Abstract
Exposure to VOCs is linked to health effects ranging from asthma to cancer and to negative impacts on the hematopoietic system. We examined the association between select blood VOC concentrations and hematological measures in a representative sample of the U.S. population from NHANES cycles spanning the years 2005 to 2010. We used Cox regression to assess the association between complete blood count with five-part differential (CBC) parameters and seven select blood VOCs, while addressing low detection rates among VOCs. Tobacco smoke exposure was classified using serum cotinine levels. The not-smoke-exposed group had lower VOC levels for most analytes compared with the smoke-exposed. Correlations between benzene, toluene, ethylbenzene, and xylenes (BTEX) were moderate to strong. Statistical associations were found between benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS) and hematocrit, hemoglobin, and white blood cell count among the smoke-exposed. Among the not-smoke-exposed, there was an association between BTEX and platelet count. We considered benzene most likely to be associated with higher levels of CBC concentrations. Our findings suggest VOC levels currently found in the general U.S. population are associated with changes in hematological measures, and smoking could be a contributor.
Keywords: Hematology, Volatile organics, BTEX, Hematological, Smoking, VOC
Introduction
Volatile organic compounds (VOCs) are a ubiquitous group of chemicals present in the environment. People are exposed to VOCs through manufactured products like paints, automobile exhaust, and solvents, among others [1]. Other sources of indoor VOCs include cleaning supplies, personal care products, air fresheners, and furniture and building materials [2]. The toxicity of individual VOCs varies, and exposure to VOCs has been linked to health effects ranging from asthma to cancer [2, 3]. Since the late 1980’s, blood levels of VOCs have been decreasing in the United States population [4, 5]. This decline is likely due to decreased exposure, as emissions have declined over time with the substitution of low-emission products and increased environmental regulations [2]. While ambient air sources may be a less significant contributor to VOC exposures, indoor air sources and personal activities have become more important in terms of their contribution to individual exposure levels [4]. Americans spend most of their time indoors, either at home, work or school. The National Human Activity Pattern Survey (NHAPS) found that respondents reported spending ~90% of their time indoors, with 44% of respondents reporting spending time with a smoker each day [6]. Tobacco smoke, both mainstream and environmental, contains high concentrations of VOCs and despite decreases in smoking rates, the general population is still exposed to tobacco smoke [7].
VOCs exposure is thought to negatively impact the hematopoietic system [8]. Most of the mechanistic research in this area has focused on benzene, which through a complicated metabolic process has cytotoxic interactions with bone marrow [9]. Benzene exposure has been linked to adverse developmental and immunological outcomes, and an increase in respiratory conditions like asthma. Benzene is also a known carcinogen [10, 11]. Benzene, toluene, ethylbenzene, and xylene are often analyzed together and are known collectively as BTEX. When styrene is added to the analysis, they are known collectively as benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS). These chemicals are thought to have some similar, non-cancer health effects [10]. Most studies examining the relationship between VOC exposure and hematological endpoints have focused on occupational exposures. These studies typically have small sample sizes and specific exposure sources, such as refineries or industrial areas. Results from these studies are varied. Koh et al. [12] and Pelallo-Martinez et al. [13] found that low-level blood benzene exposure has an inverse effect with hematological measures. However, D’Andrea et al. [14] found increased white blood cell counts (WBC) and platelet counts in smokers exposed to benzene in refinery incidents. A recent study found contrary associations between some hematological parameters and VOCs depending on tobacco smoke exposure status. For example, there were no apparent associations between blood BTEXS concentrations and either WBC or red blood cell (RBC) counts among tobacco smoke-unexposed participants, but there were associations with tobacco smoke-exposed participants. Specifically, benzene concentrations in tobacco smoke-exposed participants showed positive associations with red and white blood cell counts, hemoglobin concentration, hematocrit, mean corpuscular volume, and mean corpuscular hemoglobin concentration, while blood benzene levels in tobacco smoke-unexposed participants showed inverse associations with hemoglobin concentration and mean corpuscular hemoglobin concentration [9].
There is a lack of information in the general population on the impact of VOC exposure on the hematopoietic system. This study examines the association between blood VOC levels and hematological measures in a representative sample of the U.S. population from three combined NHANES cycles spanning the years from 2005 to 2010.
Methods
Study population
We used data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey conducted by the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS) [15]. The NHANES combines interviews and physical examinations to assess the health and nutritional status of the non-institutionalized, civilian population in the United States. NHANES uses a complex, stratified, multistage sample design to collect information, and is conducted as a continuous annual survey released in 2-year cycles. In order to improve statistical power and produce more reliable estimates for subdomains, we combined data from the 2005 to 2006, 2007 to 2008, and 2009 to 2010 cycles. The choice of NHANES cycles was driven by the availability of hematological and VOC variables in each cycle and their detection rates. Male and female NHANES participants 12 years and older in these cycles who had a lab result in the VOC subsamples (n = 10,626) were considered for inclusion in the study. Study participants who were pregnant (n = 260) or who reported being treated for anemia in the past 3 months (n = 362) were excluded from the analysis, as differences in hematological variables due to either condition could confound results. Current users of smokeless tobacco (n = 111 for chewed tobacco and n = 47 for snuff) and nicotine replacement therapy products (n = 24) were also excluded from the analysis due to the likelihood of higher serum cotinine levels without the corresponding VOC exposure from combustible tobacco products. Finally, participants with missing serum cotinine (n = 642) were excluded from analyses. The remaining 9203 participants formed our study sample. A subset of this study sample was used for generating analyte-specific results, as each analyte has a different pattern of missing values.
Survey components used in this analysis include demographics (age, sex, race, and income), health questionnaire (anemia, weight, height, body mass index (BMI), pregnancy), smoking questionnaire data, and laboratory analyses (CBC and VOCs). Six-year sample weights were calculated according to NCHS guidelines [16], using VOC subsample weight, wtsvoc2y. Each respondent was assigned a sample weight that accounted for the probability of selection into the subsample as well as for nonresponse [15]. Weights and survey design variables were used in analyses except where noted.
Laboratory analyses
Both blood VOCs and complete blood count with five-part differential (CBC) analyses are conducted in every NHANES cycle. Blood specimens were collected at NHANES Mobile Examination Centers (MECs) during the participants’ scheduled appointments. Detailed information on sample collection and analysis protocols can be found elsewhere [17]. Briefly, CBC analysis was performed on the Coulter® HMX Hematology Analyzer. Twenty CBC variables are measured in NHANES; however, for this analysis we included the following hematological parameters: white blood cell count (103 cells/μL), eosinophils number (103 cells/μL), red blood cell count (106 cells/μL), hemoglobin (g/dL), hematocrit (%), and platelet count (103 cells/μL).
