Abstract
The Vibrio cholerae SXT element encodes resistance to multiple antibiotics and is a conjugative, self-transmissible, and chromosomally integrating element (a constin). Excision and self-transfer of the SXT element require an element-encoded integrase. We now report that the SXT element can also mobilize the plasmids RSF1010 and CloDF13 in trans as well as chromosomal DNA in an Hfr-like manner. SXT element-mediated mobilization of plasmids and chromosomal DNA, unlike its self-transfer, is not dependent upon excision of the element from the chromosome. These results raise the possibility that the SXT element and other constins play a general role in horizontal gene transfer among gram-negative bacteria.
The SXT element was originally found in the chromosome of epidemic Vibrio cholerae O139 strains that arose in late 1992 on the Indian subcontinent (6, 30). This approximately 62-kbp element carries the genes encoding resistance to sulfamethoxazole and trimethoprim (SXT), chloramphenicol, and low levels of streptomycin. Despite the chromosomal location of this element, we found that the entire element is self-transmissible and can be transferred by conjugation to a variety of gram-negative bacteria including V. cholerae, Escherichia coli, and Salmonella enterica serovar Typhimurium (30). No replicative extrachromosomal form of the SXT element has been isolated.
The transfer of the SXT element has features reminiscent of both temperate bacteriophages and conjugative plasmids. The element encodes a λ family recombinase (Int) that is required for its excision from the chromosome and circularization by recombination between the right and left ends of the integrated element. Generation of this extrachromosomal intermediate is an essential step in the successful transfer of the SXT element; it must precede conjugative transfer to recipient cells. Once transferred to the recipient, the SXT element integrates site specifically into the 5′ end of prfC, the gene coding for protein chain release factor 3 (RF3). The SXT element encodes a new N terminus for RF3 and maintains the reading frame of prfC. Integration, like excision, requires the SXT element int gene (14). Since the properties of the SXT element do not precisely match those of previously described transmissible elements, we named the SXT element with an acronym for its properties as a constin, a conjugative, self-transmissible integrating element (14).
Previously described self-transmissible conjugative elements can mobilize coresiding DNA either in cis or in trans. For example, conjugative plasmids like RP4 (11) can mediate transfer of mobilizable plasmids. These mobilizable plasmids typically encode an origin of transfer (oriT) and their own relaxase and nicking accessory proteins for interaction with oriT but require a conjugative element to provide the mating pair formation functions for transfer (4). Another transfer scenario is that a chromosome can acquire an oriT by integration of a conjugative element and thereby become mobilizable. For example, integration of the F plasmid in E. coli results in formation of the so-called Hfr (high frequency of recombination) strains, which can transfer their chromosomes at high frequency (12, 32). The conjugative transposons described for Bacteroides spp. such as the Tcr Emr DOT family can also mobilize other genetic elements (23). Tcr Emr DOT-like elements can mobilize plasmids in cis (by integration into these plasmids) as well as plasmids and chromosomally integrated elements (e.g., NBUs and Tn4555) in trans (23). In this study, we explored whether the SXT element is able to mobilize plasmids and chromosomal DNA.
RSF1010 is mobilized by the SXT element.
RSF1010 is a broad-host-range plasmid that can be mobilized by conjugative plasmids, the chromosomal dot-icm virulence system of Legionella pneumophila, and the plasmid-encoded vir system of Agrobacterium tumefaciens (3, 9, 25, 29). Because RSF1010 encodes resistance to sulfonamide and streptomycin, as does the SXT element, we used an RSF1010 derivative containing a kanamycin resistance cassette, RSF1010-Kn (29), to test whether E. coli K-12 harboring the SXT element could mobilize RSF1010. Donor strains for these conjugation experiments were the E. coli K-12 MG1655 derivatives CAG18439 (27) and HW220 (CAG18439 prfC::SXT element [14]), both transformed with RSF1010-Kn. BI533, a spontaneous nalidixic acid-resistant mutant of MG1655, was used as a recipient. Matings were performed as previously described (14), and exconjugants were selected on Luria-Bertani (LB) agar containing 50 mg of kanamycin per liter, and 40 mg of nalidixic acid (NAL) per liter for RSF1010-Kn transfer and LB agar with 40 mg of NAL per liter, 160 mg of sulfamethoxazole per liter, and 32 mg of trimethoprim per liter for SXT element transfer. CAG18439 did not mobilize RSF1010. However, when its SXTr derivative HW220 was used as a donor, Knr exconjugants were obtained with a frequency of 10−7 (Table 1). This frequency was about 100-fold lower than the frequency of SXT element transfer from this strain (Table 1). Only 10% of the Knr exconjugants were also resistant to SXT. The presence of RSF1010-Kn in the exconjugants was confirmed by plasmid isolation (data not shown). Thus, in most cases the SXT element and RSF1010 were transferred independently. These results indicate that RSF1010 is mobilized in trans by the SXT element, rather than through formation of a cointegrate between the two elements.
