Abstract
Objectives
This study aimed to identify determinants of inappropriate antibiotic prescription in primary care in developed countries and to construct a framework with the determinants to help understand which actions can best be targeted to counteract development of antimicrobial resistance (AMR).
Design
A systematic review of peer-reviewed studies reporting determinants of inappropriate antibiotic prescription published through 9 September 2021 in PubMed, Embase, Web of Science and the Cochrane Library was performed.
Setting
All studies focusing on primary care in developed countries where general practitioners (GPs) act as gatekeepers for referral to medical specialists and hospital care were included.
Results
Seventeen studies fulfilled the inclusion criteria and were used for the analysis which identified 45 determinants of inappropriate antibiotic prescription. Important determinants for inappropriate antibiotic prescription were comorbidity, primary care not considered to be responsible for development of AMR and GP perception of patient desire for antibiotics. A framework was constructed with the determinants and provides a broad overview of several domains. The framework can be used to identify several reasons for inappropriate antibiotic prescription in a specific primary care setting and from there, choose the most suitable intervention(s) and assist in implementing them for combatting AMR.
Conclusions
The type of infection, comorbidity and the GPs perception of a patient’s desire for antibiotics are consistently identified as factors driving inappropriate antibiotic prescription in primary care. A framework with determinants of inappropriate antibiotic prescription may be useful after validation for effective implementation of interventions for decreasing these inappropriate prescriptions.
PROSPERO registration number
CRD42023396225.
Keywords: PRIMARY CARE, INFECTIOUS DISEASES, Public health
Strengths and limitations of this study.
This study focuses specifically on antibiotic prescription in primary care while most studies on this topic focus on hospital care.
The scope of the review was somewhat limited by only selecting studies on primary care in developed countries where general practitioners act as gatekeepers.
The framework provides a broad overview of both determinants of—and knowledge gaps regarding antibiotic prescription habits.
The information conveyed in this paper can be used for designing initiatives to improve prudent antibiotic prescription in primary care, and thereby reduce the development of antimicrobial resistance.
The proposed framework needs validation before it can be implemented.
Introduction
Antimicrobial resistance (AMR) is increasing worldwide and represents a major threat to global healthcare.1 The major driver of the rise in AMR is the use, frequently inappropriate, of antibiotics.2 Worldwide efforts are now underway to decrease unnecessary antibiotic prescribing and consequently reduce the development of AMR.1 The most common prescribers of antibiotics in developed countries are general practitioners (GPs), accounting for between 80% and 90% of all antibiotic prescriptions.3 4 As such, GPs play an important role in reducing AMR. However, there is currently insufficient insight into which potentially changeable determinants are associated with inappropriate antibiotic prescription in this setting.
GPs prescribe antibiotics for a variety of infectious diseases, ranging from respiratory tract infections (RTI) to cellulitis.5–10 However between 44% and 98% of the antibiotic prescriptions for RTIs are classified as inappropriate.11–14 The proportion of inappropriate antibiotic prescriptions for urinary tract infections is estimated at between 3% and 36.5%.15 16 Antibiotic prescriptions are generally considered inappropriate when, according to the guidelines, no or other antimicrobials should be used. The high proportion of inappropriate antibiotic prescriptions combined with the large quantity of antibiotics prescribed by GPs suggest that efforts to improve antibiotic prescribing in primary care may have a substantial effect on the development of AMR.
Determinants across several domains affect the proportion of inappropriate antibiotic prescribing in primary care. These domains include patient–doctor interactions, the organisation of primary care, the national role of primary care and the nationwide healthcare system.17 18 Reducing inappropriate antibiotic prescribing is therefore complex. To increase effectiveness, each domain should be taken into account in any intervention. However, it is still unclear which determinants play a role in each specific domain and how the different determinants may interact.
The aim of this review is to identify the determinants influencing inappropriate antibiotic prescribing by GPs, sort the determinants into a framework according to their domain and identify which determinants may be subject to antimicrobial stewardship interventions for reducing inappropriate antibiotic prescribing.
Methods
Systematic review search strategy and study selection
A systematic review was conducted. Briefly, the search included studies describing determinants in primary care in developed countries through 9 September 2021. The protocol developed to conduct this study was registered in PROSPERO (online supplemental file 1). PubMed, Embase, Web of Science and the Cochrane Library databases were searched. The full electronic search strategy can be found in online supplemental file 2. We additionally searched grey literature (ie, abstracts of conferences, symposia and meetings) and relevant references found in initially identified studies found in Embase, Web of Science and the Cochrane Library. There were no language restrictions in the search. The reporting of our systematic review was based on the protocol specified by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (online supplemental file 3).19
bmjopen-2022-065006supp001.pdf (3MB, pdf)
bmjopen-2022-065006supp002.pdf (30.7KB, pdf)
bmjopen-2022-065006supp003.pdf (50.3KB, pdf)
Studies were, regardless of their design, selected for reviewing if they provided a definition of inappropriate antibiotic prescription according to the guidelines used in that study. Only studies performed in developed countries, as defined by the United Nations (UN), in which the GP plays a ‘gatekeeper’ role in the healthcare system, were included (online supplemental files 4; 5).20 21 This gatekeeper role is defined by the UN as a compulsory GP referral to access most types of specialist care, except in case of emergency.21 Studies had to report determinants that influence the inappropriate prescribing of antibiotics as an outcome. Studies on specific subgroups of patients (eg, those with specific comorbidities) or specific diseases (such as asthma or chronic obstructive pulmonary disease) were excluded as reasons for appropriate or inappropriate antibiotic prescriptions for these groups differ, while our aim was to develop a framework for the whole population. Two reviewers (MS and FLB) independently reviewed the titles, index terms and abstracts of the identified references and rated each abstract according to the inclusion and exclusion criteria. Full texts of potentially relevant abstracts were assessed for eligibility by two reviewers (MS and FLB). Discrepancies were resolved by consensus. If consensus could not be reached, a third reviewer (MGJdB or MEN) was consulted.