Blood VOCs were collected by NHANES on participants 12 years and older from a ½ subsample of the NHANES sample. Laboratory analysis protocols are published elsewhere [18]. Briefly, 32 VOCs were analyzed in whole blood using headspace solid-phase micro extraction (SPME)/gas chromatography/isotope dilution mass spectrometry [19]. Many of the 32 blood VOCs in NHANES had extremely low detection rates. Toluene (ng/mL), m-/p-xylene (ng/mL), styrene (ng/mL), benzene (ng/mL), 1, 4-dichlorobenzene (ng/mL), ethylbenzene (ng/mL), and o-xylene (ng/mL) were selected for inclusion in this analysis based on detection rates above 35% and/or evidence from comparable studies that the analyte could impact the hematopoietic system. Since smoking is a major contributor to VOC exposure, we used serum cotinine (ng/mL) as a measure of tobacco smoke exposure. A cut-point of 14.88 (ng/mL) was used to classify study participants as smoke-exposed or not [20]. We chose this cut-point based on values used in recent literature and the distribution of serum cotinine in the bimodal distribution of serum cotinine by self-reported smoking status (Fig. 1) [20].
Fig. 1.
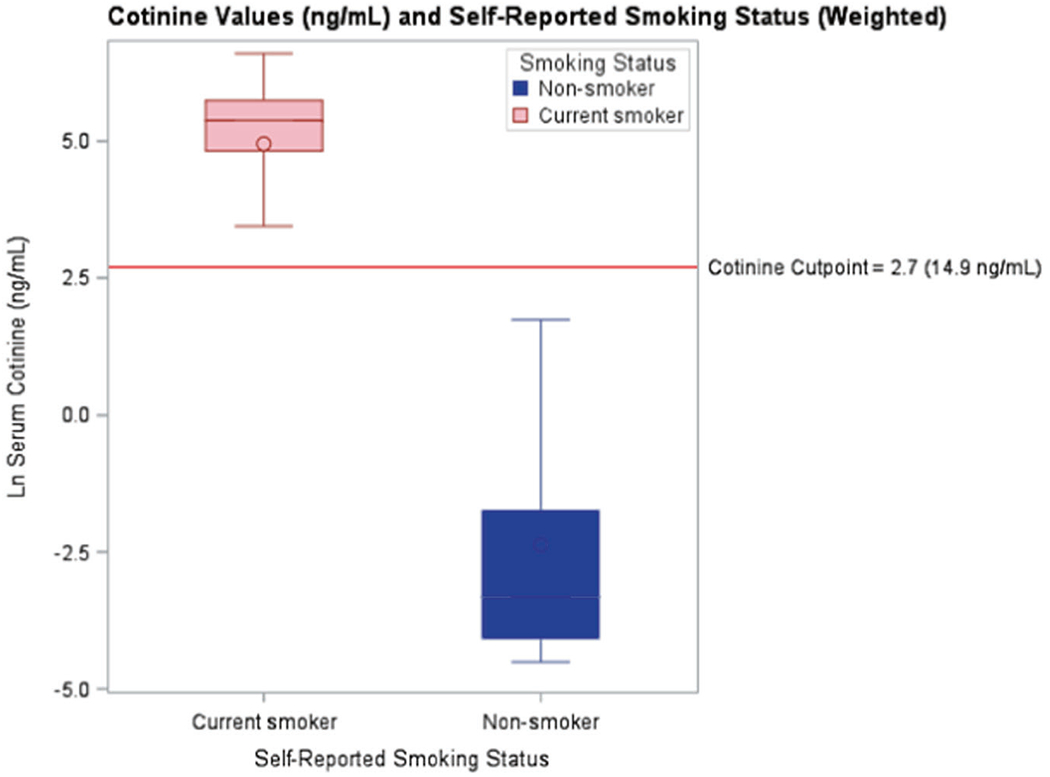
Cotinine values (ng/ml) and self-reported smoking status (weighted).
Statistical analysis
All statistical analyses were conducted using SAS Version 9.4 survey procedures (SAS Institute Inc., Cary, NC, 2012). SAS’s SURVEYFREQ, SURVEYMEANS, SURVEYREG, and SURVEYPHREG procedures were used for all weighted and design-adjusted analyses. Sample weights and design variables were used to account for NHANES’s complex sample design. SAS default Taylor series linearization was used for variance estimation [16]. SAS’s ICLIFETEST procedure was used to produce values for all reverse Kaplan–Meier plots.
Limits of detection (LODs) for each analyte are reported in NHANES documentation. For each analyte, we calculated the percent of respondents with concentrations at or above the LOD. When the concentration of an analyte was below the LOD, NHANES imputes the value of the LOD divided by the square root of 2 for that concentration [21]. This method of handling values below the LOD is referred to as substitution and is widely used, despite known limitations. To facilitate comparison to other studies, we ran all analysis using this method, as well as using methods which treat non-detects as left-censored data.
We conducted regression analyses in order to assess the association between CBC parameters and VOC variables while controlling for confounders. To address the potential bias caused when exposure biomarkers have low detection rates, we used a reverse-scale Cox regression approach proposed by Dinse et al. [22, 23]. This approach treats VOC variables as the modeled outcomes in a Cox regression, CBC parameters as independent variables, and non-detects as right-censored events after applying a scale reversal to the VOC values [22, 23]. The analyses were conducted for six CBC parameters and seven VOC variables, resulting in 42 combinations. Age, sex, race, BMI, and income were forced into each model as potential confounders. Age and BMI were modeled as continuous variables. Owing to the complex relationships between smoking, VOCs and CBC parameters, regression analyses were stratified by tobacco smoke exposure. The key parameter of interest in Cox regression is the hazard ratio. Under the proportional hazards assumption of Cox regression, the hazard ratio can be interpreted as an odds ratio representing the odds of a given VOC concentration versus all lower concentrations, for a unit change in CBC concentration.
In order to compare to other studies that use a linear regression approach with substituted values for non-detects, as well as to conduct a sensitivity analysis, we conducted corresponding linear regression analyses, where CBC values were treated as regression outcomes and where ln-VOC values were treated as independent variables. For these regression analyses, non-detect values were replaced by the . Standardized regression coefficients were calculated to account for variability in both the dependent and independent variables. The coefficients measure the change in hematological parameter (expressed in standard deviations) for a corresponding increase of 1 standard deviation in blood ln-VOC level. Though the parameters of Cox and linear regressions have different interpretations, both can be used to identify the direction, magnitude, and statistical significance of associations.
Results
There were 781 people excluded from the study due to pregnancy (n = 260), taking medication for anemia (n = 362), using smokeless tobacco (n = 111), using snuff (n = 47), or using nicotine therapy products (n = 24). Some participants fell into more than 1 exclusion category. An additional 642 were excluded due to missing serum cotinine values, resulting in a final analysis sample size of 9203. The weighted mean age of study participants was 43. NHANES self-reported race and ethnicity categories were used to classify our population by race. Because of the low counts for “Other Hispanic” responses in our sample, participants with this self-reported ethnicity were grouped with “Other Race—including Multi-Racial” to create the “Other” race variable in our study. For income, we used the ratio of family income to poverty, a calculated NHANES variable using family income and Department of Health and Human Services’ poverty guidelines [15].
