Abstract
Objective:
To study the feasibility of home-based assessment of sleep disordered breathing (SDB) on early pregnancy success after in vitro fertilization with novel wearable sensors.
Design:
Prospective observational study.
Setting:
Patients 18 to 45 years old undergoing autologous IVF at an academic infertility center.
Patients:
30 women (24–44 years old)
Intervention:
Participants provided medical history, completed sleep surveys, and a single night of home sleep monitoring prior to IVF with a novel, FDA-cleared wireless sensor system (ANNE® Sleep, Sibel Health), to collect continuous measurements of heart rate, respiratory rate, pulse oxygenation, respiratory effort/snoring, peripheral arterial tonometry, pulse arrival time, and pulse transit time, an accepted surrogate of continuous blood pressure generated by pulse arrival time and pulse transit time. Sleep nights were reviewed to derive the apnea hypopnea index (AHI), defined as the average number of apnea or hypopnea events per hour. An AHI of greater than or equal to 5 events/hour was considered abnormal.
Main outcome measure:
Rate of clinical pregnancy (defined as intrauterine gestational sac with a yolk sac) after IVF. Logistic regression models were used to estimate the unadjusted and adjusted odds ratio.
Results:
The overall rate of sleep disordered breathing of any severity was 57%. Participants with SDB had a mean AHI of 13.4 compared to 2.7 events/hr (p<0.01), were younger, and more likely to have polycystic ovary syndrome. Of the 29 patients undergoing an embryo transfer, clinical pregnancy and livebirth occurred in 35% of women with SDB compared to 58% without SDB (p = 0.22). After adjusting for age, SDB reduced pregnancy rates but was not statistically significant (aOR 0.23, 95% CI: 0.04–1.5, p = 0.12). Though polycystic ovary syndrome was associated with higher rates of SDB it was not independently associated with lower pregnancy rates.
Conclusion:
Screening for sleep disordered breathing using home-based wireless, wearable sensors was well accepted and easily performed by infertile patients in this cohort. Sleep disordered breathing of any severity was associated with an 77% (95% CI: 0.08–1.8) lower likelihood of clinical pregnancy and live birth independent of underlying diagnosis. Future larger studies will be needed to understand the role of sleep disordered breathing and IVF outcomes.
Keywords: Sleep disordered breathing, Wearables, IVF outcomes
1. Introduction
Sleep disordered breathing (SDB), caused by increased upper airway resistance, affects 5% of all adult women [1]. Once narrowly considered a disease of older, overweight men, a more inclusive understanding of SDB risk has recently emerged. Nearly one third of women with polycystic ovary syndrome (PCOS), for example, have SDB. The elevated risk among PCOS patients appears independent of underlying weight, a common risk factor for SDB [2].
Airway dysfunction causes autonomic nervous system dysregulation, sleep fragmentation, insulin resistance, systemic inflammation, oxidative stress, and vascular endothelial dysfunction [3]. These physiologic changes contribute to cardiovascular disease, metabolic disorders, motor vehicle accidents, and mood disorders [4–7]. The effects of SDB on early reproductive outcomes, including fertility, fecundity, and conception are not well understood and the majority of studies disproportionately address complications of established pregnancies, fetal or neonatal outcomes [8,9].
Despite efforts to optimize periconceptional health, such as tobacco use, obesity, or diabetes, routine screening for sleep pathology in the infertility clinic is uncommon. The paucity of both research and screening for SDB and reproduction is likely a reflection of logistical challenges to objectively studying sleep. Polysomnography (PSG), performed overnight at a certified sleep center, is currently the gold standard for diagnosing SDB. The geographical distribution of accredited centers varies considerably, perpetuating unequal accessibility and long appointment wait times [10]. Furthermore, the on-going COVID-19 pandemic has significantly disrupted the landscape of sleep diagnostics, prioritizing efforts to expand home-based screening options to minimize patient and provider exposure [11 ]. Current home sleep apnea tests (HSATs) are bulky, uncomfortable, and failure rates ranging from 2% to 33% [12–17]. Though proper patient training and correct placement may mitigate technical HSAT failures, there remains a need for new home-based diagnostic tools for SDB that improve comfort, lower cost, and equivalent accuracy to existing systems.
Advances in soft and flexible electronics have enabled a wide range of biomedical applications including comprehensive, continuous ICU-grade monitoring and mechanical imperceptibility that collectively improve home-based SDB screening and diagnosis. Previous reports validated the performance of an FDA-cleared physiological monitoring system against gold standard monitoring platforms [18–23]. The primary objective of this pilot study was to assess the feasibility of these advanced wearable sensors to assess the prevalence of SDB in an all comer infertile patient population undergoing in vitro fertilization (IVF) and quantify any differences in clinical pregnancy and livebirth rates after treatment among women exposed to SDB.
