Abstract
Organoids are three‐dimensional (3D) miniaturized versions of organs or tissues that are derived from cells with stem potential and can self‐organize and differentiate into 3D cell masses, recapitulating the morphology and functions of their in vivo counterparts. Organoid culture is an emerging 3D culture technology, and organoids derived from various organs and tissues, such as the brain, lung, heart, liver, and kidney, have been generated. Compared with traditional bidimensional culture, organoid culture systems have the unique advantage of conserving parental gene expression and mutation characteristics, as well as long‐term maintenance of the function and biological characteristics of the parental cells in vitro. All these features of organoids open up new opportunities for drug discovery, large‐scale drug screening, and precision medicine. Another major application of organoids is disease modeling, and especially various hereditary diseases that are difficult to model in vitro have been modeled with organoids by combining genome editing technologies. Herein, we introduce the development and current advances in the organoid technology field. We focus on the applications of organoids in basic biology and clinical research, and also highlight their limitations and future perspectives. We hope that this review can provide a valuable reference for the developments and applications of organoids.
Keywords: 3D culture, drug screening, precision medicine, stem cells, tumoroid
Organoids can be generated by inducing and culturing pluripotent stem cells (iPSCs), embryonic stem cells (ESCs), adult stem cells (ASCs), and tumor cells from healthy donors or patients. The potential biomedical applications for organoids include disease modeling, drug screening, toxicity assays, personalized medicine, biobank, biomarker discovery, and regenerative medicine.
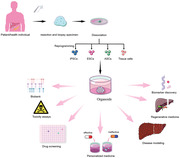
1. INTRODUCTION
Research on organoids can be considered to be traced back as far as 1907, when Wilson et al. cultivated mechanically dissociated sponge cells to form functional organisms under in vitro conditions. 1 In the decades since, organoid research has been carried out mainly through the isolation and reorganization of cells. In 1975, a study incubated primary human keratinocytes and 3T3 fibroblasts to generate stratified squamous epithelial colonies similar to those of the human epidermis. 2 By the 1980s, the first pluripotent stem cells (PSCs) were isolated from mouse embryos and obtained by humans, and mesenchymal stem cells (MSCs), human embryonic stem cells (ESCs), and induced PSCs (iPSCs) were successively discovered. 3 The development and advances of stem cell technology shed new light on the field of organoids. In 2009, intestinal adult stem cells (ASCs) were cultured in vitro to form small intestinal organoids with a crypt‐villi structure, 4 which is a landmark event in the organoid field, demonstrating the potential of stem cells to differentiate into spatial structures similar to organs in vivo. Since then, organoid culture techniques have flourished and organoids derived from various organs have been established, such as the brain, 5 , 6 retinal, 7 , 8 lung, 9 , 10 stomach, 11 , 12 liver, 13 , 14 bile duct, 15 , 16 pancreas, 17 , 18 and kidney. 19 , 20
The rapid development of three‐dimensional (3D) culture technologies exerts crucial functions in enabling organoid culture. Cells in the body reside in complex internal environments, and they are affected by multiple signals and interactions to establish, maintain, and regulate cellular phenotypes and specific functions. Cells cultured in two dimensions (2D) failed to recapitulate the normal cell morphology and interactions in vivo. Isolated tissue cells cultured in a 2D system gradually lose their morphology and become flattened, abnormally divided, and influence the cellular differentiated phenotype. 21 , 22 2D attachment tends to cause cells to lose their original shape and hierarchical structure and affect cell–cell and cell–extracellular signal transduction and interactions, resulting in cells that do not properly reproduce the cellular functions and behaviors present in tissues or organs. 23 2D tumor cell lines gradually lose their heterogeneity during long‐term culture. Meanwhile, cross‐contamination easily occurs in long‐term subculture. It has been confirmed that the genomics and metabolomics of cell lines are significantly different from those of the original tumor after long‐term passage. 24 The 3D culture system can mimic the physicochemical microenvironments and intercellular and cell–ECM interactions in which cells live in vivo. 25 Cells cultured in a 3D system can better represent the complex structure and specific cellular functions of cells, which have a high degree of fit with the parent and maintain the genetic stability and chromatin heterogeneity of the parent as well. These cells can expand rapidly in 1−2 weeks and be stably subcultured and cryopreserved. 26
Organoids can be defined as cells with stem cell potential that are incubated under 3D culture systems to aggregate by adhesion, self‐organize, and differentiate into 3D cell masses with the corresponding organ tissue morphology. 27 Organoids have a high degree of similarity to their parental cells that replicate and simulate their unique biological characteristics. 28 Additionally, organoids are able to self‐renew and self‐organize, contain various cell types, perform some specific functions, and form spatial structures similar to those of in vivo organs. Therefore, organoids are valuable models for studying the occurrence, development, and progression of diseases. Tumor organoids can be constructed by preoperative biopsy or postoperative tumor resection, which plays an important role in individualized drug sensitivity prediction. Thus, organoid models provide better options for drug screening and individualized drug therapy. 29 , 30 , 31 , 32 , 33 The potential of organoids to broaden fundamental research by complementing current model systems is becoming more generally recognized. 34
Organoid technologies pave the way for the development of new models that more closely resemble realistic physiological and pathophysiological states. In this review, we introduced the development and current advances in the field of organoids and described the main cell sources and culture methods for organoids. We focused on the application of organoids in basic biology and clinical research, such as disease modeling and precision treatment, and also highlighted their limitations and future perspectives. We hope that this review can provide a valuable reference for the application of organoid technologies.
2. CELL SOURCES OF ORGANOID
Stem cells are primitive, undifferentiated cells that are able to differentiate into many different and specialized cell types. Due to their potential for self‐renewal and multidirectional differentiation, organoids can be established from stem cells, including ESCs, iPSCs, and ASCs 4 , 35 (Figure 1). The process by which stem cells develop into organoids is similar to the way that organs acquire unique organization, mainly involving self‐organization. 36
FIGURE 1.
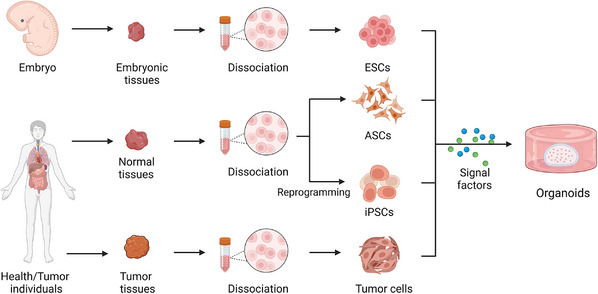
Strategies for formation of organoids in vitro. The cell sources for establishing organoids include embryonic stem cells (ESCs), adult stem cells (ASCs), induced pluripotent stem cells (iPSCs), and tumor cells. Resection and/or biopsy specimens from health/ patient individuals are dissociated into single cell to form organoids by incubating with various signal factors (Created with BioRender.com).
The following considerations need to be taken into account when cultivating organoids. First, owing to the 3D structure of organoids, the 3D culture environment should be provided, which can be achieved by embedding the organoids in a matrix, 37 or by applying the air–liquid interface (ALI) culture technique. 38 Second, establishing the appropriate regional identity is a key point in the organoid culture process, which requires the correct regulation of development and differentiation‐related signaling pathways. Finally, different types of organoids require different nutrients to develop and differentiate into the terminal stage. As a result, the medium needs to be configured according to the appropriate protocol.
2.1. PSC‐derived organoids
Since the discovery of PSCs, researchers have generated a wide range of differentiated cell types from stem cells based on theories of developmental biology. 39 , 40 iPSCs are produced by reprogramming PSCs (such as by integrating transcription factors into the target cells), and ESCs are derived from cell masses within the blastocyst, both of which have the capacity for multidirectional differentiation. 41 , 42 The establishment of PSC‐derived organoids depends on the directed differentiation of PSCs. This process requires the formation of specific germ layers (endoderm, mesoderm, or ectoderm), followed by incubating with specific growth factors, signaling molecules, and cytokines to induce cell‐directed differentiation and maturation. PSC‐derived organoids contain a richer cellular fraction, including mesenchymal, epithelial, and endothelial cells. 43 , 44 iPSC‐derived organoids were applied in modeling various human diseases in vitro. 45 , 46 , 47 , 48 , 49 However, when PSC‐derived organoids reach a certain lifespan, they lose their ability to proliferate and fail to develop to a fully mature state. 50 , 51 In addition, the limitations of current technologies cause the lack of important interactions between cells in PSC‐derived organoids with other codeveloping cells. 52 Therefore, PSC‐derived organoids are generally naïve, resembling fetal tissues, which can be considered as excellent models for organogenesis research. 53 ESC‐derived organoids are more mature than iPSC‐derived organoids, which can be used as novel models for studying later phases of organogenesis. 54 , 55 However, ESCs need to be obtained from the embryo and there are many ethical issues involved (Figure 2).
FIGURE 2.
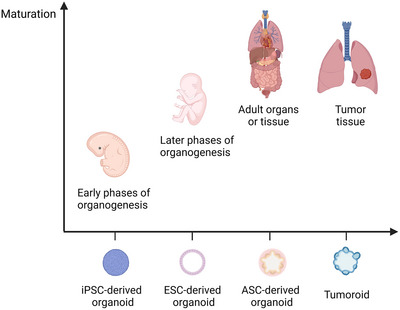
The maturation of different cell‐derived organoids. The iPSC‐derived organoids present low maturation, which are similar to the embryonic or early fetal tissues and can be used in studying organogenesis. The ESC‐derived organoids have higher maturation than iPSC‐derived organoids and are applied to model later organogenesis stage. The ASC‐derived organoids are more closely resembling adult tissue. Tumor cell‐derived organoids is a special case due to they represent adult tumor features (Created with BioRender.com).
2.2. ASC‐derived organoids
ASCs (with the exception of skin stem cells) are thought to be incapable of significant in vitro expansion. The team of Clevers applied 3D technologies to culture the leucine‐rich repeat‐containing G protein‐coupled receptor 5 positive (Lgr5+) intestinal mouse ASCs to establish intestinal organoids by adding various stem cell niche factors into the medium and manually modulating signaling pathways. This study indicated that ASCs could expand in vivo in the presence of stem cell niche factors. The construction of ASC‐derived organoids requires identifying proper ASC types. WNT pathway activation is one of the main drivers of organoid formation in epithelial ASCs, and Lgr5 was positive in almost all epithelial ASCs that could be used for organoid culture. The culture protocol for ASC‐derived organoids is simpler, shorter, and more mature, and they more closely resemble adult tissue. 56 , 57 Therefore, they can be better used in regenerative medicine and disease modeling because their cell sources are abundant. 58 , 59 , 60 However, ASC‐derived organoids have a single cellular component, mainly epithelial, 61 and prior knowledge of the medium for culturing ASC‐derived organoids from different tissues is also a limitation for ASC‐derived organoids.
2.3. Tumor cell‐derived organoids
Based on the culture protocol for ASC‐derived organoids, organoids can be generated from isolated tissues. Isolated tumor tissues after digestion and dissociation can be used to grow organoids with 3D culture conditions, and these organoids are called tumoroids. Tumoroids maintain and preserve the histological structure, molecular genetic characteristics, and heterogeneity of the original tumor, which can represent the features of the patient's tumor in some degree. 62 , 63 Tumoroids can be used as good preclinical models for basic and clinical research related to tumors. 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 Tumor liquid biopsy samples or tumor cells collected by the Pap brush method have been considered as starting material for organoid cultures. 73 , 74 , 75 , 76 De Angelis et al. 77 constructed the mouse xenograft model by using colorectal circulating tumor cells to promote these tumor cells proliferation in order to generate sufficient materials to form tumoroids. In addition, whether by surgical resection, puncture biopsy, or samples collected by Pap brush, the isolated tumor cells usually included normal cells and/or blood cells (especially red blood cells), which might influence tumoroid growth. 77 A possible solution to this problem is to reduce or delete some factors necessary for the establishment of normal cell‐derived organoids, as tumor cells reduce their dependence on them. To eliminate contamination of red blood cells, lysis is one method that can be adopted. 78
2.4. Multilineages organoids
To represent the niche of cells in an organism, the interactions between various regions and different cell populations in physiology and pathophysiology should be recapitulated as closely as possible. For example, the interactions between different brain regions contribute to the formation of certain brain regions. 79 Mesenchymal and endothelial cells have been found to exert indispensable functions in the development of the liver and are able to manipulate the behavior of hepatic progenitors. 80 Assembloids, considered as the next generation of organoids, can be established by coculturing the multiple‐type cells or combinations of organoids with different cell lineages from other tissues or organs. 79 , 81 , 82 Compared with single‐type cell‐derived organoids, assembloids can better reproduce interactions among different subregions or various cell lineages in organs.
Several studies fused cerebral organoids from different brain regions to capture the interactions between different brain regions in the process of brain development, including long‐distance projections and interneuron migration. 83 , 84 , 85 A recent study by Andersen et al. 86 formed cortical, hindbrain/cervical spinal cord, and skeletal muscle organoids, and fused the three organoids to generate cortico‐motor assembloids, which can represent the cortico‐motor pathway functions (the cortical control of muscle contraction) in vitro long term. Similarly, Ogawa et al. 87 developed a novel cancer model of gliomas that was established by the artificial combination of brain organoids and glioblastoma cells, which can be used to study tumorigenesis and metastasis mechanisms.
Research based on the assembloid has also been extended to the peripheral nervous system. It has been reported that iPSCs can differentiate into spinal cord neurons and skeletal muscle cells simultaneously, and these cells can self‐organize to form hybrid neuromuscular organoids in vitro. 88 Intestinal organoids cocultured with neural crest cells were confirmed to model the development of the enteric nervous system and motility disorders of the intestinal tract. 89
Assembloids resembling other tissues or organs have been reported by many studies. Takebe et al. 90 , 91 , 92 incubated iPSCs or human embryonic cells with human umbilical vein endothelial cells and human MSCs to generate 3D vascular and functional organ buds. Bergmann et al. 93 generated blood–brain barrier (BBB) organoids via coculturing human primary brain endothelial cells, astrocytes, and pericyte. The anterior and posterior gut spheroids derived from iPSCs were cocultured under 3D conditions to generate assembloids that could accurately mimic the continuous and dynamic process of human hepatobiliary and pancreatic organogenesis in vitro. 94 Similarly, Ng et al. 95 formed cardio‐pulmonary assembloids by inducing iPSCs to differentiate cardiac and lung epithelial cells simultaneously, which is a promising platform for studying the interactions between the human heart and lung and tissue boundary formation during embryonic development. The teams of Chan et al. and Takebe et al. established multicellular hepato‐biliary organoids from iPSCs, and these organoids have the potential to model complex liver diseases such as nonalcoholic fatty liver disease (NAFLD). 96 , 97 In addition, Tanimizu et al. 98 generated functional hepatobiliary organoids by combining EpCAM+ cholangiocytes and small hepatocytes, and found that a connection between hepatocytes and cholangiocytes was formed in the organoids.
3. CULTURE APPROACHES OF ORGANOIDS
3.1. Submerged culture
Submerged culture, developed by the group of Clevers, is the most widely used culture method for organoids. 37 In this protocol, cells or cell clusters are embedded in extracellular matrix (ECM) gels, then the mixture of cells and matrix gels is placed in a culture dish to form a dome, and medium is added to submerge the dome. ECM gels play the role of structural support and offering ECM signals, and common ECM gels include basement membrane extract, Matrigel, and Geltrex. The main components of the medium for the submerged culture include adDMEM/F12, penicillin/streptomycin, GlutaMAX, HEPES, B27, epidermal growth factor (EGF), FGF2, FGF10, Wnt3A, Noggin, R‐spodin‐1, Prostaglandin E2, N‐Acetylcysteine, Nicotinamide, Y27632, A‐8301, and SB202190. 37 , 99 The medium composition varies when forming organoids from different tissues, and which specific factors need to be added or removed usually depends on the requirements of the tissue from which they originate for the corresponding signal or hormone. 100
3.2. ALI culture
The 3D culture conditions for organoids can be achieved using ALI technology that was first proposed by Ootani et al. 101 for the establishment of pancreatic and gastrointestinal tract organoids. The mechanically separated tissue fragments were homogeneously embedded in collagen gel, the mixture was laid flatly in an inner culture dish with a porous membrane underneath, and the top of the mixture was exposed to air. The medium was added in an outer culture dish. In this system, nutrients and growth substances are transferred by diffusion from the medium in the outer dish into the inner dish to meet the requirements of organoids. 102 , 103 Due to direct exposure to oxygen, ALI cultures have a higher oxygen supply than the submerged culture method. Kuo's group later established a variety of patient‐derived tumoroids using the ALI method, including some rare tumor types such as bile duct ampullary adenocarcinoma. 38 The ALI culture system, combining two culture dishes, can support the growth of organoids and stromal cells simultaneously. It is worth noting that organoids formed with this approach are able to preserve the functional tumor‐infiltrating lymphocytes (TILs), which can represent the complex tumor immune environment. 38 , 104
3.3. Organoids‐on‐a‐chip
Organ‐on‐a‐chip is a microfluidic cell culture device that allows accurate control of the biophysical and biochemical environment for cell growth and simulates both cellular and microenvironmental conditions, as well as inter‐tissue and multiorgan interactions. 105 A variety of organ‐on‐a‐chips have been created to simulate corresponding organs in vitro, which are used for disease modeling and to study the function of related organs. 106 , 107 , 108
Although organ‐on‐a‐chip has fundamental differences from organoids, the organoids‐on‐a‐chip generated from the combination of organoid technologies and organ‐on‐a‐chip can compensate for the shortcomings of the two technologies, and thus better serve as more effective preclinical models for simulating key features of target organ tissues. Cells in organoids‐on‐a‐chip are randomly and spontaneously self‐organized into 3D structures, which differs from the carefully designed organ‐on‐a‐chip. 109 Currently, a number of studies have established organoids‐on‐a‐chip of the brain, heart, gastrointestinal tract, liver, and pancreas. 110 , 111 , 112 , 113 , 114 , 115 , 116 Cho et al. 110 developed a brain organoids‐on‐a‐chip based on PDMS chips, and this system could increase the oxygen supply and promote nutrient/waste exchange to reduce the organoid cell death. Notably, this culture system could form mature brain organoids and be used to monitor the development of the overall human brain. A recent study has shown that the organoid‐on‐a‐chip system can create a hypoxic gradient in the lumen of small intestinal organoids and maintain the intestinal microbial barrier. 117
In addition to the above culture methods, 3D systems for organoids can be prepared by the hanging drop and rotational culture methods. The hanging‐drop approach applied gravity and surface tension to hang the mixture of cells and droplets of specific medium from a plate. 118 The rotational culture method can prevent cells from settling and improve the uptake of nutrients and oxygen by constantly rotating or stirring the cells, which has been used in the formation of brain and retinal organoids. 119 , 120 Jacob et al. 121 generated patient‐derived glioblastoma organoids that preserve the histological and genetic features and part of the microvasculature and immune microenvironment.
