Summary
Octopuses coordinate their arms in a range of complex behaviors. In addition to brain-based sensorimotor integration and control, interarm coordination also occurs through a nerve ring at the arms’ base. Here, we examine responses to mechanosensory stimulation of the arms by recording neural activity in the stimulated arm, the nerve ring, and other arms in a preparation of only the ring and arms. Arm axial nerve cords show graded responses to mechanosensory input and activity is transmitted proximally and distally in the arm. Mechanostimulation of one arm generates spiking in the nerve ring and in other arms. Activity in the nerve ring decreases with distance from the stimulated arm. Spontaneous activity with a range of spiking patterns occurs in the axial nerve cords and the nerve ring. These data show rich interarm signaling that supports arm control and coordination occurring outside of the brain.
Subject areas: Mechanobiology, Marine organism, Biological sciences, Sensory neuroscience
Graphical abstract
Highlights
-
•
The axial nerve cord of the octopus arm has a graded neural response to touch
-
•
Signals are transmitted proximally and distally in the arm
-
•
Touch to one arm generates spiking in the arm-connecting nerve ring and other arms
-
•
Signal strength in the nerve ring is attenuated with distance from the touched arm
Mechanobiology; Marine organism; Biological sciences; Sensory neuroscience
Introduction
Octopuses are remarkable in their ability to coordinate their eight bendable, twistable, extensible, and sucker-bearing arms. The neural control system for arm movement includes a large axial nerve cord, which runs the arm’s length and includes ganglia and fiber tracts.1 The axial nerve cord is connected to the brain through brachial nerves that convey signals bidirectionally.2 In addition, the arms have neural connections to one another at their base. The most prominent of these structures is a nerve ring,3 which includes a set of interbrachial commissures that bridge between the axial nerve cords of neighboring arms to form a continuous morphological neural connection around all eight.
Functional studies have shown the coordination of an arm and between arms to be both arm and brain based. Individual arms removed from the rest of the body respond to simple electrical stimulation with a pattern of bending that is consistent with reaching in the behaving animal, suggesting that the neural circuits for foundational kinematics are in the arm for at least some core movements.4 In contrast, fetching, a goal directed movement, cannot be achieved with an isolated arm, requiring input from supraesophageal brain areas.5 Similar results were shown when looking broadly at behavior of all arms after lesion of supraesophageal brain areas.6 Arms would respond to scratching the mantle, bending, and reaching toward the site of stimulation, but crawling, righting, aspects of feeding, and other such complex behavior could not be elicited. Some interarm coordination was observed; when one arm was pulled, arms on the other side of the body would attach to the bottom, anchoring the animal, and when one arm grasped an object, others were drawn to the same location to participate in grasping.6 That some, limited, multi-arm coordination occurs without the presence of supraesophageal regions, highlights the potential role of the nerve ring in mediating such behavior.
Here, we examine neural responses to mechanosensory input, a press-and-hold stimulation of an arm, in the same arm, in the interbrachial commissures and in other arms in the absence of brain input. Although arms are mechanosensitive and the axial nerve cord shows a strong response to mechanosensory input,7 related neural responses in and through the interbrachial commissures and nerve ring have not been reported previously. We aimed to examine how interarm signaling through peripheral (non-brain) mechanisms could contribute to the control and coordination of the arms. To isolate and record from multiple arms and the nerve ring without descending cerebral input, we removed all neural tissue above the brachial nerves, including all supra- and subesophageal ganglia. We found, as had been assumed from anatomical investigation3 that the nerve ring is a major pathway for communicating input received by one arm to other arms and we describe the basic features of signaling within the ring. Other (smaller) interarm neuroanatomical connections besides the axial nerve cord and nerve ring have recently been described.8 These results only begin to explore the complexity of interarm circuits outside of the brain, a fascinating architecture that contrasts with those of other model systems for addressing control of limb-based behavior.
Results
Mechanosensation in the octopus arm
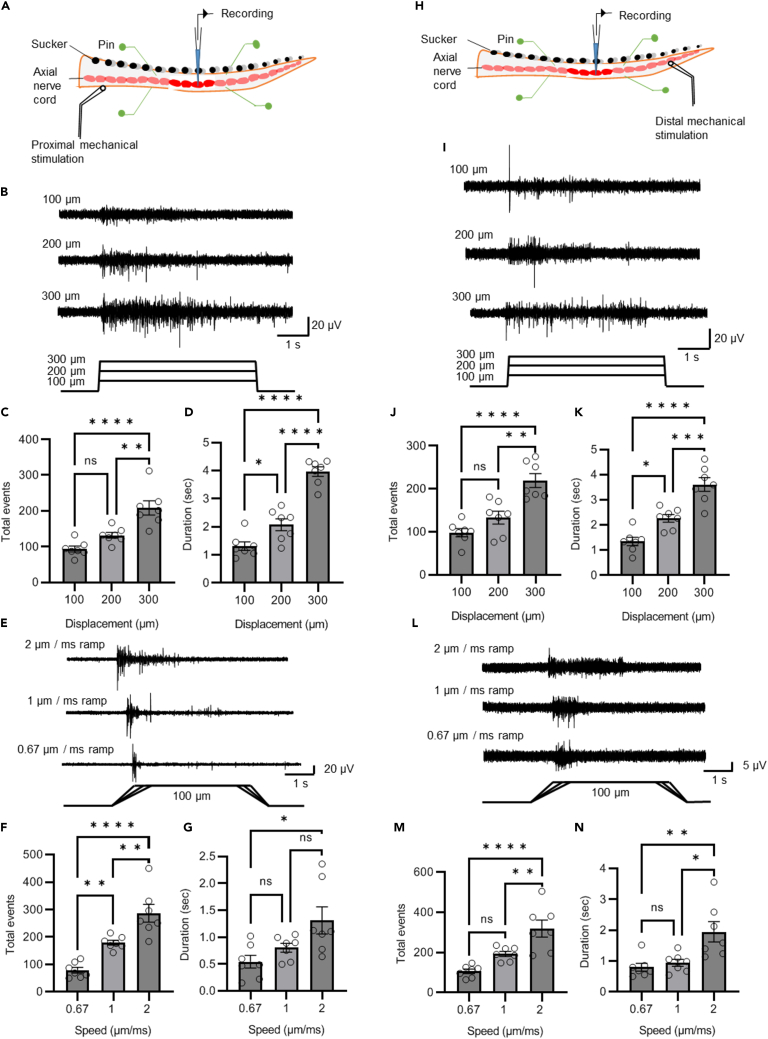
We examined how mechanosensory information is coded within an individual arm by recording activity from the arm’s axial nerve cord in response to mechanical stimulation (press-and-hold stimulus; N = 23 animals, one arm per animal). Mechanical stimulation was delivered either proximal (Figure 1A) or distal (Figure 1H) to the recording electrode. Both proximal and distal stimulation elicited sensorimotor activity in the axial nerve cord.
Figure 1.
Responses to mechanosensory input in the arm
(A) The arm was pinned down onto a Sylgard-coated chamber around the recording area to reduce the spontaneous movement. Axial nerve cord activity was recorded using a suction electrode. Mechanical stimuli were delivered by pressing on the arm proximal to the recording electrode.
(B) Representative responses to proximal stimulation amplitudes of 100, 200, and 300 μm.
(C) The total number of events recorded in response to 100, 200, and 300 μm displacements (N = 7 animals, 5 trials per animal) is significantly greater in the 300 μm displacement group.
(D) The duration of activity was significantly greater with increased stimulation amplitude.
(E) Representative responses to proximal stimulation speeds of 2, 1, and 0.67 μm/s.
(F) The total number of events in response to 2, and 0.67 μm/s deflection (N = 7 animals, 5 trials per animal) proximal to the recording site increased significantly with speed of stimulation.
