Highlights
-
•
Comprehensively explore the relationship between brewing water and tea quality.
-
•
Aroma content was significant influenced by MW.
-
•
Ca2+ and Fe3+ remarkably affected the content of aroma compounds.
-
•
Water with pH (6∼7) and lower content of mineral substances is more conducive to brew green tea.
Keywords: Brewing water, Green tea, Mineral substance dissolution, Taste, Color, Volatile compounds
Abstract
The effects of different brewing water samples, including natural drinking water (NDW), pure water (PW), mineral water (MW), distilled water (DW), and tap water (TW) on flavor and quality of green tea infusion were investigated. The results showed the dissolution rate of mineral substances varied greatly depend on the type of water used. Notably, the tea infusion brewed with MW showed the highest taste response and darker but higher brightness in color. Furthermore, the content of volatile compounds was highest in tea infusion brewed with NDW and lowest in tea infusion brewed with MW. The mineral substances content and pH were the main factors affecting volatile compounds in green tea infusion. Thereinto, Ca2+ and Fe3+ remarkably affected the content of alcohols and aldehydes in volatile compounds. These results suggested that water with a neutral pH value and lower mineral substance content is more conducive for brewing green tea.
Introduction
Tea (Camellia Sinensis), one of the three non-alcoholic beverages in the world, is widely appreciated due to its unique sensory characteristics and superior health-promoting effects. However, tea brewing is the most extensive way of tea consumption. Obviously, the brewing conditions are crucial to the quality of tea. Different brewing conditions directly affect the dissolution of non-volatile components including tea polyphenols, caffeine, amino acids, mineral elements, and the release of tea aroma components. Generally speaking, the content of tea flavor compounds increases with the increase in brewing time, brewing temperature, and tea-water ratio (Liu et al., 2018, Liu et al., 2021). Recently and more strikingly, it has been reported the types of different water samples for brewing tea also affect the quality of tea infusion (Cao et al., 2021, Xu et al., 2017). However, the market has various types of drinking water, and inappropriate brewing water would decline consumer acceptability. Therefore, it is necessary to explore the influence of brewing water on metal dissolution and quality components in tea to ensure consumer satisfaction.
Tea is usually produced as a special plant beverage by directly processing fresh leaves including buds, tender leaves, and mature leaves after being picked from tea plants without washing. Compared with other crops, the residual level of pollutants in tea leaves is higher (W. J. Gao, M. Yang, Y. Xiao, et al., 2019). Besides, the tea tree is a kind of plant containing rich Al, Mn, and a variety of mineral substances (C. Y. Peng, X. F. Xu, H. Y. Zhu, et al., 2021), and these mineral substances have different functions for human health. Some mineral substances such as Fe, Zn, Cu, and Se are beneficial to the human body. For example, Se has biological functions such as antioxidation, immunomodulation, and inhibition of cancer, and plays an important role in energy metabolism and gene expression (Q. T. Dinh, Z. W. Cui, J. Huang, et al., 2018). On the contrary, toxic mineral substances such as As, Cd, Cr, Ni, and Pb can cause neurotoxicity, nephrotoxicity, and many other adverse effects on health (Chris Cooksey, 2012). Especially, over-standard levels of As and Cd in food have caused broad attention. As is an element that can produce chronic toxicity, such as hyperpigmentation, keratinization, nausea, cancer (A. Sarkar & B. Paul, 2016). Genchi et al. (2020) showed that long-term exposure of the human body to Cd may promote the occurrence of musculoskeletal diseases, lead to placental and fetal developmental disorders, and contribute to the occurrence of various cancers such as lung cancer, breast cancer, prostate cancer, pancreatic cancer, bladder cancer, and nasopharyngeal carcinoma (G. Genchi, M. S. Sinicropi, G. Lauria, et al., 2020). Thus, a potentially viable concept has been evaluated and proposed for the decreasing the toxic mineral substances and increasing the beneficial mineral substances in tea infusion by modulating the water samples for brewing tea, thereby improve human health. Therefore, it is necessary to investigate the effect of water types on the dissolution of mineral substance in tea to seek a suitable brewing water.
Tea polyphenols, caffeine, and free amino acids are the main component of taste in tea infusion (Spjn Senanayake, 2013). It has been reported that the content of astringent substances such as epigallocatechin gallate (EGCG) and caffeine in tea infusion will increase with the increase of Ca2+ content in brewing water (J. F. Yin, Y. N. Zhang, Q. Z. Du, et al., 2014). Water-soluble pigments, such as flavonol, anthocyanin, and flavanone, are the main components of the color of tea infusion, which are also greatly influenced by brewing water of the salinity, pH value, and brewing time (L. Zeng, M. J. Ma, C. Li, et al., 2017). The turbidity of green tea infusion increases with the increase of Ca2+ concentration in brewing water (Y. Q. Xu, X. Y. Zhong, J. F. Yin, et al., 2013). Higher pH values and total dissolved solid (TDS) could produce darker tea infusions (Q. Q. Cao, et al., 2021). Tea aroma is an important quality index in sensory evaluation. The aroma compounds are mainly formed by the Maillard reaction, oxidative degradation of fatty acid derivatives and carotenoid derivatives, and hydrolysis of glycosides (Z. H. Feng, Y. F. Li, M. Li, et al., 2019). The formation of tea aroma is mainly influenced by tea varieties (C. J. He, X. M. Guo, Y. M. Yang, et al., 2016), harvest season (X. Y. Guo, C. T. Ho, W. Schwab, et al., 2021), growing environment (C. Wang, S. D. Lv, Y. S. Wu, et al., 2016), processing methods, storage time (X. M. Yang, Y. L. Liu, L. H. Mu, et al., 2018), and brewing conditions. Flavor compounds exist in the collective form of aprotic, charge-neutral substances, acids, and conjugated salts (N. Cordoba, M. Fernandez-Alduenda, F. L. Moreno, et al., 2020). Thus, the different mineral substances contents and pH values in different types of water will affect the dissolution and extraction of these organic molecules, thus affecting the sensor characteristics of tea. Franks et al. (M. Franks, P. Lawrence, A. Abbaspourrad, et al., 2019) analyzed the effects of deionized water, tap water, and bottled water on the flavor and nutrition extraction of green tea and black tea, and it was found that catechin content in tea extract decreased with the increase of water salinity. Therefore, the selection of appropriate brewing water plays an important role in the quality of tea infusion.
