Abstract
Objective
For large, integrated healthcare delivery systems, coordinating patient care across delivery systems with providers external to the system presents challenges. We explored the domains and requirements for care coordination by professionals across healthcare systems and developed an agenda for research, practice and policy.
Design
The modified Delphi approach convened a 2-day stakeholder panel with moderated virtual discussions, preceded and followed by online surveys.
Setting
The work addresses care coordination across healthcare systems. We introduced common care scenarios and differentiated recommendations for a large (main) healthcare organisation and external healthcare professionals that contribute additional care.
Participants
The panel composition included health service providers, decision makers, patients and care community, and researchers. Discussions were informed by a rapid review of tested approaches to fostering collaboration, facilitating care coordination and improving communication across healthcare systems.
Outcome measures
The study planned to formulate a research agenda, implications for practice and recommendations for policy.
Results
For research recommendations, we found consensus for developing measures of shared care, exploring healthcare professionals’ needs in different care scenarios and evaluating patient experiences. Agreed practice recommendations included educating external professionals about issues specific to the patients in the main healthcare system, educating professionals within the main healthcare system about the roles and responsibilities of all involved parties, and helping patients better understand the pros and cons of within-system and out-of-system care. Policy recommendations included supporting time for professionals with high overlap in patients to engage regularly and sustaining support for care coordination for high-need patients.
Conclusions
Recommendations from the stakeholder panel created an agenda to foster further research, practice and policy innovations in cross-system care coordination.
Keywords: health policy, quality in health care, health services administration & management
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Coordinating patient care across different healthcare delivery systems presents considerable challenges for healthcare professionals, and we convened a 2-day stakeholder panel, preceded and followed by surveys and informed by literature, to formulate concrete recommendations for research, practice and policy supporting healthcare organisations.
Some of the practice and policy recommendations may be aspirational for some organisations; however, panellists took limited organisational resources into account throughout the process and prioritised recommendations to identify the most important steps to meaningfully improve and support care coordination across healthcare systems.
While we used a structured and framework-driven approach to stakeholder selection and convened a large panel with a broad range of stakeholders, undoubtedly, some perspectives of the complex care coordination between systems will have been missed, and we hope that future research will further investigate care coordination established across healthcare systems and different care delivery organisations.
Introduction
Care coordination has received much practical and research attention, and its role in integrating clinical services is of critical importance to ensure safe and effective patient care.1 The care coordination literature derived from integrated delivery systems often focuses on coordination within a single healthcare system, such as improving interaction between primary care and specialty care providers. Efforts within systems include shared software and business processes to foster interdepartmental coordination. However, as demonstrated by network analyses that capture patient sharing between healthcare providers, care coordination across healthcare systems is an increasingly common clinical scenario.2 Such coordination may include management of chronic conditions by primary care with periodic input from external specialists.3 Other common areas of collaboration between healthcare delivery systems are cancer care4 and palliative care.5
Care coordination across healthcare systems cannot always use the same approaches as those used within a single system and instead may include introducing new software designed to facilitate coordination, such as web-based communication tools that can be accessed by healthcare professionals from all systems.6 These online tools create a secure, virtual space for care professionals and sometimes include patients and caregivers in the communication. Results across evaluation studies are mixed, with some authors concluding that tool implementation is feasible but sometimes of limited use to healthcare professionals. An evaluation of an accountable care organisation concluded that formal clinical integration may be insufficient to improve patient outcomes.7
We engaged stakeholders in an expert panel process to articulate an agenda for improving care coordination across healthcare delivery systems, from the vantage point of a large integrated healthcare system needing to coordinate care with external healthcare entities that do not share administrative or medical infrastructure. We formulated concrete recommendations for research, practice and policy.
Methods
We developed a detailed workplan that guided a 1-year process. The modified Delphi stakeholder panel used online surveys and video-assisted, moderated discussions and was informed by a rapid literature review.
Rapid review
A rapid review aimed to identify examples of care coordination approaches between healthcare systems and organisations to learn more about tested communication tools and strategies. There is no accepted nomenclature for care coordination, and the existing literature is dominated by coordination approaches within healthcare systems; hence, we applied a strategic search to identify relevant studies. We used four key publications selected by project staff to represent different aspects of care coordination research (clinical integration, implementation of technological advances, social network analysis of provider relationships and interpersonal relationships between providers) as seed articles for a forward search.1 2 6 7 We screened studies included in 127 systematic reviews on care coordination to obtain a broad range of care coordination approaches.3–5 8–131 The reviews addressed common chronic conditions managed in primary as well as specialty care, cancer care, palliative care, comorbidity and complexity, personnel specialising in care coordination, frameworks and strategies to promote coordination, technology supporting coordination, settings for temporary care such as emergency departments and care models applied to specific populations. The online supplemental appendix describes the methods and results of the rapid review in detail. We used the rapid review to prepare for the stakeholder panel meetings, made the results of the review available to stakeholders and summarised the findings during the panel meetings.
bmjopen-2021-060232supp001.pdf (1.7MB, pdf)
Analytical model
The project team designed an analytical model that anchored the care coordination discussions with three scenarios and that introduced the idea of a main integrated organisation with external healthcare professionals providing additional care outside of the primary network. Throughout the study, we used the Veterans Health Administration (VHA) as an example of a main healthcare delivery organisation that has significantly expanded its network of external providers over the years, allowing patients to use selected external healthcare providers to reduce waiting times and otherwise address patient needs, in particular since the introduction of the Maintaining Internal Systems and Strengthening Integrated Outside Networks Act.132 However, the panel’s goals were to advance care coordination across systems, an objective relevant not just to VHA but also to private-sector integrated delivery systems whose patients may receive out-of-system care (eg, for emergencies or when the main system contracts with an external organisation to deliver care).133
Our analytical framework is depicted using three scenarios in figure 1. The scenarios capture the multilevel aspect of care coordination, which includes patients, healthcare professionals, and the main healthcare delivery system or organisation. For simplicity, coordination is depicted as occurring across two systems, with the understanding that, for complex situations, coordination may need to occur across more than two entities. We also developed a glossary of key terms for panellists, also shown in the online supplemental appendix. The three care coordination scenarios include
Figure 1.

Analytical framework.
Scenario 1: intense care coordination—coordinating the care of individual, high-need patients who require frequent and high levels of care across different healthcare delivery systems.
Scenario 2: ad hoc care coordination—an unexpected need to communicate across healthcare delivery systems (eg, abnormal lab value is identified by one professional that should be communicated to the other healthcare professional).
Scenario 3: high overlap in patients—coordinating care between two healthcare professionals in different healthcare delivery systems who share a large number of patients.
We hypothesised that the scenarios may warrant different approaches to care coordination; for example, there may be differences in the amount of investment into care coordination improvement and technology.
Stakeholder panel
We reviewed stakeholder engagement models and adopted a model appropriate for public health, which includes four types of stakeholders: health services providers, decision makers, community representatives and research representatives.134 Within stakeholder categories, we approached potential representatives and ensured that the panel was multidisciplinary and represented different levels within organisations. The online supplemental appendix table documents all 16 participating panellists.
Consensus finding
The consensus-finding procedure adhered to principles of consensus methods for medical and health services research: anonymity (private ranking or voting to avoid dominance issues in the group), iteration (multiple rounds to allow individuals to change their opinions after discussions), controlled feedback (feedback of the group response after each rating round) and statistical group response (provision of summary measures of the group response).135 We used an online prepanel survey to elicit input from panellists to prepare the panel meeting. Fifteen panellists provided prepanel input (response rate 94%). The survey addressed available communication methods (formal and approved methods in the organisation as well as workarounds not sanctioned by the organisation but used to ensure communication with the external care professional), experience with approaches to foster informal interaction between healthcare professionals to support coordination, unintended consequences of care coordinated between multiple healthcare professionals, and the different aspects and layers of care coordination. All survey questions were open to all panellists, as all panellists were felt to have sufficient insight into care coordination processes (eg, patient representatives, although not engaging in ‘provider to provider’ communication, stated their preference for how they preferred their providers to communicate with one another). The panellists also received the results of the rapid review, and we made key resources available on a secure site for all team members and panellists. Results of the survey were presented during the panel in aggregate format, including points of agreement and disagreement across panellists.
