Abstract
Organising pneumonia after a mild COVID-19 infection has been increasingly reported and poses a diagnostic challenge to physicians especially in immunocompromised patients. We report a patient with a background of lymphoma in remission on rituximab who presented with prolonged and persistent fever after recovering from a mild COVID-19 infection. The initial workup showed bilateral lower zone lung consolidation; however, the infective and autoimmune workup were unremarkable. Subsequently, a bronchoscopy with transbronchial lung biopsy confirmed the diagnosis of organising pneumonia. A tapering glucocorticoid regimen was commenced with prompt resolution of the patient’s clinical symptoms, and subsequent resolution of biochemical markers and radiological lung changes 3 months later. This case highlights the importance of early recognition of the diagnosis of organising pneumonia in immunocompromised populations after a mild COVID-19 infection as it shows promising response to glucocorticoid therapy.
Keywords: COVID-19, Respiratory medicine
Background
Organising pneumonia is an established complication of severe COVID-19 infection with prevalence reported to be as high as 12.5%.1 It has also been reported in immunocompromised hosts as a late complication after a mild COVID-19 infection.2 However, little is known about the trigger, disease progression, treatment response and clinical outcome in this population. We report a case of an immunocompromised patient who presented with prolonged fever after a mild COVID-19 infection and was eventually found to have histologically confirmed organising pneumonia.
Case presentation
A man in his 50s was admitted to the hospital for persistent fever of 1 month associated with a mild cough with white sputum. He did not have any shortness of breath, chest pain, haemoptysis, night sweats, loss of weight and appetite or any other relevant contact or travel history. One week prior to the admission, he visited a general practitioner and completed a course of oral amoxicillin/clavulanic acid and clarithromycin, which did not alleviate his symptoms.
Of note, he had tested positive for COVID-19 infection 6 weeks ago via an antigen rapid test and developed a mild infection consisting of fever, dry cough, rhinorrhoea and sore throat. He did not receive any COVID-19 therapeutics and recovered quickly and uneventfully at home. He subsequently returned to his usual activities 1 week later when his antigen rapid test turned negative on day 7 of COVID-19 infection. He had previously been vaccinated with two doses of messenger RNA (mRNA) vaccines 7 months prior to the infection.
Other significant medical history included well-controlled type 2 diabetes mellitus, hypertension, hyperlipidaemia, hypothyroidism, previously treated pulmonary tuberculosis and diffuse large B cell lymphoma in remission, for which he had completed a chemotherapy regimen comprising of rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisolone and intrathecal methotrexate. He completed his treatment regimen 2 months prior to his COVID-19 infection. A recent positron emission tomography (PET)-CT scan done 3 months prior to the admission showed that the lymphoma was in remission.
On admission, he had a temperature of 39.9°C, oxygen saturation of 97% on room air and respiratory rate of 18. His oxygen saturation was above 95% on room air throughout the admission. There were crepitations auscultated over the right middle and lower lung zones. There were no palpable lymph nodes, and the rest of the physical examination was unremarkable.
Investigations
Initial blood investigations showed a high C reactive protein (CRP) of 149 mg/L, mildly raised procalcitonin level of 0.16 µg/L and normal white cell count of 5.0×109/L. Renal panel, liver function test and coagulation profile were normal. Chest X-ray showed patchy areas of consolidation in the bilateral lower zones (figure 1). A septic workup (blood, urine and sputum culture) and investigations for tuberculosis (sputum acid fast bacilli smear and culture, and Mycobacterium tuberculosis PCR) were all negative. Further microbiological tests for Legionella, pneumococcal and respiratory virus multiplex PCR all returned negative. Serum galactomannan was negative and autoimmune markers such as antinuclear antibodies, rheumatoid factor, cyclic citrullinated peptide antibody and myositis panel were unremarkable. Two sets of SARS-CoV-2 PCR done 3 days apart were positive with a high cycle threshold (CT) value of 30.9 and 36.0, respectively. Serological tests of his COVID-19 antibodies (antibody-N and antibody-S) were negative.
Figure 1.

Chest X-ray at presentation showed patchy areas of consolidation in bilateral lower zones.
Given the history of COVID-19 infection, the patient was deemed to be COVID-19 recovered with prolonged shedding of viral particles due to his immunocompromised status. He was hence treated for community-acquired pneumonia with broad-spectrum antibiotics of intravenous amoxicillin–clavulanic acid and oral clarithromycin. However, his high fever and cough persisted. CT scan of the thorax showed widespread ground-glass appearance in both lung fields, mainly in the lower zone and with a peripheral distribution (figure 2). The imaging findings were more consistent with an infection and did not suggest usual interstitial pneumonia or non‐specific interstitial pneumonia.