(G) The duration of activity also increased with significant difference in the duration of activity only being seen between lowest and highest speeds.
(H) Responses to the same stimuli distal to the recording electrode were also captured.
(I) Representative responses to distal stimulation amplitudes of 100, 200, and 300 μm.
(J) The total number of events recorded in response to 100, 200, and 300 μm displacements (N = 7 animals, 5 trials per animal) is significantly greater in the 300 μm displacement group.
(K) The duration of activity in response to 100, 200, and 300 μm displacements (N = 7 animals, 5 trials per animal) distal to the recording site is significantly greater with increasing stimulation amplitude.
(L) Representative responses to distal stimulation speeds of 2, 1, and 0.67 μm/s.
(M). The total number of events in response to 2, 1, and 0.67 μm/s deflections (N = 7 animals, 5 trials per animal) distal to the recording site increased significantly with the speed of stimulation.
(N) The duration of activity also increased with a significant difference in the duration of activity only being seen with the highest speed. Data represent the mean ± SEM; ∗∗p < 0.01, ∗∗∗∗p < 0.0001; one-way ANOVA with Bonferroni post hoc tests.
In response to mechanical stimulation proximal to the recording site there was considerable neural activity in the axial nerve cord, indicating signals traveling from the base toward the distal end of the arm. Neural activity induced by mechanical stimulation was detected using the built-in event detection function in Clampfit software. Activity recorded in the axial nerve cord had a high firing rate at the onset of stimulation with firing rate decreasing during the hold period. Impulses were consistently evoked at all deformation magnitudes applied (100, 200, and 300 μm; ramp speed 1 μm/ms; N = 7 animals, 5 trials per animal per deformation magnitude). Typical activity elicited by stimulating proximal to the recording site is shown in Figure 1B. Larger mechanical stimulation displacement steps induced more firing spikes in the axial nerve cord (100 μm: 93.5 ± 8.2 spikes; 200 μm: 131.6 ± 8.0 spikes; 300 μm: 208.4 ± 20.1 spikes, Figure 1C). The duration of axial nerve cord activity is also positively correlated with increasing deformation from 100 μm to 300 μm, 100 μm: 1314.3 ± 152.9; 200 μm: 2075.2 ± 210.5; 300 μm: 3962 ± 167.6, Figure 1D). The effects of the speed (ramp speed 0.67, 1, and 2 μm/ms for a 100 μm deformation; N = 7 animals, 5 trials per animal per speed) of the deformation to the proximal end of the arm are shown in Figure 1E. Statistical results show that higher stimulation speed generated more firing spikes in the axial nerve cord recordings (0.67 μm/ms: 78.7 ± 10.5 spikes; 1 μm/ms: 179.3 ± 8.7 spikes; 2 μm/ms: 286.4 ± 32.4 spikes, Figure 1F). Higher stimulation speed also generated longer firing duration in the axial nerve recordings; The duration of axial nerve cord activity increases significantly with increasing speed of mechanical stimulation (0.67 μm/ms: 543.4 ± 119 ms; 1 μm/ms: 803.6 ± 82 ms; 2 μm/ms: 1312 ± 250.7 ms, Figure 1G).
Axial nerve cord activity was also elicited by mechanical stimulation distal to the recording electrode, indicating signaling transmitted toward the base of the arm (Figure 1I). Similar to the response to proximal arm stimulation, the axial nerve cord continued to respond for a portion of the hold period of the mechanical stimulus. Increasing deformation steps from 100 μm to 300 μm significantly increased firing spikes in the axial nerve cord (100 μm: 98.3 ± 9.5 spikes; 200 μm: 133.3 ± 15.1 spikes; 300 μm: 219 ± 16.2 spikes, Figure 1J). The duration of axial nerve cord activity was also longer in response to larger deformation steps (100 μm: 1344.2 ± 163.5 ms; 200 μm: 2268.3 ± 154.1 ms; 300 μm: 3610.3 ± 273.7 ms, Figure 1K) (100, 200, and 300 μm; ramp speed 1 μm/ms; N = 7 animals, 5 trials per animal per deformation distance). The effects of the initial speed of the mechanical stimulation (ramp speed 0.67, 1, and 2 μm/ms for a 100 μm deformation; N = 7 animals, 5 trials per animal per speed) on the distal end of the arm are shown in Figure 1L. Higher stimulation speeds generated more firing spikes in the axial nerve recordings (0.67 μm/ms: 106.1 ± 11.2 spikes; 1 μm/ms: 192.9 ± 12.9 spikes; 2 μm/ms: 319.7 ± 42.5 spikes, Figure 1M), and also resulted in a longer duration of activity (0.67 μm/ms: 797.1 ± 129.4 ms; 1 μm/ms: 939 ± 113.3 ms; 2 μm/ms: 1949.2 ± 332.2 ms, Figure 1N).
These results show descending and ascending axial nerve cord signal transmission in response to mechanosensory inputs, which is consistent with previous findings.1 We did not observe any significant difference in axial nerve cord activity in response to stimulation either proximal or distal to the recording site (Figure S1A; two-way ANOVA p = 0.9454; and 1B; two-way ANOVA p = 0.9621). However, as the stimulation regimes were presented in different preparations, we were unable to compare responses from the same electrode and position. We found that the latency between the onset of stimulation and response in the axial nerve cord was significantly shorter when stimulating distal to the recording site (Figures S1C and S1D Proximal: 21.2 ± 2.4; Distal: 11.4 ± 1.2). This result is consistent with previous studies by Zullo et al.9
Off responses can sometimes be observed from either proximal or distal mechanical stimulation. Off responses were more frequent (Figures S2A and S2B; Proximal stimulation: 100 μm: 38.9 ± 7.2 spikes; 200 μm: 49.6 ± 6.2 spikes; 300 μm: 60.2 ± 4.0 spikes, distal stimulation: 100 μm: 36.7 ± 6.2 spikes.; 200 μm: 51.2 ± 8.8 spikes; 300 μm: 58.4 ± 3.5 spikes) and had a longer duration when mechanical stimulation steps were larger (Figure S2C; Proximal stimulation: 100 μm: 7.3 ± 2.1 spikes.; 200 μm: 13.6 ± 2.5 spikes; 300 μm: 16.7 ± 2.7 spikes, distal stimulation: 100 μm: 8.9 ± 3.2 spikes; 200 μm: 11.4 ± 2.9 spikes; 300 μm: 18.2 ± 2.6 spikes. Figure S2D; Proximal stimulation: 100 μm: 21.2 ± 4.5 spikes.; 200 μm: 25.3 ± 5.8 spikes; 300 μm: 57.5 ± 9.6 spikes, distal stimulation: 100 μm: 15.8 ± 3.2 spikes; 200 μm: 31.4 ± 6.9 spikes; 300 μm: 49.6 ± 5.7 spikes). Off responses were also more likely to occur when stimulation speeds were higher (Figures S2E and S2F; Proximal stimulation: 0.67 μm/ms: 22.3 ± 3.4%; 1 μm/ms: 37.5 ± 9.6%; 2 μm/ms: 43.8 ± 5.7%, distal stimulation: 0.67 μm/ms: 21.6 ± 3.1%; 1 μm/ms: 39.6 ± 3.4%; 2 μm/ms: 44.2 ± 2.8%) and the duration of firing was also longer in response to higher stimulation speeds (Figure S2G; Proximal stimulation: 0.67 μm/ms: 3.2 ± 1.2 ms; 1 μm/ms: 4.6 ± 1.3 ms; 2 μm/ms: 8.7 ± 2.4 ms, distal stimulation: 0.67 μm/ms: 4.2 ± 1.1 ms; 1 μm/ms: 5.1 ± 1.2 ms; 2 μm/ms: 7.9 ± 2.8 ms; Figure S2H; Proximal stimulation: 0.67 μm/ms: 5.2 ± 1.3 ms; 1 μm/ms: 8.6 ± 2.4 ms; 2 μm/ms: 12.1 ± 3.0 ms, distal stimulation: 0.67 μm/ms: 5.5 ± 1.1 ms; 1 μm/ms: 10.1 ± 1.2 ms; 2 μm/ms: 13.9 ± 2.7 ms). Note that the stimulation speed was consistent at the onset and end of the press-and-hold stimulations.
