Abstract
BACKGROUND
With the emergence of the delta variant, the United States experienced a rapid increase in Covid-19 cases in 2021. We estimated the risk of breakthrough infection and death by month of vaccination as a proxy for waning immunity during a period of delta variant predominance.
METHODS
Covid-19 case and death data from 15 U.S. jurisdictions during January 3 to September 4, 2021 were used to estimate weekly hazard rates among fully vaccinated persons, stratified by age group and vaccine product. Case and death rates during August 1 to September 4, 2021 were presented across four cohorts defined by month of vaccination. Poisson models were used to estimate adjusted rate ratios comparing the earlier cohorts to July rates.
RESULTS
During August 1 to September 4, 2021, case rates per 100,000 person-weeks among all vaccine recipients for the January to February, March to April, May to June, and July cohorts were 168.8 (95% confidence interval [CI], 167.5 to 170.1), 123.5 (95% CI, 122.8 to 124.1), 83.6 (95% CI, 82.9 to 84.3), and 63.1 (95% CI, 61.6 to 64.6), respectively. Similar trends were observed by age group for BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccine recipients. Rates for the Ad26.COV2.S (Janssen-Johnson & Johnson) vaccine were higher; however, trends were inconsistent. BNT162b2 vaccine recipients 65 years of age or older had higher death rates among those vaccinated earlier in the year. Protection against death was sustained for the mRNA-1273 vaccine recipients. Across age groups and vaccine types, people who were vaccinated 6 months ago or longer (January-February) were 3.44 (3.36 to 3.53) times more likely to be infected and 1.70 (1.29 to 2.23) times more likely to die from COVID-19 than people vaccinated recently in July 2021.
CONCLUSIONS
Our study suggests that protection from SARS-CoV-2 infection among all ages or death among older adults waned with increasing time since vaccination during a period of delta predominance. These results add to the evidence base that supports U.S. booster recommendations, especially for older adults vaccinated with BNT162b2 and recipients of the Ad26.COV2.S vaccine. (Funded by the Centers for Disease Control and Prevention.)
Introduction
Covid-19 vaccines are highly effective in preventing severe illness resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, infections among vaccinated people, so-called breakthrough infections, still occur. In addition to the underlying rate of SARS-CoV-2 transmission in the population and variation in risk behaviors between groups (e.g., differential testing or use of masks), other possible factors driving increased incidence of Covid-19 among vaccinated persons include reduced vaccine effectiveness (VE) against new SARS-CoV-2 variants such as delta1–4 and waning immunity.5,6 Six-month follow-up data from clinical trials demonstrated a 6% reduction in VE against infection every 2 months for the BNT162b2 vaccine (Pfizer–BioNTech), but no decline for the mRNA-1273 vaccine (Moderna).7,8 A study from Israel showed that in July 2021, when the delta variant was predominant, BNT162b2 vaccine protection was lower among persons vaccinated in earlier months compared with those vaccinated later, suggesting waning immunity.5,6 Data from New York State showed substantial declines in VE correlated with the increasing prevalence of the delta variant and smaller differences associated with waning immunity.1 A study from North Carolina reported waning for all three vaccines approved for use in the United States.9
Vaccine boosters have been authorized by the Food and Drug Administration (FDA). The Centers for Disease Control and Prevention (CDC) recommends that adults 18 years of age and older should receive a booster at least 6 months after the primary series for mRNA vaccines (BNT162b2 and mRNA-1273).10–12 Recipients of the adenovirus vector Ad26.COV2.S vaccine (Janssen–Johnson & Johnson) who are 18 years of age and older are also recommended to receive a booster 2 months after the first dose.10 Data on the extent of waning immunity against infection and death are scarce, especially for mRNA-1273 and Ad26.COV2.S vaccine recipients.
We analyzed Covid-19 breakthrough case and death rates among cohorts of fully vaccinated persons 12 years of age and older defined based on month of completing the primary Covid-19 vaccine series as a proxy for waning immunity during a period when the SARS-CoV-2 delta variant was predominant (>95%) in the United States. We explored variations in rates stratified by age group and vaccine product.
Methods
We analyzed data from 15 jurisdictions (Arizona, Arkansas, Florida, Georgia, Idaho, Indiana, Massachusetts, Michigan, Nebraska, New Jersey, New Mexico, New York City, Tennessee, Utah, and Wisconsin) that linked Covid-19 case surveillance records13 to immunization registry and vital statistics data reported as of September 4, 2021. Immunization registries collect and store all data on Covid-19 vaccine administration. All jurisdictions contributed data on Covid-19 cases. Wisconsin did not contribute data on deaths since the vital records system was not electronically linked with COVID-19 case surveillance data in this jurisdiction. We defined fully vaccinated persons (hereafter referred to as “vaccinated”) as those for whom 14 or more days had passed since completion of the primary series of an FDA-authorized Covid-19 vaccine. A Covid-19 case was defined as a person with detected SARS-CoV-2 RNA or antigen in a respiratory specimen. A breakthrough infection was defined as a Covid-19 case among persons for whom 14 or more days had passed since completion of the primary series of an FDA-authorized Covid-19 vaccine. A Covid-19–associated death was a person with a documented Covid-19 diagnosis who died and for whom local health authorities reviewed and determined Covid-19 as a cause of death using vital records, public health investigation, or other data sources such as electronic health records.
Two data sources were unified to conduct person-level analyses by time since vaccination. Vaccine administration data provided aggregate weekly counts of vaccinated persons stratified by the Morbidity and Mortality Weekly Report (MMWR) week of completion of the primary vaccine series, vaccine product, and age group (12 to 17, 18 to 49, 50 to 64, 65 to 79, or 80 years of age or older) and jurisdiction. These aggregate counts were converted to line-level data for each jurisdiction by replicating records for a given MMWR week, age, and vaccine product, assuming the date of the final dose to be the last day in the MMWR week.14 Counts ≤10 in the vaccine administration data were suppressed (<5% of all records) for confidentiality concerns and were assumed to be 5. Each jurisdiction provided person-level data for the Covid-19 cases among fully vaccinated persons during the study period. Results are presented across all jurisdictions and by region defined as follows: West (Arizona, Idaho, New Mexico, and Utah), Midwest (Indiana, Michigan, Nebraska, and Wisconsin), South (Arkansas, Georgia, Florida, and Tennessee), and Northeast (Massachusetts, New Jersey, and New York City). The variables used from this data source included age, vaccine product, date of vaccine doses(s), and positive specimen collection date. There were less than 0.001% missing data on these variables. The person-level vaccination data were combined with Covid-19 cases among fully vaccinated persons, after removing vaccination data corresponding to cases occurring within the MMWR week, vaccine product, and age group, so the final data comprised one record for each vaccinated person. Persons vaccinated with the Ad26.COV2.S vaccine in the January to February 2021 cohort and all persons 12 to 17 years of age vaccinated with either the mRNA-1273 or Ad26.COV2.S vaccine were excluded from this analysis, since they were not considered FDA-authorized regimens.
