Abstract
Uterine adenosarcoma is a rare gynecological malignancy with no specific symptoms, and the optimal management is still inconclusive. Herein we present a case of uterine adenosarcoma in a 38-year-old woman with a good prognosis and review of literatures. The patient presented with abnormal vaginal bleeding with no special medical history. Sonographic scan revealed a heterogeneous echoic mass in the cavity, indicating a polypus or a submucous myoma. The pathology based on the specimen after the hysteroscopic tumor excision suggested diagnosis of uterine adenosarcoma. Subsequently, the patient received pelvic MRI scan before surgery. MRI identified a patchy lesion at the cervix-lower endometrial cavity with low signal in T1WI and a mixed high T2 signal in T2WI, with no sign of metastasis. Then total abdominal hysterectomy with bilateral salpingo-oopherectomy plus pelvic lymph node dissection was performed and 6 cycles of chemotherapy were administered. The patient remains disease-free on follow-up to date, more than 15 months after chemotherapy.
Keywords: Müllerian adenosarcoma, sarcoma, uterine cancers, gynecological malignancy, Müllerian malignancy
Introduction
Uterine adenosarcoma is a rare malignancy, accounts for 5%-10% of all uterine sarcomas [1,2]. It was first reported by Clement and Scully in 1974, characterized by components of both sarcoma and benign epithelial glands [3]. The morbidity was hard to estimate worldwide but it only has an age-adjusted incidence of 2-3 per 1,000,000 in the US population [4]. Due to the rarity and the inconsistent of clinical management, here we present a case of uterine adenosarcoma in a 38-year-old woman with a good prognosis.
Case
A 38-year-old woman presented with abnormal vaginal bleeding for 1 year and a prolonged 10-day menstruation compared to the original 3-5 days with no other complaints such as menorrhagia, watery discharge, or abdominal fullness etc. She had no other medical history but caesarean section for one childbirth and she took no specific medications. The patient did not go to the clinic in the past year until her last menstrual period lasted for 15 days. In the outpatient department, no significant signs were found during the pelvic exam while transvaginal 3D ultrasound scan revealed a heterogeneous echoic mass, 3.8*2.2*2.1 cm in size with a cystic region of approximately 1.6*1.5*1.3 cm, started from the uterine cavity and descended to the cervical canal.
An endometrial polypus or a submucous myoma was suspected, the patient was admitted and hysteroscopy was performed. During the hysteroscopy, the endocervical canal was visually normal, but from the middle of endometrial cavity to the internal os, a soft, partly cystic, sessile mass about 4*2*2 cm in size was seen. It was difficult to specify whether the mass was a submucous myoma with cystic degeneration or an endometrial polypus intraoperatively, so a hysteroscopic tumor excision was done. The pathological examination found that the tumor was composed of benign glandular elements and malignant stromal components. The epithelial glands were papillary or slit-like while the stromal cells were spindle-shaped, with increased mitotic activity. The stromal cells formed intraglandular protrusions and in some areas, formed peri-glandular cuffs around the glands, which was the typical microscopic characteristic of adenosarcoma (Figure 1). The final pathological diagnosis was uterine adenosarcoma, with the malignant stromal component was low grade endometrial stromal sarcoma.
Figure 1.
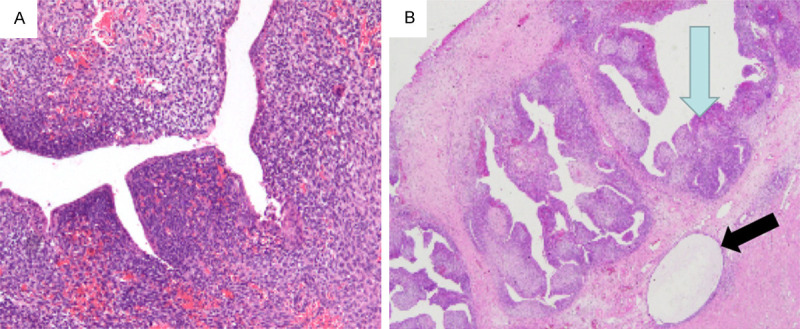
The tumor was composed of benign glandular elements and malignant stromal components. A. The slit-like epithelial glands were widely distributed in the stroma. B. The stromal cells forming intraglandular protrusions (blue arrow) and peri-glandular cuffs around the glands (black arrow).
