Take Home Message
Rectal injury is a rare, but potentially devastating, complication following radical prostatectomy. Its incidence is higher in patients ≥60 yr of age, and in those undergoing open/laparoscopic versus robotic prostatectomy or salvage prostatectomy after radiation therapy. Intraoperative detection and repair constitute the most critical step to decrease the risk of severe postoperative complications including rectourethral fistula.
Keywords: Prostate cancer, Radical prostatectomy, Rectal injury, Complications, Systematic review
Abstract
Context
Rectal injury (RI) is a dreaded complication after radical prostatectomy (RP), increasing the risk of early postoperative complications, such as bleeding and severe infection/sepsis, and late sequelae, such as a rectourethral fistula (RUF). Considering its traditionally low incidence, uncertainty remains as to predisposing risk factors and management.
Objective
To examine the incidence of RI after RP in contemporary series and to propose a pragmatic algorithm for its management.
Evidence acquisition
A systematic literature search was performed using the Medline and Scopus databases. Studies reporting data on RI incidence were selected. Subgroup analyses were conducted to assess the differential incidence by age, surgical approach, salvage RP after radiation therapy, and previous benign prostatic hyperplasia (BPH)-related surgery.
Evidence synthesis
Eighty-eight, mostly retrospective noncomparative, studies were selected. The meta-analysis obtained a pooled RI incidence of 0.58% (95% confidence interval [CI] 0.46–0.73) in contemporary series with significant across-study heterogeneity (I2 = 100%, p < 0.00001). The highest RI incidence was found in patients undergoing open RP (1.25%; 95% CI 0.66–2.38) and laparoscopic RP (1.25%; 95% CI 0.75–2.08) followed by perineal RP (0.19%; 95% CI 0–276.95) and robotic RP (0.08%; 95% CI 0.02–0.31). Age ≥60 yr (0.56%; 95% CI 0.37–06) and salvage RP after radiation therapy (6.01%; 95% CI 3.99–9.05), but not previous BPH-related surgery (4.08%, 95% CI 0.92–18.20), were also associated with an increased RI incidence. Intraoperative versus postoperative RI detection was associated with a significantly decreased risk of severe postoperative complications (such as sepsis and bleeding) and subsequent formation of a RUF.
Conclusions
RI is a rare, but potentially devastating, complication following RP. RI incidence was higher in patients ≥60 yr of age, and in those who underwent open/laparoscopic approach or salvage RP after radiation therapy. Intraoperative RI detection and repair apparently constitute the single most critical step to significantly decrease the risk of major postoperative complications and subsequent RUF formation. Conversely, intraoperatively undetected RI can lead more often to severe infective complications and RUF, the management of which remains poorly standardised and requires complex procedures.
Patient summary
Accidental rectum tear is a rare, but potentially devastating, complication in men undergoing prostate removal for cancer. It occurs more often in patients aged 60 yr or older as well as in those who underwent prostate removal via an open/laparoscopic approach and/or prostate removal after radiation therapy for recurrent disease. Prompt identification and repair of this condition during the initial operation are the key to reduce further complications such as the formation of an abnormal opening between the rectum and the urinary tract.
1. Introduction
Rectal injury (RI) is a dreaded complication after radical prostatectomy (RP). During retropubic RP (RRP), RI can occur mainly during apical dissection when attempting to develop the posterior plane between the rectum and the Denonvilliers’ fascia layers [1], while during perineal RP (PRP) the critical step is the release of the posterior prostate side from the rectum. Conversely, during laparoscopic (LRP) or robot-assisted (RARP) RP, RI is more frequently caused during seminal vesicle isolation and development of the posterior plane between the prostate and the rectal wall.
RI can be detected intraoperatively or postoperatively, or can remain undiagnosed. Regardless of the timing of detection, RI can significantly increase the risk of early postoperative complications, such as bleeding and severe infection/sepsis, and of late sequelae, such as a rectourethral fistula (RUF) [2]. For these reasons, many patients with RI after RP require a reintervention and/or complex surgical procedures with the possibility of ending with a transitory or definitive bowel and/or urinary diversion [3].
Considering the traditionally low incidence of RI after RP, uncertainty remains as to predisposing risk factors and management, which is of primary concern when RI and its sequelae become an object of medicolegal litigations.
The aim of the present systematic review with meta-analysis was to evaluate the overall incidence of RI after RP in contemporary series as well as stratified by chronological age, surgical approach, timing (primary or salvage), and previous benign prostatic hyperplasia (BPH)-related surgery status. Moreover, a pragmatic algorithm for the management of intra- and postoperatively detected RI was proposed.
2. Evidence acquisition
This systematic review with meta-analysis was conducted in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Supplementary Table 1) [4]. The protocol was registered on the International Prospective Register of Systematic Reviews PROSPERO (CRD42022381075).
There was no financial or nonfinancial support for this review, and the authors declare no competing interests.
2.1. Search strategy
A literature search was conducted up to October 15, 2022, using the Medline and Scopus databases. The search strategy is reported in Supplementary Table 2. The following limits were used: humans, gender (male), English language, and publication dates from the inception to search date.
Three independent authors manually performed initial screening of the available literature. The reasons for study exclusions were noted. Additional studies of potential interest cited in the reference list of selected papers were also screened. A critical evaluation of the selected studies was performed, and relevant reports were subjected to a full-text review. All discrepancies were resolved by consensus with all coauthors.
2.2. Inclusion and exclusion criteria, and data extraction
Studies were included if these reported on men with clinically localised prostate cancer (PCa; Patients) who underwent RP (Intervention) with different chronological age, type of surgical approach, timing (primary vs salvage RP), and previous BPH-related surgery status (Comparisons) in terms of RI incidence and management (Outcome). Meeting abstracts, case reports, editorials, letters, reviews, and articles not published in English were excluded.
Data were manually extracted independently by two authors. The following variables were collected: first author’s name, publication year, total RP cohort size, chronological age, previous BPH-related surgery status, type of surgical approach (RRP, PRP, LRP, or RARP), RP timing (primary or salvage), number of RI cases, number of RI cases detected intraoperatively and postoperatively, number of RI cases managed with a temporary colostomy, and number of RI cases developing a RUF. Retrieved data were stored in an electronic database, and quality data control was performed on a random sample of papers accounting for about 15% of the total. All discrepancies were resolved by consensus with all coauthors.
2.3. Level of evidence
The level of evidence for each study was assigned independently by two authors according to the Oxford Centre for Evidence-Based Medicine criteria [5]. Any discrepancies were resolved by consensus with all coauthors.
2.4. Statistical analyses
Statistical analyses were performed using RevMan version 5.4 (Cochrane Collaboration, Oxford, UK). RI incidence with 95% confidence intervals (CIs) was calculated using the generic inverse variance, and forest plots were generated to visually display the results. A p value of <0.05 was set as significance level when comparing studies. The Cochrane chi-square test was used to evaluate heterogeneity across studies, with p < 0.05 indicating heterogeneity. To estimate the impact of heterogeneity on the meta-analysis, I2 value was calculated. I2 value ≥50% and p < 0.05 indicated a moderate to high degree of heterogeneity among pooled studies. A fixed-effect design was used for an I2 value of <50% and p > 0.05. Otherwise, a random-effect model was adopted. We also performed a sensitivity analysis to assess the possible sources of heterogeneity. Egger’s test via Statistical Package for Social Sciences (SPSS) v.25 and visual inspection of funnel plot symmetry were used to assess the publication bias.
Subgroup analyses were preplanned based on study sample size (>500 patients) and publication decade (years before 2000, 2001–2010, and after 2011), as well as on traditional predisposing clinical factors, such as chronological age, type of RP surgical approach, RP timing (primary or salvage), and previous BPH-related surgery status.
3. Evidence synthesis
3.1. Study selection and characteristics
According to our search strategy, a total of 1806 records were identified. Of these, 1628 records were screened. After exclusion of 1556 studies, 72 full-text articles were assessed for eligibility and included in the review. Sixteen articles identified from the references of the included studies were further added. Therefore, a total of 88 out of 1806 (4.8%) studies were eventually selected for the present systematic review (Fig. 1). Twenty-eight (32%) were comparative studies, of which 24 (27%) were retrospective level 4, two (2%) were prospective nonrandomised level 3, and two (2%) were prospective randomised level 2 studies. The remaining 60 (68%) were retrospective noncomparative level 4 studies. Study characteristics are detailed in Table 1 [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93].
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart detailing the study selection process.
Table 1.