We convened two panel meetings of 5 hours each. Although originally planned as an in-person meeting, both meetings were held online due to COVID-19 restrictions. The two meetings were held in the same week, on the first and last days of the week. The first panel meeting included presentations and discussions, while the second panel meeting asked panellists to vote on themes identified in the first meeting. The first panel meeting provided panellists with some background on the topic; an introduction of all team members and panellists; the status of the Veterans Health Administration Health Information Exchange, a system designed to support care coordination across VHA and (external) community providers; the findings of the rapid literature review; a presentation of the three coordination scenarios; results from interviews conducted within VHA with a specific focus on rural health; feedback from the prepanel survey; and a summary of the panel goals.
Three formal rounds of discussions in the first panel meeting focused on strategies for informal relationship-building among providers from different healthcare systems; communication methods; and the present state of care coordination across systems, focusing on what is working and what is not working for care coordination with current methods and approaches, from the panellists’ perspectives. Five discussion rounds on the second panel meeting day addressed the domains of care coordination, unintended consequences of (poor) coordination, the future research agenda, implications for practice and recommendations for policy. Discussions were moderated by experienced moderators who ensured that a variety of perspectives were heard and panellists stayed on topic. Following an approach used for RAND appropriateness panels, no attempt was made to force the panel to consensus. Instead, the discussions explored all viewpoints and tried to clarify terms and concepts. We used online technology to provide instant feedback. The panel meetings were attended by the panellists as well as five observers and two external presenters who added to the discussions.
A postpanel survey consolidated the findings of the panel meeting. Eleven panellists (69%) completed the postpanel survey. The survey was completed by each panellist individually to avoid situational groupthink and to consolidate the panel findings independently. To orient panellists, we used VHA as an example of a main healthcare system and termed professionals and community providers delivering outside care external healthcare providers. However, the input was geared towards making recommendations that are not specific to a selected organisation but that are applicable to different healthcare delivery systems that share care. The survey included recommendations for research, practice and policy drafted by the review team following the panel discussions. The survey used a 5-point rating scale throughout that assessed the importance of the presented items, ranging from not important (1) to very important (5). We used a cut-off of a mean value of 4.5 across panellists to select items as important. For the method of communication, one-way analysis of variance was performed to test for differences in means between the three coordination scenarios.
Results
Data were collected in the prepanel meeting survey, during the meeting and the postpanel meeting. Here we present the final results, that is, those that are based on the last consolidating round of panel input, preceded by a round of prepanel ratings and video-assisted discussions during the panel meetings.
Dimensions of care coordination
We asked panellists about the importance of 16 dimensions of care coordination, all based on published literature, suggestions from individual panellists and panel discussions. Panellists rated the method of communication, the organisational mechanism, the urgency of communication, the scope of coordination, the interpersonal aspect of coordination and organisational culture (the healthcare system support for coordination) as key aspects of coordination (see figure 2).
Figure 2.
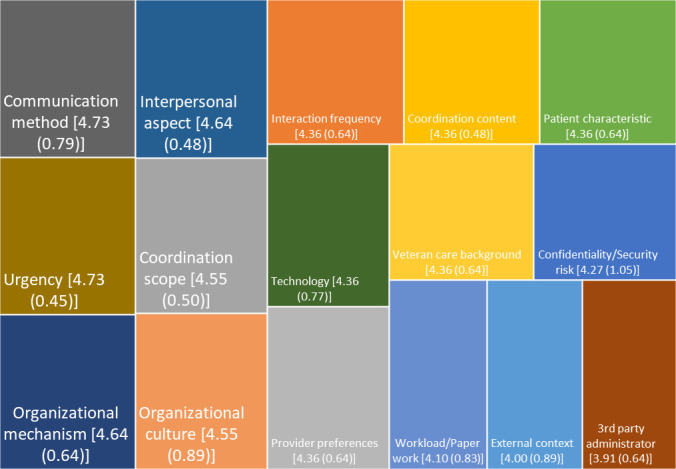
Care coordination domains. Domain content (mean (SD)) on a scale from 1 to 5.
Communication methods
The most commonly used method of communication between healthcare professionals to achieve coordination was phone calls (87%). A large proportion of the panellists used secure messaging (53%), email (47%), in-person communication (40%) and shared software (40%). The use of a web platform for care coordination (33%), call centre (33%), fax (27%), and letter/mail (27%) was also selected multiple times (table 1).
Table 1.
Frequency and preference for communication methods used in care coordination
| Modality | Frequency of use (%) | Preference ranking scenario 1 (intense coordination) |
Preference ranking scenario 2 (urgent, unplanned communication) |
Preference ranking scenario 3 (high overlap) |
| Phone call | 87 | 4.10 | 4.30 | 4.18 |
| Secure messaging | 53 | 4.60 | 4.50 | 4.60 |
| 47 | 4.10 | 4.00 | 3.91 | |
| In-person communication | 40 | 4.20 | 4.20 | 4.00 |
| Shared software | 40 | 4.40 | 4.40 | 4.36 |
| Web platform for care coordination | 33 | 4.30 | 4.50 | 4.36 |
| Call centre | 33 | 3.30 | 3.30 | 3.00 |
| Fax | 27 | 2.67 | 2.60 | 2.55 |
| Letter/mail | 27 | 2.80 | 2.50 | 2.55 |
| Email list | 20 | 3.90 | 3.90 | 3.82 |
| Pager | 13 | 2.60 | 2.70 | 2.36 |
| Text messaging (personal phone) | 20 | 2.30 | 2.60 | 2.45 |
| Text messaging (work phone) | 13 | 4.00 | 3.80 | 3.64 |
| Smart phone app | 7 | 4.30 | 4.10 | 4.00 |
| Messenger (third party) | 13 | 3.11 | 3.00 | 2.82 |
| Patient-held records | 20 | 2.60 | 2.60 | 2.64 |
| Video coordination (organisational system) | 13 | 3.90 | 3.70 | 3.55 |
| Commercial video (Zoom) | 7 | 3.70 | 3.30 | 3.36 |
| Duplicate health record | 20 | 3.40 | 3.00 | 2.64 |
| Other, query-based exchange in health information exchange system | 7 | 3.80 | 3.50 | 4.00 |
| Other, Veterans Health Information Exchange | 7 | 4.00 | 3.40 | 3.91 |
| Other, Microsoft Teams | 7 | 3.78 | 3.80 | 3.91 |
Panellists’ preferences for interprofessional communication included phone, in-person, direct communication, email, secure messaging, text, shared online portal, video, shared web-based services, commercial collaboration software (Microsoft Teams), commercial communication software (Epic messaging), and a platform that allows uploading of information and other electronic health information exchange capabilities. Some panellists indicated that the preferred method depended on the type of information, the situation or the purpose. Among all 22 communication modalities, there were no significant differences in mean preference ranking between the three scenarios; that is, we found no evidence that the communication method differed systematically by the three scenarios (intense care coordination, ad hoc care coordination and high overlap in patients). One panellist preferred phone calls for ‘real-time issues’, and another panellist responded that some conversations warrant face-to-face interaction; otherwise, calls and emails work. One panellist indicated it depends on the urgency of the need, and urgent issues required calls; otherwise, email would work. Panellists indicated it depends on whether the purpose is communication, coordination or collaboration. For collaboration, a platform that allows uploading information is needed (ideally with video or phone interface); for other purposes, phone calls are fine.