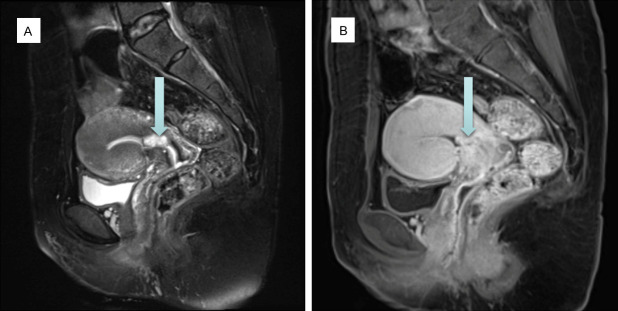
In order to understand the characteristics of the tumor and whether there was myometrial invasion or distant metastasis, pelvic Magnetic Resonance Image (MRI) with contrast enhanced was performed before reoperation. It can be observed a patchy lesion at the cervix-lower endometrial cavity with low signal in T1 Weighted Image (T1WI) and a mixed high T2 signal in T2 Weighted Image (T2WI). No obvious diffusion limitation was observed in Diffusion Weighted Image (DWI), and uneven tissue enhancement was shown in T1WI with contrast-enhanced (Figure 2). There were no obvious enlarged lymph nodes in pelvic cavity and no sign of myometrial invasion on MRI.
Figure 2.
MRI revealed a patchy lesion (arrow) of 17*9 mm at the cervix-lower endometrial cavity. A. A mixed high T2 signal in T2WI. B. Uneven tissue enhancement in T1WI with contrast-enhanced.
A subsequent laparoscopy was scheduled. There were no visible tumor and no enlarged lymph nodes detected in the surgery, then total abdominal hysterectomy with bilateral salpingo-oopherectomy (TAH with BSO) and pelvic lymphadenectomy was performed.
Macroscopic examination found a protruding adenosarcoma sized 1.8*1.2*0.8 cm at the cervix-lower uterus. The tumor is mainly exogenous, with focal invasion to the superficial myometrium (<½ myometrial thickness), and no lymphovascular space invasion (LVSI), no involvement on surgical margins, bilateral parametria, bilateral uterine horns, bilateral fallopian tubes and ovaries, and 24 resected lymph nodes were negative. Given that the risk of recurrence increased while the presence of myometrial invasion, we conducted 6 cycles of chemotherapy, with regimen of pegylated liposomal doxorubicin and carboplatin. The patient has been followed up for more than 15 months after chemotherapy, and has no signs of recurrence so far.
Discussion
Uterine adenosarcoma is a biphasic neoplasm consisting of benign or occasionally atypical glandular component and malignant stromal component. It accounts for about 5%-10% of uterine sarcomas, while uterine sarcomas are only 1% of all gynecological malignancies and 3% of all uterine cancers [1,2,4,5].
Uterine adenosarcoma occurs more frequently in postmenopausal women. According to three retrospective analyses, the median ages of patients diagnosed with uterine adenosarcoma were 54 [6], 56 [7] and 58 [8] respectively, ranging from 14 to 89 years old. Of 544 cases from the SEERs database, 51.5% of the patients were between 40 and 65 years old, and less than 10% were younger than 40 years old [9]. The patient in this study was a 38-year-old woman of reproductive age.
The most common symptom of uterine adenosarcoma is abnormal vaginal bleeding, but a small number of patients present with uterine enlargement, pelvic mass, pelvic pain, uterine prolapse, vaginal discharge, or some rare symptoms include dysmenorrhea, dysuria, infertility, abdominal distension caused by ascites [2,4,6,8,9]. During gynecological examination, tumors can be seen protruding from the external cervical orifice or even fill the vagina, which may be misdiagnosed as “cervical polyps”, especially in patients with histories of recurrent endometrial polyps or cervical canal polyps [2,6,8,10-13].
