Abstract
Antibiotics are frequently administered during pregnancy. Although necessary to address acute infections, their use facilitates antibiotic resistance. Other associations have also been found with the use of antibiotics, such as perturbations of gut bacteria, delays in microbial maturation, and increased risks of allergic and inflammatory diseases. Little is known about how the prenatal and perinatal administration of antibiotics to mothers affects the clinical outcomes of their offspring. A literature search was conducted of the Cochrane, Embase, and PubMed engines. The retrieved articles were reviewed by two authors and verified for relevance. The primary outcome was the effect of pre- and perinatal maternal antibiotic use on clinical outcomes. Thirty-one relevant studies were included in the meta-analysis. Various aspects are discussed, including infections, allergies, obesity, and psychosocial factors. In animal studies, antibiotic intake during pregnancy has been suggested to cause long-term alterations in immune regulation. In humans, associations have been found between antibiotic intake during pregnancy and different types of infections and an increased risk of pediatric infection–related hospitalization. A dose-dependent positive association between pre- and perinatal antibiotic use and asthma severity has been reported in animal and human studies, while positive associations with atopic dermatitis and eczema were reported by human studies. Multiple associations were identified between antibiotic intake and psychological problems in animal studies; however, relevant data from human studies are limited. However, one study reported a positive association with autism spectrum disorders. Multiple animal and human studies reported a positive association between pre- and perinatal antibiotic use by mothers and diseases in their offspring. Our findings have potentially significant clinical relevance, particularly considering the implications for health during infancy and later in life as well as the related economic burden.
Keywords: Microbiome, Antibiotics, Pregnancy, Neonate, Fetus, Gastroenterology
INTRODUCTION
Antibiotics are frequently used during pregnancy [1,2]. During pregnancy, various physiological changes can result in increased susceptibility to infections [2,3]. Roughly one in four pregnant women receive an antibiotic prescription [1]. Some antibiotics, such as beta-lactams, fosfomycin, metronidazole, nitrofurantoin, clindamycin, and vancomycin, are generally considered safe and effective during pregnancy. Others, such as fluoroquinolones and tetracyclines, are teratogenic and should be avoided during pregnancy [1].
Antibiotic use is necessary to address acute infections, but their long-term, frequent use creates bacterial resistance [2]. Furthermore, associations between antibiotic use and other long-term effects have been increasingly reported. Antibiotic use during infancy has been associated with an increased risk of disorders such as obesity, inflammatory bowel disease (IBD), asthma, and other allergic/inflammatory conditions [4]. Early antibiotic exposure has been hypothesized to cause a 6–12-month delay in microbial maturation [4], which normally develops over the first 18–24 months of life (Table 1) [5]. An increasing number of studies suggest that perturbations of the neonatal gut bacterial composition are a possible consequence of maternal antibiotic use [1,4,5].
Table 1. Different phases of normal initial bacterial colonization.
| Phase 0 | Partial colonization in the periuterine environment |
| Phase 1 | Acquire maternal vaginal/colonic microbiota (full-term vaginal delivery) |
| Phase 2 | Introduction of oral feedings (breast milk or formula) |
| Phase 3 | Weaning to solid foods |
| Phase 4 | Acquire complete mature colonization (12–18 mo) |
Adapted from Walker et al. (Nestle Nutr Inst Workshop Ser 2017;88:23-33) with permission [5].
Moreover, associations between antibiotic use during pregnancy and clinical outcomes in the offspring have been reported. This narrative review focuses on the different clinical outcomes in animals and children associated with the pre- and perinatal use of antibiotics.
