Abstract
Background
Depression is a common kind of mental illness, and it becomes the main health burden in the world.
Purpose
The aim of this study was to investigate the antidepressant effects of naringin and apigenin isolated from Chrysanthemum morifolium Ramatis.
Methods
Firstly, 20 mg/kg corticosterone (CORT) was injected into mice to establish an in vivo model of depression. After treated with different dosages of naringenin and apigenin for 3 weeks, the mice underwent a series of behavioral experiments. Following this, all mice were sacrificed and biochemical analyses were performed. Subsequently, CORT (500 μM) induced PC12 cells was used as an in vitro model of depression, and lipopolysaccharide (LPS) (1 μg ml−1) induced N9 microglia cells was used as an in vitro model of neuroinflammation in N9 microglia cells, to investigate the neuroprotective mechanisms of naringenin and apigenin.
Results
Results showed that the naringenin and apigenin treatment ameliorated CORT-induced sucrose preference decrease and immobility time increase, elevated the 5-hydroxytryptamine(5-HT), dopamine (DA) and norepinephrine (NE) levels, and enhanced the cAMP-response element binding protein (CREB) and brain derived neurotrophic factor (BDNF) protein expressions in the hippocampus. The results showed that the naringenin and apigenin treatment improved the PC-12 cell viability through reducing apoptosis rate induced by CORT. Furthermore, naringenin and apigenin were able to inhibit the activation of N9 cells after LPS induction, and shift microglia from proinflammatory M1 microglia toward anti-inflammatory M2 microglia, as evidenced by the decreased ratio of M1 type microglia marker CD86 and M2 type microglia marker CD86.
Conclusion
These results suggested that naringenin and apigenin may improve depressive behaviors through promoting BDNF and inhibiting neuroinflammation and neuronal apoptosis.
Keywords: Naringenin, Apigenin, cAMP-CREB-BDNF, Neuroinflammation, Neuronal apoptosis, Depression, CORT
1. Introduction
According to a 2017 WHO survey, depression is a mental disorder affecting more than 264 million people worldwide (4.4% of the world's population) and contributing to annual 800,000 suicide cases [1]. Depression can impair people's performance at work, school, and home. People with depression often feel sad, hopeless, guilty, worthless or helpless, and also lose interest or pleasure in activities and hobbies, leading to social withdrawal, changes in appetite or weight, changes in sleep habits, poor concentration, unexplained pain and distress. Severe symptoms may even lead to suicide. Persistent stress activates the HPA axis, which ultimately leads to the secretion of corticosterone (CORT). In depressive disorders, endogenous levels of neurotransmitters (NE, 5-HT and DA) are altered. Furthermore, there is a strong interaction between 5-HT and brain derived neurotrophic factor (BDNF) in the hippocampus [2]. BDNF is not only a marker of the depressive state, but is also implicated in several forms of learning, memory and neuroplasticity [2]. Some studies have reported that BDNF levels are strongly correlated with apoptosis levels in hippocampal cells [3], which may lead to neuronal loss and hippocampal atrophy. In addition, previous studies have demonstrated that exposure to high cortisol levels by repeated injection of high doses of CORT increased the number of apoptotic cells in the hippocampus and cerebral cortex [4]. Therefore, apoptosis in hippocampus neurons might be also one of the causative factors involved in the development of depression. Furthermore, neuroinflammation plays a key role in the development of central nervous system disorders, including depression, and contributes to each stage of the disease development process. Convincing evidence suggests that exposure to stress can result in elevated inflammatory responses in the brain as well as peripherally [5]. Elevated inflammatory and neuroendocrine responses might contribute to the pathogenesis of the somatic components of depression [6]. Moreover, there are studies showing that BDNF has anti-inflammatory effects, it can inhibit the expression of pro-inflammatory factors and promote the expression of anti-inflammatory factors [7,8].
The interest in evaluating the therapeutic effects of natural products, mainly plant-derived ones, in mental disorders such as depression has been a topic of research in the last decade [9]. The dry flower of Chrysanthemum morifolium Ramatis (C. morifolium) is a common medicinal and edible plant in china. The medicinal value of C. morifolium was recorded by Shennong's Classic of Materia Medica for the first time during the Qin and Han Dynasties (221 BC-220 AD). The Puji Fang compiled in 1390 recorded that the Ganju san (a traditional Chinese medicine containing C. morifolium) could be used to treat dizziness. Modern pharmacological research proved that C. morifolium has antidepressant effect [10]. Our previous study also proved that C. morifolium extract may improve depression by regulating the cAMP-response element binding protein (CREB) pathway and inhibiting neuronal apoptosis [11]. The main bioactive components of C. morifolium, including caffeoylquinic acids, flavonoids and carotenoids, were recognized to have anti-inflammatory, antibacterial, antifungal, anti-spirochetal, anti-human immunodeficiency virus and anti-oxidant characteristics properties [12]. However, the effective compounds from C. morifolium and their therapeutic mechanisms were still unknown. Components isolated from C. morifolium and screened for their antidepressant effects, naringin and apigenin were proved to be the most potential anti-depression compounds in C. morifolium. Naringenin and apigenin are the major flavonoids in extracts of C. morifolium. Flavonoids are considered as health-promoting and disease-preventing dietary supplements [13]. Clinical and animal studies have revealed that flavonoid extracts isolated from natural sources could display antidepressant properties [14,15]. Flavonoid compounds and their related analogues could also exert antidepressant activities. Rutin, quercetin, glycyrrhizin and isoglycyrrhizin have been reported to have antidepressant effects [16]. Recent studies showed that naringenin and apigenin have a wide range of biological activities. Naringin exhibits biological activities such as anti-inflammation, anti-oxidation [17], myocardial protection [18], anti-cancer [19] and so on. Apigenin possesses anti-inflammatory, antioxidant, anti-carcinogenic, and anti-kinase properties [20]. Both naringenin and apigenin were reported to have anti-inflammation effects, which might have a relationship with their anti-depression effects.
The purpose of the present study was to investigate the antidepressant effects of naringenin and apigenin from C. morifolium in the depression mouse model induced by chronic administration of CORT, and explore their possible neuroprotective mechanisms in CORT-induced PC12 cells and LPS-induced N9 microglia cells.
2. Materials and methods
2.1. Drugs and chemicals
Corticosterone (CORT) (purity: 98%) was purchased from Cayman Chemical (St. Louis, MO, USA). Fluoxetine hydrochloride (FLX) was from PATHEON FRANCE Co., Ltd. (Changzhou, China). The method of extracting apigenin (Api) and naringenin(Nar) from C. morifolium. Has been published before [21]. However, the amount of apigenin and naringenin extracted was too small, so both compounds were obtained from the distributor. The chemical formulae of apigenin (Fig. 1A) and naringenin (Fig. 1B) are shown in Fig. 1. Naringenin (Lot: X21M7C11378) and apigenin (Lot: Y27A6C1) were purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). RPMI-1640 was bought from Gibco (NY, USA). The ELISA kits of 5-HT, DA and NE were obtained from Suzhou Calvin Biotechnology Co. Ltd. (Suzhou, China). DMSO and reactive oxygen species (ROS) were from Solarbio (Beijing, China). Annexin -FITC/PI was bought from BD (USA). Caspase-3, Caspase-9, Bcl-2, Bax. cAMP, CREB, p-CREB and BDNF were purchased from Abcam (Cambridge, Cambs, UK). Cleaved Caspase-3 was purchased from CST (Boston, USA). Polyvinylidene fluoride (PVDF) membrane was from Millipore (Billerica, MA, USA). MTT was from Biosharp (Hefei, China). Griess reagent was from Solarbio (Beijing, China). IL-4, IL-10, TGF-β, IL-1β, IL-6 and TNF- α were purchased from Suzhou Calvin Biotechnology Co. Ltd. (Suzhou, China). CD11b, CD86 and CD206 were purchased from Invitrogen (Carlsbad, USA).
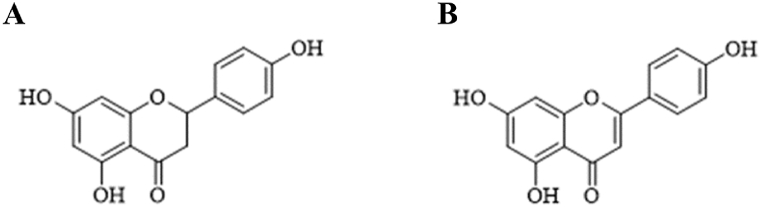
Fig. 1.
Chemical structure of Naringenin(A) and Apigenin (B).
