Highlights
-
•
Research on homogeneous, isocitrate dehydrogenase-mutant, and 1p/19q-codeleted cohorts of oligodendrogliomas remains sparse.
-
•
For WHO grade 2 oligodendrogliomas, progression-free survival was prolonged after subtotal or gross total resection as well as treatment with chemotherapy or radiotherapy.
-
•
Herein, only a combined radiochemotherapy was associated with improved progression-free survival in patients suffering from WHO grade 3 oligodendrogliomas.
Keywords: Oligodendroglioma, Radiochemotherapy, Temozolomide, PCV, IDH mutation
Abstract
Background
Oligodendrogliomas (ODG) are rare, diffusely infiltrating brain tumors, defined by their 1p/19q-codeletion and isocitrate dehydrogenase (IDH) mutation. Herein, we analyze the influence of various tumor and patient characteristics on progression-free survival (PFS) and overall survival (OS) in a homogeneous patient cohort.
Material and methods
Patients treated for a 1p/19q-codeleted and IDH-mutant ODG were evaluated. The patient and tumor characteristics were analyzed for their influence on PFS and OS.
Results
One-hundred-fourteen patients met the inclusion criteria. The median clinical and radiographic follow-up periods were 68.6 and 69.8 months. The median PFS and OS were 66.9 and 236.0 months, respectively. The 2-, 4- and 6-year PFS rates were 89.5%, 76.3%, and 46.0%. The 2-, 4- and 6-year OS rates were 99.0%, 97.9%, and 96.2%. For WHO grade 2 ODG, extent of resection (p = 0.01, hazard ratio (HR) 0.01; p = 0.02, HR 0.02), radiotherapy (p = 0.01, HR < 0.01) and chemotherapy (p = 0.01, HR < 0.01) were associated with a prolonged PFS. For WHO grade 3 ODG, only a combined radiochemotherapy (RCT) lowered the risk of progression in the multivariable analysis (p = 0.02, HR 0.09). Most RCT patients received temozolomide (TMZ) instead of procarbazine, lomustine, and vincristine.
Conclusion
Whereas previous studies often comprise tumors with IDH wild type status and without 1p/19q-codeletion, this homogeneous ODG cohort, as defined by the current WHO classification, demonstrated PFS benefits for various therapies, especially concerning RCT. While this is generally in accordance with comparable studies, more prospective work on homogeneous patient cohorts is required to refine treatment guidelines and to determine the role of TMZ in ODG.
1. Introduction
Oligodendrogliomas (ODG) are rare, malignant, diffusely infiltrating primary brain tumors. Since the update of the World Health Organization (WHO) classification of central nervous system (CNS) tumors in 2016, the diagnosis of this tumor entity requires the presence of an isocitrate dehydrogenase (IDH) gene family mutation and a 1p/19q-codeletion (LOH1p19q) [1]. The 5th edition of the WHO classification in 2021 continues to suggest using these molecular characteristics to diagnose oligodendroglial tumors.
Today, we know that molecular features determine the prognosis and response to different treatment options of gliomas. The NOA-04 randomized phase III trial showed a prognostic significance of IDH1 mutation status and O6-methylguanine-DNA-methyltransferase (MGMT) promotor methylation for anaplastic glioma [2], [3], [4]. Furthermore, it identified the extent of resection (EOR), age, and histology as prognostic factors for the time to treatment failure [2], [3]. Moreover, an association between LOH1p19q status and longer overall survival (OS) and progression-free survival (PFS) in tumors considered anaplastic oligodendroglioma (AODG) at the time was identified in the EORTC 26951 and RTOG 9402 trials [5], [6], [7].
The ideal therapy, however, remains unclear for different types of gliomas. There are several trials that have proven a combined radiochemotherapy (RCT; i.e., radiotherapy (RT) with neoadjuvant or sequential chemotherapy (CT)) effective in the treatment of ODG, which show the molecular characteristics mentioned above [3], [5], [6], [8]. Current treatment of ODG consists of maximal safe resection, followed by either a watch and wait (WW) strategy in patients with a low-risk profile or RT in combination with sequential procarbazine, lomustine (CCNU), and vincristine (PCV) for patients with unfavorable prognostic factors [4], [9]. Whether PCV can be replaced by temozolomide (TMZ) is under further investigation by the modified CODEL trial [10].
Most of the published work about oligodendroglial tumors is not based on cohorts with verified IDH mutation status and LOH1p19q. To solve this issue, several study groups have done retrospective subgroup analyses with either LOH1p19q or IDH-mutant (IDHmt) tumor patients. However, as ODG are rare brain tumors, these subgroups were usually not powered for statistical analysis [3], [5], [6], [11]. To account for the heterogeneity of previous reports, we conducted an analysis to explore the influence of several patient and tumor characteristics on PFS and OS in a homogeneous patient cohort with verified LOH1p19q and IDHmt ODG.
