006a, Wang et al., 2016). SICI and ICF are significantly reduced in FTD (Bae et al., 2016, Benussi et al., 2018a, Benussi et al., 2021a, Benussi et al., 2020a, Benussi et al., 2020b, Benussi et al., 2020c, Benussi et al., 2020d, Burrell et al., 2011, Di Lazzaro et al., 2006a, Padovani et al., 2018, Palese et al., 2020), mirroring the GABAergic and glutamatergic abnormalities which are characteristic of FTLD pathology (Benussi et al., 2019a, Murley and Rowe, 2018). Normal level of SAI was demonstrated by many studies (Benussi et al., 2018a, Benussi et al., 2016, Benussi et al., 2020b, Benussi et al., 2017, Benussi et al., 2019c, Di Lazzaro et al., 2006a, Padovani et al., 2019), confirming that cholinergic deficits are not evident in FTD.
3.1.2.3. Mild cognitive impairment
(MCI) is an intermediate condition between normal aging and dementia. Approximately 50% of patients diagnosed with mild cognitive impairment progress to dementia within 3 to 5 years of diagnosis (Albert et al., 2011, Petersen et al., 2014), underscoring the importance of effective diagnostic biomarkers at a potentially early stage of the disease (Rossini et al., 2022). Of relevance, 70–80% of patients with amnestic mild cognitive impairment have associated AD pathological changes, while 20–30% have other neuropathological processes, including frontotemporal lobar degeneration, Lewy body disease or vascular changes (Petersen and Negash, 2008). Clinical and pathological heterogeneity in mild cognitive impairment could explain the contrasting TMS findings.
In some studies, a non-significant increase in M1 excitability was reported, like that reported in AD (Benussi et al., 2021b, Nardone et al., 2012, Olazarán et al., 2010, Padovani et al., 2018, Sakuma et al., 2007, Tsutsumi et al., 2012). In contrast, no significant differences in SICI and ICF were evident in mild cognitive impairment (Benussi et al., 2021b, Nardone et al., 2012, Olazarán et al., 2010, Padovani et al., 2018, Tsutsumi et al., 2012). Most studies have reported a decrease in SAI (Benussi et al., 2017, Benussi et al., 2021b, Benussi et al., 2020c, Padovani et al., 2018, Peter et al., 2016, Tsutsumi et al., 2012), with one study reporting reduction in amnestic mild cognitive impairment patients only (Nardone et al., 2012), while another study did not show any abnormalities (Sakuma et al., 2007). LTP-like plasticity was found to be unaltered in mild cognitive impairment (Lahr et al., 2016).
A single center study reported high diagnostic accuracy (~90%) of TMS in diagnosing mild cognitive impairment related to AD when compared to non-AD related mild cognitive impairment (Padovani et al., 2018). Specifically, a novel index encompassing SICI, ICF and SAI ([SICI-ICF]/SAI) differentiated AD from non-AD mild cognitive impairment with a specificity of 87.9% and sensitivity of 94.4%. The utility of the novel TMS index was comparable to established biomarkers of amyloidosis (Padovani et al., 2019). A recent multicenter study, utilizing a machine learning algorithm approach re-affirmed a high accuracy [72 to 86%], precision [72–90%] and recall [75–98%] of TMS (SICI, ICF, SAI and LICI measures) in classifying different mild cognitive impairment phenotypes (Benussi et al., 2021b).
3.2. Movement disorders
Over the years, there has been increased interest in investigating changes of motor cortex in patients with movement disorders, particularly Parkinson’s disease (PD) and dystonia with TMS studies. Motor cortex has also been investigated in other types of movement disorders, including atypical parkinsonism, tic, and Tourette’s syndrome (TS), as well as Huntington’s disease (Bologna et al., 2022).
3.2.1. Parkinson’s disease (PD):
Parkinson’s disease is a common movement disorder affecting 1% of the population aged > 65 years, clinically presenting with rest tremor, bradykinesia, and rigidity (Bloem et al., 2021). In PD patients, RMT and AMT were normal in most studies (Chen and Rothwell, 2012), although some studies reported a reduction of RMT (Tremblay and Tremblay, 2002, Valls-Sole et al., 1994), more prominent on the side exhibiting greater rigidity (Cantello et al., 1991, Spagnolo et al., 2013). Increased AMT may correlate with bradykinesia in PD patients, which could be related to difficulties in volitional contraction (Ellaway et al., 1995), although another study did not reveal the same findings (Bologna et al., 2018). In fact, a recent study showed that PD patients with tremor-dominant subtype had both RMT and AMT reduction compared to controls and akinetic-rigid patients (Khedr et al., 2021). The MEP amplitude and the IO curve steepness were found to be increased at rest but reduced with muscle contraction in PD (Valls-Sole et al., 1994). The slope of the IO curve correlated with disease stage and severity of bradykinesia (Bologna et al., 2018, Valls-Sole et al., 1994). A compensatory increase in cortical excitability in response to bradykinesia may account for these TMS findings.
Reduced SICI (Ni et al., 2013, Ridding et al., 1995) and CSP duration (Cantello et al., 2002) have been reported in PD, indicated dysfunction of GABAergic circuits, although at least one paper reported normal SICI in PD, albeit with anterior-posterior directed currents (Hanajima et al., 2011). The degree of reduction of SICI was similar across levodopa-naïve, non-dyskinetic and dyskinetic patients (Ammann et al., 2020). Importantly, SICI was also reduced on the less affected side, even in drug-naïve patients in whom the less affected side was minimally symptomatic (Ammann et al., 2020). With disease progression, there is a further reduction in SICI (Kojovic et al., 2015). Normalization of SICI has been reported with dopaminergic medications (Ni et al., 2013, Ridding et al., 1995) and subthalamic nucleus deep brain stimulation (Cunic et al., 2002), although others have not reported any modulating effects of dopaminergic medications on SICI (Bologna et al., 2018, Lewis and Byblow, 2002, MacKinnon et al., 2005). The discordant findings may be related to heterogeneity of PD patients across different studies. Reduced LICI at ISIs 100 to 150 ms was also reported in PD patients at rest (Chu et al., 2009), although normal or increased LICI have also been reported in PD (Sailer et al., 2003, Valzania et al., 1997). Increased LICI (at ISIs 150–200 ms) with minimal muscle contraction was also reported in PD (Berardelli et al., 1996). The variable LICI findings could be related to different measurement conditions such as conditioning stimulus intensity, ISI, or target muscle status (rest or active). Of further relevance, a significant reduction of CSP duration has been reported in PD patients that were in the “OFF” compared to “ON” state, although in both states the CSP duration was not significantly different when compared to healthy controls (Ridding et al., 1995). Consequently, monitoring CSP duration could serve as a therapeutic biomarker in PD.
Normal (Ridding et al., 1995) or reduced ICF (Bares et al., 2003) has been reported in PD patients (Lefaucheur et al., 2004), while SICF is increased and was associated with reduced SICI, suggesting that SICF could partially account for decreased SICI (Ni et al., 2013). The increase in SICF was observed in de novo PD patients (Shirota et al., 2019) and is further enhanced in PD patients with dyskinesia (Guerra et al., 2019). A triple-pulse protocol reported that in the presence of SICI, SICF-1 was further facilitated in normal subjects but not in PD patients, especially in patients with greater motor impairment, and the effect may be normalized by levodopa (Saravanamuttu et al., 2021). In another study, the combined effect of SICI and SICF-1 (ISI 1.5 ms) was comparable between drug naïve PD patients and healthy controls (Shirota et al., 2019). These findings suggest that abnormal interactions between cortical circuits may be a feature of PD, and the effects may depend on disease stage.
The function of the corpus callosum is affected in PD patients. Specifically, while LIHI was reduced in PD patients with mirror movements, SIHI was normal (Li et al., 2007). Additionally, patients with tremor-dominant subtype were shown to have shorter iSP duration compared to akinetic-rigid patients, while iSP latency tended to be longer in akinetic-rigid patients compared to healthy controls (Khedr et al., 2021).
SAI was reported to be either normal or increased in PD patients off dopaminergic medications (Nardone et al., 2005), but reduced in those taking dopamine medications (Sailer et al., 2003). Stronger SAI was associated with higher gait speed and longer step length in patients receiving dopaminergic medications (Rochester et al., 2012), with SAI partially explaining the variability of gait speed (Rochester et al., 2012). Reduction of SAI was also evident in PD patients prone to falling, even after adjusting for cognitive function (Pelosin et al., 2016), suggesting a role for SAI as a predictive biomarker for gait, posture, and balance impairment. Separately, cognitive impairment in PD was also associated with reduced SAI (Celebi et al., 2012, Nardone et al., 2013a, Yarnall et al., 2013). Reduction of LAI was also reported in PD and was independent of medication states (Sailer et al., 2003). Impairment of the cerebellar-M1 connections, as reflected by reduced CBI, was reported in PD patients off medications which normalized with dopaminergic treatment (Ni et al., 2010, Shirota et al., 2010), although others have reported CBI reduction irrespective of treatment status (Carrillo et al., 2013).
3.2.2. Atypical Parkinsonism:
Atypical Parkinsonian syndromes includes multiple systemic atrophy (MSA), progressive supranuclear palsy (PSP) and diffuse Lewy body dementia. Atypical parkinsonism may be with similar clinical presentation to Parkinson’s disease, but some electrophysiological responses are different between each disorder. Several subtypes of PSP have been described and most studies focused on the typical Richardson subtype. PSP patients exhibit normal CMCT in early disease (Fisicaro et al., 2020), with evidence of CMCT prolongation with disease progression that correlates with disease duration (Abbruzzese et al., 1991, Morita et al., 2008). Moreover, PSP patients exhibit reduced SICI and steeper IO gradients at rest (Conte et al., 2012), as well as normal ICF (Bologna et al., 2017, Kuhn et al., 2004). Reduced iSP duration, implying transcallosal dysfunction, has been reported in the Richardson but not in the Parkinson subtype of PSP (Wittstock et al., 2013). SAI is unchanged in PSP, irrespective of cognitive function, suggesting that cognitive dysfunction evident in PSP is unrelated to deficits in cholinergic pathways (Nardone et al., 2005). Additionally, decreased CBI indicates involvement of the dentate-thalamo-cortical pathway in PSP (Brusa et al., 2014, Shirota et al., 2010), which is compatible with pathological findings showing dentate nucleus and superior cerebellar peduncle degeneration in PSP (Kanazawa et al., 2009).
Several studies showed prolonged CMCT in MSA (Eusebio et al., 2007, Morita et al., 2008), which may be related to UMN signs. Paired-pulse studies showed reduced SICI but normal ICF (Marchese et al., 2000, Suppa et al., 2014). Normal SICF at ISI of 3 ms was reported in both MSA parkinsonism and cerebellar (MSA-C) subtypes (Suppa et al., 2014), suggesting diagnostic utility in differentiating MSA form PD (Ni et al., 2013, Shirota et al., 2019). Prolonged contralateral and ipsilateral CSP durations were reported in MSA (Kuhn et al., 2004), although others found no significant changes (Wolters et al., 2004). Reduced SAI, elicited with digit stimulation, was reported in MSA patients, implying abnormalities in central cholinergic or GABAergic pathways (Mascia et al., 2005). SAI evoked by median nerve stimulation was reduced in MSA-C with cognitive dysfunction, as was CBI which correlated with ataxia severity (Shirota et al., 2022).
Diffuse Lewy body dementia (DLBD) manifests as parkinsonism and dementia, frequently accompanied by cognitive fluctuation, executive or visuospatial dysfunction and rapid eye movement (REM) sleep behavioral disorder (McKeith et al., 2017). In DLBD, reduction of SAI has been reported (Di Lazzaro et al., 2007b, Marra et al., 2012), and a greater SAI reduction was associated with visual hallucinations (Marra et al., 2012), implying central cholinergic deficits (Marra et al., 2012, Tiraboschi et al., 2000). More recent studies have confirmed previous findings of reduced SAI in larger cohorts, at ISIs of N20+0 ms and N20+4 ms, which may be useful to distinguish atypical parkinsonian syndrome from DLBD (Benussi et al., 2018b, Benussi et al., 2020c). The reduction in SAI, however, is not an invariable finding (Nardone et al., 2006), and larger studies with pathological confirmation used as a reference standard for DLBD diagnosis may be required to confirm a potential diagnostic utility of SAI in DLBD.
3.2.3. Huntington’s disease:
In Huntington’s disease (HD), discordant single and paired pulse TMS findings have been reported. While some studies disclosed increased RMT (Schippling et al., 2009), others have reported normal RMT values (Kamble et al., 2018). Similarly, CSP duration was reported to be either normal or reduced (Schippling et al., 2009, Tegenthoff et al., 1996). While the discordant findings were related to phenotypic heterogeneity and differences in methodology (Berardelli et al., 1999, Modugno et al., 2001, Wassermann et al., 2008), CSP shortening correlated with functional decline in HD (Lefaucheur et al., 2006b). Additionally, while some studies reported normal SICI (Hanajima et al., 1999, Priori et al., 2000), other have documented reduced SICI (Abbruzzese et al., 1997, Kamble et al., 2018) and increased ICF (Abbruzzese et al., 1997, Nardone et al., 2007), suggesting abnormalities of intracortical glutamatergic pathways. Reduced SAI was reported in HD gene carriers and early-stage HD patients, suggesting impairment of sensorimotor integration in the pre-symptomatic or early stages of disease. Greater reduction in SAI correlated with an earlier age of disease onset and more severe phenotype (Schippling et al., 2009).
3.2.4. Dystonia:
Dystonia is characterized by involuntary muscle contraction that elicits abnormal posture or irregular repetitive movements. Agonist and antagonist muscle co-contraction is typically recorded. TMS studies in dystonia have revealed normal RMT and IO curves (Kojovic et al., 2013, Quartarone et al., 2009), while most have reported reduced SICI, CSP duration and absence of ICF (Espay et al., 2006, Ridding et al., 1995). Reduced SICI may be evident in idiopathic dystonia (Gilio et al., 2003), dopa-responsive dystonia (Huang et al., 2006) or asymptomatic carriers of the dystonia-1 (DYT-1) gene mutations (Edwards et al., 2003), suggesting dysfunction of cortical inhibitory circuits. In contrast, some studies have reported increased ICF and normal SICI in cervical (Amadio et al., 2014, Ganos et al., 2018a) and focal hand dystonia (Rona et al., 1998) as well as Segawa disease (DYT5) (Hanajima et al., 2007). Differences in dystonic location does not account for TMS differences since reduced SICI was also evident in cervical dystonia when assessed from the unaffected limb (Kanovský et al., 2003). Botulinum toxin treatment could potentially account for the discordant findings (Amadio et al., 2014), since botulinum toxin may restore abnormal SICI one month after injection (Gilio et al., 2000). Methodological differences and individual variability could also contribute to discordant findings (Hanajima et al., 2008).
Separately, reduced resting LICI may be observed in the affected hemisphere in writer’s cramp (Espay et al., 2006), whereas active LICI may be normal (Espay et al., 2006), decreased (Chen R et al., 1997) or increased in dystonia (Rona et al., 1998). CSP duration may be reduced (Chen R et al., 1997), although the results seem task dependent with CSP duration reduced during pincer grasp but normal when performing full strength grip (Stinear and Byblow, 2005, Tinazzi et al., 2005). Prolonged iSP was reported in writer’s cramp, reflecting increased activation of transcallosal projections from the stimulated motor cortex to inhibitory interneurons in the non-stimulated contralateral motor cortex (Niehaus et al., 2001).
Assessment of transcallosal inhibitory connections have disclosed variable findings, depending on presence of mirror movements or whether dystonia is sporadic or familial. Reduced SIHI and LIHI in the affected but not unaffected hand was reported in focal hand dystonia (Nelson et al., 2010), being most prominent at beginning of movement and associated with mirror dystonia (Beck et al., 2009). Others have reported a reduction of SIHI and LIHI in both the affected and unaffected hands in writer’s cramp patients with mirror dystonia, and a greater decrease in IHI was evident with more severe dystonia (Sattler et al., 2014). In musician’s dystonia, IHI impairment may be an endophenotypic biomarker as its reduced in asymptomatic first-degree family members (Bäumer et al., 2016). Surround inhibition is considered a cortical physiological function that suppresses an area surrounding activated neural circuits to enable recruitment of a specific neuronal population. Dystonia patients exhibit decreased surround inhibition (Beck et al., 2008, Sohn and Hallett, 2004).
Sensory-motor integration appears to be abnormal in dystonia. LAI, generated by mixed nerve stimulation at ISI of 200 ms, reversed to facilitation in focal hand dystonia but not in cervical dystonia patients (Abbruzzese et al., 2001), and was most prominent during initiation of a phasic movement. Consequently, decreased LAI cannot explain involuntary contractions or reduced surround inhibition in dystonia patients (Pirio Richardson et al., 2009). Discordant findings have been reported for SAI in dystonia, which represent sensory-motor integration mediated by cholinergic and GABAergic pathways. With digit stimulation, reduced SAI with topographical suppression has been reported in focal hand dystonia (McDonnell et al., 2007), and only in tested muscle located near the digit that was stimulated. When SAI was tested with median nerve stimulation, facilitation was observed at ISIs of N20+10 ms (Kessler et al., 2005), but was normal at other ISIs (25 ms) (Quartarone et al., 2009).
Reduced CBI was also reported in focal hand dystonia (Brighina et al., 2009b), suggesting a pathophysiological role for cerebello-thalamo-basal ganglia pathway (Kaji et al., 2018). In contrast, CBI was reported to be normal in cervical dystonia (Koch et al., 2014b), suggesting differences in pathophysiology across the dystonic phenotypes. Additionally, the suppression of SICI and enhancement of ICF with cerebellar stimulation evident in healthy controls was absent in dystonia patients (Brighina et al., 2009b). Additionally, CBI reduction was documented in focal upper limb dystonia (Brighina et al., 2009b), but not cervical dystonia (Sondergaard et al., 2021b), although the severity of cervical dystonia significantly correlated with CBI reduction (at ISI 5 ms) implying a pathophysiological role for cerebello-thalamo-cortical tract dysfunction in focal dystonias. It should be stressed that assessment of cortical inhibition cannot distinguish organic from functional dystonia’s since CSP duration, SICI and LICI are all reduced in the latter (Avanzino et al., 2008, Espay et al., 2006). SICI and CSP duration in fixed dystonia’s, considered a subtype of functional dystonia, were also reduced.
3.2.5. Tics and Tourette’s syndrome:
Patients with tics and TS have normal RMT and IO curves (Heise et al., 2010, Orth et al., 2008). In Tourette’s syndrome, motor cortex excitability was reduced in the period immediately preceding voluntary movement and during tic suppression (Draper et al., 2015, Ganos et al., 2018b, Jackson et al., 2013). Paired pulse TMS studies have demonstrated SICI reduction in Tourette’s syndrome which correlates with motor tic severity (Gilbert et al., 2004, Orth et al., 2008, Orth and Rothwell, 2009, Ziemann et al., 1997a). CSP duration in Tourette’s syndrome patients was significantly reduced compared to controls (Ziemann et al., 1997a).
3.3. Myelopathy and spinal cord injuries
3.3.1. Cervical spondylitic myelopathy:
In chronic spondylitic myelopathy, the CMCT remains the most robust and commonly utilized parameter for evaluating the damage to the corticospinal tract due to spinal cord compression (Funaba et al., 2015, Lo, 2007, 2008). By evaluating CMCT for both proximal and distal upper limb muscles it is also possible to identify more precisely the level of cord dysfunction (Di Lazzaro et al., 1992). CMCT may be abnormal even in the absence of pyramidal signs (Lanza et al., 2020), and is particularly sensitive when MEPs were obtained from the abductor pollicis brevis (APB) muscle, at least for C6-C7 myelopathy (Imajo et al., 2018, Shibuya et al., 2014). The prolongation in CMCT has also been correlated with the ratio of flattening and anteroposterior diameter parameters visualized with MRI (Rikita et al., 2017) and kinematic CT myelography (Funaba et al., 2021), as well as long-term functional outcome after cervical spine decompression (Deftereos et al., 2015, Mazur et al., 2014, Nakanishi et al., 2014, Takahashi et al., 2008). Abnormal CMCT was also documented in other myelopathies, including those resulting from high voltage electrical burns, even if MRI was unremarkable (Seo et al., 2011), and mucopolysaccharidosis (Cantone et al., 2019).
Chronic spondylitic myelopathy exhibits similar features as chronic progressive spinal cord injury and may share similar TMS findings. Specifically, motor cortex mapping with TMS showed dynamic changes after chronic spondylitic myelopathy surgery (Green et al., 2015) and spinal cord injury (Tazoe and Perez, 2021). The findings pertaining to CSP duration have been more variable, including an increase (Barry et al., 2013), reduction (Shimizu et al., 2000) or absence of change (Nardone et al., 2013b).
3.3.2. Spinal cord injuries:
In spinal cord injuries, TMS assesses the functional integrity of the corticospinal tracts and motor control mechanisms. While no single TMS parameter may be considered a validated biomarker here, it may provide additional information on severity, prognosis and therapy. TMS provides information in four domains: assessment of residual function, cortical excitability changes, longitudinal follow up, and rehabilitation. TMS provides a useful method of assessing abdominal muscle motor preservation in spinal cord injury (Bjerkefors et al., 2015), as demonstrated in residual innervation of pelvic floor muscles by indirect cortical descending pathways (Williams et al., 2020). Additionally, TMS studies have disclosed preservation of crossed corticospinal facilitation in truncal muscles after an incomplete spinal cord injuries, as reflected in truncal control during functional arm movements (Chiou and Strutton, 2020). Another study suggested that paired corticospinal-motoneuronal stimulation may enhance spinal plasticity after spinal cord injury (Bunday et al., 2018).
Deafferentation due to acute spinal cord injuries can change the state of large cortical networks within one hour, and these changes play a critical role in the functional reorganization of central pathways (Nardone et al., 2013c). With chronic cervical spinal cord injuries, individuals have lower MEP amplitudes and a tendency toward higher TMS motor thresholds relative to healthy controls. However, no significant difference in CSP duration was observed (Sfreddo et al., 2021). A study on cortical stimulation had found prolonged MEP latencies in all coil orientations in spinal cord injuries compared to control subjects. However, the MEP latencies elicited by posterior-anterior and anterior-posterior compared to lateral-medial cortical stimulations were shorter in spinal cord injuries, particularly for MEPs elicited by anterior-posterior currents (Jo et al., 2021). MEP amplitudes remained unchanged in muscles at and within 5 segments below the cord injury during 70% of maximum voluntary contraction compared to rest. In muscles beyond the 5 segments below spinal cord injuries, MEP amplitudes were significantly higher (Bunday et al., 2013).
Paired pulse TMS studies have reported increased SICF in spinal cord injuries patients with normal CMCT, while those with abnormal CMCT showed lower SICF. The neural elements producing SICF could have increased in activity after spinal cord injuries to enhance activation of residual corticospinal tract pathways, compensating for impairment of the motor cortex in generating appropriate voluntary movements (Nardone et al., 2015a, Nardone et al., 2015b). Incomplete spinal cord injuries reduces SICI compared to controls (Roy et al., 2011), suggesting an increase in cortical excitability. In contrast, other studies have reported increased AMTs and CSP duration with spinal cord injuries (Freund et al., 2011), suggesting reduced cortical excitability. The discordant cortical excitability findings in spinal cord injuries may be related to small study cohorts and require further validation in larger sample of subjects (Nardone et al., 2015b). More recent studies have suggested that deficits in corticospinal transmission after incomplete cervical spinal cord injuries extend to the preparatory phase of upcoming movements (Federico and Perez, 2017).
Smaller MEP amplitudes and a shortening of reaction time to startle have been reported in patients with incomplete spinal cord injuries and spasticity, suggesting that imbalanced corticospinal and reticulospinal tract contributions are more pronounced in participants with chronic incomplete spinal cord injuries (Sangari and Perez, 2019). Increased reticulospinal inputs to biceps but not triceps brachii, and loss of corticospinal drive to triceps brachii in tetraplegic spinal cord injuries patients likely represent reorganization of descending motor control, thereby contributing to asymmetrical recovery between elbow flexor and extensor muscles after cervical spinal cord injuries I (Sangari and Perez, 2020). Overall, lesion studies involving corticospinal and vestibulospinal pathways, which makes differential contributions to impairment of gait ability and balance, indicate that no single electrophysiological or anatomical measure can provide an optimal prediction of clinical gait and balance disability as an ideal biomarker in spinal cord injuries (Barthélemy et al., 2015).
A long-term study of spinal cord injuries patients revealed a significant decrease in motor cortical excitability acutely, involving spinal segments below the lesion and sparing muscles rostral to the lesion, with the inhibition persisting for up to 3 years (Kriz et al., 2012). Another study reported an increase in MEP amplitude over a 12-month follow-up period which was paralleled by a significant improvement of motor and walking function (Petersen et al., 2012). Rapid motor cortical reorganization was demonstrated after spinal cord injuries which normalized at 24 months post injury (Dias Leao et al., 2020, Fassett et al., 2018). Motor maps areas are also increased when assessed at rest and decreased during voluntary contraction, with reduction being greater in patients with greater sensory deficits (Tazoe and Perez, 2021). These findings suggest that sensory input may further reshape abnormal changes in motor cortical maps in humans with chronic spinal cord injuries during voluntary contraction. Additionally, MEPs can be recorded from bulbocavernosus and external anal sphincter muscles with cortical and sacral nerve root stimulation, to assess peripheral innervation and central motor control in sacral/pudendal territories and to demonstrate the presence of neurological disorder affecting the genito-urinary tracts (Opsomer et al., 1989) or anorectal function (Lefaucheur, 2006).
3.4. Epilepsy
Cortical excitability appears to be increased in drug naïve epilepsy patients, being most prominent in generalized epilepsy. For generalized epilepsy, the majority of studies have reported lower RMTs (de Goede et al., 2016), although the reduction was only significant in specific epilepsy phenotypes including juvenile myoclonic epilepsy (Badawy et al., 2013b, Cuypers et al., 2013). Additionally, a meta-analysis reported a non-significant trend towards lower RMTs for generalized epilepsy in general, with the reduction being significant only in JME (Brigo et al., 2012). In contrast, others have reported increased RMT values, which was attributed to activation of inter-ictal compensatory mechanisms to prevent the spreading or recurrence of new seizures (Badawy et al., 2009, Lee et al., 2015) or as a result of antiepileptic drugs (AEDs) which block sodium channels (Rossini et al., 1994, Rossini et al., 2015, Ziemann et al., 2015). In focal epilepsy, RMT was not significantly different when compared to healthy controls (de Goede et al., 2016). Separately, the MEP amplitudes were within normal limits for both generalized and focal epilepsy phenotypes (de Goede et al., 2016, Klimpe et al., 2009, Lee et al., 2015).
Prolonged CSP duration has been reported in generalized epilepsy, a finding attributed to a protective hyperactivation of inhibitory circuits acting to prevent recurrence of new seizures (Cincotta et al., 2015, de Goede et al., 2016). A significant reduction in CSP duration was reported in the familial cortical myoclonic tremor with epilepsy phenotype (Suppa et al., 2009). In focal epilepsy, non-significant reduction in CSP duration has been reported (Cincotta et al., 2015, de Goede et al., 2016).
In drug naïve patient with generalized epilepsy, SICI (measured at ISI of 2 and 5 ms) was reported to be reduced or absent in the contra-and ipsilateral motor cortices (Badawy et al., 2012, 2013a, Badawy et al., 2010, Badawy et al., 2013b, de Goede et al., 2016, Werhahn et al., 2000a), although this was not a consistent finding (Cantello et al., 2006, de Goede et al., 2016, Lee et al., 2015). Although there was no significant difference in ICF measured at ISIs 10ms and 15 ms in generalized epilepsy (Badawy et al., 2012, 2013a, Badawy et al., 2010, Badawy et al., 2013b, Lee et al., 2015), averaged ICF was increased (Cantello et al., 2006). Interestingly, ICF may be decreased within 48 hours of a grand-mal seizure (Delvaux et al., 2001). For focal epilepsy, SICI was reduced while no significant changes were evident for ICF (de Goede et al., 2016).
LICI was abnormal in generalized epilepsy, with absence of inhibition evident at ISIs of 50, 150, 250 and 300 ms (Badawy and Jackson, 2012, Badawy et al., 2012, 2013a, Badawy et al., 2010, Badawy et al., 2013b). In focal epilepsy, LICI was facilitated on the ipsilateral side at ISIs of 250 and 300 ms.
Patients with some forms of epilepsy are more likely to have seizures after sleep deprivation or when assessed in the early morning period, and these factors can increase the likelihood of detecting interictal EEG epileptiform abnormalities (Badawy et al., 2006, Renganathan and Delanty, 2003). TMS studies have shown that these activating factors are associated with increased cortical excitability and reduced intracortical inhibition, potentially accounting for an increased risk of seizures in these settings (Badawy et al., 2006, Kreuzer et al., 2011, Manganotti et al., 2006, Serafini et al., 2013).
A potential limitation of using TMS in epilepsy relates to MEP variability in patients and controls (Corp et al., 2021). While MEP variability may be reduced with close attention to target muscle, pulse waveform and use of neuronavigation, current findings can only be reliably applied in a large cohort setting and not on an individual patient. Consequently, it seems unlikely that TMS will be able to be used as a diagnostic biomarker in epilepsy. In one study using LICI, the reported diagnostic sensitivity was reported to be as low as 24% (Young et al., 2009). The utility of TMS may be in evaluating the physiological effects of AEDs as a biomarker of changes in cortical excitability and future seizure risk (Badawy et al., 2012).
3.5. Migraine and other headaches
The first ten years of this century were dedicated to exploring the pathophysiology of migraine with TMS and to examine the clinical diagnostic utility of the different TMS techniques applied on distinct brain areas (Chen et al., 2008). The studies performed have provided seemingly contradictory findings, reasonably because each investigation had depicted distinct facets of a complex pathophysiological mechanism. In the last ten-to-twelve years, although great efforts have been devoted to test TMS as a possible treatment for migraine, some of the original contradictions have been clarified, whereas others remained unsolved.