MATERIALS AND METHODS
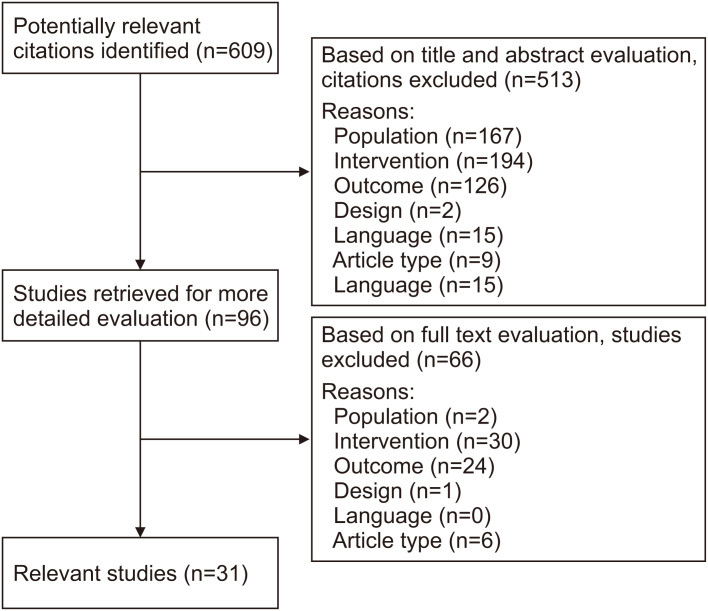
We searched the PubMed, Cochrane, and Embase databases on August 1, 2021, using the following MeSH terms combined by the Boolean operators “AND” and “OR”: “anti-bacterial agents,” “antibiotic,” “cesarean section,” “c-section,” “pregnancy,” “gastrointestinal microbiome,” and “microbiome.” We selected animal and human studies and included articles published from the day of the database creation until August 1, 2021. Only articles published in English or Dutch for which the full text was available were included. Articles on preterm neonates were excluded because of the possible impact of postnatal therapies, such as antibiotics and proton pump inhibitors, on the microbiome. The maximum age of the study population was 16 years. Editorial articles and comments were excluded from this review. A total of 609 potentially relevant articles were identified. The list of articles was reviewed by both authors and checked for relevance. After an initial screening based on the title and abstract, 96 articles were retrieved for a full-text evaluation. Studies reporting on the antibiotic intake of the child after birth were only included if relevant data on the pre- and/or perinatal antibiotic intake of the mother could be analyzed separately. Thirty-one relevant studies (Table 2) were selected and checked for confounding variables for several factors such as maternal age and body mass index (BMI), asthma, tobacco use during pregnancy, socioeconomic status, previous deliveries, past miscarriages, and delivery method (Fig. 1).
Table 2. Summary of included publications.
| First author (year of publication) | Country (total n) | Study design | Population | Sample size | Timing of antibiotic exposure | Outcome | Result |
|---|---|---|---|---|---|---|---|
| Baron et al. [26], (2020) | United States (3), Denmark (2), Canada (2), Japan (1), Iran (1), the Netherlands (1), Sweden (1) and Finland (1) | Systematic review | 0–5 years until 7–14 years | 134 (minimum) to 910,301 participants (maximum) | Pre- and perinatal | Asthma | Positive |
| Denmark (1), United States (1) and Belgium (1) | Systematic review | 18 months up to 4 years of age | 492 (minimum) to 62,560 participants (maximum) | Pre- and perinatal | Eczema | Positive | |
| Higgins et al. [20], (2021) | Isle of Wight, UK | Prospective birth cohort | 0–12 months | 412 participants | Pre- and perinatal | Infant wheezing | Negative |
| Cassidy-Bushrow et al. [28], (2018) | Detroit, US | Retrospective | 0–2 years | 527 participants | Prenatal | Early BMI Index | Positive |
| Baron et al. [29], (2020) | United States (4) and Denmark (1) | Systematic review | 2 years old up to of 7 years old | 436 (minimum) to 39,615 mother-child pairs (maximum) | Pre- and perinatal | Overweight | Positive (under certain conditions) |
| Cunha et al. [14], (2021) | Portugal | Retrospective | 0–4 years | 7,459 participants | Pre- and perinatal | Different infections: tonsillitis, otitis, pneumonia, UTI, AGE, H. pylori | Tonsillitis: positive |
| Otitis: negative | |||||||
| Pneumonia: negative | |||||||
| UTI: negative | |||||||
| AGE: negative | |||||||
| H. pylori: negative | |||||||
| Hamad et al. [36], (2019) | Manitoba, Canada | Population-based cohort | 0–13 years old | 214,834 participants | Pre- and perinatal | Autism spectrum disorders | Positive |
| Huang et al. [27], (2020) | Sweden (1), Poland (1), Belgium (2), South Korea (1), UK (1), Denmark (1) | Meta-analysis | 0–1 years old | 411 (minimum) to 62,560 participants (maximum) | Pre- and perinatal | Eczema | Positive only in first and second trimesters |
| Jess et al. [33], (2019) | Denmark | Population-based nationwide cohort | 7–11 years old | 43,365 mother-child pairs | Pre- and perinatal | Overweight | Negative |
| Leong et al. [32], (2020) | New Zealand | Cross-sectional national | 0–4 years old | 151,359 participants | Pre- and perinatal | Obesity | Negative |
| Loewen et al. [23], (2018) | Winnipeg, Canada | Population-based | 0–9 years old | 213,661 mother-child pairs | Pre- and perinatal | Asthma | Positive (and doseresponse association) |
| Metsälä et al. [21], (2015) | Finland | Population- and register-based nested case–control study | 3–11 years old | 6,690 case-control pairs | Prenatal | Asthma | Positive |
| Metz et al. [35], (2020) | USA | Retrospective cohort | 2–5 years old | 4,825 mother-child pairs | Perinatal | BMI | Negative |