4. BIOMEDICAL APPLICATIONS OF ORGANOIDS
The rapid development of hepatobiliary organoid technology has provided better options for studying cell development, tissue maintenance, and pathogenesis of the hepatobiliary system under physiological or pathological conditions closely resembling natural conditions. Organoids have the following significant advantages. First, organoids are human‐derived and can recapitulate human physiological systems. The second is high efficiency. ASC‐ or PSC‐derived organoids are quick and relatively easy to establish. Third, organoids show stability in all aspects during large‐scale genomic screening or drug screening. The last is individualization. Organoids can be generated from individual tissues or cells, contributing to the realization of precision diagnosis and treatment. These advantages endow hepatobiliary organoids with a wide range of biomedical applications (Figure 3).
FIGURE 3.
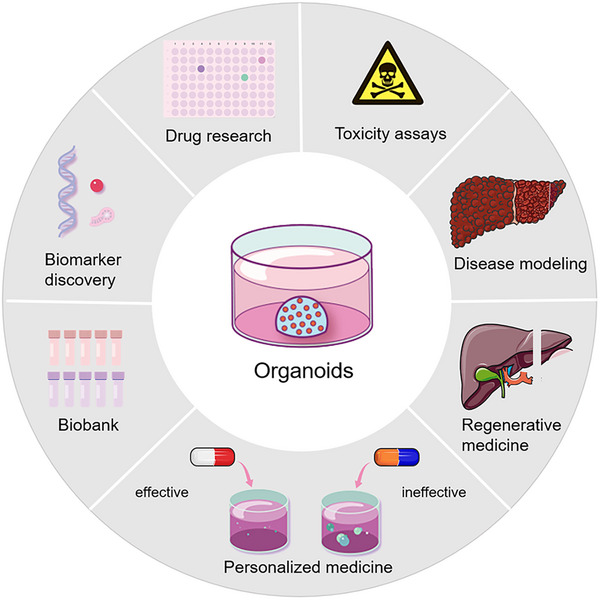
Biomedical applications of organoids. Organoids can be used as disease models to understand the mechanisms and physiopathology of human hepatobiliary diseases. Organoids are ideal models for drug screening and toxicity assays. Patient‐derived organoids can be used to predict patient‐specific responses to drugs and personalized treatment. Additionally, cryopreservation of organoids makes the establishment of biobank possible. Other biomedical applications of organoids include biomarker discovery and regenerative medicine.
4.1. Disease modeling
4.1.1. Genetic diseases
Organoids can mimic the development of organs in vitro, which can be used to model and study the mechanisms of organ‐specific genetic diseases. Liver organoids were generated from patients with α1‐antitrypsin deficiency, and the A1AT protein was found to aggregate in organoid cells, which could represent the key pathological and pathophysiological features of α1‐antitrypsin deficiency. 122 , 123 Similarly, other hepatobiliary hereditary diseases, including Alagille syndrome, Wilson's disease, and Wolman disease, have been modeled with hepatobiliary organoids. 123 , 124 , 125 , 126 , 127 The islet organoids have been unitized to model insulin secretion‐related genetic disorders such as Wolfram syndrome and congenital hyperinsulinism. 128 , 129
Cystic fibrosis (CF) is a monogenic hereditary disease, and the main clinical manifestations are dysfunction of the lungs, pancreas, liver, intestines, and reproductive system. 130 CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, and over 360 variants have been identified as pathogenic mutations. 130 , 131 CFTR, belonging to the adenosine triphosphate (ATP) binding cassette family, is a cell‐surface chloride transporter, and mutations in CFTR impair epithelial cell function, leading to the occurrence of clinical symptoms. CF organoid models of the airway, intestine, hepatobiliary system, and pancreas from humans or mice have been established. 132 , 133 , 134 , 135 , 136 Forskolin is a CFTR activator and has the ability to lead to intestinal organoid swelling. Forskolin‐induced swelling (FIS) of patient‐derived intestinal organoids has been shown to predict the response to treatment in CF patients. 137 A recent study indicated that the degree of patient‐derived rectal organoid swelling induced by forskolin was associated with the severity of clinical symptoms in CF patients. 134 Organoids can be used as a patient‐specific model for CF disease to study the molecular abnormalities associated with various CFTR mutations and develop new medicine strategies. Li et al. 138 established iPSC‐derived organoid models of autosomal dominant polycystic kidney disease. Based on the model, the authors proposed that absorptive pathways, especially glucose absorption, play an important role in the formation of cysts. More recently, Kim et al. 139 edited iPSC‐derived kidney organoids via CRISPR‐Cas9 technology to generate GLA‐mutant Fabry nephropathy models, whose applications ranged from studying pathophysiology to exploring novel therapeutic regimens in Fabry nephropathy. Cardiac organoids derived from HAND1 or NKX2‐5 knockout iPSC lines were prepared to model hypoplastic left heart syndrome. 140 Pregestational diabetes‐induced congenital heart defects were also modeled by human heart organoids. 141 Genetic diseases of organs originating from the neuroectoderm can be modeled by organoids as well. Retinal organoids have been used as powerful tools to model and investigate the mechanisms of inherited retinal diseases such as retinitis pigmentosa and Leber's congenital amaurosis. 142 , 143 , 144 , 145 Primary microcephaly is caused by single mutations or duplications of genes such as CDK5RAP2, 146 whose organoid model was developed 10 years ago. 147 In recent years, organoids have been established for different genetic mutations causing microcephaly. 148 , 149 Specific brain organoids of syndromic neurodevelopmental disorders, such as Rett syndrome, 150 Tuberous sclerosis complex, 151 , 152 fragile X syndrome, 153 Down syndrome, 154 and Angelman syndrome, 155 have been generated. The advent and rapid development of organoid technologies has provided important insights into the etiology, pathogenesis, and discovery of potential treatment alternatives for genetic disorders. The combination of gene‐editing technologies (such as CRISPR‐Cas9) and organoid models provides better options for studying genetic diseases that are complex and difficult to model in vitro.
4.1.2. Infectious diseases
Owing to the fact that infectious agents usually infect specific species or cell types, human models are superior to animal models when investigating the pathogenetic mechanisms and development of these diseases. As described above, microcephaly is a developmental brain disorder. Recent studies revealed the associations between microcephaly and Zika virus (ZIKV) infection 156 , 157 and found that several compounds could reduce the cell death induced by ZIKV. 158 Technical advances in 3D culture and organoids have made it possible to culture certain viruses in vitro. For instance, the culture system of human norovirus failed to be established for decades. Ettayebi et al. 159 first succeeded in culturing human norovirus in vitro with epithelial monolayers derived from human intestinal organoids, and they optimized the culture system to allow the cultivation of multiple strains. 160 Culture systems for influenza virus, human rotavirus, and BK virus have been established with organoids. 161 , 162 , 163
Despite advancements in therapy, liver disorders caused by viral infections continue to be a national health concern in China. When hepatitis viruses invade and attack hepatocytes, the immune response is activated to kill viruses as well as virus‐infected hepatocytes, causing degeneration and necrosis of liver parenchymal cells with reactive stromal tissue hyperplasia, leading to the accumulation of collagen fibers in the liver. The extensive liver fibrosis and hepatocyte necrosis lead to the occurrence of liver remodeling and the formation of false lobules, which ultimately bring about cirrhosis and even liver failure. 164 , 165 Additionally, viral hepatitis and liver cirrhosis are also risk factors for hepatocellular carcinoma (HCC). 166 Human liver organoids have emerged as advanced tools for studying hepatitis virus infection and several laboratories have generated liver organoids infected by hepatitis B, C, and E viruses. 167 , 168 , 169 , 170 , 171 Recent research indicated that when liver organoids derived from healthy donors were cocultured with the recombinant virus or the serum of patients with HBVs, the organoids were infected, and the virus proliferated actively. 172
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the pathogen of coronavirus disease 2019 (COVID‐19), which usually causes moderate respiratory disease such as fever and cough, and even acute respiratory syndrome. 173 Alveolar lung airway and bronchial organoids have been generated and used to mimic SARS‐CoV‐2 infection in vivo, investigate COVID‐19‐related pathophysiology, and pathology and develop novel therapeutics. 10 , 174 , 175 Additionally, COVID‐19 also leads to extrapulmonary disease, including gastrointestinal and neurological symptoms, and liver and kidney injury, and corresponding organoids have been applied in SARS‐CoV‐2‐related research. 174 , 176 , 177 , 178 , 179
The applications of organoid models in infection biology are not limited to virus research but can also be used to study bacteria and protozoan parasites. Cryptosporidium infected human small intestinal and lung organoids have been established. 180 Similarly, relationships between the BBB and Plasmodium falciparum and Lyme neuroborreliosis were explored via BBB organoids. 181 , 182 Bartfeld et al. 183 successfully established Helicobacter pylori (Hp)‐infected human gastric organoids, and inflammatory responses were observed in these infected organoid cells. The adhesion of Hp depends on the high affinity between the TlpB receptor and urea secreted by gastric cells, and Hp is likely to attach to highly differentiated pit cells with a larger volume. 184 Furthermore, the CagA factor derived from Hp was transferred into organoid cells, leading to activation of certain pathways and increased cell proliferation. 50 , 184 A recent study demonstrated that the Hp infection could activate NF‐κB signaling pathway and upregulate RAS protein activator like 2 (RASAL2) expression to promote proliferation of gastric tumor cells. Downregulation of RASAL2 could significantly inhibit the growth of gastric tumoroids. 185 Murine gallbladder organoids with Salmonella Typhi infection contributed to malignant transformation (TP53 mutations and c‐MYC amplification) and activating AKT and MAPK signal pathways, which promoted abnormal cellular proliferation and transformation to tumors. 186
To sum up, the 3D organoid models of infection biology can reflect the correlation and interaction between pathogenic microorganisms and host cells, providing crucial preclinical models for mechanistic exploration, treatment, and drug development for infectious diseases. Additionally, the establishment of a coculture system of organoids and oncogenic pathogens provides an advanced platform to further study the mechanism of tumor formation promoted by biological pathogens.
4.1.3. Metabolic diseases
Currently, metabolic diseases pose a serious risk to health worldwide. However, the absence of proper models limits the exploration of underlying mechanisms and potential therapeutics. Organoid culture technologies shed new light on the research in this filed.
Obesity is one of the common metabolic diseases that is characterized by an increase in adipose tissue, increasing the risk of type 2 diabetes and NAFLD. 187 Adipose organoids have been utilized to study fat‐related metabolism and model obesity. 188 , 189 Recent studies reported the formation of adipose organoids with immune cells or vascular systems, which could replicate the signal communication between adipocytes and other cells in vivo. 190 , 191
NAFLD is a group of disorders correlated with metabolic dysfunction, including nonalcoholic fatty liver and nonalcoholic steatohepatitis (NASH), which may eventually lead to liver cirrhosis and HCC. Additionally, it has been confirmed that NAFLD is associated with type 2 diabetes and can increase cardiovascular risk. 192 , 193 Ouchi et al. 127 generated iPSC‐derived liver organoids encompassing hepatocyte‐, stellate‐ and Kupffer‐like cells. The authors treated these organoids with free fatty acids and found that the accumulation of lipids in the organoids and the degree of organoid steatosis were aggravated with increasing lipid content in the culture environment, and the organoids manifested inflammatory and fibrotic phenotypes. Gurevich et al. 194 constructed steatosis organoid models with iPSC‐derived cryopreservable hepatocytes from patients with NASH. Intracellular lipid deposition in the organoids exhibited a dose‐dependent relationship with free fatty acids in culture, which viably conserved and recapitulated steatosis in in vitro conditions. Moreover, the organoids could be applied in drug metabolism research. Another study established bipotent ductal organoids from the hepatic explants of patients with NASH, which have impaired passaging/growth capability and NASH liver features. 178 More recently, the group of Takebe cultured human liver organoids from 24 iPSC lines with various genotypes and assembled these organoids into a population organoid panel (PoP). After incubating with oleic acid, liver organoids of PoP showed the features of NASH. Through this model, the authors indicated that under different metabolic conditions, the glucokinase regulatory protein‐rs1260326 single nucleotide polymorphism could influence mitochondrial function, which is associated with NASH severity. 97
Alcoholic liver disease (ALD) is one of the most common types of chronic liver diseases worldwide. The initial stage of ALD is alcoholic fatty liver, characterized by steatosis of liver cells. Some patients will develop into alcoholic steatohepatitis, liver fibrosis, and liver cirrhosis, which might eventually progress to liver cancer and liver failure. 195 The ALD organoid model, reported by Wang et al., 196 was generated by treating human fetal liver mesenchymal cells/human ESC‐derived expandable hepatic organoids coculture system with ethanol. This model could simulate typical characteristics of ALD pathophysiology, including increased secretion of alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase; decreased cell viability and apoptosis; increased CYP2E and CYP3A4 activity; enhanced oxidative stress; increased release of inflammatory cytokines; fibrosis; and increased deposition of ECM.
4.1.4. Cancers
Currently, the most commonly used oncology models mainly include human tumor cell lines and patient‐derived xenograft (PDX) models. However, these models have some unavoidable shortcomings. Tumor cell lines involve primary (originated from patients) and immortalized tumor cells. Although primary cancer cells preserve some features of the parental tumor, the slow growth speed, short lifespan, and lack of complexity of tumors limit their applications. The immortalized cells have unlimited proliferation capacity, but they usually fail to represent the phenotypes and lose genetic heterogeneity of the original tumor during long‐term culture and passages, which may increase the failure rate of clinical drug screening trials. 197 The 2D culture systems are unable to mimic the in vivo cellular growth conditions, and the 2D cultures fail to represent the tumor heterogeneity accurately. PDX models are constructed by transplanting human tumor cells into mice and promoting these cells to grow and form tumors. Xenograft models can maintain the relatively intact biological features of parental tumors as well as the 3D structure and tumor stroma. Studies have successfully generated several tumor PDX models, such as lung, colorectal, pancreatic, breast, prostate, and ovarian tumors. 198 , 199 , 200 , 201 , 202 However, several shortcomings restricted them to being excellent preclinical models. Xenograft models are usually established from a small number of tumor tissues, which cannot completely inherit the genetic mutations from the primary tumor, and the stroma of the human tumor is gradually substituted by murine stroma with the PDXs growth. 203 Many research results based on the PDX models have not been confirmed in human trials. 204 Furthermore, the economic and time costs of PDX models are high with a low success rate. 24 Tumoroids retain parental tumor heterogeneity and histopathological features and reserve their 3D structure after long‐term culture 32 , 33 , 205 , 206 , 207 (Table 1). Papaccio et al. 208 reported that patient‐derived colorectal tumoroids represented the morphology and immunohistochemistry characteristics and genomic and transcriptomic profile of corresponding tissues, and the response to anticancer drugs varied among these tumoroids derived from different patients.
TABLE 1.
Comparison of the current preclinical models for cancer research.
| 2D tumor cell lines | Patient‐derived xenografts | Tumoroids | |
|---|---|---|---|
| Success rate of establishment | Generally high (but some special tumor types are low) | Relatively low | Relatively high |
| Maintenance | The easiest | Little difficult | Easy |
| Genetic manipulation | Able | Unable | Able |
| Genome‐wide screening | Able | Unable | Able |
| Relative cost | Low | High | Relatively high |
| High‐throughput assay | Able | Unable | Able |
| Expansion | Quick | Relatively low | Quick |
| Reproducibility | High | Moderate | Relatively low |
| Ability to recapitulate tumor development biology | Low | Low | High |
| Tumor immune microenvironment | Unable to recapitulate | Partial recapitulation | Partial recapitulation |
| Tumor heterogeneity | Unable to recapitulate | Retain, (but heterogeneity may be lost partly in long‐term culture) | Retain parental tumor heterogeneity |
| Ability of personalized treatment | Low | Moderate | High |
| Complexity | Low complexity | High complexity (enable to reproduce the cell types and the tumor cell–stroma cell interactions of the primary tumor and can be used to study metastasis) | High complexity (recapitulate histological and genetical features of primary tumors) |
The role of mutations in genes or structures in tumor development is difficult to observe dynamically or to intervene. Therefore, the exploration of the tumorigenesis mechanism is usually performed with difficulties. Organoid technology enables in vitro simulation of the entire process of tumor development. 48 According to organoid models, the accumulation of specific tumor‐driving gene mutations has been found to play a crucial role in tumorigenesis. 209 The combination of genome editing and organoids offers new opportunities to study the role of pathogenic gene mutations. With CRISPR‐Cas9 technology, the oncogene c‐MYC associated with HCC was introduced into normal liver organoids. 210 Excessive mitochondrial endoplasmic reticulum coupling occurred in these organoids and altered mitochondrial fission and aerobic respiration, finally resulting in HCC tumorigenesis. Human colorectal tumoroids have been utilized to discover three tumor suppressor genes (Acvr1b, Acvr2a, and Arid2) and identified the function of Trp53 in liver metastasis of colorectal cancer (CRC). 211 Tumoroids serve as an advanced platform that is highly similar to the physiology and microenvironment features of in vivo tumors to propel our fundamental knowledge of tumorigenesis, progression, metastasis, and recurrence. The applications of organoids in modeling diseases are summarized in Table 2.
