Abstract
This study systematically assessed sensory processing in 34 children, aged 3–14 years, with Smith–Magenis syndrome (SMS) using the Sensory Profile Caregiver Questionnaire. Scores for the SMS cohort were significantly different from scores of the national sample of children with and without disabilities in all Sensory Profile categories and quadrants (p < .001). No main effects of age or gender were found, but an interaction effect of age by gender was found in Modulation of Sensory Input Affecting Emotional Responses, in which older females presented with the lowest scores. A significant decline over time was found in the Seeking pattern, reflecting increased vulnerability (p < .05). Nonsignificant trends suggest more vulnerabilities for older versus younger children, especially older females. The neurobehavioral phenotype in children with SMS is expanded by this description of sensory processing. How children with SMS experience and respond to everyday sensations informs multidisciplinary team decisions.
Keywords: sensory processing, sensory profile caregiver questionnaire, sensory modulation, Smith–Magenis syndrome
Smith–Magenis syndrome (SMS) is a multisystem disorder associated with a complex pattern of physical, developmental, and neurobehavioral features (Gropman, Duncan, & Smith, 2006; Smith et al., 2010; Wolters et al., 2009). Cognitive impairment occurs in all individuals with SMS but is variable with IQs ranging from 19–84 depending on the studied age group (Madduri et al., 2006; Martin, Wolters, & Smith, 2006; Udwin, Webber, & Horn, 2001). Fine and gross motor delays range from 2 to 24 months in infants and toddlers with SMS (Gropman et al., 2006; Smith, Hildenbrand, & Smith, 2009; Wolters et al., 2009) and speech/language delay, with or without associated hearing loss, occurs in over 50% of children with SMS (Di Cicco et al., 2001; Girirajan et al., 2006; Gropman et al., 2006; Madduri et al., 2006; Solomon, McCullagh, Krasnewich, & Smith, 2002). Socialization skills, though deficient, emerge as a strength in adaptive functioning relative to delay in communication and daily living skills (Madduri et al., 2006; Martin et al., 2006; Udwin et al., 2001; Wolters et al., 2009). Maladaptive behaviors are prominent in the neurobehavioral phenotype of SMS and include a unique constellation of stereotypic and self-injurious behaviors (SIB) (Dykens & Smith, 1998; Martin et al., 2006; Smith et al., 2010) (Table 1) and a chronic sleep disturbance associated with an inverted circadian melatonin rhythm (Gropman et al., 2006; Smith & Duncan, 2005) (Figure 1).
TABLE 1.
Maladaptive Behaviors in Children with Smith–Magenis Syndrome (SMS)
| Stereotypic behaviors | Bruxism (teeth grinding)a Inserts hands in moutha Inserts objects in moutha Covers eyes or ears Repetitive page turning or “lick and flip” Walks on tip toes Flaps, waves, or claps hands Purposefully drops or throws objects Taps or rubs objects or body Rocks or sways back and forth Stares closely at objects or hands Spasmodic upper body squeeze or self-hug |
| Self-injurious behaviors (SIB) | Self-bitinga Self-hittinga Hits self against surface or object |
All behaviors have a reported occurrence of ≥50% (Dykens & Smith, 1998; Martin et al., 2006; Smith et al., 2010).
Behaviors with reported occurrence of ≥80% (Martin et al., 2006).
FIGURE 1.
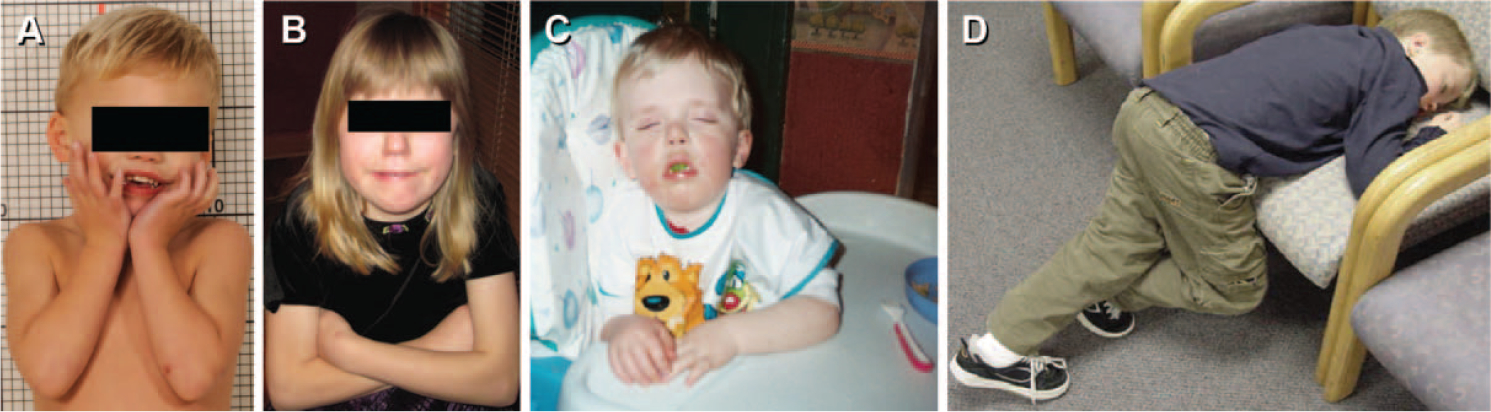
Maladaptive behaviors in SMS. “The spasmodic upper-body squeeze or “self-hug” (Finucane & Haas-Giveler, 2009; Finucane, Konar, Haas-Givler, Kurtz, & Scott, 1994) is illustrated by a preschool-age child (A) and an adolescent (B). Daytime sleepiness is illustrated by an infant asleep during dinner time (C) and a school-age child asleep while waiting for a medical appointment (D).
Well-recognized, but often under-diagnosed SMS may be as frequent as 1 in 15,000 cases (Smith, Magenis, & Elsea, 2005). The majority of SMS cases are because of interstitial deletion [del (17)(p11.2p11.2)] that includes the RAI1 gene (Vlangos, Wilson, Blancato, Smith, & Elsea, 2005). However, heterozygous mutations of the RAI1 gene also account for the phenotype in less than 10% of cases (Elsea & Girirajan, 2008). While fortuitous detection of del 17p11.2 has occurred in some SMS cases, the definitive diagnosis of SMS is often delayed beyond early childhood (18 months of age or older) because of failure to discern the deletion during the first molecular cytogenetic study and/or appreciate the phenotype (Gropman et al., 2006; Smith et al., 2005, 2010). Clinical impressions that often lead to early referral for cytogenetic investigation are complicated by the subtlety of the infantile presentation (Gropman, Elsea, Duncan, & Smith, 2007; Wolters et al., 2009).