2. Material and methods
This is a single-center retrospective analysis. Inclusion criteria were age ≥ 18 years at first diagnosis, histology of ODG, AODG, oligoastrocytoma (OA), or anaplastic OA (AOA), with the presence of LOH1p19q and IDH mutation at first diagnosis or disease progression with at least one available follow-up (FU). The diagnostic criteria are in agreement with the 4th edition update and the 5th edition of the WHO classification of CNS tumors [1], [12].
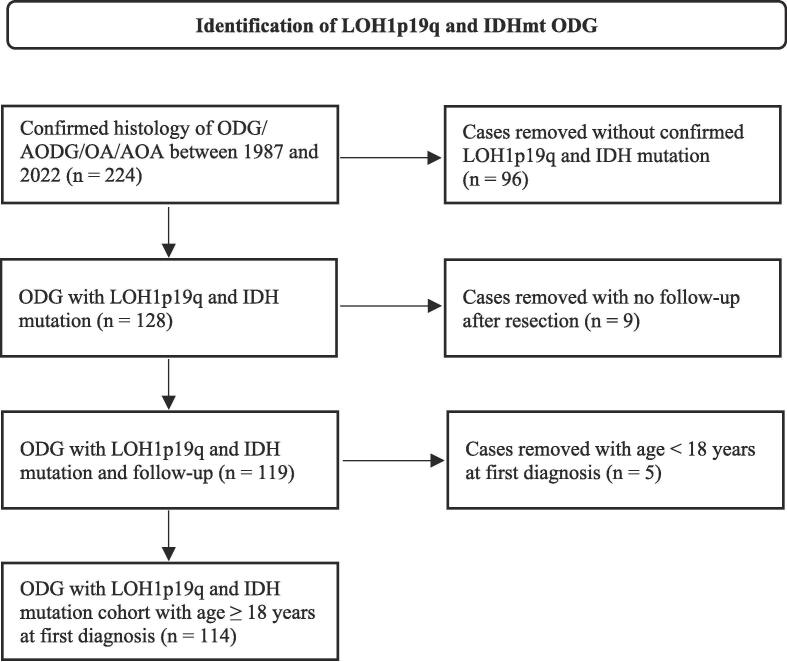
Patients treated between 1987 and 2022 were screened (Fig. 1). We obtained information including sex, age, histopathology, molecular and genetic characteristics, primary treatment, disease progression, last FU, Karnofsky performance status (KPS), and date of death. KPS was obtained after the first surgery.
Fig. 1.
Flowchart of cohort screening.
The following characteristics were analyzed for their influence on OS and PFS within the subgroups of WHO grade 2 and WHO grade 3 ODG: Age (<40 years vs. ≥40 years), EOR (gross total resection (GTR) vs. subtotal resection (STR) vs. biopsy), treatment regimen (RT vs. CT vs. RCT vs. WW), KPS after the first surgery (<90% vs. ≥90%), and neurological symptoms at first diagnosis (yes vs. no). Herein, RCT includes all patients receiving either concurrent or sequential CT with RT, RT with sequential CT, and concomitant CT with RT. For RT planning, patients underwent computed tomography imaging which was fused with pre- and postoperative contrasted-enhanced MRI whenever available. The gross tumor volume regularly included the resection cavity, contrast-enhancing tumor remnants and respective T2 signal alterations. The clinical target volume was created with a 1.5 to 2 cm isotropic margin, adjusted for anatomical barriers. OS was defined as the time from the date of first surgery to death or last FU and PFS as the time from first surgery to first progression or death or last FU. Patients were censored if no event (progression or death) occurred. The radiographic and clinical FU was defined as the time from first surgery to last available imaging and last clinical visit, respectively. Patients with grade 2 ODG underwent regular FU with MRI every six to twelve months. In case of grade 3 tumors, FU intervals were every three to six months. After two to three years, intervals were prolonged when there was no evidence of recurrence. Assessment of tumor progression was done according to the updated response assessment criteria for high-grade gliomas by the Response Assessment in Neuro-Oncology working group [13]. PFS and OS times were calculated using the Kaplan-Meier estimator. Statistical analysis was performed using IBM SPSS Statistics, version 27.0 (IBM, Armonk, NY, USA) and STATA MP 17.0 (StataCorp, College Station, TX, USA). A p-value ≤ 0.05 was considered significant. Univariable analysis (UVA) and multivariable analysis (MVA) were performed using the Cox proportional hazards model. The proportional hazards assumption was tested with scaled Schoenfeld residuals. This work was approved by the local institutional review board.
3. Results
Two hundred twenty-four patients were identified. After applying the inclusion and exclusion criteria, 114 eligible patients were included (Fig. 1).
Patient characteristics included in this study are shown in Table 1. The median age at first diagnosis was 41.5 years, and our cohort consisted of 57 men and 57 women. The vast majority of cases (n = 112, 98.2%) were treated between 2000 and 2021. Moreover, 89 patients (78.0%) received their primary therapy between 2010 and 2021. The median clinical and radiographic FU periods were 68.6 months and 69.8 months, respectively. For patients without a tumor progression or observed death, median clinical and radiographic FU times were 42.4 months and 42.0 months.