TABLE 1.
Plasmid mobilization by the SXT elementa
| Donor | Relevant genotype | Plasmid | TfSXT element | Tfmobilizable plasmid |
|---|---|---|---|---|
| CAG18439 | MG1655 lacZU118 lacI42::Tn10 | RSF1010 | ND | <10−9 |
| RSF1010ΔoriT | ND | <10−9 | ||
| HW220 | CAG18439 prfC::SXT element | RSF1010 | 4 × 10−5 | 3.5 × 10−7 |
| RSF1010ΔoriT | 5 × 10−5 | 2.6 × 10−7 | ||
| BI554 | HW220 Δint | RSF1010 | 10−9 | 1.8 × 10−7 |
| RSF1010ΔoriT | 10−9 | 0.2 × 10−7 | ||
| CAG18439 | MG1655 lacZU118 lacI42::Tn10 | pSU4628 (CloDF13) | ND | <10−9 |
| HW220 | CAG18439 prfC::SXT element | pSU4628 | 5 × 10−6 | 3.0 × 10−5 |
| BI554 | HW220 Δint | pSU4628 | 10−9 | 2.5 × 10−5 |
| HW220 | CAG18439 prfC::SXT element | pSU4601 (ColE1) | 8 × 10−5 | <10−9 |
| pSU4620 (ColE3) | <10−9 | <10−9 |
The transfer frequency was calculated by dividing the number of exconjugant cells by the number of donor cells. BI533 (MG1655 Nalr) was the recipient for all matings. ND, not done.
Since the int gene of the SXT element is required for excision and self-transfer of the element, we constructed a null mutation in int to test whether SXT element-mediated transfer of RSF1010 requires the activity of this protein. The int bp 305 to 1025 were deleted with a ClaI/NsiI digest followed by intramolecular ligation, and the intΔ305–1025 allele was cloned into the allele-exchange vector pWM91 (19), resulting in pINTΔ91. Allelic exchange was performed in HW220 as previously described (7), and an HW220Δint derivative, BI554, was isolated. Like HW514 (14), a previously described HW220 derivative with an insertion mutation in int, BI554 did not contain an extrachromosomal circular form of the SXT element detectable by PCR or transfer the SXT element to a recipient. However, BI554 could still mediate transfer of RSF1010-Kn to BI533 at nearly the same frequency as that of the int+ strain HW220 (Table 1). This indicates that the conjugative functions of the SXT element are not dependent upon a functional int gene. Therefore, excision and circularization of the SXT element are not required for expression of the SXT element-encoded transfer functions. This result also confirms that transfer of RSF1010 is not dependent on transfer of the SXT element.
To test whether the RSF1010-encoded oriT is required for its mobilization by the SXT element, we transformed CAG18439, CAG18439/RP4, HW220, and BI554 with a derivative of RSF1010-Kn carrying a 124-bp deletion extending over the oriT region (deletion Δ13 [10, 29]). As expected, CAG18439 and CAG18439 carrying RP4 could not transfer RSF1010ΔoriT. However, to our surprise, HW220 was still able to mobilize this plasmid with a frequency similar to that of RSF1010-Kn (Table 1). This indicates an alternative route of RSF1010 transfer independent of the oriT region. Mob-independent transfer of plasmids has been described for other conjugative elements like Tn916 (26), but the mechanism is not understood. Transfer of the RSF1010ΔoriT was dependent neither on cointegrate formation between the SXT element and RSF1010ΔoriT nor on recombinational repair of the oriT deletion in this plasmid by SXT element sequences. These mechanisms were excluded by our findings that, in all exconjugants tested, RSF1010ΔoriT could be isolated as a plasmid and that the oriT deletion was still present (data not shown). Furthermore, as in the previous experiment, only about 10% of the exconjugants received both the RSF1010ΔoriT and the SXT element. The int mutant BI554 was also able to mediate transfer of RSF1010ΔoriT, although with a lower frequency than HW220 (Table 1).