2.2. Experimental animals and Animal Care
C57BL/6 male mice weighing 18–22 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. They were maintained under a 12-h light/dark cycle (lights on at 08:00) and a constant temperature (22 ± 2 °C) and humidity (50–60%). All experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Henan University of Chinese Medicine. Every effort was made to minimize the number of animals used and their suffering. This experiment was conducted by the Regulations on the Management of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals of Henan University of Traditional Chinese Medicine, promulgated by the National Science and Technology Commission of the People's Republic of China. The ethical approval reference number for this research is DWLL2018080003. All procedures for the care of mice followed the institutional guidelines for using animals in the study.
After one week of adaptation, the mice were randomly divided into nine groups (n = 10), including the Control group: saline (p.o) + saline (s.c); the CORT-vehicle group: saline (p.o) + CORT (20 mg/kg,s.c) [22]; the FLX group: fluoxetine (5 mg/kg, p.o) + CORT (20 mg/kg,s.c); the Nar-L group: naringenin (25 mg/kg, p.o) + CORT (20 mg/kg,s.c); the Nar-M group: naringenin (50 mg/kg, p.o) + CORT (20 mg/kg,s.c); the Nar-H group: naringenin (100 mg/kg, p.o) + CORT (20 mg/kg,s.c); the Api-L group: apigenin (10 mg/kg, p.o) + CORT (20 mg/kg,s.c). The Api-M group: apigenin (20 mg/kg, p.o) + CORT (20 mg/kg,s.c). the Api-H group: apigenin (40 mg/kg, p.o) + CORT (20 mg/kg,s.c). After 3 weeks of treatments, the mice underwent a series of behavioral experiments. The animals were anesthetized with isoflurane, and all serum and brain samples were collected for further analysis.
2.3. Sucrose Preference Test (SPT)
After 3 weeks of treatments, all mice were allowed to be trained to consume 1% sucrose solution for 24 h without any water or food. Following the sucrose intake training, the mice were deprived of food and water for 24 h, and then two bottles with 1% sucrose (100 ml) and tip water (100 ml) were randomly placed for another 24 h. The liquids consumption volume was measured and the sucrose preference was determined using the formula: Sucrose preference (%) = [sucrose intake/(sucrose intake + water intake)] × 100% [11].
2.4. Tail suspension test (TST)
The TST immobility was performed 24 h after SPT. The mice were suspended by the tail using an adhesive tape attached to a hook connected to a strain gauge. The immobility time was recorded during a 6 min period [11].
2.5. Open field test (OFT)
The OFT was performed 1 h prior to the TST. Without any interference, each mouse was individually placed in the center of a black box (40 cm × 40cm × 30 cm) with 25 equal squares (8 cm in length and width) and allowed to explore freely for 5 min. Then, the mouse locomotors activity was recorded for 3 min. Immobility time, movement time and distance parameters were then analyzed statistically, to assess the ability of mice to explore the outer space [11].
2.6. Serum biochemical assay
The animals were anesthetized with isoflurane, and all mice were sacrificed by decapitation between 9:00–11:00 a.m. The serum was obtained by centrifugation at 1000g for 15 min at 4 °C. The serum levels of CORT,5-HT, NE and DA were detected according to the instruction of ELISA kits [11].
2.7. Western blotting
Mice in Nar-M (50 mg/kg) and Api-L (10 mg/kg) groups were used for Western blot analysis. The protein was isolated from the hippocampus using commercial RIPA Lysis buffer. The quantified proteins were separated by electrophoresis on 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto the PVDF membranes. The membranes were blocked for 1 h at room temperature using 5% skimmed milk in Tris-buffer saline (TBS), and then probed with primary antibodies raised against cAMP, CREB, p-CREB and BDNF (1:1000 dilution) overnight at 4 °C. After washing in TBST for 6 times (5min for each time), the membranes were incubated with the horseradish peroxidase-linked secondary antibody for 2 h, and washed in TBST for another 6 times. Then, immunoreactive proteins were detected by imaging systems and determined using the Image J software (NIH, Bethesda, MD, USA) [23].
2.8. MTT assay in PC12 cells
PC12 cells were cultured in RPMI-1640 containing 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine, in a humidified environment with 5% CO2 at 37 °C. The cell viability was determined by using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells were seeded in 96-well plates (4 × 104 cells/well) and co-treated with 1,3, 5, 8 or 10 μM of naringenin and apigenin and CORT (500 μM) for 24 h. Then, 5 mg/ml of MTT reagent was added to the cell. After incubation with MTT at 37 °C for 4 h, the medium was removed and 150 μL of DMSO was added to each well. Cell viability was quantified by measuring the optical density at a wavelength of 570 nm using a microplate reader.
2.9. AV-FITC/PI double staining assay
Apoptosis was quantified by staining cells with AnnexinV-FITC (AV-FITC) and Propidium Iodide (PI). Briefly, PC12 cells were seeded at a density of 8 × 104 cells/well in 6-well microplates for 24 h. After incubation, cells were co-incubated with 500 μM of CORT and different concentrations of naringenin and apigenin (1,3, 5, 8, 10 or 15 μM) for another 24 h. At the end of treatment, harvested cells were washed three times with cold PBS, and then re-suspended in 100 μL cold PBS. 5 μL of AV-FITC and 5 μL of PI were added to the cells and incubated at room temperature in the dark for 15min, and then 400 μL PBS was added to each sample. The flow cytometric analysis was performed within 1 h [24].
2.10. Measurement of intracellular reactive oxygen species (ROS)
ROS level was measured by using DCFH-DA method. In brief, PC12 cells were incubated with DCFH-DA at a final concentration of 10μΜ in the darkness for 30min at 37 °C. After the cells were washed three times with serum-free medium to remove the extracellular DCFH-DA. ROS production was evaluated by the FACS flow cytometer. The level of intracellular ROS was shown as a percentage of non-treated control [24].
2.11. In cell western
PC12 cells were seeded in a 96-well plate at 4 × 104 cells/well. After incubation, the cells were treated with 5 μM naringenin and 8 μM apigenin for 24 h. The cells were fixed with 4% paraformaldehyde for 20min and permeabilized with 0.1% Triton X for 5 times (5min for each time). Then the cells were blocked with 5% BSA for 1.5 h at room temperature. Then the cells were incubated with primary antibodies of Caspase-3, Cleaved Caspase-3, Caspase-9, Bcl-2, Bax, cAMP, CREB, p-CREB and BDNF(1:100 dilution) overnight at 4 °C. The cells were then washed with PBST and incubated in secondary antibody (1:500) for 1 h. After that, the cells were washed 5 times with PBST. Proteins were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences) [25].
2.12. MTT assay and griess assay in N9 cells
N9 cells were cultured in DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine, in a humidified environment with 5% CO2 at 37 °C. The cell viability was determined by using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Briefly, the cells were seeded in 96-well plates (2 × 104 cells/well) and co-treated with 1,5, 10, 20, 40 μM naringenin and 0.1, 0.5, 1, 2.5, 5 μM apigenin with 1 μg ml−1 LPS for 24 h. Then, 5 mg/ml of MTT reagent was added to the cell. After incubation with MTT at 37 °C for 4 h, the medium was removed and 150 μL of DMSO was added to each well. Cell viability was quantified by measuring the optical density at a wavelength of 570 nm using a microplate reader. The NO content in cell supernatant was determined by using the Griess assay. The cells were seeded in 24-well plates (1 × 105 cells/well) and co-treated with 1,5, 10, 20, 40 μM naringenin and 0.1, 0.5, 1, 2.5, 5 μM apigenin with 1 μg ml−1 LPS for 24 h. Then, add 50 μL cell supernatant and 50 μL the configured Griess reagent to a well, protect from light for 10 min, and detect the NO content of the cell supernatant at 450 nm with a microplate reader [26].
2.13. Inflammatory factors detection
N9 cells were seeded in a 24-well plate at 1 × 105 cells/well. After incubation, the cells were treated with 1 μM naringenin and 0.1 μM apigenin under 1 μg/ml LPS for 24 h. Perform the experiment according to the instructions of the inflammatory factor detection kit [24].
2.14. Microglia subtypes and markers determination
N9 cells were washed once with PBS and then collected and stained for CD11b, CD86 and CD206. The cells were protected from light for 30 min. The levels of markers were measured by flow cytometry. The degree of activation of microglia was shown by the level of CD11b, and the M1/M2 phenotype ratio of activated microglia was shown by CD86/CD206 [27].
2.15. Statistical analysis
All data were expressed as mean ± SD. The SPSS ver. 26.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A value of p < 0.05 was considered as statistically significance.