Furthermore, we asked about healthcare professionals’ use of strategies of communication and coordination that are not approved in their healthcare delivery system. Examples of these ‘workarounds’ were texting and using email. This included using day-to-day communication tools such as smartphones, for example, to check with another provider whether a patient had followed through and had made an appointment as suggested, or to check whether results of tests were coming on time for an upcoming appointment. Reasons for using workarounds were typically ease of use of the standard tools in time-pressing matters and the availability to communicate quickly without additional sign-in procedures when there were no confidentiality concerns because no details had to be shared.
Support to foster collaboration
Twenty per cent of panellists indicated that there was only minimal or no support in their respective organisations for external communication, care coordination and collaboration such as web portals, and pager and phone arrangements to make healthcare professionals accessible to partners in other health systems.
Participants reported very few informal initiatives for cross-system communication, that is, occasions characterised by informal interaction and relationship-building without focus on a particular patient or a specific care issue to solve. Similarly, only few studies identified in the rapid literature review described relevant initiatives. Where studies mentioned initiatives, these were most often informal gatherings such as meet-and-greet lunches136–139 or training and didactic sessions for topics of shared interest.140–143 Other studies relied on a shared care facility promoting communication due to proximity.144–148
Unintended consequences
While the literature cited many examples of the positive effects of care coordination, panellists also noted some unintended consequences. We differentiated between potential unintended consequences for patients and for healthcare professionals, and asked panellists to consider both perspectives. For professionals, panellists addressed burden, role confusion, miscommunication of health information leading to inaccurate care plans, patients pitting healthcare professionals against one another and healthcare professionals pitting patients against another professional.
For patients, panellists rated ambiguity of whom to contact for care needs, uncertainty of care processes, mixed messages from different professionals, delays in care (eg, professionals may wait to discuss care plans with each other first), miscommunication leading to misunderstandings, enhanced communication and collaboration disliked by patients (eg, patients not appreciating being the subject of discussion among providers), and confidentiality concerns. Although all items were rated as somewhat important, none met the prespecified threshold, indicating that the item is ‘very important’.
Recommendations
A key aim of the stakeholder panel was to identify targets for research, practice and policy. Panellists rated a large number of potential recommendations shown in the online supplemental appendix. The recommendations were based on the reviewed literature and the panel discussions on the first panel meeting day.
Figure 3 documents the 10 research recommendations selected by the panel. Recommendations for research centred around needed data on and measures of shared care and workflow, the role of patients in care coordination and explorations into better understanding healthcare provider needs. Throughout the study, recommendations stressed that needs may well be different for different care scenarios (eg, frequency of likely coordination needs). Specifically, identified research recommendations targeted the development of measures of shared care between professionals in the main healthcare delivery system and the external healthcare professionals such as the proportion or absolute numbers of patients shared between two care providers. Further research is needed that identifies coordination scenarios in which the additional expense and time associated with team care are warranted. Studies should evaluate patient experiences of care coordination across separate healthcare delivery systems. We also need more information on the proper and improper uses of patient engagement to coordinate care, that is, to determine how much we should expect patients to participate in care coordination versus how much should be owned by the providers/systems caring for the patient. Studies are further needed to understand the external professionals’ needs and preferences better in relation to interfacing with the main healthcare delivery system’s services. Furthermore, organisations should evaluate roles/responsibilities of the main healthcare delivery system’s clinicians, administrators, external care and third-party administrators, for example, look for gaps or overlap. To advance care coordination, we need to conduct ongoing, real-time evaluation of changes to care coordination processes being implemented in the field, given that care coordination and available tools is a fast-moving field. The panel also agreed that it is imperative that studies produce replicable care coordination data and data validation, such as organisations tracking referrals and follow-up. Furthermore, organisations should evaluate their workflow practices to ensure closed-loop communication. Finally, the panel agreed that evaluating the comparative effectiveness of interventions to improve care coordination is a research priority.
Figure 3.
Recommendations.
The panel also made eight recommendations that should be implemented in routine practice (see figure 3). Recommendations addressed education of healthcare professionals in the main healthcare system as well as educating the external providers. Specifically, panellists agreed that it is critical to educate main and external healthcare professionals how to use the latest communication technology, including electronic health information exchanges. Panellists also stressed that it is critical to ensure that external practices keep communication options up to date, including keeping contact details of the primary contact at the practice up to date. Furthermore, panellists agreed on the importance of determining the best point of contact phone numbers for different healthcare professionals, teams and clinics to be reached by external professionals, including embedding the contacts in the appropriate software so that they are seen by external care providers. Further recommendations included educating external healthcare professionals about patient demographics and the care approach in the main healthcare system (discussed examples included the use of opioids for pain management). The panel stressed that it is critical to educate the main healthcare delivery system providers about what the roles and responsibilities are for all parties involved with outside care coordination; multiple experiences indicated that roles and responsibilities are not always clear or do get lost over time. One concrete recommendation to increase local relationship-building activities that panellists agreed on was to initiate team meetings/huddles for professionals of both healthcare delivery systems that share a group of patients with complex care coordination needs, including determining which team members need to be involved in the meetings. Panellists also agreed that organisations should implement quality improvement routines such as audit and feedback to check that coordination mechanisms are working as intended. A final agreed recommendation was to help patients better understand the pros and cons of care within the main system and externally, in particular as patients may have unrealistic expectations about care options within or outside the healthcare system.
Finally, the panel prioritised five recommendations to direct policy (see figure 3). Panellists were aware that policy recommendations are often a trade-off between multiple important goals, and the panel determined priorities for policy by taking into account that the selected recommendations will take precedence over other targets of policy. Agreed-on recommendations included increasing investment in healthcare professionals' education about the coordination challenges between healthcare professionals. Increasing investment in healthcare professionals’ education about tools to support coordination between professionals was also agreed upon. Care coordination and available tools is a rapidly developing field, and using tools requires investing in practitioners to keep up with new developments. Panellists also agreed on dedicated time and resources for care coordination training. Further, organisations need to be prepared to support time for professionals with high patient overlap to engage regularly, for example, engaging in huddles and relationship-building activities between professionals. Finally, panellists agreed that policy makers should provide and sustain support for care coordination resources for high-need patients.
Policy recommendations included supporting time for professionals that have high overlap in patients and need to engage regularly, as well as sustaining support for care coordination for high needs patients. Such support can be reflected in workload credit, countering financial disincentives for care coordination and designating administrative staff to support providers who coordinate care.
Discussion
The stakeholder panel successfully explored many different aspects of care coordination, and panel discussions resulted in concrete recommendations for research, practice and policy. Some recommendations are general in nature, while other recommendations address specific gaps in research, practice and/or policy identified in each of the three coordination scenarios included in our analytical framework. For example, the practice recommendation to initiate team meetings or huddles for professionals in both main and external healthcare systems to coordinate care for high-need patients addresses scenario 1 (the specific challenges in coordinating care of high-need patients across systems), whereas the research recommendation to develop measures of shared care between professionals in the main healthcare system and the external system addresses scenario 3 (coordination challenges associated with high system overlap in shared patients). Recommendations relating to scenario 2 (ad hoc communication between healthcare professionals across systems) were indirectly addressed by practice recommendations relating to keeping contact information up to date and educating providers in the main healthcare system and external systems about the latest communication technology.
It was noteworthy that despite the number of publications suggesting new tools with advanced, secure, online technology for sharing information between healthcare professionals,14 33 35 50 65 73 88 102 104 122 professionals still rated phone calls as the most frequently used form of communication. This raises the question whether the mixed results seen in effects of health information exchange approaches149 are in part attributable to professionals not being ready for the technology or, alternatively, that the technology is insufficiently user friendly. The broad range of communication mechanisms used by providers is also striking. Although some of this range may be responsive to differences in patient and coordination needs and urgency, this range may also indicate the lack of best practices or standardisation for communication, coordination and collaboration across healthcare systems.
