Abstract
Background
Tuberculosis (TB) disease is the leading cause of mortality among people living with HIV (PLHIV). Interferon-gamma release assays (IGRAs) are approved for TB infection ascertainment. However, current IGRA data on the prevalence of TB infection in the context of near-universal access to antiretroviral therapy (ART) and TB preventive therapy (TPT) are lacking. We estimated the prevalence and determinants of TB infection among PLHIV within a high TB and HIV burden context.
Methods
This cross-sectional study included data from adult PLHIV age ≥18 years in whom QuantiFERON-TB Gold Plus (QFT-Plus) assay, an IGRA, was performed. TB infection was defined as a positive or indeterminate QFT-Plus test. Participants with TB and those who had previously used TPT were excluded. Regression analysis was performed to identify independent predictors of TB infection.
Results
Of 121 PLHIV with QFT-Plus test results, females were 74.4% (90/121), and the mean age was 38.4 (SD 10.8) years. Overall, 47.9% (58/121) were classified as TB infection (QFT-Plus test positive and indeterminate results were 39.7% (48/121) and 8.3% (10/121), respectively). Being obese/overweight (body mass index ≥25 kg/m2; p=0.013, adjusted OR (aOR) 2.90, 95% CI 1.25 to 6.74) and ART usage for >3 years (p=0.013, aOR 3.99, 95% CI 1.55 to 10.28) were independently associated with TB infection.
Conclusion
There was a high TB infection prevalence among PLHIV. A longer period of ART and obesity were independently associated with TB infection. The relationship between obesity/overweight and TB infection may be related to ART use and immune reconstitution and requires further investigation. Given the known benefit of test-directed TPT among PLHIV never exposed to TPT, its clinical and cost implications for low and middle-income countries should be explored further.
Keywords: Immunodeficiency, Tuberculosis, Clinical Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Among people living with HIV (PLHIV), the risk of progression to tuberculosis (TB) disease is higher with confirmed and untreated TB infection. Data on the prevalence of TB infection in the context of near-universal access to antiretroviral therapy (ART) and TB preventive therapy (TPT) are lacking in Africa.
WHAT THIS STUDY ADDS
Using the QuantiFERON-TB Gold Plus assay for diagnosis, this study provides evidence that the prevalence of TB infection remains high even with near-universal access to ART and TPT.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study should prompt larger studies to explore the prevalence and evaluate determinants of TB infection among PLHIV. A broader understanding of the clinical and cost implications of test-directed TPT for PLHIV in low and middle-income countries may better inform policy towards its utility.
Introduction
Tuberculosis (TB) continues to be a global health concern.1–3 Despite near-universal access to antiretroviral therapy (ART) and TB preventive therapy (TPT), TB remains the leading cause of disease and mortality among people living with HIV (PLHIV).3–5 In 2021, approximately 10.6 million people developed TB worldwide, 6.7% (703 000) of whom were PLHIV.3 6 In the same period, 187 000 PLHIV died from TB, accounting for one-third of all AIDS-related deaths globally.3 5 Kenya is among the 30 high-burden countries for TB and HIV-associated TB.3 In 2021, the country had 133 000 incident TB cases, and 24% (32 000) were HIV/TB coinfected, of whom 34% (11 000) died.6 TB infection, the precursor of TB disease, is a crucial focus for TB prevention strategies.3 7–9 The global burden of TB disease is estimated at a quarter of the population,10 with wide regional variation.11 12
Current information on the burden of TB infection in Africa and among PLHIV is sparse.11 12 There are also limited data on how the prevalence of TB infection has changed with near-universal access to ART and TPT. Other factors associated with TB infection among PLHIV are underexplored.13–15 Establishing the burden of TB infection and the associated risk factors in PLHIV is important for various reasons. First, the risk of progression from TB infection to TB disease is higher among PLHIV with a positive test for TB infection than among those with a negative or unknown test result.16 Second, TB risk is markedly reduced by TPT, with a 64% risk reduction among PLHIV with a positive test, compared with 14% among those with a negative or unknown test.17 This has potential implications for practice, given the WHO and national recommendation to treat all PLHIV for TB infection without the need for confirmatory testing.9 18–20
The use of gold-standard tests to establish disease burden is optimum when feasible.21 22 No gold standard exists for the diagnosis of TB infection. However, interferon-gamma release assays (IGRAs) and tuberculin skin tests are screening tests approved by the WHO.9 IGRAs are advantageous since prior BCG vaccination does not affect their performance characteristics.23 HIV impacts the QuantiFERON-TB Gold In-Tube test (QFT-GIT) less than it does the ELISPOT (T SPOT-TB).24 25 The QFT Gold Plus (QFT-Plus) and its forerunner, the QFT-GIT, have concordant performance.26 27 This paper presents the results of a study that sought to estimate TB infection prevalence among PLHIV using the QFT-Plus assay in a high TB burden context. Further, the determinants of TB infection in this population were explored.
Methods
Study setting and design
This cross-sectional study used data collected at enrolment into a prospective observational study to explore methods of monitoring response to isoniazid during TPT. The study was conducted at three HIV care and prevention centres in Nairobi, Kenya comprising Kenyatta National Hospital, Kenya’s largest national teaching and referral hospital; Pumwani maternity hospital, Kenya’s largest referral maternity hospital and the Kenya Medical Research Institute, Centre for Respiratory disease Research. The clientele is cosmopolitan, ranging from urban, periurban and rural settings.
Study period and population
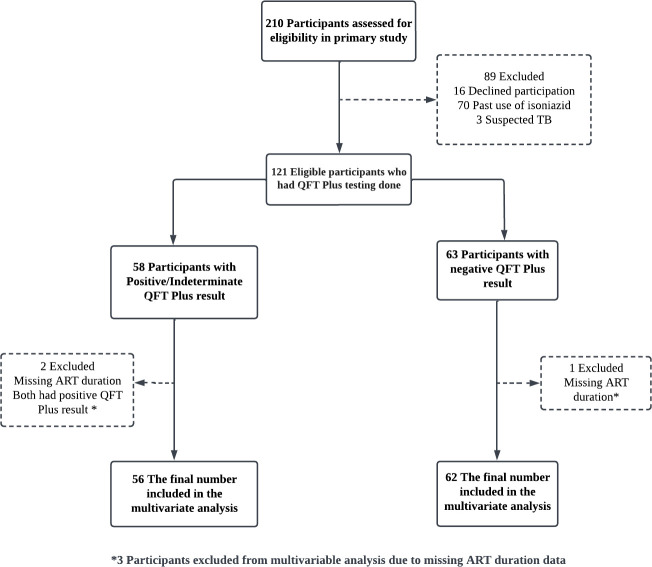
Participants aged ≥18 years seeking HIV care and prevention services between December 2019 and December 2020 were eligible to participate in the primary study if verbal and written informed consent was obtained. To reduce the chance of outcome misclassification, participants who had ever received isoniazid as a treatment for TB infection or disease and those suspected or confirmed as having TB disease were ineligible. All study participants underwent the recommended four-item TB symptom screening questions to rule out TB disease.28 Any participants with an affirmative response to any of the four questions received further evaluation, including a complete physical examination, sputum examination for cartridge-based nucleic acid amplification test and chest X-ray. This cross-sectional study includes all the 121 PLHIV that met the eligibility criteria for the primary study (figure 1).