Table 1.
Patient characteristics.
| Variables | Number of patients, median (min–max) | % |
|---|---|---|
| Total number of patients | 114 | 100.0 |
| Sex | ||
| Male | 57 | 50.0 |
| Female | 57 | 50.0 |
| Age (at first resection) | 41.5 (19–72) | |
| <40 | 48 | 42.1 |
| ≥40 | 66 | 57.9 |
| KPS (%) | 90 (50–100) | |
| <90 | 13 | 11.4 |
| ≥90 | 74 | 64.9 |
| Unknown | 27 | 23.7 |
| IDH mutation (at first resection or progression) | ||
| Mutant | 114 | 100.0 |
| 1p/19q-codeletion (at first resection or progression) | ||
| Yes | 114 | 100.0 |
| MGMT promoter (at first resection or progression) | ||
| Methylated | 47 | 41.2 |
| Unmethylated | 2 | 1.8 |
| Unknown | 65 | 57.0 |
| Resection | ||
| Biopsy | 14 | 12.3 |
| STR | 40 | 35.1 |
| GTR | 47 | 41.2 |
| No surgery | 0 | 0.0 |
| Unknown extent of resection | 13 | 11.4 |
| Grading | ||
| WHO 2 | 60 | 52.6 |
| WHO 3 | 54 | 47.4 |
| Treatment after initial surgery | ||
| WW | 40 | 35.1 |
| RT | 6 | 5.3 |
| RCT | 50 | 43.9 |
| CT | 18 | 15.7 |
| PTV (cm3) | 241.03 (63.63–577.40) | |
| <241 | 17 | 30.4 |
| ≥241 | 18 | 32.1 |
| Unknown | 21 | 37.5 |
Abbreviations: min, minimum; max, maximum; KPS, Karnofsky performance status; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA-methyltransferase; STR, subtotal resection; GTR, gross total resection; WHO, World Health Organization; WW, watch and wait; RT, radiotherapy; RCT, radiochemotherapy; CT, chemotherapy; PTV, planning target volume.
Most patients underwent a combined or sequential RCT as their primary treatment after initial resection (n = 50, 43.9%), followed by a WW strategy (n = 40, 35.1%), CT monotherapy (n = 18, 15.7%), and RT alone (n = 6, 5.3%). Forty-three patients received RT with sequential CT, six patients had RT with concurrent and sequential CT, and one patient RCT, i.e., concomitant CT while undergoing RT. The mean and median total doses were 58.9 and 59.4 Gray (Gy), respectively (total range: 54.0–67.5 Gy). Sixty percent of patients who underwent RT received a total dose ≥ 59.4 Gy. The most common fractionation schemes were 30 × 2 Gy (n = 11, 19.6%), 30 × 1.8 Gy (n = 10, 17.8%), 33 × 1.8 Gy (n = 8, 14.2%), and 37 × 1.6 Gy twice daily (n = 6, 10.7%). Total dose prescriptions were higher for grade 3 tumors (≥75% with a minimum of 59.4 Gy). The majority of patients (89%) received RT between 2009 and 2021. For concomitant CT, TMZ was the only administered drug. For adjuvant CT, most of the patients received TMZ (n = 30, 60.0%), followed by procarbazine and CCNU (PC) (n = 15, 30.0%) and PCV (n = 4, 8.0%). For CT monotherapy, TMZ was also the predominantly administered drug (n = 14, 77.8%). The median number of administered TMZ, PC, PCV cycles were 12, 6, and 4.5, respectively.
When postoperative therapy at initial diagnosis was analyzed by grading, the following treatments were chosen for grade 2 tumors: Thirty-six patients (60.0%) underwent a WW strategy, three patients received RT (5.0%), seven patients CT (11.7%), and 14 patients RCT (23.3%). Thirty-six patients with grade 3 tumors received RCT (66.7%), followed by eleven patients with CT (20.4%) and three patients with RT (5.6%). Four (7.4%) patients with high-grade ODG underwent a WW strategy.
Sixty-two patients had at least one disease progression and all of them received salvage treatment. The progressing tumors consisted of 19 grade 2 (30.6%) and 43 grade 3 tumors (69.4%). In total, seventeen cases (27.4%) of upgrading were identified, i.e., tumors with an initial grade 2 showing grade 3 histopathology at recurrence.
