Abstract
A confluence of advances in biosensor technologies, enhancements in health care delivery mechanisms, and improvements in machine learning, together with an increased awareness of remote patient monitoring, has accelerated the impact of digital health across nearly every medical discipline. Medical grade wearables—noninvasive, on-body sensors operating with clinical accuracy—will play an increasingly central role in medicine by providing continuous, cost-effective measurement and interpretation of physiological data relevant to patient status and disease trajectory, both inside and outside of established health care settings. Here, we review current digital health technologies and highlight critical gaps to clinical translation and adoption.
INTRODUCTION
Digital health encompasses mobile health, health information technology, wearable devices, and telemedicine. Advances in technology and data analytics and improvements in health care delivery mechanisms have ushered in a new era of digital health. Health data are being collected in the home at exponential rates with a rapidly expanding array of connected devices. Artificial intelligence (1) synthesizes these large datasets to guide personalized clinical decision-making, with potential for predictive value. New payment models are now reimbursing health care providers for many of these digital health services. Responses to managing the coronavirus disease 2019 (COVID-19) pandemic are further catalyzing this growth, contributing to a nearly 40-fold expansion of telemedicine adoption compared to prepandemic levels (2). Broader public health interest in wearables (noninvasive, body-worn sensors) for population-level monitoring is also accelerating. Commercialization interest has followed, with government, philanthropic, industry, and venture capital investments in digital health growing in parallel.
Essential to the success of digital health solutions is the collection, transmission, and interpretation of high-quality physiological data outside of established health care settings. Historically, health care providers have captured a relatively narrow suite of data related to key vital signs and certain other targeted parameters during acute episodes of illness or scheduled physical examinations. Wearables, originally marketed directly to consumers as fitness gadgets, are now evolving to function like medical devices by delivering accurate, multimodal capabilities to monitor and diagnose disease, continuously and with potential to engage broad classes of patients that would be otherwise difficult to reach. In parallel, prescription-only medical wearables, such as continuous glucose monitoring systems (CGMs) for diabetes management and chest-mounted electrocardiogram (ECG) patches for cardiac arrhythmia detection, are well established within the house of medicine.
For purposes of this review, we offer the following four key features that collectively define medical wearables, distinguishing them from consumer wellness devices and noncontact, implantable, or ingestible monitoring systems.
Feature 1: Intimate, noninvasive interface to the body that supports chemical, mechanical, thermal, electrical, and/or optical forms of coupling to measure a physiological parameter of clinical significance.
Feature 2: Capability for continuous, wireless monitoring over an extended period of time in both hospitals and non-clinical settings.
Feature 3: Potential to be distributed as a regulated medical device that can diagnose, treat, track, or prevent disease.
Feature 4: Interface with the broader connected health care ecosystem with data integration, interpretation, or clinical care.
This article summarizes the status of select digital health technologies, focusing on U.S. Food and Drug Administration (FDA)–cleared or CE-marked platforms, highlights critical gaps to clinical translation and adoption, describes emerging biosensing systems addressing these gaps, and presents a unifying outlook on the field. We emphasize systems providing clinical-grade biophysical and biochemical data for patient populations and conditions that offer the most compelling opportunities for digital health. We point interested readers toward existing comprehensive systematic reviews that detail subtypes of wearables and their applications (3–7).
MAINSTREAM MEDICAL WEARABLES
Biophysical sensors
Early fitness trackers (e.g., Nike+ Fuelband, Fitbit Flex, Jawbone UP, and BodyMedia armband) and smartwatches (Sony, Garmin, and Basis Health Tracker) attracted a primarily wellness-focused user base. The AppleWatch, introduced in late 2014, accelerated broader consumer adoption given its commercial success. Four years later, the AppleWatch obtained FDA clearance for ECG and arrhythmia detection. The most advanced smartwatches now include FDA-cleared sensors for both intermittent ECG and pulse oxygenation (Withings). Smart rings (e.g., Oura Ring, Movano Ring, and Bodimetrics Circul+), an evolution of these smartwatches, are also increasing in popularity. The wrist and finger are convenient and familiar mounting locations for devices marketed directly to consumers. We direct interested readers to detailed historical reviews exploring how consumer electronic innovations contributed to the current generation of consumer-oriented wearables for health (8, 9).
For prescription-only devices, chest-mounted devices enable remote cardiac telemetry and continuous ECG monitoring. Devices such as the Zio patch (iRhythm), ePatch (Biotelemetry/Philips), and BodyGuardian (Preventice/Boston Scientific) offer physicians unique options in cardiac electrophysiology given their prolonged detection periods (e.g., up to 2 weeks), greater patient comfort, and more sensitive arrhythmia detection capabilities compared to those of traditional Holter monitors (10). Many of these systems also measure respiratory rate through the ECG and plethysmograph outputs. Resulting in part from efforts to manage the COVID-19 pandemic, wireless, finger-clip pulse oximeters to detect low blood oxygenation, traditionally found only in acute care settings, are now more widespread including in the home, although their wearability for continuous monitoring is still impractical. Similarly, wireless temperature monitoring devices designed to mount in the axilla (e.g., TempTraq and Fever Scout) for measuring temperature are also generally available, although this is considered a lagging indicator of infection.
Biochemical sensors
Compared to the broad range of parameters measured by biophysical sensors, wearable biochemical sensors have a narrower repertoire. The most widespread biochemical sensor detects blood glucose concentration, a cornerstone of diabetes management. Although limited in biomarker scope and clinical applications, adoption of existing classes of biochemical sensors is rapidly growing. The prolific application of CGMs embodies the triumphs of translation of biochemical sensing technology with a clear clinical need. Most importantly, patients with CGMs achieved superior glycemic control compared to traditional glucometers (11). These results foreshadow the benefits of expanded biochemical sensing capabilities to additional markers, in form factors that encourage long-term patient use as broadly outlined in the following section.
Major translational gaps
Although wearable device technologies have improved over the past 15 years, there are many unaddressed translational gaps. Nearly 30% of users abandon smartwatches over time (12) because of motivational loss, routine disruption, perceived measurement inaccuracy, discomfort, and battery charging (13). Furthermore, skin intolerance of adhesives required for prescription medical wearables is an underappreciated limitation of current devices. More than 70% of CGM wearers experience a skin complication because of use (14, 15). In a large clinical trial of the Zio ECG patch used by older adults over a 2-week wear period, 38% reported itching, 9% indicated hindrance to daily activities, and 1.2% discontinued the study early because of skin intolerance (16). Skin intolerance is likely underreported in the literature given the high amounts of patient-reported problems in social media (17). In addition, underlying systemic or dermatological comorbidities, such as advanced age or atopic dermatitis, compound the risk for potential skin intolerance or injury. Thus, the continued growth and clinical impact of medical wearables will require advances to overcome persistent translational gaps in both measurement capabilities to drive broader utility and form factors and mounting strategies to improve patient tolerability.