STATISTICAL ANALYSES
Vaccination Cohorts and Life-Table Analyses
We defined four vaccination cohorts by calendar time as MMWR weeks of completion of a primary series: MMWR weeks 1 to 8 (January 3 to February 27, 2021), 9 to 17 (February 28 to May 1, 2021), 18 to 25 (May 2 to June 26, 2021), and 26 to 30 (June 27 to July 31, 2021), hereafter referred to as January to February, March to April, May to June, and July, respectively. Logistics of the vaccination rollout prioritized health care professionals, other high-risk groups, and older persons, suggesting that the overall results for the cohorts are likely confounded by age and vaccine type. For this reason, results are presented stratified by age and vaccine type. Factors other than age and vaccine type have not been accounted for and may result in residual confounding.
For each vaccination cohort, life-table analysis was used to estimate the hazard function at weekly and monthly intervals overall and stratified by age and vaccine product. All vaccinated persons in the data set who were not Covid-19 cases were treated as censored on September 4, 2021.
Cases and Death Rates during a Period of Delta Predominance
Subsequent statistical analyses present Covid-19 case and death rates per 100,000 vaccinated person-weeks during August 1 to September 4, 2021. This period captures the month with the highest U.S. Covid-19 incidence owing to the predominance of the SARS-CoV-2 delta variant (>95%).15 The data used for these analyses excluded all cases and deaths before August 1, 2021. Person-time was calculated as the number of days from August 1 to either a positive SARS-CoV-2 test result during that period or censored as of September 4, 2021. Rates for each vaccination cohort were calculated as the number of Covid-19 cases or deaths divided by vaccinated person-time multiplied by 100,000. Rates for counts less than 15 may be unstable and should be interpreted with caution. To compare rates between earlier vaccination cohorts and the July cohort, adjusted rate ratios were estimated using Poisson regression models. All variables (age, region, and vaccine product) were treated as categorical. An interaction between age and vaccine product and an offset using person-time was also included. To provide a summary measure of risk of infection attributable to time since vaccination, model adjusted rate ratios for January to June cohorts over the cohort vaccinated in July were also estimated.
Because of the observational nature of surveillance data, P values are not presented here. We present 95% confidence intervals (CIs) for all estimates. However, the widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible. This activity was reviewed by the CDC and was conducted consistent with applicable federal law [45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.] and CDC policy.
Results
As of October 1, 2021 across 15 jurisdictions, 411,661 cases, 6322 deaths, and 48,832,299 vaccinated persons were included for January 3 to September 4, 2021 (Table 1).
Table 1.
Characteristics of Fully Vaccinated Persons and Covid-19 Cases and Deaths for 15 U.S. Jurisdictions, from January 3 to September 4, 2021
| Covid-19 cases | Covid-19 deaths | |||||
|---|---|---|---|---|---|---|
| Characteristic | No. of total vaccinated for 15 jurisdictions | No. of cases | Case rate per 100,000 person-wk* | No. of total vaccinated for 14 jurisdictions | No. ofdeaths | Death rate per 100,000 person-wk* |
| Overall | 48,832,299 | 411,661 | 46.7 (46.6–46.9) | 45,988,351 | 6322 | 0.76 (0.75–0.78) |
| Age — yr | ||||||
| 12–17 | 2,253,130 | 8974 | 39.3 (38.5–40.1) | 2,119,998 | 1 | 0 (0–0.03)† |
| 18–49 | 20,022,549 | 189,269 | 57.5 (57.2–57.7) | 18,907,371 | 134 | 0.04 (0.04–0.05) |
| 50–64 | 12,853,359 | 103,496 | 44.9 (44.6–45.2) | 12,099,120 | 741 | 0.34 (0.32–0.37) |
| 65–79 | 10,614,732 | 80,666 | 35.4 (35.1–35.6) | 9,971,402 | 2371 | 1.11 (1.06–1.15) |
| ≥80 | 3,088,529 | 29,256 | 41.9 (41.4–42.4) | 2,890,460 | 3075 | 4.71 (4.55–4.88) |
| Vaccine product | ||||||
| BNT162b2 (Pfizer-BioNTech) | 26,269,165 | 247,051 | 53.1 (52.9–53.3) | 24,766,315 | 3701 | 0.85 (0.82–0.87) |
| mRNA-1273 (Moderna) | 18,686,366 | 113,724 | 32.4 (32.2–32.6) | 17,575,605 | 1853 | 0.56 (0.54–0.59) |
| Ad26. COV2.S (Janssen-Johnson & Johnson) | 3,876,768 | 50,886 | 79.0 (78.3–79.7) | 3,646,431 | 768 | 1.27 (1.18–1.36) |
| Vaccine product and recipient age — yr | ||||||
| BNT162b2 | ||||||
| 12–17 | 2,253,130 | 8974 | 39.3 (38.5–40.1) | 2,119,998 | 1 | 0 (0–0.03)† |
| 18–49 | 11,066,415 | 114,320 | 62.7 (62.4–63.1) | 10,480,750 | 59 | 0.03 (0.03–0.04) |
| 50–64 | 6,570,353 | 59,439 | 50.0 (49.6–50.4) | 6,196,382 | 334 | 0.30 (0.27–0.33) |
| 65–79 | 4,936,528 | 46,591 | 43.2 (42.8–43.6) | 4,617,760 | 1307 | 1.30 (1.23–1.37) |
| ≥80 | 1,442,739 | 17,727 | 52.8 (52.1–53.6) | 1,351,425 | 2000 | 6.36 (6.08–6.64) |
| mRNA-1273 | ||||||
| 18–49 | 6,901,002 | 47,468 | 41.3 (41.0–41.7) | 6,495,495 | 41 | 0.04 (0.03–0.05) |
| 50–64 | 5,077,105 | 27,701 | 30.6 (30.2–30.9) | 4,779,322 | 211 | 0.25 (0.22–0.28) |
| 65–79 | 5,189,360 | 28,523 | 25.6 (25.3–25.9) | 4,884,325 | 745 | 0.71 (0.66–0.76) |
| ≥80 | 1,518,899 | 10,032 | 29.6 (29.0–30.1) | 1,416,463 | 856 | 2.71 (2.53–2.90) |
| Ad26.COV2.S | ||||||
| 18–49 | 2,055,132 | 27,481 | 85.3 (84.3–86.3) | 1,931,126 | 34 | 0.11 (0.08–0.16) |
| 50–64 | 1,205,901 | 16,356 | 77.6 (76.5–78.8) | 1,123,416 | 196 | 1.00 (0.87–1.15) |
| 65–79 | 488,844 | 5552 | 62.6 (61.0–64.3) | 469,317 | 319 | 3.74 (3.35–4.17) |
| ≥80 | 126,891 | 1497 | 65.2 (62.0–68.6) | 122,572 | 219 | 9.86 (8.63–11.25) |
| Vaccination cohort | ||||||
| January to February | 8,366,983 | 114,195 | 50.1 (49.8–50.4) | 7,863,667 | 2692 | 1.26 (1.21–1.3) |
| March to April | 24,331,245 | 216,897 | 46.0 (45.8–46.2) | 22,755,728 | 3035 | 0.69 (0.66–0.71) |
| May to June | 13,565,104 | 72,812 | 43.2 (42.9–43.5) | 12,905,249 | 524 | 0.33 (0.30–0.36) |
| July | 2,568,967 | 7757 | 58.9 (57.6–60.2) | 2,463,707 | 71 | 0.56 (0.45–0.71) |
| Region‡ | ||||||
| South | 17,665,391 | 212,064 | 66.3 (66.0–66.6) | 17,665,391 | 4396 | 1.37 (1.33–1.42) |
| West | 6,201,697 | 48,334 | 42.2 (41.8–42.6) | 6,201,697 | 436 | 0.38 (0.35–0.42) |
| Northeast | 13,657,121 | 74,163 | 31.4 (31.1–31.6) | 13,657,121 | 653 | 0.28 (0.26–0.30) |
| Midwest | 11,308,090 | 77,100 | 36.8 (36.5–37.0) | 8,464,142 | 837 | 0.53 (0.50–0.57) |
Rates are calculated using person-weeks as the denominator, and 95% confidence intervals are provided in parentheses. The widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible. Wisconsin did not contribute deaths due to lack of linkage between vital records and COVID-19 case surveillance.