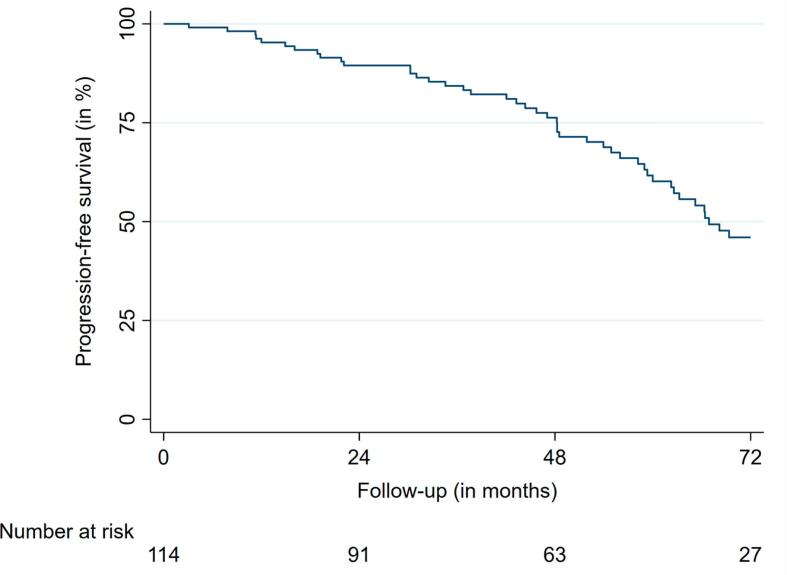
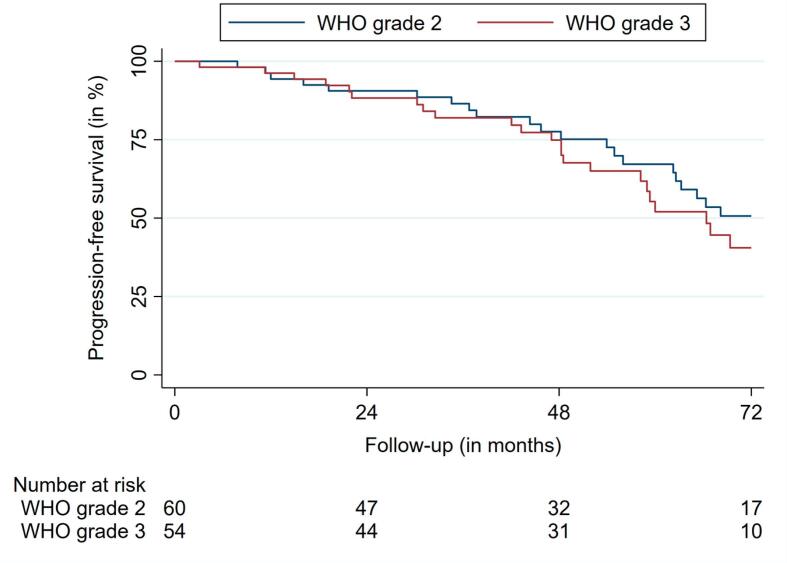
Median PFS was 66.9 months (95% confidence interval (CI): 56.0 – 77.7) for the entire cohort. The 2-, 4- and 6-year PFS rates were 89.5%, 76.3%, and 46.0%, respectively (Fig. 2a, Fig. 2b).
Fig. 2a.
Progression-free survival.
Fig. 2b.
Progression-free survival according to WHO grading.
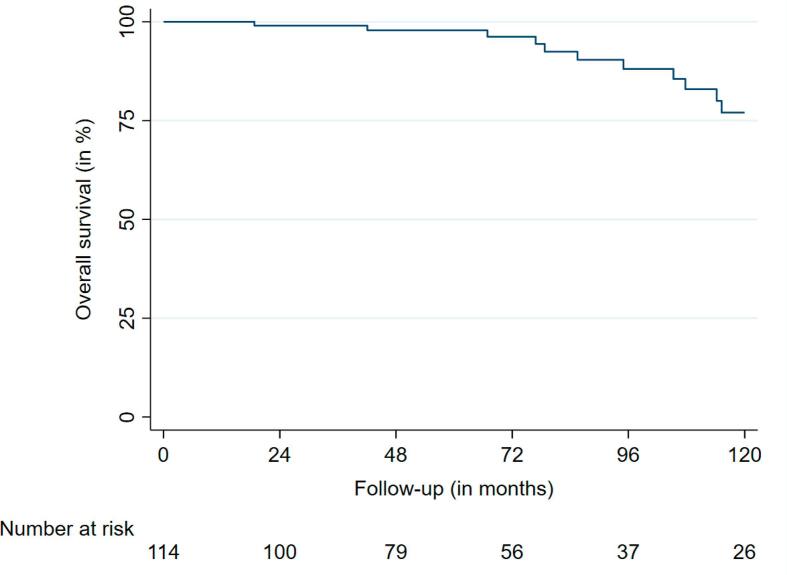
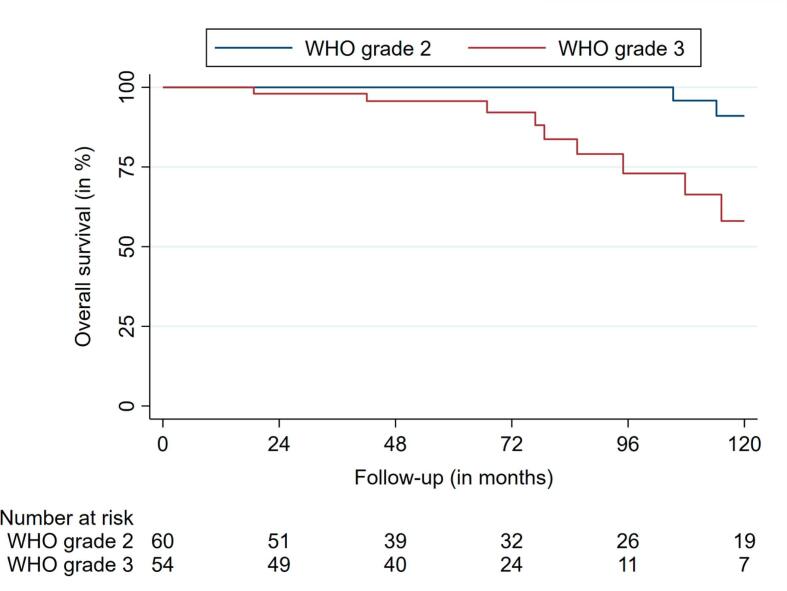
The median OS was 236.0 months (95% CI: 228.3 – 243.7). Nineteen deaths were observed during FU. The 2-, 4- and 6-year OS rates were 99.0%, 97.9%, and 96.2%, respectively (Fig. 3a, Fig. 3b).
