Abstract
Abstract
Nosocomial infections or healthcare-associated infections (HAIs) are acquired under medical care in healthcare facilities. In hospital environments, the transmission of infectious diseases through textiles such as white coats, bed linen, curtains, and towels are well documented. Textile hygiene and infection control measures have become more important in recent years due to the growing concerns about textiles as fomites in healthcare settings. However, systematic research in this area is lacking; the factors contributing to the transmission of infections through textiles needs to be better understood. The review aims to critically explore textiles as contaminants in healthcare systems, and to identify potential risks they may pose to patients and healthcare workers. It delineates different factors affecting bacterial adherence on fabrics, such as surface properties of bacteria and fabrics, and environmental factors. It also identifies areas that require further research to reduce the risk of HAIs and improve textile hygiene practices. Finally, the review elaborates on the strategies currently employed, and those that can be employed to limit the spread of nosocomial infections through fabrics. Implementing textile hygiene practices effectively in healthcare facilities requires a thorough analysis of factors affecting fabric-microbiome interactions, followed by designing newer fabrics that discourage pathogen load.
Key points
• Healthcare textiles act as a potential reservoir of nosocomial pathogens
• Survival of pathogens is affected by surface properties of fabric and bacteria
• Guidelines required for fabrics that discourage microbial load, for hospital use
Keywords: Nosocomial infections, Hospital textiles, Persistence, Biofilm, Microbial load
Introduction and scope of review
Nosocomial infections or healthcare-associated infections (HAIs), acquired when receiving healthcare treatment, are a major concern in hospitals. These infections are responsible for increased morbidity and mortality and treatment expenses due to extended stays in hospitals (Haque et al. 2018; Monegro et al. 2020; Sikora and Zahra 2021). In 2019, the World Health Organization recognized six pathogens as significant in nosocomial infections: Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Staphylococcus aureus (Murray et al. 2022). Many inanimate surfaces and medical equipments, including ultrasound machines and bedrails, have been reported to harbor drug-resistant pathogens in healthcare facilities (Kiros et al. 2021; Jablonska-Trypuc et al. 2022).
Natural fabrics are considered excellent substrates for microbial adherence because their organic constituents provide a strong base for bacterial attachment and biofilm development (Fijan et al. 2017; Achinas et al. 2019). Moreover, fabrics retain bodily fluids like human sweat, blood, sebum, and wound fluid, which provide essential nutrients for microbial growth (Mollebjerg et al. 2021). Hence, soft surfaces like fabrics act as fomites contributing to the spread of infections (Mitchell et al. 2015; Owen and Laird 2020; Jose et al. 2023). Healthcare apparel like nurses’ white coats, doctor’s coats, and surgical gowns, and other textiles such as scrubs, bedsheets, pillow covers, curtains, and towels have been reported to play an imperative role in the transfer of pathogens (Ambrosch et al. 2019; Goyal et al. 2019; Mishra et al. 2020). The contribution of fabrics in the transmission of antibiotic resistant strains including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and P. aeruginosa has also been realized (Lopez et al. 2013; Pace-Asciak et al. 2018). Several studies have reported that most transmission of infection occurs through surface contact between patients and healthcare staff or vice versa as well as through the environment (Lopez et al. 2013; Dyer et al. 2019) (Fig. 1). Transmission of bacterial infection can also occur during the collection and storage of infected hospital textiles (Abney et al. 2021; Sharma et al. 2022). Hence, controlling microbial growth on textile surfaces, and their subsequent transmission in healthcare settings, is crucial. Breaking the chain of infection can be achieved by inactivating or removing microorganisms from textiles by laundering using agents like temperature, detergents, or mechanical action (Bockmuhl et al. 2019).
Fig. 1.
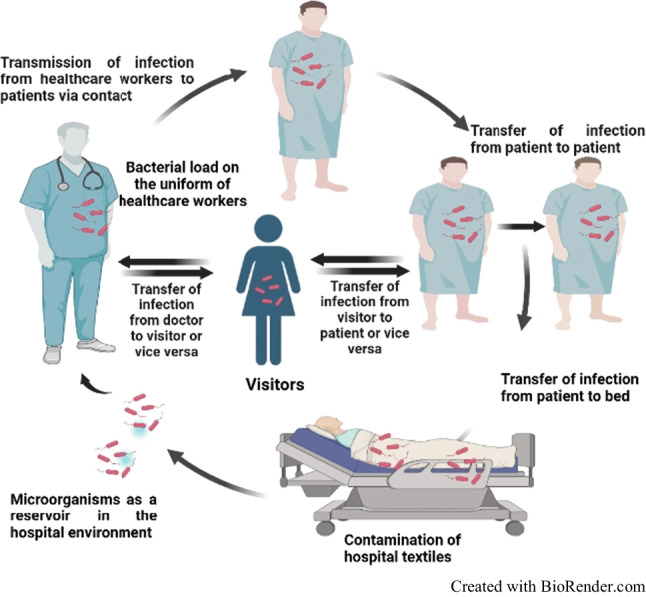
Transmission of nosocomial infections through contaminated hospital textiles
Extensive research has been performed on developing antimicrobial textiles and different biocidal nanoparticles that limit the growth and load of microorganisms (Goyal et al. 2019). While this has provided a partial solution to mitigate nosocomial infections, its applicability is not universal due to the toxic impact of antimicrobials on human health and the environment (Condo et al. 2015). A possible eco-friendly approach might be to develop fabrics that discourage microbial adherence and subsequent transmission. For this, gaining an in-depth understanding of various factors affecting fabric microbe interactions is important.
Factors contributing to the adhesion of pathogens on soft-surfaced fabrics and their transmission leading to nosocomial infections are yet to be comprehensively assessed. Several properties of fabrics and bacteria have been found to affect bacterial adhesion (Hemmatian et al. 2021; Sanders et al. 2021; Varshney et al. 2021). Understanding of the role of various abiotic and biotic factors contributing to microbial adhesion on fabrics and transmission can help develop guidelines related to types of fabrics that should be used in healthcare settings, and the laundering protocol to be followed to minimize the risks of nosocomial infections through fabrics. In many nations, including India, limited data is available on the endemic epidemiology of nosocomial infections, due to the absence of comprehensive surveillance systems, under reporting of infections, differences in reporting criteria, limited resources for data collection and analysis, and limited research (World Health Organization 2020).
Limited efforts have been made toward compiling reports related to fabric-microbe interactions and the role of fabrics as fomites in healthcare setting. While Facciola et al. (2019) brought together papers pertaining to various surfaces (mostly hard) in healthcare associated infections, Goyal et al. (2019) restricted their review to the contamination of white coats and surgical scrubs. Stephens et al. (2019) summarized the available literature on the contamination of fomites in general in the built environment, and the implications of such contamination and transmission of pathogens to human, on human health. To the best of our knowledge, the first review to comprehensively compile papers related to the contribution of textiles in transferring infections was by Owen and Laird (2020). They also outlined current laundering procedures for hospital textiles. Since then, there has been substantial development in our understanding of the interactions between fabrics and microbes in healthcare settings. An in-depth understanding of fabric-microbe interactions will help design newer fabrics that limit the pathogen load. This will also help in drafting guidelines for preferred fabrics in healthcare settings.
