Abstract
Aims
Microvascular morphology and pathological changes in gestational diabetes mellitus (GDM) placentas and normal placentas were observed via vascular casting technology, electron microscopy, and pathological detection technology. Vascular structure and histological morphology changes in GDM placentas were examined to generate basic experimental data for the diagnosis and prognostic determination of GDM.
Methods
This case–control study involving 60 placentas, 30 from healthy controls and 30 from patients with GDM. Differences in size, weight, volume, umbilical cord diameter, and gestational age were assessed. Histological changes in the placentas in the two groups were analyzed and compared. A placental vessel casting model was constructed using a self-setting dental powder technique, to compare the two groups. The placental cast microvessels of the two groups were compared using scanning electron microscopy.
Results
There were no significant differences in maternal age or gestational age between the GDM group and the control group (p > .05). The size, weight, volume, and thickness of the placentas in the GDM group were significantly greater than those in the control group, as was umbilical cord diameter (p < .05). Immature villus, fibrinoid necrosis, calcification, and vascular thrombosis were significantly greater in the placental mass in the GDM group (p < .05). The terminal branches of the microvessels in diabetic placenta casts were sparse, with significantly fewer ends and lower villous volume (p < .05).
Conclusion
Gestational diabetes can cause gross and histological changes in the placenta, particularly placental microvascular changes.
Keywords: Blood vessel casting, gestational diabetes, gross morphology, microvasculature, pathological changes, placenta
Introduction
Gestational diabetes mellitus (GDM) is a common chronic disease during pregnancy, which endangers the health of millions of women worldwide. Formally recognised by O’Sullivan and Mahan in 1964, GDM is defined as hyperglycaemia first detected during pregnancy. GDM is associated with adverse pregnancy outcomes, including premature delivery, primary cesarean delivery and preeclampsia. Prenatal exposure to maternal hyperglycemia leads to hyperinsulinemia in the fetus, which in turn increases the risk of macrosomia, neonatal hypoglycemia, hyperbilirubinemia, etc. With the incidence of obesity worldwide reaching epidemic levels, the number of pregnant women diagnosed as having GDM is growing, and these women have an increased risk of a range of complications of pregnancy. 1 According to the American Diabetes Association (2021)2,3 GDM can be diagnosed when blood glucose values exceed 5.1 mmol/L after fasting (92 mg/dL), 10.0 mmol/L (180 mg/dL) for 1 h, and 8.5 mmol/L (153 mg/dL) for 2 h.
The placenta is a complex fetal organ that plays multiple roles during fetal growth and development. It separates maternal and fetal circulation, and is connected to both through different surfaces. The syncytial trophoblasts expose the placenta to the maternal circulation, and the endothelial cells make contact with the fetal blood. Because of its unique location, the placenta is exposed to the regulation of hormones, cytokines, growth factors, and the matrix present in circulation, and may therefore be affected by changes in any of these. 4
Depending on the extent of diabetes control during pregnancy the placenta may be exposed to abnormal glucose metabolism, which may adversely affect its development. These conditions may account for the significant increase in placental weight in GDM pregnancies. 5 Metabolism and microcirculation disorders, and possible sclerosis and alteration of chorionic villi, uterine vessels, and the placenta in different types of maternal diabetes can increase the risk of circulatory failure in the maternal-fetal system. This progression can lead to the development of hypoxia, fetal growth retardation, and prenatal death. 6
During pregnancy, the incidences of hypertension, abortion, stillbirth, and puerperal sepsis are high. 7 In women with gestational diabetes the placenta becomes heavier and paler due to villous edema This is why uteroplacental blood flowdisorders should be studied in detail, including the changing tree of villi and the blood vessels of villi.
Previous studies indicate that there are differences in vascular structure between normal placentas and gestational hypertensive placentas, but there are no reports on differences between normal placentas and gestational diabetic placentas. Yin et al. 8 reported that as well as reduced vessel volume, severe preeclampsia was associated with reduced vessel diameter, which is another manifestation of insufficient perfusion. Our previous study9,10 showed that modified vascular casting technology can clearly reveal the vascular course, morphological distribution, and branching, and particularly the microvascular morphology and structure. The current study used a combination of three methods to analyze the gross vessels and microvessels of gestational diabetic placentas. The aim was to compare placental morphology, vascular morphology, and histological changes in pregnant women with GDM and normal pregnant women.
