Abstract
BACKGROUND
Age is an important prognostic factor in breast cancer. The target age to screen is under debate.
OBJECTIVE
This study aimed to assess the influence of age on the diagnosis and survival among women with breast cancer.
STUDY DESIGN
This was a retrospective cohort study of the Population-Based Cancer Registry of Campinas, Brazil, and included all women diagnosed from 2010 to 2014. The outcomes assessed were overall survival and stage. For statistical analyses, the Kaplan-Meier method, log-rank tests, and chi-square tests were used.
RESULTS
The sample comprised 1741 women aged 40 to 79 years. Diagnoses at stages 0 to II were the more frequent. In the 40 to 49 years and 50 to 59 years age groups, the frequency of stage 0 (in situ) was 20.5% and 14.9% (P=.022), respectively, and the frequency of stage I was 20.2% and 25.8% (P=.042), respectively. The mean overall survival was 8.9 years (8.6–9.2) in the 40 to 49 years age group and 7.7 years (7.3–8.1) in the 70 to 79 years age group. The 5-year overall survival was higher in the 40 to 49 years age group than in the 50 to 59 years age group for stage 0 (in situ) (100.0% vs 95.0%; P=.036) and stage III (77.4% vs 66.2%; P=.046) diagnoses. The 5-year overall survival was higher in 60 to 69 years age group than in the 70 to 79 years age group for stages I (94.6% vs 86.5%; P=.002) and III (83.5% vs 64.9%; P=.010). In all age groups, significant differences in survival were not observed for stage 0 (in situ) vs stage I diagnoses, stage 0 vs stage II diagnoses, and stage I vs stage II diagnoses.
CONCLUSION
Women aged 40 to 49 years had the highest proportion of in situ tumors, and stages III and IV accounted for about one-third of the cases in all age groups. There was no difference in the overall survival for stage 0 (in situ) vs stage I or II diagnoses in all age groups.
Key words: breast neoplasms, early detection of cancer, neoplasm staging, survival
AJOG Global Reports at a Glance.
Why was this study conducted?
The target age at which women should be screened for breast cancer is under debate. We aimed to assess the influence of age on diagnosis and survival among women with breast cancer.
Key findings
Women aged 40 to 49 years showed a higher frequency of stage 0 (in situ) cancers (20.5%).
The mean overall survival decreased with age. The 5-year overall survival was higher among those aged 40 to 49 years than among those aged 50 to 59 years for stage 0 and III diagnoses and higher among those aged 60 to 69 years than among those aged 70 to 79 years for stage I and III diagnoses.
Significant differences in survival were not observed when comparing stage 0 and I diagnoses, stage 0 and II diagnoses, and stage I and II diagnoses in the different age groups.
What does this add to what is known?
In situ and stage I cancers had the same survival as stage II cancers in all age groups, suggesting limiting screening benefits to women out of the target age.
Introduction
Breast cancer mortality rates have decreased worldwide because of screening programs and improvements in adjuvant therapies.1 Most cases are diagnosed through mammography as part of screening programs.2 Screening may detect tumors in the early stages, offering a better option for treatment and survival.3 Women who participate in a mammography screening program have a 60% risk reduction in breast cancer mortality.1 The discussion of the target age at which women should be screened is up for debate.
Women older than 50 years constitute the majority of breast cancer cases. In the United States, approximately 82% of new cases and 90% of deaths related to the disease can be attributed to this group.4 In Brazil, the median age at diagnosis is between 50 and 59 years.5 Age is an important prognostic factor, and survival decreases in older women. Over a period of 5 years, the estimated survival is 92% for women aged 45 to 55 years, 89% for those aged 55 to 65 years, 87% for those age 65 to 75 years, and 58% for those who are older than 75 years.6 However, it is important to note that there are differences in prognosis based on the frequency of biologic types by age and stage.7
The incidence of breast cancer is increasing in all age groups, including among premenopausal women aged 40 to 49 years.8 In this group, the prognosis may vary based on the setting in which the women find themselves. It is well known that tumors in young women have a more aggressive behavior because of a higher frequency of triple-negative tumors and human epidermal growth factor receptor 2 (HER2) overexpression and higher recurrence rates. However, in opportunistic screening settings, many women younger than 50 years of age practice regular screening, leading to increased detection of tumors at very early stages,5 thereby probably anticipating diagnosis and inducing lead-time bias in survival analysis.
