Abstract
Plastic pollution has become a non-negligible global pollution problem. Nanoplastics (NP) can reach the bone marrow with blood circulation and develop hematotoxicity, but potential mechanisms and prevention strategies are lacking. Here, we report the biological distribution of NP particles in the bone marrow of mice and hematopoietic toxicity after exposure to 60 μg of 80 nm NP for 42 days. NP exposure inhibited the capability of bone marrow hematopoietic stem cells to renew and differentiate. Notably, probiotics and melatonin supplementation significantly ameliorated NP-induced hematopoietic damage, and the former was superior to the latter. And interestingly, melatonin and probiotic interventions may involve different microbes and metabolites. After melatonin intervention, creatine showed a stronger correlation with NP-induced gut microbiota disorders. In contrast, probiotic intervention reversed the levels of more gut microbes and plasma metabolites. Of these, threonine, malonylcarnitine, and 3-hydroxybutyric acid might be potential performers in the regulation of hematopoietic toxicity by gut microbes, as they had a more significant relationship with the identified microbes. In conclusion, supplementation with melatonin or probiotics may be two candidates to prevent hematopoietic toxicity attributable to NP exposure. Also, the multi-omics results may lay the foundation for future investigations into in-depth mechanisms.
Keywords: gut microbiota-metabolism, melatonin, probiotics, nanoplastics exposure, hematopoietic injury
1. Introduction
Plastic pollution has become the second leading environmental problem worldwide [1]. From 1950 to 2018, plastic production increased by an average of 128 million tons per year [2]. It is estimated that by 2050, the amount of plastic waste generated globally will increase to 26 billion tons [3]. Due to the poor biodegradability and insufficient recycling of plastics, a large amount of plastic waste is retained in the atmosphere, soil, and water environment [4]. People are directly or indirectly exposed to plastic pollution via ingestion, inhalation, and dermal contact, resulting in multi-organ damage [5]. Epidemiological studies have shown that microplastics (MP) of different sizes have been found in human skin, hair, saliva, sputum, and nasal rinses [6]. However, the toxicity of plastic particles of different sizes and their corresponding targets are not well understood.
Particle size is a direct influence on the ability of micro- and nanoplastics (MNP) to reach the target organ of toxic effects from circulation. Several studies have reported substantial toxic effects of MP (size < 5 mm) and NP (size < 100 nm) exposure on marine animals, freshwater organisms, and mammalian species [7]. MP with a size of fewer than 150 μm can be transferred from the intestine to the lymphatic and circulatory systems and subsequently bind to blood proteins to form protein-plastic complexes, leading to vascular obstruction [8,9]. After 28 days of oral gavage of 0.1 mg/day 5 and 20 μm polystyrene (PS), a significant distribution was also observed in the liver, kidney, and intestine of mice [10]. Notably, there may be interactions between MP and NP, which may affect their biodistribution and toxicity in mice [11]. Yang et al. also demonstrated that although both PS-MP and NP can penetrate the mucosal barrier of the digestive tract, only the latter can cross the placental barrier into the fetal thalamus [12]. This provides evidence that NP can be transferred to distant tissues and act as a toxic agent.
Hematopoiesis is a highly regulated and complicated biological process engaging hematopoietic stem cells (HSCs) and cells of the hematopoietic niche [13]. Among them, long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs) are mainly responsible for the renewal cycle of HSCs. While multipotent progenitors (MPPs) develop into a wide range of mature blood cells via extensive proliferation and differentiation activities. Currently, very limited studies have reported adverse effects of MNP on the hematopoietic system. After 14 days of exposure to 100 nm PS-NP (concentration from 0.75 × 105–3 × 105 particles/cm3), eosinophil percentage, lymphocyte, and white blood cell (WBC) counts were significantly decreased in SD rats [14]. Sun et al. also reported that exposure to 0.5 mg 5 μm PS-MP significantly led not only to a decrease in leukocyte counts in mice, but also inhibited the colony-forming ability of HSCs in the bone marrow (BM) [15]. These studies implicated that MNP exposure might disrupt BM hematopoietic pool and hematological parameters. However, whether low doses of NP can penetrate the BM and thus affect the renewal and differentiation of HSCs and the underlying mechanisms are not clear.
Gut microbiota may affect the metabolism and physiological indicators of the body and thereby modify the progression of the disease [16]. It is convincing that MP can enter the intestine directly and accumulate significantly [17], thus MNP intake is likely to be associated with gut microbiota dysbiosis and health status. After exposure to 1,000 μg/L 5 μm PS-MP for 42 days, MP fluorescent particles were scattered in the intestine of mice, and MP induced gut microbiota disorders, intestinal barrier dysfunction, and metabolic disorders in mice [17]. Compared to MP, NP of smaller size induces more severe microbiota dysbiosis and inflammation in the intestine of adult zebrafish [18]. Recent studies have indicated that gut microbiota plays an important role in hematopoietic regulation [19]. Moreover, metabolites of gut bacteria such as amino acids and fatty acids can influence the local metabolism of BM HSCs and immune cells, directing immune cell differentiation and function [20]. Remarkably, PS-NP with less than 100 nm can penetrate into the BM micro-environment [21]. Therefore, we speculate that NP may disrupt the homeostasis of BM hematopoiesis by affecting the gut microbiota and metabolic signaling molecules.
Numerous studies revealed that melatonin or probiotics alleviated the phenotype of the disease, such as inflammation, obesity, and lung damage by modulating the gut microbiota-metabolic axis [22–25]. However, whether these nutrient regulators can alleviate NP-induced hematopoietic damage and the underlying mechanisms have not been investigated. Hence, the present study aimed to 1) explore the effects of 60 μg/day of low dose 80 nm NP exposure for 42 days on BM hematopoiesis; 2) investigate the critical gut microbes and metabolites involved in NP-induced hematopoietic damage; 3) explore the protective effects of supplementation melatonin or probiotics on NP-induced hematopoietic damage, gut microbiota disorders, and metabolic disorders. This study provides a new perspective on the underlying mechanism of NP-induced hematopoietic injury and reveals that melatonin or probiotics may potentially be effective in preventing or protecting against hematopoietic injury induced by NP exposure.
2. Materials and methods
2.1. Materials and reagents
Fluorescent and pristine 80 nm PS-NP particles (10 mg/mL, BaseLine Chromatography Technology Development Center, Tianjin, China) were used for fluorescent tracing and toxicology tests, respectively. Melatonin (Sigma, GER) was first dissolved in anhydrous ethanol as a stock solution and then diluted in sterile water as a working solution (final concentration of ethanol < 1%). Bifico (bifidobacterium longum, lactobacillus acidophilus, and enterococcus faecalis ≥ 1 × 107 colony-forming units (CFU)), a commercial probiotic reagent (Shanghai Xinyi Pharmaceutical Co., Ltd.) was dissolved in sterile water, prepared into aliquots, and stored at −20 °C.
2.2. Characterization of PS-NP
PS-NP particles were characterized in the previous study [26]. Here, the morphology and dispersion of PS-NP were observed by scanning electron microscopy (SEM, Hitachi S-4800, Japan) and transmission electron microscopy (TEM, HT7700, Japan). The zeta potential of PS-NP was detected by a nanoparticle size potentiometer (Zetasizer Nano ZSP, Malvern, UK).