3.5.1. Migraine without aura:
Abnormal cortical plasticity was reported in migraine without aura (MO) patients, and these plasticity changes were most evident on paired associative stimulation testing when the ISI was set to 10 ms, with MEP responses potentiated (Pierelli et al., 2013). Variability in RMT findings have been reported in MO patients, including normal, increased, or reduced RMT (Afra et al., 1998, Badawy and Jackson, 2012, Bettucci et al., 1992, Brighina et al., 2010, Cortese et al., 2017, Gunaydin et al., 2006, Maertens de Noordhout et al., 1992, Neverdahl et al., 2017, Pierelli et al., 2013, Siniatchkin et al., 2009, van der Kamp et al., 1996, van der Kamp et al., 1997, Werhahn et al., 2000b). The RMT appears to be influenced by the proximity of a migraine attack, being higher if measured closer to the attack (Cortese et al., 2017), potentially accounting for the variability of RMT findings. Dependance on the exact time-point at which physiological measurements are made during the migraine cycle was not confirmed in the motor cortex of children and adolescents but was replicated in the visual cortex (Siniatchkin et al., 2009). These findings confirm that excitability of motor and occipital cortex may differ in the same patient.
CSP duration, assessed interictally, was reported to be normal in MO patients (Maier et al., 2011, Siniatchkin et al., 2007), although was reduced in female interictal migraineur patients (Neverdahl et al., 2017, Yuksel and Topalkara, 2021). CSP shortening was exacerbated by a contingent negative variation task requiring focused sustained attention (Maier et al., 2011), and induced by sleep restriction especially in patients with non-sleep related migraine (Mykland et al., 2022).
In patients studied interictally, SICI was decreased when tested at ISI of 4-ms but not 2-ms (Cosentino et al., 2018, Mykland et al., 2022, Neverdahl et al., 2017). These observations underly the relevance of the stimulation parameters used when testing intracortical inhibition in migraine. Using standard stimulation paradigms, ICF was reported to be normal interictally (Neverdahl et al., 2017), albeit increased when using suprathreshold conditioning stimuli (Siniatchkin et al., 2007). The increase in ICF with subthreshold conditioning stimuli was only evident with low suprathreshold test stimuli [110%RMT] (Cosentino et al., 2018). During the preictal phase ICF decreased (Neverdahl et al., 2017), indicating that changes in migraine cycle affect ICF.
LICI studied with ISIs up to 120-ms showed no significant abnormality in migraine patients (Cosentino et al., 2018, Siniatchkin et al., 2007). Increasing the test stimulus intensity to 150% RMT leads to reduction of LICI, which positively correlates with disease duration (Cosentino et al., 2018). In a separate study, LICI was reduced at ISI of 250-ms (Badawy and Jackson, 2012), implying dysfunction of long latency intracortical inhibitory circuits in migraine. When studied interictally, SAI was found to be either normal (Alaydin et al., 2019) or reduced (Coppola et al., 2020) in MO patients. When assessed in the immediate preictal or ictal periods, SAI was reduced (Alaydin et al., 2019, Coppola et al., 2020).
3.5.2. Migraine with aura:
In migraine with aura (MA) patients, TMS studies have disclosed normal RMT (Badawy and Jackson, 2012, Brighina et al., 2011, Cosentino et al., 2011), but increased MEP amplitude in response to increasing stimulus intensity (Cosentino et al., 2011) or repetitive TMS (rTMS) [at 5Hz] delivered at 110% RMT intensity (Brighina et al., 2011). The changes in TMS variables were reversed by prophylactic levetiracetam treatment (Brighina et al., 2011, Cosentino et al., 2011). Of relevance, visual cortex exhibited reduced TMS-elicited phosphene thresholds, which was reduced by anodal transcranial direct current stimulation (Chadaide et al., 2007). Low-frequency rTMS (at 1 Hz) resulted in a reduction in TMS-elicited phosphene thresholds, an effect reverted by valproate treatment (Palermo et al., 2009).
The CSP duration was reduced in MA patients (Chen et al., 2008, Maier et al., 2011, Mykland et al., 2022), while LICI at ISI 250 ms was facilitated (Badawy and Jackson, 2012). The deficits of cortical inhibition appear to be more related to aura rather than headache mechanisms. Additionally, reduction of CSP duration was reported interictally, in females migraineurs during pre-ovulatory recordings (Yuksel and Topalkara, 2021), and may be evident when recording from facial muscles (Curra et al., 2007). Based on the calcium channel hypothesis of hemiplegic migraine and the observation that P/Q-type calcium channels are strongly expressed in the cerebellum, CBI was shown to be reduced in MA patients (Brighina et al., 2009a).
Occipital TMS may also be an effective tool in discriminating between transient ischemic attacks and migraine aura without headaches aura based both on the frequency and threshold of inducing phosphenes (Naeije et al., 2017), although further studies are required to confirm utility. Interestingly, topiramate modulates occipital cortex excitability, although the modulating effects were independent of clinical benefits, as reflected by reduction in headache frequency (Aurora et al., 2010). In chronic migraine patients without aura, RMT was reduced and SICI absent, while patients with episodic migraine exhibited normal RMT and SICI values (Valente et al., 2021). Botulinum toxin therapy resulted in partial normalisation of SICI in the chronic migraine patients after 12-months of treatment accompanied by improvement in pain (Valente et al., 2021). Taken together, it was proposed that botulinum toxin therapy resulted in long-term alteration of cortical plasticity, mainly due to effects on chronic pain.
TMS studies in episodic cluster headaches disclosed physiological RMT and CSP duration values in both hemispheres, while SICI was reduced and ICF increased in the ipsilateral hemisphere to the headache side (Cosentino et al., 2015). Of relevance, SICI was reduced when assessed ictally, whereas ICF was increased both ictally and interictally. Similarly, a reduction of SICI and increase in ICF were reported in cluster headache and allodynia (Ekizoglu et al., 2015).
In patients with medication-overuse headache, the CSP differed according to the type of medication overused (Currà et al., 2011). In patients overusing triptans alone, CSP duration was reduced, similar to migraineur patients. In contrast, overuse of non-steroidal anti-inflammatory agents alone or in combination with triptans, was not associated with abnormalities of CSP duration. The cortical changes were attributed to medication-induced neural adaptation, potentially mediated by alterations in central serotonin neurotransmission.
3.6. Neuropathic pain
In acute or tonic pain experiments, various changes in corticospinal motor excitability occur, as assessed by TMS techniques (Burns et al., 2016a, Lefaucheur, 2004). A recent meta-analysis concluded that greater reduction motor cortex excitability was associated with shorter durations and higher levels of induced experimental pain (Chowdhury et al., 2022). Additionally, corticomotor depression in the early stage of pain could indicate a higher susceptibility to development of chronic pain (Seminowicz et al., 2019). In chronic pain, measures of cortical excitability can reflect impairment of various neurotransmitter systems related to maladaptive plasticity of pain modulatory systems. Corticospinal excitability changes are not specific to pain pathophysiology. Therefore, TMS is not a relevant tool for diagnosis of chronic pain or its mechanisms (neuropathic, nociceptive, or nociplastic), but could be a biomarker to understand or monitor therapeutic analgesic interventions.
Only two meta-analyses that assessed changes of cortical excitability parameters in chronic pain have been published (Chang et al., 2018, Parker et al., 2016). A significant reduction of SICI and CSP duration was reported in one study, especially in the context of neuropathic pain (Parker et al., 2016). A trend towards increased SICF was reported, albeit from a single study, while no difference was found for RMT, IO curve, ICF, and LICI in chronic pain. A subsequent meta-analysis reported inconclusive findings regarding reduction in SICI and CSP duration, except in complex regional pain syndrome, with a trend towards increased LICI in chronic pain (Chang et al., 2018). Other reviews have also discussed cortical excitability changes in specific chronic pain conditions, such as complex regional pain syndrome (Di Pietro et al., 2013, Nardone et al., 2018), central post-stroke pain (Betancur et al., 2021), or phantom-limb pain (Nardone et al., 2019), with variable findings.
The main TMS feature most consistently associated with chronic pain is SICI reduction in the motor cortex contralateral to the painful limb (Burns et al., 2016b, Eisenberg et al., 2005, Lefaucheur et al., 2006a, Schwenkreis et al., 2010, Sorel et al., 2018), suggesting that impairment of GABAergic neurotransmission could contribute to chronic pain pathophysiology. This notion is supported by findings of SICI normalization in response to therapeutic interventions that induce analgesic effects, such as motor cortex rTMS, peripheral nerve repetitive magnetic stimulation or ketamine infusion, with the degree of pain relief correlated with SICI improvement (Fierro et al., 2010, Lefaucheur et al., 2006a, Massé-Alarie et al., 2013, Mhalla et al., 2011, Sorel et al., 2018). It remains to be determined whether SICI reduction could serve as a biomarker to select candidates for analgesic neuromodulation, especially with regard to high frequency rTMS applied over the contralateral motor cortex, a therapeutic use of rTMS with high level of evidence of efficacy (Lefaucheur et al., 2020). The response to rTMS could be used as a surrogate biomarker to predict efficacy of invasive cortical stimulation (André-Obadia et al., 2014, Lefaucheur et al., 2011, Pommier et al., 2018).
Changes in motor cortex excitability may also be related to impaired descending inhibitory pain controls, as assessed by conditioned pain modulation (CPM) protocols. In fact, defective descending inhibitory controls (no pain reduction during CPM) have been associated with increased ICF (Botelho et al., 2016), reduced CSP duration (Tarragó et al., 2016) or greater SICI in fibromyalgia (Cardinal et al., 2019, Caumo et al., 2016). The changes in cortical excitability correlated with increased serum brain-derived neurotrophic factor (BDNF) levels (Botelho et al., 2016, Cardinal et al., 2019, Caumo et al., 2016). It should be stressed that these changes in cortical excitability were opposite to that observed in chronic pain (reduced SICI and CSP duration), which cannot be explained through changes in descending controls.
Beyond cortical excitability measures, TMS can also be applied for mapping of motor cortical representation, notably using image-guided neuronavigation systems. Studies have been performed in stroke and phantom limb patients (Gunduz et al., 2020, Teixeira et al., 2021), although no clear association between motor map reorganization and presence or intensity of pain have been established beyond the plasticity of motor function in these conditions.
3.7. Multiple sclerosis
The probability that a patient with clinically definite MS (CDMS) has a prolonged CMCT is moderately high, with substantial variability across studies [56–93%] (Barker et al., 1986, Beer et al., 1995, Hess et al., 1986, Hess et al., 1987, Ingram et al., 1988, Jones et al., 1991, Kandler et al., 1991b, Mayr et al., 1991, Michels et al., 1993, Ravnborg et al., 1992, Rossini et al., 1989, van der Kamp et al., 1991). The large variability is explained by many factors, but most importantly by the selection and number of target muscles. Sensitivity increases if lower limb muscles are included (Jones et al., 1991, Jung et al., 2006, Kandler et al., 1991b, Mayr et al., 1991), and may also be influenced by the MS phenotype. Specifically, CMCT prolongation is more pronounced in progressive MS than in relapsing-remitting MS (RRMS) (Facchetti et al., 1997, Filippi et al., 1995, Humm et al., 2003, Kidd et al., 1998). Additionally, in RRMS or secondary progressive MS (SPMS) patients with lesions in the hand area of sensorimotor cortex, CMCT is prolonged (Madsen et al., 2022). Interestingly, CMCT can reveal a subclinical involvement of the corticospinal tracts in about 14% of multiple sclerosis patients (Di Lazzaro et al., 1999a).
Conventional measurements of MEP amplitude by single-pulse TMS add little to the sensitivity provided by CMCT measurements (Hess et al., 1987, Kandler et al., 1991b, Ravnborg et al., 1992). Triple-stimulation technique (TST, Figure 2), however, have revealed frequent occurrence of central conduction failure due to focal central conduction block (Humm et al., 2004a) or loss of fastest-conducting corticospinal axons, even in the presence of normal CMCT and MEP measures (Hofstadt-van Oy et al., 2015, Magistris et al., 1999). TMS abnormalities, including prolonged MEP latency and CMCT as well as reduced MEP amplitude, was evident in MS patients without pyramidal tract signs (Kale et al., 2009), implying that the diagnostic utility of TMS is possibly underestimated in the currently recommended diagnostic criteria for MS (Thompson et al., 2018).
Axial muscles such as the diaphragm, paraspinal, pelvic floor and external sphincter muscles are often affected in MS. The corticospinal projection to these muscles is more difficult to assess, but TMS measures may reveal abnormalities (Brostrom et al., 2003, Eardley et al., 1991, Garland et al., 1996, Hashimoto et al., 2000, Lagueny et al., 1998, Miscio et al., 2003, Urban and Vogt, 1994).
Many studies indicated a significant correlation between CMCT or TST abnormalities and clinical motor signs or motor disability (Britton et al., 1991, Ingram et al., 1988, Jones et al., 1991, van der Kamp et al., 1991). CMCT measures integrated into a multimodal evoked potential (EP) score revealed close correlations with the Expanded Disability Status Scale [EDSS] (Bednarik and Kadanka, 1992, Fuhr et al., 2001). With longitudinal measurements, changes in EP score correlated with changes in EDSS (Ayache et al., 2015, Fuhr et al., 2001, Schlaeger R et al., 2012).
Multimodal EP scores, including baseline CMCT measurement, predicted the EDSS score in CDMS patients 2–3 years later (Fuhr et al., 2001, Schlaeger R et al., 2012) and longterm disability 14 years later (Schlaeger R. et al., 2012). Consequently, high multimodal EP scores at the time of measurement seem to be predictive of disability development.
TMS studies may also have a positive predictive value of conversion to MS, which may be an important management issue in patients with radiologically isolated or clinically isolated syndromes (CIS), who do not fulfill the current diagnostic criteria for MS (Thompson et al., 2018). To date, only one study has addressed this issue and demonstrated a longer CSP duration in CIS patients who developed CDMS within the next 24 months compared to those that did not develop CDMS (Pallix-Guyot et al., 2011). Other TMS measures (CMCT, MEP amplitude, RMT, iSP duration, transcallosal conduction time) did not differentiate between these two CIS groups (Pallix-Guyot et al., 2011).
Monitoring or predicting treatment efficacy is another potential utility of TMS. CMCT improves during an MS relapse treated with corticosteroids and correlates with clinical improvement (Fierro et al., 2002, Kandler et al., 1991a, La Mantia et al., 1994, Salle et al., 1992). Reduced MEP amplitude and MEP map size after a first-time motor relapse improves at 6-months follow-up, and this improvement is associated with improvement of hand dexterity (Chieffo et al., 2019). Reduced SAI improves in PPMS patients over one year of treatment with ocrelizumab, and this is associated with an improvement in the 9-hole peg test, a measure of sensorimotor hand function (Dubbioso et al., 2022a). Of relevance, the treatment response to 4-aminopyridine, an agent that improves gait function in SPMS (Goodwill et al., 2013), was predicted by prolonged CMCT to lower extremities (Zeller et al., 2014) and high RMT to a hand muscle (Ahdab et al., 2019).
TMS measures such as MEP onset latency variation (Britton et al., 1991, Fujihara and Miyoshi, 1998), TMS-frequency-dependent CMCT prolongation and MEP attenuation (Claus et al., 1992, Nielsen, 1997), greater increase in CMCT and more prolonged MEP amplitude reduction in response to fatiguing exercise (Coates et al., 2020, Liepert et al., 1996, Liepert et al., 2005, Petajan and White, 2000, Russo et al., 2015, Schubert et al., 1998, White and Petajan, 2004) have also been applied in MS, although their clinical utility remains to be determined. Additionally, reduction of SICF (Dubbioso et al., 2022a, Mori et al., 2013), SICI (Belvisi et al., 2022, Caramia et al., 2004, Conte et al., 2009, Vucic et al., 2012), SAI (Cucurachi et al., 2008, Dubbioso et al., 2022a), and prolonged CSP duration (Nantes et al., 2016), prolonged transcallosal conduction time (Boroojerdi et al., 1998a) and prolonged iSP duration (Boroojerdi et al., 1998b, Höppner et al., 1999, Jung et al., 2006, Schmierer et al., 2002, Schmierer et al., 2000) have also been reported in MS.
3.8. Stroke
Stroke is associated with abnormalities in TMS measures from both the ipsilesional and contralesional primary motor cortex. Most studies record MEPs from the upper limbs, and less is known about the effects of stroke on MEPs recorded from the lower limbs or swallowing muscles. The most obvious abnormality is an absence of MEPs in response to ipsilesional M1 stimulation. The absence of MEPs prevents measurement of motor threshold, MEP amplitude or latency, but still provides clinically important information.
Absent paretic upper limb MEPs at the early sub-acute stage of stroke strongly predicts worse motor recovery and outcomes, and at the chronic stage is related to worse motor performance (Boyd et al., 2017, Cicinelli et al., 1997, Karatzetzou et al., 2022, Stinear, 2017, Talelli et al., 2006, Traversa et al., 1997). The absence of MEPs in the paretic lower limb at the early sub-acute stage is also linked to worse walking outcomes (Karatzetzou et al., 2022, Preston et al., 2021), although further research is needed. There is initial evidence that proximal upper limb muscles can recover strength despite an initial absence of MEPs, possibly via descending motor pathways that are less-readily accessed by TMS such as the reticulospinal tract (Schambra et al., 2019). Further work is needed to understand the clinical implications of MEP absences in the proximal versus distal upper limb muscles.
The presence or absence of upper limb MEPs at the early sub-acute stage is a relatively simple biomarker that can inform individualized therapy plans (Rosso and Lamy, 2020, Stinear, 2017, Stinear et al., 2017a) and improve the efficiency and sensitivity of clinical trials (Stinear et al., 2018). The MEP status biomarker, however, has yet been consistently defined. After a stroke, it is not uncommon to observe low amplitude MEPs that fail to meet traditional threshold amplitude criteria even at maximum stimulus intensity. Consequently, some studies define MEP+ status as the presence of MEPs of any amplitude, on the basis that transmission of motor output from ipsilesional M1 is more clinically relevant than threshold stimulus intensity at the early sub-acute stage (Stinear et al., 2017a, Stinear et al., 2017b). It remains unclear whether initially absent or subthreshold MEPs that eventually meet the motor threshold criterion during subsequent recovery are associated with better outcome (Schambra et al., 2019, Talelli et al., 2006).
When MEPs are present in paretic muscles, they can exhibit several abnormal features. Motor threshold is typically higher, MEP latency is delayed, MEP amplitude is smaller, and the slope of the stimulus-response curve is typically shallower (Bütefisch et al., 2008, Cicinelli et al., 1997, McDonnell and Stinear, 2017, Talelli et al., 2006, Traversa et al., 1997, Veldema et al., 2021). Motor map areas of the ipsilesional M1 are also typically smaller, and their center of gravity can be shifted anteriorly or posteriorly relative to maps of the contralesional M1 (Cicinelli et al., 1997, Lüdemann-Podubecká and Nowak, 2016). These abnormalities reflect the effects of stroke on the number of available cortical neurons and descending axons, and the excitability of surviving cortical neurons. More pronounced abnormalities in MEP threshold, latency, and amplitude, and motor map parameters are typically associated with worse motor performance at time of assessment (Boyd et al., 2017, Buetefisch et al., 2018, Duque et al., 2005, Lüdemann-Podubecká and Nowak, 2016, Talelli et al., 2006). Normalization of upper limb MEP parameters during the sub-acute stage of stroke is associated with improvements in upper limb motor performance (McDonnell and Stinear, 2017, Schambra et al., 2019, Stinear et al., 2015, Veldema et al., 2021). The clinical relevance of shifts in motor map center of gravity is not yet clear (Lüdemann-Podubecká and Nowak, 2016).
Measures of contralesional M1 excitability are typically within normal limits at the sub-acute stage of stroke (McDonnell and Stinear, 2017, Talelli et al., 2006). Contralesional excitability can become elevated at the chronic stage, perhaps reflecting prolonged asymmetric upper limb use after stroke (Xu et al., 2019). Studies at the chronic stage initially reported excessive pre-movement inhibition of the ipsilesional M1 by the contralesional M1 via the corpus callosum, and this was thought to contribute to poorer paretic upper limb performance (Duque et al., 2005, Murase et al., 2004). The resulting interhemispheric competition model posited that asymmetric interhemispheric inhibition between the M1s compounds reduced ipsilesional cortical excitability and hinders motor recovery. Subsequent studies (Bütefisch et al., 2008, Stinear et al., 2015, Xu et al., 2019) and meta-analyses (McDonnell and Stinear, 2017, Veldema et al., 2021) have shown that contralesional M1 excitability and interhemispheric inhibition of ipsilesional M1 are typically normal at the sub-acute stage of stroke. Abnormally elevated pre-movement interhemispheric inhibition of the ipsilesional M1 appears to develop as patients recover upper limb motor capacity and is therefore unlikely to be a useful therapeutic target (Xu et al., 2019). Furthermore, a recent review argues that transcallosal projections serve to shape the output of the opposite M1, rather than inhibit it (Carson, 2020).
Stimulation of the contralesional M1 can also produce MEPs and/or CSPs in paretic upper limb muscles. Ipsilateral MEPs can be more prevalent in the paretic upper limb, particularly the proximal muscles. In older adults without stroke, there is a positive association between upper limb strength and the ratio between ipsilateral and contralateral MEPs, with the former thought to be mediated by the reticulospinal tract (Maitland and Baker, 2021). There appears to be no clear relationship between ipsilateral responses to contralesional M1 stimulation and paretic upper limb performance (Hammerbeck et al., 2019). The neuronal populations responsible for ipsilateral MEPs and CSPs, and their clinical significance, remains under investigation.
Paired-pulse studies indicate that ipsilesional and contralesional SICI and LICI are typically abnormally low at the early sub-acute stage of stroke and normalize over subsequent weeks (Bütefisch et al., 2008, Dimyan and Cohen, 2010, Grigoras and Stagg, 2021, Huynh et al., 2016, McDonnell and Stinear, 2017, Talelli et al., 2006), though some longitudinal studies using threshold tracking techniques report persistently low SICI in both hemispheres (Huynh et al., 2013b, Huynh et al., 2016). Abnormally low SICI has also been reported in contralateral M1 following acute cerebellar stroke (Huynh et al., 2013a). Further research is required to establish the clinical significance of altered intracortical function after stroke, and whether it presents a viable therapeutic target (Agarwal et al., 2019).
3.9. Cerebellar disorders
In focal diseases affecting the cerebellar efferent system and comprising the cerebellar hemispheres, dentate and ventrolateral thalamic nuclei, CBI is decreased or absent (Kikuchi et al., 2012, Ugawa et al., 1997). In contrast, CBI was normal in patients with lesions of the afferent cerebellar inputs, including pontocerebellar, middle cerebellar peduncle and sensory-cerebellar pathways (Ugawa et al., 1994a, Ugawa et al., 1995a). TMS findings in chronic cerebellar neurodegenerative diseases are more complex, reflecting the underlying pathology. A reduction of CBI (at ISI 5 ms) was reported in spinocerebellar ataxia type 3 (SCA3), correlating with ataxia severity, and indicating the pathophysiological importance of cerebellothalamocortical tract dysfunction (Maas et al., 2021, Ugawa et al., 1997). CBI was normal in mildly affected SCA3 patients, suggesting that reduction of efferent pathway integrity is not a relevant feature of the earliest disease stages or that TMS only detects abnormalities after a certain threshold of dysfunction has been exceeded. A significant reduction of CBI (at ISI 5 and 6 ms) was also reported in the multiple system atrophy cerebellar subtype (MSA-C) and correlated with greater disease severity (Shirota et al., 2022). In early MSA-C with predominant dysfunction in the cerebellar afferent pathways, CBI is normal, implying a potential role for CBI as a biomarker of disease progression in neurodegenerative cerebellar diseases, although further research is required.
Reduction of CBI may herald the development of cerebellar dysfunction in other neurological disorders including progressive supranuclear palsy (PSP), essential tremor and focal dystonia. Specifically, an absence of CBI has been reported in PSP associated with degeneration of the dentate nuclei (Benussi et al., 2019b, Brusa et al., 2014, Shirota et al., 2010). While cerebellar ataxia is not a typical clinical feature of PSP, gait instability and falls could potentially be associated with dysfunction of cerebellar efferent pathways (Benussi et al., 2019b). Reduction of CBI was also reported in essential tremor but did not correlate with tremor severity (Hanajima et al., 2016), suggesting that the cerebellar efferent pathways were either a primary pathogenic event or a compensatory physiological phenomenon in response to a pathogenic process outside the cerebellum. However, another study found normal CBI in essential tremor (Pinto et al., 2003).
Increased motor threshold was reported in acute cerebellar stroke, contralateral to the effected cerebellar hemisphere (Cruz-Martínez and Arpa, 1997), although others have reported normal thresholds (Huynh et al., 2013a, Liepert et al., 2004). Transient prolongation of CMCT has also been reported after cerebellar stroke (Cruz-Martínez and Arpa, 1997), with the argument that reduced size and increased dispersion of the efferent volleys accounted for the findings. A reduction of SICI has been reported in both the ipsi- and contralesional M1 (Huynh et al., 2013a), although others have documented an increase of SICI and reduction in ICF (Liepert et al., 2004). The discordant findings could be explained by variability in the cerebellar infarct territory, suggesting that distinct cerebellar regions and projections could potentially modulate cortical inhibitory and facilitatory circuits. Whether these modulatory effects represent adaptive changes or direct cerebellar damage, remains to be determined.
In degenerative cerebellar diseases, prolonged CMCT was reported in SCA1 in the upper limbs and SCA2 in the lower limbs, but not other SCA phenotypes (Tang et al., 2020), implying diverse pathological processes. Normal level of SICI has been reported in various forms of SCA (Ugawa et al., 1994b), while ICF was reduced (Berardelli et al., 2008, Liepert et al., 1998, Schwenkreis et al., 2002, Wessel et al., 1996). The ICF reduction was evident in SCA2 and SCA3, but not in Friedreich’s ataxia, SCA1, and SCA6 patients. The reduction of ICF may be related to reduced excitatory drive form the deep cerebellar nuclei and reflecting underlying cortical pathology in SCA disorders. Reduction of SICI has also been reported in SCA 3 and SCA 14 patients (Farrar et al., 2016, Ganos et al., 2014), indicating a dysfunction of cortical inhibitory circuits, a notion supported by neuroimaging studies disclosing paracentral cortical thinning and atrophy (de Rezende et al., 2015, Etchebehere et al., 2001). The pathological and clinical heterogeneity of degenerative cerebellar disorders could account for the seemingly discordant TMS findings, although future studies should utilize multiple TMS parameters in a machine learning algorithm to develop prognostic and outcome biomarkers that can be readily translated into a clinical trial setting.
3.10. Facial nerve disorders
In unilateral idiopathic Bell’s palsy, TMS studies have disclosed a reduced or absent MEP response when recording from the facial muscles (Glocker et al., 1994, Happe and Bunten, 2012, Lin et al., 2021, Schrader and Schrader, 1995, Schriefer et al., 1988). These TMS deficits developed in acute stages of Bell’s palsy and persisted for months, even after the recovery of muscle weakness. Interestingly, the sensitivity of TMS was greater than electrical facial nerve stimulation, implying that the TMS stimulation site was proximal to the pathogenic lesion. While the prognostic utility of TMS abnormalities in Bell’s palsy is limited (Lin et al., 2021), identifying a reduced or absent MEP responses may be of diagnostic potential, particularly in setting of relatively preserved peripheral motor amplitudes and prior to development of Wallerian degeneration. A normal TMS response with facial nerve stimulation combined with reduced MEP response with cortical stimulation may argue against a peripheral facial nerve lesion (Schriefer et al., 1988, Straub et al., 2000).
A reduction of facial MEP amplitudes is not specific to Bell’s palsy and has been identified in other etiologies of facial nerve dysfunction, including infectious diseases, diabetes and neoplasms (Happe and Bunten, 2012, Nowak et al., 2005). Bilateral facial nerve dysfunction is associated with specific diseases, including Guillain–Barré syndrome, Lyme’s disease, HIV infection, or sarcoidosis (Rösler et al., 1995). Prolonged transosseal conduction time, defined as latency differences between cortical MEP responses from electrically elicited facial nerve motor responses (stylomastoid stimulation), along with simultaneous slowing of conduction in the distal segments with MEP desynchronization, are characteristic features of demyelinating neuropathies, with or without accompanying facial weakness (Rösler et al., 1995, Schriefer et al., 1988). In facial nerve trauma, the MEP responses are typically absent, although if present imply nerve continuity and thereby a better prognosis (Har-El and McPhee, 2000). Paired pulse TMS was implemented as a prognostic tool in predicting the occurrence of hemifacial spam (HFS) after microvascular decompression (Park et al., 2018). In this protocol, a subthreshold CS delivered prior to a test stimulus resulted in MEP facilitation at ISIs 20, 25, and 30s, and inhibition at ISIs 75 and 100 ms in healthy controls. Good surgical outcomes were reported after microvascular decompression when the physiological pattern of facilitation-inhibition was evident, suggesting prognostic utility, although further research is required.
3.11. Brain Tumors
Brain tumors affect standard TMS parameters to varying degrees. Tumor growth in vicinity of the primary motor cortex may be associated with a complex pattern of changes (Sollmann et al., 2018). Consideration of tumor growth dynamics is important when interpreting TMS, as its likely that effects of time-dependent compensatory mechanisms may be assessed. Due to highly variable tumor growth dynamics and individual predispositions to neuroplasticity, clinically heterogeneous findings may be evident. Increased RMT of both the tumorous and healthy hemispheres was associated with a worse motor outcome (Rosenstock et al., 2022). In patients with small tumor-to-corticospinal tract distances, a disturbance in motor excitability (interhemispheric RMT ratio < 90% or > 110%) is associated with a higher risk of new motor deficits either from surgery or tumor growth (Rosenstock et al., 2017). A greater distance between the lesion and motor hotspot as well as the presence of MEP responses 1 week after surgery have been associated with improved motor recovery, suggesting their utility in prognostication (Takakura et al., 2017).
