Abstract
The extreme acidothermophilic archaeon Sulfolobus solfataricus harbors a membrane-associated protein kinase activity. Its solubilization and stabilization required detergents, suggesting that this activity resides within an integral membrane protein. The archaeal protein kinase utilized purine nucleotides as phosphoryl donors in vitro. A noticeable preference for nucleotide triphosphates over nucleotide diphosphates and for adenyl nucleotides over the corresponding guanyl ones was observed. The molecular mass of the solubilized, partially purified enzyme was estimated to be ≈125 kDa by gel filtration chromatography. Catalytic activity resided in a polypeptide with an apparent molecular mass of ≈67 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Challenges with several exogenous substrates revealed the protein kinase to be relatively selective. Only casein, histone H4, reduced carboxyamidomethylated and maleylated lysozyme, and a peptide modeled after myosin light chains (KKRAARATSNVFA) were phosphorylated to appreciable levels in vitro. All of the aforementioned substrates were phosphorylated on threonine residues, while histone H4 was phosphorylated on serine as well. Substitution of serine for the phosphoacceptor threonine in the myosin light chain peptide produced a noticeably inferior substrate. The protein kinase underwent autophosphorylation on threonine and was relatively insensitive to a set of known inhibitors of “eukaryotic” protein kinases.
For over 3 decades following the discovery of protein phosphorylation-dephosphorylation events, a clear dichotomy between those that occur in eukaryotes and those that occur in prokaryotes was perceived. Initially, it was believed that only eukaryotes had evolved the ability to transduce signals and regulate protein function via this covalent modification process. In the late 1970s the universality of this protein phosphorylation-dephosphorylation became apparent. However, the disparity in sequence between the characteristic protein kinases from eukaryotes, i.e., the homologs of the cyclic AMP (cAMP)-dependent protein kinase, and those from prokaryotes, i.e., the histidine kinases and the isocitrate dehydrogenase kinase/phosphatase, suggested that the members of each phylogenetic domain evolved unique molecular archetypes to carry out this process.
The past several years have witnessed the demolition of this dichotomy. The “bacterial” two-component regulatory system, for example, has been discovered in several eukaryotes, while “eukaryotic” protein kinases in bacterial organisms have been identified and characterized and a number of eukaryotic protein phosphatases from members of both the Archaea and the Bacteria have been identified and characterized (reviewed in reference 19). Adding to the foment has been the recent discovery of several distinct new protein kinases, e.g., the HPr protein kinase from Bacillus subtilis (10, 28), the Rsb/Spo family protein kinases of bacteria (8, 25, 32, 43), and the myosin heavy chain kinase/eIF-2 protein kinase family in eukaryotes (5, 31). These observations demand the revision of long-standing views concerning the origins and evolution of protein phosphorylation-dephosphorylation. Knowledge of the structure and properties of the protein kinases resident in the members of the Archaea represents a key piece of this puzzle.
It has been observed that the halophilic archaeon Halobium salinarium contains a two-component histidine kinase that functions in chemo- and phototaxis (30), and the genome sequences of at least two other archaeons, Archaeoglobus fulgidus (20) and Pyrococcus horikoshii OT3 (16), contain open reading frames (ORFs) whose predicted products display homology to the histidine kinases and response regulators of the two-component signaling paradigm. It is clear that archaeal proteins also become phosphorylated on serine, threonine, and tyrosine residues (34, 35, 37, 38, 40, 41). However, little hard data concerning the enzymes responsible exist (34, 35). In this paper we describe the physical and functional properties of an archaeal protein-serine/threonine kinase from the extreme acidophilic archaeon Sulfolobus solfataricus.
MATERIALS AND METHODS
Materials.
Purchased materials included S. solfataricus (American Type Culture Collection [ATCC] accession no. 35091) from the ATCC (Manassas, Va.). [γ-32P]ATP and [γ-32P]GTP were from NEN Research Products (Boston, Mass.). [β-32P]GDP was from ICN (Costa Mesa, Calif.). Protein assay dye reagent was from Bio-Rad (Hercules, Calif.). DE-52 cellulose was from Whatman (Hillsboro, Oreg.). Partially hydrolyzed casein, DNase I, and nucleotide-5′-mono-, -di-, and -triphosphates were from Sigma Chemical Company (St. Louis, Mo.). Trichloroacetic acid (10% [wt/vol]) was from LabChem Inc. (Pittsburgh, Pa.). Myosin light chain (MLC) peptide (KKRAARATSNVFA) was prepared as described by Kennelly et al. (17). The T8S peptide (KKRAARASSNVFA) was synthesized by Genosys (The Woodlands, Tex.). Mono-P HR5/20 and Polybuffer 74 were from Pharmacia (Uppsala, Sweden). Immobilon P was from Millipore (Bedford, Mass.). Immobiline dry strips were from Pharmacia Biotech (Piscataway, N.J.). Reduced carboxyamidomethylated and maleylated (RCM) lysozyme was prepared as described by Tonks et al. (42). Unless otherwise specified, all other materials were from Fisher (Pittsburgh, Pa.) or Sigma Chemical Company.
Growth of organism.
S. solfataricus was grown in continuous culture with vigorous aeration at 70°C in de Rosa's standard medium (6) with the level of yeast extract increased to 2 g/liter. Cells were harvested by first concentrating the culture using a Pellicon cassette system membrane concentrator (Amicon, Bedford, Mass.) and then centrifuging the concentrate for 20 min at 12,000 × g. The cell pellet was stored frozen at −20°C until needed.
Routine procedures.
Protein concentrations were determined as described by Bradford (2) using premixed reagent and a standardized solution of bovine serum albumin (BSA). Sodium dodecyl sulfate (SDS)-polyacrylamide gels were produced as described by Laemmli (21) and stained with Coomassie brilliant blue as described by Fairbanks et al. (9). Two-dimensional electrophoresis was performed essentially as described by Gorg et al. (11) using Immobiline dry strips for the first dimension. Electronic autoradiography was performed using a Packard (Meriden, Conn.) Instantimager.
Assay of protein kinase activity.