Based on the above literature, lots of encouraging findings about the effect of processing conditions on tea infusion quality have been extensively obtained. Furthermore, the effect of brewing conditions on the taste and color of tea infusion has been also reported, however, the information about the dissolution rate of mineral substances and volatile compounds affected by different types of water is still limited. Thus, the objectives of this study were to (1) measure the contents of mineral substances in green tea infusion brewed by different water samples; (2) compare the effects of different water samples on the flavor (aroma, taste, and color) of tea infusion; and (3) investigate the potential factors of water samples affecting the content of volatile compounds, to provide a more reasonable way to brew tea.
Materials and methods
Materials and chemicals
Green tea was obtained from Anhui Qiyue tea Co., Ltd (Luan, China). Pure water (PW) was purchased from China Resources Yibao beverage (China) Co., Ltd (Hefei, China), natural drinking water (NDW) was purchased from Nongfu Shanquan Co., Ltd (Hefei, China), distilled water (DW) was purchased from Watsons Food & Beverage Co., Ltd. (Guangzhou, China), mineral water (MW) was purchased from Shanghai Zhengguanghe drinking water Co., Ltd. (Shanghai, China), tap water (TW) was collected from the laboratory (Hefei, China). Methanol (Aladdin Co., Ltd. Shanghai, China) is high performance liquid chromatography (HPLC) grade. Analytical chemical reagents include calcium chloride (Sinopharm Chemical Reagents Co., Ltd., Shanghai, China), sodium chloride (Sinopharm Chemical Reagents Co., Ltd., Shanghai, China), ferric chloride (McLean Biochemical Technology Co., Ltd, Shanghai, China), disodium hydrogen phosphate (Aladdin Co., Ltd., Shanghai, China) and potassium dihydrogen phosphate and potassium dihydrogen phosphate (McLean Biochemical Technology Co., Ltd, Shanghai, China). Ethyl decanoate (internal standard, 2 mg/L) was obtained from McLean (shanghai, China). The mass spectrum grade of formic acid was obtained from Aladdin Co., Ltd (Shanghai, China), and standard linear alkanes (C7-C40) were purchased from Sigma-Aldrich (St. Louis, Mo, USA), and ultrapure water prepared by the Milli-Q Advantage system (Millipore Corp., Milford, MA, USA) was used in the experiments.
Preparation of green tea infusions
The green tea infusion was prepared by adding 3 g of tea leaves into 150 mL of boiling water. After 4 min, the leaves were filtered and the infusion was quickly cooled in the ice. Different water samples namely NDW, PW, MW, DW, and TW were used to prepare tea infusion, respectively.
The continuous brewing method was an improvement on the method of Zhang et al (Haihua Zhang, Yulin Li, Yangjun Lv, et al., 2017). 3 g of tea leaves was added into 150 mL of boiling water, and brewed for 4 min. After filtration, 150 mL of boiling water was further added for the second brewing process, and repeat this process until the fourth brewing is over.
Analysis of pH and conductivity of water samples
The pH and conductivity of water samples were measured by pH meter (FiveGo F2pH Meter; Mettler Toledo Instruments Co., Ltd, Shanghai, China) and conductivity meter (DDB-303A, Indian Scientific Instrument Co., Ltd, shanghai, China), respectively.
Analysis of mineral content in tea infusions
Samples digestion
The tea leaves and tea infusion samples were digested by the microwave-assisted method according to the previous work (C. Y. Peng, et al., 2021). Briefly, 0.1 g tea leaves and 10 mL of nitric acid or 2 mL green tea infusion and 8 mL of nitric acid were added into the digestion tube, and digestion tube was put into a Teflon digestion vessel. The digestion system (MARS 6, CEM company of America) program is as follows: the temperature of digestion instrument was increased to 120 ℃ and maintained for 5 min, and increased to 150 ℃ for 10 min, and further increased to 190 ℃ for 30 min. After digestion, the digestion solution was transferred into a beaker and the acid was driven on a 180 ℃ constant temperature electric heating plate (GHP400P, Chengdu Aopule Instrument Co., Ltd). When, about 1 mL of liquid remained in the beaker, the solution was transferred into a 20 mL of volumetric flask for constant volume, which was then brought to volume by ultrapure water. After cooling at 4 ℃, the sample was passed through 0.22 μM water system filter membrane for inductively coupled plasma mass spectrometry (ICP-MS) analysis.
Analysis of mineral content in tea infusions using ICP-MS
The mineral contents in digestion solution was measured by ICP-MS (PE NexION 2000, American Platinum Elmer Co., Ltd.) according to the reported method (C. Y. Peng, et al., 2021). The operating conditions of the instrument are as follows: plasma gas flow 15.0 L/min, auxiliary gas flow 0.9 L/min, carrier gas flow 0.8 L/min, compensation gas flow 0.35 L/min, collision gas flow 4.3 mL/min (KED mode), atomizer temperature 2 ℃, high-frequency generator output power 1550 W, sampling depth 10 mm, peristaltic pump speed 0.1 R/S, the collection mode is a mass spectrum, the peak shape is 3 points, and the collection is repeated for 3 times. PerkinElmer Pure Plus ICP-MS internal standard of 20 μg /L (209Bi, 72Ge, 115In, 6Li, 45Sc, and 89Y) was used to correct matrix effect and compensate for possible changes in instrument performance, and the following isotopes were monitored: 27Al, 75As, 11B, 43Ca, 111Cd, 59Co, 52Cr, 63Cu, 57Fe, 39K, 24Mg, 55Mn, 23Na, 208Pb, 82Se, and 66Zn. The dissolution rate was calculated as follow:
where A represents the metal content in tea infusion (mg/L), B represents the metal content in water (mg/L), C represents the metal content in green tea leaves (mg/g). 0.05 represents the brewing water volume (L), 0.02 represent the digestion volume of tea leaves (L), and 0.1 represent the tea leaves weight (g).
Analysis of taste profile of tea infusions
The taste profile of green tea infusion was measured by the Insent taste system (SA402B, Insent intelligent sensor Technology Inc.). The tea infusion was cooled to 25 ℃ in the water bath for taste analysis according to the previous work (G. X. Ren, T. H. Li, Y. M. Wei, et al., 2021).
Analysis of chromatic paraments (color) of tea infusions
Tea infusion color was measured using a Spectrophotometer (Hunter Lab Color Quest XE, shanghai China), and L*, a*, and b* values were recorded. L* values range from 0 (darkness) to 100 (lightness), positive and negative a* represents redness and greenness; and positive and negative b* represents yellowness and blueness, respectively. A standard white plate (X = 91.98, Y = 93.97, and Z = 110.41) was used to standardize the instrument. Each tea infusion sample was individually measured in triplicate.
