Abstract
Objectives
Perturbations of the intestinal microbiota have been associated with mental health disorders, including major depressive disorder (MDD). Therefore, faecal microbiota transplantation (FMT) holds promise as a microbiota-modulating treatment for MDD. Yet, to date, there are no published controlled studies evaluating the use of FMT for MDD. This study aimed to address this gap by evaluating the feasibility, acceptability, and safety of FMT for MDD.
Methods
The study was an 8-week, double-blind, 2:1 parallel group, randomized controlled pilot trial (n = 15) of enema-delivered FMT (n = 10) compared with a placebo enema (n = 5) in adults with moderate-to-severe MDD.
Results
Recruitment was completed within 2 months, with 0% attrition and 100% attendance at key study appointments. There were no major protocol deviations. The placebo and blinding strategies were considered successful; nurses and participants correctly guessing their treatment allocation at a rate similar to that anticipated by chance. No serious or severe adverse events were reported in either group, and there were no significant differences in mild-to-moderate adverse events between groups (median of 2 adverse events per participant reported in both groups). Furthermore, the 12/15 participants who completed the Week 2 participant satisfaction survey agreed or strongly agreed that the enema delivery was tolerable and that they would have the treatment again if required. Whilst the study was not designed to measure clinical outcomes, exploratory data also suggested that the active FMT treatment may lead to improvements in gastrointestinal symptoms and quality of life in this population, noting that irritable bowel syndrome is commonly comorbid with MDD.
Conclusions
All feasibility targets were met or exceeded. This study found that enema-delivered FMT is feasible, acceptable, well-tolerated, and safe in patients with MDD. The findings of this study support further research to evaluate clinical efficacy, and the use of this protocol is supported.
Keywords: faecal microbiota transplantation, FMT, microbiome, psychiatry, mental disorder, depression, MDD, major depressive disorder, mood disorders, mental health
RÉSUMÉ
Objectif
Les perturbations du microbiote intestinal ont été associées aux troubles de santé mentale, notamment le trouble dépressif majeur (TDM). Par conséquent, la transplantation du microbiote fécal (TMF) est prometteuse à titre de traitement de modulation du microbiote pour le TDM. Pourtant, à ce jour il n'y a pas d'études contrôlées publiées qui évaluent l'utilisation de la TMF pour le TDM. La présente étude visait à corriger cet écart en évaluant la faisabilité, l'acceptabilité et la sécurité de la TMF pour le TDM. Méthodes : L'étude était un essai pilote randomisé contrôlé (n = 15), de 8 semaines, à double insu, en groupes parallèles 2:1, d'une TMF administrée par lavement (n = 10) comparé à un lavement placebo (n = 5) chez des adultes souffrant d'un TDM modéré à grave. Résultats : Le recrutement s'est fait en 2 mois, avec 0 % d'attrition et 100 % de participation aux principaux rendez-vous de l'étude. Il n'y a pas eu de déviation majeure du protocole. Les stratégies du placebo et du double insu ont été estimées réussies; les infirmières et les participants ont correctement deviné leur affectation de traitement à un rythme semblable à celui anticipé par hasard. Aucun événement indésirable sérieux ou grave n'a été déclaré dans aucun groupe et il n'y a pas eu de différences significatives entre les événements indésirables bénins à modérés entre les groupes (la médiane de deux événements indésirables par participant rapportée dans les deux groupes). En outre, les 12/15 participants qui ont rempli le sondage sur la satisfaction du participant à la 2e semaine étaient d'accord ou fortement d'accord que l'administration du lavement était tolérable, et qu'ils suivraient le traitement de nouveau, si nécessaire. Bien que l'étude ne soit pas conçue pour mesurer les résultats cliniques, les données exploratoires ont aussi suggéré que le traitement actif de la TMF peut entraîner des améliorations des symptômes gastro-intestinaux et de la qualité de vie dans cette population, en faisant remarquer que le syndrome du côlon irritable est communément comorbide avec le TDM. Conclusions : Toutes les cibles de faisabilité ont été satisfaites ou excédées. Cette étude a constaté que la TMF administrée par lavement est faisable, acceptable, bien tolérée et sécuritaire chez les patients souffrant du TDM. Les résultats de cette étude soutiennent plus de recherche pour évaluer l'efficacité clinique, et l'usage de ce protocole est soutenu.
Background
There is an association between mental health disorders and perturbations in intestinal microbiota composition.1,2 Various biological variables mediated by intestinal microbiota are associated with the aetiology and maintenance of major depressive disorder (MDD).1,3 A recently published systematic review described differences in the intestinal microbiota profiles of depressed compared with non-depressed humans. 4 Interventions that modify gut microbiota, such as probiotics,5–7 antibiotics,8–12 and diet 13 may also affect depression and anxiety symptoms. These data suggest the intestinal microbiota might be a modifiable target in the treatment of depression. 1
Faecal microbiota transplantation (FMT) holds promise as a potential microbiota-modulating treatment for diverse conditions 14 including depression, where current evidence-based treatments (antidepressants and psychotherapy) are ineffective in more than one-third of individuals15,16 and have limitations including access, side effects, and cost. Whilst probiotics are limited to a small number of mostly food-derived strains of bacteria, FMT encompasses the broader bacterial diversity found within the human gastrointestinal tract, including those not available in probiotics. 17 Therefore, FMT has the potential to alter the composition of the intestinal microbiota more effectively and deliver a broader range of microbial functions.
