Abstract
So far, over 20 causative genes of monogenic Parkinson’s disease (PD) have been identified. Some causative genes of non-parkinsonian entities may also manifest with parkinsonism mimicking PD. This study aimed to investigate the genetic characteristics of clinically diagnosed PD with early onset age or family history. A total of 832 patients initially diagnosed with PD were enrolled, of which, 636 were classified into the early-onset group and 196 were classified into the familial late-onset group. The genetic testing included the multiplex ligation-dependent probe amplification and next generation sequencing (target sequencing or whole-exome sequencing). The dynamic variants of spinocerebellar ataxia were tested in probands with family history. In the early-onset group, 30.03% of patients (191/636) harbored pathogenic/likely pathogenic (P/LP) variants in known PD-related genes (CHCHD2, DJ-1, GBA (heterozygous), LRRK2, PINK1, PRKN, PLA2G6, SNCA and VPS35). Variants in PRKN were the most prevalent, accounting for 15.72% of the early-onset patients, followed by GBA (10.22%), and PLA2G6 (1.89%). And 2.52% (16/636) had P/LP variants in causative genes of other diseases (ATXN3, ATXN2, GCH1, TH, MAPT, GBA (homozygous)). In the familial late-onset group, 8.67% of patients (17/196) carried P/LP variants in known PD-related genes (GBA (heterozygous), HTRA2, SNCA) and 2.04% (4/196) had P/LP variants in other genes (ATXN2, PSEN1, DCTN1). Heterozygous GBA variants (7.14%) were the most common genetic cause found in familial late-onset patients. Genetic testing is of vital importance in differential diagnosis especially in early-onset and familial PD. Our findings may also provide some clues to the nomenclature of genetic movement disorders.
Subject terms: Clinical genetics, Parkinson's disease
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative movement disorder with bradykinesia, rigidity and rest tremor as the cardinal motor symptoms. Genetic research on PD has achieved significant advance in recent years1. So far, pathogenic/likely pathogenic (P/LP) variants in over 20 genes that lead to Mendelian inheritance have been identified in PD with various evidence levels2–4 (defined as known PD-related genes in the study), accounting for 3%–5% of all PD patients1 and 7%–9% of PD patients with early onset age (<50 years old) or family history5–7.
Based on the phenotypic heterogeneity, patients with P/LP variants in causative genes of other diseases, such as ATXN2 in spinocerebellar ataxia (SCA)8, GCH1 in dopamine responsive dystonia (DRD)9 or MAPT in frontotemporal lobar degeneration (FTLD)10, might present with PD-like symptoms in the early stage or even in the late disease course11 and might be clinically misdiagnosed as PD.
Large scale genetic studies of PD have been carried out in different ethnic populations in recent years5–7,12–16. The frequency of P/LP variants ranged from 1.4 to 14%, with higher rate of 4.1–33.9% in early-onset Parkinson’s disease (EOPD) patients or patients with family history in different studies. Though P/LP variants in PRKN, LRRK2 and GBA were commonly detected, the genetic architecture differs in different ethnicities. Two large genetic studies of Chinese PD patients have been reported recently5,15. One study included familial PD, EOPD and sporadic PD and focused on 23 known PD-associated genes while the other included EOPD and focused on 26 PD-related genes and 20 other genes linked to neurodegenerative and lysosome diseases.
Here we conduct another large-scale genetic testing in Chinese Han PD patients. We investigated the 116 disease causative genes (including 22 known PD-related genes) in a cohort of clinically diagnosed PD patients with early age at onset (AAO) or family history to identify the genetic spectrum and the nomenclature of genetic movement disorders besides the genetic screen strategy.
Results
The demographic and the clinical features of the patients initially diagnosed as PD
The median AAO of the early-onset group and familial late-onset group was 39.00 (12.00) and 59.00 (10.00) years, respectively [Median (interquartile range (IQR)), p < 0.0001], and the disease duration was 46.00 (67.00) and 45.00 (57.00) months (p = 0.1371), respectively (Supplementary Table 1). The patients with family history in early-onset group account for 28.62% (181/636). There is significant difference between the two groups regarding sex (p = 0.0306) and education years (p = 0.0001). All the participants were self-reported Chinese Han ethnic.
When divided by family history, 455 patients (54.65%) were sporadic early-onset and 377 (45.31%) had family history (269 with autosomal dominant inheritance and 108 with autosomal recessive inheritance). The median AAO of sporadic early-onset patients and familial patients was 39.00 (12.00) and 50.00 (19.00) years, respectively (p = 0.0001), and the median disease duration was 44.00 (60.00) and 48.00 (71.00) months, respectively (p = 0.5297).
Distribution of causative genes in patients initially diagnosed as PD
P/LP variants responsible for the parkinsonian phenotype were found in 17 genes, including 10 known PD-related genes (CHCHD2, DJ-1, GBA, HTRA2, LRRK2, PINK1, PRKN, PLA2G6, SNCA and VPS35) and 7 causative genes of other diseases (ATXN2, ATXN3, DCTN1, GCH1, MAPT, PSEN1 and TH). P/LP variants in each patient and their genomic locations were reported in Supplementary Table 2 and Supplementary Fig. 1.