TABLE 2.
Applications of organoids in disease modeling.
| Organs or tissues | Diseases | Cell sources | References | |
|---|---|---|---|---|
| Brain | Genetic diseases | Primary microcephaly | Human iPSCs and ESCs | 148, 149 |
| Rett syndrome | Human ESCs and iPSCs | 150 | ||
| Tuberous sclerosis complex | Human iPSCs | 151, 152 | ||
| Fragile X syndrome | Human iPSCs | 153 | ||
| Down syndrome | Human iPSCs | 154 | ||
| Degenerative diseases | Parkinson's disease | Human iPSCs | 45 | |
| Alzheimer's disease | Human iPSCs | 46 | ||
| Infection | ZIKV infection | Human ESCs and iPSCs | 156, 220 | |
| HSV‐1 infection | Human ESCs | 156 | ||
| Human cytomegalovirus infection | Human iPSCs | 163 | ||
| SARS‐CoV‐2 infection | Human iPSCs | 174 | ||
| Tumor | Glioblastoma | Patient‐derived tumor cells | 64, 121 | |
| Medulloblastoma | Human iPSCs | 65 | ||
| Glioma | Patient‐derived tumor cells | 66 | ||
| Retina | Genetic diseases | Nonsyndromic CLN3 disease | Human iPSCs | 142 |
| Retinitis pigmentosa | Human iPSCs | 143 | ||
| Leber congenital amaurosis | Human iPSCs | 144, 145 | ||
| Tumor | Retinoblastoma | Human iPSCs and ESCs | 236 | |
| Air ways | Genetic diseases | CF‐related air way diseases | Human ASCs | 132 |
| Infection | Respiratory syncytial virus infection | Human ASCs | 132 | |
| SARS‐CoV‐2 infection | Human iPSCs and ASCs | 10, 161, 174, 175 | ||
| Influenza virus infection | Human ASCs | 161 | ||
| Cryptosporidium infection | Human ASCs | 180 | ||
| Tumor | Lung adenocarcinoma | Patient‐derived tumor cells | 71, 72 | |
| Nonsmall‐cell lung cancer | Patient‐derived tumor cells | 132, 216, 217 | ||
| Gastrointestinal tract | Genetic diseases | CF‐related intestinal diseases | Human ASCs | 134, 137 |
| Infection | SARS‐CoV‐2 infection | Human ASCs | 10, 177 | |
| H. pylori infection | Human iPSCs and ASCs | 50, 182 | ||
| Human norovirus infection | Human ASCs | 159, 160 | ||
| Cryptosporidium infection | Human ASCs | 180 | ||
| Tumor | Neuroendocrine carcinoma | Patient‐derived tumor cells | 33, 69 | |
| Colorectal cancer | Patient‐derived tumor cells | 38, 208, 216, 217, 232 | ||
| Gastric cancer | Patient‐derived tumor cells | 184, 251 | ||
| Hepatobiliary | Genetic diseases | Primary sclerosing cholangitis | Human ASCs | 59 |
| α1‐Antitrypsin deficiency | Human ASCs | 112, 113 | ||
| Alagille syndrome | Human ASCs and iPSCs | 113, 114 | ||
| Wilson's disease | Dog ASCs | 115, 116 | ||
| Wolman disease | Human iPSCs | 117 | ||
| CF‐related bile duct disease | Human ESCs | 135 | ||
| Metabolic diseases | Nonalcoholic fatty liver disease | Human iPSCs, ASCs | 97, 127, 194 | |
| Alcoholic liver disease | Human ESCs | 196 | ||
| Infection | Rotavirus infection | Human ESCs and ASCs | 162 | |
| Viral hepatitis | Human iPSCs, ASCs, and ESCs | 167, 168, 169, 170, 171, 172 | ||
| Salmonella Typhi infection | Mouse ASCs | 186 | ||
| Fibrosis | Liver fibrosis | Human iPSCs | 48 | |
| Tumor | Cholangiocarcinoma | Patient‐derived tumor cells | 15, 30, 33, 205, 206 | |
| Gallbladder adenoma | Human ASCs | 32 | ||
| Gallbladder carcinoma | Patient‐derived tumor cells | 32, 33 | ||
| Bile duct ampullary adenocarcinoma | Patient‐derived tumor cells | 38 | ||
| Hepatocellular carcinoma | Patient‐derived tumor cells and human iPSCs | 205, 207, 233 | ||
| Pancreas | Genetic diseases | Pancreatic dysplasia | Human iPSCs | 48 |
| Wolfram syndrome | Human iPSCs | 128 | ||
| Congenital hyperinsulinism | Human ESCs | 129 | ||
| Tumor | Pancreatic ductal adenocarcinoma | Patient‐derived tumor cells | 231, 258, 264 | |
| Thyroid | Autoimmune diseases | Hashimoto's thyroiditis | Human ASCs | 58 |
| Graves’ hyperthyroidism | Human and mouse ASCs | 60 | ||
| Tumor | Papillary thyroid cancer | Patient‐derived tumor cells | 241 | |
| Kidney | Genetic diseases | Polycystic kidney disease | Human iPSCs | 19 |
| Injury | Kidney injury | Human iPSCs | 20 | |
| Infection | SARS‐CoV‐2 infection | Human iPSCs | 176, 179 | |
| Tumor | Renal cell carcinoma | Patient‐derived tumor cells | 257 | |
| Heart | Genetic diseases | Hypoplastic left heart syndrome | Human iPSCs | 140 |
| Pregestational diabetes‐induced congenital heart defects | Human iPSCs | 141 | ||
| Salivary gland | Tumor | Salivary gland tumor | Patient‐derived tumor cells | 38, 68 |
| Mammary gland | Tumor | Breast cancer | Patient‐derived tumor cells | 62, 198, 261 |
| Prostate gland | Tumor | Prostate cancer | Patient‐derived tumor cells | 214 |
| Lacrimal gland | Dry eye disease | Mouse and human ASCs | 226 | |
| Vasculature | Metabolic diseases | Diabetic vasculopathy | Human iPSCs | 47 |
| Blood–brain barrier | Infection | Plasmodium falciparum infection | Human ASCs | 181 |
| Lyme neuroborreliosis | Human ASCs | 182 | ||
ASCs, adult stem cells; CF, cystic fibrosis; ESCs, embryonic stem cells; iPSC, induced pluripotent stem cells; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; ZIKV, Zika virus.
It has been widely accepted that the tumor immune microenvironment plays an essential role in tumor formation and progression. Based on the cancer immunoediting theory, the immune system can inhibit or promote tumor growth in different stages. 212 Despite advances in immunotherapy, a part of patients present with low responses to this treatment. One of the hot topics in the field of tumor immunotherapy is the identification of patients who are sensitive to immunotherapy. Evidence has shown that the tumor immune microenvironment is recapitulated via coculturing tumoroids and stromal cells such as TILs and cancer‐associated fibroblasts (CAFs) 213 (Figure 4). Zhang et al. 214 incubated tumoroids derived from prostate cancer patients and CAFs and found that neuregulin 1 released by CAFs could activate human EGF receptor 3 in cancer cells, leading to antiandrogen therapy resistance. Kuo et al. successfully generated patient‐derived tumoroids from 100 patients with 28 distinct tumor subtypes with ALI culture system, which retained a variety of immune cells such as T cells, B cells, NK cells, and macrophages, and the T cell receptor spectrum of parental tumors. However, with time going on, the immune components of ALI‐cultured tumoroids would decrease. Treatment with nivolumab in these tumoroids could screen for specific patients who respond to immunotherapy. 38 , 215 The interactions between tumor cells and T cells have been modeled by tumoroid and peripheral blood mononuclear cell (PBMC) coculture systems. Tumor‐reactive T cells were generated by coculturing PBMCs and tumoroids, and they have tumor‐specific T cell responses. 216 , 217 Liu et al. 218 established autologous organoid‐ killing models to confirm that high‐affinity neoantigens had higher antitumor activity in HCC patients. With a similar method, a recent RCT study (NCT03026140) demonstrated that there existed an association between induced T cell reactions in vitro and patient response. 219
FIGURE 4.
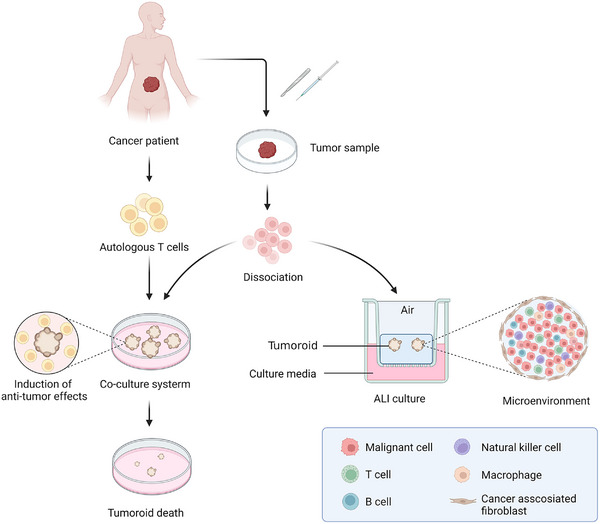
Recapitulation of tumor immune microenvironment with tumoroids. Tumor samples are obtained by resection or biopsy and dissociated into single cells to form tumoroids. Tumoroids are established from tumor cells and cocultured with autologous T cells by submerged method, which can induce the specific antitumor effects of T cells, leading to the injury and death of tumoroids. With the application of ALI method, the tumor tissues are used as the initial cell materials to generate tumoroids preserving cancer‐associated fibroblasts and immune cells, which can recapitulate the complex tumor microenvironment in vitro (Created with BioRender.com).
4.2. Drug research
Organoids have distinct advantages in that they can recapitulate in vivo physiological functions and features of organs or tissues, excelling in a wide range of models for drug‐related research. As described above, ZIKV infection contributes to birth defects and miscarriage. A work by Li et al. 220 demonstrated that methylene blue protected brain organoid cells from ZIKV infection via blocking viral protease NS3 and NS2B interactions. Another recent study represented the HEV–host interactions in liver organoids and reported that brequinar and homoharringtonine are potent anti‐HEV drugs. 171 Bacterium‐infected intestinal organoids have been utilized to serve as advanced platforms for drug discovery. Bacitracin, an antibiotic for local application, has been identified to inhibit the activity of Clostridium difficile and its toxin B. 221 Islet organoids were used for drug screening in diabetes. 222 Based on 3D culture, GLIS3−/− human ESCs (hESCs) were induced to act as a high‐throughput platform for drug identification in GLIS3‐associated diabetes. 223 High glucose metabolism could stabilize and activate HIF‐1α, and upregulate some HIF‐1α target genes. PX‐478, a HIF‐1α inhibitor, was employed to treat human islet organoids with chronic high glucose exposure, enabling the glucose‐induced insulin secretion stimulation index increase, which was considered as a potential antidiabetic agent. 224 Moreover, the lacrimal gland organoids are reported as promising tools that allow high‐content drug screening. 225 , 226
Patient‐derived organoids are advanced models for discovering and testing novel drugs. 227 Based on the results of drug sensitivity tests, patients are categorized to find the similarities in genetic mutations or epigenetics in those who respond to specific therapeutic regimens, which may help advance the precision of antitumor treatment. 228 , 229 The L1 cell adhesion molecule (L1CAM) is a trigger factor of metastasis and is upregulated in tumor that have undergone neoadjuvant chemotherapy. Ganesh et al. 230 indicated that knockdown L1CM human colorectal tumoroids presented improved sensitivity to irinotecan. The coculture system of pancreatic ductal adenocarcinoma (PDAC) organoids and CAFs showed resistance to gemcitabine, 5‐FU, and paclitaxel, with increased epithelial‐to‐mesenchymal transition (EMT) gene expression in these organoid cells. 231 Colorectal tumoroids were used to perform drug sensitivity assays and pharmacogenomics analysis, and results showed that the paclitaxel sensitivity was linked to the checkpoint with forkhead and ring finger domains (CHFR) silencing in organoid as well as in xenograft models. 232 Organoids can be cultured in a short timescale with high generation efficiency, which is valuable for extending the applicable scope of existing medicines. CF patient‐derived organoids were employed in a high‐throughput compound assay to identify potential drugs to treat individuals having rare CFTR mutations. 137 Yuan et al. 32 performed a drug sensitivity test for 29 antitumor compounds approved by the United States Food and Drug Administration (US FDA) in human gallbladder tumoroids, and histone deacetylase (HDAC) inhibitors, including vorinostat and curcumin, significantly suppressed the growth of GBC tumoroids. Furthermore, CUDC‐907, a dual PI3K/HDAC inhibitor, presented a higher level of tumor suppressive activity. PCSK9 has been reported as a therapeutic target for patients with dyslipidemia or hypercholesterolemia, and overexpression of PCSK9 is associated with oncogenesis and enhances the malignant phenotypes of CRC with APC/KRAS mutations. Wong et al. 233 found that blocking PSCK9 significantly suppressed the growth of human APC/KRAS colorectal tumoroids. The protein synthesis inhibitor omacetaxine has been approved by the US FDA to treat patients with chronic myelogenous leukemia. 234 However, in a recent study, this drug was proven to be a highly effective compound that could repress the growth of HCC patient‐derived organoids and promote tumoroid cell apoptosis. Thus, omacetaxine has the potential to be a new option for HCC treatment. 235 Watanabe et al. 236 established human PDAC tumoroids for drug screening, and the CHK inhibitors exhibited effective antitumor ability, which was found to be effective against breast and ovarian cancers and has been proven in clinical trials (NCT03495323, NCT02203513). Sunitinib has been approved to treat kidney cancer and imatinib‐resistant gastrointestinal stromal tumors. At present, sunitinib has been identified as a potential agent for the treatment of retinoblastoma with minimal toxicity to normal retinal cells. 237
Advances in organoid technologies allow for the development of efficient therapies or combination regimens. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) can produce ROS to promote EMT of cells, eventually causing cancer, and NOX4 plays an important role in the development of various tumors. 238 Fangchinoline, a small molecule extracted from Menispermaceae, was found to exert antitumor effects in nonsmall cell lung cancer (NSCLC) organoids via inhibition of NOX4 to reduce ROS, hamper Akt‐mTOR pathway activation and decrease EMT and malignant phenotypes. 239 Ghate et al treated patient‐derived colon tumoroids with the VprBP inhibitor B32B3 and found that B32B3 could restrain H2AT120 phosphorylation and restart the normal transcriptional program to impair the proliferation activity of colon tumoroids. 240 BRAFV600E inhibitor monotherapy was employed to treat human papillary thyroid tumoroids with the BRAFV600E mutation and exhibited mild antitumor activity. On the basis of BRAF inhibitors, combining MEK, RTK inhibitors, or chemotherapies could remarkably decrease the viability of tumoroid cells. 241 AT‐rich interaction domain 1A (ARID1A) is one of the subunits of the BRG1‐ or HBRM‐associated factor complex and has been demonstrated to have tumor suppressor effects. 242 , 243 ARID1A mutation or deficiency has been shown to contribute to facilitating the aggressiveness of tumors, 244 and it has been identified that most gastric cancer patients carry ARID1A mutations. Recent research by Loe et al. 245 provided a novel combination therapy of TP06 (epigenetic inhibitor) and Nutlin‐3 (p53 agonist) to treat gastric tumoroids with Arid1a heterozygosity, exhibiting a robust inhibition of tumor growth.
Other applications of organoids in drug‐related research also include toxicity assessment and drug safety evaluation. The liver and kidney are the primary organs and play essential roles in the processes of drug metabolism and excretion. Therefore, they are vulnerable to drug‐related injuries. The traditional models for toxicity assessment include 2D cell lines and animal models; however, these models have always suffered from some insurmountable drawbacks. 246 , 247 In addition, the expression and function of enzymes and proteins related to drug metabolism as well as metabolic functions, vary between species, making it impossible to accurately assess drug toxicity. 246 Organoids highly resemble the structure and metabolism characteristics of original organs or tissues, which provide excellent preclinical models for toxicity assessment and drug safety evaluation. Under the condition of differentiation into the hepatic lineage, human ASC‐derived intrahepatic cholangiocyte organoids (ICOs) can represent the metabolism features of the liver, such as the expression of CYP enzymes. 123 Shi et al. 248 identified the associations between necroptosis and bile duct disease via ICOs, which could serve as a useful ex vivo model for biliary cytotoxicity assessment. Bouwmeester et al. 249 established hepatocyte‐like ICOs and detected the expression and activity of drug metabolism‐related genes to confirm the ability of organoids in ex vivo toxicity assessment. Zhang et al. 250 developed a high throughput screening platform based on the human liver organoid‐on‐a‐chip for drug‐induced liver injury that pooled multiomics such as biomarker/analyte detection, high‐content imaging‐enabled phenotyping, and single‐cell RNA sequencing. The authors demonstrate the effectiveness of this platform by testing a set of known hepatotoxic drugs and comparing the results to clinical data. Another study demonstrated the potential utility of hepatic organoids for hepatotoxicity and drug safety assessment by conducting a functional analysis of CYP450‐mediated metabolic ability. 251 To test the potential nephrotoxicity of Esculentoside A, Gu et al. 252 exposed iPSC‐derived kidney organoids to the compound and monitored changes in cell viability, morphology, and gene expression. They found that exposure to Esculentoside A resulted in decreased cell viability and altered gene expression patterns, indicating that organoids offered a promising approach for assessing the toxic effects of compounds. What's more, the side effects of most anticancer compounds involve gastrointestinal symptoms such as abdominal pain, vomiting, nausea, and diarrhea. Serious gastrointestinal reactions may force the patient to discontinue treatment, leading to a dismal survival outcome and poor quality of life. However, the underlying mechanisms of gastrointestinal toxicity caused by many antineoplastic drugs have not been fully clarified. Recent studies forecasted the gastrointestinal toxicity caused by gefitinib and doxorubicin by evaluating the viability and apoptosis of the colon and small intestine after exposure to the two compounds. Furthermore, the possible molecular mechanisms of gefitinib and doxorubicin‐triggered intestinal toxicity were explored by transcriptomic analysis. 253 , 254
In conclusion, organoids are an important tool for assessing the effects and toxicity of drugs, understanding drug metabolism and distribution, predicting drug safety and efficacy, and providing better guidance for drug development and clinical application. The use of organoids in drug research has revolutionized the drug development process. Organoids provide a powerful tool for modeling human physiology and disease, which can improve drug discovery, reduce the failure rate of clinical trials, and accelerate the development of new therapies.
