Abstract
Radiation therapy (RT) has been the standard of care for treating a multitude of cancer types. However, ionizing radiation has adverse short and long-term side effects which have resulted in treatment complications for decades. Thus, advances in enhancing the effects of RT have been the primary focus of research in radiation oncology. To avoid the usage of high radiation doses, treatment modalities such as high-intensity focused ultrasound can be implemented to reduce the radiation doses required to destroy cancer cells. In the past few years, the use of focused ultrasound (FUS) has demonstrated immense success in a number of applications as it capitalizes on spatial specificity. It allows ultrasound energy to be delivered to a targeted focal area without harming the surrounding tissue. FUS combined with RT has specifically demonstrated experimental evidence in its application resulting in enhanced cell death and tumor cure. Ultrasound-stimulated microbubbles have recently proved to be a novel way of enhancing RT as a radioenhancing agent on its own, or as a delivery vector for radiosensitizing agents such as oxygen. In this mini-review article, we discuss the bio-effects of FUS and RT in various preclinical models and highlight the applicability of this combined therapy in clinical settings.
Keywords: focused ultrasound, radiation therapy, ultrasound-stimulated microbubbles, acid sphingomyelinase, ceramide
Introduction
Radiation therapy (RT) is a standard of care treatment for many different cancer types. Cells exposed to ionizing radiation experience increased levels of oxidative stress and DNA damage which led to cell death if repair mechanisms fail to ameliorate injury. 1 Currently, large doses of RT (> 60 Gy) are often delivered over the course of several weeks in smaller fractions (1.8-2 Gy). This enables the accumulated curative dose to be delivered to target tumor sites while reducing the radiotoxic effects of dose delivery to normal tissue.2,5 Current RT delivery methods are image-guided using a variety of modalities including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) to ensure precise targeting of the tumor region.6,9 Technological advancements have allowed for the development of integrated imaging systems such as the MRI-guided linear accelerator (MR-LINAC).7,12 Despite these advancements in RT, adverse side effects and recurrences continue to plague the field of radiation oncology.13,16 For this reason, many studies look for solutions to boost the effectiveness of radiation while maintaining or reducing the treatment doses.17,18 Recently, many studies have looked at utilizing focused ultrasound (FUS) to enhance RT treatments through a multitude of potential applications.
Focused Ultrasound
Studies as early as 1942 observed the ablative effects of FUS. Lynn et al 19 observed enhanced thermal effects at the focus of concave single-element transducers in paraffin blocks and beef liver tissue, generating lesions within the test samples. Further investigations built upon by the Fry and Fry 20 lead to the early use of FUS to treat neurological disorders in humans. Since then technological development of FUS has resulted in the successful application of this modality for clinical treatments (Figure 1A and B). Most prominently, high-intensity focused ultrasound showed great efficacy in generating lesions within tumor masses and enhancing patient response.21,31 The nonionizing nature of ultrasound provides minimal risk to organs outside of the focal region, making it a safer option than conventional RT. It has successfully been used to treat a wide variety of tumor types including prostate, liver, uterine fibroids, pancreatic, and other solid tumors accessible by FUS.32,40
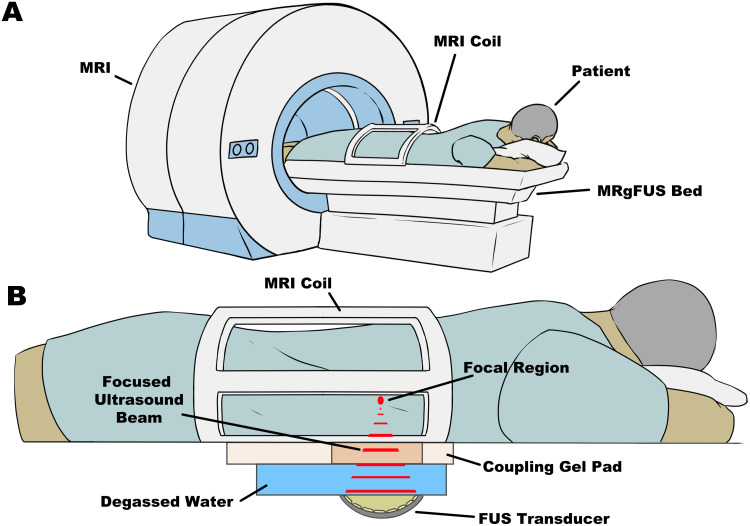
Figure 1.
(A) Physical setup of patients on the MRgFUS system with the FUS system integrated into the bed. Patients may lay on the bed as per the required orientation with the target region of interest positioned over top of the FUS transducer. (B) The FUS system is integrated into the MRI bed. The concave transducer is submerged in degassed, deionized water with a coupling gel pad to allow for the acoustic beams to traverse into the patient with minimal energy loss. The multielement transducer is capable of focusing ultrasound waves to a targeted focal region which can generate heat and induce physical damage to the targeted tissue. All of this is done while the patient is inside the MRI after thorough planning of the treatment.
Abbreviations: FUS, focused ultrasound; MRI, magnetic resonance imaging; MRgFUS, MRI-guided focused ultrasound.