The exploration of domains of care coordination showed that the organisational mechanism, the method of communication and the urgency of the communication are key components that influence care coordination. We specifically addressed support mechanisms and approaches to foster informal contact between coordinating professionals and identified approaches included shared events such as journal clubs that bring professionals together without directly discussing patients but potentially fostering relationships. These approaches need to be sufficiently attractive to draw healthcare providers in who tend to have already busy schedules (eg, offer continuing medical education credits). However, there are potential regulatory issues, given that the coordination is typically between two healthcare delivery systems, of which one serves as the external provider or ‘vendor’ offering additional care. Panellists stressed the importance of relationship-building activities towards improving care coordination for existing patients while avoiding the use of the meetings as a method of ‘advertising’ or ‘marketing’ by the ‘vendors’ (in particular, in the context of the VHA’s government contractual obligations for an external community care network). Finally, although separate systems may view themselves as competing for patients, a growing shortage of healthcare providers may foster innovative approaches for collaboration across systems.
The panel discussed potentially unintended consequences of care coordination at length, which require thoughtful consideration yet do not preclude the need for improved care coordination. Panellists debated passionately whether issues such as administrative burden and role confusion are an indication of poor coordination, simply a result of receiving care from multiple care providers, or a challenge that coordination is precisely aiming to address. These discussions did point to the need for both patients and providers to consider the pros and cons of seeking care external to the main healthcare system. The proposed research methods will support eventual analyses to inform such considerations.
This work was exploratory and therefore subject to limitation. While we used a structured and framework-driven approach to stakeholder selection and convened a large panel with a broad range of stakeholders, undoubtedly, some perspectives of the complex care coordination between systems will have been missed. In addition, the panellists were predominantly familiar with the VHA, Keck Medicine at the University of Southern California (USC) and the Los Angeles County+USC Medical Centre. Due to the small sample, we could not systematically explore differences in responses based on individual panellists’ characteristics. The panel was informed by research evidence, but the rapid review showed a research literature that is dominated by care coordination within system, and there are numerous aspects of coordination between systems that make this field even more complex. We hope that future research will further investigate care coordination established across healthcare systems and different care delivery organisations.
Panellists selected recommendations from a large pool of potential recommendations. Any recommendation needs to consider that there are usually multiple competing goals for organisations. The current study purposefully refrained from assembling a long and unrealistic ‘wish list’, and panellists discussed barriers to implementing recommendations critically. The research recommendations aimed to provide explicit direction and outlined areas for which we have little information to date, including measures of shared care, the role of patients in care coordination and exploring healthcare provider needs. Some of the practice and policy recommendations may be aspirational for some organisations (eg, providing protected time for care coordination education); however, panellists took limited organisational resources into account throughout the process and prioritised recommendations to identify the most important steps to meaningfully improve and support care coordination across healthcare systems. Our 10 concrete recommendations for research, 8 for practice and 5 for policy makers provide the first step in better understanding of care coordination between systems.
Supplementary Material
Acknowledgments
We thank Ben Dennis, Greg Foerstel, Jeffrey Anderson, Clinton Leo Greenstone, Vivian Mo, Martha Jones, Jeff Hay, Ashley Halle, Victoria Kell, Tachil Bains-Miranda, Denise Hynes and Christopher Miller for valuable contributions.
Footnotes
Twitter: @mychellss
Contributors: SH, DG and IMM-L designed the study and SH is the guarantor of the work. SH, DG, IMM-L, SS, CT, KC, AB, TP, RM, JB, MW, NF, MB, TK and AM informed recommendation statements. SH, MB, TK, AM and NF abstracted data to inform the stakeholder panel. All authors contributed to and approved the final manuscript.
Funding: This work was supported by the VA’s Care Coordination QUERI Program (QUE 15-276) and funding from VA’s Health Services Research & Development Service (RVR 19-473 and CIN 13-417).
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. The collected data are included in the online supplemental appendix. Data can be made available in different formats (eg, as Excel file) upon request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The panel included health service providers, decision makers, research and community representatives including patients. The University of Southern California’s institutional review board determined the study to be exempt. The participants gave informed consent to participate in the study before taking part.
References
- 1.Kizer KW. Clinical integration: a cornerstone for population health management. J Healthc Manag 2015;60:164–8. [PubMed] [Google Scholar]
- 2.DuGoff EH, Fernandes-Taylor S, Weissman GE, et al. A scoping review of patient-sharing network studies using administrative data. Transl Behav Med 2018;8:598–625. 10.1093/tbm/ibx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foy R, Hempel S, Rubenstein L, et al. Meta-Analysis: effect of interactive communication between collaborating primary care physicians and specialists. Ann Intern Med 2010;152:247–58. 10.7326/0003-4819-152-4-201002160-00010 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell K-AR, Brassil KJ, Rodriguez SA, et al. Operationalizing patient-centered cancer care: a systematic review and synthesis of the qualitative literature on cancer patients’ needs, values, and preferences. Psychooncology 2020;29:1723–33. 10.1002/pon.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahluwalia SC, Chen C, Raaen L, et al. A systematic review in support of the national consensus project clinical practice guidelines for quality palliative care, fourth edition. J Pain Symptom Manage 2018;56:831–70.:. 10.1016/j.jpainsymman.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res 2017;19:e219. 10.2196/jmir.7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim D, Funk RJ, Yan P, et al. Informal clinical integration in Medicare accountable care organizations and mortality following coronary artery bypass graft surgery. Med Care 2019;57:194–201. 10.1097/MLR.0000000000001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand TN, Joseph LM, Geetha AV, et al. Task-sharing interventions for cardiovascular risk reduction and lipid outcomes in low- and middle-income countries: a systematic review and meta-analysis. J Clin Lipidol 2018;12:626–42. 10.1016/j.jacl.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annis AM, Harris M, Robinson CH, et al. Do patient-centered medical home access and care coordination measures reflect the contribution of all team members? A systematic review. J Nurs Care Qual 2016;31:357–66. 10.1097/NCQ.0000000000000192 [DOI] [PubMed] [Google Scholar]
- 10.Aubin M, Giguère A, Martin M, et al. Interventions to improve continuity of care in the follow-up of patients with cancer. Cochrane Database Syst Rev 2012:CD007672. 10.1002/14651858.CD007672.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aubry RE, Scott L, Cassidy E. Lithium monitoring patterns in the united kingdom and ireland: can shared care agreements play A role in improving monitoring quality? A systematic review. Ir J Psychol Med 2017;34:127–40. 10.1017/ipm.2017.2 [DOI] [PubMed] [Google Scholar]