2. Material and methods
2.1. Study participants
Patients undergoing autologous IVF were eligible for this prospective observational cohort study. Individuals between the ages of 18 to 45 years old, with any primary infertility diagnosis were eligible, including those utilizing preimplantation genetic testing (PGT). Long-term oocyte, embryo banking, donor oocyte/embryo, or gestational carrier cycles were excluded. Individuals with SDB currently receiving treatment with either a continuous positive airway pressure (CPAP) machine or an intraoral device were also excluded.
Patients were enrolled and underwent the sleep study prior to initiation of injectable gonadotropins in the luteal phase. All participants signed written informed consent prior to enrollment. This study was approved by the University of Pennsylvania Institutional Review Board (Protocol #842992).
2.2. Primary exposure
The primary exposure of interest was sleep disordered breathing, defined by an apnea hypopnea index (AHI) greater than or equal to 5. AHI is the average number of apnea or hypopnea events per hour with a pulse oxygen desaturation of at least 3% lasting for at least 10 s, in accordance with clinical guidelines of the American Academy of Sleep Medicine (AASM) [24]. An AHI less than 5 events/hour is considered normal. An AHI between 5 and 15 events/hour is indicative of mild disease; an AHI between 15 and 30 events/hour is moderate disease, and 30 or more events/hour is severe disease. In this cohort, any elevation of AHI more than 5 events/hour was categorized as a positive exposure [24].
AHI was determined by a single home-based night of sleep monitoring using an FDA-approved, wireless two sensor system (ANNE One, Sibel Health) for both general physiological monitoring and diagnosis of sleep disordered breathing (K220095). In a multicenter study of 225 patients, the ANNE ® sensors achieved 90% sensitivity 98% specificity for detection of moderate and severe sleep disordered breathing compared to the gold standard PSG [25]. Additionally, prior performance of the system showed a high level of accuracy (94%) versus a standard Type III home sleep apnea in a high-risk SDB population [22]. One sensor was located at the suprasternal notch and the second on the index finger (Fig. 1). The chest unit measured heart rate, respiratory rate, chest wall movement, snoring, abnormal respiratory sounds, seismocardiography, body position, and core body temperature continuously. The limb unit measured oxygen saturation (SpO2), peripheral temperature, peripheral arterial tonometry (PAT), a well-established method of identifying apnea and hypopnea associated with SDB [12,26]. Time synchronization of the chest and limb units generated pulse transit time (PTT) providing surrogate markers of blood pressure [21,22,27,28]. Each night was independently scored by two reviewers and discrepancies were adjudicated by a third reviewer. All reviewers were blinded to subject characteristics and outcomes. Scorers manually reviewed multiple channel outputs from the sensors, including chest wall movement, peripheral arterial tonometry (PAT) for an attenuated signal, SpO2, heart rate, and snoring in accordance with instructions in the ANNE® Manual and manufacturing guidelines.
Fig. 1.
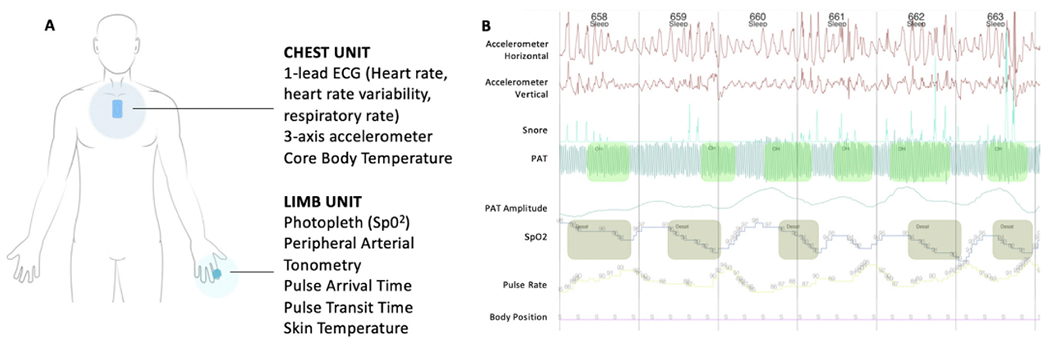
Wireless, non-invasive sensor system used for sleep monitoring and example output.
Panel A Figure demonstrating the appearance, location, and biometric parameters obtained by the wireless chest and limb units. Panel B demonstrates an example output of the sleep night data with desaturation episodes, followed by bradycardia, and concurrent irregularities in the peripheral arterial tonometry.