3. Results
3.1. Effects of naringenin and apigenin on depressive-like behaviors in CORT-induced depression mice
3.1.1. Sucrose Preference Test (SPT)
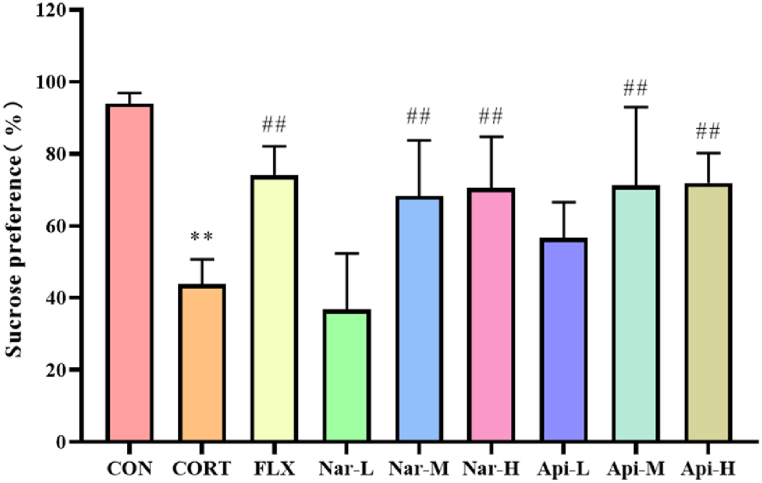
Sucrose consumption in the CORT group was significantly reduced compared with the control group (p < 0.01), while the reduction can be reversed by treatment with naringenin and apigenin in a dose-dependent manner (Fig. 2).
Fig. 2.
The effects of the naringenin and apigenin on sucrose preference index in SPT. Results are presented as means ± SD (n = 8). **p < 0.01 as compared with control group; ##p < 0.01 as compared with CORT-treated group. (single column fitting image).
3.1.2. Tail suspension test (TST)
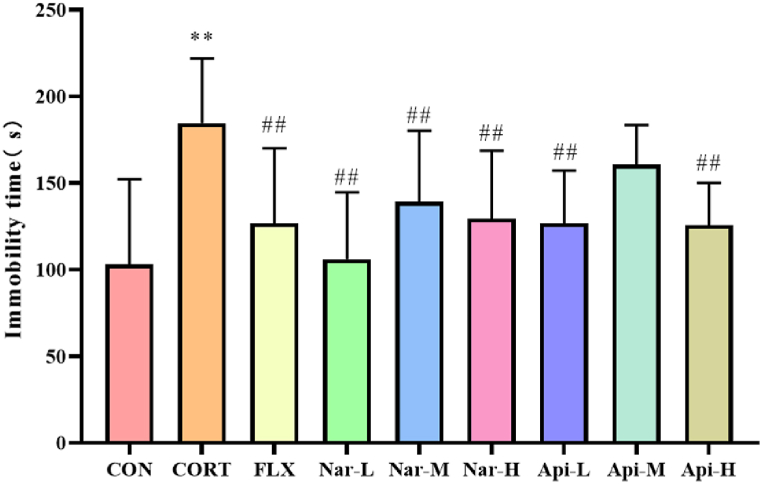
As shown in Fig. 3, the immobility time of the animals in the CORT group increased significantly compared with those of the control group in TST (p < 0.01). Compared with the CORT group, the immobility times of the 25 mg/kg, 50 mg/kg, 100 mg/kg naringenin group and 10 mg/kg and 40 mg/kg apigenin group were reduced significantly (p < 0.01).
Fig. 3.
The effects of the naringenin and apigenin on immobility time in TST. Results are presented as means ± SD (n = 8). **p < 0.01 as compared with control group; ##p < 0.01 as compared with CORT-treated group. (single column fitting image).
3.1. 3 Open-field test (OFT)
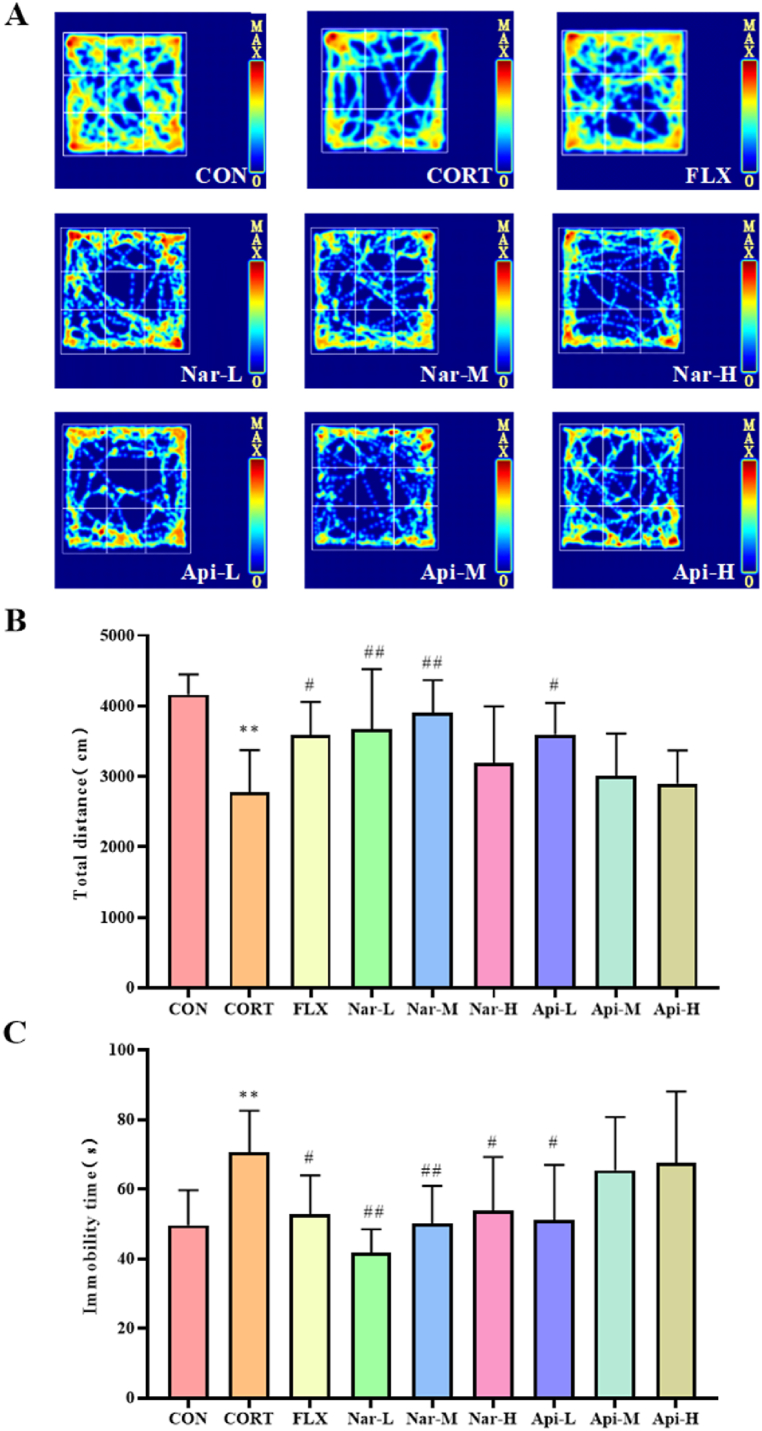
Compared to the control group, CORT treatment decreased the total moving distance (Fig. 4A and B) and increased the immobility time (Fig. 4A and C) significantly, whereas 25 mg/kg, 50 mg/kg naringenin treatment and 10 mg/kg apigenin treatment rescued the impacts of CORT on the total moving distance and decreased the immobility time.
Fig. 4.
The effects of the naringenin and apigenin on locomotor activity in OFT. (A) Individual examples of locomotor activity. (B) Total distance (cm) in traveled in OFT. (C) Immobility times (s) in traveled in OFT. Results are presented as mean ± SD. Results are presented as means ± SD (n = 8). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01as compared with CORT-treated group. (single column fitting image).
