In Brief
The authors compared changes in intellectual, adaptive, and quality-of-life (QOL) scores in children with craniopharyngioma who underwent treatment with gross-total resection (GTR) versus partial resection followed by radiotherapy (PR+RT). No significant differences in the trajectory of intellectual functioning or QOL were observed. However, patients treated with GTR exhibited significant improvements over time in overall adaptive behavior (p = 0.04) and conceptual skills (p = 0.01) compared with patients treated with PR+RT. These results provide new insight and evidence to guide the decision-making process in the management of craniopharyngiomas.
Keywords: craniopharyngioma, neurocognitive, quality of life, outcomes, pediatrics, tumor, surgical technique
ABBREVIATIONS : ABAS = Adaptive Behavior Assessment System; ABAS-II = ABAS, Second Edition; ABAS-3 = ABAS, Third Edition; DI = diabetes insipidus; GAC = General Adaptive Composite; GTR = gross-total resection; IMRT = intensity-modulated RT; PBT = proton beam therapy; PedsQL = Pediatric Quality of Life Inventory; PRI = Perceptual Reasoning Index; PR+RT = partial resection followed by RT; PRT = proton RT; PSI = Processing Speed Index; QOL = quality of life; RT = radiotherapy; VCI = Verbal Comprehension Index; WMI = Working Memory Index
Abstract
OBJECTIVE
The optimal management of pediatric craniopharyngioma patients remains controversial, shifting from radical resection (gross-total resection [GTR]) to a more conservative approach with partial resection/biopsy followed by radiotherapy (PR+RT). To the authors’ knowledge, no previous studies have compared neurocognitive and quality-of-life (QOL) outcomes between the two main treatments. In this study, the authors compared changes in intellectual, adaptive, and QOL scores in children treated for craniopharyngioma with GTR and those treated with PR+RT.
METHODS
Patients underwent annual neurocognitive and QOL evaluations for up to 10 years posttreatment, including the Full-Scale IQ, Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), and Processing Speed Index (PSI). Child- and parent-reported QOL scores and adaptive behavior in different domains were assessed. General linear mixed models were used to examine change in scores over time by treatment group with adjustment for significant covariates.
RESULTS
Scores from 43 patients treated between 2009 and 2019 (21 GTR, 22 PR+RT) were examined. Within the PR+RT group, 9 patients had intensity-modulated RT and 13 had proton beam therapy. The treatment groups were similar in sex (44% male) and age (median 7.3 years). There were no significant differences in the trajectory of intellectual functioning or QOL scale scores between the two groups. However, patients who underwent GTR exhibited significant improvement over time in overall adaptive behavior (p = 0.04) and conceptual skills (p = 0.01), which was not observed in patients treated with PR+RT.
CONCLUSIONS
Long-term pediatric craniopharyngioma survivors treated with GTR and PR+RT have similar intellectual function and QOL. Larger studies are needed to explore small but clinically significant differences between the two groups.
Craniopharyngioma is a tumor that arises from Rathke’s pouch and is typically located in the sellar, suprasellar, or parasellar region.1,2 Although craniopharyngiomas are histologically benign tumors and account for only 2%–5% of all pediatric intracranial tumors,3 these tumors are considered aggressive due to frequent recurrences and significant treatment morbidity.1,2 The proximity of craniopharyngioma to the pituitary stalk, hypothalamus, third ventricle, optic chiasm, optic nerves, and cerebral vasculature makes safe resection challenging. The optimal management of craniopharyngiomas remains controversial, shifting between gross-total resection (GTR) and a more conservative approach of partial resection followed by radiotherapy (PR+RT), with the rationale that similar tumor control rates can be achieved with lower morbidity.4–7
The acute side effects and long-term complications associated with each treatment option are well documented. Specifically, research has demonstrated changes in pituitary-hypothalamic axis function, visual outcomes, obesity risk, vasculopathy, and risk of second malignancies due to radiation.8–11
Neurobehavioral, social, and emotional impairments, with subsequent impact on quality of life (QOL), have been found in survivors of childhood craniopharyngioma.12,13 Numerous factors have been associated with these poor psychosocial and functional outcomes, such as hypothalamic involvement, diabetes insipidus (DI), hydrocephalus, number of recurrences, and socioeconomic status.14,15 Adult survivors of craniopharyngioma may have QOL scores similar to those of the general population, whereas up to 50% of childhood craniopharyngioma survivors have reported below-average QOL.15
Although numerous studies have addressed cognitive, behavioral, and QOL outcomes in children with craniopharyngioma,16–25 to our knowledge no studies have compared these outcomes by treatment type or intensity. Therefore, the aim of this study was to compare the intellectual, adaptive, and QOL outcomes in children treated for craniopharyngioma with GTR versus PR+RT.
Methods
Participants
With institutional review board approval, records were examined for pediatric brain tumor patients who met the following criteria: 1) treated for a craniopharyngioma brain tumor at Texas Children’s Hospital between 2001 and 2019, 2) ≤ 18 years of age at the time of treatment, 3) received either GTR alone or partial resection followed by focal radiotherapy (PR+RT), and 4) spoke English or Spanish. Partial resection was defined as any resection that did not result in complete removal of the tumor, from a subtotal resection to a biopsy. Patients who received RT received either intensity-modulated RT (IMRT) or proton RT (PRT). IMRT was used for patients until proton beam therapy (PBT) became available in 2006. The decision to transition from IMRT to PBT was largely made as an attempt to minimize the dose to normal surrounding brain tissue and preserve cognitive function in young children. The RT dose ranged from 50.4 to 54 Gy in 1.8-Gy fractions to the tumor, with a 0.5- to 1-cm margin (clinical target volume).