Materials and methods
Placentas
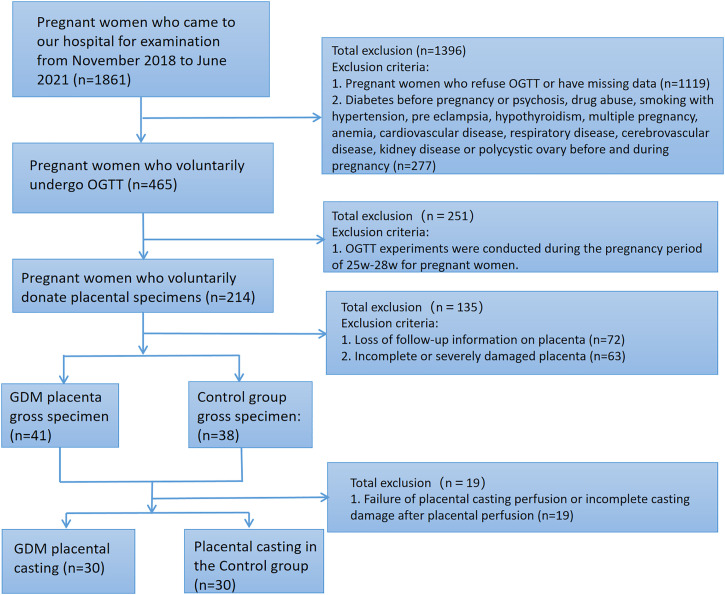
From November 2018 to June 2020, 1861 pregnant women came to the hospital. In this case–control study, oral glucose tolerance test (OGTT) was performed on 465 pregnant women who came to our hospital and signed the informed consent form. The gestation age was between 24 w and 28 w. According to the OGTT results, the pregnant women were divided into GDM group and control group. Both groups were primiparas with single pregnancy. A total of 60 placentas (30 in control group and 30 in GDM group) with gestational weeks ≥36 were collected from November 2018 to June 2020 at Xiangyang First People’s Hospital. Placentas in the control group were derived from 30 women with normal and uncomplicated pregnancies. The exclusion criteria were: (1) psychosis, substance abuse, smoking associated with hypertension, preeclampsia, hypothyroidism, multiple pregnancy, anemia, cardiovascular disease, respiratory disease, cerebrovascular disease, kidney disease, or polycystic ovary before or during pregnancy; (2) incomplete or severely damaged placenta; and (3) placenta casting model not intact. Women in the GDM group were diagnosed via the International Diabetes and Pregnancy Study Group criteria, and one or more of the following criteria were required to be met 11 : (i) Fasting blood glucose 7.0 mmol/L (126 mg/dL), (ii) 2-h post prandial blood glucose 11.1 mmol/L (200 mg/dL), and (iii) random plasma glucose 11.1 mmol/L (200 mg/dL) with diabetes symptoms. All collected placentas were treated as follows: the weight, volume, thickness and umbilical cord diameter of fresh placentas (the placentas before casting) were recorded, placental tissues were taken for pathological examination, and then the placentas were perfused (Figue 1a,d). After the placental casting was completed, the placental chorionic vessels were taken for electron microscopic scanning to observe the morphological changes of placental vessels in the pathological group and the control group. Informed consent was obtained from each patient, and the study was approved by the Ethics Committee of Xiangyang First People’s Hospital, Hubei, China (Figure 1).
Figure 1.
Flow chart of this study.
Placental gross morphology assessment
The following parameters on fresh placental specimens were recorded referring to Soma Saha et al. 7
Placental Weight: by an electronic weighing weigher.
Placental Volume: estimated by the water displacement method. Vp (Placental volume) = VAfter (Volume of compound after placenta put in)—VBefore (Volume of water in container before placental implantation)
Placental Thickness: a thin long graduated needle was inserted in to the placenta, at the centre, at the margin and midway between the centre and margin. The average of the three readings was taken as the thickness of the placenta.
Umbilical cord Diameter: measured twice with a with a ruler up to the nearest 0.5 cm. The average of the two measurements was recorded as the diameter (d) of the umbilical cord diameter.
Histologic assessment
According to the inclusion and exclusion criteria, 60 placentas with gestational weeks ≥36 were collected from November 2018 to June 2020 at Xiangyang First People’s Hospital. 30 from diabetic pregnant women (GDM group) and 30 from non-diabetic pregnant women (control group). All maternal ages were between 20 and 40 years. Sections of diabetic placenta of approximately 5 mm thick slice were taken for Histological examination. Whole-layer slices were taken from the center and periphery of the fresh placenta, then fixed the slices in 10% formaldehyde and normal saline for 3 days for routine Histological examination. All these samples are taken before casting. After that, five-μm-thick sections were taken and stained with hematoxylin and eosin, and the substrate, amniotic membrane, chorionic plate, chorionic villi, and villus gaps were observed via light microscopy.