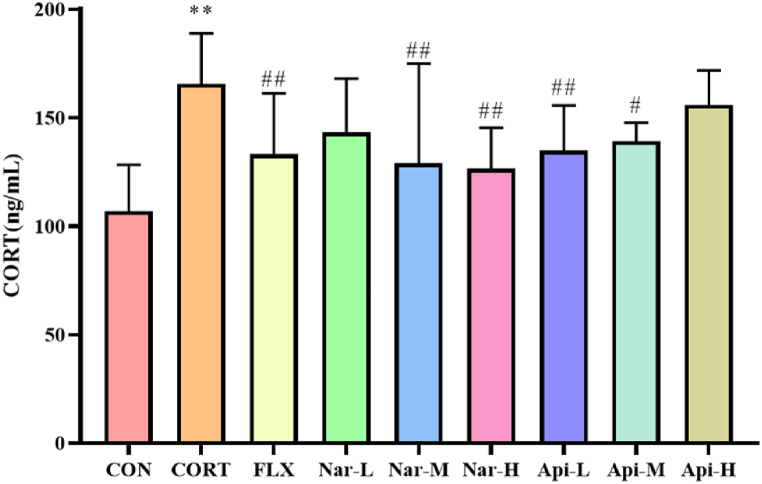
3.2. Effects of naringenin and apigenin on serum CORT level in CORT-induced depression mice
CORT-treated animals had significantly higher serum CORT levels than the control group. However, CORT -induced elevation in CORT levels could be suppressed by treatment with 50 mg/kg, 100 mg/kg of naringenin and 10 mg/kg, 20 mg/kg of apigenin for 3 weeks (Fig. 5).
Fig. 5.
The effects of the naringenin and apigenin on serum CORT levels in CORT-induced depression mice. Results are presented as means ± SD (n = 8). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (single column fitting image).
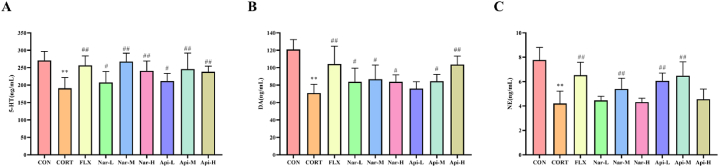
3.3. Effects of naringenin and apigenin on serum levels of neurotransmitters in CORT-induced depression mice
Result showed that the 5-HT (Fig. 6A), DA (Fig. 6B) and NE (Fig. 6C) were significantly reduced in CORT group (p < 0.01), while they were improved in Naringenin and Apigenin groups.
Fig. 6.
The effects of the naringenin and apigenin on serum neurotransmitters in CORT-induced depression mice. (A) The level of 5-HT in serum. (B) The level of DA in serum. (C) The level of NE in serum. Results are presented as means ± SD (n = 8). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (2-column fitting image).
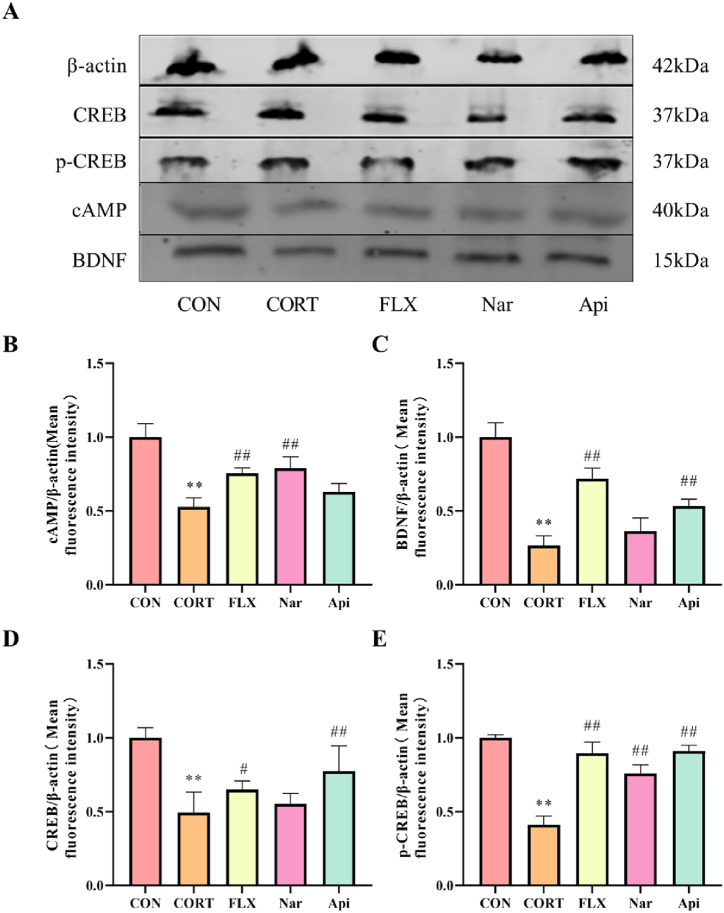
3.4. Effects of naringenin and apigenin on cAMP/CREB/BDNF signaling pathway in CORT-induced depression mice
As shown in Fig. 7A, the expressions of cAMP, BDNF, CREB and p-CREB were significantly decreased in hippocampus of CORT-induced mice. Following the treatment with the naringenin, the expressions of cAMP (Fig. 7B) and p-CREB (Fig. 7E) were markedly increased (p < 0.01). Likewise, the apigenin up-regulated the expressions of BDNF (Fig. 7C), CREB (Fig. 7D) and p-CREB (Fig. 7E) (p < 0.01).
Fig. 7.
The effects of the naringenin and apigenin on cAMP-CREB-BDNF signal pathway in CORT-induced depression mice. (A)The results of Western blot. (B)The quantitative results of cAMP. (C)The quantitative results of BDNF. (D)The quantitative results of CREB. (E)The quantitative results of p-CREB. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (single column fitting image).
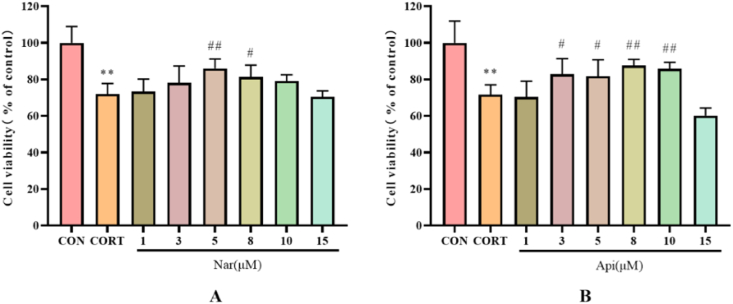
3.5. Effects of naringenin and apigenin on cell viability in CORT-induced PC12 cells
As shown in Fig. 8, 500 μM CORT inhibited the cell viability to 71.97% or 71.75% (p < 0.01). Naringenin (5, 8 μM) and apigenin (3, 5, 8, 10 μM) prevented CORT-induced neuro-toxicity in PC12 cells. 5 μM naringenin increased the cell viability to 86.03% (p < 0.01), while 8 μM apigenin increased the cell viability to 87.61% (p < 0.01), respectively (Fig. 8A and B). Therefore, 5 μM of naringenin and 8 μM of apigenin were used to subsequent studies of their neuroprotective effects.
Fig. 8.
The effects of the naringenin (A) and apigenin (B) on cell viability of CORT-treated PC12 cells. Results are presented as means ± SD (n = 6). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (1.5-column fitting image).
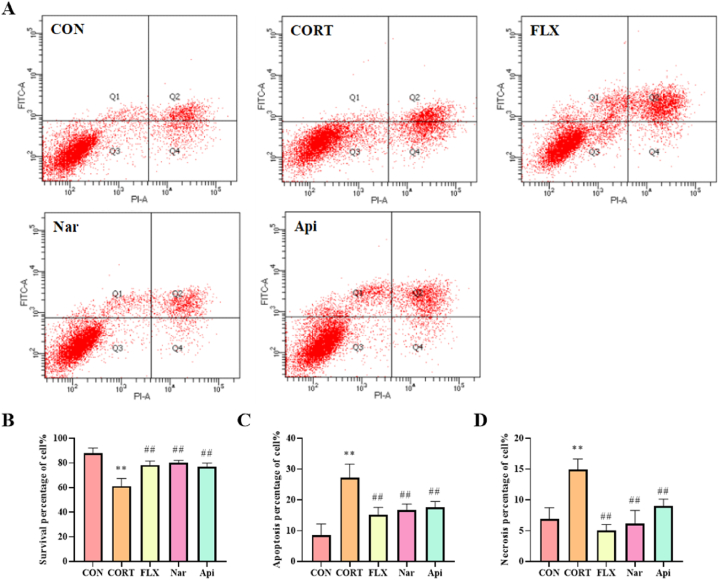
3.6. Effects of naringenin and apigenin on apoptosis rate in CORT-induced PC12 cells
As shown in Fig. 9, the percentage of survival cells (AV-FITC−/PI−) were obviously decreased to 61.27% (p < 0.01) after treated with 500 μM CORT for 24 h, and increased to 80.13% and 76.67% (p < 0.01) under pretreatment with 5 μM naringenin and 8 μM apigenin (Fig. 9A and B). The percentage of total apoptotic cells (AV-FITC+/PI−, AV-FITC+/PI+) were obviously increased to 27.30% (p < 0.01) after treated with 500 μM CORT for 24 h, whereas they were decreased to 16.63% and 17.57% (p < 0.01) under pretreatment with 5 μM naringenin and 8 μM apigenin (Fig. 9A and C). And the percentage of necrosis cells (AV-FITC−/PI+) were increased to 14.90% (p < 0.01) after treated with 500 μM CORT for 24 h, and decreased to 6.13% and 8.97% (p < 0.01) under pretreatment with 5 μM naringenin and 8 μM apigenin (Fig. 9A and D).