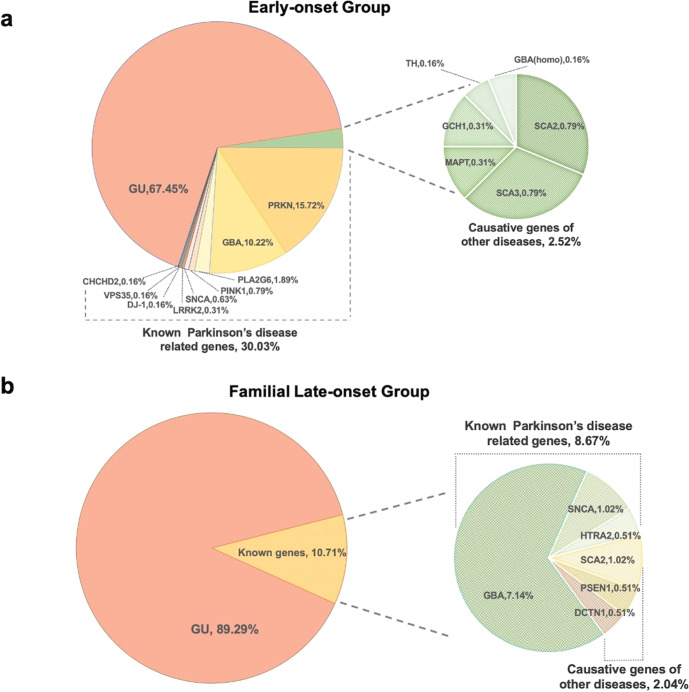
P/LP variants were found in 32.55% patients (207/636) in early-onset group and 10.71% (21/196) in familial late-onset group (Fig. 1). In early-onset group, 191 probands (30.03%) carried P/LP variants in known PD-related genes. PRKN was the most prevalent one, with 100 patients bearing two variants (15.72%, 33 patients without family segregation analysis), followed by GBA heterozygous variants (n = 65, 10.22%), PLA2G6 (n = 12, 1.89%), PINK1 (n = 5, 0.79%, 1 patient without family segregation analysis), SNCA (n = 4, 0.63%) (Supplementary Fig. 2), LRRK2 (n = 2, 0.32%), DJ-1 (n = 1, 0.16%), VPS35 (n = 1, 0.16%), and CHCHD2 (n = 1, 0.16%). Sixteen probands (2.52%) carried P/LP variants in causative genes of other diseases. Among them, 10 patients carried dynamic variants of SCA (five with ATXN3, five with ATXN2), as well as three patients with variants in DRD-related genes (two with GCH1, one with TH), two patients bearing MAPT p.Asn279Lys related with FTLD, and one carrying homozygous GBA p.Phe76Val variant causing Gaucher’s disease, respectively.
Fig. 1. The frequency of causative genes in clinical diagnosed Parkinson’s disease.
The frequency of causative genes in the early-onset patients (a) and familial late-onset patients (b) with clinical diagnosis of Parkinson’s disease.
In familial late-onset group, 17 patients (17/196, 8.67%) carried P/LP variants in known PD-related genes, lower than the early-onset group. Among them, 14 probands carried GBA heterozygous variants (7.14%), two probands carried SNCA duplication (1.02%) and one carried HTRA2 c.940-1G > C variant (0.51%). Four probands (2.04%) bore P/LP variants in causative genes of other diseases, including two with spinocerebellar ataxia type 2 (SCA2), one with Alzheimer’s disease (AD) (PSEN1 p.Leu282Arg), and one with Perry syndrome (DCTN1 p.Tyr78His).
Twenty-nine novel P/LP single nucleotide variants and short insertions/deletions were found in the cohort, with 13 in PRKN (NM_004562.3, p.Gln63Ter, p.Pro73Argfs*8, p.Val117Glyfs*9, p.Thr217Profs*8, p.Glu309Ter, p.Cys337Tyr, p.Tyr372Valfs*2, p.Ala406Glyfs*168, p.Glu426Gly, p.Trp462Ter, c.535-2A > C, c.535-3A > G, c.619-2A > G), eight in GBA (NM_000157.4, p.Ile158Serfs*42, p.Met162Val, p.Leu199Aspfs*62, p.Gly228Arg, p.Pro240Leu, p.Val414Leu, p.Trp417Ter, p.Asp419Asn), four in PLA2G6 (NM_003560.4, p.Thr518Ala, p.Phe549Ile, p.Ala633Val, p.Ala640Thr), two in PINK1 (NM_032409.3, p.Val59Serfs*48 and p.Asn521Tfs*40), one in HTRA2 (NM_001321727.1, c.940-1G > C), and one in DJ-1 (NM_007262.5, p.Val71del) (Supplementary Table 2 and Supplementary Fig. 1). In addition, one novel triplication of exon 2–4 of PRKN was also found.
The mutational frequency of all the screened genes and the genotypes were described in Supplementary Materials.
The clinical characteristics related to the known PD-related genes in the cohort
Comparing with genetic undefined (GU) patients in early-onset group, PRKN patients had an earlier AAO, longer disease duration and less levodopa equivalent daily dosage (LEDD), and tended to have more preserved olfactory function and cognitive function in domains of executive function, language, and attention. After adjusting for confounders (age, sex, education, disease duration, and LEDD), all the significance remained. More females and shorter disease duration at baseline were found in patients bearing heterozygous GBA variants. More significant olfactory dysfunction was observed in GBA patients, both before and after adjusting for confounders. Patients with P/LP variants in PLA2G6 had an earlier AAO, younger age at examination. They showed more severe motor dysfunction with higher Unified Parkinson’s Disease Rating Scale (UPDRS) III score. They were more affected in non-motor symptoms with higher Non-Motor Symptoms Questionnaire (NMSQ) scores and more depression. They also had significantly more decreased quality of life and worse cognitive function in all the 5 subdomains than GU patients both before and after adjustment (Table 1, Supplementary Table 3). P/LP PINK1 variants were detected in 5 patients. The median age at examination of PINK1 variant carriers was 31.00 (21.50) years. From the clinical observation, patients with PINK1 variants usually had a young onset age (30.00 (21.00)), and preserved olfactory function evaluated by Sniffin’ Sticks Screening 12 test (SSST-12) (9.00 (4.25)). Six patients carried SNCA variants. The median age at examination of SNCA variant carriers was 51.50 (23.50) years. The wide range of AAO (22 to 62 years) and worse scores in SSST-12 (2.00 (4.00)) were observed in SNCA patients (Supplementary Table 4).
Table 1.
Clinical features of the GU-EOPD patients, and the EOPD patients with pathogenic/likely pathogenic variants in PRKN, GBA, and PLA2G6.