Rates for counts fewer than 15 may be unstable and should be interpreted with caution.
Regions comprise the following jurisdictions: South (Arkansas, Georgia, Florida, and Tennessee), West (Arizona, Idaho, New Mexico, and Utah), Northeast (Massachusetts, New Jersey, and New York City), and Midwest (Indiana, Michigan, Nebraska, and Wisconsin).
CASES FROM JANUARY 3 TO SEPTEMBER 4, 2021
Overall case rates per 100,000 vaccinated person-weeks were higher among persons 18 to 49 years of age (57.5; 95% CI, 57.2 to 57.7) than among other age groups (range, 35.4 to 44.9), were higher in the South (66.3; 95% CI, 66.0 to 66.6) than among other regions (range, 31.4 to42.2); and were higher among Ad26.COV2.S vaccine recipients (79.0; 95% CI, 78.3 to 79.7) compared with recipients of the BNT162b2 (53.1; 95% CI, 52.9 to 53.3) and mRNA-1273 (32.4; 95% CI, 32.2 to 32.6) vaccines (Table 1). The median time to infection among vaccinated cases was 111 days (interquartile range, 79–148) after vaccination for recipients of BNT162b2, 118 days (interquartile range, 83–153) for mRNA-1273, and 102 days (interquartile range, 56–126) for Ad26.COV2.S. The distribution of cases among Ad26.COV2.S vaccine recipients showed that an initial peak in the number of cases occurred during 14 to 42 days after vaccination, with a second peak occurring at 120 to 160 days; in contrast, mRNA-1273 and BNT162b2 distributions only had one peak at approximately 120 to 160 days (Fig. S1 in the Supplementary Appendix). Larger differences in the hazard rates between vaccination cohorts were observed after July compared with January to June, corresponding with the emergence of the delta variant, which reached greater than 50% prevalence at the end of June (Fig. 1A). After July, earlier vaccinated cohorts had hazard rates that were both higher and rose faster compared with the more recently vaccinated (Fig. 1 and Table S1). Similar trends were seen by age group and vaccine product (Fig. S2).
Figure 1.
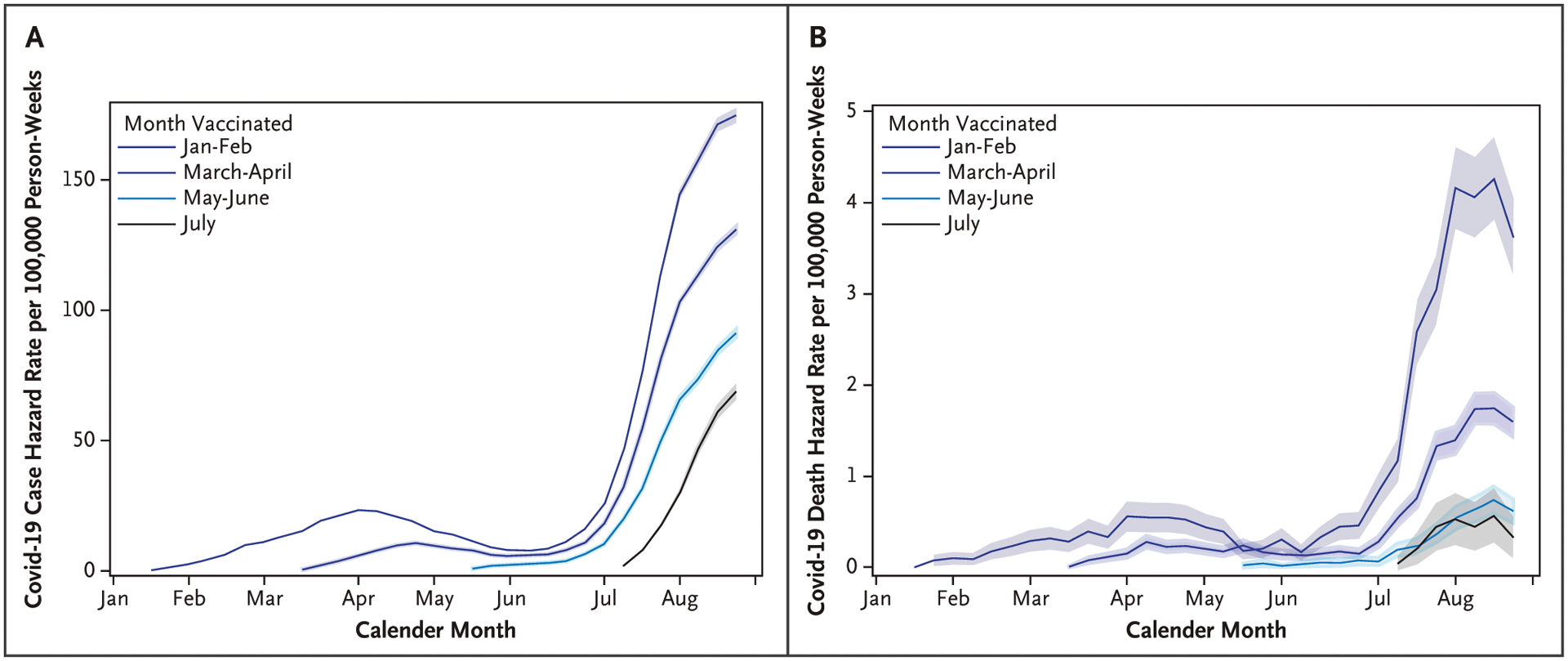
Weekly Covid-19 Hazard Rates for Cases and Deaths by Vaccination Cohort for 14 to 15 U.S. Jurisdictions, from January 3 to September 4, 2021.
Weekly Covid-19 hazard rates are presented for A) cases and B) deaths by vaccination cohort. The 95% confidence intervals are presented around hazard rates. The widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible. Fifteen jurisdictions were included for cases and 14 jurisdictions were included for deaths (Wisconsin was not included in death estimates). Persons vaccinated with the Ad26.COV2.S vaccine (Janssen–Johnson & Johnson) in the January to February cohort and all persons 12 to 17 years of age vaccinated either with the mRNA-1273 (Moderna) or Ad26.COV2.S vaccine were excluded.