Fig. 9.
The effects of the naringenin and apigenin on apoptosis in CORT-induced PC12 cells. (A) AV-FITC/PI double staining result. (B) Survival percentage of PC12 cell. (C) Apoptotic percentage of PC12 cell. (D) Necrosis percentage of PC12 cell. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; ##p < 0.01 as compared with CORT-treated group. (1.5-column fitting image).
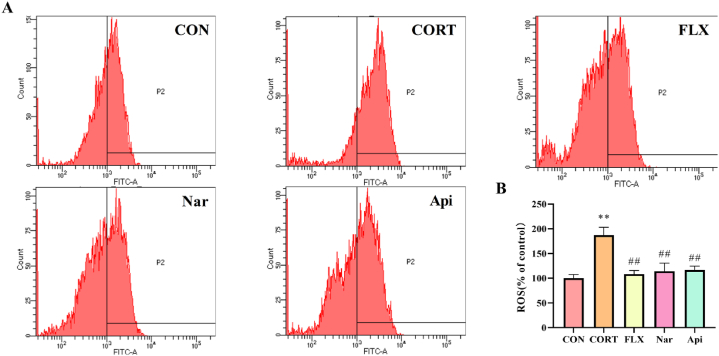
3.7. Effects of naringenin and apigenin on reactive oxygen species (ROS) level in CORT-induced PC12 cells
As shown in Fig. 10A and B, after being exposed to 500 μM CORT for 24 h, the intracellular reactive oxygen species (ROS) level of PC12 cells markedly increased to 187.12% (p < 0.01) relative to the control value, which suggests that CORT might induce oxidative stress. When the cells were incubated with 5 μM naringenin and 8 μM apigenin in the presence of 500 μM CORT for 24 h, the intracellular reactive oxygen species (ROS) levels significantly decreased to 116.48% and 131.23% (p < 0.01) of the control value, respectively.
Fig. 10.
The effects of the naringenin and apigenin on reactive oxygen species (ROS) in CORT-induced PC12 cells. (A)Results of flow cytometric analysis of ROS levels in PC12 cells. (B) Quantitative results of Figure A. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; ##p < 0.01 as compared with CORT-treated group. (1.5-column fitting image).
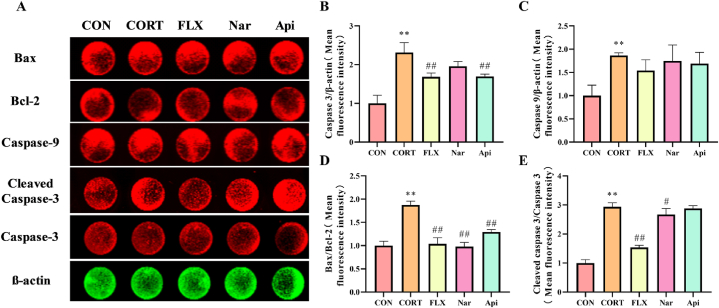
3.8. Effects of naringenin and apigenin on apoptosis pathway in CORT-induced PC12 cells
As shown in Fig. 11A, B, C, D, E, the results revealed that CORT obviously activated the apoptosis pathway in PC12 cells. Naringenin and apigenin could decrease the Bax/Bcl-2 ratio (Fig. 11D). Naringenin could increase the Cleaved caspase 3/Caspase 3 ratio (p < 0.05) (Fig. 11E), and apigenin only decreased the expression of Caspase 3 (Fig. 11B).
Fig. 11.
The effects of Naringenin and Apigenin on the apoptosis pathway in CORT induced PC12 cells. (A) The results of the detection of apoptotic protein levels in PC12 cells. (B)The quantification of caspase 3 protein content in cells. (C)The quantification of caspase 9 protein content in cells. (D)The quantification of Bax/Bcl-2 in cells. (E)The quantification of Cleaved Caspase3/Caspase3 in cells. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (2-column fitting image).
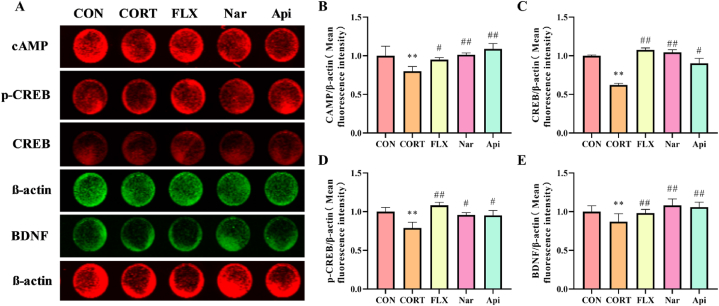
3.9. Effects of naringenin and apigenin on cAMP/CREB/BDNF pathway in CORT-induced PC12 cells
As shown in Fig. 12A, the results revealed that CORT obviously suppressed the cAMP/CREB/BDNF pathway in PC12 cells. Naringenin and apigenin could both increase the expression of cAMP (Fig. 12B), CREB (Fig. 12C), p-CREB (Fig. 12D) and BDNF (Fig. 12D).
Fig. 12.
The effects of Naringenin and Apigenin on cAMP/CREB/BDNF pathway in CORT-induced PC12 cells. (A)Results of protein levels in the cAMP/CREB/BDNF signaling pathway in PC12 cells. (B)The quantification of cAMP protein content in cells. (C)The quantification of CREB protein content in cells. (D)The quantification of p-CREB protein content in cells. (E)The quantification of BDNF protein content in cells. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with CORT-treated group. (2-column fitting image).
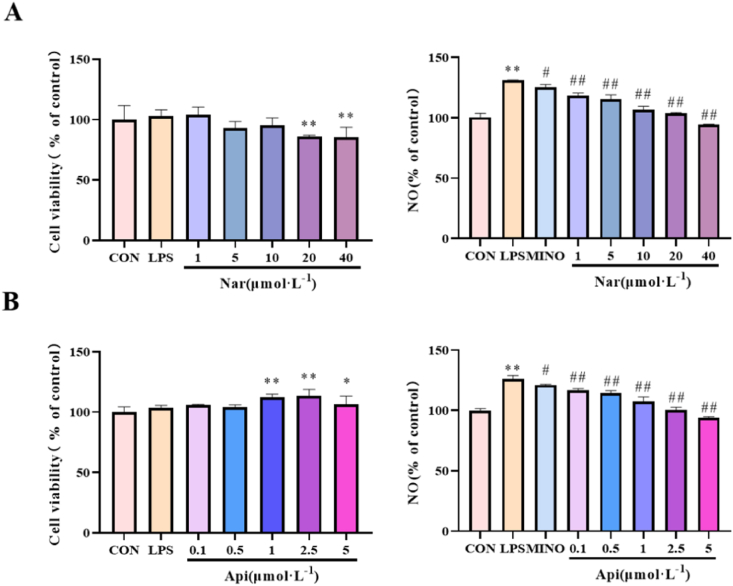
3.10. Effects of naringenin and apigenin on the cell viability and NO level in LPS-induced N9 cells
As shown in Fig. 13A and B, the level of NO in N9 cells markedly increased after being exposed to 1 μg/ml LPS for 24 h, whereas 1, 5, 10, 20, 40 μM naringenin and 0.1, 0.5, 1, 2.5, 5 μM apigenin can decreased the level of NO (p < 0.01); 1 μg/ml LPS, 1, 5 and 10 μM naringenin and 0.1 and 0.5 μM apigenin had no significant difference on cell viability in N9 cells. Therefore, 1 μΜ of naringenin and 0.1 μM of apigenin were used to subsequent studies of their neuroprotective effects.
Fig. 13.
The effects of the naringenin(A) and apigenin (B)on cell viability and the level of NO in LPS-induced N9 cells. Results are presented as means ± SD (n = 4). *p < 0.05 or **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01 as compared with LPS-treated group. (1.5-column fitting image).