2.3. Animal treatments
All animal experiments were approved by the Animal Care and Use Committee of Capital Medical University (No. AEEI-2020-168), and the mice (male, 6 weeks old, 18–22 g) were purchased and housed at the SPF-level Capital Medical University Laboratory Animal Center. After a week of adaptation, each mouse was given 250 μL of pure water (control group: n = 9) or 60 μg/day of pristine PS-NP in aqueous solution (NP exposure group: n = 9) by oral gavage for 42 days. Meanwhile, NP-exposed mice were given 10 mg/(kg·bw) melatonin (NP+Melatonin group: n = 9) or 4.2 g/(kg·bw) bifico (NP+Bifico group: n = 9) reagent by oral gavage to assess the effect of melatonin or probiotic supplementation on NP-induced hematopoietic toxicity. All mice were weighed weekly during exposure and were sacrificed after 42 days to obtain biological samples (blood and cecum contents) as well as the relative weights of each organ (organ coefficient = individual organ weight/mouse weight).
2.4. Fluorescence imaging ex vivo
Each group of three mice was also treated with 60 μg/day of NP with orange fluorescent labels (Ex/Em: 540/610 nm) to track the distribution of NP in multi-tissues by IVIS® Lumina LT Series III imaging system (PerkinElmer Ltd., USA). Mice were sacrificed at 2, 4, and 6 weeks of exposure, respectively, and individual fresh tissues were placed sequentially on the sample tray for fluorescence imaging. Mean fluorescence intensity was quantified using Image pro-plus 6.0.
2.5. Flow cytometry
After being sacrificed, the mouse BM cells were collected from femur tissue using ice-cold phosphate buffered solution (PBS) and filtered via a 70 μm cell strainer. After red blood cells (RBC) lysis, 200 μL of antibody mixture was added and mixed thoroughly and incubated on ice for 30 min. The cells were washed twice by adding 5 mL sterile PBS and centrifuged at 1,500 rpm for 5 min. Finally, the cells were resuspended in 1 mL complete RPMI 1640 medium and sorted by flow cytometry for Lin/Scal-1/c-Kit cells (LSKs), MPPs, LT-HSCs, and ST-HSCs. All antibodies were acquired from Biolegend (San Diego, California) and detailed information was described in the previous article [27].
2.6. Histopathology
Each group of three mice femurs was separated and fixed with 4% paraformaldehyde for histopathological analysis. Before hematoxylin and eosin (H&E) staining, mouse femurs were subjected to decalcification and paraffin embedding procedures. Femur sections were scanned using a Panoramic Digital Slide Scanner (3DHISTECH Ltd., Hungary) at ×20 magnification and processed using CaseViewer software.
2.7. Gut microbial profiles
Each group of six mice was randomly assigned for gut microbiota profiling. The detailed protocols were mentioned in our previous study [26]. In brief, the mouse cecum was separated on ice and the fecal contents were rinsed with 5 mL of sterile PBS. After centrifugation (1,500 rpm, 5 min), the lower fecal contents were collected for total genomic DNA extraction (PowerSoil DNA Isolation kit, MO BIO Laboratories, USA). For each fecal sample, the concentration and purify of DNA were measured, and the V1–V9 region of the bacterial 16S rRNA gene was amplified using the universal primers with the barcode (27F: AGRGTTTGATYNTGGCTCAG; 1492R: TASGGHTACCTTGTTASGACTT). All libraries were sequenced by using the Pacbio Sequel II platform at Biomarker Technologies (Beijing, China), and the α-diversity and β-diversity analyses were carried out in the BMKCloud (http://www.biocloud.net).
2.8. Plasma metabolic profile
The extraction and determination of plasma metabolites were conducted following previously described protocols [28]. Briefly, 20 μL of each plasma sample was added to 120 μL of internal standard and mixed completely. After centrifugation at 4,000g for 30 min, the supernatant was transferred to a 96-well plate for derivatization by adding 20 μL of derivatization reagent and kept at a constant temperature of 30 °C for 60 min. All quantitative targeted metabolomics analysis was done by Metabo-Proflle Biotechnology Co., Ltd. (Shanghai, China) based on ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) (ACQUITY UPLC-Xevo TQ-S, Waters Corp, Milford, MA, USA). The optimal chromatographic and MS analysis conditions were presented in Table S1 in the Electronic Supplementary Material (ESM).
2.9. Statistical analysis
All statistical parameters were presented as mean ± standard deviation (SD). Multiple comparisons of continuous variables were performed using Student’s t-test or Mann–Whitney U test. Spearman correlation was used to analyze associations among hematopoietic indicators, differential microbiota, and plasma metabolites. All data analyses were performed using SPSS26.0 or Graphpad8.3 software, and the significance level was set at p < 0.05 (two-sided).
3. Results
3.1. Characterization of NP
As examined using SEM and TEM, 80 nm NP particles showed regular, uniform, and spherical morphology (Figs. S1(a) and S1(b) in the ESM). In addition, the zeta potential value of NP dissolved in pure water was −28.93 ± 1.36 (Fig. S1(c) in the ESM), indicating that NP had favorable stability and dispersibility in pure water.
3.2. Melatonin or probiotics alleviate the overall toxic effects of NP on mice
As illustrated in Fig. 1(a), NP-exposed mice were administered melatonin or probiotics by oral gavage daily. After 42 days of exposure, we evaluated the overall toxicity of NP in mice and the treatment effect of melatonin or probiotics on organ damage. Treated mice from NP, melatonin, or probiotic showed no statistical difference in body weight compared with control mice (Fig. 1(b)). Interestingly, the relative weights of the heart and some hematopoietic organs (spleen, lung, and thymus) were significantly decreased in mice after NP exposure (Figs. 1(c), 1(e), 1(f), and 1(h)), while statistical differences were not found in the liver, kidney, and testes between the two groups (Figs. 1(d), 1(g), and 1(i)). In contrast, probiotics supplementation significantly reversed these changes (Figs. 1(c), 1(e), 1(f), and 1(h)), whereas melatonin only improved NP-induced thymic toxicity (Fig. 1(h)).
Figure 1.
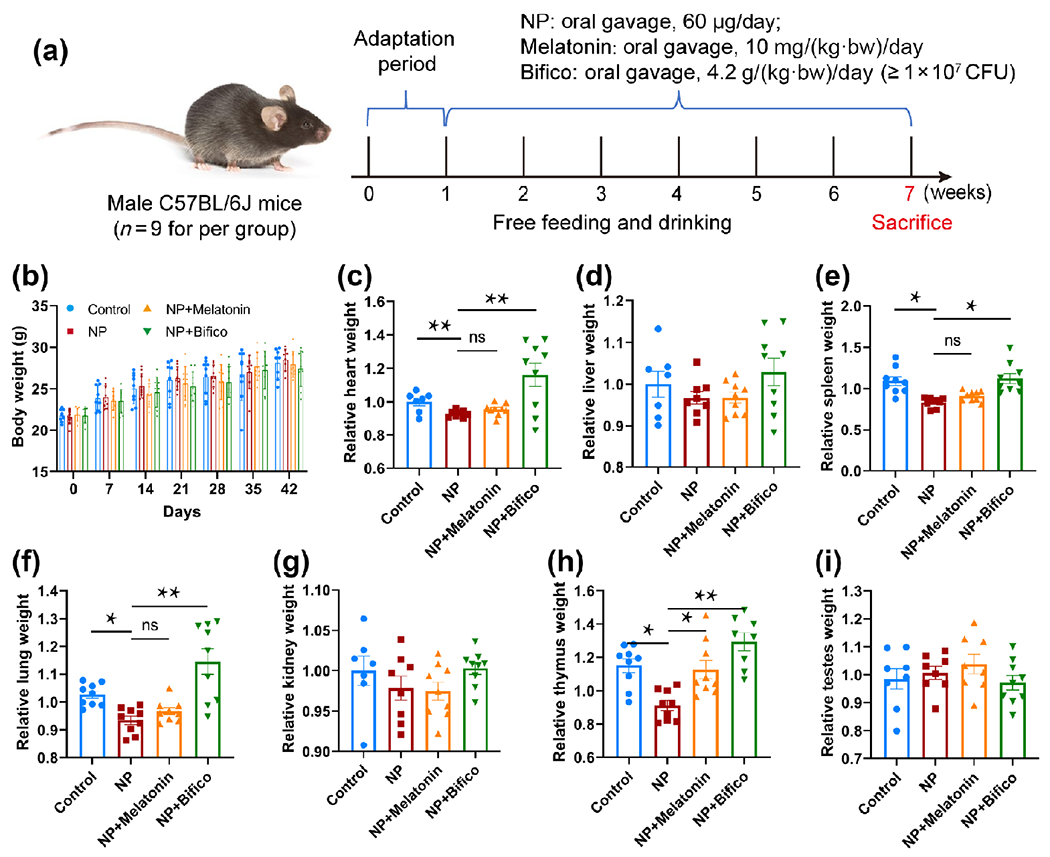
Assessment of the overall toxicity of NP exposure in mice and the treatment effect of melatonin or probiotics (n = 9). (a) Schematic diagram of animal experiment. (b) Effect of NP, melatonin, or probiotic treatment on body weight in mice. (c)–(i) Treatment effect of melatonin or probiotic supplementation on organ damage induced by NP. * p < 0.05, ** p < 0.01, ns: p > 0.05.