CASE RATES DURING A PERIOD OF DELTA PREDOMINANCE
During August 1 to September 4, 2021, rates of SARS-CoV-2 infection were highest among persons with a longer time since vaccination. Rates per 100,000 person-weeks were 168.8 (95% CI, 167.5 to 170.1), 123.5 (95% CI, 122.8 to 124.1), 83.6 (95% CI, 82.9 to 84.3), and 63.1 (95% CI, 61.6 to 64.6) among persons vaccinated in January to February, March to April, May to June, and most recently vaccinated in July, respectively (Table 2). Increases in case rates for cohorts with a longer time since vaccination were observed for all regions (Fig. S3) and for all age groups among BNT162b2 and mRNA-1273 vaccine recipients (Fig. 2A and 2B and Table S2). Among Ad26.COV2.S vaccine recipients, higher rates were observed in persons ages 18 to 49 years vaccinated in March to April compared with July, but patterns were unclear as rates were higher in July compared with May to June for some age groups (Fig. 2C and Table S2).
Table 2.
Covid-19 Case and Death Rates among Fully Vaccinated Persons by Time since Vaccination for 15 U.S. Jurisdictions, from August 1 to September 4, 2021
| Characteristic | January to February | March to April | May to June | July | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Covid-19 cases/deaths | Covid-19 case/death rate per 100,000 person-wk* | No. of Covid-19 cases/deaths | Covid-19 case/death rate per 100,000 person-wk* | No. of Covid-19 cases/deaths | Covid-19 case/death rate per 100,000 person-wk* | No. of Covid-19 cases/deaths | Covid-19 case/death rate per 100,000 person-wk* | |
| 15 jurisdictions | ||||||||
| Overall cases | 67,954 | 168.8 (167.5–170.1) | 145,059 | 123.5 (122.8–124.1) | 54,909 | 83.6 (82.9–84.3) | 7070 | 63.1 (61.6–64.6) |
| Age — yr | ||||||||
| 12–17 | 50 | 164.5 (124.7–217.1) | 1429 | 139.0 (132.0–146.4) | 5060 | 68.6 (66.7–70.5) | 1074 | 47.7 (44.9–50.6) |
| 18–49 | 25,621 | 219.8 (217.2–222.6) | 64,217 | 156.5 (155.3–157.7) | 34,157 | 91.4 (90.5–92.4) | 4036 | 68.0 (65.9–70.1) |
| 50–64 | 12,703 | 166.8 (163.9–169.7) | 43,745 | 118.1 (117.0–119.2) | 11,478 | 76.0 (74.6–77.4) | 1359 | 64.8 (61.5–68.4) |
| 65–79 | 19,989 | 134.8 (132.9–136.6) | 28,760 | 93.2 (92.1–94.3) | 3311 | 69.5 (67.2–71.9) | 461 | 61.3 (56–67.2) |
| ≥80 | 9591 | 156.7 (153.6–159.9) | 6908 | 91.8 (89.7–94) | 903 | 84.5 (79.1–90.2) | 140 | 81.8 (69.3–96.6) |
| Vaccine product | ||||||||
| BNT162b2 (Pfizer-BioNTech) | 44,898 | 201.6 (199.7–203.4) | 83,247 | 139.7 (138.8–140.7) | 34,072 | 92.2 (91.3–93.2) | 4186 | 58.0 (56.3–59.8) |
| mRNA-1273 (Moderna) | 23,056 | 128.2 (126.6–129.9) | 39,271 | 85.3 (84.5–86.2) | 14,338 | 61.4 (60.4–62.4) | 1114 | 40.5 (38.2–42.9) |
| Ad26.COV2.S (Janssen–Johnson & Johnson) | NA | 22,541 | 189.7 (187.3–192.2) | 6499 | 120.6 (117.7–123.6) | 1770 | 142.6 (136.1–149.4) | |
| Vaccine product and recipient age — yr | ||||||||
| BNT162b2 | ||||||||
| 12–17 | 50 | 164.5 (124.7–217.1) | 1429 | 139.0 (132.0–146.4) | 5060 | 68.6 (66.7–70.5) | 1074 | 47.7 (44.9–50.6) |
| 18–49 | 16,817 | 254.8 (251.0–258.7) | 38,013 | 165.2 (163.6–166.9) | 20,899 | 104.4 (103–105.8) | 2233 | 66.0 (63.3–68.8) |
| 50–64 | 8347 | 202.4 (198.1–206.8) | 24,896 | 129.2 (127.6–130.9) | 6135 | 86.5 (84.4–88.7) | 620 | 55.4 (51.2–59.9) |
| 65–79 | 13,035 | 162.5 (159.7–165.3) | 15,433 | 115.6 (113.8–117.4) | 1552 | 76.4 (72.7–80.3) | 197 | 52.1 (45.3–59.9) |
| ≥80 | 6649 | 190.1 (185.6–194.7) | 3476 | 118.6 (114.7–122.6) | 426 | 99.3 (90.3–109.1) | 62 | 76.5 (59.7–98.2) |
| mRNA-1273 | ||||||||
| 18–49 | 8804 | 174.2 (170.6–177.9) | 14,168 | 114.1 (112.2–116.0) | 9410 | 67.1 (65.7–68.4) | 699 | 41.2 (38.3–44.4) |
| 50–64 | 4356 | 124.7 (121.0–128.4) | 11,311 | 82.5 (81.0–84.0) | 3500 | 53.1 (51.4–54.9) | 265 | 37.4 (33.2–42.2) |
| 65–79 | 6954 | 102.1 (99.7–104.5) | 10,937 | 69.4 (68.1–70.7) | 1123 | 50.5 (47.6–53.5) | 109 | 38.7 (32.1–46.7) |
| ≥80 | 2942 | 112.2 (108.2–116.3) | 2855 | 68.9 (66.5–71.5) | 305 | 60.4 (54.0–67.6) | 41 | 60.2 (44.3–81.8) |
| Ad26.COV2.S | ||||||||
| 18–49 | NA | 12,036 | 214.1 (210.3–218) | 3848 | 116.1 (112.5–119.8) | 1104 | 128.7 (121.3–136.5) | |
| 50–64 | NA | 7538 | 185.5 (181.4–189.8) | 1843 | 128.6 (122.8–134.6) | 474 | 175.7 (160.6–192.3) | |