Of relevance, microstructural impairment of white matter correlates with a deterioration of the motor excitability profile, resulting in significantly higher RMTs in the ipsilateral motor cortex (Mirchandani et al., 2020). Fine granular analysis of MEPs has been shown to be more sensitive to tumor-related changes than classical analysis, with spectral analysis of EMG responses showing early impairment of cortical excitability in brain tumors (Machetanz et al., 2021). Specifically, brain tumors affect corticospinal transmission resulting in temporal and spectral MEP desynchronization which correlates with poor dexterity performance.
Recent advances in TMS technology have enabled M1 mapping with a two-coil transducer without moving the coil. This approach has resulted in electronically controlled modelling of the applied electric field over a wider cortical area allowing for improved spatial and temporal control of the stimulation (Koponen et al., 2018). Multicoil TMS systems are being developed that can tune electronic stimulation to a cortical region without moving the coil enabling time-coupled application of stimuli with unlimited E-field orientations and strengths (Nieminen et al., 2022). This would enable excitability measurements of tumorous motor cortical areas with unprecedented control and significantly improve already promising neuromodulatory interventions in brain tumor patients (Ille et al., 2021a).
Closed-loop configurations with automated modification of stimulation depending on neurophysiological and/or behavioral measures have received increasing attention in recent years. EMG-driven TMS allows online adaption of stimulation settings to allow optimized analysis of specific excitability measures (Meincke et al., 2016). EEG feedback for brain state-dependent synchronization of the TMS stimulus allows for better control of the TMS-brain interaction and therefore more reliable assessments of tumor effects on brain excitability (Zrenner et al., 2022). Given the clinical utility of RMT, novel TMS technologies have the potential to interrogate the effects of brain tumor on motor function even more accurately and at an individualized level. In the future, this could provide TMS with an even more important role for tailored treatment pathways including neuromodulatory interventions during the treatment of motor eloquent brain tumors.
Using TMS to outline language-related areas has been made possible with advances in neuronavigation (Lioumis et al., 2012). Comparison of neuronavigated TMS to intraoperative mapping showed a high negative predictive value (Krieg et al., 2014, Picht et al., 2013). Nonetheless, the combination of cortical mapping and subcortical tractography, seeded from those areas, lead to a broader clinical application for approach guidance but also risk assessment. Various studies disclosed high reliability for preoperative risk prediction, particularly for the ratio between left and right-sided language production, connectivity between hemispheres, as well as proximity between tumor and subcortical tracts (Ille et al., 2016, Sollmann et al., 2019, Sollmann et al., 2017). In adults, comparison data also provides some hint that language eloquent perisylvian tumors can be resected with the same functional and oncological results based on neuronavigated TMS data instead of performing awake surgery (Ille et al., 2021b), although further studies are required. Clinical mapping using neuronavigated TMS may be of utility in pediatric patients as well down to the age of 6 years (Rosenstock et al., 2020). Such pediatric applications further promote the spread of neuronavigated TMS in neuro-oncology.
3.12. Disorders of consciousness
As anticipated in section 2.5, directly probing the internal state of cortico-thalamic circuits with TEP provides relevant information in disorders of consciousness. TEPs reflect the reactivity of the neuronal population under the TMS coil as well as remote and re-entrant responses from connected populations with different electrophysiological properties (Massimini et al., 2005, Rosanova et al., 2009). In this way, TMS-EEG may be used to assess by a causal perspective to what extent distributed and differentiated groups of neurons interact to produce complex dynamics. Based on theoretical neuroscience (Tononi and Edelman, 1998) and empirical evidence (Sarasso et al., 2021), this kind of complexity, arising from the coexistence of functional integration and functional differentiation, is considered a fundamental prerequisite for consciousness. Consequently, specific TMS-EEG-based measures, such as Perturbational Complexity Index (PCI), have been developed to assess recovery of consciousness in patients emerging from coma. In this section, we will present the rationale and basic methods for computing PCI and describe its application in patients with disorders of consciousness, highlighting its advantages and some methodological cautionary notes.
3.12.1. Computing PCI
Upon falling asleep and with general anesthesia, the complex waveforms and spatio-temporal dynamics characterizing TEPs during wakefulness are replaced by a simpler response, characterised by a positive-negative deflection centered around the stimulated area (Ferrarelli et al., 2012, Massimini et al., 2005, Sarasso et al., 2015). Notably, a similar change in TEPs characteristics can be observed also in pathological loss of consciousness, whereas the return of complex waveforms is associated with recovery (Ragazzoni et al., 2013, Rosanova et al., 2012). PCI has been devised to capture these changes (Casali et al., 2013).
Starting from TEPs, computing PCI involves a few steps (i) performing cortical source modelling of average scalp responses (ii) applying statistics to extract a binary matrix that describes the spatial-temporal pattern of deterministic cortical activation and (iii) compressing this binary matrix using the Lempel and Ziv algorithm (Lempel and Ziv, 1976) to quantify its complexity. Local (low integration) or stereotypical (low differentiation) responses can be effectively compressed, resulting in low PCI values. On the contrary, responses that are both integrated and differentiated are less compressible, resulting in high PCI values.
3.12.2. PCI validation and application
Before applying it to disorders of consciousness patients, PCI was first calibrated in healthy subjects and patients who could report about their state of consciousness, including wakeful and dreaming experiences as well as deep sleep and general anesthesia. Such calibration in a large benchmark population (n=150) enabled a definition of an operational cut-off (PCI*=0.31) above which consciousness is invariably present. This empirical PCI cut-off was then applied to infer the presence of consciousness in challenging disorders of consciousness patients showing minimal, or no behavioural signs of consciousness (Bodart et al., 2017, Casarotto et al., 2016, Sinitsyn et al., 2020). PCI showed an unprecedented sensitivity (about 95%) in identifying Minimally Conscious State patients which show minimal behavioural outputs. In these patients, high PCI values reliably detected the presence of consciousness even when severely abnormal EEG patterns, characterized by slow waves, are present (Figure 5A, B). Crucially, PCI also allows an informative stratification of patients that are completely unresponsive, such as patients in a Vegetative State, otherwise called Unresponsiveness Wakefulness Syndrome. Within the population identified by this behavioral label, TEPs reveal the existence of three different electrophysiological states (Figure 5C): (i) a state with no significant EEG response to TMS (no response patients), (ii) a low-complexity state similar to that observed in non-rapid eye movement (NREM) sleep and anesthesia unconsciousness (low-complexity patients) and (iii) a high-complexity state similar to that observed in conscious awake or dreaming subjects (high-complexity patients) (Casarotto et al., 2016).
Figure 5: TMS-EEG.
and Perturbational Complexity Index (PCI) in a benchmark population, in minimally conscious state and Unresponsiveness Wakefulness Syndrome patients. (A) Distribution of maximum PCI values computed in the benchmark population (left) in the absence of subjective report (blue line) and in the presence of subjective report (delayed, green line; immediate, red line). The dashed horizontal line indicates the cut-off (PCI*) optimally discriminating consciousness from unconsciousness in the benchmark population. The scatter plot shows the maximum PCI values obtained in individual minimally conscious state (n = 38) patients, sorted by the Coma Recovery Scale-Revised (CRS-R). For each patient, the PCI is represented by a color-filled circle (Modified from Casarotto et al., 2016). (B) The upper row shows TMS evoked potentials [TEPs] (butterfly plot of all EEG channels superimposed, with three illustrative channels highlighted by bold red traces) together with the corresponding PCI values in three representative minimally conscious state patients with PCI higher than PCI*. The lower row shows 10 s of spontaneous EEG recorded from four bipolar EEG channels (F3-C3, P3-O1, F4-C4, and P4-O2) in the same patients. Note that despite having all PCI values above PCI*, minimally conscious state patients displayed patterns of spontaneous background EEG activity that were severely abnormal (left), moderately abnormal (center), and mildly abnormal (right) (Modified from Casarotto et al., 2016). (C) PCI-based stratification of Unresponsiveness Wakefulness Syndrome patients. The scatter plot shows all the maximum PCI values obtained in individual Unresponsiveness Wakefulness Syndrome (n = 43) patients. For each patient, the PCI is represented by a colour-filled circle. Unresponsiveness Wakefulness Syndrome patients could be stratified into three subgroups according to PCI values: high-complexity patients with PCI > PCI* (n = 9, red), low-complexity patients with PCI < PCI* (n = 21, blue), and no-response patients with PCI = 0 (n = 13, black). The lower row shows the structural MRI, the TEP and the corresponding PCI value reported for a representative subject of each subgroup (modified from Casarotto et al., 2016).
This TMS-EEG-based stratification has interesting pathophysiological implications and is clinically informative. The “no” response subgroup is typically composed of TMS of patients who suffered from post-anoxic damage and diffuse cortical necrosis, once called ‘apallic syndrome’. The low-complexity group encompasses patients of variable aetiology in whom portions of the cerebral cortex retain some degree of structural integrity, activity and reactivity. Interestingly, although these patients are awake, as judged by eye opening, residual circuits appear to be blocked in a pathological sleep-like state whereby cortical reactivity is limited to a low-complexity positive-negative deflection. Notably, in these patients, time-frequency analysis of TEPs point to specific neuronal dynamics underlying loss of complexity-the inescapable occurrence of a silent period (OFF-period) after the initial activation triggered by TMS. This tendency of cortical neurons to plunge into an OFF-period upon receiving an input, also known as cortical bistability, is a mode typical of NREM sleep, that may pathologically intrude in the awake brain after injury, leading to a massive disruption of network complexity in many unresponsive patients. As shown by longitudinal measurements, the reduction of cortical bistability parallels, and sometimes anticipates, recovery of network complexity and behavioural responsiveness (Rosanova et al., 2018, Rosanova et al., 2012). Notably, a similar intrusion of sleep-like reactivity can also be observed locally in the perilesional area of stroke patients, possibly leading to selective loss of motor/cognitive function (Sarasso et al., 2020, Tscherpel et al., 2020). Finally, the finding of a third subgroup of unresponsive patients showing high-complexity, entails important clinical and ethical implications. Indeed, this condition indicates a capacity for consciousness, not expressed in behaviour due to pathological impairment of sensory and/or motor functions, and indicates a better chance of recovery (Casarotto et al., 2016, Rosanova et al., 2018, Rosanova et al., 2012).
3.12.3. Advantages of TMS-EEG in disorders of consciousness patients and cautionary notes
The high-sensitivity of TMS-EEG above and beyond behavioural responsiveness and spontaneous EEG patterns may be ascribed to different factors. First, this technique allows by-passing sensory processing, as well as motor functions, which are often impaired after severe brain injury. Second, it does not require any active engagement in specific tasks, a demand often unmet by patients with severe cognitive impairment. Third, the high signal-to-noise ratio of TEPs allows detecting residual network complexity even when this is masked by high-amplitude delta waves in the spontaneous EEG (Frohlich et al., 2021).
The flip side is that applying TMS-EEG to disorders of consciousness demands stringent requirements and special methodological caution. A first key requirement is that TEPs must adhere to the quality criteria illustrated in section 2.5. This not only entails a high-amplitude (around 10 microvolts) initial response but also requires collecting a number of trials that is sufficient to obliterate the increased baseline variability imposed by spontaneous slow waves, which are often present after brain injury. Since collecting hundreds of trials currently takes from 5 to 10 minutes, TMS-EEG measures of complexity cannot be used to monitor short term fluctuations in the state of consciousness, such as fleeting dreams reports during sleep (Nieminen et al., 2016) and anaesthesia. Finally, given that the current calibration of PCI in subjects providing negative delayed report during sleep and anaesthesia cannot rule out the presence of fleeting dreams, low PCI values cannot be used to rule out consciousness but only to reveal the presence of it in unresponsive patients.
3.13. Functional neurological disorders
Functional neurological disorders are common and may present with almost any neurological symptom. One important feature for making the diagnosis is incongruence or incompatibility with features of other neurological disorders (Hallett et al., 2022). TMS may supplement clinical assessment in functional neurological disorders. While there are some promising small studies, none of the methods have moved into clinical practice.
In patient with functional weakness, discordant TMS findings have been reported, with some studies documenting normal RMT, SICI, and ICF (Liepert et al., 2008, 2009), while others established an increase in RMT and SICI (Benussi et al., 2020e). Differences are small and could be different from stroke patients with comparable reduction in muscle strength. Separately, an increase in MEP duration with voluntary contraction (30% maximal force) has been reported in stroke and healthy controls, decreased in MS, while no change was evident in functional neurological disorders paretic patients (Brum et al., 2015), suggesting potential utility.
Changes in MEP amplitude and RMT with movement imagery of target muscle may also be of diagnostic utility in functional neurological disorders. In healthy controls, imagining movement of congruent muscle in the contralateral limb increases the MEP amplitude and reduces RMT (Facchini et al., 2002), while negative motor imagery resulted in MEP amplitude reduction (Sohn et al., 2003). Motor imagery was associated with more pronounced motor output enhancement in the hemisphere in stroke (Cicinelli et al., 2006), contrasting with paretic functional neurological disorders patients (weak upper limb) whereby the MEP amplitude is reduced (Liepert et al., 2008, 2009, 2011).
In dystonic functional neurological disorders patients, abnormalities of TMS measures have been well documented, including a reduction of SICI, LICI, and CSP duration (Espay et al., 2006). Additionally, the cutaneous silent period was increased, and forearm reciprocal inhibition reduced. In contrast, SAI and LAI were comparable to healthy controls for functional dystonia, while a similar degree of SICI reduction was evident in both function and organic dystonia (Quartarone et al., 2009). As such, the utility of TMS in dystonic functional neurological disorders seems limited.
4. Conclusion
The various TMS techniques have demonstrated strong clinical and diagnostic utility in a variety of neurological diseases, including neurodegenerative, movement, autoimmune and episodic disorders as well as spinal cord and functional neurological diseases. Novel TMS techniques, such as threshold tracking TMS, has demonstrated diagnostic utility in ALS and has been recently commercialized. TMS-EEG is an emerging technique with the ability to directly assess cortical function, bypassing subcortical and peripheral neurological systems. In addition to established utility as a diagnostic biomarker in neurodegenerative disease, TMS measures appear suitable for incorporation as therapeutic (i.e., monitoring and outcome) biomarkers of clinical efficacy in future clinical trials. Multicenter studies incorporating larger patient cohort sizes and more homogenous study populations in terms of disease characteristics and treatment will help further clarify utility of the novel TMS techniques.
Clinical diagnostic utility of transcranial magnetic stimulation (TMS) has been established in neurological disorders.
Paired-pulse TMS exhibits utility in neurodegenerative, movement, episodic, and functional disorders.
TMS-EEG provides novel parameter (cortical excitability, effective connectivity, response complexity) for neurological diseases.
Acknowledgements
European Union’s Horizon 2020 Framework Program for Research and Innovation under the Specific Grant Agreement No. 945539 (Human Brain Project SGA3) (to MM, MR); Fondazione Regionale per la Ricerca Biomedica (Regione Lombardia), Project ERAPERMED2019-101, GA 779282 (MR); Tiny Blue Dot Foundation (to MM); Canadian Institute for Advanced Research: Azrieli Program on Brain, Mind, and Consciousness (MM).
Footnotes
Conflict of Interest
A Benussi was partially supported by the Airalzh-AGYR2020, by Fondazione Cariplo (grant n° 2021–1516), and by the Fondation pour la Recherche sur Alzheimer. M. Hallett is an inventor of a patent held by NIH for the H-coil for magnetic stimulation for which he receives license fee payments from the NIH (from Brainsway). He is on the Medical Advisory Board of Brainsway (unpaid position). S. Kreig reports being a consultant for Brainlab and receiving honorarium for lectures provided for Nexstim and Inomed. M Massimini is a co-founder and shareholder of Intrinsic Powers, a spin-off of the University of Milan, Milan, Italy. T. Picht served as a consultant for the TMS system manufacturer Nexstim Oy, Helsinki, Finland. U. Ziemann reports receiving a grant from Takeda Pharmaceutical Company Ltd., and consulting fees CorTec GmbH. The other authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruzzese G, Assini A, Buccolieri A, Schieppati M, Trompetto C. Comparison of intracortical inhibition and facilitation in distal and proximal arm muscles in humans. J Physiol (Lond) 1999;514:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbruzzese G, Buccolieri A, Marchese R, Trompetto C, Mandich P, Schieppati M. Intracortical inhibition and facilitation are abnormal in Huntington’s disease: a paired magnetic stimulation study. Neurosci Lett 1997;228(2):87–90. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain 2001;124:537–45. [DOI] [PubMed] [Google Scholar]
- Abbruzzese G, Tabaton M, Morena M, Dall’Agata D, Favale E. Motor and sensory evoked potentials in progressive supranuclear palsy. Mov Disord 1991;6(1):49–54. [DOI] [PubMed] [Google Scholar]
- Afra J, Mascia A, Gérard P, Maertens de Noordhout A, Schoenen J. Interictal cortical excitability in migraine: a study using transcranial magnetic stimulation of motor and visual cortices. Ann Neurol 1998;44(2):209–15. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Koch G, Hillis AE, Huynh W, Ward NS, Vucic S, et al. Interrogating cortical function with transcranial magnetic stimulation: insights from neurodegenerative disease and stroke. J Neurol Neurosurg Psychiatry 2019;90(1):47–57. [DOI] [PubMed] [Google Scholar]
- Ahdab R, Shatila MM, Shatila AR, Khazen G, Freiha J, Salem M, et al. Cortical Excitability Measures May Predict Clinical Response to Fampridine in Patients with Multiple Sclerosis and Gait Impairment. Brain Sci 2019;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Chalabi A, Calvo A, Chio A, Colville S, Ellis CM, Hardiman O, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 2014;13(11):1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagona G, Ferri R, Pennisi G, Carnemolla A, Maci T, Domina E, et al. Motor cortex excitability in Alzheimer’s disease and in subcortical ischemic vascular dementia. Neurosci Lett 2004;362(2):95–8. [DOI] [PubMed] [Google Scholar]
- Alaydin HC, Vuralli D, Keceli Y, Can E, Cengiz B, Bolay H. Reduced Short-Latency Afferent Inhibition Indicates Impaired Sensorimotor Integrity During Migraine Attacks. Headache 2019;59(6):906–14. [DOI] [PubMed] [Google Scholar]
- Alberici A, Bonato C, Calabria M, Agosti C, Zanetti O, Miniussi C, et al. The contribution of TMS to frontotemporal dementia variants. Acta Neurol Scand 2008;118(4):275–80. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 1989;62(3):711–22. [DOI] [PubMed] [Google Scholar]
- Amadio S, Houdayer E, Bianchi F, Tesfaghebriel Tekle H, Urban IP, Butera C, et al. Sensory tricks and brain excitability in cervical dystonia: a transcranial magnetic stimulation study. Mov Disord 2014;29(9):1185–8. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Stewart M, Quirk GJ, Rosenthal JL. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurg 1987;20:74–93. [PubMed] [Google Scholar]
- Ammann C, Dileone M, Pagge C, Catanzaro V, Mata-Marín D, Hernández-Fernández F, et al. Cortical disinhibition in Parkinson’s disease. Brain 2020;143(11):3408–21. [DOI] [PubMed] [Google Scholar]
- André-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L. Is Life better after motor cortex stimulation for pain control? Results at long-term and their prediction by preoperative rTMS. Pain Physician 2014;17(1):53–62. [PubMed] [Google Scholar]
- Antal A, Luber B, Brem AK, Bikson M, Brunoni AR, Cohen Kadosh R, et al. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract 2022;7:146–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmussen MJ, Jacobs MF, Lee KG, Zapallow CM, Nelson AJ. Short-latency afferent inhibition modulation during finger movement. PLoS One 2013;8(4):e60496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarian S, Azulay JP, Verschueren A, Pouget J. Magnetic stimulation using a triple-stimulation technique in patients with multifocal neuropathy without conduction block. Muscle Nerve 2005;32(6):710–4. [DOI] [PubMed] [Google Scholar]
- Attarian S, Franques J, Elisabeth J, Trébuchon A, Duclos Y, Wybrecht D, et al. Triple-stimulation technique improves the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 2015;51(4):541–8. [DOI] [PubMed] [Google Scholar]
- Aurora SK, Barrodale PM, Vermaas AR, Rudra CB. Topiramate modulates excitability of the occipital cortex when measured by transcranial magnetic stimulation. Cephalalgia 2010;30(6):648–54. [DOI] [PubMed] [Google Scholar]
- Avanzino L, Martino D, van de Warrenburg BP, Schneider SA, Abbruzzese G, Defazio G, et al. Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov Disord 2008;23(5):646–52. [DOI] [PubMed] [Google Scholar]
- Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol 2003;56:13–23. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H. Abnormal EPSPs evoked by magnetic brain stimulation in hand muscle motoneurons of patients with amyotrophic lateral sclerosis. Electroencephalogr Clin Neurophysiol 1993;89(6):408–14. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H, Urbach D, Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding intracortical inhibition or I-wave facilitation using a threshold-hunting paradigm. Exp Brain Res 1999;129(2):317–24. [DOI] [PubMed] [Google Scholar]
- Ayache SS, Créange A, Farhat WH, Zouari HG, Lesage C, Palm U, et al. Cortical excitability changes over time in progressive multiple sclerosis. Funct Neurol 2015;30(4):257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy R, Macdonell R, Jackson G, Berkovic S. The peri-ictal state: cortical excitability changes within 24 h of a seizure. Brain 2009;132:1013–21. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: syndrome-specific effects. Neurology 2006;67(6):1018–22. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Jackson GD. Cortical excitability in migraine and epilepsy: a common feature? J Clin Neurophysiol 2012;29(3):244–9. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Jackson GD, Berkovic SF, Macdonell RA. Inter-session repeatability of cortical excitability measurements in patients with epilepsy. Epilepsy Res 2012;98(2–3):182–6. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Jackson GD, Berkovic SF, Macdonell RA. Cortical excitability and refractory epilepsy: a three-year longitudinal transcranial magnetic stimulation study. Int J Neural Syst 2013a;23(1):1250030. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Macdonell RA, Berkovic SF, Newton MR, Jackson GD. Predicting seizure control: cortical excitability and antiepileptic medication. Ann Neurol 2010;67(1):64–73. [DOI] [PubMed] [Google Scholar]
- Badawy RA, Vogrin SJ, Lai A, Cook MJ. Patterns of cortical hyperexcitability in adolescent/adult-onset generalized epilepsies. Epilepsia 2013b;54(5):871–8. [DOI] [PubMed] [Google Scholar]
- Bae JS, Ferguson M, Tan R, Mioshi E, Simon N, Burrell J, et al. Dissociation of Structural and Functional Integrities of the Motor System in Amyotrophic Lateral Sclerosis and Behavioral-Variant Frontotemporal Dementia. J Clin Neurol 2016;12(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JS, Menon P, Mioshi E, Kiernan MC, Vucic S. Cortical hyperexcitability and the split-hand plus phenomenon: pathophysiological insights in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2014;15(3–4):250–6. [DOI] [PubMed] [Google Scholar]
- Bailey AZ, Asmussen MJ, Nelson AJ. Short-latency afferent inhibition determined by the sensory afferent volley. J Neurophysiol 2016;116(2):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bares M, Kanovsky P, Klajblova H, Rektor I. Intracortical inhibition and facilitation are impaired in patients with early Parkinson’s disease: a paired TMS study. Eur J Neurol 2003;10(4):385–9. [DOI] [PubMed] [Google Scholar]
- Barker AT, Freeston IL, Jabinous R, Jarratt JA. Clinical evaluation of conduction time measurements in central motor pathways using magnetic stimulation of human brain. Lancet 1986;1(8493):1325–6. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985;1(8437):1106–7. [DOI] [PubMed] [Google Scholar]
- Barry MD, Bunday KL, Chen R, Perez MA. Selective effects of baclofen on use-dependent modulation of GABAB inhibition after tetraplegia. J Neurosci 2013;33(31):12898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélemy D, Willerslev-Olsen M, Lundell H, Biering-Sørensen F, Nielsen JB. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. Prog Brain Res 2015;218:79–101. [DOI] [PubMed] [Google Scholar]
- Bashir S, Perez JM, Horvath JC, Pena-Gomez C, Vernet M, Capia A, et al. Differential effects of motor cortical excitability and plasticity in young and old individuals: a Transcranial Magnetic Stimulation (TMS) study. Front Aging Neurosci 2014;6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol 2006;572:857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumer T, Dammann E, Bock F, Klöppel S, Siebner HR, Münchau A. Laterality of interhemispheric inhibition depends on handedness. Exp Brain Res 2007;180(2):195–203. [DOI] [PubMed] [Google Scholar]
- Bäumer T, Schmidt A, Heldmann M, Landwehr M, Simmer A, Tönniges D, et al. Abnormal interhemispheric inhibition in musician’s dystonia - Trait or state? Parkinsonism Relat Disord 2016;25:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 2008;28(41):10363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci 2009;29(8):1634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarik J, Kadanka Z. Multimodal sensory and motor evoked potentials in a two-year follow-up study of MS patients with relapsing course. Acta Neurol Scand 1992;86(1):15–8. [DOI] [PubMed] [Google Scholar]
- Beer S, Rösler KM, Hess CW. Diagnostic value of paraclinical tests in multiple sclerosis: relative sensitivities and specificities for reclassification according to the Poser committee criteria. J Neurol Neurosurg Psychiatry 1995;59(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli P, Biabani M, Blumberger DM, Bortoletto M, Casarotto S, David O, et al. Reproducibility in TMS-EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimul 2019;12(3):787–90. [DOI] [PubMed] [Google Scholar]
- Bella R, Cantone M, Lanza G, Ferri R, Vinciguerra L, Puglisi V, et al. Cholinergic circuitry functioning in patients with vascular cognitive impairment--no dementia. Brain Stimul 2016;9(2):225–33. [DOI] [PubMed] [Google Scholar]
- Belvisi D, Tartaglia M, Borriello G, Baione V, Crisafulli SG, Zuccoli V, et al. Are Neurophysiological Biomarkers Able to Discriminate Multiple Sclerosis Clinical Subtypes? Biomedicines 2022;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Alberici A, Buratti E, Ghidoni R, Gardoni F, Di Luca M, et al. Toward a Glutamate Hypothesis of Frontotemporal Dementia. Front Neurosci 2019a;13:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Alberici A, Ferrari C, Cantoni V, Dell’Era V, Turrone R, et al. The impact of transcranial magnetic stimulation on diagnostic confidence in patients with Alzheimer disease. Alzheimers Res Ther 2018a;10(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Cantoni V, Cotelli MS, Cotelli M, Brattini C, Datta A, et al. Exposure to gamma tACS in Alzheimer’s disease: A randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul 2021a;14(3):531–40. [DOI] [PubMed] [Google Scholar]
- Benussi A, Cantoni V, Grassi M, Brechet L, Michel CM, Datta A, et al. Increasing Brain Gamma Activity Improves Episodic Memory and Restores Cholinergic Dysfunction in Alzheimer’s Disease. Ann Neurol 2022;92(2):322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Cosseddu M, Filareto I, Dell’Era V, Archetti S, Sofia Cotelli M, et al. Impaired long-term potentiation-like cortical plasticity in presymptomatic genetic frontotemporal dementia. Ann Neurol 2016;80(3):472–6. [DOI] [PubMed] [Google Scholar]
- Benussi A, Dell’Era V, Cantoni V, Cotelli MS, Cosseddu M, Spallazzi M, et al. Neurophysiological Correlates of Positive and Negative Symptoms in Frontotemporal Dementia. J Alzheimers Dis 2020a;73(3):1133–42. [DOI] [PubMed] [Google Scholar]
- Benussi A, Dell’Era V, Cantoni V, Cotelli MS, Cosseddu M, Spallazzi M, et al. TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimul 2020b;13(2):386–92. [DOI] [PubMed] [Google Scholar]