Gaps in measurement capabilities
In hospitals, biophysical measurements quantifying hemodynamic, neurological, and respiratory health are traditionally captured by expensive medical equipment reliant on invasive intravascular catheters and probes wired to external hardware. Smartwatches and smart rings may offer convenience and user familiarity, but many key measurements reported are not sufficiently reliable or are entirely immeasurable from the wrist or finger. For instance, skin temperature measured on the extremities correlates poorly with core body temperature and is susceptible to ambient temperature fluctuations. High-quality respiratory signals such as tidal volume, effort, and obstruction are substantially more difficult to quantify from the extremity.
Chest-mounted patches address some of these shortcomings, but multilead ECG (e.g., 12-lead diagnostic systems) still typically requires wired connections to large base units. Other sensing modalities measuring electroencephalography (EEG), electrooculography, and brain oxygenation, each critical for the evaluation of neurological well-being, are also not well served by mainstream wearables. Although there are a growing number of home EEG systems to assess sleep quality, they do not offer clinical grade data outputs sufficient for acute clinical decision-making. Advanced motion kinematics, currently captured in gait laboratories with sophisticated cameras and force sensors to evaluate movement disorders and guide rehabilitation protocols, likewise lie outside of the capabilities of existing wearable technologies. Other biophysical measurements fundamental for critical care that represent translational gaps include continuous blood pressure, advanced hemodynamics (e.g., cardiac output, stroke volume, and systemic vascular resistance), core body temperature, lung sounds, end tidal carbon dioxide, and arterial tonometry.
As with the biophysical measurements highlighted above, biochemical assessments represent a cornerstone of diagnostic and therapeutic treatments in hospitals. Although CGMs are highly effective at measuring blood glucose, existing versions cannot track other blood chemistries (e.g., electrolytes, hemoglobin, and neutrophil count) or inflammatory markers (e.g., C-reactive protein) that are currently processed seamlessly at scales of millions of times per day by laboratory analysis of peripherally drawn blood samples.
In addition to well-established assays, because medical wearables generate vast digital libraries of data, they may facilitate future measurements of biophysical and biochemical biomarkers that hold clinical value but are not currently assessed even in hospitals. Quantitative measurements of vocal biomarkers, for example, yield insights into emotional well-being, social isolation, and cognitive decline. Swallowing disorders (i.e., dysphagia), often observed among aging adults, stroke survivors, and patients with Parkinson’s disease and Alzheimer’s dementia, can lead to aspiration pneumonia or death. Swallowing activity and dynamics are rarely quantified, and they are never measured continuously. Similarly, the skin itself holds important clinical information—pressure, elasticity, hydration, and near-surface flow of blood or cerebrospinal fluid—with broad implications for management and measurement of wound healing, dermatological disease, skin perfusion, and hydrocephalus shunt function. Unconventional opportunities in biochemical measurements include hormones (e.g., cortisol), micronutrients (e.g., vitamins, minerals), serum cytokines, and toxins (e.g., lead, arsenic), currently only ordered as specialized tests contingent on sufficient clinical suspicion. These and other gaps in measurement capabilities constrain the use of mainstream wearables to a select group of medical indications, namely, electrophysiology, endocrinology, and fitness.
Gaps in form factors
Form factors, the hardware specifications of devices, including their composite materials, size, shape, and weight, represent another key limitation. Existing designs of wearables interfaced to the body require straps, tapes, bands, textiles, penetrating pins, and/or adhesives. These form factors and body applications are not conducive for long-term wear beyond 1 to 2 weeks as required for a typical hospital admission or ECG monitoring session. They constrain choices in mounting locations and measurement capabilities, and contribute to poor user acceptance and comfort, resulting in abandonment.
The fundamental challenge is that conventional electronic systems are planar, rigid, and physically static, whereas the human body is curvilinear, soft, and dynamic as the skin naturally stretches, bends, and flexes from routine daily activities (18). This discordance requires affixation methods that lead to skin irritation and limit natural movements. This consideration is particularly important for vulnerable populations such as older adults and premature neonates, whose skin fragility is greatest and in whom injuries lead to serious morbidity such as infection and wounds (19, 20). In addition, the global prevalence of skin conditions such as atopic dermatitis is as high as 10%, which further complicates skin tolerability (21). Thus, future form factors should aspire to achieve mechanically imperceptible interfaces to the body, ideally through mimicry of human form and mechanics, facilitated by size miniaturization and weight reduction to ensure skin tolerability and adherence for extended use. These innovations may allow for the complete elimination of adhesives by coupling to the skin via van der Waals forces alone (22). When also leveraged to enable unique mounting locations, these new form factors will create unobtrusive modes for measuring broad classes of physiological data through expanded opportunities for optical, mechanical, acoustic, and electrochemical sensing.
Overcoming these translational gaps will require fundamental advances in materials science and systems engineering, enhanced capabilities in biophysical/chemical sensing, and unusual means for coupling to the body at anatomic regions that support these measurements. The following sections highlight emerging classes of wearables that represent some of the most recent efforts, organized by devices that support biophysical and biochemical modes of operation. Here, unconventional form factors enable sophisticated measurement capabilities, realized in part by unconventional schemes for body integration and by access to underexplored classes of biofluids (Fig. 1).
Fig. 1. Unique mounting locations.
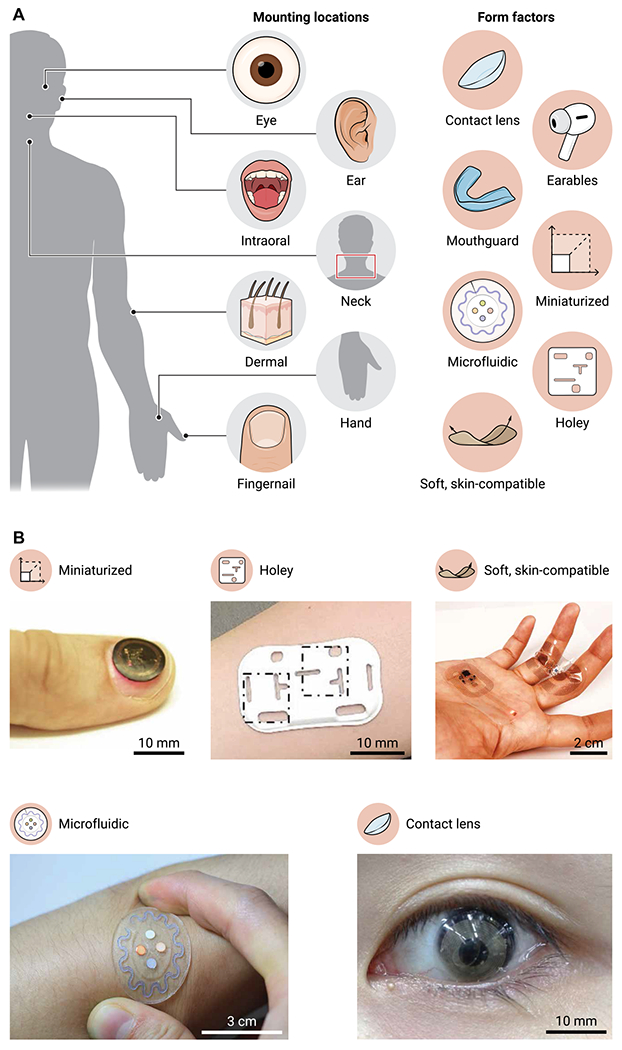
(A) An overview of emerging mounting locations and form factors for medical wearables. (B) Images of five representative form factors: a miniaturized device on a fingernail (13), a holey device on the skin (42), a binodal pair of skin-like devices on the hand, a soft microfluidic on the skin (17), and an instrumented contact lens on the eye (27). Photographs in (B) are reproduced with permission from (13, 17, 27, 42).