Phenotypically, SMS overlaps with other syndromes and developmental disorders that present with infantile hypotonia, speech and motor delays, and behavioral and sensory processing issues, including Down syndrome (in infancy); Angelman syndrome; Cornelia de Lange’s syndrome; Fragile X; Prader–Willi syndrome; Williams syndrome, and velocardiofacial syndrome/DiGeorge syndrome (Gropman et al., 2006, 2007; Smith et al., 2005). Individuals who are later confirmed with a diagnosis of SMS have often been given a functional psychiatric diagnosis of attention deficit/hyperactivity disorder, bipolar disorder, obsessive-compulsive disorder, pervasive developmental disorder, and/or autism spectrum disorder with dual diagnosis (Gropman et al., 2006; Laje et al., 2010; Levitas, Dykens, Finucane, & Kates, 2007; Smith & Duncan, 2005). In many of these syndromes and disorders, patterns of sensory processing and their relationship to behavior, affective symptoms, and functional difficulties have been described (Ashburner, Ziviani, & Rodger, 2008; Baranek et al., 2008; Ben-Sasson et al., 2008; Bruni, Cameron, Dua, & Noy, 2010; Dunn & Bennett, 2002; Rieke & Anderson, 2009; Walz & Baranek, 2006).These studies highlight the potential impact of sensory processing difficulties and the critical need for systematic studies of sensory processing in phenotypic characterization of developmental syndromes and disorders.
Research to distinguish the neurobehavioral phenotype in SMS has focused on investigation of cognitive ability, developmental profiles, maladaptive/adaptive behavior, and sleep (Dykens, Finucane, & Gayley, 1997; Dykens & Smith, 1998; Finucane, Dirrigl, & Simon, 2001; Gropman et al., 2006; Hicks, Ferguson, Bernier, & Lemay, 2008; Martin et al., 2006; Smith, Dykens, & Greenberg, 1998a, 1998b; Taylor & Oliver, 2008; Wolters et al., 2009). Sensory processing issues in SMS have been anecdotally or generally recognized by multiple authors (Gropman et al., 2006, 2007; Hicks et al., 2008; Laje et al., 2010; Smith et al., 2005, 2010), and a unique case of twins discordant for SMS, evaluated under this study, provided descriptive evidence of atypical sensory processing in an individual with SMS (Smith et al., 2009). Systematic study of the prevalence and patterns of sensory processing in a group of individuals with SMS has yet to be reported.
The purpose of this study was to systematically assess sensory processing in children with SMS using the Sensory Profile Caregiver Questionnaire (Dunn, 1999) in order to (a) describe common behaviors, performance abilities, and patterns; (b) examine age and gender differences; and (c) document change over time.
METHODS
Participants
This study was conducted at the Hatfield Clinical Research Center, National Institutes of Health (NIH) under an IRB-approved protocol (01-HG-0109) as part of an on-going multidisciplinary natural history study of SMS. Participants aged between 3 and 14 years with a confirmed diagnosis of SMS documenting interstitial deletion [del (17) (p11.2p11.2)] or RAI1 mutation were eligible for enrolment. Consent for participation was obtained from the participant’s parent or legal guardian.
Forty-one participants consecutively enrolled in this study between April 2001 and November 2008. All participants had a confirmed diagnosis of SMS: 40 documented detections (del 17p11.2) and one RAI1 mutation. Seven children were excluded from the study sample either due to failure to return the Sensory Profile (N = 4), or due to incomplete response data (≥30% of missing response items, categories, or quadrants) (N = 3). The final study sample (Table 2) consisted of 34 participants from the continental United States.
TABLE 2.
Demographics of 34 Children with Smith–Magenis Syndrome (SMS)
| Number | Gender | Mean age in years (SD) | |
|---|---|---|---|
|
| |||
| Total sample | 34 | 21 females; 13 males | 6.8 (3.6) |
| Younger (3–5 years) | 20 | 10 females; 10 males | 4.4 (0.8) |
| Older (6–14 years) | 14 | 11 females; 3 males | 10.3 (3.2) |
| SMS longitudinal group | |||
| Visit 1 | 9 | 5 females; 4 males | 3.9 (0.5) |
| Visit 2 (2–3 year follow up) | 9 | 5 females; 4 males | 6.2 (0.4) |
Measures and Procedure
The Sensory Profile along with the Sensory Profile Supplement (Dunn, 2006) were used to assess sensory processing abilities and performance patterns. While the Adolescent/Adult Sensory Profile may be viewed as the most appropriate profile for individuals aged over 10 years, this 60-item self-report was designed to actively involve the individual in assessment (Dunn, 2002). This tool was deemed inappropriate for our cognitively impaired and behaviorally challenged participant population, leading to the selection of the Sensory Profile.
The Sensory Profile is a valid and reliable parent/caregiver interview questionnaire that uses the five-point Likert scale (never = 5; seldom = 4; occasionally = 3; frequently = 2; and always = 1) to rate 125 items assessing a child’s response to daily sensory experiences. Response items are grouped into 14 categories subdivided to reflect three main areas of sensory processing: six categories of sensory processing; five categories of modulation that reflect various combinations of sensory input for use in daily life; and three categories of behavioral and emotional responses.
As a more structured way to understand patterns observed in the Sensory Profile, an additional tool, the Sensory Profile Supplement, was developed based on Dunn’s model of sensory processing (Dunn, 1997, 2001). Patterns of sensory processing were identified by groups of Sensory Profile response items that reflect four quadrants: Registration, Seeking, Sensitivity, and Avoiding. These quadrants are defined by the specific combination of neurological threshold for response and the response pattern that can be either active or passive. The registration pattern is characterized by high sensory thresholds and a passive self-regulation strategy; and the seeking pattern is associated with high sensory thresholds and an active self-regulation strategy. The sensitivity pattern reflects low neurological thresholds and a passive self-regulation strategy; the avoiding pattern represents low neurological thresholds and an active self-regulation strategy. However, Dunn (2006) clarifies that there may not be a single prominent pattern and that any combination of patterns is possible.
Sensory Profile category and quadrant scores reflect the performance level in standard deviations for a standardized sample. Both unidirectional and bidirectional scoring systems exist. The updated bi-directional scoring system, used in this study, was based on a national sample of 1,263 children, including 226 children, aged 3–14 years, with disabilities, and 1,037 children, aged 3–10 years, without disabilities. Standard deviation scores above and below the mean in the normal distribution reflect the updated expanded cut scores. These scores represent five classifications that may be used in combination with the original unidirectional system of classification to define the direction and level of difference in respective categories of sensory processing and quadrants. As 3- and 4-year-old children were found to perform differently from older children in the standardization sample, expanded cut scores were developed for the respective ages and caution is advised when interpreting the Sensory Profile for younger ages (Dunn, 1999, 2006).
Based on their age at initial assessment, participants were assigned to one of the two age groups in the SMS group: younger SMS = 3–5 years, representing preschool-aged children; older SMS = 6–14 years, representing school-aged children. Considering the known cognitive and developmental delays in SMS, 5-year-old children were included in the younger group. Nine participants in the younger SMS group who returned for a follow-up visit per convenience within 2–3 years formed the SMS longitudinal group. Parents or care givers of participants completed the Sensory Profile at initial and follow-up assessments.
Data Analysis
SAS for Windows (Version 9.1.3) was used for descriptive and statistical analysis. Univariate descriptive analyses were evaluated to identify any outliers, including the single subject with RAI1 mutation.
Frequency counts were completed for all Sensory Profile response items to identify commonly reported behavioral characteristics of sensory processing in the SMS group. The criterion for a “common behavior” was based on a previous study of children with autism (Kientz & Dunn, 1997); criterion was met if 80% or more respondents rated a single behavioral item as frequently or always. Frequency counts were also completed for category and quadrant classifications based on the standardization sample. Age-specific expanded cut scores for 3- and 4-year-old participants were used.