3.3. Melatonin or probiotics alleviate the effect of NP on bone marrow hematopoiesis
To investigate the biological distribution of NP in multiple tissues of mice, ex vivo tissue fluorescence imaging was performed. At 2 weeks of exposure, NP particles with orange fluorescence were extensively distributed in various tissues, including the brain, heart, spleen, kidney, intestines, lung, and liver. Unexpectedly, we found a weak orange fluorescence in the BM of mice, indicating that NP can already reach the BM through the blood circulation at the early stage of exposure (Fig. 2(a) and Fig. S2 in the ESM). BM is the key hematopoietic organ in mammals, and is responsible for the production and differentiation of hematopoietic cells. Here, we further examined the bioaccumulation of NP in the BM of mice. The results showed that the fluorescence intensity of NP particles in BM was enhanced with increasing exposure time (Fig. 2(a) and Fig. S3 in the ESM). To assess the negative effect of NP exposure on BM hematopoiesis, femur H&E staining and HSCs percentage were analyzed. After 42 days of exposure, mice showed disorganized cell arrangement in the BM and a significant decrease in nucleated hematopoietic cells. However, supplementation of melatonin or probiotics significantly prevented these hematopoietic damages (Fig. 2(b)). The flow results showed that NP exposure for 42 days inhibited the renewal and differentiation of HSCs in mice, with the main signatures being a decrease in LSK and MPPs and an increase in LT-HSCs and ST-HSCs (Figs. 2(c)–2(f)). Among them, MPPs are the leading regulator of differentiation into various types of blood cells, but melatonin or probiotic supplementation did not reverse the decline in MPPs (Fig. 2(d)). By contrast, there was a significant recovery of indicators associated with HSCs renewal, and the treatment effect of probiotics was superior to that of melatonin (Figs. 2(e) and 2(f)).
Figure 2.
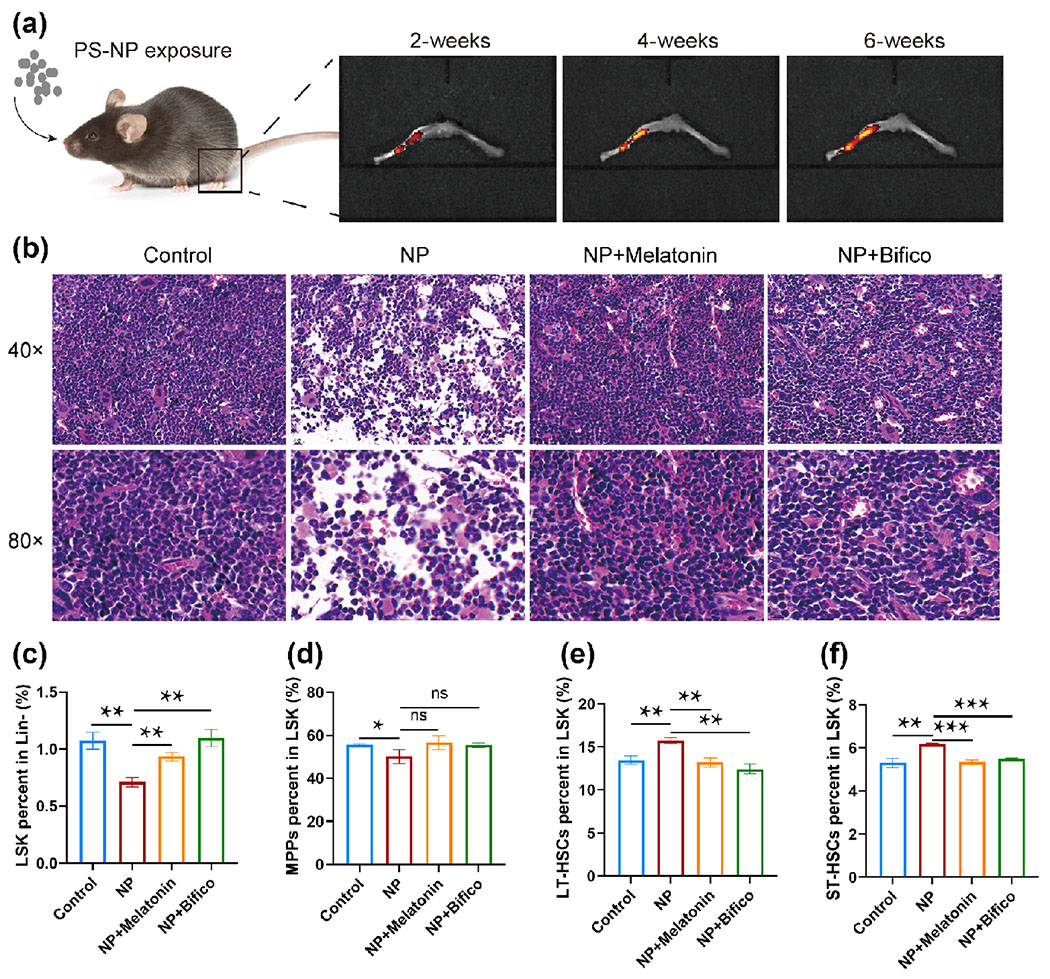
Melatonin or probiotics treatment alleviated hematopoietic damage in NP exposure mice (n = 3). (a) Distribution of 80 nm PS-NP in the bone marrow of mice. (b) Histopathological analysis of the femur. (c)–(f) Measurement of markers of hematopoietic damage by flow cytometry. *p < 0.05, **p < 0.01, ***p < 0.001, ns: p > 0.05.