Characteristics of the selected studies reporting on the incidence of rectal injury after radical prostatectomy
| No. | First author (year) | OCEBM level of evidence | RP approach | Mean age (yr) | RP cases (n) | RI cases (n) | RI incidence (%) | Comparative study | Comparison |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Neerhut (1988) [62] | 4 | RRPa | – | 16 | 3 | 18.75 | ||
| 2 | Moul (1991) [59] | 4 | RRPa | – | 10 | 1 | 10.00 | ||
| 3 | Borland (1992) [14] | 4 | RRP | 59 | 1000 | 10 | 1.00 | ||
| 4 | Stein (1992) [80] | 4 | RRPa | – | 13 | 1 | 7.69 | ||
| 5 | McLaren (1993) [57] | 4 | RRP | 60 | 2212 | 27 | 1.22 | ||
| 6 | Pontes (1993) [68] | 4 | RRPa | – | 43 | 4 | 9.30 | ||
| 7 | Zincke (1994) [93] | 4 | RRP | – | 1143 | 14 | 1.22 | ||
| 8 | Lerner (1995) [48] | 4 | RRP | – | 132 | 6 | 4.55 | ||
| 9 | Rogers (1995) [73] | 4 | RRPa | – | 29 | 6 | 20.69 | ||
| 10 | Gheiler (1998) [26] | 4 | RRPa | – | 40 | 1 | 2.50 | ||
| 11 | Lepor (2001) [47] | 4 | RRP | 60.3 | 1000 | 5 | 0.50 | ||
| 12 | Arai (2003) [6] | 4 | LRP | 68 | 148 | 10 | 6.76 | ||
| 13 | Artibani (2003) [7] | 4 | RRP (a) | 64.28 | 50 | 0 | 0 | × | RP approach |
| LRP (b) | 63.14 | 71 | 2 | 2.82 | |||||
| 14 | Augustin (2003) [8] | 4 | RRP | 62.1 | 1243 | 3 | 0.24 | ||
| 15 | Guillonneau (2003) [33] | 4 | LRP | 66.5 | 1000 | 13 | 1.30 | ||
| 16 | Maffezzini (2003) [53] | 4 | RRP | 61.83 | 300 | 1 | 0.33 | ||
| 17 | Rassweiler (2003) [70] | 4 | RRP (a) | 65 | 219 | 4 | 1.83 | × | RP approach |
| LRP (b) | 64 | 436 | 10 | 2.29 | |||||
| 18 | Tewari (2003) [85] | 3 | RRP (a) | 63.1 | 100 | 1 | 1.00 | × | RP approach |
| RARP (c) | 59.9 | 200 | 0 | 0 | |||||
| 19 | Ruiz (2004) [74] | 4 | LRP (b1) | 64.1 | 165 | 4 | 2.42 | × | Transperitoneal access |
| LRP (b2) | 62.9 | 165 | 2 | 1.21 | Extraperitoneal access | ||||
| 20 | Stephenson (2004) [81] | 4 | RRPa | 63 | 100 | 7 | 7.00 | ||
| 21 | Gonzalgo (2005) [29] | 4 | LRP | 57.6 | 246 | 2 | 0.81 | ||
| 22 | Singh (2005) [77] | 4 | LRP | 63 | 70 | 1 | 1.43 | ||
| 23 | Remzi (2005) [71] | 4 | RRP (a) | 60 | 41 | 1 | 2.44 | × | RP approach |
| LRP (b) | 60 | 80 | 1 | 1.25 | |||||
| 24 | Ward (2005) [89] | 4 | RRPa | 65.1 | 199 | 31 | 15.58 | ||
| 25 | Cohen (2006) [18] | 4 | LRP (b1) | 59.6 | 93 | 0 | 0 | × | Transperitoneal access |
| LRP (b2) | 57.9 | 172 | 1 | 0.58 | Extraperitoneal access | ||||
| 26 | Eden (2006) [23] | 4 | LRP | 62.04 | 505 | 5 | 0.99 | × | Body mass index |
| 27 | Galli (2006) [24] | 4 | LRP | 64 | 150 | 1 | 0.67 | ||
| 28 | Goeman (2006) [27] | 4 | LRP | 62.4 | 550 | 3 | 0.55 | ||
| 29 | Guazzoni (2006) [32] | 2 | RRP (a) | 62.9 | 60 | 0 | 0 | × | RP approach |
| LRP (b) | 62.29 | 60 | 1 | 1.67 | |||||
| 30 | Hu (2006) [40] | 4 | LRP (b) | 63.7 | 358 | 9 | 2.51 | × | RP approach |
| RARP (c) | 62.1 | 322 | 0 | 0 | |||||
| 31 | Lein (2006) [46] | 4 | LRP | 62 | 1000 | 47 | 4.70 | ||
| 32 | Porpiglia (2006) [69] | 4 | LRP (b1) | 64.25 | 80 | 1 | 1.25 | × | Transperitoneal access |
| LRP (b2) | 64.4 | 80 | 0 | 0 | Extraperitoneal access | ||||
| 33 | Sanderson (2006) [75] | 4 | RRPa | – | 51 | 1 | 1.96 | ||
| 34 | Badani (2007) [9] | 4 | RARP | 60.2 | 2766 | 4 | 0.14 | ||
| 35 | Boczko (2007) [13] | 4 | RARP | 59.5 | 355 | 1 | 0.28 | × | Prostate volume |
| 36 | Klevecka (2007) [45] | 4 | RRP | 65 | 1000 | 21 | 2.10 | ||
| 37 | Li (2007) [49] | 4 | LRP | 64.96 | 59 | 2 | 3.39 | ||
| 38 | Srinualnad (2007) [79] | 4 | LRP | 68.46 | 41 | 2 | 4.88 | ||
| 39 | Carini (2008) [15] | 4 | RRP | 64.5 | 488 | 3 | 0.61 | ||
| 40 | Ham (2008) [36] | 4 | RRP (a) | 66.09 | 199 | 3 | 1.51 | × | RP approach |
| RARP (c) | 67.14 | 223 | 2 | 0.90 | |||||
| 41 | Tozawa (2008) [87] | 4 | LRP | 67.2 | 160 | 4 | 2.50 | ||
| 42 | Blumberg (2009) [12] | 4 | LRP | – | 200 | 2 | 1.00 | ||
| 43 | Constantinides (2009) [19] | 4 | RRP | 63.2 | 995 | 10 | 1.01 | ||
| 44 | Gao (2009) [25] | 4 | LRP | 62.5 | 126 | 2 | 1.59 | ||
| 45 | Mariano (2009) [55] | 4 | LRP | 64.6 | 780 | 6 | 0.77 | ||
| 46 | Murphy (2009) [60] | 4 | RARP | 60.2 | 400 | 5 | 1.25 | ||
| 47 | Stolzenburg (2009) [82] | 4 | LRP | 63.3 | 2400 | 12 | 0.50 | ||
| 48 | Ou (2009) [65] | 4 | RRP (a) | 67.27 | 30 | 1 | 3.33 | × | RP approach |
| RARP (c) | 70.03 | 30 | 0 | 0 | |||||
| 49 | Paiva (2009) [67] | 2 | RRP (a) | 63.45 | 40 | 0 | 0 | × | RP approach and anaesthesia technique |
| PRP (d) | 62.55 | 40 | 2 | 5 | |||||
| 50 | Teber (2009) [84] | 4 | LRP (b3) | 66 | 55 | 1 | 1.81 | × | Previous TURP |
| LRP (b4) | 65.6 | 55 | 1 | 1.81 | No previous TURP | ||||
| 51 | Carlsson (2010) [16] | 4 | RRP (a) | 58.33 | 485 | 8 | 1.65 | × | RP approach |
| RARP (c) | 62.33 | 1253 | 2 | 0.16 | |||||
| 52 | Gotto (2010) [30] | 4 | RRP (a) | – | 3458 | 23 | 0.67 | × | RP timing (upfront vs salvage) |
| RRP (a)a | – | 98 | 9 | 9.18 | |||||
| 53 | Greco (2010) [31] | 4 | RRP (a) | 61.5 | 150 | 1 | 0.67 | × | RP approach |
| LRP (b) | 60.5 | 150 | 2 | 1.33 | |||||
| 54 | Heidenreich (2010) [37] | 4 | RRPa | – | 55 | 2 | 3.64 | ||
| 55 | Hruza (2010) [39] | 4 | LRP | 63.8 | 2200 | 33 | 1.50 | ||
| 56 | Jeong (2010) [43] | 4 | RARP | 58.8 | 200 | 2 | 1.00 | ||
| 57 | Löppenberg (2010) [51] | 4 | RRP | – | 2893 | 2 | 0.07 | ||
| 58 | Lowrance (2010) [52] | 4 | RRP (a) | – | 4697 | 115 | 2.45 | × | RP approach |
| LRP (b) | – | 1006 | 41 | 4.08 | |||||
| 59 | Masuda (2010) [56] | 4 | LRP | – | 294 | 5 | 1.70 | ||
| 60 | McNeill (2010) [58] | 4 | LRP | 62.06 | 300 | 3 | 1.00 | ||
| 61 | Novara (2010) [63] | 4 | RARP | 62.3 | 415 | 5 | 1.20 | ||
| 62 | Roberts (2010) [72] | 4 | RRP (a) | – | 10 183 | 12 | 0.12 | × | RP approach |
| LRP (b) | – | 1269 | 6 | 0.47 | |||||
| 63 | Chung (2011) [17] | 4 | RARP (c1) | 66.3 | 105 | 1 | 1 | × | Transperitoneal access |
| RARP (c2) | 65.8 | 155 | 0 | 0 | Extraperitoneal access | ||||
| 64 | Di Pierro (2011) [20] | 3 | RRP (a) | 64.3 | 75 | 1 | 1.33 | × | RP approach |
| RARP (c) | 62.8 | 75 | 0 | 0 | |||||
| 65 | Kheterpal (2011) [44] | 4 | RARP | 58.6 | 4400 | 10 | 0.23 | ||
| 66 | Ou (2011) [66] | 4 | RARP | 65.05 | 200 | 3 | 1.50 | ||
| 67 | Wedmid (2011) [91] | 4 | RARP | 62.5 | 6650 | 11 | 0.17 | ||
| 68 | Do (2012) [21] | 4 | LRP | 64 | 233 | 2 | 0.86 | ||
| 69 | Dogra (2012) [22] | 4 | RARP | 65 | 190 | 1 | 0.53 | ||
| 70 | Schmitges (2012) [76] | 4 | RRP/LRP/RARP | 61.5 | 36 699 | 264 | 0.72 | ||
| 71 | Sood (2012) [78] | 4 | PRP | 65.8 | 35 | 3 | 8.57 | ||
| 72 | Webster (2012) [90] | 4 | RRP | 63.4 | 133 | 1 | 0.75 | ||
| 73 | Yıldırım (2012) [92] | 4 | RRP (a) | – | 218 | 1 | 0.46 | × | RP approach |
| PRP (d) | 233 | 6 | 2.58 | ||||||
| 74 | Liss (2013) [50] | 4 | RARP | 61.2 | 1000 | 1 | 0.10 | ||
| 75 | Thiel (2013) [86] | 4 | RARP | 60 | 100 | 1 | 1.00 | ||
| 76 | Hung (2014) [41] | 4 | RARP (c3) | 67.5 | 16 | 3 | 18.75 | × | Previous TURP |
| RARP (c4) | 64.8 | 184 | 0 | 0 | No previous TURP | ||||
| 77 | Sugihara (2014) [83] | 4 | RRP/LRP/RARP | 66.99 | 35 099 | 151 | 0.43 | ||
| 78 | Myers (2016) [61] | 4 | RRP/PRP/LRP/RARPb | 63.31 | 2245 | 9 | 0.40 | ||
| 79 | Jakóbczyk (2017) [42] | 4 | LRP | 65.3 | 30 | 3 | 10.00 | ||
| 80 | Verze (2017) [88] | 4 | LRP (b3) | 67.2 | 98 | 2 | 2.04 | × | Previous prostate surgery |
| LRP (b4) | 65.1 | 848 | 1 | 0.12 | No previous prostate surgery | ||||
| 81 | Barashi (2018) [10] | 4 | RRP (a) | – | 414 561 | 2379 | 0.57 | × | RP approach |
| LRP (b) | – | 38 226 | 142 | 0.37 | |||||
| RARP (c) | – | 161 507 | 379 | 0.23 | |||||
| 82 | Mandel (2018) [54] | 4 | RRP (a) | 63.73 | 19 965 | 104 | 0.52 | × | RP approach and RP timing (upfront vs salvage) |
| RARP (c) | 4111 | 9 | 0.22 | ||||||
| RRPa (a) | 102 | 7 | 6.86 | ||||||
| 83 | Onaca (2018) [64] | 4 | LRP | 68 | 45 | 1 | 2.22 | ||
| 84 | Gontero (2019) [28] | 4 | RARPa (c) | 66.1 | 209 | 1 | 0.48 | × | RP approach |
| RRPa (a) | 66.43 | 186 | 5 | 2.69 | |||||
| 85 | Hajili (2019) [35] | 4 | RRP/RARP | 64 | 116 | 3 | 2.59 | Previous ADT | |
| 86 | Beech (2020) [11] | 4 | RARP | 63.5 | 2821 | 8 | 0.28 | ||
| 87 | Haeuser (2022) [34] | 4 | RRP (a) | – | 6522 | 27 | 0.41 | × | RP approach |
| LRP/RARP (b,c) | – | 6522 | 7 | 0.11 | |||||
| 88 | Hoeh (2022) [38] | 4 | RRP | 63.5 | 22 | 1 | 4.55 | Previous ADT |
ADT = androgen deprivation therapy; LRP = laparoscopic radical prostatectomy; OCEBM = Oxford Centre for Evidence-Based Medicine; PRP = perineal radical prostatectomy; RARP = robot-assisted radical prostatectomy; RI = rectal injury; RP = radical prostatectomy; RRP = retropubic radical prostatectomy; TURP = transurethral resection of the prostate.