- Benussi A, Dell’Era V, Cantoni V, Ferrari C, Caratozzolo S, Rozzini L, et al. Discrimination of atypical parkinsonisms with transcranial magnetic stimulation. Brain Stimul 2018b;11(2):366–73. [DOI] [PubMed] [Google Scholar]
- Benussi A, Dell’Era V, Cantoni V, Turrone R, Pilotto A, Alberici A, et al. Stimulation over the cerebellum with a regular figure-of-eight coil induces reduced motor cortex inhibition in patients with progressive supranuclear palsy. Brain Stimul 2019b;12(5):1290–7. [DOI] [PubMed] [Google Scholar]
- Benussi A, Di Lorenzo F, Dell’Era V, Cosseddu M, Alberici A, Caratozzolo S, et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology 2017;89(7):665–72. [DOI] [PubMed] [Google Scholar]
- Benussi A, Gazzina S, Premi E, Cosseddu M, Archetti S, Dell’Era V, et al. Clinical and biomarker changes in presymptomatic genetic frontotemporal dementia. Neurobiol Aging 2019c;76:133–40. [DOI] [PubMed] [Google Scholar]
- Benussi A, Grassi M, Palluzzi F, Cantoni V, Cotelli MS, Premi E, et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul 2021b;14(2):241–9. [DOI] [PubMed] [Google Scholar]
- Benussi A, Grassi M, Palluzzi F, Koch G, Di Lazzaro V, Nardone R, et al. Classification Accuracy of Transcranial Magnetic Stimulation for the Diagnosis of Neurodegenerative Dementias. Ann Neurol 2020c;87(3):394–404. [DOI] [PubMed] [Google Scholar]
- Benussi A, Karikari TK, Ashton N, Gazzina S, Premi E, Benussi L, et al. Diagnostic and prognostic value of serum NfL and p-Tau(181) in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2020d;91(9):960–7. [DOI] [PubMed] [Google Scholar]
- Benussi A, Premi E, Cantoni V, Compostella S, Magni E, Gilberti N, et al. Cortical Inhibitory Imbalance in Functional Paralysis. Front Hum Neurosci 2020e;14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A, Premi E, Gazzina S, Brattini C, Bonomi E, Alberici A, et al. Progression of Behavioral Disturbances and Neuropsychiatric Symptoms in Patients With Genetic Frontotemporal Dementia. JAMA Netw Open 2021c;4(1):e2030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Abbruzzese G, Chen R, Orth M, Ridding MC, Stinear C, et al. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul 2008;1(3):183–91. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Electrical and magnetic transcranial stimulation in patients with corticospinal damage due to stroke or motor neurone disease. Electroencephalogr Clin Neurophysiol 1991;81(5):389–96. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Noth J, Thompson PD, Bollen EL, Currà A, Deuschl G, et al. Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord 1999;14(3):398–403. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain 1996;119:71–7. [DOI] [PubMed] [Google Scholar]
- Betancur DFA, Tarragó M, Torres I, Fregni F, Caumo W. Central Post-Stroke Pain: An Integrative Review of Somatotopic Damage, Clinical Symptoms, and Neurophysiological Measures. Front Neurol 2021;12:678198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettucci D, Cantello R, Gianelli M, Naldi P, Mutani R. Menstrual migraine without aura: cortical excitability to magnetic stimulation. Headache 1992;32(7):345–7. [DOI] [PubMed] [Google Scholar]
- Bhandari A, Radhu N, Farzan F, Mulsant BH, Rajji TK, Daskalakis ZJ, et al. A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin Neurophysiol 2016;127(8):2834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikmullina R, Kicić D, Carlson S, Nikulin VV. Electrophysiological correlates of short-latency afferent inhibition: a combined EEG and TMS study. Exp Brain Res 2009;194(4):517–26. [DOI] [PubMed] [Google Scholar]
- Bjerkefors A, Squair JW, Chua R, Lam T, Chen Z, Carpenter MG. Assessment of abdominal muscle function in individuals with motor-complete spinal cord injury above T6 in response to transcranial magnetic stimulation. J Rehabil Med 2015;47(2):138–46. [DOI] [PubMed] [Google Scholar]
- Blair IP, Williams KL, Warraich ST, Durnall JC, Thoeng AD, Manavis J, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 2010;81:1286–8. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet 2021;397(10291):2284–303. [DOI] [PubMed] [Google Scholar]
- Bodart O, Gosseries O, Wannez S, Thibaut A, Annen J, Boly M, et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin 2017;14:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologna M, Guerra A, Paparella G, Giordo L, Alunni Fegatelli D, Vestri AR, et al. Neurophysiological correlates of bradykinesia in Parkinson’s disease. Brain 2018;141(8):2432–44. [DOI] [PubMed] [Google Scholar]
- Bologna M, Suppa A, Di Stasio F, Conte A, Fabbrini G, Berardelli A. Neurophysiological studies on atypical parkinsonian syndromes. Parkinsonism Relat Disord 2017;42:12–21. [DOI] [PubMed] [Google Scholar]
- Bologna M, Valls-Solè J, Kamble N, Pal PK, Conte A, Guerra A, et al. Dystonia, chorea, hemiballismus and other dyskinesias. Clin Neurophysiol 2022;140:110–25. [DOI] [PubMed] [Google Scholar]
- Boniface SJ, Mills KR, Schubert M. Responses of single spinal motoneurons to magnetic brain stimulation in healthy subjects and patients with multiple sclerosis. Brain 1991;114:643–62. [DOI] [PubMed] [Google Scholar]
- Bonnì S, Lupo F, Lo Gerfo E, Martorana A, Perri R, Caltagirone C, et al. Altered parietal-motor connections in Alzheimer’s disease patients. J Alzheimers Dis 2013;33(2):525–33. [DOI] [PubMed] [Google Scholar]
- Borich MR, Wheaton LA, Brodie SM, Lakhani B, Boyd LA. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS-EEG investigation. Neurosci Lett 2016;618:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Topper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol 1998a;109:230–7. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Töpper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol 1998b;109(3):230–7. [DOI] [PubMed] [Google Scholar]
- Botelho LM, Morales-Quezada L, Rozisky JR, Brietzke AP, Torres IL, Deitos A, et al. A Framework for Understanding the Relationship between Descending Pain Modulation, Motor Corticospinal, and Neuroplasticity Regulation Systems in Chronic Myofascial Pain. Front Hum Neurosci 2016;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int J Stroke 2017;12(5):480–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem AK, Atkinson NJ, Seligson EE, Pascual-Leone A. Differential pharmacological effects on brain reactivity and plasticity in Alzheimer’s disease. Front Psychiatry 2013;4:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Cosentino G, Vigneri S, Talamanca S, Palermo A, Giglia G, et al. Abnormal facilitatory mechanisms in motor cortex of migraine with aura. Eur J Pain 2011;15(9):928–35. [DOI] [PubMed] [Google Scholar]
- Brighina F, Palermo A, Daniele O, Aloisio A, Fierro B. High-frequency transcranial magnetic stimulation on motor cortex of patients affected by migraine with aura: a way to restore normal cortical excitability? Cephalalgia 2010;30(1):46–52. [DOI] [PubMed] [Google Scholar]
- Brighina F, Palermo A, Panetta ML, Daniele O, Aloisio A, Cosentino G, et al. Reduced cerebellar inhibition in migraine with aura: a TMS study. Cerebellum 2009a;8(3):260–6. [DOI] [PubMed] [Google Scholar]
- Brighina F, Romano M, Giglia G, Saia V, Puma A, Giglia F, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res 2009b;192(4):651–6. [DOI] [PubMed] [Google Scholar]
- Brigo F, Storti M, Benedetti MD, Rossini F, Nardone R, Tezzon F, et al. Resting motor threshold in idiopathic generalized epilepsies: a systematic review with meta-analysis. Epilepsy Res 2012;101(1–2):3–13. [DOI] [PubMed] [Google Scholar]
- Britton TC, Meyer BU, Benecke R. Variability of cortically evoked motor responses in multiple sclerosis. Electroencephalogr Clin Neurophysiol 1991;81(3):186–94. [DOI] [PubMed] [Google Scholar]
- Brooks B. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci 1994;124(Suppl):96 – 107. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9. [DOI] [PubMed] [Google Scholar]
- Brostrom S, Frederiksen JL, Jennum P, Lose G. Motor evoked potentials from the pelvic floor in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2003;74(4):498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum M, Cabib C, Valls-Solé J. Clinical Value of the Assessment of Changes in MEP Duration with Voluntary Contraction. Front Neurosci 2015;9:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa L, Ponzo V, Mastropasqua C, Picazio S, Bonnì S, Di Lorenzo F, et al. Theta burst stimulation modulates cerebellar-cortical connectivity in patients with progressive supranuclear palsy. Brain Stimul 2014;7(1):29–35. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM, Revill KP, Haut MW, Kowalski GM, Wischnewski M, Pifer M, et al. Abnormally reduced primary motor cortex output is related to impaired hand function in chronic stroke. J Neurophysiol 2018;120(4):1680–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler R, Magistris MR, Truffert A, Hess CW, Rosler KM. The triple stimulation technique to study central motor conduction to the lower limbs. Clin Neurophysiol 2001;112(5):938–49. [DOI] [PubMed] [Google Scholar]
- Bunday KL, Oudega M, Perez MA. Aberrant crossed corticospinal facilitation in muscles distant from a spinal cord injury. PLoS One 2013;8(10):e76747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Urbin MA, Perez MA. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul 2018;11(5):1083–92. [DOI] [PubMed] [Google Scholar]
- Burns E, Chipchase LS, Schabrun SM. Altered function of intracortical networks in chronic lateral epicondylalgia. Eur J Pain 2016a;20(7):1166–75. [DOI] [PubMed] [Google Scholar]
- Burns E, Chipchase LS, Schabrun SM. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur J Pain 2016b;20(8):1203–13. [DOI] [PubMed] [Google Scholar]
- Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain 2011;134:2582–94. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 2008;22(1):4–21. [DOI] [PubMed] [Google Scholar]
- Cahn SD, Herzog AG, Pascual-Leone A. Paired-pulse transcranial magnetic stimulation: effects of hemispheric laterality, gender, and handedness in normal controls. J Clin Neurophysiol 2003;20(5):371–4. [DOI] [PubMed] [Google Scholar]
- Cantello R, Civardi C, Varrasi C, Vicentini R, Cecchin M, Boccagni C, et al. Excitability of the human epileptic cortex after chronic valproate: a reappraisal. Brain Res 2006;1099(1):160–6. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Bettucci D, Civardi C, De Angelis MS, Mutani R. Parkinson’s disease rigidity: magnetic motor evoked potentials in a small hand muscle. Neurology 1991;41(9):1449–56. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology 1992;42(10):1951–9. [DOI] [PubMed] [Google Scholar]
- Cantello R, Tarletti R, Civardi C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Res Brain Res Rev 2002;38(3):309–27. [DOI] [PubMed] [Google Scholar]
- Cantone M, Lanza G, Le Pira A, Barone R, Pennisi G, Bella R, et al. Adjunct Diagnostic Value of Transcranial Magnetic Stimulation in Mucopolysaccharidosis-Related Cervical Myelopathy: A Pilot Study. Brain Sci 2019;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramia MD, Cicinelli P, Paradiso C, Mariorenzi R, Zarola F, Bernardi G, et al. ‘Excitability changes of muscular responses to magnetic brain stimulation in patients with central motor disorders. Electroencephalogr Clin Neurophysiol 1991;81:243–50. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Desiato MT, Cicinelli P, Iani C, Rossini PM. Latency jump of “relaxed” versus “contracted” motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalogr Clin Neurophysiol 1993;89(1):61–6. [DOI] [PubMed] [Google Scholar]
- Caramia MD, Palmieri MG, Desiato MT, Boffa L, Galizia P, Rossini PM, et al. Brain excitability changes in the relapsing and remitting phases of multiple sclerosis: a study with transcranial magnetic stimulation. Clin Neurophysiol 2004;115(4):956–65. [DOI] [PubMed] [Google Scholar]
- Caranzano L, Stephan MA, Bedulli M, Herrmann FR, Benninger DH. Peripheral stimulation affects subthreshold Triple Stimulation Technique. J Neurosci Methods 2021;347:108959. [DOI] [PubMed] [Google Scholar]
- Caranzano L, Stephan MA, Herrmann FR, Benninger DH. Desynchronization does not contribute to intracortical inhibition and facilitation: a paired-pulse paradigm study combined with TST. J Neurophysiol 2017;117(3):1052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal TM, Antunes LC, Brietzke AP, Parizotti CS, Carvalho F, De Souza A, et al. Differential Neuroplastic Changes in Fibromyalgia and Depression Indexed by Up-Regulation of Motor Cortex Inhibition and Disinhibition of the Descending Pain System: An Exploratory Study. Front Hum Neurosci 2019;13:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo F, Palomar FJ, Conde V, Diaz-Corrales FJ, Porcacchia P, Fernández-Del-Olmo M, et al. Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimul 2013;6(4):582–9. [DOI] [PubMed] [Google Scholar]
- Carson RG. Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J Physiol 2020;598(21):4781–802. [DOI] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 2013;5(198):198ra05. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Canali P, Rosanova M, Pigorini A, Fecchio M, Mariotti M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr 2013;26(2):326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, et al. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 2016;80(5):718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Fecchio M, Rosanova M, Varone G, D’Ambrosio S, Sarasso S, et al. The rt-TEP tool: real-time visualization of TMS-Evoked Potentials to maximize cortical activation and minimize artifacts. J Neurosci Methods 2022;370:109486. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Määttä S, Herukka SK, Pigorini A, Napolitani M, Gosseries O, et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport 2011;22(12):592–7. [DOI] [PubMed] [Google Scholar]
- Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One 2010;5(4):e10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Turco F, Comanducci A, Perretti A, Marotta G, Pezzoli G, et al. Excitability of the supplementary motor area in Parkinson’s disease depends on subcortical damage. Brain Stimul 2019;12(1):152–60. [DOI] [PubMed] [Google Scholar]
- Cash RF, Ziemann U, Murray K, Thickbroom GW. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol 2010;103(1):511–8. [DOI] [PubMed] [Google Scholar]
- Casula EP, Borghi I, Maiella M, Pellicciari MC, Bonnì S, Mencarelli L, et al. Regional precuneus cortical hyperexcitability in Alzheimer’s disease patients. Ann Neurol 2022;93:371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula EP, Pellicciari MC, Bonnì S, Spanò B, Ponzo V, Salsano I, et al. Evidence for interhemispheric imbalance in stroke patients as revealed by combining transcranial magnetic stimulation and electroencephalography. Hum Brain Mapp 2021;42(5):1343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula EP, Stampanoni Bassi M, Pellicciari MC, Ponzo V, Veniero D, Peppe A, et al. Subthalamic stimulation and levodopa modulate cortical reactivity in Parkinson’s patients. Parkinsonism Relat Disord 2017;34:31–7. [DOI] [PubMed] [Google Scholar]
- Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA, et al. Motor Cortex Excitability and BDNF Levels in Chronic Musculoskeletal Pain According to Structural Pathology. Front Hum Neurosci 2016;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux-Dedeystère A, Rambour M, Duhamel A, Cassim F, Derambure P, Devanne H. Task-dependent changes in late inhibitory and disinhibitory actions within the primary motor cortex in humans. Eur J Neurosci 2014;39(9):1485–90. [DOI] [PubMed] [Google Scholar]
- Celebi O, Temucin CM, Elibol B, Saka E. Short latency afferent inhibition in Parkinson’s disease patients with dementia. Mov Disord 2012;27(8):1052–5. [DOI] [PubMed] [Google Scholar]
- Chadaide Z, Arlt S, Antal A, Nitsche MA, Lang N, Paulus W. Transcranial direct current stimulation reveals inhibitory deficiency in migraine. Cephalalgia 2007;27(7):833–9. [DOI] [PubMed] [Google Scholar]
- Chandra SR, Issac TG, Nagaraju BC, Philip M. A Study of Cortical Excitability, Central Motor Conduction, and Cortical Inhibition Using Single Pulse Transcranial Magnetic Stimulation in Patients with Early Frontotemporal and Alzheimer’s Dementia. Indian J Psychol Med 2016;38(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WJ, O’Connell NE, Beckenkamp PR, Alhassani G, Liston MB, Schabrun SM. Altered Primary Motor Cortex Structure, Organization, and Function in Chronic Pain: A Systematic Review and Meta-Analysis. J Pain 2018;19(4):341–59. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve 2000;9:S26–32. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 2004;154(1):1–10. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res 1999a;129(1):77–86. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2008;119(3):504–32. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol 2000;83(3):1426–34. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 1999b;128(4):539–42. [DOI] [PubMed] [Google Scholar]
- Chen R, Rothwell JC. Cortical Connectivity: Brain Stimulation for Assessing and Modulating Cortical Connectivity and Function. Berlin-Heidelberg: Springer Science and Business Media, 2012. [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology 1997;49(3):881–3. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 1998;80(6):2870–81. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Canos M, M H. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology 1997;49:1054–9. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 2003;89(3):1256–64. [DOI] [PubMed] [Google Scholar]
- Chervyakov AV, Bakulin IS, Savitskaya NG, Arkhipov IV, Gavrilov AV, Zakharova MN, et al. Navigated transcranial magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve 2015;51(1):125–31. [DOI] [PubMed] [Google Scholar]
- Chieffo R, Straffi L, Inuggi A, Coppi E, Moiola L, Martinelli V, et al. Changes in cortical motor outputs after a motor relapse of multiple sclerosis. Mult Scler J Exp Transl Clin 2019;5(3):2055217319866480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou SY, Strutton PH. Crossed Corticospinal Facilitation Between Arm and Trunk Muscles Correlates With Trunk Control After Spinal Cord Injury. Front Hum Neurosci 2020;14:583579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury NS, Chang WJ, Millard SK, Skippen P, Bilska K, Seminowicz DA, et al. The Effect of Acute and Sustained Pain on Corticomotor Excitability: A Systematic Review and Meta-Analysis of Group and Individual Level Data . J Pain 2022;23:1680–96. [DOI] [PubMed] [Google Scholar]
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE, Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology 2009;72(9):842–9. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Marconi B, Zaccagnini M, Pasqualetti P, Filippi MM, Rossini PM. Imagery-induced cortical excitability changes in stroke: a transcranial magnetic stimulation study. Cereb Cortex 2006;16(2):247–53. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: a 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr Clin Neurophysiol 1997;105(6):438–50. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Giovannelli F, Borgheresi A, Tramacere L, Viggiano MP, Zaccara G. A Meta-analysis of the Cortical Silent Period in Epilepsies. Brain Stimul 2015;8(4):693–701. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cavalli A, Naldi P, Varrasi C, Cantello R. Hemispheric asymmetries of cortico-cortical connections in human hand motor areas. Clin Neurophysiol 2000;111(4):624–9. [DOI] [PubMed] [Google Scholar]
- Clark CM, Clark RM, Hoyle JA, Chuckowree JA, McLean CA, Dickson TC. Differential NPY-Y1 Receptor Density in the Motor Cortex of ALS Patients and Familial Model of ALS. Brain Sci 2021;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Benecke R. Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 1995;97(5):264–74. [DOI] [PubMed] [Google Scholar]
- Classen J, Knorr U, Werhahn KJ, Schlaug G, Kunesch E, Cohen LG, et al. Multimodal output mapping of human central motor representation on different spatial scales. J Physiol 1998;512:163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D. Central motor conduction: method and normal results. Muscle Nerve 1990;13(12):1125–32. [DOI] [PubMed] [Google Scholar]
- Claus D, Weis M, Jahnke U, Plewe A, Brunholzl C. Corticospinal conduction studied with magnetic double stimulation in the intact human. J Neurol Sci 1992;111(2):180–8. [DOI] [PubMed] [Google Scholar]
- Coates KD, Aboodarda SJ, Krüger RL, Martin T, Metz LM, Jarvis SE, et al. Multiple sclerosis-related fatigue: the role of impaired corticospinal responses and heightened exercise fatigability. J Neurophysiol 2020;124(4):1131–43. [DOI] [PubMed] [Google Scholar]
- Compta Y, Valls-Solé J, Valldeoriola F, Kumru H, Rumià J. The silent period of the thenar muscles to contralateral and ipsilateral deep brain stimulation. Clin Neurophysiol 2006;117(11):2512–20. [DOI] [PubMed] [Google Scholar]
- Conte A, Belvisi D, Bologna M, Ottaviani D, Fabbrini G, Colosimo C, et al. Abnormal cortical synaptic plasticity in primary motor area in progressive supranuclear palsy. Cereb Cortex 2012;22(3):693–700. [DOI] [PubMed] [Google Scholar]
- Conte A, Lenzi D, Frasca V, Gilio F, Giacomelli E, Gabriele M, et al. Intracortical excitability in patients with relapsing-remitting and secondary progressive multiple sclerosis. J Neurol 2009;256(6):933–8. [DOI] [PubMed] [Google Scholar]
- Coppola G, Di Lenola D, Abagnale C, Ferrandes F, Sebastianelli G, Casillo F, et al. Short-latency afferent inhibition and somato-sensory evoked potentials during the migraine cycle: surrogate markers of a cycling cholinergic thalamo-cortical drive? J Headache Pain 2020;21(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corp DT, Bereznicki HGK, Clark GM, Youssef GJ, Fried PJ, Jannati A, et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin Neurophysiol 2021;132(10):2639–53. [DOI] [PubMed] [Google Scholar]
- Cortes M, Thickbroom GW, Valls-Sole J, Pascual-Leone A, Edwards DJ. Spinal associative stimulation: a non-invasive stimulation paradigm to modulate spinal excitability. Clin Neurophysiol 2011;122(11):2254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese F, Coppola G, Di Lenola D, Serrao M, Di Lorenzo C, Parisi V, et al. Excitability of the motor cortex in patients with migraine changes with the time elapsed from the last attack. J Headache Pain 2017;18(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino G, Brighina F, Brancato S, Valentino F, Indovino S, Fierro B. Transcranial magnetic stimulation reveals cortical hyperexcitability in episodic cluster headache. J Pain 2015;16(1):53–9. [DOI] [PubMed] [Google Scholar]
- Cosentino G, Di Marco S, Ferlisi S, Valentino F, Capitano WM, Fierro B, et al. Intracortical facilitation within the migraine motor cortex depends on the stimulation intensity. A paired-pulse TMS study. J Headache Pain 2018;19(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino G, Fierro B, Vigneri S, Talamanca S, Palermo A, Puma A, et al. Impaired glutamatergic neurotransmission in migraine with aura? Evidence by an input-output curves transcranial magnetic stimulation study. Headache 2011;51(5):726–33. [DOI] [PubMed] [Google Scholar]
- Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis:a systematic review. Arch Neurol 2012;69(11):1410–6. [DOI] [PubMed] [Google Scholar]
- Coyle HL, Ponsford J, Hoy KE. Understanding individual variability in symptoms and recovery following mTBI: A role for TMS-EEG? Neurosci Biobehav Rev 2018;92:140–9. [DOI] [PubMed] [Google Scholar]
- Cruz-Martínez A, Arpa J. Transcranial magnetic stimulation in patients with cerebellar stroke. Eur Neurol 1997;38(2):82–7. [DOI] [PubMed] [Google Scholar]
- Cucurachi L, Immovilli P, Granella F, Pavesi G, Cattaneo L. Short-latency afferent inhibition predicts verbal memory performance in patients with multiple sclerosis. J Neurol 2008;255(12):1949–56. [DOI] [PubMed] [Google Scholar]
- Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R. Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology 2002;58(11):1665–72. [DOI] [PubMed] [Google Scholar]
- Curra A, Pierelli F, Coppola G, Barbanti P, Buzzi MG, Galeotti F, et al. Shortened cortical silent period in facial muscles of patients with migraine. Pain 2007;132(1–2):124–31. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Leenus DJ, Van Wijmeersch B, Thijs H, Levin O, Swinnen SP, et al. Anodal tDCS increases corticospinal output and projection strength in multiple sclerosis. Neurosci Lett 2013;554:151–5. [DOI] [PubMed] [Google Scholar]
- Daligadu J, Murphy B, Brown J, Rae B, Yielder P. TMS stimulus-response asymmetry in left- and right-handed individuals. Exp Brain Res 2013;224(3):411–6. [DOI] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res 2006;174(2):376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 2004;557:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson T, Tremblay F. Age and hemispheric differences in transcallosal inhibition between motor cortices: an ispsilateral silent period study. BMC Neurosci 2013;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Dick JP, Cowan JM, Berardelli A, et al. Motor cortex stimulation in intact man. 2. Multiple descending volleys. Brain 1987(110):1191–209. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, de Mendonça A, Miranda PC, Garcia C, Luís ML. Magnetic stimulation in Alzheimer’s disease. J Neurol 1997;244(5):304–7. [DOI] [PubMed] [Google Scholar]
- de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 2008;119(3):497–503. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Cristiani R, Bertini M, Curcio G, Ferrara M, Fratello F, et al. Handedness is mainly associated with an asymmetry of corticospinal excitability and not of transcallosal inhibition. Clin Neurophysiol 2004;115(6):1305–12. [DOI] [PubMed] [Google Scholar]
- de Goede AA, Ter Braack EM, van Putten M. Single and paired pulse transcranial magnetic stimulation in drug naïve epilepsy. Clin Neurophysiol 2016;127(9):3140–55. [DOI] [PubMed] [Google Scholar]
- de Rezende TJ, D’Abreu A, Guimarães RP, Lopes TM, Lopes-Cendes I, Cendes F, et al. Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol 2015;22(2):277–83, e23-4. [DOI] [PubMed] [Google Scholar]
- Deftereos SN, Kechagias E, Ioakeimidou C, Georgonikou D. Transcranial magnetic stimulation but not MRI predicts long-term clinical status in cervical spondylosis: a case series. Spinal Cord 2015;53 Suppl 1:S16–8. [DOI] [PubMed] [Google Scholar]
- Delvaux V, Alagona G, Gérard P, De Pasqua V, Delwaide PJ, Maertens de Noordhout A. Reduced excitability of the motor cortex in untreated patients with de novo idiopathic “grand mal” seizures. J Neurol Neurosurg Psychiatry 2001;71(6):772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Olivier E. Conditioning transcranial cortical stimulation (TCCS) by exteroceptive stimulation in parkinsonian patients. Adv Neurol 1990;53:175–81. [PubMed] [Google Scholar]
- Demoule A, Verin E, Ross E, Moxham J, Derenne JP, Polkey MI, et al. Intracortical inhibition and facilitation of the response of the diaphragm to transcranial magnetic stimulation. J Clin Neurophysiol 2003;20(1):59–64. [DOI] [PubMed] [Google Scholar]
- Deroide N, Uzenot D, Verschueren A, Azulay JP, Pouget J, Attarian S. Triple-stimulation technique in multifocal neuropathy with conduction block. Muscle Nerve 2007;35(5):632–6. [DOI] [PubMed] [Google Scholar]
- Dharmadasa T, Matamala JM, Howells J, Simon NG, Vucic S, Kiernan MC. The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neurosci Lett 2019;699:84–90. [DOI] [PubMed] [Google Scholar]
- Dharmadasa T, Matamala JM, Howells J, Vucic S, Kiernan MC. Early focality and spread of cortical dysfunction in amyotrophic lateral sclerosis: A regional study across the motor cortices. Clin Neurophysiol 2020;131(4):958–66. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Bella R, Benussi A, Bologna M, Borroni B, Capone F, et al. Diagnostic contribution and therapeutic perspectives of transcranial magnetic stimulation in dementia. Clin Neurophysiol 2021;132(10):2568–607. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, et al. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol 2002a;113(11):1673–9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2004;75(4):555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol, Neurosurg Psychiatry 2005;76(8):1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Insola A, Mazzone P, et al. Descending volleys evoked by transcranial magnetic stimulation of the brain in conscious humans: effects of coil shape. Clin Neurophysiol 2002b;113(1):114–9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Ferrara L, Saturno E, Pilato F, et al. The diagnostic value of motor evoked potentials. Clin Neurophysiol 1999a;110(7):1297–307. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, et al. Direct recordings of descending volleys after transcranial magnetic and electric motor cortex stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 1999b;51:120–6. [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 2000;135(4):455–61. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol 2003;547:485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology 2002c;59(3):392–7. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Marra C, Ranieri F, et al. In vivo functional evaluation of central cholinergic circuits in vascular dementia. Clin Neurophysiol 2008;119(11):2494–500. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: A TMS study. Clin Neurophysiol 2007a;118(10):2207–14. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Saturno E, Oliviero A, Marra C, et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology 2006a;66(7):111–13. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Saturno E, Profice P, Marra C, et al. Functional evaluation of cerebral cortex in dementia with Lewy bodies. Neuroimage 2007b;37(2):422–9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, et al. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol 2006b;96(4):1765–71. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, et al. I-wave origin and modulation. Brain Stim 2012;5(5):512–25. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Colosimo C, Tonali P. The contribution of magnetic stimulation of the motor cortex to the diagnosis of cervical spondylotic myelopathy. Correlation of central motor conduction to distal and proximal upper limb muscles with clinical and MRI findings. Electroencephalogr Clin Neurophysiol 1992;85(5):311–20. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol 2014;592(19):4115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, et al. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res 1999c;129(4):494–9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circuits 2013;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F, Martorana A, Ponzo V, Bonnì S, D’Angelo E, Caltagirone C, et al. Cerebellar theta burst stimulation modulates short latency afferent inhibition in Alzheimer’s disease patients. Front Aging Neurosci 2013;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F, Motta C, Bonnì S, Mercuri NB, Caltagirone C, Martorana A, et al. LTP-like cortical plasticity is associated with verbal memory impairment in Alzheimer’s disease patients. Brain Stimul 2019;12(1):148–51. [DOI] [PubMed] [Google Scholar]