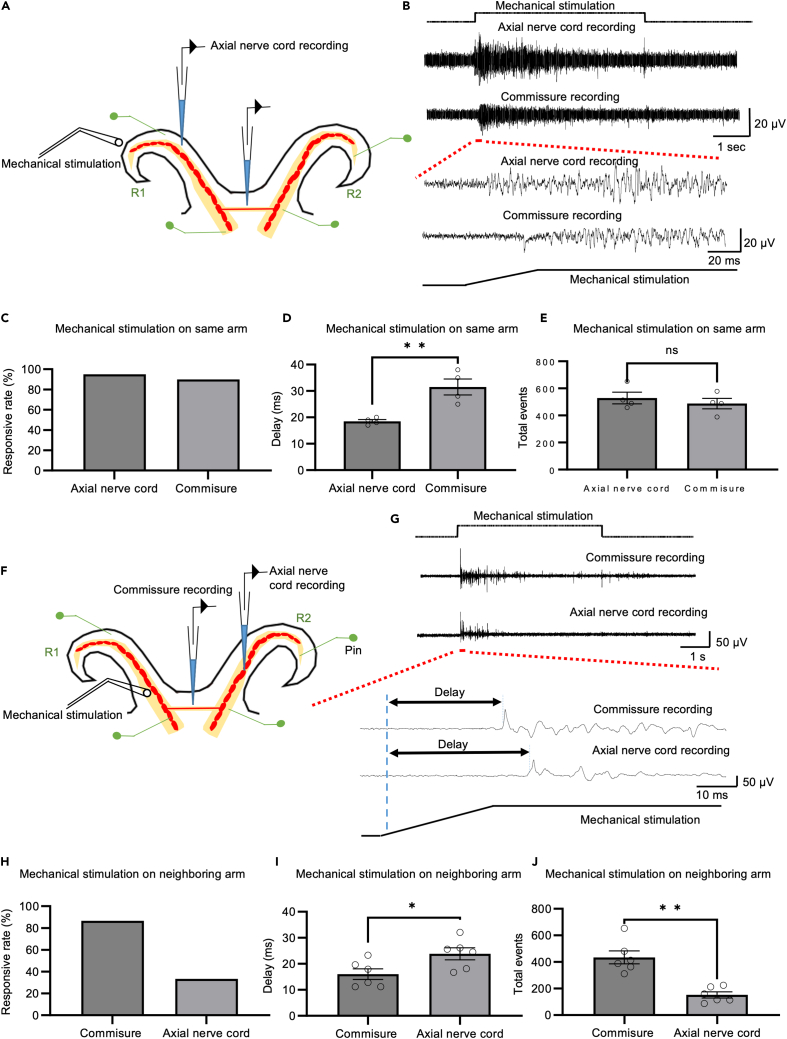
Physiological connection through the interbrachial commissures and to neighboring arms
To understand whether the mechanosensory signal is transmitted from the axial nerve cord to the nerve ring, we performed mechanostimulation on the distal end of R1 and performed double recordings on the R1 axial nerve cord, and the nerve ring between the R1 and R2 arm (Figure 2A). Mechanical stimulation from the arm can induce neural activity in the nerve ring (Figure 2B). In 19 out of 20 trials of mechanical stimulation, axial nerve cord activities were induced, and in 18 out of 20 trials of mechanical stimulation, nerve ring activities were induced, indicating that most of the nerve cord activities are transmitted to the nerve ring (N = 4 animals, 5 trials per animal; Figure 2C). The response onset of the nerve ring was always later than that recorded in the axial nerve cord, suggesting that the direction of sensorimotor signal transmission was from the axial nerve cord to the nerve ring. We found that the average delay time from the stimulation of the right-side position 1 (R1) arm to the response of the R1 axial nerve cord recording was 18.1 ± 1.4 ms, and the delay from the stimulation site to the nerve ring was 32.3 ± 3.5 ms (N = 4; 5 trials per animal; Figure 2D). The total spikes induced by mechanical stimulation recorded in the axial nerve cord and the nerve ring were not significantly different, further suggesting that considerable axial nerve cord activity is transmitted to the nerve ring (N = 4; 5 trials per animal; Figure 2E).
Figure 2.
Signal transmission between the axial nerve cord, interbrachial commissure, and neighboring arm
(A) Experimental setup of an octopus ring preparation. Mechanical stimulation was delivered to the distal end of the R1 arm while responses were recorded with suction electrodes, one in the axial nerve cord of the right-side position 1 arm (R1) and the other from the commissure between the R1 and the right-side position 2 arm (R2). The ring preparation was pinned down on a Sylgard-coated chamber to reduce movement.
(B) Mechanical stimulation of the distal end of the R1 arm can elicit neural activity in the axial nerve cord of the R1 arm and interbrachial commissure between the R1 and R2 arms (upper panel). An example of typical delay of activity onset in the axial nerve cord recording and interbrachial commissure is shown in the lower panel.
(C) In 95% of trials, mechanical stimulation could induce R1 axial nerve cord activities, and in 90% of trials, the signal was transmitted to the commissure (N = 4) when the distal end of the R1 arm was mechanically stimulated.
(D) Onset of activity in the commissure between the R1 and R2 arms is significantly delayed compared to that in the axial nerve cord of the R1 arm (N = 4) when the distal end of the R1 arm is mechanically stimulated.
(E) Summary data show that neural activity in the R1 axial nerve cord and commissure between the R1 and R2 arms are not significantly different in response to mechanical stimulation of the R1 arm (N = 4).
(F) Same as A, except that now one of the recording electrodes is moved to the R2 axial nerve cord.
(G) Mechanical stimulation of an arm can elicit neural activity in the interbrachial commissure and in the axial nerve cord of the adjacent arm (upper panel). An example of typical delay of activity onset in the interbrachial commissure and axial nerve cord recording is shown in the lower panel.
(H) In 85.7% of trials, mechanical stimulation was transmitted to the commissure, and in 35.7% of trials, the signal was transmitted to an adjacent arm (N = 6) when the neighboring arm was mechanically stimulated.
(I) Onset of activity in the axial nerve cord is significantly delayed compared to that in the commissure (N = 6) when the neighboring arm is mechanically stimulated.
(J) Summary data showed that neural activity in the commissure is significantly more robust than that in the axial nerve cord in response to mechanical stimulation of the neighboring arm (N = 6). Data represent the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01; paired Student’s t-test.