The SXT element can mobilize CloDF13.
To investigate whether the SXT element can also mediate the transfer of other mobilizable plasmids, we transformed CAG18439 and HW220 with pSU4628 (CloDF13::TnAΔEcoRV Apr [4]), pSU4601 (ColE1::Kn [4]), and pSU4620 (ColE3::Kn [4]). Matings were performed using BI533 as a recipient, and exconjugants were selected on LB agar with NAL and ampicillin (100 mg/liter) and LB plates with NAL and kanamycin, respectively (Table 1). In each experiment, SXT element transfer was also monitored. As a positive control, we used a CAG18439 derivative harboring RP4 as a donor strain and could show plasmid transfer in all cases (data not shown). As expected, CAG18439 alone could not mediate transfer of any of these plasmids. In contrast, with HW220 as the donor we could detect transfer of pSU4628 (CloDF13) but not of pSU4601 (ColE1) or pSU4620 (ColE3). None of 100 tested Apr exconjugants containing pSU4628 showed resistance to SXT, indicating that cotransfer of the SXT element with pSU4628 did not occur or occurred only at a low frequency.
As was the case for SXT element-mediated transfer of RSF1010, transfer of pSU4628 was independent of the SXT element-encoded int. The int mutant BI554 donated pSU4628 to recipient cells at approximately the same frequency as did the int+ HW220 (Table 1). Interestingly, both pSU4628 and pSU4620 influenced the SXT element transfer frequency (Table 1). With pSU4628, there was a 10-fold reduction in the transfer frequency of the SXT element. This could at least partly account for our inability to detect cotransfer of the SXT element with pSU4628. pSU4620 (ColE3), even though it was not mobilized by the SXT element, also interfered with the SXT element transfer, as no SXTr exconjugants could be isolated in these matings. Inhibition of F transfer by a copy number mutant of CloDF13 has been attributed to a reduction of the level of TraD (31). A similar mechanism might account for the reduced frequency of SXT element transfer in the presence of either pSU4628 or pSU4620. A study by Cabezón et al. (4) showed that it is primarily the TraD (in F)-TraG (in RP4) protein family that mediates the coupling between the respective conjugation systems and the mobilizable plasmids. CloDF13 encodes its own TraG homologue, but pSU4601 and pSU4620 do not. This difference could account for the efficient mobilization of CloDF13 by the SXT element and the failure to mobilize pSU4601 or pSU4620.
The SXT element can mobilize chromosomal DNA in an Hfr-like manner.
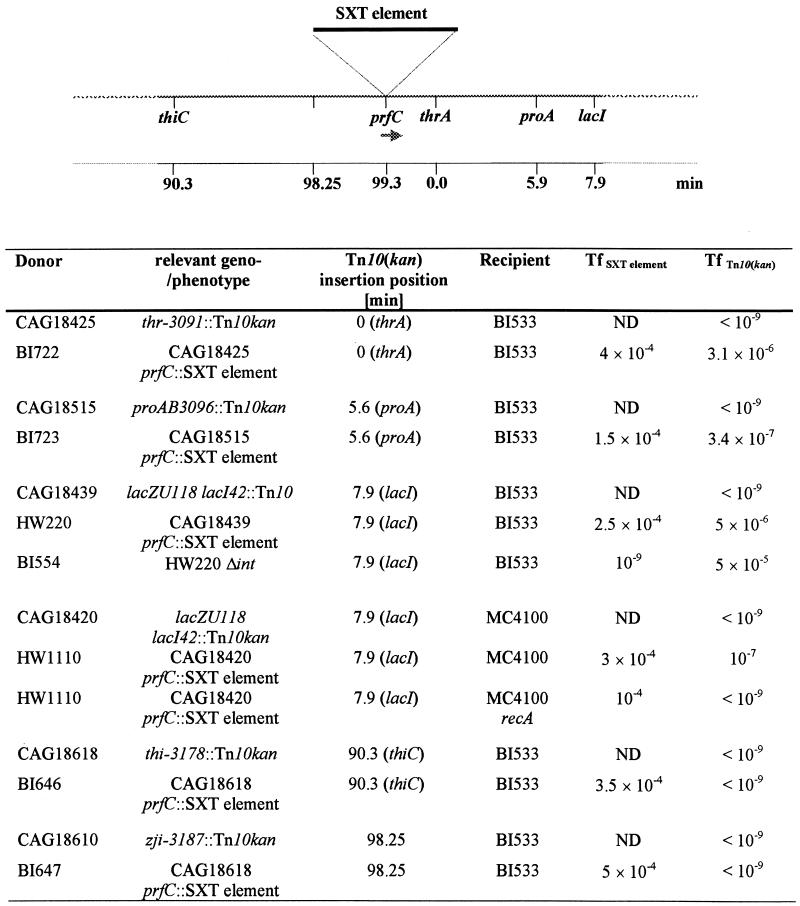
We tested whether the SXT element can, in addition, mobilize chromosomal DNA in cis. To accomplish this, we moved the SXT element into a set of MG1655 derivatives carrying single Tn10 (Tcr) or Tn10kan (Knr) insertions at different sites on the chromosome (27). The Tn10 insertions were chosen to be upstream and downstream of the SXT element insertion site in prfC, which is located at 99.3 min on the E. coli K-12 chromosome (2). We found that Tn10 insertions