Figure 2.
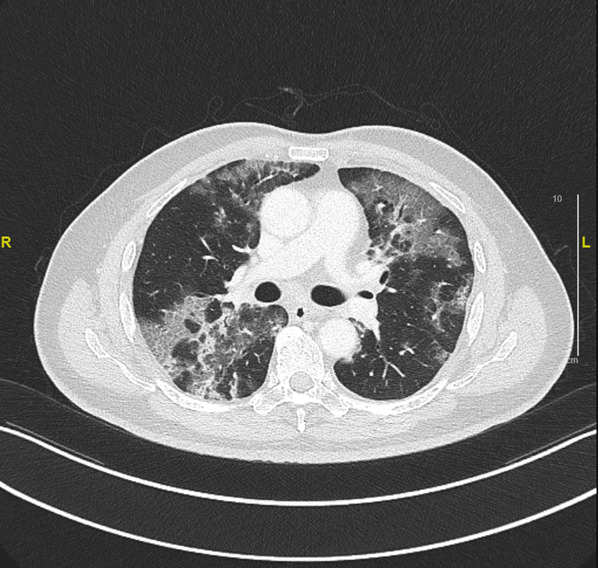
CT scan of the thorax showed widespread ground-glass appearance in both lung fields, mainly in the lower zone, and with a peripheral distribution.
In view of persistent fever, raised inflammatory markers, significant radiological changes and negative microbiological findings, a bronchoscopy with transbronchial lung biopsy of the right lobe was performed to exclude infection, lymphoma or interstitial lung disease. The bronchoalveolar lavage testing for tuberculosis, bacterial and fungal culture, respiratory virus multiplex and Aspergillus galactomannan were negative; the bronchoalveolar lavage fluid is predominantly neutrophilic and lymphocytic. Eosinophils were not elevated in the full blood count, nor were they observed in the bronchoalveolar lavage fluid or transbronchial biopsy. The tissue biopsy (figure 3) showed polypoid fibroblastic foci within alveoli as well as some foamy macrophages, consistent with a histological diagnosis of organising pneumonia. No abnormal lymphoid infiltrate, infective organisms, temporally heterogeneous areas of fibrosis, or diffuse and uniform fibrotic thickening of the alveolar septa were identified.
Figure 3.
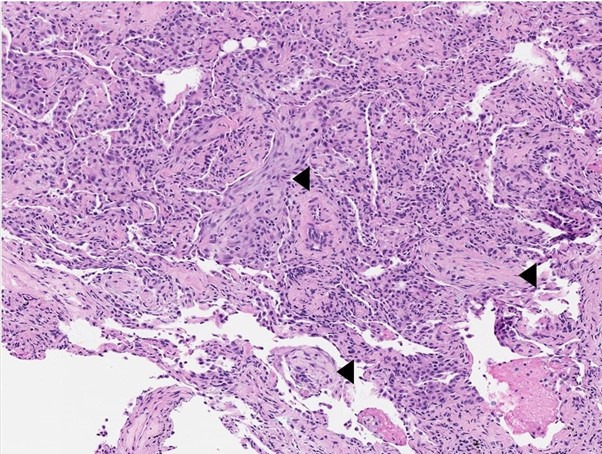
Histology image of lung tissue showing a few intra-alveolar polypoid fibroblastic foci (arrowheads) formed by spindled fibroblasts within pale-staining collagenous matrix (H&E stain, ×100).
Therefore, the overall clinical, imaging and histological findings were consistent with a clinical diagnosis of organising pneumonia.
Differential diagnosis
Subacute presentation of COVID-19 pneumonia
Patients with haematological malignancies, who recently received chemotherapy, are at an increased risk of death during hospitalisation for COVID-19 due to neutropenia.3 However, in our case study, the patient had completed chemotherapy for leukaemia and recent PET-CT scan indicated remission. The patient initially developed mild COVID-19 infection as confirmed by antigen rapid test and did not require oxygen supplementation throughout. He has recovered from COVID-19 infection and then developed fever 2 weeks later. A SARS-CoV-2 PCR showed high CT value, suggesting that he was recovering from the COVID-19 infection. The patient was also tested negative for antibodies against COVID-19 infection. Some case reports suggest that patients who have received anti-CD20 monoclonal antibody therapy may experience prolonged viral shedding and may not develop antibodies to SARS-CoV-2,4 5 which could explain normal COVID-19 antibody level in this patient. Although the CT scan indicates consolidation, the fact that no oxygen supplementation was required and considering the mild clinical features, the probability of a diagnosis of subacute COVID-19 pneumonia is less likely.