4.3. Precision medicine
Organoids have shown great potential in the field of precision medicine, which aims to tailor medical treatments to individual patients based on their specific genomics and metabolomics. 255 By providing a more physiologically relevant and personalized model of human organs or tissues, organoids can be used to predict individual patient responses to drugs and other treatments. 256 , 257
One potential application of organoids in precision medicine is in the development of personalized cancer treatments. PDOs can be used to test the efficacy of different chemotherapy drugs and identify the most effective treatment for that patient. 258 , 259 This approach is more accurate than traditional methods of testing drugs on cancer cell lines, which do not fully capture the genetic diversity of individual tumors. An article by Khan et al. described the application of organoids to predict the response of patients presenting with metastatic digestive system tumors to various chemotherapy drugs and targeted drugs. The researchers found that the organoids accurately forecasted which treatments would be effective in the patient and which would not, with significantly high specificity (93%) and sensitivity (100%). 260 Similar work was carried out by Yao et al., who established locally advanced rectal cancer patient‐derived organoids to construct a biobank. The study demonstrated that rectal tumoroids could recapitulate the patient's response to neoadjuvant chemoradiation in vitro, with an accuracy rate of 86%, and also was able to identify patients who were likely to have a complete response to chemoradiation therapy, allowing clinicians to tailor treatment to the individual patient. 261 According to the study by Lyudmyla, the drug sensitivity results of PDAC patient‐derived organoids were highly consistent with the clinical response of patients, and the PDO forecasted drug response was also linked to tumor cellularity. 262 Furthermore, studies have identified the feasibility that PDOs could serve as platforms for fast drug screening and allowed patients to achieve the most appropriate therapeutic regimens. 262 , 263 It is worth noting that, in addition to directly guiding patients on medication, the overall analysis of tumoroids can be applied in the discovery of biomarkers for clinical treatment response. The team of Burkhart suggested that the clinical sequencing results of PDAC patients who have better responses to chemotherapies tended to have fewer mutations in KRAS, TP53, and SMAD4 and the specific pharmacotype of PDOs had potential as a forecasted biomarker for the response of PDAC patients to chemotherapies via an ongoing clinical trial (NCT03563248). 264 Target therapy is a precision medicine approach that targets specific molecular markers or signaling pathways of tumors and selects specific drugs for treatment. 265 Targeted therapy is a part of precision medicine that uses specific compounds to inhibit specific molecular markers or signaling pathways of tumors or other diseases. Organoids are also used to perform target drug screening and discover potential therapeutic targets owing to the important roles of organoids in target therapy theories. 100 , 266 Ovarian tumor organoids derived from patients with three tumor types had been used as a high‐throughput screening platform to test the sensitivity to 240 protein kinase inhibitors by Phan et al., 267 and this platform allowed rapidly obtaining drug sensitivity results for ovarian cancers. Yuan et al. 32 screened 20 US FDA‐approved targeted drugs with low toxicity to normal gallbladder organoids and found that HDAC inhibitors could significantly decrease the growth of the gallbladder tumoroids. After further study, they demonstrated that the dual phosphatidylinositol‐4,5‐bisphosphate 3‐kinase (PI3K) and HDAC inhibitor CUDC‐907 had higher antitumor activity than the HDAC inhibitor alone in the gallbladder tumoroid and xenograft mouse models. 32 A novel organoid culture system combining suspension and rotating culture methods was developed to cultivate colorectal tumoroids on a large scale for drug screening with high accuracy, robustness, and reproducibility because this method could improve the scalability and decrease the variability of organoids. The study tested 56 anticancer agents, of which 47 were inhibitors with small molecules that targeted specific tumor‐associated pathways, and suggested that the tumor drug response heterogeneity was attributed to the diversity of genes or epigenetics. Furthermore, based on the gene−drug analysis, a significant correlation between the BRAFV600E mutation and vemurafenib was observed as well as TP53 mutations and the MDM2 inhibitor nutlin‐3. 232 Organoids present with the potential as promising tools or models to explore the underlying drug resistance mechanisms and help customize the optimal treatment plan. Zhao et al. 268 analyzed hepatobiliary tumoroids with various drug susceptibilities using single‐cell transcriptome sequencing technology. CD44+ hepatoma cells were found to have the ability of broad‐spectrum drug resistance and metabolic advantages. Lee et al. found that SHP099, an inhibitor of Src homology 2 domain‐containing phosphatase 2 (SHP2), could abrogate receptor tyrosine kinase (RTK)‐induced reactivation of the MAPK/ERK kinase (MEK)/extracellular regulated kinase (ERK), and protein kinase B (AKT) signaling pathways to eliminate sorafenib resistance. 269 To verify this result, they treated HCC patient‐derived organoids with SHP099 and sorafenib and showed that the combination treatment significantly inhibited tumor cell growth and that the HCC cells in these organoids were sensitive to sorafenib again. ML264, a Kruppel‐like factor 5 (KLF5) inhibitor, was found to be a suppressor of the KLF5/Bcl‐2/caspase3 signaling pathway and can restore the sensitivity to oxaliplatin in oxaliplatin‐resistant colorectal tumoroids by the inhibition of antiapoptotic effects. 270
Organoids can also be used to study rare genetic diseases and identify personalized treatments. By deriving organoids from patients with genetic diseases, researchers can study the effects of different drugs and gene therapies on these tissues, and develop tailored treatments for individual patients. Sampaziotis et al. 271 applied 3D culture technology to construct CF organoid models from iPSC‐derived cholangiocytes and explored the response of the CF organoids to VX‐809 CF therapy. As mentioned above, FIS is considered as a prospective biomarker to predict the response to CFTR‐modulating drugs. Technological advances in genetic engineering, especially CRISPR–Cas9 genome editing, allow gene repair to be a promising treatment for some diseases, and organoids provide a good platform to develop and validate novel gene therapies. 272 Schwank et al. 273 pioneered the successful application of CRISPR/Cas9 to human‐derived organoids. They restored the CFTR function of intestinal organoids by correcting the common mutation F508del in CF. More recently, Geurts et al. 274 employed CRISPR‐based adenine base editors that enabled A‐T to G‐C base changes to successfully correct the W1282X and R553X mutations and achieve functional CFTR rescue in human rectal and airway organoids. A clinical trial (NCT04254705) evaluates the response to CFTR‐modulators in intestinal organoids of CF patients with R334W mutations.
Finally, organoids can be used to study the effects of environmental toxins and other factors on human tissues. By exposing organoids to different environmental conditions, researchers can study the effects of air pollution, toxic chemicals, and other factors on human health, and identify personalized strategies for reducing exposure and preventing disease. For example, Kim et al. 275 exposed human iPSC‐derived alveolar organoids to diesel PM2.5 to investigate its developmental toxicity, and the authors showed that diesel PM2.5 could upregulate the oxidative stress and EMT expression to impair alveolar epithelium growth and contribute to high susceptibility to SARS‐CoV‐2. The lung toxicity of common air pollutants including benzo(a)pyrene, nano‐carbon black, and nano‐SiO2 has been assessed with human iPSC‐derived AT2‐like cell organoids. 276 Meanwhile, human airway organoids were employed as models for toxicological assessment of emerging inhaled pollutants of tire wear particles. 277 Nicotine exposure in pregnant women has been shown to cause harmful birth outcomes and effects on cerebral development. 278 Several studies have investigated the role of nicotine in abnormal human brain development and the potential mechanisms with organoid system models. 279 , 280 Heavy metals are pervasive and persistent environmental toxins with the feature of accumulation in organisms, which may lead to multiorgan damage. The liver and cardiac organoids have been used to evaluate the toxic effects of lead, mercury, and thallium, and these heavy metals were found to inhibit the heart rates of heart organoids. 281 Through the mouse intestinal organoid model, it was found that excessive intake of cadmium could activate the Notch pathway, increase the synthesis of ROS, cause intestinal mucosal damage, and more susceptible to Salmonella infection. 282 In addition, the brain organoids treated with Cd exhibited increased neuron apoptosis, disrupted proliferation of neural progenitor cells, and change gene expression profiles, leading to neuroinflammation and ciliogenesis restriction. 283 However, Cd was not prone to hamper the neural differentiation of cerebral organoids. Organic pollutants with chemical toxicity are difficult to be degraded, and tend to accumulate in organisms in various ways, causing dysfunction and failure of human organs. 284 Evidence has pointed out that polybrominated diphenyl ethers are more likely to disrupt the differentiated NPCs in an hESC neural differentiation model and exert their cytotoxicity relying on the developmental stage. 285 Bisphenols are widely used to produce epoxy resins, and polycarbonate plastics, which have been shown to have endocrine disruptor effects and developmental toxicity in mammalian embryos. 286 , 287 Recently, the retinotoxicity of BPA, TBBPA, and TBBPS has been evaluated with human retinal organoids derived from ESCs. 288 It was reported that all three bisphenols could adversely affect retinal organoid development and that TBBPS had higher toxicity. Furthermore, bisphenols induced retinotoxicity in a time‐dependent manner. Meanwhile, exposure to BPS and BPF, the alternative chemicals of BPA, could increase the branches and alter the protein expression of mammary organoids, which differed from those induced by estrogen and might raise the risk of breast cancer occurrence. 289 Nanomaterials can be utilized as powerful and flexible tools for the diagnosis and treatment of human diseases as well as engineering and environmental protection fields. However, nanoparticles have been pointed to lay an adverse influence on the nervous, respiratory, circulatory, and reproductive systems and could increase the risk of malformations and cancer. 290 , 291 , 292 , 293 , 294 , 295 Organoid models open a new door for studying the toxicological effects of nanomaterials. Mekky et al. 296 offered confirmation of the feasibility of liver organoids for the hepatotoxic assessment of Mg nano. The work by Zuo's team developed kidney organoids as a toxicity screening platform to test black phosphorus quantum dots. 297 Overall, the use of organoids in precision medicine has the potential to revolutionize the way we develop and deliver medical treatments, by providing more accurate and personalized models of human tissues that can be used to predict individual patient responses to different drugs and environmental factors. Given the enormous potential of organoids in biomedical applications such as drug discovery, efficacy and safety testing, and clinical personalized medicine, several organoid‐based preclinical or clinical trials are already registered or underway (Table 3).
TABLE 3.
Summary of organoid‐based clinical trials registered in the ClinicalTrials.gov database.
| Application | Condition or disease | Trial number | Phase | Recruitment Status | Study type | Treatment |
|---|---|---|---|---|---|---|
| Drug screening /Personalized treatment | Cystic fibrosis | NCT04254705 | N/A | Unknown | CT | Tezacaftor + Ivacaftor |
| Advanced inoperable abdominal tumors | NCT05378048 | 2 | Not yet recruiting | RCT | Antitumor therapy | |
| Refractory solid tumors | NCT04279509 | N/A | Unknown | CT | Chemotherapy | |
| Cholangiocarcinoma | NCT05644743 | N/A | Not yet recruiting | PSO | Gemcitabine + cisplatin | |
| NCT05634694 | N/A | Recruiting | PSO | Chemotherapy | ||
| Breast cancer | NCT03544047 | N/A | Unknown | CT | Paclitaxel | |
| NCT03925233 | N/A | Unknown | RSO | Antitumor drugs | ||
| Colorectal cancer | NCT04996355 | N/A | Recruiting | PSO | Chemotherapy | |
| NCT03577808 | N/A | Unknown | PSO | Neoadjuvant chemoradiation | ||
| NCT05352165 | N/A | Not yet recruiting | RCT | Neoadjuvant therapy | ||
| NCT05304741 | N/A | Recruiting | PSO | Chemotherapy and targeted agents | ||
| NCT05725200 | N/A | Recruiting | CT | Antitumor drugs | ||
| NCT04906733 | N/A | Recruiting | PSO | Chemotherapy with/without cetuximab | ||
| Lung cancer | NCT03979170 | N/A | Recruiting | PSO | Chemotherapy | |
| NCT05669586 | 2 | Recruiting | RCT | Antitumor therapy | ||
| Ovarian cancer | NCT05175326 | N/A | Recruiting | PSO | Chemo‐ and targeted therapy | |
| NCT05290961 | N/A | Recruiting | PSO | Antitumor drugs | ||
| Recurrent high grade astrocytic glioma | NCT05532397 | Not yet recruiting | CT | Antitumor drugs | ||
| Esophageal cancer | NCT03283527 | N/A | Unknown | PSO | Chemoradiation | |
| Pancreatic cancer | NCT05196334 | N/A | Recruiting | PSO | Antitumor therapy | |
| NCT03544255 | N/A | Unknown | PSO | Antitumor therapy | ||
| NCT04931381 | N/A | Recruiting | RCT | Chemotherapy | ||
| Gastric cancer | NCT05351398 | N/A | Not yet recruiting | RCT | Neoadjuvant therapy | |
| Gastro‐intestinal cancer | NCT05652348 | N/A | Recruiting | PSO | Hyperthermic intraperitoneal chemotherapy | |
| Nonmuscle‐invasive bladder cancer | NCT05024734 | 2 | Recruiting | CT | Epirubicin, mitomycin, gemcitabine, docetaxel | |
| Prediction of treatment response | Colorectal cancer liver metastasis | NCT05183425 | N/A | Recruiting | – | Antitumor drugs |
| Pancreatic cancer | NCT04736043 | N/A | Recruiting | – | Antitumor drugs | |
| NCT04777604 | N/A | Not yet recruiting | – | Neoadjuvant treatment | ||
| Ovarian cancer | NCT04555473 | N/A | Recruiting | – | Primary debulking surgery (PDS) + adjuvant chemotherapy and neoadjuvant chemotherapy + interval debulking surgery | |
| Gastric cancer | NCT05203549 | N/A | Recruiting | – | Neoadjuvant therapy | |
| Breast cancer | NCT05007379 | N/A | Not yet recruiting | – | New CAR‐macrophages | |
| Advanced refractory cancers | NCT05267912 | N/A | Recruiting | – | Chemotherapy, hormonal therapy, targeted therapy | |
| Intestinal irradiation and inflammatory bowel disease | NCT05425901 | N/A | Not yet recruiting | – | Irradiation | |
| Nonsmall cell lung cancer | NCT05136014 | N/A | Enrolling by invitation | – | Tyrosine kinase inhibitors | |
| NCT04826913 | N/A | Not yet recruiting | – | Chemotherapy (cisplatin, carboplatin, pemetrexed), targeted therapies or immunotherapy and radiotherapy | ||
| NCT05332925 | N/A | Recruiting | – | Immunotherapy | ||
| Head and neck cancer | NCT05400239 | N/A | Not yet recruiting | – | Chemoradiotherapy | |
| Biobank | Triple‐negative breast cancer | NCT05404321 | N/A | Recruiting | – | – |
| Breast cancer | NCT05317221 | N/A | Recruiting | – | – | |
| Neuroendocrine neoplasm | NCT04927611 | N/A | Recruiting | – | – | |
| Colorectal cancer metastases and hepatocellular carcinomas | NCT05384184 | N/A | Recruiting | – | – | |
| Liver, biliary, and pancreatic cancer | NCT02436564 | N/A | Unknown | – | – | |
| Kidney cancer | NCT04342286 | N/A | Completed | – | – | |
| Intrauterine adhesion | NCT05521932 | N/A | Not yet recruiting | – | – | |
| Head and neck cancer | NCT04261192 | N/A | Recruiting | – | – | |
| Digestive system diseases (inflammatory bowel disease, ulcerative colitis type) | NCT05294107 | N/A | Recruiting | – | – | |
| Hematologic malignancy | NCT03890614 | N/A | Recruiting | – | – | |
| Glioma tumor | NCT04865315 | N/A | Active, not recruiting | – | – | |
| Lung cancer | NCT04859166 | N/A | Completed | – | – | |
| Cancers | NCT05734963 | N/A | Recruiting | – | – | |
| Vaginal HPV infection | NCT04278326 | N/A | Recruiting | – | – | |
| Pancreatic cancer | NCT05727020 | N/A | Recruiting | – | – | |
| Models for basic research | Host–microbe interaction | NCT05323357 | N/A | Recruiting | – | – |
| Simultaneous establishment of PDAC organoids and CAFs | NCT05571956 | N/A | Recruiting | – | – | |
| The role of the immunological microenvironment in chemoresistant colorectal cancer | NCT05038358 | N/A | Not yet recruiting | – | – | |
| Characterize the level of proliferation of high‐grade astrocytoma organoids | NCT03971812 | N/A | Unknown | – | – | |
| The biology of innervated sensory epithelial cells | NCT02888587 | N/A | Completed | – | – | |
| The mechanisms of aggressive tumor growth and treatment resistance in glioblastoma | NCT04868396 | N/A | Active, not recruiting | – | – | |
| Grafts of patient‐derived glioblastoma stem cells onto autologous brain organoids for testing drugs against tumor invasion | NCT05772741 | N/A | Recruiting | – | – | |
| Human‐specific mechanisms of preimplantation embryo development and early pregnancy | NCT05231551 | N/A | Recruiting | – | – | |
| Characterization of meningioma patient‐derived organoids | NCT04478877 | N/A | Recruiting | – | – | |
| Characterization of primary sclerosing cholangitis patient‐derived organoid | NCT04753996 | N/A | Recruiting | – | – | |
| Molecular characterization of metastatic prostate cancer | NCT05577689 | N/A | Not yet recruiting | – | – | |
| Comparison of basic properties of gut epithelia of hypertensive and normotensive reference subjects | NCT04497727 | N/A | Active, not recruiting | – | – | |
| Characterization of intestinal stem cells | NCT02874365 | N/A | Recruiting | – | – | |
| Mechanisms of food sensitivity | NCT03256266 | N/A | Recruiting | – | – | |
| NCT05259826 | N/A | Recruiting | – | – |
CAFs, cancer associated fibroblasts; CT, clinical trial; N/A, not available; PDAC, pancreatic ductal adenocarcinoma; PRO, prospective observational; RSO, retrospective observational.