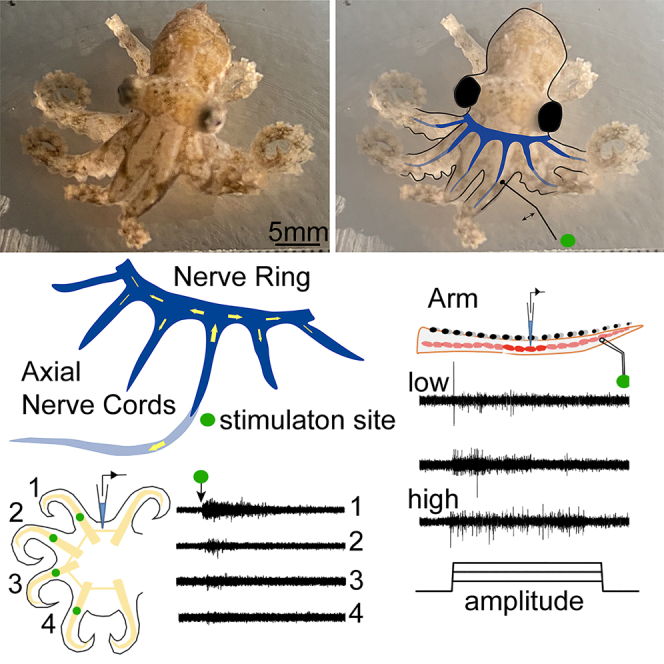
To examine signal transmission from arm to arm, we mechanically stimulated one arm and performed extracellular recordings from the nerve ring and an adjacent arm (N = 6 animals; 3–5 trials per animal). The recording and stimulation locations are shown in Figure 2A. Mechanical stimulation of one arm is capable of inducing neural activity in the nerve ring and neighboring arms (Figure 2B). Recordings were made from the right-side position two arm (R2) of four animals where R1 was stimulated and recordings were made from the R1 arm of two animals in response to mechanical stimulation of the left-side position 1 arm (L1). Mechanical stimulation to the neighboring arms elicited axial nerve cord activity in both preparations, demonstrating that information moving between arms in the nerve ring travels between arms on one side of the body (between R1 and R2) and across the midline (between L1 and R1).
The timing of onset of the axial nerve cord activity in the neighboring arm was always later than that recorded in the nerve ring, suggesting that signals from the stimulation site are first transmitted to the commissure then to the neighboring arm. We found that the average delay time from stimulation of an arm to the response in the commissure was 17.1 ± 1.8 ms and the activity was transmitted to the arms from the stimulation site with a delay of 24.3 ± 2.2 ms (N = 6; 3–5 trials per animal; Figure 2D). The number of spikes in response to arm mechanical stimulation was significantly larger in the interbrachial commissure compared to that in the neighboring arm (N = 6; 3–5 trials per animal; Figure 2E). With the application of the stimulus to the arm we recorded activity in the interbrachial commissure in 85.7% of the trials (N = 6; 3–5 trials per animal; 24 out of 28); we think the lack of signal in at least some of the remaining 14.3% (4 out of 28) of trials was due to the loss of electrode position. In 35.7% (10 out of 28) of the trials stimulating one arm signals were recorded in the neighboring arm (N = 6; 3–5 trials per animal; Figure 2C). In this case, we think that the electrode positioning was consistent but that there was variability in the signaling to the recorded arm. At the end of the deform-and-hold stimulation, when the mechanical probe was lifted from the tissue, we sometimes (5 out of 28 trials) saw an off-response in interbrachial commissure recordings, but we never observed an off response in adjacent arm axial nerve cord recording.
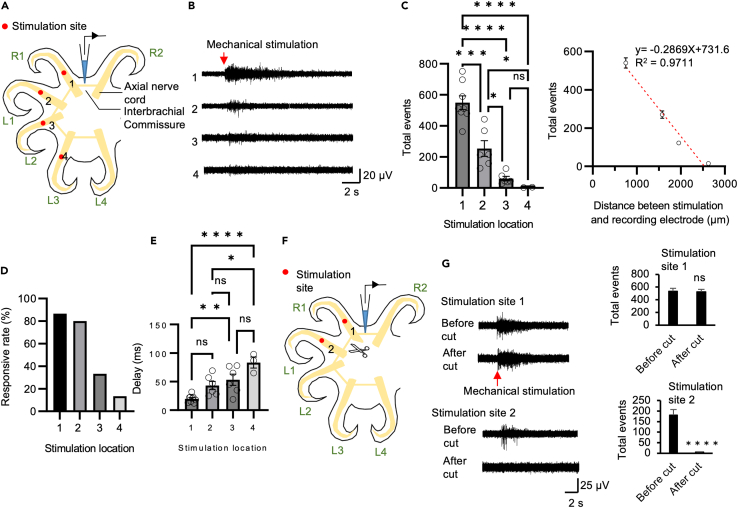
In order to understand whether the nerve ring transmits signals from distant arms, we used a probe to touch different arms (R1, L1, L2, and L3) and recorded interbrachial commissure activity between the R1 and R2 arms. The mechanical stimulation probe was placed on the neighboring arm and arms one, two, and three arm positions away from the recorded commissure (Figure 3A). We found that neighboring arm stimulation induced significantly higher activity in the interbrachial commissure than mechanical stimulation delivered to more distant arms. Activity was negatively correlated with the stimulation distance (Figures 3B and 3C). Sensorimotor signals transmitted from the axial nerve cord to the nerve ring were able to travel across the left-right midline and as far as four arms away. The delay between stimulation onset and activity onset was positively correlated with the stimulation distance (Figure 3E). As in previous experiments, not all stimulation of an arm elicited activity in the commissure (Figure 2C). Here, we found that neighboring arm stimulation had a higher chance of inducing that activity (Figure 3D, location 1: 86.7%; location 2: 80.0%; location 3: 33.0%; location 4: 13.3%).
Figure 3.
Neural activity in the nerve ring in response to mechanical stimulation of different arms
(A) Schematic drawing showed the different mechanical stimulation locations.
(B) Typical activity recorded in the commissure in response to mechanical stimulation of different arms.
(C) Summary data indicate that neural activity in the commissure in response mechanical stimulation to a neighboring arm is significantly more robust (left panel) than when stimulation occurs at more distant arms. The robustness of the neural response is negatively correlated with the distance of mechanical stimulation (right panel) (N = 5, 3–5 trials per animal).
(D) Neighboring arm stimulation also had a higher chance 86.0% of inducing neural activity in the commissure. Stimulation at location 2 has an 80.0%, location 3 has a 33.3% and location 4 has a 13.3% chance of resulting in activity in the commissure.
(E) Stimulation delivered at a shorter distance exhibits significantly less delay compared to stimulation delivered at a greater distance.
(F) Schematic drawing of the location of the commissure transection, mechanical stimulation and recording sites.
(G) Mechanical stimulation did not result in commissure activity if the interbrachial commissure was transected between the stimulation and recording sites. Statistical results showed that commissure transection completely blocked the transmission of mechanosensory information from commissure (N = 4, 3–5 trials per animal). Data represent the mean ± SEM; ∗p < 0.05, ∗∗p < 0.01; one-way ANOVA with Bonferroni post hoc tests. Transection was performed between R1 and L1, commissure recording was made between R1 and R2 arms. Mechanical stimulation was delivered on R1 and L1 arm.
We confirmed that the transmission of activity is through the nerve ring by examining activity in the nerve ring after commissure transection between the stimulating and recording locations. After a commissure is transected, mechanical stimulation of an arm on one side of the transection does not result in activity in the nerve ring on the other side of the transection. However, stimulating an arm and recording from the nerve ring adjacent to the arm (not across the transection) does generate spike activities (N = 4 animals; 3–5 trials per animal for each stimulation site; Figures 3F and 3G; stimulation site 1: before cut: 543.3 ± 42.6 spikes; after cut 530.8 ± 52.4 spikes, stimulation site 2: before cut: 187.3 ± 28.8 spikes; after cut 2 ± 1.1 spikes).