- Di Pietro F, McAuley JH, Parkitny L, Lotze M, Wand BM, Moseley GL, et al. Primary motor cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J Pain 2013;14(11):1270–88. [DOI] [PubMed] [Google Scholar]
- Dias Leao MT, Wiesinger L, Ziemann U, Tatagiba M, Naros G. Rapid motor cortical reorganization following subacute spinal cord dysfunction. Brain Stimul 2020;13(3):783–5. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair 2010;24(2):125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper A, Jude L, Jackson GM, Jackson SR. Motor excitability during movement preparation in Tourette syndrome. J Neuropsychol 2015;9(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbioso R, Bove M, Boccia D, D’Ambrosio V, Nolano M, Manganelli F, et al. Neurophysiological and behavioural correlates of ocrelizumab therapy on manual dexterity in patients with primary progressive multiple sclerosis. J Neurol 2022a;269(9):4791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbioso R, Pellegrino G, Ranieri F, Di Pino G, Capone F, Dileone M, et al. BDNF polymorphism and interhemispheric balance of motor cortex excitability: a preliminary study. J Neurophysiol 2022b;127(1):204–12. [DOI] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 2005;28(4):940–6. [DOI] [PubMed] [Google Scholar]
- Eardley I, Nagendran K, Lecky B, Chapple CR, Kirby RS, Fowler CJ. Neurophysiology of the striated urethral sphincter in multiple sclerosis. Br J Urol 1991;68(1):81–8. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Wood NW, Rothwell JC, Bhatia KP. Different patterns of electrophysiological deficits in manifesting and non-manifesting carriers of the DYT1 gene mutation. Brain 2003;126:2074–80. [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology 1996;46(5):1396–404. [DOI] [PubMed] [Google Scholar]
- Eisen A, Kim S, Pant B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron? Muscle Nerve 1992;15:219–24. [DOI] [PubMed] [Google Scholar]
- Eisen A, Shytbel W, Murphy K, Hoirch M. Cortical magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve 1990;13(2):146–51. [DOI] [PubMed] [Google Scholar]
- Eisen A, Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve 2001;24(4):564–73. [DOI] [PubMed] [Google Scholar]
- Eisenberg E, Chistyakov AV, Yudashkin M, Kaplan B, Hafner H, Feinsod M. Evidence for cortical hyperexcitability of the affected limb representation area in CRPS: a psychophysical and transcranial magnetic stimulation study. Pain 2005;113(1–2):99–105. [DOI] [PubMed] [Google Scholar]
- Ekizoglu E, Sozer-Topçular N, Baykan B, Oge AE. Assessment of excitability at the brainstem and cortex in primary headaches with allodynia. J Clin Neurophysiol 2015;32(2):119–29. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Davey NJ, Maskill DW, Dick JP. The relation between bradykinesia and excitability of the motor cortex assessed using transcranial magnetic stimulation in normal and parkinsonian subjects. Electroencephalogr Clin Neurophysiol 1995;97(3):169–78. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Morgante F, Purzner J, Gunraj CA, Lang AE, Chen R. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol 2006;59(5):825–34. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull 2006;69(1):86–94. [DOI] [PubMed] [Google Scholar]
- Etchebehere EC, Cendes F, Lopes-Cendes I, Pereira JA, Lima MC, Sansana CR, et al. Brain single-photon emission computed tomography and magnetic resonance imaging in Machado-Joseph disease. Arch Neurol 2001;58(8):1257–63. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Azulay JP, Witjas T, Rico A, Attarian S. Assessment of cortico-spinal tract impairment in multiple system atrophy using transcranial magnetic stimulation. Clin Neurophysiol 2007;118(4):815–23. [DOI] [PubMed] [Google Scholar]
- Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev 2007;31(8):1136–49. [DOI] [PubMed] [Google Scholar]
- Facchetti D, Mai R, Micheli A, Marciano N, Capra R, Gasparotti R, et al. Motor evoked potentials and disability in secondary progressive multiple sclerosis. Can J Neurol Sci 1997;24(4):332–7. [DOI] [PubMed] [Google Scholar]
- Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand 2002;105(3):146–51. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Vucic S, Nicholson G, Kiernan MC. Motor cortical dysfunction develops in spinocerebellar ataxia type 3. Clin Neurophysiol 2016;127(11):3418–24. [DOI] [PubMed] [Google Scholar]
- Fassett HJ, Turco CV, El-Sayes J, Nelson AJ. Alterations in Motor Cortical Representation of Muscles Following Incomplete Spinal Cord Injury in Humans. Brain Sci 2018;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico P, Perez MA. Altered corticospinal function during movement preparation in humans with spinal cord injury. J Physiol 2017;595(1):233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 1992;453:525–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): A systematic review. Neurosci Biobehav Rev 2018a;86:176–206. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG. The Impact of Stimulation Intensity and Coil Type on Reliability and Tolerability of Cerebellar Brain Inhibition (CBI) via Dual-Coil TMS. Cerebellum 2018b;17(5):540–9. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, et al. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry 2012;69(8):766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Määttä S, Ponzo D, Guerra A, Bressi F, et al. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation follow-up study. Neurosci Lett 2011;492(2):94–8. [DOI] [PubMed] [Google Scholar]
- Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res 2010;203(1):31–8. [DOI] [PubMed] [Google Scholar]
- Fierro B, Salemi G, Brighina F, Buffa D, Conte S, La Bua V, et al. A transcranial magnetic stimulation study evaluating methylprednisolone treatment in multiple sclerosis. Acta Neurol Scand 2002;105(3):152–7. [DOI] [PubMed] [Google Scholar]
- Filippi M, Campi A, Mammi S, Martinelli V, Locatelli T, Scotti G, et al. Brain magnetic resonance imaging and multimodal evoked potentials in benign and secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 1995;58(1):31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori F, Chiappini E, Candidi M, Romei V, Borgomaneri S, Avenanti A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: a dual site TMS study. Sci Rep 2017;7(1):14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 2004;185(2):232–40. [DOI] [PubMed] [Google Scholar]
- Fisher KM, Lai HM, Baker MR, Baker SN. Corticospinal activation confounds cerebellar effects of posterior fossa stimuli. Clin Neurophysiol 2009;120(12):2109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res 2002;143:240–8. [DOI] [PubMed] [Google Scholar]
- Fisicaro F, Lanza G, Cantone M, Ferri R, Pennisi G, Nicoletti A, et al. Clinical and Electrophysiological Hints to TMS in De Novo Patients with Parkinson’s Disease and Progressive Supranuclear Palsy. J Pers Med 2020;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SC, Luu BL, Butler JE, Taylor JL. More conditioning stimuli enhance synaptic plasticity in the human spinal cord. Clin Neurophysiol 2016;127(1):724–31. [DOI] [PubMed] [Google Scholar]
- Freund P, Rothwell J, Craggs M, Thompson AJ, Bestmann S. Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury. Eur J Neurosci 2011;34(11):1839–46. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Fox NC, Jack CR Jr., Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 2010;6(2):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect 2011;1(1):13–36. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Toker D, Monti MM. Consciousness among delta waves: a paradox? Brain 2021;144(8):2257–77. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol 1991;81(4):257–62. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Borggrefe-Chappuis A, Schindler C, Kappos L. Visual and motor evoked potentials in the course of multiple sclerosis. Brain 2001;124:2162–8. [DOI] [PubMed] [Google Scholar]
- Fujihara K, Miyoshi T. The effects of 4-aminopyridine on motor evoked potentials in multiple sclerosis. J Neurol Sci 1998;159(1):102–6. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Hikawa T, Abe T, Ishii K, Kobayashi H. Reduced short latency afferent inhibition in diffuse axonal injury patients with memory impairment. Neurosci Lett 2006;405(3):226–30. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging 2012;33(7):1484.e1–14. [DOI] [PubMed] [Google Scholar]
- Funaba M, Imajo Y, Suzuki H, Nagao Y, Sakamoto T, Nishida N, et al. Radiological factors associated with the severity of corticospinal tract dysfunctions for cervical spondylotic myelopathy: An analysis of the central motor conduction time and kinematic CT myelography. J Clin Neurosci 2021;94:24–31. [DOI] [PubMed] [Google Scholar]
- Funaba M, Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Nishida N, et al. Transcranial magnetic stimulation in the diagnosis of cervical compressive myelopathy: comparison with spinal cord evoked potentials. Spine (Phila Pa 1976) 2015;40(3):E161–7. [DOI] [PubMed] [Google Scholar]
- Ganos C, Ferrè ER, Marotta A, Kassavetis P, Rothwell J, Bhatia KP, et al. Cortical inhibitory function in cervical dystonia. Clin Neurophysiol 2018a;129(2):466–72. [DOI] [PubMed] [Google Scholar]
- Ganos C, Rocchi L, Latorre A, Hockey L, Palmer C, Joyce EM, et al. Motor cortical excitability during voluntary inhibition of involuntary tic movements. Mov Disord 2018b;33(11):1804–9. [DOI] [PubMed] [Google Scholar]
- Ganos C, Zittel S, Minnerop M, Schunke O, Heinbokel C, Gerloff C, et al. Clinical and neurophysiological profile of four German families with spinocerebellar ataxia type 14. Cerebellum 2014;13(1):89–96. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Lavoie BA, Brown WF. Motor control of the diaphragm in multiple sclerosis. Muscle Nerve 1996;19(5):654–6. [DOI] [PubMed] [Google Scholar]
- Garry MI, Thomson RH. The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res 2009;193(2):267–74. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Loy CT, Menon P, de Carvalho M, Swash M, Schrooten M, et al. Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: a systematic review using individual patient data. Clin Neurophysiol 2016;127(7):2684–91. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Menon P, Ng K, Van Den Bos M, Byth K, Kiernan MC, et al. Riluzole exerts transient modulating effects on cortical and axonal hyperexcitability in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2016a;17(7–8):580–8. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Menon P, Nicholson GA, Ng K, Howells J, Kril JJ, et al. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol 2015;72:1268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N, Menon P, Scherman DB, Simon N, Yiannikas C, Henderson RD, et al. Diagnostic criteria in amyotrophic lateral sclerosis: A multicenter prospective study. Neurology 2016b;87(7):684–90. [DOI] [PubMed] [Google Scholar]
- Geevasinga N, Menon P, Sue CM, Kumar KR, Ng K, Yiannikas C, et al. Cortical excitability changes distinguish the motor neuron disease phenotypes from hereditary spastic paraplegia. Eur J Neurol 2015;22(5):826–31, e57–8. [DOI] [PubMed] [Google Scholar]
- Gerdelat-Mas A, Loubinoux I, Tombari D, Rascol O, Chollet F, Simonetta-Moreau M. Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. Neuroimage 2005;27(2):314–22. [DOI] [PubMed] [Google Scholar]
- Giffroy X, Dive D, Kaux JF, Maes N, Albert A, Göbels C, et al. Is the triple stimulation technique a better quantification tool of motor dysfunction than motor evoked potentials in multiple sclerosis? Acta Neurol Belg 2019;119(1):47–54. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, et al. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome. Movement Disorders 2004;19(4):416–25. [DOI] [PubMed] [Google Scholar]
- Gilio F, Currà A, Inghilleri M, Lorenzano C, Suppa A, Manfredi M, et al. Abnormalities of motor cortex excitability preceding movement in patients with dystonia. Brain 2003;126:1745–54. [DOI] [PubMed] [Google Scholar]
- Gilio F, Currà A, Lorenzano C, Modugno N, Manfredi M, Berardelli A. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol 2000;48(1):20–6. [PubMed] [Google Scholar]
- Ginhoux R, Renaud P, Zorn L, Goffin L, Bayle B, Foucher J, et al. A custom robot for Transcranial Magnetic Stimulation: first assessment on healthy subjects. Annu Int Conf IEEE Eng Med Biol Soc 2013;2013:5352–5. [DOI] [PubMed] [Google Scholar]
- Glocker FX, Magistris MR, Rösler KM, Hess CW. Magnetic transcranial and electrical stylomastoidal stimulation of the facial motor pathways in Bell’s palsy: time course and relevance of electrophysiological parameters. Electroencephalogr Clin Neurophysiol 1994;93(2):113–20. [DOI] [PubMed] [Google Scholar]
- Gogulski J, Ross JM, Talbot A, Cline CC, Donati FL, Munot S, et al. Personalized Repetitive Transcranial Magnetic Stimulation for Depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2022. [DOI] [PubMed] [Google Scholar]
- Goodwill AM, Reynolds J, Daly RM, Kidgell DJ. Formation of cortical plasticity in older adults following tDCS and motor training. Front Aging Neurosci 2013;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76(11):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapperon AM, Verschueren A, Jouve E, Morizot-Koutlidis R, Lenglet T, Pradat PF, et al. Assessing the upper motor neuron in amyotrophic lateral sclerosis using the triple stimulation technique: A multicenter prospective study. Clin Neurophysiol 2021;132(10):2551–7. [DOI] [PubMed] [Google Scholar]
- Green A, Cheong PW, Fook-Chong S, Tiruchelvarayan R, Guo CM, Yue WM, et al. Cortical Reorganization Is Associated with Surgical Decompression of Cervical Spondylotic Myelopathy. Neural Plast 2015;2015:389531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoras IF, Stagg CJ. Recent advances in the role of excitation-inhibition balance in motor recovery post-stroke. Fac Rev 2021;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groiss SJ, Ugawa Y. Cerebellum . Handb Clin Neurol 2013;116:643–53. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 2012;123(5):858–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra A, Suppa A, D’Onofrio V, Di Stasio F, Asci F, Fabbrini G, et al. Abnormal cortical facilitation and L-dopa-induced dyskinesia in Parkinson’s disease. Brain Stimul 2019;12(6):1517–25. [DOI] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol 2001;112(10):1781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, et al. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry 2012;69(7):662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin S, Soysal A, Atay T, Arpaci B. Motor and occipital cortex excitability in migraine patients. Can J Neurol Sci 2006;33(1):63–7. [DOI] [PubMed] [Google Scholar]
- Gunduz ME, Pinto CB, Saleh Velez FG, Duarte D, Pacheco-Barrios K, Lopes F, et al. Motor Cortex Reorganization in Limb Amputation: A Systematic Review of TMS Motor Mapping Studies. Front Neurosci 2020;14:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson A, Norton N, Fast T, Frölich L, Georges J, Holzapfel D, et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement 2022;19:658–70. [DOI] [PubMed] [Google Scholar]
- Hallett M, Aybek S, Dworetzky BA, McWhirter L, Staab JP, Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol 2022;21(6):537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Chen R, Ziemann U, Cohen LG. Reorganization in motor cortex in amputees and in normal volunteers after ischemic limb deafferentation. Electroencephalogr Clin Neurophysiol 1999;51:183–7. [PubMed] [Google Scholar]
- Hammerbeck U, Hoad D, Greenwood R, Rothwell JC. The unsolved role of heightened connectivity from the unaffected hemisphere to paretic arm muscles in chronic stroke. Clin Neurophysiol 2019;130(5):781–8. [DOI] [PubMed] [Google Scholar]
- Hammond G, Faulkner D, Byrnes M, Mastaglia F, Thickbroom G. Transcranial magnetic stimulation reveals asymmetrical efficacy of intracortical circuits in primary motor cortex. Exp Brain Res 2004;155(1):19–23. [DOI] [PubMed] [Google Scholar]
- Hammond GR, Garvey CA. Asymmetries of long-latency intracortical inhibition in motor cortex and handedness. Exp Brain Res 2006;172(4):449–53. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Furubayashi T, Iwata NK, Shiio Y, Okabe S, Kanazawa I, et al. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res 2003;151(4):427–34. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Nomura Y, Segawa M, Ugawa Y. Intracortical inhibition of the motor cortex in Segawa disease (DYT5). Neurology 2007;68(13):1039–44. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Okabe S, Terao Y, Furubayashi T, Arai N, Inomata-Terada S, et al. Difference in intracortical inhibition of the motor cortex between cortical myoclonus and focal hand dystonia. Clin Neurophysiol 2008;119(6):1400–7. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Terao Y, Shirota Y, Ohminami S, Nakatani-Enomoto S, Okabe S, et al. Short-interval intracortical inhibition in Parkinson’s disease using anterior-posterior directed currents. Exp Brain Res 2011;214(2):317–21. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Tsutsumi R, Shirota Y, Shimizu T, Tanaka N, Ugawa Y. Cerebellar dysfunction in essential tremor. Mov Disord 2016;31(8):1230–4. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol 2001;531:849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, et al. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol 2002;538(538):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Furubayashi T, Machii K, Shiio Y, et al. Intracortical inhibition of the motor cortex is normal in chorea. J Neurol Neurosurg Psychiatry 1999;66(6):783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders J Neurol Sci 1996(140):109–16. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, et al. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol 1998;509:607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe S, Bunten S. Electrical and transcranial magnetic stimulation of the facial nerve: diagnostic relevance in acute isolated facial nerve palsy. Eur Neurol 2012;68(5):304–9. [DOI] [PubMed] [Google Scholar]
- Har-El G, McPhee JR. Transcranial magnetic stimulation in acute facial nerve injury. Laryngoscope 2000;110(7):1105–11. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul 2014;7(5):643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmelech T, Roth Y, Tendler A. Transcranial Magnetic Stimulation in Obsessive-Compulsive Disorder. Psychiatr Clin North Am 2023;46(1):133–66. [DOI] [PubMed] [Google Scholar]
- Harquel S, Bacle T, Beynel L, Marendaz C, Chauvin A, David O. Mapping dynamical properties of cortical microcircuits using robotized TMS and EEG: Towards functional cytoarchitectonics. Neuroimage 2016;135:115–24. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Uozumi T, Tsuji S. Paraspinal motor evoked potentials by magnetic stimulation of the motor cortex. Neurology 2000;55(6):885–8. [DOI] [PubMed] [Google Scholar]
- Heise KF, Steven B, Liuzzi G, Thomalla G, Jonas M, Müller-Vahl K, et al. Altered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndrome. Brain 2010;133:580–90. [DOI] [PubMed] [Google Scholar]
- Hermsen AM, Haag A, Duddek C, Balkenhol K, Bugiel H, Bauer S, et al. Test-retest reliability of single and paired pulse transcranial magnetic stimulation parameters in healthy subjects. J Neurol Sci 2016;362:209–16. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM. Measurement of central motor conduction in multiple sclerosis by magnetic brain stimulation. Lancet 1986;2(8503):355–8. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM, Schriefer TN. Magnetic brain stimulation: central motor conduction studies in multiple sclerosis. Ann Neurol 1987;22(6):744–52. [DOI] [PubMed] [Google Scholar]
- Higashihara M, Sonoo M, Imafuku I, Fukutake T, Kamakura K, Inoue K, et al. Fasciculation potentials in amyotrophic lateral sclerosis and the diagnostic yield of the Awaji algorithm. Muscle Nerve 2012;45(2):175–82. [DOI] [PubMed] [Google Scholar]
- Higashihara M, Van den Bos MAJ, Menon P, Kiernan MC, Vucic S. Interneuronal networks mediate cortical inhibition and facilitation. Clin Neurophysiol 2020;131(5):1000–10. [DOI] [PubMed] [Google Scholar]
- Hoeppner J, Wegrzyn M, Thome J, Bauer A, Oltmann I, Buchmann J, et al. Intra- and inter-cortical motor excitability in Alzheimer’s disease. J Neural Transm (Vienna) 2012;119(5):605–12. [DOI] [PubMed] [Google Scholar]
- Hofstadt-van Oy U, Keune PM, Muenssinger J, Hagenburger D, Oschmann P. Normative data and long-term test-retest reliability of the triple stimulation technique (TST) in multiple sclerosis. Clin Neurophysiol 2015;126(2):356–64. [DOI] [PubMed] [Google Scholar]
- Höppner J, Kunesch E, Buchmann J, Hess A, Grossmann A, Benecke R. Demyelination and axonal degeneration in corpus callosum assessed by analysis of transcallosally mediated inhibition in multiple sclerosis. Clin Neurophysiol 1999;110(4):748–56. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Trender-Gerhard I, Edwards MJ, Mir P, Rothwell JC, Bhatia KP. Motor system inhibition in dopa-responsive dystonia and its modulation by treatment. Neurology 2006;66(7):1088–90. [DOI] [PubMed] [Google Scholar]
- Huber R, Mäki H, Rosanova M, Casarotto S, Canali P, Casali AG, et al. Human cortical excitability increases with time awake. Cereb Cortex 2013;23(2):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 2005;11(4):357–72. [DOI] [PubMed] [Google Scholar]
- Humm AM, Beer S, Kool J, Magistris MR, Kesselring J, Rösler KM. Quantification of Uhthoff’s phenomenon in multiple sclerosis: a magnetic stimulation study. Clin Neurophysiol 2004a;115(11):2493–501. [DOI] [PubMed] [Google Scholar]
- Humm AM, Magistris MR, Truffert A, Hess CW, Rosler KM. Central motor conduction differs between acute relapsing-remitting and chronic progressive multiple sclerosis. Clin Neurophysiol 2003;114(11):2196–203. [DOI] [PubMed] [Google Scholar]
- Humm AM, Z’Graggen WJ, von Hornstein NE, Magistris MR, Rösler KM. Assessment of central motor conduction to intrinsic hand muscles using the triple stimulation technique: normal values and repeatability. Clin Neurophysiol 2004b;115(11):2558–66. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol (1985) 2006;101(4):1036–44. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol (1985) 2008;105(4):1199–209. [DOI] [PubMed] [Google Scholar]
- Hupfeld KE, Swanson CW, Fling BW, Seidler RD. TMS-induced silent periods: A review of methods and call for consistency. J Neurosci Methods 2020;346:108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh W, Krishnan AV, Vucic S, Lin CS, Kiernan MC. Motor cortex excitability in acute cerebellar infarct. Cerebellum 2013a;12(6):826–34. [DOI] [PubMed] [Google Scholar]
- Huynh W, Vucic S, Krishnan AV, Lin CS, Hornberger M, Kiernan MC. Longitudinal plasticity across the neural axis in acute stroke. Neurorehabil Neural Repair 2013b;27(3):219–29. [DOI] [PubMed] [Google Scholar]
- Huynh W, Vucic S, Krishnan AV, Lin CS, Kiernan MC. Exploring the Evolution of Cortical Excitability Following Acute Stroke. Neurorehabil Neural Repair 2016;30(3):244–57. [DOI] [PubMed] [Google Scholar]
- Hwang YT, Rocchi L, Hammond P, Hardy CJ, Warren JD, Ridha BH, et al. Effect of donepezil on transcranial magnetic stimulation parameters in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Jung P, Ziemann U. Subtle hemispheric asymmetry of motor cortical inhibitory tone. Clin Neurophysiol 2004;115(2):330–40. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Korchounov A, Ziemann U. Methylphenidate facilitates and disinhibits the motor cortex in intact humans. Neuroreport 2003;14(5):773–6. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 2002;545:153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille S, Kelm A, Schroeder A, Albers LE, Negwer C, Butenschoen VM, et al. Navigated repetitive transcranial magnetic stimulation improves the outcome of postsurgical paresis in glioma patients - A randomized, double-blinded trial. Brain Stimul 2021a;14(4):780–7. [DOI] [PubMed] [Google Scholar]
- Ille S, Kulchytska N, Sollmann N, Wittig R, Beurskens E, Butenschoen VM, et al. Hemispheric language dominance measured by repetitive navigated transcranial magnetic stimulation and postoperative course of language function in brain tumor patients. Neuropsychologia 2016;91:50–60. [DOI] [PubMed] [Google Scholar]
- Ille S, Schroeder A, Albers L, Kelm A, Droese D, Meyer B, et al. Non-Invasive Mapping for Effective Preoperative Guidance to Approach Highly Language-Eloquent Gliomas-A Large Scale Comparative Cohort Study Using a New Classification for Language Eloquence. Cancers (Basel) 2021b;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo Y, Kanchiku T, Suzuki H, Funaba M, Nishida N, Taguchi T. Utility of the central motor conduction time recorded from the abductor pollicis brevis and the abductor digiti minimi muscles in patients with C6–7 myelopathy. J Spinal Cord Med 2018;41(2):182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol 1993;466:521–34. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Conte A, Frasca V, Scaldaferri N, Gilio F, Santini M, et al. Altered response to rTMS in patients with Alzheimer’s disease. Clin Neurophysiol 2006;117(1):103–9. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Thompson AJ, Swash M. Central motor conduction in multiple sclerosis: evaluation of abnormalities revealed by transcutaneous magnetic stimulation of the brain. J Neurol Neurosurg Psychiatry 1988;51(4):487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irlbacher K, Brocke J, Mechow JV, Brandt SA. Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol 2007;118(2):308–16. [DOI] [PubMed] [Google Scholar]
- Issac TG, Chandra SR, Nagaraju BC. Transcranial magnetic stimulation in patients with early cortical dementia: A pilot study. Ann Indian Acad Neurol 2013;16(4):619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Jono Y, Mizusawa H, Kinoshita A, Hiraoka K. Interhemispheric Inhibition Induced by Transcranial Magnetic Stimulation Over Primary Sensory Cortex. Front Hum Neurosci 2016;10:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Manfredi V, Millon G, Hollis C, Jackson GM. Motor excitability is reduced prior to voluntary movements in children and adolescents with Tourette syndrome. J Neuropsychol 2013;7(1):29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 2004;115(7):1490–505. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Richardson MSA, Oudega M, Perez MA. Paired corticospinal-motoneuronal stimulation and exercise after spinal cord injury. J Spinal Cord Med 2021;44(sup1):S23–s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Streletz LJ, Raab VE, Knobler RL, Lublin FD. Lower extremity motor evoked potentials in multiple sclerosis. Arch Neurol 1991;48(9):944–8. [DOI] [PubMed] [Google Scholar]
- Jonker ZD, van der Vliet R, Hauwert CM, Gaiser C, Tulen JHM, van der Geest JN, et al. TMS motor mapping: Comparing the absolute reliability of digital reconstruction methods to the golden standard. Brain Stimul 2019;12(2):309–13. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Könönen M, Pääkkönen A, Karhu J, Soininen H. Combining transcranial magnetic stimulation and electroencephalography may contribute to assess the severity of Alzheimer’s disease. Int J Alzheimers Dis 2011;2011:654794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Westerén-Punnonen S, Pirinen E, Soininen H, Könönen M, et al. Navigated TMS combined with EEG in mild cognitive impairment and Alzheimer’s disease: a pilot study. J Neurosci Methods 2008;172(2):270–6. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Kimiskidis VK, Belardinelli P. Bridging the gap: TMS-EEG from lab to clinic. J Neurosci Methods 2022;369:109482. [DOI] [PubMed] [Google Scholar]
- Jung P, Beyerle A, Humpich M, Neumann-Haefelin T, Lanfermann H, Ziemann U. Ipsilateral silent period: a marker of callosal conduction abnormality in early relapsing-remitting multiple sclerosis? J Neurol Sci 2006;250(1–2):133–9. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage 2005;24(4):955–60. [DOI] [PubMed] [Google Scholar]
- Kaji R, Bhatia K, Graybiel AM. Pathogenesis of dystonia: is it of cerebellar or basal ganglia origin? J Neurol Neurosurg Psychiatry 2018;89(5):488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale N, Agaoglu J, Onder G, Tanik O. Correlation between disability and transcranial magnetic stimulation abnormalities in patients with multiple sclerosis. J Clin Neurosci 2009;16(11):1439–42. [DOI] [PubMed] [Google Scholar]
- Kamble N, Netravathi M, Nagaraju BC, Lenka A, Kumar K, Sowmya V, et al. Evaluation of Cognition and Cortical Excitability in Huntington’s Disease. Can J Neurol Sci 2018;45(2):176–81. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Shimohata T, Toyoshima Y, Tada M, Kakita A, Morita T, et al. Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Mov Disord 2009;24(9):1312–8. [DOI] [PubMed] [Google Scholar]
- Kandler RH, Jarratt JA, Davies-Jones GA, Gumpert EJ, Venables GS, Sagar HJ, et al. The role of magnetic stimulation as a quantifier of motor disability in patients with multiple sclerosis. J Neurol Sci 1991a;106(1):31–4. [DOI] [PubMed] [Google Scholar]
- Kandler RH, Jarratt JA, Gumpert EJ, Davies-Jones GA, Venables GS, Sagar HJ. The role of magnetic stimulation in the diagnosis of multiple sclerosis. J Neurol Sci 1991b;106(1):25–30. [DOI] [PubMed] [Google Scholar]
- Kanovský P, Bares M, Streitová H, Klajblová H, Daniel P, Rektor I. Abnormalities of cortical excitability and cortical inhibition in cervical dystonia Evidence from somatosensory evoked potentials and paired transcranial magnetic stimulation recordings. J Neurol 2003;250(1):42–50. [DOI] [PubMed] [Google Scholar]
- Karatzetzou S, Tsiptsios D, Terzoudi A, Aggeloussis N, Vadikolias K. Transcranial magnetic stimulation implementation on stroke prognosis. Neurol Sci 2022;43(2):873–88. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S, et al. Neuromagnetic evidence of pre- and post-central cortical sources of somatosensory evoked responses. Electroencephalogr Clin Neurophysiol 1996;100(1):44–50. [DOI] [PubMed] [Google Scholar]
- Keser Z, Buchl SC, Seven NA, Markota M, Clark HM, Jones DT, et al. Electroencephalogram (EEG) With or Without Transcranial Magnetic Stimulation (TMS) as Biomarkers for Post-stroke Recovery: A Narrative Review. Front Neurol 2022;13:827866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler KR, Ruge D, Ilić TV, Ziemann U. Short latency afferent inhibition and facilitation in patients with writer’s cramp. Mov Disord 2005;20(2):238–42. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Darwish ES, Ali AM. The relationship between motor cortex excitability and severity of Alzheimer’s disease: a transcranial magnetic stimulation study. Neurophysiol Clin 2011;41(3):107–13. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Lefaucheur JP, Hasan AM, Osama K. Are there differences in cortical excitability between akinetic-rigid and tremor-dominant subtypes of Parkinson’s disease? Neurophysiol Clin 2021;51(5):443–53. [DOI] [PubMed] [Google Scholar]