Fig. 3a.
Overall survival.
Fig. 3b.
Overall survival according to WHO grading.
UVA of PFS for WHO grade 2 tumors did not reveal any significant factors (Table 2). In the UVA of PFS for WHO grade 3 tumors, GTR was significantly associated with a longer PFS (p = 0.03, hazard ratio (HR) 0.17) (Table 3).
Table 2.
UVA and MVA for PFS, WHO grade 2.
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age | ||||||
| <40 | Reference | – | Reference | – | ||
| ≥40 | 1.16 | 0.59–2.27 | 0.65 | 0.75 | 0.20–2.79 | 0.67 |
| EOR | ||||||
| Biopsy | Reference | – | Reference | – | ||
| STR | 0.94 | 0.35–2.52 | 0.90 | 0.02 | 0.001–0.67 | 0.02 |
| GTR | 1.67 | 0.58–4.76 | 0.33 | 0.01 | 0.00–0.36 | 0.01 |
| Neurological symptoms preop. | ||||||
| No | Reference | – | Reference | – | ||
| Yes | 0.45 | 0.14–1.43 | 0.17 | 0.33 | 0.05–1.93 | 0.22 |
| Initial treatment | ||||||
| WW | Reference | – | Reference | – | ||
| RT | 0.78 | 0.10–5.85 | 0.81 | <0.01 | 0.00–0.33 | 0.01 |
| CT | 0.40 | 0.12–1.35 | 0.14 | <0.01 | 0.00–0.41 | 0.01 |
| RCT | 0.95 | 0.27–3.34 | 0.93 | 0.14 | 0.01–1.12 | 0.06 |
| KPS after first surgery | ||||||
| <90 | Reference | – | Reference | – | ||
| ≥90 | 0.38 | 0.13–1.06 | 0.06 | 0.31 | 0.06–1.49 | 0.14 |
Abbreviations: CI, confidence interval; EOR, extent of resection; STR, subtotal resection; GTR, gross total resection; preop., preoperative; WW, watch and wait; RT, radiotherapy; CT, chemotherapy; RCT, radiochemotherapy; KPS, Karnofsky performance status.
Table 3.
UVA and MVA for PFS, WHO grade 3.
| Variable | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age (in years) | ||||||
| <40 | Reference | – | Reference | – | ||
| ≥40 | 0.69 | 0.32–1.50 | 0.35 | 1.81 | 0.50–6.53 | 0.36 |
| EOR | ||||||
| Biopsy | Reference | – | Reference | – | ||
| STR | 0.23 | 0.04–1.12 | 0.06 | 0.35 | 0.03–4.21 | 0.41 |
| GTR | 0.17 | 0.03–0.84 | 0.03 | 0.87 | 0.06–11.10 | 0.91 |
| Neurological symptoms preop. | ||||||
| No | Reference | – | Reference | – | ||
| Yes | 1.35 | 0.39–4.69 | 0.63 | 0.65 | 0.15–2.77 | 0.56 |
| Initial treatment | ||||||
| WW | Reference | – | Reference | – | ||
| RT | 1.32 | 0.21–8.12 | 0.76 | 0.53 | 0.06–4.21 | 0.55 |
| CT | 2.39 | 0.65–8.78 | 0.18 | 3.02 | 0.37–24.17 | 0.29 |
| RCT | 0.60 | 0.16–2.21 | 0.44 | 0.09 | 0.01–0.69 | 0.02 |
| KPS after first surgery | ||||||
| <90 | Reference | – | Reference | – | ||
| ≥90 | 2.33 | 0.28–18.88 | 0.42 | >5.00 | 0.00 - * | 0.99 |
*Upper limit of the 95% CI was not determined. Abbreviations: CI, confidence interval; EOR, extent of resection; STR, subtotal resection; GTR, gross total resection; preop., preoperative; WW, watch and wait; RT, radiotherapy; CT, chemotherapy; RCT, radiochemotherapy; KPS, Karnofsky performance status.