Protein kinase activity was routinely assayed in solution by the filter paper method. Briefly, samples containing the S. solfataricus protein kinase were incubated at 25°C in a volume of 100 μl of 20 mM MES (morpholineethanesulfonic acid), pH 6.5, containing 50 μM ATP (300 μCi of [γ-32P]ATP/ml), 5 mM MnCl2, 2 mM dithiothreitol (DTT), either 0.1% (vol/vol) Triton X-100 or 12.5 mM octyl glucoside, and a phosphoacceptor substrate such as casein, 0.8 mg/ml unless otherwise indicated, or MLC peptide, 0.25 mM unless otherwise indicated. Reactions were initiated by addition of ATP. Following incubation at 25°C for periods up to 60 min, reactions were terminated by one of two methods. When a protein or amino acid copolymer (e.g., poly[Glu-Tyr]) was employed as the phosphoacceptor substrate, a 30-μl portion of each reaction mixture was spotted onto a 2- by 2-cm square of Whatman 3MM paper and immediately washed in 10% (wt/vol) trichloroacetic acid (TCA) containing 4% (wt/vol) sodium pyrophosphate for 20 min (4). Following four subsequent washes, 20 min each, in 5% (wt/vol) TCA containing 2% (wt/vol) sodium pyrophosphate, the squares were dried and the quantity of [32P]phosphate immobilized on the filter paper was determined by liquid scintillation counting in 1 ml of ScintiSafe Plus 50% (Fisher) using a Beckman model LS 5801 liquid scintillation counter. For peptide substrates, a similar procedure was followed with the following modifications: Whatman 3MM paper was replaced with Whatman P81 phosphocellulose paper and 150 mM H3PO4 was substituted for the TCA-PPi solutions (29).
In gel assays of protein kinase activity, nucleotide specificity, and autophosphorylation.
The protein kinase activities of individual polypeptides were assayed in gel following their resolution by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) or two-dimensional (isoelectric focusing [IEF] followed by SDS-PAGE) electrophoresis as described previously (1). Briefly, the running gel of the SDS-polyacrylamide gel was prepared by standard procedures with the exception that casein, 0.5 mg/ml, was added prior to initiation of polymerization. Following electrophoresis, SDS was removed from the gel by washing with 20% (vol/vol) isopropanol. Proteins then were randomized by soaking the gel in 6 M guanidine hydrochloride and then allowed to renature by incubation with several changes of 20 mM MES, pH 6.5, containing 0.1% (vol/vol) Triton X-100, 1 mM DTT, and 5 mM (each) MnCl2 and MgCl2 to remove the guanidine hydrochloride. The gels were then incubated for 1 h at 25°C in 20 mM MES, pH 6.5, containing 0.1% (vol/vol) Triton X-100, 1 mM DTT, and 5 mM (each) MnCl2 and MgCl2, to which 50 μM ATP (15 μCi of [γ-32P]ATP/ml) had been added. The gels were washed extensively in 2% (wt/vol) sodium pyrophosphate to remove excess ATP and dried, and the locations of regions in which phosphorylation of immobilized casein took place were determined by autoradiography with X-ray film and/or electronic autoradiography. The quantity of 32P transferred was quantified by electronic autoradiography. In early experiments, control gels contained 0.5 mg of BSA/ml in place of casein. Nucleotide specificity was determined by preincubation with cold nucleotides as described previously (1). Autophosphorylation of polypeptides in gel used the same procedure as that described above with the exception that no exogenous phosphoacceptor protein was copolymerized into the SDS-PAGE running gel and incubation with [γ-32P]ATP was performed at 65°C for a period of 60 min.
Solubilization and partial purification of S. solfataricus protein kinase.
Frozen S. solfataricus, 20 g wet weight, was thawed and resuspended in 2 volumes of 20 mM MES, pH 6.5, containing 0.5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 10 μg of DNase I/ml. The cells were lysed by two passages through a French pressure cell at 12,000 lb/in2. The lysate was centrifuged at 1,000 × g for 10 min at 4°C to remove debris and any remaining whole cells. The membrane fraction was then separated from the soluble fraction by centrifugation at 100,000 × g for 75 min at 4°C. The membrane fraction was washed, and integral membrane proteins were solubilized by a modification of the procedure used by Ramwani and Mishra (27). First, the membrane fraction was resuspended in 40 ml of 0.5 M NaCl in 20 mM sodium acetate, pH 5.0, to release peripherally associated proteins. The particulate material was collected by centrifugation at 100,000 × g for 60 min at 4°C. The supernatant liquid (peripheral fraction) was discarded. The pellet was then resuspended in 40 ml of 20 mM MES, pH 6.5, containing 125 mM NaCl and either 0.4% (vol/vol) Triton X-100 or 25 mM octyl glucoside. Use of the latter detergent was preferred. The mixture was then centrifuged at 100,000 × g for 75 min at 4°C, and the supernatant fluid was retained as the detergent extract.
The detergent extract was diluted fivefold by the addition of 4 volumes of 20 mM MES, pH 6.5, containing 0.5 mM EDTA and applied to a 1.5- by 18-cm column of DE-52 cellulose that had been equilibrated in 20 mM MES, pH 6.5, containing 25 mM NaCl and either 0.1% (vol/vol) Triton X-100 or 12.5 mM octyl glucoside. The column was washed with 3 column volumes of the equilibration buffer and then developed with a linear gradient, 150 ml total, of 25 to 500 mM NaCl in equilibration buffer. Fractions, 3 ml, were collected and assayed for protein kinase activity. Fractions corresponding to the major peak of protein kinase activity, which eluted at an NaCl concentration of ≈200 mM, were pooled and retained as the DE-52 fraction.
The DE-52 fraction was dialyzed against 25 mM piperazine, pH 5.5, containing 0.1% (vol/vol) Triton X-100 and applied to a Mono-P HR5/20 column that had been equilibrated in dialysis buffer using a Pharmacia fast protein liquid chromatography system. The column was developed isocratically at a flow rate of 0.5 ml/min with a solution consisting of a 1:8 dilution of Polybuffer 74, pH 4.0, containing 0.1% (vol/vol) Triton X-100. Fractions, 1 ml, were collected and assayed for protein kinase activity. Fractions corresponding to the major peak of protein kinase activity, which eluted at a pH of ≈4.5, were pooled and retained as the Mono-P fractions.
Phosphoamino acid analysis.
Phosphoamino acid analysis was performed essentially as described by Kamps and Sefton (15). Radiolabeled proteins were isolated by SDS-PAGE and transferred to Immobilon P. The portion of the membrane containing labeled product was incubated for 1 h in 6 N HCl at 95°C, and the supernatant fluid was concentrated in a Speed Vac. The hydrolysate was then applied to a 10- by 10-cm silica gel thin-layer chromatography plate, along with standards of phosphoserine, phosphothreonine, and phosphotyrosine. The plate was subjected to two-dimensional thin-layer electrophoresis. The pH of the buffer used for the first dimension was 1.9, and that of the buffer used for the second dimension was 3.5. Standards were visualized by ninhydrin staining, and the radiolabeled species were located by electronic autoradiography.
RESULTS
Detection of a membrane-associated protein kinase activity in S. solfataricus.
The presence of a protein kinase activity in the archaeon S. solfataricus was first detected when whole-cell lysates were subjected to SDS-PAGE in gels containing copolymerized casein. Following a renaturation-denaturation cycle employing guanidine hydrochloride, protein kinase activity was assayed in situ by incubating the gel with [γ-32P]ATP and the potential divalent metal ion cofactors Mn2+ and Mg2+. After the radiolabeled ATP was thoroughly washed away, autoradiography revealed the presence of a discrete area within the gel where [32P]phosphate had been retained, presumably the result of the covalent phosphorylation of those casein molecules immobilized within the gel matrix that were accessible to the protein kinase.