Figure 1.
Enrolment and analysis flow diagram. ART, antiretroviral therapy; QFT, QuantiFERON-TB; TB, tuberculosis.
Variables and definitions
The outcome variable of interest was the TB infection status. TB infection was defined as a positive or indeterminate QFT-Plus result. We included indeterminate results in the definition of TB infection for several reasons. Foremost, we were bound by the existing WHO and national recommendations to treat all PLHIV for TB infection without requiring testing.9 18 20 28 Further, guidance on handling indeterminate QFT-Plus tests among PLHIV in the context of TPT is lacking. The WHO recommends a ‘case-by-case assessment for the potential benefit and harms of TPT’ in regard to general testing for TB infection.9 Second, the manufacturer suggests repeat testing for indeterminate results when related to technical factors where instructions are not followed.29 In our case, instructions were followed per protocol. Finally, as this was a cross-sectional study and resources were limited, prospective repeat testing was not feasible, and clinical care took precedence over repeat testing.30 31 All participants with negative results were considered not to have TB infection. The explanatory variables of interest were age, sex, body mass index (BMI), cigarette smoking, alcohol use, household crowding, history of contact with a known TB case, diabetes status, duration of ART use as ≤3 vs >3 years and viral load level as ≤40 vs >40 HIV copies/mL of blood. To measure smoking and alcohol use habits, participants were asked if they had ever smoked and if they considered their alcohol use more than social. Similarly, participants were asked whether their household was crowded, whether they had been in contact with a person diagnosed with TB in the preceding 2 years, and if they had ever been diagnosed with diabetes.
Clinical and laboratory procedures
At baseline, clinical and demographic data were collected or abstracted from electronic medical records. These included age, sex, weight, height, smoking status, alcohol use, living conditions, diabetes status and other known chronic illness, history of TB infection and isoniazid use. HIV rapid testing was performed in programmatic settings for those of unknown status as part of routine HIV prevention. Where feasible, a CD4 lymphocyte count was performed. Viral load testing was performed under programmatic settings per the existing national guidelines. All participants on ART for at least 6 months had viral load testing done at the same time as the QFT-Plus test. All participants that had been on ART for less than 6 months at the time of the QFT-Plus test had viral load done after 6 months of ART use according to national guidelines. The ART regimen and initiation date were collected, and the date was used to determine the duration of ART use. Viral load level (≤40 vs >40 copies/mL) and ART duration (≤3 vs >3 years) were used as a proxy for immune reconstitution and competency in the absence of CD4 lymphocyte count.
We used the QFT-Plus assay for TB infection diagnosis according to the manufacturer’s protocol.29 Briefly, 6 mL of peripheral venous whole blood was collected into lithium heparin vacutainers and transported to the laboratory within 2 hours of collection. A 1 mL was transferred into the four QFT-Plus blood collection tubes, the Nil tube, TB1 tube, TB2 tube and the mitogen tube within 8 hours of collection and incubated at 37°C for 16 hours. The tubes were centrifuged for 15 min at 2000–3000×g RCF (g). Plasma was collected and assessed using the standard ELISA.
The results were interpreted according to the manufacturer’s instructions.29 A QFT-Plus result was interpreted as positive when the IFN-γ response to one or both MTB-specific antigens was ≥0.35 IU/mL and ≥25% of Nil value, irrespective of the IFN-γ response to the mitogen control. A QFT-Plus result was interpreted as negative when the IFN-γ response to both MTB-specific proteins was <0.35 IU/mL, or≥0.35 IU/mL and <25% of Nil value, with a response to the mitogen control ≥0.5 IU/mL. A QFT-Plus result was interpreted as indeterminate when the IFN-γ response to both MTB-specific proteins was <0.35 IU/mL, or ≥0.35 IU/mL and <25% of Nil value, with a response to the mitogen control <0.5 IU/mL. A QFT-Plus result was interpreted as indeterminate when the IFN-γ response was above the cut-off in the nil control, irrespective of the IFN-γ response to the MTB-specific antigens and the mitogen control.29
For quality control, independent study staff checked all consent forms, study questionnaires and laboratory results for accuracy and completeness on the same day of data entry.
Sample size
The sample size was determined based on an estimated TB infection prevalence of 35% in exposed32 and 10% in unexposed10 at a 95% level of confidence and 80% power. Thus, a sample of 102 with continuity correction was sufficient, and all 121 eligible participants that were HIV-infected and that consented were selected for the study.
Statistical analysis
After appropriate data cleaning, the analysis was performed using Stata Statistical Software V.17 (StataCorp). Descriptive statistics were used to summarise the sociodemographic and clinical characteristics of the participants. Pearson’s χ2 and Fisher’s exact tests were used to test for the association between categorical variables and outcomes, and the ORs were reported. For continuous variables, the Student’s t-test was used. The multivariate logistic regression analyses included factors with a p<0.10. Statistical significance was set at p<0.05. Missing data were excluded to reduce the chance of biased estimates. Specifically, CD4 lymphocyte count data were excluded from the analysis and substituted with the duration of ART use. Complete case analysis was performed during multivariate regression (figure 1).
Patient and public involvement
It was not possible to involve patients or the public in the design, conduct, reporting or dissemination plans of the study. Patients were involved to the extent to which they provided informed consent and allowed for sample collection for laboratory testing. The study results will be communicated to willing participants and community members.
