Abstract
The pine processionary moth Thaumetopoea pityocampa is a Lepidopteran pest species occurring in the Western Mediterranean. It causes heavy pine defoliations and it is a public and animal health concern because of its urticating caterpillars. Very little is known about the viruses associated to this species, as only two viruses were described so far. We here present a dataset corresponding to 34 viral transcripts, among which 27 could be confidently assigned to 9 RNA and DNA viral families (Iflaviridae, Reoviridae, Partitiviridae, Permutotetraviridae, Flaviviridae, Rhabdoviridae, Parvoviridae, Baculoviridae and PolyDNAviridae). These transcripts were identified from an original transcriptome assembled for the insect host, using both blast search and phylogenetic approaches. The data were acquired from 2 populations in Portugal and 2 populations in Italy. The transcripts were de novo assembled and used to identify viral sequences by homology searches. We also provide information about the populations and life stages in which each virus was identified. The data produced will allow to enrich the virus taxonomy in Lepidopteran hosts, and to develop PCR-based diagnostic tools to screen colonies across the range and determine the distribution and prevalence of the identified viral species.
Keywords: Transcriptome, High-throughput sequencing, De novo assembly, In silico virus identification, Pine processionary moth, Phylogeny
Specifications Table
| Subject | Virology |
| Specific subject area | Virus identification from original transcriptomic data |
| Type of data | Fastq (raw read sequences) Assemblies : de novo assembled transcriptome for the host Thaumetopoea pityocampa and 34 viral transcripts Fasta: alignments used for phylogenetic analyses Table: raw reads counts per viral transcript in each population and life stage Table: alignment statistics and hit annotations of transcripts showing significant BLAST hits with reference viral sequences Figures: phylogenetic trees and position of the Cypovirus, the Iflavirus and the Densovirus identified in this study |
| How the data were acquired | RNA-seq data were sequenced on an Illumina HiSeq2000 platform using the 2 × 100 bp paired-end protocol. Twenty-seven libraries were built, corresponding to 4 populations (2 in Italy and 2 in Portugal) and 6 to 8 life stages for each population. |
| Data format | Raw: raw read sequences Analyzed: list of annotated viral transcripts identified, alignments, read counts |
| Description of data collection | Transcriptomic data were assembled using Trinity2.0.2 and merged using CD-HIT-EST4.5.5 and CAP3 02/10/15. Assembled transcripts were first subjected to a Blastx against the reference viral RefSeq database of the NCBI, and the transcripts that were successfully aligned to a viral reference were subjected to a second Blastx search against the NCBI nr database. Virus identification was based on homology results. To identify the relative contributions of the different RNA libraries (corresponding to various populations and/or life stages) to the transcripts finally identified as viral sequences, we used Bowtie2 with default parameters to align the cleaned reads against the final set of candidate viral transcripts identified in the assembled PPM transcriptome and used the number and percentage of reads originating from each library as metrics. To further characterize the best assembled virus genomes, we conducted phylogenetic analyses for the Cypovirus, the Densovirus and the Iflavirus. |
| Data source location | • Mata Nacional de Leiria • Country: Portugal • Latitude and longitude: 39°47′ N, 8°58′ W • Cimolais • Country: Italy • Latitude and longitude: 12°27′ E, 46°19′ N • Tregnano • Country: Italy • Latitude and longitude: 11°09′ E, 45°30′ N |
| Data accessibility | Repository name for raw sequences: NCBI BioProject Data identification number: PRJNA663237 Direct URL to data: http://www.ncbi.nlm.nih.gov/bioproject/663237 Repository name for 34 viral transcripts: NCBI Genbank Data identification number: accession numbers MT796426-MT796428, MT799182-MT799183, MW584279-MW584285, MW584288-MW584291, MW584293, MW584296, MW584298- MW584302, MW584206-MW584316 |
| Repository name for the assembled transcriptome, the alignments used for phylogenetic analyses and the raw reads counts per viral transcript in each population and life stage: Institutional INRAE repository A Table containing the links to the raw data separately for each of the 27 librairies is also provided in the same repository. Direct link to data: 10.57745/AYQBQB |
Value of the Data
-
•
The data correspond to annotated viral transcripts associated to the Lepidopteran pest Thaumetopoea pityocampa, the pine processionary moth (PPM).
-
•
The data significantly enrich the list of Lepidoptera-associated viruses and provide genomic data for future exploration of their diversity.
-
•
These new sequences will serve as references to target these viruses, and will allow screening natural colonies of the PPM to determine their prevalence in the field.
-
•
Targeting larvae showing signs of viral disease will allow identifying which virus are entomopathogenic and could be candidates for future management solutions.