While the significance of fabrics in spread of infections is of utmost importance in several settings including hospitality sector, and other public places (Pillai et al. 2021), the present review has been restricted to highlight the role of fabrics in the transmission of nosocomial infections in healthcare settings. It includes an updated, extensive compilation of epidemiological research showing a direct correlation between nosocomial infections and contaminated healthcare fabrics. The review brings together the current understanding of the role of hospital fabrics in the transmission of infections, factors affecting bacterial adherence on fabrics, persistence of pathogens on fabrics, and approaches for minimizing the microbial load on fabrics in healthcare settings. The review will serve as an excellent platform for deciding the next lines of research related to the strategies to limit nosocomial infections.
Role of hospital fabrics in the transmission of infections
In healthcare settings, fabrics are integral to the patients’ and healthcare workers’ immediate environment. Uniforms of healthcare workers are made either from reusable or disposable fabrics. Reusable fabrics are woven in nature that can be laundered and sterilized to kill microorganisms; disposable fabrics, which include polypropylene, polyester, polyethylene, are non-woven and can be used for a single day/use only. It has been established that hospital fabrics serve as a reservoir for pathogens and a potential source of contamination in the healthcare system (Table 1). In fact, fabrics (cotton and polyester) have been reported to serve as better fomites for the growth of Candida albicans compared to hard surfaces like glass and metal (Traore et al. 2002). However, using two model bacteria, the transfer efficiency from porous surfaces like cotton and polyester was observed to be lower when compared to non-porous surfaces, thus indicating that microbes get embedded in the porous matrix of textile, reducing their ability to act as a fomite (Lopez et al. 2013).
Table 1.
Microorganisms reported on different apparel used in healthcare settings
| Healthcare textiles | Microbes detected | References |
|---|---|---|
| Bedsheet | Acinetobacter spp., Bacillus subtilis, Enterococcus faecalis, Streptococcus spp., Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Proteus spp., Citrobacter freundii, Klebsiella spp., Serratia spp. | Okareh 2018; Hassan et al. 2019; Varshney et al. 2022 |
| Gloves | Acinetobacter baumannii | Morgan et al. 2012 |
| Healthcare staff gowns | Bacillus spp., coliforms, Streptococcus spp., Acinetobacter baumannii, Staphylococcus aureus, Serratia rubidae, Klebsiella pneumoniae, Stenotrophomonas maltophilia | Pilonetto et al. 2004; Morgan et al. 2012; Bache et al. 2013 |
| Hospital privacy curtains | Acinetobacter spp., MRSA, VRE | Das et al. 2002 |
| Medical shoe cover | Acinetobacter baumannii, Escherichia coli, Enterococcus faecium, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis | Galvin et al. 2016 |
| Towel | MRSA, Klebsiella pneumoniae | Hassan et al. 2019 |
| White coats | Clostridium difficile, MRSA, Acinetobacter spp., Escherichia coli, Staphylococcus spp., Pseudomonas aeruginosa, Klebsiella spp., VRE, Salmonella spp., Streptococcus spp. | Gupta et al. 2017 |
Transmission of pathogens from contaminated healthcare textiles to skin as well as other surfaces has been demonstrated (Lopez et al. 2013; Lena et al. 2021). These include antibiotic resistant strains like MRSA, VRE that have been reported to be transferred from white coats, bedsheets, and towels to porcine skin (Chaoui et al. 2019). The white coat of students is considered a potential source of infection transmission in clinical settings. Different regions of the white coat, such as cuffs, sleeves, and pockets, become highly contaminated when administering care, primarily during surgeries and wound care (Loh et al. 2000; Berktold et al. 2018). Students wearing white coats throughout the day had significantly higher levels of microbes on their cuffs and side pockets than the back of the white coats; however, no variation in microbial contamination between the regions of cuffs and pockets was observed (Loh et al. 2000). As hands and cuffs regularly come in contact with patients, bacteria can be easily transmitted between the two during patient examinations. Nurses’ uniforms also transfer pathogenic microorganisms in healthcare settings when directly contacting patients, their belongings, and linen (Owen and Laird 2020). Patients, coworkers, and the public come into contact directly or indirectly with their uniforms during shifts, and serve as a vector for spreading virulent pathogens (Kanwar et al. 2019).
Another transmission route is air-borne, which is due to the generation of microbial aerosol during the movement of contaminated textiles between rooms (Ather et al. 2020). During bed preparation for patients, the dispersion of bacteria occurs through hands and clothing, which contributes to the transmission of bacteria to the surroundings (Kanwar et al. 2019; Popovich et al. 2021). The friction between the surface of the skin and the clothing may lead to the dispersion of skin scales, which in turn encourages the dispersion of bacteria (Hathway 2008). Healthcare fabrics have been associated with several outbreaks. At Jichi Medical University Hospital of Japan, an outbreak of Bacillus cereus was reported with bed linens identified as the cause; approx. 660 colony forming units (CFU) cm−2 of B. cereus were detected on them (Sasahara et al. 2011). Recently, a life-threatening healthcare-associated mucormycosis outbreak was found associated with contaminated healthcare linens (Jordan et al. 2022). In England, a Group A Streptococcus (Streptococcus pyogenes) outbreak has been associated with home healthcare (Nabarro et al. 2022).
Outbreaks have also been associated with contaminated healthcare textiles during laundering (Schmithausen et al. 2019; Boonstra et al. 2020). Washing machines have been reported to be a source of the spread of nosocomial infections when used for washing contaminated attire (Whitehead et al. 2022). The shedding of microbes colonized on washing machines, onto the fabrics during laundering, especially in domestic settings, has been observed. The transport of soiled lines and laundered textiles also lead to contamination with microbes resulting in the proliferation of infections (Owen and Laird 2020). The microbial load on laundry is influenced by several factors like type of fabrics, storage conditions, usage, type of detergent, and washing detergent (Abney et al. 2021).
Several other fabric-based patient care items like bandages, blood pressure monitors, dressings, and protective clothing serve as fomite and contribute to the transmission of infection (Kanamori et al. 2017; Akinbobola et al. 2022). Floor cleaning mops also act as reservoir for microbes; thus, proper cleaning of mops should be assured to reduce the risk of infection transmission. Singh et al. (2021) reported a significant reduction in bacterial load after mechanised laundering of mops when compared with manual washing.
Factors affecting bacterial adherence on fabrics
Several key factors affect the microbial load on textiles, including surface properties of bacteria and fabrics, and environmental conditions (Zheng et al. 2021). These factors greatly influence microbial adhesion, their growth, and subsequently their transmission from fabrics.
Bacterial surface properties
The surface charge of bacteria, determined by amino acids, carboxyl acids, and phosphate compounds on the surface, influences the type of interaction between the bacteria and textile surfaces (Poortinga et al. 2002; Tuson and Weibel 2013). Carboxyl, phosphate, and several other anionic groups dominate, conferring a net negative charge on bacterial surfaces. The hydrophobicity of bacteria is determined by their cell wall (Terada et al. 2006). Several factors, such as the type of bacterial strains, pH, and ionic concentration of the nutrient medium, affect the strength of bacterial hydrophobicity (Achinas et al. 2019; Krsmanovic et al. 2021). Depending on the type of surface, the hydrophobicity of microorganisms can affect their ability to adhere (Krasowska and Sigler 2014). Most studies on the effect of hydrophobicity have been done on hard surfaces (Krasowska and Sigler 2014; Kiros et al. 2021), with only a few assessing bacterial contamination on soft surfaces (clothing fibre/fabrics) (Gupta et al. 2017, 2019; Varshney et al. 2019, 2021). It is imperative to gain a deeper understanding of the effect of the surface chemistry of bacteria on minimizing the spread of nosocomial infections through fabrics.