The determination of the volatile compounds
Analysis of volatile compounds using gas chromatography/mass spectrometry (GC/MS)
10 mL of green tea infusion was transferred into a headspace bottle (20 mL) containing 3.0 g of sodium chloride and 1 μL of ethyl decanoate (internal standard). A 50/30 µm of CAR/PDMS/DVB SPME fiber (Supeclo Inc, Bellefonte, PA, USA) was inserted into the headspace of the vial for 30 min at 60 ℃ under the same agitation to adsorb volatile compounds. Afterward, the fiber was removed from the vial headspace and immediately inserted into the GC injection port for 5 min to release the volatiles at 250 ℃.
An Agilent Model 7890B-5977B GC–MS system (Agilent, Santa Clara, CA, USA) equipped with a DB-5MS capillary column (30 m × 0.25 mm, 0.25 μm; J&W, Folsom, CA, USA) was used for the analysis of tea infusion volatiles. The initial temperature was 45 °C, held for 3 min, ramped up to 200 °C at a rate of 8 °C/min, then increased to 280 °C at 10 °C/min, and subsequently held at a final temperature of 280 °C for 5 min. High purity helium (greater than99.999 %) was used as the carrier gas at a flow rate of 1.0 mL/min. The mass spectrometer was operated in electron ionization mode at 70 eV. The transfer line and ion source temperatures were 150 °C and 230 °C, respectively. The mass spectra were recorded in full scan mode with the scan range set to m/z 30–400. Retention indices (RI) of the detected volatile components were calculated by linear interpolation from the retention times of the standard linear alkanes (C7-C40). The concentrations of volatile compounds in tea infusions were quantified based on their peak areas and the peak area of the internal standard compound.
Volatile aroma compounds analyzed by aroma dilution analysis (ADA) and gas chromatography/olfactometry (GC-O/MS)
GC-O/MS analytical method was similar with the method for GC/MS analysis described above, and headspace-solid phase microextraction (HS-SPME) was used to extract highly volatile aroma compounds from green tea infusions. After separation by GC, the volatile compounds were split between the mass detector and the olfactory detector from low dilution ratio to high dilution ratio until no odor was smelled (split ratio from 0, 21, 22, 23, 24….…) and the airflow rate was 60 mL/min (humidification), and the temperature of the smelling mouth was 180 ◦C (Yun et al., 2021). Finally, three trained sensory evaluators sniffed the odor and recorded the odor time.
The effect of Fe3+, Ca2+, and pH on the flavor and quality of tea infusion
The control group (CK) was distilled water. Fe3+ treatment: ferric chloride was added to distilled water at four levels: c1 = 0.1 mg/L, c2 = 0.2 mg/L, c3 = 0.3 mg/L, c4 = 0.4 mg/L, Ca2+ treatment: calcium chloride was added to distilled water at four levels: c1 = 10 mg/L, c2 = 20 mg/L, c3 = 30 mg/L, c4 = 40 mg/L, and pH was adjusted by dilute hydrochloric acid to make pH equal to the control; the pH treatment: dilute hydrochloric acid and sodium hydroxide were added to the distilled water to adjust the pH values to 4.5, 5.5, 6.5, 7.5, respectively, and pH = 5.65 was used for the control.
Statistical analysis
All samples were replicated three times, and the data was recorded in the table as mean ± standard deviation. Statistical analysis was carried out by one-way analysis of the variance (ANOVA) followed by Tukey test. Principal component analysis (PCA) and orthogonal partial least squares – discriminant analysis (OPLS-DA) were performed using SIMCA 14.1 software. RI, matching degree, and peak purity were used to identify aroma compounds.
Results and discussion
Physicochemical properties of water samples
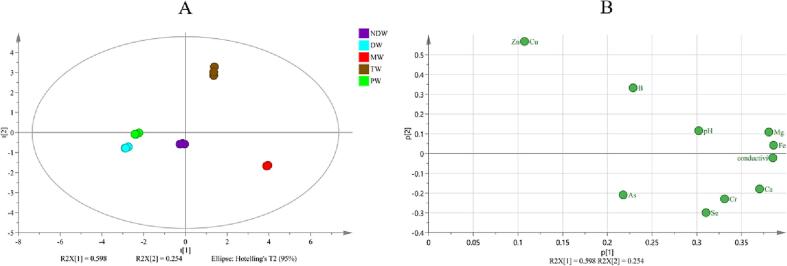
Five different water samples, including NDW, PW, MW, DW, and TW, which are usually used to brew tea, were chosen to evaluate the effect of different types of brewing water on quality of tea infusion in this work. The physicochemical properties of five water samples, including on pH, conductivity, and mineral contents, were shown in Table S1. The pH value, conductivity, and mineral in water samples showed significant difference. The pH values of the five water samples ranged from 5.65 to 7.40, and the conductivity ranged from 1.70 ± 0.72 to 377.0 ± 0.7 S/m. Thereinto, the pH and conductivity values in DW and PW samples were much lower than those in NDW, MW and TW samples. Furthermore, there was a significant correlation between pH value and conductivity in different type of brewing water (r = 0.686, p<0.05). Furthermore, the mineral concentration in water samples also varied significantly. The metal content in MW was obviously higher than those in other water samples. It was obvious that Ca (0–63.006 ± 1.331 mg/L), Na (0–10.856 ± 0.336 mg/L) and Mg (0–5.565 ± 0.039 mg/L) showed highest contents in five samples. Then, the features of brewing water including metal content, pH and conductivity were also involved in PCA analysis as the raw data. It was obvious that the first two PCs in PCA result of brewing water, accounting for 59.8% and 25.4% of the total variance, respectively, could reflect the difference of structure of mineral content in brewing water (Fig. 1A). Based on the loading plot (Fig. 1B) analysis, it was concluded that Zn, Cu, Se, Fe, Mg and conductivity might be the main variables for distinguish the difference between brewing water samples. These results are consistent with many reported brewing water phases (Q. Q. Cao, et al., 2021). The high mineral contents also mainly contribute to the high conductivity in MW and TW groups, which may thereby affect the flavor and quality of tea infusion.
Fig. 1.
PCA analysis based on metals content, pH, and conductivity in five water samples, (A) the score scatter plots, (B) the loading scatter plots. NDW: natural drinking water. DW: distilled water. MW: mineral water. TW: tap water. PW: pure water.