downstream of prfC in donor strains BI722 (0 min), BI723 (5.6 min), and HW220 (7.9 min) could be donated to a recipient if the SXT element was integrated at prfC (Fig. 1). Transfer of Tn10 was absolutely dependent on the presence of the SXT element in the donor strains (Fig. 1), and no transposition of Tn10 was evident in the exconjugants. Like their respective donor strains, exconjugants derived from BI722 were threonine auxotrophs, and the exconjugants derived from BI723 were proline auxotrophs. Similarly, 98% of 100 tested exconjugants derived from HW220 were LacZ− (white on plates containing 0.02% 5-bromo-4-chloro-indolyl-β-d-galactoside), indicating cotransfer of the lacZU118 allele along with the lacI42::Tn10 to the recipient strain.
FIG. 1.
Mobilization of chromosomal DNA by the SXT element. The transfer frequency was calculated by dividing the number of exconjugant cells by the number of donor cells. All donor strains are derivatives of E. coli K-12 MG1655. Locations of the Tn10 insertions in the donor strains were determined by Singer et al. (27) and Nichols et al. (20). ND, not done.
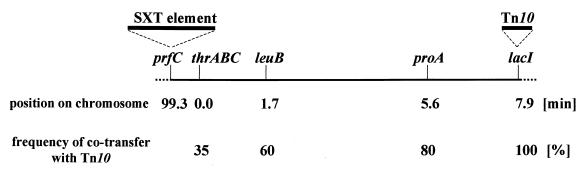
In contrast to Tn10 insertions downstream of prfC, the two Tn10 insertions upstream of prfC in donor strains BI646 (90.3 min) and BI647 (98.25 min) could not be transferred by the SXT element. The lack of detectable transfer from insertions upstream of prfC suggests that imprecise excision of the SXT element, as seen with specialized transducing phages or an F′ plasmid, does not constitute the mechanism of transfer of chromosomal DNA by this element. Instead, it appears that the SXT element mobilizes chromosomal DNA in a directional manner, similar to Hfr mobilization of chromosomal DNA. To investigate this further, we carried out conjugation experiments using HW220 as a donor and a Nalr derivative of AB1157 [E. coli K-12; thr-1, leuB6 Δ(gpt-proA)62 (1)] as a recipient and selected for Tcr (lacI::Tn10) AB1157 (Nalr) exconjugants. The frequency of cotransfer of the thrABC, leuB, and proA alleles of HW220 to AB1157 was determined (Fig. 2). These frequencies were dependent on the distance of these markers relative to the selected marker (lacI::Tn10). Thus, Tcr AB1157 exconjugants were proline auxotrophs more frequently than leucine or threonine auxotrophs, respectively (Fig. 2). These data indicate that the transferred DNA is integrated by homologous recombination into the recipient's chromosome. Additional evidence that the mechanism of SXT element-mediated transfer of chromosomal DNA is Hfr-like and dependent on homologous recombination was the finding that transfer of Tn10 markers to recipients was RecA dependent. When we compared the transfer frequency of lacI::Tn10kan from HW1110 (MG1655 lacI::Tn10kan prfC::SXT element) to either MC4100 or MC4100 recA, we found that the Tn10kan insertion in lacI could not be transferred successfully to the recA mutant of MC4100 (Fig. 1). In contrast, RecA is not required in the recipient for SXT element transfer (Fig. 1).