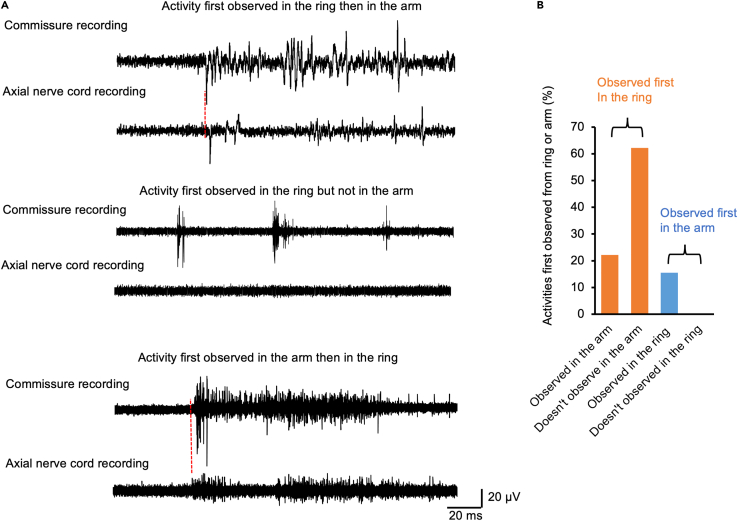
Spontaneous neural activity in the arm and interbrachial commissures
Spontaneous neural activity in the axial nerve cord and nerve ring was recorded. Since the delay in activity between the interbrachial commissure and the axial nerve cord in response to mechanical stimulation is 7.2 ms, we used an 8 ms time window to consider spontaneous activity in the nerve ring and axial nerve cord correlated. We found that activity first seen in the nerve ring wasn’t always associated with activity in the arm while activity first seen in the arm was always associated with activity in the nerve ring (Figure 4A). In all double recording trials (N = 6 animals; 232 bouts of spontaneous activity total across all animals), 22% of the bouts showed activity first seen in the nerve ring that was followed by activity in the arm, 62% showed bouts of activity in the nerve ring that were not followed by activity in the arm, and the remaining 16% of activity bouts were first observed in the arm and subsequent activity was seen in the nerve ring (Figure 4B).
Figure 4.
Spontaneous neural activity in the arm and interbrachial commissures
(A) Typical examples of spontaneous activity recorded in the interbrachial commissure and axial nerve cord. Spontaneous activity first observed in the arms was always transmitted to the commissure, while spontaneous activity first observed in the commissure was not always transmitted to the arms, as observable in our recordings.
(B) 22.3% (52/232) of the activity first observed in the commissure was transmitted to the arm and 62.1% (144/232) of the activity first observed in the commissure was not transmitted to the arm, while 15.5% (36/232) of activity first observed in the arm was not transmitted to the commissure.
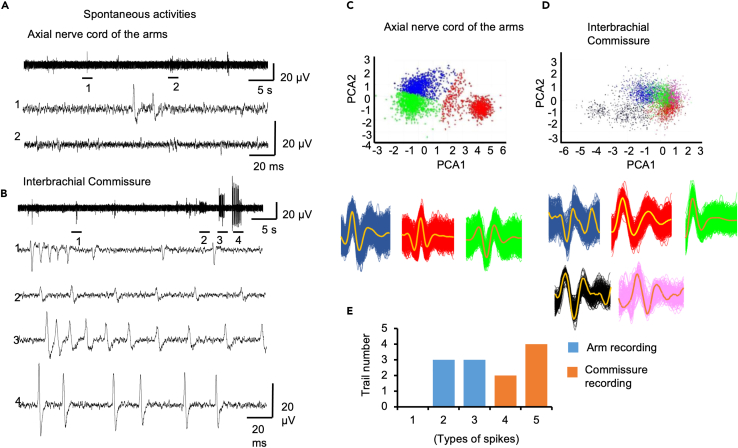
We examined the spike characteristics of the spontaneous activity we recorded. This was possible because the spontaneous firing was sparser than the spiking from our stimulated arm recording. We found that the spontaneous activity we recorded in the arms and nerve ring consisted of multiple types of spikes (Figure 5A). Spike sorting showed that there were 2–3 types of spikes in axial nerve cord recordings, while 4–5 types of spikes were detected in commissure recordings (Figures 5B–5D, N = 6 animals/trials for simultaneous axial nerve cord and commissure recording). We aimed to determine if there were consistent associations between spikes in the arm and spikes in the commissure. Spike detection was performed using threshold methods (Figure S3A), and automatic sorting was carried out in an unsupervised manner using MATLAB R2022a (Figure S3B). In the example shown in Figure S3, spikes from the axial nerve cord recording were sorted into three groups, while spikes from the nerve ring recording were sorted into five groups. We analyzed the cross-correlation within a ±50 ms time window of the detected spikes. Some group comparisons are missing because no spikes were detected within the ±50 ms time window. We found that group 1 of the axial nerve cord spikes had a high correlation coefficient with group 4 spikes from the nerve ring, while group 3 of the axial nerve cord spikes had a high correlation coefficient with group 5 spikes from the nerve ring (Figure S3C). The latency of the peak was within 10 ms, which falls within the range of the average delay from the axial nerve cord to the nerve ring (approximately 8 ms). The delay time observed between the axial nerve cord and the nerve ring, along with the correlated activities of certain spikes in both structures, suggests that some of the spikes recorded in the nerve ring may be transmitted from the axial nerve cord via synapses.
Figure 5.
Spontaneous activities in the commissure and the axial nerve cord
(A) Typical spontaneous activities recorded in the axial nerve cord of the arms. Two segments were selected and magnified in the lower panel.
(B) Typical spontaneous activity recorded in the commissure, four segments were selected and magnified in the lower panel.
(C and D) Spike sorting showed that, in our recordings, spontaneous activity in the interbrachial commissure contained more types of spikes than in the arms.
(E) In six paired recordings, three trials from axial nerve recording had two types of spikes and another three trials had three types of spikes; while in commissure recording, two trials have four types of spikes and four trials have five types of spikes.
Signal transmission in the interbrachial commissure
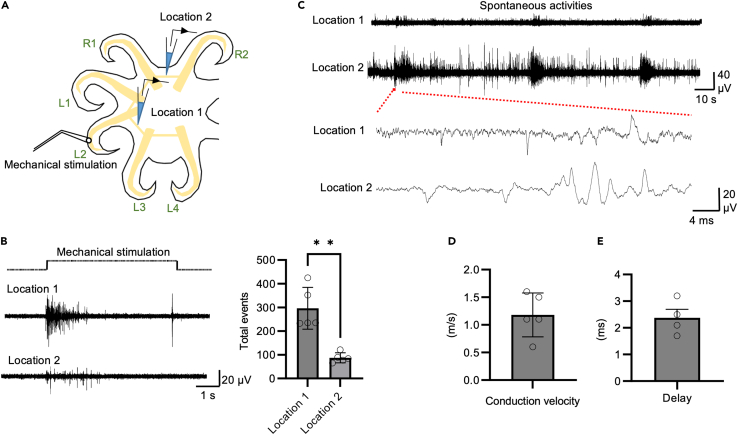
In order to understand how signals are transmitted inside the nerve ring, we performed double recordings from the nerve ring, with recording sites that were two arms away from each other. A mechanical stimulation electrode was placed on the L2 arm. A recording electrode was inserted into the interbrachial commissure between the L2 and L3 arms, which is located posterior to the stimulation electrode. Another recording electrode was inserted into the interbrachial commissure between the R1 and R2 arms, which is located anterior to the stimulation electrode (Figure 6A). Both sites showed activity in response to mechanical stimulation (Figure 6B; N = 5 animals; 2–5 trials per animal, location 1: 296 ± 39.4; location 2: 87 ± 9.4). The electrode between the R1 and R2 arms could detect axial nerve cord sensorimotor signals from L2, indicating that the signals can cross the midline. Both location 1 and 2 electrodes can detect signals transmitted from the axial nerve cord, indicating that sensorimotor signals from the axial nerve cord to the nerve ring can travel in both anterior and posterior directions. Typical spontaneous activity recorded from the two sites is shown in Figure 6C. By calculating the distance between the two electrodes divided by the time delay between the spikes, the conduction speed is around 1.18 ± 0.19 m/s, which falls into the category of unmyelinated axons (Figure 6D; N = 5 animals, 50 bouts of activity per animal). The delay between the two recording sites is 2 ± 0.13 ms in response to the mechanical stimulation, suggesting that the recordings were made on the same nerve tract or at most, a single synapse is involved in this signal transmission (Figure 6E, 50 bouts of activity per animal, N = 4).