- Kidd D, Thompson PD, Day BL, Rothwell JC, Kendall BE, Thompson AJ, et al. Central motor conduction time in progressive multiple sclerosis. Correlations with MRI and disease activity. Brain 1998;121:1109–16. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet 2011;377(9769):942–55. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Talbot K, McDermott CJ, Hardiman O, Shefner JM, et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 2020;17:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 1993;89:415–23. [DOI] [PubMed] [Google Scholar]
- Kikuchi S, Mochizuki H, Moriya A, Nakatani-Enomoto S, Nakamura K, Hanajima R, et al. Ataxic hemiparesis: neurophysiological analysis by cerebellar transcranial magnetic stimulation. Cerebellum 2012;11(1):259–63. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Papagiannopoulos S, Sotirakoglou K, Kazis DA, Kazis A, Mills KR. Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res 2005;163(1):21–31. [DOI] [PubMed] [Google Scholar]
- Kimiskidis VK, Tsimpiris A, Ryvlin P, Kalviainen R, Koutroumanidis M, Valentin A, et al. TMS combined with EEG in genetic generalized epilepsy: A phase II diagnostic accuracy study. Clin Neurophysiol 2017;128(2):367–81. [DOI] [PubMed] [Google Scholar]
- Kleine BU, Schelhaas HJ, van Elswijk G, de Rijk MC, Stegeman DF, Zwarts MJ. Prospective, blind study of the triple stimulation technique in the diagnosis of ALS. Amyotroph Lateral Scler 2010;11(1–2):67–75. [DOI] [PubMed] [Google Scholar]
- Klimpe S, Behrang-Nia M, Bott MC, Werhahn KJ. Recruitment of motor cortex inhibition differentiates between generalized and focal epilepsy. Epilepsy Res 2009;84(2–3):210–6. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. Alzheimer disease. Nat Rev Dis Primers 2021;7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Bonnì S, Giacobbe V, Bozzali M, Caltagirone C, et al. Dopaminergic modulation of cortical plasticity in Alzheimer’s disease patients. Neuropsychopharmacology 2014a;39(11):2654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Di Lorenzo F, Del Olmo MF, Bonní S, Ponzo V, Caltagirone C, et al. Reversal of LTP-Like Cortical Plasticity in Alzheimer’s Disease Patients with Tau-Related Faster Clinical Progression. J Alzheimers Dis 2016;50(2):605–16. [DOI] [PubMed] [Google Scholar]
- Koch G, Porcacchia P, Ponzo V, Carrillo F, Cáceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul 2014b;7(4):564–72. [DOI] [PubMed] [Google Scholar]
- Kohara N, Kaji R, Kojima Y, Hamano T, Fujii H, Katayama M, et al. Magnetic stimulation in ALS--a single motor unit study. Electroencephalogr Clin Neurophysiol 1996a;46:327–36. [PubMed] [Google Scholar]
- Kohara N, Kaji R, Kojima Y, Mills KR, Fujii H, Hamano T, et al. Abnormal excitability of the corticospinal pathway in patients with amyotrophic lateral sclerosis: a single motor unit study using transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 1996b;101(1):32–41. [DOI] [PubMed] [Google Scholar]
- Kojovic M, Kassavetis P, Bologna M, Pareés I, Rubio-Agusti I, Berardelli A, et al. Transcranial magnetic stimulation follow-up study in early Parkinson’s disease: A decline in compensation with disease progression? Mov Disord 2015;30(8):1098–106. [DOI] [PubMed] [Google Scholar]
- Kojovic M, Pareés I, Kassavetis P, Palomar FJ, Mir P, Teo JT, et al. Secondary and primary dystonia: pathophysiological differences. Brain 2013;136:2038–49. [DOI] [PubMed] [Google Scholar]
- Komissarow L, Rollnik JD, Bogdanova D, Krampfl K, Khabirov FA, Kossev A, et al. Triple stimulation technique (TST) in amyotrophic lateral sclerosis. Clin Neurophysiol 2004;115(2):356–60. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp 2004;21(3):154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen LM, Goetz SM, Tucci DL, Peterchev AV. Sound comparison of seven TMS coils at matched stimulation strength. Brain Stimul 2020;13(3):873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen LM, Nieminen JO, Ilmoniemi RJ. Multi-locus transcranial magnetic stimulation-theory and implementation. Brain Stimul 2018;11(4):849–55. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology 2011;36(9):1894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer P, Langguth B, Popp R, Raster R, Busch V, Frank E, et al. Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neurosci Lett 2011;493(3):63–6. [DOI] [PubMed] [Google Scholar]
- Krieg SM, Tarapore PE, Picht T, Tanigawa N, Houde J, Sollmann N, et al. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage 2014;100:219–36. [DOI] [PubMed] [Google Scholar]
- Kriz J, Kozak J, Zedka M. Primary motor cortex inhibition in spinal cord injuries. Neuro Endocrinol Lett 2012;33(4):431–41. [PubMed] [Google Scholar]
- Kuhn AA, Grosse P, Holtz K, Brown P, Meyer BU, Kupsch A. Patterns of abnormal motor cortex excitability in atypical parkinsonian syndromes. Clin Neurophysiol 2004;115(8):1786–95. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YL, Dubuc T, Boufadel DF, Fisher BE. Measuring ipsilateral silent period: Effects of muscle contraction levels and quantification methods. Brain Res 2017;1674:77–83. [DOI] [PubMed] [Google Scholar]
- La Mantia L, Riti F, Milanese C, Salmaggi A, Eoli M, Ciano C, et al. Serial evoked potentials in multiple sclerosis bouts. Relation to steroid treatment. Ital J Neurol Sci 1994;15(7):333–40. [DOI] [PubMed] [Google Scholar]
- Lagueny A, Arnaud A, Le Masson G, Burbaud P, Deliac P, Marthan R. Study of central and peripheral conductions to the diaphragm in 22 patients with definite multiple sclerosis. Electromyogr Clin Neurophysiol 1998;38(6):333–42. [PubMed] [Google Scholar]
- Lahr J, Peter J, Minkova L, Lauer E, Reis J, Heimbach B, et al. No difference in paired associative stimulation induced cortical neuroplasticity between patients with mild cognitive impairment and elderly controls. Clin Neurophysiol 2016;127(2):1254–60. [DOI] [PubMed] [Google Scholar]
- Lanza G, Puglisi V, Vinciguerra L, Fisicaro F, Vagli C, Cantone M, et al. TMS Correlates of Pyramidal Tract Signs and Clinical Motor Status in Patients with Cervical Spondylotic Myelopathy. Brain Sci 2020;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Joo EY, Seo DW, Hong SB. Lateralizing Cortical Excitability in Drug Naïve Patients with Generalized or Focal Epilepsy. J Epilepsy Res 2015;5(2):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Harrison LM, Mechelli A. A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage 2003;19(2 Pt 1):457–65. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Transcranial magnetic stimulation in the management of pain. Suppl Clin Neurophysiol 2004;57:737–48. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Excitability of the motor cortical representation of the external anal sphincter. Exp Brain Res 2005;160(2):268–72. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Neurophysiological testing in anorectal disorders. Muscle Nerve 2006;33(3):324–33. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Neurophysiol Clin 2010;40(1):1–5. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin Neurophysiol 2020;131(2):474–528. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125(11):2150–206. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006a;67(9):1568–74. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Von Raison F, Ménard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol 2004;115(11):2530–41. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Lofaso F. Diaphragmatic silent period to transcranial magnetic cortical stimulation for assessing cortical motor control of the diaphragm. Exp Brain Res 2002;146(3):404–9. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Lucas B, Andraud F, Hogrel JY, Bellivier F, Del Cul A, et al. Inter-hemispheric asymmetry of motor corticospinal excitability in major depression studied by transcranial magnetic stimulation. J Psychiatr Res 2008;42(5):389–98. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Ménard-Lefaucheur I, Goujon C, Keravel Y, Nguyen JP. Predictive value of rTMS in the identification of responders to epidural motor cortex stimulation therapy for pain. J Pain 2011;12(10):1102–11. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Ménard-Lefaucheur I, Maison P, Baudic S, Cesaro P, Peschanski M, et al. Electrophysiological deterioration over time in patients with Huntington’s disease. Mov Disord 2006b;21(9):1350–4. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Picht T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin 2016;46(2):125–33. [DOI] [PubMed] [Google Scholar]
- Lempel A, Ziv J. On the Complexity of Finite Sequences. IEEE Trans Inform Theory 1976;22:75–81. [Google Scholar]
- Leodori G, De Bartolo MI, Guerra A, Fabbrini A, Rocchi L, Latorre A, et al. Motor Cortical Network Excitability in Parkinson’s Disease. Mov Disord 2022;37(4):734–44. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Altered sensorimotor integration in Parkinson’s disease. Brain 2002;125:2089–99. [DOI] [PubMed] [Google Scholar]
- Li JY, Espay AJ, Gunraj CA, Pal PK, Cunic DI, Lang AE, et al. Interhemispheric and ipsilateral connections in Parkinson’s disease: relation to mirror movements. Mov Disord 2007;22(6):813–21. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bar KJ, Meske U, Weiller C. Motor cortex disinhibition in Alzheimer’s disease. Clin Neurophysiol 2001;112(8):1436–41. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hassa T, Tüscher O, Schmidt R. Electrophysiological correlates of motor conversion disorder. Mov Disord 2008;23(15):2171–6. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hassa T, Tüscher O, Schmidt R. Abnormal motor excitability in patients with psychogenic paresis. A TMS study. J Neurol 2009;256(1):121–6. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hassa T, Tüscher O, Schmidt R. Motor excitability during movement imagination and movement observation in psychogenic lower limb paresis. J Psychosom Res 2011;70(1):59–65. [DOI] [PubMed] [Google Scholar]
- Liepert J, Kotterba S, Tegenthoff M, Malin JP. Central fatigue assessed by transcranial magnetic stimulation. Muscle Nerve 1996;19(11):1429–34. [DOI] [PubMed] [Google Scholar]
- Liepert J, Kucinski T, Tüscher O, Pawlas F, Bäumer T, Weiller C. Motor cortex excitability after cerebellar infarction. Stroke 2004;35(11):2484–8. [DOI] [PubMed] [Google Scholar]
- Liepert J, Mingers D, Heesen C, Baumer T, Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler 2005;11:316–21. [DOI] [PubMed] [Google Scholar]
- Liepert J, Wessel K, Schwenkreis P, Trillenberg P, Otto V, Vorgerd M, et al. Reduced intracortical facilitation in patients with cerebellar degeneration. Acta Neurol Scand 1998;98(5):318–23. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Chen PC, Tsai TT, Hsu SP. Comparison of nerve conduction study and transcranial magnetic stimulation for early diagnosis and prognosis prediction of idiopathic facial palsy. Neurol Sci 2021;42(10):4149–54. [DOI] [PubMed] [Google Scholar]
- Lioumis P, Rosanova M. The role of neuronavigation in TMS-EEG studies: Current applications and future perspectives. J Neurosci Methods 2022;380:109677. [DOI] [PubMed] [Google Scholar]
- Lioumis P, Zhdanov A, Mäkelä N, Lehtinen H, Wilenius J, Neuvonen T, et al. A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. J Neurosci Methods 2012;204(2):349–54. [DOI] [PubMed] [Google Scholar]
- Livingston SC, Goodkin HP, Ingersoll CD. The influence of gender, hand dominance, and upper extremity length on motor evoked potentials. J Clin Monit Comput 2010;24(6):427–36. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 1999;96(26):15222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL. The role of electrophysiology in the diagnosis and management of cervical spondylotic myelopathy. Ann Acad Med Singap 2007;36(11):886–93. [PubMed] [Google Scholar]
- Lo YL. How has electrophysiology changed the management of cervical spondylotic myelopathy? Eur J Neurol 2008;15(8):781–6. [DOI] [PubMed] [Google Scholar]
- Lüdemann-Podubecká J, Nowak DA. Mapping cortical hand motor representation using TMS: A method to assess brain plasticity and a surrogate marker for recovery of function after stroke? Neurosci Biobehav Rev 2016;69:239–51. [DOI] [PubMed] [Google Scholar]
- Ly JQM, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, et al. Circadian regulation of human cortical excitability. Nat Commun 2016;7:11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R, van de Warrenburg BPC, Schutter D. Inverse associations between cerebellar inhibition and motor impairment in spinocerebellar ataxia type 3. Brain Stimul 2021;14(2):351–7. [DOI] [PubMed] [Google Scholar]
- Maccabee PJ, Eberle LP, Stein IA, Willer JA, Lipitz ME, Kula RW, et al. Upper leg conduction time distinguishes demyelinating neuropathies. Muscle Nerve 2011;43(4):518–30. [DOI] [PubMed] [Google Scholar]
- Machetanz K, Wiesinger L, Leao MT, Liebsch M, Trakolis L, Wang S, et al. Interhemispheric differences in time-frequency representation of motor evoked potentials in brain tumor patients. Clin Neurophysiol 2021;132(11):2780–8. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol 2005;58(4):516–24. [DOI] [PubMed] [Google Scholar]
- Madsen MAJ, Wiggermann V, Marques MFM, Lundell H, Cerri S, Puonti O, et al. Linking lesions in sensorimotor cortex to contralateral hand function in multiple sclerosis: a 7 T MRI study. Brain 2022;145(10):3522–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens de Noordhout A, Pepin JL, Schoenen J, Delwaide PJ. Percutaneous magnetic stimulation of the motor cortex in migraine. Electroencephalogr Clin Neurophysiol 1992;85(2):110–5. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rösler KM. The triple stimulation technique to study corticospinal conduction. Suppl Clin Neurophysiol 2003;56:24–32. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Landis T, Hess CW. A clinical study of motor evoked potentials using a triple stimulation technique. Brain 1999;122:265–79. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rosler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 1998a;121:437–50. [DOI] [PubMed] [Google Scholar]
- Magistris MR, Rösler KM, Truffert A, Myers JP. Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 1998b;121 (121):437–50. [DOI] [PubMed] [Google Scholar]
- Maidan I, Zifman N, Hausdorff JM, Giladi N, Levy-Lamdan O, Mirelman A. A multimodal approach using TMS and EEG reveals neurophysiological changes in Parkinson’s disease. Parkinsonism Relat Disord 2021;89:28–33. [DOI] [PubMed] [Google Scholar]
- Maier J, Sebastian I, Weisbrod M, Freitag CM, Resch F, Bender S. Cortical inhibition at rest and under a focused attention challenge in adults with migraine with and without aura. Cephalalgia 2011;31(8):914–24. [DOI] [PubMed] [Google Scholar]
- Maitland S, Baker SN. Ipsilateral Motor Evoked Potentials as a Measure of the Reticulospinal Tract in Age-Related Strength Changes. Front Aging Neurosci 2021;13:612352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganotti P, Bongiovanni LG, Fuggetta G, Zanette G, Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: a combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry 2006;77(1):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano Y, Nakamuro T, Ikoma K, Sugata T, Morimoto S, Takayanagi T, et al. Central motor conductivity in aged people. Intern Med 1992;31(9):1084–7. [DOI] [PubMed] [Google Scholar]
- Marchese R, Trompetto C, Buccolieri A, Abbruzzese G. Abnormalities of motor cortical excitability are not correlated with clinical features in atypical parkinsonism. Mov Disord 2000;15(6):1210–4. [DOI] [PubMed] [Google Scholar]
- Mariorenzi R, Zarola F, Caramia MD, Paradiso C, Rossini PM. Non-invasive evaluation of central motor tract excitability changes following peripheral nerve stimulation in healthy humans. Electroencephalogr Clin Neurophysiol 1991;81(2):90–101. [DOI] [PubMed] [Google Scholar]
- Marra C, Quaranta D, Profice P, Pilato F, Capone F, Iodice F, et al. Central cholinergic dysfunction measured “in vivo” correlates with different behavioral disorders in Alzheimer’s disease and dementia with Lewy body. Brain Stimul 2012;5(4):533–8. [DOI] [PubMed] [Google Scholar]
- Martorana A, Di Lorenzo F, Esposito Z, Lo Giudice T, Bernardi G, Caltagirone C, et al. Dopamine D₂-agonist rotigotine effects on cortical excitability and central cholinergic transmission in Alzheimer’s disease patients. Neuropharmacology 2013;64:108–13. [DOI] [PubMed] [Google Scholar]
- Martorana A, Mori F, Esposito Z, Kusayanagi H, Monteleone F, Codecà C, et al. Dopamine modulates cholinergic cortical excitability in Alzheimer’s disease patients. Neuropsychopharmacology 2009;34(10):2323–8. [DOI] [PubMed] [Google Scholar]
- Martorana A, Stefani A, Palmieri MG, Esposito Z, Bernardi G, Sancesario G, et al. L-dopa modulates motor cortex excitability in Alzheimer’s disease patients. J Neural Transm (Vienna) 2008;115(9):1313–9. [DOI] [PubMed] [Google Scholar]
- Mascia MM, Valls-Solé J, Martí MJ, Salazar G. Sensorimotor integration in patients with parkinsonian type multisystem atrophy. J Neurol 2005;252(4):473–81. [DOI] [PubMed] [Google Scholar]
- Massé-Alarie H, Flamand VH, Moffet H, Schneider C. Peripheral neurostimulation and specific motor training of deep abdominal muscles improve posturomotor control in chronic low back pain. Clin J Pain 2013;29(9):814–23. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science 2005;309(5744):2228–32. [DOI] [PubMed] [Google Scholar]
- Matamala JM, Howells J, Dharmadasa T, Trinh T, Ma Y, Lera L, et al. Inter-session reliability of short-interval intracortical inhibition measured by threshold tracking TMS. Neurosci Lett 2018;674:18–23. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Hamada M, Terao Y, Yugeta A, Inomata-Terada S, et al. Double-pulse magnetic brain stem stimulation: mimicking successive descending volleys. J Neurophysiol 2008;100(6):3437–44. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Shirota Y, Hamada M, Terao Y, Ohminami S, et al. Cortico-conus motor conduction time (CCCT) for leg muscles. Clin Neurophysiol 2010a;121(11):1930–3. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Hashida H, Ugawa Y. Cauda equina conduction time in Guillain-Barré syndrome. J Neurol Sci 2015;351(1–2):187–90. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Ugawa Y. Magnetic-motor-root stimulation: review. Clin Neurophysiol 2013a;124(6):1055–67. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Hanajima R, Terao Y, Yugeta A, Hamada M, Shirota Y, et al. Prominent cauda equina involvement in patients with chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci 2010b;290(1–2):112–4. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Konoma Y, Fujii K, Hanajima R, Terao Y, Ugawa Y. A conduction block in sciatic nerves can be detected by magnetic motor root stimulation. J Neurol Sci 2013b;331(1–2):174–6. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Konoma Y, Shimizu T, Okabe S, Shirota Y, Hanajima R, et al. Aging influences central motor conduction less than peripheral motor conduction: a transcranial magnetic stimulation study. Muscle Nerve 2012;46(6):932–6. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Octaviana F, Hanajima R, Terao Y, Yugeta A, Hamada M, et al. Magnetic lumbosacral motor root stimulation with a flat, large round coil. Clin Neurophysiol 2009a;120(4):770–5. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Octaviana F, Terao Y, Hanajima R, Yugeta A, Hamada M, et al. Magnetic stimulation of the cauda equina in the spinal canal with a flat, large round coil. J Neurol Sci 2009b;284(1–2):46–51. [DOI] [PubMed] [Google Scholar]
- Matsumoto L, Hanajima R, Matsumoto H, Ohminami S, Terao Y, Tsuji S, et al. Supramaximal responses can be elicited in hand muscles by magnetic stimulation of the cervical motor roots. Brain Stimul 2010;3(3):153–60. [DOI] [PubMed] [Google Scholar]
- Mayr N, Baumgartner C, Zeitlhofer J, Deecke L. The sensitivity of transcranial cortical magnetic stimulation in detecting pyramidal tract lesions in clinically definite multiple sclerosis. Neurology 1991;41(4):566–9. [DOI] [PubMed] [Google Scholar]
- Mazur MD, White A, McEvoy S, Bisson EF. Transcranial magnetic stimulation of the motor cortex correlates with objective clinical measures in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2014;39(14):1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res 2006;173(1):86–93. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul 2017;10(4):721–34. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Thompson PD, Ridding MC. The effect of cutaneous input on intracortical inhibition in focal task-specific dystonia. Mov Disord 2007;22(9):1286–92. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meincke J, Hewitt M, Batsikadze G, Liebetanz D. Automated TMS hotspot-hunting using a closed loop threshold-based algorithm. Neuroimage 2016;124:509–17. [DOI] [PubMed] [Google Scholar]
- Menon P, Geevasinga N, van den Bos M, Yiannikas C, Kiernan MC, Vucic S. Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis. Eur J Neurol 2017;24(6):816–24. [DOI] [PubMed] [Google Scholar]
- Menon P, Geevasinga N, Yiannikas C, Howells J, Kiernan M, Vucic S. The sensitivity and specificity of threshold-tracking transcranial magnetic stimulation for the diagnosis of amyotrophic lateral sclerosis: a prospective study Lancet Neurol 2015;14:478–84. [DOI] [PubMed] [Google Scholar]
- Menon P, Geevasinga N, Yiannikas C, Kiernan MC, Vucic S. Cortical contributions to the flail leg syndrome: Pathophysiological insights. Amyotroph Lateral Scler Frontotemporal Degener 2016;17(5–6):389–96. [DOI] [PubMed] [Google Scholar]
- Menon P, Kiernan MC, Vucic S. Cortical dysfunction underlies the development of the split-hand in amyotrophic lateral sclerosis. PLoS One 2014;9(1):e87124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P, Kiernan MC, Vucic S. Cortical excitability varies across different muscles. J Neurophysiol 2018;120(3):1397–403. [DOI] [PubMed] [Google Scholar]
- Menon P, Yiannikas C, Kiernan MC, Vucic S. Regional motor cortex dysfunction in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2019;6(8):1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsomaa J, Belardinelli P, Ermolova M, Ziemann U, Zrenner C. Causal decoding of individual cortical excitability states. Neuroimage 2021;245:118652. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 1995;118:429–40. [DOI] [PubMed] [Google Scholar]
- Mhalla A, Baudic S, de Andrade DC, Gautron M, Perrot S, Teixeira MJ, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain 2011;152(7):1478–85. [DOI] [PubMed] [Google Scholar]
- Michels R, Wessel K, Klöhn S, Kömpf D. Long-latency reflexes, somatosensory evoked potentials and transcranial magnetic stimulation: relation of the three methods in multiple sclerosis. Electroencephalogr Clin Neurophysiol 1993;89(4):235–41. [DOI] [PubMed] [Google Scholar]
- Mills KR. Motor neuron disease. Studies of the corticospinal excitation of single motor neurons by magnetic brain stimulation. Brain 1995;118:971–82. [DOI] [PubMed] [Google Scholar]
- Mills KR. Magnetic brain stimulation: a review after 10 years experience. Electroencephalogr Clin Neurophysiol Suppl 1999;49:239–44. [PubMed] [Google Scholar]
- Mills KR. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain 2003;126:2558–66. [DOI] [PubMed] [Google Scholar]
- Mills KR, Murray NM. Electrical stimulation over the human vertebral column: which neural elements are excited? Electroencephalogr Clin Neurophysiol 1986;63(6):582–9. [DOI] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve 1997a;20:1137–41. [DOI] [PubMed] [Google Scholar]
- Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve 1997b;20(5):570–6. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Nishida H, Nakajima S, Tsugawa S, Morita S, Yoshida K, et al. Neurophysiological biomarkers using transcranial magnetic stimulation in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev 2021;121:47–59. [DOI] [PubMed] [Google Scholar]
- Mirchandani AS, Beyh A, Lavrador JP, Howells H, Dell’Acqua F, Vergani F. Altered corticospinal microstructure and motor cortex excitability in gliomas: an advanced tractography and transcranial magnetic stimulation study. J Neurosurg 2020;134(5):1368–76. [DOI] [PubMed] [Google Scholar]
- Miscio G, Guastamacchia G, Priano L, Baudo S, Mauro A. Are the neurophysiological techniques useful for the diagnosis of diaphragmatic impairment in multiple sclerosis (MS)? Clin Neurophysiol 2003;114(1):147–53. [DOI] [PubMed] [Google Scholar]
- Miscio G, Pisano F, Mora G, Mazzini L. Motor neuron disease: usefulness of transcranial magnetic stimulation in improving the diagnosis. Clin Neurophysiol 1999;110(5):975–81. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol 2004;561:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modugno N, Currà A, Giovannelli M, Priori A, Squitieri F, Ruggieri S, et al. The prolonged cortical silent period in patients with Huntington’s disease. Clin Neurophysiol 2001;112(8):1470–4. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol 2003;553:665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RA, Ni Z, Shirota Y, Chen R, Ugawa Y, Celnik PA. Age-related strengthening of cerebello-cortical motor circuits. Neurobiol Aging 2022;118:9–12. [DOI] [PubMed] [Google Scholar]
- Mori F, Kusayanagi H, Monteleone F, Moscatelli A, Nicoletti CG, Bernardi G, et al. Short interval intracortical facilitation correlates with the degree of disability in multiple sclerosis. Brain Stimul 2013;6(1):67–71. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci 2009;12(1):85–91. [DOI] [PubMed] [Google Scholar]
- Morita Y, Osaki Y, Doi Y. Transcranial magnetic stimulation for differential diagnostics in patients with parkinsonism. Acta Neurol Scand 2008;118(3):159–63. [DOI] [PubMed] [Google Scholar]
- Motolese F, Capone F, Di Lazzaro V. New tools for shaping plasticity to enhance recovery after stroke. Handb Clin Neurol 2022;184:299–315. [DOI] [PubMed] [Google Scholar]
- Motta C, Di Lorenzo F, Ponzo V, Pellicciari MC, Bonnì S, Picazio S, et al. Transcranial magnetic stimulation predicts cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2018;89(12):1237–42. [DOI] [PubMed] [Google Scholar]
- Mueller JK, Grigsby EM, Prevosto V, Petraglia FW 3rd, Rao H, Deng ZD, et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat Neurosci 2014;17(8):1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Abe D, Matsumoto H, Tokimura R, Abe M, Tiksnadi A, et al. A patient with McLeod syndrome showing involvement of the central sensorimotor tracts for the legs. BMC Neurol 2019;19(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 2004;55(3):400–9. [DOI] [PubMed] [Google Scholar]
- Murley AG, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration. Brain 2018;141(5):1263–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutanen TP, Metsomaa J, Makkonen M, Varone G, Marzetti L, Ilmoniemi RJ. Source-based artifact-rejection techniques for TMS-EEG. J Neurosci Methods 2022;382:109693. [DOI] [PubMed] [Google Scholar]
- Mykland MS, Uglem M, Neverdahl JP, Øie LR, Meisingset TW, Dodick DW, et al. Sleep restriction alters cortical inhibition in migraine: A transcranial magnetic stimulation study. Clin Neurophysiol 2022;139:28–42. [DOI] [PubMed] [Google Scholar]
- Naeije G, Fogang Y, Ligot N, Mavroudakis N. Occipital transcranial magnetic stimulation discriminates transient neurological symptoms of vascular origin from migraine aura without headache. Neurophysiol Clin 2017;47(4):269–74. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Eisen A, Stewart H. Diverse abnormalities of corticomotoneuronal projections in individual patients with amyotrophic lateral sclerosis. Electroencephalogr Clin Neurophysiol 1997;105(6):451–7. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol 1997;498:817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Tanaka N, Kamei N, Ohta R, Fujioka Y, Hiramatsu T, et al. Electrophysiological evidence of functional improvement in the corticospinal tract after laminoplasty in patients with cervical compressive myelopathy: clinical article. J Neurosurg Spine 2014;21(2):210–6. [DOI] [PubMed] [Google Scholar]
- Nantes JC, Zhong J, Holmes SA, Whatley B, Narayanan S, Lapierre Y, et al. Intracortical inhibition abnormality during the remission phase of multiple sclerosis is related to upper limb dexterity and lesions. Clin Neurophysiol 2016;127(2):1503–11. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Brigo F, Christova M, Kunz A, Seidl M, et al. Functional evaluation of central cholinergic circuits in patients with Parkinson’s disease and REM sleep behavior disorder: a TMS study. J Neural Transm (Vienna) 2013a;120(3):413–22. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, et al. Short latency afferent inhibition differs among the subtypes of mild cognitive impairment. J Neural Transm (Vienna) 2012;119(4):463–71. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Kronbichler M, Kunz A, Klein S, Caleri F, et al. Abnormal short latency afferent inhibition in early Alzheimer’s disease: a transcranial magnetic demonstration. J Neural Transm (Vienna) 2008;115(11):1557–62. [DOI] [PubMed] [Google Scholar]
- Nardone R, Bratti A, Tezzon F. Motor cortex inhibitory circuits in dementia with Lewy bodies and in Alzheimer’s disease. J Neural Transm (Vienna) 2006;113(11):1679–84. [DOI] [PubMed] [Google Scholar]
- Nardone R, Brigo F, Höller Y, Sebastianelli L, Versace V, Saltuari L, et al. Transcranial magnetic stimulation studies in complex regional pain syndrome type I: A review. Acta Neurol Scand 2018;137(2):158–64. [DOI] [PubMed] [Google Scholar]
- Nardone R, Florio I, Lochner P, Tezzon F. Cholinergic cortical circuits in Parkinson’s disease and in progressive supranuclear palsy: a transcranial magnetic stimulation study. Exp Brain Res 2005;163(1):128–31. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Bathke AC, Orioli A, Schwenker K, Frey V, et al. Spinal cord injury affects I-wave facilitation in human motor cortex. Brain Res Bull 2015a;116:93–7. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Brigo F, Höller P, Christova M, Tezzon F, et al. Fatigue-induced motor cortex excitability changes in subjects with spinal cord injury. Brain Res Bull 2013b;99:9–12. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Brigo F, Orioli A, Tezzon F, Schwenker K, et al. Descending motor pathways and cortical physiology after spinal cord injury assessed by transcranial magnetic stimulation: a systematic review. Brain Res 2015b;1619:139–54. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res 2013c;1504:58–73. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Thomschewski A, Kunz AB, Lochner P, Golaszewski S, et al. Dopamine differently modulates central cholinergic circuits in patients with Alzheimer disease and CADASIL. J Neural Transm (Vienna) 2014;121(10):1313–20. [DOI] [PubMed] [Google Scholar]
- Nardone R, Lochner P, Marth R, Ausserer H, Bratti A, Tezzon F. Abnormal intracortical facilitation in early-stage Huntington’s disease. Clin Neurophysiol 2007;118(5):1149–54. [DOI] [PubMed] [Google Scholar]
- Nardone R, Versace V, Sebastianelli L, Brigo F, Christova M, Scarano GI, et al. Transcranial magnetic stimulation in subjects with phantom pain and non-painful phantom sensations: A systematic review. Brain Res Bull 2019;148:1–9. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R. Impaired interhemispheric inhibition in writer’s cramp. Neurology 2010;75(5):441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 1995;104(3):527–33. [DOI] [PubMed] [Google Scholar]