The data collection method has been previously described in detail in our prior publications.11 Clinical data collected included date of diagnosis, age, and sex. Recurrence was defined as any tumor progression on MRI determined by a neuroradiologist that necessitated further treatment. MRI findings that did not change or influence patient management were not classified as recurrence. Anterior pituitary dysfunction or just pituitary dysfunction was defined as the need for steroid, thyroid hormone, growth hormone, or gonadotropic hormone replacement. DI was determined by the need for regular replacement of antidiuretic hormone with desmopressin.
Measures
Intellectual, adaptive, and QOL scores were abstracted from performance-based, parent-reported, and child-reported measures administered as part of the clinical and research neurocognitive evaluation process. Serial neurocognitive surveillance is a standard clinical protocol for pediatric craniopharyngioma patients at our institution. Additionally, most patients treated after 2007 are enrolled in a larger prospective, longitudinal research study examining long-term neurocognitive and behavioral outcomes in survivors of pediatric brain tumor.
Intellectual Functioning
IQ scores were derived from the age-appropriate version of the Wechsler Scales of Intelligence26–30 (92.9%) or the Leiter International Performance Scale (7.1%).31 IQ score correlations across these tests range from 0.71 to 0.86. IQ scores provide a measure of global intellectual functioning. The Wechsler Scales of Intelligence also yield index scores. The Verbal Comprehension Index (VCI) measures verbal reasoning ability, the Perceptual Reasoning Index (PRI) measures nonverbal and fluid reasoning ability, the Working Memory Index (WMI) measures the ability to store and manipulate information in short-term memory, and the Processing Speed Index (PSI) measures the speed, efficiency, and accuracy of information processing. Age-normalized standard scores have a mean ± SD of 15, with lower scores indicating worse functioning. The Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V), does not generate a PRI score. At our request, NCS Pearson Inc. (publisher of the WISC-V) generated the norms for calculating PRI scores from WISC-V subtests to enable comparison of scores across versions. WISC-V PRI reliabilities ranged from 0.93 to 0.95 across ages 6–16 years.32
Adaptive Functioning
Adaptive functioning scores were derived from the Adaptive Behavior Assessment System, Second Edition (ABAS-II) or Third Edition (ABAS-3), Parent Form.33,34 The ABAS generates a General Adaptive Composite (GAC) score, which is a broad measure of overall adaptive functioning. Additionally, the measure provides scores that represent functioning in specific adaptive domains: conceptual (communication, functional academics, self-direction), social (social/leisure skills), and practical (self-care, home/school living, community use, health/safety). High correlations have been reported between ABAS-II and ABAS-3 GAC and domain scores (0.83–0.88). Age-normalized standard scores have a mean ± SD of 100 ± 15, with lower scores indicating worse functioning.
Quality of Life
Health-related QOL was assessed with the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scale.35 A parent proxy report was obtained from all patients, and a child report was obtained from patients ≥ 5 years of age. The Total Scale Score measures global QOL. Subscale scores measure QOL in specific domains: physical functioning, emotional functioning, social functioning, and school functioning. Average intraclass correlations between parent proxy and child reports across all ages ranged from 0.49 to 0.64.35 Scale scores ranged from 0 to 100, with lower scores indicating worse QOL.
Statistical Analyses
Summary statistics were stratified by treatment group (GTR vs PR+RT) and compared using Wilcoxon rank-sum or Fisher’s exact tests. A general linear mixed model was used to compare changes in intellectual, adaptive, and QOL scores between treatment groups over time. The model included fixed effects for the treatment group, time, and group-by-time interaction terms, as well as a random intercept and slope. The model was used to estimate the mean change per year (95% CI) by treatment group. Regression coefficients were assessed at the 0.05 level of significance (two-sided). In this pilot study, p values were not adjusted for multiple hypothesis testing, and the values for the comparison between both treatment modalities (GTR vs PR+RT) are for the time interaction in each group.
Results
We identified 63 eligible patients. Neurocognitive evaluation records were available for 43 patients (21 GTR, 22 PR+RT), resulting in an overall inclusion rate of 71.7%. Scores were unavailable for patients who were deceased (n = 1), refused the parent study (n = 3), or did not receive neurocognitive testing for reasons not specified in the medical chart (n = 13). There were no significant differences in treatment group, age at treatment, or sex between patients without scores and patients included in analyses. Most patients (95.3%) received the same IQ test across serial evaluations. There were no statistically significant differences between treatment groups for years of follow-up (GTR 3.9 ± 3.0, PR+RT 6.3 ± 5.6 years) or number of evaluations per patient (GTR: median 2, range [minimum–maximum] 1–8; PR+RT: median 2, range 1–7) (both p > 0.05). Individual patients had between 1 and 8 evaluations contributing scores to this study (median 2).