The weighted race breakdown of our study population was 68.43% non-Hispanic white, 11.17% non-Hispanic black, 9.02 % Mexican American, and 11.38% other (Table 1). Nearly 63% were overweight or obese with BMI greater than or equal to 25. The percentage of tobacco smoke-exposed as determined by serum cotinine levels was 22.61%.
Table 1.
Selected characteristics of all study participants.
| n | % (weighted) | |
|---|---|---|
| Race/ethnicity | ||
| Non-Hispanic white | 4006 | 68.43 |
| Non-Hispanic Black | 1976 | 11.17 |
| Mexican American | 1964 | 9.02 |
| Other | 1257 | 11.38 |
| Sex | ||
| Male | 4906 | 49.09 |
| Female | 4596 | 50.91 |
| BMI | ||
| Underweight | 366 | 3.22 |
| Normal | 3010 | 33.53 |
| Overweight | 2852 | 31.99 |
| Obese | 2861 | 31.26 |
| Age (years) | ||
| 12–17 | 1513 | 10.18 |
| 18–29 | 1651 | 18.74 |
| 30–59 | 3571 | 50.20 |
| 60–80 | 2468 | 20.89 |
| Smoke exposed | ||
| Cotinine ≤14.88 | 7256 | 77.39 |
| Cotinine >14.88 | 1947 | 22.61 |
| Poverty income ratio | ||
| 1st Tertile | 4008 | 32.45 |
| 2nd Tertile | 2549 | 32.72 |
| 3rd Tertile | 1969 | 34.82 |
Detection rates (Table 2) ranged from a low of 37.7% for benzene to a high of 93.6% for Toluene. Figure 2 shows unweighted reverse Kaplan–Meier plots for the study participants by VOC and smoke exposure status. These plots graphically depict left-censoring among study participants due to values below the LOD by smoke exposure status. VOC concentrations are lower among our study participants for non-smoke-exposed than for smoke-exposed for all analytes other than 1, 4-Dichlorobenzene, for which the concentrations are nearly equal.
Table 2.
Detection rates, geometric means and 95% confidence intervals for selected VOC blood levels in the study population, stratified by gender and smoke exposure status.a
| VOC | LOD (ng/mL) | Detection rate (%) | Males |
Females |
||
|---|---|---|---|---|---|---|
| n | Geometric mean (95% CI) | n | Geometric mean (95% CI) | |||
| Toluene | 0.0250 | 93.56 | 3705 | 0.124 (0.115, 0.132) | 3627 | 0.100 (0.092, 0.110) |
| m-/p-Xylene | 0.0340 | 89.54 | 4209 | 0.108 (0.100, 0.116) | 4211 | 0.089 (0.082, 0.097) |
| o-Xylene | 0.0240 | 57.50 | 4140 | 0.034 (0.032, 0.036) | 4116 | 0.030 (0.028, 0.032) |
| Styrene | 0.0300 | 39.14 | 3151 | 0.036 (0.034, 0.038) | 3134 | 0.032 (0.030, 0.034) |
| Benzene | 0.0240 | 37.73 | 4170 | 0.035 (0.033, 0.038) | 4141 | 0.031 (0.029, 0.032) |
| Ethylbenzene | 0.0240 | 52.47 | 4205 | 0.035 (0.033, 0.037) | 4194 | 0.030 (0.029, 0.032) |
| 1,4-Dichlorobenzene | 0.0400 | 55.35 | 3987 | 0.119 (0.107, 0.132) | 4015 | 0.104 (0.094, 0.115) |
| VOC | LOD (ng/mL) | Detection rate (%) | Smoke exposed (>2.7) |
Not smoke exposed (<−2.7) |
||
| n | Geometric mean (95% CI) | n | Geometric mean (95% CI) | |||
|
| ||||||
| Toluene | 0.0250 | 93.56 | 1565 | 0.392 (0.365, 0.422) | 5767 | 0.077 (0.071, 0.083) |
| m-/p-Xylene | 0.0340 | 89.54 | 1780 | 0.206 (0.192, 0.222) | 6640 | 0.079 (0.072, 0.085) |
| o-Xylene | 0.0240 | 57.50 | 1723 | 0.05 (0.046, 0.054) | 6533 | 0.028 (0.026, 0.03) |
| Styrene | 0.0300 | 39.14 | 1288 | 0.079 (0.075, 0.084) | 4997 | 0.026 (0.025, 0.028) |
| Benzene | 0.0240 | 37.73 | 1804 | 0.148 (0.137, 0.16) | 6507 | 0.021 (0.02, 0.022) |
| Ethylbenzene | 0.0240 | 52.47 | 1785 | 0.075 (0.069, 0.08) | 6614 | 0.025 (0.024, 0.027) |
| 1,4-Dichlorobenzene | 0.0400 | 55.35 | 217 | 0.115 (0.103, 0.128) | 6323 | 0.11 (0.099, 0.122) |
Analyte values below LOD were replaced with LOD/√2. Low detection rates can result in biased estimates but are presented to facilitate comparison to other studies. Means are weighted and design-adjusted.
Fig. 2.
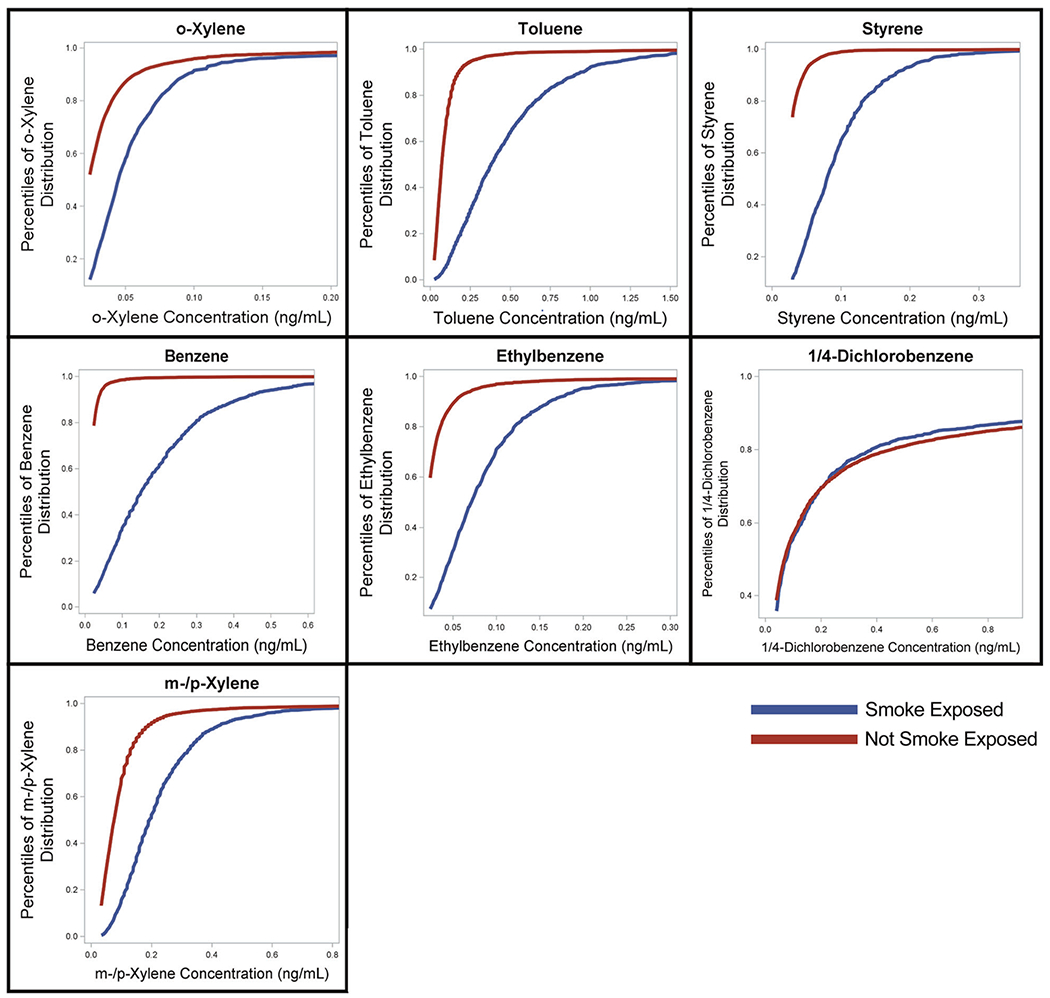
Differences in VOC concentration percentiles by smoke exposure status for non-survey adjusted NHANES data.