FIG. 2.
Frequency of cotransfer of unselected markers. The relative organization and map locations of the genes prfC (SXT element integration site), thrABC, leuB, proA, and lacI on the E. coli K-12 chromosome are depicted. Matings were performed with HW220 (lacI::Tn10 prfC::SXT element) as donor and AB1157 Nalr as recipient cells. Exconjugants (which had obtained Tn10) were selected as Nalr Tcr CFU and subsequently scored for the presence of functional thrABC, leuB, and proA alleles. The frequency of cotransfer of thrABC, leuB, and proA along with lacI::Tn10 is given as a percentage (bottom line).
Chromosomal DNA transfer mediated by the SXT element, like transfer of plasmids by this element, appears to be independent of transfer of the element itself. Only a fraction of the Tn10-containing exconjugants derived from BI722, BI723, and HW220 were also SXTr (41, 36, and 14%, respectively). Also, BI554, the Δint derivative of HW220, was capable of donating the lacI::Tn10 to the recipient. In fact, the frequency of transfer of lacI::Tn10 was even higher from BI554 than from HW220 (Fig. 1), suggesting that transfer of the SXT element DNA may interfere with transfer of chromosomal DNA.
Conclusions.
In this study, we found that the gene transfer capacity of the SXT element goes beyond its self-transfer. In E. coli K-12, we showed that the SXT element also mobilizes certain plasmids in trans and transfers chromosomal DNA in a directional fashion in cis. These findings confirm that conjugation is the mechanism of SXT element transfer. However, as a conjugative gene transfer system, the SXT element has distinct features compared with conjugative plasmids and conjugative transposons. First, compared to other known conjugation systems in gram-negative bacteria (4), the SXT element transfer system is more selective and less efficient with regard to the plasmids it can mobilize. Second, the SXT element mobilized RSF1010 in an oriT-independent manner. As we excluded cointegrate formation between RSF1010 and the SXT element, other mechanisms must account for this oriT-independent mobilization of RSF1010. One possibility is that either the SXT element or the RSF1010 MobA can recognize and nick a different RSF1010 sequence that can serve as an alternative origin of transfer. Since other conjugative transfer systems such as RP4 (10) and the icm-dot system (29) cannot mobilize an oriT-deleted RSF1010, it seems more likely that an alternative oriT is recognized by an SXT element-encoded nickase rather than by the RSF1010 MobA. It is also possible that SXT element-mediated transfer of RSF1010 proceeds via a mechanism independent of an oriT and results in the transfer of a double-stranded plasmid to recipient cells. If such a process occurs, there must be some specific interaction between the SXT element-encoded conjugative machinery and RSF1010, because we did not observe transfer of other mobilizable plasmids like pSU4601 and pSU4620. We are currently investigating which sequences of RSF1010 and the SXT element are required for this unexpected oriT-independent transfer of RSF1010.
We found that the SXT element int is not required for mobilization of plasmids or chromosomal DNA. Thus, similar to integrated conjugative plasmids such as F (8), but unlike other obligate integrated elements such as Tn916 (5), the expression of SXT element transfer functions is not dependent on its excision. For Tn916, the most thoroughly studied conjugative transposon of gram-positive bacteria, excision is required for expression of the transposon-encoded transfer functions (5). However, the transfer frequency of Tn916 is not determined by its frequency of excision, indicating that other factors in addition to excision regulate transfer of this conjugative transposon (18). The coupling of excision and transfer could explain why transfer of chromosomal DNA has not been reported for Tn916. It will be interesting to see whether other constins, such as CTnscr94 from enterobacteria (13), Tn5276 from Lactococcus lactis (21), the clc element from Pseudomonas putida (22), the Mesorhizobium loti symbiosis island (28), and the Tcr elements (24), will also be found to be capable of transfer of chromosomal DNA in a manner similar to the SXT element.