Because of the broad application of FUS, many studies have investigated its implementation in, but not limited to, the opening of the blood–brain barrier,41,44 neuromodulation,45,47 enhancing chemotherapy drug delivery,48,50 immunomodulation,51,54 and enhancing RT response.55,58
Focused Ultrasound and Radiation Therapy
The combination of FUS alongside RT has only seen preliminary studies indicating potential synergy between the 2 different modalities.59,64 An early study conducted by Jernberg et al 59 looked at the effects of FUS (1.1 MHz) and RT (2 Gy) in the Chinese hamster cell line V79-379A in vitro and observed reduced cell survival in the combination treatment compared to FUS or RT alone. It was postulated by Borasi et al61,62 that high-power FUS can complement RT in treating tumors by causing ablations and hyperthermia in the typically avascular and hypoxic core regions of the tumor where RT is potentially less effective, while RT targets the invasive margins of the tumor, a region often missed by FUS treatments. This concept was further explored by Borasi et al 63 as a mathematical model to describe the effect of RT and FUS in treating glioblastoma multiforme. The results indicate that patient survival could potentially be significantly improved with combined FUS and different fractionated radiation regimens. While these results were generated through a theoretical pipeline, they reflect the tangible effects that FUS and RT could have while suggesting safety margins similar to FUS or RT alone.
With the advancement of MRI-Guided FUS (MRgFUS) systems, Zhang et al 64 recently showed an enhanced impact of 10 Gy of RT combined with FUS in suppressing tumor growth and proliferation while increasing levels of apoptosis in PC-3 mouse xenografts. Applications of FUS and RT in clinical settings have also seen success in enhancing RT. Wu et al 65 evaluated the feasibility of replacing reduced field boost irradiation with FUS in treating patients with locally advanced and metastatic prostate cancer. Combination treatment showed a greater overall survival in patients compared to low-dose external beam radiotherapy alone. These promising results show potential for applying this combination treatment modality in a clinical setting.
FUS has only recently begun to become a standard of care treatment in the clinical setting as a monotherapy system. Interest in expanding its application is often overshadowed by optimization and integration into a clinical setting. Currently, much of FUS + RT research revolves around the use of microbubbles (MBs) to induce effects within the tumor microenvironment to enhance RT effects.
Ultrasound-Stimulated MBs
MBs have primarily been utilized as an ultrasound contrast agent to enhance the imaging of a multitude of organ structures.66,69 MBs are often 0.5 to 10 µm in diameter with a core composed of high molecular weight and low solubility gas. Often, bubbles are coated in a lipid or protein shell to enhance stability.68,70 When placed in an acoustic field of a resonant frequency, MB expands and compresses in response to the acoustic pressure applied in a process known as cavitation. MBs undergo one of 2 cavitation states; steady symmetrical oscillations at lower amplitudes (stable cavitation), or rapid, irregular oscillations leading to bubble collapse at higher amplitudes (inertial cavitation).71,72 Both forms of cavitation can generate effects on the surrounding environment such as pushing, pulling, microstreaming, generating shockwaves, and micro-jetting.71,75 These effects are capable of generating perforations in the plasma membrane, in a process known as sonoporation,74,76 which can be exploited to induce endothelial cell apoptosis or enhance the delivery of radiosensitizing agents, such as oxygen.
Ultrasound Stimulated MB (USMB) Mediated Vascular Disruption and RT
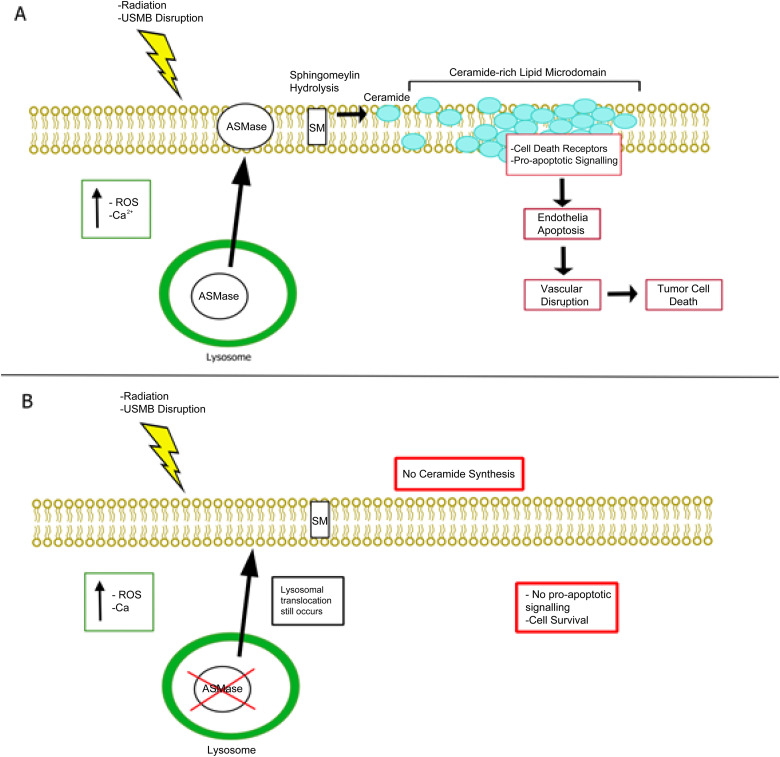
The tumor vasculature is a key component to ensuring tumor growth and survival. High doses of RT (> 8 Gy) induce increased oxidative stress to vascular endothelial cells, resulting in the activation of pro-apoptotic pathways such as the acid sphingomyelinase (ASMase)-ceramide pathway (Figure 2A).77,86 Many in vivo studies involving ASMase knockout mice (ASMase −/−) demonstrate that radiation exposure showed significant levels of endothelial cell apoptosis in wild-type (WT) mice, but not in ASMase −/− groups.77,78,84,87,92 The ASMase-Ceramide pathway was later found to also be activated through USMB mechanotransduction.93,97 This led to the idea of using USMB to enhance lower doses of RT (< 8 Gy) through ASMase activation. Combination treatments of USMB and single doses of 2 and 8 Gy in murine models demonstrated elevated levels of tumor cell death and reduced microvascular density in a dose-dependent manner.56,98,102 Furthermore, tumors in ASMase −/− mice exhibited a radioresistant phenotype against USMB + RT treatments with low single doses (2 and 8 Gy) compared to WT mice bearing fibrosarcoma tumors 56 (Figure 2B). This effect was also observed in fractionated RT regimens in mouse and rabbit xenografts where tumor growth delay and proliferation reduction were observed when combined with USMB treatments.55,100 Furthermore, in a clinical trial conducted by Eisenbrey et al, 103 the efficacy and safety of USMB alongside transarterial radioembolization (TARE) were demonstrated in treating patients with hepatocellular carcinoma. The results showed that 14 of 15 patients treated with TARE + USMB showed a partial or complete response compared to TARE alone, in which only 5 out of 10 patients showed partial or complete response, with no adverse effects. These studies offer great promise in applying USMB treatments alongside standard clinical RT regimens.