Within the PR+RT group, 9 patients underwent IMRT and 13 PBT. The GTR and PR+RT treatment groups were similar for sex (44% male), age at first evaluation (median 7.3 years), and length of follow-up from first evaluation (median 3.6 years). Among the baseline clinical features, the shunted hydrocephalus rate was found to be significantly higher in the PR+RT group (GTR 9.5%, PR+RT 59.1%, p < 0.001). The recurrence rate, time from treatment to evaluation, and tumor volume did not significantly differ between the treatment groups (Table 1). In addition, tumor volume did not have an effect for any of the domains within the cognitive and QOL outcomes.
TABLE 1.
Demographic and clinical characteristics by treatment group (n = 43)
| GTR (n = 21) | PR+RT (n = 22) | p Value | |
|---|---|---|---|
| Sex |
|
|
0.364 |
| Male |
11 (52.4) |
8 (36.4) |
|
| Female |
10 (47.6) |
14 (63.6) |
|
| Shunted hydrocephalus |
|
|
0.001 |
| No |
19 (90.5) |
9 (40.9) |
|
| Yes |
2 (9.5) |
13 (59.1) |
|
| Recurrence |
|
|
>0.999 |
| No |
14 (66.7) |
15 (68.2) |
|
| Yes |
7 (33.3) |
7 (31.8) |
|
| Pituitary dysfunction |
20 (95.2) |
15 (68.2) |
0.046 |
| DI |
19 (90.5) |
12 (54.5) |
0.016 |
| Hypothalamic obesity |
14 (66.7) |
14 (63.6) |
>0.999 |
| Age at 1st posttreatment evaluation, yrs |
6.4 (2.2 to 16.8) |
8.5 (3.8 to 16.4) |
0.177 |