Uterine adenosarcomas occur typically in the endometrium (87%) and may also be confined to the internal os (9%) or myometrium (4%) [6,8-10,14]. Similar tumors can originate in the cervix [12,13,15-17], ovaries [6,15,18-24], pelvis [25-28], and vagina [29-31]. The diameter of adenosarcoma varied from 0.1 cm to >20 cm, with an average of 5 cm [8,10,32]. Most tumors are lobulated and granular, grown as solitary polypoid, but multiple polypoid masses within the uterine cavity have also been seen [2,6,8-10,32].
Due to well performance on soft tissue resolution, MRI can display the internal structure and changes such as hemorrhage or necrosis of the tumor, and can accurately determine the relationship between the lesion and the endometrium or myometrium. Typical MRI manifestations of uterine adenosarcoma are huge polypoid masses arising from the uterine cavity, protruding to the vagina through the cervical canal, with clear or blurred borders, and multiple small cystic areas inside [33-36]. Metastasis and enlarged lymph nodes could also be revealed on MRI in advanced patients. Compared with the signal of myometrium, the masses of uterine adenosarcoma often showed slightly high signal on T2WI and low or high signal on T1WI, which was quite similar to that of endometrial polyps or submucosal fibroids [34,36]. Usually, there is heterogeneous enhancement in T1WI with contrast-enhanced [34,35]. However, with regard to DWI, the results are inconsistent. Some researchers reported that adenosarcomas usually presented relatively low DWI signal intensity, especially low-grade adenosarcomas [35-38], while some other researchers found that uterine adenosarcomas showed slightly high signal or high signal on DWI [39-41]. The reasons for the different DWI signals are still unclear but could be explained by the different proportions of benign epithelial and malignant mesenchymal components to some extent. Other imaging modalities can also be used, such as sonography, CT or Positron emission tomography-computed tomography (PET-CT). Sonography and CT are nonspecific but most reachable, usually revealed enlarged uterine with a heterogeneous mass inside the cavity [34,42]. PET/CT can be used to identify distant metastases or relapse locations [43].
Without specific immunohistochemical markers, the diagnosis of uterine adenosarcomas mainly relies on morphological features. The typical microscopic characteristic of adenosarcoma is peri-glandular cuffs composed of benign epithelial glands and malignant stromal components [2,6,8,44,45]. The mitotic activity of the stroma cells varies greatly, ranging from 0 to 24 MF/10 high-power fields (hpf) [8,10,45,46]. About 30% of cases were accompanied by endometrial dysplasia or endometrial adenocarcinoma [8]. Most uterine adenosarcomas were confined to the endometrium [6,44,45,47], but Zaloudek et al. reported 7/25 cases of myometrial invasion [10], Clement et al. found that 15/100 cases had superficial myometrial invasion, while 4 cases had deep invasion [8]. The immunophenotype of most uterine adenosarcomas is similar to that of endometrial stromal sarcomas (positive ER, PR, WT1, and CD10), while patients with sarcoma overgrowth have loss of ER, PR, and CD10 expression [48-50].