The soluble fraction and the detergent extract of the membrane fraction were tested using the in gel assay technique to further localize the putative protein kinase activity. In this experiment, a second SDS gel in which BSA was copolymerized into the running gel in place of casein was used to distinguish phosphotransfer to casein from other potential mechanisms of phosphate incorporation, such as autophosphorylation or the trapping of a phosphoenzyme intermediate. As can be seen in Fig. 1, a distinct band of casein phosphorylation was observed in the detergent extract of the membrane fraction, while little if any phosphorylation could be observed in the gel where BSA was substituted for casein. Little or no cytosolic protein kinase activity was evident. In this early experiment the band of activity comigrated just below the position of an 85-kDa standard. However, later experiments using more highly purified preparations indicated that the active polypeptide species possessed a molecular mass of ≈67 kDa. We hypothesize that the retarded electrophoretic migration observed in this early experiment reflected incomplete coating of the polypeptide with SDS as a consequence of the presence of the large quantities of Triton X-100 in the detergent extract.
FIG. 1.
Detection of protein kinase activity in the membrane fraction of S. solfataricus. Shown are the results of an in gel assay of S. solfataricus protein kinase activity following SDS-PAGE. Portions, containing 20 μg of total protein each, of the cytosolic fraction (C) and the detergent extract of the membrane fraction (M) from S. solfataricus were electrophoresed into an SDS–10% (wt/vol) polyacrylamide gel in which either BSA or casein had been copolymerized into the gel matrix at a concentration of 1 mg/ml. Following electrophoresis, the gel was washed free of SDS and the proteins within it were renatured using guanidine hydrochloride. The gels were each then incubated for 60 min in 30 ml of 20 mM MES, pH 6.5, containing 0.5 mM EDTA, 5 mM Mg2+, 5 mM Mn2+, and 50 μM [γ-32P]ATP (specific activity, 30 Ci/mol). The gels then were washed to remove free ATP, and regions of each gel in which [32P]phosphate was incorporated into protein were visualized by autoradiography using X-ray film. Shown are the autoradiograms of each gel. The positions and molecular masses (in kilodaltons) of the protein standards are indicated at the left.
The putative protein kinase activity in the detergent extract was next assayed using a conventional solution assay, once again using casein as a potential phosphoacceptor substrate. When the assay mixture was subjected to SDS-PAGE and the phosphorylated species were visualized by autoradiography, it was observed that [32P]phosphate indeed had been transferred to the casein. Subsequent phosphoamino acid analysis revealed that the phosphate was bound as phosphothreonine (Fig. 2), establishing that a covalent protein-phosphate bond had been formed and that the activity detected displayed the essential features of a protein kinase.
FIG. 2.
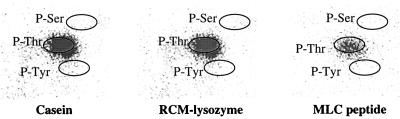
The S. solfataricus protein kinase exhibits a tendency to phosphorylate threonine residues in vitro. Several of the protein and peptide substrates phosphorylated by the S. solfataricus protein kinase were subjected to phosphoamino acid analysis to determine the type of amino acid modified. Shown are the electronic autoradiograms of the thin-layer plates from the analyses of casein, RCM lysozyme, and MLC peptide. The migration positions of ninhydrin-stained amino acid standards are circled. Phosphoamino acids were resolved by two-dimensional thin-layer electrophoresis by the procedure of Kamps and Sefton (15). The origin was in the lower left corner. First-dimension electrophoresis, at pH 1.9, proceeded from left to right, while second-dimension electrophoresis, at pH 3.5, proceeded from bottom to top.
Solubilization and partial purification of the S. solfataricus protein kinase.
Efforts to solubilize the S. solfataricus protein kinase by alternative means such as extraction with mildly acidic buffers and/or high salt concentrations proved unsuccessful. Only nonionic detergents such as Triton X-100 and octyl glucoside were effective at extracting the enzyme. Following solubilization, the presence of the detergent was required to maintain catalytic activity. We therefore concluded that the S. solfataricus protein kinase was an integral membrane protein.
Attempts to purify the S. solfataricus protein kinase met with limited success (Table 1). The protein could be bound to and eluted from DE-52 cellulose in high yield, a process which also resulted in significant purification. The protein could be further purified by chromatofocusing on a Mono-P column. However, while significant purification once again was achieved, yields were poor. Thus, many of the experiments described below were performed with only the DE-52 fraction. It should be noted that, where reported in the figures and tables, the performance of the Mono-P fraction paralleled that observed with the DE-52 fraction. The degrees of purification of the DE-52 fraction and Mono-P fraction over cell lysates could not be calculated as we were unable to perform quantitative solution assays until after the enzyme had been solubilized with detergents. Thus, the significant enrichment that undoubtedly resulted from the isolation, washing, and detergent extraction of the membrane fraction remained unaccounted for.
TABLE 1.
Partial purification of S. solfataricus protein kinasea
| Fraction | Vol (ml) | Amt of protein (mg) | Activity (pmol/min) | Sp act (pmol/min/ mg) | Recovery (%) | Enrichment (fold) |
|---|---|---|---|---|---|---|
| Detergent extract | 25 | 40 | 1,375 | 34.4 | 100 | 1.0 |
| DE-52 | 22 | 7.5 | 1,632 | 218 | 119 | 6.3 |
| Mono-P | 2.6 | 0.065 | 156 | 2,400 | 11 | 70 |
Summary of a representative preparation of the protein kinase activity from a detergent extract (Triton X-100) of the membrane fraction from 20 g, wet weight, of S. solfataricus. All steps were performed as described in Materials and Methods. Protein kinase activity was measured using casein as the phosphoacceptor substrate.
Gel filtration chromatography of the DE-52 fraction consistently revealed the presence of protein kinase activity that eluted with an apparent molecular mass of ≈125 kDa, suggesting that the enzyme exists as a heteroligomer or, perhaps, a homodimer. The physiological relevance of the ≈125-kDa oligomer must be viewed with some caution, however, as the protein kinase activity present in the crude detergent extract always eluted in the void volume (>250 kDa) of our gel filtration column. The ability to recover protein kinase activity following either one-dimensional SDS-PAGE or two-dimensional IEF/SDS-PAGE indicates that, if the protein does exist as a heteroligomer in vivo, the essential determinants for catalytic function reside within a single ≈67-kDa subunit.
Phosphorylation of exogenous proteins and peptides by the S. solfataricus protein kinase.