| Time from treatment to 1st posttreatment evaluation, yrs |
0.5 (−0.6 to 4.0) |
0.3 (−0.6 to 4.2) |
0.448 |
| Tumor vol, ml | 39.0 (0.7 to 164.5) | 22.2 (0.3 to 119.7) | 0.209 |
Values are presented as number (%) of patients or median (range) unless otherwise indicated.
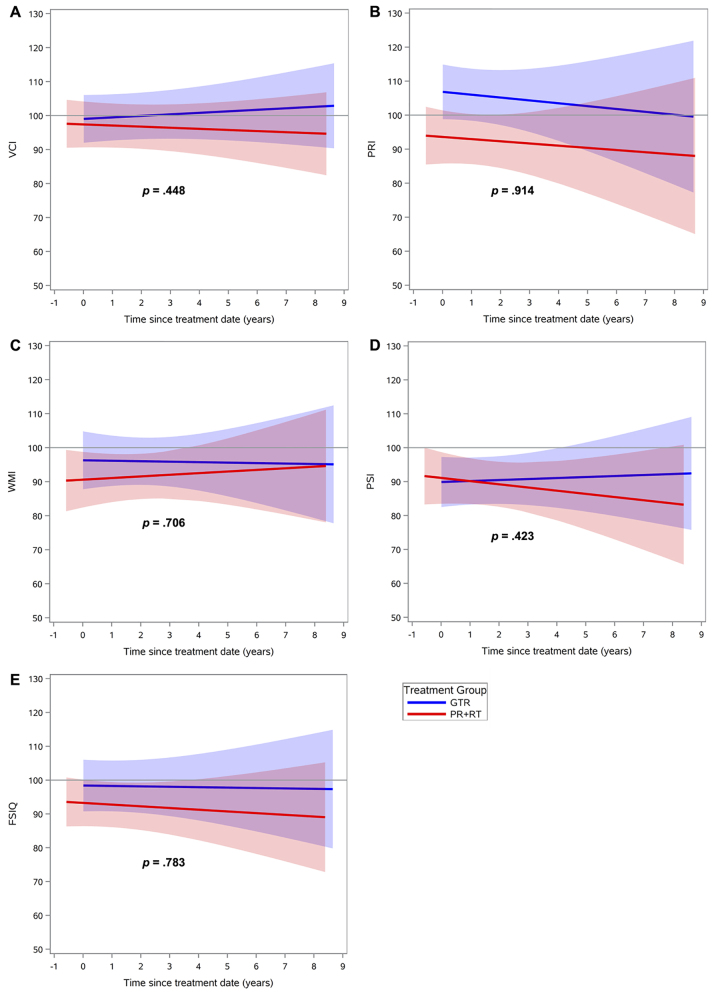
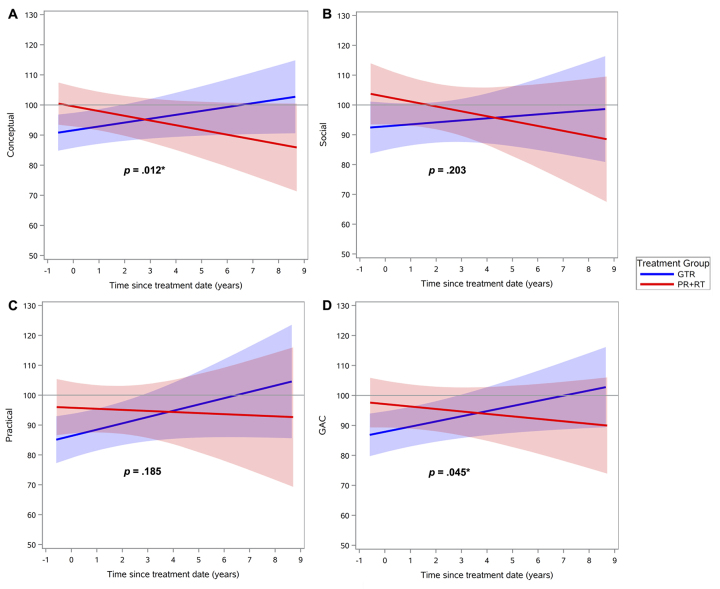
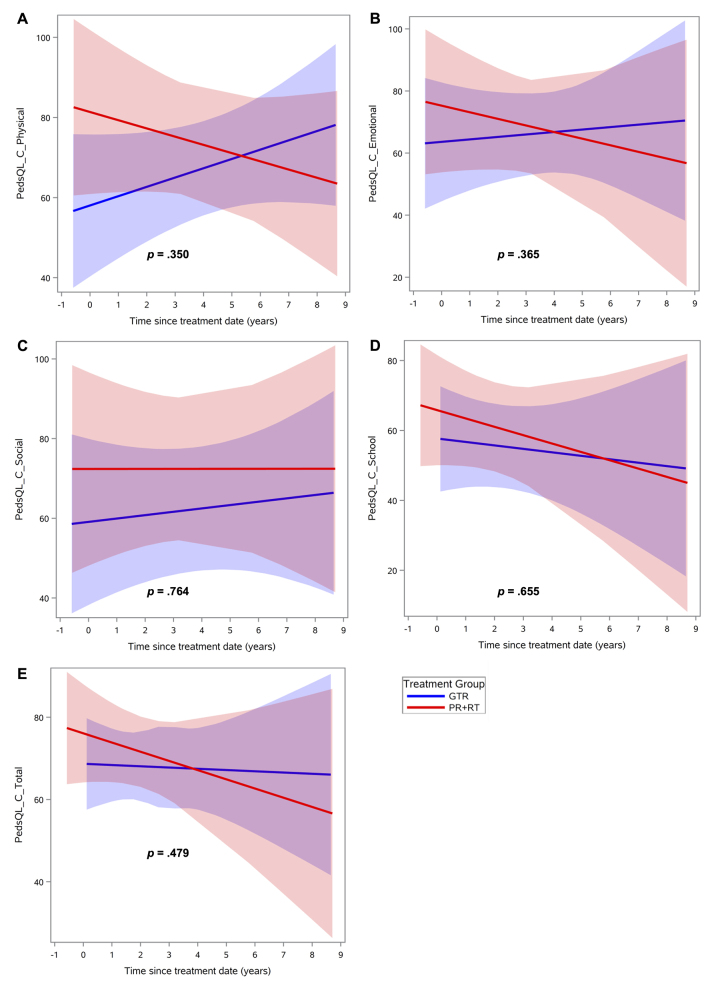
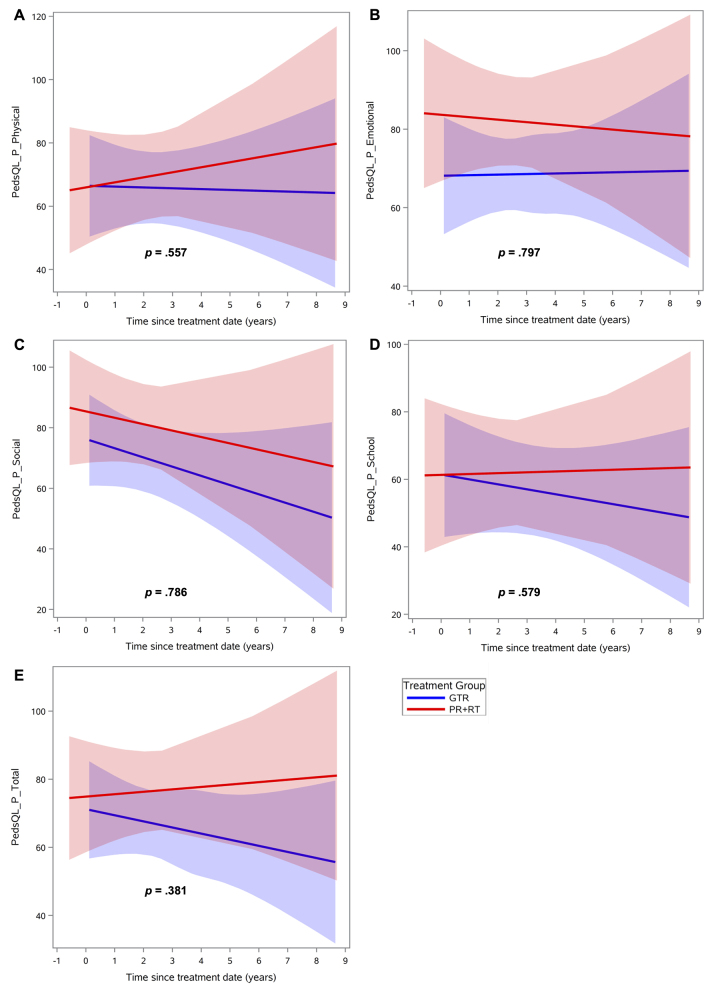
Regarding intellectual functioning, neither treatment group demonstrated a significant change over time on any index score (Fig. 1, Supplementary Table 1). Regarding adaptive behavior, the GTR group exhibited significant improvement over time in the general (p = 0.04) and conceptual (p = 0.01) domains compared with the PR+RT group (Fig. 2, Table 2). Social and practical domains were found to have no significant differences between the two groups. Finally, there were no significant differences in change of QOL scores over time, in both the global and specific domains, for parent or child reports (Figs. 3 and 4, Supplementary Table 2). Regarding the mean baseline scores for each domain, there were no significant differences in the mean baseline scores for any of the domains except for the PRI, for which the baseline score in the surgery group was significantly higher than the score in the radiation group (p = 0.02).