Results
Sociodemographic and clinical characteristics of participants
Among the 121 study participants living with HIV included in this analysis, females were the majority at 74.4% (90/121). The mean age of all participants was 38.4 years with a standard deviation (SD) of 10.8, and 51.2% (62/121) of the participants were ≥40 years. By BMI, 15.7% (19/121) of the participants were categorised as obese, 30.6% (37/121) as overweight, 49.6% (60/121) as normal and 4.1% (5/121) as underweight. Among the social risk factors, 18.2% (22/121) were cigarette smokers, 20.7% (25/121) used alcohol, 27.3% (33/121) reported living in a crowded place and 26.4% (32/121) had known contact with TB cases. Regarding clinical and laboratory characteristics, only 2.5% (3/121) had diabetes, and 38.1% (45 of 118 with complete ART duration data) had been on ART for >3 years. All participants were on the recommended first-line regimens with a backbone of tenofovir and lamivudine, combined with dolutegravir in 95% (115) and efavirenz in the remaining 5%. The participants with viral load <40 copies/mL were 85.7% (102 of 119 with complete viral load data). Table 1 shows the participants’ sociodemographic and clinical characteristics.
Table 1.
Sociodemographic and clinical characteristics of participants (n=121)
| Variable | Frequency (n=121) |
No TB infection* (n=63) |
TB infection† (n=58) |
| Demographic variables | |||
| Gender (n, %) | |||
| Male | 31 | 16 (51.6) | 15 (48.4) |
| Female | 90 | 47 (52.2) | 43 (47.8) |
| Age (mean, SD) | 38.4, 10.8 | (38.2, 11.3) | (38.7, 10.3) |
| Age groups (n, %) | |||
| <30 | 26 | 15 (57.7) | 11 (42.3) |
| 30–39 | 33 | 19 (57.6) | 14 (42.4) |
| ≥40 | 62 | 29 (46.8) | 33 (53.2) |
| BMI (n, %) | |||
| <18.5 | 5 | 3 (60.0) | 2 (40.0) |
| 18.5 to <25.0 | 60 | 37 (61.7) | 23 (38.3) |
| 25.0 to 30.0 | 37 | 12 (32.4) | 25 (67.6) |
| ≥30 | 19 | 11 (57.9) | 8 (42.1) |
| Social risk factors | |||
| History of smoking (n, %) | |||
| No | 99 | 57 (57.6) | 42 (42.4) |
| Yes | 22 | 6 (27.3) | 16 (72.7) |
| Alcohol use (n, %) | |||
| No | 96 | 54 (56.3) | 42 (43.8) |
| Yes | 25 | 9 (36.0) | 16 (64.0) |
| Household crowding (n, %) | |||
| Not crowded | 88 | 47 (53.4) | 41 (46.6) |
| Crowded | 33 | 16 (48.5) | 17 (51.5) |
| History of contact with TB case (n, %) | |||
| No | 89 | 51 (57.3) | 38 (42.7) |
| Yes | 32 | 12 (37.5) | 20 (62.5) |
| Clinical and laboratory characteristics | |||
| Diabetes status (n, %) | |||
| No | 118 | 60 (50.9) | 58 (49.2) |
| Yes | 3 | 3 (100.0) | 0 (0.0) |
| Duration of ART use (n, %)‡ | |||
| ≤3 years | 73 | 45 (61.6) | 28 (38.4) |
| >3 years | 45 | 17 (37.8) | 28 (62.2) |
| Viral load level (copies/mL; n, %)§ | |||
| ≤40 | 102 | 52 (51.0) | 50 (49.0) |
| >40 | 17 | 9 (52.9) | 8 (47.1) |
*Negative QFT-Plus test.
†Positive and indeterminate QFT-Plus test.
‡n=118, 3 participants did not have a documented ART start date.
§n=119, 2 participants did not have a valid viral load report.
ART, antiretroviral therapy; BMI, body mass index; QFT, QuantiFERON-TB; TB, tuberculosis.
Prevalence of TB infection estimated by QFT-Plus test results
The prevalence of TB infection estimated by QFT-Plus test results in this population of PLHIV was 47.9% (58/121; table 1). This comprised 39.7% (48/121) with a positive QFT-Plus test report and 8.3% (10/121) with an indeterminate QFT-Plus test report. Participants with an indeterminate QFT-Plus are described in table 2. Among the participants with indeterminate results, 60% (6/10) were female, and 80% (8/10) were ≥30 years old. All the participants reported having HIV as the only underlying chronic illness known to them. Only 30% (3/10) reported cigarette use, and only 40% (4/10) reported alcohol use. Sixty per cent (6/10) of participants had been on ART for less than 1 year. In 8 of the 10 participants with indeterminate results, the interferon-gamma (IFN-γ) level in response to mitogen was below the cut-off of 0.5 IU/mL for mitogen minus Nil (table 2). Three of the 10 participants had the IFN-γ response in the Nil tube above the cut-off of 8.0 IU/mL (table 2), signifying an ongoing immune response.
Table 2.
Characteristics of participants with indeterminate QFT-Plus test results (n=10)
| Patient | Age | Sex | Duration on ART (years) | BMI | History of smoking | Alcohol use | IFN-level (IU/mL) | |||
| Nil | TB1-Nil | TB2-Nil | Mitogen-Nil | |||||||
| 1 | 30–39 | F | <1 | 25 | No | Yes | 0.08 | −0.03 | −0.03 | 0.02 |
| 2 | 30–39 | F | <1 | 16 | Yes | No | >10 | >10 | >10 | >10 |
| 3 | 30–39 | F | <1 | 18 | Yes | Yes | >10 | −15.63 | −59.3 | −66.84 |
| 4 | <30 | M | <1 | 22 | No | Yes | 0.09 | 0.04 | −0.03 | 0.04 |
| 5 | ≥40 | F | 11 | 24 | No | No | 0.62 | −0.34 | −0.36 | 0.15 |
| 6 | ≥40 | M | 11 | 27 | No | No | 0.09 | 0.06 | −0.02 | 0.14 |
| 7 | ≥40 | F | 4 | 30 | No | No | >10 | >10 | >10 | >10 |
| 8 | 30–39 | F | <1 | 26 | No | No | 0.06 | 0 | −0.01 | 0.11 |
| 9 | <30 | M | <1 | 27 | Yes | Yes | 1.18 | −0.16 | −0.35 | 0.02 |
| 10 | ≥40 | M | 11 | 21 | No | No | 0.07 | −0.04 | −0.02 | 0.18 |
ART, antiretroviral therapy; BMI, body mass index; F, female; M, male; QFT, QuantiFERON-TB.