Placental casting
The method of placental casting is similar to that reported by J.L. James et al. 12 Take fresh placental samples, wash the blood stain on the surface and place them in heparin normal saline (add 100 IU heparin to every 500 mL of normal saline). Prepare 30 g of self-made denture powder, 60 mL of self-made denture water, 30 mL of dibutyl phthalate mixed casting materials, glass cannula, plastic syringe, and appropriate red and blue oil paints. Place the placenta in a square plate containing water, find the umbilical cord, separate two arteries and one vein, and connect 18 g blunt needle to a tube about 15–20 cm with an inner diameter of 1.57 mm. This setting allows the syringe/solution to be replaced without moving the needle. Then connect the tube to a 50 mL syringe, and insert the tube at the placental vein and artery respectively. First, inject the umbilical vein, and then inject heparin normal saline into the artery to flush the blood in the blood vessel until the color of the placenta becoming pale. The speed of 0.5 mL/min is appropriate. After one tube is injected, the other tube is quickly connected. Fluid leakage of the placenta and placental vessels should be monitored to ensure that the perfusion circulation area is intact. In all steps, care should be taken to ensure that there are no bubbles in the perfusion solution or the placenta vascular cast. After the perfusion of one umbilical vein and two umbilical arteries is completed, take out the tube which inserted in the blood vessel, and ligate the umbilical artery and vein. After the filling agent in specimen is fully hardened and fixed, the specimen is placed in 20% concentrated hydrochloric acid in parallel for about 3–5 days, then take out the specimen to washing the surface. After all these steps, the complete placenta casting specimen is made.
Scanning electron microscopy
After the completion of placental casting, take the terminal villous vessels of all placental casting specimens (on our placental casting, it shows the fifth grade of placental artery vessels and the seventh grade of placental veins, which may be related to the precision of casting), and a specimen approximately the size of half a mung bean was put into an EP tube. Pre-cooled 4% paraformaldehyde was added, and the specimen was left to fix for more than 12 h. It was then dried using a critical point Leica EM CPD 300 dryer, and the surface was sprayed using a high vacuum Leica EM ACE 600 coating instrument. The prepared samples were put into a Hitachi SU 8010 scanning electron microscope for observation, with a magnification of 30 and a voltage of 3.0 kV.
Statistics
Whether datasets conformed to a normal distribution was determined via the Shapiro-Wilk test, and data from the normal control group and the GDM group were compare via the t test for independent samples. p < .05 was deemed to indicate statistical significance. Non-normal variables were compared using the nonparametric Mann-Whitney test. Normally distributed data are expressed as means and standard deviations, and non-normally distributed data are expressed as medians ranges.
Results
General placenta morphology
In the control group the mean age was 29.8 years (range 24–37 years), mean gestational age was 38.17 weeks (range 36–40 weeks), mean placental weight was 0.41 kg (range 0.32–0.53 kg), mean placental volume was 339.41 cm3 (range 229.75–424.2 cm3), mean placental thickness was 2.06 cm (range 1.6–2.9 cm), and mean umbilical cord diameter was 1.06 cm (range 0.8–1.5 cm) (Table 1).
Table 1.
Placental morphology in the control group (n = 30).
| Parameters | mean | Maximum | Minimum | Standard deviation |
|---|---|---|---|---|
| Age (years) | 29.8 | 37 | 24 | 3.88 |
| Pregnancy age (weeks) | 38.17 | 40 | 36 | 1.26 |
| Umbilical cord diameter (cm) | 1.06 | 1.5 | 0.8 | 0.15 |
| Placental weight (kg) | 0.41 | 0.53 | 0.32 | 0.06 |
| Placental volume (cm3) | 339.41 | 424.20 | 299.75 | 36.66 |
| Placental | 2.06 | 2.9 | 1.6 | 0.24 |
In the GDM group the mean age was 30.9 years (range 25–38 years), mean gestational age was 38.23 weeks (range 36–40 weeks), mean placental weight was 0.66 kg (range 0.55–0.99 kg), mean placental volume was 470.9 cm3 (range 400.4–530.0 cm3), mean thickness of the placenta was 2.99 cm (range 2.3–3.8 cm), and mean diameter of the umbilical cord was 1.4 cm (range 1.0–2.0 cm) (Table 2).
Table 2.