bmjopen-2022-065006supp004.pdf (46.4KB, pdf)
bmjopen-2022-065006supp005.pdf (42.7KB, pdf)
Data extraction and quality assessment
The determinants of inappropriate prescription of antibiotics were extracted from the included studies, along with the study design, geographical location, disease group, definition of inappropriate prescribing, study population and research period. ORs describing associations between determinants and inadequate prescription were extracted where provided. Study quality was assessed using the National Heart and Lung Institute (NHLI) study quality assessment tool22 for quantitative studies and the Critical Appraisal Skills Programme (CASP)23 for qualitative studies.
Framework
Determinants were placed in a framework by a reviewer (MS) which was thereafter reviewed by the research group and adapted based on consensus in the groups’ discussion. We used a practical framework set-up as described by Morgan et al.17 This framework is specifically designed for understanding and reducing medical overuse in primary care and takes all relevant domains of influence into account, including the culture of healthcare consumption, patient factors and experiences, the culture of professional medicine, clinician attitudes and beliefs, practice environments and patient–clinician interactions. The domain ‘government’ was left out of the framework as it was found to be redundant owing to our selection of studies from developed countries in which GPs play a gatekeeper role.
If the definition of determinants showed large similarity, we choose to combine the determinants to prevent overlap in our framework. Determinants were eligible to be added to the framework if they had a positive or negative impact on inappropriate antibiotic prescribing. The determinants were classified as having either a positive or negative influence on inappropriate antibiotic prescription according to the findings and description in their study. Subsequently, each determinant was noted in the framework with a plus or minus sign. The identified determinants were categorised and attributed to the framework domains specified by a method described by Morgan et al.17 Determinants specific to one country, as well as those on which studies reported conflicting results, were included to create a complete framework appropriate to various settings. Determinants on which studies returned conflicting results were noted in the framework with a plus or minus sign (±).
Patient and public involvement
Patients were not involved in designing the review, data collection, interpretation or write-up of this review.
Results
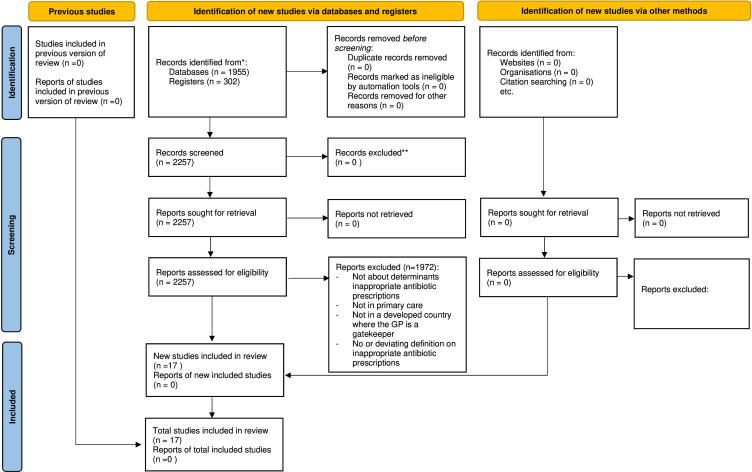
The literature search identified 2257 studies. Following screening of titles and abstracts, 285 studies were retained for full-text review, of which 17 were ultimately included in the review as they specified determinants of inappropriate antibiotic prescription (figure 1).24–40 Characteristics of the selected studies are presented in the online supplemental materials S6a and S6b. The studies were conducted in six countries: Australia, Canada, Ireland, The Netherlands, Spain and the UK. Four studies25 32 33 38 had a qualitative design (one explorative qualitative design, one cross-sectional survey, one focus group and one questionnaire) while 13 studies had a quantitative design (all observational in nature). The methodologies of the included studies as assessed by the NHLI or CASP tool all had a low risk of bias. Quality assessment tables are presented in the online supplemental materials S7; S8.
Figure 1.