Studies are listed by publication year.
In the table, (a), (b), (c), and (d) denote study subgroups as follows: (a) = RRP; (b) = LRP, (b1) = transperitoneal access, (b2) = extraperitoneal access, (b3) = previous prostate surgery, (b4) = no previous prostate surgery; (c) = RARP, (c1) = transperitoneal access, (c2) = extraperitoneal access, (c3) = previous prostate surgery, (c4) = no previous prostate surgery; and (d) = PRP.
Salvage prostatectomy.
Including salvage prostatectomy.
3.2. RI incidence
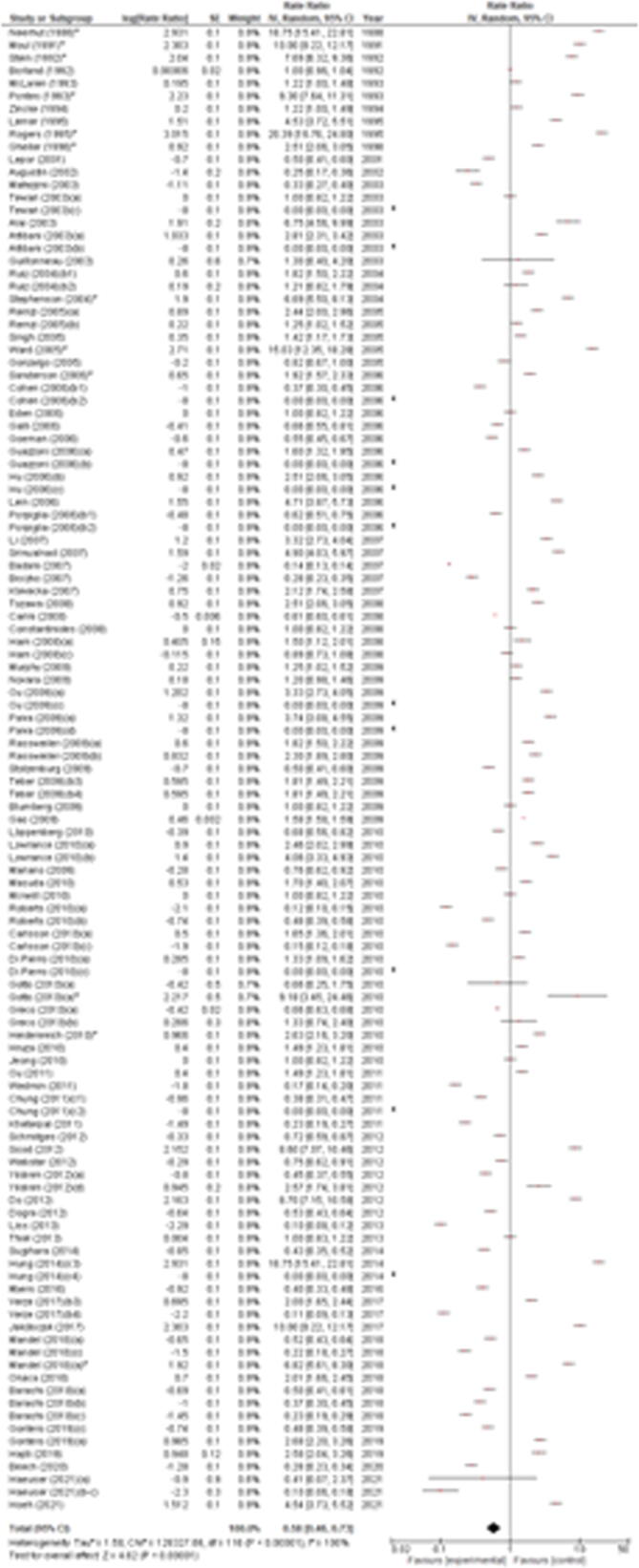
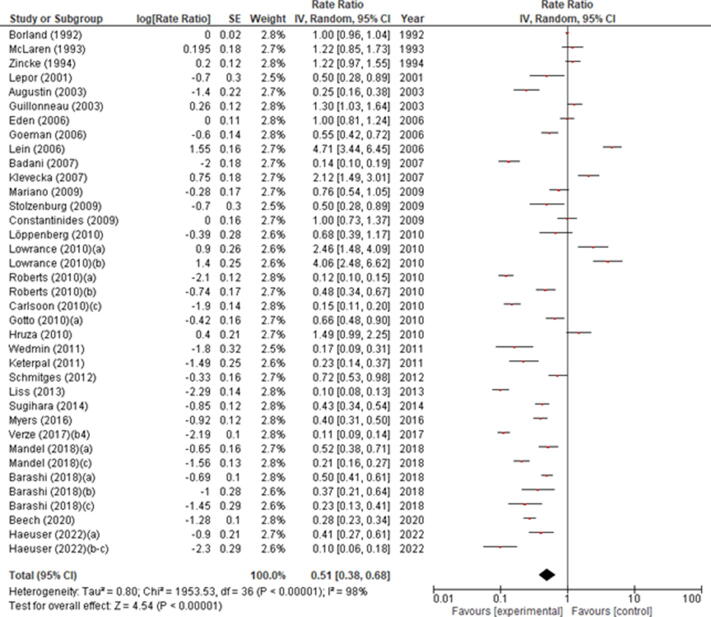
RI incidence ranged from 0% to 20.39%, with significant differences between studies (p < 0.00001). Based on the random-effect model, the meta-analysis of all included studies showed a pooled estimated incidence of 0.58% (95% CI 0.46–0.73) with significant across-study heterogeneity (I2 = 100%, p < 0.00001; Fig. 2). In the subgroup analysis of studies including >500 cases only, RI incidence ranged from 0.10% to 4.71%, with significant differences between studies (p < 0.0001). Based on the random-effect model, the meta-analysis of only these studies showed a pooled estimated incidence of 0.51% (95% CI 0.38–0.68) with significant across-study heterogeneity (I2 = 98%, p < 0.00001; Supplementary Fig. 1). The leave-one-out sensitivity analysis showed that RI incidence ranged from 1.25% (95% CI 1.06–1.46) to 1.31% (95% CI 1.14–1.51), indicating that the meta-analysis had strong reliability (Supplementary Table 3). This was confirmed when considering only studies including >500 cases, where RI incidence ranged from 0.52% (95% CI 0.39–0.69) to 0.59% (95% CI 0.44–0.78; Supplementary Table 4).
Fig. 2.
Forest plot of the meta-analysis of the incidence of rectal injury after radical prostatectomy (all studies). Studies are listed by publication year. The letters a, b, c, and d denote study subgroups as per Table 1. CI = confidence interval; IV = inverse variance. *Salvage prostatectomy.
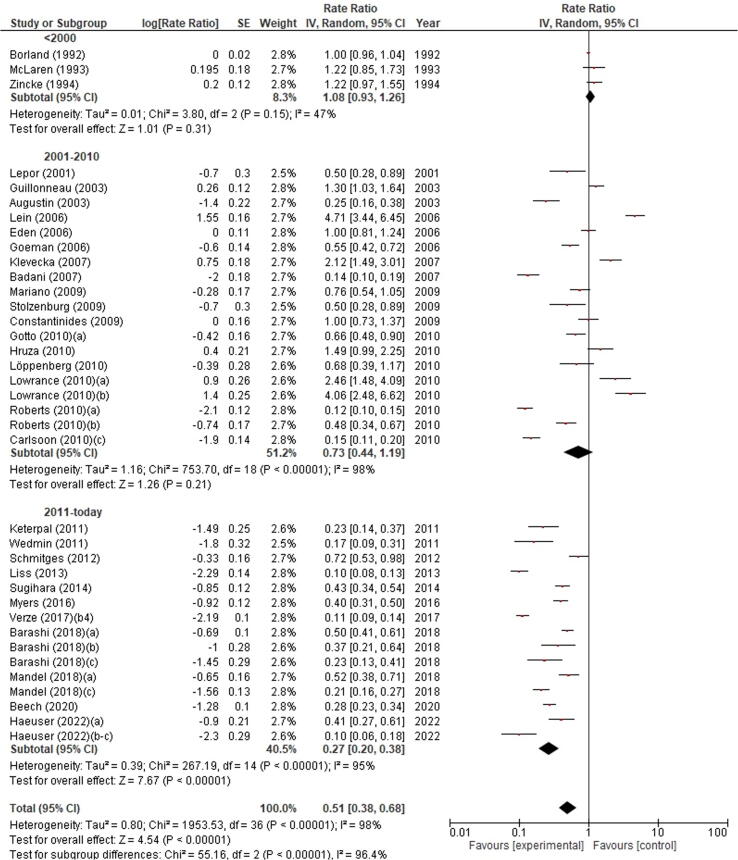
Based on the random-effect model, the forest plot analysis including only studies with >500 cases revealed that RI incidence was significantly different according to the decade of publication of selected studies (p < 0.00001). Indeed, the highest RI incidence was observed in studies published before 2000 (1.08%, 95% CI 0.93–1.26), followed by studies published between 2001 and 2010 (0.73%, 95% CI 0.44–1.19), and then studies published after 2011 (0.27%, 95% CI 0.20–0.38; Fig. 3).
Fig. 3.
Forest plot of the meta-analysis of the incidence of rectal injury after radical prostatectomy stratified by publication decade. Studies are listed by publication year. The letters a, b, c denote study subgroups as per Table 1. CI = confidence interval; IV = inverse variance.
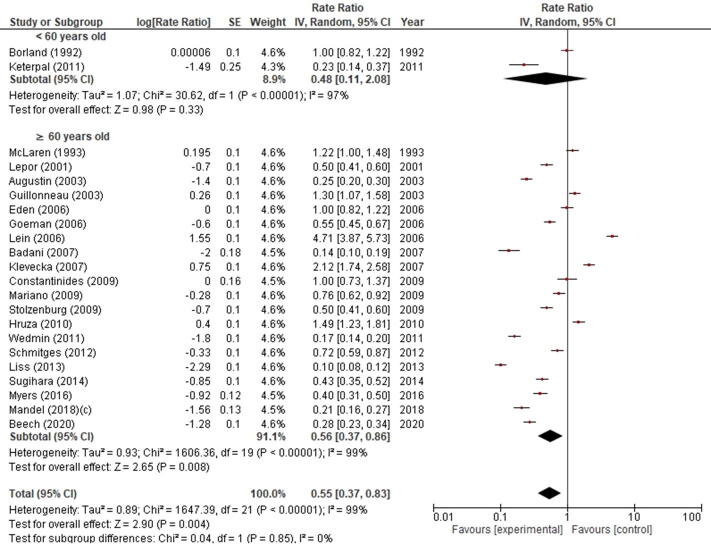
RI incidence was significantly different when patients were stratified by chronological age (p = 0.004). Indeed, RI incidence was 0.56% (95% CI 0.37–0.86) in patients ≥60 yr of age and 0.48% (95% CI 0.11–2.08) in their younger counterparts (Supplementary Fig. 2).