- Neverdahl JP, Omland PM, Uglem M, Engstrøm M, Sand T. Reduced motor cortical inhibition in migraine: A blinded transcranial magnetic stimulation study. Clin Neurophysiol 2017;128(12):2411–8. [DOI] [PubMed] [Google Scholar]
- Ngomo S, Leonard G, Moffet H, Mercier C. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods 2012;205(1):65–71. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology 2013;80(19):1746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ, et al. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol 2011;105(2):749–56. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, et al. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex 2009;19(7):1654–65. [DOI] [PubMed] [Google Scholar]
- Ni Z, Leodori G, Vial F, Zhang Y, Avram AV, Pajevic S, et al. Measuring latency distribution of transcallosal fibers using transcranial magnetic stimulation. Brain Stimul 2020;13(5):1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Pinto AD, Lang AE, Chen R. Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 2010;68(6):816–24. [DOI] [PubMed] [Google Scholar]
- Niehaus L, von Alt-Stutterheim K, Röricht S, Meyer BU. Abnormal postexcitatory and interhemispheric motor cortex inhibition in writer’s cramp. J Neurol 2001;248(1):51–6. [DOI] [PubMed] [Google Scholar]
- Nielsen CS, Samusyte G, Pugdahl K, Blicher JU, Fuglsang-Frederiksen A, Cengiz B, et al. Test-Retest Reliability of Short-Interval Intracortical Inhibition Assessed by Threshold-Tracking and Automated Conventional Techniques. eNeuro 2021;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JF. Frequency-dependent conduction delay of motor-evoked potentials in multiple sclerosis. Muscle Nerve 1997;20(10):1264–74. [DOI] [PubMed] [Google Scholar]
- Nieminen JO, Gosseries O, Massimini M, Saad E, Sheldon AD, Boly M, et al. Consciousness and cortical responsiveness: a within-state study during non-rapid eye movement sleep. Sci Rep 2016;6:30932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen JO, Sinisalo H, Souza VH, Malmi M, Yuryev M, Tervo AE, et al. Multi-locus transcranial magnetic stimulation system for electronically targeted brain stimulation. Brain Stimul 2022;15(1):116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei K, McKee AC, Kowall NW. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathologica 1993;86:55–64. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Linder S, Topka H. Diagnostic relevance of transcranial magnetic and electric stimulation of the facial nerve in the management of facial palsy. Clin Neurophysiol 2005;116(9):2051–7. [DOI] [PubMed] [Google Scholar]
- Olazarán J, Hernández-Tamames JA, Molina E, García-Polo P, Dobato JL, Álvarez-Linera J, et al. Clinical and anatomical correlates of gait dysfunction in Alzheimer’s disease. J Alzheimers Dis 2013;33(2):495–505. [DOI] [PubMed] [Google Scholar]
- Olazarán J, Prieto J, Cruz I, Esteban A. Cortical excitability in very mild Alzheimer’s disease: a long-term follow-up study. J Neurol 2010;257(12):2078–85. [DOI] [PubMed] [Google Scholar]
- Olivier E, Baker SN, Lemon RN. Comparison of direct and indirect measurements of the central motor conduction time in the monkey. Clin Neurophysiol 2002;113(4):469–77. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, et al. Effects of aging on motor cortex excitability. Neurosci Res 2006;55(1):74–7. [DOI] [PubMed] [Google Scholar]
- Opsomer RJ, Caramia MD, Zarola F, Pesce F, Rossini PM. Neurophysiological evaluation of central-peripheral sensory and motor pudendal fibres. Electroencephalogr Clin Neurophysiol 1989;74(4):260–70. [DOI] [PubMed] [Google Scholar]
- Ortega JD, Fehlman LA, Farley CT. Effects of aging and arm swing on the metabolic cost of stability in human walking. J Biomech 2008;41(16):3303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Münchau A, Rothwell JC. Corticospinal system excitability at rest is associated with tic severity in tourette syndrome. Biol Psychiatry 2008;64(3):248–51. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol 2004;115(5):1076–82. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry 2009;80(1):29–34. [DOI] [PubMed] [Google Scholar]
- Padovani A, Benussi A, Cantoni V, Dell’Era V, Cotelli MS, Caratozzolo S, et al. Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease with Transcranial Magnetic Stimulation. J Alzheimers Dis 2018;65(1):221–30. [DOI] [PubMed] [Google Scholar]
- Padovani A, Benussi A, Cotelli MS, Ferrari C, Cantoni V, Dell’Era V, et al. Transcranial magnetic stimulation and amyloid markers in mild cognitive impairment: impact on diagnostic confidence and diagnostic accuracy. Alzheimers Res Ther 2019;11(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo A, Fierro B, Giglia G, Cosentino G, Puma AR, Brighina F. Modulation of visual cortex excitability in migraine with aura: effects of valproate therapy. Neurosci Lett 2009;467(1):26–9. [DOI] [PubMed] [Google Scholar]
- Palese F, Bonomi E, Nuzzo T, Benussi A, Mellone M, Zianni E, et al. Anti-GluA3 antibodies in frontotemporal dementia: effects on glutamatergic neurotransmission and synaptic failure. Neurobiol Aging 2020;86:143–55. [DOI] [PubMed] [Google Scholar]
- Pallix-Guyot M, Guennoc AM, Blasco H, de Toffol B, Corcia P, Praline J. Predictive value of motor evoked potentials in clinically isolated syndrome. Acta Neurol Scand 2011;124(6):410–6. [DOI] [PubMed] [Google Scholar]
- Paradiso GO, Cunic DI, Gunraj CA, Chen R. Representation of facial muscles in human motor cortex. J Physiol 2005;567:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee S, Park SK, Lee JA, Park K. Facial motor evoked potential with paired transcranial magnetic stimulation: prognostic value following microvascular decompression for hemifacial spasm. J Neurosurg 2018;131(6):1780–7. [DOI] [PubMed] [Google Scholar]
- Parker RS, Lewis GN, Rice DA, McNair PJ. Is Motor Cortical Excitability Altered in People with Chronic Pain? A Systematic Review and Meta-Analysis. Brain Stimul 2016;9(4):488–500. [DOI] [PubMed] [Google Scholar]
- Paulus W, Rothwell JC. Membrane resistance and shunting inhibition: where biophysics meets state-dependent human neurophysiology. J Physiol 2016;594(10):2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond B Biol Sci 2005;360(1457):1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J Neurophysiol 2001;86(4):1983–90. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Bonnì S, Ponzo V, Cinnera AM, Mancini M, Casula EP, et al. Dynamic reorganization of TMS-evoked activity in subcortical stroke patients. Neuroimage 2018;175:365–78. [DOI] [PubMed] [Google Scholar]
- Pelosin E, Ogliastro C, Lagravinese G, Bonassi G, Mirelman A, Hausdorff JM, et al. Attentional Control of Gait and Falls: Is Cholinergic Dysfunction a Common Substrate in the Elderly and Parkinson’s Disease? Front Aging Neurosci 2016;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepin JL, Bogacz D, de Pasqua V, Delwaide PJ. Motor cortex inhibition is not impaired in patients with Alzheimer’s disease: evidence from paired transcranial magnetic stimulation. J Neurol Sci 1999;170(2):119–23. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 2009;587:725–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petajan JH, White AT. Motor-evoked potentials in response to fatiguing grip exercise in multiple sclerosis patients. Clin Neurophysiol 2000;111(12):2188–95. [DOI] [PubMed] [Google Scholar]
- Peter J, Lahr J, Minkova L, Lauer E, Grothe MJ, Teipel S, et al. Contribution of the Cholinergic System to Verbal Memory Performance in Mild Cognitive Impairment. J Alzheimers Dis 2016;53(3):991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JA, Spiess M, Curt A, Dietz V, Schubert M. Spinal cord injury: one-year evolution of motor-evoked potentials and recovery of leg motor function in 255 patients. Neurorehabil Neural Repair 2012;26(8):939–48. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med 2014;275(3):214–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr 2008;13(1):45–53. [DOI] [PubMed] [Google Scholar]
- Petitjean M, Ko JY. An age-related change in the ipsilateral silent period of a small hand muscle. Clin Neurophysiol 2013;124(2):346–53. [DOI] [PubMed] [Google Scholar]
- Peurala S, Muller-Dahlhaus JFM, Arai N, Ziemann U. Interference of short-interval intracortical inbition (SICI) and short-interval intracortical facilitation. Clin Neurophysiol 2008;119(10):2291–7. [DOI] [PubMed] [Google Scholar]
- Picht T, Krieg SM, Sollmann N, Rösler J, Niraula B, Neuvonen T, et al. A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery 2013;72(5):808–19. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Marciani MG, Palmieri MG, Brusa L, Galati S, Caramia MD, et al. Effect of Vigabatrin on motor responses to transcranial magnetic stimulation: an effective tool to investigate in vivo GABAergic cortical inhibition in humans. Brain Res 2004a;1028(1):1–8. [DOI] [PubMed] [Google Scholar]
- Pierantozzi M, Panella M, Palmieri MG, Koch G, Giordano A, Marciani MG, et al. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin Neurophysiol 2004b;115(10):2410–8. [DOI] [PubMed] [Google Scholar]
- Pierelli F, Iacovelli E, Bracaglia M, Serrao M, Coppola G. Abnormal sensorimotor plasticity in migraine without aura patients. Pain 2013;154(9):1738–42. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord: spinal and corticospinal mechanisms of movement: Cambridge University Press, 2012. [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Experimental brain research 2001;140(4):505–10. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 2001;140(4):505–10. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Lang AE, Chen R. The cerebellothalamocortical pathway in essential tremor. Neurology 2003;60(12):1985–7. [DOI] [PubMed] [Google Scholar]
- Pirio Richardson S, Bliem B, Voller B, Dang N, Hallett M. Long-latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res 2009;193(2):173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol 2003;546:605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier B, Quesada C, Fauchon C, Nuti C, Vassal F, Peyron R. Added value of multiple versus single sessions of repetitive transcranial magnetic stimulation in predicting motor cortex stimulation efficacy for refractory neuropathic pain. J Neurosurg 2018:1–12. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, et al. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci 2014;34(16):5603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston E, Ada L, Stanton R, Mahendran N, Dean CM. Prediction of Independent Walking in People Who Are Nonambulatory Early After Stroke: A Systematic Review. Stroke 2021;52(10):3217–24. [DOI] [PubMed] [Google Scholar]
- Priori A, Oliviero A, Donati E, Callea L, Bertolasi L, Rothwell JC. Human handedness and asymmetry of the motor cortical silent period. Exp Brain Res 1999;128(3):390–6. [DOI] [PubMed] [Google Scholar]
- Priori A, Polidori L, Rona S, Manfredi M, Berardelli A. Spinal and cortical inhibition in Huntington’s chorea. Mov Disord 2000;15(5):938–46. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Rizzo V, Terranova C, Morgante F, Schneider S, Ibrahim N, et al. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain 2009;132:2871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzoni A, Pirulli C, Veniero D, Feurra M, Cincotta M, Giovannelli F, et al. Vegetative versus minimally conscious states: a study using TMS-EEG, sensory and event-related potentials. PLoS One 2013;8(2):e57069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 2007;68(19):1571–5. [DOI] [PubMed] [Google Scholar]
- Ravnborg M, Liguori R, Christiansen P, Larsson H, Sørensen PS. The diagnostic reliability of magnetically evoked motor potentials in multiple sclerosis. Neurology 1992;42(7):1296–301. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris‐Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 2008;586(2):325–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan R, Delanty N. Juvenile myoclonic epilepsy: under-appreciated and under-diagnosed. Postgrad Med J 2003;79(928):78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol 1995;37(2):181–8. [DOI] [PubMed] [Google Scholar]
- Rikita T, Tanaka N, Nakanishi K, Kamei N, Sumiyoshi N, Kotaka S, et al. The relationship between central motor conduction time and spinal cord compression in patients with cervical spondylotic myelopathy. Spinal Cord 2017;55(4):419–26. [DOI] [PubMed] [Google Scholar]
- Rimpiläinen I, Karma P, Eskola H, Häkkinen V. Magnetic facial nerve stimulation in normal subjects. Three groups of responses. Acta Otolaryngol Suppl 1992;492:99–102. [DOI] [PubMed] [Google Scholar]
- Rimpiläinen I, Pyykkö I, Blomstedt G, Kuurne T, Karma P. The site of impulse generation in transcranial magnetic stimulation of the facial nerve. Acta Otolaryngol 1993;113(3):339–44. [DOI] [PubMed] [Google Scholar]
- Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain 2012;135:2779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero Lauro LJ, Rosanova M, Mattavelli G, Convento S, Pisoni A, Opitz A, et al. TDCS increases cortical excitability: direct evidence from TMS-EEG. Cortex 2014;58:99–111. [DOI] [PubMed] [Google Scholar]
- Romero MC, Davare M, Armendariz M, Janssen P. Neural effects of transcranial magnetic stimulation at the single-cell level. Nat Commun 2019;10(1):2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rona S, Berardelli A, Vacca L, Inghilleri M, Manfredi M. Alterations of motor cortical inhibition in patients with dystonia. Mov Disord 1998;13(1):118–24. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. J Neurosci 2009;29(24):7679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Fecchio M, Casarotto S, Sarasso S, Casali AG, Pigorini A, et al. Sleep-like cortical OFF-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat Commun 2018;9(1):4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno MA, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain 2012;135:1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J Physiol 2004;561:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock T, Grittner U, Acker G, Schwarzer V, Kulchytska N, Vajkoczy P, et al. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J Neurosurg 2017;126(4):1227–37. [DOI] [PubMed] [Google Scholar]
- Rosenstock T, Häni L, Grittner U, Schlinkmann N, Ivren M, Schneider H, et al. Bicentric validation of the navigated transcranial magnetic stimulation motor risk stratification model. J Neurosurg 2022;136(4):1194–206. [DOI] [PubMed] [Google Scholar]
- Rosenstock T, Picht T, Schneider H, Vajkoczy P, Thomale UW. Pediatric navigated transcranial magnetic stimulation motor and language mapping combined with diffusion tensor imaging tractography: clinical experience. J Neurosurg Pediatr 2020;26(5):583–93. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res 2003;151(3):330–7. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Hess CW, Schmid UD. Investigation of facial motor pathways by electrical and magnetic stimulation: sites and mechanisms of excitation. J Neurol Neurosurg Psychiatry 1989;52(10):1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler KM, Magistris MR, Glocker FX, Kohler A, Deuschl G, Hess CW. Electrophysiological characteristics of lesions in facial palsies of different etiologies. A study using electrical and magnetic stimulation techniques. Electroencephalogr Clin Neurophysiol 1995;97(6):355–68. [DOI] [PubMed] [Google Scholar]
- Rosler KM, Petrow E, Mathis J, Aranyi Z, Hess CW, Magistris MR. Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin Neurophysiol 2002;113(11):1680–7. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Truffert A, Hess CW, Magistris MR. Quantification of upper motor neuron loss in amyotrophic lateral sclerosis. Clin Neurophysiol 2000;111(12):2208–18. [DOI] [PubMed] [Google Scholar]
- Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmöller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol 2021;132(1):269–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994;91(2):79–92. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126(126):1071–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Caramia MD, Zarola F. Mechanisms of nervous propagation along central motor pathways: noninvasive evaluation in healthy subjects and in patients with neurological disease. Neurosurgery 1987;20(1):183–91. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Di Iorio R, Bentivoglio M, Bertini G, Ferreri F, Gerloff C, et al. Methods for analysis of brain connectivity: An IFCN-sponsored review. Clin Neurophysiol 2019;130(10):1833–58. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Miraglia F, Vecchio F. Early dementia diagnosis, MCI-to-dementia risk prediction, and the role of machine learning methods for feature extraction from integrated biomarkers, in particular for EEG signal analysis. Alzheimers Dement 2022;18:2699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Zarola F, Floris R, Bernardi G, Perretti A, Pelosi L, et al. Sensory (VEP, BAEP, SEP) and motor-evoked potentials, liquoral and magnetic resonance findings in multiple sclerosis. Eur Neurol 1989;29(1):41–7. [DOI] [PubMed] [Google Scholar]
- Rosso C, Lamy JC. Prediction of motor recovery after stroke: being pragmatic or innovative? Curr Opin Neurol 2020;33(4):482–7. [DOI] [PubMed] [Google Scholar]
- Rothwell J, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl 1999;52:97–103. [PubMed] [Google Scholar]
- Roy FD, Zewdie ET, Gorassini MA. Short-interval intracortical inhibition with incomplete spinal cord injury. Clin Neurophysiol 2011;122(7):1387–95. [DOI] [PubMed] [Google Scholar]
- Rurak BK, Rodrigues JP, Power BD, Drummond PD, Vallence AM. Reduced Cerebellar Brain Inhibition Measured Using Dual-Site TMS in Older Than in Younger Adults. Cerebellum 2022;21(1):23–38. [DOI] [PubMed] [Google Scholar]
- Russo M, Crupi D, Naro A, Avanzino L, Buccafusca M, Dattola V, et al. Fatigue in patients with multiple sclerosis: from movement preparation to motor execution. J Neurol Sci 2015;351(1–2):52–7. [DOI] [PubMed] [Google Scholar]
- Russo S, Sarasso S, Puglisi GE, Dal Palù D, Pigorini A, Casarotto S, et al. TAAC - TMS Adaptable Auditory Control: A universal tool to mask TMS clicks. J Neurosci Methods 2022;370:109491. [DOI] [PubMed] [Google Scholar]
- Rusu CV, Murakami M, Ziemann U, Triesch J. A model of TMS-induced I-waves in motor cortex. Brain Stimul 2014;7(7):401–14. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain 2003;126:1883–94. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Adachi Y, Fukuda H, Kai T, Nakashima K. Triple stimulation technique in patients with spinocerebellar ataxia type 6. Clin Neurophysiol 2005;116(11):2586–91. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Murakami T, Nakashima K. Short latency afferent inhibition is not impaired in mild cognitive impairment. Clin Neurophysiol 2007;118(7):1460–3. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol (1985) 2005;99(4):1483–93. [DOI] [PubMed] [Google Scholar]
- Salle JY, Hugon J, Tabaraud F, Boulesteix JM, Vallat JM, Dumas M, et al. Improvement in motor evoked potentials and clinical course post-steroid therapy in multiple sclerosis. J Neurol Sci 1992;108(2):184–8. [DOI] [PubMed] [Google Scholar]
- Sangari S, Perez MA. Imbalanced Corticospinal and Reticulospinal Contributions to Spasticity in Humans with Spinal Cord Injury. J Neurosci 2019;39(40):7872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangari S, Perez MA. Distinct Corticospinal and Reticulospinal Contributions to Voluntary Control of Elbow Flexor and Extensor Muscles in Humans with Tetraplegia. J Neurosci 2020;40(46):8831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 2001;530:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, Boly M, Napolitani M, Gosseries O, Charland-Verville V, Casarotto S, et al. Consciousness and Complexity during Unresponsiveness Induced by Propofol, Xenon, and Ketamine. Curr Biol 2015;25(23):3099–105. [DOI] [PubMed] [Google Scholar]
- Sarasso S, Casali AG, Casarotto S, Rosanova M, Sinigaglia C, Massimini M. Consciousness and complexity: a consilience of evidence. Neurosci Conscious 2021;niab023. 10.1093/nc/niab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso S, D’Ambrosio S, Fecchio M, Casarotto S, Viganò A, Landi C, et al. Local sleep-like cortical reactivity in the awake brain after focal injury. Brain 2020;143(12):3672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanamuttu J, Radhu N, Udupa K, Baarbé J, Gunraj C, Chen R. Impaired motor cortical facilitatory-inhibitory circuit interaction in Parkinson’s disease. Clin Neurophysiol 2021;132(10):2685–92. [DOI] [PubMed] [Google Scholar]
- Sattler V, Dickler M, Michaud M, Meunier S, Simonetta-Moreau M. Does abnormal interhemispheric inhibition play a role in mirror dystonia? Mov Disord 2014;29(6):787–96. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Xu J, Branscheidt M, Lindquist M, Uddin J, Steiner L, et al. Differential Poststroke Motor Recovery in an Arm Versus Hand Muscle in the Absence of Motor Evoked Potentials. Neurorehabil Neural Repair 2019;33(7):568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet 2021;397(10284):1577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippling S, Schneider SA, Bhatia KP, Munchau A, Rothwell JC, Tabrizi SJ, et al. Abnormal motor cortex excitability in preclinical and very early Huntington’s disease. Biol Psychiatry 2009;65(11):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi T, Di Lorenzo F, Sancesario GM, Di Lazzaro G, Ponzo V, Pisani A, et al. Amyloid-Mediated Cholinergic Dysfunction in Motor Impairment Related to Alzheimer’s Disease. J Alzheimers Dis 2018;64(2):525–32. [DOI] [PubMed] [Google Scholar]
- Schlaeger R, D’Souza M, Schindler C, Grize L, Dellas S, Radue EW, et al. Prediction of long-term disability in multiple sclerosis. Mult Scler 2012;18(1):31–8. [DOI] [PubMed] [Google Scholar]
- Schlaeger R, D’Souza M, Schindler C, Grize L, Kappos L, Fuhr P. Combined Evoked Potentials as Markers and Predictors of Disability in Early Multiple Sclerosis. Clin Neurophysiol 2012;123:406–10. [DOI] [PubMed] [Google Scholar]
- Schmid UD, Møller AR, Schmid J. Transcranial magnetic stimulation of the facial nerve: intraoperative study on the effect of stimulus parameters on the excitation site in man. Muscle Nerve 1992;15(7):829–36. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Irlbacher K, Grosse P, Röricht S, Meyer BU. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology 2002;59(8):1218–24. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Niehaus L, Röricht S, Meyer BU. Conduction deficits of callosal fibres in early multiple sclerosis. J Neurol Neurosurg Psychiatry 2000;68(5):633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Schrader V. [Reliability of magnetic stimulation in the diagnosis of peripheral facial paralysis of idiopathic origin]. Rev Laryngol Otol Rhinol (Bord) 1995;116(2):123–7. [PubMed] [Google Scholar]
- Schriefer TN, Mills KR, Murray NM, Hess CW. Evaluation of proximal facial nerve conduction by transcranial magnetic stimulation. J Neurol Neurosurg Psychiatry 1988;51(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Wohlfarth K, Rollnik JD, Dengler R. Walking and fatigue in multiple sclerosis: the role of the corticospinal system. Muscle Nerve 1998;21(8):1068–70. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Scherens A, Rönnau AK, Höffken O, Tegenthoff M, Maier C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 2010;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P, Tegenthoff M, Witscher K, Börnke C, Przuntek H, Malin JP, et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain 2002;125:301–9. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Thapa T, Schabrun SM. Corticomotor Depression is Associated With Higher Pain Severity in the Transition to Sustained Pain: A Longitudinal Exploratory Study of Individual Differences. J Pain 2019;20(12):1498–506. [DOI] [PubMed] [Google Scholar]
- Senocak O, Hürel DM, Sener U, Uğurel B, Oztura I, Ertekin C. Motor conduction time along the cauda equina in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 2009;34(13):1410–4. [DOI] [PubMed] [Google Scholar]
- Seo CH, Jang KU, Lee BC, Choi IG, Kim JH, Chun W, et al. Transcranial magnetic stimulation can diagnose electrical burn-induced myelopathy. Burns 2011;37(4):687–91. [DOI] [PubMed] [Google Scholar]
- Serafini A, Rubboli G, Gigli GL, Koutroumanidis M, Gelisse P. Neurophysiology of juvenile myoclonic epilepsy. Epilepsy Behav 2013;28 Suppl 1:S30–9. [DOI] [PubMed] [Google Scholar]
- Sfreddo HJ, Wecht JR, Alsalman OA, Wu YK, Harel NY. Duration and reliability of the silent period in individuals with spinal cord injury. Spinal Cord 2021;59(8):885–93. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Al-Chalabi A, Baker MR, Cui LY, de Carvalho M, Eisen A, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol 2020;131:1975–8. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Park SB, Geevasinga N, Huynh W, Simon NG, Menon P, et al. Threshold tracking transcranial magnetic stimulation: Effects of age and gender on motor cortical function. Clin Neurophysiol 2016a;127(6):2355–61. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Park SB, Geevasinga N, Menon P, Howells J, Simon NG, et al. Motor cortical function determines prognosis in sporadic ALS. Neurology 2016b;87(5):513–20. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Simon NG, Geevasinga N, Menon P, Howells J, Park SB, et al. The evolution of motor cortical dysfunction in amyotrophic lateral sclerosis. Clin Neurophysiol 2017;128(6):1075–82. [DOI] [PubMed] [Google Scholar]
- Shibuya R, Wada E, Iwasaki M, Yonenobu K, Yoshikawa H. Motor conduction measurement in myelopathy hand. Funct Neurol 2014;29(3):177–82. [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hino T, Komori T, Hirai S. Loss of the muscle silent period evoked by transcranial magnetic stimulation of the motor cortex in patients with cervical cord lesions. Neurosci Lett 2000;286(3):199–202. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Hamada M, Hanajima R, Terao Y, Matsumoto H, Ohminami S, et al. Cerebellar dysfunction in progressive supranuclear palsy: a transcranial magnetic stimulation study. Mov Disord 2010;25(14):2413–9. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Hanajima R, Hamada M, Terao Y, Matsumoto H, Tsutsumi R, et al. Inter-individual variation in the efficient stimulation site for magnetic brainstem stimulation. Clin Neurophysiol 2011;122(10):2044–8. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Hanajima R, Shimizu T, Terao Y, Tsuji S, Ugawa Y. Quantitative Evaluation of Cerebellar Function in Multiple System Atrophy with Transcranial Magnetic Stimulation. Cerebellum 2022;21(2):219–24. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Ohminami S, Tsutsumi R, Terao Y, Ugawa Y, Tsuji S, et al. Increased facilitation of the primary motor cortex in de novo Parkinson’s disease. Parkinsonism Relat Disord 2019;66:125–9. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Conde V, Tomasevic L, Thielscher A, Bergmann TO. Distilling the essence of TMS-evoked EEG potentials (TEPs): A call for securing mechanistic specificity and experimental rigor. Brain Stimul 2019;12(4):1051–4. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Funke K, Aberra AS, Antal A, Bestmann S, Chen R, et al. Transcranial magnetic stimulation of the brain: What is stimulated? - A consensus and critical position paper. Clin Neurophysiol 2022;140:59–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert BI, Patterson HI, Pevcic DD, Windnagel KA, Thickbroom GW. A comparison of relative-frequency and threshold-hunting methods to determine stimulus intensity in transcranial magnetic stimulation. Clin Neurophysiol 2013;124(4):708–12. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Kröner-Herwig B, Kocabiyik E, Rothenberger A. Intracortical inhibition and facilitation in migraine--a transcranial magnetic stimulation study. Headache 2007;47(3):364–70. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Reich AL, Shepherd AJ, van Baalen A, Siebner HR, Stephani U. Peri-ictal changes of cortical excitability in children suffering from migraine without aura. Pain 2009;147(1–3):132–40. [DOI] [PubMed] [Google Scholar]
- Sinitsyn DO, Poydasheva AG, Bakulin IS, Legostaeva LA, Iazeva EG, Sergeev DV, et al. Detecting the Potential for Consciousness in Unresponsive Patients Using the Perturbational Complexity Index. Brain Sci 2020;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, Stinear CM. Transcranial magnetic stimulation (TMS) in stroke: Ready for clinical practice? J Clin Neurosci 2016;31:10–4. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Dang N, Hallett M. Suppression of corticospinal excitability during negative motor imagery. J Neurophysiol 2003;90(4):2303–9. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol 2004;56(4):595–9. [DOI] [PubMed] [Google Scholar]
- Soininen H, Reinikainen K, Partanen J, Mervaala E, Paljärvi L, Helkala EL, et al. Slowing of the dominant occipital rhythm in electroencephalogram is associated with low concentration of noradrenaline in the thalamus in patients with Alzheimer’s disease. Neurosci Lett 1992;137(1):5–8. [DOI] [PubMed] [Google Scholar]
- Sollmann N, Fratini A, Zhang H, Zimmer C, Meyer B, Krieg SM. Associations between clinical outcome and tractography based on navigated transcranial magnetic stimulation in patients with language-eloquent brain lesions. J Neurosurg 2019;132(4):1033–42. [DOI] [PubMed] [Google Scholar]
- Sollmann N, Negwer C, Tussis L, Hauck T, Ille S, Maurer S, et al. Interhemispheric connectivity revealed by diffusion tensor imaging fiber tracking derived from navigated transcranial magnetic stimulation maps as a sign of language function at risk in patients with brain tumors. J Neurosurg 2017;126(1):222–33. [DOI] [PubMed] [Google Scholar]
- Sollmann N, Wildschuetz N, Kelm A, Conway N, Moser T, Bulubas L, et al. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: a combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J Neurosurg 2018;128(3):800–10. [DOI] [PubMed] [Google Scholar]
- Somaa FA, de Graaf TA, Sack AT. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front Neurol 2022;13:793253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Ciocca M, Chieffo R, Hammond P, Neef A, Paulus W, et al. TMS of primary motor cortex with a biphasic pulse activates two independent sets of excitable neurones. Brain stimulation 2018;11(3):558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Tergau F, Wischer S, Reimers CD, Beuche W, Paulus W. Riluzole does not have an acute effect on motor thresholds and the intracortical excitability in amyotrophic lateral sclerosis. J Neurol 1999;246 Suppl 3:III22–6. [DOI] [PubMed] [Google Scholar]
- Sondergaard RE, Martino D, Kiss ZHT, Condliffe EG. TMS Motor Mapping Methodology and Reliability: A Structured Review. Front Neurosci 2021a;15:709368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard RE, Strzalkowski NDJ, Gan LS, Jasaui Y, Furtado S, Pringsheim TM, et al. Cerebellar Brain Inhibition Is Associated With the Severity of Cervical Dystonia. J Clin Neurophysiol 2021b;0:1–8. [DOI] [PubMed] [Google Scholar]