Several surface features of bacterial cells, like adhesins, lipoteichoic acid, surface fibrils, outer membrane protein, and oligosaccharides may impact bacterial adherence on distinct surfaces (Krasowska and Sigler 2014). Bacterial EPS mainly consists of polysaccharides, with small fractions of proteins, lipids, and phospholipids (Simoes et al. 2010). P. aeruginosa PA14 strain has a Pel gene cluster that plays a crucial role in forming glucose-rich matrix essential for forming thick pellicle and resistance to biofilms. Mutation in Pel genes has been reported to adversely affect the adhesion capability of P. aeruginosa on solid surfaces (Vasseur et al. 2005). The outer layer of gram-negative bacteria comprises lipopolysaccharide (LPS) that impacts the process of bacterial adherence on surfaces (Donlan 2002). In E. coli, truncated LPS inhibits the synthesis of fimbriae type I and flagella, reducing attachment (Wang et al. 2021). EPS has also been reported to obstruct the initial adherence of E. coli O157: H7 to stainless steel (SS) (Ryu et al. 2004).
Bacterial appendages
Flagella act as a virulence factor, which enables the colonization of textile surfaces by pathogens (Belas 2014). Flagella facilitate adhesion, biofilm formation, and secretion of virulent factors. Surface interactions between cells and textiles are facilitated by adhesins present on the surface of flagella (Haiko and Westerlund-Wikstrom 2013). Adhesins help overcome repulsive forces that may prevent cell-surface interaction. Fimbriae (or pili) are made up of a cluster of straight and filamentous structures consisting of a protein subunit known as pilin (Dhakal et al. 2019). Pili or fimbriae are associated with the outer membrane of the bacterial surface and play an essential role in the initial adhesion of the bacterial cell to surfaces (Dhakal et al. 2019). Fimbriae types 1 and 3 present on the surface of K. pneumoniae encourage the bacteria’s adhesion and aid in forming biofilms on a variety of surfaces (Murphy et al. 2013). In E. coli, surface adhesion is directly correlated with fimbrial type 1 expression levels (Blumer et al. 2005). The fimbriae of P. aeruginosa facilitate their adhesion to polystyrene, SS, and polyvinyl chloride surfaces (Zheng et al. 2021).
Surface properties of fabrics
Surface roughness may affect bacterial adhesion to textile surfaces (Zheng et al. 2021). During biofilm formation, topography significantly influences bacterial adhesion (Feng et al. 2014). Different mechanisms appear to be responsible for the adhesion on textile surfaces at the nanometric vs. micrometric scale (Cheng et al. 2019). A high roughness level of textile materials protects bacteria from shear forces and provides a greater surface area (Teughels et al. 2006). When bacteria adhere to rough surfaces, the irregularities on the textile surfaces create microenvironments in which they can attach themselves more strongly, thus preventing them from dislodging by shear forces. However, smoothening of the textile surface decreases surface area and surface roughness (Ionescu et al. 2012). As the surface area decreases, there are fewer attachment sites for bacteria to colonize, further limiting the growth of biofilms.
Bacterial adhesion also depends on the hydrophobicity and hydrophilicity of the surfaces (Bruinsma et al. 2001). A reduction in the hydrophobicity has been observed to increase the bacterial load on plastics (Ganesan et at. 2022). Microbes adhere to textiles based on the latter’s surface charge (Zheng et al. 2021). E. coli, Pseudomonas, and S. aureus are more attracted to positively charged poly (acrylic acid) and poly (diallyldimethylammonium chloride) surfaces (Zhu et al. 2015). Polyelectrolyte multilayers with positive charges have been reported to enhance the adhesion of bacteria such as S. aureus and E. coli (Guo et al. 2018). P. aeruginosa was observed to adhere more readily to positively charged poly(allylamine hydrochloride) surfaces than to negatively charged poly(styrenesulfonate) surfaces (Kovacevic et al. 2016). In an Indian hospital, bacterial contamination on nurses’ white coats was investigated on different fabrics and polyester:cotton blend patches had higher levels than polyester patches (Gupta et al. 2017). Various physicochemical properties of fabrics affecting bacterial load have been listed in Table 2.
Table 2.
Major pathogens reported on different types of fabrics used in healthcare settings
| Textile | Primary composition of fabrics | Characteristics of fabrics | Major microbes reported on fabrics | References |
|---|---|---|---|---|
| Cotton | Cellulose | Fluffy staple fibre, crystalline structure, rough surface, good absorbent, poor resistance to acids and alkali | Acinetobacter, Cellulomonas spp., Bacillus spp., Enterococcus faecalis, Escherichia coli, Microbispora bispora, Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus spp., Aspergillus spp., Cladosporium spp., Trichoderma spp., Memnoniella spp. | Gutarowska and Michalski 2012; Varshney et al. 2019; Chiereghin et al. 2020; Varshney et al. 2021 |
| Linen | Mixture of cellulose, hemicellulose, lignin and pectin | Bast fibre, high strength with high capacity of moisture retention | Acinetobacter baumannii, Bacillus amylobacter, B. subtilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, E. coli, P. putida, Staphylococcus spp., Aspergillus spp., Penicillium spp., Rhizopus spp. | Dunn 2022; Sharma et al. 2022 |
| Polyester | An ester of a dihydric alcohol and terephthalic acid | Strong, low moisture retention, accumulates electrostatic charges on the surface, resistant to acids and alkalis | B. subtilis, E. coli, Enterococcus, Pseudomonas aeruginosa, Staphylococcus spp., Aspergillus oryzae, Candida antarctica, Penicillium citrinum, Thermomyces spp. | Varshney et al. 2019; Chiereghin et al. 2020; Varshney et al. 2021 |
| Silk | Fibroin connected through a protein called sericin | Crystalline structure, linked by disulfide bridges, intra and intercellular hydrogen bonds | Pseudomonas cepacia, Streptomyces spp., Staphylococcus spp., Variovorax paradoxus, Aspergillus. niger, Chaetomium spp., Penicillium spp. Enterococcus, Pseudomonas aeruginosa | Szostak-Kotowa 2004; Varshney et al. 2019; Sanders et al. 2021; Varshney et al. 2021 |
| Wool | Keratin | Highly cross-linked structure, disulfide bridges, large portion of cysteine residues in keratin | Bacillus cereus, B. mesentericus, B. mycoides B. subtilis, Streptomyces spp., Aspergillus spp., Microsporum, Chrysosporium spp., Trichophyton, Fusarium, Pseudomonas aeruginosa, E. coli | Gutarowska and Michalski 2012; Varshney et al. 2019; Sanders et al. 2021; Varshney et al. 2021 |
Environmental factors
Environmental factors like pH, temperature, and incubation time affect bacterial adherence on textile substrate. Variations in pH values affect bacterial adherence (McWhirter et al. 2002) as it causes alterations in the hydrophobicity of bacterial cell surface. At an isoelectric point, bacterial cells have zero net charge, which favors better bacterial adherence on hydrophobic surfaces (Palmer et al. 2007). Temperature is considered one of the most essential factors determining bacterial adherence and biofilm development (Abdallah et al. 2014; Fijan et al. 2017). The growth of microbes is adversely affected by temperatures lower or higher than their optimum. The polymer composition on the surface of bacteria changes because of temperature fluctuation, which reduces bacterial adhesion to polymer surfaces at low temperatures (Garrett et al. 2008). A study has reported longer survivability of nosocomial pathogens on cotton at room temperature compared to extremes of temperatures (Fijan et al. 2017). Relative humidity (RH) is essential for the survival of microorganisms on various surfaces (Hokunan et al. 2016; Igo and Schaffner 2019). Increased survival rate of gram-negative bacteria such as Pseudomonas spp. and Klebsiella spp. is reported at higher RH (Tang 2009), while gram-positive bacteria, such as Staphylococcus spp. and Streptococcus spp., have enhanced survival at lower RH (Kramer and Assadian 2014). This change in bacterial behavior is attributed to their cell wall composition, due to which gram-positive bacteria can tolerate dry conditions better than gram-negative bacteria (Kramer and Assadian 2014). Moisture in the fabrics acts as a lubricant and means of transport, thereby promoting the transfer of bacteria from one surface to another, compared to dry fabrics (Sattar et al. 2001; Varshney et al. 2020).