There is evidence supporting the use of FMT for MDD.18,19 FMT-induced changes in intestinal microbiota composition can influence rodent models of psychiatric disorders and behaviour.20–22 Compellingly, 4 studies have now demonstrated that FMT from depressed humans into rodents induces depression-like behaviours in the rodents.23–26 Similar outcomes have been observed in the rodent models of schizophrenia. 27 In humans, FMT has shown promise in a range of other disorders, 14 including autism spectrum disorder. 28 Case studies also suggest promising outcomes with respect to the use of FMT for the treatment of depressive symptoms 29–31 and bipolar disorder.32,33 Studies of FMT for irritable bowel syndrome (IBS), which is commonly comorbid with MDD, have shown concurrent improvements in mental health symptomatology.14,34–36
The acceptability and feasibility of FMT have been evaluated across a range of disorders and vary widely depending on the disorder. One study surveyed 887 patients in which 50% of patients with ulcerative colitis and Crohn's disease stated they would be interested or very interested in undergoing this type of procedure, but this figure was 25% for patients with psoriasis. 37
To our knowledge, there are no published controlled studies specifically evaluating the use of FMT for mental illness in humans. Current evidence is limited to uncontrolled open-label studies and case reports. Thus, the safety, tolerability, acceptability, and feasibility of FMT for mental illnesses, including MDD, is yet to be established. The study presented here represents the first reported trial of FMT for MDD using a double-blind randomized controlled trial (RCT) design. The primary objective of this study (The Moving Moods Pilot Study) was to establish the feasibility and safety of FMT as an adjunctive treatment for MDD in adults. The secondary aim of the Moving Moods Pilot Study was to assess the degree of microbial engraftment of donor stool in recipients, and changes in intestinal microbiota composition following FMT (these data will be published separately). Further exploratory aims included assessing changes in depressive and other mental health symptoms, gut symptoms, sleep quality, quality of life, and cardiometabolic risk factors. The outcomes of this pilot study will help inform and support further trials in this emerging field.
Methods
Trial Design and Setting
The Moving Moods Pilot Study was an 8-week, double-blind, 2:1 parallel group, randomized controlled pilot trial (n = 15) of enema-delivered FMT (henceforward referred to as “active”) (n = 10) compared with a placebo enema (n = 5) in adults with moderate-to-severe MDD, with a 26-week follow-up for safety data. Participants, investigators, raters, and staff delivering the intervention were blinded to allocation. The study was conducted at University Hospital Geelong (Barwon Health Research Ethics, Governance and Integrity Number: 21/56). The full protocol is submitted for publication (currently under review) and registered with the Australian and New Zealand Clinical Trials Register (ANZCTR): ACTRN12621000932864. Results are reported in accordance with the CONSORT randomized pilot and feasibility trials extension. 38
One amendment was made to the eligibility criteria following the commencement of recruitment. Our original protocol excluded those with any previous history of inflammatory bowel disease (IBD)—which is commonly comorbid with MDD. 39 However, this was changed to only exclude those with “active” IBD. This was done because FMT is considered safe in individuals with IBD, 14 but also due to an open-label study showing FMT improved depression symptoms in some IBD patients. 40
Participants
Details of eligibility criteria are available in the registered protocol. To summarize, participants were considered eligible if they were adults (age 18–65 years), with a diagnosis of MDD according to the Structured Clinical Interview for DSM-5 (SCID-5) MDD module 41 ; had moderate-to-severe depressive symptomatology (Montgomery Asberg Depression Rating Scale [MADRS] 42 score ≥20); and had been on stable treatment for their mental health (pharmacotherapy and psychotherapy) for at least 1 month prior to commencing the trial. Exclusion criteria were: active suicidality; use of probiotics, antibiotics, or any experimental drug in the 1 month prior to study entry; serious gastrointestinal conditions (including active IBD, bowel cancer, or a history of major bowel surgery, but not including IBS, chronic diarrhoea, or constipation); pregnancy or breastfeeding; major comorbid psychiatric disorders (including bipolar disorder, a primary psychotic disorder, obsessive compulsive disorder, bulimia nervosa, or anorexia nervosa); active substance-use disorder; inability to read and understand the participant information and informed consent form (PICF); and a history of severe anaphylactic or anaphylactoid food allergy.
Study Intervention and Procedures
Regarding recruitment, advertisements were placed on social media, which generated media attention. Further planned recruitment strategies outlined in the registered study protocol were not required due to sizeable interest in the study arising from the media exposure. Some participants learned of the study through the media attention and social media advertisements, whilst others heard about it from their treating doctor (e.g., gastroenterologist or psychiatrist).
Potential participants registered an expression of interest via the study website and were then contacted by a member of the study team. Potential participants were provided with a PICF and booked for a screening assessment. A signed copy of the PICF and verbal consent were required prior to screening and study enrolment. Consent was again verbally confirmed at the initial screening appointment. Participants were made aware that they could withdraw consent at any time.
Given the extensive screening requirements, screening for eligibility was conducted in a 2-part assessment to reduce participant burden. Appointment 1A was conducted by a research assistant and covered general eligibility criteria and informed consent. Appointment 1B was conducted by the study psychiatrist and involved confirmation of MDD diagnosis (using SCID 5-MDD module), and assessment of severity (using MADRS). Following Appointment 1B, eligible participants provided further baseline data including medical history, medication history, family history, and psychiatric history, which was also collected separately by the study psychiatrist within the diagnostic interview.
Participants were randomly assigned to receive a total of 4 doses of active or placebo FMT via enema over 4 consecutive days as an adjunct to treatment as usual, delivered by a qualified nurse in a hospital setting. The active FMT enema comprised of syringes supplied by BiomeBank (Adelaide, Australia) containing a 50 mL total volume of donor faeces, normal saline, and 10% glycerol. The placebo consisted of a visually identical placebo product containing 50 mL total of normal saline, 10% glycerol, and brown dye. In Australia, the manufacture of FMT is regulated by the Therapeutic Goods Association (TGA), ensuring compliance with strict, comprehensive, and detailed donor screening and FMT manufacturing requirements. 43 The FMT product utilized in the Moving Moods Pilot Study met these requirements. As required by the TGA, each participant allocated to active FMT received all 4 enemas from a single donor.