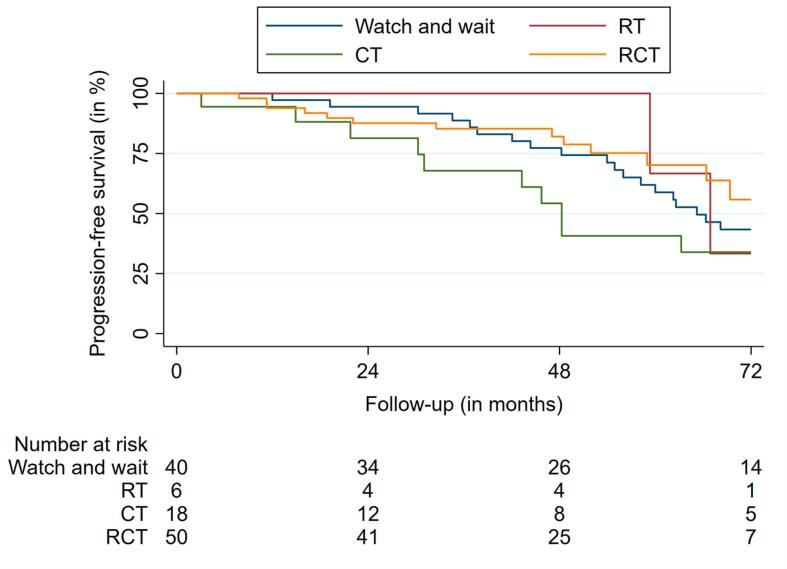
In MVA for WHO grade 2 ODG, GTR, and STR led to prolonged PFS (p = 0.01, HR 0.01; p = 0.02, HR 0.02). RT and CT were also associated with longer PFS (p = 0.01, HR 0.01; p = 0.01, HR 0.01) (Table 2). RCT did not show a benefit (p = 0.06, HR 0.14). For grade 3 tumors, RCT led to prolonged PFS (p = 0.02, HR 0.09) (Table 3). UVA and MVA for OS did not reveal any results for both tumor grades due to the small number of events (n = 19) observed. PFS outcomes according to treatment group are shown in Fig. 4.
Fig. 4.
Progression-free survival according to treatment group.
4. Discussion
This study examined the influence of various patient and tumor characteristics on PFS and OS in a cohort of verified 1p/19q-codeleted and IDHmt ODG. The MVA for WHO grade 2 tumors suggests a prolonged PFS after GTR or STR. Moreover, RT and CT prolonged PFS in comparison to WW, while RCT did not formally decrease the risk of progression (p = 0.06, HR 0.14). For WHO 3 ODG, the MVA suggests a benefit of RCT. UVA and MVA for OS did not reveal any significant risk factors regardless of tumor grading, which is not surprising given the low number of events. Several points of our work have to be addressed in light of the observed results.
First, we decided to perform separate MVA analyses depending on the WHO grading as treatment choices after surgery were based on tumor grading. Therefore, confounding of the MVA of the overall cohort (especially in relation to adjuvant therapies) could not be ruled out. Subsequently, the lack of a statistically significant effect by RCT in grade 2 tumors may be caused by the low number of patients that received this treatment (n = 14) and respective events, i.e., small sample size. Grade 3 tumors are characterized by a worse outcome [14], [15]. The European Association of Neuro-Oncology guidelines define grade 3 histology as an unfavorable prognostic factor with a differing treatment recommendation [4]. This is in agreement with the clinical practice guideline of the American Society for Radiation Oncology [9]. There is no recommendation for a potential WW strategy. Instead, RT with sequential PCV is recommended as the preferred therapy [4].
Grade 3 tumor patients in our cohort received more aggressive treatments. Nevertheless, we did not clearly identify a worse PFS with grade 3 tumors, but this interpretation must be done with caution given the differing treatment regimens, sample size, and underlying patient characteristics. The grading of ODG and its impact on outcomes remains a subject of ongoing debates [14], [15], [16], [17].
Moreover, PFS herein was neither influenced by the patients’ age or presence of preoperative neurological symptoms nor by KPS, which was unexpected, as all these factors besides KPS are considered to be relevant prognostic factors [4]. To calculate the influence of age on patients’ outcomes, we have used the cutoff age of 40 years [4]. Our findings, however, do not clearly support the impact of this cutoff for PFS.
For the calculations of the influence of KPS, we used the median KPS of our cohort (90%). Most of our patients barely had physical limitations before surgery and, therefore, we were not able to detect any influence of KPS on patient outcomes. Other authors were able to show a connection between a lower KPS and OS [6]. However, one must note the underlying heterogeneity of the analyzed patient cohorts described by other authors.