The secretion of sweat, skin shedding, naturally occurring particles in the garment and/or nutrients from the surrounding environment, all contribute to bacterial growth on clothing (Szostak-Kotowa 2004). Sweating from the underarm region of the body facilitates the transfer of bacteria to textile surfaces (van Herreweghen et al. 2020). Other body fluids like blood, sebum, wound fluid, and other materials like starch and dust also provide essential nutrients for microbial growth (Mollebjerg et al. 2021).
Persistence of pathogens on fabrics
The persistence of pathogens on hospital textiles has been well established (Lopez-Gigosos et al. 2019; Kampf 2020; Owen and Laird 2020; Akinbobola et al. 2022). Wißmann et al. (2021) reported that hospital textiles harboured pathogens for periods ranging from hours to months under controlled conditions. Pathogens like Acinetobacter spp., P. aeruginosa, E. coli, S. aureus, K. pneumoniae, Enterococcus spp., and Streptococcus pyogenes have been reported to survive on cotton, polyester, blend, and wool from several days to a few months at room temperature (Koca et al. 2012; Riley et al. 2017; Kampf 2020). MRSA has the longest persistence time on wool compared to other fabrics used in hospital (Koca et al. 2012). It is reported that microbial load on nurses’ white coats is significantly higher after the second shift. Thus, the duration of wear can be directly correlated with microbial load and persistence time (Varshney et al. 2019). Fungal strains, such as Candida tropicalis, Candida krusei, C. albicans, C. parapsilosis, Candida glabrata, Candida parapsilosis, and Cryptococcus neoformans, have shown persistence from few days to a month on cotton, whereas C. albicans and C. parapsilosis survived for 14 days on blend fabric (cotton-polyester) (Traore et al. 2002). Most viruses, including coronavirus, influenza A virus, metapneumovirus, and ebolavirus, were observed to lose their infectious properties after one day on cotton and after 2 to 4 weeks on other materials (Kampf 2020; Kampf et al. 2020). Ideal growth conditions, including large surface area, high moisture content (increased during sweat production), ambient room temperature, moderate humidity, and biofilm formation (Szostak-Kotowa 2004; Mollebjerg et al. 2021), may lead to a longer persistence time of microbes on textiles (Kampf 2020).
Approaches for minimizing the microbial load on fabrics
World Health Organization has released several infection prevention and control (IPC) guidelines since the COVID-19 outbreak in 2020, highlighting the significance of the prevention of nosocomial infections, but these policies have not settled the existing gap between scientific evidence and clinical practice (Monegro et al. 2020; Honghui et al. 2022). In healthcare settings, contaminated fabrics act as a vehicle for infection transmission, which necessitates the implementation of proper hygiene, frequent washing of fabrics and use of disinfectant on a regular basis. Besides, several strategies to prevent the growth and spread of pathogens on textiles are either in practice or proposed (Fig. 2).
Fig. 2.
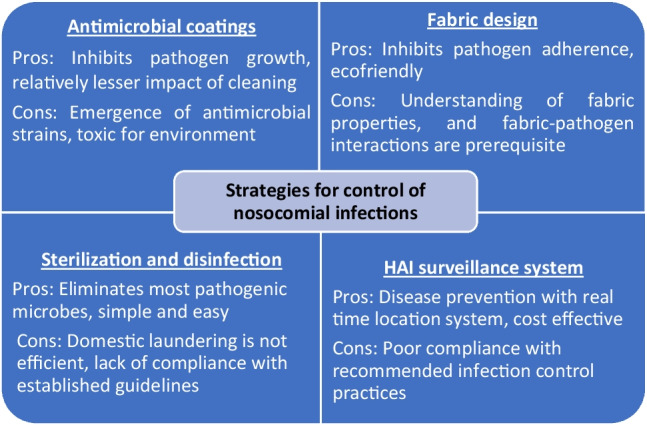
Current strategies to control nosocomial infections, with their challenges and scope of improvement
The survival of pathogens on white coats, even after laundering, indicates that domestic and commercial laundering is insufficient for sterilizing healthcare uniforms (Tarrant et al. 2018; Bockmuhl et al. 2019; Gupta et al. 2019). Thus, proper laundering guidelines are needed for complete decontamination. Healthcare textiles should be laundered after every shift at required temperatures (37–60 °C) following proper laundry instructions (type of detergent, quantity of detergent) depending on the number of clothes, type of bleaching agents, the number of drying cycles, etc. (Bockmuhl et al. 2019; Abney et al. 2021). Ironing, an essential part of the laundering process, effectively decreases the microbial load on healthcare uniforms. Hence it is considered one of the critical processes to ensure the safety of patients and healthcare staffs (Owen and Laird 2020).
Antimicrobial coatings on textile effectively discourage microbial colonization (Ibrahim et al. 2021). Such fabrics are currently in use by healthcare workers and doctors to prevent the spread of infections (Gulati et al. 2022). Inorganic nanoparticles and their composites are the most commonly used antibacterial compounds for textile coatings but are associated with several limitations as they are expensive, and unstable, thus easy to release into the immediate surroundings resulting in toxicity (Nawab et al. 2022). Antimicrobial silver ions at high concentrations have been reported to cause cytotoxicity with the emergence of silver-resistant bacteria (Silver et al. 2006; Wu et al. 2018). The development of antibiotic resistance in bacteria has also been reported due to the extensive use of antibiotics in surface coating under release-based coating strategies (Simoncic and Tomsic 2010). In developing countries, there is no actual information and/or statistical data on the causes of antimicrobial susceptibility (Khan et al. 2015). Thus, it is incredibly challenging to develop and implement an effective plan to control infections when dealing with antimicrobial or antibiotic resistant pathogens (Obiero et al. 2015).
Another method includes textile surface modification by nanotechnologies, plasma treatment, microencapsulation to impart microbe repellent properties (Mahmud and Nabi 2017; Peng et al. 2023). Advanced, eco-friendly, and non-toxic approaches like Sharklet technology are urgently needed to combat these issues (Mann et al. 2014).
It is generally agreed upon that surveillance systems facilitate the assessment of the regional burden of nosocomial infections and aid in early detection, including major outbreaks (Murhekar and Kumar 2022). Conclusively, there is an urgent need for best practices and effective HAI surveillance that should be followed in hospitals to limit the spread of nosocomial infections.
