Key Points
Question
Are single-nucleotide variants associated with transient acantholytic dermatosis (Grover disease)?
Findings
In a retrospective case series employing archival tissue from 15 participants with Grover disease, we found that 12 (80%) were associated with damaging single-nucleotide variants in ATP2A2. All variants were C>T or G>A substitutions, suggesting that UV light-induced mutagenesis may contribute to the development of lesions.
Meaning
Genetic analysis of this cohort of patients suggests that somatic single-nucleotide variants in ATP2A2 are often associated with Grover disease.
This case series examines whether damaging somatic single-nucleotide variants in the ATP2A2 gene are associated with Grover disease.
Abstract
Importance
Grover disease (GD), a truncal eruption that typically occurs in older individuals, is exacerbated by sweating, irradiation, cancers, medications, kidney failure, and organ transplantation. The pathobiology of GD remains unknown.
Objective
To determine if damaging somatic single-nucleotide variants (SNVs) are associated with GD.
Design, Setting, and Participants
In this retrospective case series, we identified consecutive patients from a dermatopathology archive over a 4-year period (January 2007 to December 2011) who had 1 biopsy with a clinical diagnosis of GD confirmed via histopathologic findings and another non-GD biopsy. Participant DNA was extracted from both biopsy tissues and sequenced to high depth with a 51-gene panel to screen for SNVs in genes previously associated with acantholysis and Mendelian disorders of cornification. Analysis took place between 2021 and 2023.
Main Outcomes and Measures
Comparative analysis of sequencing data from paired GD and control tissue was employed to identify SNVs predicted to affect gene function, which were exclusive to, or highly enriched in, GD tissue.
Results
Overall, 12 of 15 cases of GD (12 men and 3 women; mean [SD] age, 68.3 [10.0] years) were associated with C>T or G>A ATP2A2 SNVs in GD tissue; all were predicted to be highly damaging via combined annotation dependent depletion (CADD) scores, and 4 were previously associated with Darier disease. In 9 cases (75%), the GD-associated ATP2A2 SNV was absent from control tissue DNA, and in 3 cases (25%), ATP2A2 SNVs were enriched 4- to 22-fold in GD vs control tissue.
Conclusions and Relevance
In this case series study of 15 patients, damaging somatic ATP2A2 SNVs were associated with GD. This discovery expands the spectrum of acantholytic disorders associated with ATP2A2 SNVs and highlights the role of somatic variation in acquired disorders.
Introduction
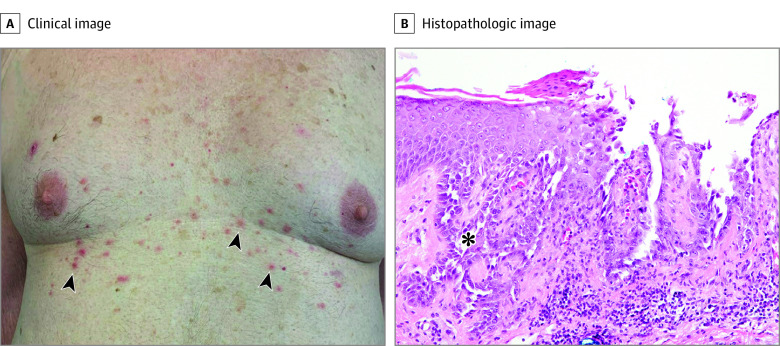
Transient acantholytic dermatosis, commonly referred to as Grover disease (GD), manifests as papules, papulovesicles, and small nodules that appear on the torso more often than limbs of affected patients (Figure 1). The molecular pathogenesis of GD is unknown, but flares are more common in sun-damaged skin and are often attributed to sweating and other environmental factors.1,2 Histopathologic features of GD are variable and can include acantholysis, dyskeratosis, spongiosis, and a perivascular lymphocytic infiltrate.2
Figure 1. Clinical Features of Grover Disease (GD).