For the seven VOC analytes included in this analysis, weighted and design-adjusted geometric means by sex and by smoke exposure status and associated 95% confidence intervals were calculated (Table 2). Men had higher geometric means for blood VOC levels for every analyte (Table 2). Non-Hispanic whites had higher geometric means for ethylbenzene, m-/p-xylene, o-xylene, styrene, and toluene than all other races. African-Americans had the highest levels of benzene and 1, 4-dichlorobenzene. Pairwise correlations between log-transformed concentrations of benzene, toluene, ethylbenzene, and xylenes (BTEX) were moderate to strong with weighted Pearson Correlation Coefficients ranging from 0.44 to 0.91. 1, 4-Dichlorobenzene had weak correlations to all other analytes. Adjusted geometric means of each of our analytes are also presented by smoke exposure category in Table 2.
Regression results for linear and reverse-scale Cox regression are shown in Tables 3 and 4, respectively. All regression analyses were stratified by smoke exposure status as determined by serum cotinine levels, and all regression models adjust for age, sex, race, household income, and BMI. Standardized beta coefficients are shown in Table 3 and can be interpreted as the change in CBC concentration (expressed in standard deviations) for a corresponding increase of 1 standard deviation in natural log blood VOC level. For Cox regression results, there is a role reversal between VOCs and CBCs, with CBCs acting as independent variables in the models. Hazard ratios reported in Table 4 are calculated for a 1 standard deviation increase in CBC concentration. Therefore they can be interpreted as the confounder-adjusted odds of a given VOC concentration versus all lower concentrations for a one standard deviation increase in CBC concentration.
Table 3.
Summary table of changes in hematological measures per unit increase of log blood VOC concentration.
| Hematological parameter | VOC | Not smoke exposed |
Smoke exposed |
||||
|---|---|---|---|---|---|---|---|
| S Beta | Beta (95% CI) | P value | S Beta | Beta (95% CI) | P value | ||
| Eosinophils | Benzene | 0.018 | 0.006 (−0.003, 0.015) | 0.2118 | −0.020 | −0.005 (−0.018, 0.009) | 0.4944 |
| Ethylbenzene | 0.002 | 0 (−0.007, 0.008) | 0.9051 | −0.032 | −0.009 (−0.021, 0.003) | 0.1507 | |
| m-/p-Xylene | 0.010 | 0.002 (−0.004, 0.008) | 0.5421 | −0.041 | −0.012 (−0.023,−0.001) | 0.0403 | |
| o-Xylene | 0.001 | 0 (−0.007, 0.007) | 0.9617 | −0.025 | −0.008 (−0.025, 0.01) | 0.3736 | |
| 1,4-Dichlorobenzene | 0.008 | 0.001 (−0.003, 0.004) | 0.6293 | 0.015 | 0.002 (−0.008, 0.012) | 0.6431 | |
| Styrene | 0.010 | 0.004 (−0.008, 0.016) | 0.4909 | 0.000 | 0 (−0.015, 0.015) | 0.9857 | |
| Toluene | 0.012 | 0.002 (−0.004, 0.008) | 0.4785 | −0.031 | −0.008 (−0.022, 0.006) | 0.2452 | |
| Hematocrit | Benzene | 0.033 | 0.277 (−0.001, 0.555) | 0.0505 | 0.109 | 0.458 (0.182, 0.734) | 0.0017 |
| Ethylbenzene | 0.028 | 0.159 (−0.06, 0.378) | 0.1506 | 0.073 | 0.38 (0.126, 0.633) | 0.0041 | |
| m-/p-Xylene | 0.038 | 0.182 (−0.01, 0.373) | 0.0622 | 0.092 | 0.5 (0.254, 0.747) | 0.0002 | |
| o-Xylene | 0.014 | 0.077 (−0.153, 0.308) | 0.5034 | 0.073 | 0.414 (0.106, 0.723) | 0.0095 | |
| 1,4-Dichlorobenzene | 0.004 | 0.011 (−0.086, 0.108) | 0.8152 | 0.008 | 0.022 (−0.12, 0.163) | 0.7577 | |
| Styrene | 0.024 | 0.233 (−0.142, 0.608) | 0.2167 | 0.076 | 0.433 (0.023, 0.843) | 0.0391 | |
| Toluene | 0.036 | 0.174 (−0.017, 0.365) | 0.0727 | 0.087 | 0.405 (0.094, 0.716) | 0.0119 | |
| Hemoglobin | Benzene | 0.026 | 0.074 (0.001, 0.148) | 0.0468 | 0.125 | 0.185 (0.095, 0.275) | 0.0001 |
| Ethylbenzene | 0.016 | 0.033 (−0.034, 0.1) | 0.3245 | 0.087 | 0.157 (0.066, 0.249) | 0.0011 | |
| m-/p-Xylene | 0.012 | 0.02 (−0.038, 0.077) | 0.4925 | 0.088 | 0.168 (0.077, 0.259) | 0.0005 | |
| o-Xylene | 0.001 | 0.001 (−0.068, 0.071) | 0.9742 | 0.072 | 0.142 (0.038, 0.247) | 0.0083 | |
| 1,4-Dichlorobenzene | −0.017 | −0.016 (−0.047, 0.016) | 0.3320 | −0.017 | −0.017 (−0.063, 0.029) | 0.4652 | |
| Styrene | 0.014 | 0.047 (−0.034, 0.128) | 0.2510 | 0.089 | 0.179 (0.042, 0.316) | 0.0115 | |
| Toluene | 0.017 | 0.029 (−0.03, 0.088) | 0.3288 | 0.112 | 0.183 (0.068, 0.299) | 0.0025 | |
| Platelet count | Benzene | 0.047 | 6.787 (1.35, 12.224) | 0.0155 | 0.072 | 5.437 (0.567, 10.307) | 0.0294 |
| Ethylbenzene | 0.050 | 4.933 (1.69, 8.177) | 0.0037 | 0.030 | 2.819 (−3.886, 9.523) | 0.4020 | |
| m-/p-Xylene | 0.040 | 3.271 (0.158, 6.384) | 0.0399 | 0.039 | 3.863 (−3.038, 10.764) | 0.2658 | |
| o-Xylene | 0.030 | 2.843 (−0.562, 6.248) | 0.0996 | 0.047 | 4.724 (−2.255, 11.704) | 0.1798 | |
| 1,4-Dichlorobenzene | 0.032 | 1.485 (−0.135, 3.105) | 0.0715 | 0.047 | 2.357 (−0.727, 5.441) | 0.1309 | |