Determinants of TB infection estimated by QFT-Plus test results
The mean age of participants with TB infection was 38.7 years (SD 10.3) compared with 38.2 years (SD 11.3) among those without TB infection (p=0.6022) (table 3). On bivariate analysis, increased odds of having TB infection were observed among participants aged ≥40 years (p=0.353, OR 1.55, 95% CI 0.61 to 3.95), those with reported alcohol use (p=0.071, OR 2.29, 95% CI 0.90 to 5.78), those living in a crowded place (p=0.631, OR 1.22, 95% CI 0.54 to 2.72) and those with history of contact with a case of TB (p=0.056, OR 2.24, 95% CI 0.96 to 5.21). However, these findings were not statistically significant at p<0.05 (table 3). BMI (p=0.025, OR 2.30, 95% CI 1.09 to 4.85), a history of smoking cigarettes (p=0.010, OR 3.62, 95% CI 1.26 to 10.37) and duration of ART use >3 years (p=0.012, OR 2.65, 95% CI 1.20 to 5.83) were significantly associated with TB infection (table 3).
Table 3.
Determinants of TB infection among PLHIV—bivariate analysis (n=121)
| Variable | No TB infection* (n=63) |
TB infection† (n=58) |
P value | OR‡ (95% CI) | χ2 |
| Baseline characteristics | |||||
| Gender (n, %) | |||||
| Male Female |
16 (51.6) 47 (52.2) |
15 (48.4) 43 (47.8) |
0.953 | Ref 0.98 (0.43 to 2.22) |
0.00 |
| Age (mean, SD) | (38.2, 11.3) | (38.7, 10.3) | 0.602 | – | – |
| Age groups (n, %) | |||||
| <30 30–39 ≥40 |
15 (57.7) 19 (57.6) 29 (46.8) |
11 (42.3) 14 (42.4) 33 (53.2) |
Ref 0.993 0.353 |
Ref 1.00 (0.35 to 2.87) 1.55 (0.61 to 3.95) |
1.43 |
| BMI (n, %) | |||||
| <25 ≥25.0 |
40 (61.5) 23 (41.1) |
25 (38.5) 33 (58.9) |
0.025 | Ref 2.30 (1.09 to 4.85) |
5.01 |
| Social risk factors | |||||
| History of smoking (n, %) | |||||
| No Yes |
57 (57.6) 6 (27.3) |
42 (42.4) 16 (72.7) |
0.010 | Ref 3.62 (1.26 to 10.37) |
6.57 |
| Alcohol use (n, %) | |||||
| No Yes |
54 (56.3) 9 (36.0) |
42 (43.8) 16 (64.0) |
0.071 | Ref 2.29 (0.90 to 5.78) |
3.23 |
| Household crowding (n, %) | |||||
| Not crowded Crowded |
47 (53.4) 16 (48.5) |
41 (46.6) 17 (51.5) |
0.631 | Ref 1.22 (0.54 to 2.72) |
0.23 |
| Contact with TB§ case (n, %) | |||||
| No Yes |
51 (57.3) 12 (37.5) |
38 (42.7) 20 (62.5) |
0.056 | Ref 2.24 (0.96 to 5.21) |
3.67 |
| Clinical and laboratory characteristics | |||||
| Diabetes status (n, %) | |||||
| No Yes |
60 (50.9) 3 (100.0) |
58 (49.2) 0 (0.0) |
0.094 | Ref 0.00(-) |
2.81 |
| Duration of ART use (n, %)¶ | |||||
| ≤3 years >3 years |
45 (61.6) 17 (37.8) |
28 (38.4) 28 (62.2) |
0.012 | Ref 2.65 (1.20 to 5.83) |
6.31 |
| Viral load level (copies/ml; n, %)** | |||||
| ≤40 >40 |
52 (51.0) 9 (52.9) |
50 (49.0) 8 (47.1) |
0.881 | Ref 0.92 (0.33 to 2.60) |
0.02 |
The bold values are factors with a p<0.10 included in multivariate analyses.
*Negative QFT-Plus test.
†Positive & indeterminate QFT-Plus test.
‡Odds ratio.
§Tuberculosis.
¶n=118, 3 participants did not have a documented ART start date.
**n=119, 2 participants did not have a valid viral load report.
ART, antiretroviral therapy; BMI, body mass index; TB, tuberculosis.
In a subanalysis, we found that among factors associated with being on ART>3 years were age >40 years old (p=0.005, OR 4.57, 95% CI 1.42 to 14.7) and having a positive QFT-Plus test (p=0.009, OR 2.89, 95% CI 1.25 to 6.65). Although increased odds of being on ART>3 years were observed among participants with indeterminate results (p=0.420, OR 1.76, 95% CI 0.44 to 7.15), this finding was not statistically significant. Reduced odds of being on ART>3 years were observed among participants having a viral load of >40 copies/mL (p=0.772, OR 0.85, 95% CI 0.29 to 2.51) (online supplemental table 1).
bmjresp-2022-001581supp001.pdf (44.6KB, pdf)
On multivariate analysis including factors with p<0.10 (table 4), there were increased odds of TB infection among those using alcohol (p=0.066, adjusted OR (aOR) 2.88, 95% CI 0.93 to 8.91). Being obese/overweight (BMI≥25 kg/m2; p=0.013, aOR 2.90, 95% CI 1.25 to 6.74) and being on ART for >3 years (p=0.013, aOR 3.99, 95% CI 1.55 to 10.28) were independently associated with TB infection (table 4). We performed sensitivity analyses in which we excluded the category with BMI<18.5 kg/m2 (online supplemental table 2) and again considered indeterminate QFT-Plus tests as negative (online supplemental table 3). The observed associations remained.
Table 4.
Logistic regression analysis for factors associated with TB infection (n=118)
| Variable | No TB infection* (n=62) |
TB infection† (n=56) |
P value | cOR‡ (95% CI) | aOR§ (95% CI) |
| Baseline characteristics | |||||
| BMI (n, %) | |||||
| <25 ≥25.0 |
39 (61.9) 23 (41.8) |
24 (38.1) 32 (58.2) |
0.013 | Ref 2.26 (1.08 to 4.73) |
Ref 2.90 (1.25 to 6.74) |
| Social risk factors | |||||
| History of smoking (n, %) | |||||
| No Yes |
56 (57.7) 6 (28.6) |
41 (42.3) 15 (71.4) |
0.253 | Ref 3.41 (1.22 to 9.55) |
Ref 1.72 (0.44 to 6.67) |
| Alcohol use (n, %) | |||||
| No Yes |
53 (57.0) 9 (36.0) |
40 (43.0) 16 (64.0) |
0.066 | Ref 2.36 (0.94 to 5.88) |
Ref 2.88 (0.93 to 8.91) |
| Contact with TB¶ case (n, %) | |||||
| No Yes |
51 (57.3) 11 (37.9) |
38 (42.7) 18 (62.1) |
0.367 | Ref 2.20 (0.93 to 5.19) |
Ref 1.69 (0.54 to 5.33) |
| Clinical and laboratory characteristics | |||||
| Duration of ART use (n, %) | |||||
| ≤3 years >3 years |
45 (61.6) 17 (37.8) |
28 (38.4) 28 (62.2) |
Ref 0.013 |
Ref 2.65 (1.23 to 5.69) |
Ref 3.99 (1.55 to 10.28) |
The bold values are factors meeting statistical significance at p<0.05.