Figure 6.
Communication within the interbrachial commissures
(A) The location of stimulation and recording electrodes in the interbrachial commissures.
(B) Typical neural activity recorded in different locations in the interbrachial commissures in response to mechanical stimulation of an arm. Statistical results showed that mechanical stimulation elicit significantly more robust SA activities in closer recording location.
(C) Typical spontaneous activity recorded in two locations within the commissure.
(D and E) Statistical result of propagation velocity (N = 5) (E) Statistical result showed that the delay between two recording site is 2.3 ± 0.31 ms (N = 4). Data represent the mean ± SEM; ∗∗p < 0.01; paired Student’s t test.
Discussion
These findings contribute to the understanding of the physiological responses to mechanostimulation of the arms and show signaling through the nerve ring and to other arms. While the neuroanatomy of the nerve ring,3 and the behavioral experiments of Boycott and Young,6 in which brain regions were removed, indicated that the commissures were sites of information flow among the arms, this has not previously been shown physiologically. In addition, Boycott and Young6 retained some subesophageal brain tissue to provide respiratory function in their preparations, leaving the question of whether the behaviors they recorded might have involved neural control from those areas. In experiments presented here, the brachial nerves were severed and only arm/arm base material remained, preventing any such activity. In these experiments the arm and body tissues generally remained healthy for many hours despite the loss of respiratory centers, perhaps owing to the small size of the animals studied. Controls were also conducted in which an interbrachial commissure was transected. Cutting the commissure removed all responses on one side of the transection to stimulation of the other, indicating that there were no other pathways responsible for the responses recorded here, such as through networks of nerve fibers in the skin or through the intramuscular nerve cords.8 As previously reported for arm movement behaviors,6,10,11 there was no indication of rhythmic neuronal activity generated by a central pattern generator between the arms, as is the case in numerous vertebrate and invertebrate preparations examining motor control in the absence of brain activity (e.g.,12,13,14).
There are other animals with a limb-connecting nerve ring but with no brain. A comparison of particular relevance is the brittle star, an echinoderm, which does not have a brain but does have nerve cords in their five arms that are connected together through a ring at their base. Despite lacking a central brain structure, brittle stars can perform multiple strong locomotor gaits oriented to the direction of movement.15,16,17 Organized locomotion is lost with transection of many nerve ring connectives, though it is generally retained with transection of one or two.18 In contrast, the octopus does not perform organized locomotor movements with the loss of the brain, even when the nerve ring is intact.6 Relatedly some octopuses, at least Octopus bimaculoides and Octopus vulgaris, also do not appear to generate locomotor rhythms when fully intact.19,20 These lines of evidence suggest that central limb-coordinating locomotor rhythm generating circuits that can drive locomotion without a brain in some animals (as in the nerve ring of echinoderms or spinal cord of vertebrates) did not evolve in octopuses but rather another circuit solution has arisen that only functions with brain input.
The nervous system of each arm is capable of driving some movement of the arm without specific input from the brain. Sumbre and colleagues4 showed that an isolated arm can perform extension motions that are consistent with those of an arm with brain connections intact, showing that basic elements of arm motor programs can be accomplished by arm circuits.4 Here we add to these explorations of arm control by examining the response of the axial nerve cord to mechanical stimuli using a deform-and-hold stimulation with different parameters for the deformation (varying amplitude and speed). We presented stimuli proximal and distal to the recording site and found responses to those stimulations to be consistent, with both resulting in strong responses in the axial nerve cord. Both increasing the magnitude of the deformation of the arm tissue and the speed of application of the deformation led to a stronger response, namely increased the number of activity spikes and relatedly increased overall duration of post-stimulus activity during the hold period. Mechanosensory activity in response to larger deformation could last longer; this activity resembles slowly adapting responses found in various species including mammals,21 Xenopus laevis,22 and drosophila.23 In a previous cephalopod study, only relatively fast adapting on-off responses were recorded in the arm when muscles were stimulated in an isolated axial nerve cord preparation.24 We used a whole arm preparation in this study and found that mechanical stimulation could induce slow adapting activity during the hold period. This result is consistent with previous research on mechanosensory response in the brachial connective, in which touch stimulation induces long lasting activity.25
Mechanostimulation of an arm results in activity of nerve fibers in the nerve ring and in other arms. These results contribute to our understanding of interarm coordination outside of the brain, reinforcing the work by Boycott and Young6 that shows behavioral responses of multiple arms from a local stimulation to one arm without major brain input. That we found information from arm stimulation to be carried through the nerve ring, including across the body midline, and across the connections with multiple arms, shows that the response is not simply to neighboring arms, but to a larger set of the arms and potentially all eight arms. Although circuit connections remain to be determined, we examined relative activity (presence and timing) of paired electrodes to begin to gain insight into neuron connectivity. We found that in response to mechanostimulation of an arm, some trials included activity of both the commissure and adjacent arm, while others only showed activity in the commissure. It is possible that there was a problem with the arm recording electrode, such as a shift to a different population of nerve fibers, but nothing about the preparation suggested this was the case. It is also possible that we were seeing alternative pathways for mechanosensory information of an arm with differences in the precise location of the stimulus or other factors leading to different downstream activity. Examining responses from two electrodes positioned in the nerve ring, we estimated the conduction velocity in the nerve ring to be about 1.2 m/s in our preparation, which falls within the range expected for unmyelinated nerve fibers. For double recording on interbrachial commissure nerve fibers separated by two arms (direct distance between two electrodes: 2.5 ± 0.29 mm), the average delay between activity in the two electrodes is within 2 ms. This suggests that the signals might transmitted by the same nerve fiber or a single synapse is involved. Among cold blooded animals, zebrafish has the synaptic delay of around 3 ms26,27; C. elegans and frogs have a synaptic delay within 3 ms.28 The average delay between the nerve ring activity and the arm’s axial nerve cord activity recorded was around 8 ms, and the distance between the recording pipettes was about 2.5 ± 0.29 mm. In our preparation, the nerve conducting velocity is around 1.2 m/s, which means that to travel 2.5 mm would only take 2.1 ms. These results suggest that the sensorimotor signal transmitted across from the interbrachial commissure to the axial nerve cord likely involves multiple synapses.
Limb neural control of octopuses’ shares with many other animals the basic nervous system elements—a brain and nerve cords—built into an overall bilaterally symmetrical system. As in other systems, the brain has been shown to be critical for complex behaviors,29,30,31 but basic movements and limb coordination can occur without the brain (e.g.,12,13). However, key differences in the octopus nervous systems compared to those of other model species make the octopus particularly interesting for comparative study. For example, in many animals, limb coordination occurs through one cord/set of ganglia to which all limbs are connected by nerves while in the octopus each arm has its own separate nerve cord. This structure sets up a very different circumstance for control of an individual arm and for coordination among arms.
The nerve ring was believed, and has been shown here, to be major paths for flow of neural information among arms. That the interbrachial connections are in the form of a ring and the arms are oriented outward from the body, rather than with an anteroposterior orientation, are very different approach to interarm circuits and function that is typical. It seems to be the case that there is more symmetry in connections between any arm and the others in association to their relative position than seen in other animals where limbs are connected in cross-midline pairs, as well as being coordinated in series along the anterior-posterior axis. Kuuspalu and colleagues8 found additional neuroanatomical connections between arms and their third arms over to each side through intramuscular nerve cords, suggesting even greater complexity and divergence in the octopus nervous system.