EMERGING WEARABLES
Biophysical sensors
Emerging biophysical sensors offer unique measurements and associated opportunities for clinical benefit. Advanced patches that mount on the chest capitalize on innovations in soft electronics, characterized by an ability to bend, stretch, and conform to the skin, for enhanced signal fidelity and reduced skin irritation for long-term wear. Several patches are FDA-cleared and commercially available (e.g., VitalConnect’s VitalPatch, Philip’s BX100, VivaLNK’s VV330 Continuous ECG Platform, and Sibel Health’s ANNE One) for continuous vital sign streaming in hospital settings that extend beyond storing ECG recordings. Newer miniaturized systems, although still rigid in nature, that can mount on the chest (e.g., BioIntellisense) or the arm (Current Health) are being increasingly used for remote patient monitoring. However, more validation on the value of continuous monitoring in both hospital and home settings needs to be done (5, 23).
As an important extension in cardiopulmonary monitoring, newer FDA-cleared or CE-marked systems provide continuous blood pressure measurements via nonoscillometric techniques (Caretaker Medical, Biobeat, and Aktiia) in smaller form factors compared to older systems (Sotera Wireless), although broader scale validation is still needed to ensure accuracy and performance compared to traditional blood pressure measurement techniques (24). Another FDA-cleared system, the CoVa wearable necklace (toSense/Baxter), noninvasively measures estimated stroke volume and cardiac output through thoracic impedance measurements. Emerging wrist-mounted wearables are providing measurements beyond pulse rate and activity to include more advanced clinical assessments. For instance, there is an FDA-cleared watch (Empatica) that can detect active generalized tonic-clonic seizures on the basis of electrodermal activity and motion outputs (25) and can even forecast seizures in the future using machine learning (25). Others have shown that outputs from wrist-mounted wearables can be used to correlate to clinical laboratory values such as hematocrit and blood glucose (26, 27).
Other emerging classes of soft, skin-interfaced wearables address measurement gaps in skin hydration to track barrier function, skin modulus for diagnostic and esthetic applications, and skin surface pressure to assess wound healing and prosthetic skin (28–33). Other unconventional applications use thermal sensing to quantify the near surface flow of cerebrospinal fluid for hydrocephalic patients with shunts (34) and blood for evaluation of peripheral circulation disorders, fistula, and skin graft viability. Monitoring of cerebral hemodynamics, systemic vascular resistance, vascular stiffness, and pulse wave velocities can be captured using skin-mounted spectroscopic sensors (29) or ultrathin arrays of ultrasound transducers (28). Soft devices that mount on the neck, the suprasternal notch, or the manubrium measure respiratory sounds, cycles of inspiration/expiration, swallowing events, vocal biomarkers, cardiac auscultations, and others.
In parallel to skin-like form factors, efforts in miniaturization, enhanced by options in power harvesting, wireless power transfer, and integration with clothing (e.g., Spire) and other body-mounted products, create additional possibilities. For instance, millimeter-scale, battery-free sensors placed on the fingernail, earlobe, or nasal ala can measure blood oxygenation and pulse rate (35). Related devices can be embedded in headphones, eyewear, and clothing for measurements of vital signs such as heart rate, respiratory rate, and physical activity.
Biochemical sensors
Many of these same advances in form factors and expanded choices in mounting locations also have implications for biochemical sensing. The latest CGMs analyze glucose concentration in interstitial fluid for diabetes management, thereby suggesting additional opportunities for validation and translation of assays of alternative biofluids (e.g., sweat, saliva, and tears) and new biomarker targets. Emerging biochemical systems in this context combine multifunctional sensing capabilities in soft and miniaturized designs with microfluidic handling capabilities (36). Parallel to these technology development efforts are important programs of research in establishing correlations between biomarker concentrations in these relatively underexplored but easily accessed biofluids to those in interstitial fluid or blood serum.
Wearable biochemical detection via sweat is the most mature of these options. Sweat emerges through micropores of the epidermis and, much like blood, contains multiple important biomarkers, including electrolytes (e.g., Na+, Cl−, K+, Zn2+, Fe2+, and Ca2+), metabolites (e.g., lactate, creatinine, glucose, alcohol, and urea), and small biomolecules (e.g., amino acids and microribonucleic acids) (15–18). Skin-interfaced platforms that sample and analyze sweat can support biochemical means for real-time tracking of health status, complementary to the biophysical options described in the preceding section. Sweat biomarker concentrations can diagnose and monitor disease progression in certain cases. For example, evaluations of chloride concentrations in cystic fibrosis patients represent the clinical standard for screening (37, 38). During physical activity, lactate concentration differentiates aerobic and anaerobic respiration (39). The latest platforms integrate intricate microfluidic channel networks and reservoirs to capture and route sweat from the epidermis to target areas within the device structure in a time sequential and programmable manner. Methods to initiate release of sweat include physical exercise, environmental exposure (e.g., warm water shower), or pharmacological induction (e.g., iontophoretic delivery of pilocarpine) (21, 22). Rates of sweating induced by these mechanisms span the entire physiological range (0.5 to 2 liters/hour), where volumes as small as 10 to 15 μl are sufficient for colorimetric or electrochemical analyses. Commercial sweat sensors in the form of thin, flexible microfluidic devices that incorporate colorimetric assays and integrate with the skin are now available for both consumer health (Gx Sweat Patch System, Epicore Biosystems) and medical applications (Discovery Patch Sweat Collection System, Epicore Biosystems). Notable additional developments are the integration of electrochemical sensing capabilities with wireless communications, on-board memory, power, data processing subsystems, and real-time alerts for applications in sports, industrial safety, and health care. Future advances include devices passively capturing and analyzing insensible sweat released without deliberate means of initiation (14). Last, trace or ultratrace concentrations (nanograms per milliliter or less) of biomarkers (e.g., vitamins, minerals, proteins, and hormones) are found in sweat with important health implications. Recent technical advances have shown the ability to measure vitamin C, levodopa, and cortisol with applications in sports science, nutrition, and stress monitoring (40–42). Future advances in sweat sensing with enzymatic, ion-selective matrices, or electrode capabilities will enable broader detection ranges, increased sensitivity and specificity, and corrections for variations in sweat flow, temperature, and pH.