Participants refrained from caffeine, alcohol, and napping for 8 h prior to going to bed and any use of sleep aids. The monitored sleep night was performed in the week preceding IVF stimulation to avoid any effects from exogenous hormones on the sleep study and perceived ill effects of wearing the sleep sensors on cycle stimulation or pregnancy outcome and have as accurate a reflection of sleep as possible. Following completion of the night of sleep monitoring, subjects completed a System Usability Scale (SUS) with respect to their experience using the sensors.
2.3. Covariates
Sociodemographic information including age, gender, race/ethnicity, and education level, habits (previous and current tobacco, vaping, or alcohol use), past medical and obstetrical history were collected. Participants completed a battery of baseline sleep questionnaires. The STOP-BANG questionnaire identified obstructive sleep apnea risk (range 0–8), with a score of 3 or more predicting moderate sleep apnea with 93% sensitivity [29]. The Epworth Sleepiness Survey (ESS) assessed daytime somnolence [30]; scores (range 0–24) greater than 10 were considered abnormal levels of daytime sleepiness. The Pittsburgh Sleep Quality Index (PSQI) assessed global sleep quality. Scores range from 0 to 21 and a score greater than or equal to 5 is indicative of poor sleep quality. The Insomnia Severity Index (ISI) identified evidence of clinically significant insomnia with scores ranging from 0 to 28. Scores between 8 and 14 are considered subclinical insomnia, while scores 15 or greater suggest moderate to severe insomnia [31]. Height and weight were also measured.
Patients underwent IVF stimulation per standard of clinical care and discretion of their reproductive endocrinologist after completion of the monitored sleep night and surveys. Additional variables from the IVF cycle, including protocol type, length of stimulation, total amount of gonadotropins required, day and type of trigger, peak estrogen levels, endometrial thickness and endometrial stripe pattern were noted. The type of transfer (fresh versus frozen), day 3 versus day 5 blastocyst transfer, number and grade of embryos transferred were recorded.
2.4. Outcome
The primary outcome of interest was clinical pregnancy after in vitro fertilization. Clinical pregnancy was defined as an intrauterine gestational sac with a yolk sac. Additional pregnancy outcomes including the initial level of human chorionic gonadotropin (HCG) measured 10 days after embryo transfer. Embryology outcomes including total number of oocytes retrieved at the time of ultrasound-guided oocyte retrieval, number of mature oocytes (MII), fertilization rate, number and grade of blastocysts were collected.
2.5. Statistical analysis
Participant baseline characteristics, IVF cycle parameters, embryology, and early pregnancy outcomes were compared between patients with and without exposure to sleep disordered breathing. Continuous variables were compared using Student’s t-test or Mann-Whitney U test as appropriate, while x2 or Fisher’s exact tests were used to compare categorical variables among those participants with or without sleep disordered breathing. The risk difference, unadjusted and adjusted risk ratio, and odds ratio were directly calculated.
Multivariable logistic regression was used to further evaluate the association between achievement of a clinical pregnancy and sleep disordered breathing, while adjusting for relevant confounders. The covariates in the final model included age. Other potential covariates were tested, including BMI, diagnosis of polycystic ovary syndrome, type of embryo transfer (fresh transfer performed immediately after an oocyte retrieval versus frozen transfer of a thawed cryopreserved embryo), day 3 versus day 5 blastocyst transfer, number and grade of embryos transferred, use of preimplantation genetic testing, and prior parity. Given the small sample size, our intention was to generate the most parsimonious model to avoid overfitting the regression model. None of the tested covariates meet our pre-determined threshold for inclusion in the analysis of changing the odds ratio by at least 10%.
All statistical analyses were performed with STATA version 15.1 (Stata-Corp).
3. Results
A total of 30 subjects were recruited from an academic fertility center between July 30th, 2020 to March 1st, 2021. Overall, the mean age was 33 years old (SD 4.6). The cohort was diverse with 77% self-identifying as White, 17% as Black and 7% as Asian. Overall, 40% of participants were diagnosed with unexplained infertility, 27% with polycystic ovary syndrome, 20% with male factor, and 7% with diminished ovarian reserve. All participants completed baseline surveys and the monitored sleep night, but one patient never had an embryo transfer.
The prevalence of sleep disordered breathing (AHI ≥ 5 events/hour) was 57% among the entire cohort with an average AHI of 8.7 (95% CI 6.02 to 11.4) events per hour. Among those with sleep disordered breathing, 37% (n = 11) had mild, 13% had moderate (n = 5) and 3% (n = 1) had severe SDB based on AHI cutoffs. Self-reported sleep pathology was also prevalent. Overall, 10% (n = 3) of participants reported an abnormal level of daytime somnolence, 63% (n = 19) reported poor sleep quality (PSQI score greater than or equal to 5), and 43% (n = 13) of participants had at least subclinical insomnia, while 7% (n = 2) had moderate to severe insomnia.