Means and standard deviations were calculated for each of the 14 categories of sensory processing and the four sensory processing quadrants (see Table 4). These means and standard deviations were used to describe the SMS group. Two tailed Z-tests (p < .05) were used to determine differences between the SMS group and the national sample with respect to the mean category and quadrant scores. The Z-test was used as a one sample location test comparing the means and standard deviations of Sensory Profile category and quadrant scores obtained from a very large and nationally representative sample of children (referred to as the national sample) to the means and standard deviations derived from our sample of children with SMS.
TABLE 4.
Sensory Profile in 34 Children with Smith–Magenis Syndrome (SMS) and the National Sample of 1,263 Children, 3–14 Years Old
| Sensory Profile Categories and Quadrants | Children with SMS Mean (SD)* | National Sample (Dunn, 2006) Mean (SD) |
|---|---|---|
|
| ||
| Sensory profile categories (A–N) | ||
| Sensory processing | ||
| A. Auditory processing | 26.0 (5.7)a | 33.6 (4.0) |
| B. Visual processing | 31.7 (5.7) | 36.7 (4.6) |
| C. Vestibular processing | 40.9 (6.5) | 51.2 (3.5) |
| D. Touch processing | 60.9 (10.3) | 80.6 (7.4) |
| E. Multisensory processing | 20.9 (4.7) | 30.2 (2.9) |
| F. Oral sensory processing | 44.1 (8.2) | 52.5 (6.5) |
| Modulation | ||
| G. Sensory processing related to endurance/tone | 26.6 (7.8) | 42.7 (3.3) |
| H. Modulation related to body position and movement | 34.2 (4.8) | 44.9 (4.0) |
| I. Modulation of movement affecting activity level | 20.3 (3.5) | 26.5 (3.4) |
| J. Modulation of sensory input affecting emotional responses | 11.0 (3.0) | 18.1 (1.9) |
| K. Modulation of visual input affecting emotional responses and activity level | 13.3 (2.9) | 16.7 (2.2) |
| Behavioral and emotional responses | ||
| L. Emotional/social responses | 57.5 (8.8)a | 70.9 (8.1) |
| M. Behavioral outcomes of sensory processing | 13.5 (3.2)a | 24.9 (3.0) |
| N. Items indicating thresholds for response | 10.1 (2.2)a | 13.1 (1.6) |
| Sensory profile quadrants (1–4) | ||
| 1. Registration | 45.7 (9.4) | 68.2 (5.1) |
| 2. Seeking | 86.0 (14.3)a | 113.3 (11.4) |
| 3. Sensitivity | 69.1 (11.7)a | 87.9 (7.8) |
| 4. Avoiding | 96.2 (12.8)a | 123.1 (10.8) |
Significant difference (p < .001) by 2-tailed Z-tests were demonstrated for the 14 categories and 4 quadrants.
Lower scores indicate engagement in respective behaviors more often (always = 1, never = 5). Italicized and bold figures are greater than 1 and 2 SD, respectively, below the national sample means.
Missing values included in category (Ca) and quadrant (Q) estimates (CaA, Q3, Q4 − N = 1; CaN, Q2 − N = 2; CaL − N = 4; CaM − N = 5).
For age (younger SMS, older SMS) and gender comparisons, ANOVAs were conducted on each of the 14 categories and four quadrants of the Sensory Profile. Post hoc testing using the Tukey–Kramer test was done to examine interaction effects. For the SMS longitudinal group, repeated measures ANOVAs were conducted on each Sensory Profile category and quadrant.
RESULTS
All category and quadrant scores were normally distributed. One participant with RAI1 mutation (N = 1) had scores that fell within the range of scores for participants with deletions (N = 33) in all 14 categories and four quadrants, permitting this participant’s inclusion in all group results. A single behavior met established criterion for a “common behavior”; 29 participants from the SMS group (N = 32) were found to have trouble staying between the lines when coloring or when writing. Thirty-three behavioral response items were rated as occurring frequently or always by 50–76% of our study respondents (see Appendix). Raw scores for 12 out of 14 performance categories and all four quadrants for more than half of the participant group fell within the definite difference/much more than others or the probable difference/more than others ranges (Table 3).
TABLE 3.
Frequency of Sensory Profile Performance Classifications in Children with Smith–Magenis Syndrome (N = 34)
| Much less than others | Less than others | Similar to othersa | More than othersa | Much more than othersa | |
|---|---|---|---|---|---|
| Sensory Profile Categories and Quadrants | Definite differencea | Probable differencea | Typical performanceb | Probable differenceb | Definite differenceb |
|
| |||||
| Sensory profile categories (A–N) | |||||
| Sensory processing | |||||
| A. Auditory processingc | 0 | 1 | 8 | 6 | 18 |
| B. Visual processing | 0 | 3 | 15 | 10 | 6 |
| C. Vestibular processing | NA | 0 | 8 | 7 | 19 |
| D. Touch processing | NA | 0 | 8 | 6 | 20 |
| E. Multisensory processing | NA | 0 | 6 | 4 | 24 |
| F. Oral sensory processing | NA | 0 | 18 | 8 | 8 |
| Modulation | |||||
| G. Sensory processing related to endurance/tone | NA | NA | 2 | 1 | 31 |
| H. Modulation related to body position and movement | NA | 0 | 7 | 9 | 18 |
| I. Modulation of movement affecting activity level | 0 | 0 | 8 | 16 | 10 |
| J. Modulation of sensory input affecting emotional responses | NA | NA | 2 | 3 | 29 |
| K. Modulation of visual input affecting emotional responses and activity level | NA | 1 | 12 | 11 | 10 |
| Behavior and emotional responses | |||||
| L. Emotional/social responsesc | 0 | 1 | 7 | 9 | 13 |
| M. Behavioral outcomes of sensory processingc | 0 | 0 | 1 | 2 | 26 |
| N. Items indicating thresholds for responsec | NA | 1 | 9 | 11 | 11 |
| Sensory profile quadrants (1–4) | |||||
| 1. Registration | NA | 0 | 2 | 1 | 31 |
| 2. Seekingc | NA | 0 | 4 | 5 | 23 |
| 3. Sensitivityc | NA | 0 | 7 | 7 | 19 |
| 4. Avoidingc | 0 | 0 | 3 | 7 | 23 |
Indicates bidirectional classifications.
Indicates unidirectional classifications.
Missing values included in frequency count of categories (Ca) and quadrants (Q) (CaA, Q3, Q4 − N = 1; CaN, Q2 − N = 2; CaL − N = 4; CaM − N = 5).
NA indicates not applicable; no score is possible for the respective categories and quadrants.
Means and standard deviations for the group of children with SMS and the national sample are shown in Table 4. Two-tailed Z-tests demonstrate a significant difference in mean scores (p <. 001) for the SMS group compared to the national sample for all 14 categories and four quadrants.