1. Objective
The main objective of this work was to explore the virome associated to natural populations of T. pityocampa by mining large RNAseq datasets corresponding to all developmental stages, various phenologies and geographic origins. We first assembled a PPM transcriptome and developed bio-informatics analyses to specifically identify viral genomes and transcripts. This approach allowed to identify new viruses associated with the PPM and assign their origin. It was very important to expand the knowledge about the viruses associated to the PPM because only 2 exogenous and one endogenous viruses were described so far, from the sister species T. wilkinsoni [1]. We provide the list of identified viruses, their annotation and best blast-hit as well as the corresponding transcript sequences.
2. Data Description
We first assembled a PPM reference transcriptome from the raw RNAseq data generated from 27 libraries; this transcriptome was comprised of 198,336 transcripts corresponding to 88,121 unigenes. Concerning quality assessment, the Cegma procedure allowed recovering the 248 genes at full length (100%). The Busco approach also confirmed that completeness was very high, as it retrieved 100% of the 303 eukaryote genes at full length, 99.5% of the arthropod genes (1061 at full length, 3 fragmented and 2 missing genes) and 98.8% of the insect genes (1638 complete, 14 fragmented and 6 missing genes). The raw data corresponding to this transcriptome were deposited in the NCBI BioProject PRJNA663237 and the transcriptome assembly is available in the INRAE institutional dataverse at 10.57745/AYQBQB. Table 1 shows the number of raw and cleaned paired reads per population and developmental stage (27 libraries), and proportion of mapped reads on the assembled transcripts.
Table 1.
Number of raw and cleaned paired reads per population and developmental stage, and proportion of mapped reads on the assembled transcripts.
| Population Library code |
Stage | # raw read pairs | # cleaned read pairs | % reads mapped back to transcripts |
|---|---|---|---|---|
| Cimolais (Italy) | ||||
| 1 | L1 larvae | 38,661,411 | 35,564,006 | 81.32% |
| 2 | L2 larvae | 41,021,841 | 37,709,113 | 82.52% |
| 3 | L3 larvae | 41,010,324 | 37,713,232 | 83.61% |
| 4 | L4 larvae | 13,690,903 | 12,643,194 | 84.42% |
| 5 | L5 larvae | 50,736,491 | 44,626,888 | 84.59% |
| 6 | Early pupae | 42,449,326 | 39,187,603 | 84.62% |
| 7 | Late pupae | 54,739,835 | 50,323,960 | 84.52% |
| 8 | Adults | 34,251,971 | 31,602,186 | 81.41% |
| Tregnano (Italy) | ||||
| 9 | L1 larvae | 51,314,703 | 47,158,804 | 82.96% |
| 10 | L2 larvae | 29,668,151 | 27,188,282 | 84.86% |
| 11 | L3 larvae | 24,789,340 | 22,615,028 | 83.41% |
| 12 | L4 larvae | 33,608,031 | 30,912,034 | 84.23% |
| 13 | L5 larvae | 29,293,135 | 26,804,959 | 84.07% |
| 14 | Early pupae | 40,739,299 | 37,669,684 | 83.95% |
| 15 | Adults | 55,893,599 | 51,181,710 | 81.14% |
| Leiria SP (Portugal) | ||||
| 16 | Eggs | 38,803,348 | 34,478,275 | 81.42% |
| 17 | L3 larvae | 40,550,188 | 36,300,947 | 83.74% |
| 18 | L5 larvae | 43,752,664 | 40,660,297 | 91.70% |
| 19 | Early pupae | 55,936,647 | 52,278,035 | 90.75% |
| 20 | Late pupae | 57,463,974 | 53,488,169 | 86.74% |
| 21 | Adults | 43,248,551 | 40,265,025 | 87.61% |
| Leiria WP (Portugal) | ||||
| 22 | Eggs | 41,464,384 | 37,438,856 | 79.08% |
| 23 | L3 larvae | 35,699,902 | 31,709,969 | 85.06% |
| 24 | L5 larvae | 44,360,149 | 41,117,253 | 91.38% |
| 25 | Early pupae | 43,469,212 | 40,440,752 | 89.86% |
| 26 | Late pupae | 56,184,645 | 52,026,117 | 89.99 % |
| 27 | Adults | 55,480,253 | 51,785,133 | 87.08% |
The bioinformatic procedure described below then allowed to identify and annotate 34 viral transcripts. Among those, 27 were assigned to 9 virus families and 7 remained unclassified due to poor taxonomic information available in the public databases. Table 2 summarizes the characteristics and annotation of each viral transcript and gives the corresponding NCBI accession number and Blast results. RNA viruses were the most represented, with 6 virus families, namely Iflaviridae, Reoviridae, Partitiviridae, Permutotetraviridae, Flaviviridae and Rhabdoviridae. We also found DNA viruses from 3 families, both with small (Parvoviridae) and large genomes (Baculoviridae and PolyDNAviridae). The numbers of viral reads obtained from the different transcriptome libraries can be found in the INRAE repository (10.57745/AYQBQB), and allow to determine to which sampling site and life stage each virus was associated.