Figure 2.
Mechanism of USMB + RT action. (A) Radiation or plasma membrane disruption induces an increase in oxidative stress and calcium influx resulting in lysosomal translocation and fusion to the plasma membrane. ASMase release into the plasma membrane initiates sphingomyelin hydrolyzation to generate ceramide, resulting in the localization of ceramide-rich lipid microdomains. Plasma membrane alteration in these microdomains result in the localization of cell death receptors and factors resulting in pro-apoptotic signaling, leading to endothelial cell apoptosis, vascular disruption, and eventually tumor cell death. (B) ASMase −/− can resist radiation and USMB leading to vascular preservation and tumor cell survival. When ASMase is knocked out, ceramide is not synthesized, thus no pro-apoptotic signaling occurs in endothelial cells.
Abbreviations: ASMase, acid sphingomyelinase; ASMase −/−, acid sphingomyelinase knockout mice; USMB, ultrasound stimulated microbubble; RT, radiation therapy; Ca2 + , calcium ion; SM, Sphingomyelin.
Oxygen Loaded MBs (OMBs) and RT
In the past few years, the use of MBs as a delivery vector has been explored in great depth for chemotherapy to enhance targeting and treatment efficacy.104,105 In the context of FUS + RT, this technique can be applied in the delivery of radiosensitizers, such as oxygen, to elicit greater tumor responses to RT.
Oxygen has been of great interest as a radiosensitizing agent, as hypoxia is a known phenomenon that hinders RT efficacy.106,107 To overcome this, OMBs were developed to allow for reoxygenation in otherwise hypoxic tumor regions. Though there have been no clinical trials as of the date of this publication, many in vitro and in vivo studies have demonstrated the feasibility of utilizing OMB to elevate oxygen levels in a variety of tumors including breast, fibrosarcoma, prostate, head and neck squamous cell carcinoma, and nasopharyngeal carcinoma.108,114 Eisenbrey et al 108 demonstrated, in immunodeficient nude mice, that ultrasound-activated OMB + 5 Gy RT, resulting in breast tumor growth delay at 25 to 35 days with significantly lower growth rates and greater survival. This effect was also seen in studies conducted using higher doses of radiation (> 10 Gy), in which RT + OMB treated tumors showed lower growth rates compared to untreated tumors, demonstrating the range of doses in which OMB can enhance RT effects in single dose in external beam,109,111 and brachytherapy. 114
Overall, these studies show great promise in the efficacy of utilizing OMB to overcome hypoxia and have potential applications to be used in a clinical setting.
Current Limitations of FUS and RT
Though much of the groundwork in the preclinical studies have established the efficacy of combining FUS + RT while demonstrating its safety and application, several limitations must be considered before full implementation in the clinic. For instance, attenuation, reflections, and scattering must be accounted for when planning treatments as these effects can reduce the efficacy of the ultrasound treatment.115,116 Additionally, there are currently several commercially available USMBs with different coating and gas compositions. These properties can affect the efficacy of RT enhancement and there have yet to be studies that compare the difference between them.68,103 Furthermore, adverse effects related to FUS (e.g., thermal damage resulting in organ dysfunction) or RT (e.g., off-target exposure leading to tissue damage) must be taken into great consideration to prevent a reduction of quality of life as a result of the combination treatment.35,60,117
Motion during treatments can also provide additional challenges as there is an added risk of off-target effects in ablative FUS and RT treatments.118,121 While there have been many strategies implemented to overcome this in RT,122,124 further studies would need to be conducted to determine whether they are applicable in a FUS + RT regimen or if other strategies would be needed.
Current MRgFUS systems are used to allow for adequate planning and treatment and have been used primarily for their ablative capabilities. 125 With the state of this current technology, FUS + RT treatments would require excessive planning to ensure that targeting of the tumor is accurate as patients would be required to be moved between different systems. This can potentially be overcome by integrating FUS into MR-LINAC systems, allowing for rapid treatment of FUS and RT using the same treatment plan for precise targeting. However, as of the date of this publication, there are no such systems in place used in clinical settings.