Baseline patient characteristics were compared between those women with and without SDB. Women with sleep disordered breathing (AHI ≥ 5 events/hour) were younger (31.4 vs 35.4 years old, p = 0.02) (Table 1). There was not a statistically significant difference in BMI between the two groups, though unexpectedly the mean BMI was lower in the group with sleep disordered breathing (28.0 vs 31.6 kg/m2, p = 0.17). Rates of hypertensive disorders, depression, anxiety, and current alcohol, tobacco, or vaping use did not differ between groups. Women with sleep disordered breathing were more likely to carry a primary infertility diagnosis of PCOS (41% vs 8%, p = 0.04). Antral follicle count was higher in the sleep disordered breathing group (26 vs 18, p = 0.04).
Table 1.
Patient and cycle characteristics compared between participants exposed and unexposed to sleep disordered breathing.
| Factor | Normal Breathing (AHI <5) | Sleep Disordered Breathing (AHI ≥5) | p-value |
|---|---|---|---|
| N | 13 | 17 | |
| Age, mean (SD) | 35.4 (5.1) | 31.4 (3.4) | 0.02 |
| BMI, mean (SD) | 31.6 (8.7) | 28.0 (5.3) | 0.17 |
| Race, n (%) | 0.41 | ||
| Asian | 0 (0%) | 2 (12%) | |
| Black/African American | 2 (15%) | 3 (18%) | |
| White | 11 (85%) | 12 (71%) | |
| Ethnicity, n (%) | 3 (23%) | 0 (0%) | 0.04 |
| Hispanic/Latino/Spanish origin | |||
| Primary Diagnosis | 0.21 | ||
| Diminished ovarian reserve | 1 (8%) | 1 (6%) | |
| Male factor | 2 (15%) | 4 (24%) | |
| Polycystic Ovary Syndrome | 1 (8%) | 7 (41%) | |
| Recurrent Pregnancy Loss | 1 (8%) | 0 (0%) | |
| Tubal factor | 1 (8%) | 0 (0%) | |
| Unexplained | 7 (54%) | 5 (29%) | |
| Polycystic Ovary Syndrome | 1 (8%) | 7 (41%) | 0.04 |
| Regular Cyclesa | 11 (85%) | 10 (59%) | 0.13 |
| Prior Parity, n (%) | 3 (23%) | 1 (6%) | 0.17 |
| Current Tobacco Use, n (%) | 0 | 1 (6%) | 0.37 |
| History of Tobacco Use, n (%) | 1 (8%) | 2 (12%) | 0.71 |
| Current Alcohol Use, n (%) | 5 (38%) | 5 (29%) | 0.60 |
| Number of Alcoholic Drinks per Week, n (%) | 0.23 | ||
| 0 | 8 (62%) | 12 (71%) | |
| 1 | 3 (23%) | 3 (18%) | |
| 2 | 2 (15%) | 0 (0%) | |
| 3+ | 0 (0%) | 2 (12%) | |
| Current Vape User, n (%) | 0 (0%) | 0 (0%) | |
| Anti-Mullerian Hormone, mean (SD) | 2.9 (1.9) | 4.5 (4.2) | 0.22 |
| Antral Follicle Count, mean (SD) | 18 (10) | 26 (11) | 0.04 |
| Cycle Numberb | 0.55 | ||
| 1 | 11 (85%) | 15 (88%) | |
| 2 | 1 (8%) | 1 (6%) | |
| 3 | 1 (8%) | 0 (0%) | |
| 4 | 0 (0%) | 1 (6%) | |
| Total Oocytesc, mean (SD) | 17 (10) | 14 (10) | 0.58 |
| Total Number of Metaphase II oocytesd, mean (SD) | 13 (8.6) | 12 (7.8) | 0.60 |
| Fertilization Ratee, mean (SD) | 0.90 (0.11) | 0.75 (0.27) | 0.08 |
| Total Embryos, mean (SD) | 8 (5.5) | 6 (5.6) | 0.24 |
| Proportion Undergoing an Embryo Transfer, n (%) | 11 (0.85) | 16 (0.94) | 0.39 |
| Fresh Transferf, n (%) | 9 (0.69) | 12 (0.71) | 0.94 |
| Day 3 Embryo Transfer, n (%) | 3 (0.23) | 3 (0.23) | 0.71 |
| Number of Embryos Transferred, mean (SD) | 1.12 (0.6) | 1.0 (0.63) | 0.46 |
| Preimplantation Genetic Testingg, n (%) | 1 (8%) | 1 (6%) | 0.84 |
Regular cycles defined as cycle length ranging between 21 and 35 days in length.