| Metzler et al. [25], (2019) | Austria, Finland, France, Germany, and Switzerland | Prospective birth cohort | 0–6 years old | 1,080 participants | Prenatal | AD, food allergy, asthma, atopic sensitization and allergic rhinitis | AD: positive Food allergy: positive |
| Asthma: negative Atopic sensitization: negative | |||||||
| Allergic rhinitis: negative | |||||||
| Miller et al. [16], (2018) | Denmark | Population-based cohort | 0–14 years old | 443,546 participants | Pre- and perinatal | Infection-related hospitalization | Positive |
| Mor et al. [30], (2015) | Denmark | Prevalence | 7–16 years old | 9,886 participants | Pre- and perinatal | Overweight and obesity | Positive |
| Mulder et al. [22], (2016) | The Netherlands | Case-sibling and case-control | 0–5 years old | 2,456 participants | Pre- and perinatal | Asthma | Case-sibling: positive only 3rd trimester |
| Case-control study: positive in any trimester | |||||||
| Örtqvist et al. [17], (2019) | Sweden | Population-based | 0–6 years old | 827,239 participants | Pre- and perinatal | IBD | Positive |
| Pedersen et al. [15], (2017) | Denmark | Birth cohort | 0–3 years old | 514 (OM) and 699 (VT) participants | Pre- and perinatal | OM and VT | OM: positive (and doseresponse association) |
| VT: positive | |||||||
| Timm et al. [18], (2017) | Denmark | Birth cohort | 18 months old | 62,560 mother-child pairs | Prenatal | AD | Positive |
| Turi et al. [24], (2021) | Tennessee (United States) | Population-based cohort | 0–8 years old | 84,214 mother-child pairs | Pre- and perinatal | Asthma | Positive (and dose-response association) |
| Wan et al. [31], (2020) | Hungary, South Korea, Denmark, New Zealand, UK, Canada, United States, Finland, The Netherlands | Systematic review and meta-analysis | 0–11 years old | 1,253,035 participants total | Pre- and perinatal | Childhood overweight and obesity | Negative |
| Wohl et al. [19], (2015) | Pennsylvania (United States) | Retrospective analysis | 0–2 years old | 492 mother-child pairs | Prenatal | AD | Positive |
OM: otitis media, VT: ventilation tubes, BMI: body mass index, UTI: urinary tract infection, AGE: acute gastroenteritis, AD: atopic dermatitis, IBD: inflammatory bowel disease, H. pylori: Helicobacter pylori.
Fig. 1. Flow chart of literature review process.
RESULTS
Animals
Alhasan et al. [6] demonstrated that vancomycin intake in pregnant mice is associated with increased asthma severity in a dose-dependent manner. Moreover, mice with the highest antibiotic concentration showed reduced offspring weights and increased miscarriages [6]. When given a diet high in saturated fat later in life, low-dose antibiotics during pregnancy cause weight gain in newborn rat pups, eventually leading to obesity [3]. Maternal intake of the antibiotic neomycin during pregnancy was found to accelerate the development of diabetes mellitus (DM) in diabetic mice compared to the offspring of untreated control mice [7]. In neomycin-treated mice, immune-tolerant antigen-presenting cells with diminished specific autoantigen-presenting capability have been detected both in vitro and in vivo [7]. Tormo-Badia et al. [8] showed that a mixture of metronidazole, neomycin, and polymyxin ingested by pregnant mice is a potential risk factor for type 1 DM.
Male mice treated with prenatal penicillin developed long-lasting alterations in immune regulation, including a significant decline in T-reg cells and CD3+ CD4+ CD25+ lymphocytes [9]. In another study, the offspring of mice treated with a combination of ampicillin, streptomycin, and clindamycin showed reduced interferon-γ production from CD8+ T cells and exhibited alterations in dendritic cell and natural killer cell populations during infection that could contribute to the poor antiviral immunity seen among them [10].
Multiple animal studies reported that antibiotics administered during pregnancy have a psychosocial impact on offspring [9,11,12,13]. In mice, Champagne-Jorgensen et al. [9] showed that the administration of low-dose penicillin during the last week of pregnancy had sex-specific long-term effects on the offspring. Female offspring of the treated group showed decreased anxiety-like behavior, whereas male mice showed abnormal social behavior on a sociability test. Furthermore, male offspring did not exhibit a strong preference for a mouse versus empty chamber compared with the control group [9].