FIG. 1.
Neurocognitive scores over time after treatment, by treatment group: GTR versus PR+RT, in different domains: VCI (A), PRI (B), WMI (C), PSI (D), and Full-Scale IQ (FSIQ) (E). Lines indicate means and shaded areas 95% confidence intervals. Figure is available in color online only.
FIG. 2.
Adaptative behavior scores over time after treatment, by treatment group: GTR versus PR+RT, in different domains: conceptual (A), social (B), practical (C), and GAC (D). Lines indicate means and shaded areas 95% confidence intervals. *p < 0.05. Figure is available in color online only.
TABLE 2.
Linear mixed models of adaptive behavior change over time by treatment group and ABAS-3 composite score
| Estimate | 95% CI | p Value | |
|---|---|---|---|
| ABAS-3 conceptual |
|
|
|
| Slope: GTR |
1.29 |
−0.07 to 2.64 |
0.062 |
| Slope: PR+RT |
−1.57 |
−3.30 to 0.16 |
0.074 |
| Slope difference: GTR vs PR+RT |
2.86 |
0.66 to 5.05 |
0.012 |
| ABAS-3 social |
|
|
|
| Slope: GTR |
0.67 |
−1.74 to 3.08 |
0.553 |
| Slope: PR+RT |
−1.64 |
−4.47 to 1.20 |
0.236 |
| Slope difference: GTR vs PR+RT |
2.31 |
−1.41 to 6.03 |
0.203 |
| ABAS-3 practical |
|
|
|
| Slope: GTR |
2.11 |
−0.42 to 4.63 |
0.087 |
| Slope: PR+RT |
−0.36 |
−3.47 to 2.75 |
0.791 |
| Slope difference: GTR vs PR+RT |
2.47 |
−1.54 to 6.47 |
0.185 |
| ABAS-3 GAC |
|
|
|
| Slope: GTR |
1.72 |
0.18 to 3.27 |
0.030 |
| Slope: PR+RT |
−0.82 |
−2.77 to 1.12 |
0.401 |
| Slope difference: GTR vs PR+RT | 2.55 | 0.06 to 5.03 | 0.045 |
Estimates reported for each slope represent the increase/decrease in points per year on each ABAS-3 composite score by treatment group. Slope difference is the result of comparison of slopes between treatment groups on each ABAS-3 composite.
FIG. 3.
QOL scores over time after treatment, according to assessment by the child, by treatment group: GTR versus PR+RT, in different domains: physical (A), emotional (B), social (C), school (D), and total (E). Lines indicate means and shaded areas 95% confidence intervals. Figure is available in color online only.
FIG. 4.
QOL scores over time after treatment, according to assessment by the parent, by treatment group: GTR versus PR+RT, in different domains: physical (A), emotional (B), social (C), school (D), and total (E). Lines indicate means and shaded areas 95% confidence intervals. Figure is available in color online only.
Discussion
It is well known that children with craniopharyngioma are at risk for cognitive decline and poor QOL.16–25,36 In fact, one of the primary arguments used to support a conservative surgical approach with radiation (vs radical resection) is the intended preservation of cognitive and functional outcomes for these patients, especially by reducing the risk of hypothalamic injury. Currently, it is accepted that GTR offers the same event-free survival as radiation at 5 and even 10 years posttreatment, but with higher incidences of hypopituitarism and DI.7,9–11,37,38 Alternatively, radiation for craniopharyngioma also imparts risk, including moyamoya syndrome and second malignancies.11 To the best of our knowledge, this is the first study that presents a direct comparison of neurocognitive functioning, adaptive behavior, and QOL outcomes between children treated for craniopharyngioma with GTR and those treated with PR+RT.
In the present study, patients in the GTR and PR+RT groups presented similar outcomes in neurocognitive functioning. There were no statistically significant differences in any of the domains, even in those known to be particularly radiosensitive or that have been previously described as impaired in children with craniopharyngioma, such as VCI or PSI. In addition, cognitive functioning remained grossly stable over time with both modalities.17,22,36 Of note, examination of VCI slopes in our sample showed that the GTR and PR+RT group scores diverged over time, with an increasing trend in the GTR group and a declining trend in the PR+RT group. However, this divergence did not reach statistical significance. Overall, our findings are consistent with those of a previously reported study showing comparable neurocognitive outcomes among children with brain tumors treated with focal proton therapy or surgery without RT.39
Similarly, no statistically significant change over time was identified for QOL assessments in either treatment group. Even so, parent-reported QOL trended lower for overall QOL in the GTR group, a trend that appeared to be driven mostly by worsening social and school functioning. In fact, patients in both the GTR and PR+RT groups appeared to decline over time in parent-reported social functioning even though this trend did not reach statistical significance. This same pattern was not observed on child-reported QOL assessments. However, even though no statistically significant changes in functioning were identified over time for child-reported QOL scores, patients in the GTR group appeared to remain stable or improve in all domains, while patients in the PR+RT group declined in all domains except social functioning.