Data source: ClinicalTrials.gov website (https://clinicaltrials.gov/ct2/home).
4.4. Regenerative medicine
Regenerative medicine refers to biological and engineering methods to repair, regenerate, or replace damaged or absent cells, tissues, or organs to restore normal structure and function. Organ transplantation is crucial for treating patients suffering from organ failure, but it faces various challenges, such as severe graft rejection and a growing shortage of donors. To overcome these obstacles, in vitro‐cultured organoids have emerged as a promising alternative, offering an unlimited supply of potential donors for autologous transplantation therapy and tissue repair. Several experiments have clarified that organoids transplanted into animals could restore impaired organ function. 298 , 299 , 300 , 301 Yang et al. 302 constructed bioprinted hepatorganoids using 3D printing technology and transplanted them into immunodeficient mice with tyrosinemia type I and liver failure. The organoids could form functional vasculature systems after transplantation. The serum levels of liver function biomarkers significantly declined, indicating alleviation of liver injury and an apparent therapeutic effect of hepatorganoids on tyrosine metabolism defects. Implantation of pancreatic islet‐like organoids generated by planting pancreatic islet cells into a bioscaffold has the ability to restore insulin secretion and decrease glucose levels in type 1 diabetic mice. 303 Human brain organoids could keep survival, form long lateral hypothalamus projections and functionally incorporate into the brain circuits after transplantation into the medial prefrontal cortex of mice. 304
Moreover, there are still some patients presenting with the dysfunction or defects of certain tissues or organs such as biliary atresia, bile tract injury, and short bowel syndrome due to congenital and acquired factors, for which effective therapeutic regimens are usually unavailable. The emergence and advancement of organoid technology provides powerful support for the generalization and application of tissue repair and regeneration. Sampaziotis et al. 305 , 306 pioneered the use of human bile duct epithelial organoids to repair the gallbladder and bile duct of mice. Cholangiocyte organoids were generated using normal human bile duct tissue. After being marked by a green fluorescent protein, these organoids were seeded on polyglycolic acid or densified collagen scaffolds to repair the gallbladder or bile duct of immunodeficient mice. The results showed that the mice transplanted with cholangiocyte organoids had extended survival, and their bile ducts were unobstructed, without manifestations of obstructive jaundice and increased bilirubin and alkaline phosphatase. More recently, a study injected human‐derived gallbladder organoids marked by red fluorescent protein into the intrahepatic ducts of isolated human livers. 307 The authors discovered red fluorescent cells in the injected bile ducts, without duct dilatation or obstruction, and the transplanted cholangiocytes expressed key biliary markers. The results implied that organoids can be applied to repair human bile ducts. Similar findings were described in a study by Roos et al. 308 Bile cholangiocyte organoids rapidly repopulate decellularized extrahepatic biliary duct scaffolds and restore the monolayer of cholangiocyte‐like cells in vitro. Jejunum organoids derived from 12 kids with intestinal failure were seeded into human intestinal scaffolds and able to differentiate into columnar epithelial cells with crypt units that fully covered the scaffolds, which could reappear several physiological jejunal functions. 309 Mouse transplantation experiments exhibited that these grafts survived and built a luminal structure. Similarly, Sato's team recently reported a small intestinalized colon (SIC) generated by transplanting small intestinal epithelium onto the colon surface. 310 The authors suggested that SIC could form the nascent villus and subepithelial lymphatic structures that are only observed in the small intestine. Furthermore, in SBS rats, xenotransplanted SIC could establish a complete vascular system and exert small intestine function, leading to improved survival and a low risk of intestinal failure.
5. LIMITATIONS AND FUTURE PERSPECTIVES
The past decade has witnessed dramatic progress in organoid technologies. Organoids have unique strengths in that they mimic nearly all physiological conditions and conserve parental genetic stability. They can be generated from a few cells or tissues for disease modeling and drug screening studies and can be used to reverse the disease‐causing mutation to treat disorders resulting from mutations. Furthermore, organoids have fast growth and a high culture success rate, which might solve the problem of the low tumor‐forming efficiency of patient‐derived tumor xenograft models. However, the technology is not yet mature, and many hurdles still exist that need to be overcome.
First, only a few laboratories are able to perform organoid culture, and experimental repeatability between different research groups is low. The reason is that the development of organoids requires the timely activation of morphogenetic signaling pathways to induce cell fate and promote the separation of different types of cells, thereby jointly completing the self‐organization process. Therefore, it is necessary to add a variety of growth factors or nutritional components to simulate the microenvironment of cell growth in vivo and use corresponding techniques to maintain the unique 3D spatial structure of the organoids. However, the high price of growth factors and medium additives restrict the popularization of organoid culture technology, and the types and doses of growth factors and nutrients used in organoid culture media also vary from laboratory to laboratory. The addition of a variety of growth factors might also cause gene mutations, resulting in a gap between the results of mechanism exploration or drug susceptibility testing and the real situation. The batch and quality of original cells and the changes in gene expression and transcriptomics in the culture and passage process also contributed to confounding effects. 311 Krefft et al. 312 proposed a protocol that enabled the generation of forebrain cerebral organoids with low heterogeneity by controlling the patterning factors of iPSCs. The matrix softening method was employed to specify the organoid geometry to control their patterning, achieving spatiotemporal control over organoids. 313 Future efforts should be devoted to developing inexpensive and high‐quality media and establishing procedures and quality control standards to increase the popularity of organoid technologies and the reproducibility of organoid generation.
Second, another factor hindering the organoid from being an excellent tissue biology model is its limited cellular development and low cell maturity (especially for PSC‐derived organoids). PSC‐derived organoids resemble fetal tissue rather than adult tissue. Two reasons are involved in this phenomenon: on the one hand, organoids are unable to develop to the maturation stage because of their short life span. As the organoid volume increases, the demand for organoids to acquire nutrients and oxygen and expel metabolites cannot be met, which results in cell death and tissue necrosis because of hypoxic metabolite accumulation. Consequently, current organoids cannot replace actual tissues and organs in terms of size and physiological function. The application of bioreactors may solve this problem, which can improve nutrient supply. 314 , 315 , 316 In addition, it can also extend the lifespan of organoids by promoting vascularization, enabling the transport of nutrients and metabolic wastes. Coculture of organoid cells with endothelial cells or their progenitors can promote vascularization during organoid formation. 3D‐vascularized liver organoids, which were cultured with organoid chip technology, significantly improved intercellular interactions and metabolic activity. 317 Besides, bioengineering methods, including sacrificial molds, 318 , 319 laser ablation, 320 , 321 and bioprinting, 322 , 323 can also produce vessel‐like structures. On the other hand, the cells used to generate organoids that are mainly epithelium‐originated cells, including normal or tumor cells. The microenvironment in which cells in the body are located comprises interstitial cells, immune cells, and matrix components. The current organoid field fails to reflect the complexity and cell–cell interactions of the native tissue. Unique in vivo environmental factors that encourage organoid maturation may be absent from in vitro conditions. Organoids tend to develop a more mature phenotype when implanted in vivo, indicating that exposure to the in vivo microenvironment will increase the development and maturity of organoids. When constructing hepatobiliary organoids, it is better to coculture them with mesenchymal cells. Because they can better promote organoid maturation, 196 and can provide certain paracrine signals to induce cell differentiation and the formation of the 3D spatial structure of the corresponding organ. 324
Finally, the ECM, which plays a role in supporting the 3D structure and providing ECM signals, impacts the development of organoids. The most commonly used is Matrigel, an ECM protein mixture secreted by mouse Englebreth‐Holm‐Swarm sarcoma cells, which contains many growth factors, such as insulin‐like growth factor 1 and EGF. However, different batches or the same batch of Matrigel might have inconsistent biochemical properties. As a nonhuman‐derived material, Matrigel might introduce viral or xenogenic contaminants to induce immune responses, which can interfere with organoid behavior and constrain the capacity to induce organoid morphogenesis. 325 Matrigel leads to slower penetration of exogenous substances, which is not conducive to transfection and drug screening. Besides, Matrigel cannot yet fully mimic the true tumor stroma because tumor cells have features of anchorage‐independent growth. The above shortcomings may restrict its clinical applications. Therefore, significant efforts have been devoted to developing artificially synthesized ECM or hydrogels for organoid culture, which are chemically defined with clear components, and their chemical and physical features can be adjusted on the basis of different requirements. 326 , 327 , 328 , 329 , 330 , 331 The advantages of these novel hydrogels have been demonstrated. A nanofibrillar hydrogel proposed by Prince et al. 332 could support the initiation and growth of breast tumoroids and protect the PDOs from murine contamination. Decellularized ECM hydrogels derived from various species have been employed in 3D culture, because they can promote proliferation and differentiation and enhance the attachment ability of organoid cells. 333 , 334 , 335 , 336 Liu's group recently developed a hydrogel based on the decellularized uvea with excellent cytocompatibility with iPSC‐derived retinal organoids. Animal experiment results indicated that after transplantation, retinal organoids cultured in this hydrogel showed an improved survival rate and were able to restore the vision of retinal degeneration in rats. 337
Organoids provide unprecedented opportunities to study the development, physiology, and diseases of humans, comprising novel preclinical models with promising application prospects. Organoid models conserve the histological and gene expression properties of native tissue in vivo, and their high similarity to native tissue makes them a valuable addition to cell lines and animal models, which have a high clinical and scientific value. However, the organoid technique is still in the initial stage and has some shortcomings. Researchers should focus on combining this technique with other advanced technologies and models to further improve the accuracy of research in the future.
AUTHOR CONTRIBUTION
Siqi Yang and Haijie Hu contributed equally to the manuscript and were the first coauthors. Siqi Yang and Haijie Hu contributed to data acquisition and drafted the manuscript. Yafei Hu, Yushi Dai, Ruiqi Zou, Tiantian Wang, Tianrun Lv and Hengchung Kung contributed to data acquisition. Fuyu Li, Jun Yu, and Haijie Hu contributed to the study design and revision of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21046); 1.3.5 project for disciplines of excellence‐Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001); Natural Science Foundation of Sichuan Province (2022NSFSC0806); National Natural Science Foundation of China for Young Scientists Fund (82203782), Sichuan Science and Technology Program (2021YJ0132, 2021YFS0100); The fellowship of China Postdoctoral Science Foundation (2021M692277); Sichuan University‐Zigong School‐local Cooperation project (2021CDZG‐23); Science and Technology project of the Health planning committee of Sichuan (21PJ046); Post‐Doctor Research Project, West China Hospital, Sichuan University (2021HXBH127).
Yang S, Hu H, Kung H, et al. Organoids: The current status and biomedical applications. MedComm. 2023;4:e274. 10.1002/mco2.274
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Corrò C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol. 2020;319(1):C151‐C165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331‐343. [DOI] [PubMed] [Google Scholar]
- 3. Yu J, Vodyanik MA, Smuga‐Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917‐1920. [DOI] [PubMed] [Google Scholar]
- 4. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262‐265. [DOI] [PubMed] [Google Scholar]
- 5. Altinisik N, Rathinam D, Tran M, Gopalakrishnan J. Brain organoids restore cortical damage. Cell Stem Cell. 2023;30(3):241‐242. [DOI] [PubMed] [Google Scholar]
- 6. Jgamadze D, Lim JT, Zhang Z, et al. Structural and functional integration of human forebrain organoids with the injured adult rat visual system. Cell Stem Cell. 2023;30(2):137‐152.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finkbeiner C, Ortuño‐Lizarán I, Sridhar A, Hooper M, Petter S, Reh TA. Single‐cell ATAC‐seq of fetal human retina and stem‐cell‐derived retinal organoids shows changing chromatin landscapes during cell fate acquisition. Cell Rep. 2022;38(4):110294. [DOI] [PubMed] [Google Scholar]
- 8. Saha A, Capowski E, Fernandez Zepeda MA, Nelson EC, Gamm DM, Sinha R. Cone photoreceptors in human stem cell‐derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell. 2022;29(3):460‐471.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokota E, Iwai M, Yukawa T, et al. Clinical application of a lung cancer organoid (tumoroid) culture system. NPJ Precis Oncol. 2021;5(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han Y, Duan X, Yang L, et al. Identification of SARS‐CoV‐2 inhibitors using lung and colonic organoids. Nature. 2021;589(7841):270‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song H, Park JY, Kim JH, et al. Establishment of patient‐derived gastric cancer organoid model from tissue obtained by endoscopic biopsies. J Korean Med Sci. 2022;37(28):e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eicher AK, Kechele DO, Sundaram N, et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell. 2022;29(1):36‐51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hendriks D, Artegiani B, Hu H, Chuva de Sousa Lopes S, Clevers H. Establishment of human fetal hepatocyte organoids and CRISPR‐Cas9‐based gene knockin and knockout in organoid cultures from human liver. Nat Protoc. 2021;16(1):182‐217. [DOI] [PubMed] [Google Scholar]
- 14. Shinozawa T, Kimura M, Cai Y, et al. High‐fidelity drug‐induced liver injury screen using human pluripotent stem cell‐derived organoids. Gastroenterology. 2021;160(3):831‐846.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roos FJM, van Tienderen GS, Wu H, et al. Human branching cholangiocyte organoids recapitulate functional bile duct formation. Cell Stem Cell. 2022;29(5):776‐794.e13. [DOI] [PubMed] [Google Scholar]
- 16. Hallett JM, Ferreira‐Gonzalez S, Man TY, et al. Human biliary epithelial cells from discarded donor livers rescue bile duct structure and function in a mouse model of biliary disease. Cell Stem Cell. 2022;29(3):355‐371.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Randriamanantsoa S, Papargyriou A, Maurer HC, et al. Spatiotemporal dynamics of self‐organized branching in pancreas‐derived organoids. Nat Commun. 2022;13(1):5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang L, Desai R, Conrad DN, et al. Commitment and oncogene‐induced plasticity of human stem cell‐derived pancreatic acinar and ductal organoids. Cell Stem Cell. 2021;28(6):1090‐1104.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tran T, Song CJ, Nguyen T, et al. A scalable organoid model of human autosomal dominant polycystic kidney disease for disease mechanism and drug discovery. Cell Stem Cell. 2022;29(7):1083‐1101.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta N, Matsumoto T, Hiratsuka K, et al. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci Transl Med. 2022;14(634):eabj4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015‐3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saraswathibhatla A, Indana D, Chaudhuri O. Cell‐extracellular matrix mechanotransduction in 3D. Nat Rev Mol Cell Biol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scalise M, Marino F, Salerno L, et al. From spheroids to organoids: the next generation of model systems of human cardiac regeneration in a dish. Int J Mol Sci. 2021;22(24):13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bresnahan E, Ramadori P, Heikenwalder M, Zender L, Lujambio A. Novel patient‐derived preclinical models of liver cancer. J Hepatol. 2020;72(2):239‐249. [DOI] [PubMed] [Google Scholar]
- 25. Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol. 2019;12(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;18(7):407‐418. [DOI] [PubMed] [Google Scholar]
- 27. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. [DOI] [PubMed] [Google Scholar]
- 28. Brevini T, Tysoe OC, Sampaziotis F. Tissue engineering of the biliary tract and modelling of cholestatic disorders. J Hepatol. 2020;73(4):918‐932. [DOI] [PubMed] [Google Scholar]
- 29. Perez‐Lanzon M, Kroemer G, Maiuri MC. Organoids for modeling genetic diseases. Int Rev Cell Mol Biol. 2018;337:49‐81. [DOI] [PubMed] [Google Scholar]
- 30. Broutier L, Mastrogiovanni G, Verstegen MM, et al. Human primary liver cancer‐derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23(12):1424‐1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Guo Y, Jin Y, et al. Establishment and drug screening of patient‐derived extrahepatic biliary tract carcinoma organoids. Cancer Cell Int. 2021;21(1):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan B, Zhao X, Wang X, et al. Patient‐derived organoids for personalized gallbladder cancer modelling and drug screening. Clin Transl Med. 2022;12(1):e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saito Y, Muramatsu T, Kanai Y, et al. Establishment of patient‐derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27(4):1265‐1276.e4. [DOI] [PubMed] [Google Scholar]
- 34. Bahmad HF, Elajami MK, Daouk R, et al. Stem cells: in sickness and in health. Curr Stem Cell Res Ther. 2021;16(3):262‐276. [DOI] [PubMed] [Google Scholar]
- 35. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self‐renewal in the stomach and build long‐lived gastric units in vitro. Cell Stem Cell. 2010;6(1):25‐36. [DOI] [PubMed] [Google Scholar]
- 37. Driehuis E, Kretzschmar K, Clevers H. Establishment of patient‐derived cancer organoids for drug‐screening applications. Nat Protoc. 2020;15(10):3380‐3409. [DOI] [PubMed] [Google Scholar]