*Negative QFT-Plus test
†Positive & indeterminate QFT-Plus test
‡crude OR
§adjusted OR
¶Tuberculosis
ART, antiretroviral therapy; BMI, body mass index; QFT-Plus, QuantiFERON-TB; TB, tuberculosis.
bmjresp-2022-001581supp002.pdf (127KB, pdf)
bmjresp-2022-001581supp003.pdf (54.7KB, pdf)
Discussion
We used the QFT-Plus test to estimate the prevalence and determinants of TB infection among PLHIV in the context of high TB/HIV burden and near-universal ART and TPT access. We found the prevalence of TB infection estimated by QFT-Plus test results, comprising positive and indeterminate test results, to be 47.9%. We found that the QFT-Plus test positivity in this population of PLHIV was 39.7%, a rate corresponding to estimates from the general population in an equivalent context.33 This differs slightly from a similar study conducted among pregnant women in Kenya, where 35.8% of the pregnant women living with HIV had TB infection by QFT-Plus test positivity rate equivalent to those without HIV.32 In Nigeria, in a similar high HIV/TB burden setup, Aladesanmi et al found that 53% of PLHIV had TB infection.34 Globally, TB infection is estimated at 25% of the world population.1 2 This variability, both geographically and by population, underscores the need for regular context-specific determination of the prevalence of TB infection for policy-making and clinical practice.
The most significant benefit following TPT is among PLHIV with confirmed TB infection.17 Further, a recent review demonstrated higher proportions of PLHIV starting and completing TPT with testing for TB infection.35 The current WHO and local guidelines indicate that confirming TB infection is not a prerequisite for TPT among PLHIV.9 18–20 28 It is essential to consider the clinical and cost implications of treating individuals who may not benefit, either because they are uninfected or because of non-adherence to TPT.17 35 From our findings, approximately 60% of PLHIV receiving TPT may not benefit, potentially exposing them to avoidable adverse drug events, drug-drug interactions, polypharmacy and increased risk of non-adherence. Although a recent review concluded that providing TPT to PLHIV is cost-effective for preventing TB disease,36 only two studies assessing the incremental cost of test-directed treatment from lower-income and middle-income countries were included.37 38 Furthermore, these were conducted among pregnant women, and the findings may not be generalisable. This, therefore, remains an area for further investigation.
We found that indeterminate results were reported in 8.3% of the cases, a rate similar to that of the general population but lower than those reported among PLHIV.39–41 An altered immune response increases the likelihood of indeterminate results.13 39 40 42–44 In HIV, a low CD4 cell count is often implicated.41 45 46 However, HIV can lead to indeterminate results even with normal CD4 cell counts due to altered T-cell function.47–49 The rate of indeterminate result findings comparable to that of the average population in our study can be explained in two ways. First, although CD4 counts were unavailable, most participants-initiated ART early in the ‘test and treat’ era. We speculated that most had competent immunity.50 Second, most participants had been on ART long enough (median duration on ART being 3.9 years), and ART duration influences immune recovery.50–53
A QFT-Plus test result is interpreted as indeterminate when there is a decrease in IFN-γ production in the mitogen tube (positive control) or/and an increase in the Nil tube (negative control).29 In our study, we found decreased production of IFN-γ in the mitogen tube in 8 out of 10 cases. An altered immune response will lead to an insufficient reaction in the mitogen tube, corresponding to the incapacity of lymphocytes to secrete IFN-γ.46 54–58 Possibly, the T lymphocytes among the participants with indeterminate results were compromised in quality and were thus unable to produce sufficient levels of IFN-γ.46 57 In support, most of the participants with indeterminate results had been on ART for less than a year, all were ambulatory, and none had a known illness other than HIV. In cases of immune suppression, repeat testing is advised after recovery.54 55 Further, with indeterminate results, TB infection cannot be ruled out with certainty, and the prognosis may be poorer.54 55 The increased response in the Nil tube found in three participants may signify residual IFN-γ due to ongoing infection,58 and TB infection could not be ruled out.
Interpreting indeterminate results is a clinical dilemma. The WHO and local Kenyan guidelines do not require testing for TB infection before initiating TPT, and there is no guidance for handling indeterminate results where testing is done.9 18 20 28 Given the clinical implication and potential benefit of TPT in PLHIV,30 31 the possibility of immune suppression among participants with indeterminate results, the uncertainty around prognostic implications of indeterminate results54 55 and the resource limitation that precluded repeat testing during follow-up, the operational definition of TB infection used in the present study was justified. Indeterminate results were included in the definition of TB infection, bringing the proportion of PLHIV classified as having TB infection to 47.9%.
TB infection was significantly higher among participants who were obese/overweight (BMI≥25 kg/m2) and who had been on ART for over 3 years. The association with obesity differed from previous findings, although PLHIV were not explicitly considered in these studies.59–61 Obesity is associated with an increased risk of diabetes,61 which increases the risk of TB infection and progression to active TB.62 63 In addition, ART is implicated in various components of metabolic syndrome, including an increased risk of hyperglycaemia and diabetes.41 However, our study only assessed self-reported diabetes and did not measure the haemoglobin A1c level to determine its association with TB infection. We hypothesised that being on ART for over 3 years resulted in CD4 recovery to at least the lower end of the normal range with increased yield from QFT-Plus testing.46 There was a non-significant association of viral load with ART duration. Since the viral load was not done at the point of QFT-Plus testing, it was unsuitable for assessing immune reconstitution, explaining our findings. Although the age category was not associated with TB infection,14 64 65 those on ART longer were also older. Longer cumulative exposure time expected with increasing age could be a source of residual confounding. Compared with those without TB infection, those on ART longer had higher odds of being indeterminate. This is contrary to what we would expect; however, the study was not sufficiently powered to assess this association, given that the numbers with indeterminate results were small.