Flow diagram of study selection. GP, general practitioner.
bmjopen-2022-065006supp006.pdf (149.1KB, pdf)
bmjopen-2022-065006supp007.pdf (55.6KB, pdf)
bmjopen-2022-065006supp008.pdf (42.2KB, pdf)
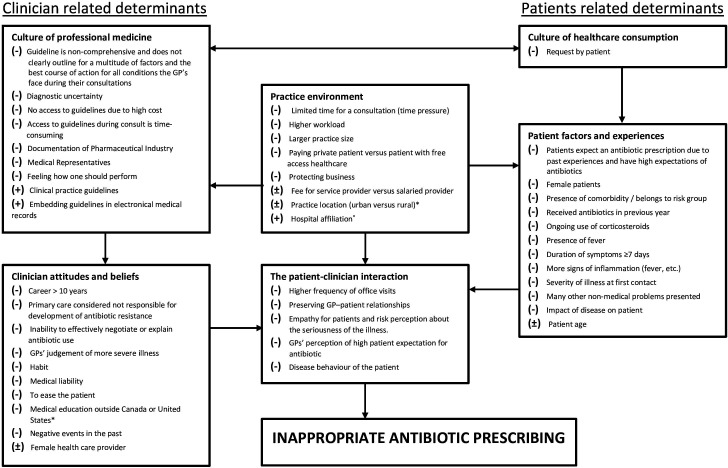
Framework determinants of inappropriate prescriptions
In total, 54 determinants were identified from 17 studies. Seven determinants were directly not included in the framework as they showed no association with inappropriate antibiotic prescribing, either positive or negative (online supplemental materials S6b). Forty-five determinants were included and are presented in a framework (figure 2). There were five determinants with conflicting results from the included studies and three determinants with a positive impact on inappropriate antibiotic prescribing. Three determinants showed similarity and were combined with each other to one determinant.34 Silverman et al compared careers of between 11 and 24 years with careers shorter than 11 years and careers longer than 25 years with careers less than 11 years.34 These outcomes were combined to form one determinant, a career longer than 10 years.
Figure 2.
Framework for determinants of inappropriate antibiotic prescribing in primary care in developed countries. Determinants associated with more inappropriate antibiotic prescribing. ±Determinants with conflicting results on inappropriate antibiotic prescribing. *Determinants specific for a country. GPs, general practitioners.
Discussion
We systematically reviewed the determinants of inappropriate antibiotic prescription in developed countries in which GPs act as the gatekeepers. Comorbidity and GPs’ perceptions of a patient’s expectation for antibiotics were consistently identified as main factors that drive inappropriate prescription of antibiotics in primary care. There were no restrictions on the design of the study for the inclusion as our aim was to include as many determinants as possible.
Determinants of inappropriate antibiotic prescription in primary care
Comorbidity was the most frequently found determinant of inappropriate antibiotic prescription.25–27 29 35 37 40 However, it is not clear to what extent prescribing an antibiotic for a patient with one or more comorbidities is inappropriate. The guidelines for appropriate antibiotic use are largely based on studies of patients without comorbidities. Consideration of antibiotic prescription is also advised by guidelines in cases of comorbidity.5 9 GPs may quickly choose to prescribe an antibiotic to be on the safe side with regard to complications, leading to more antibiotic prescriptions for patients presumably at risk for complications.
Another important determinant was the GPs perception of a patient’s expectation of getting antibiotics.24–26 30 GPs may assume the reason for a patient’s visit is an antibiotic prescription, but may not verify this with the patient. Thus, more effort focused towards verifying the specific reason for the encounter may represent a typical primary care approach to further reducing inappropriate antibiotic prescriptions. Inability to effectively negotiate or explain antibiotic use also leads to more inappropriate prescriptions.32 Both determinants illustrate the benefits of the availability of time to communicate with patients and efficient communication skills. This was confirmed by a recent review of communication training aimed at reduction of antibiotic prescriptions for RTIs.41
Remarkably, some GPs did not consider themselves responsible for antibiotic resistance.32 In their opinion, their prescribing at an individual level did not contribute to AMR. Rather, they believe AMR is mainly driven by antibiotic prescriptions in hospitals or those in veterinary use. This notion was confirmed by a study performed by the European Centre for Disease Control.42 In reality, up to 90% of antibiotic prescriptions find their origin in primary care.3 4 Furthermore, according to the one health concept, antibiotic prescriptions from all sectors contribute to antibiotic selection pressure.43 Additionally, more (inappropriate) antibiotic prescription is the cause of a vicious cycle of increasing AMR which leads to prescribing of second choice, mostly broad-spectrum antibiotics leading to increasing AMR. This points to the need for continuous education which emphasises that inappropriate antibiotic prescriptions give unnecessary antibiotic selection pressure and thus lead to more AMR.
There were conflicting results on some determinants. A study by Eggermont et al specifically designed to investigate gender differences in inappropriate antibiotic prescriptions failed to detect any such association with gender.27 However, there were three studies reporting a gender association. Therefore, we included female gender as a determinant associated with more inappropriate antibiotic prescribing in our framework.26 29 30
Two studies found an association between larger practice size and inappropriate antibiotic prescription29 31 while a third study found no association with practice size.35 A higher daily patient load was associated with more inappropriate prescription of antibiotics in one study.34 As practice size and patient load are generally related, a larger practice was included in the framework.