Among older women, there is a concern for overdiagnosis. In this group, tumors are expected to be more indolent.9 A diagnosis may represent a tumor that would never cause harm or be clinically detectable in a woman's lifetime. These women may be exposed to unnecessary treatments that induce morbidities. In this sense, an indication for screening among women older than 70 years is controversial.9, 10, 11
The differences observed in breast cancer survival when adjusted by stage may reflect the impact of screening and early detection practices in different age groups. This study aimed to assess the influence of age on the diagnosis and survival in women with breast cancer in a setting where opportunistic screening is mainly available.
Material and Methods
This study was a retrospective cohort of women diagnosed with breast cancer and used secondary data from the Population-Based Cancer Registry (PBCR) and the Mortality Information System (MIS) of Campinas City, São Paulo state, Brazil. Women were diagnosed from 2010 to 2014. The PBCR covers all breast cancer cases of women living in the city regardless of the type of healthcare provider (public or private). After combining the primary data from the PBCR and MIS, a manual search was conducted of the physical and digital medical records of the main city's facilities to find missing information on the variables selected. In the study period, 2715 cases were registered in the PBCR. This study included only women aged 40 to 79 years. Cases diagnosed through death certificates (DC) and those for which the stage at diagnosis could not be identified were excluded.
The variables that were analyzed were age and stage at diagnosis, date of death, and date of censorship or last follow-up. Stage was registered according to the American Joint Committee on Cancer.12 The study's end for censorship was March 31, 2020, which was the first month of the isolation period in Brazil because of the COVID-19 pandemic. The MIS in Campinas is very accurate and gets updated periodically with the MIS of surrounding cities, a well-established linkage in the region. It is improbable that the MIS would not identify a death of a woman.
The overall survival (OS) at 2 and 5 years was calculated for the time between the date of diagnosis (histopathologic result) and the date of death or censorship using the Kaplan-Meier method and was expressed as a percentage. Survival curves were compared using the log-rank test. Pairwise comparisons were made to see if there was a difference in the survival by stage within each age group. Stage categories were compared using chi-square tests. The Statistical Analysis System (SAS) program for Windows (version 9.2) was used for statistical analysis (SAS Institute Inc, Cary, NC).
This study was approved by the Ethics and Research Committee of UNICAMP, University of Campinas, under the number CAAE 89399018. 2.0000.5404. The committee waived the need for informed consent because of the study's retrospective nature. Confidentiality was guaranteed. Personal data were treated only by teams from the Department of Population Registration of Cancer and Mortality Surveillance of Campinas as part of their routine. The first author used an active search in medical records from the city.
Results
In the study period, 2715 breast cancer cases were registered in the PBCR. The stage was determined in 2054 cases. Of them, 1741 cases were from women aged 40 to 79. Diagnoses of stages 0 (in situ), I, or II were more frequent in all age groups than stage III and IV, with stage III and IV diagnoses comprising 35.1%, 34.9%, 31.5%, and 30.7% of cases in the age groups 40 to 49 years, 50 to 59 years, 60 to 69 years, and 70 to 79 years, respectively.
The description and comparison of case distribution by age groups and stages are shown in Table 1. Comparing the age group of 40 to 49 years with 50 to 59 years, the proportion of stage 0 (in situ) diagnoses was higher among the younger women (20.5% vs 14.9%; P=.022). For stage I diagnoses, there was a higher rate among the older group of women (20.2% vs 25.8%; P=.042). For other stages and age groups, no difference was observed.
Table 1.