Adaptive functioning (in the general and conceptual domains) improved over time among patients treated with GTR, while functioning remained stable among patients treated with PR+RT. This finding indicates that patients who undergo surgery with GTR experience better global adaptive functioning and improved ability to communicate and direct their own behavior over time from this treatment. It seems possible that GTR imparts an initial impact on patient functioning, which improves over time as the brain recovers and undergoes functional reorganization. Although a statistically significant decline in adaptive functioning was not detected in the PR+RT group, examination of adaptive functioning slopes in this group was suggestive of adaptive decline. If, in fact, adaptive functioning decline was present in the PR+RT group, it is possible that the initial impact of RT on functioning is not as substantial as that with GTR, but there is a protracted, progressive impact on functioning that is unique to RT.
Importantly, the PR+RT group included patients treated with IMRT or with PRT. We have previously reported that for brain tumor treatment focal proton therapy may yield better cognitive outcomes than focal conventional RT with photons;38 however, no statistically significant differences in these outcomes were observed between IMRT and PRT groups in this study (data not shown). Given the small sample size and lack of differences by RT modality, we analyzed IMRT and proton patients together in the PR+RT group. Future studies with larger samples and longer follow-up are needed to evaluate whether protons are significantly superior to photons for the treatment of pediatric craniopharyngioma.
Study limitations must be considered, including the retrospective study design and small sample size. Additionally, pretreatment neurocognitive assessment was not available for this sample. Each case was discussed in a multidisciplinary conference to determine treatment strategy; usually, large tumors and tumors that cause hydrocephalus were more likely to be treated with a radical resection than with RT. Tumor resection addresses hydrocephalus in most cases, which was the reason why the shunted hydrocephalus rate was higher in the PR+RT group. Further, we did not find an effect of the tumor volume on the outcomes (data not shown), and even though the difference between groups for the mean tumor volume was not statistically significant, hydrocephalus at presentation and larger tumor volumes have been associated with poorer QOL outcomes15 and may have penalized outcomes in the surgery group. Additionally, partial resection is a broad category that includes a range of interventions from cyst decompression to near-total resection (NTR). Thus, the morbidity of NTR is certainly different from that for a conservative debulking and may have penalized outcomes in the radiation group. These differences could explain why the DI and pituitary dysfunction rates in the PR+RT group (54.5% and 68%, respectively) were higher than those in a previously reported series in which RT was used following very conservative surgery or biopsy.11
The main mechanism that has been accepted as the cause of the negative impacts on cognitive and QOL outcomes in craniopharyngioma is hypothalamic injury.4,20,21,36 Hypothalamic obesity is one of the first and main signs of hypothalamic injury.14,36,40 In the present study, the hypothalamic obesity rate was similar in both groups (GTR 66.6% vs PT+RT 63.6%). This result may explain why there were no differences in cognitive and QOL outcomes between these two groups in the present study. However, between diagnosis and late survivorship for children with craniopharyngioma, there are multiple factors that can impact cognitive development and QOL (e.g., hydrocephalus and subsequent shunt revisions, vision impairment, DI, ischemia secondary to moyamoya, radiation-related white matter disruption, recurrence, and tumor location). Thus, the incidence of visual dysfunction or moyamoya, for example, was too low for any meaningful analysis regarding their impact on cognitive or QOL outcomes in this sample. Additionally, information regarding interventions (e.g., speech/occupational/physical therapy) received by individual patients was not available for analysis. Larger prospective studies will help to adjust and control for confounding factors. One of the most important confounding factors associated with meningioma is the tumor location and its relationship with the stalk, hypothalamus, or third ventricle, but analysis of this variable in our recently published largest series of 63 patients did not show any significant influence on endocrine or visual outcomes. In retrospective studies, when the tumor features drive the treatment choice, the outcomes among the different treatment options are biased by the tumor location itself. Thus, the cognitive and QOL outcomes of an endoscopic endonasal approach for a purely sellar craniopharyngioma may be vastly different compared with the outcomes for a craniopharyngioma with hypothalamic involvement treated with RT. In this context, the relative contribution to outcomes of tumor location versus treatment modality remains unknown. This inherent selection bias in retrospective series is especially important in craniopharyngioma because it is a tumor with a heterogenous presentation. Despite these limitations, many that are inherent with studying a rare, heterogeneous disease in children, this study is to our knowledge the first to attempt to identify the optimal treatment approach for craniopharyngioma based on a comparison of outcomes that matter the most to these survivors, their families, and their physicians.
The shifting attitude in the management of craniopharyngiomas in the last few years toward a more conservative surgical approach should be assessed cautiously. In our series, the cognitive and QOL outcomes were similar between both groups, with some indication of improving adaptive functioning scores among GTR patients. Therefore, GTR may represent a valid alternative for a subset of children with craniopharyngiomas. This tumor represents one of the most difficult challenges we face in pediatric neurosurgery-neurooncology, and a multidisciplinary approach and individualized treatment, based on the features of the tumor as well as the personal and social circumstances of the patient, are key to achieving the best possible outcome. Additional research with larger samples and longer follow-up is needed and ongoing.