| Styrene | −0.011 | −1.843 (−11.252, 7.567) | 0.6948 | 0.044 | 4.366 (−3.352, 12.083) | 0.2603 | |
| Toluene | 0.070 | 5.729 (2.281, 9.177) | 0.0017 | 0.040 | 3.386 (−3.421, 10.192) | 0.3218 | |
| Red cell count | Benzene | 0.014 | 0.013 (−0.014, 0.041) | 0.3287 | 0.004 | 0.002 (−0.031, 0.035) | 0.9127 |
| Ethylbenzene | −0.001 | −0.001 (−0.021, 0.02) | 0.9342 | −0.001 | 0 (−0.031, 0.031) | 0.9747 | |
| m-/p-Xylene | 0.002 | 0.001 (−0.019, 0.022) | 0.8995 | 0.021 | 0.014 (−0.013, 0.04) | 0.3050 | |
| o-Xylene | −0.008 | −0.005 (−0.027, 0.017) | 0.6512 | 0.014 | 0.01 (−0.023, 0.042) | 0.5441 | |
| 1,4-Dichlorobenzene | 0.008 | 0.003 (−0.007, 0.012) | 0.5976 | −0.005 | −0.002 (−0.018, 0.015) | 0.8395 | |
| Styrene | 0.012 | 0.014 (−0.031, 0.058) | 0.5417 | −0.005 | −0.004 (−0.05, 0.043) | 0.8793 | |
| Toluene | 0.009 | 0.005 (−0.017, 0.026) | 0.6517 | −0.005 | −0.003 (−0.042, 0.037) | 0.8954 | |
| White cell count | Benzene | 0.016 | 0.067 (−0.069, 0.203) | 0.3248 | 0.202 | 0.518 (0.377, 0.659) | <0.0001 |
| Ethylbenzene | 0.024 | 0.069 (−0.017, 0.154) | 0.1116 | 0.165 | 0.525 (0.31, 0.739) | <0.0001 | |
| m-/p-Xylene | 0.011 | 0.026 (−0.053, 0.106) | 0.5064 | 0.130 | 0.436 (0.228, 0.643) | 0.0001 | |
| o-Xylene | 0.018 | 0.053 (−0.045, 0.15) | 0.2815 | 0.111 | 0.384 (0.142, 0.626) | 0.0025 | |
| 1,4-Dichlorobenzene | 0.004 | 0.005 (−0.038, 0.048) | 0.8153 | −0.006 | −0.01 (−0.094, 0.075) | 0.8180 | |
| Styrene | 0.010 | 0.05 (−0.083, 0.183) | 0.4505 | 0.206 | 0.695 (0.484, 0.907) | <0.0001 | |
| Toluene | −0.018 | −0.046 (−0.133, 0.042) | 0.3013 | 0.186 | 0.543 (0.385, 0.7) | <0.0001 | |
Table 4.
Reverse-scale cox regression results and hazard ratios.
| Parameter | Dependent | Not smoke exposed |
Smoke exposed |
||||
|---|---|---|---|---|---|---|---|
| Cox Beta | P value | Hazard Ratio per 1 SD Increase in CBC | Cox Beta | P value | Hazard Ratio per 1 SD Increase in CBC | ||
| Eosinophils | Benzene | 0.449 | 0.0201 | 1.08 (1.01, 1.15) | −0.012 | 0.9067 | 1.00 (0.97, 1.03) |
| Ethylbenzene | 0.180 | 0.2171 | 1.03 (0.98, 1.08) | −0.001 | 0.9912 | 1.00 (0.97, 1.03) | |
| M-/p-Xylene | 0.159 | 0.1512 | 1.03 (0.99, 1.06) | −0.034 | 0.6948 | 0.99 (0.97, 1.02) | |
| O-Xylene | 0.108 | 0.5047 | 1.02 (0.97, 1.07) | −0.050 | 0.6905 | 0.99 (0.95, 1.03) | |
| 1,4-Dichlorobenzene | 0.048 | 0.7119 | 1.01 (0.97, 1.05) | −0.176 | 0.5275 | 0.97 (0.89, 1.06) | |
| Styrene | 0.128 | 0.4905 | 1.02 (0.96, 1.09) | 0.127 | 0.2198 | 1.02 (0.99, 1.06) | |
| Toluene | 0.013 | 0.9052 | 1.00 (0.97, 1.04) | −0.011 | 0.9041 | 1.00 (0.97, 1.03) | |
| Hematocrit | Benzene | 0.038 | 0.0194 | 1.16 (1.03, 1.31) | 0.041 | 0.0014 | 1.18 (1.07, 1.3) |
| Ethylbenzene | 0.026 | 0.0604 | 1.11 (1.00, 1.24) | 0.035 | 0.0014 | 1.15 (1.06, 1.25) | |
| M-/p-Xylene | 0.021 | 0.0394 | 1.09 (1.00, 1.17) | 0.042 | <0.0001 | 1.18 (1.1, 1.26) | |
| O-Xylene | 0.015 | 0.2321 | 1.06 (0.96, 1.17) | 0.042 | 0.0002 | 1.18 (1.09, 1.28) | |
| 1,4-Dichlorobenzene | −0.012 | 0.2248 | 0.95 (0.89, 1.03) | −0.017 | 0.1358 | 0.94 (0.86, 1.02) | |
| Styrene | 0.009 | 0.5441 | 1.04 (0.92, 1.16) | 0.034 | 0.0118 | 1.14 (1.03, 1.27) | |
| Toluene | 0.018 | 0.0791 | 1.07 (0.99, 1.16) | 0.033 | 0.0158 | 1.14 (1.03, 1.26) | |
| Hemoglobin | Benzene | 0.077 | 0.0408 | 1.11 (1.00, 1.23) | 0.140 | 0.0002 | 1.21 (1.1, 1.34) |
| Ethylbenzene | 0.042 | 0.2513 | 1.06 (0.96, 1.17) | 0.114 | 0.0004 | 1.17 (1.08, 1.27) | |
| M-/p-Xylene | 0.016 | 0.5014 | 1.02 (0.96, 1.09) | 0.110 | 0.0002 | 1.16 (1.08, 1.26) | |
| O-Xylene | 0.011 | 0.7308 | 1.02 (0.93, 1.11) | 0.114 | 0.0004 | 1.17 (1.08, 1.27) | |
| 1,4-Dichlorobenzene | −0.041 | 0.1078 | 0.94 (0.88, 1.01) | −0.056 | 0.0852 | 0.93 (0.85, 1.01) | |
| Styrene | 0.001 | 0.9585 | 1.00 (0.93, 1.08) | 0.112 | 0.0035 | 1.17 (1.06, 1.29) | |
| Toluene | 0.023 | 0.3622 | 1.03 (0.96, 1.11) | 0.119 | 0.0034 | 1.18 (1.06, 1.31) | |
| Platelet count | Benzene | 0.002 | 0.0001 | 1.16 (1.08, 1.25) | 0.001 | 0.0208 | 1.09 (1.01, 1.16) |
| Ethylbenzene | 0.002 | <0.0001 | 1.15 (1.09, 1.21) | 0.001 | 0.1839 | 1.05 (0.98, 1.14) | |
| M-/p-Xylene | 0.001 | 0.0161 | 1.06 (1.01, 1.12) | 0.001 | 0.1529 | 1.05 (0.98, 1.14) | |
| O-Xylene | 0.001 | 0.0016 | 1.10 (1.04, 1.17) | 0.001 | 0.0890 | 1.07 (0.99, 1.16) | |
| 1,4-Dichlorobenzene | −0.000 | 0.2539 | 0.97 (0.91, 1.02) | 0.000 | 0.9792 | 1.00 (0.9, 1.11) | |
| Styrene | −0.000 | 0.7175 | 0.98 (0.88, 1.09) | 0.001 | 0.2502 | 1.06 (0.96, 1.16) | |
| Toluene | 0.001 | 0.0030 | 1.09 (1.03, 1.15) | 0.001 | 0.2040 | 1.06 (0.97, 1.15) | |
| Red cell count | Benzene | 0.149 | 0.1421 | 1.07 (0.98, 1.18) | 0.041 | 0.6560 | 1.02 (0.94, 1.11) |