Limitations of the study
One limitation of the current study is that mechanical stimulation sometimes induced motor responses and it was impossible to separate signal in those periods from what might have been movement artifact with the movements generated sometimes masking the sensory signals. We excluded activities with obvious movements in response to mechanical stimulation. An additional limitation is that we were working on very small axial nerve cords and interbrachial commissures. While we tried to record from consistent locations in large axial tracts at the dorsal margin of the axial nerve cord, to be conservative we did not attempt to interpret the data at the level of a particular location within the axial nerve cord or an interbrachial commissure.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Magnesium chloride | Sigma- Aldrich | Cat# M8266 |
| Artificial sea water | Instant Ocean, S.G. | N/A |
| M2-222 | Sigma- Aldrich | Cat# E10521 |
| Borosilicate glass capillaries | Harvard Apparatus, Holliston, MA, USA | Cat# GC150F-7.5 1.5 mm OD, 0.86ID |
| Sylgard | Dow Corning Corporation, USA | Cat# Sylgard 184 |
| Experimental models: Organisms/strains | ||
| Octopus bimaculoides | Marine Biological Laboratory, Woods Hole, MA, USA. | N/A |
| Software and algorithms | ||
| Prism9 | GraphPad Software, Inc | https://www.graphpad.com/scie ntific-software/prism/ |
| MATLAB R2022b | Mathworks | https://www.mathworks.com/products/matlab.html |
| Spike sorting | Toosi et al.32 | https://github.com/ramintoosi/ROSS |
| Clampfit11 | Molecular device | https://www.moleculardevices.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Melina E Hale (mhale@uchicago.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
During all animal procedures, the most stringent regulations for cephalopod research worldwide, as outlined in EU Directive 63/2010/EU,33 were followed. The work was performed on young octopuses (2-4 months post hatching, species: Octopus bimaculoides) that were obtained from the Marine Biological Laboratory, Woods Hole, MA, USA. Octopuses (N=23, average length between the eyes / head vertical length, 4.0±0.15 / 8.1± 0.16 mm) were used in experiments. This species sexually mature many months after the ages studied here. Determining the gender of hatchling and other very young octopuses through external inspection is not possible as they lack sexually dimorphic traits and internal anatomical evidence remains unclear.
Animals were kept individually in artificial seawater (ASW) (Instant Ocean, S.G. 1.023, pH 8.1-8.2) with standard recirculating water filtration. The water temperature was maintained at approximately 25°C and there was natural lighting. Octopuses were fed daily with pieces of shrimp, and the housing was cleaned daily. All efforts were made to utilize only the minimum number of experimental animals necessary to obtain reliable scientific data.
Method details
Electrophysiology recording
Octopuses were euthanized with MS222 (tricaine methanesulfonate, Sigma, St Louis, MO, USA) at a concentration of 0.8 mg /ml or 330 mM MgCl2. (Sigma-Aldrich). We switched euthanasia to MgCl2 based on Butler-Struben et al34 which showed that it was preferable for animal welfare. We didn’t see significant differences in our recordings between the two euthanasia solutions and so pooled the data. After the cessation of any response to tactile stimulation, the head and much of the body were removed by cutting just below the eye level under the stereomicroscope (Leica MZFLIII, Leica Microsystems, Germany) and the arms and connected tissue were returned to ASW. For recordings on individual arms, arms were excised from the octopus and pinned to the recording chamber coated with Sylgard (Sylgard 184, Dow Corning Corporation, USA). Tissues dorsal and lateral to the axial nerve cord were removed, exposing the cord for recording. Axial nerve cords of L1, L2, R1 and R2 arms were selected for recording. A preparation of the nerve ring was made for interbrachial commissure and multiarm recording. The internal organ and beak of the octopus were removed, and the nerve ring cut between the position four arms. The nerve ring could then be spread and pinned onto Sylgard and the interbrachial commissures and bases of the axial nerve cords were exposed. The tissue was placed onto the recording chamber and the ASW was changed frequently for 20 minutes before the recording began.
Electrophysiological responses to mechanostimulation were recorded. The recording chamber was placed on a custom-built inverted microscope built on an upright compound base (Olympus, USA) with a motorized XY stage (Prior Scientific., USA). The mechanical stimulation probe and recording electrodes were positioned by manipulators (Scientifica Inc., USA). A fire-polished blunted glass probe with tip size of 500 μm in diameter was used for delivering mechanical stimuli at the body surface on either proximal or distal ends of the octopus’s arm (proximal or distal to the recording electrode, Figure 1A) and was controlled by a piezo device (E-727; Physik Instrumente, Auburn, MA, USA). The mechanical stimulation probe was gently touched to the surface of the skin before deformation steps started. Unless otherwise indicated, tissue deformation was induced by a 100μm forward step for the durations of 5 second; the step had a ramp at the speed of 1μm/ms before reaching the 100-μm step and a reversed ramp to its original position at the end of the step. For ramps at different speeds, the hold period shortened so that the entire duration from the start of the stimulus to the end was 5s. Thus, hold duration was 4.7s for 0.67 μm/ms ramps, 4.8 s for 1 μm/ms ramps and 4.9s for 2 μm/ms ramps. The analog outputs from the piezo device were used to monitor the actual movement of the piezo probe and were recorded together with the electrophysiological signals.
Air-bubbled seawater was changed regularly throughout the duration of physiology experiments. An extracellular glass suction electrode was connected to axial nerve cord and interbrachial commissure to record multi-unit response to mechanical stimulation. The diameter of axial nerve cord of a two month-old octopus is less than 200 μm.8 We use suction electrodes with a relative small tip diameter, 20 μm, to suck a portion of the axial nerve and interbrachial commissure to record electrical signals. The electrodes were made by pulling borosilicate glass capillaries (GC150F-7.5 1.5 mm OD, 0.86 ID, Harvard Apparatus, Holliston, MA, USA) in a P-97 Flaming/Brown micropipette puller (Sutter Instrument Co., Novato, CA, USA). An Ag/AgCl reference electrode was placed in the recording chamber. Signals were amplified using a Multiclamp 700B amplifier (Molecular Devices, Foster City, CA, USA), and sampled 10 KHz with low-pass filter set at 1 KHz. The analogue voltage signals were digitized with a DigiData 1440A digitizing board (Molecular Devices) and acquired using pClamp 10 Clampex software (Molecular Devices).
Quantification and statistical analysis
Firing frequency and spike events were analyzed using Clampfit software. Clampfit built in threshold detection function were used to detect mechanical stimulation induced spikes. Threshold for spike detection was set to 5xSD of the background noise. All statistical analyses were performed in GraphPad Prism (GraphPad Software 9.4.1). We used D’Agostino-Pearson method to test the normality of the data. Data are presented as mean ± SEM. Statistical significance was evaluated using Student’s t test; one-way or two-way ANOVA with Bonferroni post hoc tests for multiple groups, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. The recorded potential signals were automated sorted, in an unsupervised approach using MATLAB R2022a (MathWorks, Natick, MA, USA) with an algorithm published by Toosi et al.32 We used the xcorr MATLAB function to compute the cross-correlation of two signals recorded from the axial nerve cord and the nerve ring. For the analysis, a window of ±50 milliseconds was used when spikes were detected in the axial nerve recording electrode. Cross-correlation analyses were only conducted if spikes were detected in the nerve ring recording electrode within the same 50 millisecond time window.
Acknowledgments
This research was funded by the US Office of Naval Research (Tom McKenna, Program Manager, ONR 341), grant number N00014-22-1-2208. The work used equipment funded by the US Department of Defense DURIP grant N00014-19-1-2219. The authors would like to thank members of the Hale Laboratory for their input on the project and broader octopus discussions and the papers reviewers for their effort and expertise. M. E. H. would like to thank collaborators Roger Hanlon (The Marine Biological Laboratory, Woods Hole, MA), Trevor Wardill (University of Minnesota) and Bret Grasse and Taylor Sakmar (both of the Marine Resource Center, The Marine Biological Laboratory, Woods Hole, MA) for introduction to the remarkable world of cephalopods and ongoing conversations and support. The authors would like to thank the Hale Laboratory, particularly Adam Hardy and Adam Kuuspalu, for their input on the initial development of physiology preparations (AH) and octopus anatomy and care (AK).
