Abstract
Although constipation is a common complication of chronic kidney disease (CKD), there is no animal model that can be used to study the association between renal impairment and gastrointestinal function without interfering with the gastrointestinal tract of the model. Therefore, we determined whether adenine could induce CKD in association with gastrointestinal dysfunction. Six-week-old ICR mice were intraperitoneally injected with saline, 25, 50, or 75 mg adenine/kg body weight for 21 days. Blood urea nitrogen (BUN), plasma creatinine, and renal histopathology were evaluated. Defecation status was evaluated from defecation frequency and fecal water content. Colonic smooth muscle contraction was measured by the organ bath technique, and transepithelial electrical resistance (TEER) was measured using an Ussing chamber. In the 50 mg/kg treatment group, BUN and creatinine were significantly increased compared with control, and inflammatory cell infiltration, glomerular necrosis, tubular dilatation, and interstitial fibrosis were observed in renal tissues. Mice in this group also showed a significant decrease in defecation frequency, fecal water content, colonic motility index, and TEER. Overall, 50 mg/kg of adenine was the best dose to induce CKD with associated constipation and intestinal barrier impairment. Therefore, this adenine administration model can be recommended for CKD-associated gastrointestinal dysfunction research.
Keywords: Constipation, Gastrointestinal motility, Intestinal barrier impairment, Renal impairment
1. Introduction
Chronic kidney disease (CKD) has become one of the world’s major health issues. It is characterized by kidney damage or a progressive decline of kidney functions for at least three months (Meijers et al., 2018, Wilson et al., 2021). In 2017, the global prevalence of CKD was 9.1% of the population (695.7 million cases), which represented an increase of 29.3% compared with 1990. Similarly, the incidence of end-stage kidney disease (ESKD) has increased year on year. In 2022, the prevalence of CKD was still increasing, affecting > 10% of the global population. Although the mortality rate has decreased in ESKD patients because of improved treatment and prevention, it has been predicted that CKD will be the fifth most common cause of death worldwide (Kovesdy 2022). However, CKD is a preventable and treatable disease, and with suitable research-based therapeutic strategies, it is possible to slow the progression of the condition (Bikbov et al. 2020).
In the advanced stage of CKD, many associated health conditions are present, including cardiovascular disease (CVD), anemia, and gastrointestinal dysfunction (Liu et al. 2018). In ESKD patients, constipation is one of the most common complications and the prevalence of this symptom in CKD patients is higher than in the general population (Ramos et al., 2019, Sumida et al., 2020). Constipation in CKD is complicated by pathophysiological mechanisms such as dietary restriction (fiber and fluid intake limitation), low physical activity, comorbidity, medication, intestinal dysbiosis, and uremic toxins. Even though constipation is not a life-threatening disease, it does affect the quality of life. Nevertheless, in CKD patients, constipation affects more than that; it is associated with other health problems such as systemic inflammation and CVD, and triggering the progression of CKD to ESKD (Hoibian et al., 2018, Koppe et al., 2015, Sumida et al., 2020).
Prolonged gastrointestinal transit causes a high production and accumulation of uremic toxins and endotoxins. The major endotoxin is lipopolysaccharide, which is known to impair intestinal barrier integrity, resulting in bacterial translocation and increased production of endotoxins, uremic toxins, and pro-inflammatory cytokines in the systemic circulation (Cigarran Guldris, González Parra, and Cases Amenós 2017). These toxins induce local intestinal inflammation and chronic systemic inflammation, which are related to the high levels of pro-inflammatory cytokines and activated component markers associated with CKD (Pan and Kang 2018). Furthermore, uremic toxins such as indoxyl sulfate and p-cresyl sulfate could induce tubulointerstitial fibrosis, glomerular sclerosis, and severe renal function impairment, resulting in the progression of CKD. Therefore, constipation is one of the therapeutic targets in the fight to slow the progression of CKD (Barreto, Fellype C., 2009, Mikusic et al., 2020).
In CKD research, progressive renal function decline is usually induced in one of two ways: 5/6 nephrectomy and intake of adenine-containing food (Diwan, Brown, and Gobe 2018). The surgery is, however, limited by its high mortality rate and inflammation, which can alter the gut microbiota. On the other hand, an adenine-containing diet is an invasive model. This diet could induce CKD when there is an accumulation of adenine and its metabolite, 2,8-dihydroxyadenine, which forms crystals in the proximal tubule and causes tubulointerstitial fibrosis and tubular atrophy (Diwan et al., 2018, Rahman et al., 2018). A recent study involving C57BL/6 mice and Wistar rats successfully induced CKD-associated anemia by 28-day oral gavage with 50 and 200 mg/kg, respectively, of adenine suspended in 0.5% carboxymethylcellulose (CMC) (Rahman et al. 2018). Unfortunately, CMC has been shown to directly alter gut microbiota and induce chronic intestinal inflammation (Naimi et al. 2021). In addition, animals in oral administration models are reluctant to eat powdered food, and food intake may reduce not because of disease but because of the powdered form of the administered adenine. A previous study reported that intraperitoneal (i.p.) injection of adenine at 50 and 100 mg/kg for 4 weeks could induce CKD in Wistar rats (Al Za’abi et al. 2015). In a more recent study, i.p. injection of 300 mg/kg adenine twice a week for 4 weeks induce CKD in Wistar rats similarly to oral administration (Said, Atwa, and Khalifa 2019). Increased levels of blood urea nitrogen (BUN), plasma creatinine, and changes in renal morphology and histopathology confirmed that i.p. injection of adenine is a valid alternative method of inducing CKD in an animal model. The method is especially suited to studies of enteral agents in CKD because adenine directly enters the systemic circulation and does not directly alter intestinal organs (Ali et al. 2013).
As mentioned above, gastrointestinal dysfunction is one of the complications of CKD that trigger the progression of the condition. Several studies have proposed the use of antioxidative agents, laxative agents, or absorbent agents to slow the progression of CKD by improving gastrointestinal function (Chen et al., 2021, Van Hung and Suzuki, 2018, Mafra et al., 2019, Mishima et al., 2015). Thus, the use of an animal model is obviously the important first step in studying the progression of CKD. However, an animal model for CKD associated with gastrointestinal dysfunction is unavailable. Therefore, the purpose of this study was to determine whether the i.p. injection of adenine could induce CKD-associated gastrointestinal dysfunction in a mouse model.