Periconceptional exposure to succinylsulfathiazole in Wistar rats reduced social interactions, increased anxiety, and altered sensorimotor gating in male and female offspring [11]. Persistent alterations in anxiety (increased anxiety), sociability (deficits in social recognition), and cognitive behaviors (cognitive deficits) were observed in the offspring of mice when antibiotics were administered during pregnancy [12]. These effects were greater in the group of mice whose mothers were treated with a cocktail of ampicillin, vancomycin, metronidazole, ciprofloxacin, and imipenem than in the group treated with penicillin only [12]. Sulfamonomethoxine ingestion in pregnant mice reportedly increase anxiety among offspring, and spatial learning and memory were impaired more severely in male versus female offspring and control groups [13]. Furthermore, significantly increased blood glucose levels were observed in pups whose mothers received a high sulfamonomethoxine dose [13].
Human
1. Infections
In a Portuguese birth cohort of 7,459 children, Cunha et al. [14] found an association between prenatal antibiotic use in all trimesters and a higher occurrence of tonsillitis at 4 years of age even after controlling for potential confounders. However, this association could have been coincidental since no association was found with other infections (gastroenteritis, otitis, pneumonia, urinary tract infection, gastroenteritis) [14]. Prenatal antibiotic exposure in all trimesters was associated with an increased risk of otitis media [15]. The risk of ventilation tubes was particularly associated with antibiotic use during the third trimester. The risk of otitis media increases with a higher number of prenatal exposures to antibiotics; however, this association was not identified with ventilation tubes [15]. In a Danish population-based cohort study, all children were tracked from birth until the date of their first hospitalization due to an infection, death, 14th birthday, emigration, or December 31, 2009, whichever occurred first [16]. Antibiotic exposure during pregnancy is associated with a higher incidence of pediatric infection–related hospitalizations. This elevated risk persisted throughout childhood. Overall, hospitalization owing to infection was more likely to occur in boys [16]. When mothers received more antibiotic prescriptions throughout pregnancy and were closer to delivery, there was a higher risk of infection–related hospitalization [16]. A population-based study found a positive association between prenatal antibiotic exposure and very early onset IBD [17]. This increased risk was higher for Crohn’s disease than for ulcerative colitis [17].
In summary, studies have shown positive associations between pre- and perinatal antibiotic use and different infections (tonsillitis, otitis media, and IBD) and an increased risk of childhood infection–related hospitalization.
2. Allergy, asthma, atopic dermatitis, eczema, and wheezing
Timm et al. [18] investigated the association between atopic dermatitis (AD) and prenatal antibiotics among 18-month-old children within the Danish National Birth Cohort, which included 62,560 mother–child pairs. The authors found that prenatal antibiotic exposure throughout pregnancy was associated with an increased risk of AD, but only when the mother was atopic. In a study by Wohl et al. [19], perinatal antibiotic exposure for more than 24 hours before birth was found to be a risk factor for the development of AD by 2 years of age, whereas that for less than 24 hours before vaginal delivery did not increase the risk of AD.
A prospective birth cohort study showed no significant association between prenatal antibiotic exposure and wheezing (infectious and noninfectious) at 3, 6, and 12 months and prenatal antibiotic exposure [20]. According to a population- and register-based nested case-control study, the risk of asthma in the offspring was linked to maternal antibiotic use during pregnancy [21]. Cephalosporins and macrolides were the most strongly associated drugs. Antibiotics for gram-positive bacterial infections are associated with a higher risk of asthma [21]. In the Netherlands, a case-sibling study together with a case-control study was conducted to examine the association between prenatal antibiotic use and asthma in preschoolers [22]. The use of antibiotics during the third trimester of pregnancy has been linked to a higher incidence of asthma in preschool-aged children in both the case-sibling and case-control analyses [22].
Only one case-control study showed a significant association between antibiotic exposure during any trimester of pregnancy and the onset of asthma in preschoolers [22]. A population-based study in Canada found that children born to mothers who received antibiotics during pregnancy had significantly higher rates of asthma than their unexposed counterparts [23]. This association persisted after the adjustment for different confounding factors. Moreover, a dose-dependent increase in the risk of asthma was observed. However, the relationship between maternal antibiotic use and childhood asthma was unaffected by the timing of the mother’s exposure [23]. Similar associations were observed for maternal antibiotic use during the first, second, and third trimesters, but also 9 months before and after pregnancy, suggesting that the association is either not directly causal or not specific to pregnancy [23].