In the 14 sensory processing categories and four quadrants, two-way ANOVAs demonstrated no main effects for age or gender. An interaction effect on age by gender was demonstrated in Modulation of Sensory Input Affecting Emotional Responses, F(3,30)=9.54,p=.004.Post hoc testing revealed that older females had the lowest scores. Significant differences (p < .05) were found between the mean scores for younger females and younger males and between younger females and older females (Table 5).
TABLE 5.
Sensory Profile in Children with Smith-Magenis Syndrome (SMS) by Age and Gender
| 3–5 Years Females N = 10 |
3–5 Years Males N = 10 |
6–14 Years Females N = 11 |
6–14 Years Males N = 3 |
|
|---|---|---|---|---|
| Sensory Profile Categories and Quadrants | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
|
| ||||
| Sensory profile categories (A–N) | ||||
| Sensory processing | ||||
| A. Auditory processing | 28.2 (5.2) | 25.0 (4.0) | 24.8 (6.6) | 27.0 (12.7)a |
| B. Visual processing | 33.4 (5.4) | 30.2 (6.5) | 30.6 (5.1) | 35.3 (5.5) |
| C. Vestibular processing | 42.9 (5.7) | 42.6 (5.8) | 36.9 (7.0) | 43.3 (6.0) |
| D. Touch processing | 66.2 (7.2) | 62.0 (8.1) | 55.2 (11.6) | 60.0 (15.4) |
| E. Multisensory processing | 22.5 (4.2) | 21.2 (5.1) | 19.7 (4.5) | 18.3 (6.4) |
| F. Oral sensory processing | 42.6 (8.4) | 47.7 (5.1) | 43.2 (7.6) | 40.3 (16.9) |
| Modulation | ||||
| G. Sensory processing related to endurance/tone | 28.0 (7.2) | 28.3 (8.8) | 22.9 (7.1) | 29.3 (8.1) |
| H. Modulation related to body position and movement | 34.9 (5.0) | 34.2 (4.2) | 32.7 (5.1) | 36.7 (5.1) |
| I. Modulation of movement affecting activity level | 21.1 (3.8) | 20.5 (4.0) | 19.6 (3.4) | 19.7 (0.6) |
| J. Modulation of sensory input affecting emotional responses | 13.7 (2.7)* | 10.7 (1.3)* | 8.6 (2.4)* | 11.3 (3.5) |
| K. Modulation of visual input affecting emotional responses and activity level | 14.7 (2.3) | 13.5 (2.8) | 11.6 (2.7) | 14.3 (3.5) |
| Behavioral and emotional responses | ||||
| L. Emotional/social responses | 61.1 (9.0) | 59.9 (8.0)b | 53.5 (8.5) | 53.5 (6.4)a |
| M. Behavioral outcomes of sensory processing | 14.1 (4.3)c | 14.1 (2.9)b | 12.5 (3.1) | 14.0 (2.0) |
| N. Items indicating thresholds for response | 11.4 (2.3)c | 9.6 (1.4) | 9.9 (2.7) | 9.0 (2.0) |
| Sensory profile quadrants (1–4) | ||||
| 1. Registration | 48.7 (9.0) | 46.7 (10.5) | 41.5 (8.9) | 47.3 (6.5) |
| 2. Seeking | 92.3 (15.9)c | 88.2 (12.2) | 80.2 (14.6) | 83.0 (13.8) |
| 3. Sensitivity | 71.8 (10.7) | 73.9 (10.7) | 62.7 (12.3) | 66.5 (7.8)a |
| 4. Avoiding | 100.4 (13.3) | 96.8 (12.6)b | 92.0 (14.0) | 96.3 (5.5) |
Lower scores indicate engagement in respective behaviors more often (always = 1, never = 5). Italicized and bold figures are greater than 1 and 2 SD, respectively, below the national sample means.
Missing values included in category (Ca) and quadrant (Q) estimates
(CaA, CaL, Q3 − N = 1)
(Q4 − N = 1; CaM − N = 2; CaL − N = 3), and
(CaN, Q2 − N = 2; CaM − N = 3).
Significant differences (p < .05) were found between the mean scores for younger females and younger males and for younger females and older females.
For the SMS longitudinal group (N = 9) evaluated at two time intervals, mean category and quadrant scores are shown in Table 6. Repeated measures of ANOVA revealed significant differences in the mean quadrant score for Seeking, F(9,8) = 5.57, p = .05.
TABLE 6.
Comparison of the Sensory Profile in the SMS Longitudinal Group, Visits 1 and 2 and the National Sample
| Mean Scores (SD) SMS Longitudinal Group Visit 1 N = 9 |
Mean Scores (SD) SMS Longitudinal Group Visit 2 N = 9 |
Mean Scores (SD) National Sample (Dunn, 2006) N = 1,263 |
|
|---|---|---|---|
| Sensory Profile Categories and Quadrants | (3–5 years) | (6–8 years) | (3–14 years) |
|
| |||
| Sensory profile categories (A–N) | |||
| Sensory processing | |||
| A. Auditory processing | 28.0 (5.1) | 26.4 (4.5) | 33.6 (4.0) |
| B. Visual processing | 31.8 (5.8) | 31.7 (5.2) | 36.7 (4.6) |
| C. Vestibular processing | 43.0 (5.3) | 40.2 (4.9) | 51.2 (3.5) |
| D. Touch processing | 67.1 (9.1) | 65.7 (9.5) | 80.6 (7.4) |
| E. Multisensory processing | 22.0 (4.9) | 21.2 (4.0) | 30.2 (2.9) |
| F. Oral sensory processing | 44.2 (7.8) | 43.3 (8.1) | 52.5 (6.5) |
| Modulation | |||
| G. Sensory processing related to endurance/tone | 26.1 (5.4) | 23.7 (5.5) | 42.7 (3.3) |
| H. Modulation related to body position and movement | 35.9 (4.0) | 34.3 (3.7) | 44.9 (4.0) |
| I. Modulation of movement affecting activity level | 21.3 (3.1) | 19.3 (3.8) | 26.5 (3.4) |
| J. Modulation of sensory input affecting emotional responses | 12.9 (2.7) | 10.8 (3.7) | 18.1 (1.9) |
| K. Modulation of visual input affecting emotional responses and activity level | 14.4 (3.2) | 14.7 (2.9) | 16.7 (2.2) |
| Behavioral and emotional responses | |||
| L. Emotional/social responses | 63.1 (5.7)a | 60.0 (8.4) | 70.9 (8.1) |
| M. Behavioral outcomes of sensory processing | 15.3 (4.2)a | 14.7 (2.2) | 24.9 (3.0) |
| N. Items indicating thresholds for response | 11.0 (2.4) | 11.2 (1.9) | 13.1 (1.6) |
| Sensory profile quadrants (1–4) | |||
| 1. Registration | 46.0 (8.0) | 44.1 (7.0) | 68.2 (5.1) |
| 2. Seeking | 96.9 (15.5)* | 86.6 (11.5)* | 113.3 (11.4) |
| 3. Sensitivity | 73.2 (7.9) | 70.6 (7.2) | 87.9 (7.8) |
| 4. Avoiding | 99.3 (5.5) | 99.7 (5.9) | 123.1 (10.8) |
Lower scores indicate engagement in respective behaviors more often (always = 1; never = 5). Italicized and bold figures are greater than 1 and 2 SD, respectively, below mean for national sample.