2. Methods
2.1. Animals and experimental design
The six-week-old male ICR mice used in this study were purchased from Nomura Siam International Co., Ltd., Thailand. The animals were housed at the Southern Laboratory Animal Facility, Prince of Songkla University, Thailand, in controlled environments with a 12-hour light/dark cycle, at temperatures ranging from 23 to 27℃ and humidity levels ranging from 50 to 55%. They received standard chow from Perfect Companion Group Co., Ltd., Thailand, and water ad libitum. After 7 days of acclimatization, the mice were divided into four groups, which were control, 25, 50, and 75 mg/kg adenine. Mice were i.p. injected once a day for 21 days with normal saline, 25, 50, or 75 mg adenine/kg body weight (BW). Alertness, BW, and food and water intake were recorded every day during treatment. After 21 days of treatment, the mice were anesthetized by i.p. injection of 70 mg thiopental sodium/kg BW (K-da et al. 2020). Thoracic and abdominal incisions were made to collect blood, heart, liver, spleen, kidneys, and intestines. Finally, mice were euthanatized by cervical distraction. All experimental procedures in this study were guided and approved by the Animal Ethics Committee of Prince of Songkla University, Thailand (Ethical clearance MHESI 6800.11/911).
2.2. Drugs and reagents
Adenine (Sigma-Aldrich, Inc., Saint Louis, MO, USA) was freshly prepared by heating at 60℃ in normal saline. Before injection, the solution was allowed to cool down to room temperature. Thiopental sodium (Jagsonpal Pharmaceutical Ltd., India) was prepared for injection in sterile water. Krebs solution contained 119 mM NaCl, 4.5 mM KCl, 2.5 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, 11.1 mM glucose, and 2.5 mM CaCl2, all purchased from Merck Co., Ltd., Darmstadt, Germany (Khuituan et al. 2019).
2.3. Blood biochemistry
The deep anesthesia of mice was ensured before the thoracic incision was made. Blood was collected by cardiac puncture. Complete blood count (CBC) was measured by BC-2800Vet Auto Hematology Analyzer (Mindray, Shenzhen, China). The whole blood was centrifuged at 4000 rpm for 10 min. Plasma (250–300 µL) was used to measure BUN, creatinine, aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) by BS-20 Chemistry Analyzer (Mindray, Shenzhen, China).
2.4. Intestinal length and the weight of vital organs
After the abdomen was opened, the large intestines were resected and immediately measured from the cecum to the anus. The heart, liver, spleen, and kidney were removed and relative organ weight (%) was calculated by (organ weight/BW) × 100.
2.5. Fecal water content and frequency of defecation
On day 21, before anesthetization, the defecation status of the mice was observed. The frequency of defecation was measured by collecting fecal pellets every 10 min for 4 h. Fecal water content was measured from fecal wet weight and fecal dried weight (the fecal pellet was dried at 100℃ for 30 min), and calculated by fecal water content (%) = (wet weight – dried weight/wet weight) × 100 (Hayeeawaema, Wichienchot, and Khuituan 2020).
2.6. Ex vivo colonic smooth muscle contraction
The mice were deeply anesthetized, and an abdominal incision was made. The colon was removed and immediately placed in a cold Krebs solution. To evaluate smooth muscle contractility, 1 cm of the distal colon was longitudinally suspended in an organ bath containing Krebs solution at 37℃ continuously oxygenated with carbogen. The contraction parameters were amplitude, duration, and frequency of contraction, which were detected by a force transducer (Model FT03, Grass, MA, USA) and recorded by the PowerLab System (AD Instruments, New South Wales, Australia). The signal was analyzed by LabChat7 software (Khuituan et al. 2019). The motility index was calculated as motility index = Ln ((number of peaks × sum of peak amplitudes) + 1) (Hoibian et al. 2018).
2.7. Ex vivo colonic epithelial permeability
Colonic epithelial permeability was investigated using an Ussing chamber (Physiologic Instrument, San Diego, USA). The distal colon was longitudinally divided and tissue was pinned on a 0.3 cm2 disk. The disk was inserted into the Ussing chamber, which contained oxygenated Krebs solution at 37℃. Transepithelial electrical resistance (TEER) was measured when a current of 3 µA was passed. TEER was calculated according to Ohm’s law (Siringoringo et al. 2021).
2.8. Histopathological examination
After euthanasia, the kidney was excised and immediately fixed in 10% formalin for 24 h. The tissue was dehydrated in graded concentrations of ethanol and embedded in paraffin. The tissue was sectioned at 5 µm and stained with hematoxylin and eosin (H&E), Masson’s trichrome, and picrosirius red following the standard protocols. Histopathology was examined and photographed under a light microscope (Olympus DP73). The area of collagen fiber deposition was quantified using the ImageJ software.
2.9. Statistical analysis
The data were shown as means ± the standard error of the mean (SEM). Significant differences were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test and Tukey multiple comparison tests in GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Effect of adenine injection on survival rate and BW
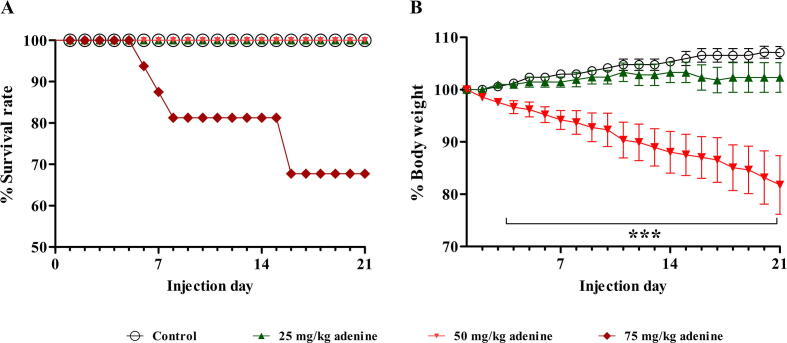
The general health status of all mice was observed daily by considering signs such as alertness and grooming. It was found that mice in the 75 mg/kg adenine group showed signs of weight loss, ceased grooming, and lacked alertness from day 3 of treatment. Furthermore, these mice gradually weakened and died, and from days 5, 7, and 15, their survival rate decreased to <90%, 80%, and 70%, respectively (Fig. 1A). The survival rate of the 25 and 50 mg/kg adenine groups was not affected. This result indicated that an adenine dose of 75 mg/kg was intolerable for ICR mice. The study, therefore, continued by investigating BW change and blood biochemistry of the 25 or 50 mg/kg adenine groups to determine which dose could more effectively induce CKD-associated gastrointestinal dysfunction. It was found that the BW of mice in the 50 mg/kg adenine group continually and significantly decreased from day 3 to day 21 when compared to the control group (p < 0.001) (Fig. 1B). The BW of mice in the 25 mg/kg treatment and the control group did not significantly differ. This result indicated that a dose of 50 mg/kg adenine could affect the normal physiological condition of the mice.
Fig. 1.
Effect of adenine injection on survival rate and body weight (BW). ICR mice were i.p. injected with normal saline (control) or adenine at 25, 50, and 75 mg/kg for 21 days to induce chronic kidney disease (CKD). A: Survival rate (%) of the mice during 21 days of adenine induction. Adenine at 75 mg/kg gradually reduced the survival rate of the mice to<90%, 80%, and 70% from days 5, 7, and 15, respectively. B: BW (%) of the mice during adenine induction. Adenine at 50 mg/kg significantly decreased the percent BW from day 3 to day 21 when compared to the control group. Data are shown as means ± SEM (n = 3–5). ***p < 0.001 when compared to the control group (one-way ANOVA followed by Bonferroni test).