A, A man in his 80s presented with numerous pruritic red papules (black arrowheads), some with excoriation, after a medication change led to daily night sweats. These resolved after medication was discontinued, and night sweats ceased. B, Histopathologic examination revealed acantholysis (asterisk) and parakeratosis with an inflammatory infiltrate, consistent with a diagnosis of GD (hematoxylin-eosin stain, original magnification ×20).
Darier disease (DD), which features keratotic papules in seborrheic areas including the central trunk, scalp, forehead, and flexures, has similar histologic findings to GD with focal loss of adhesion between cells and abnormal keratinization. Darier disease is an autosomal-dominant disorder associated with germline variants in the ATP2A2.3 Sweating, UV irradiation, friction, medications, and bacterial or viral infection are some triggers of DD flares.4
Given the clinical and histologic similarities between GD and DD, we hypothesized that somatic damaging single-nucleotide variants (SNVs) in ATP2A2 or other genes associated with acantholytic disorders including ATP2C1, DSG, DSP, KRT1, KRT2, and KRT10 may be associated with GD.
Methods
We identified consecutive patients with GD from our dermatopathology archives with accessions between January 2007 and December 2011 for which a separate biopsy of skin without GD was also available. Analysis took place between 2021 and 2023. All specimens were formalin-fixed and paraffin embedded. Three to 5 1-mm tissue cores were collected using hematoxylin-eosin–stained slides to guide site selection. Genomic DNA was isolated using the Qiagen DNA FFPE kit, and high-depth multiplex amplicon-based screening (Generead, Qiagen) of a 51-gene panel (eTable 1 in Supplement 1) was performed on paired tissue DNA samples. Damaging SNVs were identified and assessed in GD and control tissue and the burden of UV signature C>T and G>A substitutions was examined. We identified somatic damaging SNVs unique to, or significantly enriched in, GD vs unaffected tissue (eMethods in Supplement 1). This study was approved by the ethics committee of the Yale School of Medicine. A waiver of written informed consent was approved for the study of deidentified archival tissue.
Results
Overall, 15 patients with paired GD and control tissue were identified. The cohort included 12 men and 3 women in their 50s to 80s; all patients were White. All GD biopsies were located on the torso, were submitted with a clinical diagnosis of GD by 14 different experienced dermatologists, and showed histologic findings consistent with GD (Figure 2; eFigure 1 in Supplement 1). Control tissue included many sun-induced lesions. In paired analysis of GD and control tissue we found ATP2A2 SNVs in 12 participants (80%) at allele fractions of 8% to 40% admixed with the wild-type allele (Table). All were predicted to alter the encoded protein and had Combined Annotation Dependent Depletion (CADD) scores greater than 25, strongly supporting pathogenicity; 4 were previously associated with DD.5,6,7,8 Of the 12 cases with ATP2A2 damaging SNVs, 9 (75%) showed ATP2A2 somatic variants that were absent from unaffected tissue DNA, and in 3 cases (25%), ATP2A2 variants were enriched 4- to 22-fold in GD vs control tissue. In all cases, the ATP2A2 SNVs identified had the highest CADD scores of all high-quality variants. The age of these patients and the finding that all SNVs were C>T or G>A transitions raised the possibility of UV light–induced mutagenesis. We assessed the mutation burden in GD and control tissue, finding a higher overall burden with 57% UV-signature SNVs in control tissue, whereas GD tissue harbored 90% UV-signature SNVs. After filtering for rare, damaging, coding SNVs, we found that control tissue had an average of 3 SNVs, none of which resided in ATP2A2, in contrast to a single, somatic ATP2A2 SNV identified in 11 of 12 GD samples (eFigure 2 in Supplement 1). One of the 12 ATP2A2-positive samples, case 6, contained 2 additional somatic variants, both of which had CADD scores and allele fractions lower than the high-quality ATP2A2 SNV (eTable 2 in Supplement 1).