| Clinical features | GU-EOPD (N = 429) |
PRKN (N = 100) |
GBA (N = 65) |
PLA2G6 (N = 12) | P | ||
|---|---|---|---|---|---|---|---|
| PRKN | GBA | PLA2G6 | |||||
| Sex, female (%) | 160 (37.30) | 41 (41.00) | 36 (55.38) | 5 (41.67) | 0.4920 | 0.0055** | 0.7576 |
| Education, years | 12.00 (6.00) | 12.00 (6.00) | 11.00 (7.00) | 12.50 (8.00) | 0.9253 | 0.6765 | 0.6058 |
| AAO, years | 41.00 (9.00) | 27.00 (11.00) | 42.00 (8.00) | 27.00 (8.00) | 0.0001** | 0.4419 | 0.0001** |
| Age at examination, years | 45.00 (11.00) | 37.00 (13.50) | 45.00 (10.00) | 30.00 (4.50) | 0.0001** | 0.7449 | 0.0001** |
| Disease duration, month | 43.00 (60.00) | 93.00 (123.50) | 31.00 (54.00) | 33.00 (39.00) | 0.0001** | 0.0314* | 0.3928 |
| LEDD, mg | 450.00 (400.00) | 350.00 (400.00) | 475.00 (375.00) | 350.00 (400.00) | 0.0034* | 0.1294 | 0.8828 |
| UPDRS III, score (Med off) | 27.25 (21.00) | 30.00 (17.00) | 28.50 (20.00) | 39.00 (15.75) | 0.2598 | 0.9394 | 0.0148*a |
| Non-motor features | |||||||
| BDI, score | 12.00 (12.00) | 14.00 (13.00) | 11.00 (17.00) | 23.00 (12.00) | 0.1838 | 0.5385 | 0.0001**a |
| PDQ39, score | 29.00 (34.00) | 38.00 (32.00) | 28.00 (46.00) | 59.00 (44.00) | 0.0542 | 0.5028 | 0.0012**a |
| NMSQ, score | 10.00 (10.00) | 9.00 (5.00) | 9.00 (10.00) | 15.00 (6.50) | 0.3362 | 0.3336 | 0.0266* |
| ESS, score | 6.00 (6.00) | 5.00 (5.00) | 5.00 (5.00) | 7.00 (4.00) | 0.7024 | 0.4791 | 0.3527 |
| RBDSQ, score | 3.00 (3.00) | 3.00 (3.00) | 3.00 (3.00) | 3.00 (0.00) | 0.4316 | 0.9808 | 0.7485 |
| SSST-12, score | 6.00 (3.00) | 8.50 (3.00) | 5.00 (3.00) | 5.00 (4.00) | 0.0001**a | 0.0003**a | 0.1838a |
| Cognitive domains | |||||||
| Memory | −0.07 (1.08) | 0.07 (1.08) | −0.08 (1.18) | −1.10 (0.86) | 0.2463 | 0.7969 | 0.0017**a |
| Visuospatial function | 0.22 (0.76) | 0.21 (0.82) | 0.06 (1.22) | −0.78 (1.79) | 0.8576 | 0.2634 | 0.0022**a |
| Language | −0.02 (1.06) | 0.27 (1.29) | −0.25 (1.13) | −1.22 (2.54) | 0.0034**a | 0.2124 | 0.0434*a |
| Attention | 0.09 (0.98) | 0.45 (0.93) | 0.30 (1.23) | −0.47 (1.76) | 0.0001**a | 0.1113 | 0.0308*a |
| Executive function | 0.08 (0.78) | 0.31 (0.83) | 0.13 (0.82) | −0.36 (1.69) | 0.0001**a | 0.4364 | 0.0515a |
| MMSE, score | 28.00 (2.00) | 29.00 (1.00) | 28.50 (3.00) | 28.00 (2.00) | 0.3661 | 0.9885 | 0.0722a |
The continuous data are presented as median (interquartile range (IQR)), and the categorical data are presented as n (%).
AAO age at onset, EOPD early-onset Parkinson’s disease, GU genetically undefined, BDI beck depression inventory, ESS Epworth Sleepiness Score, LEDD Levodopa equivalent dose daily, MMSE mini mental state examination, NMSQ non-motor symptoms questionnaire, PDQ39 39-item Parkinson’s disease questionnaire, RBDSQ rapid-eye-movement sleep behavior disorder screening questionnaire, SSST-12 Sniffin’ Sticks screening 12 test, UPDRS Unified Parkinson’s Disease Rating Scale.
aSignificant after adjustment for age, gender, education, disease duration, and levodopa dose equivalents.
P: Comparison between the early-onset group with variants within a specific gene and GU-EOPD group. *P < 0.05, **P < 0.001.
The clinical characteristics related to the causative genes of other diseases in the cohort
As for the patients carrying P/LP variants in causative genes of other diseases, all the patients met the clinical diagnostic criteria of PD at the first clinical visit. All the affected family members of the probands with family history were diagnosed or suspected with PD. No cerebellar signs were observed in all of the SCA patients at the baseline visit, and only one SCA2 patient showed mild nystagmus at follow-up. As for the noteworthy features, three out of seven SCA2 patients had dystonia, with one patient having foot dystonia and blepharospasm at the first year of disease and 2 patients developing blepharospasm, and spastic torticollis/upper limb dystonia respectively during follow-up. One spinocerebellar ataxia type 3 (SCA3) patient had peripheral neuropathy and one patient with PSEN1 variant had a medical history of epilepsy and stroke. Among the 12 patients followed up in our center, 10 patients still met the clinical diagnostic criteria of PD with the disease duration from 4 to 15 years, and the other two with MAPT variant developed progressive supranuclear palsy (PSP) or frontotemporal dementia like symptoms at the third and fourth year of disease (Supplementary Table 5).
Discussion
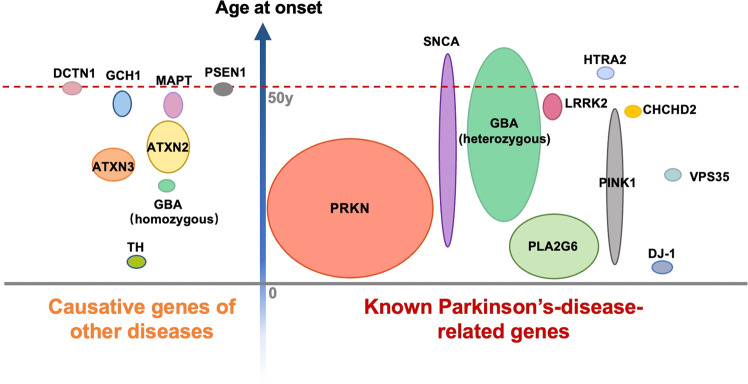
In the current study, the genetic spectrum was investigated in a cohort of clinically diagnosed PD patients from mainland China. We found 26.92% patients (224/832) carrying P/LP variants in known causative genes in a cohort of patients with an early AAO or family history. Of note, twenty patients (20/832, 2.15%) carried P/LP variants in causative genes of other diseases, including ATXN2, ATXN3, GCH1, MAPT, PSEN1, TH, GBA (homozygous variants) and DCTN1. The genetic architecture in our cohort and its relationship with clinical diagnosis and AAO were summarized in Fig. 217,18.
Fig. 2. The genetic architecture of clinical diagnosed Parkinson’s disease in the study.
The vertical axis represents the age at onset. The size of a certain gene illustrated in the figure represents the frequency identified in our study.