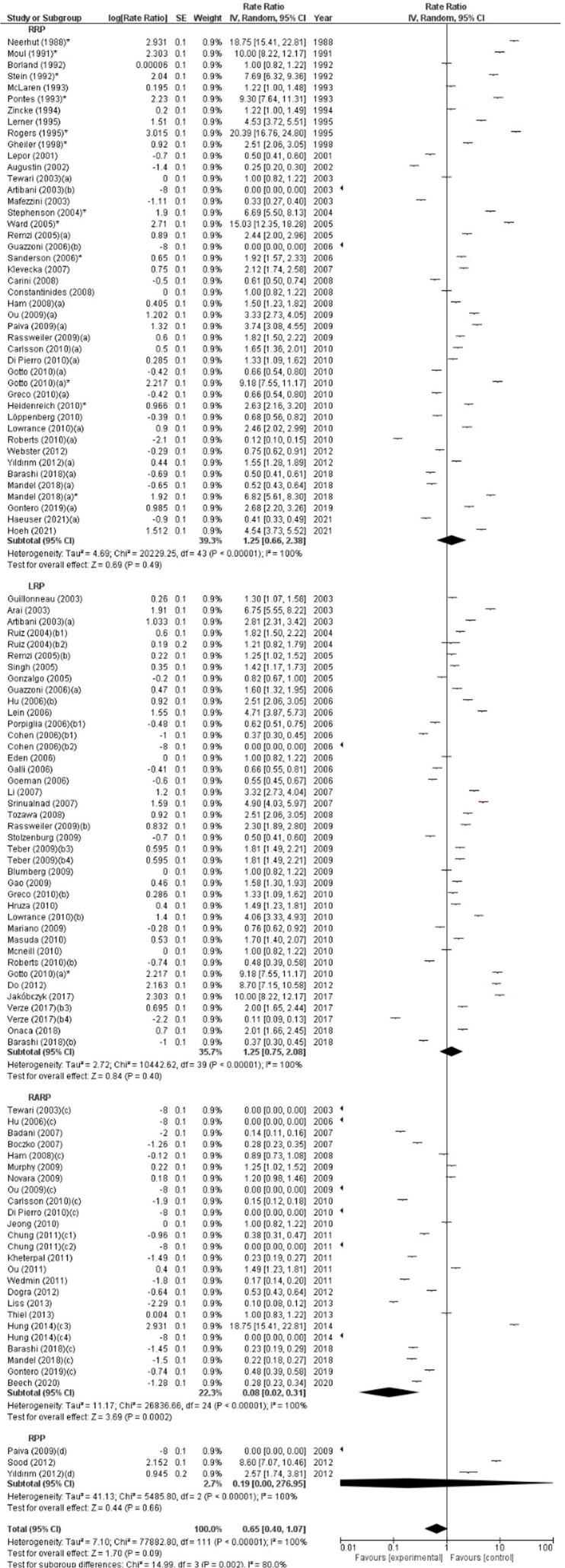
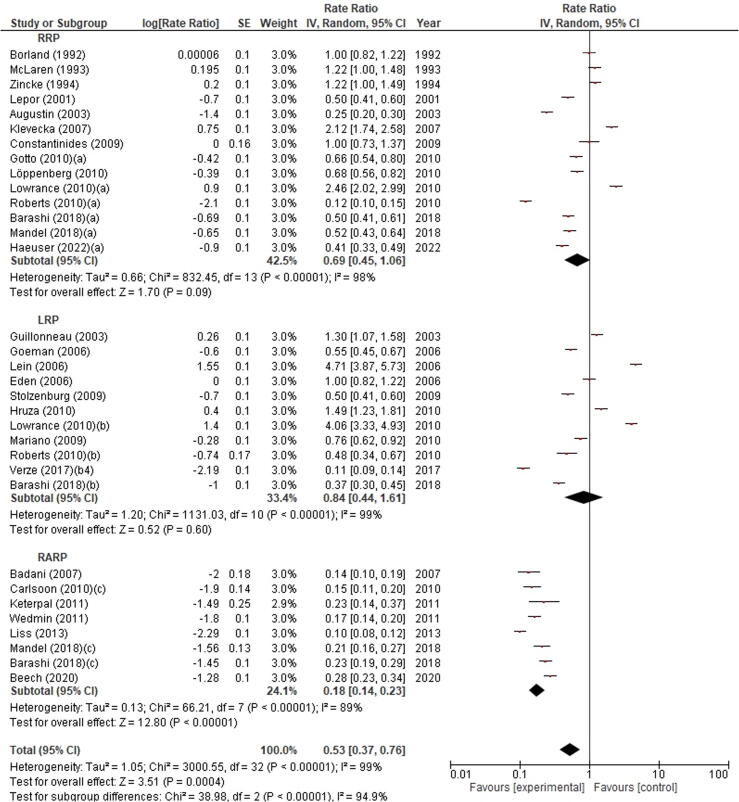
In the subgroup analysis by surgical approach used to perform RP, the highest RI incidence was observed in patients who underwent RRP (1.25%, 95% CI 0.66–2.38) and LRP (1.25%, 95% CI 0.75–2.08), followed by PRP (0.19%, 95% CI 0.00–276.95), while the lowest RI incidence was found with RARP (0.08%, 95% CI 0.02–0.31). Significant differences in RI incidence were found between the different surgical approaches (p = 0.002). The meta-analysis showed significant across-study heterogeneity (I2 = 100%, p < 0.00001; Fig. 4). A subgroup analysis including only studies with >500 cases confirmed that RI incidence was significantly influenced by surgical approach (p = 0.0004). In particular, the highest RI incidence was found with LRP (0.84%, 95% CI 0.44–1.61), followed by RRP (0.69%, 95% CI 0.45–1.06), while the lowest RI incidence was observed with RARP (0.108%, 95% CI 0.14–0.23; Fig. 5).
Fig. 4.
Forest plot of the meta-analysis of the incidence of rectal injury after radical prostatectomy stratified by surgical approach (all studies). Studies are listed by publication year. The letters a, b, c, d denote study subgroups as per Table 1. CI = confidence interval; IV = inverse variance; LRP = laparoscopic radical prostatectomy; PRP = perineal radical prostatectomy; RARP = robot-assisted radical prostatectomy; RRP = retropubic radical prostatectomy. *Salvage prostatectomy.
Fig. 5.
Forest plot of the meta-analysis of the incidence of rectal injury after radical prostatectomy stratified by surgical approach (only studies including >500 patients). Studies are listed by publication year. The letters a, b, c denote study subgroups as per Table 1. CI = confidence interval; IV = inverse variance; LRP = laparoscopic radical prostatectomy; RARP = robot-assisted radical prostatectomy; RRP = retropubic radical prostatectomy.
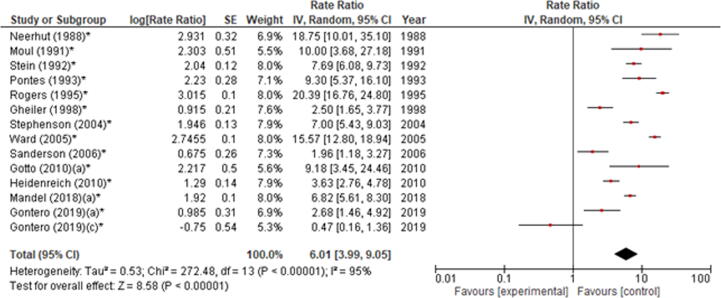
In the subgroup analysis including patients receiving a salvage RP after radiation therapy, RI incidence was 6.01% (95% CI 3.99–9.05). However, significant across-study heterogeneity was found (chi-square: 272.48, I2 = 95%, p < 0.00001; Fig. 6).
Fig. 6.
Forest plot of the meta-analysis of the incidence of rectal injury after salvage radical prostatectomy. Studies are listed by publication year. The letters a, c denote study subgroups as per Table 1. CI = confidence interval; IV = inverse variance. *Salvage prostatectomy.
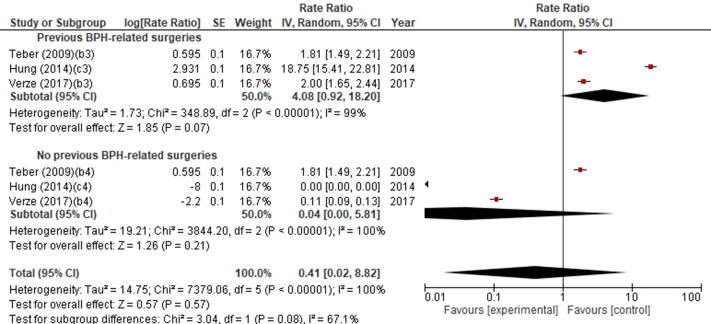
Although the meta-analysis showed that RI incidence was 4.08% (95% CI 0.92–18.20) in patients with previous BPH-related surgery and only 0.04% (95% CI 0.02–5.81) in those without, this difference was not statistically significant (p = 0.57; Supplementary Fig. 3).
Egger’s regression test failed to reveal a publication bias for RI incidence when considering both all studies (p = 0.15; Supplementary Fig. 4) and only studies with >500 patients (p = 0.11; Supplementary Fig. 5). Moreover, visual inspection revealed funnel plot symmetry in both cases.
3.3. Discussion
The incidence of RI after RP declined by 26% from years 2003–2006 to 2009–2012, with most recent studies reporting values <0.5% [10], [34], [54]. Our meta-analysis showed an RI incidence of 0.58% when all studies were considered and 0.51% in the subgroup analysis including only studies with >500 cases. According to our meta-analysis, some studies showed that RI incidence was significantly higher in low-volume centres [10] and in low-volume surgeons [54].
Although RI can occur regardless of the surgical approach used, our data showed that the incidence was higher in patients treated with open RP than in those treated with minimally invasive RP. In a recent population-based study, Barashi et al. [10] reported an RI incidence of 0.6%, 0.4%, and 0.3% after RRP, LRP, and RARP, respectively. Moreover, Mandel et al. [54] observed RI in 0.27% of patients treated with RARP and in 0.55% of those treated with RRP. Similarly, Haeuser et al. [34] reported an RI incidence of 0.41% after RRP and 0.11% after minimally invasive RP. Better vision due to three-dimensional vision and optical magnification as well as the position of the 0° or 30° lens in the operative field together with the availability of Endowrist instruments may help robotic surgeons develop the posterior plane at the level of the seminal vesicles, posterior prostatic surface, and apex, under direct vision, thus preventing RI. Our meta-analysis showed a higher RI risk in patients who underwent RRP and LRP than in those who underwent RARP. This difference was confirmed in a subgroup analysis including larger series. Most studies exploring the perioperative outcomes of Retzius-sparing RARP (RS-RARP) did not specifically address the issue of RI. Galfano et al. [94] in 2021 reported a 0.2% RI rate in a large series including 626 patients who underwent RS-RARP, with all lesions detected in patients treated by surgeons in the learning curve. Similarly, Raheem et al. [95] in 2018 reported a 0.3% RI rate in a series of 359 patients treated with RS-RARP.
Previous pelvic radiation therapy for PCa or other conditions was associated with an increased RI risk, with several authors reporting an incidence between 2% and 15% in patients who underwent salvage RP [28], [54], [96]. Notably, in a multicentre, multisurgeon study, including 395 patients undergoing salvage RP (186 RRP and 209 RARP), Gontero et al. [28] reported an RI incidence of 2.96% after RRP and 0.48% after RARP. Our meta-analysis confirmed the higher RI risk in patients undergoing salvage versus primary RP. Literature data are still immature to demonstrate whether RARP is associated with a lower RI incidence than RRP in this patient subgroup.