Comparison of breast cancer stage at diagnosis among women at the extremes of age and women from the target group for screening
| Stage |
40–49 y |
50–59 y |
P value |
60–69 y |
70–79 y |
P value |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| 0 (in situ) | 88 | 20.5 | 82 | 14.9 | .022 | 72 | 15.5 | 45 | 15.2 | .907 |
| I | 87 | 20.2 | 142 | 25.8 | .042 | 131 | 28.2 | 98 | 33.1 | .153 |
| II | 104 | 24.2 | 135 | 24.5 | .909 | 115 | 24.8 | 62 | 21.0 | .222 |
| III | 68 | 15.8 | 86 | 15.6 | .930 | 57 | 12.3 | 37 | 12.5 | .930 |
| IV | 83 | 19.3 | 106 | 19.2 | .980 | 89 | 19.2 | 54 | 18.2 | .747 |
| All | 430 | 100.0 | 551 | 100.0 | .110 | 464 | 100.0 | 296 | 100.0 | .614 |
P values were determined using chi-square tests.
Fernandes. Breast cancer survival by age and stage. Am J Obstet Gynecol Glob Rep 2023.
The mean OS was 8.9 years (95% confidence interval [CI], 8.6–9.2) among women aged 40 to 49 years, 8.6 years (95% CI, 8.4–8.9) among those aged 50 to 59 years, 8.6 years (95% CI, 8.3–8.9) among those aged 60 to 69 years, and 7.7 years (95% CI, 7.3–8.1) among those aged 70 to 79 years. Table 2 presents the 2-year OS and 5-year OS for the age groups 40 to 49 years and 50 to 59 years. A higher survival was observed among younger women with stage 0 (in situ) and III diagnoses. The pairwise comparisons between the age groups showed a significant difference in survival between early breast cancer (stages 0 and I and II) and stages III and IV (P<.001), but not among stages 0 or I or II.
Table 2.
Comparison of breast cancer survival by stage at diagnosis among women aged 40 to 49 years and women in the target group for screening aged 50 to 59 years
| Stage | 40–49 y | 50–59 y | P valuea | ||
|---|---|---|---|---|---|
| 2-y OS | 5-y OS | 2-y OS | 5-y OS | ||
| 0 (in situ)b | 100.0 | 100.0 | 98.8 | 95.0 | .036c |
| Id | 98.9 | 96.6 | 99.3 | 94.3 | .854 |
| IIe | 95.1 | 93.1 | 98.5 | 94.0 | .991 |
| III | 91.0 | 77.4 | 80.2 | 66.2 | .046c |
| IV | 77.1 | 56.6 | 69.7 | 55.3 | .652 |
| All | 94.0 | 87.7 | 90.7 | 83.7 | |
OS, overall survival.
Log-rank tests were used to determine P values
A significant pairwise comparison among women aged 40–49 years was obtained when stage 0 was compared with stage III (P<.001) and stage IV (P<.001). A significant pairwise comparison among women aged 50–59 years was obtained when stage 0 was compared with stage III (P<.001) and stage IV (P<.001)
XXX
A significant pairwise comparison among women aged 40–49 years was obtained when stage I was compared with stage III (P<.001) and stage IV (P<.001). A significant pairwise comparison among women aged 50–59 years was obtained when stage I was compared with stage III (P<.001) and stage IV (P<.001)
A significant pairwise comparison among women aged 40–49 years was obtained when stage II was compared with stage III (P<.001) and stage IV (P<.001). A significant pairwise comparison among women aged 50–59 years was obtained when stage II was compared with stage III (P<.001) and stage IV (P<.001).
Fernandes. Breast cancer survival by age and stage. Am J Obstet Gynecol Glob Rep 2023.