3.2. Effect of adenine injection on BUN and plasma creatinine levels.
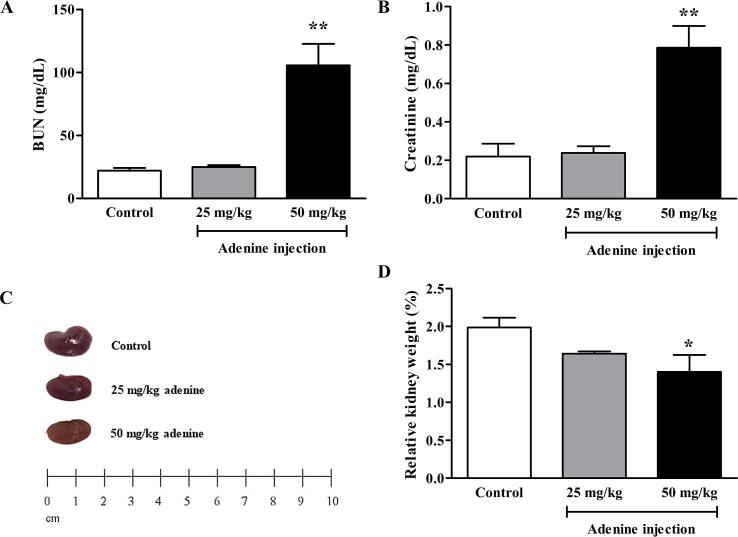
Blood was collected to measure BUN and plasma creatinine levels after 21 days of adenine treatment. BUN in the 50 mg/kg adenine group (102.30 ± 23.85 mg/dL) was significantly higher than in the control group (22.12 ± 2.17 mg/dL) (p < 0.01) but there was no significant difference in BUN between the 25 mg/kg group (24.94 ± 1.47 mg/dL) and the control group (Fig. 2A). Plasma creatinine levels were also significantly higher in the 50 mg/kg treatment (0.73 ± 0.17 mg/dL) than in the control group (0.22 ± 0.06 mg/dL) (p < 0.01), whereas there was no significant difference between the 25 mg/kg adenine group (0.24 ± 0.03 mg/dL) and control (Fig. 2B). These results suggested that adenine injection could induce CKD in ICR mice by increasing BUN and plasma creatinine levels, which are potential indicators of CKD.
Fig. 2.
Effect of adenine injection on BUN and plasma creatinine levels and the relative kidney weight. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). A: BUN level after 21 days of adenine injection. Adenine at 50 mg/kg significantly increased BUN compared to the control group. B: Plasma creatinine level after 21 days of adenine injection. Adenine at 50 mg/kg significantly increased creatinine compared to the control group. C: The representative images are of the kidney of control, 25, and 50 mg/kg adenine groups. D: Relative kidney weight was significantly lower in the 50 mg/kg adenine group when compared to the control group. Data are shown as means ± SEM (n = 3–5). *p < 0.05 and **p < 0.01 when compared to the control group (one-way ANOVA followed by Bonferroni test).
3.3. Effect of adenine injection on kidney gross morphology, weight, and histopathology.
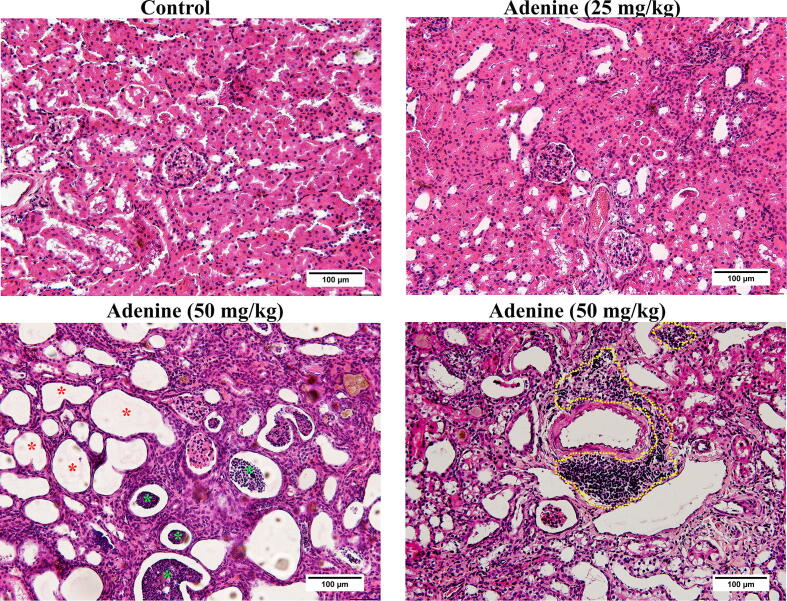
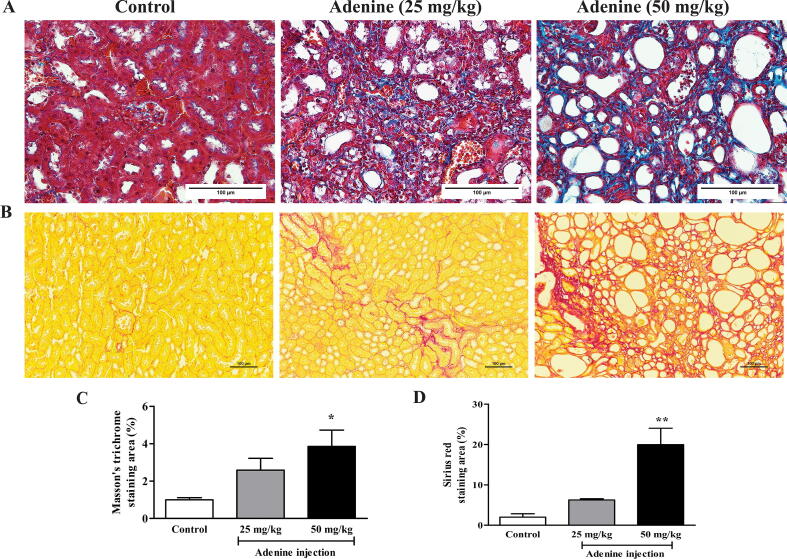
Kidneys in the control group were normal size and reddish-brown. However, the kidneys in the treatment groups were smaller and pale, especially in the 50 mg/kg adenine group (Fig. 2C). Relative kidney weight in the 50 mg/kg adenine group (1.53 ± 0.21%) was significantly lower than in the control group (1.99 ± 0.13%) (p < 0.05), whereas relative kidney weight in the 25 mg/kg adenine group (1.64 ± 0.03%) was lower but not significantly different (Fig. 2D). Under H&E staining, the kidney of mice injected with adenine at 50 mg/kg showed obvious glomerular necrosis (green asterisk), tubular dilatation, and loss of brush border (red asterisk), but the architecture of the kidney tissue of mice in the 25 mg/kg adenine group remained normal, showing intact glomerulus and renal tubules. In addition, the kidney tissue of mice in the 50 mg/kg treatment revealed an inflammatory response characterized by extensive infiltration of inflammatory cells (dash line, Fig. 3). Moreover, both Masson’s trichrome and picrosirius red staining showed extensive deposition of collagen fiber in both treatment groups (Fig. 4A and B). The percent area of collagen fiber deposition in the 50 mg/kg group was significantly higher compared to the control group (p < 0.05 and p < 0.01), which was consistent with the observed fibrosis of renal interstitium (Fig. 4C and D). Overall, the results showed that renal function and renal tissue histology had been altered in the 50 mg/kg adenine group. The results, therefore, indicated that adenine injection could induce CKD in mice.