| Ethylbenzene | 0.035 | 0.6923 | 1.02 (0.94, 1.1) | 0.027 | 0.7358 | 1.01 (0.94, 1.09) | |
| M-/p-Xylene | 0.019 | 0.7872 | 1.01 (0.94, 1.08) | 0.088 | 0.1474 | 1.04 (0.99, 1.1) | |
| O-Xylene | 0.006 | 0.9387 | 1.00 (0.93, 1.08) | 0.091 | 0.2295 | 1.04 (0.97, 1.12) | |
| 1,4-Dichlorobenzene | −0.078 | 0.2478 | 0.96 (0.91, 1.03) | −0.220 | 0.0228 | 0.90 (0.83, 0.99) | |
| Styrene | 0.004 | 0.9709 | 1.00 (0.9, 1.11) | 0.052 | 0.5858 | 1.02 (0.94, 1.12) | |
| Toluene | 0.032 | 0.6228 | 1.02 (0.96, 1.08) | −0.003 | 0.9742 | 1.00 (0.92, 1.09) | |
| White cell count | Benzene | 0.017 | 0.1829 | 1.04 (0.98, 1.09) | 0.081 | <0.0001 | 1.19 (1.13, 1.25) |
| Ethylbenzene | 0.010 | 0.4582 | 1.02 (0.97, 1.08) | 0.076 | <0.0001 | 1.18 (1.13, 1.23) | |
| M-/p-Xylene | 0.002 | 0.8341 | 1.01 (0.96, 1.06) | 0.062 | <0.0001 | 1.14 (1.09, 1.19) | |
| O-Xylene | 0.012 | 0.3433 | 1.03 (0.97, 1.08) | 0.056 | <0.0001 | 1.12 (1.07, 1.18) | |
| 1,4-Dichlorobenzene | −0.001 | 0.9576 | 1.00 (0.94, 1.06) | −0.009 | 0.6269 | 0.98 (0.91, 1.06) | |
| Styrene | 0.001 | 0.9354 | 1.00 (0.94, 1.07) | 0.082 | <0.0001 | 1.19 (1.17, 1.22) | |
| Toluene | −0.012 | 0.3134 | 0.98 (0.93, 1.02) | 0.079 | <0.0001 | 1.18 (1.14, 1.22) | |
Among the smoke-exposed group, a pattern of statistically significant associations was found between BTEXS VOCs and hematocrit, hemoglobin, and white blood cell count. P values ranged from <0.0001 to 0.016, and hazard ratios ranged from 1.12 to 1.21 for a 1 standard deviation increase in CBC concentration, indicating positive association between BTEXS and CBC concentrations. For each of these blood parameters, the strongest associations (as measured by the hazard ratio) were with benzene. Among the not-smoke-exposed group, there was strong statistical evidence of an association between BTEX (without styrene) and platelet count. P values ranged from <0.0001 to 0.02, and hazard ratios ranged from 1.09 to 1.16 for a 1 standard deviation increase in CBC concentration. As with smoke-exposed, the strongest association (as measured by the hazard ratio) was for benzene. In addition, there was some evidence of an association between benzene and eosinophils, hematocrit, and hemoglobin among not-smoke-exposed. P values ranged from 0.02 to 0.04, and hazard ratios ranged from 1.08 to 1.16 for a 1 standard deviation increase in CBC concentration. Of note, the finding for eosinophils and hematocrit did not meet with p = 0.05 threshold when using the linear regression with substitution method.
Generally the linear regression with substitution results mirrored those of the Cox regression. Though interpretation of regression parameters differ, the identical pattern of key associations between VOC and CBC levels was observed regardless of regression method. As Cox Regression has been suggested for analyzing data with lower detection rates, we feel it’s helpful to demonstrate that either method yields similar if not identical results to ease comparisons between studies.
Discussion
The majority of studies investigating the relationship between VOC exposure and hematological measures are analyses of occupational cohorts, with most focusing on the gasoline refining and manufacturing industries. In addition, most of these studies have small sample sizes and are not reflective of the general population. Due to the lack of studies of VOC exposure in the general population, we analyzed three combined cycles of NHANES data to study potential relationships between blood VOCs and hematological measures. A major source of VOC exposure in the general population is cigarette smoking [24]. To ease comparisons to other studies, we stratified our analysis using serum cotinine levels. We chose to use a cut-point of 14.88 (ng/mL) to classify study participants as tobacco smoke-exposed or not rather than self-reported smoking exposure to minimize survey bias. Certain groups, like Mexican-Americans, may underreport smoking status due to fear of stigma [25]. Using serum cotinine concentrations eliminates this bias. Further, there were 6714 participants with missing self-reported smoking status in the NHANES dataset prior to eligibility exclusions. By using cotinine, we were able to reduce the number of participants without a smoking status to 691. Any cut-point will result in some misclassification of smoking status, regardless of chosen cut-point (Fig. 1). Other studies have used different cut-points based on their data sets [20]. Using our chosen cut-point, we estimate that 127 self-reported smokers are classified as not-smoke-exposed (3.7%), and 113 self-reported non-smokers are classified as smoke-exposed (3.3%) among study participants with non-missing values on each variable.