Table 2.
Transcripts showing similarity to viral references. Library # refers to the codes given in Table 1. VP: viral protein; NSP: non-structural protein; CPV: Cypovirus; RdRp: RNA-dependent RNA polymerase. The genome range size for viruses in each family is given between brackets.
| Virus | Trancript ID | Seq. length | GenBank accession number | Blast hit description | Alignment length (% identity) | e-value | obs. in library # |
|---|---|---|---|---|---|---|---|
| Iflavirus (8.8-9.7 kb) | TR140100|c0_g1_i1 | 608 bp | MW584290 | polyprotein, Lysiphlebus fabarum RNA virus, Iflaviridae | 203 aa (47%) | 7.51e-64 | 19 |
| TR143551|c0_g1_i1 | 448 bp | MW584291 | polyprotein, partial, Bee iflavirus 1 | 141 aa (44%) | 6.04e-28 | 19 | |
| TR73151|c0_g2_i1 | 1098 bp | MW584279 | polyprotein, Lysiphlebus fabarum RNA virus type A, Iflaviridae | 369 aa (52%) | 1.25e-118 | 19 | |
| TR73151|c1_g1_i1 | 309 bp | MW584293 | polyprotein, Lysiphlebus fabarum RNA virus, Iflaviridae | 107 aa (44%) | 2.18e-22 | 19 | |
| TR10271|c1_g1_i1 | 518 bp | MW584296 | RdRp, Venturia canescens picorna-like virus | 172 aa (67%) | 1.77e-79 | 19 | |
| Cypovirus (CPV) (25 kb, segmented genome; 10 segments 1-4 kb) | TR63295|c0_g2_i1 | 3690 bp | MW584298 | VP3, Erinnyis ello CPV2 | 1168 aa (34%) | 0.0 | 24,25,26,27 |
| TR90152|c0_g1_i1 | 3360 bp | MW584280 | VP4, Erinnyis ello CPV2 | 1134 aa (34%) | 9.64e-174 | 24,25,26,27 | |
| TR92789|c0_g2_i1 | 3697 bp | MW584281 | RdRp, Erinnyis ello CPV2 | 1238 aa (44%) | 0.0 | 24,25,26,27 | |
| TR93454|c0_g1_i1 | 2112 bp | MW584299 | VP5, Inachis io CPV2 | 622 aa (31%) | 2.52e-61 | 24,25,26,27 | |
| TR98576|c0_g1_i1 | 2042 bp | MW584300 | Unknown protein, Choristoneura occidentalis CPV16 | 288 aa (33%) | 5.15e-36 | 24,25,26,27 | |