Alcohol use increases the likelihood of TB infection and disease.66 67 Although not significantly associated with TB infection on multivariable analysis, there were increased odds of TB infection with a trend towards significance. Structurally, people who consume alcohol are more likely to congregate in crowded places with high TB transmission rates. Alcohol is also known to cause immune suppression, which increases the risk of infection.67 There was no association between TB infection and living in a crowded place or contact with a person with TB compared with findings from other studies.33 This could be due to the small sample size and the reduced power required to detect an association. In addition, household crowding and the history of contact with a TB case were measured by self-report. This is subjective and disposed to social desirability and recall bias.
Study strengths and limitations
Our study had several strengths. The involvement of participants visiting the national referral hospital, a cosmopolitan group, increased our ability to generalise our findings. We excluded participants with prior exposure to TPT, thus reducing the chance of outcome misclassification. We considered the clinical implications of indeterminate QFT-Plus results for a resource-limited setup. We confirmed the robustness of our results by performing a sensitivity analysis, assuming indeterminate results were negative. A limitation of our study is that we did not collect data on CD4 counts and could not account for the association of immune status with TB infection. This was due to changes in the guidelines, precluding the use of CD4 for HIV monitoring. Since the viral load was not done at the point of QFT-Plus testing, there was a misclassification bias. To address this limitation, we used years of ART as a proxy for immune recovery and competency.
Second, alcohol use, cigarette use, household crowding, history of contact with a TB case and presence of other chronic conditions, including autoimmune diseases, were based on self-reporting. This is subject to error and social desirability, and recall bias. Third, obesity was not correlated with other metabolic dysfunctions, and diabetes status was not objectively ascertained. Finally, this was a cross-sectional study; no long-term follow-up was conducted to see those who developed TB disease. A larger sample size would have had more power to detect associations. Therefore, studies with larger sample sizes are necessary to elucidate better the factors affecting indeterminate QFT-plus tests.
Conclusion and recommendation
The prevalence of TB infection in this population of PLHIV who had not received TPT is higher than the global estimate, depicting high levels of exposure and better performance of the QFT-Plus test with early and longer ART use. The rates of indeterminate results were the same as those reported for the general population, further supporting the effect of ART on immunity and QFT-Plus test sensitivity. Given the existing evidence, it is important to consider the clinical and cost implications of test-directed TPT for PLHIV in low-income and middle-income countries. This will better inform policies towards recommending test-directed TPT in PLHIV never exposed to TPT. Clear directives on handling indeterminate QFT-Plus test results in PLHIV are needed. People with vulnerabilities, such as obesity and alcoholism, need to be targeted for TPT. A more in-depth analysis of the determinants of TB infection using a larger sample size is recommended.
Acknowledgments
LNN was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Centre and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No. G-19–57145), Sida (Grant No:54100113), Uppsala Monitoring Centre, Norwegian Agency for Development (Norad), the Wellcome Trust (reference no. 107768/Z/15/Z) and the UK Foreign, Commonwealth & Development Office, with support from the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme. The statements made, and views expressed are solely the responsibility of the Fellow. For open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. LNN acknowledges Babatunde Adedokun of CARTA for the support during training and data analysis. LNN also received training from the NIH/Fogarty HIV Research Training Program (D43 TW011817 Tuberculosis & HIV Co-Infection Training Program in Kenya) during manuscript development. We thank the patients who volunteered for this study and the research team involved in data collection. We also thank the staff and administration of the Kenyatta National Hospital’s comprehensive care clinic, KEMRI Center for Respiratory Disease Research and Pumwani Maternity Hospital for supporting this work. We also thank the University of Nairobi, Faculty of Health Sciences, for supporting doctoral training. We thank Editage (www.editage.com) for English language editing.
Footnotes
Twitter: @DrLiliannjagi
Contributors: Concept development and study design: LNN, JOM and VN. Supervision of the study: LNN, JOM, VN and MWM. Data analysis: LNN and VN. Critically revised manuscript: LNN, JOM, VN and MWM. All authors have read and approved the final draft for publication. LNN is responsible for the overall content as the guarantor.
Funding: This work was supported by funding from the Consortium for Advanced Research Training in Africa (CARTA), the Royal Society of Tropical Medicine and Hygiene (RSTMH) small grants, and the Africa Centre of Excellence, Materials, Products and Nano Technology (ACE-MAPRONANO) project.
Disclaimer: The funders had no role in study design, data collection and analysis, publication decisions, or manuscript preparation
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data generated or analysed during this study are not publicly available due to privacy policy regulations but are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Kenyatta National Hospital/University of Nairobi Institutional Review Board (KNH/UON ERB) under Ref. KNH-ERC/A/375. Participants gave informed consent to participate in the study before taking part.
References
- 1.Dye C, Scheele S, >Dolin P, et al. Global burden of tuberculosis. JAMA 1999;282:677. 10.1001/jama.282.7.677 [DOI] [PubMed] [Google Scholar]
- 2.Dye C, Scheele S, Dolin P, et al. Consensus statement. global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. who global surveillance and monitoring project. JAMA 1999;282:677–86. 10.1001/jama.282.7.677 [DOI] [PubMed] [Google Scholar]
- 3.WHO . Global tuberculosis report. Geneva, Available: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 [Google Scholar]
- 4.Ford N, Matteelli A, Shubber Z, et al. Tb as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016;19:20714. doi:20714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS . Aids info (website). Geneva:; 2022. Available: https://aidsinfo.unaids.org [Accessed 18 Jan 2023]. [Google Scholar]
- 6.World Health Organisation . Tuberculosis profile: global (website). 2021. Available: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&lan=%22EN%22&entity_type=%22group%22&group_code=%22global%22 [Accessed 24 Jan 2023].