Fig. 3.
Effect of adenine injection on kidney histopathology. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). The representative images are of kidney tissue of control, 25, and 50 mg/kg adenine groups stained with hematoxylin and eosin (H&E). Red and green asterisks indicate glomerular necrosis and tubular dilatation, respectively. Inflammatory cell infiltration is shown by the yellow dashed line.
Fig. 4.
Effect of adenine injection on renal collagen fiber deposition. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). A and B: Representative images are of the kidney tissue stained with Masson’s trichrome and Sirius red, respectively. C and D: The percent area of collagen fiber deposition revealed by Masson’s trichrome and picrosirius red staining, respectively. Data are shown as means ± SEM (n = 3–5). *p < 0.05 and **p < 0.01 when compared to the control group (one-way ANOVA followed by Turkey’s multiple comparison test).
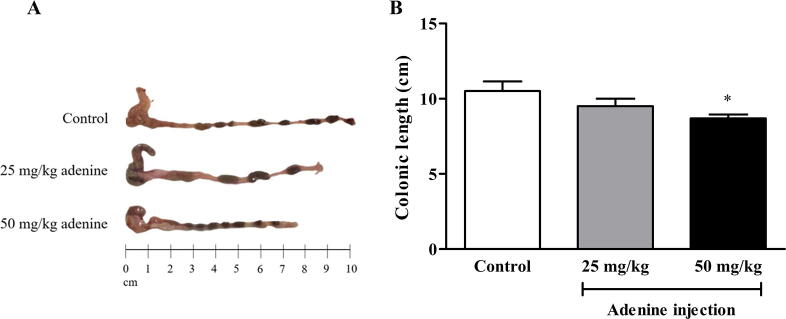
3.4. Colonic length in adenine-induced CKD mice
To determine the effect of CKD induction on gastrointestinal structure, colonic length was measured. The length of the large intestine was affected in both 25 and 50 mg/kg adenine groups (Fig. 5A). A significant shortening of the colon was found in the 50 mg/kg treatment (8.70 ± 0.25 cm) compared to the control group (10.50 ± 0.65 cm) (p < 0.05). In the 25 mg/kg group (9.50 ± 0.50 cm), intestinal length was shorter than in the control group but not significantly different (Fig. 5B). This result indicated that adenine induced gastrointestinal structural abnormality.
Fig. 5.
Effect of adenine injection on colonic length. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). A: Representative images of colon length. B: Large intestinal length was significantly decreased in the 50 mg/kg adenine group when compared to the control group. Data are shown as means ± SEM (n = 3–5). *p < 0.05 when compared to the control group (one-way ANOVA followed by Bonferroni test).
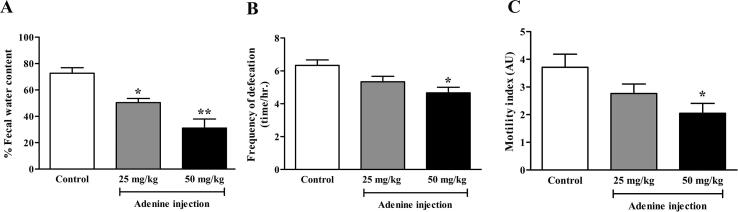
3.5. Constipation in adenine-induced CKD mice
This study aimed to determine whether systemic adenine injection could affect gastrointestinal function. The defecation status of mice was determined after 21 days of treatment and was based on the frequency of defecation and fecal water content. Fecal water content was significantly lower in the 25 and 50 mg/kg adenine groups (50.40 ± 3.12 and 31.17 ± 6.80%, respectively) than in the control group (72.68 ± 4.23%), (p < 0.05 and p < 0.01, respectively) (Fig. 6A). The frequency of defecation was significantly lower in the 50 mg/kg adenine group (4.66 ± 0.33 time/h.) than in the control group (6.33 ± 0.33 time/h.) (p < 0.05) but there was no significant difference between the 25 mg/kg group (5.33 ± 0.33 time/h.) and the control group (Fig. 6B).This result indicated that constipation could be associated with adenine injection.
Fig. 6.
Effect of adenine injection on constipation and colonic motility. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). A: Fecal water content (%) was significantly decreased in both 25 and 50 mg/kg adenine groups when compared to the control group. B: Frequency of defecation was significantly reduced in the 50 mg/kg adenine group when compared to the control group. C: The motility index was significantly lower in the 50 mg/kg adenine group. Data are shown as means ± SEM (n = 3–5). *p < 0.05 and **p < 0.01 when compared to the control group (one-way ANOVA followed by Bonferroni test).
3.6. Colonic motility in adenine-induced CKD mice
The frequency and amplitude of colonic contraction were used to calculate a colonic motility index (as described in the method). Colonic motility was significantly lower in the 50 mg/kg adenine group (2.04 ± 0.35 AU) than in the control group (3.70 ± 0.47 AU), (p < 0.05) but there was no significant difference between the 25 mg/kg group (2.76 ± 0.34 AU) and the control group (Fig. 6C). This result suggested that adenine administration by i.p. injection affected gastrointestinal function by reducing colonic motility which led to the slow propagation of colonic luminal contents; one of the criteria for constipation.
3.7. Colonic barrier integrity in adenine-induced CKD mice
In addition to colonic motility, colonic epithelial barrier function is also thought to be affected by CKD. Colonic epithelial barrier function is one of the factors that contributes to local and systemic inflammations, and renal fibrosis in CKD. Therefore, in this experiment, we measured the TEER of colonic tissue to determine whether adenine injection alters colonic barrier integrity. Colonic TEER was significantly lower in the 50 mg/kg adenine group (69 ± 3.51 O.cm2) (p < 0.05) than in the control group (102 ± 10.15 O.cm2), whereas the TEER of the 25 mg/kg adenine group (75 ± 5.00 O.cm2) was lower than control but not significantly different (Fig. 7). This result indicated that adenine-induced gastrointestinal dysfunction affected not only contractility but also colonic epithelial integrity.
Fig. 7.