3.4. Melatonin or probiotics alleviate NP-induced gut microbiota dysbiosis
Gut microbiota is strongly associated with environmental pollutant-induced hematopoietic damage. Hence, we further examined the microbial composition in the cecum contents after 42 days of NP exposure in mice. In the present study, we found 413, 420, 388, and 412 operational taxonomic units (OTUs) in the control, NP exposure, NP+Melatonin, and NP+Bifico groups, respectively, with 327 OTUs being common to all four groups (Fig. 3(a) and Table S2 in the ESM). The smooth Shannon–Wiener curves indicated that a sufficient number of sequences were acquired in all samples (Fig. 3(b)). As displayed in Figs. 3(c) and 3(d), the number of sequences in each group differed significantly and similar samples were clustered in the near distance, indicating a significant between-group difference in the gut microbial composition of mice, and less individual variation within groups. Further LEfSe analysis showed that NP exposure was indeed disrupting intestinal microbial homeostasis in mice, and 29 bacterial species were found to be significantly different in the four groups (Figs. 3(e) and 3(f)). Of these, 12 bacteria were statistically associated with markers of hematopoietic damage (Fig. 3(g)). Among these potential microbes, those in which both melatonin and probiotics play a reversal role include erysipelotrichaceae, lactobacillus taiwanensis, dubosiella, anaerotruncus, lactobacillus, and lactobacillales (Figs. 3(h)–3(k)). In addition, the probiotics also improved the disorder of prevotellaceae at the family level and muribaculum at the genus level (Figs. 3(h) and 3(i)).
Figure 3.
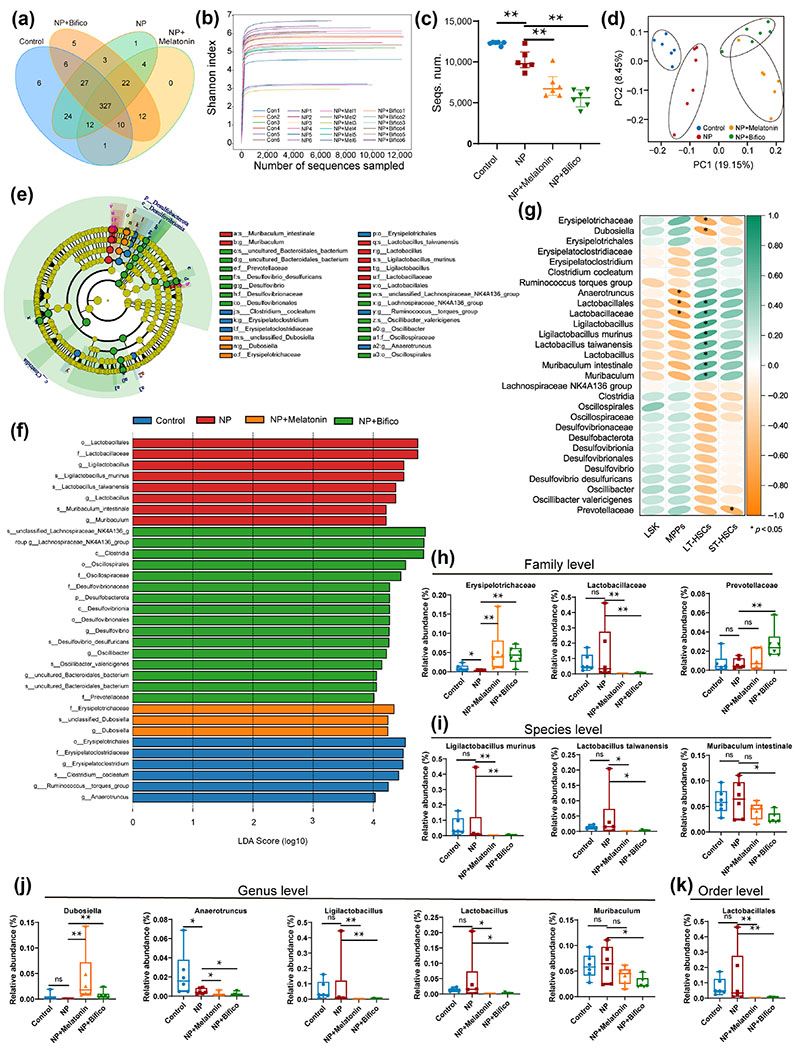
Melatonin or probiotics treatment alleviated gut microbiota disorders in NP exposure mice (n = 6). (a) Distribution of OTUs in the four groups. (b) The sequencing depth was characterized by Shannon–Wiener curves for all samples. (c) Number of sequences detected in each group. (d) PCoA plot analysis. (e) and (f) Differential gut microbial identification by LEfSe analysis (linear discriminant analysis (LDA) > 4). (g) Correlation between differential gut microbiota and hematopoietic damage by Spearman analysis. (h)–(k) Relative abundances of key microbiota at the family, species, genus, and order level, respectively. Values were presented as median ± quartile. Differences were assessed by Mann Whitney test and denoted as follows: *p < 0.05, **p < 0.01, ns: p > 0.05.
These results suggested that melatonin or probiotic supplementation prevented mice from NP exposure-induced hematopoietic damage by modulating specific gut microbes.
3.5. Melatonin or probiotics alleviate NP-related metabolic disorders
Metabolite alterations associated with the gut microbiome had been recognized as a possible mechanism by which microbes affect host health [29]. Therefore, we used full quantitative metabolomics to detect the active metabolites in mouse plasma in the present study. As shown in Fig. 4(a), the scatters of samples from the same group were presented as distinct clusters, indicating the heterogeneity of the effects of different treatments on plasma metabolites in mice. In a mouse model of NP-induced hematopoietic injury, a total of 26 differential metabolites were identified by UPLC-MS/MS after oral administration of melatonin or probiotics (Fig. 4(b) and Table S3 in the ESM). Spearman analysis was used to further understand the relationship between metabolites and hematopoietic damage. 12 of these metabolites were closely associated with hematopoietic damage markers, especially LT-HSCs (Fig. 4(c)). Notably, melatonin administration could effectively reverse the abnormal metabolite changes caused by NP, such as creatine and xylose. In contrast, probiotics reversed the levels of seven metabolites after NP exposure, which was adipoylcarnitine, malonylcarnitine, threonine, 3-hydroxybutyric acid, palmitoylcarnitine, 4-hydroxycinnamic acid, and homocitrulline (Fig. 4(d)). This evidence supported the hypothesis that metabolic alterations might be a key mechanism by which melatonin or probiotics improve NP-induced hematopoietic damage.
Figure 4.
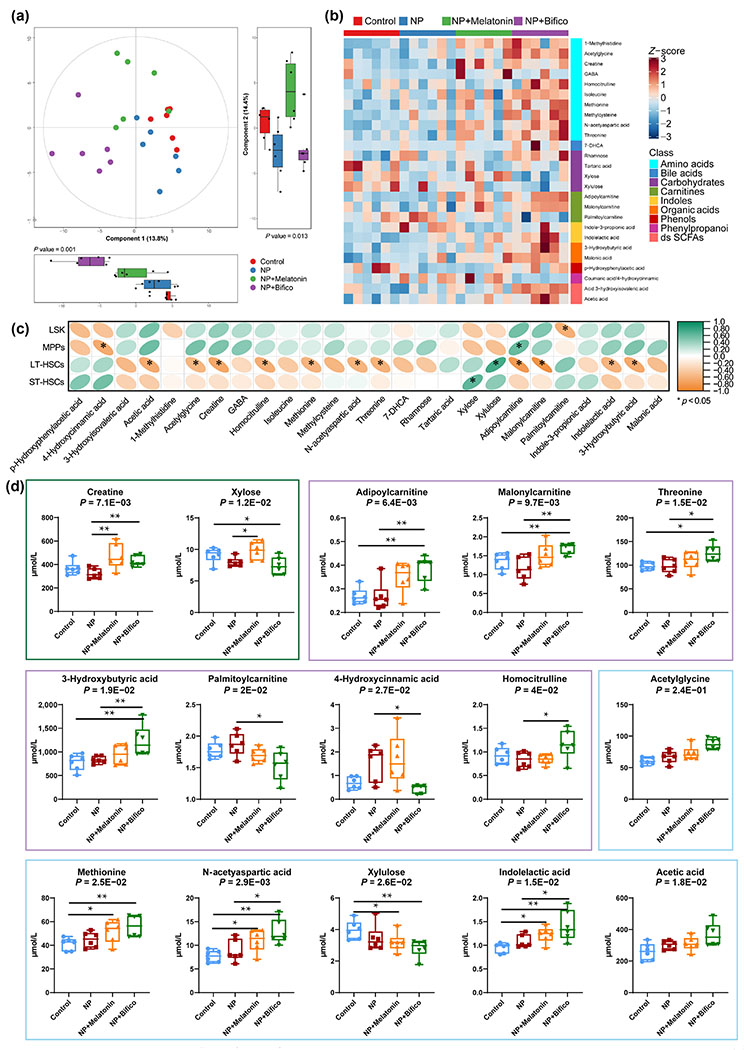
Melatonin or probiotics treatment alleviated metabolic phenotypes in NP exposure mice (n = 6). (a) Principal component analysis (PCA) score plot with principal component boxplot. (b) 25 identified differential plasma metabolite levels. (c) Correlation between differential metabolite and hematopoietic damage by Spearman analysis. (d) The levels of differential metabolites associated with hematopoiesis. Green boxes indicate metabolites reversed by melatonin, purple boxes indicate metabolites reversed by probiotics, and blue boxes indicate metabolites with no reversal by melatonin or probiotics. Values were presented as median ± quartile. Differences were assessed by Mann Whitney test or Kruskal–Wallis test and denoted as follows: *p < 0.05, **p < 0.01, ns: p > 0.05.