- 12.Backhouse A, Richards DA, McCabe R, et al. Stakeholders perspectives on the key components of community-based interventions coordinating care in dementia: a qualitative systematic review. BMC Health Serv Res 2017;17:767. 10.1186/s12913-017-2725-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backhouse A, Ukoumunne OC, Richards DA, et al. The effectiveness of community-based coordinating interventions in dementia care: a meta-analysis and subgroup analysis of intervention components. BMC Health Serv Res 2017;17:717. 10.1186/s12913-017-2677-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartoli L, Zanaboni P, Masella C, et al. Systematic review of telemedicine services for patients affected by chronic obstructive pulmonary disease (COPD). Telemed J E Health 2009;15:877–83. 10.1089/tmj.2009.0044 [DOI] [PubMed] [Google Scholar]
- 15.Bee P, Playle J, Lovell K, et al. Service user views and expectations of UK-registered mental health nurses: a systematic review of empirical research. Int J Nurs Stud 2008;45:442–57. 10.1016/j.ijnurstu.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 16.Beesley VL, Alemayehu C, Webb PM. A systematic literature review of trials of survivorship interventions for women with gynaecological cancer and their caregivers. Eur J Cancer Care (Engl) 2019;28:e13057. 10.1111/ecc.13057 [DOI] [PubMed] [Google Scholar]
- 17.Berning MJ, Oliveira J. e Silva L, Suarez NE, et al. Interventions to improve older adults’ emergency department patient experience: a systematic review. The American Journal of Emergency Medicine 2020;38:1257–69. 10.1016/j.ajem.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 18.Bowser D, Marqusee H, El Koussa M, et al. Health system barriers and enablers to early access to breast cancer screening, detection, and diagnosis: a global analysis applied to the MENA region. Public Health 2017;152:58–74. 10.1016/j.puhe.2017.07.020 [DOI] [PubMed] [Google Scholar]
- 19.Brennan ME, Gormally JF, Butow P, et al. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer 2014;111:1899–908. 10.1038/bjc.2014.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmont S-A, Mitchell G, Senior H, et al. Systematic review of the effectiveness, barriers and facilitators to general practitioner engagement with specialist secondary services in integrated palliative care. BMJ Support Palliat Care 2018;8:385–99. 10.1136/bmjspcare-2016-001125 [DOI] [PubMed] [Google Scholar]
- 21.Cassarino M, Robinson K, Quinn R, et al. Impact of early assessment and intervention by teams involving health and social care professionals in the emergency department: a systematic review. PLoS One 2019;14:e0220709. 10.1371/journal.pone.0220709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuah FLH, Haldane VE, Cervero-Liceras F, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan 2017;32(suppl_4):iv27–47. 10.1093/heapol/czw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciapponi A, Lewin S, Herrera CA, et al. Delivery arrangements for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev 2017;2017. 10.1002/14651858.CD011083.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemente D, Leon L, Foster H, et al. Systematic review and critical appraisal of transitional care programmes in rheumatology. Semin Arthritis Rheum 2016;46:372–9. 10.1016/j.semarthrit.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Coller RJ, Nelson BB, Sklansky DJ, et al. Preventing hospitalizations in children with medical complexity: a systematic review. Pediatrics 2014;134:e1628–47. 10.1542/peds.2014-1956 [DOI] [PubMed] [Google Scholar]
- 26.Colombel J-F, D’haens G, Lee W-J, et al. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis 2020;14:254–66. 10.1093/ecco-jcc/jjz131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway A, O’Donnell C, Yates P. The effectiveness of the nurse care coordinator role on patient-reported and health service outcomes: A systematic review. Eval Health Prof 2019;42:263–96. 10.1177/0163278717734610 [DOI] [PubMed] [Google Scholar]
- 28.Costa DK, White MR, Ginier E, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: A systematic review. Chest 2017;152:304–11. 10.1016/j.chest.2017.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damarell RA, Morgan DD, Tieman JJ. General practitioner strategies for managing patients with multimorbidity: a systematic review and thematic synthesis of qualitative research. BMC Fam Pract 2020;21:131.:131. 10.1186/s12875-020-01197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Regge M, De Pourcq K, Meijboom B, et al. The role of hospitals in bridging the care continuum: a systematic review of coordination of care and follow-up for adults with chronic conditions. BMC Health Serv Res 2017;17:550.:550. 10.1186/s12913-017-2500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiMartino LD, Weiner BJ, Mayer DK, et al. Do palliative care interventions reduce emergency department visits among patients with cancer at the end of life? A systematic review. J Palliat Med 2014;17:1384–99. 10.1089/jpm.2014.0092 [DOI] [PubMed] [Google Scholar]
- 32.Dossett LA, Hudson JN, Morris AM, et al. The primary care provider (PCP) -cancer specialist relationship: a systematic review and mixed-methods meta-synthesis. CA Cancer J Clin 2017;67:156–69. 10.3322/caac.21385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Inform 2015;84:87–100. 10.1016/j.ijmedinf.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 34.Eklund K, Wilhelmson K. Outcomes of coordinated and integrated interventions targeting frail elderly people: a systematic review of randomised controlled trials. Health Soc Care Community 2009;17:447–58. 10.1111/j.1365-2524.2009.00844.x [DOI] [PubMed] [Google Scholar]
- 35.Falconer E, Kho D, Docherty JP. Use of technology for care coordination initiatives for patients with mental health issues: a systematic literature review. Neuropsychiatr Dis Treat 2018;14:2337–49. 10.2147/NDT.S172810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fathi R, Sheehan OC, Garrigues SK, et al. Development of an interdisciplinary team communication framework and quality metrics for home-based medical care practices. J Am Med Dir Assoc 2016;17:725–9. 10.1016/j.jamda.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 37.Fiorino G, Allocca M, Chaparro M, et al. “Quality of care” standards in inflammatory bowel disease: a systematic review. J Crohns Colitis 2019;13:127–37. 10.1093/ecco-jcc/jjy140 [DOI] [PubMed] [Google Scholar]
- 38.Flodgren G, Gonçalves-Bradley DC, Summerbell CD. Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in children and adults with overweight or obesity. Cochrane Database Syst Rev 2017;11:CD000984. 10.1002/14651858.CD000984.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaebel W, Kerst A, Janssen B, et al. Epa guidance on the quality of mental health services: a systematic meta-review and update of recommendations focusing on care coordination. Eur Psychiatr 2020;63:1–47. 10.1192/j.eurpsy.2020.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gall A, Leske S, Adams J, et al. Traditional and complementary medicine use among Indigenous cancer patients in Australia, Canada, New Zealand, and the United States: a systematic review. Integr Cancer Ther 2018;17:568–81. 10.1177/1534735418775821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardiner C, Gott M, Ingleton C. Factors supporting good partnership working between generalist and specialist palliative care services: a systematic review. Br J Gen Pract 2012;62:e353–62. 10.3399/bjgp12X641474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gervès-Pinquié C, Girault A, Phillips S, et al. Economic evaluation of patient navigation programs in colorectal cancer care, a systematic review. Health Econ Rev 2018;8:12. 10.1186/s13561-018-0196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorin SS, Haggstrom D, Han PKJ, et al. Cancer care coordination: a systematic review and meta-analysis of over 30 years of empirical studies. Ann Behav Med 2017;51:532–46. 10.1007/s12160-017-9876-2 [DOI] [PubMed] [Google Scholar]
- 44.Grant SJ, Frawley J, Bensoussan A. Process of care in outpatient integrative healthcare facilities: a systematic review of clinical trials. BMC Health Serv Res 2015;15:322. 10.1186/s12913-015-0976-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunn J, Diggens J, Hegarty K, et al. A systematic review of complex system interventions designed to increase recovery from depression in primary care. BMC Health Serv Res 2006;6:88. 10.1186/1472-6963-6-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halpern MT, Viswanathan M, Evans TS, et al. Models of cancer survivorship care: overview and summary of current evidence. JOP 2015;11:e19–27. 10.1200/JOP.2014.001403 [DOI] [PubMed] [Google Scholar]