The S. solfataricus protein kinase was challenged with a variety of exogenous protein, polymer, and peptide substrates (Table 2). Of these, casein proved to be the most effective substrate. No phosphorylation could be detected with poly(Glu4:Tyr), poly(Glu:Tyr), or myelin basic protein. Lower, but still detectable, rates of phosphorylation could be measured using RCM lysozyme, mixed histones or histone H4, or MLC peptide (KKRAARATSNVFA), a short peptide modeled after the phosphorylation site sequence in MLCs (17), as phosphoacceptor substrates. Phosphoamino acid analysis revealed that casein, RCM lysozyme, and MLC peptide were phosphorylated exclusively on threonine residues (Fig. 2). Histone H4 was phosphorylated on serine in addition to threonine (data not shown).
TABLE 2.
Activity of S. solfataricus protein kinase toward exogenous proteins, amino acid copolymers, and peptidesa
| Protein or peptide | Concn (mg/ml) | Activity (pmol/min/mg) with:
|
Relative activity (% that of casein) with:
|
||
|---|---|---|---|---|---|
| DE-52 | Mono-P | DE-52 | Mono-P | ||
| Casein | 0.60 | 44.0 ± 6.5 | 78.8 ± 3.9 | 100 | 100 |
| RCM lysozyme | 0.80 | 7.5 ± 1.2 | 13.9 ± 0.4 | 17 | 18 |
| Mixed histones | 0.20 | 1.8 ± 0.5 | 17.5 ± 0.5 | 4 | 22 |
| Histone H4 | 0.43 | 6.6 ± 1.5 | 20.4 ± 1.3 | 15 | 26 |
| MLC peptide | 0.25c | 4.2 ± 0.4 | 1.9 ± 0.3 | 10 | 2 |
| Myelin basic protein | 1.0 | n.d.b | n.d. | ||
| Poly(Glu4:Tyr) | 1.0 | n.d. | n.d. | ||
| Poly(Glu:Tyr) | 1.0 | n.d. | n.d. | ||
| BSA | 1.0 | n.d. | n.d. | ||
The activities of the S. solfataricus protein kinase, 3.2 to 5.1 μg of the DE-52 fraction or 0.14 μg of the Mono-P fraction, toward a range of exogenous proteins, amino acid copolymers, and peptides were determined using the solution assay technique described in Materials and Methods. Substrate concentrations were selected as those which yielded the highest activity in preliminary experiments. In those instances where no activity was detected in preliminary experiments, a concentration of 1.0 mg/ml was selected. Shown are the averages of triplicate determinations ± standard errors.
n.d., not detectable (<1.0 pmol/min/mg).
Units are millimolar.
MLC peptide provided a vehicle for exploring the observed tendency of the S. solfataricus protein kinase to phosphorylate threonine residues. A variant of MLC peptide, called T8S peptide, in which the threonine at position 8 was replaced by serine, was synthesized. The sequence of the T8S peptide was thus KKRAARASSNVFA. Phosphorylation of T8S peptide was undetectable at 25°C. However, phosphorylation of T8S peptide did take place at measurable rates (i) if the assay temperature was raised to 65°C (Table 3) or (ii) if poly(Glu4:Tyr) was included in the assay mixture. Poly(Glu4:Tyr) had been observed to stimulate protein kinase activity toward MLC (Table 3) and T8S peptide but not toward macromolecular substrates such as casein and myelin basic protein.
TABLE 3.
Kinetic constants describing the phosphorylation of peptide substrates by the S. solfataricus protein kinasea
| Variable substrate | Temp (°C) | Presence of Poly(Glu4:Tyr) | Vmax (pmol/min/mg) | Km (μM) |
|---|---|---|---|---|
| MLC peptide | 25 | − | 3.88 ± 0.09 | 30 ± 1 |
| MLC peptide | 25 | + | 102 ± 13 | 13 ± 2 |
| MLC peptide | 65 | − | 333 ± 11 | 45 ± 5 |
| T8S peptide | 65 | − | 13.9 ± 1.4 | 20 ± 2 |
| ATP | 25 | − | 5.43 ± 0.94 | 0.74 ± 0.19 |
| ATP | 65 | − | 345 ± 23 | 15.7 ± 1.8 |
Kinetic constants for the phosphorylation of the MLC peptide (KKRAARATSNVFA) and the T8S peptide (KKRAARASSNVFA) at both 25 and 65°C were determined using the solution assay procedure described in Materials and Methods. Each assay mixture contained 3.2 μg of the DE-52 fraction. Peptide concentrations were varied over the range 5 to 700 μM for the MLC peptide and 5 to 600 μM for the T8S peptide with ATP as the phosphoryl donor at a concentration of 50 μM. The ATP concentration was varied over the range 0.1 to 200 μM with the MLC peptide as the phosphoacceptor substrate at a concentration of either 0.25 (25°C) or 0.75 mM (65°C). Where indicated, poly(Glu4:Tyr) was added to a final concentration of 0.5 mg/ml. All kinetic constants were estimated using Lineweaver-Burk plots. Shown are the averages of duplicate determinations, except that with ATP as the variable substrate at 25°C, which was performed in triplicate, ± standard errors.
An examination of the kinetic parameters for the phosphorylation of MLC and T8S peptides was conducted at both 25 and 65°C, the latter temperature being the highest at which enzyme stability could be maintained for a significant period. (The optimal temperature for growth of S. solfataricus is 70 to 75°C [3, 6].) These analyses revealed that while the Kms for the two peptides were quite similar, with that for T8S peptide in fact being the lower of the two, the Vmax for phosphorylation of the MLC peptide was over 20-fold higher. The effect of heparin on phosphorylation of the MLC peptide also was attributable entirely to an enhancement of Vmax. The Km values for ATP, both at 25 and 65°C, were in the micromolar range and thus fell well within the range of typical physiological concentrations.
Metal ion and nucleotide preferences of the S. solfataricus protein kinase.