Placental morphology in the GDM group (n = 30).
| Parameters | Mean | Maximum | Minimum | Standard deviation |
|---|---|---|---|---|
| Age (years) | 30.9 | 38 | 25 | 3.23 |
| Pregnancy age (weeks) | 38.23 | 40 | 36 | 1.19 |
| Umbilical cord diameter (cm) | 1.40 | 2 | 1 | 0.32 |
| Placental weight (kg) | 0.66 | 0.99 | 0.55 | 0.10 |
| Placental volume (cm3) | 470.90 | 530.00 | 400.40 | 40.82 |
| Placental thickness (cm) | 2.99 | 3.8 | 2.3 | 0.22 |
There were no significant differences in age, gestational week, placental volume, umbilical cord diameter, placental weight, and placental thickness between the control group and the GDM group (Tables 1 and 2). The size, weight, volume, thickness, and umbilical cord diameter of the placenta were significantly larger in the GDM group than in the control group (Table 3).
Table 3.
Comparison between the control group and the GDM group.
| Parameters | Control group | GDM group | p |
|---|---|---|---|
| (n = 30) | (n = 30) | ||
| Age (years) | 29.8 ± 3.88 | 30.9 ± 3.20 | 0.20 |
| Pregnancy age (weeks) | 38.0 (37.0, 39.0) | 38.5 (37.0, 39.0) | 0.81 |
| Umbilical cord diameter (cm) | 1.0 (1.0, 1.2) | 1.2 (1.2, 1.6) | <0.001 |
| Placental weight (kg) | 0.41 ± 0.06 | 0.64 (0.59, 0.72) | <0.001 |
| Placental volume (cm3) | 339.41 ± 36.66 | 470.90 ± 40.82 | <0.001 |
| Placental thickness (cm) | 2.0 (2.0, 2.2) | 2.99 ± 0.22 | <0.001 |
Placental vessel casting and scanning electron microscopy
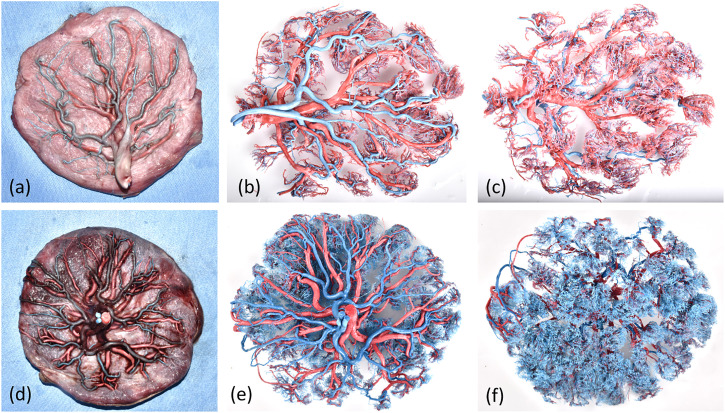
There were significant differences in blood vessels, microvessels, and villi between the control group and the GDM group. Dry villous blood vessel models of placentas in the normal group were generally composed of five or six bundles of parallel intermediate microarteries located close together, with a straight course and smooth surfaces, and they tended to consistently branch outward to form villous branches. As shown in Figure 2(b), (c), (e), (f), corrosion casting can provide vascular structure information on the fetal artery and vein tree. Compared to GDM placentas (Figures 2(b) and (c)), the vessels of normal placentas (Figures 2(e) and (f)) were copiously branched, closely constructed, and thick and uniform, and they exhibited smooth surfaces and were rich in terminal villous branches. The placental blood vessels in the GDM group were rough and uneven, and the terminal branch blood vessels were sparse. There were significantly fewervilli, and the cast surface was dry, rough, and uneven. This was due to increased swelling of vascular endothelial cells, a thickened basement membrane, and endothelial cells protruding into the lumen resulting in a rough vascular wall, stenosis, and even occlusion of the lumen. The above changes in villous blood vessels could cause placental hemodynamic changes, increased blood flow resistance, reduced blood perfusion, and placental fetal blood circulation disorders, thus affecting material exchange between the mother and fetus, ultimately leading to fetal growth and developmental disorders.
Figure 2.
Visualization of the stages of placental vessel casting. (a, b) Placental microvessels in the GDM group were characterized by a significant reduction in branched vessels, uneven thickness, rigid casting, and dry surfaces, and some branched vessels followed a disordered snake-like course or exhibited reticulate distortion. (c, d) Placental microvessels in the normal group were characterized by a soft and straight course and smooth surfaces, and branches that consistently projected outward to form villous branching vessels.