| TR77729|c0_g1_i1 | 3597 bp | MW584301 | Unknown protein, Choristoneura occidentalis CPV16 | 1206 aa (34%) | 5.17e-177 | 24,25,26,27 | |
|
Rhabdovirus (11-15 kb) |
TR101106|c2_g3_i1 | 1514 bp | MW584302 | polymerase, Yinshui bat virus | 265 aa (61%) | 6.70e-125 | All |
| TR103332|c1_g2_i2 | 2222 bp | MW584282 | RdRp, partial, Muscina stabulans sigmavirus | 141 aa (60%) | 1.87e-46 | All | |
| TR104746|c0_g1_i1 | 1028 bp | MW584283 | L protein, Orgyia pseudotsugata Orgi virus | 145 aa (38%) | 1.49e-21 | All | |
| TR105009|c0_g2_i1 | 5765 bp | MW584284 | RdRp, Bactrocera dorsalis sigmavirus | 322 aa (58%) | 0.0 | All | |
|
Betapartitivirus (6 kb, segmented genome; 3 segments 1.7-2.2 kb) |
TR105233|c0_g1_i1 | 2164 bp | MT799183 | Coat protein, Plasmopara viticola lesion associated Partitivirus 7 | 593 aa (30%) | 2.16e-70 | 22 |
| TR85286|c0_g1_i1 | 926 bp | MW584306 | Coat protein, Plasmopara viticola lesion associated Partitivirus 7 | 197 aa (37%) | 2.93e-30 | 22 | |
| TR85286|c1_g1_i1 | 1214 bp | MW584285 | Capsid protein, Heterobasidion partitivirus 8 | 410 aa (33%) | 5.19e-42 | 22 | |
| TR91956|c0_g1_i1 | 2210 bp | MT799182 | RdRp, Soybean thrips partiti-like virus 8 | 696 aa (56%) | 0.0 | 22 | |
|
Iteradensovirus (5 kb) |
TR17180|c0_g1_i1 | 2310 bp | MT796426 | Non structural protein NS1, Iteravirus sp from bat guano | 750 aa (98%) | 0.0 | 5,16,17,22,23 |
| TR46092|c0_g1_i1 | 2040 bp | MT796427 | Structural protein VP, Iteravirus sp from bat guano | 679 aa (93%) | 0.0 | 5,16,17,22,23 | |
|
Betabaculovirus (80-180 kb) |
TR5412|c0_g1_i1 | 439 bp | MW584307 | Hypothetical protein AsGV069, Agrotis segetum granulovirus | 143 aa (42%) | 5.02e-27 | 5,11,13,16 |
| TR81448|c0_g1_i1 | 1178 bp | MW584308 | Hypothetical protein AsGV069, Agrotis segetum granulovirus | 208 aa (43%) | 1.17e-42 | 1,2,4,5,7,11,13,16,17 | |