There is no consensus on the optimal approach to treatment of uterine adenosarcoma. According to the published data, the recommended management for adenosarcoma is TAH with BSO [9,51], but for women with fertility requirements, fertility sparing surgery (FSS) can be considered for appropriate selected patients [52-54]. ZiZolfi et al. reported one 23-year-old woman with Stage IA uterine adenosarcoma underwent hysteroscopic tumor resection followed by 160 mg/d oral megestrol acetate, and got full-term infant four years after diagnosis [54]. Goh et al. reported a 21-year-old patient with Stage I low-grade uterine adenosarcoma, underwent dilatation and curettage (D&C) and polypectomy, and got spontaneous conception and delivery four years later, but she received TAH+BSO+ lymphadenectomy when recurrence was confirmed 96 months after initial diagnosis [55]. L’Heveder et al. reported an 18-year-old girl who diagnosed as low-grade adenosarcoma after polypectomy, she conceived and delivered twins at 28 weeks, and underwent hysterectomy 20 years postoperatively with no recurrence [53]. Lee et al. reported seven cases, the largest case series to date, of Stage I uterine adenosarcoma managed with FSS, but two of them suffered recurrence while only one woman got livebirth [56]. Zaloudek et al. reported three cases of 14 to 15-year-old patients, one underwent TAH with ovary preservation, the second only received cervixectomy, and the third underwent only tumor excision. All three patients received radiotherapy after surgery, and no recurrence was observed during follow-up, but only the third woman got a normal pregnancy [10]. Michener et al. reported a 25-year-old female diagnosed with Stage IB uterine adenosarcoma who underwent hysterectomy but preserved ovaries, and was suggested to in vitro fertilization using a surrogate [52]. Due to the extremely rarity, it cannot to conclude a standard procedure nor unified criteria of FSS, but it seems it can be performed in young patients with low-grade, stage IA disease without sarcomatous overgrowth, and close long-term follow-up is needed. However, fertility-sparing surgery is still not the recommended approach due to the risk of recurrence, and hysterectomy should be considered after completion of childbearing.
Lymphadenectomy is still controversial. It has been reported that the incidence of lymph node metastasis in uterine adenosarcoma is very low, ranging from 0 to 6.5% [2,6,7,9,57]. Furthermore, some researchers found that lymphadenectomy did not show overall or progression-free survival benefit [6,7]. But meanwhile, some studies found patients underwent lymphadenectomy showed a better survival [57,58]. In a retrospective study of 994 cases and a systematic review of 230 surgically treated adenosarcomas, lymph node involvement revealed the greatest impact on progression-free survival compared to other factors such as deep myometrial invasion or sarcomatous overgrowth [57]. So, the question of whether lymph nodes should be removed during the primary surgery remained unanswered. But in any case, enlarged lymph nodes should be excised to rule out or confirm metastasis [57,59]. Although the mainstream guidelines like the NCCN guideline suggested TH+BSO as standard surgery for uterine adenosarcoma, lymphadenectomy is practiced widely. Of 1027 cases from the SEERs database, 53.1% of patients underwent lymph node dissection [7]. In this case, considering the involvement of lymph node metastasis in tumor staging and it may be an indicator of poor prognosis, we performed lymphadenectomy during the surgery.
The general 5-year overall survival of uterine adenosarcoma is about 60-87%, and the most important prognostic factors are tumor stage, presence of sarcomatous overgrowth and myometrial invasion [2,4-7,9,46,47,51,57,60,61]. Survival analyses from the SEERs database indicated that the 5-year survival for patients with stage I was 79% (95% CI, 75-84%) and for patients with stage III was 48% (95% CI, 29-65%) [9]. However, the 2-year PFS and OS were only 20%-50% with sarcomatous overgrowth while both 100% without sarcomatous overgrowth [6,45,60]. Myometrial invasion is related with a more aggressive behavior and a high rate of recurrence, the overall survival for early stage patients with myometrial invasion was only 63% [9]. The case reported here was stage IB with myometrial invasion, so the risk of recurrence cannot be ignored. Therefore, we administered 6 cycles of adjuvant chemotherapy. The patient was disease-free to date.
Conclusion
Most patients with uterine adenosarcoma are in the early stage and with relatively good prognosis. The recommended management is TAH with BSO, lymphadenectomy may not be routinely performed but enlarged lymph nodes should be resected. Sarcoma-specified chemotherapy regimens (eg, doxorubicin or ifosfamide) may be considered in patients at high risk of recurrence or death, such as myometrial invasion, sarcomatous overgrowth and advanced stage.
Disclosure of conflict of interest
None.