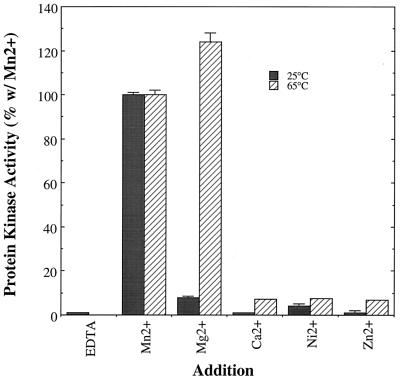
Analysis of the cofactor requirements for the enzyme revealed a strong preference for Mn2+ over other divalent metal ions when assays were performed at 25°C (Fig. 3). However, at a temperature within the physiological range for S. solfataricus, 65°C, Mg2+ proved equally effective (Fig. 3). A variation of the in gel assay technique was used to examine the nucleotide specificity of the S. solfataricus protein kinase. Briefly, samples of the protein kinase were subjected to SDS-PAGE and renatured by standard procedures. Next, the gel was divided into individual lanes and each section was incubated overnight with a nucleotide triphosphate or other potential nucleotide donor. These compounds were not radiolabeled; hence any phosphate transferred to the casein during this stage would be undetectable by autoradiography. The purity of all nucleotide preparations was verified by thin-layer chromatography on polyethyleneimine cellulose to verify that the nucleotide diphosphates were free of contamination by nucleotide triphosphates. After a washing, a standard in gel assay was performed using [γ-32P]ATP. If substantial phosphotransfer took place during the first incubation, transfer of radiophosphate from [γ-32P]ATP should be attenuated for lack of available phosphorylation sites in the immobilized casein. As can be seen in Table 4, purine nucleotide tri- and diphosphates served as phosphodonor substrates in vitro, with the apparent order of preference being ATP > GTP > ADP > GDP. Neither of the pyrimidine nucleotide triphosphates tested, CTP and UTP, served as substrates and neither did AMP, GMP, or pyrophosphate.
FIG. 3.
Survey of potential metal ion cofactors for the S. solfataricus protein kinase. The activity of the S. solfataricus protein kinase was assayed in the presence of the indicated divalent metal ions at both 25 and 65°C. Conditions were as described in Materials and Methods with the exception that, in place of the standard divalent metal ion cofactor, Mn2+, the compounds listed were added at a final concentration of 5 mM. Casein was used as the phosphoacceptor substrate, and the Mono-P fraction, 0.25 to 0.50 μg, was used as the source of protein kinase for the assays performed at 25°C. At 65°C, the MLC peptide was used as the phosphoacceptor substrate and the DE-52 fraction, 3.0 μg, was used as the source of protein kinase activity. Shown are the averages of the duplicate determinations ± standard errors.
TABLE 4.
Nucleotide specificity of the S. solfataricus protein kinasea
| Nucleotide | Phosphate incorporation into casein (% control) (n) |
|---|---|
| ATP | 32 ± 5 (3) |
| ADP | 70 ± 17 (3) |
| AMP | 96 ± 4 (3) |
| GTP | 50 ± 12 (3) |
| GDP | 85 ± 14 (2) |
| PPi | 96 ± 4 (2) |
Samples of the DE-52 fraction, 12.5 μg each, were subjected to SDS-PAGE in gels containing copolymerized casein. The protein kinase was renatured within the gel, the gels were sectioned into individual lanes, and the lanes were incubated overnight with the indicated nucleotides at a concentration of 50 mM. Afterwards, the nonlabeled nucleotides were washed away and an in gel assay was performed using [γ-32P]ATP. The gels were then washed free of [γ-32P]ATP, and the incorporation of [32P]phosphate into protein was determined by electronic autoradiography. Phosphorylation of casein by unlabeled phosphate from the nucleotide present during the overnight preincubation period should be manifested as an attenuation of [32P]phosphate during the subsequent incubation with [γ-32P]ATP. Shown are the levels of [32P]radioactivity incorporated into casein relative to those for controls in which no nucleotide was present during preincubation. All values are averages of multiple (n) determinations ± standard errors.
To assess the validity of this inferential method for assessing nucleotide specificity, the ability of both the DE-52 fraction and the Mono-P fraction to catalyze the transfer of [32P]phosphate from [γ-32P]GTP or [β-32P]GDP was assayed in solution. As shown in Table 5, both sources of S. solfataricus protein kinase catalyzed the phosphorylation of casein or MLC peptide using either guanine nucleotide, as predicted. In addition, transfer of [32P]phosphate to casein by the DE-52 fraction could be detected via the in gel technique using [γ-32P]ATP, [γ-32P]GTP, or [β-32P]GDP as the phosphodonor substrate. Each nucleotide donor manifested a single catalytically active species of identical molecular mass, ≈67 kDa, with the degree of phosphoryl transfer exhibiting the order ATP > GTP ≫ GDP (data not shown).
TABLE 5.
Phosphoryl transfer by the S. solfataricus protein kinase from GTP or GDP in vitroa
| Protein kinase fraction | Phosphoacceptor | Activity (pmol/min/mg) (% that with ATP) with nucleotide donor:
|
||
|---|---|---|---|---|
| ATP | GTP | GDP | ||
| DE-52 | Casein | 36.9 ± 1.8 (100) | 24.1 ± 0.1 (65) | 3.0 ± 0.1 (8) |
| DE-52 | MLC peptide | 45.9 ± 0.1 (100) | 20.0 ± 2.8 (44) | 1.4 ± 0.3 (3) |
| Mono-P | Casein | 57.0 ± 5.1 (100) | 40.2 ± 2.1 (70) | 0.6 ± 0.5 (1) |
| Mono-P | MLC peptide | 55.4 ± 2.2 (100) | 89.2 ± 8.0 (161) | 23.0 ± 4.3 (26) |
The activity of the S. solfataricus protein kinase, 4.0 μg of the DE-52 fraction or 0.28 μg of the Mono-P fraction, toward casein and the MLC peptide was determined using the solution assay technique described in Materials and Methods. Where indicated, [γ-32P]GTP or [β-32P]GDP was substituted for the normal phosphoryl donor, [γ-32P]ATP. Casein phosphorylation was measured at 25°C, and that of the MLC peptide was measured at 65°C. Shown are the averages of duplicate (DE-52 fraction) or triplicate (Mono-P fraction) analyses ± standard errors.
Sensitivity of S. solfataricus protein kinase to inhibitors of eukaryotic protein kinases.
The S. solfataricus protein kinase was challenged in vitro with several compounds known to inhibit the catalytic activity of members of the eukaryotic protein kinase superfamily. These included PKI peptide, a highly specific inhibitor of the cAMP-dependent protein kinase; genistein, an inhibitor of protein-tyrosine kinases; tamoxifen, an inhibitor of protein kinase C; and the relatively broadly specific or nonspecific inhibitors H7, H89, ML-9, and staurosporine (reviewed in reference 12). All compounds, with the exception of PKI peptide, were tested at millimolar concentrations, well above those at which they were known to inhibit their eukaryotic target enzymes.
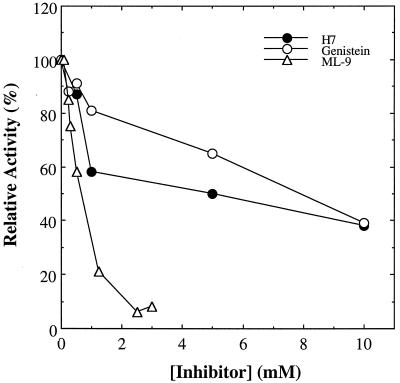
Only three of these compounds inhibited the activity of the S. solfataricus protein kinase in a concentration-dependent manner: genistein, ML-9, and H7 (Fig. 4). By far the most effective of these three was ML-9. Even so, the apparent 50% inhibitory concentration exhibited by this compound fell between 0.5 and 1.0 mM, a range well above the concentrations at which it inhibits eukaryotic protein kinases such as MLC kinase (Ki = 3.8 μM), protein kinase C (Ki = 54 μM), and the cAMP-dependent protein kinase (Ki = 32 μM) (13).