The determinant age of the patient was investigated by seven studies.24–27 29 30 37 Two studies found that an age between 18 and 65 years was associated with increased inappropriate antibiotic prescription,26 29 one study concluded increasing age to be associated with greater inappropriate antibiotic prescription37 and two studies failed to find any such association.24 27 Two studies focusing on otitis media found inappropriate antibiotic prescription more commonly occurred with children younger than 2 years of age as compared with children 2 years and older.25 30 This was therefore included in the framework as a determinant with conflicting results.
The healthcare payment model was researched in several studies exploring various determinants, with some finding an association with inappropriate antibiotic prescription.32–35 An explorative study in Ireland from O’Doherty et al reported a higher rate of inappropriate antibiotic prescriptions in self-paying or fee-for-service insured patients versus patients with free access to healthcare.33 Likewise, a study in Canada found fee-for-service providers more commonly inappropriately prescribed antibiotics than salaried providers.35 Another study from Canada failed to detect this association,34 and likewise found no association between inappropriate antibiotic prescription and a healthcare capitation payment system. Protecting business was singled out as a reason for inappropriate antibiotic prescription in a cross-sectional survey study in Australia.32
Framework determinants of inappropriate antibiotic prescribing
As our aim was to construct a comprehensive framework as possible. The determinants practice location (rural vs urban), hospital affiliation and medical education outside the USA and Canada were put in the framework despite being specific to a country or setting.29 31 34 35 Rural locations in Canada have a different context than rural locations in Europe and this determinant should be used in that context.29 One study found that physicians trained outside Canada or USA prescribed more inappropriate antibiotics while working in Canada.31 The constructed framework provides a broad overview of all determinants by domain and can be used, after validation, to design interventions intended to reduce inappropriate prescriptions in primary care. For example, the framework shows that clinical judgement differs between GPs due to different interpretations of the severity of the symptoms.24 26 30 A career longer than 10 years was associated with more inappropriate antibiotic prescription,29 31 34 with a possible cause being that they are less familiar with guidelines and rely more on their clinical experience. This illustrates that a more objective tool for judgement of severity is needed. A possible solution could be using C-reactive protein (CRP) and other point of care tests for patients with RTIs. CRP-guided treatment has been proven effective in reducing inappropriate antibiotic prescription for patients with RTIs.44 More examples of effective interventions per determinant are presented in table 1. Only determinants associated with inappropriate antibiotic prescriptions that can be influenced by effective interventions were included (table 1). Studies on effective interventions for reducing antibiotic prescriptions in primary care show that multifaceted interventions thus covering more determinants seem to be more effective in reducing antibiotic prescribing.44–48
Table 1.
Overview determinants with examples of potential effective interventions
| Determinants associated with inappropriate antibiotic prescribing | Examples of potential effective interventions |
| Culture of professional medicine | |
| Diagnostic uncertainty. | CRP POCT.*44–46 53–57 |
| No access to guidelines due to high cost. | Free access to guidelines.58 |
| Access to guidelines during consult is time-consuming. | CDSS.†47 58 |
| Culture of healthcare consumption | |
| Request by patient. | Patient education.‡45 59–61 Mass media campaign.§62 Delayed antibiotic prescription.¶44 63–65 |
| Clinician attitudes and beliefs | |
| Career >10 years. | Feedback on antibiotic prescribing.45 65–68 |
| Primary care considered not responsible for development of antibiotic resistance. | |
| Habit. | |
| Inability to effectively negotiate or explain antibiotic use. | CST.**53 66 69 70 |
| GPs’ judgement of more severe illness. | CRP POCT.*44–46 53–57 |
| Medical liability. | Physician education.††45 67 70 71 |
| Delayed antibiotic prescription.¶44 63–65 | Delayed antibiotic prescription.¶44 63–65 |
| The patient–clinician interaction | |
| Preserving GP–patient relationships. | Delayed antibiotic prescription.¶44 63–65 |
| Empathy for patients and risk perception about the seriousness of the illness. | Physician education.††45 67 70 71 |
| GPs’ perception of high patient expectation for antibiotic. | CST.**53 66 69 70 |
| Disease behaviour of the patient. | Patient education.‡45 59–61 |
| Patient factors and experiences patient | |
| Patients expect an antibiotic prescription due to past experiences and have high expectations of antibiotics. Received antibiotics in previous year. |
Patient education.‡45 59–61 |
| Presence of comorbidity/belongs to risk group. Ongoing use of corticosteroids. Presence of fever. Duration of symptoms ≥7 days. More signs of inflammation (fever, etc). Severity of illness at first contact. |
Physician education.††45 67 70 71 |
*CRP POCT: C-reactive protein point-of-care testing for patients with a respiratory tract infection divers between uncomplicated and complicated respiratory tract infections and reduces antibiotic prescriptions.
†CDSS: Clinical decision support system is integrated in an electronic medical system. It gives direct access to guidelines and supports clinical decision-making.
‡Patient education: Patient can be educated through handout/leaflets and waiting room posters on the limited effect of antibiotics for a viral infection.
§Mass media campaign: Mass media campaign providing information on the appropriate use of antibiotic and reduces antibiotic prescriptions.