Author contributions
Conceptualization, M.E.H.; methodology, W.C., M.E.H.; electrophysiology recording and analysis, W.C. and M.E.H.; data interpretation, writing, editing, W.C., M.E.H.; funding acquisition, M.E.H.
Declaration of interests
The authors declare no competing interests.
Published: April 24, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106722.
Supplemental information
Data and code availability
-
•
All raw data or information related to electrophysiology as well as analysis presented in this paper is available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Zullo L., Eichenstein H., Maiole F., Hochner B. Motor control pathways in the nervous system of Octopus vulgaris arm. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2019;205:271–279. doi: 10.1007/s00359-019-01332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shigeno S., Andrews P.L.R., Ponte G., Fiorito G. Cephalopod brains: an overview of current knowledge to facilitate comparison with vertebrates. Front. Physiol. 2018;9:952. doi: 10.3389/fphys.2018.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graziadei P. In the Anatomy of the Nervous System of Octopus Vulgaris. Oxford University Press; 1971. Chapter 3: the nervous system of the arms; pp. 45–61. [Google Scholar]
- 4.Sumbre G., Gutfreund Y., Fiorito G., Flash T., Hochner B. Control of octopus arm extension by a peripheral motor program. Science. 2001;293:1845–1848. doi: 10.1126/science.1060976. [DOI] [PubMed] [Google Scholar]
- 5.Sumbre G., Fiorito G., Flash T., Hochner B. Octopuses use a human-like strategy to control precise point-to-point arm movements. Curr. Biol. 2006;16:767–772. doi: 10.1016/j.cub.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 6.Boycott B., Young J. Vol. 4. Symposia of the Society For Experimental Biology; 1950. pp. 432–453. (The Comparativ Study of Learning). [Google Scholar]
- 7.Rowell C.H. Activity of interneurones in arm of octopus in response to tactile stimulation. J. Exp. Biol. 1966;44:589–605. doi: 10.1242/jeb.44.3.589. [DOI] [PubMed] [Google Scholar]
- 8.Kuuspalu A., Cody S., Hale M.E. Multiple nerve cords connect the arms of octopuses, providing alternative paths for inter-arm signaling. Curr. Biol. 2022;32:5415–5421.e3. doi: 10.1016/j.cub.2022.11.007?. [DOI] [PubMed] [Google Scholar]
- 9.Zullo L., Fossati S., Benfenati F. Vol. 61. Vie Et Milieu-Life and Environment; 2011. pp. 197–201. (Transmission of Sensory Responses in the Peripheral Nervous System of the Arm of Octopus Vulgaris). [Google Scholar]
- 10.Levy G., Hochner B. Embodied organization of. Front. Physiol. 2017;8:164. doi: 10.3389/fphys.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzner H., Gutfreund Y., Hochner B. Neuromuscular system of the flexible arm of the octopus: physiological characterization. J. Neurophysiol. 2000;83:1315–1328. doi: 10.1152/jn.2000.83.3.1315. [DOI] [PubMed] [Google Scholar]
- 12.Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 13.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016;17:224–238. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marder E., Bucher D., Schulz D.J., Taylor A.L. Invertebrate central pattern generation moves along. Curr. Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Arshavskii Y.I., Kashin S.M., Litvinova N.M., Orlovskii G.N., Fel'dman A.G. Types of locomotion in ophiurans. Neurophysiology. 1977;8:398–404. [PubMed] [Google Scholar]
- 16.Arshavskii Y.I., Kashin S.M., Litvinova N.M., Orlovskii G.N., Fel'dman A.G. Coordination of arm movement during locomotion in ophiurans. Neurophysiology. 1977;8:404–410. [PubMed] [Google Scholar]
- 17.Astley H.C. Getting around when you're round: quantitative analysis of the locomotion of the blunt-spined brittle star, Ophiocoma echinata. J. Exp. Biol. 2012;215:1923–1929. doi: 10.1242/jeb.068460. [DOI] [PubMed] [Google Scholar]
- 18.Clark E.G., Kanauchi D., Kano T., Aonuma H., Briggs D.E.G., Ishiguro A. The function of the ophiuroid nerve ring: how a decentralized nervous system controls coordinated locomotion. J. Exp. Biol. 2019;222:jeb192104. doi: 10.1242/jeb.192104. [DOI] [PubMed] [Google Scholar]
- 19.Levy G., Flash T., Hochner B. Arm coordination in octopus crawling involves unique motor control strategies. Curr. Biol. 2015;25:1195–1200. doi: 10.1016/j.cub.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 20.Hale M., Goolsbee A. Substrate-based locomotion in young octopuses. Integr. Comp. Biol. 2020;60:E93. [Google Scholar]
- 21.Ikeda R., Cha M., Ling J., Jia Z., Coyle D., Gu J.G. Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saccomanno V., Love H., Sylvester A., Li W.C. The early development and physiology of. J. Neurophysiol. 2021;126:1814–1830. doi: 10.1152/jn.00618.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y., Cao L.H., Sui X.W., Guo X.Q., Luo D.G. Mechanosensory circuits coordinate two opposing motor actions in. Sci. Adv. 2019;5:eaaw5141. doi: 10.1126/sciadv.aaw5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutfreund Y., Matzner H., Flash T., Hochner B. Patterns of motor activity in the isolated nerve cord of the octopus arm. Biol. Bull. (Woods Hole) 2006;211:212–222. doi: 10.2307/4134544. [DOI] [PubMed] [Google Scholar]
- 25.Crook R.J. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience. 2021;24:102229. doi: 10.1016/j.isci.2021.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang W., Pedroni A., Hohendorf V., Giacomello S., Hibi M., Köster R.W., Ampatzis K. Functionally distinct Purkinje cell types show temporal precision in encoding locomotion. Proc. Natl. Acad. Sci. USA. 2020;117:17330–17337. doi: 10.1073/pnas.2005633117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J., Ampatzis K., Björnfors E.R., El Manira A. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature. 2016;529:399–402. doi: 10.1038/nature16497. [DOI] [PubMed] [Google Scholar]
- 28.Li L., Liu H., Wang W., Chandra M., Collins B.M., Hu Z. SNT-1 functions as the Ca. J. Neurosci. 2018;38:5313–5324. doi: 10.1523/JNEUROSCI.3097-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grillner S., El Manira A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 2020;100:271–320. doi: 10.1152/physrev.00015.2019. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy E.B.L., Buresch K.C., Boinapally P., Hanlon R.T. Octopus arms exhibit exceptional flexibility. Sci. Rep. 2020;10:20872. doi: 10.1038/s41598-020-77873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zullo L., Sumbre G., Agnisola C., Flash T., Hochner B. Nonsomatotopic organization of the higher motor centers in octopus. Curr. Biol. 2009;19:1632–1636. doi: 10.1016/j.cub.2009.07.067. [DOI] [PubMed] [Google Scholar]
- 32.Toosi R., Akhaee M.A., Dehaqani M.R.A. An automatic spike sorting algorithm based on adaptive spike detection and a mixture of skew-t distributions. Sci. Rep. 2021;11:13925. doi: 10.1038/s41598-021-93088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorito G., Affuso A., Basil J., Cole A., de Girolamo P., D'Angelo L., Dickel L., Gestal C., Grasso F., Kuba M., et al. Guidelines for the care and welfare of cephalopods in research -A consensus based on an initiative by CephRes, FELASA and the boyd group. Lab. Anim. 2015;49:1–90. doi: 10.1177/0023677215580006. [DOI] [PubMed] [Google Scholar]
- 34.Butler-Struben H.M., Brophy S.M., Johnson N.A., Crook R.J. Recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front. Physiol. 2018;9:109. doi: 10.3389/fphys.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All raw data or information related to electrophysiology as well as analysis presented in this paper is available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.