FIG. 4.
Sensitivity of S. solfataricus protein kinase to inhibitors of eukaryotic protein kinases. The DE-52 fraction, 3.2 μg of protein, was assayed for protein kinase activity toward the MLC peptide under standard conditions with the exception that, where indicated, the following compounds were present at the concentrations indicated on the ordinate: H7, genistein, and ML-9. Shown is activity relative to that measured in the absence of inhibitor, 4.0 pmol/min/mg, which was set equal to 100%.
Autophosphorylation of the S. solfataricus protein kinase.
When the catalytic performance of the S. solfataricus protein kinase at elevated (65°C) temperatures was examined, it was noted that the levels of radiophosphate present in controls lacking an exogenous phosphoacceptor substrate were much higher than expected. This behavior indicated that the S. solfataricus protein kinase was phosphorylating some component within the DE-52 fraction itself, and may in fact be undergoing autophosphorylation, under these conditions. An in gel assay was therefore performed on an SDS-polyacrylamide gel, but with the modification that the temperature was increased from the 25°C used in previous experiments to 65°C. As expected, an active species of ≈67 kDa was apparent in a gel containing copolymerized casein as an exogenous phosphoacceptor. However, a species of similar size also was apparent in a gel in which no exogenous protein had been copolymerized. While this behavior was highly suggestive of an autophosphorylation event, the possibility remained that the S. solfataricus protein kinase had phosphorylated an archaeal protein(s) of similar Mr that was accessible to it within the one-dimensional gel. Therefore, the protein kinase was completely resolved from other proteins by two-dimensional IEF/SDS-PAGE. The subsequent in gel assay revealed the presence of a diffuse band of phosphate incorporation matching the position of the protein kinase as established by parallel in gel assays in gels containing casein. It was therefore concluded that the protein kinase itself was the phosphorylated species and that autophosphorylation had taken place.
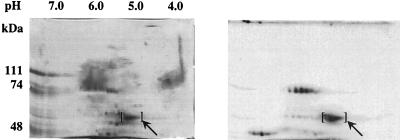
By contrast with the results obtained with SDS-PAGE, the zones of phosphorylating activity detected with in gel assays of two-dimensional gels were observed to be fairly diffuse, particularly in the first dimension. This behavior was observed regardless of whether the phosphorylation of copolymerized casein or protein kinase autophosphorylation was monitored. Such behavior might reflect diffusion of the enzyme through the gel matrix during the extended time period over which the gels were subjected to renaturation-denaturation, etc. Alternatively, it may be indicative of the presence of multiple active polypeptides of similar Mr but slightly differing pI. To further investigate this phenomenon, the DE-52 fraction was incubated with [γ-32P]ATP at 65°C to radiolabel the protein kinase by autophosphorylation prior to two-dimensional electrophoresis. The gels were fixed and stained immediately following electrophoresis to minimize the opportunity for protein diffusion. The results of this experiment are shown in Fig. 5. As indicated, a cluster of two or three distinct phosphorylated polypeptides that migrated to the position corresponding to the previously observed Mr and pI of the S. solfataricus protein kinase were apparent. Each phosphorylated species corresponded to a distinct polypeptide, as visualized by staining with Coomassie blue. In addition, two other sets of phosphoproteins with apparent molecular masses of 45 to 50 kDa and ≈85 kDa were apparent. These may represent trapped phosphoenzyme intermediates or autophosphorylated proteins that do not become manifest during in gel assays because they do not renature in an active form following SDS-PAGE. Alternatively, these proteins may have been phosphorylated by the S. solfataricus protein kinase prior to electrophoresis.
FIG. 5.
Analysis of autophosphorylated S. solfataricus protein kinase by two-dimensional electrophoresis. The DE-52 fraction, 150 μg, was incubated for 60 min at 65°C in a volume of 100 μl of 20 mM MES, pH 6.5, containing 50 μM ATP (300 μCi of [γ-32P]ATP/ml), 5 mM MnCl2, 2 mM DTT, and 12.5 mM octyl glucoside. The reaction was quenched by adding 3 volumes of ice-cold acetone. The precipitated protein was collected by centrifugation and resuspended in rehydration solution (Pharmacia Biotech). The protein mixture was analyzed by two-dimensional electrophoresis using an Immobiline gel with a linear pH gradient of 4.0 to 7.0 for the first dimension followed by SDS-PAGE through a 6% (wt/vol) gel for the second. The gel was stained for protein, and 32P-phosphorylated species were visualized by autoradiography. Shown are a picture of the Coomassie-stained gel (left) and an autoradiogram of the gel (right). The brackets mark the position of the S. solfataricus protein kinase. The pH gradient of the first-dimension IEF gel is shown at the top of the Coomassie-stained gel, and the molecular masses of protein standards used for SDS-PAGE are at the left.
The resolution of the S. solfataricus protein kinase into multiple phosphoproteins by two-dimensional electrophoresis may reflect the presence of proteolytically nicked forms of the protein or a covalent modification process such as phosphorylation at multiple sites. One argument in favor of the latter explanation was the appearance of two-dimensional gels in which prior incubation with ATP was omitted. Under those circumstances, only a single major spot of Coomassie-stained protein was apparent at the position predicted for the S. solfataricus protein kinase. Phosphoamino acid analysis indicated that autophosphorylation took place on threonine. Addition of high concentrations of an exogenous substrate, MLC peptide, reduced the degree of phosphate incorporation into the protein kinase in a concentration-dependent manner (Fig. 6), consistent with catalysis of peptide phosphorylation by the same protein that underwent autophosphorylation. Incubation of autophosphorylated protein kinase that had been renatured in gel with diffusible phosphoacceptor substrate MLC peptide had no effect on the level of phosphate associated with the protein (data not shown). This behavior indicates that the protein-bound phosphoryl group was not a catalytic intermediate, as it could not be transferred to an exogenous substrate.
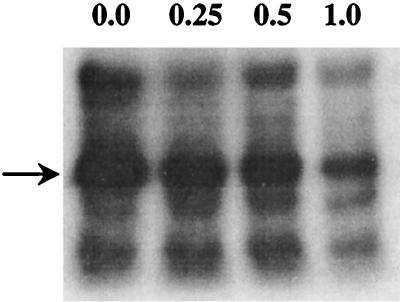
FIG. 6.