¶Delayed antibiotic prescription is prescribed directly at a consult but the patient is advised to use the antibiotic only when the symptoms persist or become more severe. It reduces antibiotic use by patients while maintaining patient satisfaction.
**CST: Communication skills training helps a physician to explain the limited effect of antibiotics to a patient and is effective in reducing antibiotic prescriptions.
††Physician education: Education of physicians about guidelines for infectious diseases, the limited effect of antibiotics for viral infections and which diagnostic tools can help to differ between a self-limiting infection and a more severe infectious diseases, such as a CRP POCT.
Feedback: Feedback on antibiotic prescribing provides insight in the number of antibiotic prescriptions by a physician and the impact on antibiotic resistance which stimulates a physician to reflect on his own antibiotic prescription habits.
GP, general practitioner.
The focus and interpretation of the framework, and hence the needed interventions, differ by country. For example, patient expectations of an antibiotic may stem from local beliefs and attitudes and be more common in cultures placing an emphasis on masculinity as antibiotic prescription tends to be higher in such societies.49 A priority in a masculine society is an early return to work and antibiotics are seen as an important facilitator therefore.50 In societies in which this effect is smaller, illness is considered a legitimate reason for absence from work. Ireland, Spain and the UK have much higher masculinity scores than The Netherlands,51 and antibiotic prescription rates are indeed higher in those three countries as compared with The Netherlands.3 Interventions should focus on informing patients about the mild natural course of most infectious diseases and the low value of antibiotic use.
Strength and limitations
The strengths of our study include that our review summarises determinants covering many domains, thus providing a broad overview. Additionally, the Morgan et al framework was specifically designed to reduce overuse in primary care,17 making it particularly useful when designing and/or implementing interventions to reduce inappropriate antibiotic prescription. Only studies from developed countries where GPs act as gatekeepers were included as both influence the level of appropriate antibiotic prescriptions in a country.52 This choice reduced the number of eligible studies and may have concurrently reduced the number of detected determinants. Our framework has not been validated in this study, which is needed before it can be implemented. Another limitation was the lack of objective measure of the effect size due to the inclusion of qualitative studies. This makes it not possible to determine which determinants are more relevant.
Conclusions
The most important determinants of inappropriate antibiotic prescribing are comorbidity, diagnostic uncertainty, the GPs perception of a patient’s wish for antibiotics, an inability to effectively negotiate or explain appropriate use of antibiotics and a direct request for an antibiotic by a patient. Although our framework needs validation before it can be used. It may provide a viable starting point for designing, implementing and conducting interventions aimed at evidence-based reduction of antibiotic prescriptions in primary care.
Supplementary Material
Acknowledgments
We thank Jan Schoones of the Walaeus library LUMC for his expertise in setting up the literature search.
Footnotes
Contributors: All authors read, commented on and approved the final manuscript. MS: Conception and design, literature search, selection and review, data extraction and analysis, framework development and revisions, first draft of the manuscript and revisions. FLB: Literature selection and review, data analysis, framework development, manuscript revisions. NHS: Data analysis, manuscript revisions. MEN: Framework development, manuscript revisions, guarantor. MGJdB: Conception and design, framework development, manuscript revisions.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no data sets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Worldwide country situation analysis: Response to antimicrobial resistance 2015 [World Health Organisation]. Available: https://www.who.int/drugresistance/documents/situationanalysis/en/ [Accessed May 2019].
- 2.Bell BG, Schellevis F, Stobberingh E, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014;14:13. 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control report . Antimicrobial consumption ECDC Website2020. Available: https://ecdc.europa.eu/en/antimicrobial-consumption/surveillance-and-disease-data/database [Accessed Dec 2020].
- 4.National Institute for Public health and the . Consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands in 2020. n.d. Available: https://swab.nl/nl/nethmap
- 5.NHG_workgroup . Acute coughing 2011. Dutch society of General practitioners guideline. Available: https://richtlijnen.nhg.org/standaarden/acuut-hoesten [Accessed Jun 2020].
- 6.NHG_workgroup . Dutch society of General practitioners guideline: Bacterial skin infections. 2019. Available: https://richtlijnen.nhg.org/standaarden/bacteriele-huidinfecties
- 7.NHG_workgroup . Dutch society of General practitioners guideline: Acute Rhinosinusitis. 2014. Available: https://richtlijnen.nhg.org/standaarden/acute-rhinosinusitis2020
- 8.NHG_workgroup . Dutch society of General practitioners guideline: Urinary tract infections. 2020. Available: https://richtlijnen.nhg.org/standaarden/urineweginfecties2020
- 9.National Institute for Health Care and Excellence (NICE) . Self-limiting respiratory tract and ear infections– antibiotic Prescribing overview. Available: https://pathways.nice.org.uk/pathways/self-limiting-respiratory-tract-and-ear-infections-antibiotic-prescribing#path=view%3A/pathways/self-limiting-respiratory-tract-and-ear-infections-antibiotic-prescribing/self-limiting-respiratory-tract-and-ear-infections-antibiotic-prescribing-overview.xml&content=view-index [Accessed Jun 2020].