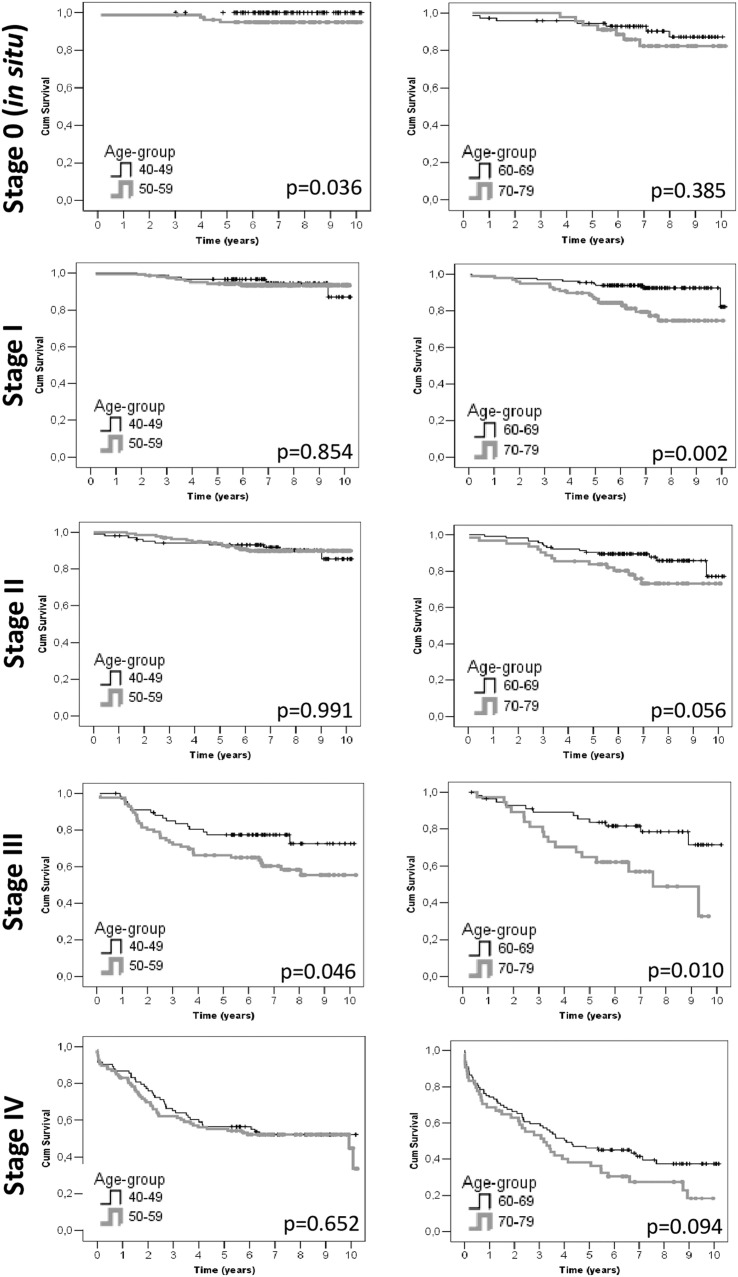
Table 3 presents the 2-year and 5-year OS for the age groups 60 to 69 years and 70 to 79 years. A higher survival was observed among younger women diagnosed at stages I and III. The pairwise comparisons in 60- to 69-year-olds showed a significant difference in survival for all stages when compared with stage IV (P<.001) and when stage I was compared with stage III (P=.005). Among those aged 70 to 79 years, a significant difference in survival was observed when all stages were compared with stage IV (P<.001) and when stages 0 and I were compared with stage III (P<.01). Kaplan-Meyer curves for survival between the groups can be seen in the Figure.
Table 3.
Comparison of breast cancer survival by stage at diagnosis among women aged 70 to 79 years and women in the target group for screening aged 60 to 69 years
| Stage | 60–69 y | 70–79 y | P valuea | ||
|---|---|---|---|---|---|
| 2-y OS | 5-y OS | 2-y OS | 5-y OS | ||
| 0 (in situ)b | 95.8 | 94.3 | 100.0 | 93.3 | .385 |
| Ic | 97.7 | 94.6 | 95.9 | 86.5 | .002d |
| IIe | 98.3 | 90.4 | 95.2 | 83.8 | .056 |
| IIIf | 92.8 | 83.5 | 89.2 | 64.9 | .010d |
| IV | 66.3 | 46.1 | 62.9 | 38.1 | .094 |
| All | 91.5 | 83.8 | 87.6 | 75.5 | |
OS, overall survival.