A profound analysis for OS was not possible due to the small number of events. A long FU period is necessary to retrieve reliable results for PFS and OS in ODG, as the RTOG 9402, RTOG 9802, and EORTC 26951 trials demonstrated [6], [7], [18]. Thus, our obtained results have to be carefully compared to the outcomes of these trials. We noted that patients with LOH1p19q and IDHmt WHO grade 3 tumors seem to benefit from a combined RCT. This beneficial effect was not formally detected in grade 2 tumors. Still, chances are high that with a larger sample size and more events as well as less missing information, the effect could become significant. Our observed findings are supported by the results of other studies. For example, the RTOG 9402 and EORTC 26951 trials also showed that patients with AODG benefit from a RT with neoadjuvant and sequential PCV regarding PFS [6], [18].
The 2-, 4-, and 6-year PFS rates for patients treated with RCT herein were 87.6%, 82.0%, and 55.8%, respectively. RTOG 9802, RTOG 9402, and EORTC 26951 were not able to base their statistical calculations on purely verified LOH1p19q and IDHmt ODG at first. Within the RTOG 9802 trial, which analyzed high-risk grade 2 gliomas, there were 37 patients (35.0%) with LOH1p19q and IDH mutation. Nineteen of them received RT and eighteen RT with adjuvant PCV. The 2-, 4-, and 6-year PFS rates for the verified LOH1p19q and IDHmt group were approximately 92%, 84%, and 68%, and 95%, 95%, and 89%, respectively, for patients who received RCT [7]. The RTOG 9402 trial, which investigated AODG (grade 3) just like the EORTC 26951, was able to identify 58 patients (20.0%) with LOH1p19q in the RCT arm and 67 patients (23.1%) with LOH1p19q in the RT arm in their final report. The 2-, 4-, and 6-year PFS rates for the verified LOH1p19q group treated with RCT were approximately 81%, 65%, and 60% [18]. In the EORTC 26951, 43 patients (11.6%) with LOH1p19q received adjuvant RCT, showing 2-, 4-, and 6-year PFS rates of approximately 81%, 71%, and 61%, respectively [18]. Not purely verified cohorts within the other trials and the small number of patients within each treatment arm, as well as the retrospective nature of our work, could explain the differences in PFS between our work and the trials mentioned above. Nevertheless, our results are fairly comparable to the data of these trials. Our study provides further evidence on how verified LOH1p19q and IDHmt ODG respond to different treatment regimens, especially concerning the benefit that RCT may provide. This is noteworthy given the considerable number of cases treated with TMZ instead of PCV in our cohort.
Nonetheless, our study has several limitations. First, the retrospective nature weakens the data quality, i.e., missing information, which is especially important regarding the MVA. Moreover, KPS was not documented for several patients, and the proportion of patients with a favorable performance status was relatively high in our cohort. In addition, an underlying sampling bias given the study type of a retrospective cohort study cannot be excluded.
Furthermore, there was a variety of postoperative treatment regimens. According to the current recommendations, patients with less favorable prognostic characteristics should receive RCT with PCV [4], [5], [6]. However, there was only a small number of patients in our cohort treated with PCV (n = 4). This might be explained by the presumably higher toxicity of PCV or the necessity of intravenous application of vincristine which may impose a logistical burden for patients and physicians [19]. In our cohort, there were more patients treated with TMZ, demonstrating solid OS and PFS outcomes. In this regard, the results of the modified CODEL trial are eagerly awaited [10]. Moreover, the ongoing POLCA trial, comparing PCV alone with RT and sequential PCV, will also determine whether RT can be safely deferred with the sole use of the system therapy standard of care (NCT02444000). The field of targeted therapies may hold the potential to improve outcomes, with ongoing trials and research in IDHmt tumors utilizing IDH and glutaminase inhibitors, demethylation agents, poly-ADP-ribose-polymerase (PARP) inhibitors, and vaccines [20]. Overall, various questions concerning the ideal management of ODG remain unanswered and will be the subject of future molecular and clinical research [21].
Finally, further limitations of this work have to be considered when interpreting the presented results. First, molecular information was missing at the time of the initial diagnosis in some cases. This is due to molecular profiling not being part of the clinical routine until a few years ago. When available, nearly all tumors showed MGMT promoter methylation (95.9%). However, this finding is expected as nearly all ODG show such methylation. Thus, dedicated testing for MGMT promoter methylation status is generally not recommended [22]. The molecular analysis of cyclin-dependent kinase inhibitor 2A/B, whose homozygous deletion is associated with worse outcomes, is missing entirely, but its deletions are also relatively rare in this tumor entity [23]. Furthermore, missing data were also an issue concerning treatment toxicity and the use and tapering of corticosteroids and anticonvulsant medication. Finally, not all patients with RCT received their CT sequentially, which may account for undetected variations in outcomes due to the effect heterogeneity concerning cases with RT and concurrent CT and patients receiving concomitant and sequential CT with their RT.