Figure 2. Histopathologic Images.
Histopathologic features of index Grover disease cases (A and B) show suprabasal acantholysis (asterisk), and dyskeratosis in the form of corps ronds and grains (hematoxylin-eosin stain, original magnification ×20).
Table. Clinical Features and ATP2A2 Damaging SNV Status in Grover Disease (GD) Cases.
| Case | Age, y | GD clinical impression | Site | Pathologic diagnosis | Somatic ATP2A2 SNV | SNV type | CADD score | Variant allele fraction | SNV reads | Normal reads | Control clinical impression | Site | Pathologic diagnosis | Variant allele fraction | SNV reads | Normal reads |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70s | GD | Lower back | GD | c.1645C>T, p.R549* | Nonsense | 38 | 0.21 | 194 | 719 | BCC vs SCCIS | Neck | BCC | 0 | 0 | 15 |
| 2 | 70s | GD | Abdomen | GD | c.2305G>A, p.G769Ra | Missense | 30 | 0.29 | 3878 | 9496 | Sebaceous hyperplasia vs BCC | Cheek | Hidrocystoma | 0 | 0 | 1217 |
| 3 | 70s | GD | Abdomen | GD | c.1018G>A, p.E340K | Missense | 27.7 | 0.35 | 363 | 683 | Actinic keratosis vs BCC | Hand | Actinic keratosis | 0 | 0 | 436 |
| 4 | 60s | GD vs lichen planus | Breast | GD | c.748C>T, p.Q250* | Nonsense | 36 | 0.27 | 22 | 60 | r/o lichen planus | Leg | Seborrheic keratosis | 0 | 0 | 17 |
| 5 | 60s | GD vs hypersensitivity | Abdomen | GD | c.391C>T, p.R131*a | Nonsense | 35 | 0.38 | 273 | 439 | Seborrheic keratosis | Forehead | Seborrheic keratosis | 0 | 0 | 48 |
| 6 | 60s | GD | Upper abdomen | GD | c.1268C>T, p.S423F | Missense | 36 | 0.41 | 43 | 63 | Atypical nevus | Elbow | CN | 0 | 0 | 201 |
| 7 | 50s | GD vs hypersensitivity | Mid-back | GD | ND | NA | NA | NA | NA | NA | r/o MIS | Upper arm | Lentigo | NA | NA | NA |
| 8 | 60s | GD vs mastocytosis | Mid-abdomen | GD | c.479C>T, p.P160La | Missense | 32 | 0.08 | 633 | 6954 | Mastocytosis, histiocytosis, GD | Forearm | BCC | 0 | 0 | 2620 |
| 9 | 50s | GD vs folliculitis | Clavicle | GD | c.1429C>T, p.Q477* | Nonsense | 41 | 0.09 | 166 | 1695 | r/o folliculitis, dermatitis | Shoulder | Dermatitis | 0.02 | 2 | 87 |
| 10 | 70s | GD vs folliculitis | Upper abdomen | GD | ND | NA | NA | NA | NA | NA | r/o GD | Abdomen | Hidrocystomano GD | NA | NA | NA |
| 11 | 60s | GD vs eczema | Chest | GD | c.2747C>T, p.S916F | Missense | 28.7 | 0.09 | 401 | 3987 | Eczema vs GD | Forearm | Dermatitis no GD | 0 | 0 | 424 |
| 12 | 80s | GD vs hypersensitivity | Chest | GD | c.391C>T, p.R131*a | Nonsense | 36 | 0.08 | 703 | 8458 | Seborrheric keratosis vs NMSC | Lower back | LPLK | 0.004 | 6 | 1699 |
| 13 | 50s | GD vs hypersensitivity | Back | GD | c.1178G>A, p.G393E | Missense | 28.7 | 0.08 | 26 | 293 | Bite | Lower back | Bite | 0 | 0 | 0 |
| 14 | 80s | GD vs folliculitis | Abdomen | GD | c.557C>T, p.S186Fa | Missense | 32 | 0.15 | 37 | 201 | Seborrheic keratosis vs SCC | Cheek | SCC | 0.02 | 2 | 122 |
| 15 | 70s | GD | Chest | GD | ND | NA | NA | NA | NA | NA | r/s SCC | Cheek | Actinic keratosis | NA | NA | NA |
Abbreviations: BCC, basal cell carcinoma; GD, Grover disease; LPLK, lichen planus like keratosis; NA, not applicable; ND, not detected; NMSC, nonmelanoma skin cancer; SCCIS, squamous cell carcinoma; SNV, single-nucleotive variant.