Cytotoxic-induced organising pneumonia
According to Pneumotox, it is known that cytotoxic agents, such as rituximab, cyclophosphamide, vincristine and methotrexate, can cause various lung injuries including organising pneumonia, interstitial lung disease and lymphoproliferative lung disease. Methotrexate may cause lung injury after several weeks or months of use, especially in people with leukaemia.6 Rituximab can cause infusion reactions within the first 30–120 min of the first exposure, and about 50% of patients may experience it. About 8% of patients receiving rituximab-containing chemotherapy may develop interstitial pneumonia (interstitial lung disease).7 The incidence of cyclophosphamide and vincristine causing organising pneumonia is not well established in the literature. Our patient had received a chemotherapy regimen consisting of rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisolone and methotrexate, which was completed 2 months before the current presentation.
COVID-19 vaccination causing organising pneumonia
There has been a reported case of a patient experiencing respiratory symptoms approximately 10 days after receiving mRNA COVID-19 vaccination. A transbronchial lung biopsy confirmed the diagnosis of organising pneumonia, which was attributed to the COVID-19 vaccination as no other triggering factors were identified.8 The Center for Disease Control and Prevention has cited the possibility of allergic reactions induced by the mRNA or other ingredients in the COVID-19 vaccine as a potential cause of pneumonitis. Our patient had received two doses of mRNA COVID-19 vaccine at least 7 months before the onset of respiratory symptoms. However, since there was a long interval between vaccination and the development of organising pneumonia, this makes COVID-19 vaccination a less likely cause.
Treatment
The patient was started on prednisolone 30 mg per day (0.5 mg/kg/day) for 1 week and the dose was reduced by 5 mg every week and maintained at 10 mg per day thereafter. His fever lysed within 48 hours after commencement of glucocorticoid therapy, with complete resolution of his upper respiratory tract symptoms.
Outcome and follow-up
He was followed up at 6 weeks after and reported complete resolution of symptoms. However, the CRP level is still elevated at 98.5 mg/L. The chest X-ray showed interval improvement but there were still residual ill-defined and irregular foci of airspace opacification. His prednisolone dosage was increased to 15 mg daily for 1 month followed by 12.5 mg daily.
Eight weeks later, he continued to be asymptomatic and the chest X-ray findings had improved completely (figure 4). Blood markers showed improvement as the CRP reduced to 16 mg/L. His prednisolone dose was tapered off further to 10 mg daily for 1 month then 5 mg daily until the subsequent review.
Figure 4.

Chest X-ray repeated 3 months after glucocorticoid therapy showed significant improvement.
Discussion
Limited but emerging evidence suggests that organising pneumonia may occur as a respiratory complication following COVID-19 infection, with reported prevalence as high as 12.5% in one study.1 A number of clinical trials have demonstrated that the severity of COVID-19 infection is a key factor in determining the development of organising pneumonia.9 Currently, evidence is lacking for the treatment of organising pneumonia following COVID-19 infection. However, corticosteroid is generally considered the standard treatment for organising pneumonia in non-COVID-19 infection and it has been shown to result in rapid and complete recovery in most cases.10 There is limited understanding of the variations in clinical manifestations, laboratory findings and medication dosages for organising pneumonia. Typically, corticosteroid use in immunocompromised individuals should be determined on a case-by-case basis, taking into account the assessment of the clinician. Caution should be exercised when administering corticosteroids to immunocompromised patients due to their heightened risk of infections and other complications. Since there are few studies reporting the outcomes of organising pneumonia following a mild COVID-19 infection, especially in immunocompromised patients, further research is needed to compare these two populations.
Persistent symptoms after COVID-19 infection such as fatigue, shortness of breath, chest tightness and cough lasting 2–12 months pose a diagnostic and treatment challenge especially in an immunocompromised host.11 Persistent fever, as with our clinical case, would rightly raise concerns for an infective aetiology. Although broad-spectrum antibiotic coverage may be an appropriate initial management strategy, it is important to have early consideration for an alternative non-infectious aetiology, such as organising pneumonia. Timely transbronchoscopic biopsy with a confirmed histopathological diagnosis of organising pneumonia will allow early administration of steroid therapy, which could be curative.