3.6. Metabolites act as mediators of gut microbes to regulate the host hematopoietic phenotype
The crosstalk of gut microbes with specific metabolites has emerged as a potential pathogenic mechanism for environmental pollutants [26]. Therefore, we further analyzed the relationship between gut microbes and metabolites effectively reversed by melatonin or probiotics and explored the mediating effects of key metabolites in the association between microbial and hematopoietic damage (Fig. 5(a)). After melatonin intervention, two metabolites were found to be potentially involved in NP-induced hematopoietic toxicity, and creatine showed a stronger correlation with gut microbes (Fig. 5(b)). In contrast, probiotic intervention reversed the levels of more gut microbes and metabolites. Of these, threonine, malonylcarnitine, and 3-hydroxybutyric acid might be potent performers in the regulation of hematopoietic toxicity by gut flora, as they had a more significant relationship with the identified microbes (Fig. 5(c)). In conclusion, melatonin and probiotics ameliorate NP-induced hematopoietic toxicity possibly by modulating different gut microbial-metabolic pathways, which may lay the foundation for an in-depth investigation into the mechanisms of NP-induced health damage in the future (Fig. 6).
Figure 5.
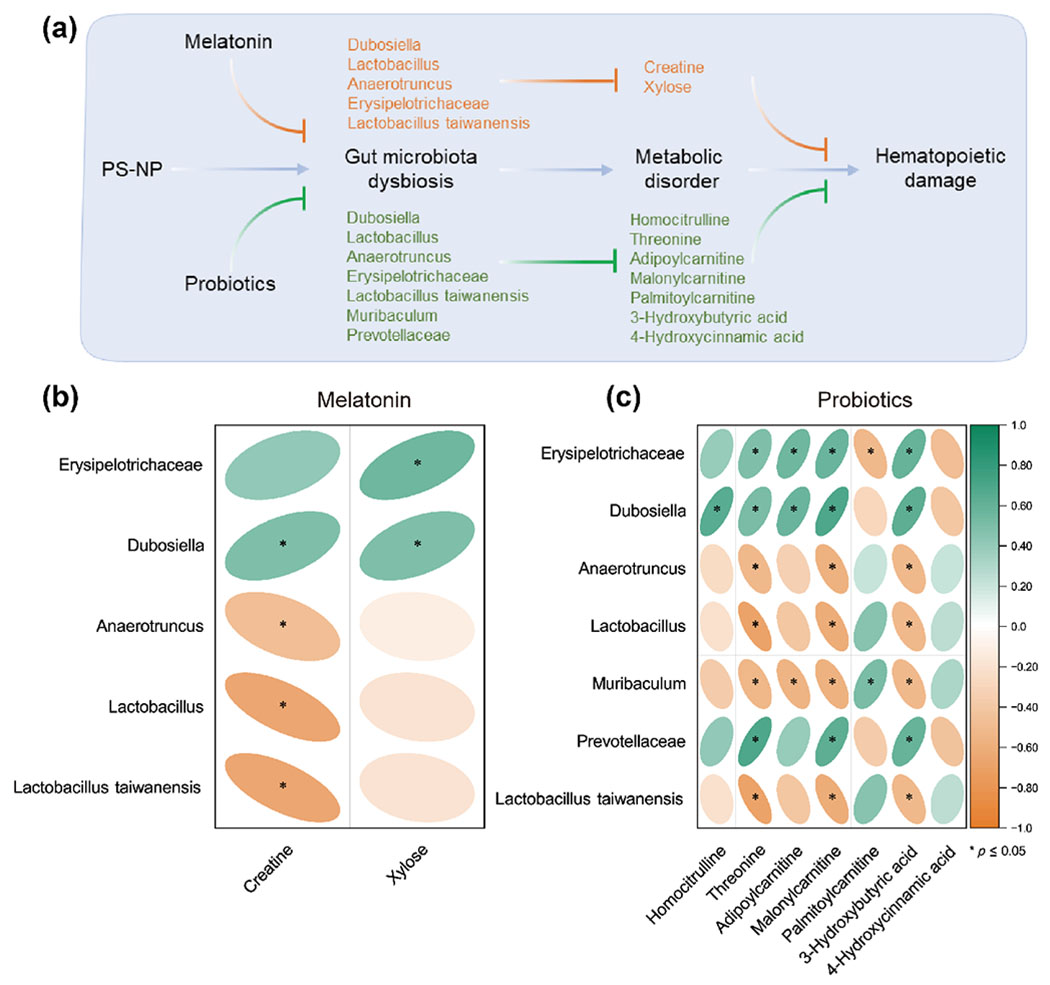
Connection of key gut microbes to metabolites in the process of melatonin or probiotics ameliorating NP-induced hematopoietic damage. (a) Potential microbes and metabolites that may be involved in the process of melatonin or probiotics ameliorating NP hematopoietic damage. (b) and (c) Spearman analysis reveals key microbes and metabolites involved in melatonin or probiotics ameliorating NP hematopoietic damage. *p < 0.05.
Figure 6.