- Sorel M, Zrek N, Locko B, Armessen C, Ayache SS, Lefaucheur JP. A reappraisal of the mechanisms of action of ketamine to treat complex regional pain syndrome in the light of cortical excitability changes. Clin Neurophysiol 2018;129(5):990–1000. [DOI] [PubMed] [Google Scholar]
- Souza VH, Nieminen JO, Tugin S, Koponen LM, Baffa O, Ilmoniemi RJ. TMS with fast and accurate electronic control: Measuring the orientation sensitivity of corticomotor pathways. Brain Stimul 2022;15(2):306–15. [DOI] [PubMed] [Google Scholar]
- Spagnolo F, Coppi E, Chieffo R, Straffi L, Fichera M, Nuara A, et al. Interhemispheric balance in Parkinson’s disease: a transcranial magnetic stimulation study. Brain Stimul 2013;6(6):892–7. [DOI] [PubMed] [Google Scholar]
- Spampinato DA, Celnik PA, Rothwell JC. Cerebellar-Motor Cortex Connectivity: One or Two Different Networks? J Neurosci 2020;40(21):4230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Classen J. Effects of riluzole on cortical excitability in patients with amyotrophic lateral sclerosis. Ann Neurol 2001;49:536–9. [PubMed] [Google Scholar]
- Stetkarova I, Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin Neurophysiol 2013;124(2):339–45. [DOI] [PubMed] [Google Scholar]
- Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol 2017;16(10):826–36. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol 2003;89(4):2014–20. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD. Task-dependent modulation of silent period duration in focal hand dystonia. Mov Disord 2005;20(9):1143–51. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Ackerley SJ, Barber PA, Smith MC. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke 2017a;48(4):1011–9. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Ackerley SJ, Smith MC, Borges VM, Barber PA. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol 2017b;4(11):811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Barber PA, Ackerley SJ, Smith M-C, Cramer SC. Biomarker-based patient selection improves stroke rehabilitation trial efficiency. bioRxiv 2018:1–8. [Google Scholar]
- Stinear CM, Petoe MA, Byblow WD. Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation. Brain Stimul 2015;8(6):1183–90. [DOI] [PubMed] [Google Scholar]
- Straub J, Magistris MR, Delavelle J, Landis T. Facial palsy in cerebral venous thrombosis: transcranial stimulation and pathophysiological considerations. Stroke 2000;31(7):1766–9. [DOI] [PubMed] [Google Scholar]
- Suppa A, Berardelli A, Brancati F, Marianetti M, Barrano G, Mina C, et al. Clinical, neuropsychological, neurophysiologic, and genetic features of a new Italian pedigree with familial cortical myoclonic tremor with epilepsy. Epilepsia 2009;50(5):1284–8. [DOI] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Di Stasio F, Latorre A, Parvez AK, Colosimo C, et al. Primary motor cortex long-term plasticity in multiple system atrophy. Mov Disord 2014;29(1):97–104. [DOI] [PubMed] [Google Scholar]
- Swash M. Why are upper motor neuron signs difficult to elicit in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry 2012;83(6):659–62. [DOI] [PubMed] [Google Scholar]
- Taieb G, Grapperon AM, Duclos Y, Franques J, Labauge P, Renard D, et al. Proximal conduction block in the pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Muscle Nerve 2015;52(6):1102–6. [DOI] [PubMed] [Google Scholar]
- Takahashi J, Hirabayashi H, Hashidate H, Ogihara N, Yamazaki I, Kamimura M, et al. Assessment of cervical myelopathy using transcranial magnetic stimulation and prediction of prognosis after laminoplasty. Spine (Phila Pa 1976) 2008;33(1):E15–20. [DOI] [PubMed] [Google Scholar]
- Takakura T, Muragaki Y, Tamura M, Maruyama T, Nitta M, Niki C, et al. Navigated transcranial magnetic stimulation for glioma removal: prognostic value in motor function recovery from postsurgical neurological deficits. J Neurosurg 2017;127(4):877–91. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol 2006;117(8):1641–59. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Fiaschi A, Andreoli A, Marani S, Zanette G. Sensorimotor integration to cutaneous afferents in humans: the effect of the size of the receptive field. Exp Brain Res 2005;167(3):362–9. [DOI] [PubMed] [Google Scholar]
- Tamburin S, Manganotti P, Zanette G, Fiaschi A. Cutaneomotor integration in human hand motor areas: somatotopic effect and interaction of afferents. Exp Brain Res 2001;141(2):232–41. [DOI] [PubMed] [Google Scholar]
- Tang ZC, Chen Z, Shi YT, Wan LL, Liu MJ, Hou X, et al. Central motor conduction time in spinocerebellar ataxia: a meta-analysis. Aging (Albany NY) 2020;12(24):25718–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankisi H, Cengiz B, Howells J, Samusyte G, Koltzenburg M, Bostock H. Short-interval intracortical inhibition as a function of inter-stimulus interval: Three methods compared. Brain Stimul 2021;14(1):22–32. [DOI] [PubMed] [Google Scholar]
- Tankisi H, Pia H, Strunge K, Howells J, Cengiz B, Samusyte G, et al. Three different short-interval intracortical inhibition methods in early diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2022:1–9. [DOI] [PubMed] [Google Scholar]
- Tarragó M, Deitos A, Brietzke AP, Vercelino R, Torres ILS, Fregni F, et al. Descending Control of Nociceptive Processing in Knee Osteoarthritis Is Associated With Intracortical Disinhibition: An Exploratory Study. Medicine (Baltimore) 2016;95(17):e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 2006;16(3):215–23. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol 2002;541:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe T, Perez MA. Abnormal changes in motor cortical maps in humans with spinal cord injury. J Physiol 2021;599(22):5031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegenthoff M, Vorgerd M, Juskowiak F, Roos V, Malin JP. Postexcitatory inhibition after transcranial magnetic single and double brain stimulation in Huntington’s disease. Electroencephalogr Clin Neurophysiol 1996;101(4):298–303. [DOI] [PubMed] [Google Scholar]
- Teixeira PEP, Pacheco-Barrios K, Gunduz ME, Gianlorenço AC, Castelo-Branco L, Fregni F. Understanding intracortical excitability in phantom limb pain: A multivariate analysis from a multicenter randomized clinical trial. Neurophysiol Clin 2021;51(2):161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res 2009;193(4):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Braack EM, de Vos CC, van Putten MJ. Masking the Auditory Evoked Potential in TMS-EEG: A Comparison of Various Methods. Brain Topogr 2015;28(3):520–8. [DOI] [PubMed] [Google Scholar]
- Terranova C, SantAngelo A, Morgante F, Rizzo V, Allegra R, Arena MG, et al. Impairment of sensory-motor plasticity in mild Alzheimer’s disease. Brain Stimul 2013;6(1):62–6. [DOI] [PubMed] [Google Scholar]
- Tervo AE, Metsomaa J, Nieminen JO, Sarvas J, Ilmoniemi RJ. Automated search of stimulation targets with closed-loop transcranial magnetic stimulation. Neuroimage 2020;220:117082. [DOI] [PubMed] [Google Scholar]
- Tervo AE, Nieminen JO, Lioumis P, Metsomaa J, Souza VH, Sinisalo H, et al. Closed-loop optimization of transcranial magnetic stimulation with electroencephalography feedback. Brain Stimul 2022;15(2):523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund The and Groups Manchester. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994;57(4):416–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;17(2):162–73. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Farina S, Edwards M, Moretto G, Restivo D, Fiaschi A, et al. Task-specific impairment of motor cortical excitation and inhibition in patients with writer’s cramp. Neurosci Lett 2005;378(1):55–8. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Sabbagh MN, Schoos B, Masliah E, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology 2000;54(2):407–11. [DOI] [PubMed] [Google Scholar]
- Toepp SL, Turco CV, Rehsi RS, Nelson AJ. The distribution and reliability of TMS-evoked short- and long-latency afferent interactions. PLoS One 2021;16(12):e0260663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 2000;523 Pt 2:503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol 1996;101(4):263–72. [DOI] [PubMed] [Google Scholar]
- Tokimura R, Murakami T, Ugawa Y. Central motor conduction time reveals upper motor neuron involvement masked by lower motor neuron impairment in a significant portion of patients with amyotrophic lateral sclerosis. Clin Neurophysiol 2020;131(8):1896–901. [DOI] [PubMed] [Google Scholar]
- Tokushige S, Sonoo T, Maekawa R, Shirota Y, Hanajima R, Terao Y, et al. Isolated pyramidal tract impairment in the central nervous system of adult-onset Krabbe disease with novel mutations in the GALC gene. Brain Dev 2013;35(6):579–81. [DOI] [PubMed] [Google Scholar]
- Toleikis JR, Sloan TB, Ronai AK. Optimal transcranial magnetic stimulation sites for the assessment of motor function. Electroencephalogr Clin Neurophysiol 1991;81(6):443–9. [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Consciousness and complexity. Science 1998;282(5395):1846–51. [DOI] [PubMed] [Google Scholar]
- Torrecillos F, Falato E, Pogosyan A, West T, Di Lazzaro V, Brown P. Motor Cortex Inputs at the Optimum Phase of Beta Cortical Oscillations Undergo More Rapid and Less Variable Corticospinal Propagation. J Neurosci 2020;40(2):369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke. A brain stimulation study with focal magnetic pulses. Stroke 1997;28(1):110–7. [DOI] [PubMed] [Google Scholar]
- Trebbastoni A, Gilio F, D’Antonio F, Cambieri C, Ceccanti M, de Lena C, et al. Chronic treatment with rivastigmine in patients with Alzheimer’s disease: a study on primary motor cortex excitability tested by 5 Hz-repetitive transcranial magnetic stimulation. Clin Neurophysiol 2012;123(5):902–9. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Tremblay LE. Cortico-motor excitability of the lower limb motor representation: a comparative study in Parkinson’s disease and healthy controls. Clin Neurophysiol 2002;113(12):2006–12. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, et al. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol 2019;130(5):802–44. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Macdonell RA, Cros D, Chiappa KH, Shahani BT, Day BJ. Motor inhibition and excitation are independent effects of magnetic cortical stimulation. Ann Neurol 1992;32(3):345–51. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Menkes D, Onorato J, Yan RS, Young MS, Newell K, et al. Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology 1999;53:605–11. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Buccolieri A, Marinelli L, Abbruzzese G. Differential modulation of motor evoked potential and silent period by activation of intracortical inhibitory circuits. Clin Neurophysiol 2001;112(10):1822–7. [DOI] [PubMed] [Google Scholar]
- Tscherpel C, Dern S, Hensel L, Ziemann U, Fink GR, Grefkes C. Brain responsivity provides an individual readout for motor recovery after stroke. Brain 2020;143(6):1873–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Hanajima R, Hamada M, Shirota Y, Matsumoto H, Terao Y, et al. Reduced interhemispheric inhibition in mild cognitive impairment. Exp Brain Res 2012;218(1):21–6. [DOI] [PubMed] [Google Scholar]
- Turco CV, El-Sayes J, Fassett HJ, Chen R, Nelson AJ. Modulation of long-latency afferent inhibition by the amplitude of sensory afferent volley. J Neurophysiol 2017;118(1):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco CV, El-Sayes J, Locke MB, Chen R, Baker S, Nelson AJ. Effects of lorazepam and baclofen on short- and long-latency afferent inhibition. J Physiol 2018a;596(21):5267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco CV, El-Sayes J, Savoie MJ, Fassett HJ, Locke MB, Nelson AJ. Short- and long-latency afferent inhibition; uses, mechanisms and influencing factors. Brain Stimul 2018b;11(1):59–74. [DOI] [PubMed] [Google Scholar]
- Turco F, Canessa A, Olivieri C, Pozzi NG, Palmisano C, Arnulfo G, et al. Cortical response to levodopa in Parkinson’s disease patients with dyskinesias. Eur J Neurosci 2018c;48(6):2362–73. [DOI] [PubMed] [Google Scholar]
- Türk U, Rösler KM, Mathis J, Müllbacher W, Hess CW. Assessment of motor pathways to masticatory muscles: an examination technique using electrical and magnetic stimulation. Muscle Nerve 1994;17(11):1271–7. [DOI] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol 2009;8:94–109. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol 1991a;441:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Genba-Shimizu K, Rothwell JC, Iwata M, Kanazawa I. Suppression of motor cortical excitability by electrical stimulation over the cerebellum in ataxia. Ann Neurol 1994a;36(1):90–6. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Genba K, Mannen T, Kanazawa I. Stimulation of corticospinal pathways at the level of the pyramidal decussation in neurological disorders. Brain 1992;115:1947–61. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Hanajima R, Kanazawa I. Motor cortex inhibition in patients with ataxia. Electroencephalogr Clin Neurophysiol 1994b;93(3):225–9. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Magnetic stimulation over the spinal enlargements. J Neurol Neurosurg Psychiatry 1989;52(9):1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol 1991b;29(4):418–27. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Furubayashi T, Machii K, et al. Magnetic stimulation over the cerebellum in patients with ataxia. Electroencephalogr Clin Neurophysiol 1997;104(5):453–8. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Nagai C, Nakamura K, Kanazawa I. Electrical stimulation of the cerebellum normally suppresses motor cortical excitability in a patient with ataxia due to a lesion of the middle cerebellar peduncle. Eur Neurol 1995a;35(4):243–4. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol 1994c;36(36):618–24. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol 1995b;37(6):703–13. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Suzuki M, Sakai K, Hanajima R, et al. Clinical utility of magnetic corticospinal tract stimulation at the foramen magnum level. Electroencephalogr Clin Neurophysiol 1996;101(3):247–54. [DOI] [PubMed] [Google Scholar]
- Urban P, Wicht S, Hopf H. Sensitivity of transcranial magnetic stimulation of cortico-bulbar vs. cortico-spinal tract involvement in ALS. J Neurol 2001;248(248):850–5. [DOI] [PubMed] [Google Scholar]
- Urban PP, Beer S, Hopf HC. Cortico-bulbar fibers to orofacial muscles: recordings with enoral surface electrodes. Electroencephalogr Clin Neurophysiol 1997;105(1):8–14. [DOI] [PubMed] [Google Scholar]
- Urban PP, Vogt T. Conduction times of cortical projections to paravertebral muscles in controls and in patients with multiple sclerosis. Muscle Nerve 1994;17(11):1348–9. [DOI] [PubMed] [Google Scholar]
- Valente M, Lettieri C, Russo V, Janes F, Gigli GL. Clinical and Neurophysiological Effects of Botulinum Neurotoxin Type A in Chronic Migraine. Toxins (Basel) 2021;13(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A, Arunachalam R, Mesquita-Rodrigues A, Garcia Seoane JJ, Richardson MP, Mills KR, et al. Late EEG responses triggered by transcranial magnetic stimulation (TMS) in the evaluation of focal epilepsy. Epilepsia 2008;49(3):470–80. [DOI] [PubMed] [Google Scholar]
- Vallence AM, Schneider LA, Pitcher JB, Ridding MC. Long-interval facilitation and inhibition are differentially affected by conditioning stimulus intensity over different time courses. Neurosci Lett 2014;570:114–8. [DOI] [PubMed] [Google Scholar]
- Vallence AM, Smalley E, Drummond PD, Hammond GR. Long-interval intracortical inhibition is asymmetric in young but not older adults. J Neurophysiol 2017;118(3):1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology 1994;44(44):735–41. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 1992;85(6):355–64. [DOI] [PubMed] [Google Scholar]
- Valzania F, Strafella AP, Quatrale R, Santangelo M, Tropeani A, Lucchi D, et al. Motor evoked responses to paired cortical magnetic stimulation in Parkinson’s disease. Electroencephalogr Clin Neurophysiol 1997;105(1):37–43. [DOI] [PubMed] [Google Scholar]
- van den Bos MAJ, Geevasinga N, Higashihara M, Menon P, Vucic S. Pathophysiology and Diagnosis of ALS: Insights from Advances in Neurophysiological Techniques. Int J Mol Sci 2019;20(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos MAJ, Higashihara M, Geevasinga N, Menon P, Kiernan MC, Vucic S. Pathophysiological associations of transcallosal dysfunction in ALS. Eur J Neurol 2021;28:1172–80. [DOI] [PubMed] [Google Scholar]
- van den Bos MAJ, Menon P, Geevasinga N, Kiernan MC, Vucic S. ALS disability is predicted by a shift in the balance between short latency facilitatory and inhibitory circuits. Clin Neurophysiol 2018;129(4):e3. [Google Scholar]
- Van den Bos MAJ, Menon P, Howells J, Geevasinga N, Kiernan MC, Vucic S. Physiological Processes Underlying Short Interval Intracortical Facilitation in the Human Motor Cortex. Front Neurosci 2018;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos MAJ, Menon P, Vucic S. Cortical hyperexcitability and plasticity in Alzheimer’s disease: developments in understanding and management. Expert Rev Neurother 2022;22(11–12):981–93. [DOI] [PubMed] [Google Scholar]
- van der Kamp W, Maassen VanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical hyperexcitability in migraine patients demonstrated with transcranial magnetic stimulation. J Neurol Sci 1996;139(1):106–10. [DOI] [PubMed] [Google Scholar]
- van der Kamp W, MaassenVanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical excitability to magnetic stimulation in familial hemiplegic migraine. Neurology 1997;48(5):1462–4. [DOI] [PubMed] [Google Scholar]
- van der Kamp W, Maertens de Noordhout A, Thompson PD, Rothwell JC, Day BL, Marsden CD. Correlation of phasic muscle strength and corticomotoneuron conduction time in multiple sclerosis. Ann Neurol 1991;29(1):6–12. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Sadikot AF, Strafella AP, Paus T. The neural response to transcranial magnetic stimulation of the human motor cortex. II. Thalamocortical contributions. Exp Brain Res 2006;175(2):246–55. [DOI] [PubMed] [Google Scholar]
- Vastano R, Perez MA. Changes in motoneuron excitability during voluntary muscle activity in humans with spinal cord injury. J Neurophysiol 2020;123(2):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldema J, Nowak DA, Gharabaghi A. Resting motor threshold in the course of hand motor recovery after stroke: a systematic review. J Neuroeng Rehabil 2021;18(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos I, Kugiumtzis D, Tsalikakis DG, Kimiskidis VK. TMS-induced brain connectivity modulation in Genetic Generalized Epilepsy. Clin Neurophysiol 2022;133:83–93. [DOI] [PubMed] [Google Scholar]
- Vucic S, Burke T, Lenton K, Ramanathan S, Gomes L, Yannikas C, et al. Cortical dysfunction underlies disability in multiple sclerosis. Mult Scler 2012;18(4):425–32. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Kiernan MC. Dissecting the mechanisms underlying short-interval intracortical inhibition using exercise. Cereb Cortex 2011a;21(7):1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D, Kiernan MC. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res 2009;1273:39–47. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Yiannikas C, Kiernan MC. Cortical excitability distinguishes ALS from mimic disorders. Clin Neurophysiol 2011b;122(9):1860–6. [DOI] [PubMed] [Google Scholar]
- Vucic S, Higashihara M, Sobue G, Atsuta N, Doi Y, Kuwabara S, et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 2020;94(15):e1657–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Howells J, Trevillion L, Kiernan MC. Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve 2006;33(4):477–86. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain 2006;129:2436–46. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Abnormalities in cortical and peripheral excitability in flail arm variant amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2007;78:849–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Cortical excitability testing distinguishes Kennedy’s disease from amyotrophic lateral sclerosis. Clin Neurophysiol 2008;119:1088–96. [DOI] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Upregulation of persistent sodium conductances in familial ALS. J Neurol Neurosurg Psychiatry 2010;81(2):222–7. [DOI] [PubMed] [Google Scholar]
- Vucic S, Lin CS-Y, Cheah BC, Murray J, Menon P, Krishnan AV, et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain 2013a;136(5):1361–70. [DOI] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 2008;131:1540–50. [DOI] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical excitability in hereditary motor neuronopathy with pyramidal signs: comparison with ALS. J Neurol Neurosurg Psychiatry 2010;81(1):97–100. [DOI] [PubMed] [Google Scholar]
- Vucic S, van den Bos M, Menon P, Howells J, Dharmadasa T, Kiernan MC. Utility of threshold tracking transcranial magnetic stimulation in ALS. Clin Neurophysiol Pract 2018;3:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Westeneng HJ, Al-Chalabi A, Van Den Berg LH, Talman P, Kiernan MC. Amyotrophic lateral sclerosis as a multi-step process: an Australia population study. Amyotroph Lateral Scler Frontotemporal Degener 2019;20(7–8):532–7. [DOI] [PubMed] [Google Scholar]
- Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry 2013b;84(84):1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle-Shukla A, Ni Z, Gunraj CA, Bahl N, Chen R. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol 2009;587:5665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang H, Han L, Zhou Y. Cortical function in Alzheimer’s disease and frontotemporal dementia. Transl Neurosci 2016;7(1):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Niu Z, Xia X, Bai Y, Liang Z, He J, et al. Application of Fast Perturbational Complexity Index to the Diagnosis and Prognosis for Disorders of Consciousness. IEEE Trans Neural Syst Rehabil Eng 2022;30:509–18. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang H, Cui LY. Triple Stimulation Technique in Amyotrophic Lateral Sclerosis. J Clin Neurophysiol 2019;36(2):87–92. [DOI] [PubMed] [Google Scholar]
- Wassermann E, Epstein C, Ziemann U, Walsh V, Paus T, Lisanby S. Oxford handbook of transcranial stimulation: Oxford University Press, 2008. [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 1992;85(1):1–8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, et al. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 1996;109(1):158–63. [DOI] [PubMed] [Google Scholar]
- Weber M, Eisen A. Assessment of upper and lower motor neurons in Kennedy’s disease: implications for corticomotoneuronal PSTH studies. Muscle Nerve 1999;22:299–306. [DOI] [PubMed] [Google Scholar]
- Weber M, Eisen A. Peristimulus time histograms (PSTHs)--a marker for upper motor neuron involvement in ALS? Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1 Suppl 2:S51–6. [DOI] [PubMed] [Google Scholar]
- Weber M, Eisen A, Nakajima M. Corticomotoneuronal activity in ALS: changes in the peristimulus time histogram over time. Clin Neurophysiol 2000;111(1):169–77. [DOI] [PubMed] [Google Scholar]
- Weber M, Ferreira V, Eisen A. Determinants of double discharges in amyotrophic lateral sclerosis and Kennedy disease. Clin Neurophysiol 2009;120(11):1971–7. [DOI] [PubMed] [Google Scholar]
- Wegrzyn M, Teipel SJ, Oltmann I, Bauer A, Thome J, Großmann A, et al. Structural and functional cortical disconnection in Alzheimer’s disease: a combined study using diffusion tensor imaging and transcranial magnetic stimulation. Psychiatry Res 2013;212(3):192–200. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Classen J, Benecke R. The silent period induced by transcranial magnetic stimulation in muscles supplied by cranial nerves: normal data and changes in patients. J Neurol Neurosurg Psychiatry 1995;59(6):586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Lieber J, Classen J, Noachtar S. Motor cortex excitability in patients with focal epilepsy. Epilepsy Res 2000a;41(2):179–89. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr Clin Neurophysiol 1996;101(1):58–66. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Wiseman K, Herzog J, Förderreuther S, Dichgans M, Straube A. Motor cortex excitability in patients with migraine with aura and hemiplegic migraine. Cephalalgia 2000b;20(1):45–50. [DOI] [PubMed] [Google Scholar]
- Wessel K, Tegenthoff M, Vorgerd M, Otto V, Nitschke MF, Malin JP. Enhancement of inhibitory mechanisms in the motor cortex of patients with cerebellar degeneration: a study with transcranial magnetic brain stimulation. Electroencephalogr Clin Neurophysiol 1996;101(4):273–80. [DOI] [PubMed] [Google Scholar]
- White AT, Petajan JH. Physiological measures of therapeutic response to interferon beta-1a treatment in remitting-relapsing MS. Clin Neurophysiol 2004;115(10):2364–71. [DOI] [PubMed] [Google Scholar]
- Williams AMM, Eginyan G, Deegan E, Chow M, Carpenter MG, Lam T. Residual Innervation of the Pelvic Floor Muscles in People with Motor-Complete Spinal Cord Injury. J Neurotrauma 2020;37(21):2320–31. [DOI] [PubMed] [Google Scholar]
- Williams KL, Fifita JA, Vucic S, Durnall JC, Kiernan MC, Blair IP, et al. Pathophysiological insights into ALS with C9ORF72 expansions. J Neurol Neurosurg Psychiatry 2013;84:931–5. [DOI] [PubMed] [Google Scholar]
- Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci 1999;2(1):50–6. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Haigh ZJ, Shirinpour S, Alekseichuk I, Opitz A. The phase of sensorimotor mu and beta oscillations has the opposite effect on corticospinal excitability. Brain Stimul 2022;15(5):1093–100. [DOI] [PubMed] [Google Scholar]
- Wittstock M, Pohley I, Walter U, Grossmann A, Benecke R, Wolters A. Interhemispheric inhibition in different phenotypes of progressive supranuclear palsy. J Neural Transm (Vienna) 2013;120(3):453–61. [DOI] [PubMed] [Google Scholar]
- Wochnik-Dyjas D, Głazowski C, Niewiadomska M. Segmental conduction times in the motor nervous system. Electromyogr Clin Neurophysiol 1997;37(3):155–67. [PubMed] [Google Scholar]
- Wolf SR, Strauss C, Schneider W. On the site of transcranial magnetic stimulation of the facial nerve: electrophysiological observations in two patients after transection of the facial nerve during neuroma removal. Neurosurgery 1995;36(2):346–9. [DOI] [PubMed] [Google Scholar]
- Wolters A, Classen J, Kunesch E, Grossmann A, Benecke R. Measurements of transcallosally mediated cortical inhibition for differentiating parkinsonian syndromes. Mov Disord 2004;19(5):518–28. [DOI] [PubMed] [Google Scholar]
- Xu D, Ding Q, Wang H. Corticospinal Tract Impairment of Patients With Parkinson’s Disease: Triple Stimulation Technique Findings. Front Aging Neurosci 2020;12:588085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Branscheidt M, Schambra H, Steiner L, Widmer M, Diedrichsen J, et al. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann Neurol 2019;85(4):502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Murakami T, Hattori N, Miyai I, Ugawa Y. Intensity dependency of peripheral nerve stimulation in spinal LTP induced by paired associative corticospinal-motoneuronal stimulation (PCMS). PLoS One 2021;16(11):e0259931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Baker MR, David R, Khoo TK, Duncan GW, et al. Short latency afferent inhibition: a biomarker for mild cognitive impairment in Parkinson’s disease? Mov Disord 2013;28(9):1285–8. [DOI] [PubMed] [Google Scholar]
- Yildiz FG, Saka E, Elibol B, Temucin CM. Modulation of Cerebellar-Cortical Connections in Multiple System Atrophy Type C by Cerebellar Repetitive Transcranial Magnetic Stimulation. Neuromodulation 2018;21(4):402–8. [DOI] [PubMed] [Google Scholar]
- Yokota T, Yoshino A, Inaba A, Saito Y. Double cortical stimulation in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 1996;61:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Newton M, Edmonds M, Badawy R, Macdonell R. Transcranial Magnetic Stimulation as a Diagnostic Tool in First Seizure Patients. J Clin Neuroscience 2009(16):1523–4. [Google Scholar]
- Yuksel H, Topalkara KK. Increased Cortical Excitability in Female Migraineurs: A Transcranial Magnetic Stimulation Study Conducted in the Preovulatory Phase. J Clin Neurol 2021;17(2):236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z’Graggen WJ, Humm AM, Durisch N, Magistris MR, Rösler KM. Repetitive spinal motor neuron discharges following single transcranial magnetic stimuli: a quantitative study. Clin Neurophysiol 2005;116(7):1628–37. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J Neurol 2002a;249(12):1723–8. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol 2002b;113(11):1688–97. [DOI] [PubMed] [Google Scholar]
- Zeller D, Reiners K, Bräuninger S, Buttmann M. Central motor conduction time may predict response to fampridine in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2014;85(6):707–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang L, Liang B, Schroeder D, Zhang Z, Cox GA, et al. Hyperactive Somatostatin Interneurons Contribute to Excitotoxicity in Neurodegenerative Disorders. Nat Neurosci 2016;19(4):557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology 1998a;51(5):1320–4. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ilić TV, Alle H, Meintzschel F. Cortico-motoneuronal excitation of three hand muscles determined by a novel penta-stimulation technique. Brain 2004;127:1887–98. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 1996a;40(3):367–78. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett 1993;156(1–2):167–71. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry 1997a;154(9):1277–84. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol 2015;126(10):1847–68. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 1996b;496:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 1998b;511:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 1998c;511:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Winter M, Reimers CD, Reimers K, Tergau F, Paulus W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology 1997b;49(5):1292–8. [DOI] [PubMed] [Google Scholar]
- Zipser CM, Premoli I, Belardinelli P, Castellanos N, Rivolta D, Heidegger T, et al. Cortical Excitability and Interhemispheric Connectivity in Early Relapsing-Remitting Multiple Sclerosis Studied With TMS-EEG. Front Neurosci 2018;12:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi M, Pearce SL, Nordstrom MA. Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol 2003;550:933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner C, Belardinelli P, Ermolova M, Gordon PC, Stenroos M, Zrenner B, et al. μ-rhythm phase from somatosensory but not motor cortex correlates with corticospinal excitability in EEG-triggered TMS. J Neurosci Methods 2022;379:109662. [DOI] [PubMed] [Google Scholar]
- Zrenner C, Desideri D, Belardinelli P, Ziemann U. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul 2018;11(2):374–89. [DOI] [PubMed] [Google Scholar]