Saliva, another readily accessible biofluid, also shares biochemical markers found in blood, such as exogenous drug concentrations, endogenous hormones, metabolites, proteins, and antibodies (43). Biosensing systems for salivary biomarker tracking that exist as sensors embedded in soft polymers shaped into mouth guards or pacifiers have some promise (44). A disadvantage is in measurement inaccuracies and sensor malfunctions that can follow from biofouling secondary to food or drink contamination. As a result, current platforms for saliva-based biochemical sensing are best suited for use in controlled athletic activity and clinical monitoring.
Tears offer another noninvasive option in biochemical sensing, given close concordance with blood across several biomarkers, including catecholamines and lysozymes (45). In addition, tears contain electrolytes (e.g., Na+, Cl−, and K+), metabolites (e.g., lactate, cortisol, and glucose), and proteins (e.g., antibodies, neuropeptides, and protective proteins) (46). Although tear sensing remains in the early stages of translation, the latest advances in instrumented contact lens development show promise for eye disease management, as well as a range of other applications, such as diabetes, glaucoma, and stress monitoring (47–49). In particular, recent work combining wireless power transfer antenna and near-field communication (NFC) chips on contact lens biosensors transported tear biomarkers response to a smartphone in real time (48). This device offered comfortable wearability through stretchable and transparent materials. In addition, human trials demonstrated safety of the contact lens platform. Although tear sensing remains in early stages, the contact lens platform has an advantage of continuous access to the biofluid without the need for induction or extraction compared with sweat biochemical sensors. Figure 2 summarizes emerging opportunities for measurements from medical wearables.
Fig. 2. Emerging applications of medical wearables.
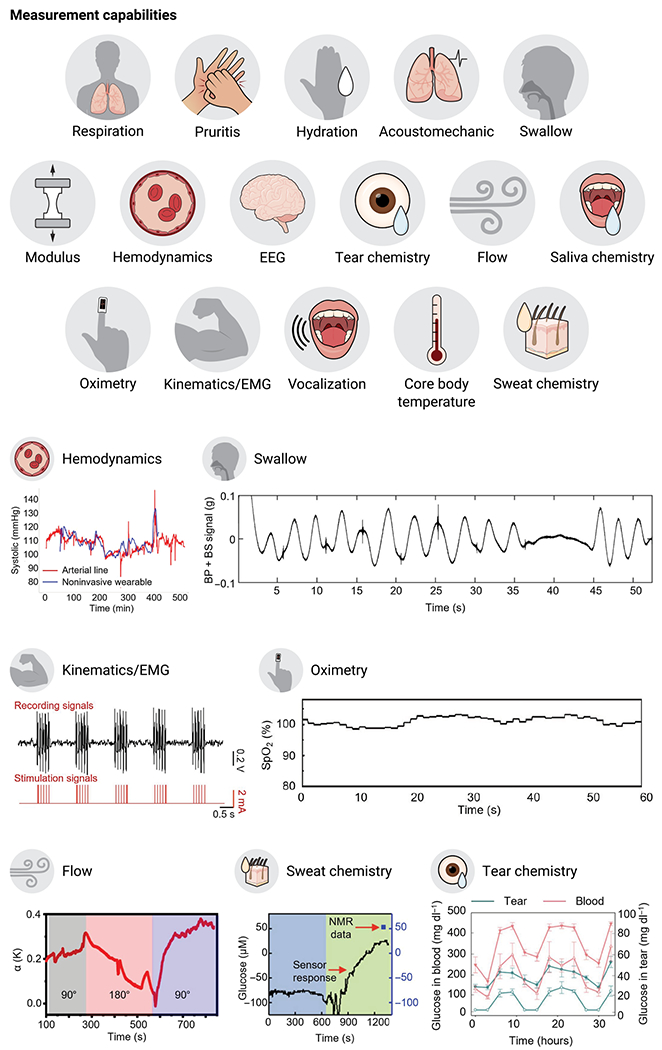
Representative data to illustrate measurement capabilities of clinical importance using emerging medical wearables: blood pressure, swallow activity, EMG signals (43), blood oxygen concentration (13), flow of cerebrospinal fluid (CSF) (12), glucose concentration in sweat (18), and glucose concentration in tear fluid (28). Graphs are reproduced with permission from (12, 13, 18, 28, 43).
AREAS OF OPPORTUNITY
Innovations like those described in the previous sections position emerging medical wearables to address some of the most urgent and compelling needs in health care at a population scale, in even the most resource-constrained regions of the globe. We highlight three such opportunities here: beginning of life, end of life, and the spread of infectious diseases.
Care of premature neonates represents an excellent example of the opportunities for wearables to improve health. Current monitoring systems adapted technologies originally developed for adults. When applied to neonates, this hardware often leads to iatrogenic skin injuries, constrains natural motions, frustrates clinical care, and prevents therapeutic skin-to-skin bonding between parent and child. After discharge, premature neonates face readmission rates as high as 30% in the first year of life due to respiratory infections (50), thereby highlighting a need for medical wearables to facilitate management in neonatal intensive care units and maintain a safe transition to the home. Recent work in this area demonstrates many of these benefits through soft, skin-like wireless technologies, deployed in both high- and low-resource settings. The results not only address requirements in monitoring traditional vital signs, without any of the drawbacks of conventional systems, but also offer additional capabilities in monitoring body motions, orientation, cardiac sounds, vocal biomarkers, pulse wave dynamics, and surrogates of blood pressure (22, 51).
A natural extension of beginning-of-life technologies is in skin-interfaced wireless platforms for tracking of health during pregnancy and childbirth. The United States has the highest rate of maternal mortality among industrialized countries (52), and two-thirds of these deaths are considered preventable given preceding quantifiable vital sign abnormalities (53). A recent FDA-approved abdominal band (INVU, Nuvo) monitors fetal and maternal heart rate for 5 min at a time in the home setting. A wireless network of three soft electronic devices offered by Sibel Health reproduces all of the monitoring capabilities available with wired systems used during childbirth in advanced hospital settings continuously. Studies of these devices with expecting mothers (n = 576) throughout the intrapartum period in high- and low-income countries demonstrate the ability of technologies to operate reliably, with high accuracy even in the most challenging settings (54).