- 10.National Institute for Health Care and Excellence (NICE) . Urinary tract infections overview. Available: https://pathways.nice.org.uk/pathways/urinary-tract-infections [Accessed Jun 2020].
- 11.Bianco A, Papadopoli R, Mascaro V, et al. Antibiotic prescriptions to adults with acute respiratory tract infections by Italian general practitioners. Infect Drug Resist 2018;11:2199–205. 10.2147/IDR.S170349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jørgensen LC, Friis Christensen S, Cordoba Currea G, et al. Antibiotic Prescribing in patients with acute Rhinosinusitis is not in agreement with European recommendations. Scand J Prim Health Care 2013;31:101–5. 10.3109/02813432.2013.788270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hek K, van Esch TEM, Lambooij A, et al. n.d. Guideline adherence in antibiotic Prescribing to patients with respiratory diseases in primary care: Prevalence and practice variation. Antibiotics;9:571. 10.3390/antibiotics9090571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howarth T, Brunette R, Davies T, et al. Antibiotic use for Australian aboriginal children in three remote Northern territory communities. PLoS One 2020;15:e0233533. 10.1371/journal.pone.0233533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debets VE, Verheij TJ, van der Velden AW, et al. Antibiotic Prescribing during office hours and out-of-hours: A comparison of quality and quantity in primary care in the Netherlands. Br J Gen Pract 2017;67:e178–86. 10.3399/bjgp17X689641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peñalva G, Fernández-Urrusuno R, Turmo JM, et al. Long-term impact of an educational antimicrobial stewardship programme in primary care on infections caused by extended-spectrum Β-Lactamase-producing Escherichia coli in the community: An interrupted time-series analysis. Lancet Infect Dis 2020;20:199–207. 10.1016/S1473-3099(19)30573-0 [DOI] [PubMed] [Google Scholar]
- 17.Morgan DJ, Leppin AL, Smith CD, et al. A practical framework for understanding and reducing medical Overuse: Conceptualizing Overuse through the patient-clinician interaction. J Hosp Med 2017;12:346–51. 10.12788/jhm.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: A systematic review and synthesis of Frameworks and Taxonomies of factors that prevent or enable improvements in Healthcare professional practice. Implement Sci 2013;8:35. 10.1186/1748-5908-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nations U . World economic situation and prospect; 2015.
- 21.OECD health system characteristics survey 2019 [Organisation for Economic Co-operation and Development report]. Available: http://www.oecd.org/ [Accessed May 2019].
- 22.National heart lung and blood Institute: Study quality assesment tools. Available: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Accessed May 2019].
- 23.CASP Chekclist. Available: https://casp-uk.net/casp-tools-checklists/ [Accessed 7 May 2019].
- 24.Akkerman AE, Kuyvenhoven MM, van der Wouden JC, et al. Determinants of antibiotic Overprescribing in respiratory tract infections in general practice. J Antimicrob Chemother 2005;56:930–6. 10.1093/jac/dki283 [DOI] [PubMed] [Google Scholar]
- 25.Damoiseaux RA, de Melker RA, Ausems MJ, et al. Reasons for non-guideline-based antibiotic prescriptions for acute Otitis media in the Netherlands. Fam Pract 1999;16:50–3. 10.1093/fampra/16.1.50 [DOI] [PubMed] [Google Scholar]
- 26.Dekker ARJ, Verheij TJM, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: Most prominent in adult patients. Fam Pract 2015;32:401–7. 10.1093/fampra/cmv019 [DOI] [PubMed] [Google Scholar]
- 27.Eggermont D, Smit MAM, Kwestroo GA, et al. The influence of gender Concordance between general practitioner and patient on antibiotic Prescribing for sore throat symptoms: A retrospective study. BMC Fam Pract 2018;19:175. 10.1186/s12875-018-0859-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouwels KB, Dolk FCK, Smith DRM, et al. Actual versus 'ideal' antibiotic Prescribing for common conditions in English primary care. J Antimicrob Chemother 2018;73(suppl_2):19–26. 10.1093/jac/dkx502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer A, Fanella S, Kosowan L, et al. Informing antimicrobial stewardship: Factors associated with inappropriate antimicrobial Prescribing in primary care. Fam Pract 2018;35:455–60. 10.1093/fampra/cmx118 [DOI] [PubMed] [Google Scholar]
- 30.Akkerman AE, Kuyvenhoven MM, van der Wouden JC, et al. Analysis of Under- and Overprescribing of antibiotics in acute Otitis media in general practice. J Antimicrob Chemother 2005;56:569–74. 10.1093/jac/dki257 [DOI] [PubMed] [Google Scholar]