Missing values included in category (Ca) and quadrant (Q) estimates (CaL − N = 2; CaM − N = 3).
Significant difference (p < .05) between visit 1 and visit 2 in the longitudinal SMS group.
DISCUSSION
This study provides the first systematic description of sensory processing, based on parent or caregiver report, in a group of children with SMS and validates previous descriptions of sensory processing difficulties in children with SMS (Gropman et al. 2006, 2007; Hicks et al., 2008; Smith et al., 2005, 2009, 2010). Our results, demonstrate significant differences in performance abilities and patterns of sensory processing between children with SMS and the sample used to norm the Sensory Profile and a characteristic pattern of sensory processing.
Only one behavioral response item, trouble staying between the lines when coloring or writing, was found to be a “common behavior” based on our established criterion of 80% or more. This is consistent with fine motor deficits reported in children with SMS and may be associated with fine motor tremor and related hand anomalies, i.e., brachydactyly, short broad hands, and ligamentous laxity in SMS (Gropman et al., 2006; Hicks et al., 2008; Madduri et al., 2006; Smith et al., 2009, 2010; Wolters et al., 2009). Acknowledging that this behavior is not solely reflective of sensory processing difficulties, the clinical significance of this finding should be interpreted in the context of characteristic developmental disabilities, neurological dysfunction, and specific hand anomalies in SMS.
The frequencies of three of the 33 behaviors, reported as frequently or always occurring in 50–76% of respondents (see Appendix), are within the reported range of relevant stereotypic behaviors in children with SMS (Table 1). Two Sensory Profile behaviors, chews or licks on nonfood objects and mouths objects (for example, pencil, hands), clearly overlap with the stereotypic behaviors of inserts hands in mouth and inserts objects in mouth; the third behavior, touches people and objects, reflects similar tendencies to the stereotypic behaviors of taps or rubs objects or body. Nine other Sensory Profile behaviors that share similar tendencies to the stereotypic behaviors in Table 1 include holds hands over ears to protect ears from sound; covers eyes or squints to protect eyes from light; looks carefully or intensely at objects/people (for example, stares); rocks unconsciously (for example, while watching TV); rocks in desk/chair/on floor; rubs or scratches out a spot that has been touched; touches people/objects to the point of irritating others; walks on toes; and stares intensely at objects or people.
In contrast to published rates of occurrence of stereotypic behaviours (Dykens & Smith, 1998; Martin et al., 2006; Smith et al., 2010), the nine Sensory Profile behaviors were reported as frequently or always observed by less than 50% of our parent respondents. This may reflect different measures used to characterize the neurobehavioral phenotype in this population. The 36-item Stereotypy Checklist (SCL) (Bodfish et al., 1995) uses a yes/no response for parents to report stereotypical behaviors or repetitive movements, whereas the Sensory Profile uses the five-point Likert scale to report the frequency of behavioral responses to sensory experiences in context to daily activities. The addition of a five-point subscale (0 = no interference to 4 = extreme interference) to the SCL in one study (Martin et al., 2006) provided a valuable measure of the extent to which stereotypic behaviors impacted the daily life of children with SMS. Most of the stereotypic behaviors assessed by Martin et al. (2006) ranged from interfering minimally or not at all; the only stereotypic behaviors rated by parents as moderately interfering in the child’s life were inserting hands and inserting objects in mouth. Finally, a large number of children in our cohort reported decreased awareness of pain and temperature, which validates reports of signs of peripheral neuropathy in children with SMS, including decreased pain or temperature sensation (Greenberg et al., 1996; Gropman, Smith, Allanson, & Greenberg, 1998; Smith et al., 1998a).
Although 21 out of the 34 children with SMS were reported as frequently or always having difficulty putting puzzles together, Visual Processing emerged as a relative strength in our cohort. Dykens et al. (1997) reported alertness to the environment and attention to meaningful visual details as relative strengths in individuals with SMS (14–51 years). While results from our study of younger individuals are consistent with these findings, the discrete difficulties noted in responding to visual stimuli and modulating visual input in our study group warrant consideration and further investigation (see Appendix, items 13 and 98).
Oral Sensory Processing was a relative strength: 18 of 34 children were reported as having typical responses to touch and taste stimuli to the mouth. The few atypical mouthing behaviors reported may be understood to represent persistent, but adaptive tendencies that support overall function. Though age-inappropriate in pre-school and school-aged children, children with SMS who chew, lick, or mouth nonfood objects may be exhibiting behaviors that accommodate developmental deficits. This developmental perspective coincides with a previous suggestion that other unusual or stereotypic behaviors in children with SMS, including the self-hug may be adaptive (Finucane & Haas-Givler, 2009). Prior to interpreting the function or impact of these mouthing behaviors and other unusual or stereotypic behaviors, further study is needed across a broader age of children with SMS to characterize developmental abilities and to examine associations with sensory processing behaviors and functional abilities.
The scores for Sensory Processing Related to Endurance/Tone and Modulation of Sensory Input Affecting Emotional Responses in our cohort may provide insight into previous descriptions of neurological characteristics and social–emotional behaviors of children with SMS. In at least half of our sample, difficulties were reported for tires easily, seems to have weak muscles, and poor endurance (see Appendix, items 67, 69, and 73).Difficulties regulating or sustaining performance aligns with infantile hypotonia, generalized lethargy in infancy, and signs of peripheral neuropathy in SMS (Gropman et al., 2006; Smith et al., 2005, 2010). More than half of the children in our study were reported to need more protection from life than other children and to be overly affectionate with others (Appendix, items 92 and 94). This weakness in using body senses to generate emotional response is congruent with the adult-oriented, attention-seeking, and affectionate nature of individuals with SMS (Finucane, Konar, Haas-Givler, Kurtz, & Scott, 1994; Gropman et al., 2006; Martin et al., 2006; Smith et al., 1998a; Taylor & Oliver, 2008).
The underlying behaviors and poor performance of our cohort in the category Behavioral Outcomes of Sensory Processing are consistent with characteristics and behaviors reported previously for children with SMS. The only behavior not rated as frequently or always occurring by more than 50% of our respondents was talks self through tasks. The infrequency of this behavior may reflect the language delay characteristic of children with SMS (Di Cicco et al., 2001; Girirajan et al., 2006; Gropman et al., 2006; Madduri et al., 2006; Solomon et al., 2002). Representative of the problems in Behavioral Outcomes of Sensory Processing in our cohort are two behaviors characterized as difficulties meeting performance demands when writing and/or coloring (Appendix, items 118 and 119). These findings substantiate fine- and visual-motor delays reported for children with SMS (Gropman et al., 2006; Hicks et al., 2008; Madduri et al., 2006; Smith et al., 2009; Wolters et al., 2009). Again, interpretation of these findings must consider developmental disabilities, neurological dysfunction, and specific hand anomalies in this population. The remaining three behaviors that reflect problems in Behavioral Outcomes of Sensory Processing include inefficient ways of doing things, difficulty tolerating changes in plans and expectations, and difficulty tolerating changes in routines (Appendix, items 120–122).