Log-rank tests were used to determine P values
A significant pairwise comparison among women aged 60–69 years was obtained when stage 0 was compared with stage IV (P<.001). A significant pairwise comparison among women aged 70–79 years was obtained when stage 0 was compared with stage III (P=.001) and stage IV (P<.001)
A significant pairwise comparison among women aged 60–69 years was obtained when stage I was compared with stage III (P=.005) and stage IV (P<.001). A significant pairwise comparison among women aged 70–79 years was obtained when stage I was compared with stage III (P=.002) and stage IV (P<.001)
XXX
A significant pairwise comparison among women aged 60–69 years was obtained when stage II was compared with stage IV (P<.001). A significant pairwise comparison among women aged 70–79 years was obtained when stage II was compared with stage III (P=.017) and stage IV (P<.001)
A significant pairwise comparison among women aged 60–69 years was obtained when stage III was compared with stage IV (P<.001). A significant pairwise comparison among women aged 70–79 years was obtained when stage III was compared with stage IV (P=.003).
Fernandes. Breast cancer survival by age and stage. Am J Obstet Gynecol Glob Rep 2023.
Figure.
Survival curves by age groups and breast cancer stage
The data are presented for cases recorded in Campinas, Brazil, between 2010 and 2014. Kaplan-Meyer curves are presented. P values were obtained using log-rank tests.
Fernandes. Breast cancer survival by age and stage. Am J Obstet Gynecol Glob Rep 2023.
Discussion
In this population-based study that evaluated the diagnosis and survival of Brazilian women with breast cancer, the mean OS decreased with advancing age and stage. Women aged 40 to 49 years had the highest proportion of in situ tumors (20.5%), and stages III and IV accounted for about one-third of the cases in all age groups. The survival of women aged 40 to 49 years with a stage 0 (in situ) diagnosis was significantly higher than that of women aged 50 to 59 years. It was also significantly higher among women aged 60 to 69 years with a stage I diagnosis than among those aged 70 to 79 years.
This study showed that most women older than 50 years are diagnosed at stage I in the study region, which is a populated urban area. A recent study from Brazil found a nationwide prevalence of stage 0 (in situ) or stage I breast cancer of 31% among women aged 50 to 70 years and a prevalence of 36% in the state of São Paulo.12 In our study based in Campinas, a municipality of the state of São Paulo, tumors staged 0 (in situ) or I accounted for 40% of women aged 50 to 59 years and 44% of women aged 60 to 69 years. There are 2 possible explanations for this higher prevalence. First, is the origin of the data. The nationwide survey collected data from the Hospital-based Cancer Registry from which most of the public health system data were derived. Our data came from the PBCR, which merged data from the public and private services. In Campinas, half of the population of women use private care, thereby improving access to diagnostic services.13 The second explanation is also related to access to care. Campinas city has a Human Development Index that is higher than the nationwide index and that of the state of São Paulo, implying a more structured health system.14 It is well described how access to care is related to early cancer diagnosis.15
Early tumors are usually diagnosed through mammographic screening, reinforcing the importance of screening in early detection. In the United States, from 1976 to 2008, mammographic screening increased early-stage breast cancer detection from 112 to 234 per 100,000 women and reduced detection at later stages by 8%.2 The detection of tumors smaller than 2 cm and in situ carcinomas increased from 36% to 68% after the dissemination of mammographic screening. In comparison, detection of tumors larger than 2 cm decreased from 64% to 32% from 1975 to 2012 mainly because of early diagnosis.16 Low- and middle-income countries do not have organized screening programs or no programs at all, and diagnosis is usually made when tumors are advanced and symptomatic.17 In situ tumors comprise only 1% of the cases India and Pakistan and 7% of the cases in Iran. In these same countries, the percentage of cases with stage I tumors is 5%, 10%, and 14%, respectively.18, 19, 20 In Brazil, the mammographic screening program led to an increase in the diagnosis of early-stage tumors from 14.5% to 43.2%, and in recent years, a trend of downstaging could be observed.5,21
Although most cases in this study were early tumors, approximately one-third were advanced neoplasms (stages III and IV) in all age groups. It may indicate difficulty in accessing health services for cases with symptomatic tumors. At the population level, care should be integrated and mammography should be offered systematically. Caring for symptomatic patients is a more complex health action at the individual level. Healthcare providers should be aware of admitting these women, and the referral structure must be organized. Fragile health systems’ healthcare usually fail in this process. To give adequate care for symptomatic women and to reduce the detection rate at more advanced stages, the diagnosis of symptomatic breast cancer must be agile and effective.