Variants have been previously reported in patients with Darier disease.
Discussion
Prior reports have established that normal-appearing skin can have a high mutation burden, including pathogenic variants, which give rise to the development of isolated clones that can undergo positive selection.9 These are only detected when a clinically evident lesion results, such as seborrheic keratoses associated with acquired FGFR mutations.10 The resulting patchwork mosaic of clones is subject to environmental exposures and host factors, which can elicit disease for certain genotypes. Grover disease is a disorder with multiple known triggers, and in 15 participants with GD, we identified 12 with damaging SNVs in ATP2A2 and found no comparable high-quality, damaging variants in other genes. All were UV-signature variants, correlating with later age of onset observed in our cohort and greater prevalence in sun-damaged skin.4
The ATP2A2 gene encodes SERCA2, a sarco/endoplasmic reticulum Ca2+-adenosine triphosphate (ATP)ase pump (SERCA2) with a role in maintaining homeostatic cytoplasmic Ca2+ levels. ATP2A2 SNVs associated with DD cause both acantholysis and apoptosis, in part due to defects in synthesis, trafficking and folding of desmosomal proteins, and abnormal cytokeratin expression.11
In contrast to DD, which presents in childhood or early adulthood, the mean age of GD participants in our cohort was 68 years. Later age at presentation is consistent with increasing cutaneous mutation burden over time and is underscored by the finding of recurrent UV-associated mutations, emphasizing sun damage as a considerable risk factor for GD.1 Because of the low representation of ATP2A2 single-nucleotide variants in control tissue in this cohort, our analyses suggest that most GD lesions arise from a single acquired genetic variants. In a small subset of other cases with a low fraction of mutant reads occurring at a biopsy site distant from the GD-affected tissue, there may be low-level, widespread mosaicism. In either case, lesions remain indolent but undergo inflammatory, acantholytic changes in synchrony on exposure to environmental triggers. To better understand the molecular pathogenesis of GD, further analysis of ATP2A2 damaging SNVs across multiple lesions from a single individual is warranted.
Limitations
Limitations of this study include the retrospective design, which prevented review of clinical presentation and course of GD, the use of a targeted sequencing panel that could not fully characterize genome-wide variation contributing to disease phenotypes, and the examination of unilesional GD samples. Although 9 of 12 cases showed UV-induced damaging somatic SNVs absent from control tissue, in 3 of 12, control tissue showed low-frequency reads of the same SNV. This raises the possibility that a fraction of GD cases may represent mosaic DD, with environmental factors driving presentation with a GD clinical appearance. We were unable to examine multiple lesions from an individual participant with GD to determine if distinct lesions resulted from independent damaging somatic variants.