There is currently a lack of data and thus consensus regarding a suitable dosing strategy for health conditions other than Clostridioides difficile. 14 Our 4-enema dosing strategy, delivering a total of 50 g stool, was based on the knowledge that while 30–50g of stool is a widely accepted dose in the treatment of C. difficile, 44 the broader data on FMT for use in other conditions suggests that a higher dose is likely required for non-C. difficile health conditions conditions. 14 Hence, we selected the upper limit of the dosing strategy.
Following the intervention, participants were assessed every 2 weeks until the Week 8 primary endpoint. A final follow-up phone call assessment will be conducted at Week 26 to assess long-term safety.
Participants provided stool samples and blood samples at baseline, Week 2, and Week 8. Participants self-collected stool samples using a Microba stool collection kit 45 according to manufacturer's protocol. Venipuncture was completed by research nurses and processed by Australian Clinical Labs (Geelong, Australia). Additional blood samples were collected and retained for future analyses.
Outcomes
Primary, secondary, and exploratory outcomes are described below. A more detailed description of the study outcome measures is available in the registered protocol.
Primary Outcome Measures
The primary outcome of this study was the feasibility and safety of FMT as an adjunctive treatment for MDD in adults.
Feasibility was measured by the ability to meet recruitment targets, participant retention and completion rates, adherence to the intended study protocol, participant acceptability, and robustness of study methodology including effectiveness of blinding. Acceptability was measured using a modified version of the Study Participant Feedback Questionnaire (SPFQ). 46 Data are reported descriptively. Participants completed the SPFQ 2, 4, and 8 weeks following the intervention.
Safety of FMT was measured by assessment of adverse events (AE). Feasibility targets, together with the feasibility outcomes, are summarized in Table 2. If satisfied, these criteria would suggest it is feasible to proceed with a future efficacy trial.
Table 2.
Feasibility Targets and Outcomes.
| Target area | Feasibility target | Outcome achieved | Target achieved (Yes/no) |
|---|---|---|---|
| Recruitment | Successful enrolment of n = 15 participants across a 6-month active recruitment period | 15 participants were enrolled in a 2-month period | Yes |
| Retention | A minimum of n = 10 participants to complete study until the 8-week primary endpoint (i.e., a 33% attrition rate). | All 15 participants completed the study until the 8-week primary endpoint | Yes |
| Adherence to protocol | Participants should: 1. Receive 2 of the total 4 enemas 2. Attend their baseline appointments (in-person) and Week 2 and Week 8 appointments (via telehealth or in-person) 3. Provide baseline and Week 2 stool samples |
1. All 15 participants received all 4 enemas 2. Attendance rate of 100% at key appointments. 3. All participants provided baseline stool samples. 14/15 Week-2 stool samples were returned. |
1. Yes 2. Yes 3. Yes |
| Safety | Nil severe and/or serious AEs rated as likely due to study intervention in the active FMT group. | No serious or severe AEs were reported in either group during the 8-week study period. | Yes |
| Adequacy of blinding | The best achievable outcome for adequacy blinding is participants and researchers correctly guessing allocation at a rate of 50% (the rate due to chance). Whilst we are not likely to be statistically powered to measure this outcome, we aim to reach an outcome approaching 50%. | 35.7% of participants correctly guessed treatment allocation (Fisher's Exact P = 0.45). | Yes |
Secondary Outcome Measures
The secondary outcome measures were changes in intestinal microbiota and associated biomarkers. The results of these analyses will be published separately.
Exploratory Outcome Measures
The following data were collected; however, given the modest sample size, the study was expected to be underpowered to measure significant changes in any of these outcome measures. As such, these outcomes were considered exploratory. The exploratory outcome measures included between-groups differential changes post-intervention compared with baseline in the following outcome measures: (a) mental health symptoms as measured by the MADRS and the Depression-Anxiety Stress Scale (DASS) 47 ; (b) quality of life as measured by the Assessment of Quality of Life-8 Dimension (AQoL-8D) with unweighted scoring 48 ; (c) sleep, as measured by the Pittsburgh Sleep Quality Index 49 ; (d) level of function as measured by the Sheehan Disability Scale 50 ; (e) gut symptomatology as measured by the Gastrointestinal Symptom Rating Scale (GSRS) 51 ; (f) cardiometabolic blood parameters (random lipid profile, random blood sugar levels, and HbA1c); (g) metabolic and cardiovascular risk factors assessed via physical exam, including heart rate, blood pressure, height, and weight; and (h) overall improvement assessed using the Patient Global Impression of Change. 52 Outcomes of exploratory blood biomarkers, such as brain-derived neurotrophic factor, markers of inflammation (e.g., macrophage inhibitory factor, interleukins 1b, 1ra, 6, and 10, soluble CD14, and high-sensitivity C-reactive protein), and markers gut permeability (lipopolysaccharide binding protein and zonulin) will be published separately along with the intestinal microbiota data.
Statistical Analyses
Feasibility Analysis
As this was a feasibility study, a power calculation was not performed, the sample size was normative for a study of this type and available budget. 53 Feasibility data were reported descriptively. A qualitative synthesis of feasibility data was reported in accordance with the pre-determined feasibility targets provided in Table 2.
Safety was reported as the number of AEs of each type and severity experienced per participant.
Exploratory Outcomes Analyses
The exploratory outcome data are summarized in Table 4. Between-groups differential changes from baseline to Week 2 and Week 8 using generalized estimation equation (GEE) technique were estimated. To avoid Type I error inflation, all p-values reflect the overall comparisons for time points and group allocation from the 2-way interaction between time point and group allocation.
Table 4.