Average NHANES blood VOC levels were comparable to other exposure studies as well as Canada’s National Biomoriting Program [9, 26]. However, some occupational studies reported much higher values for benzene and styrene [27, 28]. The evidence for associations between VOC exposure and hematological measures was stronger in the smoke-exposed group than in not-smoke-exposed group. This finding is also consistent with other studies [9, 29].
The smoke-exposed group was found to have higher hematocrit and hemoglobin levels with increasing blood concentrations of benzene, ethylbenzene, m-/p-xylene, o-xylene, styrene, and toluene. Doherty et al. had similar findings in smokers [9]. Higher hematocrit and hemoglobin counts in the smoke-exposed group could be evidence of early lung disease as the lungs of smokers cannot efficiently absorb oxygen and the hematopoietic system may produce more red blood cells to compensate [30]. Malenica et al. [30] also observed increased hemoglobin and hematocrit in smokers but did not specifically examine the role of VOCs present in smoke [30]. Our findings could provide evidence that increased VOC exposure in smokers plays a role in the known effect of hematological stimulation by cigarette smoking. However, our analysis did not control for potential confounders found in cigarette smoke like carbon monoxide, which can also affect oxygen absorption through the formation of carboxyhemoglobin [30]. The smoke-exposed group also had significantly increased white blood cell counts with increased exposure to benzene, ethylbenzene, m-/p-xylene, o-xylene, styrene, and toluene. One might expect lowered white blood cell counts with chronic exposure to VOCs due to suppression of the immune system through the well-known negative interaction of benzene with bone marrow [31]. However, smokers likely suffer from chronic inflammatory responses due to the thousands of other toxins present in tobacco smoke, so the impact of other smoke constituents cannot be ruled out.
The not-smoke-exposed group had increased platelet counts with increasing exposure to half of the VOCs included in this analysis. This result is counterintuitive as VOCs, especially benzene, are thought to negatively interact with the hematopoietic system, resulting in a decreased platelet count. However, Krishnan et al. found increased platelet counts in healthy subjects after inhaling diesel exhaust [32]. BTEX are an important component of traffic-related air pollution and the personal vehicle commute is likely one of the few opportunities for environmental VOC exposure for most participants in the hours leading up to the blood draw at the MEC. This increase in platelets was not seen in the smoke-exposed-group (with the exception of benzene). While tobacco smoke exposure is known to increase platelet activation, nicotine has been shown to moderate this effect [33]. Nicotine could have confounded platelet counts for smokers included in this study. Further, increased platelet count can be a sign of anemia, or a number of health conditions like cancer, infectious disease, recent surgery, and other conditions that were not controlled for in this analysis. The majority of the outliers for high platelet count were in the not-smoker-exposed group, so we cannot rule out other potential explanations of this finding in this group.
Benzene blood level was found to have the greatest number of associations with CBCs in this study (Tables 3 and 4). Benzene exposure was associated with increases in eosinophils, hematocrit, hemoglobin, and platelet counts in the not-smoker-group. In the smoke-exposed group, benzene exposure was associated with increases in hematocrit, hemoglobin, platelet count, and WBCs. The statistical associations between benzene and hematocrit and hemoglobin were stronger among the smoke-exposed group compared with the not-smoke-exposed group. The detrimental effects of benzene exposure on the hematopoietic system are well documented in occupational studies [8, 12, 14, 31, 34]. However, there is currently limited evidence in the literature for benzene and other VOCs increasing hematopoietic activity. Most occupational studies have found low-level benzene exposure decreases some hematological measures [12, 14, 27, 34]. Our study population differs from occupational studies in a number of ways. First, our study has a comparable number of males and females, whereas occupational studies tend to have majority male participants. Further, our population is younger, and most are non-occupationally exposed. Some studies have found that low-level benzene-induced hematoxicity is dependent on the number of years exposed [35]. We chose to include adolescents in the analysis as median cotinine levels in this group were higher than the 60+ group. E-cigarette use in this age group has rapidly increased since the time of the NHAHES cycles included here and has been identified as a source of VOC exposure [36]. Reporting on the associations with this age group included could inform future studies. However, because of this inclusion we were unable to add the education variable to the analysis and this is a limitation of the study. Finally, our population is more diverse than nearly all relevant studies as we sought to analyze the general population. Some associations found in this analysis were greater for certain racial groups such as African-Americans. In our analysis, being male was also a risk factor for increased VOC exposure; men had higher geometric means for blood VOC levels for every analyte, as has been reported previously [37]. Future research could more narrowly focus on the hematological impact of VOC exposure by race, gender and occupations other than those typically associated with VOC exposure.
In the current study, we analyzed data from a large, representative sample of the U.S. population. As such, we were able to examine VOC exposures typically experienced in the general population, not just occupational. The inclusion of extensive survey data from NHANES allowed for the control of potential confounders from the population. Participants with conditions or diseases that could affect hematological measures like pregnant women or those taking medication for anemia were excluded from the analysis. Other factors, like self-reported exposure to VOCs through commercial items (e.g., nail polish or paint) were considered as well but did not have a significant impact on blood VOC levels. However, this study still has several limitations. VOCs, especially those included in this data set, have very short half-lives [38]. Because of the short half-life, the VOC levels measured at the time of the exam are not necessarily indicative of lifetime exposure and represent only a snapshot in time. For example, benzene’s half-life has been observed to be as short as 42 min [39]. The limitation of the short half-life of VOCs is present in comparable studies and the cross-sectional design is a well-known limitation of NHANES. Blood VOC levels in the general population have been decreasing over the past 30 years [4]. Very low-level blood VOCs combined with low detection rates for some analytes made finding more meaningful associations challenging. Therefore, other confounding exposures not examined here should not be ruled out. The findings from this study suggests that VOC exposure at the levels currently found in the U.S. are statistically associated with differences in hematological measures, and smoking could be a contributor to this relationship.
Acknowledgements
The authors would like to acknowledge the contributions of CDC Division of Laboratory Science, the Tobacco and Volatiles branch.
Funding
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Technical overview of volatile organic compounds. 2017. https://www.epa.gov/indoor-air-quality-iaq/technical-overview-volatile-organic-compounds.
- 2.Volatile organic compounds’ impact on indoor air quality. 2017. https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality.