Previous BPH-related surgery and large prostate volume have been considered as further patient-related factors associated with an increased RI risk. However, in their single-centre study, Mandel et al. [54] did not find any correlation between prostate volume and RI. Notably, our meta-analysis confirms an association between previous BPH-related surgery and RI risk.
Other potential factors increasing the RI risk have been reported, such as previous androgen deprivation therapy, pelvic fracture, and rectal surgery, since periprostatic fibrosis could render the development of posterior surgical plane more challenging. [35] In particular, patients with a hostile abdomen due to inflammatory bowel disease, previous colorectal or pelvic surgeries including colostomy reversal might have an increased risk of RI during RP. Recently, Luciani et al. [97] reported on 14 RARP cases performed after colorectal surgery with a 21% conversion rate to open surgery, but with no RI. However, in view of the risk of intestinal injury during conversion, the authors suggest a direct retropubic approach in case of previous multiple or complicated abdominal interventions. Interestingly, obesity seems to be a protective factor against RI. The perirectal adipose tissue might, in fact, act as a physical barrier granting an additional space between the rectum and the prostate [10]. However, Mandel et al. [54] did not find any correlation between body mass index and RI.
Considering tumour-related risk factors, high-risk category according to D’Amico classification and preoperative total prostate-specific antigen were considered as possible risk factors for RI in a recent large surgical series including 24 178 RPs [54]. Considering pathological variables, RI is apparently more frequent in patients with locally advanced disease, a high Gleason score, lymph node involvement, and positive surgical margins [10], [54], [57].
3.4. Proposal for a pragmatic algorithm for RI management
No preoperative measures focused to avoid the risk of RI or minimise its potential infective consequences are currently recommended. In detail, no specific antibiotic prophylaxis is indicated for patients potentially at risk for RI during RP. Moreover, some authors proposed to use mechanical bowel preparation to reduce the severity of RI, if ever occurring, by reducing the risk of subsequent infectious complications and/or delayed colostomy [44], [91]. However, an analysis of 35 099 RP cases included in the Japanese Diagnosis Procedure Combination database between 2007 and 2012 showed that mechanical bowel preparation did not positively affect perioperative morbidity associated with RI during RP [83]. Therefore, mechanical bowel preparation can safely be omitted [98].
The management and prognosis of RI after RP significantly differ by timing of detection, that is, intra- versus postoperative. Intra- versus postoperative detection of RI has strongly been associated with a significantly decreased risk of severe postoperative complications and subsequent formation of a RUF [72]. Therefore, all measures aimed at identifying the presence of RI intraoperatively should strongly be considered and applied. Recently, Canda et al. [99] identified some surgical manoeuvres limiting the risk of RI during RP above all in the context of robotic surgery. Careful dissection and development of the posterior plane between the prostate and rectal wall favouring the use of cold scissors and avoiding monopolar cautery close to the rectal wall, visualisation of the periprostatic fat tissue during extrafascial posterior dissection, and minimal traction on the rectum wall in the presence of periprostatic adhesions might represent important measures to reduce RI risk [100], [101]. Moreover, a cautious dissection under clear and magnified vision is most important during RPs performed for locally advanced and extracapsular disease or in presence of severe adhesions [99].
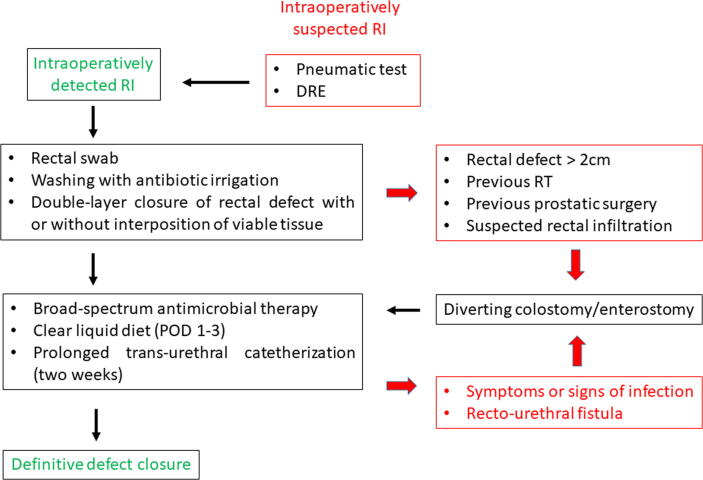
A probe or a sponge stick can be placed in the rectum at the beginning of the surgical procedure to help the surgeon identify any rectal mucosal perforation. If RI is suspected, or in the presence of a visible thin rectal wall, the pneumatic test must always be done. Preliminarily, the pelvis should be irrigated with normal saline solution to remove blood clots and identify any actively bleeding vessels, and then filled with standard saline solution. Air can be insufflated into the rectum using the rectal probe. The detection of air bubbles escaping from the rectum is clearly due to the presence of a small rectal lesion. In absence of a preliminarily placed rectal probe, air can be insufflated into the rectum through a rectal Foley catheter, while the pelvis is filled with irrigation fluid [56], [102]. Digital rectal examination (DRE) with two fingers allows complete identification of the injury’s extent, improving certainty of the defect closure [14]. Performing an intraoperative local swab to eventually identify pathogens resistant to the antimicrobials used for prophylaxis could be an option as to minimise the risk of severe sepsis. Then, the operative field should be washed abundantly with antimicrobial irrigation [100].
During LRP or RARP, RI can occur at the beginning of the procedure during the seminal vesicle dissection or at the development of the posterior plane between the prostate and the rectum wall. In this case, some authors have proposed to repair the lesion at this stage before further mobilisation of the prostate [93]. Regardless of the approach used, the rectal defect must be closed before completing the vesicourethral anastomosis [72]. Usually, the rectal defect can be closed using absorbable (Monocryl or Vycril) 2-0 or 3-0 sutures in two layers (mucosa and seromuscular) [54], [72]. The use of endoscopic clips as well as the application of the fibrin glue has only anecdotally been described, mostly during LRP [103]. The interposition of viable and vascularised tissues between the injured rectal wall and the vesicourethral anastomosis should strongly be considered to create a barrier improving the rectal wound healing, with the omentum being the most frequently used tissue [102]. However, in clinical scenarios where omentum mobilisation is challenging (short omentum, previous surgery, or steep Trendelenburg position), a full-layer peritoneal graft obtained from the pelvic side wall can be used alternatively as a third layer to cover the area of RI repair [99].
Controversy exists on the indication for a contextual colostomy in patients with RI during RP. Recent literature data showed that only 10% of patients with RI received a concomitant colostomy [10], [54], [76]. Indeed, diverting colostomy should not be considered as the standard of care in all patients with intraoperatively detected RI. However, it should strongly be considered in patients with large rectal defects (>2 cm) and/or previous pelvic radiotherapy and/or previous BPH-related surgery and/or suspected PCa infiltration of the rectum. A suction drain should be placed especially if the vesicourethral anastomosis is not watertight and if the suture line for RI repair is under tension [102].
As for the postoperative management, a clear liquid diet can be started on postoperative day 1 and shifted stepwise to a regular diet on day 3. The indication for a low-fibre diet remains debatable. Broad-spectrum antibiotic therapy (penicillin or cefuroxime plus metronidazole) is strongly recommended [54]. Moreover, a longer catheterisation time should strongly be considered (>2 wk) and the catheter removal should be planned only after a negative cystogram [54], [101], [102]. Nevertheless, 10% of patients with intraoperatively detected RI develop a RUF during the postoperative period [3].
Based on these data, we propose an algorithm for the management of intraoperatively detected RI during RP (Fig. 7).
Fig. 7.
Pragmatic algorithm for the management of intraoperatively detected rectal injury during radical prostatectomy. DRE = digital rectal examination; POD = postoperative day; RI = rectal injury; RT = radiotherapy.
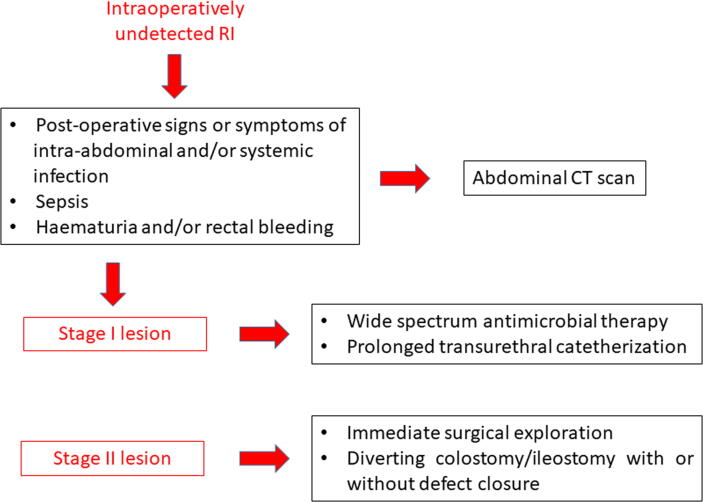
Intraoperatively undetected RI can be responsible for early and late severe postoperative complications, which may be fatal if not treated promptly. An undetected abnormal communication between the rectal lumen and the clean-contaminated surrounding pelvic space can be responsible for abdominal pain associated with systemic signs of inflammation (fever, tachycardia, tachypnoea, hypotension, and oliguria). Moreover, laboratory tests can show an increased white blood cell count with neutrophilia, a low platelet count, a rapid increase of inflammatory markers, and an increased serum lactate level. Moreover, undetected RI can evolve into severe infective complications such as abscess formation, peritonitis (if a transperitoneal route for RP was used), septic sequelae, and even death [102]. Moreover, several cases of RI were associated with bleeding (haematuria and/or rectal bleeding). Symptoms and signs of RI should prompt DRE and further investigations such as an abdominal computed tomographic scan. According to DRE findings, RI after RP can be categorised in three different stages: stage 1: <1.5 cm (when the tip of a finger does not pass through the opening); stage 2: >1.5 cm (when the tip of a finger passes quickly through the opening); and stage 3: any diameter with urethral sphincter damage [104].
Stage 1 lesions could be treated conservatively with large spectrum antibiotic therapy, long-term transurethral catheterisation (1–3 mo), and initial parenteral nutrition if signs of peritonitis or infection are not present. However, persistent fever or any unfavourable clinical condition (peritonitis, pelvic abscess, or persistent rectal bleeding) require an immediate surgical exploration, faecal diversion (colostomy/enterostomy), and eventual closure of the rectal defect to avoid the risk of severe sepsis, as for stage 2 lesions [3], [105], [106]. A consistent proportion of patients treated conservatively or surgically for RI develop a RUF in the following weeks.
Based on these data, we propose a pragmatic algorithm for the management of postoperatively detected RI after RP (Fig. 8).
Fig. 8.
Pragmatic algorithm for the management of postoperatively detected rectal injury after radical prostatectomy. CT = computed tomography; RI = rectal injury.