Exploratory Outcomes Including Mental Health Outcome Measures, Other Questionnaire Data, and Physical Examination Data.
| Group allocation | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | FMT | Placebo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome measure | MADRS | DASS | GSRS | PGI | SDS | PSQI | AQoL-8D | Weight (kg) | Systolic BP | Diastolic BP | |||||||||||
| Week 0 (baseline), mean (s.d.) | 29.5 (±5.8) | 30.0 (±4.1) | 34.3 (±11.1) | 38.2 (±9.2) | 2.2 (±0.9) | 2.3 (±0.7) | 22.3 (±6.3) | 18.4 (±5.9) | 13.5 (±3.8) | 11.2 (±1.9) | 115.0 (±14.2) | 101.6 (±7.8) | 87.4 (±23.4) | 87.5 (±26.2) | 126.2 (±17.4) | 132.6 (±12.1) | 75.6 (±11.2) | 80.8 (±6.6) | |||
| Week 0, n | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | 10 | 5 | |||
| Week 2, mean (s.d.) | 21.3 (±10.7) | 22.0(±9.8) | 21.5 (±12.5) | 27.5 (±16.4) | 2.0 (±0.5) | 2.4 (±0.8) | 2.4 (±0.7) | 3.5 (±1.3) | 11.3 (±1.9) | 9.0 (±0.0) | 95.9 (±20.4) | 88.8 (±16.4) | 87.8 (±23.2) | 83.3 (±23.7) | 131.2 (±22.7) | 132.7 (±11.2) | 75.7 (±8.7) | 87.7 (±6.0) | |||
| Week 2, n | 10 | 5 | 10 | 4 | 10 | 4 | 8 | 4 | 9 | 4 | 8 | 4 | 10 | 3 | 10 | 3 | 10 | 3 | |||
| Between group differential change from baseline to Week 2, Hedges g effect size (CI) | -0.02 (-1.1-1.1) | -0.2 (-1.3-1.0) | -0.2 (-1.4-1.0) | -1.1 (-2.5-0.1) | 0.2 (-0.9-1.4) | -0.3 (-1.6-0.9) | 1.0 (-1.2-1.4) | 0.4 (-0.9-1.7) | -1.4 (-2.9- -0.05) | ||||||||||||
| Week 8, mean (s.d.) | 16.6 (±11.8) | 16.6 (±6.9) | 18.8 (±12.8) | 19.3 (±16.4) | 1.6 (±0.4) | 2.6 (±0.3) | 2.9 (±1.5) | 2.8 (±1.0) | 17.1 (±9.6) | 14.0 (±8.3) | 9.6 (±3.5) | 9.3 (±2.2) | 91.6 (±22.7) | 81.8 (±24.2) | 83.5 (±19.4) | 88.6 (±27.0) | 126.9 (±18.7) | 134.4 (±8.9) | 79.1 (±14.4) | 86.2 (±6.1) | |
| Week 8, n | 10 | 5 | 9 | 4 | 10 | 4 | 9 | 4 | 9 | 4 | 8 | 4 | 8 | 4 | 9 | 5 | 9 | 5 | 9 | 5 | |
| Between group differential change from baseline to Week 8, Hedges g effect size (CI) | 0.05 (-1.0-1.1) | 0.2 (-1.0-1.4) | -0.8 (-2.0-0.4) | 0.1 (-1.1-1.3) | -0.3 (-1.5-0.9) | -0.2 (-1.4-1.0) | -0.3 (-1.5-0.9) | -0.2 (-1.3-1.0) | -0.07 (-1.2-1.0) | -0.07 (-1.2-1.0) | |||||||||||
| P-value* | 0.67 | 0.19 | 0.001 | 0.87 | 0.86 | 0.592 | 0.068 | 0.476 | 0.527 | 0.253 | |||||||||||
Note. FMT = faecal microbiota transplantation.
*All P-values reflect the overall values for time points and group allocation 2-way interaction.
Trial Allocation, Sequence Generation, and Blinding
Allocation to treatment arms was randomly assigned by an independent statistician external to the study in a 2:1 ratio using a simple randomization method. Unblinded researchers independent to the study team allocated and packaged enema kits sequentially, using identical packaging to conceal treatment allocation and blinding. The study staff, investigators, nurses delivering the enemas, and participants were blinded to group allocations. Given the unequal treatment group sizes, it was not possible to blind the study statistician.
Results
Baseline Demographics
Table 1 summarizes baseline demographic data.
Table 1.
Baseline Characteristics.
| Characteristic | FMT (n = 10) | Placebo (n = 5) | Total (n = 15) | |
|---|---|---|---|---|
| Age, mean (s.d.) | 47.23 (±6.51) | 38.44 (±2.14) | 44.09 (±6.83) | |
| Sex, n (%) | Female | 6 (60) | 3 (60) | 9 (60) |
| Marital status, n (%) | Divorced/separated/widowed | 3 (30) | 0 | 3 (20) |
| Married/de facto relationship | 3 (30) | 3 (60) | 6 (40) | |
| Single (never married) | 4 (40) | 2 (40) | 6 (40) | |
| Highest level of education, n (%) | Completed secondary school/some secondary school | 2 (20) | 0 | 2 (13.33) |
| TAFE/trade/apprenticeship/other post-secondary training | 3 (30) | 0 | 3 (20) | |
| University | 5 (50) | 5 (100) | 10 (66.67) | |
| Employment status, n (%) | Casual | 2 (20) | 1 (20) | 3 (20) |
| Part-time or full time work/student | 6 (60) | 4 (80) | 10 (66.67) | |
| Unemployed/unable to work | 2 (20) | 0 | 2 (13.33) | |
| Household income, n (%) | <$25,000 | 3 (30) | 1 (20) | 4 (26.67) |
| $25,000–$100,000 | 2 (20) | 3 (60) | 5 (33.33) | |
| >$100,000 | 5 (50) | 0 | 5 (33.33) | |
| Medical history, n (%) | Current smoker | 1 (10) | 1 (20) | 2 (13.33) |
| Atopic condition | 8 (80) | 4 (80) | 12 (80) | |
| Food intolerances | 5 (50) | 3 (60) | 8 (53.33) | |
| IBD | 2 (20) | 1 (20) | 3 (20) | |
| IBS | 4 (40) | 3 (60) | 7 (46.67) | |
| Psychiatric history, n (%) | Treatment-resistant depression | 7 (70) | 4 (80) | 11 (73.33) |
| Dysthymia | 5 (50) | 4 (80) | 9 (60) | |
| Family history (psychiatric), n (%) | 8/8 (100) | 5 (100) | 13 (100) | |
| SAPAS score, mean (s.d.) | 2.70 (±1.77) | 2.60 (±0.89) | 2.67 (±1.50) | |
| Treatment, n (%) | Antidepressant use (current) | 5 (50) | 5 (100) | 10 (66.67) |
| Psychologist (current) | 5 (50) | 2 (40) | 7 (46.67) | |
| TMS/ECT (current or past) | 2 (20) | 1 (20) | 3 (20) | |
Note. ECT = electroconvulsive therapy; FMT = faecal microbiota transplantation; SAPAS = standardized assessment of personality abbreviated assessment; TAFE = technical and further education; TMS = transcranial magnetic stimulation.