Effects of five water samples on dissolution of mineral substance of tea
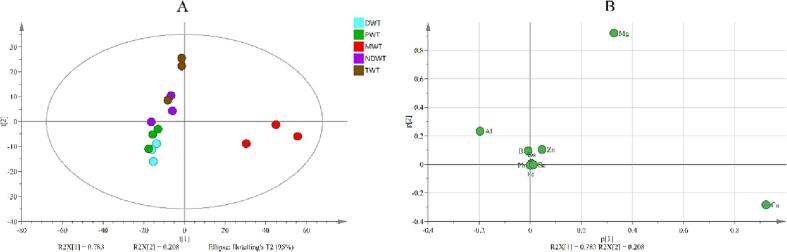
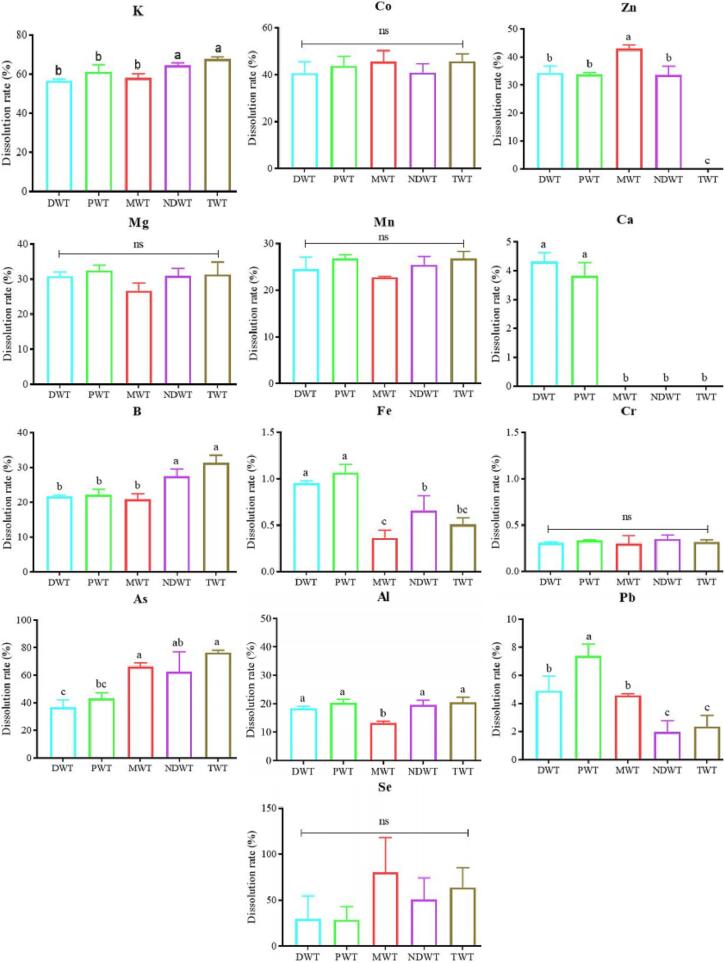
Three major elements (Ca, K and Mg) and eleven trace elements (Al, As, B, Cd, Co, Cr, Fe, Mn, Pb, Se, and Zn) in green tea leaves were determined by ICP-MS (Table S2). The contents of K (19950.138 ± 428.510 mg/kg), Ca (3448.001 ± 81.654 mg/kg) and Mg (2069.303 ± 44.108 mg/kg) in green tea leaves were the highest. The contents of Cd (0.015 ± 0.004 mg/kg), Se (0.022 ± 0.010 mg/kg) and As (0.044 ± 0.008 mg/kg) were the lowest. As expected, the contents of metals in green tea leaves were all lower than the limits specified in Chinese standard NY 659–2003 (i.e., 5 mg/kg for Cd, 1 mg/kg for Cr, 2 mg/kg for As in tea). The previous research has reported that significant differences in the dissolutions of F, Pb, and Cu were observed when tea was brewed with different water samples (Johanna Brinkel, Mobarak H. Khan, & Alexander Kraemer, 2009), indicated that the dissolved mineral substance content is greatly influenced by water. The effect of brewing water on the mineral content of tea infusion was investigated using PCA analysis. The result showed that the first two PCs accounting for 78.3% and 20.3% of the total variance in PCA of tea infusion also displayed the difference of structure of mineral content in tea infusions (Fig. 2A). According to loading plot (Fig. 2B) analysis, Ca, Mg and Al might contribute to the difference between tea infusions. Thus, the dissolution rate of Ca, Mg and Al from tea leaves might be affected by brewing water. Furthermore, the mineral substance had different dissolution rates (Table S3) in different types of water samples, including NDWT (0.00%-62.51%), MWT (0.00%-66.46%), PWT (0.34%-61.23%), DWT (0.31%-56.62%) and TWT (0.31%-56.62%), respectively. The dissolution rate of different mineral substance in tea infusion brewed with five types water was compared in Fig. 3. As expected, the dissolution rate of different mineral substance showed great difference. For example, the dissolution rate of Cr was the lowest with lower than 0.5%, while that the dissolution rate of K was the highest with more than 65% in green tea infusions. It was obvious that the dissolution rate of Co, Mg, Mn, and Cr in the different brewing waters showed no difference (Fig. 3), whereas, the significant differences in the dissolution rates of K, Zn, B, Fe, As, Al, and Pb were observed in different groups (p < 0.05). Thereinto, As, Cr, and Pb are harmful mineral substance to the human body, and the dissolution rates of As and Pb were different in all groups. The dissolution rates of As in MWT, NDWT and TWT were higher, whereas, the dissolution rate of Pb in PWT group was higher than those of other group. Fe and Al were also important mineral substances in tea, and it has been reported that higher level of Fe and Al could affect the human health. In the present work, the dissolution rates of Fe and Al in MWT group were the lowest in these groups. However, the dissolution rate of Zn, considered as health-promoting mineral substance in tea, was the highest in MWT group. To better understand the possible difference in metal dissolution trend caused by several times brewing, ICP-MS was used to determine the mineral substance content in tea infusion brewed four times (Table S4). The result showed that most mineral substance can be dissolved in the first brewing. Apart from Ca, the contents of mineral substance decreased significantly in all water samples as number of brewing times increased. After fourth brewing, Pb and Zn could not be detected by ICP-MS. Thus, the water sources may affect the dissolution rate of mineral substance in tea. However, it was hard to obtain the most suitable water sample for preparing tea infusion based on mineral substance content in tea infusion in the present work. Most mineral substances in tea are complexed with flavanols, catechol, tannins, and polyphenols. The metals dissolution depends on the combination form with organic compounds and the total concentration of metal in tea (M. Memic, D. Mahic, S. Zero, et al., 2014).