Turi et al. [24] investigated the dose, timing, and spectrum of prenatal antibiotic exposure and the risk of developing childhood asthma in a population-based cohort study and found a significant dose-response association between the number of prenatal antibiotic courses and childhood asthma. Based on the trimester of prenatal antibiotic exposure, the odds of childhood asthma increased by 17% for first-trimester-only exposure, 9% for second-trimester-only exposure, 11% for third-trimester-only exposure, and 38% for multiple-trimester exposure compared with non-exposed children [24]. The effect of timing on the first course was moderated by the total number of maternal courses in children exposed to at least one treatment in utero. The timing of exposure had no effect on the likelihood of developing asthma of the offspring of pregnant women receiving a single course of antibiotics.
Among women who received more than one course of treatment, early exposure to the first course of treatment was linked to a higher chance of developing childhood asthma. A higher risk of childhood asthma was observed in the subgroup analysis of a specific number of courses when the first course was administered early in pregnancy. There was no significant association between the number and spectrum of prenatal antibiotic courses and the risk of developing asthma. Broad-spectrum antibiotics significantly increased the risk of childhood asthma compared with narrow-spectrum antibiotics among children exposed to only one course of antibiotics [24].
Data from 1,080 children who participated in a European birth cohort study (PASTURE), a prospective birth cohort of children living in rural regions of five European countries (Switzerland, Germany, France, Finland, and Austria), revealed an association between prenatal antibiotic exposure, AD, and food allergies [25]. Diseases that began within the first year of life were the most common. Asthma, atopic sensitization, and allergic rhinitis were not associated with prenatal antibiotic exposure [25].
A systematic review showed that, in most studies (9/12), a significant relationship was found between asthma and the prenatal use of antibiotics [26]. For eczema (three studies total), there was an overall significant effect in one study and in two other studies only when prenatal antibiotic exposure was prolonged (>24 hours) or when there was antibiotic exposure occurred in the first or second and third trimesters [26]. Prenatal antibiotic use in the first or second trimester was positively associated with eczema before 1 year of age according to a meta-analysis [27]. However, there was no association between third-trimester antibiotic exposure and infant eczema [27].
In summary, the role of antibiotics in the development of allergies indicates a moderate amount of evidence of the relationship between early life antibiotics and childhood asthma, AD, and eczema. The results were inconsistent; however, a positive association was generally found. Factors such as genetic predisposition or environmental exposure are possible confounding factors and merit additional studies addressing antibiotic use during pregnancy, including the role of family history of atopic disease, pollution, and socioeconomic factors.
3. Overweight and obesity
A growing body of research suggests that antibiotic use is associated with childhood BMI, possibly through processes mediated by gut microbiome alterations [28]. Five studies of prenatal antibiotic exposure and its effect on body weight were identified in a systematic review. All five studies indicated a positive correlation between prenatal antibiotic exposure and overweight/obesity [29]. Two studies reported a significant overall relationship, while the other three reported significant relationships when the frequency of antibiotic administration, antibiotic exposure only during the first or second trimester, and/or overweight status of the mothers were included [29]. Prenatal antibiotic use is reportedly associated with a higher mean BMI at 2 years of age [28]. Associations between prenatal antibiotic usage and childhood BMI varied by trimester of exposure, with first- or second-trimester exposure being more strongly associated with a higher BMI at age 2 years for overweight/obesity. A Danish prevalence study revealed a sex-specific adjusted prevalence with a higher prevalence of overweight and obesity in children with prenatal antibiotic exposure [30]. Among girls, the ratio was 1.16 for overweight and 1.27 for obesity. Among boys, the ratios were 1.37 and 1.29, respectively [30].
A systematic review and meta-analysis investigated whether antibiotic exposure during pregnancy and childhood was associated with childhood overweight or obesity [31]. The results of 23 observational studies of 1,253,035 participants showed that prenatal exposure to antibiotics was not significantly associated with childhood overweight or obesity, whereas an increased risk of overweight or obesity was observed in a subgroup analysis when antibiotics were administered during the second trimester [31]. A dose-dependent relationship between prenatal antibiotic exposure and obesity at 4 years of age was discovered in a cross-sectional national study using covariate-adjusted analysis [32]. Despite evidence linking antibiotic exposure to an increased risk of obesity, subsequent investigations of twins and siblings with discordant results reported no correlation. Thus, antibiotics are unlikely to be major contributors to childhood obesity and these discordant results most likely reflect unmeasured confounding factors [32].