|
PolyDNAvirus (6-20 kb) |
TR95017|c0_g1_i1 | 2490 bp | MW584309 | N-gene1, Hyposoter didymator ichnovirus | 415 aa (51%) | 7.62e-107 | All |
|
Permutotetravirus (5.6 kb) |
TR46077|c0_g1_i1 | 5621 bp | MT796428 | Capsid protein precursor, Thosea asigna alphapermutotetravirus | 555 aa (49%) | 1.79e-161 | 23 |
|
Flavivirus (10-11 kb) |
TR50290|c0_g1_i1 | 368 bp | MW584310 | Putative polyprotein, Lampyris noctiluca flavivirus 1 | 123 aa (49%) | 2.07e-25 | 22 |
| TR50290|c1_g1_i1 | 610 bp | MW584311 | Putative polyprotein, Lampyris noctiluca flavivirus 1 | 202 aa (55%) | 1.23e-61 | 22 | |
| Unclassified viruses | TR98942|c7_g1_i1 | 2054 bp | MW584288 | Polymerase Acidic protein, Lepidopteran orthomyxo-related virus OKIAV178 | 543 aa (43%) | 3.32e-99 | All but 4 |
| TR62311|c0_g1_i1 | 1261 bp | MW584312 | Glycoprotein, Hymenopteran phasma-related virus OKIAV229 | 412 aa (33%) | 5.26e-69 | 26 | |
| TR12337|c0_g2_i1 | 407 bp | MW584313 | Putative RdRp, Notori virus | 134 aa (46%) | 6.16e-29 | 22 | |
| TR26798|c0_g1_i1 | 356 bp | MW584314 | Glycoprotein precursor, Kaisodi virus | 119 aa (37%) | 1.06e-19 | 22 | |
| TR93735|c0_g1_i1 | 2723 bp | MW584315 | Putative RdRp, Raphidiopteran tombus-related virus | 430 aa (50%) | 7.92e-134 | 1,2,4-6,9,11,13,16,17,23 | |
| TR93735|c2_g1_i1 | 785 bp | MW584316 | RdRp, Diaphorina citri associated C-virus | 213 aa (53%) | 2.47e-68 | 11,13,16 | |
| TR80667|c2_g1_i1 | 975 bp | MW584289 | Glycoprotein, Hymenopteran phasma-related virus OKIAV244 | 313 aa (52%) | 4.70e-90 | 22 | |
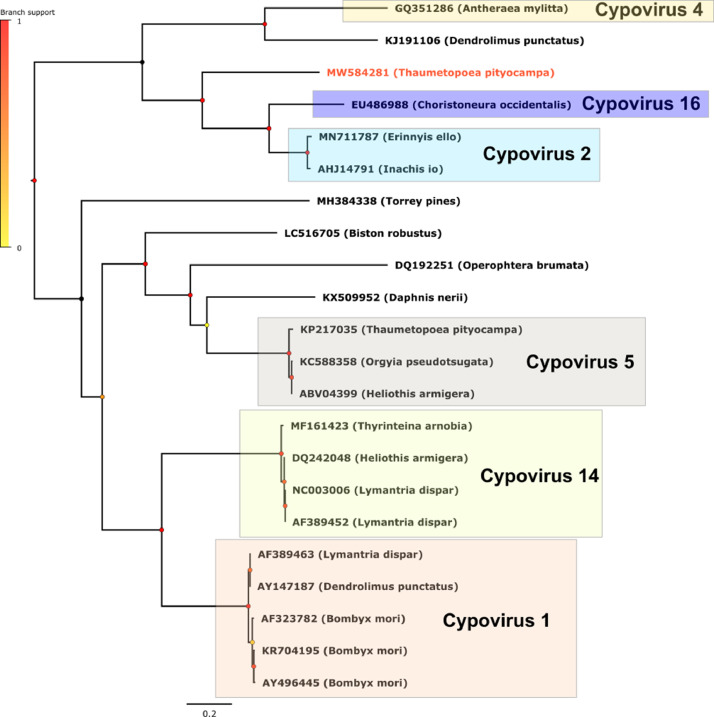
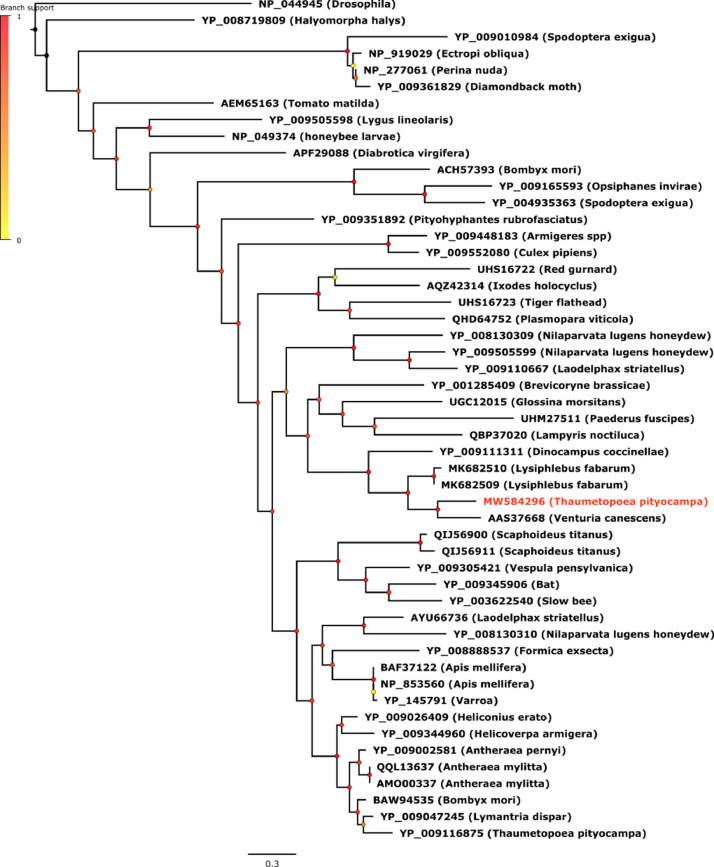
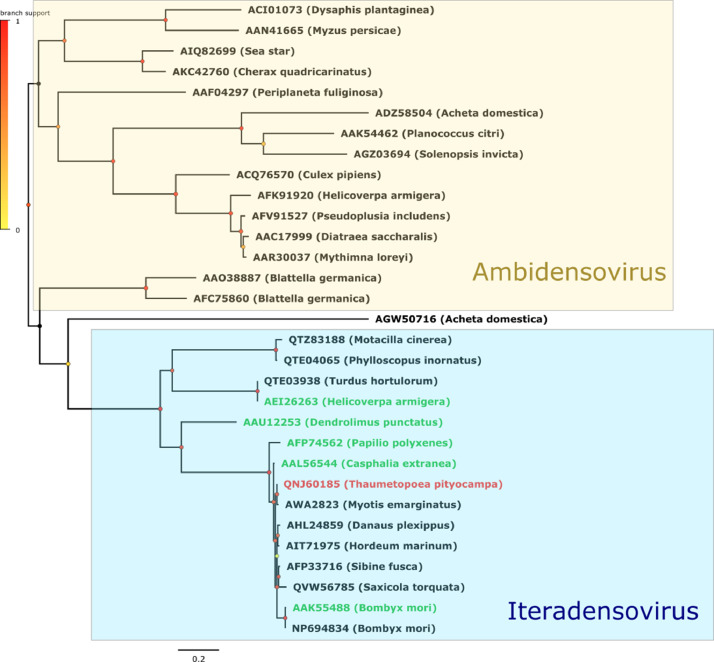
The three figures we provide show the phylogenetic placements of the Cypovirus, the Iflavirus and the Densovirus we identified in the present work.