Contributing to the urgency of these opportunities are data indicating that people in low-income countries, those in remote regions of the globe, or those subject to health care inequities bear the vast majority of the burden of neonatal and maternal morbidity and mortality (55). These patients lack access to the physical and data infrastructure to support traditional medical monitoring systems. Wearables thus offer the potential to improve health in these settings and address inherent inequities through broader accessibility, lower cost, and natural compatibility with ubiquitous mobile devices to support advanced monitoring functionality continuously, anywhere.
The second broad area of compelling need is the end of life, where medical wearables are designed for the unique needs of frail, older patients who are susceptible to skin injury and often have limited technical literacy. As with neonates, advances in form factor and function are essential, and capabilities can include but are not limited to monitoring of standard vital signs. For instance, dysphagia (difficulty swallowing), the most common cause of lethal aspiration pneumonia, affects more than 50% of nursing home residents (56). Wearables assessing swallow dynamics relative to the phase of the respiratory cycle could strongly benefit these patients. Furthermore, aphasia (difficulty speaking or understanding language) is common in this patient population, and loneliness is known to adversely affect mental and physical well-being. Investigational efforts with a single, soft mechanoacoustic sensor that mounts on the suprasternal notch and imposes minimal burden on the user demonstrate the ability to track vital signs, activity, falls, and vocalizations as a measure of social interaction and tool for rehabilitation of aphasia, without the confounding effects of ambient sounds (57). For interested readers, recent reviews focus on the unique challenges facing older adults in regard to wearables, from technical literacy to the lack of direct validation in the population (6).
A third urgent opportunity for medical wearables is use in population-scale tracking of the spread of infectious diseases to guide public health interventions, most prominently illustrated by challenges associated with the COVID-19 pandemic (58). Even years into the pandemic, supply chain, distribution, and manufacturing challenges have limited the availability of widespread and low-cost molecular tests (59)—this illustrates how medical wearables can serve as alternative diagnostic platforms as future pandemics will likely face similar testing challenges. Physiological data already being generated from millions of existing, commercially available wearables may be helpful as early warning systems for asymptomatic or presymptomatic infection at the scale of tens of thousands of individuals (60, 61). Extending beyond population surveillance, customized systems such as Tiger Tech’s COVID Plus Monitor and Empatica’s detection system Aria offer early warning alerts of COVID-19 infection. The COVID Plus Monitor uses two embedded photoplethysmography sensors and a probabilistic machine learning model to identify biomarkers in asymptomatic and afebrile individuals that may be associated with COVID-19 infection. The FDA granted Emergency Use Authorization to Tiger Tech on the basis of an n = 467 validation study in which the system demonstrated a positive percentage agreement of 98.6% and a negative percent agreement of 94.5% for asymptomatic COVID-19 infection (62). The Aria system derives pulse rate, pulse rate variability, skin temperature, and electrodermal activity (EDA) from a smartwatch to yield 94% sensitivity with an average of 2.6 days of early warning for COVID-19 infection (63). Beyond diagnostics, clinical studies indicate that home monitoring with a simple pulse oximeter reduces the risk of death for COVID-19–infected patients by allowing for earlier diagnosis and presentation to the hospital (64). For future pandemics, medical wearables will likely play a greater role in extending the availability of remote monitoring to reduce the burden of health care systems and act as a population-level surveillance network for outbreaks, with the potential to save lives and reduce adverse economic impact through early detection, strategic isolation, and individualized care escalation. We direct readers to additional comprehensive reviews on the use of consumer wearables for the COVID-19 pandemic (65).
ECOSYSTEM CHALLENGES
Continued proliferation of medical wearables depends on several factors beyond the translational gaps highlighted previously; these include (i) controls to ensure privacy and data protection, (ii) regulatory mechanisms that keep pace with technology advances, and (iii) large-scale clinical studies that prove cost savings and/or improved patient outcomes leading to meaningful reimbursement.
First, privacy and user permission controls must be comprehensive and transparent, allowing users to opt-in to terms and services easily. Historically, technology companies have a poor track record of protecting user data, suggesting the need for broader government oversight. Although the European Union has implemented the General Data Protection Regulation (GPDR) requirements that govern consumer data protection and privacy that applies to wearables, no such federal protections exist in the United States. Although the Protecting Personal Data Health Act (S. 1842) was introduced to the U.S. Senate in 2019 to create uniform standards for data sharing consent, set minimum security standards, and establish a national task force, it has not moved forward. Second, digital health has evolved as a discipline of medicine faster than any other specialty in history. Regulatory science must keep pace while also protecting public health and preventing the dissemination of unproven technologies. Within the past 3 years, the FDA has initiated multiple efforts including a digital health software precertification program, a new Digital Health Center of Excellence, and a new office of digital transformation. In addition, the FDA has exerted more authority on wearables marketed to consumers that blur the line between medical device and wellness tool, as in the case of a popular baby monitor (66, 67). Third, medical wearables must demonstrate clinical benefit via well-conducted, adequately powered trials in specific patient use cases to drive reimbursement by payors. In the case of extended ECG monitoring, reimbursement codes were essential to adoption by the medical establishment. However, recent reductions in reimbursement by regional Medicare administrators in 2021 have created uncertainty in the space. Ultimately, consistent and meaningful reimbursement justified by well-designed trials showing meaningful clinical benefit is essential to ensure both a cost-effective public health benefit and a supportive innovation ecosystem.
FUTURE OUTLOOK
Beyond monitoring and diagnostics, future wearables will combine important biophysical and biochemical parameters in clinically rational ways, offer therapeutic capabilities as stand-alone systems or as closed-loop wearable drug delivery platforms, and leverage methods to fuse multimodal and multinodal data streams to power new artificial intelligence and machine learning engines. The continuous, time-synchronized linking of biomechanical and biophysical measurements will allow for more predictive and personalized assessments of human health. For instance, patients with congestive heart failure will benefit from a future wearable that offers continuous thoracic impedance of cardiac output and serum biomarkers of fluid overload (e.g., B-type natriuretic peptide). Combination measurements may be able to better characterize difficult-to-quantify clinical symptoms such as stress (e.g., sweat cortisol and heart rate variability), pain, depression, anxiety, and inflammation. For nearterm therapeutic benefits, wearables will offer vibratory biofeedback, heat, or neurostimulation. Existing transcutaneous electrical nerve stimulation systems for pain (68) will continue to improve in form factor with an opportunity to deliver therapeutic stimulation in a closed-loop manner on the basis of electromyography (EMG), electroencephalogram, EDA, or other physiological aberrations (69). Closed-loop wearable drug delivery platforms may include sensors that track biochemical markers (e.g., micronutrients) and release transdermal vitamins in proportion to the quantity measured in real time. Other examples include wearables that measure continuous closed-loop hemodynamics and deliver pharmacologic agents transdermally (e.g., continuous blood pressure sensing and transdermal clonidine release). As an extension of CGMs and insulin pumps, more wearables will integrate with implantable drug delivery systems to manage a wider range of clinical use cases. In 2022, Johnson & Johnson announced FDA clearance of the world’s first drug-eluting contact lens for allergic eye itch. Future innovations may include closed-loop contact lenses that both track tear pH and cytokines and deliver therapy automatically. Last, rapid developments in data analytics, dominated in many cases by applications in consumer, automotive, and manufacturing sectors, will deliver predictive capabilities that extend far beyond what is possible today. For instance, emerging applications include alerts for silent cardiac arrhythmias hidden in normal sinus rhythms identified with deep learning (70). Ultimately, future medical wearables and the data insights they generate will operate within an ecosystem where remote monitoring, clinician engagement, telemedicine, and even therapy integrate seamlessly with the traditional health care system.