Several patients in whom RI does not heal spontaneously or is not repaired develop a RUF over a variable time ranging between few days and several weeks after RP [3], [10]. Faecaluria, pneumaturia, and drainage of urine per anus pose a strong suspicion for the presence of a RUF. Usually, faecaluria is considered a poor prognostic sign [106]. RUF management still represents a challenging situation for academic and nonacademic urologists. Retrograde urethrocystography, flexible or rigid urethrocystoscopy, and rectoscopy or colonoscopy should strongly be considered to plan the most appropriate surgical treatment.
Although several approaches, including transperineal, transanal, transabdominal, transvesical, trans-sphincterial, or combined, have been proposed, there is no standardised treatment for a RUF because of its rarity. Nevertheless, the York-Mason procedure is considered most appropriate according to its high success rate and low morbidity [107], [108]. Although several studies reported high success rates after the most popular surgical techniques, a recurrent RUF occurs and represents a very challenging issue in clinical practice because repeated surgical failures significantly increase morbidity and mortality in these patients. Indeed, a recurrent RUF represents the greatest challenge for surgeons [104]. Patients with a recurrent RUF who had previously undergone pelvic radiation therapy and/or those with unfavourable anatomical conditions (low bladder capacity) and/or a high risk of infective/general complications should be considered for permanent urinary and/or faecal diversion.
3.5. Study limitations
There are several limitations to our systematic review, mainly related to the low quality of the available literature in view of the rarity of this complication. Most studies were retrospective and noncomparative, and in several of these intra- and postoperative complications were not assessed using a standardised classification and reporting system. Only few studies were focused on RI as the primary outcome of interest. Moreover, among the potential predisposing factors, the role of surgeon’s expertise and learning curve effect could not be investigated. Finally, as for the management of RI and RUF, only limited expert opinion data were available; thus, strong recommendations cannot be made. Prospective, possibly multicentre, registries of complications after RP should be established, and patients diagnosed with RI should ideally be referred to multidisciplinary reconstructive teams in high-volume centres.
4. Conclusions
RI is a rare, but potentially devastating, complication following RP. Its potential occurrence should always be discussed during the preoperative patient counselling and promptly investigated during surgery, especially when certain clinical factors are present, such as age ≥60 yr and previous radiation therapy to the pelvis. RRP and LRP are apparently associated with a higher RI incidence than RARP.
Intraoperative detection and repair of RI during RP are apparently the most critical step to significantly decrease the risk of severe postoperative complications and subsequent RUF formation. Conversely, intraoperatively undetected RI can be responsible for severe infectious complications and can lead to a RUF in a high percentage of cases.
Author contributions: Vincenzo Ficarra had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Romito, Ficarra.
Acquisition of data: Romito, Rossanese, Mucciardi.
Analysis and interpretation of data: Ficarra.
Drafting of the manuscript: Giannarini, Ficarra.
Critical revision of the manuscript for important intellectual content: Giannarini, Simonato, Ficarra.
Statistical analysis: Romito, Ficarra.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Ficarra.
Other: None.
Financial disclosures: Vincenzo Ficarra certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.03.017.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Forest plot of the meta-analysis of incidence of rectal injury after radical prostatectomy (only studies including >500 patients). Studies are listed by publication year. [a, b, c denote study subgroups as per Table 1].
Supplementary Figure 2.
Forest plot of the meta-analysis of incidence of the rectal injury stratified by patient age. Studies are listed by publication year. [c denotes study subgroup as per Table 1].
Supplementary Figure 3.
Forest plot of the meta-analysis of incidence of the rectal injury after radical prostatectomy stratified by previous benign prostatic hyperplasia-related surgery. Studies are listed by publication year. BPH = benign prostatic hyperplasia [b, c denote study subgroups as per Table 1].
Supplementary Figure 4.
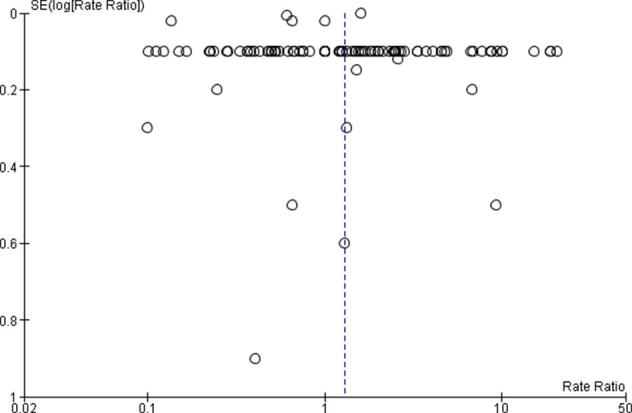
Funnel plot showing no evidence of publication bias (all studies).
Supplementary Figure 5.
Funnel plot showing no evidence of publication bias (only studies including >500 patients).
References
- 1.Walsh P.C., DeWeese T.L., Eisenberger M.A. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–2705. doi: 10.1056/NEJMcp0706784. [DOI] [PubMed] [Google Scholar]
- 2.Hechenbleikner E.M., Buckley J.C., Wick E.C. Acquired rectourethral fistulas in adults: a systematic review of surgical repair techniques and outcomes. Dis Colon Rectum. 2013;56:374–383. doi: 10.1097/DCR.0b013e318274dc87. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura H., Tsukamoto T. Rectourinary fistula after radical prostatectomy: review of the literature for incidence, etiology, and management. Prostate Cancer. 2011;2011 doi: 10.1155/2011/629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Howick J, Chalmers I, Glasziou P, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) levels of evidence (background document). Oxford Centre for Evidence-Based Medicine. https://www.cebm.net.
- 6.Arai Y., Egawa S., Terachi T., et al. Morbidity of laparoscopic radical prostatectomy: summary of early multi-institutional experience in Japan. Int J Urol. 2003;10:430–434. doi: 10.1046/j.1442-2042.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- 7.Artibani W., Grosso G., Novara G., et al. Is laparoscopic radical prostatectomy better than traditional retropubic radical prostatectomy? An analysis of peri-operative morbidity in two contemporary series in Italy. Eur Urol. 2003;44:401–406. doi: 10.1016/s0302-2838(03)00315-4. [DOI] [PubMed] [Google Scholar]
- 8.Augustin H., Hammerer P., Graefen M., et al. Intraoperative and perioperative morbidity of contemporary radical retropubic prostatectomy in a consecutive series of 1243 patients: results of a single center between 1999 and 2002. Eur Urol. 2003;43:113–118. doi: 10.1016/s0302-2838(02)00495-5. [DOI] [PubMed] [Google Scholar]
- 9.Badani K.K., Kaul S., Menon M. Evolution of robotic radical prostatectomy: assessment after 2766 procedures. Cancer. 2007;110:1951–1958. doi: 10.1002/cncr.23027. [DOI] [PubMed] [Google Scholar]
- 10.Barashi N.S., Pearce S.M., Cohen A.J., Pariser J.J., Packiam V.T., Eggener S.E. incidence, risk factors, and outcomes for rectal injury during radical prostatectomy: a population-based study. Eur Urol Oncol. 2018;1:501–506. doi: 10.1016/j.euo.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Beech B., Follett G., Ghosh S., et al. Are urologic surgeons performing robot-assisted radical prostatectomy at the University of Alberta meeting surgical quality performance benchmarks? The PROCURE-02 quality assurance study. Can Urol Assoc J. 2020;14:E369–E372. doi: 10.5489/cuaj.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumberg J.M., Lesser T., Tran V.Q., Aboseif S.R., Bellman G.C., Abbas M.A. Management of rectal injuries sustained during laparoscopic radical prostatectomy. Urology. 2009;73:163–166. doi: 10.1016/j.urology.2008.08.473. [DOI] [PubMed] [Google Scholar]
- 13.Boczko J., Erturk E., Golijanin D., Madeb R., Patel H., Joseph J.V. Impact of prostate size in robot-assisted radical prostatectomy. J Endourol. 2007;21:184–188. doi: 10.1089/end.2006.0163. [DOI] [PubMed] [Google Scholar]
- 14.Borland R.N., Walsh P.C. The management of rectal injury during radical retropubic prostatectomy. J Urol. 1992;147:905–907. doi: 10.1016/s0022-5347(17)37418-9. [DOI] [PubMed] [Google Scholar]
- 15.Carini M., Masieri L., Minervini A., Lapini A., Serni S. Oncological and functional results of antegrade radical retropubic prostatectomy for the treatment of clinically localised prostate cancer. Eur Urol. 2008;53:554–561. doi: 10.1016/j.eururo.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson S., Nilsson A.E., Schumacher M.C., et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital. Sweden. Urology. 2010;75:1092–1097. doi: 10.1016/j.urology.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 17.Chung J.S., Kim W.T., Ham W.S., et al. Comparison of oncological results, functional outcomes, and complications for transperitoneal versus extraperitoneal robot-assisted radical prostatectomy: a single surgeon's experience. J Endourol. 2011;25:787–792. doi: 10.1089/end.2010.0222. [DOI] [PubMed] [Google Scholar]
- 18.Cohen M.S., Triaca V., Silverman M.L., Tuerk I.A. Progression of laparoscopic radical prostatectomy: improved outcomes with the extraperitoneal approach and a running anastomosis. J Endourol. 2006;20:574–579. doi: 10.1089/end.2006.20.574. [DOI] [PubMed] [Google Scholar]
- 19.Constantinides C.A., Tyritzis S.I., Skolarikos A., Liatsikos E., Zervas A., Deliveliotis C. Short- and long-term complications of open radical prostatectomy according to the Clavien classification system. BJU Int. 2009;103:336–340. doi: 10.1111/j.1464-410X.2008.08080.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Pierro G.B., Baumeister P., Stucki P., Beatrice J., Danuser H., Mattei A. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011;59:1–6. doi: 10.1016/j.eururo.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Do M., Ragavan N., Dietel A., et al. Feasibility of minimally invasive radical prostatectomy in prostate cancer patients with high prostate-specific antigen: feasibility and 1-year outcomes. Int J Urol. 2012;19:923–927. doi: 10.1111/j.1442-2042.2012.03068.x. [DOI] [PubMed] [Google Scholar]
- 22.Dogra P.N., Javali T.D., Singh P., et al. Perioperative outcome of initial 190 cases of robot-assisted laparoscopic radical prostatectomy—a single-center experience. Indian J Urol. 2012;28:159–163. doi: 10.4103/0970-1591.98454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eden C.G., Chang C.M., Gianduzzo T., Moon D.A. The impact of obesity on laparoscopic radical prostatectomy. BJU Int. 2006;98:1279–1282. doi: 10.1111/j.1464-410X.2006.06443.x. [DOI] [PubMed] [Google Scholar]