- 31.Cadieux G, Tamblyn R, Dauphinee D, et al. Predictors of inappropriate antibiotic Prescribing among primary care physicians. CMAJ 2007;177:877–83. 10.1503/cmaj.070151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher-Lartey S, Yee M, Gaarslev C, et al. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: A mixed methods study. BMJ Open 2016;6:e012244. 10.1136/bmjopen-2016-012244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Doherty J, Leader LFW, O’Regan A, et al. Over Prescribing of antibiotics for acute respiratory tract infections; a qualitative study to explore Irish general practitioners' perspectives. BMC Fam Pract 2019;20:27. 10.1186/s12875-019-0917-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman M, Povitz M, Sontrop JM, et al. Antibiotic Prescribing for Nonbacterial acute upper respiratory infections in elderly persons. Ann Intern Med 2017;166:765. 10.7326/M16-1131 [DOI] [PubMed] [Google Scholar]
- 35.Singer A, Kosowan L, Katz A, et al. Prescribing and testing by primary care providers to assess adherence to the choosing wisely Canada recommendations: A retrospective cohort study. CMAJ Open 2018;6:E603–10. 10.9778/cmajo.20180053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Esch TEM, Brabers AEM, Hek K, et al. Does shared decision-making reduce antibiotic Prescribing in primary care J Antimicrob Chemother 2018;73:3199–205. 10.1093/jac/dky321 [DOI] [PubMed] [Google Scholar]
- 37.Malo S, Poblador-Plou B, Prados-Torres A, et al. Poor congruence with guidelines in the use of antibiotics for acute Bronchitis: A descriptive study based on electronic health records. Fam Pract 2016;33:471–5. 10.1093/fampra/cmw037 [DOI] [PubMed] [Google Scholar]
- 38.Biezen R, Roberts C, Buising K, et al. How do general practitioners access guidelines and Utilise electronic medical records to make clinical decisions on antibiotic use? results from an Australian qualitative study. BMJ Open 2019;9:e028329. 10.1136/bmjopen-2018-028329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-Álvarez I, Zapata-Cachafeiro M, Vázquez-Lago J, et al. Pharmaceutical companies information and antibiotic prescription patterns: A follow-up study in Spanish primary care. PLoS One 2019;14:e0221326. 10.1371/journal.pone.0221326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowakowska M, van Staa T, Mölter A, et al. Antibiotic choice in UK general practice: Rates and drivers of potentially inappropriate antibiotic Prescribing. J Antimicrob Chemother 2019;74:3371–8. 10.1093/jac/dkz345 [DOI] [PubMed] [Google Scholar]
- 41.Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: A systematic review. Implement Sci 2018;13:47. 10.1186/s13012-018-0732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Centre for Disease Prevention and Control Report . Survey of Healthcare workers’ knowledge, attitudes and Behaviours on antibiotics, antibiotic use and antibiotic resistance in the EU/EEA 2019. Available: https://www.ecdc.europa.eu/sites/default/files/documents/survey-of-healthcare-workersknowledge-attitudes-behaviours-on-antibiotics.pdf [Accessed Sep 2020].
- 43.One health 2022 [World Health Organization report]. n.d. Available: https://www.who.int/news-room/questions-and-answers/item/one-health
- 44.Cals JWL, Schot MJC, de Jong SAM, et al. Point-of-care C-reactive protein testing and antibiotic Prescribing for respiratory tract infections: A randomized controlled trial. Ann Fam Med 2010;8:124–33. 10.1370/afm.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjerrum L, Munck A, Gahrn-Hansen B, et al. Health Alliance for prudent antibiotic Prescribing in patients with respiratory tract infections (HAPPY AUDIT) -Impact of a non-randomised Multifaceted intervention programme. BMC Fam Pract 2011;12:52. 10.1186/1471-2296-12-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cals JWL, Butler CC, Hopstaken RM, et al. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: Cluster randomised trial. BMJ 2009;338(may05 1):b1374. 10.1136/bmj.b1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute Bronchitis. JAMA Intern Med 2013;173:267. 10.1001/jamainternmed.2013.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Little P, Hobbs FDR, Moore M, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: Randomised controlled trial of PRISM (primary care Streptococcal management). BMJ 2013;347(oct10 3):f5806. 10.1136/bmj.f5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Touboul-Lundgren P, Jensen S, Drai J, et al. Identification of cultural determinants of antibiotic use cited in primary care in Europe: A mixed research synthesis study of integrated design "culture is all around us. BMC Public Health 2015;15:908. 10.1186/s12889-015-2254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borg MA. National cultural dimensions as drivers of inappropriate ambulatory care consumption of antibiotics in Europe and their relevance to awareness campaigns. J Antimicrob Chemother 2012;67:763–7. 10.1093/jac/dkr541 [DOI] [PubMed] [Google Scholar]
- 51.Hofstede G. Compare countries tool 2020. Available: https://www.hofstede-insights.com/product/compare-countries/ [Accessed Oct 2020].