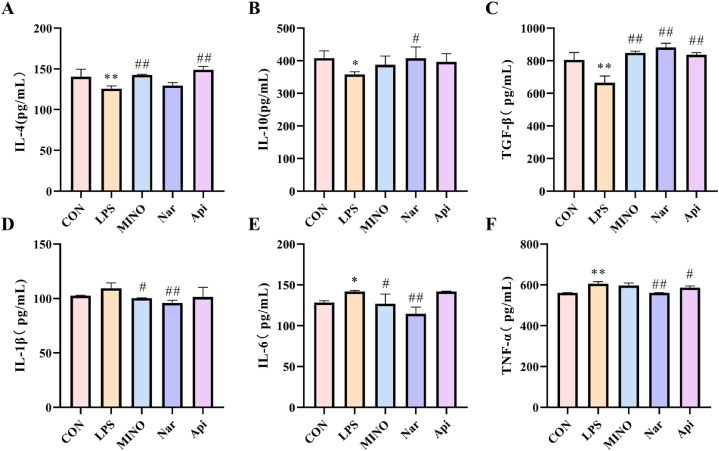
3.11. Effects of naringenin and apigenin on the inflammatory factors in LPS-induced N9 cells
As shown in Fig. 14, the content of anti-inflammatory factors such as IL-4 (Fig. 14A), IL-10 (Fig. 14B) and TGF-β (Fig. 14C) were decreased whereas the content of pro-inflammatory factors such as IL-1β (Fig. 14D), IL-6 (Fig. 14E) and TNF- α (Fig. 14F) were increased after exposure to 1 μg/ml LPS for 24 h. The content of anti-inflammatory factors increased under treatment with 1 μM naringenin and 0.1 μM apigenin in the presence of 1 μg/ml LPS for 24 h, and the content of pro-inflammatory factors decreased under treatment with 1 μM naringenin and 0.1 μM apigenin.
Fig. 14.
The effects of the naringenin and apigenin on the level of Inflammatory factors in LPS-induced N9 cells. (A)The levels of IL-4 in N9 cells. (B)The levels of IL-10 in N9 cells. (C)The levels of TGF-β in N9 cells. (D)The levels of IL-1β in N9 cells. (E)The levels of IL-6 in N9 cells. (F)The levels of TNF-α in N9 cells. Results are presented as means ± SD (n = 3). *p < 0.05 or **p < 0.01 as compared with control group; #p < 0.05 or ##p < 0.01as compared with LPS-treated group. (1.5-column fitting image).
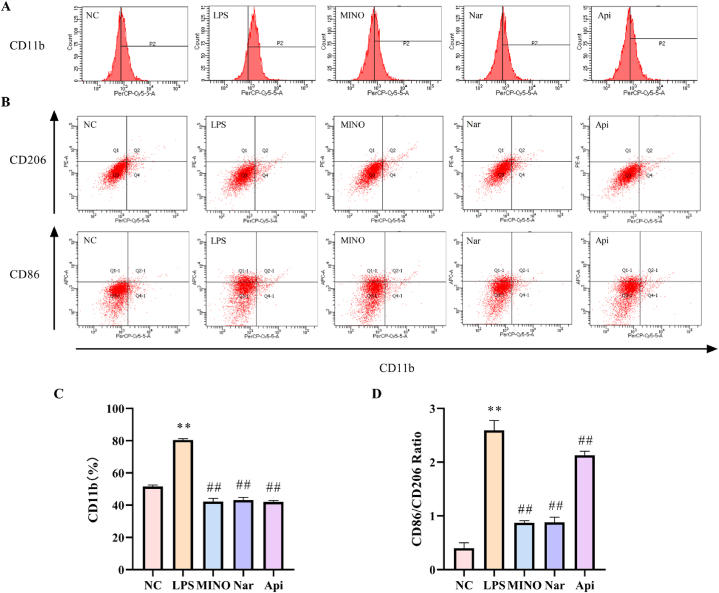
3.12. Effects of naringenin and apigenin on the M1/M2 phenotype in LPS-induced N9 cells
As shown in Fig. 15, after being exposed to 1 μg·ml-1 LPS for 24 h, The level of microglia activation marker CD11b was significantly increased (Fig. 15A and C), and the ratio of M1-type microglia marker CD86 to M2-type microglia marker CD206 was significantly increased in activated microglia (Fig. 15B and D). However, 1 μM naringenin and 0.1 μM apigenin can effectively reduce the activation of microglia after LPS administration, and inhibit the conversion of microglia M2 type to M1 type after activation, leading to reducing the level of inflammation.
Fig. 15.
The effects of the naringenin and apigenin on the M1/M2 phenotype in LPS-induced N9 cells. (A)The results of flow cytometric analysis of CD11b content in N9 cells. (B)The results of flow cytometric analysis of CD86 and CD206 content in N9 cells. (C)The quantification data of CD11b content in N9 cells. (D)The quantification data of CD86/CD206 in N9 cells. Results are presented as means ± SD (n = 3). **p < 0.01 as compared with control group; ##p < 0.01as compared with LPS-treated group. (1.5-column fitting image).
4. Discussion
Childhood abuse, and adult stressful life events such as unemployment or financial and relationship problems are associated with an enhanced risk for depression. Activation of the hypothalamic-pituitary-adrenal (HPA) axis is a hallmark of the stress response and represents an important indicator of psychosocial stress [28]. Continuous stress induces alterations in the endogenous stress response system, activating the HPA axis [29], and ultimately leads to the secretion of CORT, which is the principal glucocorticoid synthesized in the rodent adrenal cortex in response to stress. As shown in many studies, CORT administration for 21 days was effective in inducing depresson-like behavior in mice [30,31]. Loss of anhedonia is considered to be the core symptom of depression, and the sucrose preference index is an indicator of the lack of pleasure. CORT-treated animals have been reported with several depression-like behavioral features, including augmented behavioral despair in the Forced Swim Test (FST) and anhedonia in Sucrose Preference Test (SPT), as well as an increased latency to approach the food in the Novelty Suppressed Feeding (NSF) [32]. The state of immobility in the TST or FST is reported to mimic the depression phenotypes in humans and sucrose consumption in animals has been used as an indicator of anhedonia-like behavior [33]. The Open-Field Test (OFT) was also commonly used to assess the locomotor activity as well as depression/anxiety-like behavior in rodents. In our study, the animal behavioral tests including SPT, TST and OFT were used to assess the depressive symptoms. Mice in the CORT group showed increased immobility times in the TST and OFT and decreased sucrose consumption in the SPT. Naringenin and apigenin improved depressive symptoms in CORT-treated mice, as evidenced by a decreased immobility time in TST and OFT (Fig. 3, Fig. 4) and increased sucrose consumption in the SPT (Fig. 2). 5-HT, DA, and NE are monoamine neurotransmitters which had been regarded as the main pathogenesis of depression [34], both naringenin and apigenin administration markedly restored CORT-induced changes of neurotransmitters (NE, 5-HT and DA) and CORT levels. These results positively demonstrated the antidepressant-like effects of naringenin and apigenin in vivo. Many studies extensively adopted high concentrations of CORT-induced rat adrenal pheochromocytoma (PC12) cells as an in vitro model to study neurons damage and depression-like syndromes [35]. In the present study, after treatment with 500 μM CORT, the cell viability of PC12 cells were markedly decreased compared to the untreated control cells in the MTT assay. Naringenin (5, 8 μM) and apigenin (3, 5, 8, 10 μM) were able to increase the cell viability by protecting them from CORT-induced injury. These results suggest that naringenin and apigenin have antidepressant effects on CORT-induced depression in vitro.
A recent hypothesis suggests that depressive disorders are associated with altered levels of neurotrophins in the hippocampus, including a deficiency of brain-derived neurotrophic factor (BDNF) [36]. BDNF is one of the neurotrophic factors responsible for regulating neuronal survival, differentiation and synaptic plasticity, and is involved in cognitive dysfunction and mood alterations [[37], [38], [39]]. Indeed, CORT treatment reduced the amount of BDNF, whereas treatment with antidepressants has been shown to enhance BDNF expression in adult mice [40,41]. BDNF expression is regulated by the cAMP-CREB-BDNF signaling pathway. cAMP can activate PKA, which is subsequently able to activate CREB through CREB phosphorylation, thus inducing CREB-dependent BDNF expression in the hippocampus [42]. In the present study, the effects of naringenin and apigenin on the cAMP-CREB-BDNF signaling pathway in vitro and in vivo were investigated (Fig. 6, Fig. 12). The result showed that naringenin and apigenin regulated the cAMP-CREB-BDNF signaling pathway in both CORT-induced male mice depression model and CORT-induced PC12 cells.