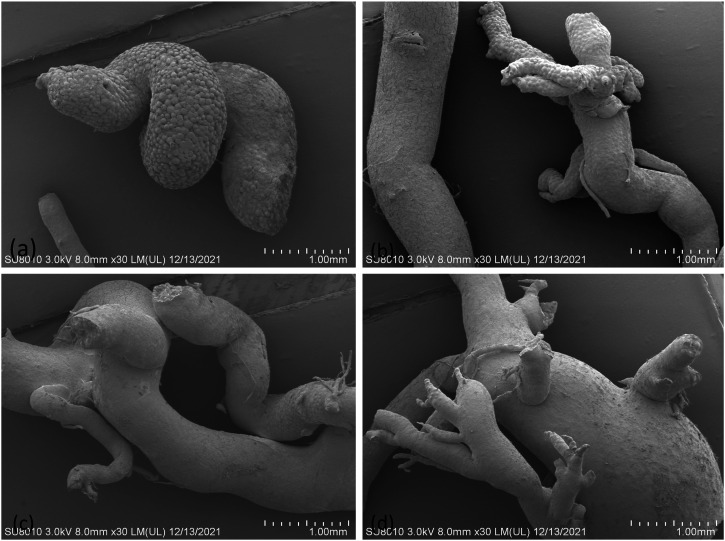
Scanning electron microscopy (SEM) of placental casts in the GDM group revealed vessels with uneven vascular thickness of placental villi, reduced branches, and rough inner walls. Only parts of the vessels were captured in some castings because of broken cast specimens, particularly at the branching level of small, fragile vessels. Villous branched blood vessels in casts from control placentas were full, and exhibited vascular endothelial cell compression impression, compact branched blood vessel structure, uniform blood vessel thickness, and gentle and natural courses in (Figures 3(c) and (d)). In the GDM group branched vessels were significantly reduced, and exhibited uneven thickness, a rigid cast shape, and dry surfaces, and some branched vessels followed a bizarre snake-like course or exhibited reticulate distortion.in (Figures 3(a) and (b))
Figure 3.
Pictures of placental microvessels in the two groups. (a, b) Placental microvessels in the GDM group were characterized by a significant reduction in branched vessels, uneven thickness, rigid casting, and dry surfaces, and some branched vessels followed a disordered snake-like course or exhibited reticulate distortion. (c, d) Placental microvessels in the normal group were characterized by a soft and straight course and smooth surfaces, and branches that consistently projected outward to form villous branching vessels.
Placental pathological histology
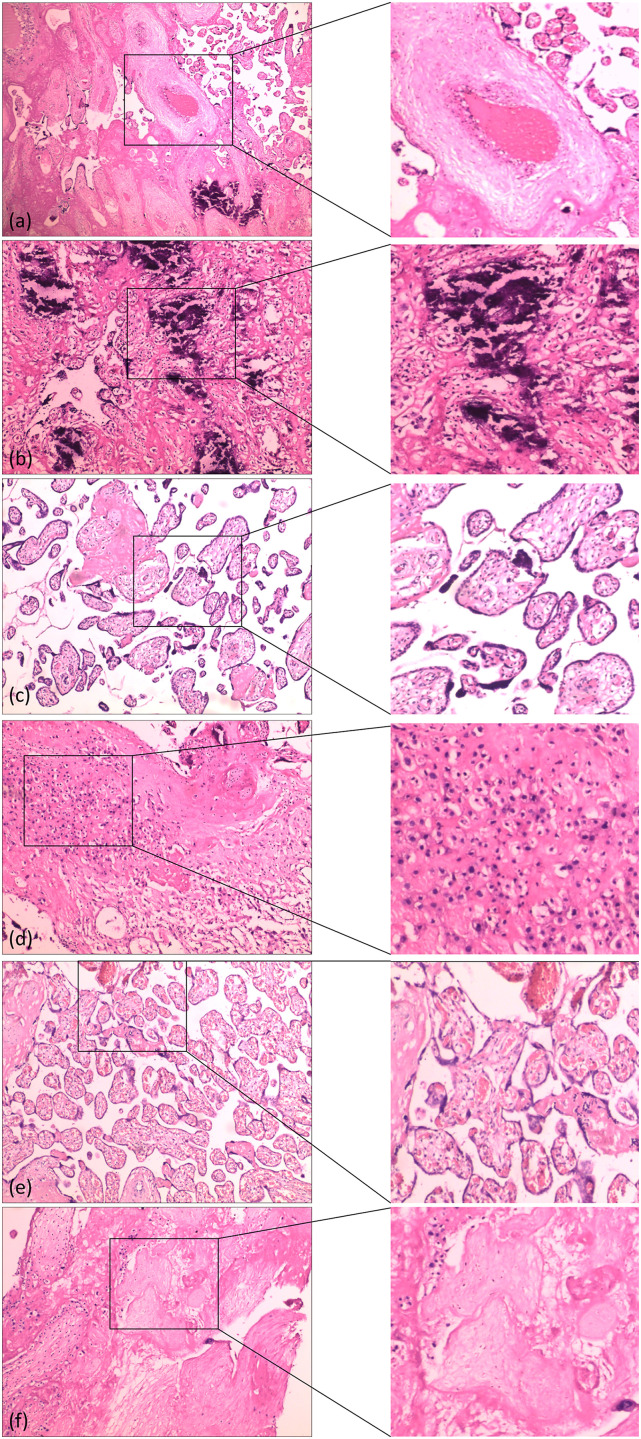
Placental pathological tissue in the control group differed significantly from that in the GDM group. Histological features in the GDM group included immature villi, a significant increase in vascular thrombosis in (Figure 4(a)). A diffuse calcification in (Figure 3(b)) and syncytia in (Figure 3(c)), inflammation in (Figure 4(d)), a villous congestion in (Figure 3(e)) and a fibroprotein necrosis in (Figure 4(f)) When stained with hematoxylin and eosin, calcification appeared as intracellular and extracellular basophilic deposits, respectively. These features were found in placentas from both groups, but they were more common in the GDM group.