Cycle numbers refer to the IVF cycle performed, 1 indicating the patient’s first IVF cycle.
Total oocytes is the total number of oocytes obtained at the time of ultrasound-guided egg retrieval.
Metaphase II oocytes refers to the total number mature oocytes capable of being fertilized.
Fertilization rate refers to the total number of mature oocyte (Metaphase II oocytes) that successfully fertilize.
Fresh Transfer refers to an embryo transfer performed 5 days following oocyte retrieval of an embryo that was never cryopreserved.
Preimplantation genetic testing (PGT) is the pre-planned process of performing a multi-cellular biopsy of a day 5, 6, or 7 blastocyst-stage embryo to assess for chromosomal abnormalities.
Total days of stimulation, total amount of gonadotropins received, and peak estradiol did not differ between groups. Though not statistically significant, women with sleep disordered breathing on average had fewer oocytes retrieved, lower fertilization rates and fewer embryos following their cycle. There were no differences in the proportion of patients undergoing a day 3 or day 5 embryos transfer or total number of embryos transferred. Only 2 patients in the cohort utilized preimplantation genetic testing.
Sleep surveys mean score or proportion with abnormal scores did not differ significantly between the two groups (Table 2). The experimental sensors successfully collected high-quality data on all patients with total mean observation data points of 30,000. The average AHI in the sleep disordered breathing group was 13.4 events/hour (95% CI: 10 to 16.8) versus 2.7 events/hour (95% CI: 2.05 to 3.35) (p < 0.001).
Table 2.
Sleep survey results characterizing patient-reported symptoms of obstructive sleep apnea, excessive daytime somnolence, poor sleep quality and insomnia. Mean vital sign results from sleep monitoring night prior to IVF.
| Factor | Normal Breathing (AHI <5) | Sleep Disordered Breathing (AHI ≥5) | p-value |
|---|---|---|---|
| N | 13 | 17 | |
| Apnea Hypopnea Index, mean (SD) | 2.7 (1.2) | 13.4 (7.1) | <0.001 |
| STOP Bang ≥3 | 2 (15%) | 3 (18%) | 0.87 |
| Abnormal Daytime Sleepiness (ESS >10) | 1 (8%) | 2 (12%) | 0.71 |
| Poor Sleep Quality (PSQI ≥ 5) | 7 (54%) | 12 (71%) | 0.35 |
| Subclinical Insomnia (ISI ≥8) | 4 (31%) | 9 (53%) | 0.22 |
| Total sleep time (mins), mean (SD) | 533.5 (93.6) | 479.0 (92.8) | 0.12 |
| SpO2 (%; mean), mean (SD) | 93.9 (2.0) | 92.7 (3.7) | 0.31 |
| Heart Rate (bpm), mean (SD) | 63 (5.6) | 66 (7.0) | 0.30 |
| Heart Rate Variabilitya, mean (SD) | 69.6 (84.1) | 56.4 (35.7) | 0.59 |
| Respiratory Rate (rpm), mean (SD) | 17 (2.1) | 17 (2.3) | 0.90 |
| Pulse Transit Timeb (ms), mean (SD) | 217.3 (20.9) | 208.2 (17.8) | 0.23 |
| Perfusion Indexc (PI %), mean (SD) | 5.6 (1.7) | 3.5 (1.8) | 0.005 |
| Chest Temperature (degrees Celsius), mean (SD) | 35.2 (0.55) | 35.1 (0.44) | 0.74 |
HRV was generated by determination of the root mean square of successive differences between normal heartbeats was found by determination of the successive time differences between heartbeats in milliseconds [34].
Pulse Transit Time is the time for the pulse to travel from the heart to the index finger, detected through time synchronization of the chest and limb sensors, which provides a surrogate for systolic blood pressure [21,28].
Perfusion index was determined by the ratio of pulsatile blood flow to non-pulsatile blood flow as measured at the index finger [35].
Among the 29 patients undergoing an embryo transfer, a smaller proportion of women with sleep disordered breathing achieved a clinical pregnancy (35% vs 58%, p = 0.22) (Table 3). When further stratifying by normal (AHI <5 events/hour), mild (5–15 events/hour), and moderate to severe (at least 15 events/hour), the proportion of patients achieving a clinical pregnancy was 58% (n = 7 of 12) versus 36% (n = 4 of 11) versus 33% (n = 2 of 6) respectively.
Table 3.