The presence of a substrate peptide inhibits autophosphorylation. The DE-52 fraction, 20 μg, was incubated for 60 min at 65°C in a volume of 100 μl of 20 mM MES, pH 6.5, containing 50 μM ATP (300 μCi of [γ-32P]ATP/ml), 5 mM MnCl2, 2 mM DTT, 12.5 mM octyl glucoside, and the indicated concentrations of the MLC peptide. The reaction was stopped by precipitating the proteins with 3 volumes of cold acetone. The precipitated proteins were collected by centrifugation and resuspended in SDS-sample buffer and analyzed by SDS-PAGE using an 8% (wt/vol) gel. Shown is an autoradiogram of the gel. Arrow, position of the S. solfataricus protein kinase. The concentration of the MLC peptide present in each sample (millimolar) is indicated at the top of each lane.
DISCUSSION
We have detected and examined various properties of a membrane-associated protein-serine/threonine kinase from the extreme acidothermophilic archaeon S. solfataricus. Enzymatic activity resides in an ≈67-kDa polypeptide that can only be extracted from membranes using nonionic detergents, indicating that it is an integral membrane protein. Gel filtration chromatography suggested that the protein exists in an oligomeric form. Among its more striking properties was a marked preference for phosphorylating threonine residues in vitro. While phosphorylation of serine residues on histone H4 and the T8S peptide was observed, this was the exception to a pattern in which exogenous peptide and protein substrates were consistently and exclusively phosphorylated on threonine. It must be noted that all of these substrates were nonphysiological. However, autophosphorylation of the S. solfataricus protein kinase also took place on threonine. More compellingly, kinetic analysis indicated a dramatic difference, greater than 20-fold, in the Vmax for phosphorylation of the threonine residue in the MLC peptide as opposed to the T8S peptide, which differs only in the substitution of serine for the phosphoacceptor threonine of the former.
Another unusual property of the S. solfataricus protein kinase was its utilization of both purine nucleotide di- and triphosphates as phosphoryl donor substrates in vitro. The utilization of nucleotides other than ATP as substrates by protein kinases of any kind has been reported only rarely, the best-documented example being casein kinase II, which can use either GTP or ATP (14). It also has been reported that pyrophosphate can serve as a phosphoryl donor for protein kinase activities in crude fractions of Escherichia coli (7) and that polyphosphate can serve a similar function for the archaeon Sulfolobus acidocaldarius (35). Pyrophosphate did not, however, serve as a phosphoryl donor substrate for the S. solfataricus protein kinase. The utilization of Mn2+ as a cofactor by the enzyme, a fairly infrequent occurrence among protein kinases, reflected the pattern observed previously with protein-serine/threonine phosphatases from the Archaea (18, 24, 39) and in experiments probing protein kinase activity in S. acidocaldarius (35). However, Mn2+ may not serve as the prime cofactor in vivo, as the effectiveness of Mg2+ becomes greatly enhanced at the high temperatures under which the organism lives.
It is difficult to classify the S. solfataricus protein kinase based on the functional properties examined in this study. Very few threonine-specific or threonine-preferring protein kinases have been reported in the literature. Their numbers include one enzyme that conforms to the eukaryotic protein kinase paradigm, PYT (23), and a second enzyme that does not (26). The tepid response of the S. solfataricus protein kinase to known inhibitors of eukaryotic protein kinases also offers little indication as to its nature. Inspections of archaeal genome sequences by a number of laboratories (22, 33, 36) have revealed the presence of ORFs that exhibit faint resemblance to the eukaryotic protein kinase paradigm, whose prime exemplar is the cAMP-dependent protein kinase. However, in general the degree of resemblance was minimal, and many of the sequences predicted products lacking the conserved GXGXXG sequence of subdomain I and/or subdomain X of these eukaryotic protein kinases (33). In some cases, subdomain XI was missing as well. It is thus difficult to predict whether the hypothetical products of these ORFs constitute competent, physiologically relevant protein kinases and, if so, the degree to which they will resemble their eukaryotic counterparts in form and function. None of the archaeal genomes thus far examined have been reported to contain ORFs homologous to those encoding other known protein-serine/threonine kinases such as the HPr kinase, Rsb/Spo kinases, and the myosin heavy chain kinase/eIF-2 protein kinase family. However, because few examples of each of these novel types have been identified to date, no clear consensus as to the minimum necessary features for such enzymes has been developed. Thus, contemporary genome searches would potentially reveal only those ORFs that exhibit a relatively high degree of overall sequence homology to the available bacterial and eukaryotic examples. Regardless of whether the S. solfataricus protein kinase proves to be a highly divergent member of an established protein kinase family or the defining member of a unique new family, the complete resolution of its structure and properties will provide important insights into the origins and evolution of protein-serine/threonine kinases.
ACKNOWLEDGMENTS
This work was supported by grant R01 GM55067 from the National Institutes of Health (to P.J.K.) and an NSF and Alfred P. Sloan Foundation Postdoctoral Research Fellowship in Molecular Evolution (to K.M.B.).