Conclusions
In healthcare settings, apart from direct contact with patients and body fluids (blood, saliva, sweat, urine), common hospital fabrics are a major contributor to nosocomial infections. This review highlights the significance of fabric-mediated transmission of nosocomial infections to healthcare workers, patients, and other environmental surfaces. This type of transmission is generally overlooked and may pose a significant burden on the entire healthcare system. Healthcare workers’ attires, soiled linens, and surroundings near patients remain associated with several pathogens and are reported to be a risk factor for outbreaks. A major limitation of research related to the contamination of healthcare textiles is that they do not differentiate between endogenous and exogenous microorganisms, leading to a blurred picture of fabrics serving as fomites. Moreover, studies have not been performed to correlate the microbial load of textiles with the rate of nosocomial infections. It is only recently that this area has gained appreciable attention from the research community worldwide. Further research is warranted since various aspects of healthcare textiles serving as a significant infection source are currently inconclusive. The adherence of bacteria onto textiles subsequently leads to biofilm formation that helps in the transfer of bacteria; thus, the study of biofilm formation on different fabrics should be one of the subjects of future research. Thorough investigations on the infectivity of microbes adhered onto textiles for a specific period need to be performed, as this information may give further insight into the transmission of pathogens from textiles to hospital staff and patients. While fabrics coated with antimicrobials can limit microbial growth, they can also pose health risks and environmental problems. Hence, another crucial area of study is designing newer fabrics by surface modifications that discourage microbial load. Additionally, implementation of textile hygiene practices, training of hospital staff, analysis of fabric-microbe interactions, framing guidelines for preferred fabrics in healthcare settings, and advancement of infection control programs using efficient epidemiological surveillance systems are some suggested future research areas to manage and control healthcare-associated infections in clinical settings.
Author contribution
SS conceived the idea; SD and SV performed literature survey and compiled and synthesized the papers; SD, SV, and SS wrote the manuscript; SS and DG were awarded funding, and critically reviewed the manuscript. All authors read and approved the manuscript.
Funding
SD received funding from the Science and Engineering Research Board, Government of India (Reference no.PDF/2021/001456). The work was supported by the Grand Challenge scheme (MI1798G) of Indian Institute of Technology Delhi, New Delhi, India.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shweta Dixit and Swati Varshney have equal contribution.
References
- Abdallah M, Benoliel C, Jama C, Drider D, Dhulster P, Chihib NE. Thermodynamic prediction of growth temperature dependence in the adhesion of Pseudomonas aeruginosa and Staphylococcus aureus to stainless steel and polycarbonate. J Food Prot. 2014;77:1116–1126. doi: 10.4315/0362-028X.JFP-13-365. [DOI] [PubMed] [Google Scholar]
- Abney SE, Ijaz MK, McKinney J, Gerba CP. Laundry hygiene and odor control: state of the science. Appl Environ Microbiol. 2021;87:1–12. doi: 10.1128/AEM.03002-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achinas S, Charalampogiannis N, Euverink GJW. A brief recap of microbial adhesion and biofilms. Appl Sci. 2019;9:1–15. doi: 10.3390/app9142801. [DOI] [Google Scholar]
- Akinbobola AB, Osunla AC, Bello OM, Ajayi OA. Study of the persistence of selected Gram-negative bacteria pathogens of healthcare-associated infections on hospital fabrics. Am J Infect Cont. 2022;50:755–757. doi: 10.1016/j.ajic.2021.11.023. [DOI] [PubMed] [Google Scholar]
- Ambrosch A, Wahrburg K, Klawonn F. Bacterial load and pathogenic species on healthcare personnel attire: implications of alcohol hand-rub use, profession, and time of duty. J Hosp Infect. 2019;101:414–421. doi: 10.1016/j.jhin.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Ather B, Mirza TM, Edemekong PF (2020) Airborne precautions. StatPearls [Internet]. Treasure Island, FL: StatPearls. Available online: http://www.ncbi.nlm.nih.gov/books/NBK531468 (Accessed on 16 April 2023).
- Bache SE, Maclean M, Gettinby G, Anderson JG, MacGregor SJ, Taggart I. Quantifying bacterial transfer from patients to staff during burns dressing and bed changes: implications for infection control. Burns. 2013;39:220–228. doi: 10.1016/j.burns.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Belas R. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol. 2014;22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Berktold M, Mayr A, Obwegeser A, Lass-Flörl C, Kreidl P, Orth-Holler D. Long-sleeved medical workers’ coats and their microbiota. Am J Infect Cont. 2018;46:1408–1410. doi: 10.1016/j.ajic.2018.04.230. [DOI] [PubMed] [Google Scholar]
- Blumer C, Kleefeld A, Lehnen D, Heintz M, Dobrindt U, Nagy G, Unden G. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology. 2005;15:3287–3298. doi: 10.1099/mic.0.28098-0. [DOI] [PubMed] [Google Scholar]
- Bockmuhl DP, Schages J, Rehberg L. Laundry and textile hygiene in healthcare and beyond. Microb Cell. 2019;6:299–306. doi: 10.15698/mic2019.07.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra MB, Spijkerman DC, Voor AF, van der Laan RJ, Bode LG, van Vianen W, Severin JA. An outbreak of ST307 extended-spectrum beta-lactamase (ESBL)–producing Klebsiella pneumoniae in a rehabilitation center: an unusual source and route of transmission. Infect Cont Hosp Epidemiol. 2020;41:31–36. doi: 10.1017/ice.2019.304. [DOI] [PubMed] [Google Scholar]
- Bruinsma GM, Van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–3224. doi: 10.1016/S0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- Chaoui L, Mhand R, Mellouki F, Rhallabi N. Contamination of the surfaces of a healthcare environment by multidrug-resistant (MDR) bacteria. Int J Microbiol. 2019;2019:1–8. doi: 10.1155/2019/3236526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Feng G, Moraru CI. Micro-and nanotopography sensitive bacterial attachment mechanisms: a review. Front Microbiol. 2019;10:1–17. doi: 10.3389/fmicb.2019.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiereghin A, Felici S, Gibertoni D, Foschi C, Turello G, Piccirilli G, Gabrielli L, Clerici P, Landini MP, Lazzarotto T. Microbial contamination of medical staff clothing during patient care activities: performance of decontamination of domestic versus industrial laundering procedures. Curr Microbiol. 2020;77:1159–1166. doi: 10.1007/s00284-020-01919-2. [DOI] [PubMed] [Google Scholar]
- Condo C, Messi P, Anacarso I, Sabia C, Iseppi R, Bondi M, De Niederhausern S. Antimicrobial activity of silver doped fabrics for the production of hospital uniforms. New Microbiol. 2015;38:551–558. [PubMed] [Google Scholar]
- Das I, Lambert P, Hill D, Noy M, Bion J, Elliott T. Carbapenem-resistant Acinetobacter and role of curtains in an outbreak in intensive care units. J Hosp Infect. 2002;50:110–114. doi: 10.1053/jhin.2001.1127. [DOI] [PubMed] [Google Scholar]
- Dhakal BK, Bower JM, Mulvey MA, Yang XH. Pili Fimbriae In Encyclopedia of Microbiology. 4. Oxford, UK: Academic Press; 2019. pp. 595–613. [Google Scholar]
- Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn D. Linen: The New Frontier in Infection Control and Prevention. AORN J. 2022;115:310–324. doi: 10.1002/aorn.13643. [DOI] [PubMed] [Google Scholar]