For the known PD-related genes, we found a higher frequency of P/LP variants in early-onset patients (30.03%) than familial late-onset patients (8.67%), this finding is consistent with previous reports that the genetic factors play a major role in the disease onset in EOPD5. The frequency of P/LP variants (8.67%) was much lower in familial late-onset patients comparing with that in all the included familial patients regardless of onset age (27.05%), though all the probands had family history of parkinsonism. Notably, two third late-onset patients in the cohort had a family history of late-onset PD. The familial aggregation of late-onset PD might partly be explained by the genetic burden of rare variants or common variants, the similar environmental exposures in one family, and the prevalence with ageing, rather than a single strong genetic effector19.
The frequency of causative genes or the P/LP variant spectrum within a certain gene varied among different geographical regions and populations12,20,21. LRRK2 variants were reported as the leading genetic cause of PD in European population, with LRRK2 G2019S explaining about 5–6% familial PD and 1% sporadic cases13. The frequency of LRRK2 G2019S in PD can surge to almost 14% in Ashkenazi Jews population5. However, no LRRK2 G2019S were identified in our cohort in line with previous reports in Asian population22. In Chinese EOPD and familial PD patients, P/LP variants in PRKN were the most prevalent with 4.3–5.7% in different studies, followed by heterogenous GBA variants (2.1–7.2%)5–7,15. The P/LP variants of PINK1 (0.4–1.2%), PLA2G6 (0.3–0.6%), SNCA (0.2–0.7%), LRRK2 (0.4–2.0%), VPS35 (0.06–0.3%) were reported with a lower frequency. However, in this study, the proportion of patients carrying PRKN (15.72%), GBA (9.50%) and PLA2G6 (1.89%) variants was higher than previously reported, while the proportion of patients carrying the other PD causative genes was similar. Accordingly, the frequency of known PD-related genes excluding GBA was 19.81% in early-onset group, higher than other findings (11.6%15, 9.3%6, 8.74%5, 7.5%7). It could be interpreted to the distribution of AAO and the selective bias of a single center of tertiary hospital. The percentage of patients with AAO ≤ 30, 30 < AAO ≤ 40, and 40 < AAO ≤ 50 was 19.6%, 37.2%, 45.1% in our cohort, with much more patients with younger AAO than those in Zhao’s study (6.6%, 19.6% and 73.8%)5. This may explain the higher molecular diagnosis rate of our study, since the lower AAO was related with a higher molecular diagnosis rate5.
Dynamic variants of ATXN2 and ATXN3 explained 1.4% of familial PD in this study, which indicated the importance of genetic testing of dynamic variants in patients with parkinsonian symptoms. Though autosomal dominant cerebellar ataxia was recognized as the predominant phenotype of SCA2, parkinsonism was also common in SCA223. The SCA2 patients predominantly manifesting parkinsonism might be reminiscent of PD patients and should be detected by genetic testing24,25. Similarly, parkinsonian phenotype was also frequently described in SCA3, albeit less prevalent than ataxia26,27. Intrafamilial phenotypic heterogeneity was found in reported SCA3 cases with parkinsonism28, but the affected family members of four SCA3 probands in this study were all PD phenotype, resulting in further setbacks to the exact diagnosis.
SCA2 or SCA3 patients with parkinsonism being the dominant symptoms could have unremarkable findings in brain magnetic resonance images (MRI)25,29, which indicated the brain MRI may or may not be the clues to differential PD from SCA. One patient with SCA3 dynamic variant had peripheral neuropathy, which was commonly observed in ataxia predominant SCA2 and SCA330,31 and was also reported in parkinsonism dominant SCA2 and SCA326,32. Though the percentage of SCA dynamic variants in patients with parkinsonism plus peripheral neuropathy was unknown, we suggested besides family history, peripheral neuropathy can be another important hint to the SCA diagnosis.
The P/LP variants of GCH1 were initially detected in the patients with DRD. Besides classic phenotype of DRD, the broadened clinical spectrum of GCH1 can include adult-onset parkinsonism, focal dystonia, DRD-simulating cerebral palsy or spastic paraplegia33. The P/LP variants of GCH1 were later reported to be associated with DRD and PD in different family members in the same pedigree, and even with sporadic PD patients9,34,35, indicating the GCH1- related mechanism might contribute to the pathogenesis of PD and tend to increase the risk of PD34. The patients in our study showed PD-like symptoms. Significant sleep benefit and good response to levodopa treatment was observed but no dystonia. They might be PD or DRD since the two GCH1 variants were reported both in DRD and PD2,34,35. The positron emission computed tomography (PET) for dopamine transporter could be helpful to the diagnosis, which, however, was not applied in the patients.
Biallelic TH and GBA variants were identified as the genetic cause of DRD and Gaucher’s disease. For phenotypic spectrum, patients with homozygous/compound heterozygous variants of TH or GBA could also present PD-like characteristics36–38. DCTN1 was initially identified as the genetic cause of Perry syndrome, but adult-onset (atypical) parkinsonism was later found prominent in patients bearing DCTN1 variants39. Patients with MAPT or PSEN1 variants usually present with FTLD and AD respectively, but can have prominent parkinsonism10,40. In the early disease stage, these patients might manifest characterized symptoms of PD and be misdiagnosed as PD10,40–43. With the progression of the diseases, more atypical symptoms emerge which indicate other diagnosis instead of PD. Parkinsonism is one of the core phenotypes of the frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) spectrum caused by MAPT variants10, which should be differentiated from PD in the first place especially in cases with the autosomal dominant inherited family history. Besides, the atrophy pattern on brain MRI may give clues to the diagnosis of FTLD or AD, but the genetic testing should be implemented early whenever possible.
Our work may provide important implication for the application and interpretation of genetic testing in clinically diagnosed PD patients, especially in Chinese Han population. The genetic testing will improve the diagnostic accuracy of PD in the early-onset and familial patients, especially those with onset age below 50. Besides known PD-related genes, causative genes of other diseases should also be attached importance during interpretating the results of genetic testing, as they were also the indispensable genetic contributor to the PD-like phenotype.
Our findings would also refine the patient recruitment for clinical trials. The “one-size-fits all” patient recruitment approach contributed a lot to the high rate of failure in previous PD clinical trials44,45. The genetic status determined by proper genetic testing can lead to quick access to eligible candidates for new treatment options. Genetically targeted clinical trials and the implementation of personalized medicine may bring new opportunities to PD disease-modifying therapy.