- 47.Hamline MY, Speier RL, Vu PD, et al. Hospital-to-home interventions, use, and satisfaction: a meta-analysis. Pediatrics 2018;142:e20180442. 10.1542/peds.2018-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey EL, Glenny A, Kirk SF, et al. Improving health professionals’ management and the organisation of care for overweight and obese people. Cochrane Database Syst Rev 2001:CD000984. 10.1002/14651858.CD000984 [DOI] [PubMed] [Google Scholar]
- 49.Harvey EL, Glenny AM, Kirk SFL, et al. An updated systematic review of interventions to improve health professionals’ management of obesity. Obes Rev 2002;3:45–55. 10.1046/j.1467-789x.2002.00053.x [DOI] [PubMed] [Google Scholar]
- 50.Hawley G, Janamian T, Jackson C, et al. In a maternity shared-care environment, what do we know about the paper hand-held and electronic health record: a systematic literature review. BMC Pregnancy Childbirth 2014;14:52. 10.1186/1471-2393-14-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Høeg BL, Bidstrup PE, Karlsen RV, et al. Follow-up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database Syst Rev 2019;2019:CD012425. 10.1002/14651858.CD012425.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hohmann NS, McDaniel CC, Mason SW, et al. Patient perspectives on primary care and oncology care coordination in the context of multiple chronic conditions: a systematic review. Research in Social and Administrative Pharmacy 2020;16:1003–16. 10.1016/j.sapharm.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 53.Hollowell J, Oakley L, Kurinczuk JJ, et al. The effectiveness of antenatal care programmes to reduce infant mortality and preterm birth in socially disadvantaged and vulnerable women in high-income countries: a systematic review. BMC Pregnancy Childbirth 2011;11:13. 10.1186/1471-2393-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson F. Systematic review of oncology nurse practitioner navigation metrics. Clin J Oncol Nurs 2015;19:308–13. 10.1188/15.CJON.308-313 [DOI] [PubMed] [Google Scholar]
- 55.Jones A, Hannigan B, Coffey M, et al. Traditions of research in community mental health care planning and care coordination: a systematic meta-narrative review of the literature. PLoS ONE 2018;13:e0198427. 10.1371/journal.pone.0198427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joo JY, Huber DL. Community-based case management effectiveness in populations that abuse substances. Int Nurs Rev 2015;62:536–46. 10.1111/inr.12201 [DOI] [PubMed] [Google Scholar]
- 57.Joo JY, Liu MF. Case management effectiveness in reducing hospital use: a systematic review. Int Nurs Rev 2017;64:296–308. 10.1111/inr.12335 [DOI] [PubMed] [Google Scholar]
- 58.Joo JY, Liu MF. Case management effectiveness for managing chronic illnesses in korea: a systematic review. Int Nurs Rev 2019;66:30–42. 10.1111/inr.12472 [DOI] [PubMed] [Google Scholar]
- 59.Kastner M, Cardoso R, Lai Y, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: a systematic review and meta-analysis. CMAJ 2018;190:E1004–12. 10.1503/cmaj.171391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kastner M, Hayden L, Wong G, et al. Underlying mechanisms of complex interventions addressing the care of older adults with multimorbidity: a realist review. BMJ Open 2019;9:e025009. 10.1136/bmjopen-2018-025009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katz EB, Carrier ER, Umscheid CA, et al. Comparative effectiveness of care coordination interventions in the emergency department: a systematic review. Ann Emerg Med 2012;60:12–23. 10.1016/j.annemergmed.2012.02.025 [DOI] [PubMed] [Google Scholar]
- 62.Ke Y, Ng T, Chan A. Survivorship care models for breast cancer, colorectal cancer, and adolescent and young adult (AYA) cancer survivors: a systematic review. Support Care Cancer 2018;26:2125–41. 10.1007/s00520-018-4197-y [DOI] [PubMed] [Google Scholar]
- 63.Khan-Neelofur D, Gülmezoglu M, Villar J. Who should provide routine antenatal care for low-risk women, and how often? A systematic review of randomised controlled trials. who antenatal care trial Research Group. Paediatr Perinat Epidemiol 1998;12 Suppl 2:7–26. 10.1046/j.1365-3016.12.s2.6.x [DOI] [PubMed] [Google Scholar]
- 64.Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int J Geriatr Psychiatry 2013;28:551–61. 10.1002/gps.3864 [DOI] [PubMed] [Google Scholar]
- 65.Kooij L, Groen WG, van Harten WH. The effectiveness of information technology-supported shared care for patients with chronic disease: a systematic review. J Med Internet Res 2017;19:e221. 10.2196/jmir.7405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kroll-Desrosiers AR, Crawford SL, Moore Simas TA, et al. Improving pregnancy outcomes through maternity care coordination: a systematic review. Women’s Health Issues 2016;26:87–99. 10.1016/j.whi.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 67.Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PLoS One 2017;12:e0186315. 10.1371/journal.pone.0186315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langberg EM, Dyhr L, Davidsen AS. Development of the concept of patient-centredness - a systematic review. Patient Educ Couns 2019;102:1228–36. 10.1016/j.pec.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 69.Lawrence M, Kerr S, McVey C, et al. The effectiveness of secondary prevention lifestyle interventions designed to change lifestyle behavior following stroke: summary of a systematic review. Int J Stroke 2012;7:243–7. 10.1111/j.1747-4949.2012.00771.x [DOI] [PubMed] [Google Scholar]
- 70.Lawrence RA, McLoone JK, Wakefield CE, et al. Primary care physicians’ perspectives of their role in cancer care: a systematic review. J Gen Intern Med 2016;31:1222–36. 10.1007/s11606-016-3746-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee D-CA, Tirlea L, Haines TP. Non-pharmacological interventions to prevent hospital or nursing home admissions among community-dwelling older people with dementia: a systematic review and meta-analysis. Health Soc Care Community 2020;28:1408–29. 10.1111/hsc.12984 [DOI] [PubMed] [Google Scholar]
- 72.Lemoyne SE, Herbots HH, De Blick D, et al. Appropriateness of transferring nursing home residents to emergency departments: a systematic review. BMC Geriatr 2019;19:17.:17. 10.1186/s12877-019-1028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leniz J, Weil A, Higginson IJ, et al. Electronic palliative care coordination systems (epaccs): a systematic review. BMJ Support Palliat Care 2020;10:68–78. 10.1136/bmjspcare-2018-001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis ME, Myhra LL. Integrated care with indigenous populations: A systematic review of the literature. Am Indian Alsk Native Ment Health Res 2017;24:88–110. 10.5820/aian.2403.2017.88 [DOI] [PubMed] [Google Scholar]
- 75.Lion KC, Mangione-Smith R, Britto MT. Individualized plans of care to improve outcomes among children and adults with chronic illness: a systematic review. Care Manag J 2014;15:11–25. 10.1891/1521-0987.15.1.11 [DOI] [PubMed] [Google Scholar]
- 76.Low J, Pattenden J, Candy B, et al. Palliative care in advanced heart failure: an international review of the perspectives of recipients and health professionals on care provision. J Card Fail 2011;17:231–52. 10.1016/j.cardfail.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 77.Low JTS, Rohde G, Pittordou K, et al. Supportive and palliative care in people with cirrhosis: international systematic review of the perspective of patients, family members and health professionals. J Hepatol 2018;69:1260–73. 10.1016/j.jhep.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 78.Lynch S, Greeno C, Teich JL, et al. Pediatric integrated behavioral health service delivery models: using a federal framework to assess levels of integration. Social Work in Health Care 2019;58:32–59. 10.1080/00981389.2018.1531104 [DOI] [PubMed] [Google Scholar]
- 79.Macdonald D, Snelgrove-Clarke E, Campbell-Yeo M, et al. The experiences of midwives and nurses collaborating to provide birthing care: a systematic review. JBI Database System Rev Implement Rep 2015;13:74–127. 10.11124/jbisrir-2015-2444 [DOI] [PubMed] [Google Scholar]
- 80.Majka AJ, Wang Z, Schmitz KR, et al. Care coordination to enhance management of long-term enteral tube feeding: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014;38:40–52. 10.1177/0148607113482000 [DOI] [PubMed] [Google Scholar]