- Dyer C, Hutt LP, Burky R, Joshi LT. Biocide resistance and transmission of Clostridium difficile spores spiked onto clinical surfaces from an American health care facility. Appl Environ Microbiol. 2019;85:1–11. doi: 10.1128/AEM.01090-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciola A, Pellicanò GF, Visalli G, Paolucci IA, Venanzi Rullo E, Ceccarelli M, ... Nunnari G (2019) The role of the hospital environment in the healthcare-associated infections: a general review of the literature. Eur Rev Med Pharmacol Sci 23:1266–1278. [DOI] [PubMed]
- Feng G, Cheng Y, Wang SY, Hsu LC, Feliz Y, Borca-Tasciuc DA, Moraru CI. Alumina surfaces with nanoscale topography reduce attachment and biofilm formation by Escherichia coli and Listeria spp. Biofouling. 2014;30:1253–1268. doi: 10.1080/08927014.2014.976561. [DOI] [PubMed] [Google Scholar]
- Fijan S, Pahor D, Sostar Turk S. Survival of Enterococcus faecium, Staphylococcus aureus and Pseudomonas aeruginosa on cotton. Text Res J. 2017;87:1711–1721. doi: 10.1177/0040517516658514. [DOI] [Google Scholar]
- Galvin J, Almatroudi A, Vickery K, Deva A, Lopes LKO, de MeloCosta D, Hu H. Patient shoe covers: transferring bacteria from the floor onto surgical bedsheets. Am J Infect Cont. 2016;44:1417–1419. doi: 10.1016/j.ajic.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Ruendee T, Kimura SY, Chawengkijwanich C, Janjaroen D. Effect of biofilm formation on different types of plastic shopping bags: structural and physicochemical properties. Environ Res. 2022;206:112542. doi: 10.1016/j.envres.2021.112542. [DOI] [PubMed] [Google Scholar]
- Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog Nat Sci. 2008;18:1049–1056. doi: 10.1016/j.pnsc.2008.04.001. [DOI] [Google Scholar]
- Goyal S, Khot SC, Ramachandran V, Shah KP, Musher DM. Bacterial contamination of medical providers’ white coats and surgical scrubs: a systematic review. Am J Infect Cont. 2019;47:994–1001. doi: 10.1016/j.ajic.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Gulati R, Sharma S, Sharma RK. Antimicrobial textile: recent developments and functional perspective. Polym Bull. 2022;79:5747–5771. doi: 10.1007/s00289-021-03826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kwek MY, Toh ZQ, Pranantyo D, Kang ET, Loh XJ, Neoh KG. Tailoring polyelectrolyte architecture to promote cell growth and inhibit bacterial adhesion. ACS Appl Mater Interfaces. 2018;10:7882–7891. doi: 10.1021/acsami.8b00666. [DOI] [PubMed] [Google Scholar]
- Gupta P, Bairagi N, Priyadarshini R, Singh A, Chauhan D, Gupta D. Bacterial contamination of nurses’ white coats after first and second shift. Am J Infect Cont. 2017;45:86–88. doi: 10.1016/j.ajic.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Gupta P, Bairagi N, Gupta D (2019) Effect of domestic laundering on removal of bacterial contamination from nurses’ white coats. Functional Textiles and Clothing. Springer Singapore. 67–73.
- Gutarowska B, Michalski A (2012) Microbial degradation of woven fabrics and protection against biodegradation. IntechOpen, Rijeka, Croatia. Available online: https://www.intechopen.com/citation-pdf-url/36909. Accessed on 18 January 2023
- Haiko J, Westerlund-Wikström B. The role of the bacterial flagellum in adhesion and virulence. Biology. 2013;2:1242–1267. doi: 10.3390/biology2041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Sartelli M, McKimm J, Bakar MA. Health care-associated infections–an overview. Infect Drug Resist. 2018;11:2321–2333. doi: 10.2147/IDR.S177247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MZ, Sturm-Ramirez K, Rahman MZ, Hossain K, Aleem MA, Bhuiyan MU, Gurley ES. Contamination of hospital surfaces with respiratory pathogens in Bangladesh. Plos One. 2019;14:1–10. doi: 10.1371/journal.pone.0224065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathway EA (2008) CFD modelling of pathogen transport due to human activity. Dissertation, University of Leeds
- Hemmatian T, Lee H, Kim J. Bacteria adhesion of textiles influenced by wettability and pore characteristics of fibrous substrates. Polymers. 2021;13:1–14. doi: 10.3390/polym13020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokunan H, Koyama K, Hasegawa M, Kawamura S, Koseki S. Survival kinetics of Salmonella enterica and enterohemorrhagic Escherichia coli on a plastic surface at low relative humidity and on low–water activity foods. J Food Prot. 2016;79:1680–1692. doi: 10.4315/0362-028X.JFP-16-081. [DOI] [PubMed] [Google Scholar]
- Honghui Z, Qingting L,Yao C, Hua P, Chunmi G, Qing L, Jia G (2022) Prevention and control of nosocomial infections among hospital logistic staff during the COVID-19 pandemic. J Healthc Eng 1–5. [DOI] [PMC free article] [PubMed]
- Ibrahim A, Laquerre JE, Forcier P, Deregnaucourt V, Decaens J, Vermeersch O (2021) Antimicrobial agents for textiles: types, mechanisms and analysis standards. Text Funct Appl London, UK Intech Open, pp 13–42.
- Igo MJ, Schaffner DW. Quantifying the influence of relative humidity, temperature, and diluent on the survival and growth of Enterobacter aerogenes. J Food Prot. 2019;82:2135–2147. doi: 10.4315/0362-028X.JFP-19-261. [DOI] [PubMed] [Google Scholar]
- Ionescu A, Wutscher E, Brambilla E, Schneider-Feyrer S, Giessibl FJ, Hahnel S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur J Oral Sci. 2012;120:458–465. doi: 10.1111/j.1600-0722.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- Jablonska-Trypuc A, Makuła M, Włodarczyk-Makuła M, Wołejko E, Wydro U, Serra-Majem L, Wiater J. Inanimate surfaces as a source of hospital infections caused by fungi, bacteria and viruses with particular emphasis on SARS-CoV-2. Int J Environ Res Public Health. 2022;19:8121. doi: 10.3390/ijerph19138121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, James AE, Gold JA, Wu K, Glowicz J, Wolfe F, Toda M. Investigation of a prolonged and large outbreak of healthcare-associated mucormycosis cases in an acute care hospital—Arkansas, June 2019–May 2021. Open Forum Infect Dis. 2022;9:ofac510. doi: 10.1093/ofid/ofac510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose A, Gizdavic-Nikolaidis M, Swift S. Antimicrobial coatings: reviewing options for healthcare applications. Appl Microbiol. 2023;3:145–174. doi: 10.3390/applmicrobiol3010012. [DOI] [Google Scholar]
- Kampf G (2020) How long can nosocomial pathogens survive on textiles? A systematic review. GMS Hygiene Infect Cont 15. [DOI] [PMC free article] [PubMed]
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori H, Rutala WA, Weber DJ. The role of patient care items as a fomite in healthcare-associated outbreaks and infection prevention. Clin Infect Dis. 2017;65:1412–1419. doi: 10.1093/cid/cix462. [DOI] [PubMed] [Google Scholar]
- Kanwar A, Thakur M, Wazzan M, Satyavada S, Cadnum JL, Jencson AL, Donskey CJ. Clothing and shoes of personnel as potential vectors for transfer of health care–associated pathogens to the community. Am J Infect Cont. 2019;47:577–579. doi: 10.1016/j.ajic.2019.01.028. [DOI] [PubMed] [Google Scholar]
- Khan HA, Ahmad A, Mehboob R. Nosocomial infections and their control strategies. Asian Pac J Trop Biomed. 2015;5:509–514. doi: 10.1016/j.apjtb.2015.05.001. [DOI] [Google Scholar]
- Koca O, Altoparlak U, Ayyildiz A, Kaynar H. Persistence of nosocomial pathogens on various fabrics. Eurasian J Emerg Med. 2012;44:28–31. doi: 10.5152/eajm.2012.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic D, Pratnekar R, Godicc Torkar K, Salopek J, Drazic G, Abram A, Bohinc K. Influence of polyelectrolyte multilayer properties on bacterial adhesion capacity. Polymers. 2016;8:1–12. doi: 10.3390/polym8100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Assadian O (2014) Survival of microorganisms on inanimate surfaces. Use of biocidal surfaces for reduction of healthcare acquired infections. Springer, 7–26.
- Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:1–7. doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsmanovic M, Biswas D, Ali H, Kumar A, Ghosh R, Dickerson AK. Hydrodynamics and surface properties influence biofilm proliferation. Adv Colloid Interface Sci. 2021;288:1–26. doi: 10.1016/j.cis.2020.102336. [DOI] [PubMed] [Google Scholar]
- Kiros T, Damtie S, Eyayu T, Tiruneh T, Hailemichael W, Workineh L (2021) Bacterial pathogens and their antimicrobial resistance patterns of inanimate surfaces and equipment in Ethiopia: a systematic review and meta-analysis. Biomed Res Int 1–25. [DOI] [PMC free article] [PubMed]
- Lena P, Karageorgos SA, Loutsiou P, Poupazi A, Lamnisos D, Papageorgis P, Tsioutis C. Multidrug-resistant bacteria on healthcare workers’ uniforms in hospitals and long-term care facilities in cyprus. Antibiotics. 2021;11:1–9. doi: 10.3390/antibiotics11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W, Ng VV, Holton J. Bacterial flora on the white coats of medical students. J Hosp Infect. 2000;45:65–68. doi: 10.1053/jhin.1999.0702. [DOI] [PubMed] [Google Scholar]
- Lopez GU, Gerba CP, Tamimi AH, Kitajima M, Maxwell SL, Rose JB. Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Appl Environ Microbiol. 2013;79:5728–5734. doi: 10.1128/AEM.01030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gigosos RM, Mariscal A, Gutierrez-Bedmar M, Real M, Mariscal-López E. Carbapenem resistance in Acinetobacter baumannii is associated with enhanced survival on hospital fabrics. Acta Microbiol Immunol Hung. 2019;66:143–154. doi: 10.1556/030.65.2018.043. [DOI] [PubMed] [Google Scholar]
- Mahmud R, Nabi, F (2017) Application of nanotechnology in the field of textile. IOSR J Polym Text Eng 4.
- Mann EE, Mettetal MR, May RM, Drinker MC, Stevenson BC, Baiamonte VL, Sande MK. Surface micropattern resists bacterial contamination transferred by healthcare practitioners. J Microbiol Exp. 2014;1:1–7. [Google Scholar]
- McWhirter MJ, McQuillan AJ, Bremer PJ. Influence of ionic strength and pH on the first 60 min of Pseudomonas aeruginosa attachment to ZnSe and to TiO2 monitored by ATR-IR spectroscopy. Colloids Surf B. 2002;26:365–372. doi: 10.1016/S0927-7765(02)00017-6. [DOI] [Google Scholar]
- Mishra SK, Maharjan S, Yadav SK, Sah NP, Sharma S, Parajuli K, Sherchand JB (2020) Bacteria on medical professionals’ white coats in a university hospital. Can J Infect Dis Med Microbiol 1–6. [DOI] [PMC free article] [PubMed]
- Mitchell A, Spencer M, Edmiston C., Jr Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: a review of the literature. J Hosp Infect. 2015;90:285–292. doi: 10.1016/j.jhin.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollebjerg A, Palmén LG, Gori K, Meyer RL. The bacterial life cycle in textiles is governed by fiber hydrophobicity. Microbiol Spectr. 2021;9:1–15. doi: 10.1128/Spectrum.01185-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monegro AF, Muppidi V, Regunath H (2020) Hospital acquired infections. In StatPearls (Internet), StatPearls Publishing. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441857 (Accessed 20 February 2023). [PubMed]
- Morgan DJ, Rogawski E, Thom KA, Johnson JK, Perencevich EN, Shardell M, Leekha S, Harris AD. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40:1045. doi: 10.1097/CCM.0b013e31823bc7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Kumar CG. Health-care-associated infection surveillance in India. Lancet Glob Health. 2022;10:1222–1223. doi: 10.1016/S2214-109X(22)00317-5. [DOI] [PubMed] [Google Scholar]
- Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect Immun. 2013;81:3009–3017. doi: 10.1128/IAI.00348-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, GrayA NM. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabarro LE, Brown CS, Balasegaram S, Decraene V, Elston J, Kapadia S, Lamagni T. Invasive Group A Streptococcus outbreaks associated with home healthcare, England, 2018–2019. Emerg Infect Dis. 2022;28:915–923. doi: 10.3201/eid2805.211497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawab R, Iqbal A, Niazi F, Iqbal G, Saleem A, Munis MFH (2022) Review featuring the use of inorganic nano-structured material for anti-microbial properties in textile. Polym Bull 1–25.
- Obiero CW, Seale AC, Berkley JA. Empiric treatment of neonatal sepsis in developing countries. Pediatr Infect Dis J. 2015;34:659–661. doi: 10.1097/INF.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okareh OT. Bacterial pathogens from bed linen used in secondary and tertiary health facilities in Benin city Nigeria. J Microbiol Exp. 2018;6:84–87. [Google Scholar]
- Owen L, Laird K. The role of textiles as fomites in the healthcare environment: a review of the infection control risk. PeerJ. 2020;8:1–35. doi: 10.7717/peerj.9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak P, Bhimrao SK, Kozak FK, Westerberg BD. Health care professionals’ neckties as a source of transmission of bacteria to patients: a systematic review. Can Med Assoc J. 2018;6:26–30. doi: 10.9778/cmajo.20170126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Flint S, Brooks J. Bacterial cell attachment the beginning of a biofilm. J Ind Microbiol Biotechnol. 2007;34:577–588. doi: 10.1007/s10295-007-0234-4. [DOI] [PubMed] [Google Scholar]
- Peng X, Umer M, Pervez MN, Hasan KF, Habib MA, Islam MS, Lin L, Xiong X, Naddeo V, Cai Y (2023) Biopolymers-based microencapsulation technology for sustainable textiles development: a short review. Case Stud Chem Environ Eng 100349.