References
- 1.Ulrich UA, Denschlag D. Uterine adenosarcoma. Oncol Res Treat. 2018;41:693–696. doi: 10.1159/000494067. [DOI] [PubMed] [Google Scholar]
- 2.Nathenson MJ, Ravi V, Fleming N, Wang WL, Conley A. Uterine adenosarcoma: a review. Curr Oncol Rep. 2016;18:68. doi: 10.1007/s11912-016-0552-7. [DOI] [PubMed] [Google Scholar]
- 3.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus. A clinicopathologic analysis of ten cases of a distinctive type of mullerian mixed tumor. Cancer. 1974;34:1138–1149. doi: 10.1002/1097-0142(197410)34:4<1138::aid-cncr2820340425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 6.Carroll A, Ramirez PT, Westin SN, Soliman PT, Munsell MF, Nick AM, Schmeler KM, Klopp AH, Fleming ND. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol. 2014;135:455–461. doi: 10.1016/j.ygyno.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H, Wei Z, Zhao H, Yuan G. Epidemiology of adenosarcoma and the inverse probability of treatment weighting (IPTW) adjusted survival analysis of lymph node dissection in uterine adenosarcoma. Medicine (Baltimore) 2022;101:e30607. doi: 10.1097/MD.0000000000030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990;21:363–381. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- 9.Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, Herzog TJ, Wright JD. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol. 2010;119:305–308. doi: 10.1016/j.ygyno.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Zaloudek CJ, Norris HJ. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer. 1981;48:354–366. doi: 10.1002/1097-0142(19810715)48:2<354::aid-cncr2820480222>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Kerner H, Lichtig C. Mullerian adenosarcoma presenting as cervical polyps: a report of seven cases and review of the literature. Obstet Gynecol. 1993;81:655–659. [PubMed] [Google Scholar]
- 12.Garcia-Rostan y P erez GM, Troyas RG, Bercero EA. Mullerian adenosarcoma of the cervix: differential diagnosis, histogenesis and review of the literature. Pathol Int. 1995;45:890–894. doi: 10.1111/j.1440-1827.1995.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 13.Chin PS, Chia YN, Lim YK, Yam KL. Diagnosis and management of Mullerian adenosarcoma of the uterine cervix. Int J Gynaecol Obstet. 2013;121:229–232. doi: 10.1016/j.ijgo.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Oda Y, Nakanishi I, Tateiwa T. Intramural müllerian adenosarcoma of the uterus with adenomyosis. Arch Pathol Lab Med. 1984;108:798–801. [PubMed] [Google Scholar]
- 15.Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009;33:278–288. doi: 10.1097/PAS.0b013e318181a80d. [DOI] [PubMed] [Google Scholar]
- 16.Seagle BL, Falter KJ 2nd, Lee SJ, Frimer M, Samuelson R, Shahabi S. Mullerian adenosarcoma of the cervix: report of two large tumors with sarcomatous overgrowth or heterologous elements. Gynecol Oncol Case Rep. 2014;9:7–10. doi: 10.1016/j.gynor.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoharan M, Azmi MA, Soosay G, Mould T, Weekes AR. Mullerian adenosarcoma of uterine cervix: report of three cases and review of literature. Gynecol Oncol. 2007;105:256–260. doi: 10.1016/j.ygyno.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Kao GF, Norris HJ. Benign and low grade variants of mixed mesodermal tumor (adenosarcoma) of the ovary and adnexal region. Cancer. 1978;42:1314–1324. doi: 10.1002/1097-0142(197809)42:3<1314::aid-cncr2820420342>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Eichhorn JH, Young RH, Clement PB, Scully RE. Mesodermal (mullerian) adenosarcoma of the ovary: a clinicopathologic analysis of 40 cases and a review of the literature. Am J Surg Pathol. 2002;26:1243–1258. doi: 10.1097/00000478-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shetty M, Lal N, Vu NH. Mullerian adenosarcoma of the ovary: case report and review of the literature. Ultrasound Q. 2007;23:189–191. doi: 10.1097/RUQ.0b013e31814b94f7. [DOI] [PubMed] [Google Scholar]