- 38. Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175(7):1972‐1988.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014;14(1):13‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cherry AB, Daley GQ. Reprogramming cellular identity for regenerative medicine. Cell. 2012;148(6):1110‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145‐1147. [DOI] [PubMed] [Google Scholar]
- 42. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663‐676. [DOI] [PubMed] [Google Scholar]
- 43. McCracken KW, Aihara E, Martin B, et al. Wnt/β‐catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541(7636):182‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brassard JA, Lutolf MP. Engineering stem cell self‐organization to build better organoids. Cell Stem Cell. 2019;24(6):860‐876. [DOI] [PubMed] [Google Scholar]
- 45. Kim H, Park HJ, Choi H, et al. Modeling G2019S‐LRRK2 sporadic Parkinson's disease in 3D midbrain organoids. Stem Cell Reports. 2019;12(3):518‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen X, Sun G, Tian E, et al. Modeling sporadic Alzheimer's disease in human brain organoids under serum exposure. Adv Sci (Weinh). 2021;8(18):2101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wimmer RA, Leopoldi A, Aichinger M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019;565(7740):505‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Breunig M, Merkle J, Wagner M, et al. Modeling plasticity and dysplasia of pancreatic ductal organoids derived from human pluripotent stem cells. Cell Stem Cell. 2021;28(6):1105‐1124.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan Y, Enejder A, Wang M, et al. A human multi‐lineage hepatic organoid model for liver fibrosis. Nat Commun. 2021;12(1):6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCracken KW, Catá EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem‐cell‐derived gastric organoids. Nature. 2014;516(7531):400‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eiraku M, Takata N, Ishibashi H, et al. Self‐organizing optic‐cup morphogenesis in three‐dimensional culture. Nature. 2011;472(7341):51‐56. [DOI] [PubMed] [Google Scholar]
- 52. McCauley HA, Wells JM. Pluripotent stem cell‐derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144(6):958‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586‐1597. [DOI] [PubMed] [Google Scholar]
- 54. Liang J, Li X, Dong Y, Zhao B. Modeling human organ development and diseases with fetal tissue‐derived organoids. Cell Transplant. 2022;31:096368972211244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fordham RP, Yui S, Hannan NR, et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13(6):734‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schutgens F, Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol. 2020;15(1):211‐234. [DOI] [PubMed] [Google Scholar]
- 57. Günther C, Winner B, Neurath MF, Stappenbeck TS. Organoids in gastrointestinal diseases: from experimental models to clinical translation. Gut. 2022;71(9):1892‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiao H, Liang J, Liu S, et al. Proteomics and organoid culture reveal the underlying pathogenesis of hashimoto's Thyroiditis. Front Immunol. 2021;12:784975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soroka CJ, Assis DN, Alrabadi LS, et al. Bile‐derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology. 2019;70(3):871‐882. [DOI] [PubMed] [Google Scholar]
- 60. van der Vaart J, Bosmans L, Sijbesma SF, et al. Adult mouse and human organoids derived from thyroid follicular cells and modeling of Graves' hyperthyroidism. Proc Natl Acad Sci USA. 2021;118(51):e2117017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sato T, Stange DE, Ferrante M, et al. Long‐term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141(5):1762‐1772. [DOI] [PubMed] [Google Scholar]
- 62. Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1‐2):373‐386.e10. [DOI] [PubMed] [Google Scholar]
- 63. Lee SH, Hu W, Matulay JT, et al. Tumor evolution and drug response in patient‐derived organoid models of bladder cancer. Cell. 2018;173(2):515‐528.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krieger TG, Tirier SM, Park J, et al. Modeling glioblastoma invasion using human brain organoids and single‐cell transcriptomics. Neuro Oncol. 2020;22(8):1138‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ballabio C, Anderle M, Gianesello M, et al. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat Commun. 2020;11(1):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Golebiewska A, Hau AC, Oudin A, et al. Patient‐derived organoids and orthotopic xenografts of primary and recurrent gliomas represent relevant patient avatars for precision oncology. Acta Neuropathol. 2020;140(6):919‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Norrie JL, Nityanandam A, Lai K, et al. Retinoblastoma from human stem cell‐derived retinal organoids. Nat Commun. 2021;12(1):4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oridate KTASOSUYASKHTANT . Establishment of PDX‐derived salivary adenoid cystic carcinoma cell lines using organoid culture method. Int J Cancer. 2021;148(1):193‐202. [DOI] [PubMed] [Google Scholar]
- 69. Dijkstra KK, van den Berg JG, Weeber F, et al. Patient‐derived organoid models of human neuroendocrine carcinoma. Front Endocrinol (Lausanne). 2021;12:627819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kawasaki K, Toshimitsu K, Matano M, et al. An organoid biobank of neuroendocrine neoplasms enables genotype‐phenotype mapping. Cell. 2020;183(5):1420‐1435.e21. [DOI] [PubMed] [Google Scholar]
- 71. Kim SY, Kim SM, Lim S, et al. Modeling clinical responses to targeted therapies by patient‐derived organoids of advanced lung adenocarcinoma. Clin Cancer Res. 2021;27(15):4397‐4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li Z, Qian Y, Li W, et al. Human lung adenocarcinoma‐derived organoid models for drug screening. iScience. 2020;23(8):101411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159(1):176‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kopper O, de Witte CJ, Lõhmussaar K, et al. An organoid platform for ovarian cancer captures intra‐ and interpatient heterogeneity. Nat Med. 2019;25(5):838‐849. [DOI] [PubMed] [Google Scholar]
- 75. Mazzocchi A, Dominijanni A, Soker S. Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. Methods Mol Biol. 2022;2394:471‐483. [DOI] [PubMed] [Google Scholar]
- 76. Lõhmussaar K, Oka R, Espejo Valle‐Inclan J, et al. Patient‐derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell. 2021;28(8):1380‐1396.e6. [DOI] [PubMed] [Google Scholar]
- 77. De Angelis ML, Francescangeli F, Nicolazzo C, et al. An organoid model of colorectal circulating tumor cells with stem cell features, hybrid EMT state and distinctive therapy response profile. J Exp Clin Cancer Res. 2022;41(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nguyen HTL, Soragni A. Patient‐derived tumor organoid rings for histologic characterization and high‐throughput screening. STAR Protoc. 2020;1(2):100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Paşca SP. Assembling human brain organoids. Science. 2019;363(6423):126‐127. [DOI] [PubMed] [Google Scholar]
- 80. Ober EA, Lemaigre FP. Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 2018;68(5):1049‐1062. [DOI] [PubMed] [Google Scholar]
- 81. Sharma A, Sances S, Workman MJ, Svendsen CN. Multi‐lineage human iPSC‐derived platforms for disease modeling and drug discovery. Cell Stem Cell. 2020;26(3):309‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pașca SP, Arlotta P, Bateup HS, et al. A nomenclature consensus for nervous system organoids and assembloids. Nature. 2022;609(7929):907‐910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiang Y, Tanaka Y, Cakir B, et al. hESC‐derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 2019;24(3):487‐497.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bagley JA, Reumann D, Bian S, Lévi‐Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14(7):743‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miura Y, Li MY, Birey F, et al. Generation of human striatal organoids and cortico‐striatal assembloids from human pluripotent stem cells. Nat Biotechnol. 2020;38(12):1421‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Andersen J, Revah O, Miura Y, et al. Generation of functional human 3D cortico‐motor assembloids. Cell. 2020;183(7):1913‐1929.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep. 2018;23(4):1220‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Faustino Martins JM, Fischer C, Urzi A, et al. Self‐organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 2020;26(2):172‐186.e6. [DOI] [PubMed] [Google Scholar]
- 89. Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent‐stem‐cell‐derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC‐derived organ bud transplant. Nature. 2013;499(7459):481‐484. [DOI] [PubMed] [Google Scholar]
- 91. Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell‐driven condensation. Cell Stem Cell. 2015;16(5):556‐565. [DOI] [PubMed] [Google Scholar]
- 92. Takebe T, Sekine K, Suzuki Y, et al. Self‐organization of human hepatic organoid by recapitulating organogenesis in vitro. Transplant Proc. 2012;44(4):1018‐1020. [DOI] [PubMed] [Google Scholar]
- 93. Bergmann S, Lawler SE, Qu Y, et al. Blood‐brain‐barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc. 2018;13(12):2827‐2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Koike H, Iwasawa K, Ouchi R, et al. Modelling human hepato‐biliary‐pancreatic organogenesis from the foregut‐midgut boundary. Nature. 2019;574(7776):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ng WH, Johnston EK, Tan JJ, et al. Recapitulating human cardio‐pulmonary co‐development using simultaneous multilineage differentiation of pluripotent stem cells. Elife. 2022;11:e67872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ramli MNB, Lim YS, Koe CT, et al. Human pluripotent stem cell‐derived organoids as models of liver disease. Gastroenterology. 2020;159(4):1471‐1486.e12. [DOI] [PubMed] [Google Scholar]
- 97. Kimura M, Iguchi T, Iwasawa K, et al. En masse organoid phenotyping informs metabolic‐associated genetic susceptibility to NASH. Cell. 2022;185(22):4216‐4232.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tanimizu N, Ichinohe N, Sasaki Y, et al. Generation of functional liver organoids on combining hepatocytes and cholangiocytes with hepatobiliary connections ex vivo. Nat Commun. 2021;12(1):3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Meran L, Tullie L, Eaton S, De Coppi P, Li VSW. Bioengineering human intestinal mucosal grafts using patient‐derived organoids, fibroblasts and scaffolds. Nat Protoc. 2023;18(1):108‐135. [DOI] [PubMed] [Google Scholar]
- 100. Wang Q, Guo F, Jin Y, Ma Y. Applications of human organoids in the personalized treatment for digestive diseases. Signal Transduct Target Ther. 2022;7(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt‐dependent stem cell niche. Nat Med. 2009;15(6):701‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lamers MM, van der Vaart J, Knoops K, et al. An organoid‐derived bronchioalveolar model for SARS‐CoV‐2 infection of human alveolar type II‐like cells. Embo J. 2021;40(5):e105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wakamatsu T, Ogawa H, Yoshida K, et al. Establishment of organoids from human epithelioid sarcoma with the air‐liquid interface organoid cultures. Front Oncol. 2022;12:893592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hong HK, Yun NH, Jeong YL, et al. Establishment of patient‐derived organotypic tumor spheroid models for tumor microenvironment modeling. Cancer Med. 2021;10(16):5589‐5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Xue Y, Seiler MJ, Tang WC, et al. Retinal organoids on‐a‐chip: a micro‐millifluidic bioreactor for long‐term organoid maintenance. Lab Chip. 2021;21(17):3361‐3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Huang D, Liu T, Liao J, et al. Reversed‐engineered human alveolar lung‐on‐a‐chip model. Proc Natl Acad Sci USA. 2021;118(19):e2016146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bircsak KM, DeBiasio R, Miedel M, et al. A 3D microfluidic liver model for high throughput compound toxicity screening in the OrganoPlate®. Toxicology. 2021;450:152667. [DOI] [PubMed] [Google Scholar]
- 108. Xie X, Maharjan S, Kelly C, et al. Customizable microfluidic origami liver‐on‐a‐chip (oLOC). Adv Mater Technol. 2022;7(5):2100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saorin G, Caligiuri I, Rizzolio F. Microfluidic organoids‐on‐a‐chip: the future of human models. Semin Cell Dev Biol. 2022;144:41‐54. [DOI] [PubMed] [Google Scholar]
- 110. Cho AN, Jin Y, An Y, et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun. 2021;12(1):4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Khan I, Prabhakar A, Delepine C, Tsang H, Pham V, Sur M. A low‐cost 3D printed microfluidic bioreactor and imaging chamber for live‐organoid imaging. Biomicrofluidics. 2021;15(2):024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang F, Qu KY, Zhou B, et al. Design and fabrication of an integrated heart‐on‐a‐chip platform for construction of cardiac tissue from human iPSC‐derived cardiomyocytes and in situ evaluation of physiological function. Biosens Bioelectron. 2021;179:113080. [DOI] [PubMed] [Google Scholar]
- 113. Abulaiti M, Yalikun Y, Murata K, et al. Establishment of a heart‐on‐a‐chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci Rep. 2020;10(1):19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shin YC, Shin W, Koh D, et al. Three‐dimensional regeneration of patient‐derived intestinal organoid epithelium in a physiodynamic mucosal interface‐on‐a‐chip. Micromachines (Basel). 2020;11(7):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Teng Y, Zhao Z, Tasnim F, Huang X, Yu H. A scalable and sensitive steatosis chip with long‐term perfusion of in situ differentiated HepaRG organoids. Biomaterials. 2021;275:120904. [DOI] [PubMed] [Google Scholar]
- 116. Wiedenmann S, Breunig M, Merkle J, et al. Single‐cell‐resolved differentiation of human induced pluripotent stem cells into pancreatic duct‐like organoids on a microwell chip. Nat Biomed Eng. 2021;5(8):897‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shin W, Kim HJ. 3D in vitro morphogenesis of human intestinal epithelium in a gut‐on‐a‐chip or a hybrid chip with a cell culture insert. Nat Protoc. 2022;17(3):910‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging‐drop method. Methods Mol Med. 2007;140:141‐151. [DOI] [PubMed] [Google Scholar]
- 119. Romero‐Morales AI, O'Grady BJ, Balotin KM, Bellan LM, Lippmann ES, Gama V. Spin∞: an updated miniaturized spinning bioreactor design for the generation of human cerebral organoids from pluripotent stem cells. HardwareX. 2019;6:e00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region‐specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 2018;13(3):565‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jacob F, Salinas RD, Zhang DY, et al. A patient‐derived glioblastoma organoid model and biobank recapitulates inter‐ and intra‐tumoral heterogeneity. Cell. 2020;180(1):188‐204.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gómez‐Mariano G, Matamala N, Martínez S, et al. Liver organoids reproduce alpha‐1 antitrypsin deficiency‐related liver disease. Hepatol Int. 2020;14(1):127‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huch M, Gehart H, van Boxtel R, et al. Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell. 2015;160(1‐2):299‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Guan Y, Xu D, Garfin PM, et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2017;2(17):e94954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nantasanti S, Spee B, Kruitwagen HS, et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. 2015;5(5):895‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kruitwagen HS, Oosterhoff LA, van Wolferen ME, et al. Long‐term survival of transplanted autologous canine liver organoids in a COMMD1‐deficient dog model of metabolic liver disease. Cells. 2020;9(2):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ouchi R, Togo S, Kimura M, et al. Modeling steatohepatitis in humans with pluripotent stem cell‐derived organoids. Cell Metab. 2019;30(2):374‐384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Maxwell KG, Augsornworawat P, Velazco‐Cruz L, et al. Gene‐edited human stem cell‐derived β cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci Transl Med. 2020;12(540):eaax9106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Guo D, Liu H, Ruzi A, et al. Modeling congenital hyperinsulinism with ABCC8‐deficient human embryonic stem cells generated by CRISPR/Cas9. Sci Rep. 2017;7(1):3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dickinson KM, Collaco JM. Cystic fibrosis. Pediatr Rev. 2021;42(2):55‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. de Melo ACV, de Souza KSC, da Silva HPV, et al. Screening by high‐throughput sequencing for pathogenic variants in cystic fibrosis: benefit of introducing personalized therapies. J Cell Mol Med. 2022;26(23):5943‐5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sachs N, Papaspyropoulos A, Zomer‐van Ommen DD, et al. Long‐term expanding human airway organoids for disease modeling. Embo J. 2019;38(4):e100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sette G, Lo Cicero S, Blaconà G, et al. Theratyping cystic fibrosis in vitro in ALI culture and organoid models generated from patient‐derived nasal epithelial conditionally reprogrammed stem cells. Eur Respir J. 2021;58(6):2100908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ramalho AS, Fürstová E, Vonk AM, et al. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. Eur Respir J. 2021;57(1):1902426. [DOI] [PubMed] [Google Scholar]
- 135. Verstegen MMA, Roos FJM, Burka K, et al. Human extrahepatic and intrahepatic cholangiocyte organoids show region‐specific differentiation potential and model cystic fibrosis‐related bile duct disease. Sci Rep. 2020;10(1):21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shik Mun K, Arora K, Huang Y, et al. Patient‐derived pancreas‐on‐a‐chip to model cystic fibrosis‐related disorders. Nat Commun. 2019;10(1):3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Spelier S, de Poel E, Ithakisiou GN, et al. High‐throughput functional assay in cystic fibrosis patient‐derived organoids allows drug repurposing. ERJ Open Res. 2023;9(1):00495‐02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Li SR, Gulieva RE, Helms L, et al. Glucose absorption drives cystogenesis in a human organoid‐on‐chip model of polycystic kidney disease. Nat Commun. 2022;13(1):7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim JW, Kim HW, Nam SA, et al. Human kidney organoids reveal the role of glutathione in Fabry disease. Exp Mol Med. 2021;53(10):1580‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hofbauer P, Jahnel SM, Papai N, et al. Cardioids reveal self‐organizing principles of human cardiogenesis. Cell. 2021;184(12):3299‐3317.e22.e22. [DOI] [PubMed] [Google Scholar]