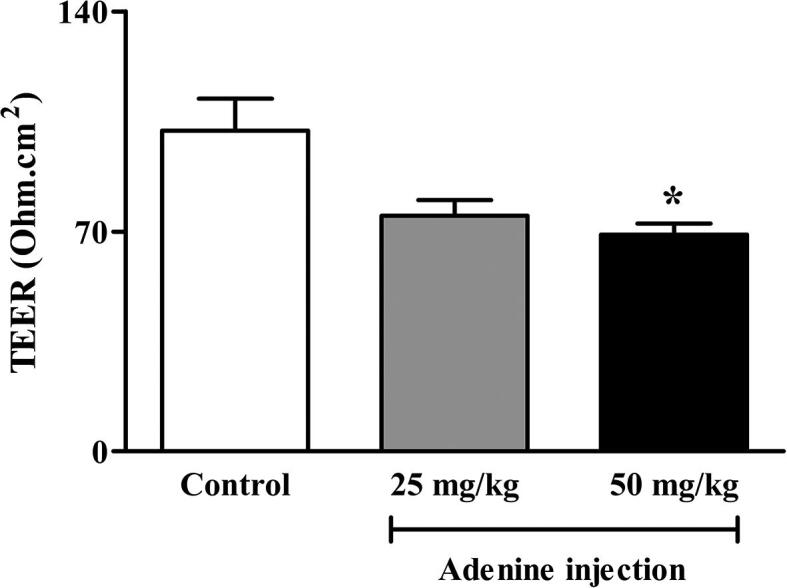
Effect of adenine injection on colonic barrier integrity. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). Colonic barrier integrity was significantly impaired in the 50 mg/kg adenine group when compared to the control group. Data are shown as means ± SEM (n = 3–5). *p < 0.05 when compared to the control group (one-way ANOVA followed by Bonferroni test).
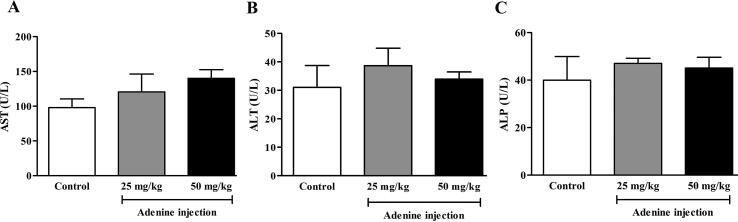
3.8. Liver function in adenine-induced CKD mice
AST, ALT, and ALP are used to identify heart and liver injury. These enzymes are released into the circulation when tissue is damaged. It was found that AST, ALT, and ALP levels in plasma were not affected in either treatment group (Fig. 8A, B, and C). This result indicated that 50 mg/kg of adenine is the lowest dose that could induce CKD without affecting liver function.
Fig. 8.
Effect of adenine injection on liver function test. ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). A, B, and C: Adenine injections at 25 and 50 mg/kg did not affect AST, ALT, and ALP levels, respectively. Data are shown as means ± SEM (n = 3–5) (one-way ANOVA followed by Bonferroni test).
3.9. CBC in adenine-induced CKD mice
A CBC was used to determine whether adenine injection caused the infection, inflammation, or anemia. The results showed no significant differences between groups in counts of red blood cells (RBC), white blood cells (WBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), and mean platelet volume (MPV) (Table 1). These parameters are indicators of cytotoxicity or anemia and the results indicated that injections of 25 and 50 mg of adenine/kg of BW for 21 days did not cause infection, inflammation, or anemia in ICR mice.
Table 1.
Effect of adenine injection on complete blood count (CBC).
| CBC / groups | Control | 25 mg/kg adenine | 50 mg/kg adenine |
|---|---|---|---|
| RBC (×106/µL) | 9.05 ± 1.1.28 | 8.14 ± 0.86 | 8.88 ± 0.0.62 |
| WBC (×103/µL) | 1.82 ± 0.25 | 1.77 ± 0.22 | 2.85 ± 0.50 |
| HGB (g/dL) | 12.15 ± 1.49 | 13.20 ± 0.30 | 13.54 ± 0.90 |
| HCT (%) | 42.95 ± 3.22 | 46.47 ± 1.73 | 46.22 ± 2.83 |
| MCV (fL) | 54.00 ± 1.62 | 52.07 ± 0.60 | 51.93 ± 2.18 |
| MCH (pg) | 15.33 ± 0.15 | 14.72 ± 0.26 | 15.20 ± 0.50 |
| MCHC (g/dL) | 28.52 ± 0.72 | 28.32 ± 0.34 | 29.40 ± 0.37 |
| PLT (×103/µL) | 1328 ± 189.1 | 1509 ± 182.9 | 1782 ± 344.1 |
| MPV (fL) | 5.42 ± 0.15 | 5.42 ± 0.37 | 5.40 ± 0.10 |
ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease (CKD). CBC in adenine-induced CKD mice revealed no significant difference in any parameter between groups. Data are shown as means ± SEM (n = 5).
3.10. Relative vital organ weight in adenine-induced CKD mice
After euthanization, the heart, spleen, and liver, were collected and weighed to further assess the toxicity of adenine. It was found that the heart, spleen, and liver in both treatments were not affected (Table 2).
Table 2.
Effect of adenine injection on relative vital organ weight.
| Control | 25 mg/kg adenine | 50 mg/kg adenine | |
|---|---|---|---|
| Heart (%) | 0.46 ± 0.03 | 0.50 ± 0.01 | 0.46 ± 0.01 |
| Spleen (%) | 0.26 ± 0.01 | 0.28 ± 0.03 | 0.29 ± 0.03 |
| Liver (%) | 5.45 ± 0.17 | 5.00 ± 0.33 | 4.49 ± 0.13 |
ICR mice were i.p. injected with normal saline (control) or adenine at 25 and 50 mg/kg for 21 days to induce chronic kidney disease. Relative vital organ weights were not significantly different between groups. Data are shown as means ± SEM (n = 5).
4. Discussion
Gastrointestinal dysfunction is a common complication of CKD. It was not thought a serious health problem until it was found that the cause of constipation in CKD patients was gut microbiota alteration and that may trigger CKD progression (Sumida, Yamagata, and Kovesdy 2020). To more clearly understand the association between kidney function impairment and gastrointestinal dysfunction, the approach chosen to induce CKD in the present study was one that deliberately avoided surgical procedures and oral administration of adenine. Although partial nephrectomy is known to induce CKD, it may also cause abdominal cavity inflammation which might then affect the gastrointestinal tract. In a more novel approach, adenine was administered orally with a vehicle; CMC. However, CMC was reported to directly affect gut microbiota and aggravate colitis symptoms (Naimi et al. 2021). Therefore, this study investigated whether the administration of adenine by i.p. injection could induce CKD in ICR mice. With this approach, any alteration of gastrointestinal function would not be a direct consequence of the substance used to induce CKD.