This table demonstrates the differential cycle outcomes following embryo transfer after a single IVF cycle among participants with and without sleep disordered breathing. 1 of the original participants has not yet had an embryo transfer.
| Factor | Normal Breathing (AHI <5) | Sleep Disordered Breathing (AHI ≥5) | p-value |
|---|---|---|---|
| N | 13 | 17 | |
| Cycle Outcome | 0.28 | ||
| Not Pregnant | 5 (38%) | 9 (53%) | |
| Biochemical Pregnancy | 0 (0%) | 2 (12%) | |
| Clinical Pregnancy | 7 (54%) | 6 (38%) | |
| No Transfer | 1 (8%) | 0 (0%) | |
| Clinical Pregnancy (of those completing an embryo transfer) | 7 (58%) | 6 (38%) | 0.18 |
| Hypertensive Disorder of Pregnancya | 3 (43%) | 2 (33%) | 0.92 |
| Preterm Deliveryb | 0 (0%) | 2 (33%) | 0.12 |
| Live Birth | 7 (58%) | 6 (38%) | 0.24 |
Hypertensive disorders of pregnancy include gestational hypertension and preeclampsia.
Preterm delivery is defined as delivery prior to 37 weeks gestational age.
The overall unadjusted risk difference of achieving a clinical pregnancy in the setting of concurrent sleep disordered breathing was −0.23 (95% CI: −0.59, 0.13). The unadjusted risk ratio was 0.60 (95% CI: 0.27–1.35) and the odds ratio was 0.39 (95% CI: 0.09 to 1.78). Logistic regression was used to further characterize the relationship between rate of achieving a clinical pregnancy and sleep disordered breathing. After adjusting for age, sleep disordered breathing was not significantly associated with likelihood of achieving a clinical pregnancy, adjusted OR of 0.23 (95% CI: 0.04–1.5, p = 0.12).
All patients achieving a clinical pregnancy went on to have a live birth. Overall, 5 pregnancies were complicated by hypertensive disorders of pregnancy and 2 patients delivered prematurely.
Sleep disordered breathing was more commonly encountered among patients with PCOS (87.5% versus 45.5%, p = 0.04), but PCOS was not associated with reduced pregnancy rates—thus not meeting the statistical criteria for a confounder. Even when stratifying the sample based on PCOS diagnosis and adjusting for it in the regression model, the association of adverse outcome with SDB exposure persisted independent of PCOS (Mantel-Haenszel Test: OR 0.34 (95% CI: 0.06–2.00, 2 = 1.58, p = 0.47 and test of homogeneity 2 = 0.53, p = 0.21).
The sensor system scored favorably among users with the average SUS score reported as 75.4 (SD 14). A score over 68 is considered above average [32].
4. Discussion
This is the first prospective study to investigate the relationship between SDB and early pregnancy outcomes in an IVF population. Overall, this study suggests a high rate of self-reported and objective evidence of sleep pathology in this patient population. The presence of SDB was high in this cohort—57% (n = 17 out of 30 subjects studied). This pilot study provides preliminary evidence that SDB of any severity is a negative risk factor for clinical pregnancy with a possible trend towards worsening pregnancy outcomes with more severe SDB. This effect appears to be potentially large with the likelihood of achieving a clinical pregnancy reduced by 84% with SDB of any severity when adjusted for the age. Additionally, though PCOS appeared to be a risk factor for the presence of sleep disordered breathing, abnormal breathing remained detrimental independent of underlying fertility diagnosis. This is particularly critical because even among patients with ovulatory dysfunction known to have an increased risk of SDB, expert consensus currently does not recommend treatment for SDB if the patient is asymptomatic [33]. Studies that demonstrate a potential adverse impact on early pregnancy could challenge this recommendation.
An unexpected observation of the cohort was that age and BMI were lower in the group of patients with SDB. This finding is possibly driven by the patients in the cohort with PCOS, who may be accessing reproductive care at a younger age due to their underlying diagnosis and have higher rates of SDB independent of BMI. These findings underscore the need for future research in the reproductive age population to challenge historically common risk factors (older age and elevated BMI), that may be less relevant among patients with other predisposing conditions, such as the hyperandrogenism of PCOS.
SDB may be associated with adverse early pregnancy rates given increases in stress, fatigue, overnight hypoxemia and systemic inflammation [3]. Future studies will be required to further explore the phenotype of infertility patients with sleep disordered breathing to facilitate expedited diagnosis and treatment. Additionally, understanding the implications of preexisting dysfunctional breathing on the course of fertility treatments and pregnancy will be critical. More extensive preliminary studies will inform future interventional, randomized controlled trials, to study if treating SDB with either intraoral devices or continuous positive airway pressure, improves clinical pregnancy rates and pregnancy outcomes. These studies will also help to determine an age-specific, and population-derived pathogenic level of sleep disordered breathing for fertility. It remains to be understood if an AHI of greater than or equal to 5 events per hour is the most appropriate cut off for reproductive age individuals attempting pregnancy, or if there is a different threshold, or dose-dependent relationship between dysfunctional breathing, lower fertility rates, and adverse pregnancy outcomes.