- 21.Shah A, Rekhi B, Maheshwari A, Jambhekar NA. Low-grade Mullerian adenosarcoma with prominent decidualization, involving bilateral ovaries against a background of endometriosis: a diagnostic and treatment challenge. J Postgrad Med. 2013;59:149–152. doi: 10.4103/0022-3859.113833. [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa E, Yamamoto Y, Fujimoto C, Kobayashi S, Haba R, Ishikawa M, Imaida K. Aggressive adenosarcoma of the ovary. Histopathology. 2003;42:202–203. doi: 10.1046/j.1365-2559.2003.01532_5.x. [DOI] [PubMed] [Google Scholar]
- 23.Garalejic E, Arsic B, Perovic M, Vasiljevic M, Usaj SK, Stanojevic D. Adenosarcoma ovarii in a 51-year-old woman: case report. Eur J Gynaecol Oncol. 2012;33:543–545. [PubMed] [Google Scholar]
- 24.Lee TY, Lee C, Choi WJ, Lee JY, Kim HY. Synchronous occurrence of primary malignant mixed mullerian tumor in ovary and uterus. Obstet Gynecol Sci. 2013;56:269–272. doi: 10.5468/ogs.2013.56.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerner H, Lichtig C, Beck D. Extrauterine müllerian adenosarcoma of the peritoneal mesothelium: a clinicopathologic and electron microscopic study. Obstet Gynecol. 1989;73:510–513. [PubMed] [Google Scholar]
- 26.Mills SE, Sugg NK, Mahnesmith RC. Endometrial adenosarcoma with pelvic involvement following uterine perforation. Diagn Gynecol Obstet. 1981;3:149–154. [PubMed] [Google Scholar]
- 27.Dincer AD, Timmins P, Pietrocola D, Fisher H, Ambros RA. Primary peritoneal mullerian adenosarcoma with sarcomatous overgrowth associated with endometriosis: a case report. Int J Gynecol Pathol. 2002;21:65–68. doi: 10.1097/00004347-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Visvalingam S, Jaworski R, Blumenthal N, Chan F. Primary peritoneal mesodermal adenosarcoma: report of a case and review of the literature. Gynecol Oncol. 2001;81:500–505. doi: 10.1006/gyno.2001.6165. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Leng J, Guo L, Xiang Y, Lang J. Vaginal adenosarcoma arising from refractory endometriosis: a case report. Aust N Z J Obstet Gynaecol. 2010;50:574–576. doi: 10.1111/j.1479-828X.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 30.Toyoshima M, Akahira J, Moriya T, Hayakawa S, Yaegashi N. Primary vaginal adenosarcoma with sarcomatous overgrowth. Gynecol Oncol. 2004;95:759–761. doi: 10.1016/j.ygyno.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Huang YW, Li YF. Primary vaginal sarcoma: experience of a regional cancer center in China. J Obstet Gynaecol Res. 2015;41:1463–1468. doi: 10.1111/jog.12746. [DOI] [PubMed] [Google Scholar]
- 32.McCluggage WG. Mullerian adenosarcoma of the female genital tract. Adv Anat Pathol. 2010;17:122–129. doi: 10.1097/PAP.0b013e3181cfe732. [DOI] [PubMed] [Google Scholar]