5. Conclusion
Our data suggest a beneficial role of RCT in prolonging PFS in a homogeneous cohort of patients with LOH1p19q and IDHmt WHO grade 3 ODG. Most RCT patients received TMZ instead of PCV as their systemic therapy. Besides, surgical resection, RT, and CT reduced the risk of progression in corresponding WHO grade 2 tumors, whereas RCT formally failed to show such an effect. These findings generally correspond to current treatment guidelines for managing oligodendroglial tumors and the long-term results of the RTOG 9402, RTOG 9802, and EORTC 26951 trials. Further prospective research in homogeneous tumor cohorts is needed to refine treatment algorithms and resolve ongoing controversies, e.g., the use of TMZ instead of PCV.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Felix Ehret is a participant in the BIH Charité Junior Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and Berlin Institute of Health (BIH).
References
- 1.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Wick W., Hartmann C., Engel C., Stoffels M., Felsberg J., Stockhammer F., et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 3.Wick W., et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016;18(11):1529–1537. doi: 10.1093/neuonc/now133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. doi: 10.1038/s41571-020-00447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Bent M.J., Brandes A.A., Taphoorn M.J.B., Kros J.M., Kouwenhoven M.C.M., Delattre J.-Y., et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 6.Cairncross G., Wang M., Shaw E., Jenkins R., Brachman D., Buckner J., et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell E.H., Zhang P., Shaw E.G., Buckner J.C., Barger G.R., Bullard D.E., et al. Comprehensive Genomic Analysis in NRG Oncology/RTOG 9802: A Phase III Trial of Radiation Versus Radiation Plus Procarbazine, Lomustine (CCNU), and Vincristine in High-Risk Low-Grade Glioma. J Clin Oncol. 2020;38(29):3407–3417. doi: 10.1200/JCO.19.02983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassman A.B., Iwamoto F.M., Cloughesy T.F., Aldape K.D., Rivera A.L., Eichler A.F., et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol. 2011;13(6):649–659. doi: 10.1093/neuonc/nor040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halasz L.M., Attia A., Bradfield L., Brat D.J., Kirkpatrick J.P., Laack N.N., et al. Radiation Therapy for IDH-Mutant Grade 2 and Grade 3 Diffuse Glioma: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2022;12(5):370–386. doi: 10.1016/j.prro.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Jaeckle K.A., Ballman K.V., van den Bent M., Giannini C., Galanis E., Brown P.D., et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 2021;23(3):457–467. doi: 10.1093/neuonc/noaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner J.C., Shaw E.G., Pugh S.L., Chakravarti A., Gilbert M.R., Barger G.R., et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374(14):1344–1355. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi E., Tosoni A., Bartolini S., Minichillo S., Mura A., Asioli S., et al. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur J Cancer. 2020;137:10–17. doi: 10.1016/j.ejca.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Cimino P.J., Zager M., McFerrin L., Wirsching H.-G., Bolouri H., Hentschel B., et al. Multidimensional scaling of diffuse gliomas: application to the 2016 World Health Organization classification system with prognostically relevant molecular subtype discovery. Acta Neuropathol Commun. 2017;5(1) doi: 10.1186/s40478-017-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olar A., Wani K.M., Alfaro-Munoz K.D., Heathcock L.E., van Thuijl H.F., Gilbert M.R., et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129(4):585–596. doi: 10.1007/s00401-015-1398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki H., Aoki K., Chiba K., Sato Y., Shiozawa Y., Shiraishi Y., et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458–468. doi: 10.1038/ng.3273. [DOI] [PubMed] [Google Scholar]
- 18.Lassman A.B., Hoang-Xuan K., Polley M.-Y., Brandes A.A., Cairncross J.G., Kros J.M., et al. Joint Final Report of EORTC 26951 and RTOG 9402: Phase III Trials With Procarbazine, Lomustine, and Vincristine Chemotherapy for Anaplastic Oligodendroglial Tumors. J Clin Oncol. 2022;40(23):2539–2545. doi: 10.1200/JCO.21.02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruff M.W., Buckner J.C., Johnson D.R., van den Bent M.J., Geurts M. Neuro-Oncology Clinical Debate: PCV or temozolomide in combination with radiation for newly diagnosed high-grade oligodendroglioma. Neurooncol Pract. 2019;6(1):17–21. doi: 10.1093/nop/npy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J.J. Targeting IDH-Mutant Glioma. Neurotherapeutics. 2022;19(6):1724–1732. doi: 10.1007/s13311-022-01238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penas-Prado M., Armstrong T.S., Gilbert M.R. Progress in rare central nervous system tumors. Curr Opin Neurol. 2019;32(6):895–906. doi: 10.1097/WCO.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 22.Brat D.J., Aldape K., Bridge J.A., Canoll P., Colman H., Hameed M.R., et al. Molecular Biomarker Testing for the Diagnosis of Diffuse Gliomas. Arch Pathol Lab Med. 2022;146(5):547–574. doi: 10.5858/arpa.2021-0295-CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appay R., Dehais C., Maurage C.-A., Alentorn A., Carpentier C., Colin C., et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019 doi: 10.1093/neuonc/noz124. [DOI] [PMC free article] [PubMed] [Google Scholar]