Despite these limitations, the successful application of ultrasound combined with MB together with RT to enhance tumor response has been well documented and continues to suggest viability in safe implementation in the clinical setting.
Conclusion
FUS has progressed significantly since the initial studies began to explore its potential. While the majority of studies involving FUS have been focused on its optimization and implementation in the clinic, the value of it alongside RT must also be recognized. The use of MB cavitation has provided greater promise for FUS + RT applications as many studies have shown the effectiveness of enhancing radiation through vascular disruption or enhancing radiosensitization delivery. Thus, further studies are warranted to explore the upper boundaries of utilizing FUS by exploiting its biological effects in conjunction with RT. All of this taken together shows the feasibility of applying FUS and RT together to enhance patient quality of life while potentially reducing radiation exposure during treatments.
Footnotes
Abbreviations: ASMase, acid sphingomyelinase; ASMase −/−, acid sphingomyelinase knockout mice; FUS, focused ultrasound; LRT, low dose external beam radiation therapy; MR-LINAC, MRI-guided linear accelerator; MRgFUS, MRI-guided focused ultrasound; OMB, oxygen-loaded microbubbles; PC-3, human prostate cancer cell line; TARE, transarterial radioembolization; USMB, ultrasound stimulated microbubble; WT, wild type; RT, radiation therapy
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Statement: Not applicable.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kai Xuan Leong https://orcid.org/0000-0003-3100-8219
Deepa Sharma https://orcid.org/0000-0002-0475-1755
Gregory J. Czarnota https://orcid.org/0000-0002-0519-2182
References
- 1.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Withers HR, Lett JT, Adler H. Advances in radiation biology. 1975; 241-271.
- 3.Haque W, Butler EB, Teh BS. Stereotactic body radiation therapy for prostate cancer-a review. Chin Clin Oncol. 2017;6(Suppl 2):S10. [DOI] [PubMed] [Google Scholar]
- 4.Gensheimer MF, Loo BW, Jr. Optimal radiation therapy for small cell lung cancer. Curr Treat Options Oncol. 2017;18(4):21. [DOI] [PubMed] [Google Scholar]
- 5.Alfouzan AF. Radiation therapy in head and neck cancer. Saudi Med J. 2021;42(3):247‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sterzing F, Engenhart-Cabillic R, Flentje M, Debus J. Image-guided radiotherapy: a new dimension in radiation oncology. Dtsch Arztebl Int. 2011;108(16):274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henke LE, Contreras JA, Green OL, et al. Magnetic resonance image-guided radiotherapy (MRIgRT): a 4.5-year clinical experience. Clin Oncol (R Coll Radiol). 2018;30(11):720‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt A, Hanson I, Dunlop A, et al. Feasibility of magnetic resonance guided radiotherapy for the treatment of bladder cancer. Clin Transl Radiat Oncol. 2020;25:46‐51. Published 2020 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dyk S, Khaw P, Lin MY, Chang D, Bernshaw D. Ultrasound-guided brachytherapy for cervix cancer. Clin Oncol (R Coll Radiol). 2021;33(9):e403‐e411. [DOI] [PubMed] [Google Scholar]
- 10.Raaymakers BW, Lagendijk JJ, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54(12):N229‐N237. [DOI] [PubMed] [Google Scholar]
- 11.Fallone BG, Murray B, Rathee S, et al. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med Phys. 2009;36(6):2084‐2088. [DOI] [PubMed] [Google Scholar]
- 12.Corradini S, Alongi F, Andratschke N, et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother Oncol. 2021;159:146‐154. [DOI] [PubMed] [Google Scholar]
- 13.Powell S, Cooke J, Parsons C. Radiation-induced brachial plexus injury: follow-up of two different fractionation schedules. Radiother Oncol. 1990;18(3):213‐220. [DOI] [PubMed] [Google Scholar]
- 14.Chargari C, Riet F, Mazevet M, Morel E, Lepechoux C, Deutsch E. Complications of thoracic radiotherapy. Presse Med. 2013;42(9 Pt 2):e342‐e351. [DOI] [PubMed] [Google Scholar]
- 15.Ingle M, Lalondrelle S. Current status of anatomical magnetic resonance imaging in brachytherapy and external beam radiotherapy planning and delivery. Clin Oncol (R Coll Radiol). 2020;32(12):817‐827. [DOI] [PubMed] [Google Scholar]
- 16.Hall WA, Bedi M, Kilari D, et al. Long-term outcomes of dose-escalated pelvic lymph node intensity-modulated radiation therapy (IMRT) with a simultaneous hypofractionated boost to the prostate for very high-risk adenocarcinoma of the prostate: a prospective phase II clinical trial. Pract Radiat Oncol. 2021;11(6):527‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Mu X, He H, Zhang XD. Cancer radiosensitizers. Trends Pharmacol Sci. 2018;39(1):24‐48. [DOI] [PubMed] [Google Scholar]
- 18.Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy [published correction appears in Int J Nanomedicine. 2021 Dec 16;16:8139-8140]. Int J Nanomedicine. 2021;16:1083‐1102. Published 2021 Feb 12. doi: 10.2147/IJN.S290438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26(2):179‐193. doi: 10.1085/jgp.26.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7(3):166‐181. [DOI] [PubMed] [Google Scholar]
- 21.Huber PE, Jenne JW, Rastert R, et al. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res. 2001;61(23):8441‐8447. [PubMed] [Google Scholar]