- 141. Lewis‐Israeli YR, Wasserman AH, Gabalski MA, et al. Self‐assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12(1):5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang X, Zhang D, Thompson JA, et al. Gene correction of the CLN3 c.175G>A variant in patient‐derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol Genet Genomic Med. 2021;9(3):e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Diakatou M, Dubois G, Erkilic N, Sanjurjo‐Soriano C, Meunier I, Kalatzis V. Allele‐specific knockout by CRISPR/Cas to treat autosomal dominant retinitis pigmentosa caused by the G56R mutation in NR2E3. Int J Mol Sci. 2021;22(5):2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kruczek K, Qu Z, Gentry J, et al. Gene therapy of dominant CRX‐Leber congenital amaurosis using patient stem cell‐derived retinal organoids. Stem Cell Rep. 2021;16(2):252‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lukovic D, Artero Castro A, Kaya KD, et al. Retinal organoids derived from hiPSCs of an AIPL1‐LCA patient maintain cytoarchitecture despite reduced levels of mutant AIPL1. Sci Rep. 2020;10(1):5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bhattacharya A, Choi WWY, Muffat J, Li Y. Modeling developmental brain diseases using human pluripotent stem cells‐derived brain organoids ‐ progress and perspective. J Mol Biol. 2022;434(3):167386. [DOI] [PubMed] [Google Scholar]
- 147. Lancaster MA, Renner M, Martin CA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Dhaliwal N, Choi WWY, Muffat J, Li Y. Modeling PTEN overexpression‐induced microcephaly in human brain organoids. Mol Brain. 2021;14(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Esk C, Lindenhofer D, Haendeler S, et al. A human tissue screen identifies a regulator of ER secretion as a brain‐size determinant. Science. 2020;370(6519):935‐941. [DOI] [PubMed] [Google Scholar]
- 150. Samarasinghe RA, Miranda OA, Buth JE, et al. Identification of neural oscillations and epileptiform changes in human brain organoids. Nat Neurosci. 2021;24(10):1488‐1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dooves S, van Velthoven AJH, Suciati LG, Heine VM. Neuron‐glia interactions in tuberous sclerosis complex affect the synaptic balance in 2D and organoid cultures. Cells. 2021;10(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Eichmüller OL, Corsini NS, Vértesy Á, et al. Amplification of human interneuron progenitors promotes brain tumors and neurological defects. Science. 2022;375(6579):eabf5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Raj N, McEachin ZT, Harousseau W, et al. Cell‐type‐specific profiling of human cellular models of fragile X syndrome reveal PI3K‐dependent defects in translation and neurogenesis. Cell Rep. 2021;35(2):108991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tang XY, Xu L, Wang J, et al. DSCAM/PAK1 pathway suppression reverses neurogenesis deficits in iPSC‐derived cerebral organoids from patients with Down syndrome. J Clin Invest. 2021;131(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sun AX, Yuan Q, Fukuda M, et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science. 2019;366(6472):1486‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Krenn V, Bosone C, Burkard TR, et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus‐specific responses leading to microcephaly. Cell Stem Cell. 2021;28(8):1362‐1379.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Esper NB, Franco AR, Soder RB, et al. Zika virus congenital microcephaly severity classification and the association of severity with neuropsychomotor development. Pediatr Radiol. 2022;52(5):941‐950. [DOI] [PubMed] [Google Scholar]
- 158. Xu M, Lee EM, Wen Z, et al. Identification of small‐molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22(10):1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ettayebi K, Crawford SE, Murakami K, et al. Replication of human noroviruses in stem cell‐derived human enteroids. Science. 2016;353(6306):1387‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ettayebi K, Tenge VR, Cortes‐Penfield NW, et al. New insights and enhanced human norovirus cultivation in human intestinal enteroids. mSphere. 2021;6(1):e01136‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ekanger CT, Zhou F, Bohan D, et al. Human organotypic airway and lung organoid cells of bronchiolar and alveolar differentiation are permissive to infection by influenza and SARS‐CoV‐2 respiratory virus. Front Cell Infect Microbiol. 2022;12:841447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen S, Li P, Wang Y, et al. Rotavirus infection and cytopathogenesis in human biliary organoids potentially recapitulate biliary atresia development. mBio. 2020;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. O'Brien BS, Mokry RL, Schumacher ML, et al. Downregulation of neurodevelopmental gene expression in iPSC‐derived cerebral organoids upon infection by human cytomegalovirus. iScience. 2022;25(4):104098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Odenwald MA, Paul S. Viral hepatitis: past, present, and future. World J Gastroenterol. 2022;28(14):1405‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Shiha G, Soliman R, Serwah A, Mikhail NNH, Asselah T, Easterbrook P. A same day ‘test and treat’ model for chronic HCV and HBV infection: results from two community‐based pilot studies in Egypt. J Viral Hepat. 2020;27(6):593‐601. [DOI] [PubMed] [Google Scholar]
- 166. Zhang X, Guan L, Tian H, et al. Risk factors and prevention of viral hepatitis‐related hepatocellular carcinoma. Front Oncol. 2021;11:686962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cao D, Ge JY, Wang Y, Oda T, Zheng YW. Hepatitis B virus infection modeling using multi‐cellular organoids derived from human induced pluripotent stem cells. World J Gastroenterol. 2021;27(29):4784‐4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hossain T, Romal S, Mahmoudi T. Production of recombinant hepatitis B virus (HBV) and detection of HBV in infected human liver organoids. Bio Protoc. 2022;12(8):e4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Romal S, Hossain T, Mahmoudi T. Generation, maintenance and HBV infection of human liver organoids. Bio Protoc. 2022;12(6):e4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Natarajan V, Simoneau CR, Erickson AL, et al. Modelling T‐cell immunity against hepatitis C virus with liver organoids in a microfluidic coculture system. Open Biol. 2022;12(3):210320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Li P, Li Y, Wang Y, et al. Recapitulating hepatitis E virus‐host interactions and facilitating antiviral drug discovery in human liver‐derived organoids. Sci Adv. 2022;8(3):eabj5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. De Crignis E, Hossain T, Romal S, et al. Application of human liver organoids as a patient‐derived primary model for HBV infection and related hepatocellular carcinoma. Elife. 2021;10:e60747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Ochani R, Asad A, Yasmin F, et al. COVID‐19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29(1):20‐36. [PubMed] [Google Scholar]
- 174. Tiwari SK, Wang S, Smith D, Carlin AF, Rana TM. Revealing tissue‐specific SARS‐CoV‐2 infection and host responses using human stem cell‐derived lung and cerebral organoids. Stem Cell Rep. 2021;16(3):437‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Duan X, Tang X, Nair MS, et al. An airway organoid‐based screen identifies a role for the HIF1α‐glycolysis axis in SARS‐CoV‐2 infection. Cell Rep. 2021;37(6):109920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wysocki J, Ye M, Hassler L, et al. A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS‐CoV‐2 infection in human kidney organoids. J Am Soc Nephrol. 2021;32(4):795‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Krüger J, Groß R, Conzelmann C, et al. Drug inhibition of SARS‐CoV‐2 replication in human pluripotent stem cell‐derived intestinal organoids. Cell Mol Gastroenterol Hepatol. 2021;11(4):935‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McCarron S, Bathon B, Conlon DM, et al. Functional characterization of organoids derived from irreversibly damaged liver of patients With NASH. Hepatology. 2021;74(4):1825‐1844. [DOI] [PubMed] [Google Scholar]
- 179. Jansen J, Reimer KC, Nagai JS, et al. SARS‐CoV‐2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29(2):217‐231.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Heo I, Dutta D, Schaefer DA, et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol. 2018;3(7):814‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Adams Y, Jensen AR. 3D organoid assay of the impact of infected erythrocyte adhesion on the blood‐brain barrier. Methods Mol Biol. 2022;2470:587‐599. [DOI] [PubMed] [Google Scholar]
- 182. Adams Y, Clausen AS, Jensen P, et al. 3D blood‐brain barrier‐organoids as a model for Lyme neuroborreliosis highlighting genospecies dependent organotropism. iScience. 2023;26(1):105838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Bartfeld S, Bayram T, van de Wetering M, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148(1):126‐136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Aguilar C, Pauzuolis M, Pompaiah M, et al. Helicobacter pylori shows tropism to gastric differentiated pit cells dependent on urea chemotaxis. Nat Commun. 2022;13(1):5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Cao L, Zhu S, Lu H, et al. Helicobacter pylori‐induced RASAL2 through activation of nuclear factor‐κB promotes gastric tumorigenesis via β‐catenin signaling axis. Gastroenterology. 2022;162(6):1716‐1731.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Scanu T, Spaapen RM, Bakker JM, et al. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe. 2015;17(6):763‐774. [DOI] [PubMed] [Google Scholar]
- 187. Hu W, Lazar MA. Modelling metabolic diseases and drug response using stem cells and organoids. Nat Rev Endocrinol. 2022;18(12):744‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhang L, Avery J, Yin A, et al. Generation of functional brown adipocytes from human pluripotent stem cells via progression through a paraxial mesoderm state. Cell Stem Cell. 2020;27(5):784‐797.e11. [DOI] [PubMed] [Google Scholar]
- 189. Bahmad HF, Daouk R, Azar J, et al. Modeling adipogenesis: current and future perspective. Cells. 2020;9(10):2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Taylor J, Sellin J, Kuerschner L, et al. Generation of immune cell containing adipose organoids for in vitro analysis of immune metabolism. Sci Rep. 2020;10(1):21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Muller S, Ader I, Creff J, et al. Human adipose stromal‐vascular fraction self‐organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 2019;9(1):7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Powell EE, Wong VW, Rinella M. Non‐alcoholic fatty liver disease. Lancet. 2021;397(10290):2212‐2224. [DOI] [PubMed] [Google Scholar]
- 193. Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691‐1705. [DOI] [PubMed] [Google Scholar]
- 194. Gurevich I, Burton SA, Munn C, et al. iPSC‐derived hepatocytes generated from NASH donors provide a valuable platform for disease modeling and drug discovery. Biol Open. 2020;9(12):bio055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Singal AK, Mathurin P. Diagnosis and treatment of alcohol‐associated liver disease: a review. Jama. 2021;326(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 196. Wang S, Wang X, Tan Z, et al. Human ESC‐derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ren X, Chen W, Yang Q, Li X, Xu L. Patient‐derived cancer organoids for drug screening: basic technology and clinical application. J Gastroenterol Hepatol. 2022;37(8):1446‐1454. [DOI] [PubMed] [Google Scholar]
- 198. Rivera M, Fichtner I, Wulf‐Goldenberg A, et al. Patient‐derived xenograft (PDX) models of colorectal carcinoma (CRC) as a platform for chemosensitivity and biomarker analysis in personalized medicine. Neoplasia. 2021;23(1):21‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Yang G, Guan W, Cao Z, et al. Integrative genomic analysis of gemcitabine resistance in pancreatic cancer by patient‐derived xenograft models. Clin Cancer Res. 2021;27(12):3383‐3396. [DOI] [PubMed] [Google Scholar]
- 200. Guillen KP, Fujita M, Butterfield AJ, et al. A human breast cancer‐derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 2022;3(2):232‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Gitto SB, George E, Medvedev S, Simpkins F. Humanized patient‐derived xenograft models of ovarian cancer. Methods Mol Biol. 2022;2424:255‐274. [DOI] [PubMed] [Google Scholar]
- 202. Chen X, Shen C, Wei Z, et al. Patient‐derived non‐small cell lung cancer xenograft mirrors complex tumor heterogeneity. Cancer Biol Med. 2021;18(1):184‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Morgan KM, Riedlinger GM, Rosenfeld J, Ganesan S, Pine SR. Patient‐derived xenograft models of non‐small cell lung cancer and their potential utility in personalized medicine. Front Oncol. 2017;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bleijs M, van de Wetering M, Clevers H, Drost J. Xenograft and organoid model systems in cancer research. Embo J. 2019;38(15):e101654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Xian L, Zhao P, Chen X, et al. Heterogeneity, inherent and acquired drug resistance in patient‐derived organoid models of primary liver cancer. Cell Oncol (Dordr). 2022;45(5):1019‐1036. [DOI] [PubMed] [Google Scholar]
- 206. Maier CF, Zhu L, Nanduri LK, et al. Patient‐derived organoids of cholangiocarcinoma. Int J Mol Sci. 2021;22(16):8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dekkers JF, van Vliet EJ, Sachs N, et al. Long‐term culture, genetic manipulation and xenotransplantation of human normal and breast cancer organoids. Nat Protoc. 2021;16(4):1936‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Papaccio F, García‐Mico B, Gimeno‐Valiente F, et al. Proteotranscriptomic analysis of advanced colorectal cancer patient derived organoids for drug sensitivity prediction. J Exp Clin Cancer Res. 2023;42(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Blokzijl F, de Ligt J, Jager M, et al. Tissue‐specific mutation accumulation in human adult stem cells during life. Nature. 2016;538(7624):260‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sun L, Wang Y, Cen J, et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat Cell Biol. 2019;21(8):1015‐1026. [DOI] [PubMed] [Google Scholar]
- 211. Takeda H, Kataoka S, Nakayama M, et al. CRISPR‐Cas9‐mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc Natl Acad Sci USA. 2019;116(31):15635‐15644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell‐based immunotherapy. Nat Rev Clin Oncol. 2019;16(3):151‐167. [DOI] [PubMed] [Google Scholar]
- 213. Bar‐Ephraim YE, Kretzschmar K, Clevers H. Organoids in immunological research. Nat Rev Immunol. 2020;20(5):279‐293. [DOI] [PubMed] [Google Scholar]
- 214. Zhang Z, Karthaus WR, Lee YS, et al. Tumor microenvironment‐derived NRG1 promotes antiandrogen resistance in prostate cancer. Cancer Cell. 2020;38(2):279‐296.e9.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Yuki K, Cheng N, Nakano M, Kuo CJ. Organoid models of tumor immunology. Trends Immunol. 2020;41(8):652‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor‐reactive T cells by co‐culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586‐1598.e12.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cattaneo CM, Dijkstra KK, Fanchi LF, et al. Tumor organoid‐T‐cell coculture systems. Nat Protoc. 2020;15(1):15‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Liu T, Tan J, Wu M, et al. High‐affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39(+)CD8(+) T cells. Gut. 2021;70(10):1965‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR‐proficient and MMR‐deficient early‐stage colon cancers. Nat Med. 2020;26(4):566‐576. [DOI] [PubMed] [Google Scholar]
- 220. Li Z, Lang Y, Sakamuru S, et al. Methylene blue is a potent and broad‐spectrum inhibitor against Zika virus in vitro and in vivo. Emerg Microbes Infect. 2020;9(1):2404‐2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zhu Z, Schnell L, Müller B, Müller M, Papatheodorou P, Barth H. The antibiotic bacitracin protects human intestinal epithelial cells and stem cell‐derived intestinal organoids from clostridium difficile toxin TcdB. Stem Cells Int. 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Zhang X, Ma Z, Song E, Xu T. Islet organoid as a promising model for diabetes. Protein Cell. 2022;13(4):239‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Amin S, Cook B, Zhou T, et al. Discovery of a drug candidate for GLIS3‐associated diabetes. Nat Commun. 2018;9(1):2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Ilegems E, Bryzgalova G, Correia J, et al. HIF‐1α inhibitor PX‐478 preserves pancreatic β cell function in diabetes. Sci Transl Med. 2022;14(638):eaba9112. [DOI] [PubMed] [Google Scholar]
- 225. Rodboon T, Yodmuang S, Chaisuparat R, Ferreira JN. Development of high‐throughput lacrimal gland organoid platforms for drug discovery in dry eye disease. SLAS Discov. 2022;27(3):151‐158. [DOI] [PubMed] [Google Scholar]
- 226. Bannier‐Hélaouët M, Post Y, Korving J, et al. Exploring the human lacrimal gland using organoids and single‐cell sequencing. Cell Stem Cell. 2021;28(7):1221‐1232.e7. [DOI] [PubMed] [Google Scholar]
- 227. Li X, Fu G, Zhang L, et al. Assay establishment and validation of a high‐throughput organoid‐based drug screening platform. Stem Cell Res Ther. 2022;13(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hirt CK, Booij TH, Grob L, et al. Drug screening and genome editing in human pancreatic cancer organoids identifies drug‐gene interactions and candidates for off‐label treatment. Cell Genom. 2022;2(2):100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hennig A, Baenke F, Klimova A, et al. Detecting drug resistance in pancreatic cancer organoids guides optimized chemotherapy treatment. J Pathol. 2022;257(5):607‐619. [DOI] [PubMed] [Google Scholar]
- 230. Ganesh K, Basnet H, Kaygusuz Y, et al. L1CAM defines the regenerative origin of metastasis‐initiating cells in colorectal cancer. Nat Cancer. 2020;1(1):28‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Schuth S, Le Blanc S, Krieger TG, et al. Patient‐specific modeling of stroma‐mediated chemoresistance of pancreatic cancer using a three‐dimensional organoid‐fibroblast co‐culture system. J Exp Clin Cancer Res. 2022;41(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Toshimitsu K, Takano A, Fujii M, et al. Organoid screening reveals epigenetic vulnerabilities in human colorectal cancer. Nat Chem Biol. 2022;18(6):605‐614. [DOI] [PubMed] [Google Scholar]
- 233. Wong CC, Wu JL, Ji F, et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS‐mutant colorectal cancer. Nat Commun. 2022;13(1):3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Cortes J, Lang F. Third‐line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Li L, Halpert G, Lerner MG, et al. Protein synthesis inhibitor omacetaxine is effective against hepatocellular carcinoma. JCI Insight. 2021;6(12):e138197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Watanabe S, Yogo A, Otsubo T, et al. Establishment of patient‐derived organoids and a characterization‐based drug discovery platform for treatment of pancreatic cancer. BMC Cancer. 2022;22(1):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Srimongkol A, Laosillapacharoen N, Saengwimol D, et al. Sunitinib efficacy with minimal toxicity in patient‐derived retinoblastoma organoids. J Exp Clin Cancer Res. 2023;42(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Dasgupta A, Shukla SK, Vernucci E, et al. SIRT1‐NOX4 signaling axis regulates cancer cachexia. J Exp Med. 2020;217(7):e20190745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Chen B, Song Y, Zhan Y, et al. Fangchinoline inhibits non‐small cell lung cancer metastasis by reversing epithelial‐mesenchymal transition and suppressing the cytosolic ROS‐related Akt‐mTOR signaling pathway. Cancer Lett. 2022;543:215783. [DOI] [PubMed] [Google Scholar]