CONCLUSION
Medical wearables are rapidly emerging as one of the most exciting aspects of the future digital health space. Advances in engineering science, from unconventional hardware platforms to advanced sensor technologies and data analytics, will ideally produce devices that operate seamlessly and in a burden-free manner at any relevant anatomical location of the body, tailored to address specific patient needs and conditions, minimizing the risks of abandonment and skin irritation. Results will not only improve health outcomes and reduce costs but also address the needs of the most vulnerable populations in the most resource-constrained and remote areas. This digital health revolution, driven in a large part by a growing ecosystem of medical wearable technologies, will affect all aspects of medicine with transformative benefits, both planned and unplanned, to our global community.
Footnotes
Competing interests: J.A.R. is a co-founder and advisor to Sibel Health, Sonica, and Epicore Biosystems and holds patents associated with these companies.
REFERENCES AND NOTES
- 1.Kim J, Imani S, de Araujo WR, Warchall J, Valdes-Ramirez G, Paixao TR, Mercier PP, Wang J, Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron 74, 1061–1068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bestsennyy O, Gilbert G, Harris A, Rost J, “Telehealth: A quarter-trillion-dollar post-COVID-19 reality?” (McKinsey and Company, 2021). [Google Scholar]
- 3.Ray TR, Choi J, Bandodkar AJ, Krishnan S, Gutruf P, Tian L, Ghaffari R, Rogers JA, Bio-integrated wearable systems: A comprehensive review. Chem. Rev 119, 5461–5533 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Ghaffari R, Yang DS, Kim J, Mansour A, Wright JA Jr., Model JB, Wright DE, Rogers JA, Ray TR, State of sweat: Emerging wearable systems for real-time, noninvasive sweat sensing and analytics. ACS Sens. 6, 2787–2801 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leenen JPL, Leerentveld C, van Dijk JD, van Westreenen HL, Schoonhoven L, Patijn GA, Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: Systematic review. J. Med. Internet Res 22, e18636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alharbi M, Straiton N, Smith S, Neubeck L, Gallagher R, Data management and wearables in older adults: A systematic review. Maturitas 124, 100–110 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Zhang J, Xie Y, Gao F, Xu S, Wu X, Ye Z, Wearable health devices in health care: Narrative systematic review. JMIR Mhealth Uhealth 8, e18907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gretton M, Frans Pauwels P, Position, Navigation, and Timing Technologies in the 21st Century: Integrated Satellite Navigation, Sensor Systems, and Civil Applications, Volume 2, Morton YTJ, van Diggelen F, Spilker JJ Jr., Parkinson BW, Lo S, Gao G, Eds., Wearables (Wiley, 2020). [Google Scholar]
- 9.Ferrone A, Napier C, Menon C, Wearable technology to increase self-awareness of low back pain: A survey of technology needs among health care workers. Sensors (Basel) 21, 8412 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yenikomshian M, Jarvis J, Patton C, Yee C, Mortimer R, Birnbaum H, Topash M, Cardiac arrhythmia detection outcomes among patients monitored with the Zio patch system: A systematic literature review. Curr. Med. Res. Opin 35, 1659–1670 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Rodbard D, Continuous glucose monitoring: A review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol. Ther 19, S25–S37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre A, “User survey analysis: Wearables need to be more useful” (Gartner Research, 2016). [Google Scholar]
- 13.Attig C, Franke T, Abandonment of personal quantification: A review and empirical study investigating reasons for wearable activity tracking attrition. Comput. Hum. Behav 102, 223–237 (2020). [Google Scholar]
- 14.Berg AK, Olsen BS, Thyssen JP, Zachariae C, Simonsen AB, Pilgaard K, Svensson J, High frequencies of dermatological complications in children using insulin pumps or sensors. Pediatr. Diabetes 19, 733–740 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Berg AK, Norgaard K, Thyssen JP, Zachariae C, Hommel E, Rytter K, Svensson J, Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: A cross-sectional study. Diabetes Technol. Ther 20, 475–482 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, von Grunhagen D, McIntyre WF, Benz AP, Wong JA, Merali F, Henein S, Nichol C, Connolly SJ, Healey JS, Investigators S-A, Screening for atrial fibrillation in the older population: A randomized clinical trial. JAMA Cardiol. 6, 558–567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khatsenko K, Khin Y, Maibach H, Allergic contact dermatitis to components of wearable adhesive health devices. Dermatitis 31, 283–286 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Rogers JA, Electronics for the human body. JAMA 313, 561–562 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Blume-Peytavi U, Tan J, Tennstedt D, Boralevi F, Fabbrocini G, Torrelo A, Soares-Oliveira R, Haftek M, Rossi AB, Thouvenin MD, Mangold J, Galliano MF, Hernandez-Pigeon H, Aries MF, Rouvrais C, Bessou-Touya S, Duplan H, Castex-Rizzi N, Mengeaud V, Ferret PJ, Clouet E, Aroman MS, Carrasco C, Coutanceau C, Guiraud B, Boyal S, Herman A, Delga H, Biniek K, Dauskardt R, Fragility of epidermis in newborns, children and adolescents. J. Eur. Acad. Dermatol. Venereol 30Suppl 4, 3–56 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Katoh N, Tennstedt D, Abellan van Kan G, Saint Aroman M, Loir A, Bacqueville D, Duprat L, Guiraud B, Bessou-Touya S, Duplan H, Gerontodermatology: The fragility of the epidermis in older adults. J. Eur. Acad. Dermatol. Venereol 32(Suppl. 