- 22.Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176‐185. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz MD, Ghanouni P, Kanaev SV, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5):dju082. doi: 10.1093/jnci/dju082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deckers R, Merckel LG, Denis de Senneville B, et al. Performance analysis of a dedicated breast MR-HIFU system for tumor ablation in breast cancer patients. Phys Med Biol. 2015;60(14):5527‐5542. [DOI] [PubMed] [Google Scholar]
- 25.Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31(3):302‐309. [DOI] [PubMed] [Google Scholar]
- 26.Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74(4):422‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knuttel FM, van den Bosch MA. Magnetic resonance-guided high intensity focused ultrasound ablation of breast cancer. Adv Exp Med Biol. 2016;880:65‐81. doi: 10.1007/978-3-319-22536-4_4 [DOI] [PubMed] [Google Scholar]
- 28.Lyon PC, Rai V, Price N, Shah A, Wu F, Cranston D. Ultrasound-guided high intensity focused ultrasound ablation for symptomatic uterine fibroids: preliminary clinical experience. Ultraschallgesteuerte hochintensive fokussierte ultraschallablation bei symptomatischen uterusmyomen: eine vorläufige klinische erfahrung. Ultraschall Med. 2020;41(5):550‐556. [DOI] [PubMed] [Google Scholar]
- 29.Yee CH, Chiu PK, Teoh JY, Ng CF, Chan CK, Hou SM. High-intensity focused ultrasound (HIFU) focal therapy for localized prostate cancer with MRI-US fusion platform. Adv Urol. 2021;2021:ID:7157973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feril LB, Fernan RL, Tachibana K. High-intensity focused ultrasound in the treatment of breast cancer. Curr Med Chem. 2021;28(25):5179‐5188. [DOI] [PubMed] [Google Scholar]
- 31.Bachu VS, Kedda J, Suk I, Green JJ, Tyler B. High-intensity focused ultrasound: a review of mechanisms and clinical applications. Ann Biomed Eng. 2021;49(9):1975‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leslie T, Ritchie R, Illing R, et al. High-intensity focused ultrasound treatment of liver tumours: post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br J Radiol. 2012;85(1018):1363‐1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y, Hu K, Zhang Y, et al. High-intensity focused ultrasound (HIFU) treatment for uterine fibroids: a meta-analysis. Arch Gynecol Obstet. 2017;296(6):1181‐1188. [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Jiang Y. Comparison of efficiency of TACE plus HIFU and TACE alone on patients with primary liver cancer. J Coll Physicians Surg Pak. 2019;29(5):414‐417. [DOI] [PubMed] [Google Scholar]
- 35.Napoli A, Alfieri G, Scipione R, et al. High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices. 2020;17(5):427‐433. [DOI] [PubMed] [Google Scholar]
- 36.Marinova M, Feradova H, Gonzalez-Carmona MA, et al. Improving quality of life in pancreatic cancer patients following high-intensity focused ultrasound (HIFU) in two European centers. Eur Radiol. 2021;31(8):5818‐5829. [DOI] [PubMed] [Google Scholar]
- 37.Fergadi MP, Magouliotis DE, Rountas C, et al. A meta-analysis evaluating the role of high-intensity focused ultrasound (HIFU) as a fourth treatment modality for patients with locally advanced pancreatic cancer. Abdom Radiol (NY). 2022;47(1):254‐264. [DOI] [PubMed] [Google Scholar]
- 38.Klotz L, Pavlovich CP, Chin J, et al. Magnetic resonance imaging-guided transurethral ultrasound ablation of prostate cancer. J Urol. 2021;205(3):769‐779. [DOI] [PubMed] [Google Scholar]
- 39.Ghai S, Finelli A, Corr K, et al. MRI-guided focused ultrasound ablation for localized intermediate-risk prostate cancer: early results of a phase II trial [published correction appears in Radiology. 2021 May;299(2):E258]. Radiology. 2021;298(3):695‐703. [DOI] [PubMed] [Google Scholar]
- 40.Yang T, Ng DM, Du N, et al. HIFU for the treatment of difficult colorectal liver metastases with unsuitable indications for resection and radiofrequency ablation: a phase I clinical trial. Surg Endosc. 2021;35(5):2306‐2315. [DOI] [PubMed] [Google Scholar]
- 41.Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rezai AR, Ranjan M, D'Haese PF, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer's disease with focused ultrasound. Proc Natl Acad Sci USA. 2020;117(17):9180‐9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, et al. Blood–brain barrier opening with focused ultrasound in Parkinson's disease dementia. Nat Commun. 2021;12(1):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinh D, Nash J, Goertz D, et al. Microbubble drug conjugate and focused ultrasound blood brain barrier delivery of AAV-2 SIRT-3. Drug Deliv. 2022;29(1):1176‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Airan RD, Meyer RA, Ellens NP, et al. Noninvasive targeted transcranial neuromodulation via focused ultrasound gated drug release from nanoemulsions. Nano Lett. 2017;17(2):652‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meng Y, Pople CB, Lea-Banks H, Hynynen K, Lipsman N, Hamani C. Focused ultrasound neuromodulation. Int Rev Neurobiol. 2021;159:221‐240. [DOI] [PubMed] [Google Scholar]