- 240. Ghate NB, Kim S, Spiller E, et al. VprBP directs epigenetic gene silencing through histone H2A phosphorylation in colon cancer. Mol Oncol. 2021;15(10):2801‐2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Chen D, Su X, Zhu L, et al. Papillary thyroid cancer organoids harboring BRAF(V600E) mutation reveal potentially beneficial effects of BRAF inhibitor‐based combination therapies. J Transl Med. 2023;21(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Wang SC, Nassour I, Xiao S, et al. SWI/SNF component ARID1A restrains pancreatic neoplasia formation. Gut. 2019;68(7):1259‐1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Guo B, Friedland SC, Alexander W, et al. Arid1a mutation suppresses TGF‐β signaling and induces cholangiocarcinoma. Cell Rep. 2022;40(9):111253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Sun X, Wang SC, Wei Y, et al. Arid1a has context‐dependent oncogenic and tumor suppressor functions in liver cancer. Cancer Cell. 2017;32(5):574‐589.e6.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Loe AKH, Francis R, Seo J, et al. Uncovering the dosage‐dependent roles of Arid1a in gastric tumorigenesis for combinatorial drug therapy. J Exp Med. 2021;218(6):e20200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic Transl Sci. 2019;4(7):845‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Segovia‐Zafra A, Di Zeo‐Sánchez DE, López‐Gómez C, et al. Preclinical models of idiosyncratic drug‐induced liver injury (iDILI): moving towards prediction. Acta Pharm Sin B. 2021;11(12):3685‐3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Shi S, Verstegen MMA, Roest HP, et al. Recapitulating cholangiopathy‐associated necroptotic cell death in vitro using human cholangiocyte organoids. Cell Mol Gastroenterol Hepatol. 2022;13(2):541‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bouwmeester MC, Tao Y, Proença S, et al. Drug metabolism of hepatocyte‐like organoids and their applicability in in vitro toxicity testing. Molecules. 2023;28(2):621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Zhang CJ, Meyer SR, O'Meara MJ, et al. A human liver organoid screening platform for DILI risk prediction. J Hepatol. 2023;78:998‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kim H, Im I, Jeon JS, et al. Development of human pluripotent stem cell‐derived hepatic organoids as an alternative model for drug safety assessment. Biomaterials. 2022;286:121575. [DOI] [PubMed] [Google Scholar]
- 252. Gu S, Wu G, Lu D, et al. Nephrotoxicity assessment of Esculentoside A using human‐induced pluripotent stem cell‐derived organoids. Phytother Res. 2023. [DOI] [PubMed] [Google Scholar]
- 253. Rodrigues D, Coyle L, Füzi B, et al. Unravelling mechanisms of doxorubicin‐induced toxicity in 3d human intestinal organoids. Int J Mol Sci. 2022;23(3):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Rodrigues D, Herpers B, Ferreira S, et al. A Transcriptomic approach to elucidate the mechanisms of gefitinib‐induced toxicity in healthy human intestinal organoids. Int J Mol Sci. 2022;23(4):2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Hamilton JG, Banerjee SC, Carlsson SV, et al. Clinician perspectives on communication and implementation challenges in precision oncology. Per Med. 2021;18(6):559‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Li G, Ma S, Wu Q, et al. Establishment of gastric signet ring cell carcinoma organoid for the therapeutic drug testing. Cell Death Discov. 2022;8(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Li Z, Xu H, Yu L, et al. Patient‐derived renal cell carcinoma organoids for personalized cancer therapy. Clin Transl Med. 2022;12(7):e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Grossman JE, Muthuswamy L, Huang L, et al. Organoid sensitivity correlates with therapeutic response in patients with pancreatic cancer. Clin Cancer Res. 2022;28(4):708‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Pettersen S, Øy GF, Egeland EV, et al. Breast cancer patient‐derived explant cultures recapitulate in vivo drug responses. Front Oncol. 2023;13:1040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Vlachogiannis G, Hedayat S, Vatsiou A, et al. Patient‐derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359(6378):920‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Yao Y, Xu X, Yang L, et al. Patient‐derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26(1):17‐26.e6. [DOI] [PubMed] [Google Scholar]
- 262. Demyan L, Habowski AN, Plenker D, et al. Pancreatic cancer patient‐derived organoids can predict response to neoadjuvant chemotherapy. Ann Surg. 2022;276(3):450‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Steele NG, Chakrabarti J, Wang J, et al. An organoid‐based preclinical model of human gastric cancer. Cell Mol Gastroenterol Hepatol. 2019;7(1):161‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Seppälä TT, Zimmerman JW, Suri R, et al. Precision medicine in pancreatic cancer: patient‐derived organoid pharmacotyping is a predictive biomarker of clinical treatment response. Clin Cancer Res. 2022;28(15):3296‐3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Carneiro BA, El‐Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Xiao Y, Ma D, Yang YS, et al. Comprehensive metabolomics expands precision medicine for triple‐negative breast cancer. Cell Res. 2022;32(5):477‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Phan N, Hong JJ, Tofig B, et al. A simple high‐throughput approach identifies actionable drug sensitivities in patient‐derived tumor organoids. Commun Biol. 2019;2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Zhao Y, Li ZX, Zhu YJ, et al. Single‐cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids. Adv Sci (Weinh). 2021;8(11):2003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Leung CON, Tong M, Chung KPS, et al. Overriding adaptive resistance to sorafenib through combination therapy with Src homology 2 domain‐containing phosphatase 2 blockade in hepatocellular carcinoma. Hepatology. 2020;72(1):155‐168. [DOI] [PubMed] [Google Scholar]
- 270. Shen X, Zhang Y, Xu Z, et al. KLF5 inhibition overcomes oxaliplatin resistance in patient‐derived colorectal cancer organoids by restoring apoptotic response. Cell Death Dis. 2022;13(4):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Sampaziotis F, de Brito MC, Madrigal P, et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33(8):845‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Nie J, Hashino E. Organoid technologies meet genome engineering. EMBO Rep. 2017;18(3):367‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13(6):653‐658. [DOI] [PubMed] [Google Scholar]
- 274. Geurts MH, de Poel E, Amatngalim GD, et al. CRISPR‐based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell. 2020;26(4):503‐510.e7. [DOI] [PubMed] [Google Scholar]
- 275. Kim JH, Kim J, Kim WJ, Choi YH, Yang SR, Hong SH. Diesel particulate matter 2.5 induces epithelial‐to‐mesenchymal transition and upregulation of SARS‐CoV‐2 receptor during human pluripotent stem cell‐derived alveolar organoid development. Int J Environ Res Public Health. 2020;17(22):8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Liu S, Yang R, Chen Y, et al. Development of human lung induction models for air pollutants' toxicity assessment. Environ Sci Technol. 2021;55(4):2440‐2451. [DOI] [PubMed] [Google Scholar]
- 277. Jiang Y, Lu L, Du C, et al. Human airway organoids as 3D in vitro models for a toxicity assessment of emerging inhaled pollutants: tire wear particles. Front Bioeng Biotechnol. 2022;10:1105710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Hernandez Mejia M, Wade NE, Baca R, Diaz VG, Jacobus J. The influence of cannabis and nicotine co‐use on neuromaturation: a systematic review of adolescent and young adult studies. Biol Psychiatry. 2021;89(2):162‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Notaras M, Lodhi A, Barrio‐Alonso E, et al. Neurodevelopmental signatures of narcotic and neuropsychiatric risk factors in 3D human‐derived forebrain organoids. Mol Psychiatry. 2021;26(12):7760‐7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Wang Y, Wang L, Zhu Y, Qin J. Human brain organoid‐on‐a‐chip to model prenatal nicotine exposure. Lab Chip. 2018;18(6):851‐860. [DOI] [PubMed] [Google Scholar]
- 281. Forsythe SD, Devarasetty M, Shupe T, et al. Environmental toxin screening using human‐derived 3D bioengineered liver and cardiac organoids. Front Public Health. 2018;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Xie S, Jiang L, Wang M, et al. Cadmium ingestion exacerbates Salmonella infection, with a loss of goblet cells through activation of Notch signaling pathways by ROS in the intestine. J Hazard Mater. 2020;391:122262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Huang Y, Dai Y, Li M, et al. Exposure to cadmium induces neuroinflammation and impairs ciliogenesis in hESC‐derived 3D cerebral organoids. Sci Total Environ. 2021;797:149043. [DOI] [PubMed] [Google Scholar]
- 284. Li M, Gong J, Gao L, Zou T, Kang J, Xu H. Advanced human developmental toxicity and teratogenicity assessment using human organoid models. Ecotoxicol Environ Saf. 2022;235:113429. [DOI] [PubMed] [Google Scholar]
- 285. Chen H, Seifikar H, Larocque N, et al. Using a multi‐stage hESC model to characterize BDE‐47 toxicity during neurogenesis. Toxicol Sci. 2019;171(1):221‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Sirasanagandla SR, Al‐Huseini I, Sakr H, et al. Natural products in mitigation of bisphenol a toxicity: future therapeutic use. Molecules. 2022;27(17):5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Yang R, Liu S, Liang X, et al. TBBPA, TBBPS, and TCBPA disrupt hESC hepatic differentiation and promote the proliferation of differentiated cells partly via up‐regulation of the FGF10 signaling pathway. J Hazard Mater. 2021;401:123341. [DOI] [PubMed] [Google Scholar]
- 288. Li M, Gong J, Ge L, et al. Development of human retinal organoid models for bisphenol toxicity assessment. Ecotoxicol Environ Saf. 2022;245:114094. [DOI] [PubMed] [Google Scholar]
- 289. Winkler J, Liu P, Phong K, et al. Bisphenol A replacement chemicals, BPF and BPS, induce protumorigenic changes in human mammary gland organoid morphology and proteome. Proc Natl Acad Sci USA. 2022;119(11):e2115308119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Yousef MI, Roychoudhury S, Jafaar KS, Slama P, Kesari KK, Kamel MA. Aluminum oxide and zinc oxide induced nanotoxicity in rat brain, heart, and lung. Physiol Res. 2022;71(5):677‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Grebowski J, Konopko A, Krokosz A, DiLabio GA, Litwinienko G. Antioxidant activity of highly hydroxylated fullerene C(60) and its interactions with the analogue of α‐tocopherol. Free Radic Biol Med. 2020;160:734‐744. [DOI] [PubMed] [Google Scholar]
- 292. Su J, Duan X, Qiu Y, et al. Pregnancy exposure of titanium dioxide nanoparticles causes intestinal dysbiosis and neurobehavioral impairments that are not significant postnatally but emerge in adulthood of offspring. J Nanobiotechnology. 2021;19(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Vassal M, Rebelo S, Pereira ML. Metal oxide nanoparticles: evidence of adverse effects on the male reproductive system. Int J Mol Sci. 2021;22(15):8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Nirmal NK, Awasthi KK, John PJ. Hepatotoxicity of graphene oxide in Wistar rats. Environ Sci Pollut Res Int. 2021;28(34):46367‐46376. [DOI] [PubMed] [Google Scholar]
- 295. Casals E, Zeng M, Parra‐Robert M, et al. Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small. 2020;16(20):1907322. [DOI] [PubMed] [Google Scholar]
- 296. Mekky G, Seeds M, Diab AEA, et al. The potential toxic effects of magnesium oxide nanoparticles and valproate on liver tissue. J Biochem Mol Toxicol. 2021;35(3):e22676. [DOI] [PubMed] [Google Scholar]
- 297. He C, Ruan F, Jiang S, et al. Black phosphorus quantum dots cause nephrotoxicity in organoids, mice, and human cells. Small. 2020;16(22):2001371. [DOI] [PubMed] [Google Scholar]
- 298. Georgakopoulos N, Prior N, Angres B, et al. Long‐term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev Biol. 2020;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Peng WC, Logan CY, Fish M, et al. Inflammatory cytokine TNFα promotes the long‐term expansion of primary hepatocytes in 3D culture. Cell. 2018;175(6):1607‐1619.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Ribeiro J, Procyk CA, West EL, et al. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell‐derived cone photoreceptors. Cell Rep. 2021;35(3):109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Tanigawa S, Tanaka E, Miike K, et al. Generation of the organotypic kidney structure by integrating pluripotent stem cell‐derived renal stroma. Nat Commun. 2022;13(1):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Yang H, Sun L, Pang Y, et al. Three‐dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021;70(3):567‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Elizondo DM, Brandy NZD, da Silva RLL, et al. Pancreatic islets seeded in a novel bioscaffold forms an organoid to rescue insulin production and reverse hyperglycemia in models of type 1 diabetes. Sci Rep. 2020;10(1):4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Dong X, Xu SB, Chen X, et al. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol Psychiatry. 2021;26(7):2964‐2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Sampaziotis F, Justin AW, Tysoe OC, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23(8):954‐963. [DOI] [PubMed] [Google Scholar]
- 306. Tysoe OC, Justin AW, Brevini T, et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat Protoc. 2019;14(6):1884‐1925. [DOI] [PubMed] [Google Scholar]
- 307. Sampaziotis F, Muraro D, Tysoe OC, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371(6531):839‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Reich M, Spomer L, Klindt C, et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J Hepatol. 2021;75(3):634‐646. [DOI] [PubMed] [Google Scholar]
- 309. Meran L, Massie I, Campinoti S, et al. Engineering transplantable jejunal mucosal grafts using patient‐derived organoids from children with intestinal failure. Nat Med. 2020;26(10):1593‐1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Sugimoto S, Kobayashi E, Fujii M, et al. An organoid‐based organ‐repurposing approach to treat short bowel syndrome. Nature. 2021;592(7852):99‐104. [DOI] [PubMed] [Google Scholar]
- 311. Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6(5):402‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Krefft O, Jabali A, Iefremova V, Koch P, Ladewig J. Generation of standardized and reproducible forebrain‐type cerebral organoids from human induced pluripotent stem cells. J Vis Exp. 2018(131):56768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Gjorevski N, Nikolaev M, Brown TE, et al. Tissue geometry drives deterministic organoid patterning. Science. 2022;375(6576):eaaw9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Eremeev A, Belikova L, Ruchko E, et al. Brain Organoid generation from induced pluripotent stem cells in home‐made mini bioreactors. J Vis Exp. 2021(178). [DOI] [PubMed] [Google Scholar]
- 315. Arthur P, Kandoi S, Sun L, et al. Biophysical, molecular and proteomic profiling of human retinal organoid‐derived exosomes. Pharm Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Suong DNA, Imamura K, Inoue I, et al. Induction of inverted morphology in brain organoids by vertical‐mixing bioreactors. Commun Biol. 2021;4(1):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Jin Y, Kim J, Lee JS, et al. Vascularized liver organoids generated using induced hepatic tissue and dynamic liver‐specific microenvironment as a drug testing platform. Adv Funct Mater. 2018;28(37):1801954. [Google Scholar]
- 318. Eltaher HM, Abukunna FE, Ruiz‐Cantu L, Stone Z, Yang J, Dixon JE. Human‐scale tissues with patterned vascular networks by additive manufacturing of sacrificial sugar‐protein composites. Acta Biomater. 2020;113:339‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Kinstlinger IS, Saxton SH, Calderon GA, et al. Generation of model tissues with dendritic vascular networks via sacrificial laser‐sintered carbohydrate templates. Nat Biomed Eng. 2020;4(9):916‐932. [DOI] [PubMed] [Google Scholar]
- 320. Verit I, Gemini L, Preterre J, et al. Vascularization of cell‐laden microfibres by femtosecond laser processing. Int J Mol Sci. 2022;23(12):6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Brandenberg N, Lutolf MP. In situ patterning of microfluidic networks in 3D cell‐laden hydrogels. Adv Mater. 2016;28(34):7450‐7456. [DOI] [PubMed] [Google Scholar]
- 322. Neufeld L, Yeini E, Reisman N, et al. Microengineered perfusable 3D‐bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci Adv. 2021;7(34):eabi9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Anada T, Pan CC, Stahl AM, et al. Vascularized bone‐mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci. 2019;20(5):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Asai A, Aihara E, Watson C, et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 2017;144(6):1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5(7):539‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Hipwood L, Clegg J, Weekes A, et al. Semi‐synthetic click‐gelatin hydrogels as tunable platforms for 3D cancer cell culture. Gels. 2022;8(12):821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Monferrer E, Dobre O, Trujillo S, et al. Vitronectin‐based hydrogels recapitulate neuroblastoma growth conditions. Front Cell Dev Biol. 2022;10:988699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Mosquera MJ, Kim S, Bareja R, et al. Extracellular matrix in synthetic hydrogel‐based prostate cancer organoids regulate therapeutic response to EZH2 and DRD2 inhibitors. Adv Mater. 2022;34(2):2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Kim S, Min S, Choi YS, et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat Commun. 2022;13(1):1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Willemse J, van Tienderen G, van Hengel E, et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284:121473. [DOI] [PubMed] [Google Scholar]
- 331. Candiello J, Grandhi TSP, Goh SK, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27‐39. [DOI] [PubMed] [Google Scholar]
- 332. Prince E, Cruickshank J, Ba‐Alawi W, et al. Biomimetic hydrogel supports initiation and growth of patient‐derived breast tumor organoids. Nat Commun. 2022;13(1):1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Peng Y, Qing X, Lin H, et al. Decellularized Disc Hydrogels for hBMSCs tissue‐specific differentiation and tissue regeneration. Bioact Mater. 2021;6(10):3541‐3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Simsa R, Rothenbücher T, Gürbüz H, et al. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS One. 2021;16(1):e0245685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Padma AM, Alsheikh AB, Song MJ, et al. Immune response after allogeneic transplantation of decellularized uterine scaffolds in the rat. Biomed Mater. 2021;16(4):045021. [DOI] [PubMed] [Google Scholar]
- 336. Xu Y, Zhou J, Liu C, et al. Understanding the role of tissue‐specific decellularized spinal cord matrix hydrogel for neural stem/progenitor cell microenvironment reconstruction and spinal cord injury. Biomaterials. 2021;268:120596. [DOI] [PubMed] [Google Scholar]
- 337. Li G, Liu S, Chen W, et al. Acellularized uvea hydrogel as novel injectable platform for cell‐based delivering treatment of retinal degeneration and optimizing retinal organoids inducible system. Adv Healthc Mater. 2022;11(23):2202114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