REFERENCES
- 1.Bischoff K M, Kennelly P J. An ‘in-gel’ assay for alternative nucleotide donors for protein kinases. Anal Biochem. 1999;271:199–202. doi: 10.1006/abio.1999.4150. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 3.Brock T D, Brock K M, Belly R T, Weiss R L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Microbiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 4.Corbin J D, Reimann E M. Assay of cAMP-dependent protein kinases. Methods Enzymol. 1971;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- 5.Cote G P, Luo X, Murphy M B, Egelhoff T T. Mapping of the novel protein kinase catalytic domain of Dictyostelium myosin II heavy chain kinase A. J Biol Chem. 1997;272:6846–6849. doi: 10.1074/jbc.272.11.6846. [DOI] [PubMed] [Google Scholar]
- 6.de Rosa M, Gambacorta A, Bullock J D. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus acidocaldarius. J Gen Microbiol. 1975;86:156–164. doi: 10.1099/00221287-86-1-156. [DOI] [PubMed] [Google Scholar]
- 7.Duclos B, Vaganay E, Dadssi M, Cozzone A J. Pyrophosphate is a source of phosphoryl groups for Escherichia coli protein phosphorylation. FEMS Microbiol Lett. 1996;145:49–54. doi: 10.1111/j.1574-6968.1996.tb08555.x. [DOI] [PubMed] [Google Scholar]
- 8.Dufour A, Haldenwang W G. Interactions between a Bacillus subtilis anti-ς factor (RsbW) and its antagonist (RsbV) J Bacteriol. 1994;176:1813–1820. doi: 10.1128/jb.176.7.1813-1820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairbanks G, Steck T L, Wallace D F H. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 10.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer M-C, Deutscher J, Haiech J. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorg A, Postel W, Gunther S, Weser J. Improved horizontal two-dimensional electrophoresis with hybrid isoelectric focusing in immobilized pH gradients in the first dimension and laying-on transfer to the second dimension. Electrophoresis. 1985;6:599–604. [Google Scholar]
- 12.Hemmings H C., Jr Protein kinase and protein phosphatase inhibitors. Neuromethods. 1997;30:121–218. [Google Scholar]
- 13.Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 14.Jakobi R, Traugh J A. Characterization of the phosphotransferase domain of casein kinase II by site-directed mutagenesis and expression in Escherichia coli. J Biol Chem. 1992;267:23894–23902. [PubMed] [Google Scholar]
- 15.Kamps M P, Sefton B M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989;176:22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- 16.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S-I, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K-I, Yoshizawa T, Nakamura Y, Robb F T, Horikoshi K, Masuchi Y, Shizuya H, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 17.Kennelly P J, Edelman A M, Blumenthal D K, Krebs E G. Rabbit skeletal muscle myosin light chain kinase: the calmodulin-binding domain as a potential active site-directed inhibitory domain. J Biol Chem. 1987;262:11958–11963. [PubMed] [Google Scholar]
- 18.Kennelly P J, Oxenrider K A, Leng J, Cantwell J S, Zhao N. Identification of a serine/threonine-specific protein phosphatase from the archaebacterium Sulfolobus solfataricus. J Biol Chem. 1993;268:6505–6510. [PubMed] [Google Scholar]
- 19.Kennelly P J, Potts M. Fancy meeting you here! A fresh look at “prokaryotic” protein phosphorylation. J Bacteriol. 1996;178:4759–4764. doi: 10.1128/jb.178.16.4759-4764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwin M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Overbeek B, Gocayne J D, Weidman J F, McDonald L, Utterback T, Cotton M D, Spriggs T, Artiach P, Kaine B P, Sykes S M, Dadow P W, D'Andrea K P, Bowman C, Fujii C, Garland S A, Mason T M, Olson G J, Fraser C M, Smith H O, Woese C R, Ventner J C. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–379. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Leonard C J, Aravind L, Koonin E V. Novel families of putative protein kinases in bacteria and archaea: evolution of the “eukaryotic” protein kinase superfamily. Genome Res. 1998;8:1038–1047. doi: 10.1101/gr.8.10.1038. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg R A, Fischer W H, Hunter T. Characterization of a human protein threonine kinase isolated by screening an expression library with antibodies to phosphotyrosine. Oncogene. 1993;8:351–359. [PubMed] [Google Scholar]
- 24.Mai B, Frey G, Swanson R V, Mathur E J, Stetter K O. Molecular cloning and functional expression of a protein-serine/threonine phosphatase from the hyperthermophilic archaeon Pyrodictium abyssi TAG11. J Bacteriol. 1998;180:4030–4035. doi: 10.1128/jb.180.16.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. Sigma-F, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 26.Oda Y, Kuo M-D, Huang S S, Huang J S. The plasma cell membrane glycoprotein, PC-1, is a threonine-specific protein kinase stimulated by acidic fibroblast growth factor. J Biol Chem. 1991;266:16791–16795. [PubMed] [Google Scholar]
- 27.Ramwani J, Mishra R K. Purification of bovine striatal dopamine D2 receptor by affinity chromatography. J Biol Chem. 1986;261:8894–8898. [PubMed] [Google Scholar]
- 28.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stulke J, Karamata D, Saier M H, Jr, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 29.Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph J, Osesterhelt D. Chemotaxis and phototaxis require a CheA histidine kinase in the archaeon Halobacterium salinarium. EMBO J. 1995;14:667–673. doi: 10.1002/j.1460-2075.1995.tb07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryazanov A G, Ward M D, Mendola C E, Pavur K S, Dorovkov M V, Wiedmann M, Erdjument-Bromage H, Tempst P, Parmer T G, Protsko C R, Germino F J, Hait W N. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc Natl Acad Sci USA. 1997;94:4884–4889. doi: 10.1073/pnas.94.10.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Bischoff K M, Kennelly P J. The icfG gene cluster of Synechocystis sp. strain PCC6803 encodes an Rsb/Spo-like protein kinase, protein phosphatase, and two phosphoproteins. J Bacteriol. 1999;181:4761–4767. doi: 10.1128/jb.181.16.4761-4767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Potts M, Kennelly P J. The serine, threonine, and/or tyrosine-specific protein kinases and protein phosphatases of prokaryotic organisms. A family portrait. FEMS Microbiol Rev. 1998;22:229–253. doi: 10.1111/j.1574-6976.1998.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 34.Skorko R. Protein phosphorylation in the archaebacterium Sulfolobus acidocaldarius. Eur J Biochem. 1984;145:617–622. doi: 10.1111/j.1432-1033.1984.tb08601.x. [DOI] [PubMed] [Google Scholar]
- 35.Skorko R. Polyphosphate as a source of phosphoryl group in protein modification in the archaebacterium Sulfolobus acidocaldarius. Biochimie. 1989;71:1089–1093. doi: 10.1016/0300-9084(89)90115-6. [DOI] [PubMed] [Google Scholar]
- 36.Smith R F, King K Y. Identification of a eukaryotic-like protein kinase gene in the Archaebacteria. Protein Sci. 1995;4:126–129. doi: 10.1002/pro.5560040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith S C, McCartney B, Kennelly P J, Potts M. Protein-tyrosine phosphorylation in the Archaea. J Bacteriol. 1997;179:2418–2420. doi: 10.1128/jb.179.7.2418-2420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solow B, Bischoff K M, Zylka M J, Kennelly P J. Archaeal phosphoproteins. Identification of a hexosephosphate mutase and the α-subunit of succinyl-CoA synthetase in the extreme acidothermophile Sulfolobus solfataricus. Protein Sci. 1998;7:105–111. doi: 10.1002/pro.5560070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solow B, Young J C, White R H, Kennelly P J. Gene cloning and expression and characterization of a toxin-sensitive protein phosphatase from the methanogenic archaeon Methanosarcina thermophila TM-1. J Bacteriol. 1997;179:5072–5075. doi: 10.1128/jb.179.16.5072-5075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spudich E A, Spudich J L. Photosensitive phosphoproteins in halobacteria: regulatory coupling of transmembrane proton flux and protein dephosphorylation. J Cell Biol. 1981;1981:895–900. doi: 10.1083/jcb.91.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spudich J L, Stoeckenius W. Light-regulated retinal-dependent reversible phosphorylation of Halobacterium proteins. J Biol Chem. 1980;255:5501–5503. [PubMed] [Google Scholar]
- 42.Tonks N K, Diltz C D, Fischer E H. Purification of the major protein-tyrosine phosphatases of human placenta. J Biol Chem. 1988;263:6722–6730. [PubMed] [Google Scholar]
- 43.Yang X, Kang C M, Brody M S, Price C W. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 1996;10:2265–2275. doi: 10.1101/gad.10.18.2265. [DOI] [PubMed] [Google Scholar]