- 7.Ai J-W, Ruan Q-L, Liu Q-H, et al. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect 2016;5:e10. 10.1038/emi.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 1997;119:183–201. 10.1017/s0950268897007917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation . Consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva, 2020. Available: https://www.who.int/publications/i/item/9789240001503(accessed [accessed Jul 2022]. [PubMed] [Google Scholar]
- 10.Houben R, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLOS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen A, Mathiasen VD, Schön T, et al. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019;54:1900655. 10.1183/13993003.00655-2019 [DOI] [PubMed] [Google Scholar]
- 12.Basera TJ, Ncayiyana J, Engel ME. Prevalence and risk factors of latent tuberculosis infection in Africa: a systematic review and meta-analysis protocol. BMJ Open 2017;7:e012636. 10.1136/bmjopen-2016-012636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Moreno J, García-Gasalla M, Losada-López I, et al. IGRA testing in patients with immune-mediated inflammatory diseases: which factors influence the results? Rheumatol Int 2018;38:267–73. 10.1007/s00296-017-3852-9 [DOI] [PubMed] [Google Scholar]
- 14.Ncayiyana JR, Bassett J, West N, et al. Prevalence of latent tuberculosis infection and predictive factors in an urban informal settlement in Johannesburg, South Africa: a cross-sectional study. BMC Infect Dis 2016;16. 10.1186/s12879-016-1989-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos JA, Duarte R, Nunes C. Host factors associated to false negative and indeterminate results in an interferon-γ release assay in patients with active tuberculosis. Pulmonology 2020;26:353–62. 10.1016/j.pulmoe.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Antonucci G, Girardi E, Raviglione MC, et al. Risk factors for tuberculosis in HIV-lnfected persons: a prospective cohort study. Jama 1995;274:143–8. [DOI] [PubMed] [Google Scholar]
- 17.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010;2010:CD000171. 10.1002/14651858.CD000171.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health, National AIDS & STI Control Program . Kenya HIV Prevention and Treatment Guidelines. Nairobi, Kenya: NASCOP, [Google Scholar]
- 19.World health organisation . Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, 2018. Available: https://apps.who.int/iris/handle/10665/260233 [accessed Jul 2022]. [PubMed] [Google Scholar]
- 20.Kenya Ministry of Health . Integrated guideline for tuberculosis, leprosy and lung disease. 2021. Available: https://chskenya.org/wp-content/uploads/2022/04/integrated-guideline-for-tuberculosis-leprosy-and-lung-disease-2021.pdf [Accessed 24 Jan 2023].
- 21.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol 1995;141:263–72. 10.1093/oxfordjournals.aje.a117428 [DOI] [PubMed] [Google Scholar]
- 22.Rogan WJ, Gladen B. Estimating prevalence from the results of a screening test. Am J Epidemiol 1978;107:71–6. 10.1093/oxfordjournals.aje.a112510 [DOI] [PubMed] [Google Scholar]
- 23.Wolf T, Goetsch U, Oremek G, et al. Tuberculosis skin test, but not interferon-γ-releasing assays is affected by BCG vaccination in HIV patients. J Infect 2013;66:376–80. 10.1016/j.jinf.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 24.Sultan B, Benn P, Mahungu T, et al. Comparison of two interferon-gamma release assays (QuantiFERON-TB gold in-tube and T-SPOT.TB) in testing for latent tuberculosis infection among HIV-infected adults. Int J STD AIDS 2013;24:775–9. 10.1177/0956462413486459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klautau GB, da Mota NVF, Salles MJC, et al. Interferon-Γ release assay as a sensitive diagnostic tool of latent tuberculosis infection in patients with HIV: a cross-sectional study. BMC Infect Dis 2018;18:585. 10.1186/s12879-018-3508-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafeque A, Bigio J, Hogan CA, et al. Fourth-Generation QuantiFERON-TB gold plus: what is the evidence? J Clin Microbiol 2020;58. 10.1128/JCM.01950-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Yang Q, Zhou J, et al. Comparison of QuantiFERON-TB gold in-tube and QuantiFERON-TB gold-plus in the diagnosis of Mycobacterium tuberculosis infections in immunocompromised patients: a real-world study. Microbiol Spectr 2022;10:e01870-21. 10.1128/spectrum.01870-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministry of Health, National AIDS & STI Control Program . Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018 Edition. Nairobi, Kenya: NASCOP, 2018. [Google Scholar]
- 29.QuantiFERON-TB Gold Plus (QFT-Plus) . Elisa package insert 02/2016. n.d. Available: http://www.quantiferon.com/wp-content/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf
- 30.Badje A, Moh R, Gabillard D, et al. Effect of isoniazid preventive therapy on risk of death in West African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the temprano ANRS 12136 trial. Lancet Glob Health 2017;5:e1080–9. 10.1016/S2214-109X(17)30372-8 [DOI] [PubMed] [Google Scholar]
- 31.The TEMPRANO ANRS 12136 Study Group . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015;373:808–22. 10.1056/NEJMoa1507198 [DOI] [PubMed] [Google Scholar]
- 32.Kaplan SR, Escudero JN, Mecha J, et al. Interferon gamma release assay and tuberculin skin test performance in pregnant women living with and without HIV. J Acquir Immune Defic Syndr 2022;89:98–107. 10.1097/QAI.0000000000002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen AV, Jensen L, Faurholt-Jepsen D, et al. The prevalence of latent Mycobacterium tuberculosis infection based on an interferon-γ release assay: a cross-sectional survey among urban adults in mwanza, Tanzania. PLoS One 2013;8:e64008. 10.1371/journal.pone.0064008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aladesanmi AO, Ojuawo OB, Aladesanmi OO, et al. Diagnosis of latent tuberculosis among HIV infected patients in ilorin, Nigeria using tuberculin skin test and interferon gamma release assay. Pan Afr Med J 2021;38:24. 10.11604/pamj.2021.38.24.24039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastos ML, Melnychuk L, Campbell JR, et al. The latent tuberculosis cascade-of-care among people living with HIV: a systematic review and meta-analysis. PLoS Med 2021;18:e1003703. 10.1371/journal.pmed.1003703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uppal A, Rahman S, Campbell JR, et al. Economic and modeling evidence for tuberculosis preventive therapy among people living with HIV: a systematic review and meta-analysis. PLOS Med 2021;18:e1003712. 10.1371/journal.pmed.1003712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapoor S, Gupta A, Shah M. Cost-Effectiveness of isoniazid preventive therapy for HIV-infected pregnant women in India. Int J Tuberc Lung Dis 2016;20:85–92. 10.5588/ijtld.15.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H-Y, Hanrahan CF, Martinson N, et al. Cost-Effectiveness of universal isoniazid preventive therapy among HIV-infected pregnant women in South Africa. Int J Tuberc Lung Dis 2018;22:1435–42. 10.5588/ijtld.18.0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira S do V de, Trajman A, Paniago AMM, et al. Frequency of indeterminate results from an interferon-gamma release assay among HIV-infected individuals. J Bras Pneumol 2017;43:215–8. 10.1590/S1806-37562016000000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oni T, Gideon HP, Bangani N, et al. Risk factors associated with indeterminate gamma interferon responses in the assessment of latent tuberculosis infection in a high-incidence environment. Clin Vaccine Immunol 2012;19:1243–7. 10.1128/CVI.00166-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007;175:737–42. 10.1164/rccm.200608-1088OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong SJ, Han SH, Kim CO, et al. Predictive factors for indeterminate result on the quantiferon test in an intermediate tuberculosis-burden country. J Infect 2011;62:347–54. 10.1016/j.jinf.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 43.Darby J, Black J, Buising K. Interferon-Gamma release assays and the diagnosis of tuberculosis: have they found their place? Intern Med J 2014;44:624–32. 10.1111/imj.12469 [DOI] [PubMed] [Google Scholar]