Transfer of linked chromosomal DNA by the SXT element may be an important mechanism of cross-species gene transfer. Presumably, any DNA sequence within about 500 kbp of the 3′ end of prfC could be mobilized by the SXT element. In the era of sequenced microbial genomes, this large stretch of DNA can be examined in a number of bacterial species to identify genes that could have been mobilized by the SXT element. For example, we wondered whether the wfb gene cluster, which is thought to be horizontally transmitted in V. cholerae populations (16), is closely linked to the V. cholerae prfC. This turned out not to be the case, as the wfb region maps about 460 kbp 5′ of prfC. Therefore, it seems unlikely that the SXT element played a role in the mobilization of the O139 wfb cluster from some donor strain into an El Tor V. cholerae O1 strain to give rise to V. cholerae O139. However, the V. cholerae pathogenicity island, which encodes TCP pili, maps only about 200 kbp 3′ of prfC. Although the entire V. cholerae pathogenicity island has recently been reported to be self-transmissible as a bacteriophage (17), the SXT element could provide an alternative pathway for mobilization of this virulence gene cluster. Similarly, the S. enterica serovar Typhimurium pathogenicity island encoding SigE, a factor required for invasion of host cells, is located at about 25 min (15). We were able to transfer a marked version of sigE at a very low frequency, in an SXT element-dependent fashion between S. enterica serovar Typhimurium strains (our unpublished results). This raises the possibility that the SXT element or perhaps similar constins may play a role in the mobilization of pathogenicity islands (whose mechanism of mobility is generally not understood) as well as other chromosomally encoded virulence genes. Finally, the SXT element may be a useful tool for mobilization of linked chromosomal genes in bacterial species like V. cholerae where there are currently no Hfr-like elements available. The expanded potential of bacteria harboring the SXT element to engage in horizontal gene transfer may be an explanation for the widespread dissemination of the SXT element and similar elements in bacterial populations.
Acknowledgments
We are grateful to J. P. Vogel, F. de la Cruz, V. L. Miller, and C. A. Lee for kindly providing us with plasmids and strains. We appreciate the helpful suggestions of A. Wright. We also thank A. Kane, B. Davis, M. Malamy, D. RayChaudhuri, A. Camilli, and H. Kimsey for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (B.H.), NIH grant AI42347 (M.K.W.), a PEW Scholar Award (M.K.W.), and P30DK-34928 (for the NEMC GRASP Center). J.M. was supported by the NIH Short-Term Training Program for minority students (2 T35 HL07785-06).
REFERENCES
- 1.Anilionis A, Ostapchuk P, Riley M. Identification of a second cryptic lambdoid prophage locus in the E. coli K-12 chromosome. Mol Gen Genet. 1980;180:479–481. doi: 10.1007/BF00425865. [DOI] [PubMed] [Google Scholar]
- 2.Berlyn M K B. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchanan-Wollaston V, Passiatore J E, Cannon F. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature. 1987;328:172–175. [Google Scholar]
- 4.Cabezón E, Sastre J I, de la Cruz F. Genetic evidence of a coupling role of the TraG protein family in bacterial conjugation. Mol Gen Genet. 1997;254:400–406. doi: 10.1007/s004380050432. [DOI] [PubMed] [Google Scholar]
- 5.Celli J, Trieu-Cout P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–117. doi: 10.1046/j.1365-2958.1998.00778.x. [DOI] [PubMed] [Google Scholar]
- 6.Cholera Working Group. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firth N, Ippen-Ihler K, Skurray R A. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 2377–2401. [Google Scholar]
- 9.Frey J, Bagdasarian M. The molecular biology of the IncQ plasmids. In: Thomas C M, editor. Promiscous plasmids of Gram-negative bacteria. London, United Kingdom: Academic Press Ltd.; 1989. pp. 79–94. [Google Scholar]
- 10.Frey J, Bagdasarian M M, Bagdasarian M. Replication and copy number control of the broad-host-range plasmid RSF1010. Gene. 1992;113:101–106. doi: 10.1016/0378-1119(92)90675-f. [DOI] [PubMed] [Google Scholar]
- 11.Guiney D. Broad host range conjugative and mobilizable plasmids in Gram-negative bacteria. In: Clewell D, editor. Bacterial conjugation. New York, N.Y: Plenum Publishing Corp.; 1993. pp. 75–102. [Google Scholar]