According to a population-based national cohort study, prenatal exposure to narrow-spectrum antibiotics was not associated with overweight in offspring until the age of 11 years [33]. By 7 years of age, exposure to broad-spectrum antibiotics was associated with a higher odds of overweight, but this association disappeared as the children aged. Antibiotic prophylaxis for group B streptococcus (GBS) was not associated with a higher BMI in young children [34,35].
In summary, the current literature shows inconsistent results regarding the relationship between prenatal antibiotic use, overweight, and obesity. These outcomes reflect unmeasured confounding factors and show that pre- and perinatal contact with antibiotics are small contributors to childhood obesity if at all. More well-designed studies are needed to address potential confounders.
4. Psychosocial effects
In a population-based cohort study conducted by Hamad et al. [36] of 214,834 children born in Canada, 37.6% of the individuals had prenatal exposure to antibiotics. There were no differences in the antibiotic dose. During follow-up, 2,965 children were diagnosed with autism spectrum disorder (ASD), and those exposed to antibiotics had a higher risk of ASD, with a hazard ratio of 1.10. An association was observed in women exposed to antibiotics during the second or third trimesters. Hence, exposure to prenatal antibiotics is associated with a slight increase in the risk of ASD.
DISCUSSION
Multiple animal and human studies evaluated the effects of pre- and perinatal antibiotic use on and outcomes of offspring. In animal studies, specific lymphocyte subset typing has shown long-term altered immune regulation [9,10] and profound alterations in the composition of the gut microbiota in mothers and infants [9]. Due to the altered gut microbiome, pathogens can interact with the intestinal surface, resulting in an inflammatory response. These factors may play a significant role in epigenetic and/or immunological alterations, another possible mechanism affecting long-term outcomes. There was also a positive association between autoimmune diseases and pre- and perinatal antibiotic use as a potential risk factor for type 1 DM. Human studies have shown positive associations between pre- and perinatal antibiotics and different infections; however, not all confirmed these associations [14,15,17]. A recent systematic review found that GBS perinatal antibiotic prophylaxis had profound effects on the intestinal microbiota of infants by diminishing beneficial commensals [34], although GBS prophylaxis was not associated with the risk of overweight [35]. Nevertheless, changes in the intestinal microbiota composition during early life may impact the development of the immune system.
Allergies, asthma, and eczema are serious public health issues whose prevalence is increasing worldwide. Animal and human studies reported a dose-dependent association between pre- and perinatal antibiotics and asthma severity. Human studies also reported a positive association between AD and eczema [26,27].
Limited data suggest a positive association between overweight/obesity and antibiotic use during pregnancy. One animal study found an increase in weight only when the offspring were fed a diet high in high saturated fat [5]. The results of human studies reflect unmeasured confounding factors and suggest that antibiotics are, if at all, small contributors to childhood obesity [28,29,30,31,32,33,34,35].
Many animal studies have analyzed the association between pre- and perinatal antibiotic use and psychological problems in offspring, showing that long-term effects can be sex-specific. Associations with abnormal social behavior, cognitive deficits, increased anxiety, and altered sensorimotor gating have been reported. To date, only a few human studies have analyzed the influence of pre- and perinatal antibiotics at the psychological level. One study reported that prenatal antibiotic exposure was associated with a low but increased risk of ASD [36].
This study had some limitations. However, these are association studies, and no human studies have shown causality. The importance of confounding factors cannot be underestimated. However, in animal studies, in which confounding factors can be easily eliminated and causality can be better investigated, multiple effects of antibiotic intake during pregnancy on various health aspects have been demonstrated. Due to interstudy heterogeneity, clear definitions such as childhood asthma and perinatal antibiotic use were not possible. However, this study provides a good summary of the different outcomes.
Given the high prevalence of maternal antibiotic intake during pregnancy [31], our findings have potentially significant translational relevance, particularly considering the implications for health from infancy to later life. This review emphasizes the possible consequences for the child, including an increased risk of asthma and atopy, increased susceptibility to infections with longer hospital stays, and a possible negative effect on the psyche. No or dubious associations were found between antibiotic use during pregnancy and overweight and obesity in childhood. More well-designed studies are needed to better address important potential confounders, such as environmental exposure, familial factors, or genetic predisposition. Other aspects to be addressed in future studies include the influence of different antibiotics, as some studies have shown different effects of specific antibiotics.