Previous researches have shown that BDNF levels are closely related to apoptosis levels in hippocampal cells [43]. Previous studies have also confirmed that exposure to high cortisol levels through repeated injection of high doses of CORT increased the number of apoptotic cells in the hippocampus and cerebral cortex [44], which could be reduced by antidepressant treatments [45]. Therefore, apoptosis of hippocampus neurons might be one of the causative factors involved in the development of depression. In this study, 5 μM of naringenin and 8 μM of apigenin inhibited CORT-induced apoptosis/necrosis (Fig. 9), decreased the intracellular reactive oxygen species (ROS) levels (Fig. 10), and reduced the Bcl-2/Bax in PC12 cells (Fig. 11) compared with the CORT-treated group. These results indicated that naringenin and apigenin may exert a neuroprotective effect by inhibiting neuronal apoptosis through regulating the mitochondrial apoptotic pathway. The BDNF signaling pathway activation by naringenin and apigenin may led to the inhibition of neuronal apoptosis.
Neuroinflammation plays a key role in the development and contributes to each stage of the disease development process of central nervous system (CNS) diseases, such as depression, ischaemic stroke, Alzheimer's disease (AD), and Parkinson's disease (PD). Previous studies have shown that microglia are the key to the pathogenesis of neuroinflammatory diseases [46]. Microglia respond to various micro-environmental signals with two functional polarization states to mediate immune and inflammatory responses of the central nervous systems. The M1 phenotype of microglia releases a variety of pro-inflammatory mediators and free radicals that impair brain repair. In contrast, the M2 phenotype of microglia phagocytoses damaged nerve cell debris through scavenger receptors and matrix-degrading enzymes, inhibiting excessive inflammatory responses, promoting tissue repair and neuronal regeneration and avoiding secondary inflammatory damage. There are some studies shown that BDNF has anti-inflammatory effects, it can inhibit the expression of pro-inflammatory factors and promote the expression of anti-inflammatory factors, and can promote the transformation of microglia from M1 to M2 in the injured area [7]. Thus, in this experiment, LPS-induced N9 microglia cells were used as neuroinflammation models to explore the anti-neuroinflammation effects of naringenin and apigenin [47]. After being treated with 1 μg/ml LPS for 24 h, the viability of cells had no significant change compared to the control group in the MTT assay, indicating that 1 μg/ml LPS had no cytotoxic activity against N9 cells. In addition, 1 μg/ml LPS increased the NO level, whereas naringenin (1,5, 10 μm) and apigenin (0.1, 0.5 μM) reduced the NO level without cytotoxic activity (Fig. 13). Furthermore, naringenin and apigenin reduced the release of pro-inflammatory factors and increased the release of anti-inflammatory factors (Fig. 14). Moreover, naringenin and apigenin were able to inhibit the activation of N9 cells after LPS induction by reducing the level of microglia activation marker CD11b. Activated microglia are classified into M1 phenotypes and M2 phenotypes, with M1 phenotypes having pro-inflammatory and M2 phenotypes playing anti-inflammatory. Further results (Fig. 15) showed the pro-inflammatory M1 microglia could be transferred to anti-inflammatory M2 microglia by naringenin and apigenin, as evidenced by the decreased ratio of M1 type microglia marker CD86 and M2 type microglia marker CD206. It has been reported that M1 phenotype glial cells are neurotoxic, promoting the release of large amounts of inflammatory factors, reactive oxygen species (ROS), NO, etc., which have a neuronal killing effect. Therefore, naringenin and apigenin reduce the proportion of M1 phenotype glial cells in order to reduce the level of neuronal apoptosis [48].
In conclusion, naringenin and apigenin may improve depressive behaviors through promoting BDNF and inhibiting neuroinflammation and neuronal apoptosis.
5. Conclusions
To our knowledge, there are relatively few researches on the antidepressant effects of naringin and apigenin. In this research, naringenin and apigenin could reverse the depressive-like behaviors and metabolic abnormalities induced by chronic corticosterone exposure by inhibiting neuronal apoptosis, reducing neuroinflammation and promoting neurotrophic factors. Based on these findings, we speculated that naringenin and apigenin from Chrysanthemum morifolium may be useful in the treatment of depression.
Author contribution statement
Li Zhang: Conceived and designed the experiments; Wrote the paper. Rui-Hao Xu: Conceived and designed the experiments; Performed the experiments. Ren-Rui Lu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Hui-Hui Wang: Analyzed and interpreted the data. Xiaoke Zheng, Wei-Sheng Feng: Contributed reagents, materials, analysis tools or data.
Funding statement
Prof Xiaoke Zheng was supported by Ministry of Science and Technology of the People's Republic of China [2019YFC1708802]; Henan province high-level personnel special support “ZhongYuan One Thousand People Plan” [ZYQR201810080]. Wei-Sheng Feng was supported by Ministry of Science and Technology of the People's Republic of China [2017YFC1702800]. Li Zhang was supported by Health Commission of Henan Province [20-21ZY2148]; Doctoral Fund of Henan University of Chinese Medicine [RSBSJJ2018-04]. This work was supported by the education department of henan province [21B360003].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
Conceived and designed the experiments, L.Z. and R-H.X.; Performed the experiments, R-R.L. and R-H.X.; Analyzed and interpreted the data, R-R.L. and H-H.W. ; Contributed reagents, materials, analysis tools or data, X-K.Z. and W-S.F.; Wrote the paper, L.Z. and R-R.L. L.Z. and R-R.L. contributed equally to this work and should be considered co-first authors. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
This experiment was conducted by the Regulations on the Management of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals of Henan University of Traditional Chinese Medicine, promulgated by the National Science and Technology Commission of the People's Republic of China. The ethical approval reference number for this research is DWLL2018080003. All procedures for the care of mouse were following the institutional guidelines for the use of animals in the study.
Contributor Information
Wei-Sheng Feng, Email: fwsh@hactcm.edu.cn.
Xiao-Ke Zheng, Email: zhengxk.2006@163.com.
References
- 1.Gal Z., Huse R.J., Gonda X., et al. Anxiety and depression - the role of blood-brain barrier integrity. Neuropsychopharmacol. Hung. 2019;21(1):19–25. [PubMed] [Google Scholar]
- 2.Peritore A.F., Crupi R., Scuto M., et al. The role of annexin A1 and formyl peptide receptor 2/3 signaling in chronic corticosterone-induced depression-like behaviors and impairment in hippocampal-dependent memory. CNS Neurol. Disord.: Drug Targets. 2020;19(1):27–43. doi: 10.2174/1871527319666200107094732. [DOI] [PubMed] [Google Scholar]
- 3.Eker C., Kitis O., Taneli F., et al. Correlation of serum BDNF levels with hippocampal volumes in first episode, medication-free depressed patients. Eur. Arch. Psychiatr. Clin. Neurosci. 2010;260(7):527–533. doi: 10.1007/s00406-010-0110-5. [DOI] [PubMed] [Google Scholar]
- 4.Jeon S.C., Kim H.J., Ko E.A., et al. Prenatal exposure to high cortisol induces ADHD-like behaviors with delay in spatial cognitive functions during the post-weaning period in rats. Experimental Neurobiology. 2021;30(1):87–100. doi: 10.5607/en20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcia M.A., Bonsall D.R., Bloomfield P.S., et al. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233(9):1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu A.L., Stochl J., Lewis G., et al. Longitudinal association between inflammatory markers and specific symptoms of depression in a prospective birth cohort. Brain Behav. Immun. 2019;76:74–81. doi: 10.1016/j.bbi.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B., Zhang Y., Yang Z., et al. ω-3 DPA protected neurons from neuroinflammation by balancing microglia M1/M2 polarizations through inhibiting NF-κB/MAPK p38 signaling and activating neuron-BDNF-PI3K/AKT pathways. Mar. Drugs. 2021;19(11) doi: 10.3390/md19110587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D., Lian D., Wu J., et al. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflammation. 2017;14(1):156. doi: 10.1186/s12974-017-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umukoro S., Akinyinka A.O., Aladeokin A.C. Antidepressant activity of methyl jasmonate, a plant stress hormone in mice. Pharmacol. Biochem. Behav. 2011;98(1):8–11. doi: 10.1016/j.pbb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 10.MJ, Y.; ZR, L.; F, L., et al. Pharmacological action research progress of chrysanthemi indici fols with meridian tropism in liver. Res. Pract. Chin. Med. 2020;34(5):77–81. [Google Scholar]
- 11.Renrui L., Li Z., Ruihao X., et al. Protective effect and mechanistic of Huai Chrysanthemi Flos extract on corticosterone-induced depression model. Chin. Tradit. Herb. Drugs. 2022;53(18):5750–5758. [Google Scholar]
- 12.Chen S., Liu J., Dong G., et al. Flavonoids and caffeoylquinic acids in Chrysanthemum morifolium Ramat flowers: a potentially rich source of bioactive compounds. Food Chem. 2021;344:128733. doi: 10.1016/j.foodchem.2020.128733. [DOI] [PubMed] [Google Scholar]
- 13.Joo-Shin K. Flavonoids, an overview: chemical structures, dietary sources, and biological properties. Food Eng. Prog. 2020;24(3):151–163. [Google Scholar]
- 14.Guan L.P., Liu B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016;121:47–57. doi: 10.1016/j.ejmech.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Wenzhi, H.; Lu, W.; Junqing, H., et al., Research progress on the pharmacodynamic mechanism of antidepressant compound prescriptions and its flavonoids active ingredients. Acta Pharm. Sin., 1-29.