Doses of 25, 50, and 75 mg of adenine/kg of BW were chosen with reference to previous reports that described the induction of CKD by oral administration and i.p. injection. In those reports, CKD was induced in mice by oral administration of 50 mg/kg adenine for 28 days, and in rats by i.p. injection of 50 and 100 mg/kg adenine for 28 days (Rahman et al., 2018, Al Za’abi et al., 2015). The results of the present study showed that 75 mg/kg was an intolerable adenine dosage since the survival rate of the mice in this group significantly decreased. After 21 days of treatment with 50 mg/kg adenine, all mice were alive but their BW was significantly reduced. The reduction in BW was consistent with a CKD animal model that received a diet containing adenine and another that underwent 5/6 nephrectomy (Ali et al., 2013, Rahman et al., 2018, Yang et al., 2018). In addition to reductions in BW and food intake, an increase in BUN and plasma creatinine levels occurred from an early stage of adenine administration. In a recent long-term adenine administration study, crystal deposition in the kidney resulted in progressive renal dysfunction and the development of CKD (Santos et al. 2019).
The accumulated BUN and plasma creatinine of the 50 mg/kg adenine group indicated a decline in kidney function. A histopathological study revealed marked renal tubular dilatation, glomerular necrosis, cellular infiltration, and interstitial fibrosis in this group. Urea and creatinine are the nitrogenous end products of dietary and tissue protein metabolism. BUN level indicates the nitrogenous component of the urea in the blood and creatinine is derived from muscle creatine catabolism. Both urea and creatinine, which are distributed in the body's circulation, are normally eliminated by the kidneys, but when kidney function declines by up to 50%, they accumulate in the bloodstream, and levels of BUN and plasma creatinine are elevated (Hosten 1990). This elevation is the earliest sign of the effect of adenine on renal function and the onset of CKD induction. Adenine causes massive fluid volume depletion by downregulation of water channel aquaporin 2 and sodium–potassium chloride cotransporter. The massive fluid depletion causes renal fluid loss, resulting in the first development of early renal failure, and then CKD in long-term adenine administration. At a pharmacological dose, adenine is catalyzed by the xanthine pathway resulting in 2,8-dihydroxyadenine (DHA) which is poorly soluble in water. If urine pH is normal, DHA forms crystal deposits in the renal tubule, leading to renal cellular infiltration and renal fibrosis, and subsequently, progressive renal failure accompanied by renal fibrosis (Santos et al., 2019, Tamura et al., 2009). Furthermore, the uremic toxins indoxyl sulfate and p-cresyl sulfate can induce tubulointerstitial fibrosis, glomerular sclerosis, and severe renal function impairment, which advance the development of CKD (Barreto, Fellype C., 2009, Mikusic et al., 2020).
Since the kidney’s normal function is to maintain fluid, electrolyte, and waste product homeostasis, when kidney function is reduced, urea accumulates in the circulation and enters the gastrointestinal tract, causing uremic dysbiosis or alteration of gut microbiota. These changes can lead to gastrointestinal tract dysfunction, including constipation and intestinal epithelial barrier damage (Castillo-Rodriguez, Fernandez-Prado, and Esteras 2019). To investigate gastrointestinal tract symptoms associated with adenine injection, the present study determined defecation status, intestinal motility, and intestinal permeability. We also measured intestinal length, which provides the first sign of colonic structural abnormality and inflammation (Chen et al., 2017, K-da et al., 2020). The colon of mice in the 50 mg/kg adenine group was shorter than the colon in the control group, which indicated an association between CKD and the gastrointestinal tract. In this CKD model, the associated gastrointestinal dysfunction may be quite significant.
Mice in both treatment groups showed a reduction in the defecation frequency and fecal water content. The possible cause of these changes could be increased populations of urease bacteria in the modified gut microbiota. Urease bacteria can hydrolyze urea to ammonium and ammonium hydroxide. This alteration to the gut microbiota increases luminal pH and inhibits intestinal smooth muscle function (Vaziri, 2012, Wong et al., 2014). Smooth muscle dysfunction can reduce intestinal motility which slows gastrointestinal transit and prolongs luminal content. In turn, water absorption in the gut increases, fecal water content is reduced and stool becomes harder (Hayeeawaema et al., 2020, Mikusic et al., 2020). These results are consistent with reports of increasing complaints of constipation associated with a progressive decline in kidney function among ESKD patients (Lu et al., 2019, Zha and Qian, 2017). Constipation is a vicious cycle that alters gut microbiota. This cycle affects active substances in the lumen and influences bowel motor, secretory, and integrity functions (Khalif et al. 2005).
The significant reduction in TEER found in the 50 mg/kg adenine group indicates an impairment of intestinal epithelial integrity. When urea enters the gastrointestinal lumen, it alters the balance of gut microbiota. In CKD patients, obligate anaerobic bacteria such as Lactobacillus and Bifidobacterium genera were seen to decrease, but pathogenic bacteria such as the Enterobacteriaceae family, which generate toxins such as amines, indoles, and p-cresol, increased (Zha and Qian 2017). Pathogenic bacteria can also generate bacterial urease, which can elevate ammonia and ammonium hydroxide levels. These toxins would disrupt the intestinal epithelial tight junctions and eventually interrupt the intestinal barrier (Ramos et al., 2019, Sumida et al., 2019). Gastrointestinal dysbiosis, constipation, and impaired intestinal integrity may well affect the onset and progression of CKD over the neurogenic, endocrine, and inflammatory pathways (Al Khodor and Shatat, 2017, Mikusic et al., 2020).
Our results showed no alteration in the activities of AST, ALT, and ALP, which are important biomarkers of liver injury. Vital organs and the CBC parameters were unaffected, which suggested that, except for the kidney and gastrointestinal tract, this adenine injection model did not cause tissue injury, infection, or anemia in the ICR mice. Therefore, this novel adenine injection model might be a good alternative approach to the study of CKD-associated gastrointestinal dysfunction and the development of therapeutic strategies to slow the progression of CKD by improving the gastrointestinal tract function or rebalancing gut microbiota (Barreto, Fellype C., 2009, Mikusic et al., 2020). The great advantage of this model is that adenine administered by injection does not directly alter the agents of interest or supplementary products given to the mice (Ali et al. 2013).
5. Conclusion
Adenine intraperitoneally injected at 50 mg/kg was the lowest dose of adenine to induce CKD. This route of administration could induce CKD with the associated gastrointestinal dysfunctions of constipation and intestinal barrier impairment. Thus, this method of inducing CKD could be useful in studying CKD-associated gastrointestinal problems but could also be useful to those developing new treatments aimed at slowing down the progression of the condition and alleviating associated complications by improving gastrointestinal functions.
Funding
This research was supported by the National Research Council of Thailand (NRCT): NRCT5-RGJ63-160, and National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No VET6405007S).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors are grateful to Mr. Thomas Coyne, Faculty of Science, Prince of Songkla University for providing assistance in English proofreading and providing feedback on the manuscript.
Author’s contribution
Research project conception and experimental design: F. Hayeeawaema, P. Khuituan.