Fig. 1 showed that the Cypovirus identified in the present study is distantly related to the TpCPV5 described in Ref. [1] from the sister host species T. wilkinsoni. It branches as a sister group close to Cypovirus 2 and Cypovirus 16, in the same clade as Cypovirus 4.
Fig. 1.
Cypovirus phylogeny reconstructed by Maximum Likelihood method (midpoint rooted tree). For each virus, the name of the host is indicated in parenthesis. The main clades of Cypovirus are indicated and the newly identified cypovirus of this study is written in red. Scale bar indicates number of amino acid changes per site and aLRT branch support values at each node are indicated by a color code.
Fig. 2 shows the phylogenetic tree of Iflavirus. The virus we identified from T. pityocampa belongs to the main Iflavirus lineage including the type species "Infectious flacherie virus" and did not fall in the same clade as the TpIV1 identified in Ref. [1] but appeared closely related to Iflavirus species associated to parasitoids (namely Venturia canescens picorna-like virus, Dinocampus coccinellae paralysis virus and Lysiphlebus fabarum Iflavirus).
Fig. 2.
Iflavirus phylogeny reconstructed by Maximum Likelihood method (midpoint rooted tree). For each virus, the name of the host is indicated in parenthesis. The newly identified iflavirus of this study is written in red. Scale bar indicates number of amino acid changes per site and aLRT branch support values at each node are indicated by a color code.
The phylogeny shown in Fig. 3 shows the monophyly of the genus Iteradensovirus, which formed a strongly supported clade differentiated from the Ambidensoviruses included in the analysis. As we could include the 5 Iteradensovirus species recognized by the ICTV, we conclude that the Iteradensovirus we identified from T. pityocampa grouped with Lepidoptera Iteradensovirus 2 (from Casphalia castanea) together with 5 viruses isolated either from Lepidopterans (Danaus plexippus or Sibine fusca) or from bat and bird feces, or from a plant (Hordeum marinum). This group of 7 closely related sequences falls within a clade including also Lepidoptera iteradensovirus 1 and 4. The identity levels were above 85% when comparing the 7 NS1 cited above, and even reached 98% between the transcript we identified in the present study and the virus identified in a bat guano in Croatia.
Fig. 3.
Densovirus phylogeny reconstructed by Maximum Likelihood method (midpoint rooted tree). For each virus, the name of the host is indicated in parenthesis. Ambidensovirus and Iteradensovirus are indicated. The newly identified iteradensovirus of this study is written in red while iteradensovirus reference strains available from ICTV are written in green. Scale bar indicates number of amino acid changes per site and aLRT branch support values at each node are indicated by a color code.
3. Experimental Design, Materials and Methods
3.1. Sampling and Raw Data Acquisition
Raw paired-end reads were obtained from Illumina HiSeq2000 RNA sequencing of individuals sampled from Italy (populations Cimolais, 12°27′ E, 46°19′ N; Tregnago, 11°09′ E, 45°30′ N) and Portugal (summer (SP) and winter (WP) populations from Leiria, 39°47′ N, 8°58′ W). The different developmental stages analyzed in each population are shown in Table 1. All molecular procedures were the same as described in Ref. [2].
3.2. Transcriptome Assembly and Quality Assessment
The raw reads were trimmed using Trimmomatic v.0.33 [3] using the following parameters: ILLUMINACLIP: adaptors_file.fa: 2: 40: 15; HEADCROP: 12; SLIDINGWINDOW: 4: 15 and MINLEN: 30 combined with Prinseq-lite v.0.20.2 [4] to eliminate polyA tails (parameters -trim_tail_left 5, -trim_ tail_right 5, -min_len 30, -out_format 3). The remaining reads were processed with Flash v.1.2.11 (Fast Length Adjustment of SHort reads) [5] to merge r1 and r2 reads.
The cleaned reads, obtained after the quality filtering step described above, were used for de novo transcript assembly using Trinity v.2.0.2 [6] with the normalization option on and default kmer value. To reduce redundancy, the transcripts were further merged into clusters using Cd-Hit-Est v.4.5.4 [7,8] with an identity threshold of 98 (parameters: -c 21 0.98 -l 20 -M 0 -B 1). These clusters were then analyzed with Cap3 v.02/10/15 [9] using parameters -o 200 -p 99. Finally, we excluded transcripts with low coverage by using the FPKM metric (Fragments Per Kilobase transcript length per million fragments Mapped) from the Rsem v.1.3.0 program [10]. Transcripts were removed when FPKM was lower than 1.