Furthermore, we demonstrate the utility of advanced wearable sensor technology that enables sleep diagnosis in a patient’s home setting reducing access barriers and mitigating potential COVID-19 exposure. The unique design of the experimental system allows for mechanical deformation with natural body movement and lower skin contact stress enabling high fidelity monitoring and comfort [19]. In addition, the low-profile nature and placement of the experimental chest unit mounted on the suprasternal notch allows for prone sleeping positions unlike most existing home sleep testing systems with large central processing units affixed to the front of the chest. The ability of the experimental system to allow for more natural sleeping positions and automatically determine body position over a sleep night may reflect a more accurate assessment of AHI that patients may be more willing to use.
There are several limitations that should be discussed. First, this study was not designed to find a hypothesized effect on pregnancy rate and the sample size was small. However, an association was demonstrated. The strength of the association can be assessed and confirmed in future larger studies. Additionally, our study was a sample of convenience. The findings may not be generalizable to other populations. Our cohort was too small to assess variable outcomes in different subgroups. Additionally, the study was not powered to evaluate any potential interactions between insomnia, sleep disordered breathing, and the establishment of pregnancy which will be essential given high observed rates of subclinical insomnia. Further studies will be needed to confirm the presence of a differential association or effect modification based on underlying patient characteristics, such as infertility diagnosis, or race/ethnicity. Third, this is a single-site study which limits broader generalizability. However, the study did include a diverse patient population. The presented data do not address potential causality. The underlying mechanism and temporality of how disordered sleep may influence IVF success were not assessed and warrant additional exploration. Finally, while this sensor system is FDA-cleared for the diagnosis of SDB, its validation was performed in a patient population with an expected higher baseline risk of SDB and its positive predictive value may be reduced in a patient population with a lower underlying prevalence of disease, particularly given such a large proportion of patients demonstrating some evidence of SDB. In validation studies, the diagnostic performance of ANNE® Sleep compared to polysomnography for any evidence of SDB (AHI>5 events/hr) demonstrated a sensitivity of 96% (95% CI 92–99%) and a specificity of 86% (77%–92%). However, given the lack of studies of the underlying rates of SDB among infertile patients, it is possible that infertility is actually a signal of dysfunctional breathing and the true rate of SDB among infertile patients may be profoundly elevated, particularly those requiring the most aggressive available treatment of IVF.
Given the high prevalence of SDB in the infertile population and the limited number of modifiable risk factors to improve pregnancy outcomes in IVF, the ability to increase the chances for successful pregnancy by treating SDB via well accepted therapeutic modalities such as continuous positive airway pressure therapy is highly attractive. There is a need for larger prospective, inclusive cohort studies to confirm the findings of this pilot study. If proven, therapeutic interventional trials may demonstrate that treating SDB in this population will lead directly to improved pregnancy outcomes as well as long term maternal health benefits.
5. Conclusion
This single-arm, prospective, observational pilot study provides preliminary evidence that SDB is prevalent and may be a risk factor for treatment failure in an IVF population. By leveraging a novel sensor system, women undergoing IVF were able to successfully use the system overnight at home with a high degree of comfort. Thus, SDB may represent a new modifiable risk factor for infertility patients. The opportunity to intervene by treating SDB in this population may improve early pregnancy rates and IVF success warranting the need for larger confirmatory and interventional studies.
Funding
University of Pennsylvania Graduate and Professional Student Assembly (GAPSA) Provost Fellowship for Interdisciplinary Innovation Grant Award (JRW), NIH T32 Training Grant T32-HD007440 (JRW), NICHD 5K12HD050121-18 (JRW)
Footnotes
Credit author statement
JRW- conceptualization, methodology, investigation, data curation, project administration, data collection, data analysis, writing of original manuscript.
JYL/BS/JBP, DHK- data analysis, software.
SX-methodology, data analysis, critical revision of manuscript.
KB- study design, supervision, visualization, critical revision (review and editing) of the manuscript.