- 3.Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health. 2007;80:711–9. [DOI] [PubMed] [Google Scholar]
- 4.Su F-C, Mukherjee B, Batterman S. Trends of VOC exposures among a nationally representative sample: Analysis of the NHANES 1988 through 2004 data sets. Atmos Environ. 2011;45:4858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RB. Detection rates, trends in and factors affecting observed levels of selected volatile organic compounds in blood among US adolescents and adults. Environ Toxicol Pharm. 2017;56:21–28. [DOI] [PubMed] [Google Scholar]
- 6.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11:231–52. [DOI] [PubMed] [Google Scholar]
- 7.García-Esquinas E, Jiménez A, Pastor-Barriuso R, Jones MR, Perez-Gomez B, Navas-Acien A, et al. Impact of declining exposure to secondhand tobacco smoke in public places to decreasing smoking-related cancer mortality in the US population. Environ Int. 2018;117:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeng HA, Lee IL, Gau YY, Yang CT, Lin C, Hong YJ. Changes in immunological and hematological parameters of female residents exposed to volatile organic compounds in the city of Kaohsiung, Taiwan. J Environ Health. 2006;69:20–25. [PubMed] [Google Scholar]
- 9.Doherty BT, Kwok RK, Curry MD, Ekenga C, Chambers D, Sandler DP, et al. Associations between blood BTEXS concentrations and hematologic parameters among adult residents of the U.S. Gulf States. Environ Res. 2017;156:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolden AL, Kwiatkowski CF, Colborn T. New look at BTEX: are ambient levels a problem? Environ Sci Technol. 2015;49:5261–76. [DOI] [PubMed] [Google Scholar]
- 11.Humans; IWGotEoCRt. Chemical agents and related occupations. Vol 100F. Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- 12.Koh DH, Jeon HK, Lee SG, Ryu HW. The relationship between low-level benzene exposure and blood cell counts in Korean workers. Occup Environ Med. 2015;72:421–7. [DOI] [PubMed] [Google Scholar]
- 13.Pelallo-Martinez NA, Batres-Esquivel L, Carrizales-Yanez L, Diaz-Barriga FM. Genotoxic and hematological effects in children exposed to a chemical mixture in a petrochemical area in Mexico. Arch Environ Contam Toxicol. 2014;67:1–8. [DOI] [PubMed] [Google Scholar]
- 14.D’Andrea MA, Reddy GK. Benzene exposure from the BP refinery flaring incident alters hematological and hepatic functions among smoking subjects. Int J Occup Med Environ Health. 2017;30:849–60. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics Survey methods and analytic guidelines. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Health Statistics; 2008. [Google Scholar]
- 16.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital-Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. NHANES 2009–2010 Labratory Methods. 2009. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/LabMethods.aspx?.
- 18.National Center for Health Statistics. National Health and Nutrition Examination Survey; 2009–2010 Data Documentation, Codebook, and Frequencies; Volatile Organic Compounds (VOCs)-Blood (VOCWB_G). National Center for Health Statistics. https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/VOC_F.htm. [Google Scholar]
- 19.Blount BC, Kobelski RJ, McElprang DO, et al. Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2006;832:292–301. [DOI] [PubMed] [Google Scholar]
- 20.Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. 2016;13:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 22.Dinse GE, Jusko TA, Ho LA, Annam K, Graubard BI, Hertz-Picciotto I, et al. Accommodating measurements below a limit of detection: a novel application of Cox regression. Am J Epidemiol. 2014;179:1018–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie BW, Chen Q, Reichert H, Franzblau A, Hedgeman E, Lepkowski J, et al. Estimating population distributions when some data are below a limit of detection by using a reverse Kaplan-Meier estimator. Epidemiology. 2010;21:S64–S70. [DOI] [PubMed] [Google Scholar]
- 24.Chambers DM, Ocariz JM, McGuirk MF, Blount BC. Impact of cigarette smoking on volatile organic compound (VOC) blood levels in the U.S. population: NHANES 2003–2004. Environ Int. 2011;37:1321–8. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Stable EJ, Marin BV, Marin G, Brody DJ, Benowitz NL. Apparent underreporting of cigarette consumption among Mexican American smokers. Am J Public Health. 1990;80:1057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canada H. Third report on human biomonitoring of environmental chemicals in Canada. Ottawa, Ontario: Government of Canada; 2017. [Google Scholar]
- 27.Casale T, Sacco C, Ricci S, Loreti B, Pacchiarotti A, Cupelli V, et al. Workers exposed to low levels of benzene present in urban air: Assessment of peripheral blood count variations. Chemosphere. 2016;152:392–8. [DOI] [PubMed] [Google Scholar]
- 28.Tulinska J, Dusinska M, Jahnova E, Liskova A, Kuricova M, Vodicka P, et al. Changes in cellular immunity among workers occupationally exposed to styrene in a plastics lamination plant. Am J Ind Med. 2000;38:576–83. [DOI] [PubMed] [Google Scholar]
- 29.Akbas E, Derici E, Soylemez F, Kanik A, Polat F. An investigation of effects of toluene and cigarette smoking on some blood parameters and lymphocyte life span. Cell Biol Toxicol. 2004;20:33–40. [DOI] [PubMed] [Google Scholar]
- 30.Malenica M, Prnjavorac B, Bego T, Dujic T, Semiz S, Skrbo S, et al. Effect of cigarette smoking on haematological parameters in healthy population. Med Arch. 2017;71:132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder R. Recent developments in the understanding of benzene toxicity and leukemogenesis. Drug Chem Toxicol. 2000;23:13–25. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, et al. A randomized cross-over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol. 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girdhar G, Xu S, Bluestein D, Jesty J. Reduced-nicotine cigarettes increase platelet activation in smokers in vivo: a dilemma in harm reduction. Nicotine Tob Res. 2008;10:1737–44. [DOI] [PubMed] [Google Scholar]
- 34.Robert Schnatter A, Kerzic PJ, Zhou Y, Chen M, Nicolich MJ, Lavelle K, et al. Peripheral blood effects in benzene-exposed workers. Chem Biol Interact. 2010;184:174–81. [DOI] [PubMed] [Google Scholar]
- 35.Uzma N, Salar BM, Kumar BS, Aziz N, David MA, Reddy VD. Impact of organic solvents and environmental pollutants on the physiological function in petrol filling workers. Int J Environ Res Public Health. 2008;5:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein ML, Delucchi K, Benowitz NL, Ramo DE. Adolescent exposure to toxic volatile organic chemicals from E-cigarettes. Pediatrics. 2018;141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churchill JE, Ashley DL, Kaye WE. Recent chemical exposures and blood volatile organic compound levels in a large population-based sample. Arch Environ Health: Int J. 2001;56:157–66. [DOI] [PubMed] [Google Scholar]
- 38.Lin YS, Egeghy PP, Rappaport SM. Relationships between levels of volatile organic compounds in air and blood from the general population. J Expo Sci Environ Epidemiol. 2008;18:421–9. [DOI] [PubMed] [Google Scholar]
- 39.Agency for Toxic Substances and Disease Registry. Toxicological profile for Benzene. Atlanta, GA: U.S. Department of Health and Human Services PHS; 2007. [PubMed] [Google Scholar]