- 52.Blommaert A, Marais C, Hens N, et al. Determinants of between-country differences in ambulatory antibiotic use and antibiotic resistance in Europe: A longitudinal observational study. J Antimicrob Chemother 2014;69:535–47. 10.1093/jac/dkt377 [DOI] [PubMed] [Google Scholar]
- 53.Cals JWL, de Bock L, Beckers P-JHW, et al. Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract infection: 3.5-Year follow-up of a cluster randomized trial. Ann Fam Med 2013;11:157–64. 10.1370/afm.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreeva E, Melbye H. Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: An open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam Pract 2014;15:80. 10.1186/1471-2296-15-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Little P, Stuart B, Francis N, et al. Effects of Internet-based training on antibiotic Prescribing rates for acute respiratory-tract infections: A multinational, cluster, randomised, factorial, controlled trial. Lancet 2013;382:1175–82. 10.1016/S0140-6736(13)60994-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Little P, Stuart B, Francis N, et al. Antibiotic Prescribing for acute respiratory tract infections 12 months after communication and CRP training: A randomized trial. Ann Fam Med 2019;17:125–32. 10.1370/afm.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Llor C, Bjerrum L, Munck A, et al. Access to point-of-care tests reduces the prescription of antibiotics among antibiotic-requesting subjects with respiratory tract infections. Respir Care 2014;59:1918–23. 10.4187/respcare.03275 [DOI] [PubMed] [Google Scholar]
- 58.Fernández Urrusuno R, Flores Dorado M, Vilches Arenas A, et al. Improving the appropriateness of antimicrobial use in primary care after implementation of a local antimicrobial guide in both levels of care. Eur J Clin Pharmacol 2014;70:1011–20. 10.1007/s00228-014-1704-z [DOI] [PubMed] [Google Scholar]
- 59.Francis NA, Butler CC, Hood K, et al. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on Reconsulting and antibiotic Prescribing: A cluster randomised controlled trial. BMJ 2009;339(jul29 2):b2885. 10.1136/bmj.b2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macfarlane J, Holmes W, Gard P, et al. Reducing antibiotic use for acute Bronchitis in primary care: Blinded, randomised controlled trial of patient information leaflet. BMJ 2002;324:91–4. 10.1136/bmj.324.7329.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Småbrekke L, Berild D, Giaever A, et al. Educational intervention for parents and healthcare providers leads to reduced antibiotic use in acute Otitis media. Scand J Infect Dis 2002;34:657–9. 10.1080/00365540210147651 [DOI] [PubMed] [Google Scholar]
- 62.Lambert MF, Masters GA, Brent SL. Can mass media campaigns change antimicrobial Prescribing? A regional evaluation study. J Antimicrob Chemother 2007;59:537–43. 10.1093/jac/dkl511 [DOI] [PubMed] [Google Scholar]
- 63.Little P, Gould C, Williamson I, et al. Pragmatic randomised controlled trial of two Prescribing strategies for childhood acute Otitis media. BMJ 2001;322:336–42. 10.1136/bmj.322.7282.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mas-Dalmau G, Villanueva López C, Gorrotxategi Gorrotxategi P, et al. Delayed antibiotic prescription for children with respiratory infections: A randomized trial. Pediatrics 2021;147:e20201323. 10.1542/peds.2020-1323 [DOI] [PubMed] [Google Scholar]
- 65.McNulty C, Hawking M, Lecky D, et al. Effects of primary care antimicrobial stewardship outreach on antibiotic use by General practice staff: Pragmatic randomized controlled trial of the TARGET antibiotics workshop. J Antimicrob Chemother 2018;73:1423–32. 10.1093/jac/dky004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of Multifaceted educational programme to reduce antibiotic dispensing in primary care: Practice based randomised controlled trial. BMJ 2012;344(feb02 1):d8173. 10.1136/bmj.d8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dyrkorn R, Gjelstad S, Espnes KA, et al. Peer academic detailing on use of antibiotics in acute respiratory tract infections. A controlled study in an urban Norwegian out-of-hours service. Scand J Prim Health Care 2016;34:180–5. 10.3109/02813432.2016.1163035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Persell SD, Doctor JN, Friedberg MW, et al. Behavioral interventions to reduce inappropriate antibiotic Prescribing: A randomized pilot trial. BMC Infect Dis 2016;16:373. 10.1186/s12879-016-1715-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Briel M, Langewitz W, Tschudi P, et al. Communication training and antibiotic use in acute respiratory tract infections. A cluster randomised controlled trial in general practice. Swiss Med Wkly 2006;136:241–7. 10.4414/smw.2006.11342 [DOI] [PubMed] [Google Scholar]
- 70.Welschen I, Kuyvenhoven MM, Hoes AW, et al. Effectiveness of a multiple intervention to reduce antibiotic Prescribing for respiratory tract symptoms in primary care: Randomised controlled trial. BMJ 2004;329:431. 10.1136/bmj.38182.591238.EB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gjelstad S, Høye S, Straand J, et al. Improving antibiotic Prescribing in acute respiratory tract infections. BMJ 2013;347:f4403. 10.1136/bmj.f4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065006supp001.pdf (3MB, pdf)
bmjopen-2022-065006supp002.pdf (30.7KB, pdf)
bmjopen-2022-065006supp003.pdf (50.3KB, pdf)
bmjopen-2022-065006supp004.pdf (46.4KB, pdf)
bmjopen-2022-065006supp005.pdf (42.7KB, pdf)
bmjopen-2022-065006supp006.pdf (149.1KB, pdf)
bmjopen-2022-065006supp007.pdf (55.6KB, pdf)
bmjopen-2022-065006supp008.pdf (42.2KB, pdf)
Data Availability Statement
Data sharing not applicable as no data sets generated and/or analysed for this study. All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information.