| 65–79 | NA | 2390 | 137.0 (131.6–142.6) | 636 | 125.4 (116.0–135.5) | 155 | 168.9 (144.3–197.7) | |
| ≥80 | NA | 577 | 127.8 (117.8–138.7) | 172 | 127.5 (109.8–148) | 37 | 167.9 (121.6–231.7) | |
| Region† | ||||||||
| South | 34,973 | 221.4 (219.1–223.8) | 74,808 | 178.9 (177.6–180.2) | 28,941 | 128.9 (127.4–130.4) | 4266 | 94.3 (91.5–97.2) |
| West | 9138 | 164.7 (161.3–168.1) | 16,396 | 105.3 (103.7–106.9) | 5562 | 76.5 (74.5–78.5) | 788 | 55.8 (52.0–59.8) |
| Northeast | 9208 | 104.9 (102.8–107) | 25,758 | 80.9 (79.9–81.9) | 10,602 | 48.5 (47.6–49.5) | 987 | 30.1 (28.2–32.0) |
| Midwest | 14,635 | 144.4 (142.1–146.8) | 28,097 | 99.4 (98.3–100.6) | 9804 | 69.5 (68.1–70.9) | 1029 | 51.8 (48.7–55.1) |
| 14 jurisdictions | ||||||||
| Overall deaths | 1512 | 4.00 (3.80–4.20) | 1779 | 1.62 (1.55–1.7) | 376 | 0.6 (0.54–0.67) | 54 | 0.5 (0.38–0.66) |
| Age — yr | ||||||||
| 12–17 | 0 | 0‡ | 1 | 0.11 (0.01–0.75)‡ | 0 | 0‡ | 0 | 0‡ |
| 18–49 | 9 | 0.08 (0.04–0.16)‡ | 44 | 0.11 (0.09–0.15) | 17 | 0.05 (0.03–0.08) | 5 | 0.09 (0.04–0.21)‡ |
| 50–64 | 58 | 0.81 (0.63–1.05) | 286 | 0.82 (0.73–0.92) | 90 | 0.63 (0.51–0.78) | 11 | 0.55 (0.3–0.99)‡ |
| 65–79 | 462 | 3.29 (3.00–3.60) | 754 | 2.62 (2.44–2.82) | 151 | 3.3 (2.81–3.87) | 26 | 3.59 (2.44–5.27) |
| ≥80 | 983 | 17.06 (16.02–18.16) | 694 | 9.96 (9.25–10.73) | 118 | 11.46 (9.57–13.72) | 12 | 7.28 (4.13–12.82)‡ |
| Vaccine product | ||||||||
| BNT162b2 | 1135 | 5.49 (5.18–5.82) | 869 | 1.55 (1.45–1.66) | 148 | 0.42 (0.36–0.49) | 15 | 0.22 (0.13–0.36) |
| mRNA-1273 | 377 | 2.2 (1.99–2.43) | 600 | 1.4 (1.3–1.52) | 127 | 0.57 (0.48–0.68) | 9 | 0.34 (0.18–0.65)‡ |
| Ad26.COV2.S | NA | 310 | 2.8 (2.5–3.13) | 101 | 1.97 (1.62–2.39) | 30 | 2.52 (1.76–3.6) | |
| Vaccine product and recipient age — yr | ||||||||
| BNT162b2 | ||||||||
| 12–17 | 0 | 0‡ | 1 | 0.11 (0.01–0.75)‡ | 0 | 0‡ | 0 | 0‡ |
| 18–49 | 5 | 0.08 (0.03–0.20)‡ | 20 | 0.09 (0.06–0.14) | 6 | 0.03 (0.01–0.07)‡ | 2 | 0.06 (0.02–0.25)‡ |
| 50–64 | 38 | 0.99 (0.72–1.36) | 124 | 0.68 (0.57–0.81) | 34 | 0.51 (0.36–0.71) | 6 | 0.56 (0.25–1.25)‡ |
| 65–79 | 318 | 4.26 (3.82–4.76) | 378 | 3.03 (2.74–3.35) | 59 | 3.02 (2.34–3.90) | 5 | 1.37 (0.57–3.3)‡ |
| ≥80 | 774 | 23.55 (21.94–25.26) | 346 | 12.72 (11.45–14.13) | 49 | 11.89 (8.98–15.73) | 2 | 2.56 (0.64–10.26)‡ |
| mRNA-1273 | ||||||||
| 18–49 | 4 | 0.08 (0.03–0.22)‡ | 10 | 0.09 (0.05–0.16)‡ | 10 | 0.07 (0.04–0.14)‡ | 0 | 0‡ |
| 50–64 | 20 | 0.60 (0.39–0.94) | 70 | 0.54 (0.43–0.69) | 28 | 0.45 (0.31–0.66) | 0 | 0‡ |
| 65–79 | 144 | 2.19 (1.86–2.57) | 252 | 1.73 (1.53–1.95) | 47 | 2.19 (1.65–2.91) | 6 | 2.20 (0.99–4.89)‡ |
| ≥80 | 209 | 8.44 (7.37–9.67) | 268 | 7.03 (6.24–7.93) | 42 | 8.60 (6.36–11.64) | 3 | 4.55 (1.47–14.11)‡ |
| Ad26.COV2.S | ||||||||
| 18–49 | NA | 14 | 0.27 (0.16–0.45)‡ | 1 | 0.03 (0–0.22)‡ | 3 | 0.36 (0.12–1.12)‡ | |
| 50–64 | NA | 92 | 2.44 (1.99–3.00) | 28 | 2.08 (1.43–3.01) | 5 | 1.96 (0.82–4.71)‡ | |
| 65–79 | NA | 124 | 7.38 (6.19–8.80) | 45 | 9.33 (6.97–12.5) | 15 | 17.24 (10.39–28.59) | |
| ≥80 | NA | 80 | 18.29 (14.69–22.77) | 27 | 20.9 (14.33–30.48) | 7 | 33.37 (15.91–69.99)‡ | |
| Region | ||||||||
| South | 1142 | 7.23 (6.82–7.66) | 1285 | 3.07 (2.91–3.25) | 280 | 1.25 (1.11–1.4) | 44 | 0.97 (0.72–1.31) |
| West | 102 | 1.84 (1.51–2.23) | 130 | 0.83 (0.7–0.99) | 31 | 0.43 (0.3–0.61) | 3 | 0.21 (0.07–0.66)‡ |
| Northeast | 92 | 1.05 (0.85–1.29) | 164 | 0.51 (0.44–0.60) | 34 | 0.16 (0.11–0.22) | 4 | 0.12 (0.05–0.32)‡ |
| Midwest | 176 | 2.28 (1.97–2.65) | 200 | 0.97 (0.84–1.11) | 31 | 0.28 (0.20–0.40) | 3 | 0.20 (0.06–0.61)‡ |
Rates for counts less than 15 may be unstable and should be interpreted with caution. NA denotes not applicable.
Regions comprise the following jurisdictions: South (Arkansas, Georgia, Florida, and Tennessee), West (Arizona, Idaho, New Mexico, and Utah), Northeast (Massachusetts, New Jersey, and New York City), and Midwest (Indiana, Michigan, Nebraska, and Wisconsin). Wisconsin did not contribute death data due to lack of linkage between vital records and COVID-19 case surveillance.
Rates were calculated using person-weeks as denominator, and 95% confidence intervals provided in parentheses. The widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible.
Figure 2.