Figure 4.
Photomicrographs of hematoxylin and eosin-stained specimens showing pathologic changes in the placenta. (a) Vessel thrombosis (100× magnification). (b) Villous stroma with diffuse calcifications (200× magnification). (c) Immaturity of chorial villosities with increased villous lumen, with numerous syncytial nodes (200× magnification). (d) Diffuse inflammation (200× magnification). (e) Congestion of villi (200× magnification). (f) Villous fibrinoid necrosis (200× magnification).
Discussion
GDM is the most prevalent metabolic disorder during pregnancy, and is defined as “diabetes diagnosed in the second or third trimester with no apparent diabetes prior to pregnancy.” The incidence of GDM varies widely between countries and even within states of the same country, mainly due to the use of different criteria or other reasons such as ethnicity and economic level. In Asian countries the prevalence of GDM ranges from 3.0% to 21.2%. In the United States it exceeds 9.0% and is increasing at a rate of nearly 6.3% per year. Once GDM is diagnosed both the pregnant woman and the fetus face multiple risks arising from blood sugar disorders. For the mother, complications include a need for caesarean section, polyhydramnios, preeclampsia, shoulder dystocia, and gestational hypertension. For the baby, common complications include neonatal hypoglycemia, birth injury, macrosomia, neonatal hospitalization, preterm birth, and respiratory distress.
To date the specific mechanisms underlying GDM have not been confirmed. Insulin resistance accounts for its basic pathogenesis and has been confirmed to be the initiating factor of GDM. Numerous studies have shown that inflammation plays a key role in insulin resistance and pancreatic beta cell failure. Once pregnant, the body gradually enters a state of low-grade systemic inflammation. 13 Inflammatory factors initiate the occurrence and development of insulin resistance. Placental tissue exhibits good endocrine function and can synthesize and secrete a variety of inflammatory cytokines, aggravating the maternal chronic inflammatory response and the degree of insulin resistance. Lastly, there are detrimental effects on the general morphology of the placenta and the umbilical cord, ultrastructure, blood vessels, and immunohistochemistry.
There are reports that placental weight may be the most important factor affecting fetal growth. 7 Wong, Kadivar et al.11,14 showed that gross morphological features such as enlarged cord diameter and thick edematous cord were more common in women with GDM. Abnormal placental and umbilical cord diameters can lead to increased fetal morbidity and mortality. Macroscopically the gestational diabetic placenta is large, plethoric, edematous, and attached to a thick edematous cord. In the present study the size, weight, volume, thickness, and cord diameter of the placenta in diabetic pregnant women were significantly larger than those in normal pregnant women. Many reports suggest that placenta size increases with increasing blood glucose, 6 and GDM may aggravate placental overgrowth via hyperglycemia and other unknown metabolic or endocrine mechanisms such as insulin resistance, leading to placental overgrowth.
The present study is the first to compare the vascular structures of GDM placentas and normal placentas via vascular casting and SEM. It is currently difficult to clearly demonstrate changes in placental blood vessels at all levels, so we used vascular casting methodology that can reveal the characteristics of all levels of placental vascular morphology. 15 Our previous research demonstrates that modified vascular casting technology can clearly reveal vascular courses, morphological distributions, and branching. 16 It has unique advantages with respect to determining the form and structure of microvessels. Ultrasound in Obstetrics and Gynecology’s editor-in-chief commented on our cardiovascular casting as follows:“Your vascular casting is special and can perfectly show the morphology and progression of the fetal microvessels that are not anatomically visible.”