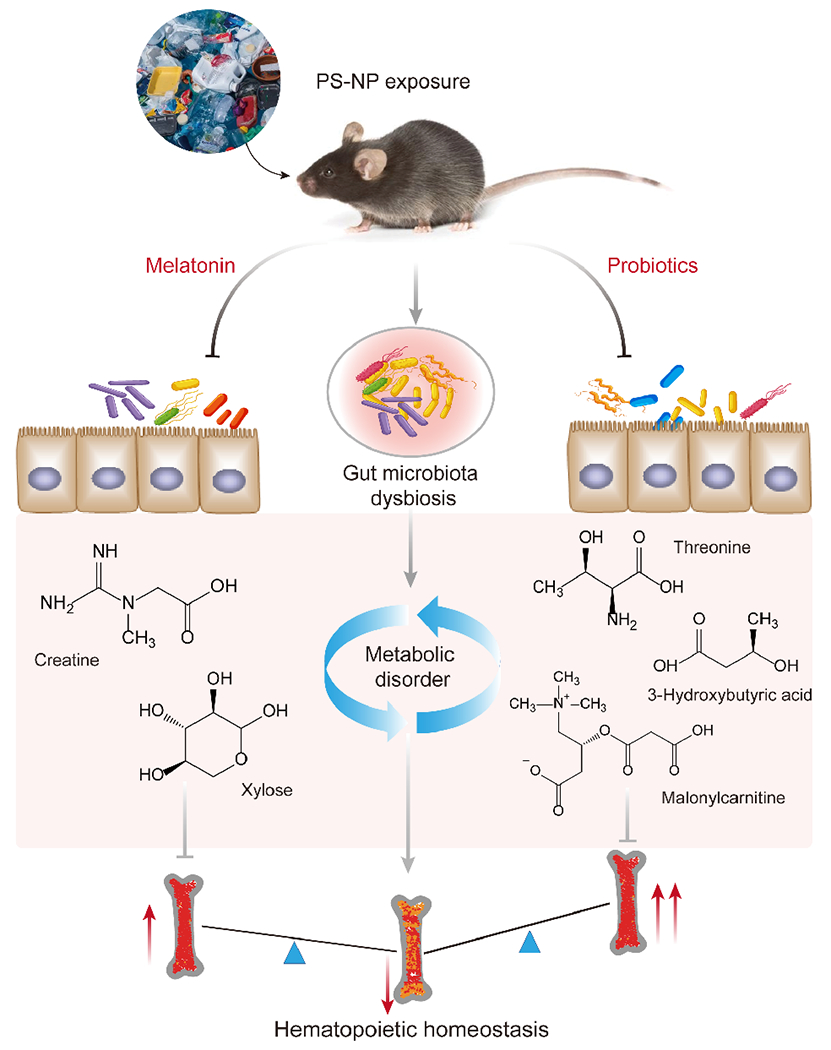
Schematic diagram of the mechanism by which melatonin and probiotics reverse NP-induced hematopoietic toxicity via specific gut microbes and metabolites.
4. Discussion
An increasing body of evidence suggests that people are commonly exposed to MNP in various food, drinking water, and air [30]. As a novel contaminant, MNP had been reported to produce significant multi-organ adverse effects, including liver damage [31], immune disorders [4], and neurological and reproductive toxicity [32,33]. It can penetrate into more tissues and produce more severe bioaccumulation as the diameter of plastic particles decreases. To date, numerous in vivo studies had proven that plastic particles larger than 50 μm were primarily concentrated in the intestine, while plastics of 5 μm could enter the liver [8,10]. Nanoscale plastics (< 100 nm) could even penetrate the barrier to reach the circulation and BM, thus affecting hematological parameters and even the capacity of hematopoietic progenitor cell colony formation [21,26]. These studies indicated that NP was a potential threat to the BM hematopoietic system. However, the potential mechanisms and prevention strategies for NP exposure-induced hematopoietic damage are not fully clear. In this study, we observed that low doses of 80 nm NP were able to reach the BM with circulation and inhibited the renewal and differentiation potential of HSCs. Whereas melatonin and probiotics effectively reversed the NP-induced decrease in HSCs renewal capacity with no effect on differentiation capacity. This may act through specific gut microbes and metabolites. In general, probiotics are more effective than melatonin for NP-induced hematopoietic toxicity from a pathological, hematological, and microbiomic and metabolomic perspective.
As a plastic polymer, PS is extensively utilized in industrial products (such as food packaging, household appliances, and automotive industry) and biomedical applications (such as medical devices, petri dishes, and diagnostic components) [4]. In recent studies, quantifiable MP was found for the first time in human blood and 36% of them was PS [34]. MP in the environment can be decomposed into smaller micron-sized or nanometer-sized plastic particles under long-term light irradiation [35]. Several studies had reported that a large number of NP particles less than 100 nm were detected in environmental and marine organisms [36–38]. Plastic particles are probably transported through the bloodstream to arrive at the distal organs. While PS particles less than 100 nm have been shown to penetrate into the mammalian BM [21,26]. Besides the above factors, the strain and sex of the animals are also factors that must be considered in toxicology tests. Because of their susceptibility to hematological tumors, C57BL/6J mice are often considered as experimental animals in environmental pollutants and hematotoxicity studies [15,27]. In addition, male mice are also the most widely used animal model in recent studies of MNP, except for reproductive and developmental toxicity studies [15,26,39, 40]. Taken together, the hematopoietic toxicity of 80 nm PS-NP was investigated in this study.
Limited studies have investigated the effects of MNP on the hematological system, but in-depth mechanisms and interventions are lacking. After 15 days of exposure to MP (> 100 nm), several hematological parameters in tilapia showed significant alterations (decrease in RBC, blood hemoglobin concentration, hematocrit, mean corpuscular hemoglobin concentration, platelets, WBC, and percent of monocytes; increase in mean corpuscular volume and mean corpuscular hemoglobin) with increasing exposure dose and were irrecoverable except for WBC and RBC [41]. HSCs strictly regulated self-renewal and balanced spectrum differentiation by generating hematopoietic progenitor cells and further downstream cells, thus providing a continuous supply of blood cells. A preliminary study has also demonstrated a significant inhibitory effect of 5 μm PS-MP on WBC counts and BM hematopoietic progenitor cell differentiation in C57BL/6J mice [15]. 1.6 μg of MP per mL of blood was detected in human volunteers, which further raised concerns about the long-term effects of MP on human health [34]. These shreds of evidence confirm that the disturbance of the hematopoietic pool by MNP in mammals is an important mechanism for the progression of hematological diseases. However, as yet no studies were published regarding prevention or treatment protocols for hematopoietic damage caused by MNP.
MNP that migrate into the blood circulation might be accompanied by lipid peroxidation, metabolic disorders, and inflammatory responses, in addition to the destruction of blood cell components [42]. Melatonin is known to be a powerful immunomodulator for anti-oxidation, anti-inflammatory, immune enhancing, cardiovascular protective, and anti-cancer [43]. In a mouse model of PM2.5-induced lung injury, 20 mg/kg/day melatonin treatment for 56 consecutive days alleviated PM2.5-induced inflammatory cell infiltration, pathological lung damage, and edema by activating Nrf2 to inhibit ferroptosis in lung epithelial cells [23]. Furthermore, given the important role of gut microbiota and metabolism in disease development, this would also be a new target for melatonin intervention. In titanium particle-induced osteolysis [44], dextran sodium sulfate-induced neuroinflammation [45], and high-fat diet-induced obesity and lipid metabolism disorders [22,46], the superior therapeutic effect of melatonin had been demonstrated by modulating the intestinal microbiota. In contrast to the healthy state, the development of blood disorders is frequently accompanied by disturbances in intestinal microbes and metabolites [27,28]. Probiotics, a nutritional supplement, are receiving increasing attention for their potential in the prevention and treatment of intestinal flora dysbiosis. In diesel exhaust particles (DEPs)-exposed mice, oral probiotic administration significantly increased the relative abundance of lactobacillus and protected mice from DEPs-induced colonic epithelial damage [47]. Animal studies revealed that lactobacillus supplementation improved the survival rate of mice and reduced the adverse effects of hematopoietic cell transplantation, such as graft-versus-host disease and intestinal inflammation [48,49]. In conclusion, these evidences suggested that melatonin or probiotic supplementation might be effective measures to ameliorate the hematopoietic damage induced by MNP and that the intestinal flora-metabolic axis is an important intermediate regulator.