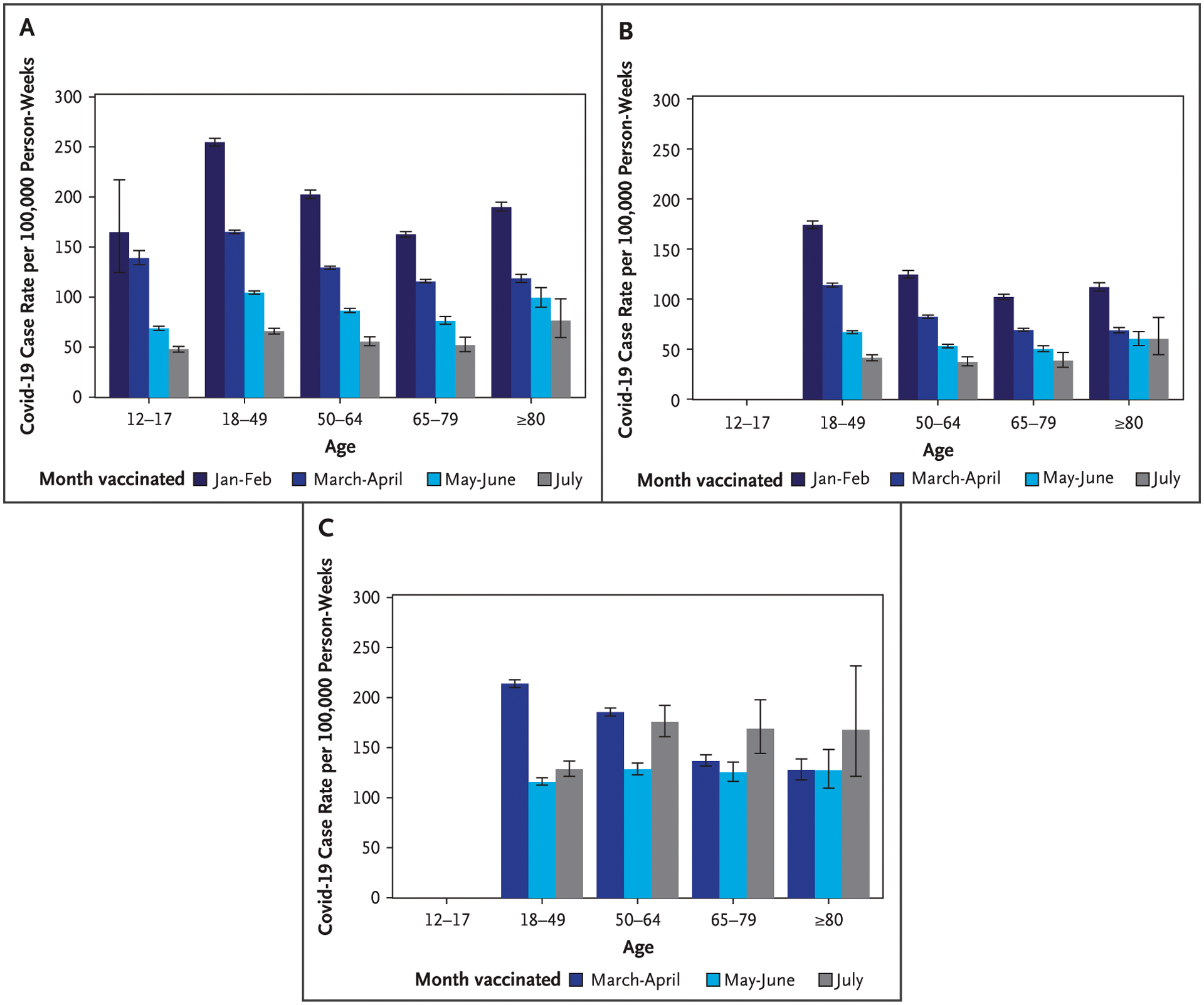
Covid-19 Case Rates by Vaccination Cohort and Age for the BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines for 15 U.S. Jurisdictions, from August 1 to September 4, 2021.
Covid-19 case rates are presented by vaccination cohort and age for the A) BNT162b2 (Pfizer–BioNTech), B) mRNA-1273 (Moderna), and C) Ad26.COV2.S (Janssen–Johnson & Johnson) vaccines. Error bars represent 95% confidence intervals. The widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible. Persons vaccinated with the Ad26.COV2.S vaccine in the January to February cohort and all persons 12 to 17 years of age vaccinated with either the mRNA-1273 or Ad26.COV2.S vaccine were excluded.
Model adjusted case rates during August 1 to September 4 among those vaccinated in January to February, March to April, and May to June were 3.44 (95% CI, 3.36 to 3.53), 2.29 (95% CI, 2.23 to 2.34), and 1.44 (95% CI, 1.41 to 1.48) times higher than among those vaccinated in July, respectively (Table S2).
DEATH RATES FROM JANUARY 3 TO SEPTEMBER 4, 2021
Death rates per 100,00 person-weeks were 0.76 (95% CI,0.75 to 0.78) across all ages and vaccine products during January 3 to September 4, 2021 (Table 1). There was only one death among vaccinated children and adolescents 12 to 17 years of age. Death rates per 100,000 person-weeks were highest among persons 80 years of age or older(4.71; 95% CI, 4.55 to 4.88) compared with younger age groups (range, 0 to 1.11), were highest in the South (1.37; 95% CI, 1.33 to 1.42) compared with other regions (range,0.28 to 0.53), and were highest among recipients of the Ad26.COV2.S vaccine (1.27; 95% CI, 1.18 to 1.36), followed by recipients of the BNT162b2 (0.85; 95% CI, 0.82 to 0.87) and mRNA-1273 (0.56; 95% CI, 0.54 to 0.59) vaccines (Table 1). Larger differences in the mortality hazard rates between vaccination cohorts were observed after July 2021 compared with before delta circulation (Figs. 1B and S4A and Table S1).
DEATH RATES DURING A PERIOD OF DELTA PREDOMINANCE
Death rates from August 1 to September 4, 2021 were similar by vaccination cohort for mRNA-1273 vaccine recipients in all age groups and BNT162b2 vaccine recipients 12 to 64 years of age (Fig. 3A and 3B). BNT162b2 vaccine recipients 65 to 79 and 80 years of age or older had higher death rates among those vaccinated earlier in the year compared with those vaccinated in July (Fig. 3A and Tables 2 and S3). Similar patterns were observed by region (Fig. S5). Death rates by age group among Ad26.COV2.S vaccine recipients were higher among those vaccinated in July, specifically among those 65 to 79 years of age (Fig. 3C and Table S3). Death rates during August 1 to September 4 among those vaccinated 6 months ago or longer (January to February) were 1.7 (95% CI, 1.29 to 2.23) times higher than among those vaccinated more recently in July (Table S3).
Figure 3.