The timing of developing organising pneumonia after COVID-19 infection is not well established, but may be related to the severity of the initial COVID-19 infection. While one study showed that CT changes suggestive of organising pneumonia occur as early as 3 weeks after the onset of COVID-19 infection,12 in severe COVID-19 infection, the diagnosis of organising pneumonia is often reached via CT scan at time of the initial COVID-19 infection.13 Organising pneumonia following severe COVID-19 infection also presents primarily with hypoxaemia. A retrospective case–control study in Portugal found that the odds of developing organising pneumonia were 7.03 times higher if a patient still required supplemental oxygen on day 21 of illness.14 However, following a mild COVID-19 infection, limited cases (table 1), including this case report, suggest that organising pneumonia presents in a delayed fashion instead. Delayed organising pneumonia typically presents 4–8 weeks after recovery following initial mild COVID-19 infection, with new onset of fever, cough, breathlessness and chest pain.
Table 1.
Summary of case reports of organising pneumonia (OP) following mild COVID-19 infection
| Case | Gender | Presenting symptoms | Duration from initial COVID-19 infection | Prolonged viral shedding (demonstrated by positive SARS-CoV-2 PCR at time of diagnosing OP) | Risk factors for immunocompromised status | Treatment plan | Duration to radiological response |
| Golbets et al2 | Male | Fever | 40 days | Yes | Yes, regular rituximab therapy | IV methylprednisolone for 3 days, then oral prednisolone (dose and duration not disclosed) | Not available |
| Ng et al23 | Female | Exertional dyspnoea with hypoxaemia | 2 months | Yes | No | IV methylprednisolone 500 mg/day for 3 days, IV dexamethasone 6 mg/day for 10 days and oral prednisolone 0.5 mg/kg/day tapered over 3 months | 6 weeks |
| Ng et al23 | Male | Pleuritic chest pain and cough | 2 months | No | No | Oral prednisolone 0.5 mg/kg/day tapered over 5 months | 12 weeks |
| Present case | Male | Fever | 6 weeks | Yes | Yes, chemotherapy including rituximab | Oral prednisolone 0.5 mg/kg/day for 1 week, reduced by 5 mg every week and maintained at 10 mg per day thereafter | 14 weeks |
IV, intravenous.
As to the aetiology of organising pneumonia, insults such as viral infection, toxic agents and medications have been established to cause alveolar-epithelial injury leading to organising pneumonia.15 In immunocompromised hosts, it is believed that accelerated viral mutagenesis and changes within the spike protein contribute to prolonged viral shedding.16 While it is known that the median time from initial COVID-19 infection to SARS-CoV-2 PCR clearance is found to be longer in immunocompromised population (20–22 days) compared with immunocompetent population (16 days),17 its clinical significance is uncertain. We hypothesise that prolonged viral shedding phase may pose a risk of developing delayed organising pneumonia, especially in the immunocompromised population. Our patient was significantly immunocompromised, having recently received a dose of rituximab, which is known to deplete B cells and causes hypogammaglobulinaemia. Studies have also highlighted that patients on rituximab treatment tend to have prolonged viral shedding interval18 and poor antibody development against COVID-19 infection,19 as was also demonstrated in this case. At present, patients with chronic COVID-19 infection show a worse disease trajectory and higher mortality rate than the general population20; however, the pathogenesis and association between the immunocompromised status and risk of developing organising pneumonia require further study.
With regard to treatment for organising pneumonia, the decision on whether to initiate glucocorticoid treatment is based on a case-to-case basis. In our case, the patient had persistent symptoms; hence, glucocorticoid therapy was initiated. Eighty-seven per cent of the patients who develop organising pneumonia from miscellaneous causes showed response to glucocorticoid therapy,21 and the duration of treatment varies from 6 to 12 months.22 Our patient had significant clinical improvement of symptoms within a few days of glucocorticoid treatment; however, the radiological findings took 14 weeks for complete resolution. The timing for complete resolution of radiological findings of organising pneumonia was not clear; however, case reports, as shown in table 1, showed varying radiological responses from 6 weeks to 12 weeks.2 23
A multicentre and large case–control research, especially in the immunocompromised population, to study the risk factors of developing organising pneumonia following mild COVID-19 infection is warranted. Early consideration of transbronchoscopic biopsy to diagnose organising pneumonia in this population is recommended as the initial treatment (glucocorticoid therapy) is easily accessible and has promising response.
Learning points.
Consider COVID-19-related organising pneumonia as a possible non-infectious cause of fever.
Organising pneumonia following mild COVID-19 infection may present in a delayed manner.
Immunocompromised health status may be a risk factor for developing delayed organising pneumonia after mild COVID-19 infection.
Treatment of organising pneumonia possibly requires a prolonged course of glucocorticoids to achieve clinical, biochemical and radiological improvement.