Feasibility Outcomes
Table 2 presents the results of pre-specified targets considered as a minimum standard that would indicate that the study design was feasible. All targets were met or exceeded, and as such, the study was considered feasible.
Recruitment and Enrolment
The recruitment target of 15 participants in 6 months was met within a 2-month time frame, including completion of the 2-stage screening protocol requiring approximately a 2–2.5-h time commitment per participant. Expressions of interest (n = 164) greatly exceeded the 15-participant requirement, and additional participants were placed on a waitlist.
Eligibility
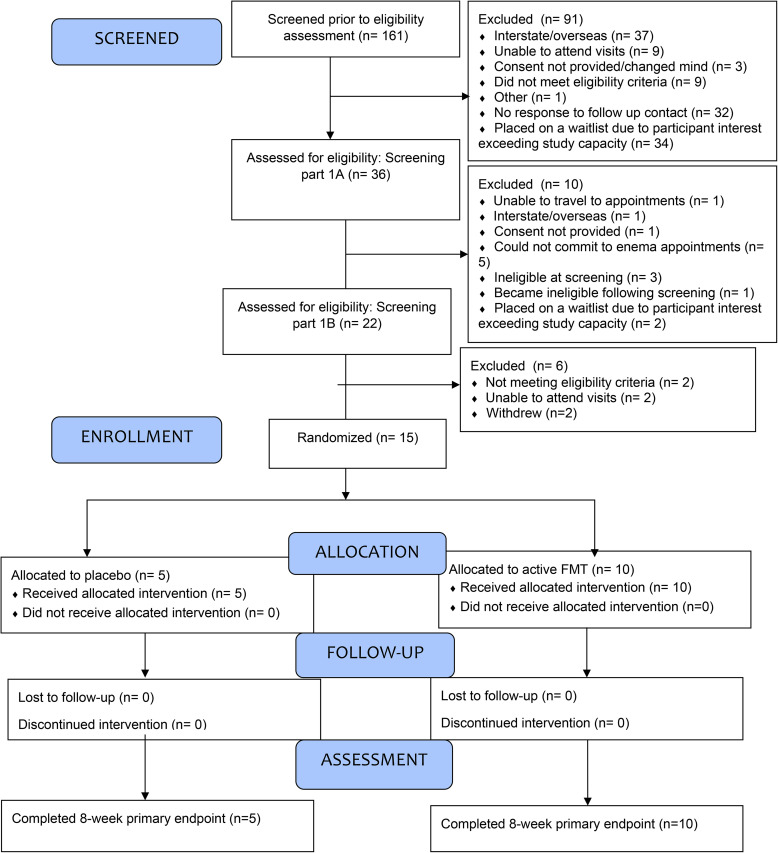
Eligibility is summarized in the CONSORT Flow diagram (see Figure 1).
Figure 1.
Consort flow diagram.
Retention Rates
No participants were lost to follow-up or withdrew from the study following enrolment.
Adherence to Study Methodology
Regarding delivery of the intervention, no major protocol deviations were reported. All participants received 4 enemas as intended. Of the 60 enemas delivered to the 15 participants, 57 were retained for 30 min or longer, and the remaining 3 were retained for 26–30 min, representing a minor protocol deviation, which was not considered clinically significant.
With respect to attendance at key appointments, feasibility targets were met. There was an overall 93% in-person attendance rate; all participants attended their baseline appointments (15/15; 100%), and majority attended their Week 2 (13/15; 87%) and Week 8 (14/15; 93%) appointments. In-person attendance was necessary to conduct a physical examination.
All participants provided baseline stool samples (15/15; 100%), and majority provided Week 2 (14/15; 93%) and Week 8 (14/15; 93%) stool samples. A total of 58/60 (97%) stool samples across the 3 timepoints were returned, which was considered adequate adherence to the study protocol.
Adequacy of Blinding
Adequacy of blinding was assessed in both participants and nursing staff administering the enemas. Data were missing for 1 participant. Of the remaining 14 participants, 28.6% were unsure of their allocation, 35.7% believed they were allocated to placebo, and 35.7% believed they were allocated to active treatment.
With respect to maintaining blindness, 5 (35.7%) participants correctly guessed allocation, 5 (35.7%) guessed incorrectly, and 4 (28.6%) were unsure (i.e., they did not guess) (Fisher's exact test P = 0.45). Participants who correctly guessed allocation were, on average, 80% confident in their decision, whilst those who were incorrect were on average 69.8% confident.
Acceptability
Week 2 Survey (1 Week Following Intervention)
Of the 12/15 (80%) participants who completed the Week 2 survey, 8 (66%) agreed or strongly agreed that they would prefer to receive enema-delivered FMT over orally delivered encapsulated FMT, and 6 (50%) agreed or strongly agreed that they would prefer enema-delivered FMT over medication. Regarding the number of treatments, 10 respondents (83%) agreed or strongly agreed that 4 enema appointments was not too many. Nine respondents (75%) agreed or strongly agreed that they would recommend the intervention to others.