- 33.Ueda M, Otsuka M, Hatakenaka M, Sakai S, Ono M, Yoshimitsu K, Honda H, Torii Y. MR imaging findings of uterine endometrial stromal sarcoma: differentiation from endometrial carcinoma. Eur Radiol. 2001;11:28–33. doi: 10.1007/s003300000541. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Kim SH, Cho JY, Yeon KM. Uterine adenofibroma and adenosarcoma: CT and MR findings. J Comput Assist Tomogr. 1998;22:314–316. doi: 10.1097/00004728-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizako T, Wada A, Kitagaki H, Ishikawa N, Miyazaki K. MR imaging of uterine adenosarcoma: case report and literature review. Magn Reson Med Sci. 2011;10:251–254. doi: 10.2463/mrms.10.251. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi M, Matsuzaki K, Yoshida S, Kudo E, Bando Y, Hasebe H, Kamada M, Nishitani H. Adenosarcoma of the uterus: magnetic resonance imaging characteristics. Clin Imaging. 2009;33:244–247. doi: 10.1016/j.clinimag.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, Togashi K. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723–730. doi: 10.1007/s00330-007-0787-7. [DOI] [PubMed] [Google Scholar]
- 38.Namimoto T, Yamashita Y, Awai K, Nakaura T, Yanaga Y, Hirai T, Saito T, Katabuchi H. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2009;19:2756–2764. doi: 10.1007/s00330-009-1471-x. [DOI] [PubMed] [Google Scholar]
- 39.Sumi A, Terasaki H, Sanada S, Uchida M, Tomioka Y, Kamura T, Yano H, Abe T. Assessment of MR imaging as a tool to differentiate between the major histological types of uterine sarcomas. Magn Reson Med Sci. 2015;14:295–304. doi: 10.2463/mrms.2014-0023. [DOI] [PubMed] [Google Scholar]
- 40.Sousa FAE, Ferreira J, Cunha TM. MR imaging of uterine sarcomas: a comprehensive review with radiologic-pathologic correlation. Abdom Radiol (NY) 2021;46:5687–5706. doi: 10.1007/s00261-021-03263-w. [DOI] [PubMed] [Google Scholar]
- 41.Fujii S, Nosaka K, Mukuda N, Fukunaga T, Sato S, Ogawa T. MR imaging of an intramural adenosarcoma with pathologic correlation. Magn Reson Med Sci. 2018;17:1–2. doi: 10.2463/mrms.ci.2017-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chourmouzi D, Boulogianni G, Zarampoukas T, Drevelengas A. Sonography and MRI of tamoxifen-associated mullerian adenosarcoma of the uterus. AJR Am J Roentgenol. 2003;181:1673–1675. doi: 10.2214/ajr.181.6.1811673. [DOI] [PubMed] [Google Scholar]
- 43.Morales FD, Medina RM, Trujillo LM, Beltran MI, Dulcey IC. Mullerian adenosarcoma of the uterine cervix with sarcomatous overgrowth: a case report of aggressive disease in a young patient. Int J Surg Case Rep. 2016;27:155–161. doi: 10.1016/j.ijscr.2016.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czernobilsky B, Hohlweg-Majert P, Dallenbach-Hellweg G. Uterine adenosarcoma: a clinicopathologic study of 11 cases with a reevaluation of histologic criteria. Arch Gynecol. 1983;233:281–294. doi: 10.1007/BF02133803. [DOI] [PubMed] [Google Scholar]
- 45.Kaku T, Silverberg SG, Major FJ, Miller A, Fetter B, Brady MF. Adenosarcoma of the uterus: a Gynecologic Oncology Group clinicopathologic study of 31 cases. Int J Gynecol Pathol. 1992;11:75–88. [PubMed] [Google Scholar]
- 46.Nathenson MJ, Conley AP, Lin H, Fleming N, Lazar A, Wang WL, Ravi V. The importance of lymphovascular invasion in uterine adenosarcomas: analysis of clinical, prognostic, and treatment outcomes. Int J Gynecol Cancer. 2018;28:1297–1310. doi: 10.1097/IGC.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 47.Bernard B, Clarke BA, Malowany JI, McAlpine J, Lee CH, Atenafu EG, Ferguson S, Mackay H. Uterine adenosarcomas: a dual-institution update on staging, prognosis and survival. Gynecol Oncol. 2013;131:634–639. doi: 10.1016/j.ygyno.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Soslow RA, Ali A, Oliva E. Mullerian adenosarcomas: an immunophenotypic analysis of 35 cases. Am J Surg Pathol. 2008;32:1013–1021. doi: 10.1097/PAS.0b013e318161d1be. [DOI] [PubMed] [Google Scholar]