In situ tumors were more frequent among those in the 40 to 49 year age group than among those in the 50 to 59 year age group. If the natural history of breast cancer were well defined, this could indicate screening protection. However, such linearity is not well defined for breast cancer. Therefore, our results may indicate overdiagnosis among women aged 40 to 49 years because the proportion of in situ tumors was similar in other age groups. A Canadian study showed that mammographic screening of women aged 40 to 49 years did not promote breast cancer-specific mortality reduction in this group and estimated a 22% rate of overdiagnosis.22 Another result that restricts mammographic benefits is that there was no significant difference in the survival between those with stage I and those with stage II tumors in all age groups. Stage I is usually diagnosed by mammography, and stage II includes more women diagnosed with symptoms. In this region where opportunistic screening is widespread, screen-detected cancer probably has the same survival as early-stage cancer that was detected following symptomatic presentation.
In Brazil, mammographic screening is performed until 69 years of age.11 One argument for not including women older than 69 years in screening is the overdiagnosis of indolent tumors in this group.9,11 This study did not detect a significant difference in the staging of women aged 60 to 69 years and 70 to 79 years, which may not support this argument. This study showed that women aged 70 to 79 years with stage I breast tumors have a worse prognosis than those aged 60 to 69 years (86.5% and 94.6% in 5 years, respectively; P=.002), and those with stage III breast cancer (64.9% and 83.5% in 5 years; P=.010). This effect can be explained by aging, reducing OS, and limited therapeutics. However, the better prognosis in early stages observed among older women is an argument to include them in mammographic screening.
This study has a main limitation, namely stage was unknown in 25% of the sample, which may slightly influence the results. This is a problem inherent to population-based studies. The database did not have information about the tumor immunohistochemical profile and histologic grade, which are well-known and important prognostic factors. That information would have helped to improve the discussion. The main strength of this study lies in the number of patients and follow-up reliability and the high quality and consistency of data that the active search for vital status provided. Despite their limitations, we believe the data presented are relevant because of the lack of population-based studies in low- and middle-income countries.
Conclusion
In this population-based study in a big Brazilian metropole, the survival rate of women aged 40 to 49 years with stage 0 (in situ) or III breast cancer diagnoses was higher than the survival rate of those aged 50 to 59 years. Women aged 40 to 49 years had the highest proportion of in situ tumors, and stages III and IV diagnoses accounted for about one-third of the cases in all age groups. There was no difference in the survival when patients with stage 0 (in situ) breast cancer were compared with those with stage I or when those with stage II were compared with all age groups.
Acknowledgments
The authors would like to thank the Department of Population Registration of Cancer and Mortality Surveillance of the Campinas team.
This study was performed during the tenure of the research funding of the “Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP),” registered under the number 2017/21908-1. The study sponsors had no involvement in the study other than funding.
Footnotes
The authors report no conflict of interest.
Patient consent was not required because no personal information or details were included.
Cite this article as: Fernandes JO, Cardoso-Filho C, Kraft MB, et al. Differences in breast cancer survival and stage by age in off-target screening groups: a population-based retrospective study. Am J Obstet Gynecol Glob Rep 2023;XX:x.ex–x.ex.