Due to the clinical heterogeneity of the neurogenetic diseases, the broad and complex phenotypes-including the parkinsonian characteristics, can be associated with a specific causative gene, sometimes it is a causative gene of other disease. Meanwhile, the P/LP variants in the known PD-related genes might present other phenotypes rather than the characterized PD symptoms. For example, LRRK2 variant carriers can present with PSP phenotype46, PRKN variant carriers with DRD, ATP13A2/PLA2G6/FBXO7/SYNJ1/VPS13C variant carriers with atypical Parkinsonian syndromes11. These confusions raise the question of the nomenclature of genetic movement disorders which has been discussed for a long time47–49. Thus in 2016, the international Parkinson and movement disorder society task force recommended a 2-axis nomenclature system with the phenotype followed by the gene, and divided the hereditary parkinsonism-related genes into 3 categories based on phenotype besides parkinsonism. The recommendation was updated in 202239,47, in which, most mutated genes found in the study were included in the hereditary parkinsonism (DCTN1 in atypical parkinsonism or complex phenotypes; GCH1, PLA2G6, and TH in combined phenotypes; GBA, ATXN2 and MAPT in disorders that usually present with other phenotypes but can have predominant parkinsonism) except for PSEN1 and ATXN3. Our work might improve the understanding of nomenclature system and offer further clues to the system.
This study has some limitations. Firstly, we did not apply whole-exome sequencing (WES) and dynamic variant testing of SCA to all the patients which might bias the frequency of causative genes in other diseases in the cohort. Secondly, a PET for dopamine transporter was not available in some patients carrying causative genes of other diseases. A follow-up study was important to these patients. Thirdly, this study was conducted in a single center of tertiary hospital which may inevitably result in bias, thus more multicenter studies are necessary in the future to verify our findings.
In conclusion, we investigated the genetic characteristics of a group of clinically diagnosed PD patients. This study demonstrated the importance of genetic testing in the diagnosis in clinically diagnosed PD patients, especially in the early-onset patients or patients with family history. It provided some clues to the nomenclature of genetic movement disorders. This will be crucial in grouping the patients in clinical trials and developing the treatment for the etiology.
Methods
Participants
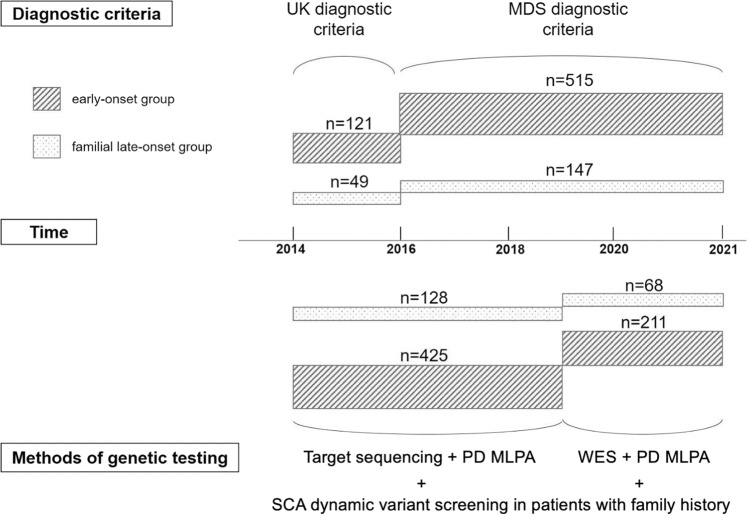
Patients meeting the following criteria from February 2014 to December 2020 were investigated retrospectively: (1) a diagnosis of PD at the initial clinical visit; (2) AAO < 50, or AAO ≥ 50 and with family history of PD (defined as having at least one other affected relative in the family); and (3) consent to genetic testing related to PD. The diagnostic criteria used for PD were the United Kingdom PD Society Brain Bank Clinical Diagnostic Criteria50 (for patients recruited before 2016) or the 2015 Movement Disorder Society Clinical Diagnostic Criteria for PD51 (for patients recruited from 2016 on) (Fig. 3). The affected relatives were not included in the cohort.
Fig. 3. Procedure of the study.
The upper part of the diagram indicated the diagnostic criteria used in patients of early-onset group and familial late-onset group clinically diagnosed as PD according to the time line. The bottom part of the diagram indicated the genetic testing carried out in the patients of different groups according to the time line. MDS diagnostic criteria: 2015 Movement Disorder Society Clinical Diagnostic Criteria for Parkinson’s disease, MLPA multiplex ligation-dependent probe amplification, PD Parkinson’s disease, SCA spinocerebellar ataxia, UK diagnostic criteria: The United Kingdom Parkinson’s disease Society Brain Bank Clinical Diagnostic Criteria, WES whole-exome sequencing.
Eight hundred and thirty-two patients were included. They were divided into “early-onset group” (AAO < 50, n = 636) and “familial late-onset group” (AAO ≥ 50 and with family history of PD, n = 196).
The study was approved by the Institutional Review Board of Huashan Hospital and the China human genetic resources management office. Written informed consent was obtained from all study participants.
Clinical assessments of the patients initially diagnosed with PD
The clinical assessments were performed through a face-to-face interview with all patients. Baseline data were collected including demographic profiles (age, sex, education), disease history, family history, clinical signs, comorbidities, medications, and neurological examination results. An established method was used to calculate the LEDD52.
The Hoehn and Yahr (H&Y) scale and UPDRS motor examination (items 18–31) were conducted during the off-medication state, defined as withdrawal of anti-PD medications for at least 12 hours, except in those who could not tolerate it (n = 9). A battery of neuropsychological tests was performed to assess cognitive function and affected subdomains, as indicated in our previous study53. The non-motor symptoms were investigated by Epworth sleepiness scale (ESS), rapid eye movement sleep behavior disorder screening questionnaire (RBDSQ), Beck Depression Inventory (BDI), NMSQ, 39 item Parkinson’s disease questionnaire (PDQ39), and SSST-12, as indicated in our previous study53.
Genetic testing and variants screening
Genomic deoxyribonucleic acid (DNA) samples were extracted from peripheral blood leukocytes of the probands and family members if necessary (Qiagen, Germany). The patients enrolled before January 2019 had genetic testing by target sequencing of a panel containing 116 movement-disorder-related genes (Supplementary Table 6), including 22 known PD-related genes (ATP13A2, CHCHD2, DJ-1, DNAJC13, DNAJC6, EIF4G1, FBXO7, GBA, GIGYF2, HTRA2, LRRK2, PINK1, PLA2G6, POLG, PRKN, RAB39B, SNCA, SYNJ1, TMEM230, UCHL1, VPS13C, VPS35)3,4,54, while the patients enrolled after that had genetic testing by WES (Fig. 1).