- 44.Papay P, Eser A, Winkler S, et al. Predictors of indeterminate IFN-γ release assay in screening for latent TB in inflammatory bowel diseases. Eur J Clin Invest 2011;41:1071–6. 10.1111/j.1365-2362.2011.02502.x [DOI] [PubMed] [Google Scholar]
- 45.Brock I, Ruhwald M, Lundgren B, et al. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir Res 2006;7:56. 10.1186/1465-9921-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raby E, Moyo M, Devendra A, et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One 2008;3:e2489. 10.1371/journal.pone.0002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhosale R, Alexander M, Deshpande P, et al. Stages of pregnancy and HIV affect diagnosis of tuberculosis infection and Mycobacterium tuberculosis (MTB) -induced immune response: findings from prachiti, a cohort study in Pune, India. Int J Infect Dis 2021;112:205–11. 10.1016/j.ijid.2021.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroon EE, Kinnear CJ, Orlova M, et al. An observational study identifying highly tuberculosis-exposed, HIV-1-positive but persistently TB, tuberculin and IGRA negative persons with M. tuberculosis specific antibodies in Cape town, South Africa. EBioMedicine 2020;61:103053. 10.1016/j.ebiom.2020.103053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? implications for tuberculosis control. AIDS 2005;19:1113–24. 10.1097/01.aids.0000176211.08581.5a [DOI] [PubMed] [Google Scholar]
- 50.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013;368:218–30. 10.1056/NEJMoa1110187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kufa T, Shubber Z, MacLeod W, et al. Cd4 count recovery and associated factors among individuals enrolled in the South African antiretroviral therapy programme: an analysis of national laboratory based data. PLoS One 2019;14:e0217742. 10.1371/journal.pone.0217742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisk TL, Hon H-M, Lennox JL, et al. Detection of latent tuberculosis among HIV-infected patients after initiation of highly active antiretroviral therapy. AIDS 2003;17:1102–4. 10.1097/00002030-200305020-00027 [DOI] [PubMed] [Google Scholar]
- 53.Bishop JD, DeShields S, Cunningham T, et al. Cd4 count recovery after initiation of antiretroviral therapy in patients infected with human immunodeficiency virus. The American Journal of the Medical Sciences 2016;352:239–44. 10.1016/j.amjms.2016.05.032 [DOI] [PubMed] [Google Scholar]
- 54.Sester M, van Leth F, Bruchfeld J, et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med 2014;190:1168–76. 10.1164/rccm.201405-0967OC [DOI] [PubMed] [Google Scholar]
- 55.Jacquier M, Binquet C, Manoha C, et al. n.d. Beyond QuantiFERON-TB results, the added value of a weak mitogen response. Front Med;9. 10.3389/fmed.2022.876864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belliere J, Blancher A. Quantiferon test interpretation in patients receiving immunosuppressive agents: an alert. Eur Respir J 2017;49:1602102. 10.1183/13993003.02102-2016 [DOI] [PubMed] [Google Scholar]
- 57.Aabye MG, Ravn P, PrayGod G, et al. The impact of HIV infection and CD4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS ONE 2009;4:e4220. 10.1371/journal.pone.0004220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ariga H, Nagai H, Kurashima A, et al. Stratified threshold values of quantiferon assay for diagnosing tuberculosis infection in immunocompromised populations. Tuberc Res Treat 2011;2011:940642. 10.1155/2011/940642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badawi A, Liu CJ. Obesity and prevalence of latent tuberculosis: a population-based survey. Infect Dis (Auckl) 2021;14:117863372199460. 10.1177/1178633721994607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronacher K, van Crevel R, Critchley JA, et al. Defining a research agenda to address the converging epidemics of tuberculosis and diabetes: Part 2: underlying biologic mechanisms. Chest 2017;152:174–80. 10.1016/j.chest.2017.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roth J, Sahota N, Patel P, et al. Obesity paradox, obesity orthodox, and the metabolic syndrome: an approach to unity. Mol Med 2017;22:873–85. 10.2119/molmed.2016.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barron MM, Shaw KM, Bullard KM, et al. Diabetes is associated with increased prevalence of latent tuberculosis infection: findings from the National health and nutrition examination survey, 2011-2012. Diabetes Res Clin Pract 2018;139:366–79. 10.1016/j.diabres.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 63.Badawi A, Sayegh S, Sallam M, et al. The global relationship between the prevalence of diabetes mellitus and incidence of tuberculosis: 2000-2012. Glob J Health Sci 2014;7:183–91. 10.5539/gjhs.v7n2p183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin W-C, Lin H-H, Lee S-J, et al. Prevalence of latent tuberculosis infection in persons with and without human immunodeficiency virus infection using two interferon-gamma release assays and tuberculin skin test in a low human immunodeficiency virus prevalence, intermediate tuberculosis-burden country. J Microbiol Immunol Infect 2016;49:729–36. 10.1016/j.jmii.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 65.Kizza FN, List J, Nkwata AK, et al. Prevalence of latent tuberculosis infection and associated risk factors in an urban African setting. BMC Infect Dis 2015;15:165. 10.1186/s12879-015-0904-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imtiaz S, Shield KD, Roerecke M, et al. Alcohol consumption as a risk factor for tuberculosis: meta-analyses and burden of disease. Eur Respir J 2017;50:1700216. 10.1183/13993003.00216-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 2009;9:450. 10.1186/1471-2458-9-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001581supp001.pdf (44.6KB, pdf)
bmjresp-2022-001581supp002.pdf (127KB, pdf)
bmjresp-2022-001581supp003.pdf (54.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data generated or analysed during this study are not publicly available due to privacy policy regulations but are available from the corresponding author on reasonable request.