- 24.Galli S., Simonato A., Bozzola A., et al. Oncologic outcome and continence recovery after laparoscopic radical prostatectomy: 3 years' follow-up in a “second generation center”. Eur Urol. 2006;49:859–865. doi: 10.1016/j.eururo.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Gao X., Zhou J.H., Li L.Y., Qiu J.G., Pu X.Y. Laparoscopic radical prostatectomy: oncological and functional results of 126 patients with a minimum 3-year follow-up at a single Chinese institute. Asian J Androl. 2009;11:548–556. doi: 10.1038/aja.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheiler E.L., Tefilli M.V., Tiguert R., et al. Predictors for maximal outcome in patients undergoing salvage surgery for radio-recurrent prostate cancer. Urology. 1998;51:789–795. doi: 10.1016/s0090-4295(98)00096-x. [DOI] [PubMed] [Google Scholar]
- 27.Goeman L., Salomon L., La De T.A., et al. Long-term functional and oncological results after retroperitoneal laparoscopic prostatectomy according to a prospective evaluation of 550 patients. World J Urol. 2006;24:281–288. doi: 10.1007/s00345-006-0054-6. [DOI] [PubMed] [Google Scholar]
- 28.Gontero P., Marra G., Alessio P., et al. Salvage radical prostatectomy for recurrent prostate cancer: morbidity and functional outcomes from a large multicenter series of open versus robotic approaches. J Urol. 2019;202:725–731. doi: 10.1097/JU.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalgo M.L., Pavlovich C.P., Trock B.J., Link R.E., Sullivan W., Su L.M. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005;174:135–139. doi: 10.1097/01.ju.0000161607.04334.26. [DOI] [PubMed] [Google Scholar]
- 30.Gotto G.T., Yunis L.H., Vora K., Eastham J.A., Scardino P.T., Rabbani F. Impact of prior prostate radiation on complications after radical prostatectomy. J Urol. 2010;184:136–142. doi: 10.1016/j.juro.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 31.Greco F., Wagner S., Hoda M.R., et al. Laparoscopic vs open retropubic intrafascial nerve-sparing radical prostatectomy: surgical and functional outcomes in 300 patients. BJU Int. 2010;106:543–547. doi: 10.1111/j.1464-410X.2009.09157.x. [DOI] [PubMed] [Google Scholar]
- 32.Guazzoni G., Cestari A., Naspro R., et al. Intra- and peri-operative outcomes comparing radical retropubic and laparoscopic radical prostatectomy: results from a prospective, randomised, single-surgeon study. Eur Urol. 2006;50:98–104. doi: 10.1016/j.eururo.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 33.Guillonneau B., Gupta R., El Fettouh H., Cathelineau X., Baumert H., Vallancien G. Laparoscopic [correction of laproscopic] management of rectal injury during laparoscopic [correction of laproscopic] radical prostatectomy. J Urol. 2003;169:1694–1696. doi: 10.1097/01.ju.0000059860.00022.07. [DOI] [PubMed] [Google Scholar]
- 34.Haeuser L., Reese S.W., Paciotti M., et al. Surgical complications requiring intervention in open versus minimally invasive radical prostatectomy. Urol Int. 2022;106:51–55. doi: 10.1159/000515618. [DOI] [PubMed] [Google Scholar]
- 35.Hajili T., Ohlmann C.H., Linxweiler J., et al. Radical prostatectomy in T4 prostate cancer after inductive androgen deprivation: results of a single-institution series with long-term follow-up. BJU Int. 2019;123:58–64. doi: 10.1111/bju.14393. [DOI] [PubMed] [Google Scholar]
- 36.Ham W.S., Park S.Y., Kim W.T., Koo K.C., Lee Y.S., Choi Y.D. Open versus robotic radical prostatectomy: a prospective analysis based on a single surgeon's experience. J Robot Surg. 2008;2:235–241. doi: 10.1007/s11701-008-0111-9. [DOI] [PubMed] [Google Scholar]
- 37.Heidenreich A., Richter S., Thüer D., Pfister D. Prognostic parameters, complications, and oncologic and functional outcome of salvage radical prostatectomy for locally recurrent prostate cancer after 21st-century radiotherapy. Eur Urol. 2010;57:437–443. doi: 10.1016/j.eururo.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 38.Hoeh B., Preisser F., Wenzel M., et al. Feasibility and outcome of radical prostatectomy following inductive neoadjuvant therapy in patients with suspicion of rectal infiltration. Urol Oncol. 2022;40:59.e7–59.e12. doi: 10.1016/j.urolonc.2021.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Hruza M., Weiss H.O., Pini G., et al. Complications in 2200 consecutive laparoscopic radical prostatectomies: standardised evaluation and analysis of learning curves. Eur Urol. 2010;58:733–741. doi: 10.1016/j.eururo.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Hu J.C., Nelson R.A., Wilson T.G., et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541–546. doi: 10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 41.Hung C.F., Yang C.K., Ou Y.C. Robotic assisted laparoscopic radical prostatectomy following transurethral resection of the prostate: perioperative, oncologic and functional outcomes. Prostate Int. 2014;2:82–89. doi: 10.12954/PI.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakóbczyk B., Wrona M., Wrona-Lis M., et al. Endoscopic extraperitoneal radical prostatectomy: An initial report following the first 30 cases. Cent European J Urol. 2017;70:48–52. doi: 10.5173/ceju.2017.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong J., Choi E.Y., Kim I.Y. Clavien classification of complications after the initial series of robot-assisted radical prostatectomy: the Cancer Institute of New Jersey/Robert Wood Johnson Medical School experience. J Endourol. 2010;24:1457–1461. doi: 10.1089/end.2010.0027. [DOI] [PubMed] [Google Scholar]
- 44.Kheterpal E., Bhandari A., Siddiqui S., Pokala N., Peabody J., Menon M. Management of rectal injury during robotic radical prostatectomy. Urology. 2011;77:976–979. doi: 10.1016/j.urology.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 45.Klevecka V., Burmester L., Musch M., Roggenbuck U., Kroepfl D. Intraoperative and early postoperative complications of radical retropubic prostatectomy. Urol Int. 2007;79:217–225. doi: 10.1159/000107953. [DOI] [PubMed] [Google Scholar]
- 46.Lein M., Stibane I., Mansour R., et al. Complications, urinary continence, and oncologic outcome of 1000 laparoscopic transperitoneal radical prostatectomies—experience at the Charité Hospital Berlin. Campus Mitte. Eur Urol. 2006;50:1278–1282. doi: 10.1016/j.eururo.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 47.Lepor H., Nieder A.M., Ferrandino M.N. Intraoperative and postoperative complications of radical retropubic prostatectomy in a consecutive series of 1,000 cases. J Urol. 2001;166:1729–1733. [PubMed] [Google Scholar]
- 48.Lerner S.E., Blute M.L., Lieber M.M., Zincke H. Morbidity of contemporary radical retropubic prostatectomy for localized prostate cancer. Oncology (Williston Park) 1995;9:379–382. [PubMed] [Google Scholar]
- 49.Li B., Suzuki K., Tsuru N., Ushiyama T., Ozono S. Retrospective comparative study of 59 cases of laparoscopic radical prostatectomy: transperitoneal anterior versus transperitoneal posterior approach. Int J Urol. 2007;14:1005–1008. doi: 10.1111/j.1442-2042.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 50.Liss M.A., Skarecky D., Morales B., Osann K., Eichel L., Ahlering T.E. Preventing perioperative complications of robotic-assisted radical prostatectomy. Urology. 2013;81:319–323. doi: 10.1016/j.urology.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Löppenberg B., Noldus J., Holz A., Palisaar R.J. Reporting complications after open radical retropubic prostatectomy using the Martin criteria. J Urol. 2010;184:944–948. doi: 10.1016/j.juro.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Lowrance W.T., Elkin E.B., et al. Comparative effectiveness of prostate cancer surgical treatments: a population based analysis of postoperative outcomes. J Urol. 2010;183:1366–1372. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maffezzini M., Seveso M., Taverna G., Giusti G., Benetti A., Graziotti P. Evaluation of complications and results in a contemporary series of 300 consecutive radical retropubic prostatectomies with the anatomic approach at a single institution. Urology. 2003;61:982–986. doi: 10.1016/s0090-4295(02)02517-7. [DOI] [PubMed] [Google Scholar]
- 54.Mandel P., Linnemannstöns A., Chun F., et al. Incidence, risk factors, management, and complications of rectal injuries during radical prostatectomy. Eur Urol Focus. 2018;4:554–557. doi: 10.1016/j.euf.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Mariano M.B., Tefilli M.V., Fonseca G.N., Goldraich I.H. Laparoscopic radical prostatectomy: 10 years’ experience. Int Braz J Urol. 2009;35:565–571. doi: 10.1590/s1677-55382009000500008. [DOI] [PubMed] [Google Scholar]
- 56.Masuda T., Kinoshita H., Nishida S., Kawa G., Kawakita M., Matsuda T. Rectal injury during laparoscopic radical prostatectomy: detection and management. Int J Urol. 2010;17:492–495. doi: 10.1111/j.1442-2042.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- 57.McLaren R.H., Barrett D.M., Zincke H. Rectal injury occurring at radical retropubic prostatectomy for prostate cancer: etiology and treatment. Urology. 1993;42:401–405. doi: 10.1016/0090-4295(93)90366-i. [DOI] [PubMed] [Google Scholar]
- 58.McNeill A.S., Nabi G., McLornan L., Cook J., Bollina P., Stolzenberg J.U. Endoscopic extraperitoneal radical prostatectomy: critical analysis of outcomes and learning curve. BJU Int. 2010;106:1537–1543. doi: 10.1111/j.1464-410X.2010.09322.x. [DOI] [PubMed] [Google Scholar]
- 59.Moul J.W., Paulson D.F. The role of radical surgery in the management of radiation recurrent and large volume prostate cancer. Cancer. 1991;68:1265–1271. doi: 10.1002/1097-0142(19910915)68:6<1265::aid-cncr2820680615>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 60.Murphy D.G., Kerger M., Crowe H., Peters J.S., Costello A.J. Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol. 2009;55:1358–1366. doi: 10.1016/j.eururo.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 61.Myers S.N., Ghani K.R., Dunn R.L., et al. Notable outcomes and trackable events after surgery: evaluating an uncomplicated recovery after radical prostatectomy. J Urol. 2016;196:399–404. doi: 10.1016/j.juro.2016.02.083. [DOI] [PubMed] [Google Scholar]
- 62.Neerhut G.J., Wheeler T., Cantini M., Scardino P.T. Salvage radical prostatectomy for radiorecurrent adenocarcinoma of the prostate. J Urol. 1988;140:544–549. doi: 10.1016/s0022-5347(17)41714-9. [DOI] [PubMed] [Google Scholar]
- 63.Novara G., Ficarra V., D'Elia C., Secco S., Cavalleri S., Artibani W. Prospective evaluation with standardised criteria for postoperative complications after robotic-assisted laparoscopic radical prostatectomy. Eur Urol. 2010;57:363–370. doi: 10.1016/j.eururo.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 64.Onaca M., Nita G., Manu M., Adou L., Tie G., Copaescu C. Retroperitoneal laparoscopic radical prostatectomy. Chirurgia (Bucur) 2018;113:542–550. doi: 10.21614/chirurgia.113.4.542. [DOI] [PubMed] [Google Scholar]