Performance difficulties coupled with impaired behavioral regulation and cognitive inflexibility may be the underlying factors in problems that have been previously reported in children with SMS, such as poor functional abilities and life skills (Smith et al., 2009; Udwin et al., 2001). Difficulties tolerating changes assessed in our cohort are analogous with difficulties with transitions and changes in routines previously reported in children with SMS (Smith et al., 1998a); these difficulties may also be linked to relevant challenging behaviors (i.e., disobedience, impulsivity, aggression, tantrums, and hyperactivity) and flight reactions described as part of the behavioral phenotype of SMS (Dykens & Smith, 1998; Gropman et al., 2006; Martin et al., 2006; Taylor & Oliver, 2008).
Registration, which reflects a pattern of high neurological thresholds with passive self-regulation (Dunn, 2006), presents as the most prevalent quadrant (31/34) in our cohort. However, this should also be interpreted in the context of frequency of single-quadrant and multi-quadrant patterns. Compared to six of our group participants that presented with Registration as the single extreme pattern, 16 participants presented with definite difference/much more than others scores across all four quadrants. A four-quadrant pattern of sensory processing is defined as high and low neurological thresholds with a tendency to fluctuate between active and passive self-regulation strategies and responding (Dunn, 1997, 2006). Children with this pattern present with an imbalance between habituation and sensitization, missing sensory input, as well as being highly sensitive to sensory input. Management of their needs fluctuates between active self-regulation, adding to or reducing sensory input, and passive self-regulation, not noticing what is going on around them or letting things happen and then responding.
While the Registration pattern may align with a few neurological characteristics and generalized lethargy reported in SMS (Gropman et al., 2006; Smith et al., 2005, 2010), the four-quadrant pattern mirrors a larger range of maladaptive behaviors described in children with SMS: lethargy, sensory seeking, impulsivity, hyperactivity, distractibility, outbursts/tantrums, flight reactions, aggression, and emotional lability (Dykens & Smith, 1998; Gropman et al., 2006; Martin et al., 2006; Taylor & Oliver, 2008). Knowledge of how adaptive and maladaptive behaviors impact function and participation of children with SMS will require further research.
The single interaction effect of age and gender indicate that females aged 3–5 years were closer to the typical performance range in Modulation of Sensory Input Affecting Emotional Responses and females aged 6–14 years were further from typical (more vulnerable). Older females, for example, were observed to be more defenseless physically or emotionally and overly affectionate with others. Although not statistically significant, younger females had more mean scores closer to the typical range compared to younger males; in contrast, older females had more mean scores further from the typical range compared to older males (Table 5). The few studies examining age or gender differences in children with the SMS neurobehavioral phenotype report variable findings (Dykens & Smith, 1998; Edelman et al., 2007; Finucane et al., 2001; Laje et al., 2010; Martin et al., 2006; Wolters et al., 2009). Relevant to our results are previous findings of females with SMS exhibiting significantly more frustration with communication and greater impulsivity and having significantly more impairment in interpreting social cues and in stereotypic behavior and restricted interests; nonsignificant trends have also been demonstrated toward increased hyperactivity and more severe impairments of social responsiveness in females with SMS (Edelman et al., 2007; Laje et al., 2010; Martin et al., 2006). It should be noted that the studies done by Laje et al. (2010) and Martin et al. (2006) included a subset of participants in our study.
Several age-related trends emerged in our study of which some were not of statistical significance; however, these trends suggest direction for further research. Compared to younger children, children aged 6–14 years tended to present with lower mean scores across all categories and quadrants. Between visit one and visit two in the SMS longitudinal group, all but three mean scores decreased (increased vulnerability); the exceptions, categories K and N and quadrant 4 (Table 6), showed only marginal increase. Among the mean scores that decreased in the SMS longitudinal group, only the Seeking pattern demonstrated a statistically significant change (p < .05), representing increased vulnerability. The presentation of more extreme sensory processing behaviors in older children with SMS and for the same child over time aligns with reports of increased maladaptive behaviors in older as compared to younger individuals with SMS (Finucane et al., 2001; Smith et al., 1998a; Wolters et al., 2009). However, in at least two studies no age-related associations with maladaptive behavior were found (Dykens & Smith, 1998; Martin et al., 2006). It is possible that the emergence of more extreme sensory processing behaviors with age is associated with the decline or plateau of cognitive and developmental skills, underlying developmental asynchrony, and/or a gene–environment interaction suggested by others (Finucane & Haas-Givler, 2009; Martin et al., 2006; Taylor & Oliver, 2008; Wolters et al., 2009), warranting further longitudinal investigation.
STUDY LIMITATIONS AND DIRECTIONS FOR FUTURE RESEARCH
This study has several limitations. While this sample may be considered small for a group of children with developmental disabilities, it represents the largest group of children with SMS in which sensory processing has been systematically assessed. Since the sample size of the longitudinal group was very small, findings should be interpreted with caution. Several potential moderators, including developmental or mental age, genotype (deletion size vs. RAI1 mutation), and sleep disturbance were not examined. Outside of using the national sample for comparative analysis, comparison to an age-matched group of typically developing or other diagnoses was not feasible.
We recommend that future research in sensory processing in children with SMS investigate potential moderators, specifically developmental or mental age, genotype, and sleep disturbance, to determine the impact on sensory processing. Administering alternative questionnaires as well as observational and physiological measures of sensory processing is suggested to provide a more comprehensive and clinically applicable description. Research is needed to determine if behaviors, abilities, or patterns of sensory processing differentiate children with SMS from children with autism and other disorders or syndromes in order to support differential diagnosis and guide more specific clinical intervention. Lastly, studies examining the relationship between sensory processing and adaptive/maladaptive behaviors and areas of occupational performance are needed to identify the impact of sensory processing on social participation and performance of daily activities, and thereby inform occupational therapy and multidisciplinary interventions.
Implications for Practice
Identifying and describing comorbid impairments of behavior and understanding how children experience and respond to sensations in the context of their daily life leads to more evidence-based interventions by health care providers. Based on our findings and corroborating studies, in addition to administering the Sensory Profile, it would be prudent to conduct clinical observation and individualized parent interviews to assess the functional impact of reported high or moderately high frequency behaviors and patterns of sensory processing in a child with SMS. This would assist in designing more effective interventions, including parental education on a child’s behavioral patterns and on providing safe and supportive activities and environments.
In addition, performance-based assessments and interviews by various clinical and educational team members for a child with SMS are recommended to allow a broader perspective of behavior in various settings. Synthesis of strengths and problems in sensory processing with findings from other assessments and observations would allow caregivers and occupational and physical therapists to adapt activity demands, modify the environment, and facilitate social interactions. For example, from a sensory processing frame of reference, a child with SMS who has markedly low endurance/tone yet seeks movement and touch, is distracted by extraneous sounds and actions, has difficulty with changes, and jumps from one activity to another may benefit from activities of shorter duration to accommodate tolerance or with built-in breaks by which to meet other sensory needs. Environmental modifications for such a child could include reducing auditory and visual distractions, providing “fidgets,” and increasing postural supports during seated work. In addition, making activity schedules, transitional supports, and social models would support the child’s emotional and social functioning. Therapists and caregivers are encouraged to consider how the relative strengths (i.e., visual processing and oral sensory processing) of a child with SMS can be used to increase motivation, participation, and performance in therapy and daily life activities.