Conclusions
GTR and PR+RT treatments for craniopharyngioma present comparable cognitive and QOL outcomes in children with craniopharyngioma. However, in this study treatment with GTR presented a statistically significant improvement in aspects of adaptive functioning scores over time compared with treatment with radiation. Further prospective and larger studies are necessary to confirm these results.
Acknowledgments
This study was supported by NIH grants K07CA157923 (PI: Lisa S. Kahalley) and R01CA187202 (PI: Lisa S. Kahalley).
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Aldave, Okcu, Kahalley. Acquisition of data: Aldave, Chintagumpala, Ruggieri, Paulino, Kahalley. Analysis and interpretation of data: Aldave, Okcu, Chintagumpala, Minard, Paulino, McGovern, Ramaswamy, Kahalley. Drafting the article: Aldave, Minard, Mash, Kahalley. Critically revising the article: Aldave, Okcu, Chintagumpala, Minard, Malbari, Mash, Paulino, McGovern, Ramaswamy, Whitehead, Kahalley. Reviewed submitted version of manuscript: Aldave, Chintagumpala, Ruggieri, Minard, Malbari, Paulino, McGovern, Ramaswamy, Whitehead. Approved the final version of the manuscript on behalf of all authors: Aldave. Statistical analysis: Aldave, Minard. Administrative/technical/material support: Aldave, Ramaswamy, Kahalley. Study supervision: Aldave, Kahalley.
Supplemental Information
- Supplementary Tables 1 and 2. https://thejns.org/doi/suppl/10.3171/2022.12.PEDS22367.
Abstract Presentations
This work has been accepted as an oral presentation in the North American Skull Base Society Annual Meeting scheduled for February 17–19, 2023, in Tampa, Florida.
References
- 1. Clark AJ, Cage TA, Aranda D, et al. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst. 2013;29(2):231–238. doi: 10.1007/s00381-012-1926-2. [DOI] [PubMed] [Google Scholar]
- 2. Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. J Neurosurg. 1998;89(4):547–551. doi: 10.3171/jns.1998.89.4.0547. [DOI] [PubMed] [Google Scholar]
- 3. Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75. doi: 10.1038/s41572-019-0125-9. [DOI] [PubMed] [Google Scholar]
- 4. Müller HL. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm Res. 2008;69(4):193–202. doi: 10.1159/000113019. [DOI] [PubMed] [Google Scholar]
- 5. Merchant TE, Edmonston DY, Wu S, Li Y, Boop FA, Lustig RH. Endocrine outcomes after limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 protocol. Neuro Oncol. 2022;24(12):2210–2220. doi: 10.1093/neuonc/noac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edmonston DY, Wu S, Li Y, Khan RB, Boop FA, Merchant TE. Limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 protocol. Neuro Oncol. 2022;24(12):2200–2209. doi: 10.1093/neuonc/noac124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young M, Delaney A, Jurbergs N, et al. Radiotherapy alone for pediatric patients with craniopharyngioma. J Neurooncol. 2022;156(1):195–204. doi: 10.1007/s11060-021-03908-2. [DOI] [PubMed] [Google Scholar]
- 8. Fouda MA, Scott RM, Marcus KJ, et al. Sixty years single institutional experience with pediatric craniopharyngioma: between the past and the future. Childs Nerv Syst. 2020;36(2):291–296. doi: 10.1007/s00381-019-04294-x. [DOI] [PubMed] [Google Scholar]
- 9. Patel KS, Raza SM, McCoul ED, et al. Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg. 2015;123(3):571–580. doi: 10.3171/2014.12.JNS141591. [DOI] [PubMed] [Google Scholar]
- 10. Madsen PJ, Buch VP, Douglas JE, et al. Endoscopic endonasal resection versus open surgery for pediatric craniopharyngioma: comparison of outcomes and complications. J Neurosurg Pediatr. 2019;24(3):236–245. doi: 10.3171/2019.4.PEDS18612. [DOI] [PubMed] [Google Scholar]
- 11. Ravindra VM, Okcu MF, Ruggieri L, et al. Comparison of multimodal surgical and radiation treatment methods for pediatric craniopharyngioma: long-term analysis of progression-free survival and morbidity. J Neurosurg Pediatr. 2021;28(2):152–159. doi: 10.3171/2020.11.PEDS20803. [DOI] [PubMed] [Google Scholar]
- 12. Heinks K, Boekhoff S, Hoffmann A, et al. Quality of life and growth after childhood craniopharyngioma: results of the multinational trial KRANIOPHARYNGEOM 2007. Endocrine. 2018;59(2):364–372. doi: 10.1007/s12020-017-1489-9. [DOI] [PubMed] [Google Scholar]
- 13. Eveslage M, Calaminus G, Warmuth-Metz M, et al. The postoperative quality of life in children and adolescents with craniopharyngioma. Results of a prospective multicenter study. Dtsch Arztebl Int. 2019;116(18):321–328. doi: 10.3238/arztebl.2019.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poretti A, Grotzer MA, Ribi K, Schönle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol. 2004;46(4):220–229. doi: 10.1017/s0012162204000374. [DOI] [PubMed] [Google Scholar]