Footnotes
Contributors: HWT discussed the case presentation, investigations and management of the patient with JWPT and CHYT. HWT wrote the case under continuous supervision of JWPT.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Vadász I, Husain-Syed F, Dorfmüller P, et al. Severe organising pneumonia following covid-19. Thorax 2021;76:201–4. 10.1136/thoraxjnl-2020-216088 [DOI] [PubMed] [Google Scholar]
- 2.Golbets E, Kaplan A, Shafat T, et al. Secondary organizing pneumonia after recovery of mild covid-19 infection. J Med Virol 2022;94:417–23. 10.1002/jmv.27360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21:1309–16. 10.1016/S1470-2045(20)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kos I, Balensiefer B, Roth S, et al. Prolonged course of COVID-19-associated pneumonia in a B-cell depleted patient after rituximab. Front Oncol 2020;10:1578. 10.3389/fonc.2020.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-cov-2 after immunosuppressive therapy for cancer. N Engl J Med 2020;383:2586–8. 10.1056/NEJMc2031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Imokawa S, Colby TV, Leslie KO, et al. Methotrexate pneumonitis: review of the literature and histopathological findings in nine patients. Eur Respir J 2000;15:373–81. 10.1034/j.1399-3003.2000.15b25.x [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Hong X-N, Gu Y-J, et al. Interstitial pneumonitis during rituximab-containing chemotherapy for non-Hodgkin lymphoma. Leuk Lymphoma 2008;49:1778–83. 10.1080/10428190802270886 [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa T, Tomomatsu K, Okazaki E, et al. COVID-19 vaccine-associated organizing pneumonia. Respirol Case Rep 2022;10:e0944. 10.1002/rcr2.944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazdyrev E, Panova M, Zherebtsova V, et al. The hidden pandemic of covid-19-induced organizing pneumonia. Pharmaceuticals (Basel) 2022;15:1574. 10.3390/ph15121574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radzikowska E, Wiatr E, Langfort R, et al. Cryptogenic organizing pneumonia-results of treatment with clarithromycin versus corticosteroids-observational study. PLoS ONE 2017;12:e0184739. 10.1371/journal.pone.0184739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Q, Xu M, Li J, et al. Clinical sequelae of covid-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021;27:89–95. 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieksiene K, Zaveckiene J, Malakauskas K, et al. Post covid-19 organizing pneumonia: the right time to interfere. Medicina (Kaunas) 2021;57:283. 10.3390/medicina57030283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocha AS, Meireles M, Vilaça H, et al. Outcomes of covid-19 organizing pneumonia in critically ill patients. J Infect 2021;83:496–522. 10.1016/j.jinf.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinde J, Teixeira T, Figueiredo C, et al. Secondary organising pneumonia among COVID-19 patients: a retrospective case-control study. Cureus 2022;14:e26230. 10.7759/cureus.26230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra D, Maini R, Pneumonia DM, et al. StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. [Google Scholar]
- 16.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of sars-cov-2 in an immunocompromised host. N Engl J Med 2020;383:2291–3. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein RL, Sperring H, Hofman M, et al. Time to sars-cov-2 PCR clearance in immunocompromising conditions: is test-based removal from isolation necessary in severely immunocompromised individuals? Open Forum Infect Dis 2021;8:ofab164. 10.1093/ofid/ofab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton CS, Huntley K, Berenger BM, et al. Prolonged sars-cov-2 infection following rituximab treatment: clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control 2022;11:28. 10.1186/s13756-022-01067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levavi H, Lancman G, Gabrilove J. Impact of rituximab on covid-19 outcomes. Ann Hematol 2021;100:2805–12. 10.1007/s00277-021-04662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbasi J. Researchers tie severe immunosuppression to chronic covid-19 and virus variants. JAMA 2021;325:2033–5. 10.1001/jama.2021.7212 [DOI] [PubMed] [Google Scholar]
- 3.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British thoracic Society in collaboration with the thoracic Society of Australia and New Zealand and the Irish thoracic Society. Thorax 2008;63 Suppl 5:v1–58. 10.1136/thx.2008.101691 [DOI] [PubMed] [Google Scholar]
- 1.Cordier JF. Organising pneumonia. Thorax 2000;55:318–28. 10.1136/thorax.55.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng BH, Ban AY-L, Nik Abeed NN, et al. Organising pneumonia manifesting as a late-phase complication of covid-19. BMJ Case Rep 2021;14:e246119. 10.1136/bcr-2021-246119 [DOI] [PMC free article] [PubMed] [Google Scholar]