Footnotes
Funding: None.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M. A review of antibiotic use in pregnancy. Pharmacotherapy. 2015;35:1052–1062. doi: 10.1002/phar.1649. [DOI] [PubMed] [Google Scholar]
- 2.Ghouri F, Hollywood A, Ryan K. A systematic review of non-antibiotic measures for the prevention of urinary tract infections in pregnancy. BMC Pregnancy Childbirth. 2018;18:99. doi: 10.1186/s12884-018-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27:89–94. doi: 10.5830/CVJA-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong CYL, Bloomfield FH, O’Sullivan JM. Factors affecting gastrointestinal microbiome development in neonates. Nutrients. 2018;10:274. doi: 10.3390/nu10030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker WA. Bacterial colonization of the newborn gut, immune development, and prevention of disease. Nestle Nutr Inst Workshop Ser. 2017;88:23–33. doi: 10.1159/000455210. [DOI] [PubMed] [Google Scholar]
- 6.Alhasan MM, Cait AM, Heimesaat MM, Blaut M, Klopfleisch R, Wedel A, et al. Antibiotic use during pregnancy increases offspring asthma severity in a dose-dependent manner. Allergy. 2020;75:1979–1990. doi: 10.1111/all.14234. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Jin P, Peng J, Zhang X, Wong FS, Wen L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J Autoimmun. 2016;72:47–56. doi: 10.1016/j.jaut.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tormo-Badia N, Håkansson Å, Vasudevan K, Molin G, Ahrné S, Cilio CM. Antibiotic treatment of pregnant non-obese diabetic mice leads to altered gut microbiota and intestinal immunological changes in the offspring. Scand J Immunol. 2014;80:250–260. doi: 10.1111/sji.12205. [DOI] [PubMed] [Google Scholar]
- 9.Champagne-Jorgensen K, Mian MF, Kay S, Hanani H, Ziv O, McVey Neufeld KA, et al. Prenatal low-dose penicillin results in long-term sex-specific changes to murine behaviour, immune regulation, and gut microbiota. Brain Behav Immun. 2020;84:154–163. doi: 10.1016/j.bbi.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, Lamousé-Smith ES. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J Immunol. 2016;196:3768–3779. doi: 10.4049/jimmunol.1502322. [DOI] [PubMed] [Google Scholar]
- 11.Degroote S, Hunting DJ, Baccarelli AA, Takser L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:76–82. doi: 10.1016/j.pnpbp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor R, Moloney GM, Fulling C, O’Riordan KJ, Fitzgerald P, Bastiaanssen TFS, et al. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav Brain Res. 2021;404:113156. doi: 10.1016/j.bbr.2021.113156. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Zhang D, Ye K, Liu K, Sheng J, Liu Y, et al. Physiological and behavioral responses in offspring mice following maternal exposure to sulfamonomethoxine during pregnancy. Neurosci Lett. 2016;624:8–16. doi: 10.1016/j.neulet.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Cunha AJLA, Santos AC, Medronho RA, Barros H. Use of antibiotics during pregnancy is associated with infection in children at four years of age in Portugal. Acta Paediatr. 2021;110:1911–1915. doi: 10.1111/apa.15733. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen TM, Stokholm J, Thorsen J, Mora-Jensen AC, Bisgaard H. Antibiotics in pregnancy increase children’s risk of otitis media and ventilation tubes. J Pediatr. 2017;183:153–8.e1. doi: 10.1016/j.jpeds.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Miller JE, Wu C, Pedersen LH, de Klerk N, Olsen J, Burgner DP. Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring: a population-based cohort study. Int J Epidemiol. 2018;47:561–571. doi: 10.1093/ije/dyx272. [DOI] [PubMed] [Google Scholar]
- 17.Örtqvist AK, Lundholm C, Halfvarson J, Ludvigsson JF, Almqvist C. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut. 2019;68:218–225. doi: 10.1136/gutjnl-2017-314352. [DOI] [PubMed] [Google Scholar]
- 18.Timm S, Schlünssen V, Olsen J, Ramlau-Hansen CH. Prenatal antibiotics and atopic dermatitis among 18-month-old children in the Danish National Birth Cohort. Clin Exp Allergy. 2017;47:929–936. doi: 10.1111/cea.12916. [DOI] [PubMed] [Google Scholar]