- 15. Zada G, Kintz N, Pulido M, Amezcua L. Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS One. 2013;8(11):e76562. doi: 10.1371/journal.pone.0076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pedreira CC, Stargatt R, Maroulis H, et al. Health related quality of life and psychological outcome in patients treated for craniopharyngioma in childhood. J Pediatr Endocrinol Metab. 2006;19(1):15–24. doi: 10.1515/jpem.2006.19.1.15. [DOI] [PubMed] [Google Scholar]
- 17. Fjalldal S, Holmer H, Rylander L, et al. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab. 2013;98(8):3253–3262. doi: 10.1210/jc.2013-2000. [DOI] [PubMed] [Google Scholar]
- 18. Fournier-Goodnight AS, Ashford JM, Merchant TE, et al. Neurocognitive functioning in pediatric craniopharyngioma: performance before treatment with proton therapy. J Neurooncol. 2017;134(1):97–105. doi: 10.1007/s11060-017-2492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laffond C, Dellatolas G, Alapetite C, et al. Quality-of-life, mood and executive functioning after childhood craniopharyngioma treated with surgery and proton beam therapy. Brain Inj. 2012;26(3):270–281. doi: 10.3109/02699052.2011.648709. [DOI] [PubMed] [Google Scholar]
- 20. Niel KA, Klages KL, Merchant TE, et al. Impact of sleep, neuroendocrine, and executive function on health-related quality of life in young people with craniopharyngioma. Dev Med Child Neurol. 2021;63(8):984–990. doi: 10.1111/dmcn.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Özyurt J, Müller HL, Thiel CM. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J Neurooncol. 2015;125(1):9–21. doi: 10.1007/s11060-015-1885-z. [DOI] [PubMed] [Google Scholar]
- 22. Özyurt J, Thiel CM, Lorenzen A, et al. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J Pediatr. 2014;164(4):876–881.e4. doi: 10.1016/j.jpeds.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 23. Peterson RK, Ashford JM, Scott SM, et al. Predicting parental distress among children newly diagnosed with craniopharyngioma. Pediatr Blood Cancer. 2018;65(10):e27287. doi: 10.1002/pbc.27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheetham T, Wood C. Quality of life in craniopharyngioma: sorting out cause from association. Dev Med Child Neurol. 2021;63(8):895. doi: 10.1111/dmcn.14902. [DOI] [PubMed] [Google Scholar]
- 25. Toumba M, Stanhope R. Quality of life and psychological outcome in patients treated for craniopharyngioma. J Pediatr Endocrinol Metab. 2006;19(1):11–13. doi: 10.1515/jpem.2006.19.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. 5th ed. NCS Pearson Inc; 2014. Intelligence Scale for Children. [Google Scholar]
- 27.Wechsler D. 4th ed. NCS Pearson Inc; 2012. Preschool and Primary Scale of Intelligence. [Google Scholar]
- 28.Wechsler D. 4th ed. NCS Pearson Inc; 2008. Adult Intelligence Scale. [Google Scholar]
- 29.Wechsler D. 4th ed. The Psychological Corporation; 2003. Intelligence Scale for Children. [Google Scholar]
- 30.Wechsler D. 3rd ed. Pearson; 2002. Preschool and Primary Scale of Intelligence. [Google Scholar]
- 31. Roid GH, Miller LJ. Leiter International Performance Scale-Revised (Leiter-R) Stoelting; 1997:10. [Google Scholar]
- 32.NCS Pearson Inc; 2014. Standardization Data from the Wechsler Intelligence Scale for Children (WISC-V) [Google Scholar]
- 33.Harrison PL, Oakland T. Western Psychological Services; 2015. ABAS-3. [Google Scholar]
- 34.Harrison PL, Oakland T. Adaptive Behavior Assessment System-II. Elsevier; 2008. ABAS-II assessment methods; pp. 37–49. [Google Scholar]
- 35. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otte A, Müller HL. Childhood-onset craniopharyngioma. J Clin Endocrinol Metab. 2021;106(10):e3820–e3836. doi: 10.1210/clinem/dgab397. [DOI] [PubMed] [Google Scholar]
- 37. Soldozy S, Yeghyayan M, Yağmurlu K, et al. Endoscopic endonasal surgery outcomes for pediatric craniopharyngioma: a systematic review. Neurosurg Focus. 2020;48(1):E6. doi: 10.3171/2019.10.FOCUS19728. [DOI] [PubMed] [Google Scholar]
- 38. Child AE, Warren EA, Grosshans DR, et al. Long-term cognitive and academic outcomes among pediatric brain tumor survivors treated with proton versus photon radiotherapy. Pediatr Blood Cancer. 2021;68(9):e29125. doi: 10.1002/pbc.29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kahalley LS, Douglas Ris M, Mahajan A, et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro Oncol. 2019;21(6):809–818. doi: 10.1093/neuonc/noz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fouda MA, Zurakowski D, Scott RM, et al. Novel predictive scoring system for morbid hypothalamic obesity in patients with pediatric craniopharyngioma. Childs Nerv Syst. 2021;37(2):403–410. doi: 10.1007/s00381-020-04877-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- Supplementary Tables 1 and 2. https://thejns.org/doi/suppl/10.3171/2022.12.PEDS22367.