For the first time we have observed the cast structure of placentas from patients with GDM combined with SEM, and accurately observed the placental microvessels, revealing the structure of the microvessel surface and the degree of vascular smoothness. The microvilli of the placental microvessels were clearly displayed in the placental cast specimens, and were mainly distributed in the free surface of the villous compound trophoblast in the normal placentas. Studies have shown that microvilli directly affect the uptake of nutrients and the excretion of metabolites between the placenta and the fetus. 17 Significant changes in microvilli size, morphology, and number are not conducive to normal fetal growth. Bedell et al. 18 found that GDM causes villus immaturity, characterized by reduced terminal villus branches and an increased capillary count in the villi. The increased number of capillaries within the villus limits blood flow within the villus space, thereby reducing nutrient and gas exchange between the mother and the fetus.
Under SEM, vessel casts of GDM placentas showed that the villous branched vessels were thinner than normal and were more sparsely distributed than normal. Vascular branches were very sparse, and the cast surface was dry and rough. There was obvious visual vascular endothelial cell compression and dot depression, and this was due to vascular endothelial cell swelling and basement membrane thickening. There were endothelial cells protruding into the lumen resulting in vascular lining roughening, lumen stenosis, and even occlusion. The above changes in villous vessels could cause changes in placental hemodynamics, increased blood flow resistance, reduced blood perfusion, and the development of placental fetal blood circulation disorders, thus affecting material exchange between the mother and fetus, ultimately leading to fetal growth and development disorders. This study shows that microvascular scanning based on the placenta casting technique is an ideal method for studying the three-dimensional construction of microvessels in organs, and is broadly applicable for the morphological study of microcirculation.
Hyperglycemia in GDM is a procontractile, procoagulatory, proinflammatory, and proangiogenetic factor affecting angiogenesis and vascular system maturation dysfunction during critical periods of placental development. 3 Most previous histopathological studies of GDM placentas have been retrospective or observational, and no studies have investigated the effects of GDM on the ultrastructure of the placenta.
Changes in placental morphology due to hypoxia in the GDM include villus immaturity, ischemia, villus fibrosis, and necrotic plaques. Choroidal vascular proliferation, calcification, and syncytial junction formation are also important markers of placental structural changes. Increases in these hypoxic parameters in placental tissue are associated with elevated fetal and maternal mortality and morbidity, and correct diagnosis and treatment can significantly reduce the risk of complications. 19 Wong, Akarsu, Gauster et al.5,11,20 have reported increased formation of syncytial nodules, peri-villous fibrin deposition, trophoblast basement membrane thickening, villous interstitial fibrosis, villous edema, vascular thickening, and the presence of nucleated erythrocytes. In such cases the terminal villi exhibit more branches per capillary, which is known as vasculopathy. Antoine et al. 21 reported immature villus, Hofbauer cell proliferation, stromal fibroblast proliferation, and excess trophoblast in gestational diabetic placenta, and other studies have revealed specific changes in the placenta despite improvements in maternal glycemic control. According to Soma 7 and Yun Han et al., 22 gestational diabetes mellitus caused profound, gross, and histologically altered placenta. 23
In the current histological study there were immature villi, fibrin necrosis inflammation, diffuse calcification and syncytium within the villous mucosa, villous congestion, and significantly increased vascular thrombosis in GDM placentas. When stained with hematoxylin and eosin, calcification appeared as intracellular and extracellular basophilic deposits, respectively. These characteristics occurred more frequently in the GDM group. GDM placentas had fewer microvilli than normal placentas, consistent with Arshad. 21
This is the first study combining three methods of morphological investigation; gross analysis, microvascular analysis, and SEM. The study has updated the data on GDM placenta, and supports the importance of ultrasonography, particularly with respect to placental structure. The results show that GDM can cause significant macroscopic and histological changes in the placenta, and placental microvascular changes are also particularly significant. The study provides a theoretical basis for early accurate warning and intervention in GDM. Notably however, the number of positive cases in the study was not sufficient, and large-scale analysis and molecular studies are necessary to assess the short-term and long-term effects of placental ultrastructural changes in GDM on offspring and pregnant women, which may have important clinical implications.
Acknowledgements
Patients are warmly thanked for consenting to participate in the study. We are also thankful to Dr Jingxing Dai for his work of preparation the placental vascular casts.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Hubei Clinical Medical Research Center for accurate diagnosis of fetal complex malformations, Grant/Award Number: 2021LC002, Key R&D Plan of Hubei Province (grant no: 2022BCE004) and Key Projects in Xiangyang Medical and Health Field (grant no:2022YL26 A).