Fig. 2.
PCA analysis of metals dissolution content in green tea infusions brewed in five water samples, (A) the score scatter plots, (B) the loading scatter plots. DWT: green tea infusions brewed with distilled water; PWT: green tea infusions brewed with pure water; MWT: green tea infusions brewed with mineral water; NDWT: green tea infusions brewed with natural drinking water; TWT: green tea infusions brewed with tap water. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig.3.
The dissolution rate of metals in green tea infusion brewed in five types of water. “ns” means no significant difference. DWT: green tea infusions brewed with distilled water; PWT: green tea infusions brewed with pure water; MWT: green tea infusions brewed with mineral water; NDWT: green tea infusions brewed with natural drinking water; TWT: green tea infusions brewed with tap water. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Effects of different types of water samples on taste and color of tea infusion
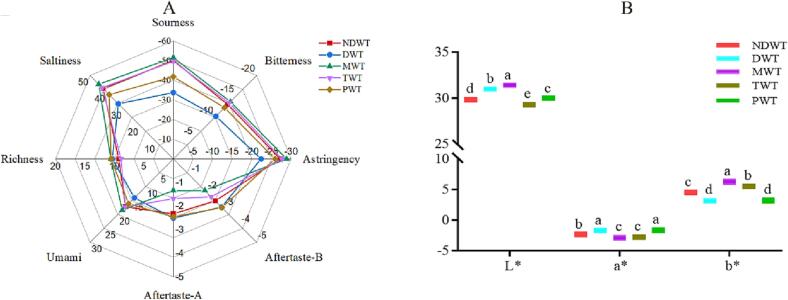
The electronic tongue simulates the human tongue to analyze the taste of food sample, which can quickly and objectively respond to the overall quality information of the samples, and has been applied in the tea research fields of quality grade, class identification and origin (G. X. Ren et al., 2021, Li et al., 2015, Xu et al., 2019). Thus, the electronic tongue was used to investigate the effect of different types of water samples on taste of tea infusions in this work, and the result was shown in Fig. 4A. It was found that the different brewing water could affect the taste quality of tea infusion. The sourness of the tea infusion was highly differentiated, and DWT and PWT were clearly separated from other groups, which might be due to the low pH values of DW and PW water samples. The previous study has showed that the tea infusion of higher grade had higher umami and lower bitterness and astringency (G. X. Ren, et al., 2021), which can be also considered as signature of high quality tea. In this work, the green tea brewed with MW showed higher responses in sourness, bitterness, astringency, umami and saltiness, and DW showed the least response. Therefore, MW is suitable for prepare heavy taste of tea infusion, whereas, DW could be used to obtain light taste of tea infusion.
Fig.4.
Distribution of taste feature of green tea infusion brewed with different waters, (A) taste radar map, (B) color difference distribution. DWT: green tea infusions brewed with distilled water; PWT: green tea infusions brewed with pure water; MWT: green tea infusions brewed with mineral water; NDWT: green tea infusions brewed with natural drinking water; TWT: green tea infusions brewed with tap water. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The chromatic difference analysis is a technique to evaluate the color of green tea infusion. Brewing water is one of the main factors affecting the color of tea infusion, and the results were shown in Fig. 4B. The L* value of tea infusion indicated that MWT was brighter than other waters, whereas, the color of tea infusion brewed with TW was the darkest. The a* value of tea infusion with PW and DW was the highest, while the b* value was lower than those in others group. The a* values and b* values indicated that tea infusion brewed with MW and TW showed greener and yellower color than other water samples. Therefore, green tea brewed with MW was superior to other types of water samples in brightness. The color of tea infusion was highly related to pH value of water, and the catechins could be oxidized and degraded in higher pH water, resulting in darker color of the tea infusion (Y. Zou, W. J. Ma, Q. Tang, et al., 2020). PW and DW samples were weakly acidic, which can reduce the oxidation of catechins, thus, the color of tea infusions was not significantly deepened in PWT and DWT groups. In addition, the higher content of mineral substances in the water sample could also deep the color of the tea infusions. Xu et al. (2013) have found that the L* was mainly related to the pH of the water sample, and the additions of Ca, Mg, Fe, and Zn could enhance the formation of tea cream in green tea infusions (Y. Q. Xu, et al., 2013). Namely, the a* value and b* value was mainly related to TDS, and higher TDS can deepen color in green tea.