References
- 1.Tabár L, Dean PB, Chen TH-H, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer. 2019;125:515–523. doi: 10.1002/cncr.31840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 3.Shen Y, Yang Y, Inoue LYT, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97:1195–1203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 5.Vale DB, Filho CC, Shinzato JY, Spreafico FS, Basu P, Zeferino LC. Downstaging in opportunistic breast cancer screening in Brazil: a temporal trend analysis. BMC Cancer. 2019;19:432. doi: 10.1186/s12885-019-5647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank S, Carton M, Dubot C, et al. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast. 2020;52:50–57. doi: 10.1016/j.breast.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rueda OM, Sammut SJ, Seoane JA, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567:399–404. doi: 10.1038/s41586-019-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Agency for Research on Can- cer. Breast cancer screening, IARC handbooks of cancer prevention. 1st ed. IARC: 2016.
- 9.Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311:1336–1347. doi: 10.1001/jama.2014.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-cancer screening - viewpoint of the IARC working group. N Engl J Med. 2015;372:2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 11.Migowski A, Silva GAE, Dias MBK, Diz MDPE, Sant'Ana DR, Nadanovsky P. Guidelines for early detection of breast cancer in Brazil. II - new national recommendations, main evidence, and controversies. Cad Saude Publica. 2018;34 doi: 10.1590/0102-311X00074817. [DOI] [PubMed] [Google Scholar]
- 12.Cuoghi IC, da Silva Soares MF, Dos Santos GMC, et al. 10-year opportunistic mammographic screening scenario in Brazil and its impact on breast cancer early detection: a nationwide population-based study. J Glob Health. 2022;12:04061. doi: 10.7189/jogh.12.04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brasil. Agência nacional de saúde suplementar. ANS Tabnet – informações em saúde suplementar: Beneficiários. 2023. Available at: http://www.ans.gov.br/anstabnet/cgi-bin/dh?dados/tabnet_br.def. Accessed June 12, 2015.
- 14.PNUD Brasil. Por um desenvolvimento humano, inclusivo e sustentave. 2023. Available at: https://www.br.undp.org/. Accessed November 19, 2020.
- 15.Gonzaga CMR, Freitas-Junior R, Curado MP, Sousa A-LL, Souza-Neto JA, Souza MR. Temporal trends in female breast cancer mortality in Brazil and correlations with social inequalities: ecological time-series study. BMC Public Health. 2015;15:96. doi: 10.1186/s12889-015-1445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welch HG, Prorok PC, O'Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–1447. doi: 10.1056/NEJMoa1600249. [DOI] [PubMed] [Google Scholar]
- 17.Vieira RADC, Formenton A, Bertolini SR. Breast cancer screening in Brazil. Barriers related to the health system. Rev Assoc Med Bras (1992) 2017;63:466–474. doi: 10.1590/1806-9282.63.05.466. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PSY. Spectrum of breast cancer in Asian women. World J Surg. 2007;31:1031–1040. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 19.Khokher S, Qureshi MU, Riaz M, Akhtar N, Saleem A. Clinicopathologic profile of breast cancer patients in Pakistan: ten years data of a local cancer hospital. Asian Pac J Cancer Prev. 2012;13:693–698. doi: 10.7314/apjcp.2012.13.2.693. [DOI] [PubMed] [Google Scholar]
- 20.Afsharfard A, Mozaffar M, Orang E, Tahmasbpour E. Trends in epidemiology, clinical and histopathological characteristics of breast cancer in Iran: results of a 17 year study. Asian Pac J Cancer Prev. 2013;14:6905–6911. doi: 10.7314/apjcp.2013.14.11.6905. [DOI] [PubMed] [Google Scholar]
- 21.Vieira RAda C, Lourenço TS, Mauad EC, et al. Barriers related to non-adherence in a mammography breast-screening program during the implementation period in the interior of São Paulo State, Brazil. J Epidemiol Glob Health. 2015;5:211–219. doi: 10.1016/j.jegh.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]