- Pillai SG, Haldorai K, Seo WS, Kim WG. COVID-19 and hospitality 5.0: Redefining hospitality operations. Int J Hosp Manag. 2021;94:102869. doi: 10.1016/j.ijhm.2021.102869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilonetto M, Rosa EAR, Brofman PRS, BaggioD CF, Schelp C, Messias-Reason I. Hospital gowns as a vehicle for bacterial dissemination in an intensive care unit. Braz J Infect Dis. 2004;8:206–210. doi: 10.1590/S1413-86702004000300003. [DOI] [PubMed] [Google Scholar]
- Poortinga AT, Bos R, Norde W, Busscher HJ. Electric double layer interactions in bacterial adhesion to surfaces. Surf Sci Rep. 2002;47:1–32. doi: 10.1016/S0167-5729(02)00032-8. [DOI] [Google Scholar]
- Popovich KJ, Green SJ, Okamoto K, Rhee Y, Hayden MK, Schoeny M, Weinstein RA. MRSA transmission in intensive care units: genomic analysis of patients their environments and healthcare workers. Clin Infect Dis. 2021;72:1879–1887. doi: 10.1093/cid/ciaa731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley K, Williams J, Owen L, Shen J, Davies A, Laird K. The effect of low-temperature laundering and detergents on the survival of Escherichia coli and Staphylococcus aureus on textiles used in healthcare uniforms. J Appl Microbiol. 2017;123:280–286. doi: 10.1111/jam.13485. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Kim H, Beuchat LR. Attachment and biofilm formation by Escherichia coli O157: H7 on stainless steel as influenced by exopolysaccharide production nutrient availability and temperature. J Food Prot. 2004;67:2123–2131. doi: 10.4315/0362-028X-67.10.2123. [DOI] [PubMed] [Google Scholar]
- Sanders D, Grunden A, Dunn RR. A review of clothing microbiology: the history of clothing and the role of microbes in textiles. Biol Lett. 2021;17:1–13. doi: 10.1098/rsbl.2020.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimura A, Hirai Y. Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis. 2011;30:219–226. doi: 10.1007/s10096-010-1072-2. [DOI] [PubMed] [Google Scholar]
- Sattar SA, Springthorpe S, Mani S, Gallant M, Nair RC, Scott E, Kain J. Transfer of bacteria from fabrics to hands and other fabrics: development and application of a quantitative method using Staphylococcus aureus as a model. J Appl Microbiol. 2001;90:962–970. doi: 10.1046/j.1365-2672.2001.01347.x. [DOI] [PubMed] [Google Scholar]
- Schmithausen RM, Sib E, Exner M, Hack S, Rosing C, Ciorba P, Exner D. The washing machine as a reservoir for transmission of extended-spectrum-beta-lactamase (CTX-M-15)-producing Klebsiella oxytoca ST201 to newborns. Appl Environ Microbiol. 2019;85:1–11. doi: 10.1128/AEM.01435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Krishnamoorthi S, Kumar A, Biswal M, Koushal V. Bacterial counts of hospital linen and effectiveness of laundry process: a need for consensus on microbial sterility of hospital linen. J Patient Saf Infect Cont. 2022;10:6. doi: 10.4103/jpsic.jpsic_1_22. [DOI] [Google Scholar]
- Sikora A, Zahra F (2021) Nosocomial infections. In StatPearls (Internet), StatPearls Publishing: http://www.ncbi.nlm.nih.gov/books/NBK559312 (Accessed 13 February 2023).
- Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006;33:627–634. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- Simoes M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT-Food Sci Technol. 2010;43:573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- Simoncic B, Tomsic B. Structures of novel antimicrobial agents for textiles-a review. Text Res J. 2010;80:1721–1737. doi: 10.1177/0040517510363193. [DOI] [Google Scholar]
- Singh K, Siddharth V, Singh G. Mechanized laundering of mops for floor cleaning can reduce infection transmission through hospital floor. Indian J Med Microbiol. 2021;39:224–227. doi: 10.1016/j.ijmmb.2021.03.009. [DOI] [PubMed] [Google Scholar]
- Stephens B, Azimi P, Thoemmes MS, Heidarinejad M, Allen JG, Gilbert JA. Microbial exchange via fomites and implications for human health. Curr Pollut Rep. 2019;5:198–213. doi: 10.1007/s40726-019-00123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak-Kotowa J. Biodeterioration of textiles. Int Biodeterior Biodegrad. 2004;53:165–170. doi: 10.1016/S0964-8305(03)00090-8. [DOI] [Google Scholar]
- Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;6:S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant J, Jenkins RO, Laird KT. From ward to washer: the survival of Clostridium difficile spores on hospital bed sheets through a commercial UK NHS healthcare laundry process. Infect Control Hosp Epidemiol. 2018;39:1406–1411. doi: 10.1017/ice.2018.255. [DOI] [PubMed] [Google Scholar]
- Terada A, Yuasa A, Kushimoto T, Tsuneda S, Katakai A, Tamada M. Bacterial adhesion to and viability on positively charged polymer surfaces. Microbiology. 2006;152:3575–3583. doi: 10.1099/mic.0.28881-0. [DOI] [PubMed] [Google Scholar]
- Teughels W, van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Impl Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- Traore O, Springthorpe VS, Sattar SA. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J Appl Microbiol. 2002;92:549–555. doi: 10.1046/j.1365-2672.2002.01560.x. [DOI] [PubMed] [Google Scholar]
- Tuson HH, Weibel DB. Bacteria–surface interactions. Soft Matter. 2013;9:4368–4380. doi: 10.1039/c3sm27705d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herreweghen F, Amberg C, Marques R, Callewaert C. Biological and chemical processes that lead to textile malodour development. Microorganisms. 2020;8:1709. doi: 10.3390/microorganisms8111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney S, Sharma S, Gupta D. Factors affecting bacterial load on nurses’ white coats. J Hosp Infect. 2019;102:470–471. doi: 10.1016/j.jhin.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Varshney S, Pandey P, Gupta D, Sharma S. Role of fabric properties, moisture, and friction in transfer of bacteria from fabric to fabric. Text Res J. 2020;90:478–485. doi: 10.1177/0040517519866956. [DOI] [Google Scholar]
- Varshney S, Sain A, Gupta D, Sharma S. Factors affecting bacterial adhesion on selected textile fibres. Ind J Microbiol. 2021;61:31–37. doi: 10.1007/s12088-020-00903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney S, Sharma S, Gupta D (2022) Surveillance of bacterial load and multi-drug resistant bacteria on bedsheets in a primary health care unit. Int J Environ Health Res 1–12. [DOI] [PubMed]
- Vasseur P, Vallet-Gely I, Soscia C, Genin S, Filloux A. The pel genes of the Pseudomonas aeruginosa PAK strain is involved at early and late stages of biofilm formation. Microbiology. 2005;151:985–997. doi: 10.1099/mic.0.27410-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma W, Wang X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb Cell Factories. 2021;20:1–17. doi: 10.1186/s12934-021-01565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K, Eppinger J, Srinivasan V, Ijaz MK, Nims RW, McKinney J. Potential for microbial cross contamination of laundry from public washing machines. Microbiol Res. 2022;13(4):995–1006. doi: 10.3390/microbiolres13040072. [DOI] [Google Scholar]
- Wißmann JE, Kirchhoff L, Brüggemann Y, Todt D, Steinmann J, Steinmann E. Persistence of pathogens on inanimate surfaces: a narrative review. Microorganisms. 2021;9:343. doi: 10.3390/microorganisms9020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020) Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance 6 April 2020 (No WHO/2019-nCov/IPC PPE_use/2020 3) World Health Organization.
- Wu J, Zhang C, Xu S, Pang X, Cai G, Wang J. Preparation of zwitterionic polymer-functionalized cotton fabrics and the performance of anti-biofouling and long-term biofilm resistance. Colloid Interface Sci Commun. 2018;24:98–104. doi: 10.1016/j.colcom.2018.02.001. [DOI] [Google Scholar]
- Xu H, Murdaugh AE, Chen W, Aidala KE, Ferguson MA, Spain EM, Nunez ME. Characterizing pilus-mediated adhesion of biofilm-forming E. coli to chemically diverse surfaces using atomic force microscopy. Langmuir. 2013;29:3000–3011. doi: 10.1021/la304745s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Bawazir M, Dhall A, Kim HE, He L, Heo J, Hwang G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front Bioeng Biotechnol. 2021;9:643722. doi: 10.3389/fbioe.2021.643722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jańczewski D, Guo S, Lee SSC, Parra Velandia FJ, Teo SLM, Vancso GJ. Polyion multilayers with precise surface charge control for antifouling. ACS Appl Mater Interfaces. 2015;7:852–861. doi: 10.1021/am507371a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