ORCID iD
References
- 1.Sweeting A, Wong J, Murphy HR, et al. A clinical update on gestational diabetes mellitus. Endocr Rev 2022; 43: 763–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Pasquel FJ. Management of inpatient hyperglycemia and diabetes in older adults. Diabetes Care 2017; 40: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng Q, Shao L, Luo X, et al. Ultrastructure of placenta of gravidas with gestational diabetes mellitus. Obstet Gynecol Int 2015; 2015: 283124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care 2007; 30(Suppl 2): S120–S126. [DOI] [PubMed] [Google Scholar]
- 5.Gauster M, Desoye G, Totsch M, et al. The placenta and gestational diabetes mellitus. Curr Diab Rep 2012; 12: 16–23. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers E, Talton OO, Schust DJ, et al. Placental structural abnormalities in gestational diabetes and when they develop: a scoping review. Placenta 2021; 116: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha S, Biswas S, Mitra D, et al. Histologic and morphometric study of human placenta in gestational diabetes mellitus. Ital J Anat Embryol 2014; 119: 1–9. [PubMed] [Google Scholar]
- 8.Yin G, Chen M, Li J, et al. Vascular corrosion casting of normal and pre-eclamptic placentas. Exp Ther Med 2017; 14: 5535–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang J, Feng W, et al. Description of misdiagnosis and missed diagnosis of fetal complex heart malformations by prenatal echocardiography combined with postnatal cardiovascular casting. Prenat Diagn 2020; 40: 792–802. [DOI] [PubMed] [Google Scholar]
- 10.Peng A, Ye Y, Feng W, et al. Left atrial isomerism with complex cardiovascular malformation on prenatal ultrasound and vascular casting. Ultrasound Obstet Gynecol 2019; 54: 285–287. [DOI] [PubMed] [Google Scholar]
- 11.Carrasco-Wong I, Moller A, Giachini FR, et al. Placental structure in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis 2020; 1866: 165535. [DOI] [PubMed] [Google Scholar]
- 12.James JL, Tongpob Y, Srinivasan V, et al. Three-dimensional visualisation of the feto-placental vasculature in humans and rodents. Placenta 2021; 114: 8–13. [DOI] [PubMed] [Google Scholar]
- 13.Pan X, Jin X, Wang J, et al. Placenta inflammation is closely associated with gestational diabetes mellitus. Am J Transl Res 2021; 13: 4068–4079. [PMC free article] [PubMed] [Google Scholar]
- 14.Kadivar M, Khamseh ME, Malek M, et al. Histomorphological changes of the placenta and umbilical cord in pregnancies complicated by gestational diabetes mellitus. Placenta 2020; 97: 71–78. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann M, Konerding MA. Vascular casting for the study of vascular morphogenesis. Methods Mol Biol 2015; 1214: 49–66. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang J, Zeng H, et al. Utility of modified vascular corrosion casting technique in the diagnosis of fetal ductus arteriosus abnormalities. Sci Rep 2020; 10: 13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto T, Sato Y, Kodama Y, et al. Association between fetal vascular malperfusion and gestational diabetes. J Obstet Gynaecol Res 2022; 48: 80–86. [DOI] [PubMed] [Google Scholar]
- 18.Bedell S, Hutson J, de Vrijer B, et al. Effects of maternal obesity and gestational diabetes mellitus on the placenta: current knowledge and targets for therapeutic interventions. Curr Vasc Pharmacol 2021; 19: 176–192. [DOI] [PubMed] [Google Scholar]
- 19.Arshad R, Kanpurwala MA, Karim N, et al. Effects of diet and metformin on placental morphology in gestational diabetes mellitus. Pak J Med Sci 2016; 32: 1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akarsu S, Bagirzade M, Omeroglu S, et al. Placental vascularization and apoptosis in Type-1 and gestational DM. J Matern Fetal Neonatal Med 2017; 30: 1045–1050. [DOI] [PubMed] [Google Scholar]
- 21.Edu A, Teodorescu C, Dobjanschi CG, et al. Placenta changes in pregnancy with gestational diabetes. Rom J Morphol Embryol 2016; 57: 507–512. [PubMed] [Google Scholar]
- 22.Han Y, Zheng YL, Wu AM, et al. Effects of management in gestational diabetes mellitus with normal prepregnancy body mass index on pregnancy outcomes and placental ultrastructures: a prospective cohort study. Endocrine 2016; 54: 691–699. [DOI] [PubMed] [Google Scholar]
- 23.Singh V, Ranjan K, Tewarson SL. A study of microscopic changes in the placenta in gestational diabetes mellitus. J Anat Soc India 2020; 69: 166. [Google Scholar]