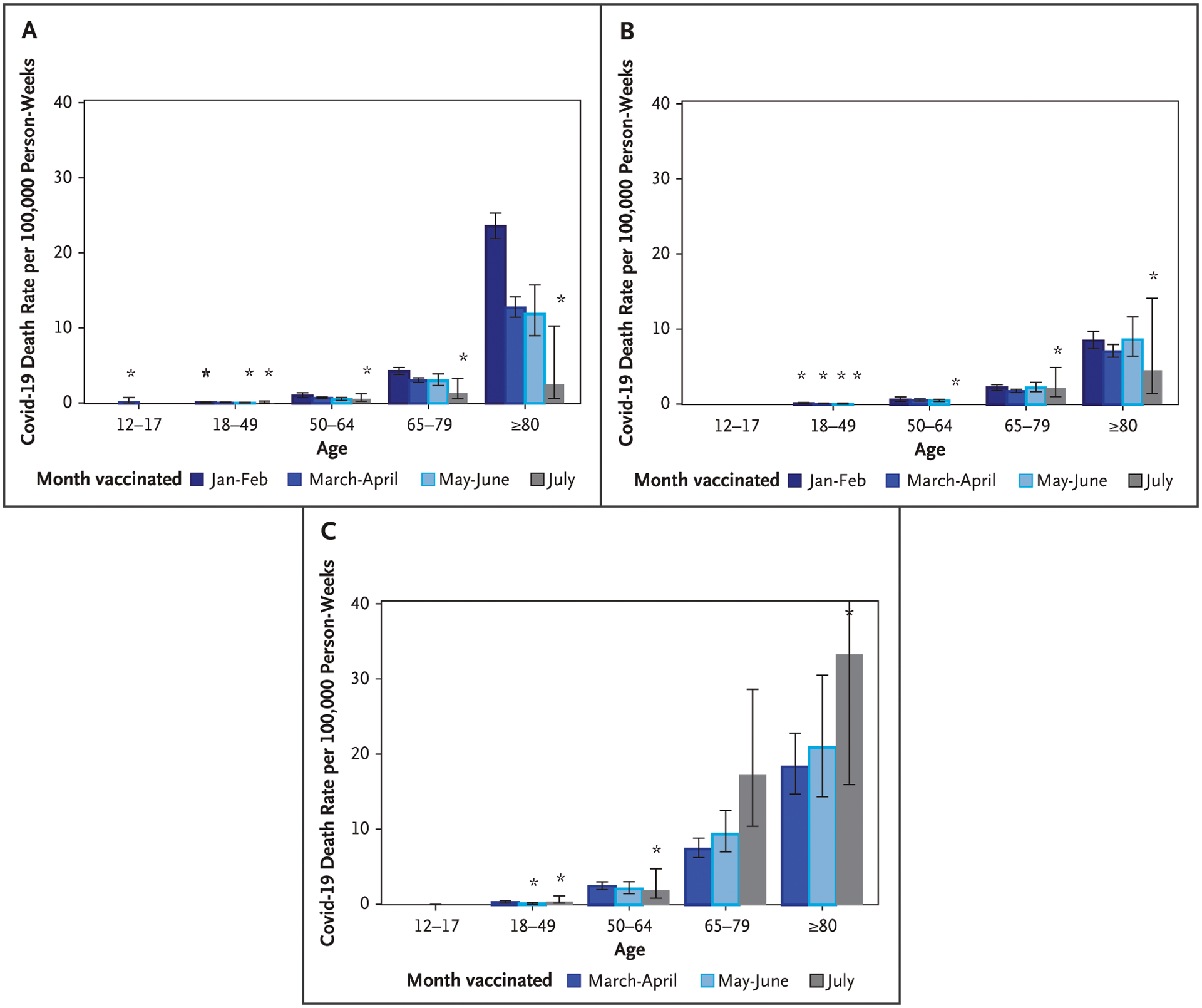
Covid-19 Death Rates by Vaccination Cohort and Age for the BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines for 14 U.S. Jurisdictions, from August 1 to September 4, 2021.
Covid-19 death rates are presented by vaccination cohort and age for the A) BNT162b2 (Pfizer–BioNTech), B) mRNA-1273 (Moderna), and C) Ad26.COV2.S (Janssen–Johnson & Johnson) vaccines. Only 14 jurisdictions contributed deaths. Wisconsin did not contribute death data due to lack of linkage between vital records and COVID-19 case surveillance. Error bars represent 95% confidence intervals. The widths of the intervals have not been adjusted for multiplicity and any of the inferences drawn may not be reproducible. Persons vaccinated with the Ad26.COV2.S vaccine in the January to February cohort and all persons 12 to 17 years of age vaccinated with either the mRNA-1273 or Ad26.COV2.S vaccine were excluded. *For a death count that is 15 or fewer, the rate may be unstable and should be interpreted with caution.
Discussion
Among 15 geographically diverse jurisdictions representing approximately 32% of the U.S. population and including nearly 50 million vaccinated persons and close to half a million Covid-19 cases, we found higher cumulative case rates among vaccinated persons ages 18 to 49 years compared with vaccinated persons of other ages; this is similar to what has been observed in the total vaccinated and unvaccinated population in August 2021.15 We also found higher rates among recipients of the Ad26.COV2.S vaccine compared with recipients of the BNT162b2 and mRNA-1273 vaccines. Overall death rates were higher among persons 80 years of age or older compared with younger age groups and among those who received the Ad26.COV2.S vaccine compared with the BNT162b2 and mRNA-1273 vaccinees, as reported elsewhere.16 Differences in case rates by vaccination cohort were relatively minor before delta became the predominant variant in July 2021. Subsequently, case rates were higher among mRNA-1273 and BNT162b2 vaccine recipients who were vaccinated in earlier months compared with recently vaccinated people, suggesting waning protection against infection. Although Covid-19 case rates for recipients of the Ad26.COV2.S vaccine during the entire study period were highest, a consistent pattern of waning immunity was not observed. While death rates were low among all vaccinated persons, we observed increased death rates among recipients of the BNT162b2 vaccine 65 years of age or older who were vaccinated in earlier months. Death rates among recipients of the Ad26.COV2.S vaccine were also higher overall. There was less evidence of waning protection against death with the mRNA-1273 vaccine.
Across all age groups, rates of SARS-CoV-2 infection among recipients of mRNA vaccines who were vaccinated at different times showed an increase in infection rates among persons vaccinated earlier. A similar analytical approach from Israel suggested waning immunity among BNT162b2 vaccine recipients,5,6 whereas a study from New York State showed small differences in VE associated with waning.1,17 These apparently contradictory results when evaluating rates among vaccinated individuals versus vaccine efficacy provide complementary perspectives on the data. The former perspective emphasizes rates that differ over time since vaccination, focusing on the relative differences among vaccination cohorts; the latter perspective shows that these differences are relatively small when compared with the unvaccinated population. Like other studies,18 we showed that despite waning of protection against infection, vaccine protection is largely sustained against death, even when delta was the predominant variant. However, our findings suggest waning protection against death among BNT162b2 vaccine recipients 65 years of age or older.
Unlike for the mRNA vaccines, rates among Ad26.COV2.S vaccination cohorts did not follow a consistent pattern across the entire period that was suggestive of waning immunity, except for vaccine recipients 18 to 49 years of age. Higher case and death rates among recently vaccinated Ad26.COV2.S vaccine recipients may be explained by the single vaccine dose and relatively limited time for this group to develop robust antibody and cellular immune responses compared with the mRNA vaccines. Because the CDC defines a person fully vaccinated as 14 days or more after completion of the full primary series, BNT162b2 and mRNA-1273 vaccine recipients have 35 and 42 days, respectively, to develop immunity from their first dose, whereas Ad26.COV2.S vaccine recipients have only 14 days. This explanation is consistent with the bimodal distribution observed for breakthrough cases among Ad26.COV2.S vaccine recipients, in which initial increases in breakthrough cases were observed up to 42 days after receipt of the Ad26.COV2.S vaccine and a second peak was observed at 4 to 5 months; by comparison, BNT162b2 and mRNA-1273 vaccine recipients had a single peak at approximately 4 to 5 months.
Studies have shown differential kinetics of immune responses induced by the mRNA and adenovirus vector vaccines, with lower overall antibody levels and less evidence of decay 6 to 8 months after vaccination for the Ad26.COV2.S vaccine compared with the mRNA vaccines.19,20 The Ad26.COV2.S phase 3 trial also suggested lower vaccine efficacy for moderate to severe disease at 14 days postvaccination, which increased gradually until about 30 days and then remained stable. For severe to critical disease, the highest vaccine efficacy was observed even later at 56 days.21 Importantly, our study found higher relative case and death rates among Ad26.COV2.S vaccine recipients compared with other vaccine product recipients.