Week 4 Survey (Mid-point)
Of the 14/15 (93%) participants who completed the mid-point survey, 12 (87%) agreed or strongly agreed that the amount of time spent on data collection was acceptable.
Week 8 Survey (End-point)
At the conclusion of the study, 9 (67%) of the 12/15 (80%) participants that completed the final survey agreed or strongly agreed that overall they were satisfied with their trial experience. With respect to the time commitment, 9 respondents (67%) reported this was the same as expected. Participants were also given the opportunity to provide written feedback, and this was mainly positive. One participant emailed separately to the SPFQ providing feedback that the number of appointments and surveys was too burdensome and time consuming.
Safety and Tolerability
The intervention was considered safe, as no serious or severe AEs were reported in either group. According to the Week 2 SPFQ, all 12 respondents agreed or strongly agreed that the enema delivery was tolerable, and that they would have the treatment again if required.
The median number of mild-moderate AEs reported per participant was 2 in both the placebo and active FMT groups (see Table 3).
Table 3.
Mild-to-Moderate Adverse Events.
| Symptom | FMT (n = 10) | Placebo (n = 5) | Total (n = 15) |
|---|---|---|---|
| Number of participants who reported mild AE, n (%) | |||
| Abdominal pain | 2 (20) | 0 | 2 (13.33) |
| Belching | 0 | 1 (20) | 1 (6.67) |
| Cramping | 3 (30) | 2 (40) | 5 (33.33) |
| Constipation | 3 (30) | 0 | 3 (20) |
| Diarrhoea | 1 (10) | 1 (20) | 2 (13.33) |
| Distension/abdominal discomfort | 2 (20) | 1 (20) | 3 (20) |
| Excessive flatulence | 2 (20) | 2 (40) | 4 (26.67) |
| Nausea | 2 (20) | 3 (60) | 5 (33.33) |
| Vomiting | 1 (10) | 0 | 1 (6.67) |
| Other gastrointestinal | 1 (10) | 0 | 1 (6.67) |
| Headache | 1 (10) | 0 | 1 (6.67) |
| Rectal bleeding | 1 (10) | 0 | 1 (6.67) |
| Fatigue/malaise | 3 (30) | 1 (20) | 4 (26.67) |
| Other systemic | 3 (30) | 1 (20) | 4 (26.67) |
| Mild AE subtotal, n | 25 | 12 | 37 |
| Median mild AEs reported per participant [IQR] | 2 [2] | 2 [3] | 2 [3] |
| Number of participants who reported moderate AE, n (%) | |||
| Cramping | 1 (10) | 1 (20) | 2 (13.33) |
| Other gastrointestinal | 0 | 1 (20) | 1 (6.67) |
| Infection (including COVID-19) | 1 (10) | 1 (20) | 2 (13.33) |
| Moderate AE subtotal, n | 2 | 3 | 5 |
| Median moderate AEs reported per participant [IQR] | 1 [1] | 1.5 [1] | 1 [0.5] |
| Total AEs, n | 27 | 14 | 41 |
| Median total AEs reported per participant [IQR] | 2 [2] | 2 [3] | 2 [2] |
Note. AE = adverse events; FMT = faecal microbiota transplantation.
Exploratory Outcomes
The following outcomes are considered exploratory and were included to help inform the design of a full-scale RCT, albeit 2 outcomes met or approached statistical significance. The active FMT group had a greater improvement in average gastrointestinal symptom scores (measured by GSRS) from baseline to Week 8 (Hedges g = −0.77; 95%CI: −2.0, −0.4, P < 0.001). The active FMT group also reported a greater improvement in quality of life compared with the placebo group at both the Week 2 and Week 8 timepoints (Hedges g = −0.3 (95%CI: −1.6–0.9) and −0.3 (95%CI: −1.5–0.9), respectively, P = 0.068). The remaining exploratory outcomes are reported in Table 4. The blood biomarkers including lipid profile and blood glucose levels are not included in this table, as no significant within- or between-group changes were observed in either group. CRP results are not reported due to missing data arising from a lab error.
Discussion
To our knowledge, this study represents the first published study of FMT for MDD using a randomized, double-blinded, placebo-controlled design. We found that enema-delivered FMT is a feasible, acceptable, well tolerated, and safe treatment strategy for patients with MDD. A priori stated targets for feasibility outcomes were all met.
We note the high proportion of participants reporting a history of IBS (50%) and IBD (20%) in our study population. There are several possible explanations. Firstly, several participants were encouraged to participate in the study by local gastroenterologists, who were aware of the study. Gastroenterologists have greater awareness and acceptance of FMT, as it is a mainstay treatment for C. difficile. 54 Secondly, the acceptability of FMT has been reported to vary widely by indication, with highest acceptability reported in gastrointestinal disorders such as IBD. 37 As such, participants with gastrointestinal disorders may have been more interested in participating in FMT research. Thirdly, depression is highly comorbid with both IBS 55 and IBD. 39 However, whilst the prevalence of MDD in an IBS/IBD population has been widely reported, there are sparse literature around the prevalence of IBS/IBD in a population with MDD. It is unclear if our study cohort represents something close to a typical population prevalence of IBS/IBD in MDD. The population prevalence of IBS is reported to be 10–20%, 56 and 1 small study (n = 40) reported a prevalence of IBS in 27% of participants with MDD. 56 Given the prevalence of IBS/IBD in a MDD population is not well documented, but probably higher than the population average, it is also possible that the figures observed in our study are within typical limits for this cohort.
Whilst this study was not designed to measure efficacy, an improvement in gastrointestinal symptoms (large effect) was observed. This finding of significant improvement in gastrointestinal symptoms in our study population is also of importance given our cohort had a high proportion of gastrointestinal disorders at baseline.