A growing number of studies suggested that metabolites may be involved as mediators in the relationship between gut microbiota and disease [50,51]. Creatine has attracted a lot of attention as a nutritional supplement because of its antioxidant, neuroprotective, anti-lactate, and calcium homeostatic effects [52]. Physiologically, creatine serves as an atypical energy fuel that can be used to supply cellular energy requirements acutely through the creatine–phosphocreatine cycle with ATP–ADP conversion in the brain and muscle. Peng et al. revealed that reduced expression of SLC6A8 leads to reduced creatine input and ATP production, thereby detracting from CD8+ T cell function. This study highlights the importance of creatine metabolism for T-cell function in leukemia [53]. These evidences supported the results of the present study that melatonin reversed NP-induced hematopoietic damage, which was significantly associated with creatine regression.
Unlike melatonin, the reversal of NP-induced hematopoietic damage by probiotics is mainly attributed to threonine, malonylcarnitine, and 3-hydroxybutyric. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is known to play an important regulatory role in cell growth, proliferation, metabolism, and survival [54]. Notably, mTOR activity is a possible key checkpoint for HSCs quiescence and self-renewal [55]. Therefore, threonine may regulate NP-induced hematopoietic injury by activating mTOR activity. Malonylcarnitine is a metabolite that accumulates due to a disorder in fatty acid oxidation (FAO) caused by malonyl-CoA decarboxylase deficiency [56]. Previous studies also indicated that FAO might be a potential key metabolic pathway for hematopoietic injury [28]. In addition, 3-hydroxybutyric, a byproduct of FAO, is a fuel source for peripheral tissues including the brain, heart, and skeletal muscle, and also acts as a signaling molecule regulating lipolysis, oxidative stress, and neuroprotection [57]. The above studies suggested that these metabolites might be critical mediators in the regulation of hematopoiesis.
5. Conclusions
In summary, this study revealed that 80 nm of PS-NP could enter the BM and affect the hematopoietic capacity of mice. Furthermore, we provided preliminary evidence that probiotics and melatonin ameliorate NP-induced hematopoietic damage, which may involve different gut microbiota and metabolites. Our study uncovered a promising individual intervention candidate against novel contaminant-associated hematopoietic disorders.
Supplementary Material
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Nos. 82073520 and 81773397), the Beijing Natural Science Program and Sci-entific Research Key Program of Beijing Municipal Commission of Ed-ucation (No. KZ201810025032), and the Support Project of High-level Teachers in Beijing Municipal Universities in the Period of 13th Five-year Plan (No. CIT&TCD 20170323).
Footnotes
Electronic Supplementary Material: Supplementary material (PS-NP characterization, fluorescence imaging, UPLC-MS/MS conditions) is available in the online version of this article at https://doi.org/10.1007/s12274-022-5032-9.
References
- [1].Kershaw PKS; Leemseth J Woodring plastic debris in the ocean UNEP Yearbook emerging issues in our global environment; United Nations Environment Programme: Nairobi, 2011. [Google Scholar]
- [2].Wang CH; Zhao J; Xing BS Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater 2021, 407, 124357. [DOI] [PubMed] [Google Scholar]
- [3].Geyer R; Jambeck JR; Law KL Production, use, and fate of all plastics ever made. Sci. Adv 2017, 3, e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hirt N; Body-Malapel M Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol 2020, 77, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prata JC; da Costa JP; Lopes I; Duarte AC; Rocha-Santos T Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ 2020, 702, 134455. [DOI] [PubMed] [Google Scholar]
- [6].Abbasi S; Turner A Human exposure to microplastics: A study in Iran. J. Hazard. Mater 2021, 403, 123799. [DOI] [PubMed] [Google Scholar]
- [7].Jiang BR; Kauffman AE; Li L; McFee W; Cai B; Weinstein J; Lead JR; Chatterjee S; Scott GI; Xiao S Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med 2020, 25, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hussain N; Jaitley V; Florence AT Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev 2001, 50, 107–142. [DOI] [PubMed] [Google Scholar]
- [9].Gopinath PM; Saranya V; Vijayakumar S; Mythili Meera M; Ruprekha S; Kunal R; Pranay A; Thomas J; Mukherjee A; Chandrasekaran N Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep 2019, 9, 8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Deng YF; Zhang Y; Lemos B; Ren HQ Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep 2017, 7, 46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liang BX; Zhong YZ; Huang YJ; Lin X; Liu J; Lin L; Hu MJ; Jiang JY; Dai ΜZ; Wang B et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol 2021, 18, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang DQ; Zhu JD; Zhou XS; Pan D; Nan S; Yin RL; Lei QH; Ma N; Zhu HM; Chen JG et al. Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ. Int 2022, 166, 107362. [DOI] [PubMed] [Google Scholar]
- [13].Zhang YF; Gao S; Xia J; Liu F Hematopoietic hierarchy—An updated roadmap. Trends Cell Biol. 2018, 28, 976–986. [DOI] [PubMed] [Google Scholar]
- [14].Lim D; Jeong J; Song KS; Sung JH; Oh SM; Choi J Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere 2021, 262, 128330. [DOI] [PubMed] [Google Scholar]
- [15].Sun RL; Xu K; Yu LL; Pu YQ; Xiong F; He YH; Huang QC; Tang MJ; Chen MJ; Yin LH et al. Preliminary study on impacts of polystyrene microplastics on the hematological system and gene expression in bone marrow cells of mice. Ecotoxicol. Environ. Saf 2021, 218, 112296. [DOI] [PubMed] [Google Scholar]
- [16].Adak A; Khan MR An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci 2019, 76, 473–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jin YX; Lu L; Tu WQ; Luo T; Fu ZW Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ 2019, 649, 308–317. [DOI] [PubMed] [Google Scholar]
- [18].Xie SL; Zhou AG; Wei TL; Li SY; Yang B; Xu GH; Zou JX Nanoplastics induce more serious microbiota dysbiosis and inflammation in the gut of adult zebrafish than microplastics. Bull. Environ. Contam. Toxicol 2021, 107, 640–650. [DOI] [PubMed] [Google Scholar]
- [19].Yan H; Baldridge ΜT; King KY Hematopoiesis and the bacterial microbiome. Blood 2018, 132, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Richter FC; Obba S; Simon AK Local exchange of metabolites shapes immunity. Immunology 2018, 155, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jani P; Halbert GW; Langridge J; Florence AT Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol 1990, 42, 821–826. [DOI] [PubMed] [Google Scholar]
- [22].Xu PF; Wang JL; Hong F; Wang S; Jin X; Xue TT; Jia L; Zhai YG Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal. Res 2017, 62, e12399. [DOI] [PubMed] [Google Scholar]
- [23].Fan GH; Zhu TY; Min XP; Xiong J Melatonin protects against PM2.5-induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2-dependent manner. Ecotoxicol. Environ. Saf 2021, 223, 112588. [DOI] [PubMed] [Google Scholar]