- 49.Amant F, Steenkiste E, Schurmans K, Verbist L, Abeler VM, Tulunay G, de Jonge E, Massuger L, Moerman P, Vergote I. Immunohistochemical expression of CD10 antigen in uterine adenosarcoma. Int J Gynecol Cancer. 2004;14:1118–1121. doi: 10.1111/j.1048-891X.2004.14610.x. [DOI] [PubMed] [Google Scholar]
- 50.Amant F, Schurmans K, Steenkiste E, Verbist L, Abeler VM, Tulunay G, De Jonge E, Massuger L, Moerman P, Vergote I. Immunohistochemical determination of estrogen and progesterone receptor positivity in uterine adenosarcoma. Gynecol Oncol. 2004;93:680–685. doi: 10.1016/j.ygyno.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Friedlander ML, Covens A, Glasspool RM, Hilpert F, Kristensen G, Kwon S, Selle F, Small W, Witteveen E, Russell P. Gynecologic Cancer InterGroup (GCIG) consensus review for mullerian adenosarcoma of the female genital tract. Int J Gynecol Cancer. 2014;24:S78–82. doi: 10.1097/IGC.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 52.Michener CM, Simon NL. Ovarian conservation in a woman of reproductive age with mullerian adenosarcoma. Gynecol Oncol. 2001;83:424–427. doi: 10.1006/gyno.2001.6398. [DOI] [PubMed] [Google Scholar]
- 53.L’Heveder A, Jones BP, Saso S, Barcroft J, Richardson R, Kaur B, Ghaem-Maghami S, Yazbek J, Smith JR. Conservative management of uterine adenosarcoma: lessons learned from 20 years of follow-up. Arch Gynecol Obstet. 2019;300:1383–1389. doi: 10.1007/s00404-019-05306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zizolfi B, Foreste V, Di Spiezio Sardo A, Manzi A, Bifulco G, Carugno J. Fertility sparing management of uterine adenosarcoma: case report and literature review. Facts Views Vis Obgyn. 2021;12:315–318. [PMC free article] [PubMed] [Google Scholar]
- 55.Goh C, Lin XH, Chin PS, Lim YK. Uterine preservation in a young patient with adenosarcoma of the uterus - Case report and review of literature. Gynecol Oncol Rep. 2018;25:27–29. doi: 10.1016/j.gore.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YJ, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Feasibility of uterine preservation in the management of early-stage uterine adenosarcomas: a single institute experience. World J Surg Oncol. 2017;15:87. doi: 10.1186/s12957-017-1137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machida H, Nathenson MJ, Takiuchi T, Adams CL, Garcia-Sayre J, Matsuo K. Significance of lymph node metastasis on survival of women with uterine adenosarcoma. Gynecol Oncol. 2017;144:524–530. doi: 10.1016/j.ygyno.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoellen F, Waldmann A, Benthin S, Hanker L, Rody A, Fischer D. The role of lymphadenectomy in uterine sarcoma: a clinical practical approach based on retrospective analysis. Anticancer Res. 2014;34:985–993. [PubMed] [Google Scholar]
- 59.Trope CG, Abeler VM, Kristensen GB. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol. 2012;51:694–705. doi: 10.3109/0284186X.2012.689111. [DOI] [PubMed] [Google Scholar]
- 60.Tanner EJ, Toussaint T, Leitao MM Jr, Hensley ML, Soslow RA, Gardner GJ, Jewell EL. Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol Oncol. 2013;129:140–144. doi: 10.1016/j.ygyno.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 61.Benito V, Lubrano A, Arencibia O, Andujar M, Alvarez E, Medina N, Falcon JM, Falcon O. Clinicopathologic analysis of uterine sarcomas from a single institution in the Canary Islands. Int J Gynaecol Obstet. 2009;107:44–49. doi: 10.1016/j.ijgo.2009.05.020. [DOI] [PubMed] [Google Scholar]