- 47.Yoo S, Mittelstein DR, Hurt RC, Lacroix J, Shapiro MG. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat Commun. 2022;13(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roovers S, Segers T, Lajoinie G, et al. The role of ultrasound-driven microbubble dynamics in drug delivery: from microbubble fundamentals to clinical translation. Langmuir. 2019;35(31):10173‐10191. doi: 10.1021/acs.langmuir.8b03779 [DOI] [PubMed] [Google Scholar]
- 49.Phenix CP, Togtema M, Pichardo S, Zehbe I, Curiel L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci. 2014;17(1):136‐153. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Lin Z, Zeng L, et al. Ultrasound-induced biophysical effects in controlled drug delivery. Sci China Life Sci. 2022;65(5):896‐908. [DOI] [PubMed] [Google Scholar]
- 51.Chen KT, Chai WY, Lin YJ, et al. Neuronavigation-guided focused ultrasound for transcranial blood–brain barrier opening and immunostimulation in brain tumors. Sci Adv. 2021;7(6):eabd0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cirincione R, Di Maggio FM, Forte GI, et al. High-Intensity focused ultrasound- and radiation therapy-induced immuno-modulation: comparison and potential opportunities. Ultrasound Med Biol. 2017;43(2):398‐411. [DOI] [PubMed] [Google Scholar]
- 53.Sheybani ND, Price RJ. Perspectives on recent progress in focused ultrasound immunotherapy. Theranostics. 2019;9(25):7749‐7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim C, Lim M, Woodworth GF, Arvanitis CD. The roles of thermal and mechanical stress in focused ultrasound-mediated immunomodulation and immunotherapy for central nervous system tumors. J Neurooncol. 2022;157(2):221‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNabb E, Al-Mahrouki A, Law N, et al. Ultrasound-stimulated microbubble radiation enhancement of tumors: single-dose and fractionated treatment evaluation. PLoS One. 2020;15(9):e0239456. Published 2020 Sep 25. doi: 10.1371/journal.pone.0239456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Kaffas A, Al-Mahrouki A, Hashim A, Law N, Giles A, Czarnota GJ. Role of acid sphingomyelinase and ceramide in mechano-acoustic enhancement of tumor radiation responses. J Natl Cancer Inst. 2018;110(9):1009‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Y, Dong XH, Zhu Q, Xu YL, Chen ML, Liu Z. Ultrasound-triggered microbubble destruction enhances the radiosensitivity of glioblastoma by inhibiting PGRMC1-mediated autophagy in vitro and in vivo. Mil Med Res. 2022;9(1):9. Published 2022 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCorkell G, Nakayama M, Feltis B, Piva T, Geso M. Ultrasound-stimulated microbubbles enhance radiation-induced cell killing. Ultrasound Med Biol. 2022;48(12):2449‐2460. [DOI] [PubMed] [Google Scholar]
- 59.Jernberg A, Edgren MR, Lewensohn R, Wiksell H, Brahme A. Cellular effects of high-intensity focused continuous wave ultrasound alone and in combination with x-rays. Int J Radiat Biol. 2001;77(1):127‐135. [DOI] [PubMed] [Google Scholar]
- 60.Liu CX, Gao XS, Xiong LL, et al. A preclinical in vivo investigation of high-intensity focused ultrasound combined with radiotherapy. Ultrasound Med Biol. 2011;37(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 61.Borasi G, Russo G, Alongi F, et al. High-intensity focused ultrasound plus concomitant radiotherapy: a new weapon in oncology? J Ther Ultrasound. 2013;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borasi G, Russo G, Vicari F, et al. Experimental evidence for the use of ultrasound to increase tumor-cell radiosensitivity. Trans Cancer Res. 2014;3(5):512‐520. [Google Scholar]
- 63.Borasi G, Nahum A, Paulides MM, et al. Fast and high temperature hyperthermia coupled with radiotherapy as a possible new treatment for glioblastoma. J Ther Ultrasound. 2016;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Greiser S, Roy U, et al. Evaluation of a developed MRI-guided focused ultrasound system in 7 T small animal MRI and proof-of-concept in a prostate cancer xenograft model to improve radiation therapy. Cells. 2023;12(3):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu RY, Wang GM, Xu L, et al. The feasibility and safety of high-intensity focused ultrasound combined with low-dose external beam radiotherapy as supplemental therapy for advanced prostate cancer following hormonal therapy. Asian J Androl. 2011;13(3):499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Correas JM, Claudon M, Tranquart F, Hélénon AO. The kidney: imaging with microbubble contrast agents. Ultrasound Q. 2006;22(1):53‐66. [PubMed] [Google Scholar]
- 67.Medellin A, Merrill C, Wilson SR. Role of contrast-enhanced ultrasound in evaluation of the bowel. Abdom Radiol (NY). 2018;43(4):918‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: current status and future. Abdom Radiol (NY). 2018;43(4):762‐772. [DOI] [PubMed] [Google Scholar]
- 69.Barr RG, Huang P, Luo Y, et al. Contrast-enhanced ultrasound imaging of the liver: a review of the clinical evidence for SonoVue and Sonazoid. Abdom Radiol (NY). 2020;45(11):3779‐3788. [DOI] [PubMed] [Google Scholar]
- 70.Versluis M, Stride E, Lajoinie G, Dollet B, Segers T. Ultrasound contrast agent modeling: a review. Ultrasound Med Biol. 2020;46(9):2117‐2144. [DOI] [PubMed] [Google Scholar]
- 71.Vignon F, Shi WT, Powers JE, et al. Microbubble cavitation imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(4):661‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chowdhury SM, Abou-Elkacem L, Lee T, Dahl J, Lutz AM. Ultrasound and microbubble mediated therapeutic delivery: underlying mechanisms and future outlook. J Control Release. 2020;326:75‐90. [DOI] [PubMed] [Google Scholar]