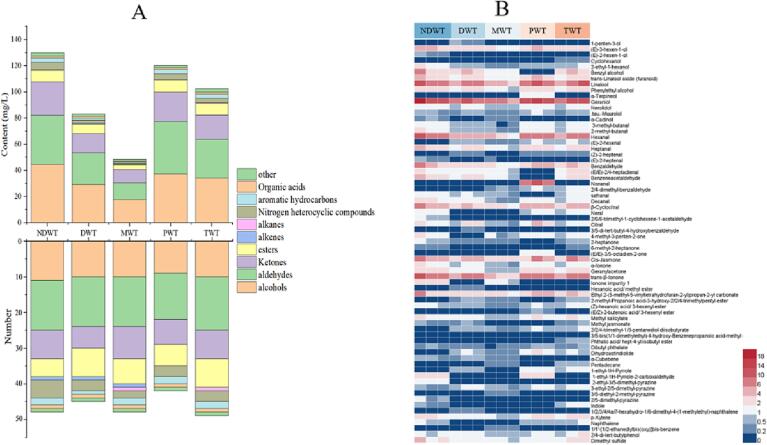
Effects of different water samples on volatile components of tea infusion
The effects of different water samples on volatile components of tea infusion were analyzed by GC–MS (Fig. 5). There were 41–49 volatile compounds in five kinds of green tea infusions. Furthermore, the odor and dilution factor of volatile compounds were analyzed by GC-O/MS (Table S5). Among these volatile compounds, (Z)-2-heptenal, linalool, phenylethyl alcohol, geraniol, citral, indole, α-ionone, 2,4-di-tert-butyl phenol, and (Z)-hexanoic acid, 3-hexenyl ester mainly contributed to the tea aroma. The main characteristics of these compounds were fishy, smoky, floral, fruity, leather, and sweet. The green tea brewed by different water samples showed significantly different contents of the volatile compound, and the total volatile compounds content ranged from 32.15 to 86.74 mg/L, including alcohols (12.50–29.68 mg/L), aldehydes (7.86–26.99 mg/L), ketones (7.20–16.96 mg/L), esters (2.28–5.87 mg/L), and alkenes (0.00–0.37 mg/L). Thereinto, the content of volatile components was the highest in green tea infusion brewed with NDW (86.75 mg/L) and the lowest brewed with MW (32.15 mg/L). Among five kinds of brewing tea infusions, there were 49 volatile components in green tea brewed by TW. The content of esters and nitrogenous compounds was the highest, 5.87 mg/L and 2.21 mg/L, respectively. Esters and nitrogen-containing heterogeneous compounds are mainly described as sweet and nutty. There were 48 volatile compounds in green tea brewed with NDW. Among these volatile compounds, the contents of alcohols, aldehydes, aromatic hydrocarbons, and ketones were the highest. Among the alcohols, the contents of linalool (6.96 mg/L) and geraniol (9.52 mg/L) were higher, and these compounds are mainly described as citrus or floral. Acetaldehyde had a higher content of hexanal (5.31 mg/L), which is described as grassy flavor. Among the ketone compounds, the contents of jasmonate (4.32 mg/L) and trans-β-ionone (5.74 mg/L) were higher in tea infusions, and these components are mainly described as floral flavor. Therefore, green tea infusion brewed with NDW may have a better floral flavor than those brewed by water samples. There were 41 volatile compounds in green tea brewed with PW. Compared to green tea infusions brewed with other water types, the content of 2,4-di-tert-butylphenol in PWT group was the highest with a level of 1.31 mg/L, which is described as a leather flavor. Lastly, the green tea infusion brewed with MW contains 46 types of aroma compounds, and the content of volatile compounds was the least. Overall, the aroma content of green tea brewed with NDW was higher than other brewing water samples, while the aroma compound content of MW was the lowest, which may be affected by minerals content and water pH. It has reported that the addition of cations (such as Na, K, Ca, Mg, Al, and Fe) in white wine promotes the hydrolysis of ester components, and level of esters and alcohols decrease with the increase of mineral substance concentration (Z. J. Huang, Y. H. Zeng, W. H. Liu, et al., 2020). Similarly, pH regulates the activity of enzymes, which can affect the metabolism of volatile precursors, thus producing new volatile compounds (S. Y. Wang, Y. Q. Li, J. Ren, et al., 2018). As described above, a large difference in the mineral content among different water types was observed. For example, MW had the highest content of mineral substances (76.661 mg/L), especially Ca (63.006 mg/L). An increasing number of studies have showed that water with high Ca content could deepen color and aggravate bitterness for tea infusion (J. F. Yin, et al., 2014; Y. Q. Xu, et al., 2013). However, the effect of mineral substance on the content of aroma compounds is still unclear. At the same time, the previous study has shown that ferrous ions could enhance the loss of grass flavor and produce new volatile compounds (Y. Gao, J. Q. Wang, J. X. Chen, et al., 2021). Besides, the pH values of water samples were also different, ranging from weakly acidic distilled water (pH 5.65) to weakly alkaline natural drinking water (pH 7.40), which results in the changes of volatile compounds in tea infusions. Therefore, it is necessary to further verify whether the mineral substances content and pH value in water could affect the aroma content.
Fig.5.
The effect of different water samples on volatile compounds in green tea infusion (A) the content and number of each chemical category of volatile compounds, and (B) heat map of the content of 69 volatile compounds. DWT: green tea infusions brewed with distilled water; PWT: green tea infusions brewed with pure water; MWT: green tea infusions brewed with mineral water; NDWT: green tea infusions brewed with natural drinking water; TWT: green tea infusions brewed with tap water. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
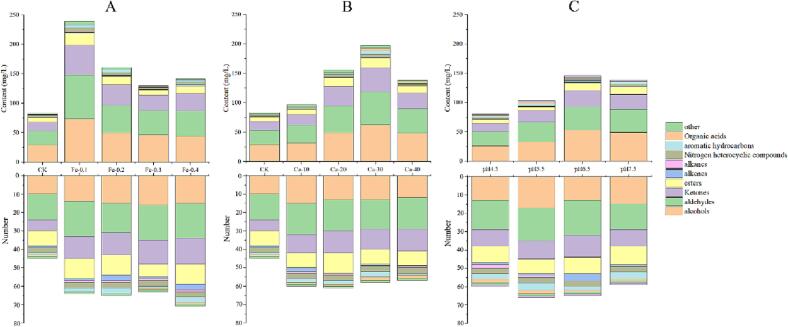
Effect of Fe3+, Ca2+, and pH in water on volatile compounds in green tea infusion
Ca, Fe, and pH in brewing water were reported to have a great influence on the aroma, and the five types of water were significantly different. To further investigate the potential factors that may affect the aroma in tea infusion, the influence of Fe3+, Ca2+, and pH on volatile compounds was analyzed by GC–MS (Table S6-S8). By changing the levels of Fe3+, Ca2+ and pH values, a lot of 106 volatile compounds (Fig. 6) were identified and quantified in tea infusion. Among them, 60 volatile compounds were detected in CK group, 80 volatile compounds were detected in tea infusion with water containing different Fe3+ contents, 76 volatile compounds were detected in tea infusion with water containing different Ca2+ contents, and 89 volatile compounds were detected in green tea infusion with different pH values. Specially, the aroma content in green tea infusion was the highest after addition of 0.1 mg/L of Fe3+, and the aroma content in other group decreased with the increase of Fe3+ content. The previous studies have shown that 4 μM of Fe2+ could lead to catechins degradation to produce new volatile compounds, and Fe3+ could be complexed with amino acids, the complexes further induce the oxidative decarboxylation and promote the formation of strecker aldehydes (Y. Gao et al., 2021, Nashalian and Yaylayan, 2014). Our result also showed that the types of aroma compounds increased after adding Fe3+. Compared with CK group (192.73 ± 35.10 mg/L), the aroma content of tea infusion prepared by brewing water containing 30 mg/L of Ca2+ was the highest with content of 297.53 ± 128.13 mg/L. When the content of Ca2+ was lower than 30 mg/L, the aroma contents increased with the increase of Ca2+ contents. Thus, the content of Ca2+ in water could largely affect the aroma content in tea infusion. The previous study has showed CaCl2 could enhance ester volatile release in apples, and the ester content increased in pectin with the increase of Ca content (Ortiz et al., 2011, Kim et al., 2016). Meanwhile, the content of aroma compounds in tea infusion with brewing water of pH 4.5 was the lowest (121.91 ± 49.26 mg/L) in this work. The aroma content tea increased when the pH values increased from 4.5 to 6.5, and then decreased with the pH value of 7.5. It has been reported that both acidic and alkaline brewing water could reduce the aroma content of tea infusion (O. Nashalian & V. A. Yaylayan, 2014). Likewise, the lower pH in wine will accelerate the hydrolysis of esters to decrease the content of aroma, which is consistent with our study (I. Talaverano, C. Ubeda, A. Caceres-Mella, et al., 2018). In the present study, the content of ester volatile compounds in tea infusion brewed with a weak acid (pH 4.5 and 5.5) was lower than those in pH 6.5 and 7.5 group. Our results indicated that the different content of Fe3+ and Ca2+, and pH values could modulate the content of aroma in green tea infusion, which may mainly contribute to the different effects of different water samples on volatile components of tea infusion. It is well known that the factors affecting aroma change are complicated, which should be further investigated.