Finally, it is clear that children with SMS present with atypical patterns of sensory processing that appear more prominent with increased age, especially in females. The potential for more atypical and problematic behaviors with increased age emphasizes the need for early and ongoing monitoring.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Human Genome Research Institute (NHGRI), and a Bench-to-Bedside Award from the NIH Clinical Center to the Medical Genetics Branch of the NHGRI. The authors would like to thank Donna Krasnewich, MD, Wendy Introne, MD, and Bonnie Hodsdon, OTR/L, NIH Chief OT who supported our work on this study; Joanne E. Flanagan, MS, OTR/L for initial work on this study; Rebecca Morse, MA, for assistance with data entry; Elizabeth K. Rasch, PT, PhD, for data analysis; Captain Rebecca A. Parks, MS, OTR/L, BCP, FAOTA, and Maryanne Sacco-Peterson, MA, OTR/L, for internal reviewing; and the families who participated in this research.
Biographies
Hanna L. Hildenbrand, is a senior staff occupational therapist in the Department of Rehabilitation Medicine, National Institutes of Health, Bethesda, Maryland.
Ann C. M. Smith, is a senior genetic counselor in the Office of the Clinical Director, Human Genome Research Institute, National Institutes of Health, Bethesda, Maryland.
APPENDIX:
Sensory Profile Behaviors Displayed Always or Frequently in Children with Smith–Magenis Syndrome (N = 34)
| Sensory Profile Item Number | Frequency |
|---|---|
|
| |
| Sensory processing | |
| A. Auditory processing | |
| 4. Is distracted or has trouble functioning if there is a lot of noise around. | 21 |
| B. Visual processing | |
| 13. Has difficulty putting puzzles together (as compared to same age children). | 21 |
| C. Vestibular processing | |
| 24. Seeks all kinds of movement and this interferes with daily routines (for example, can’t sit still, fidgets). | 17 |
| D. Touch processing | |
| 30. Expresses distress during grooming (for example, fights or cries during haircutting, face washing, fingernail cutting). | 17 |
| 32. Expresses discomfort at dental work or toothbrushing (for example, cries or fights). | 17 |
| 42. Decreased awareness of pain and temperature. | 26 |
| 44. Avoids wearing shoes; loves to be barefoot. | 18 |
| 45. Touches people and objects. | 26 |
| 46. Doesn’t seem to notice when face or hands are messy. | 17 |
| E. Multisensory processing | |
| 48. Has difficulty paying attention. | 22 |
| 49. Looks away from tasks to notice all actions in the room. | 24 |
| F. Oral Sensory processing | |
| 64. Chews or licks on nonfood objects. | 17 |
| 65. Mouths objects (for example, pencil, hands). | 25 |
| Modulation | |
| G. Sensory processing related to endurance/tone | |
| 67. Tires easily, especially when standing or holding particular body position. | 17 |
| 69. Seems to have weak muscles. | 21 |
| 73. Poor endurance/tires easily. | 21 |
| H. Modulation related to body position and movement | |
| 76. Hesitates going up or down curbs or steps (for example, is cautious, stops before moving). | 21 |
| 79. Holds onto walls or banisters (for example, clings). | 18 |
| I. Modulation of movement affecting activity level | |
| 86. Prefers quiet, sedentary play (for example, watching TV, books, computers). | 22 |
| 87. Seeks sedentary play options. | 17 |
| 88. Prefers sedentary activities. | 19 |
| J. Modulation of sensory input affecting emotional responses | |
| 92. Needs more protection from life than other children (for example, defenseless physically or emotionally). | 25 |
| 94. Is overly affectionate with others. | 20 |
| K. Modulation of visual input affecting emotional responses and activity level | |
| 98. Watches everyone when they move around the room. | 21 |
| Behavioral and Emotional Responses | |
| L. Emotional/social responses | |
| 101. Has trouble “growing up” (for example, reacts immaturely to situations). | 18c |
| 105. Displays excessive emotional outbursts when unsuccessful at a task. | 24 |
| 108. Has temper tantrums. | 20 |
| 109. Poor frustration tolerance. | 22 |
| M. Behavioral outcomes of sensory processing | |
| 118. Writing is illegible. | 19d |
| 119.Has trouble staying between the lines when coloring or when writing. | 29b |
| 120. Uses inefficient ways of doing things (for example, wastes time, moves slowly, does things a harder way than is needed). | 20c |
| 121. Has difficulty tolerating changes in plans and expectations. | 22a |
| 122. Has difficulty tolerating changes in routines. | 22a |
| N. Items indicating thresholds for response | |
| 123. Jumps from one activity to another so that it interferes with play. | 22b |
All behaviors listed occurred frequently or always in at least 50% of the SMS group.
Missing values included in item estimates:
N = 1
N = 2
N = 3
N = 5.
Footnotes
Declaration of interest: The authors report no declarations of interest.
REFERENCES
- Ashburner J, Ziviani J, & Rodger S (2008). Sensory processing and classroom emotional, behavioral, and educational outcomes in children with autism spectrum disorder. American Journal of Occupational Therapy, 62(5), 564–573. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, et al. (2008). Developmental trajectories and correlates of sensory processing in young boys with fragile x syndrome. Physical & Occupational Therapy in Pediatrics, 28(1), 79–98. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, & Carter AS (2008). Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry, 49(8), 817–825. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Crawford TW, Powell SB, Parker DE, Golden RN, & Lewis MH (1995). Compulsions in adults with mental retardation: Prevalence, phenomenology, and comorbidity with stereotypy and self-injury. American Journal of Mental Retardation, 100(2), 183–192. [PubMed] [Google Scholar]
- Bruni M, Cameron D, Dua S, & Noy S (2010). Reported sensory processing of children with Down syndrome. Physical & Occupational Therapy in Pediatrics, 30(4), 280–293. [DOI] [PubMed] [Google Scholar]
- Di Cicco M, Padoan R, Felisati G, Dilani D, Moretti E, Guerneri S, et al. (2001). Otorhinolaringologic manifestation of smith-magenis syndrome. International Journal of Pediatric Otorhinolaryngology, 59(2), 147–150. [DOI] [PubMed] [Google Scholar]
- Dunn W (1997). The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants and Young Children, 9(4), 23–35. [Google Scholar]
- Dunn W (1999). Sensory profile user’s manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Dunn W (2001). The sensations of everyday life: Empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy, 55(6), 608–620. [DOI] [PubMed] [Google Scholar]
- Dunn W (2002). Adolescent/adult sensory profile user’s manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Dunn W (2006). Sensory profile supplement user’s manual. San Antonio, TX: Harcourt Assessment, Inc. [Google Scholar]