- 73.Collis J, Manasseh R, Liovic P, et al. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics. 2010;50(2):273‐279. [DOI] [PubMed] [Google Scholar]
- 74.Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CT. Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev. 2014;72:49‐64. [DOI] [PubMed] [Google Scholar]
- 75.Guo G, Ma Y, Guo Y, et al. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason Sonochem. 2017;37:279‐285. [DOI] [PubMed] [Google Scholar]
- 76.Escoffre JM, Bouakaz A. Minireview: biophysical mechanisms of cell membrane sonopermeabilization. Knowns and unknowns. Langmuir. 2019;35(31):10151‐10165. [DOI] [PubMed] [Google Scholar]
- 77.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293‐297. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155‐1159. [DOI] [PubMed] [Google Scholar]
- 79.Sathishkumar S, Boyanovsky B, Karakashian AA, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4(9):979‐986. [DOI] [PubMed] [Google Scholar]
- 80.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89‐91. [DOI] [PubMed] [Google Scholar]
- 81.Wortel RC, Mizrachi A, Li H, et al. Sildenafil protects endothelial cells from radiation-induced oxidative stress. J Sex Med. 2019;16(11):1721‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim EJ, Lee H, Lee YJ, Sonn JK, Lim YB. Ionizing radiation regulates vascular endothelial growth factor—a transcription in cultured human vascular endothelial cells via the PERK/eIF2α/ATF4 pathway. Int J Radiat Oncol Biol Phys. 2020;107(3):563‐570. [DOI] [PubMed] [Google Scholar]
- 83.Li X, Gulbins E, Zhang Y. Oxidative stress triggers ca-dependent lysosome trafficking and activation of acid sphingomyelinase. Cell Physiol Biochem. 2012;30(4):815‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferranti CS, Cheng J, Thompson C, et al. Fusion of lysosomes to plasma membrane initiates radiation-induced apoptosis. J Cell Biol. 2020;219(4):e201903176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest. 2012;122(5):1786‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hua G, Kolesnick R. Using ASMase knockout mice to model human diseases. Handb Exp Pharmacol. 2013;216(216):29‐54. doi: 10.1007/978-3-7091-1511-4_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.García-Barros M, Thin TH, Maj J, et al. Impact of stromal sensitivity on radiation response of tumors implanted in SCID hosts revisited. Cancer Res. 2010;70(20):8179‐8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stancevic B, Varda-Bloom N, Cheng J, et al. Adenoviral transduction of human acid sphingomyelinase into neo-angiogenic endothelium radiosensitizes tumor cure. PLoS One. 2013;8(8):e69025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu H, Deng K, Zhao YQ, et al. The effects of ASMase mediated endothelial cell apoptosis in multiple hypofractionated irradiations in CT26 tumor bearing mice. Asian Pac J Cancer Prev. 2015;16(11):4543‐4548. [DOI] [PubMed] [Google Scholar]
- 90.Bodo S, Campagne C, Thin TH, et al. Single-dose radiotherapy disables tumor cell homologous recombination via ischemia/reperfusion injury. J Clin Invest. 2019;129(2):786‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ketteler J, Wittka A, Leonetti D, et al. Caveolin-1 regulates the ASMase/ceramide-mediated radiation response of endothelial cells in the context of tumor-stroma interactions. Cell Death Dis. 2020;11(4):228. Published 2020 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leonetti D, Estéphan H, Ripoche N, et al. Secretion of acid sphingomyelinase and ceramide by endothelial cells contributes to radiation-induced intestinal toxicity. Cancer Res. 2020;80(12):2651‐2662. [DOI] [PubMed] [Google Scholar]
- 93.Juffermans LJ, Dijkmans PA, Musters RJ, Visser CA, Kamp O. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2006;291(4):H1595‐H1601. [DOI] [PubMed] [Google Scholar]
- 94.Juffermans LJ, van Dijk A, Jongenelen CA, et al. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol. 2009;35(11):1917‐1927. [DOI] [PubMed] [Google Scholar]
- 95.Kooiman K, van der Steen AF, de Jong N. Role of intracellular calcium and reactive oxygen species in microbubble-mediated alterations of endothelial layer permeability. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60(9):1811‐1815. [DOI] [PubMed] [Google Scholar]
- 96.Jia C, Xu L, Han T, Cai P, Yu ACH, Qin P. Generation of reactive oxygen species in heterogeneously sonoporated cells by microbubbles with single-pulse ultrasound. Ultrasound Med Biol .2018;44(5):1074‐1085. [DOI] [PubMed] [Google Scholar]
- 97.Lacerda Q, Tantawi M, Leeper DB, Wheatley MA, Eisenbrey JR. Emerging applications of ultrasound-contrast agents in radiation therapy. Ultrasound Med Biol. 2021;47(6):1465‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Czarnota GJ, Karshafian R, Burns PN, et al. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc Natl Acad Sci U S A. 2012;109(30):E2033‐E2041. doi: 10.1073/pnas.1200053109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tran WT, Iradji S, Sofroni E, Giles A, Eddy D, Czarnota GJ. Microbubble and ultrasound radioenhancement of bladder cancer. Br J Cancer. 2012;107(3):469‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Czarnota GJ. Ultrasound-stimulated microbubble enhancement of radiation response. Biol Chem. 2015;396(6-7):645‐657. [DOI] [PubMed] [Google Scholar]