Fig. 6.
The effects of (A) Fe3+, (B) Ca2+, and (C) pH on each chemical category of volatile compounds in green tea infusion. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the present work, it was found that the qualities of tea infusion such as taste, color and volatile compounds were largely affected by sources of brewing waters. Thus, it was meaningful to choose the proper brewing water to decrease the dissolution of toxic mineral, and improve the qualities of tea infusion. The mineral substances content and pH values of brewing water were the main factors that affect volatile compounds of the green infusion. More specifically, it is suggested that water with a neutral pH value and lower content of mineral substance is more conducive to brew green tea based on our encouraging result.
Conclusion
Five types of water with different contents of mineral substances and pH values were used to brew green tea, and their effects on the dissolution of mineral substances, taste, color, and volatile components of tea infusion were investigated in this work. Firstly, the mineral substances dissolution rate of TW in the five brewing waters was higher, and the dissolution rate of As in TW was the higher than those in DW and PW for toxic mineral. Therefore, choosing proper brewing water (PW and DW) can reduce the mineral substance content in tea infusion. Secondly, the green tea infusion brewed by MW had higher bitterness and astringency in taste and darker color due to promoting catechins polymerization with the help of minerals. Thirdly, MW with higher minerals content and conductivity greatly reduced the content of volatile compounds in green tea infusion, and higher Ca2+ content and lower Fe3+ content increased the content of volatile compounds. The aroma content in green tea infusion brewed using weak acidic and alkaline water was lower than those brewed by neutral water. Notably, the mineral content in brewing water showed a more significant effect on the aroma compound content of green tea infusion compared to pH. Therefore, to maximize the sensory flavor of tea infusion, it is recommended to use NDW with lower mineral contents and pH between 6 and 7. These findings provide simple and affordable guidance for consumers to choose brewing water and for tea beverage manufacturers to achieve better tea flavor.
CRediT authorship contribution statement
Fuqing Bai: Methodology, Visualization, Formal analysis, Investigation, Writing – original draft. Guijie Chen: Methodology, Validation, Formal analysis, Writing – review & editing. Huiliang Niu: Methodology, Formal analysis. Hongliang Zhu: Methodology, Validation. Ying Huang: Methodology, Formal analysis. Mingming Zhao: Methodology, Formal analysis. Ruyan Hou: Formal analysis, Writing – review & editing. Chuanyi Peng: Conceptualization, Writing – review & editing. Hongfang Li: Methodology, Writing – review & editing. Xiaochun Wan: Conceptualization, Formal analysis, Supervision, Writing – review & editing. Huimei Cai: Conceptualization, Formal analysis, Investigation, Resources, Validation, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the financial support from the National Key Research and Development Program of China (2021YFD1601102), Anhui Excellent Scientific Research and Innovation Team (2022AH010055), and the Modern Agroindustry Technology Research System in Tea Industry (CARS-19).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100681.
Contributor Information
Xiaochun Wan, Email: xcwan@ahau.edu.cn.
Huimei Cai, Email: chm@ahau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Brinkel J., Khan M.H., Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. International Journal of Environmental Research and Public Health. 2009;6(5):1609–1619. doi: 10.3390/ijerph6051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q.Q., Wang F., Wang J.Q., et al. Effects of brewing water on the sensory attributes and physicochemical properties of tea infusions. Food Chemistry. 2021;364 doi: 10.1016/j.foodchem.2021.130235. [DOI] [PubMed] [Google Scholar]
- Cooksey C. Health concerns of heavy metals and metalloids. Science Progress. 2012;95(Pt 1):73–88. doi: 10.3184/003685012X13286247093244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba N., Fernandez-Alduenda M., Moreno F.L., et al. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends in Food Science & Technology. 2020;96:45–60. doi: 10.1016/j.tifs.2019.12.004. [DOI] [Google Scholar]
- Dinh Q.T., Cui Z.W., Huang J., et al. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environment International. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. [DOI] [PubMed] [Google Scholar]
- Feng Z.H., Li Y.F., Li M., et al. Tea aroma formation from six model manufacturing processes. Food Chemistry. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- Franks M., Lawrence P., Abbaspourrad A., et al. The influence of water composition on flavor and nutrient extraction in green and black tea. Nutrients. 2019;11(1):80. doi: 10.3390/nu11010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Wang J.Q., Chen J.X., et al. Effect of ferrous ion on heat-induced aroma deterioration of green tea infusion. Molecules. 2021;26(14):4255. doi: 10.3390/molecules26144255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W.J., Yang M., Xiao Y., et al. Rinsing tea before brewing decreases pesticide residues in tea infusion. Journal of Agricultural and Food Chemistry. 2019;67(19):5384–5393. doi: 10.1021/acs.jafc.8b04908. [DOI] [PubMed] [Google Scholar]
- Genchi G., Sinicropi M.S., Lauria G., et al. The effects of cadmium toxicity. International Journal of Environmental Research and Public Health. 2020;17(11):3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.Y., Ho C.T., Schwab W., et al. Aroma profiles of green tea made with fresh tea leaves plucked in summer. Food Chemistry. 2021;363 doi: 10.1016/j.foodchem.2021.130328. [DOI] [PubMed] [Google Scholar]