We mapped the filtered reads back to the assembled transcripts using Bowtie2 v.2.2.4 with default parameters [11] and we used the number of such reads as a quality assessment estimator. The completeness of the transcriptome assembly was assessed using the Cegma (Core Eukaryotic Genes Mapping Approach) pipeline v.2.5 [12], which searches for 248 orthologous groups of proteins [13]. We also used the Busco v.3.0 program [14,15] to search for 303 conserved eukaryotic genes, 1,066 conserved arthropod genes and 1,658 insect genes.
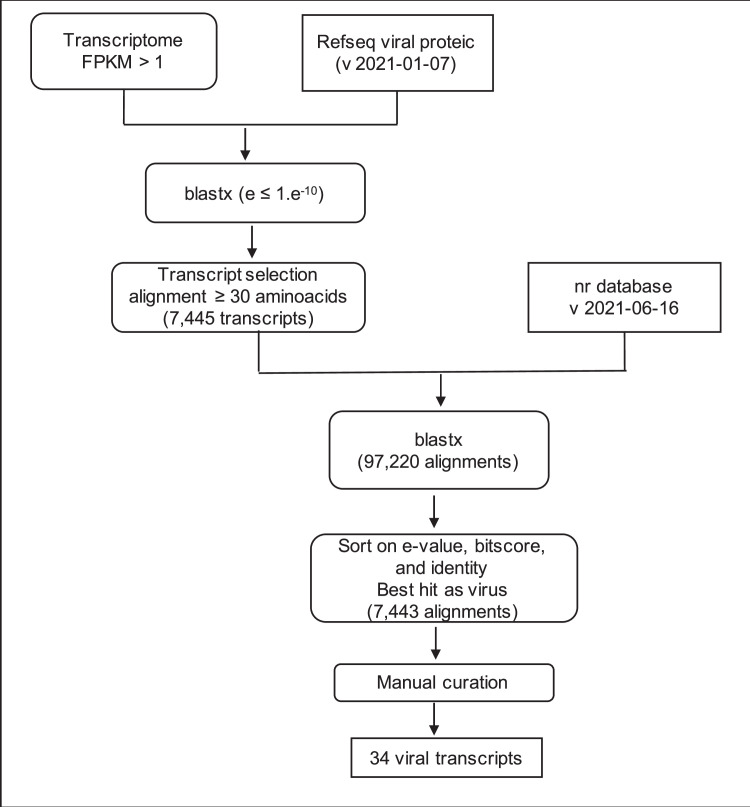
3.3. Bioinformatic Procedures for Virus Identification
The pipeline is summarized in Fig. 4. First, we searched for homology between de novo transcripts and the viral proteins present in the viral RefSeq database of NCBI (available at https://www.ncbi.nlm.nih.gov/refseq, version refseq_viral_proteic_2021-01-07) using the Blastx program [16] with an e-value threshold of 1.e-10 (parameters –outfmt 5; -gapopen 11; -gapextend 1; -word_size 3; -matrix BLOSUM62). Second, the subset of transcripts that could successfully be aligned against a viral protein in the viral RefSeq database and that were longer than 30 amino acids were subjected to a second Blastx search against the NCBI nr database (release 2021-06-16). They were retained only if this Blast search retrieved a viral protein as best Blast Hit in nr and if the alignment between the query and the subject was longer that 100 amino acids. We then restricted the results to the best alignments based on lowest E-values, highest bitscores and highest identities. Virus identification was based on homology results when Blast search provided consistent results. In some cases, the viral sequences found in the GenBank database originated from large metabarcoding programs that did not provide any taxonomic information. We then selected the most informative taxonomy within the search results when bit score and e-values were equivalent and the query start/end were equal.
Fig. 4.
Bio-informatics workflow developed to identify potential viral transcripts.
To identify the relative contributions of the different RNA libraries (corresponding to various populations and life stages) to the viral transcripts, we used Bowtie2 with default parameters to align the cleaned reads against the final set of candidate viral transcripts. We used the number of reads originating from each library as metrics.
3.4. Phylogenetic Analyses
Analyses corresponding to the genus cypovirus were carried out on a set of aminoacid sequences of the RNA-dependent RNA polymerase (RdRp). The alignment included representative members of the genus recognized by the ICTV (6 of the 16 species), the putative Cypovirus 19 isolated from Operophtera brumata, 14 sequences identified in GenBank as corresponding to undescribed Cypovirus and the Cypovirus identified in the present study (TR92789, Genbank accession number MW584281). Analyses corresponding to the genus iflavirus were carried out on a dataset corresponding to the aminoacid sequences of the polyprotein of 50 representative members of the genus and the iflavirus identified in the present study (TR10271, Genbank accession number MW584296). Analyses corresponding to the genus densovirus were carried out on an alignment of the aminoacid sequences of the NS1 protein of 30 representative members of the genus including the 5 Iteradensovirus species recognized by the ICTV and the iteradensovirus identified in the present study (TR17180, Genbank accession number QNJ60185).