4), 1–20 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Hanifin JM, Reed ML, Eczema P, Impact Working G, A population-based survey of eczema prevalence in the United States. Dermatitis 18, 82–91 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Chung HU, Kim BH, Lee JY, Lee J, Xie Z, Ibler EM, Lee K, Banks A, Jeong JY, Kim J, Ogle C, Grande D, Yu Y, Jang H, Assem P, Ryu D, Kwak JW, Namkoong M, Park JB, Lee Y, Kim DH, Ryu A, Jeong J, You K, Ji B, Liu Z, Huo Q, Feng X, Deng Y, Xu Y, Jang KI, Kim J, Zhang Y, Ghaffari R, Rand CM, Schau M, Hamvas A, Weese-Mayer DE, Huang Y, Lee SM, Lee CH, Shanbhag NR, Paller AS, Xu S, Rogers JA, Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mecklai K, Smith N, Stern AD, Kramer DB, Remote patient monitoring—Overdue or overused? N. Engl. J. Med 384, 1384–1386 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Kario K, Management of hypertension in the digital era: Small wearable monitoring devices for remote blood pressure monitoring. Hypertension 76, 640–650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasseri M, Pal Attia T, Joseph B, Gregg NM, Nurse ES, Viana PF, Worrell G, Dumpelmann M, Richardson MP, Freestone DR, Brinkmann BH, Ambulatory seizure forecasting with a wrist-worn device using long-short term memory deep learning. Sci. Rep 11, 21935 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bent B, Cho PJ, Henriquez M, Wittmann A, Thacker C, Feinglos M, Crowley MJ, Dunn JP, Engineering digital biomarkers of interstitial glucose from noninvasive smartwatches. NPJ Digit. Med 4, 89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn J, Kidzinski L, Runge R, Witt D, Hicks JL, Schussler-Fiorenza Rose SM, Li X, Bahmani A, Delp SL, Hastie T, Snyder MP, Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med 27, 1105–1112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Li X, Hu H, Zhang L, Huang Z, Lin M, Zhang Z, Yin Z, Huang B, Gong H, Bhaskaran S, Gu Y, Makihata M, Guo Y, Lei Y, Chen Y, Wang C, Li Y, Zhang T, Chen Z, Pisano AP, Zhang L, Zhou Q, Xu S, Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng 2, 687–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rwei AY, Lu W, Wu C, Human K, Suen E, Franklin D, Fabiani M, Gratton G, Xie Z, Deng Y, Kwak SS, Li L, Gu C, Liu A, Rand CM, Stewart TM, Huang Y, Weese-Mayer DE, Rogers JA, A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl. Acad. Sci. U.S.A 117, 31674–31684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon K, Wang H, Lim J, Chun KS, Jang H, Yoo I, Wu D, Chen AJ, Gu CG, Lipschultz L, Kim JU, Kim J, Jeong H, Luan H, Park Y, Su CJ, Ishida Y, Madhvapathy SR, Ikoma A, Kwak JW, Yang DS, Banks A, Xu S, Huang Y, Chang JK, Rogers JA, Wireless, soft electronics for rapid, multisensor measurements of hydration levels in healthy and diseased skin. Proc. Natl. Acad. Sci. U.S.A 118, e2020398118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park Y, Kwon K, Kwak SS, Yang DS, Kwak JW, Luan H, Chung TS, Chun KS, Kim JU, Jang H, Ryu H, Jeong H, Won SM, Kang YJ, Zhang M, Pontes D, Kampmeier BR, Seo SH, Zhao J, Jung I, Huang Y, Xu S, Rogers JA, Wireless, skin-interfaced sensors for compression therapy. Sci. Adv 6, eabe1655 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iversen M, Monisha M, Agarwala S, Flexible, wearable and fully-printed smart patch for pH and hydration sensing in wounds. Int. J. Bioprint 8, 447 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao S, Myers A, Malhotra A, Lin F, Bozkurt A, Muth JF, Zhu Y, A wearable hydration sensor with conformal nanowire electrodes. Adv. Healthc. Mater 6, 1601159 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Krishnan SR, Arafa HM, Kwon K, Deng Y, Su CJ, Reeder JT, Freudman J, Stankiewicz I, Chen HM, Loza R, Mims M, Mims M, Lee K, Abecassis Z, Banks A, Ostojich D, Patel M, Wang H, Borekci K, Rosenow J, Tate M, Huang Y, Alden T, Potts MB, Ayer AB, Rogers JA, Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. NPJ Digit Med. 3, 29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Gutruf P, Chiarelli AM, Heo SY, Cho K, Xie Z, Banks A, Han S, Jang KI, Lee JW, Lee KT, Feng X, Huang Y, Fabiani M, Gratton G, Paik U, Rogers JA, Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater 27, 1604373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunmair J, Gotsmy M, Niederstaetter L, Neuditschko B, Bileck A, Slany A, Feuerstein ML, Langbauer C, Janker L, Zanghellini J, Meier-Menches SM, Gerner C, Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun 12, 5993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Han H, Naw HPP, Lammy AV, Goh CH, Boujday S, Steele TWJ, Real-time colorimetric hydration sensor for sport activities. Mater. Des 90, 1181–1185 (2016). [Google Scholar]
- 38.Ray TR, Ivanovic M, Curtis PM, Franklin D, Guventurk K, Jeang WJ, Chafetz J, Gaertner H, Young G, Rebollo S, Model JB, Lee SP, Ciraldo J, Reeder JT, Hourlier-Fargette A, Bandodkar AJ, Choi J, Aranyosi AJ, Ghaffari R, McColley SA, Haymond S, Rogers JA, Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med 13, eabd8109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamont LS, Sweat lactate secretion during exercise in relation to women’s aerobic capacity. J. Appl. Physiol. (1985) 62, 194–198 (1987). [DOI] [PubMed] [Google Scholar]
- 40.Cheng C, Li X, Xu G, Lu Y, Low SS, Liu G, Zhu L, Li C, Liu Q, Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens. Bioelectron 172, 112782 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Goud KY, Moonla C, Mishra RK, Yu C, Narayan R, Litvan I, Wang J, Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: Toward parkinson management. ACS Sens. 4, 2196–2204 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Wu Y, Luan H, Yang DS, Cho D, Kwak SS, Liu S, Ryu H, Ghaffari R, Rogers JA, A skin-interfaced, miniaturized microfluidic analysis and delivery system for colorimetric measurements of nutrients in sweat and supply of vitamins through the skin. Adv. Sci. (Weinh) 9, e2103331 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang A, Wang CP, Tu M, Wong DT, Oral biofluid biomarker research: Current status and emerging frontiers. Diagnostics (Basel) 6, 45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Valdes-Ramirez G, Bandodkar AJ, Jia W, Martinez AG, Ramirez J, Mercier P, Wang J, Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 139, 1632–1636 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Kim AR, Nodel MR, Pavlenko TA, Chesnokova NB, Yakhno NN, Ugrumov MV, Tear fluid catecholamines as biomarkers of the Parkinson’s disease: A clinical and experimental study. Acta Naturae 11, 99–103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Cha E, Park JU, Recent advances in smart contact lenses. Adv. Mater. Technol 5, 1900728 (2020). [Google Scholar]