- He C.J., Guo X.M., Yang Y.M., et al. Characterization of the aromatic profile in “zijuan” and “pu-erh” green teas by headspace solid-phase microextraction coupled with GC-O and GC-MS. Analytical Methods. 2016;8(23):4727–4735. doi: 10.1039/c6ay00700g. [DOI] [Google Scholar]
- Huang Z.J., Zeng Y.H., Liu W.H., et al. Effects of metals released in strong-flavor baijiu on the evolution of aroma compounds during storage. Food Science & Nutrition. 2020;8(4):1904–1913. doi: 10.1002/fsn3.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Ju H.K., Kim Y., et al. Effects of amidation and/or methylesterification of pectin on aroma release at different calcium concentration. Food Hydrocolloids. 2016;52:343–349. doi: 10.1016/j.foodhyd.2015.07.006. [DOI] [Google Scholar]
- Li Y.J., Lei J.C., Liang D.W. Identification of fake green tea by sensory assessment and electronic tongue. Food Science and Technology Research. 2015;21(2):207–212. doi: 10.3136/fstr.21.207. [DOI] [Google Scholar]
- Liu Y., Luo L.Y., Liao C.X., et al. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chemistry. 2018;269:24–34. doi: 10.1016/j.foodchem.2018.06.130. [DOI] [PubMed] [Google Scholar]
- Liu H.C., Xu Y.J., Wen J., et al. A comparative study of aromatic characterization of Yingde Black Tea infusions in different steeping temperatures. Lwt-Food Science and Technology. 2021;143 doi: 10.1016/j.lwt.2021.110860. [DOI] [Google Scholar]
- Memic M., Mahic D., Zero S., et al. Comparison of different digestion methods of green and black tea at the Sarajevo market for the determination of the heavy metal content. Journal of Food Measurement and Characterization. 2014;8(2):149–154. doi: 10.1007/s11694-014-9175-6. [DOI] [Google Scholar]
- Nashalian O., Yaylayan V.A. Thermally induced oxidative decarboxylation of copper complexes of amino acids and formation of strecker aldehyde. Journal of Agricultural and Food Chemistry. 2014;62(33):8518–8523. doi: 10.1021/jf502751n. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Graell J., Lara I. Preharvest calcium sprays improve volatile emission at commercial harvest of 'Fuji Kiku-8' apples. Journal of Agricultural and Food Chemistry. 2011;59(1):335–341. doi: 10.1021/jf1035959. [DOI] [PubMed] [Google Scholar]
- Peng C.Y., Xu X.F., Zhu H.Y., et al. Metabolics and ionomics responses of tea leaves (Camellia sinensis (L.) O. Kuntze) to fluoride stress. Plant Physiology and Biochemistry. 2021;158:65–75. doi: 10.1016/j.plaphy.2020.11.024. [DOI] [PubMed] [Google Scholar]
- Ren G.X., Li T.H., Wei Y.M., et al. Estimation of Congou black tea quality by an electronic tongue technology combined with multivariate analysis. Microchemical Journal. 2021;163 doi: 10.1016/j.microc.2020.105899. [DOI] [Google Scholar]
- Sarkar A., Paul B. The global menace of arsenic and its conventional remediation – A critical review. Chemosphere. 2016;158:37–49. doi: 10.1016/j.chemosphere.2016.05.043. [DOI] [PubMed] [Google Scholar]
- Senanayake S. Green tea extract: Chemistry, antioxidant properties and food applications – A review. Journal of Functional Foods. 2013;5(4):1529–1541. doi: 10.1016/j.jff.2013.08.011. [DOI] [Google Scholar]
- Talaverano I., Ubeda C., Caceres-Mella A., et al. Water stress and ripeness effects on the volatile composition of Cabernet Sauvignon wines. Journal of the Science of Food and Agriculture. 2018;98(3):1140–1152. doi: 10.1002/jsfa.8565. [DOI] [PubMed] [Google Scholar]
- Wang S.Y., Li Y.Q., Ren J., et al. Comparison on evolution of volatile compounds and aroma attributes in different pH-adjusted fermented bog bilberry syrup wines during bottle-aging period. Food Bioscience. 2018;22:121–128. doi: 10.1016/j.fbio.2018.01.003. [DOI] [Google Scholar]
- Wang C., Lv S.D., Wu Y.S., et al. Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics. Springerplus. 2016;5(1):1–15. doi: 10.1186/s40064-016-2229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Wang J., Zhu L.Y. The qualitative and quantitative assessment of tea quality based on E-nose, E-tongue and E-eye combined with chemometrics. Food Chemistry. 2019;289:482–489. doi: 10.1016/j.foodchem.2019.03.080. [DOI] [PubMed] [Google Scholar]
- Xu Y.Q., Zhong X.Y., Yin J.F., et al. The impact of Ca2+ combination with organic acids on green tea infusions. Food Chemistry. 2013;139(1–4):944–948. doi: 10.1016/j.foodchem.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Xu Y.Q., Zou C., Gao Y., et al. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chemistry. 2017;236:142–151. doi: 10.1016/j.foodchem.2016.11.110. [DOI] [PubMed] [Google Scholar]
- Yang X.M., Liu Y.L., Mu L.H., et al. Discriminant research for identifying aromas of non-fermented Pu-erh tea from different storage years using an electronic nose. Journal of Food Processing and Preservation. 2018;42(10):e13721. [Google Scholar]
- Yin J.F., Zhang Y.N., Du Q.Z., et al. Effect of Ca2+ concentration on the tastes from the main chemicals in green tea infusions. Food Research International. 2014;62:941–946. doi: 10.1016/j.foodres.2014.05.016. [DOI] [Google Scholar]
- Yun J., Cui C.J., Zhang S.H., et al. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chemistry. 2021;360 doi: 10.1016/j.foodchem.2021.130033. [DOI] [PubMed] [Google Scholar]
- Zeng L., Ma M.J., Li C., et al. Stability of tea polyphenols solution with different pH at different temperatures. International Journal of Food Properties. 2017;20(1):1–18. doi: 10.1080/10942912.2014.983605. [DOI] [Google Scholar]
- Zhang H., Li Y., Lv Y., et al. Influence of brewing conditions on taste components in Fuding white tea infusions. Journal of the Science of Food and Agriculture. 2017;97(9):2826–2833. doi: 10.1002/jsfa.8111. [DOI] [PubMed] [Google Scholar]
- Zou Y., Ma W.J., Tang Q., et al. A high-precision method evaluating color quality of Sichuan Dark Tea based on colorimeter combined with multi-layer perceptron. Journal of Food Process Engineering. 2020;43(8):e13444. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.