For each dataset, multiple protein alignments were generated with the MAFFT v.7 alignment program [17] with default parameters, using a G-INS-i iterative refinement method. Gblocks method [18] implemented in SEAVIEW v5.0.4 [19] was used to eliminate poorly aligned positions and divergent regions, resulting in 690, 136 and 250 amino acids for respectively cypovirus, iflavirus and densovirus final sequence alignments. Optimal substitution models were identified using the SMS program [20] as the LG +G+I+F model (cypovirus and densovirus) or as the LG +G+I model (iflavirus).
Phylogenetic reconstruction was performed by a maximum likelihood (ML) approach in PHYML v3.0 [21] implemented in SEAVIEW v5.0.4, with a statistical approximate likelihood ratio test (aLRT) for branch support. Unrooted phylogenetic trees were visualized and edited with FIGTREE v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/) using the midpoint rooting option.
Ethics Statements
This study did not involve any experiment conducted on human or vertebrate animals.
CRediT authorship contribution statement
Franck Dorkeld: Software, Formal analysis, Data curation, Visualization. Réjane Streiff: Conceptualization, Writing – review & editing. Laure Sauné: Investigation, Resources. Guillaume Castel: Formal analysis, Writing – review & editing, Visualization. Mylène Ogliastro: Conceptualization, Writing – original draft, Supervision. Carole Kerdelhué: Conceptualization, Writing – original draft, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to Manuela Branco and Susana Rocha (University of Lisbon, Portugal) and to Andrea Battisti and Laura Berardi (University of Padua, Italy) for providing the PPM samples. The authors would also like to thank the bioinformatics platforms ABiMS (Roscoff, France) for providing access to their computational resources.
This research was funded by the French National Agency for Research (ANR, project GenoPheno, grant ANR-10-JCJC-1705-01) and by a support by INRA, EFPA (ECODIV) department (projet innovant 2019 EVIL).
Data Availability
References
- 1.Jakubowska A.K., Nalcacioglu R., Millán-Leiva A., Sanz-Carbonell A., Muratoglu H., Herrero S., Demirbag Z. In search of pathogens: transcriptome-based identification of viral sequences from the pine processionary moth (Thaumetopoea pityocampa) Viruses. 2015;7(2):456–479. doi: 10.3390/v7020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gschloessl B., Dorkeld F., Berges H., Beydon G., Bouchez O., Branco M., Bretaudeau A., Burban C., Dubois E., Gauthier P., Lhuillier E., Nichols J., Nidelet S., Rocha S., Sauné L., Streiff R., Gautier M., Kerdelhué C. Draft genome and reference transcriptomic resources for the urticating pine defoliator Thaumetopoea pityocampa (Lepidoptera: Notodontidae) Mol. Ecol. Res. 2018;18(3):602–619. doi: 10.1111/1755-0998.12756. [DOI] [PubMed] [Google Scholar]
- 3.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magoč T., Salzberg S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., Chen Z., Mauceli E., Hacohen N., Gnirke A., Rhind N., di Palma F., Birren B.W., Nusbaum C., Lindblad-Toh K., Friedman N., Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu L., Niu B., Zhu Z., Wu S., Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 9.Huang X., Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9(9):868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra G., Bradnam K., Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- 13.Tatusov R.L., Fedorova N.D., Jackson J.D., Jacobs A.R., Kiryutin B., Koonin E.V., Krylov D.M., Mazumder R., Mekhedov S.L., Nikolskaya A.N., Rao B.S., Smirnov S., Sverdlov A.V., Vasudevan S., Wolf Y.I., Yin J.J., Natale D.A. The COG database: an updated version includes eukaryotes. BMC Bioinform. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simao F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse R.M., Seppey M., Simao F.A., Manni M., Ioannidis P., Klioutchnikov G., Kriventseva E.V., Zdobnov E.M. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol. Biol. Evol. 2017;35(3):543–548. doi: 10.1093/molbev/msx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Katoh K., Rozewicki J., Yamada K.D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019;20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17(4):540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 19.Gouy M., Guindon S., Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 20.Lefort V., Longueville J.E., Gascuel O. SMS: smart model selection in PhyML. Mol. Biol. Evol. 2017;34(9):2422–2424. doi: 10.1093/molbev/msx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.