- 81.Manderson B, McMurray J, Piraino E, et al. Navigation roles support chronically ill older adults through healthcare transitions: a systematic review of the literature. Health Soc Care Community 2012;20:113–27. 10.1111/j.1365-2524.2011.01032.x [DOI] [PubMed] [Google Scholar]
- 82.Mapp F, Hutchinson J, Estcourt C. A systematic review of contemporary models of shared HIV care and HIV in primary care in high-income settings. Int J STD AIDS 2015;26:991–7. 10.1177/0956462415577496 [DOI] [PubMed] [Google Scholar]
- 83.Mazzarello S, McIsaac DI, Montroy J, et al. Postoperative shared-care for patients undergoing non-cardiac surgery: a systematic review and meta-analysis. Can J Anaesth 2019;66:1095–105. 10.1007/s12630-019-01433-5 [DOI] [PubMed] [Google Scholar]
- 84.Meiklejohn JA, Mimery A, Martin JH, et al. The role of the GP in follow-up cancer care: a systematic literature review. J Cancer Surviv 2016;10:990–1011. 10.1007/s11764-016-0545-4 [DOI] [PubMed] [Google Scholar]
- 85.Meiqari L, Al-Oudat T, Essink D, et al. How have researchers defined and used the concept of “continuity of care” for chronic conditions in the context of resource-constrained settings? a scoping review of existing literature and a proposed conceptual framework. Health Res Policy Syst 2019;17:27. 10.1186/s12961-019-0426-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell GK, Brown RM, Erikssen L, et al. Multidisciplinary care planning in the primary care management of completed stroke: a systematic review. BMC Fam Pract 2008;9:44. 10.1186/1471-2296-9-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mitchell GK, Burridge L, Zhang J, et al. Systematic review of integrated models of health care delivered at the primary-secondary interface: how effective is it and what determines effectiveness? Aust J Prim Health 2015;21:391–408. 10.1071/PY14172 [DOI] [PubMed] [Google Scholar]
- 88.Narasimha S, Madathil KC, Agnisarman S, et al. Designing telemedicine systems for geriatric patients: a review of the usability studies. Telemedicine and E-Health 2017;23:459–72. 10.1089/tmj.2016.0178 [DOI] [PubMed] [Google Scholar]
- 89.Nejati M, Razavi M, Harirchi I, et al. The impact of provider payment reforms and associated care delivery models on cost and quality in cancer care: A systematic literature review. PLoS One 2019;14:e0214382. 10.1371/journal.pone.0214382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng CH, Ong ZH, Koh JWH, et al. Enhancing interprofessional communications training in internal medicine. lessons drawn from a systematic scoping review from 2000 to 2018. J Contin Educ Health Prof 2020;40:27–35. 10.1097/CEH.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 91.Nguyen J, Smith L, Hunter J, et al. n.d. Conventional and complementary medicine health care practitioners’ perspectives on interprofessional communication: a qualitative rapid review. Medicina;55:650. 10.3390/medicina55100650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oishi A, Murtagh FEM. The challenges of uncertainty and interprofessional collaboration in palliative care for non-cancer patients in the community: a systematic review of views from patients, carers and health-care professionals. Palliat Med 2014;28:1081–98. 10.1177/0269216314531999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax 2009;64:749–56. 10.1136/thx.2008.109330 [DOI] [PubMed] [Google Scholar]
- 94.Overbeck G, Davidsen AS, Kousgaard MB. Enablers and barriers to implementing collaborative care for anxiety and depression: a systematic qualitative review. Implement Sci 2016;11:165. 10.1186/s13012-016-0519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parker VA, Lemak CH. Navigating patient navigation: crossing health services research and clinical boundaries. Adv Health Care Manag 2011;11:149–83. 10.1108/s1474-8231(2011)0000011010 [DOI] [PubMed] [Google Scholar]
- 96.Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact 2016;32:530–41. 10.1177/0890334415618668 [DOI] [PubMed] [Google Scholar]
- 97.Perfors IAA, May AM, Boeijen JA, et al. Involving the general practitioner during curative cancer treatment: a systematic review of health care interventions. BMJ Open 2019;9:e026383. 10.1136/bmjopen-2018-026383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peterson K, Anderson J, Bourne D, et al. Health care coordination theoretical frameworks: a systematic scoping review to increase their understanding and use in practice. J Gen Intern Med 2019;34:90–8. 10.1007/s11606-019-04966-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ponka D, Agbata E, Kendall C, et al. The effectiveness of case management interventions for the homeless, vulnerably housed and persons with lived experience: A systematic review. PLoS One 2020;15:e0230896. 10.1371/journal.pone.0230896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ranaghan C, Boyle K, Meehan M, et al. Effectiveness of a patient navigator on patient satisfaction in adult patients in an ambulatory care setting: a systematic review. JBI Database System Rev Implement Rep 2016;14:172–218. 10.11124/JBISRIR-2016-003049 [DOI] [PubMed] [Google Scholar]
- 101.Rogers K, Zeni MB. Systematic review of medical home models to promote transitions to primary adult health care for adolescents living with autism spectrum disorder. Worldviews Evid Based Nurs 2015;12:98–107. 10.1111/wvn.12085 [DOI] [PubMed] [Google Scholar]
- 102.Rouleau G, Gagnon M-P, Côté J, et al. Impact of information and communication technologies on nursing care: results of an overview of systematic reviews. J Med Internet Res 2017;19:e122. 10.2196/jmir.6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Runtu TM, Novieastari E, Handayani H. How does organizational culture influence care coordination in hospitals? A systematic review. Enfermería Clínica 2019;29:785–802. 10.1016/j.enfcli.2019.04.119 [DOI] [Google Scholar]
- 104.Russell C, Sandu V, Moroz I, et al. Key components of traditional consultation letters and their relevance to electronic consultation replies: a systematic review. Telemed J E Health 2020;26:689–99. 10.1089/tmj.2019.0161 [DOI] [PubMed] [Google Scholar]
- 105.Senior H, Grant M, Rhee JJ, et al. General practice physicians’ and nurses’ self-reported multidisciplinary end-of-life care: a systematic review. BMJ Support Palliat Care 2019:bmjspcare-2019-001852. 10.1136/bmjspcare-2019-001852 [DOI] [PubMed] [Google Scholar]
- 106.Shaw J, Sethi S, Vaccaro L, et al. Is care really shared? a systematic review of collaborative care (shared care) interventions for adult cancer patients with depression. BMC Health Serv Res 2019;19:120. 10.1186/s12913-019-3946-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singer S, Gianinazzi ME, Hohn A, et al. General practitioner involvement in follow-up of childhood cancer survivors: a systematic review. Pediatr Blood Cancer 2013;60:1565–73. 10.1002/pbc.24586 [DOI] [PubMed] [Google Scholar]
- 108.Smith SM, Allwright S, O’Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev 2007:CD004910. 10.1002/14651858.CD004910.pub2 [DOI] [PubMed] [Google Scholar]
- 109.Smith SM, Allwright S, O’Dowd T. Does sharing care across the primary-specialty interface improve outcomes in chronic disease? A systematic review. Am J Manag Care 2008;14:213–24. [PubMed] [Google Scholar]
- 110.Smith SM, Cousins G, Clyne B, et al. Shared care across the interface between primary and specialty care in management of long term conditions. Cochrane Database Syst Rev 2017;2017. 10.1002/14651858.CD004910.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snaterse M, Dobber J, Jepma P, et al. Effective components of nurse-coordinated care to prevent recurrent coronary events: a systematic review and meta-analysis. Heart 2016;102:50–6. 10.1136/heartjnl-2015-308050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stocker R, Close H. Assessing the uptake of the liverpool care pathway for dying patients: a systematic review. BMJ Support Palliat Care 2013;3:399–404. 10.1136/bmjspcare-2012-000406 [DOI] [PubMed] [Google Scholar]
- 113.Storm M, Husebø AML, Thomas EC, et al. Coordinating mental health services for people with serious mental illness: A scoping review of transitions from psychiatric hospital to community. Adm Policy Ment Health 2019;46:352–67. 10.1007/s10488-018-00918-7 [DOI] [PubMed] [Google Scholar]