For target sequencing, genomic DNA was fragmented into 150–200 bp length by sonication. The DNA fragments were processed by end-repairing and enriched by a panel capturing the coding exons and corresponding flanking regions of 116 genes related to movement disorders (Supplementary Table 6). Paired-end sequencing was performed on lllumina HiSeq2000 platform to provide a mean read depth of over 100X and a coverage of at least 20X in more than 95% of targeted areas. Raw data was processed by the Illumina pipeline (version 1.3.4) for image analysis, error estimation, base calling and generating the primary sequence data. Variant calling was performed using Genome Analysis Toolkit (GATK) “Best Practices” workflow55,56. Briefly, after the removal of 3′-/5′- adapters and low-quality reads for the quality control, the clean reads were aligned to the human reference genome (Genome Reference Consortium Human Build 37 (GRCh37)/ human genome, version 19 (hg19)) using Burrow-Wheeler Aligner (BWA) (version: dynamic update with the time, http://bio-bwa.sourceforge.net) with default parameter settings. Polymerase chain reaction (PCR) duplicates were removed by Picard (version: dynamic update with the time, http://picard.sourceforge.net). Then we followed GATK (version: dynamic update with the time, https://software.broadinstitute.org/gatk/) standard pipelines to call germline variants.
As for WES, DNA libraries were prepared with KAPA Library Preparation Kit (Kapa Biosystems, KR0453) following the manufacturer’s instructions and DNA libraries were sequenced on the Illumina Novaseq platform with 200-bp paired-end mode. Similarly, we used GATK “Best Practices” workflow for germline variant calling, as previously described for target sequencing.
After variant calling steps above, all variants were annotated with ANNOtate VARiation (ANNOVAR) (https://annovar.openbioinformatics.org/en/latest/) tool57, and were further filtered with additional considerations from allele frequencies, variant damage predictions, and related phenotypes of corresponding genes. More specifically, variants with frequencies >1% were filtered according to 1000 Genomes Project (http://www.internationalgenome.org/data), Exome Sequencing Project v. 6500 (ESP6500) (evs.gs.washington.edu/EVS/), Exome Aggregation Consortium (ExAC) (EXac.broadinstitute.org/) and our inhouse database. Then, damaging missense variants were predicted by Sorting Intolerant From Tolerant (SIFT)58 (sift.bii.a-star.edu.sg/), Polymorphism Phenotyping v2 (PolyPhen-2)59 (genetics.bwh.harvard.edu/pph2/), and MutationTaster60 (www.mutationtaster.org/). The synonymous variants were excluded, and predicted damaging variants were passed as candidate variants. Besides, the phenotypes of the screened genes were compared with the clinical manifestations of the proband, and inherited modes were also taken into consideration in order to further exclude irrelevant genes.
The copy number variants (CNVs) in the 116 genes were analyzed by a bioinformatic tool of CNVkit61. The suspected CNV would be confirmed by real-time PCR.
The candidate variants with unknown pathogenicity were rated according to the American College of Medical Genetics (ACMG) guidelines62.
All the patients had multiplex ligation-dependent probe amplification (MLPA) of 8 PD-related genes (ATP13A2, GCH1, LRRK2, PARK7, PINK1, PRKN, SNCA and UCHL1) to investigate the CNVs. MLPA was performed using a SALSA MLPA Probemix P051-D1/P052-D2 Parkinson kit (MRC-Holland, the Netherland) according to the standard protocols provided by the manufacturer.
The candidate variants and any CNVs detected were further tested in the proband’s parents, other affected or unaffected family members for segregation and co-segregation by Sanger sequencing, real-time PCR or MLPA respectively, if necessary and possible, during which the homozygous or compound heterozygous status of the variants in genes with autosomal recessive inheritance would be confirmed.
The testing of dynamic variants was applied to the patients with family history, including SCA type 1, 2, 3, 6, 7, 8, 10, 12, 17 and Dentatorubral-pallidoluysian atrophy (DRPLA) by triplet repeat primed polymerase chain reaction (TP-PCR) and capillary electrophoresis techniques.
The quality of the sequencing
As for the panel, the average sequencing depth of the target region was 393.65× and the mean percentage of the target region covered at least 20× was 99.35%. A total of 2153 regions were sequenced; 14 regions (0.65%) had read depths below 20×, which were mainly in GC-rich areas.
As for the WES, 251779 regions were sequenced. The average sequencing depth of the target region was 101.3x and the mean percentage of the target regions covered at least 20x was 80.6%. As for the 2235 regions in the 115 genes of interest, the average sequencing depth was 108.6x and the mean percentage covered at least 20x was 87.5%. The regions with sequencing depth below 20x in both target sequencing and WES were listed in Supplementary Table 7.
Statistical analysis
All measurements were taken from distinct samples. The Shapiro–Wilk test was applied to test the normality. While categorical variables were demonstrated as frequencies (%), continuous variables were demonstrated as the median and IQR. The Chi-squared test, or Fisher’s exact test was used for comparing the categorical variables, and the Kruskal-Wallis test was used for comparing the continuous variables. Raw scores of neuropsychological tests were transformed into Z-scores as previously described53. The mean Z-scores of each domain from individual tests were computed. The generalized linear model (GLM) was used to evaluate the association between motor or non-motor scales and genetic status, which adjusted for age, sex, education, disease duration, and LEDD. Two-tailed P values were presented. Differences were considered statistically significant at P < 0.05. The data analysis was conducted by STATA 17.0 (StataCorp).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
J.W. received research funding from the Shanghai Municipal Science and Technology Project (21S31902200, 2018SHZDZX01), National Health Commission of China (Pro20211231084249000238), and the National Nature Science Foundation of China (91949118, 82171421, 92249302). Y.A. was supported by Shanghai Municipal Science and Technology Major Project (2017SHZDZX01). B.L. received research funding from the National Nature Science Foundation of China (81870990). L.H.G. received research funding from China Postdoctoral Science Foundation (KLF151042).
Author contributions
Study concept and design: Y.M.S., Y.A., J.J.W., and J.W. Data acquisition and analysis: Y.M.S., X.Y.Z., X.N.L., J.R.L., Y.D.X., C.C., S.D.W., Q.S.C., F.T.L., J.Z, Y.L.T., B.S., L.H.G., B.L. and Z.T.D. Statistical analysis: X.Y.Z. and X.N.L. Manuscript drafting: Y.M.S., X.Y.Z., and X.N.L. Review and Critique: Y.M.S., Y.A., J.J.W., and J.W. These co-first authors contributed equally: Y.M.S., X.Y.Z., X.N.L., and J.R.L. All authors contributed to the article and approved the submitted version.