- 16.Dimpfel W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine. 2009;16(4):287–294. doi: 10.1016/j.phymed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.J., Kim B.J. Naringenin inhibits pacemaking activity in interstitial cells of Cajal from murine small intestine. Integr. Med. Res. 2017;6(2):149–155. doi: 10.1016/j.imr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You Q., Wu Z., Wu B., et al. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016;230(2):197–214. doi: 10.1530/JOE-16-0004. [DOI] [PubMed] [Google Scholar]
- 19.Cai L., Wu H., Tu C., et al. Naringin inhibits ovarian tumor growth by promoting apoptosis: an in vivo study. Oncol. Lett. 2018;16(1):59–64. doi: 10.3892/ol.2018.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshihisa Y., Andoh T., Rehman M.U., et al. The regulation of protein kinase casein kinase II by apigenin is involved in the inhibition of ultraviolet B-induced macrophage migration inhibitory factor-mediated hyperpigmentation. Phytother Res. : PT. 2020;34(6):1320–1328. doi: 10.1002/ptr.6597. [DOI] [PubMed] [Google Scholar]
- 21.Feng W.S., Chen W.J., Zheng X.K., et al. Flavonoids from the flowers of Chrysanthemum morifolium Ramat. Chin. Pharmaceut. J. 2017;52(17):1497–1502. [Google Scholar]
- 22.Zhao Y., Ma R., Shen J., et al. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. 2008;581(1–2):113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Zeng M., Xu R., et al. Inhibitory activity of acteoside in melanoma via regulation of the ERβ-Ras/Raf1-STAT3 pathway. Arch. Biochem. Biophys. 2021;710:108978. doi: 10.1016/j.abb.2021.108978. [DOI] [PubMed] [Google Scholar]
- 24.Guo P., Zeng M., Wang S., et al. Eriodictyol and homoeriodictyol improve memory impairment in aβ25-35-induced mice by inhibiting the NLRP3 inflammasome. Molecules. 2022;27(8) doi: 10.3390/molecules27082488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renrui L., Huihui W., Li Z., et al. Effect of echinacoside extracted from Dihuang on glutamate-induced oxidative stress and NMDAR1 expression in PC-12 cells. Pharmacology and Clinics of Chinese Materia. 2021;37(5):45–48. [Google Scholar]
- 26.Huihui W., Renrui L., Li Z., et al. Rehmannioside D in Dihuang inhibits neuroinflammation by regulating M1/M2 polarization in microglia. J. Chin. Med. Mater. 2021;44(11):2683–2687. [Google Scholar]
- 27.Liu M., Zeng M., Wang S., et al. Thymidine and 2'-deoxyuridine reduce microglial activation and improve oxidative stress damage by modulating glycolytic metabolism on the Aβ25-35-induced brain injury. Arch. Biochem. Biophys. 2022;729:109377. doi: 10.1016/j.abb.2022.109377. [DOI] [PubMed] [Google Scholar]
- 28.Iob E., Kirschbaum C., Steptoe A. Positive and negative social support and HPA-axis hyperactivity: evidence from glucocorticoids in human hair. Psychoneuroendocrinology. 2018;96:100–108. doi: 10.1016/j.psyneuen.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Keller J., Gomez R., Williams G., et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatr. 2017;22(4):527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo A., Dalmagro A.P., Rikel L., et al. Cholecalciferol counteracts depressive-like behavior and oxidative stress induced by repeated corticosterone treatment in mice. Eur. J. Pharmacol. 2018;833:451–461. doi: 10.1016/j.ejphar.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y., Cong Y., Liu H. Folic acid ameliorates depression-like behaviour in a rat model of chronic unpredictable mild stress. BMC Neurosci. 2020;21(1):1. doi: 10.1186/s12868-020-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger S., Gureczny S., Reisinger S.N., et al. Effect of chronic corticosterone treatment on depression-like behavior and sociability in female and male C57bl/6N mice. Cells. 2019;8(9) doi: 10.3390/cells8091018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renard C.E., Dailly E., David D.J., et al. Monoamine metabolism changes following the mouse forced swimming test but not the tail suspension test. Fund. Clin. Pharmacol. 2003;17(4):449–455. doi: 10.1046/j.1472-8206.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 34.Shao X., Zhu G. Associations among monoamine neurotransmitter pathways, personality traits, and major depressive disorder. Front. Psychiatr. 2020;11:381. doi: 10.3389/fpsyt.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi X., Zhou N., Cheng J., et al. Chlorogenic acid protects PC12 cells against corticosterone-induced neurotoxicity related to inhibition of autophagy and apoptosis. BMC BMC Pharmacol. Toxicol. 2019;20(1):56. doi: 10.1186/s40360-019-0336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017;2017:7260130. doi: 10.1155/2017/7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim D.W., Han T., Um M.Y., et al. Administration of asian herb bennet (geum japonicum) extract reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Nutrients. 2019;11(12) doi: 10.3390/nu11122841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esvald E.E., Tuvikene J., Sirp A., et al. CREB family transcription factors are major mediators of BDNF transcriptional autoregulation in cortical neurons. J. Neurosci. 2020;40(7):1405–1426. doi: 10.1523/JNEUROSCI.0367-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai Y., Ma Y., Liu J., et al. Brain-derived neurotrophic factor alleviates ropivacaine-induced neuronal damage by enhancing the akt signaling pathway. Med. Sci. Monit. 2019;25:10154–10163. doi: 10.12659/MSM.918479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng L., Guo X., Li Y., et al. Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur. J. Pharmacol. 2016;774:50–410. doi: 10.1016/j.ejphar.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Yan T., Xu M., Wan S., et al. Schisandra chinensis produces the antidepressant-like effects in repeated corticosterone-induced mice via the BDNF/TrkB/CREB signaling pathway. Psychiatr. Res. 2016;243:135–4210. doi: 10.1016/j.psychres.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Capibaribe V.C.C., Vasconcelos Mallmann A.S., Lopes I.S., et al. Thymol reverses depression-like behaviour and upregulates hippocampal BDNF levels in chronic corticosterone-induced depression model in female mice. J. Pharm. Pharmacol. 2019;71(12):1774–1783. doi: 10.1111/jphp.13162. [DOI] [PubMed] [Google Scholar]
- 43.Ding Y., Zhu W., Kong W., et al. Edaravone attenuates neuronal apoptosis in hippocampus of rat traumatic brain injury model via activation of BDNF/TrkB signaling pathway. Arch. Med. Sci. : AMS. 2021;17(2):514–522. doi: 10.5114/aoms.2019.89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen D.N., Huyghens L., Zhang H., et al. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. BioMed Res. Int. 2014;2014:712742. doi: 10.1155/2014/712742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He D., Wang N., Sai X., et al. Camellia euphlebia protects against corticosterone-induced apoptosis in differentiated PC12 cells by regulating the mitochondrial apoptotic pathway and PKA/CREB/BDNF signaling pathway. Food Chem. Toxicol. 2019;126:211–222. doi: 10.1016/j.fct.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 46.Wang X.L., Chen F., Shi H., et al. Oxymatrine inhibits neuroinflammation byRegulating M1/M2 polarization in N9 microglia through the TLR4/NF-κB pathway. Int. Immunopharm. 2021;100:108139. doi: 10.1016/j.intimp.2021.108139. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L., Zhang J., You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front. Cell. Neurosci. 2018;12:306. doi: 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C., Liu C., Li F., et al. Extracellular mitochondria activate microglia and contribute to neuroinflammation in traumatic brain injury. Neurotox. Res. 2022;40(6):2264–2277. doi: 10.1007/s12640-022-00566-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.