Investigation and methodology: F. Hayeeawaema, P. Muangnil, J. Jiangsakul, C. Tipbunjong.
Data analysis and interpretation: F. Hayeeawaema, P. Muangnil, J. Jiangsakul, C. Tipbunjong, N. Huipao, P. Khuituan.
Drafting and revising the article: F. Hayeeawaema, P. Muangnil, C. Tipbunjong, N. Huipao, P. Khuituan.
Providing intellectual content of critical importance to the work described: F. Hayeeawaema, P. Muangnil, C. Tipbunjong, N. Huipao, P. Khuituan.
Final approval of the version to be published: F. Hayeeawaema, P. Muangnil, J. Jiangsakul, C. Tipbunjong, N. Huipao, P. Khuituan
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Fittree Hayeeawaema, Email: 6110230031@email.psu.ac.th.
Paradorn Muangnil, Email: paradorn.m@psu.ac.th.
Julaluk Jiangsakul, Email: julalak.j@psu.ac.th.
Chittipong Tipbunjong, Email: chittipong.t@psu.ac.th.
Nawiya Huipao, Email: nawiya.h@psu.ac.th.
Pissared Khuituan, Email: pissared.k@psu.ac.th.
References
- Al Khodor, Souhaila, and Ibrahim F. Shatat. 2017. “Gut Microbiome and Kidney Disease: A Bidirectional Relationship.” Pediatric Nephrology 32 (6), 921–31. Doi: 10.1007/s00467-016-3392-7. [DOI] [PMC free article] [PubMed]
- Al Za’abi, Mohammed, Badreldin Ali, Javed Yasin, Nicole Schupp, and Abderrahim Nemmar. 2015. “Development of a New Model for the Induction of Chronic Kidney Disease via Intraperitoneal Adenine Administration, and the Effect of Treatment with Gum Acacia Thereon.” The FASEB Journal 29 (S1). Doi: 10.1096/fasebj.29.1_supplement.938.3. [PMC free article] [PubMed]
- Ali, Badreldin H., Suhail Al-Salam, Mohammed Al Za’abi, Mostafa I. Waly, Aishwarya Ramkumar, Sumyia Beegam, Intisar Al-Lawati, Sirin A. Adham, and Abderrahim Nemmar. 2013. “New Model for Adenine-Induced Chronic Renal Failure in Mice, and the Effect of Gum Acacia Treatment Thereon: Comparison with Rats.” Journal of Pharmacological and Toxicological Methods 68 (3), 384–93. Doi: 10.1016/j.vascn.2013.05.001. [DOI] [PubMed]
- Barreto, Fellype C., Daniela V. Barreto, Sophie Liabeuf, Natalie Meert, Griet Glorieux, Mohammed Temmar, Gabriel Choukroun, Raymond Vanholder, Ziad A. Massy, and European Uremic Toxin Work Group (EUTox). 2009. “Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients.” Clinical Journal of the American Society of Nephrology: CJASN 4 (10), 1551–58. Doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed]
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. The Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Rodriguez, Esmeralda, Raul Fernandez-Prado, and Raquel Esteras. 2019. “Diseases | Free Full-Text | Impact of Gut Dysbiosis on Neurohormonal Pathways in Chronic Kidney Disease.” December 4, 2019. https://www.mdpi.com/2079-9721/7/1/21. [DOI] [PMC free article] [PubMed]
- Chen Y.-C., Cheng C.-Y., Liu C.-T., Sue Y.-M., Chen T.-H., Hsu Y.-H., Huang N.-J., Chen C.-H. Combined Protective Effects of Oligo-Fucoidan, Fucoxanthin, and L-Carnitine on the Kidneys of Chronic Kidney Disease Mice. European Journal of Pharmacology. 2021;892(February) doi: 10.1016/j.ejphar.2020.173708. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhao X., Wang H., Yang Z., Li J., Suo H. Prevent Effects of Lactobacillus fermentum HY01 on Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients. 2017;9(May):545. doi: 10.3390/nu9060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan V., Brown L., Gobe G.C. Adenine-Induced Chronic Kidney Disease in Rats. Nephrology. 2018;23(1):5–11. doi: 10.1111/nep.13180. [DOI] [PubMed] [Google Scholar]
- Cigarran Guldris, Secundino, Emilio González Parra, and Aleix Cases Amenós. 2017. “Gut Microbiota in Chronic Kidney Disease.” Nefrologia: Publicacion Oficial De La Sociedad Espanola Nefrologia 37 (1), 9–19. Doi: 10.1016/j.nefro.2016.05.008. [DOI] [PubMed]
- Hayeeawaema, Fittree, Santad Wichienchot, and Pissared Khuituan. 2020. “Amelioration of Gut Dysbiosis and Gastrointestinal Motility by Konjac Oligo-Glucomannan on Loperamide-Induced Constipation in Mice.” Nutrition (Burbank, Los Angeles County, Calif.) 73 (May), 110715. Doi: 10.1016/j.nut.2019.110715. [DOI] [PubMed]
- Hoibian E., Florens N., Koppe L., Vidal H., Soulage C.O. Distal Colon Motor Dysfunction in Mice with Chronic Kidney Disease: Putative Role of Uremic Toxins. Toxins. 2018;10(5) doi: 10.3390/toxins10050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosten, Adrian O. 1990. “BUN and Creatinine.” In Clinical Methods: The History, Physical, and Laboratory Examinations, edited by H. Kenneth Walker, W. Dallas Hall, and J. Willis Hurst, 3rd ed. Boston: Butterworths. http://www.ncbi.nlm.nih.gov/books/NBK305/. [PubMed]
- K-da S., Peerakietkhajorn S., Siringoringo B., Muangnil P., Wichienchot S., Khuituan P. Oligosaccharides from Gracilaria Fisheri Ameliorate Gastrointestinal Dysmotility and Gut Dysbiosis in Colitis Mice. Journal of Functional Foods. 2020;71(August) doi: 10.1016/j.jff.2020.104021. [DOI] [Google Scholar]
- Khalif I.L., Quigley E.M.M., Konovitch E.A., Maximova I.D. Alterations in the Colonic Flora and Intestinal Permeability and Evidence of Immune Activation in Chronic Constipation. Digestive and Liver Disease. 2005;37(11):838–849. doi: 10.1016/j.dld.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Khuituan P., K-da S., Bannob K., Hayeeawaema F., Peerakietkhajorn S., Tipbunjong C., Wichienchot S., Charoenphandhu N. Prebiotic Oligosaccharides from Dragon Fruits Alter Gut Motility in Mice. Biomedicine & Pharmacotherapy. 2019;114(June) doi: 10.1016/j.biopha.2019.108821. [DOI] [PubMed] [Google Scholar]
- Koppe L., Mafra D., Fouque D. Probiotics and Chronic Kidney Disease. Kidney International. 2015;88(5):958–966. doi: 10.1038/ki.2015.255. [DOI] [PubMed] [Google Scholar]
- Kovesdy C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney International Supplements. 2022;12(1):7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li J., Jingao Y.u., Wang Y., Jingbo L.u., Shang E.-X., Zhu Z., Guo J., Duan J. Disorder of Gut Amino Acids Metabolism during CKD Progression Is Related with Gut Microbiota Dysbiosis and Metagenome Change. Journal of Pharmaceutical and Biomedical Analysis. 2018;149(February):425–435. doi: 10.1016/j.jpba.2017.11.040. [DOI] [PubMed] [Google Scholar]