- 19.Wohl DL, Curry WJ, Mauger D, Miller J, Tyrie K. Intrapartum antibiotics and childhood atopic dermatitis. J Am Board Fam Med. 2015;28:82–89. doi: 10.3122/jabfm.2015.01.140017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins D, Karmaus W, Jiang Y, Banerjee P, Sulaiman IM, Arshad HS. Infant wheezing and prenatal antibiotic exposure and mode of delivery: a prospective birth cohort study. J Asthma. 2021;58:770–781. doi: 10.1080/02770903.2020.1734023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metsälä J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy. 2015;45:137–145. doi: 10.1111/cea.12356. [DOI] [PubMed] [Google Scholar]
- 22.Mulder B, Pouwels KB, Schuiling-Veninga CC, Bos HJ, de Vries TW, Jick SS, et al. Antibiotic use during pregnancy and asthma in preschool children: the influence of confounding. Clin Exp Allergy. 2016;46:1214–1226. doi: 10.1111/cea.12756. [DOI] [PubMed] [Google Scholar]
- 23.Loewen K, Monchka B, Mahmud SM, ’t Jong G, Azad MB. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J. 2018;52:1702070. doi: 10.1183/13993003.02070-2017. [DOI] [PubMed] [Google Scholar]
- 24.Turi KN, Gebretsadik T, Ding T, Abreo A, Stone C, Hartert TV, et al. Dose, timing, and spectrum of prenatal antibiotic exposure and risk of childhood asthma. Clin Infect Dis. 2021;72:455–462. doi: 10.1093/cid/ciaa085. Erratum in: Clin Infect Dis 2022;74:1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzler S, Frei R, Schmaußer-Hechfellner E, von Mutius E, Pekkanen J, Karvonen AM, et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr Allergy Immunol. 2019;30:423–433. doi: 10.1111/pai.13039. [DOI] [PubMed] [Google Scholar]
- 26.Baron R, Taye M, Besseling-van der Vaart I, Ujčič-Voortman J, Szajewska H, Seidell JC, et al. The relationship of prenatal antibiotic exposure and infant antibiotic administration with childhood allergies: a systematic review. BMC Pediatr. 2020;20:312. doi: 10.1186/s12887-020-02042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang FQ, Lu CY, Wu SP, Gong SZ, Zhao Y. Maternal exposure to antibiotics increases the risk of infant eczema before one year of life: a meta-analysis of observational studies. World J Pediatr. 2020;16:143–151. doi: 10.1007/s12519-019-00301-y. [DOI] [PubMed] [Google Scholar]
- 28.Cassidy-Bushrow AE, Burmeister C, Havstad S, Levin AM, Lynch SV, Ownby DR, et al. Prenatal antimicrobial use and early-childhood body mass index. Int J Obes (Lond) 2018;42:1–7. doi: 10.1038/ijo.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron R, Taye M, Besseling-van der Vaart I, Ujčič-Voortman J, Szajewska H, Seidell JC, et al. The relationship of prenatal and infant antibiotic exposure with childhood overweight and obesity: a systematic review. J Dev Orig Health Dis. 2020;11:335–349. doi: 10.1017/S2040174419000722. [DOI] [PubMed] [Google Scholar]
- 30.Mor A, Antonsen S, Kahlert J, Holsteen V, Jørgensen S, Holm-Pedersen J, et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int J Obes (Lond) 2015;39:1450–1455. doi: 10.1038/ijo.2015.129. [DOI] [PubMed] [Google Scholar]
- 31.Wan S, Guo M, Zhang T, Chen Q, Wu M, Teng F, et al. Impact of exposure to antibiotics during pregnancy and infancy on childhood obesity: a systematic review and meta-analysis. Obesity (Silver Spring) 2020;28:793–802. doi: 10.1002/oby.22747. [DOI] [PubMed] [Google Scholar]
- 32.Leong KSW, McLay J, Derraik JGB, Gibb S, Shackleton N, Taylor RW, et al. Associations of prenatal and childhood antibiotic exposure with obesity at age 4 years. JAMA Netw Open. 2020;3:e1919681. doi: 10.1001/jamanetworkopen.2019.19681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jess T, Morgen CS, Harpsøe MC, Sørensen TIA, Ajslev TA, Antvorskov JC, et al. Antibiotic use during pregnancy and childhood overweight: a population-based nationwide cohort study. Sci Rep. 2019;9:11528. doi: 10.1038/s41598-019-48065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmermann P, Curtis N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2020;105:201–208. doi: 10.1136/archdischild-2018-316659. [DOI] [PubMed] [Google Scholar]
- 35.Metz TD, McKinney J, Allshouse AA, Knierim SD, Carey JC, Heyborne KD. Exposure to group B Streptococcal antibiotic prophylaxis and early childhood body mass index in a vaginal birth cohort. J Matern Fetal Neonatal Med. 2020;33:3318–3323. doi: 10.1080/14767058.2019.1571575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF. Prenatal antibiotics exposure and the risk of autism spectrum disorders: a population-based cohort study. PLoS One. 2019;14:e0221921. doi: 10.1371/journal.pone.0221921. [DOI] [PMC free article] [PubMed] [Google Scholar]