- [24].Li DC; Zhang R; Cui LH; Chu C; Zhang HY; Sun H; Luo J; Zhou LX; Chen LP; Cui J et al. Multiple organ injury in male C57BL/6J mice exposed to ambient particulate matter in a real-ambient PM exposure system in Shijiazhuang, China. Environ. Pollut 2019, 248, 874–887. [DOI] [PubMed] [Google Scholar]
- [25].Hardeland R Melatonin and inflammation-story of a double-edged blade. J. Pineal. Res 2018, 65, e12525. [DOI] [PubMed] [Google Scholar]
- [26].Jing JR; Zhang L; Han L; Wang JY; Zhang W; Liu ZY; Gao A Polystyrene micro-/nanoplastics induced hematopoietic damages via the crosstalk of gut microbiota, metabolites, and cytokines. Environ. Int 2022, 161, 107131. [DOI] [PubMed] [Google Scholar]
- [27].Zhang L; Jing JR; Han L; Wang JY; Zhang W; Liu ZY; Gao A Characterization of gut microbiota, metabolism and cytokines in benzene-induced hematopoietic damage. Ecotoxicol. Environ. Saf 2021, 225, 112956. [DOI] [PubMed] [Google Scholar]
- [28].Guo XL; Zhang L; Wang JY; Zhang W; Ren J; Chen YJ; Zhang YL; Gao A Plasma metabolomics study reveals the critical metabolic signatures for benzene-induced hematotoxicity. JCI Insight 2022, 7, e154999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blacher E; Bashiardes S; Shapiro H; Rothschild D; Mor U; Dori-Bachash M; Kleimeyer C; Moresi C; Hamik Y; Zur M et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 2019, 572, 474–480. [DOI] [PubMed] [Google Scholar]
- [30].Vethaak AD; Legler J Microplastics and human health. Science 2021, 371, 672–674. [DOI] [PubMed] [Google Scholar]
- [31].Zheng HB; Wang J; Wei XY; Chang L; Liu S Pro inflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ 2021, 750, 143085. [DOI] [PubMed] [Google Scholar]
- [32].Rafiee M; Dargahi L; Eslami A; Beirami E; Jahangiri-Rad M; Sabour S; Amereh F Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere 2018, 193, 745–753. [DOI] [PubMed] [Google Scholar]
- [33].Jin HB; Ma T; Sha XX; Liu ZY; Zhou Y; Meng XN; Chen YB; Han XD; Ding J Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater 2021, 401, 123430. [DOI] [PubMed] [Google Scholar]
- [34].Leslie HA; van Velzen MJM; Brandsma SH; Vethaak AD; Garcia-Vallejo JJ; Lamoree ΜH Discovery and quantification of plastic particle pollution in human blood. Environ. Int 2022, 163, 107199. [DOI] [PubMed] [Google Scholar]
- [35].Lv YD; Huang YJ; Kong MQ; Yang Q; Li GX Multivariate correlation analysis of outdoor weathering behavior of polypropylene under diverse climate scenarios. Polym. Test 2017, 64, 65–76. [Google Scholar]
- [36].Yang Q; Zhang SY; Su J; Li S; Lv XC; Chen J; Lai YC; Zhan JH Identification of trace polystyrene nanoplastics down to 50 nm by the hyphenated method of filtration and surface-enhanced Raman spectroscopy based on silver nanowire membranes. Environ. Sci. Technol 2022, 56, 10818–10828. [DOI] [PubMed] [Google Scholar]
- [37].Morgana S; Casentini B; Amalfitano S Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J. Hazard. Mater 2021, 419, 126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou XX; He S; Gao Y; Chi HY; Wang DJ; Li ZC; Yan B Quantitative analysis of polystyrene and poly(methyl methacrylate) nanoplastics in tissues of aquatic animals. Environ. Sci. Technol 2021, 55, 3032–3040. [DOI] [PubMed] [Google Scholar]
- [39].Wang LX; Xu M; Chen JM; Zhang X; Wang QS; Wang YX; Cui JS; Zhang SP Distinct adverse outcomes and lipid profiles of erythrocytes upon single and combined exposure to cadmium and microplastics. Chemosphere 2022, 307, 135942. [DOI] [PubMed] [Google Scholar]
- [40].Zhao LT; Shi WY; Hu FF; Song XJ; Cheng ZJ; Zhou JH Prolonged oral ingestion of microplastics induced inflammation in the liver tissues of C57BL/6J mice through polarization of macrophages and increased infiltration of natural killer cells. Ecotoxicol. Environ. Saf 2021, 227, 112882. [DOI] [PubMed] [Google Scholar]
- [41].Hamed M; Soliman HAM; Osman AGM; Sayed AEDH Assessment the effect of exposure to microplastics in Nile Tilapia (oreochromis niloticus) early juvenile: I. Blood biomarkers. Chemosphere 2019, 228, 345–350. [DOI] [PubMed] [Google Scholar]
- [42].Kim JH; Yu YB; Choi JH Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater 2021, 413, 125423. [DOI] [PubMed] [Google Scholar]
- [43].Meng X; Li Y; Li S; Zhou Y; Gan RY; Xu DP; Li HB Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wu YL; He F; Zhang CH; Zhang Q; Su XL; Zhu X; Liu A; Shi WD; Lin WF; Jin ZQ et al. Melatonin alleviates titanium nanoparticles induced osteolysis via activation of butyrate/GPR109A signaling pathway. J. Nanobiotechnol 2021, 19, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lv WJ; Liu C; Yu LZ; Zhou JH; Li Y; Xiong Y; Guo A; Chao LM; Qu Q; Wei GW et al. Melatonin alleviates neuroinflammation and metabolic disorder in DSS-induced depression rats. Oxid. Med. Cell. Longev 2020, 2020, 1241894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yin J; Li YY; Han H; Chen S; Gao J; Liu G; Wu X; Deng JP; Yu QF; Huang X et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal. Res 2018, 65, e12524. [DOI] [PubMed] [Google Scholar]
- [47].Li XB; Sun H; Li B; Zhang XW; Cui J; Yun J; Yang YP; Zhang LE; Meng QT; Wu SS et al. Probiotics ameliorate colon epithelial injury induced by ambient ultrafine particles exposure. Adv. Sci 2019, 6, 1900972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jenq RR; Ubeda C; Taur Y; Menezes CC; Khanin R; Dudakov JA; Liu C; West ML; Singer NV; Equinda MJ et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med 2012, 209, 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vossen JM; Heidt PJ; van den Berg H; Gerritsen EJA; Hermans J; Dooren LJ Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur. J. Clin. Microbiol. Infect. Dis 1990, 9, 14–23. [DOI] [PubMed] [Google Scholar]
- [50].Agus A; Planchais J; Sokol H Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [DOI] [PubMed] [Google Scholar]
- [51].Inoue K; Yan Q; Arah OA; Paul K; Walker DI; Jones DP; Ritz B Air pollution and adverse pregnancy and birth outcomes: Mediation analysis using metabolomic profiles. Curr. Environ. Health Rep 2020, 7, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Marshall RP; Droste JN; Giessing J; Kreider RB Role of creatine supplementation in conditions involving mitochondrial dysfunction: A narrative review. Nutrients 2022, 14, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Peng ΜX; Ren J; Jing YP; Jiang XK; Xiao QL; Huang JP; Tao YH; Lei L; Wang X; Yang ZL et al. Tumour-derived small extracellular vesicles suppress CD8+ T cell immune function by inhibiting SLC6A8-mediated creatine import in NPM1-mutated acute myeloid leukaemia. J. Extracell. Vesicles 2021, 10, e12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mossmann D; Park S; Hall ΜN mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [DOI] [PubMed] [Google Scholar]
- [55].Liu L; Inoki A; Fan K; Mao FB; Shi GJ; Jin X; Zhao ML; Ney G; Jones M; Sun SY et al. ER-associated degradation preserves hematopoietic stem cell quiescence and self-renewal by restricting mTOR activity. Blood 2020, 136, 2975–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Weeghel M; Abdurrachim D; Nederlof R; Argmann CA; Houtkooper RH; Hagen J; Nabben M; Denis S; Ciapaite J; Kolwicz SC et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc. Res 2018, 114, 1324–1334. [DOI] [PubMed] [Google Scholar]
- [57].Wang L; Chen PJ; Xiao WH β-Hydroxybutyrate as an anti-aging metabolite. Nutrients 2021, 13, 3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