- Lu C.-Y., Chen Y.-C., Yu-Wen L.u., Muo C.-H., Chang R.-E. Association of Constipation with Risk of End-Stage Renal Disease in Patients with Chronic Kidney Disease. BMC Nephrology. 2019;20(1):304. doi: 10.1186/s12882-019-1481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra D., Borges N., Alvarenga L., Esgalhado M., Cardozo L., Lindholm B., Stenvinkel P. Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease. Nutrients. 2019;11:(3). doi: 10.3390/nu11030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijers B., Farré R., Dejongh S., Vicario M., Evenepoel P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins. 2018;10(7) doi: 10.3390/toxins10070298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikusic R., Lucía N., Kouyoumdzian N.M., Choi M.R. Gut Microbiota and Chronic Kidney Disease: Evidences and Mechanisms That Mediate a New Communication in the Gastrointestinal-Renal Axis. Pflügers Archiv - European Journal of Physiology. 2020;472(3):303–320. doi: 10.1007/s00424-020-02352-x. [DOI] [PubMed] [Google Scholar]
- Mishima E., Fukuda S., Shima H., Hirayama A., Akiyama Y., Takeuchi Y., Fukuda N.N., et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. Journal of the American Society of Nephrology. 2015;26(8):1787–1794. doi: 10.1681/ASN.2014060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi S., Viennois E., Gewirtz A.T., Chassaing B. Direct Impact of Commonly Used Dietary Emulsifiers on Human Gut Microbiota. Microbiome. 2021;9(1):66. doi: 10.1186/s40168-020-00996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Kang Y. Gut Microbiota and Chronic Kidney Disease: Implications for Novel Mechanistic Insights and Therapeutic Strategies. International Urology and Nephrology. 2018;50(2):289–299. doi: 10.1007/s11255-017-1689-5. [DOI] [PubMed] [Google Scholar]
- Rahman A., Yamazaki D., Sufiun A., Kitada K., Hitomi H., Nakano D., Nishiyama A. A Novel Approach to Adenine-Induced Chronic Kidney Disease Associated Anemia in Rodents. PLOS ONE. 2018;13(2):e0192531. doi: 10.1371/journal.pone.0192531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, Christiane Ishikawa, Rachel Gatti Armani, Maria Eugenia Canziani, Carla Juliana Ribeiro Dolenga, Lia Sumie Nakao, Katrina Louise Campbell, and Lilian Cuppari. 2019. “Bowel Habits and the Association With Uremic Toxins in Non-Dialysis-Dependent Chronic Kidney Disease Patients.” Journal of Renal Nutrition: The Official Journal of the Council on Renal Nutrition of the National Kidney Foundation, April. Doi: 10.1053/j.jrn.2019.02.004. [DOI] [PubMed]
- Said A.M., Atwa S.A.E., Khalifa O.A. Ameliorating Effect of Gum Arabic and Lemongrass on Chronic Kidney Disease Induced Experimentally in Rats. Bulletin of the National Research Centre. 2019;43(1):47. doi: 10.1186/s42269-019-0086-x. [DOI] [Google Scholar]
- Santos D., Ingrid F., Sheriff S., Amlal S., Ahmed R.P.H., Thakar C.V., Amlal H. Adenine Acts in the Kidney as a Signaling Factor and Causes Salt- and Water-Losing Nephropathy: Early Mechanism of Adenine-Induced Renal Injury. American Journal of Physiology-Renal Physiology. 2019;316(4):F743–F757. doi: 10.1152/ajprenal.00142.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siringoringo B., Huipao N., Tipbunjong C., Nopparat J., Wichienchot S., Hutapea A.M., Khuituan P. Gracilaria Fisheri Oligosaccharides Ameliorate Inflammation and Colonic Epithelial Barrier Dysfunction in Mice with Acetic Acid-Induced Colitis. Asian Pacific Journal of Tropical Biomedicine. 2021;11(10):440. doi: 10.4103/2221-1691.326098. [DOI] [Google Scholar]
- Sumida K., Molnar M.Z., Potukuchi P.K., Thomas F., Jun Ling L.u., Yamagata K., Kalantar-Zadeh K., Kovesdy C.P. Constipation and Risk of Death and Cardiovascular Events. Atherosclerosis. 2019;281(February):114–120. doi: 10.1016/j.atherosclerosis.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida K., Yamagata K., Kovesdy C.P. Constipation in CKD. Kidney International Reports. 2020;5(2):121–134. doi: 10.1016/j.ekir.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Aizawa R., Hori M., Ozaki H. Progressive Renal Dysfunction and Macrophage Infiltration in Interstitial Fibrosis in an Adenine-Induced Tubulointerstitial Nephritis Mouse Model. Histochemistry and Cell Biology. 2009;131:483–490. doi: 10.1007/s00418-009-0557-5. [DOI] [PubMed] [Google Scholar]
- Van Hung T., Suzuki T. Dietary Fermentable Fibers Attenuate Chronic Kidney Disease in Mice by Protecting the Intestinal Barrier. The Journal of Nutrition. 2018;148(4):552–561. doi: 10.1093/jn/nxy008. [DOI] [PubMed] [Google Scholar]
- Vaziri N.D. CKD Impairs Barrier Function and Alters Microbial Flora of the Intestine: A Major Link to Inflammation and Uremic Toxicity. Current Opinion in Nephrology and Hypertension. 2012;21(6):587–592. doi: 10.1097/MNH.0b013e328358c8d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S., Mone P., Jankauskas S.S., Gambardella J., Santulli G. Chronic Kidney Disease: Definition, Updated Epidemiology, Staging, and Mechanisms of Increased Cardiovascular Risk. The Journal of Clinical Hypertension. 2021;23(4):831–884. doi: 10.1111/jch.14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Piceno Y.M., DeSantis T.Z., Pahl M., Andersen G.L., Vaziri N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. American Journal of Nephrology. 2014;39(3):230–327. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Li Q., Henning S.M., Zhong J., Hsu M., Lee R., Long J., et al. Effects of Prebiotic Fiber Xylooligosaccharide in Adenine-Induced Nephropathy in Mice. Molecular Nutrition & Food Research. 2018;62(15):1800014. doi: 10.1002/mnfr.201800014. [DOI] [PubMed] [Google Scholar]
- Zha Y., Qian Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients. 2017 doi: 10.3390/nu9030208. [DOI] [PMC free article] [PubMed] [Google Scholar]