Declaration of competing ineterst
JRW: Spouse with equity interest in company commercializing technology
JYL, JBP, DHK: Sibel Health employees have interest in the company commercializing technology
SX: Equity interest in company commercializing technology. Royalty interest in patents associated with technology
References
- [1].Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5(2):136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril 2018;110(5):794–809. [DOI] [PubMed] [Google Scholar]
- [3].Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA 2020;323(14):1389–400. [DOI] [PubMed] [Google Scholar]
- [4].Bonsignore MR, Baiamonte P, Mazzuca E, Castrogiovanni A, Marrone O. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med 2019;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 2008;177(4):369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shaw JE, Punjabi NM, Wilding JP, et al. Sleep-disordered breathing and type 2 diabetes: a report from the international diabetes federation taskforce on epidemiology and prevention. Diabetes Res Clin Pract 2008;81(1):2–12. [DOI] [PubMed] [Google Scholar]
- [7].Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med 2009;5(6):573–81. [PMC free article] [PubMed] [Google Scholar]
- [8].Fung AM, Wilson DL, Lappas M, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One 2013;8(7):e68057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bublitz MH, Monteiro JF, Caraganis A, et al. Obstructive sleep apnea in gestational diabetes: a pilot study of the role of the hypothalamic-pituitary-adrenal Axis. J Clin Sleep Med 2018;14(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tachibana N, Ayas NT, White DP. A quantitative assessment of sleep laboratory activity in the United States. J Clin Sleep Med 2005;1(1):23–6. [PubMed] [Google Scholar]
- [11].Patel SR, Donovan LM. The COVID-19 pandemic presents an opportunity to reassess the value of polysomnography. Am J Respir Crit Care Med 2020;202(3):309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007;3(7):737–47. [PMC free article] [PubMed] [Google Scholar]
- [13].Ken He RK, Kapur Vishesh K. Home- vs. Laboratory-based management of OSA: an economic review. Current Sleep Medicine Reports 2016;2:107–223. [Google Scholar]
- [14].Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep 2011;34(6):695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rundo JV, Downey 3rd R. Polysomnography. Handb Clin Neurol. 2019;160:381–92. [DOI] [PubMed] [Google Scholar]
- [16].Golpe R, Jimenez A, Carpizo R. Home sleep studies in the assessment of sleep apnea/hypopnea syndrome. Chest 2002;122(4):1156–61. [DOI] [PubMed] [Google Scholar]
- [17].He KM, Atwood M, W C. Comparison of sleep specialist vs primary care driven home sleep apnea testing in routine practice. SLEEP 2020;43:A231. [Google Scholar]
- [18].Chung HU, Kim BH, Lee JY, et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019;363(6430). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chung HU, Rwei AY, Hourlier-Fargette A, et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat Med 2020;26(3):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu S, Jayaraman A, Rogers JA. Skin sensors are the future of health care. Nature 2019;571(7765):319–21. [DOI] [PubMed] [Google Scholar]
- [21].Ryu D, Kim DH, Price JT, et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc Natl Acad Sci U S A 2021;118(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JY, Kim Donghyun, Blake Stefanida, Kalluri Lakshmi, Walter Jessica, Davies Charles, Zee Phyllis, Xu Shuai, Power Thomas. Comparative study of wireless sensors versus type III home sleep apnea test for home-based diagnosis of obstructive sleep apnea. Sleep 2021;44(2). A160–A160. [Google Scholar]
- [23].Lee K, Ni X, Lee JY, et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat Biomed Eng 2020;4(2):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of sleep medicine. J Clin Sleep Med 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Charles Davies, Jessica Walter, Donghyun Kim, Lian Yu, Stefanida Blake, Lakshmi Kalluri, et al. A single arm, open-label, multi-center, and comparative study of the anne sleep system versus polysomnography to diagnose obstructive sleep apnea. J Clin Sleep Med 2022. In press, 10.5664/jcsm.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep 2006;29(3):367–74. [DOI] [PubMed] [Google Scholar]
- [27].Ma Y, Choi J, Hourlier-Fargette A, et al. Relation between blood pressure and pulse wave velocity for human arteries. Proc Natl Acad Sci U S A 2018;115(44):11144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu C, Kim JT, Kwak SS, et al. Wireless, skin-interfaced devices for pediatric critical care: application to continuous, noninvasive blood pressure monitoring. Adv Healthc Mater 2021;10(17):e2100383. [DOI] [PubMed] [Google Scholar]
- [29].Chung F, Abdullah HR, Liao P. STOP-bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149(3):631–8. [DOI] [PubMed] [Google Scholar]
- [30].Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- [31].Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- [32].Ni X, Ouyang W, Jeong H, et al. Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc Natl Acad Sci U S A 2021;118(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33(9):1602–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hales JR, Stephens FR, Fawcett AA, et al. Observations on a new non-invasive monitor of skin blood flow. Clin Exp Pharmacol Physiol 1989;16(5):403–15. [DOI] [PubMed] [Google Scholar]