- 12.Hayes W. The mechanism of genetic recombination in E. coli. Cold Spring Harbor Symp Quant Biol. 1953;18:75–93. doi: 10.1101/sqb.1953.018.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Hochhut B, Jahreis K, Lengeler J W, Schmid K. CTnscr94, a conjugative transposon found in enterobacteria. J Bacteriol. 1997;179:2097–2102. doi: 10.1128/jb.179.7.2097-2102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochhut B, Waldor M K. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol Microbiol. 1999;32:99–110. doi: 10.1046/j.1365-2958.1999.01330.x. [DOI] [PubMed] [Google Scholar]
- 15.Hong K H, Miller V L. Identification of a novel Salmonella invasion locus homologous to Shigella ipgDE. J Bacteriol. 1998;180:1793–1802. doi: 10.1128/jb.180.7.1793-1802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaolis D K, Lan R, Reeves P R. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol. 1995;177:3191–3198. doi: 10.1128/jb.177.11.3191-3198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaolis D K R, Somara S, Maneval D R, Jr, Johnson J A, Kaper J B. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature. 1999;399:375–379. doi: 10.1038/20715. [DOI] [PubMed] [Google Scholar]
- 18.Marra D, Pethel B, Churchward G G, Scott J R. The frequency of conjugative transposition of Tn916 is not determined by the frequency of excision. J Bacteriol. 1999;181:5414–5418. doi: 10.1128/jb.181.17.5414-5418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalf W W, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 20.Nichols B P, Shafiq O, Meiners V. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain construction. J Bacteriol. 1998;180:6408–6411. doi: 10.1128/jb.180.23.6408-6411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauch P J G, de Vos W M. Identification and characterization of genes involved in excision of the Lactococcus lactis conjugative transposon Tn5276. J Bacteriol. 1994;176:2165–2171. doi: 10.1128/jb.176.8.2165-2171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravatn R, Studer S, Springael D, Zehnder A J B, van der Meer J R. Chromosomal integration, tandem amplification, and deamplification in Pseudomonas putida F1 of a 105-kilobase genetic element containing the chlorocatechol degradative genes from Pseudomonas sp. strain B13. J Bacteriol. 1998;180:4360–4369. doi: 10.1128/jb.180.17.4360-4369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salyers A A, Shoemaker N B, Li L-Y. In the driver's seat: the Bacteroides conjugative transposons and the elements they mobilize. J Bacteriol. 1995;177:5727–5731. doi: 10.1128/jb.177.20.5727-5731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salyers A A, Shoemaker N B, Stevens A M, Li L-Y. Conjugative transposons: an unusual and diverse set of integrated gene transfer elements. Microbiol Rev. 1995;59:579–590. doi: 10.1128/mr.59.4.579-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal G, Purcell M W, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22 kb region of the Legionella pneumophila genome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Showsh S A, Andrews R E., Jr Analysis of the requirement for a pUB110 mob region during Tn916-dependent mobilization. Plasmid. 1999;41:179–186. doi: 10.1006/plas.1999.1398. [DOI] [PubMed] [Google Scholar]
- 27.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:6408–6411. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan J T, Ronson C W. Evolution of rhizobia by acquisition of a 500 kb symbiosis island that integrates into a phe-tRNA gene. Proc Natl Acad Sci USA. 1998;95:5145–5149. doi: 10.1073/pnas.95.9.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel P J, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 30.Waldor M K, Tschäpe H, Mekalanos J J. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996;178:4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willetts N. Interactions between the F conjugal transfer system and CloDF13::TnA plasmids. Mol Gen Genet. 1980;180:213–217. doi: 10.1007/BF00267372. [DOI] [PubMed] [Google Scholar]
- 32.Wollman E-L, Jacob F, Hayes W. Conjugation and genetic recombination in Escherichia coli K-12. Cold Spring Harbor Symp Quant Biol. 1956;21:141–162. doi: 10.1101/sqb.1956.021.01.012. [DOI] [PubMed] [Google Scholar]