Data availability
The cloud data sharing of the raw DNA sequencing data was not included in informed consent signed by the participants. The sequencing data can be available once the applicant’s institution and the research objective are verified by the corresponding author Jian Wang. The other data that support the findings in the study can be available from the request of the corresponding author Jian Wang. All the data should be applied for non-commercial purposes only, and sharing restrictions may be applied to sensitive data to preserve the participants’ privacy.
Code availability
The bioinformatic analysis of the sequncing data was conducted as the previously reported sequencing analysis pipline, which is available at https://www.protocols.io/view/adbs-whole-exome-sequencing-wes-analysis-pipeline-8epv56rz6g1b/v1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi-Min Sun, Xin-Yue Zhou, Xiao-Niu Liang, Jin-Ran Lin.
Contributor Information
Yu An, Email: anyu@fudan.edu.cn.
Jian-Jun Wu, Email: wujianjun@fudan.edu.cn.
Jian Wang, Email: wangjian_hs@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-023-00518-9.
References
- 1.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–2303. doi: 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 2.Cao L, et al. Four novel mutations in the GCH1 gene of Chinese patients with dopa-responsive dystonia. Mov. Disord. 2010;25:755–760. doi: 10.1002/mds.22646. [DOI] [PubMed] [Google Scholar]
- 3.Wilson GR, et al. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am. J. Hum. Genet. 2014;95:729–735. doi: 10.1016/j.ajhg.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funayama M, et al. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14:274–282. doi: 10.1016/S1474-4422(14)70266-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, et al. The role of genetics in Parkinson’s disease: a large cohort study in Chinese mainland population. Brain. 2020;143:2220–2234. doi: 10.1093/brain/awaa167. [DOI] [PubMed] [Google Scholar]
- 6.Lin CH, et al. A clinical and genetic study of early-onset and familial parkinsonism in taiwan: An integrated approach combining gene dosage analysis and next-generation sequencing. Mov. Disord. 2019;34:506–515. doi: 10.1002/mds.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, et al. Whole-exome sequencing in early-onset Parkinson’s disease among ethnic Chinese. Neurobiol. aging. 2020;90:150 e155–150.e111. doi: 10.1016/j.neurobiolaging.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Kim YE, et al. SCA2 family presenting as typical Parkinson’s disease: 34 year follow up. Parkinsonism Relat. Disord. 2017;40:69–72. doi: 10.1016/j.parkreldis.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino H, et al. GCH1 mutations in dopa-responsive dystonia and Parkinson’s disease. J. Neurol. 2018;265:1860–1870. doi: 10.1007/s00415-018-8930-8. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda A, et al. Clinical heterogeneity of frontotemporal dementia and Parkinsonism linked to chromosome 17 caused by MAPT N279K mutation in relation to tau positron emission tomography features. Mov. Disord. 2019;34:568–574. doi: 10.1002/mds.27623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissbach A, Wittke C, Kasten M, Klein C. ‘Atypical’ Parkinson’s disease - genetic. Int. Rev. Neurobiol. 2019;149:207–235. doi: 10.1016/bs.irn.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Milanowski ŁM, et al. Genetics of Parkinson’s disease in the Polish population. Neurol. Neurochir. Pol. 2021;55:241–252. doi: 10.5603/PJNNS.a2021.0013. [DOI] [PubMed] [Google Scholar]
- 13.Tan MMX, et al. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson’s disease study. Brain. 2019;142:2828–2844. doi: 10.1093/brain/awz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milanowski ŁM, et al. Frequency of mutations in PRKN, PINK1, and DJ1 in Patients With Early-Onset Parkinson Disease from neighboring countries in Central Europe. Parkinsonism Relat. Disord. 2021;86:48–51. doi: 10.1016/j.parkreldis.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YP, et al. The mutation spectrum of Parkinson-disease-related genes in early-onset Parkinson’s disease in ethnic Chinese. Eur. J. Neurol. 2022;29:3218–3228. doi: 10.1111/ene.15509. [DOI] [PubMed] [Google Scholar]
- 16.Skrahina V, et al. The Rostock International Parkinson’s Disease (ROPAD) Study: Protocol and Initial Findings. Mov. Disord. 2021;36:1005–1010. doi: 10.1002/mds.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang T, et al. Han Chinese family with early-onset Parkinson’s disease carries novel compound heterozygous mutations in the PARK2 gene. Brain Behav. 2019;9:e01372. doi: 10.1002/brb3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montaut S, et al. Assessment of a targeted gene panel for identification of genes associated with movement disorders. JAMA Neurol. 2018;75:1234–1245. doi: 10.1001/jamaneurol.2018.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball N, Teo WP, Chandra S, Chapman J. Parkinson’s disease and the environment. Front. Neurol. 2019;10:218. doi: 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesage, S. et al. Characterization of recessive Parkinson’s disease in a large multicenter study. Annals of neurology, 10.1002/ana.25787 (2020). [DOI] [PMC free article] [PubMed]
- 21.Benitez BA, et al. Resequencing analysis of five Mendelian genes and the top genes from genome-wide association studies in Parkinson’s Disease. Mol. neurodegeneration. 2016;11:29. doi: 10.1186/s13024-016-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan EK, et al. The G2019S LRRK2 mutation is uncommon in an Asian cohort of Parkinson’s disease patients. Neurosci. Lett. 2005;384:327–329. doi: 10.1016/j.neulet.2005.04.103. [DOI] [PubMed] [Google Scholar]
- 23.Furtado S, et al. Profile of families with parkinsonism-predominant spinocerebellar ataxia type 2 (SCA2). Mov. Disord. 2004;19:622–629. doi: 10.1002/mds.20074. [DOI] [PubMed] [Google Scholar]
- 24.Wang JL, et al. Analysis of SCA2 and SCA3/MJD repeats in Parkinson’s disease in mainland China: genetic, clinical, and positron emission tomography findings. Mov. Disord. 2009;24:2007–2011. doi: 10.1002/mds.22727. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, et al. Importance of low-range CAG expansion and CAA interruption in SCA2 Parkinsonism. Arch. Neurol. 2007;64:1510–1518. doi: 10.1001/archneur.64.10.1510. [DOI] [PubMed] [Google Scholar]
- 26.Gwinn-Hardy K, et al. Spinocerebellar ataxia type 3 phenotypically resembling parkinson disease in a black family. Arch. Neurol. 2001;58:296–299. doi: 10.1001/archneur.58.2.296. [DOI] [PubMed] [Google Scholar]
- 27.Pedroso JL, Braga-Neto P, de Souza PV, Barsottini OG. The cerebellum in Parkinson’s disease and Parkinsonism in cerebellar disorders. Brain. 2013;136:e248. doi: 10.1093/brain/awt089. [DOI] [PubMed] [Google Scholar]
- 28.Socal MP, et al. Intrafamilial variability of Parkinson phenotype in SCAs: Novel cases due to SCA2 and SCA3 expansions. Parkinsonism Relat. Disord. 2009;15:374–378. doi: 10.1016/j.parkreldis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Chen SJ, Lee NC, Chien YH, Hwu WL, Lin CH. Heterogeneous nonataxic phenotypes of spinocerebellar ataxia in a Taiwanese population. Brain Behav. 2019;9:e01414. doi: 10.1002/brb3.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linnemann C, et al. Peripheral neuropathy in spinocerebellar ataxia type 1, 2, 3, and 6. Cerebellum. 2016;15:165–173. doi: 10.1007/s12311-015-0684-6. [DOI] [PubMed] [Google Scholar]
- 31.Gentile F, Bertini A, Priori A, Bocci T. Movement disorders and neuropathies: overlaps and mimics in clinical practice. J. Neurol. 2022;269:4646–4662. doi: 10.1007/s00415-022-11200-0. [DOI] [PubMed] [Google Scholar]
- 32.Payami H, et al. SCA2 may present as levodopa-responsive parkinsonism. Mov. Disord. 2003;18:425–429. doi: 10.1002/mds.10375. [DOI] [PubMed] [Google Scholar]
- 33.Furukawa, Y. in GeneReviews((R)) (eds M. P. Adam et al.) (1993).