Conclusions
In this retrospective case series, the finding that 20% of GD cases did not have associated ATP2A2 damaging SNVs suggests that there may be undetected somatic variants in ATP2A2 or other genes and may explain the absence of ATP2A2-damaging SNVs found in prior genetic studies of GD pathogenesis.12,13 Future GD studies may reveal additional somatic damaging SNVs in other genes associated with acantholysis. The findings of this study suggest that GD may be frequently associated with somatic ATP2A2-damaging SNVs and highlight the contribution of somatic variants to acquired dermatoses.
eTable 1. 51 Gene Panel for Mendelian Disorders of Cornification
Table 2. High Quality Variants in GD Case 6
eMethods
eFigure 1. Histopathology of GD Cases
eFigure 2. Somatic SNV Burden in GD samples
Data Sharing Statement
References
- 1.Quirk CJ, Heenan PJ. Grover’s disease: 34 years on. Australas J Dermatol. 2004;45(2):83-86. doi: 10.1111/j.1440-0960.2004.054_1.x [DOI] [PubMed] [Google Scholar]
- 2.Davis MD, Dinneen AM, Landa N, Gibson LE. Grover’s disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999;74(3):229-234. doi: 10.4065/74.3.229 [DOI] [PubMed] [Google Scholar]
- 3.Dhitavat J, Dode L, Leslie N, Sakuntabhai A, Lorette G, Hovnanian A. Mutations in the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase isoform cause Darier’s disease. J Invest Dermatol. 2003;121(3):486-489. doi: 10.1046/j.1523-1747.2003.12410.x [DOI] [PubMed] [Google Scholar]
- 4.Shi BJ, Xue M, Zhong GS, et al. The ATP2A2 gene in patients with Darier’s disease: one novel splicing mutation. Int J Dermatol. 2012;51(9):1074-1077. doi: 10.1111/j.1365-4632.2012.05514.x [DOI] [PubMed] [Google Scholar]
- 5.Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem. 2003;278(48):47877-47889. doi: 10.1074/jbc.M306784200 [DOI] [PubMed] [Google Scholar]
- 6.Agematsu A, Kamata M, Uchida H, et al. A case of type 1 segmental Darier disease showing widespread Blaschkoid skin lesions with p.P160L mutation in ATP2A2. J Eur Acad Dermatol Venereol. 2020;34(10):e633-e635. doi: 10.1111/jdv.16506 [DOI] [PubMed] [Google Scholar]
- 7.Amichai B, Karpati M, Goldman B, Peleg L. Novel mutations in two families with Darier’s disease. Int J Dermatol. 2007;46(1):64-67. doi: 10.1111/j.1365-4632.2006.03049.x [DOI] [PubMed] [Google Scholar]
- 8.Sakuntabhai A, Dhitavat J, Burge S, Hovnanian A. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol. 2000;115(6):1144-1147. doi: 10.1046/j.1523-1747.2000.00182.x [DOI] [PubMed] [Google Scholar]
- 9.Martincorena I, Roshan A, Gerstung M, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880-886. doi: 10.1126/science.aaa6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafner C, Hartmann A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20(8):895-903. doi: 10.1038/modpathol.3800837 [DOI] [PubMed] [Google Scholar]
- 11.Hobbs RP, Amargo EV, Somasundaram A, et al. The calcium ATPase SERCA2 regulates desmoplakin dynamics and intercellular adhesive strength through modulation of PKC&α; signaling. FASEB J. 2011;25(3):990-1001. doi: 10.1096/fj.10-163261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell J, Sakuntabhai A, James M, Burge S, Hovnanian A. Grover’s disease, despite histological similarity to Darier’s disease, does not share an abnormality in the ATP2A2 gene. Br J Dermatol. 2000;143(3):658. doi: 10.1111/j.1365-2133.2000.03736.x [DOI] [PubMed] [Google Scholar]
- 13.Asahina A, Ishiko A, Saito I, Hasegawa K, Sawamura D, Nakano H. Grover’s disease following multiple bilateral Blaschko lines: a rare clinical presentation with genetic and electron microscopic analyses. Dermatology. 2012;225(2):183-187. doi: 10.1159/000343172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. 51 Gene Panel for Mendelian Disorders of Cornification
Table 2. High Quality Variants in GD Case 6
eMethods
eFigure 1. Histopathology of GD Cases
eFigure 2. Somatic SNV Burden in GD samples
Data Sharing Statement