- Dunn W, & Bennett D (2002). Patterns of sensory processing in children with attention deficit hyperactivity disorder. OTJR-Occupation Participation and Health, 22(1), 4–15. [Google Scholar]
- Dykens EM, Finucane BM, & Gayley C (1997). Brief report: Cognitive and behavioral profiles in persons with Smith–Magenis syndrome. Journal of Autism and Developmental Disorders, 27(2), 203–211. [DOI] [PubMed] [Google Scholar]
- Dykens EM, & Smith AC (1998). Distinctiveness and correlates of maladaptive behaviour in children and adolescents with Smith–Magenis syndrome. Journal of Intellectual Disabilities Research, 42(Pt 6), 481–489. [DOI] [PubMed] [Google Scholar]
- Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, et al. (2007). Gender, genotype, and phenotype differences in Smith–Magenis syndrome: A meta-analysis of 105 cases. Clinical Genetics, 71(6), 540–550. [DOI] [PubMed] [Google Scholar]
- Elsea SH, & Girirajan S (2008). Smith–Magenis syndrome. European Journal of Human Genetics, 16(4), 412–421. [DOI] [PubMed] [Google Scholar]
- Finucane B, Dirrigl KH,&Simon EW (2001).Characterization of self-injurious behaviors in children and adults with Smith–Magenis syndrome. American Journal of Mental Retardation, 106(1), 52–58. [DOI] [PubMed] [Google Scholar]
- Finucane B, & Haas-Givler B (2009). Smith–Magenis syndrome: Genetic basis and clinical implications. Journal of Mental Health Research, 2, 134–148. [Google Scholar]
- Finucane BM, Konar D, Haas-Givler B, Kurtz MB, & Scott CI Jr. (1994). The spasmodic upper-body squeeze: A characteristic behavior in Smith–Magenis syndrome. Developmental Medicine & Child Neurology, 36(1), 78–83. [DOI] [PubMed] [Google Scholar]
- Girirajan S, Vlangos CN, Szomju BB, Edelman E, Trevors CD, Dupuis L, et al. (2006). Genotype–phenotype correlation in Smith–Magenis syndrome: Evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genetic Medicine, 8(7), 417–427. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, et al. (1996). Multidisciplinary clinical study of Smith–Magenis syndrome (deletion 17p11.2). American Journal of Medical Genetics, 62(3), 247–254. [DOI] [PubMed] [Google Scholar]
- Gropman A, Smith ACM, Allanson JE, & Greenberg F (1998). Smith–Magenis syndrome: Aspects of the infant phenotype. American Journal of Human Genetics, 63, A19. [Google Scholar]
- Gropman AL, Duncan WC, & Smith AC (2006). Neurologic and developmental features of the Smith–Magenis syndrome (del 17p11.2). Pediatric Neurology, 34(5), 337–350. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Elsea S, Duncan WC Jr., & Smith AC (2007). New developments in Smith–Magenis syndrome (del 17p11.2). Current Opinions in Neurology, 20(2), 125–134. [DOI] [PubMed] [Google Scholar]
- Hicks M, Ferguson S, Bernier F, & Lemay JF (2008). A case report of monozygotic twins with Smith–Magenis syndrome. Journal of Development and Behavioral Pediatrics, 29(1), 42–46. [DOI] [PubMed] [Google Scholar]
- Kientz MA, & Dunn W (1997). A comparison of the performance of children with and without autism on the sensory profile. American Journal of Occupational Therapy, 51(7), 530–537. [DOI] [PubMed] [Google Scholar]
- Laje G, Morse R, Richter W, Ball J, Pao M, & Smith AC (2010). Autism spectrum features in Smith–Magenis syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 154C(4), 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitas A, Dykens E, Finucane B, & Kates WR (2007). Behavioral phenotypes of genetic disorders. In Fletcher R, Loschen E, Stavrakaki C, & First M (Eds.), Diagnostic manual-intellectual disability: A textbook of diagnosis of mental disorders in persons with intellectual disability (pp. 42–43). Kingston, NY: NADD Press. [Google Scholar]
- Madduri N, Peters SU, Voigt RG, Llorente AM, Lupski JR, & Potocki L (2006). Cognitive and adaptive behavior profiles in Smith–Magenis syndrome. Journal of Development and Behavioral Pediatrics, 27(3), 188–192. [DOI] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, & Smith AC (2006). Adaptive and maladaptive behavior in children with Smith–Magenis syndrome. Journal of Autism and Developmental Disorders, 36(4), 541–552. [DOI] [PubMed] [Google Scholar]
- Rieke EF, & Anderson D (2009). Adolescent/adult sensory profile and obsessive-compulsive disorder. American Journal of Occupational Therapy, 63(2), 138–145. [DOI] [PubMed] [Google Scholar]
- Smith ACM, Boyd K, Elsea SH, Finucane BM, Haas-Givler B, Gropman A, et al. (2010). Smith–Magenis syndrome [del(17)(p11.2)]. GeneReviews. Retrieved March 16, 2010, from http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=sms
- Smith ACM, & Duncan WC (2005). Smith–Magenis syndrome—a developmental disorder with circadian dysfunction. In Butler MG & Meaney FJ (Eds.), Genetics of developmental disabilities (pp. 419–475). New York: Taylor and Francis. [Google Scholar]
- Smith AC, Dykens E, & Greenberg F (1998a). Behavioral phenotype of Smith–Magenis syndrome (del 17p11.2). American Journal of Medical Genetics, 81(2), 179–185. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, & Greenberg F (1998b). Sleep disturbance in Smith–Magenis syndrome (del 17 p11.2). American Journal of Medical Genetics, 81(2), 186–191. [PubMed] [Google Scholar]
- Smith MR, Hildenbrand H, & Smith ACM (2009). Sensory motor and functional skills of dizygotic twins: One with Smith–Magenis syndrome and a twin control. Physical & Occupational Therapy in Pediatrics, 29(3), 239–257. [DOI] [PubMed] [Google Scholar]
- Smith AC, Magenis RE, & Elsea SH (2005). Overview of Smith–Magenis syndrome. Journal of the Association of Genetic Technologists, 31(4), 163–167. [PubMed] [Google Scholar]
- Solomon B, McCullagh L, Krasnewich D, & Smith ACM (2002). Oral motor, speech and voice functions in Smith–Magenis syndrome children: A research update. American Journal of Human Genetics, 71, 271. [Google Scholar]
- Taylor L, & Oliver C (2008). The behavioural phenotype of Smith–Magenis syndrome: Evidence for a gene–environment interaction. Journal of Intellectual Disabilities Research, 52(10), 830–841. [DOI] [PubMed] [Google Scholar]
- Udwin O, Webber C, & Horn I (2001). Abilities and attainment in Smith–Magenis syndrome. Developmental Medicine and Child Neurology, 43(12), 823–828. [DOI] [PubMed] [Google Scholar]
- Vlangos CN, Wilson M, Blancato J, Smith AC, & Elsea SH (2005). Diagnostic fish probes for del(17)(p11.2p11.2) associated with Smith–Magenis syndrome should contain the rai1 gene. American Journal of Medical Genetics A, 132A(3), 278–282. [DOI] [PubMed] [Google Scholar]
- Walz NC, & Baranek GT (2006). Sensory processing patterns in persons with angelman syndrome. American Journal of Occupational Therapy, 60(4), 472–479. [DOI] [PubMed] [Google Scholar]
- Wolters PL, Gropman AL, Martin SC, Smith MR, Hildenbrand HL, Brewer CC, et al. (2009). Neurodevelopment of children under 3 years of age with Smith–Magenis syndrome. Pediatric Neurology, 41(4), 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]