A trend toward improvement in quality of life (small effect) was also observed in the active FMT group, compared with the placebo group. The improvement in quality of life may reflect the improvement in gastrointestinal symptoms reported in the active FMT group.
Whilst this study had significant strengths as described above, the following limitations should also be noted. A small sample size meant that exploratory outcomes were overtly underpowered to demonstrate efficacy. Therefore, at this stage it is not be possible to draw any conclusions with respect to the efficacy of FMT for MDD. With respect to the mental health outcomes (MADRS and DASS), these were no significant differences between groups. We also note that the mental health outcomes might have been strongly influenced by factors unrelated to the intervention such as participants changing their psychotropic treatments during the study or contracting COVID, making the results impossible to interpret. A larger, fully powered RCT would be expected to control for such problems.
In addition, the primary statistician was unavoidably unblinded due to the unequal group allocations. The study was therefore only double blinded, rather than triple blinded as intended. A 1:1 allocation ratio in future studies would allow for triple blinding.
With respect to the design of a larger RCT, the question remains of whether the dosing strategy used in this study was sufficient or optimal. The intestinal microbiota analysis results from this study are still pending at the time of publication and will be published separately. It is expected that these data may help to inform dosing strategy. In addition, participant feedback suggested that, whilst the dosing strategy (i.e., 4 enemas over 4 days) was acceptable, the subsequent appointment burden should be reduced. In future studies, we would recommend removal of the 4- and 6-week follow-up appointments as these did not add significant value to the study outcomes, and were burdensome to participants.
Conclusions
This study was considered successful in that all feasibility targets were exceeded, and acceptability was high across all measured domains. In addition, the intervention appeared safe and well tolerated, with no serious or severe AEs recorded. The rates of mild-to-moderate AEs including constipation and nausea were similar in both treatment groups, and in-keeping with those reported with FMT in general.14,57 Blinding by placebo was also successful. Exploratory data also suggested that the treatment may lead to improvements in gastrointestinal symptoms and quality of life in this population. The findings of this study support further research to evaluate clinical efficacy, and the use of this protocol is recommended given its established safety, acceptability, tolerability, and feasibility.
Supplemental Material
Supplemental material, sj-docx-1-cpa-10.1177_07067437221150508 for Feasibility, Acceptability, and Safety of Faecal Microbiota Transplantation in the Treatment of Major Depressive Disorder: A Pilot Randomized Controlled Trial by Jessica Emily Green, Michael Berk, Mohammadreza Mohebbi, Amy Loughman, Amelia J. McGuinness, David Castle, Mary Lou Chatterton, Joahna Perez, Philip Strandwitz, Eugene Athan, Christopher Hair, Andrew A. Nierenberg, John F. Cryan and Felice Jacka in The Canadian Journal of Psychiatry
Footnotes
Contributors: JG was responsible for study design, writing and registering the protocol, psychiatric clinical oversight, rating, and review of psychiatric AEs, writing, editing the manuscript, and data analysis. FJ was the initiator and primary investigator for the study. MB, FJ, DC, EA, PS, and CH provided oversight and advice around study design. MB, MM, AL, and FJ contributed to data interpretation. MM was the study statistician and oversaw JG in performing the statistical analyses. CH provided gastrointestinal clinical oversight and rating of gastrointestinal AEs. MLC and JP performed scoring and analysis of the AQoL-8D. EA, MB, CH, and JG were additional investigators for the study. AM was the study coordinator. All authors contributed to and approved the final manuscript.
Conflict of Interest: Jessica Green is supported by an IMPACT Part-time PhD Scholarship.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Holobiome, Wilson Foundation (grant number: Funding for faecal microbiota analyses, Philanthropic donation).
ORCID iDs: Jessica Emily Green https://orcid.org/0000-0002-1219-8910
Michael Berk https://orcid.org/0000-0002-5554-6946
David Castle https://orcid.org/0000-0002-3075-1580
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Cryan JF, O'Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 2.Dash S, Clarke G, Berk M, et al. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. 2015;28:1–6. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BW, Milaneschi Y, Lamers F, et al. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuinness AJ, Davis JA Dawson SL, et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol Psychiatry. 2022;27:1920–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson F, Adamos M, Katsafanas E, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. 2018;20:614–621. [DOI] [PubMed] [Google Scholar]
- 7.Minayo MS, Miranda I, Telhado RS. A systematic review of the effects of probiotics on depression and anxiety: an alternative therapy? Cien Saude Colet. 2021;26:4087–4099. [DOI] [PubMed] [Google Scholar]
- 8.Heijtz R D, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crumeyrolle-Arias M, Jaglin M, Bruneau A, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. [DOI] [PubMed] [Google Scholar]
- 10.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 11.Leclercq S, Mian FM, Stanisz AM, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köhler-Forsberg O, Petersen L, Gasse C, et al. A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry. 2019;76:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green JE, Davis JA, Berk M, et al. Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes. 2020;12:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. [DOI] [PubMed] [Google Scholar]
- 16.Sinyor M, Schaffer A, Levitt A. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can J Psychiatry. 2010;55:126–135. [DOI] [PubMed] [Google Scholar]
- 17.Strandwitz P, Kim KH Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2018;4:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green J, Castle D, Berk M, et al. Faecal microbiota transplants for depression - who gives a crapsule? Aust N Z J Psychiatry. 2019;53:732–734. [DOI] [PubMed] [Google Scholar]
- 19.Green JE, Berk M, Loughman A, et al. FMT For psychiatric disorders: following the brown brick road into the future. Bipolar Disord. 2021;23:651–655. [DOI] [PubMed] [Google Scholar]
- 20.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. [DOI] [PubMed] [Google Scholar]