- 47.Ku M, Kim J, Won JE, Kang W, Park YG, Park J, Lee JH, Cheon J, Lee HH, Park JU, Smart, soft contact lens for wireless immunosensing of cortisol. Sci. Adv 6, eabb2891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Park J, Park YG, Cha E, Ku M, An HS, Lee KP, Huh MI, Kim J, Kim TS, Kim DW, Kim HK, Park JU, A soft and transparent contact lens for the wireless quantitative monitoring of intraocular pressure. Nat. Biomed. Eng 5, 772–782 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Park J, Kim J, Kim SY, Cheong WH, Jang J, Park YG, Na K, Kim YT, Heo JH, Lee CY, Lee JH, Bien F, Park JU, Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv 4, eaap9841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Underwood MA, Danielsen B, Gilbert WM, Cost, causes and rates of rehospitalization of preterm infants. J. Perinatol 27, 614–619 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Chung HU, Rwei AY, Hourlier-Fargette A, Xu S, Lee K, Dunne EC, Xie Z, Liu C, Carlini A, Kim DH, Ryu D, Kulikova E, Cao J, Odland IC, Fields KB, Hopkins B, Banks A, Ogle C, Grande D, Park JB, Kim J, Irie M, Jang H, Lee J, Park Y, Kim J, Jo HH, Hahm H, Avila R, Xu Y, Namkoong M, Kwak JW, Suen E, Paulus MA, Kim RJ, Parsons BV, Human KA, Kim SS, Patel M, Reuther W, Kim HS, Lee SH, Leedle JD, Yun Y, Rigali S, Son T, Jung I, Arafa H, Soundararajan VR, Ollech A, Shukla A, Bradley A, Schau M, Rand CM, Marsillio LE, Harris ZL, Huang Y, Hamvas A, Paller AS, Weese-Mayer DE, Lee JY, Rogers JA, Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med 26, 418–429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Declercq E, Zephyrin L, “Maternal Mortality in the United States: A Primer” (The Commonwealth Fund, 2020). [Google Scholar]
- 53.Berg CJ, Harper MA, Atkinson SM, Bell EA, Brown HL, Hage ML, Mitra AG, Moise KJ Jr., Callaghan WM, Preventability of pregnancy-related deaths: Results of a state-wide review. Obstet. Gynecol 106, 1228–1234 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Ryu D, Kim DH, Price JT, Lee JY, Chung HU, Allen E, Walter JR, Jeong H, Cao J, Kulikova E, Abu-Zayed H, Lee R, Martell KL, Zhang M, Kampmeier BR, Hill M, Lee J, Kim E, Park Y, Jang H, Arafa H, Liu C, Chisembele M, Vwalika B, Sindano N, Spelke MB, Paller AS, Premkumar A, Grobman WA, Stringer JSA, Rogers JA, Xu S, Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc. Natl. Acad. Sci. U.S.A 118, 2100466118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trends in maternal mortality: 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization; 2019. [Google Scholar]
- 56.Park YH, Han HR, Oh BM, Lee J, Park JA, Yu SJ, Chang H, Prevalence and associated factors of dysphagia in nursing home residents. Geriatr. Nurs 34, 212–217 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Lee K, Ni X, Lee JY, Arafa H, Pe DJ, Xu S, Avila R, Irie M, Lee JH, Easterlin RL, Kim DH, Chung HU, Olabisi OO, Getaneh S, Chung E, Hill M, Bell J, Jang H, Liu C, Park JB, Kim J, Kim SB, Mehta S, Pharr M, Tzavelis A, Reeder JT, Huang I, Deng Y, Xie Z, Davies CR, Huang Y, Rogers JA, Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng 4, 148–158 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeong H, Rogers JA, Xu S, Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities. Sci. Adv 6, eabd4794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z, Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol 19, 171–183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quer G, Radin JM, Gadaleta M, Baca-Motes K, Ariniello L, Ramos E, Kheterpal V, Topol EJ, Steinhubl SR, Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med 27, 73–77 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Zargaran A, Radenkovic D, Theodoulou I, Paraboschi A, Trengrove C, Colville G, Arbane G, El-Boghdadly K, Nebbia G, Martinez-Nunez R, Greenough A, The COVID-19 early detection in doctors and healthcare workers. medRxiv 10.1101/2020.08.11.20172502 (2020). 10.1101/2020.08.11.20172502. [DOI] [Google Scholar]
- 62.Coronavirus (COVID-19) Update: FDA authorizes first machine learning-based screening device to identify certain biomarkers that may indicate COVID-19 infection (2021); https://www.empatica.com/blog/aura-and-care-receive-ce-mark-for-early-detection-of-covid-19-and-other-respiratory-infections.html, Date: 9 March 2021 (Accessed: 15 February 2022).
- 63.Empatica receives first of its kind European CE mark for early detection of COVID-19 (2021); https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-machine-learning-based-screening-device-identify, Date: 9 March 2021 (Accessed: 15 February 2022).
- 64.Nematswerani N, Collie S, Chen T, Cohen M, Champion J, Feldman C, Richards G, The impact of routine pulse oximetry use on outcomes in COVID-19-infected patients at increased risk of severe disease: A retrospective cohort analysis. SSRN: 10.2139/ssrn.3804758 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Channa A, Popescu N, Rehman Malik N, Managing COVID-19 global pandemic with high-tech consumer wearables: A comprehensive review, in 2020 12th International Congress on Ultra Modern Telecommunications and Control Systems and Workshops (ICUMT) (IEEE, 2020), pp. 222–228. [Google Scholar]
- 66.Bonafide CP, Localio AR, Ferro DF, Orenstein EW, Jamison DT, Lavanchy C, Foglia EE, Accuracy of pulse oximetry-based home baby monitors. JAMA 320, 717–719 (2018). [DOI] [PubMed] [Google Scholar]
- 67.FDA, Warning Letter Owlet Baby Care Inc. (FDA, 2021). [Google Scholar]
- 68.Vance CG, Dailey DL, Rakel BA, Sluka KA, Using TENS for pain control: The state of the evidence. Pain Manag. 4, 197–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y, Ling J, Yu L, Liu P, Jiang M, Closed-loop transcutaneous auricular vagal nerve stimulation: Current situation and future possibilities. Front. Hum. Neurosci 15, 785620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baek YS, Lee SC, Choi W, Kim DH, A new deep learning algorithm of 12-lead electrocardiogram for identifying atrial fibrillation during sinus rhythm. Sci. Rep 11, 12818 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