As of mid-November 2021, 58% of the U.S. population had been fully vaccinated against Covid-19 and 68% had received at least one vaccine dose.22 It seems reasonable to believe that booster doses could reduce the risk of severe disease in elderly persons and potentially help reduce transmission of SARS-CoV-2 among other age groups.17,23–25 However, vaccinating the unvaccinated continues to be a public health priority. A recent modeling study suggests that increasing vaccine coverage from current levels to 84% could reduce the reproductive number, which quantifies the average number of cases that each case goes on to infect by 37%.26 Furthermore, reaching persons who have not been vaccinated could prevent more severe disease and deaths than booster doses among young and healthy populations, since we observe waning of protection against infection but sustained protection against death among persons younger than 65 years of age.26 Data from 24 jurisdictions in the United States highlight the enormous impact of vaccination in reducing infection and death risk. Incidence rates for the month of August 2021 show that unvaccinated people had 7 times the risk of being infected with SARS-CoV-2 and 14 times the risk of dying from Covid-19 compared with vaccinated people. The rates among unvaccinated individuals compared with vaccinated persons were 116.0 and 783.5 Covid-19 cases per 100,000 and 1.2 and 17.2 deaths per 100,000 persons.16
Among the study limitations, we were unable to adjust for different testing and prevention behaviors by age and over time or for differences in local SARS-CoV-2 transmission to account for geographic heterogeneity, although we did adjust rate ratios by region. We were not able to identify reinfections in the study database and such cases were therefore not excluded from the analyses. Reduced access to testing earlier in 2021 may have resulted in a greater number of unrecognized infections in earlier cohorts. Persons of the same group vaccinated earlier may be different from those vaccinated more recently in ways we cannot control for in the analyses, as early vaccine rollouts were targeted toward potentially higher-risk exposure settings (e.g., health care workers, long-term care facility residents) and older adults who were often frail and had a higher probability of dying over the next 9 months, whereas later vaccine recipients may be more likely to represent the general population. Different case surveillance, testing, immunization, and vital records databases were integrated for these analyses. These databases typically lack a common unique identifier to match records using patient-specific information; thus, linkage may be inaccurate or incomplete. As we only analyzed breakthrough cases occurring 14 days after completion of the primary series as defined, overall rates may be underestimated because some persons may have had a positive test result 0 to 13 days after the primary series and should have been removed from the at-risk set. This underestimation likely has a differential impact on the cohorts more recently vaccinated, as the risk of an early infection tracks with varying SARS-CoV-2 transmission risk over time.
Monitoring of trends and timing of Covid-19 infections, hospitalizations,27 and deaths16 among vaccinated persons is needed to inform vaccination strategies and recommendations for booster doses. The CDC continues to collaborate with health departments to monitor these trends.16,28 Findings from this study are consistent with what has been reported elsewhere1–6,9 and underscore the likely value of boosters for certain high-risk groups17,23–25 and continued use of nonpharmaceutical interventions, including among vaccinated persons.
Supplementary Material
Acknowledgments
We thank the contributions of the Vaccine Breakthrough Unit in the Epidemiology Task Force, the Data Monitoring and Reporting Section in the Vaccine Task Force, the Vaccine Data Section, and Adam Langer, D.V.M., and Victoria Seffren, M.P.H., from the line-level data team in the Case Data Section, Data Analytics and Visualization Task Force, COVID-19 Response, Centers for Disease Control and Prevention, Atlanta.
Funded by the Centers for Disease Control and Prevention.
Footnotes
Disclosures
Disclosure forms provided by the authors are available with the full text of this article at evidence.nejm.org.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author disclosures and other supplementary material are available with the full text of this article at evidence.nejm.org.
References
- 1.Rosenberg ES, Dorabawila V, Easton D, et al. COVID-19 vaccine effectiveness by product and timing in New York State. October 9, 2021. (https://www.medrxiv.org/content/10.1101/2021.10.08.21264595v1).
- 2.Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status — New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1150–5 10.15585/mmwr.mm7034e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med 2021;27: 1614–21 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585–94 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y, Mandel M, Bar-On Y, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. August 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1). [DOI] [PMC free article] [PubMed]
- 6.Goldberg Y, Mandel M, Bar-On Y, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021;385:e85 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, Moreira ED, Kitchin N. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. July 28, 2021. (https://www.medrxiv.org/content/10.1101/2021.07.28.21261159v1/?ipid=post_link_2). [DOI] [PMC free article] [PubMed]
- 8.El Sahly HM, Baden LR, Essink B, et al. ; COVE Study Group. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774–85 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Y, Gu YB, Wheeler B, et al. Effectiveness of Covid-19 vaccines in the United States over 9 months: surveillance data from the State of North Carolina. October 26, 2021. (https://www.medrxiv.org/content/10.1101/2021.10.25.21265304v1).
- 10.Centers for Disease Control and Prevention. CDC expands eligibility for COVID-19 booster shots. October 21, 2021. (https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html).
- 11.Centers for Disease Control and Prevention. CDC statement on ACIP booster recommendations. September 24, 2021. (https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html).
- 12.Centers for Disease Control and Prevention. COVID-19 vaccine booster shots. Updated December 9, 2021. (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html).
- 13.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): 2021 case definition. August 24, 2021. (https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2021/).
- 14.Centers for Disease Control and Prevention. MMWR weeks. 2021. (https://ndc.services.cdc.gov/wp-content/uploads/MMWR_Week_overview.pdf).
- 15.Centers for Disease Control and Prevention. COVID-19 weekly cases and deaths per 100,000 population by age, race/ethnicity, and sex. 2021. (https://covid.cdc.gov/covid-data-tracker/#demographicsovertime).
- 16.Centers for Disease Control and Prevention. Rates of COVID-19 cases and deaths by vaccination status. COVID Data Tracker. 2021. (https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status).
- 17.Levine-Tiefenbrun M, Yelin I, Alapi H, et al. Viral loads of delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med 2021. November 2 (Epub ahead of print) 10.1038/s41591-021-01575-4. [DOI] [PubMed] [Google Scholar]
- 18.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. November 18, 2021. (https://ssrn.com/abstract=3961378). [DOI] [PMC free article] [PubMed]
- 19.Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021;385:951–3 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier AY, Yu J, McMahan K, et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med 2021;385:2010–2 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadoff J, Gray G, Vandebosch A, et al. ; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–201 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Trends in number of COVID-19 vaccinations in the US. COVID Data Tracker. 2021. (https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-onedose-cum).
- 23.Saciuk Y, Kertes J, Shamir Stein N, Ekka Zohar A. Effectiveness of a third dose of BNT162b2 mRNA vaccine. J Infect Dis 2021;jiab556 10.1093/infdis/jiab556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews N, Stowe J, Kirsebom F, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of BNT162b2 (Comirnaty, Pfizer-BioNTech) COVID-19 booster vaccine against covid-19 related symptoms in England: test negative case-control study. November 15, 2021. (https://www.medrxiv.org/content/10.1101/2021.11.15.21266341v1).
- 25.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385: 1393–400 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner BJ, Kilpatrick AM. Third doses of COVID-19 vaccines reduce infection and transmission of SARS-CoV-2 and could prevent future surges in some populations: a modeling study. November 29, 2021. (https://www.medrxiv.org/content/10.1101/2021.10.25.21265500v3).
- 27.Centers for Disease Control and Prevention. Rates of laboratory-confirmed COVID-19 hospitalizations by vaccination status. COVID Data Tracker. 2021. (https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination).
- 28.Scobie HM, Johnson AG, Suthar AB, et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status — 13 U.S. jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1284–90 10.15585/mmwr.mm7037e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