- 114.Street TD, Somoray K, Richards GC, et al. Continuity of care for patients with chronic conditions from rural or remote australia: A systematic review. Aust J Rural Health 2019;27:196–202. 10.1111/ajr.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suva G, Sharma T, Campbell KE, et al. Strategies to support pressure injury best practices by the inter-professional team: A systematic review. Int Wound J 2018;15:580–9. 10.1111/iwj.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tho PC, Ang E. The effectiveness of patient navigation programs for adult cancer patients undergoing treatment: a systematic review. JBI Database System Rev Implement Rep 2016;14:295–321. 10.11124/jbisrir-2016-2324 [DOI] [PubMed] [Google Scholar]
- 117.Tomasone JR, Brouwers MC, Vukmirovic M, et al. Interventions to improve care coordination between primary healthcare and oncology care providers: a systematic review. ESMO Open 2016;1:e000077. 10.1136/esmoopen-2016-000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tradewell M, Pariser JJ, Nimeh T, et al. Systematic review and practice policy statements on urinary tract infection prevention in adults with spina bifida. Transl Androl Urol 2018;7(Suppl 2):S205–19. 10.21037/tau.2018.04.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trivedi D, Goodman C, Gage H, et al. The effectiveness of inter-professional working for older people living in the community: a systematic review. Health Soc Care Community 2013;21:113–28. 10.1111/j.1365-2524.2012.01067.x [DOI] [PubMed] [Google Scholar]
- 120.Valentijn PP, Pereira FA, Ruospo M, et al. Person-centered integrated care for chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2018;13:375–86. 10.2215/CJN.09960917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vogel EA, Ly K, Ramo DE, et al. Strategies to improve treatment utilization for substance use disorders: A systematic review of intervention studies. Drug Alcohol Depend 2020;212:108065. 10.1016/j.drugalcdep.2020.108065 [DOI] [PubMed] [Google Scholar]
- 122.Vyas KS, Hambrick HR, Shakir A, et al. A systematic review of the use of telemedicine in plastic and reconstructive surgery and dermatology. Ann Plast Surg 2017;78:736–68. 10.1097/SAP.0000000000001044 [DOI] [PubMed] [Google Scholar]
- 123.Wang V, Diamantidis CJ, Wylie J, et al. Minding the gap and overlap: a literature review of fragmentation of primary care for chronic dialysis patients. BMC Nephrol 2017;18:274.:274. 10.1186/s12882-017-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weaver SJ, Che XX, Petersen LA, et al. Unpacking care coordination through A multiteam system lens: A conceptual framework and systematic review. Med Care 2018;56:247–59. 10.1097/MLR.0000000000000874 [DOI] [PubMed] [Google Scholar]
- 125.Welbourn R, Hopkins J, Dixon JB, et al. Commissioning guidance for weight assessment and management in adults and children with severe complex obesity. Obes Rev 2018;19:14–27. 10.1111/obr.12601 [DOI] [PubMed] [Google Scholar]
- 126.Wiktorowicz M, Abdulle A, Di Pierdomenico K, et al. Models of concurrent disorder service: policy, coordination, and access to care. Front Psychiatry 2019;10:61.:61. 10.3389/fpsyt.2019.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong CK, O’Rielly CM, Teitge BD, et al. The characteristics and effectiveness of interventions for frequent emergency department utilizing patients with chronic noncancer pain: a systematic review. Acad Emerg Med 2020;27:742–52. 10.1111/acem.13934 [DOI] [PubMed] [Google Scholar]
- 128.Wong WCW, Luk CW, Kidd MR. Is there a role for primary care clinicians in providing shared care in HIV treatment? A systematic literature review. Sex Transm Infect 2012;88:125–31. 10.1136/sextrans-2011-050170 [DOI] [PubMed] [Google Scholar]
- 129.Wood E, Ohlsen S, Ricketts T. What are the barriers and facilitators to implementing collaborative care for depression? A systematic review. Journal of Affective Disorders 2017;214:26–43. 10.1016/j.jad.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 130.Ye F, Winchester D, Jansen M, et al. Assessing prognosis of acute coronary syndrome in recent clinical trials: a systematic review. Clin Med Res 2019;17:11–9. 10.3121/cmr.2019.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Y, Brettle A, Qiu L. The effectiveness of shared care in cancer survivors-a systematic review. Int J Integr Care 2018;18:2.:2. 10.5334/ijic.3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.U.S. Department of Veterans Affairs . Available: https://missionact.va.gov/ [Accessed 15 Jun 2021].
- 133.Raven MC, Guzman D, Chen AH, et al. Out-of-network emergency department use among managed Medicaid beneficiaries. Health Serv Res 2017;52:2156–74. 10.1111/1475-6773.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Council on Health Research for Development (COHRED) . Essential national health research and priority setting: lessons learned. 1997. Available: http://www.cohred.org/downloads/586.pdf [Accessed 24 Jul 2019].
- 135.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80. 10.1136/bmj.311.7001.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sarvet B, Gold J, Bostic JQ, et al. Improving access to mental health care for children: the massachusetts child psychiatry access project. Pediatrics 2010;126:1191–200. 10.1542/peds.2009-1340 [DOI] [PubMed] [Google Scholar]
- 137.Morita T, Miyashita M, Yamagishi A, et al. Effects of a programme of interventions on regional comprehensive palliative care for patients with cancer: a mixed-methods study. Lancet Oncol 2013;14:638–46. 10.1016/S1470-2045(13)70127-X [DOI] [PubMed] [Google Scholar]
- 138.Greene CA, Ford JD, Ward-Zimmerman B, et al. Strengthening the coordination of pediatric mental health and medical care: piloting a collaborative model for freestanding practices. Child Youth Care Forum 2016;45:729–44. 10.1007/s10566-016-9354-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borgermans L, Goderis G, Van Den Broeke C, et al. Interdisciplinary diabetes care teams operating on the interface between primary and specialty care are associated with improved outcomes of care: findings from the Leuven diabetes project. BMC Health Serv Res 2009;9. 10.1186/1472-6963-9-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fleury M-J, Perreault M, Grenier G, et al. Implementing key strategies for successful network integration in the quebec substance-use disorders programme. Int J Integr Care 2016;16:7.:7. 10.5334/ijic.2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Iddins BW, Frank JS, Kannar P, et al. Evaluation of team-based care in an urban free clinic setting. Nurs Adm Q 2015;39:254–62. 10.1097/NAQ.0000000000000103 [DOI] [PubMed] [Google Scholar]
- 142.Stewart B, Allan S, Keane B, et al. Palliative care partnership: a successful model of primary/secondary integration. N Z Med J 2006;119:U2235. [PubMed] [Google Scholar]
- 143.Bouis S, Reif S, Whetten K, et al. An integrated, multidimensional treatment model for individuals living with HIV, mental illness, and substance abuse. Health Soc Work 2007;32:268–78. 10.1093/hsw/32.4.268 [DOI] [PubMed] [Google Scholar]
- 144.Grace S, Higgs J. Interprofessional collaborations in integrative medicine. The Journal of Alternative and Complementary Medicine 2010;16:1185–90. 10.1089/acm.2009.0402 [DOI] [PubMed] [Google Scholar]
- 145.van Orden M, Hoffman T, Haffmans J, et al. Collaborative mental health care versus care as usual in a primary care setting: a randomized controlled trial. Psychiatr Serv 2009;60:74–9. 10.1176/ps.2009.60.1.74 [DOI] [PubMed] [Google Scholar]
- 146.Maar MA, Erskine B, McGregor L, et al. Innovations on a shoestring: a study of a collaborative community-based aboriginal mental health service model in rural canada. Int J Ment Health Syst 2009;3:27. 10.1186/1752-4458-3-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knowles SE, Chew-Graham C, Coupe N, et al. Better together? a naturalistic qualitative study of inter-professional working in collaborative care for co-morbid depression and physical health problems. Implement Sci 2013;8:110. 10.1186/1748-5908-8-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nel P, Pashen D. Shared antenatal care for indigenous patients in a rural and remote community. Aust Fam Physician 2003;32:127–31. [PubMed] [Google Scholar]
- 149.Menachemi N, Rahurkar S, Harle CA, et al. The benefits of health information exchange: an updated systematic review. J Am Med Inform Assoc 2018;25:1259–65. 10.1093/jamia/ocy035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060232supp001.pdf (1.7MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. The collected data are included in the online supplemental appendix. Data can be made available in different formats (eg, as Excel file) upon request.