- 101.Lai P, Tarapacki C, Tran WT, et al. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo [published correction appears in Oncoscience. 2017 Jan 30;4(1-2):14]. Oncoscience. 2016;3(3-4):98‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng H, Cai Y, Feng Q, et al. Ultrasound-stimulated microbubbles enhance radiosensitization of nasopharyngeal carcinoma. Cell Physiol Biochem. 2018;48(4):1530‐1542. [DOI] [PubMed] [Google Scholar]
- 103.Eisenbrey JR, Forsberg F, Wessner CE, et al. US-triggered microbubble destruction for augmenting hepatocellular carcinoma response to transarterial radioembolization: a randomized pilot clinical trial. Radiology. 2021;298(2):450‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omata D, Munakata L, Maruyama K, Suzuki R. Enhanced vascular permeability by microbubbles and ultrasound in drug delivery. Biol Pharm Bull. 2021;44(10):1391‐1398. [DOI] [PubMed] [Google Scholar]
- 105.Yamaguchi K, Matsumoto Y, Suzuki R, et al. Enhanced antitumor activity of combined lipid bubble ultrasound and anticancer drugs in gynecological cervical cancers. Cancer Sci. 2021;112(6):2493‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan MS, Hwang J, Lee K, et al. Oxygen-Carrying micro/nanobubbles: composition, synthesis techniques and potential prospects in photo-triggered theranostics. Molecules. 2018;23(9):2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57 Suppl 1(Suppl 1):i90‐i98. doi: 10.1093/jrr/rrw007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eisenbrey JR, Shraim R, Liu JB, et al. Sensitization of hypoxic tumors to radiation therapy using ultrasound-sensitive oxygen microbubbles. Int J Radiat Oncol Biol Phys. 2018;101(1):88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Delaney LJ, Ciraku L, Oeffinger BE, et al. Breast cancer brain metastasis response to radiation after microbubble oxygen delivery in a murine model. J Ultrasound Med. 2019;38(12):3221‐3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Drzał A, Delalande A, Dziurman G, Fournié M, Pichon C, Elas M. Increasing oxygen tension in tumor tissue using ultrasound sensitive O2 microbubbles. Free Radic Biol Med. 2022;193(Pt 2):567‐578. [DOI] [PubMed] [Google Scholar]
- 111.Fix SM, Papadopoulou V, Velds H, et al. Oxygen microbubbles improve radiotherapy tumor control in a rat fibrosarcoma model – a preliminary study. PLoS One. 2018;13(4):e0195667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho YJ, Thao DT, Yeh CK. Overcoming hypoxia-induced drug resistance via promotion of drug uptake and reoxygenation by acousto-mechanical oxygen delivery. Pharmaceutics. 2022;14(5):902. Published 2022 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacerda Q, Rochani A, Oeffinger B, et al. Tumoral oxygenation and biodistribution of ionidamine oxygen microbubbles following localized ultrasound-triggered delivery. Int J Pharm. 2022;625:122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng S, Song R, Lin Q, et al. A robust oxygen microbubble radiosensitizer for iodine-125 brachytherapy. Adv Sci (Weinh). 2021;8(7):2002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosseini SH, Zheng X, Vaezy S. Effects of gas pockets on high-intensity focused ultrasound field. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(6):1203‐1210. [DOI] [PubMed] [Google Scholar]
- 116.Zhang H, Zhang Y, Xu M, et al. The effects of the structural and acoustic parameters of the skull model on transcranial focused ultrasound. Sensors (Basel). 2021;21(17):5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bond AE, Shah BB, Huss DS, et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: a randomized clinical trial. JAMA Neurol. 2017;74(12):1412‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bour P, Ozenne V, Marquet F, Denis de Senneville B, Dumont E, Quesson B. Real-time 3D ultrasound based motion tracking for the treatment of mobile organs with MR-guided high-intensity focused ultrasound. Int J Hyperthermia. 2018 Dec;34(8):1225‐1235. doi: 10.1080/02656736.2018.1433879 [DOI] [PubMed] [Google Scholar]
- 119.Celicanin Z, Manasseh G, Petrusca L, et al. Hybrid ultrasound-MR guided HIFU treatment method with 3D motion compensation. Magn Reson Med. 2018;79(5):2511‐2523. [DOI] [PubMed] [Google Scholar]
- 120.Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(4):822‐834. [DOI] [PubMed] [Google Scholar]
- 121.Cole AJ, Hanna GG, Jain S, O'Sullivan JM. Motion management for radical radiotherapy in non-small cell lung cancer. Clin Oncol (R Coll Radiol). 2014;26(2):67‐80. [DOI] [PubMed] [Google Scholar]
- 122.Li W, Ye X, Huang Y, Dong Y, Chen X, Yang Y. An integrated ultrasound imaging and abdominal compression device for respiratory motion management in radiation therapy. Med Phys. 2022;49(10):6334‐6345. [DOI] [PubMed] [Google Scholar]
- 123.Piruzan E, Vosoughi N, Mahdavi SR, Khalafi L, Mahani H. Target motion management in breast cancer radiation therapy. Radiol Oncol. 2021;55(4):393‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen H, Zhong Z, Yang Y, et al. Internal motion estimation by internal-external motion modeling for lung cancer radiotherapy. Sci Rep. 2018;8(1):3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50(2):221‐229. [DOI] [PubMed] [Google Scholar]