- 21.Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt EKA, Torres-Espin A, Raposo PJF, et al. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One. 2020;15:e0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21:786–796. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JR, Borre Y COB, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Yang X, Zeng B, et al. Proteomic analysis of olfactory bulb suggests CACNA1E as a promoter of CREB signaling in microbiota-induced depression. J Proteomics. 2019;194:132–147. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Guo R, Liu F, et al. Gut Microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat. 2020;16:859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5:eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang D-W, Adams JB, Gregory AC, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai T, Shi X, Yuan LZ, et al. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr. 2019;31:1525–1526. [DOI] [PubMed] [Google Scholar]
- 30.Xie WR, Yang XY, Xia HH, et al. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: a case report and review of the literature. World J Clin Cases. 2019;7:3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doll JPK, Vázquez-Castellanos JF Schaub A-C, et al. Fecal microbiota transplantation (FMT) as an adjunctive therapy for depression—case report. Front Psychiatry. 2022;13:815422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker G, Spoelma MJ Rhodes N. Faecal microbiota transplantation for bipolar disorder: a detailed case study. Bipolar Disord. 2022;24:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinton R. A case report looking at the effects of faecal microbiota transplantation in a patient with bipolar disorder. Aust N Z J Psychiatry. 2020;54:649–650. [DOI] [PubMed] [Google Scholar]
- 34.El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurokawa S, Kishimoto T, Mizuno S, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord. 2018;235:506–512. [DOI] [PubMed] [Google Scholar]
- 36.Huang HL, Chen HT, Luo QL, et al. Relief of irritable bowel syndrome by fecal microbiota transplantation is associated with changes in diversity and composition of the gut microbiota. J Dig Dis. 2019;20:401–408. [DOI] [PubMed] [Google Scholar]
- 37.Benech N, Legendre P, Radoszycki L, et al. Patient knowledge of gut microbiota and acceptability of fecal microbiota transplantation in various diseases. Neurogastroenterol Motility. 2022;34:e14320. [DOI] [PubMed] [Google Scholar]
- 38.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Br Med J. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhamre R, Sawrav S, Adarkar S, et al. Psychiatric comorbidities in patients with inflammatory bowel disease. Indian J Gastroenterol. 2018;37:307–312. [DOI] [PubMed] [Google Scholar]
- 40.Kilinçarslan S, Evrensel A. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with inflammatory bowel disease: an experimental study. Actas Esp Psiquiatr. 2020;48:1–7. [PubMed] [Google Scholar]
- 41.Osório FL, Loureiro SR, Hallak JEC, et al. Clinical validity and intrarater and test-retest reliability of the structured clinical interview for DSM-5 - clinician version (SCID-5-CV). Psychiatry Clin Neurosci. 2019;73:754–760. [DOI] [PubMed] [Google Scholar]
- 42.Davidson J, Turnbull CD, Strickland R, et al. The montgomery-asberg depression scale: reliability and validity. Acta Psychiatr Scand. 1986;73:544–548. [DOI] [PubMed] [Google Scholar]
- 43.Administration TG. Faecal microbiota transplant products regulation, https://www.tga.gov.au/faecal-microbiota-transplant-products-regulation (2021, accessed 16/7/22 2022).
- 44.Gweon TG, Lee YJ, Kim KO, et al. Clinical practice guidelines for fecal microbiota transplantation in Korea. J Neurogastroenterol Motil. 2022;28:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alena L, Pribyl KDT Donovan H. et al. Benchmarking Microba’s sample collection device. Queensland, Australia: Microba Life Sciences Ltd; Epub ahead of print December 2020. [Google Scholar]
- 46.Nieboer AP, Cramm JM. Validation of the SPF-Q, an instrument to assess the quality of production functions to achieve well-being, among multimorbid patients. Health Qual Life Outcomes. 2020;18:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng F, Trauer T, Dodd S, et al. The validity of the 21-item version of the depression anxiety stress scales as a routine clinical outcome measure. Acta Neuropsychiatr. 2007;19:304–310. [DOI] [PubMed] [Google Scholar]
- 48.Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res. 1999;8:209–224. [DOI] [PubMed] [Google Scholar]
- 49.Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh sleep quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. [DOI] [PubMed] [Google Scholar]
- 50.Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan disability scale. Int Clin Psychopharmacol. 2008;23:70–83. [DOI] [PubMed] [Google Scholar]
- 51.Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual Life Res. 1997;7:75–83. [DOI] [PubMed] [Google Scholar]
- 52.Mohebbi M, Dodd S, Dean OM, et al. Patient centric measures for a patient centric era: agreement and convergent between ratings on the patient global impression of improvement (PGI-I) scale and the Clinical Global Impressions – Improvement (CGI-S) scale in bipolar and major depressive disorder. Eur Psychiatry. 2018;53:17–22. [DOI] [PubMed] [Google Scholar]
- 53.Bell ML, Whitehead AL, Julious SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol. 2018;10:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paramsothy S, Walsh AJ, Borody T, et al. Gastroenterologist perceptions of faecal microbiota transplantation. World J Gastroenterol. 2015;21:10907–10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fadgyas-Stanculete M, Buga AM, Popa-Wagner A, et al. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J Mol Psychiatry. 2014;2(4):20140627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masand PS, Kaplan DS, Gupta S, et al. Major depression and irritable bowel syndrome: is there a relationship? J Clin Psychiatry. 1995;56:363–367. [PubMed] [Google Scholar]
- 57.Michailidis L, Currier AC, Le M, et al. Adverse events of fecal microbiota transplantation: a meta-analysis of high-quality studies. Ann Gastroenterol. 2021;34:802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cpa-10.1177_07067437221150508 for Feasibility, Acceptability, and Safety of Faecal Microbiota Transplantation in the Treatment of Major Depressive Disorder: A Pilot Randomized Controlled Trial by Jessica Emily Green, Michael Berk, Mohammadreza Mohebbi, Amy Loughman, Amelia J. McGuinness, David Castle, Mary Lou Chatterton, Joahna Perez, Philip Strandwitz, Eugene Athan, Christopher Hair, Andrew A. Nierenberg, John F. Cryan and Felice Jacka in The Canadian Journal of Psychiatry