- 65.Ou Y.C., Yang C.R., Wang J., Cheng C.L., Patel V.R. Comparison of robotic-assisted versus retropubic radical prostatectomy performed by a single surgeon. Anticancer Res. 2009;29:1637–1642. [PubMed] [Google Scholar]
- 66.Ou Y.C., Yang C.R., Wang J., et al. The learning curve for reducing complications of robotic-assisted laparoscopic radical prostatectomy by a single surgeon. BJU Int. 2011;108:420–425. doi: 10.1111/j.1464-410X.2010.09847.x. [DOI] [PubMed] [Google Scholar]
- 67.Paiva C.S., Andreoni C., Cunha G.P., Khalil W., Ortiz V. Differences among patients undergoing perineal or retropubic radical prostatectomy in pain and perioperative variables: a prospective study. BJU Int. 2009;104:1219–1226. doi: 10.1111/j.1464-410X.2009.08551.x. [DOI] [PubMed] [Google Scholar]
- 68.Pontes J.E., Montie J., Klein E., Huben R. Salvage surgery for radiation failure in prostate cancer. Cancer. 1993;71:976–980. doi: 10.1002/1097-0142(19930201)71:3+<976::aid-cncr2820711413>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 69.Porpiglia F., Terrone C., Tarabuzzi R., et al. Transperitoneal versus extraperitoneal laparoscopic radical prostatectomy: experience of a single center. Urology. 2006;68:37680. doi: 10.1016/j.urology.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 70.Rassweiler J., Seemann O., Schulze M., Teber D., Hatzinger M., Frede T. Laparoscopic versus open radical prostatectomy: a comparative study at a single institution. J Urol. 2003;169:1689–1693. doi: 10.1097/01.ju.0000062614.56629.41. [DOI] [PubMed] [Google Scholar]
- 71.Remzi M., Klingler H.C., Tinzl M.V., et al. Morbidity of laparoscopic extraperitoneal versus transperitoneal radical prostatectomy versus open retropubic radical prostatectomy. Eur Urol. 2005;48:83–89. doi: 10.1016/j.eururo.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 72.Roberts W.B., Tseng K., Walsh P.C., Han M. Critical appraisal of management of rectal injury during radical prostatectomy. Urology. 2010;76:1088–1091. doi: 10.1016/j.urology.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 73.Rogers E., Ohori M., Kassabian V.S., Wheeler T.M., Scardino P.T. Salvage radical prostatectomy: outcome measured by serum prostate specific antigen levels. J Urol. 1995;153:104–110. doi: 10.1097/00005392-199501000-00037. [DOI] [PubMed] [Google Scholar]
- 74.Ruiz L., Salomon L., Hoznek A., et al. Comparison of early oncologic results of laparoscopic radical prostatectomy by extraperitoneal versus transperitoneal approach. Eur Urol. 2004;46:50–54. doi: 10.1016/j.eururo.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 75.Sanderson K.M., Penson D.F., Cai J., et al. Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol. 2006;176:2025–2031. doi: 10.1016/j.juro.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 76.Schmitges J., Trinh Q.D., Sun M., et al. Annual prostatectomy volume is related to rectal laceration rate after radical prostatectomy. Urology. 2012;79:796–803. doi: 10.1016/j.urology.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 77.Singh A., Fagin R., Shah G., Shekarriz B. Impact of prostate size and body mass index on perioperative morbidity after laparoscopic radical prostatectomy. J Urol. 2005;173:552–554. doi: 10.1097/01.ju.0000150101.95236.35. [DOI] [PubMed] [Google Scholar]
- 78.Sood R., Khattar N., Nayyar R., Kathuria S., Narang V., Kaushal D. Case for resurgence of radical perineal prostatectomy in Indian subcontinent. Indian J Urol. 2012;28:418–423. doi: 10.4103/0970-1591.105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinualnad S., Udompunturak S. Extraperitoneal laparoscopic radical prostatectomy: early experience in Thailand. Asian J Surg. 2007;30:272–277. doi: 10.1016/S1015-9584(08)60038-X. [DOI] [PubMed] [Google Scholar]
- 80.Stein A., Smith R.B., deKernion J.B. Salvage radical prostatectomy after failure of curative radiotherapy for adenocarcinoma of prostate. Urology. 1992;40 doi: 10.1016/0090-4295(92)90473-a. [DOI] [PubMed] [Google Scholar]
- 81.Stephenson A.J., Scardino P.T., Bianco F.J., Jr, DiBlasio C.J., Fearn P.A., Eastham J.A. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004;172:2239–2243. doi: 10.1097/01.ju.0000140960.63108.39. [DOI] [PubMed] [Google Scholar]
- 82.Stolzenburg J.U., Kallidonis P., Minh D., et al. Endoscopic extraperitoneal radical prostatectomy: evolution of the technique and experience with 2400 cases. J Endourol. 2009;23:1467–1472. doi: 10.1089/end.2009.0336. [DOI] [PubMed] [Google Scholar]
- 83.Sugihara T., Yasunaga H., Horiguchi H., et al. Does mechanical bowel preparation ameliorate damage from rectal injury in radical prostatectomy? Analysis of 151 rectal injury cases. Int J Urol. 2014;21:566–570. doi: 10.1111/iju.12368. [DOI] [PubMed] [Google Scholar]
- 84.Teber D., Cresswell J., Ates M., et al. Laparoscopic radical prostatectomy in clinical T1a and T1b prostate cancer: oncologic and functional outcomes—a matched-pair analysis. Urology. 2009;73:577–581. doi: 10.1016/j.urology.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 85.Tewari A., Srivasatava A., Menon M., Members of the VIP Team A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 86.Thiel D.D., Chavez M., Brisson T.E. Transition from resident robotic training program to clinical practice: robotic-assisted radical prostatectomy benchmark for perioperative safety. J Laparoendosc Adv Surg Tech A. 2013;23:516–520. doi: 10.1089/lap.2012.0503. [DOI] [PubMed] [Google Scholar]
- 87.Tozawa K., Hashimoto Y., Yasui T., et al. Evaluation of operative complications related to laparoscopic radical prostatectomy. Int J Urol. 2008;15:222–225. doi: 10.1111/j.1442-2042.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 88.Verze P., Greco F., Scuzzarella S., et al. The impact of previous prostate surgery on the outcomes of laparoscopic radical prostatectomy. Minerva Urol Nefrol. 2017;69:76–84. doi: 10.23736/S0393-2249.16.02612-6. [DOI] [PubMed] [Google Scholar]
- 89.Ward J.F., Sebo T.J., Blute M.L., Zincke H. Salvage surgery for radiorecurrent prostate cancer: contemporary outcomes. J Urol. 2005;173:1156–1160. doi: 10.1097/01.ju.0000155534.54711.60. [DOI] [PubMed] [Google Scholar]
- 90.Webster T.M., Newell C., Amrhein J.F., Newell K.J. Cancer Care Ontario guidelines for radical prostatectomy: striving for continuous quality improvement in community practice. Can Urol Assoc J. 2012;6:442–445. doi: 10.5489/cuaj.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wedmid A., Mendoza P., Sharma S., et al. Rectal injury during robot-assisted radical prostatectomy: incidence and management. J Urol. 2011;186:1928–1933. doi: 10.1016/j.juro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Yıldırım M., Göktaş C., Horuz R., et al. Rectal injury during radical prostatectomy. Ulus Travma Acil Cerrahi Derg. 2012;18:250–254. doi: 10.5505/tjtes.2012.04379. [DOI] [PubMed] [Google Scholar]
- 93.Zincke H., Bergstralh E.J., Blute M.L., et al. Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol. 1994;12:2254–2263. doi: 10.1200/JCO.1994.12.11.2254. [DOI] [PubMed] [Google Scholar]
- 94.Galfano A., Secco S., Dell'Oglio P., et al. Retzius-sparing robot-assisted radical prostatectomy: early learning curve experience in three continents. BJU Int. 2021;127:412–417. doi: 10.1111/bju.15196. [DOI] [PubMed] [Google Scholar]
- 95.Raheem A.A., Chang K.D., Alenzi M.J., et al. Predictors of biochemical recurrence after Retzius-sparing robot-assisted radical prostatectomy: analysis of 359 cases with a median follow-up period of 26 months. Int J Urol. 2018;25:1006–1014. doi: 10.1111/iju.13808. [DOI] [PubMed] [Google Scholar]
- 96.Kimura M., Mouraviev V., Tsivian M., Mayes J.M., Satoh T., Polascik T.J. Current salvage methods for recurrent prostate cancer after failure of primary radiotherapy. BJU Int. 2010;105:191–201. doi: 10.1111/j.1464-410X.2009.08715.x. [DOI] [PubMed] [Google Scholar]
- 97.Luciani L.G., Mattevi D., Puglisi M., et al. Robotic-assisted radical prostatectomy following colo-rectal surgery: a user's guide. J Robot Surg. 2022;16:189–192. doi: 10.1007/s11701-021-01228-1. [DOI] [PubMed] [Google Scholar]
- 98.Güenaga K.F., Matos D., Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011; 2011::CD001544. doi: 10.1002/14651858.CD001544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Canda A.E., Tilki D., Mottrie A. Rectal injury during radical prostatectomy: focus on robotic surgery. Eur Urol Oncol. 2018;1:507–509. doi: 10.1016/j.euo.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Katz R., Borkowski T., Hoznek A., Salomon L., de la Taille A., Abbou C.C. Operative management of rectal injuries during laparoscopic radical prostatectomy. Urology. 2003;62:310–313. doi: 10.1016/s0090-4295(03)00326-1. [DOI] [PubMed] [Google Scholar]
- 101.Castillo O.A., Bodden E., Vitagliano G. Management of rectal injury during laparoscopic radical prostatectomy. Int Braz J Urol. 2006;32:428–433. doi: 10.1590/s1677-55382006000400007. [DOI] [PubMed] [Google Scholar]
- 102.Yee D.S., Ornstein D.K. Repair of rectal injury during robotic-assisted laparoscopic prostatectomy. Urology. 2008;72:428–431. doi: 10.1016/j.urology.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 103.Dolay K., Aras B., Tuğcu V., Ozbay B., Aygün E., Taşçi A.I. Combined treatment of iatrogenic rectourethral fistula with endoscopic fibrin glue application and clipping. J Endourol. 2007;21:433–436. doi: 10.1089/end.2006.0302. [DOI] [PubMed] [Google Scholar]
- 104.Martini A., Gandaglia G., Nicita G., Montorsi F. A novel classification proposal for rectourethral fistulas after primary treatment of prostate cancer. Eur Urol Oncol. 2018;1:510–511. doi: 10.1016/j.euo.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 105.Zinman L. The management of the complex recto-urethral fistula. BJU Int. 2004;94:1212–1213. doi: 10.1111/j.1464-410X.2004.05225.x. [DOI] [PubMed] [Google Scholar]
- 106.Thomas C., Jones J., Jäger W., Hampel C., Thüroff J.W., Gillitzer R. Incidence, clinical symptoms and management of rectourethral fistulas after radical prostatectomy. J Urol. 2010;183:608–612. doi: 10.1016/j.juro.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 107.Fengler S.A., Abcarian H. The York Mason approach to repair of iatrogenic rectourinary fistulae. Am J Surg. 1997;173:213–217. doi: 10.1016/s0002-9610(96)00015-3. [DOI] [PubMed] [Google Scholar]
- 108.Renschler T.D., Middleton R.G. 30 years of experience with York-Mason repair of recto-urinary fistulas. J Urol. 2003;170:1222–1225. doi: 10.1097/01.ju.0000082013.58783.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.