- 34.Mencacci NE, et al. Parkinson’s disease in GTP cyclohydrolase 1 mutation carriers. Brain. 2014;137:2480–2492. doi: 10.1093/brain/awu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan YP, et al. Study of GCH1 and TH genes in Chinese patients with Parkinson’s disease. Neurobiol. aging. 2018;68:159 e153–159.e156. doi: 10.1016/j.neurobiolaging.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Fossbakk A, Kleppe R, Knappskog PM, Martinez A, Haavik J. Functional studies of tyrosine hydroxylase missense variants reveal distinct patterns of molecular defects in Dopa-responsive dystonia. Hum. Mutat. 2014;35:880–890. doi: 10.1002/humu.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 38.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange, L. M. et al. Nomenclature of Genetic Movement Disorders: Recommendations of the International Parkinson and Movement Disorder Society Task Force - An Update. Mov. Disord., 10.1002/mds.28982 (2022). [DOI] [PubMed]
- 40.Jo H, et al. Dopa responsive Parkinsonism in an early onset Alzheimer’s disease patient with a presenilin 1 mutation (A434T) J. Alzheimers Dis. 2019;71:7–13. doi: 10.3233/JAD-190469. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Genetic identification is critical for the diagnosis of Parkinsonism: A Chinese pedigree with early onset of Parkinsonism. PLoS One. 2015;10:e0136245. doi: 10.1371/journal.pone.0136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuboi Y, Mishima T, Fujioka S. Perry disease: concept of a new disease and clinical diagnostic criteria. J. Mov. Disord. 2021;14:1–9. doi: 10.14802/jmd.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takao M, et al. A novel mutation (G217D) in the Presenilin 1 gene (PSEN1) in a Japanese family: presenile dementia and parkinsonism are associated with cotton wool plaques in the cortex and striatum. Acta Neuropathol. 2002;104:155–170. doi: 10.1007/s00401-002-0536-6. [DOI] [PubMed] [Google Scholar]
- 44.Sjögren, M., Huttunen, H. J., Svenningsson, P. & Widner, H. Genetically targeted clinical trials in Parkinson’s disease: Learning from the successes made in oncology. Genes (Basel)12, 10.3390/genes12101529 (2021). [DOI] [PMC free article] [PubMed]
- 45.Schneider SA, Alcalay RN. Precision medicine in Parkinson’s disease: emerging treatments for genetic Parkinson’s disease. J. Neurol. 2020;267:860–869. doi: 10.1007/s00415-020-09705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen Y, Zhou Y, Jiao B, Shen L. Genetics of progressive supranuclear palsy: a review. J. Parkinson’s Dis. 2021;11:93–105. doi: 10.3233/JPD-202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marras C, et al. Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force. Mov. Disord. 2016;31:436–457. doi: 10.1002/mds.26527. [DOI] [PubMed] [Google Scholar]
- 48.Lee WW, Jeon B, Kim R. Expanding the spectrum of dopa-responsive dystonia (DRD) and proposal for new definition: DRD, DRD-plus, and DRD Look-alike. J. Korean Med. Sci. 2018;33:e184. doi: 10.3346/jkms.2018.33.e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albanese A, et al. Phenomenology and classification of dystonia: a consensus update. Mov. Disord. 2013;28:863–873. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol., Neurosurg., psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postuma RB, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 52.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 53.Fan Y, et al. Determinants of quality of life according to cognitive status in Parkinson’s disease. Front Aging Neurosci. 2020;12:269. doi: 10.3389/fnagi.2020.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–178. doi: 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Auwera GA, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinforma. 2013;43:11.10.11–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganesh S, et al. Exome sequencing in families with severe mental illness identifies novel and rare variants in genes implicated in Mendelian neuropsychiatric syndromes. Psychiatry Clin. Neurosci. 2019;73:11–19. doi: 10.1111/pcn.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adzhubei, I., Jordan, D. M. & Sunyaev, S. R. Predicting functional effect of human missense mutations using PolyPhen-2. Curr. Protoc. Hum Genet Chapter 7, Unit7.20, 10.1002/0471142905.hg0720s76 (2013). [DOI] [PMC free article] [PubMed]
- 60.Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 61.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: Genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol. 2016;12:e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The cloud data sharing of the raw DNA sequencing data was not included in informed consent signed by the participants. The sequencing data can be available once the applicant’s institution and the research objective are verified by the corresponding author Jian Wang. The other data that support the findings in the study can be available from the request of the corresponding author Jian Wang. All the data should be applied for non-commercial purposes only, and sharing restrictions may be applied to sensitive data to preserve the participants’ privacy.
The bioinformatic analysis of the sequncing data was conducted as the previously reported sequencing analysis pipline, which is available at https://www.protocols.io/view/adbs-whole-exome-sequencing-wes-analysis-pipeline-8epv56rz6g1b/v1.