In Brief
Surgeons in the Hydrocephalus Clinical Research Network implemented a simplified infection prevention protocol for shunt surgery. Compliance was 79.4% and nonprotocol factors were recorded. In 4913 procedures, the infection rate was 5.1%. The presence of = 2 complex chronic conditions and recent shunt surgery (within 12 weeks) were independent predictors of infection. The use of antibiotic-impregnated catheters and vancomycin irrigation were independent factors protective against shunt infection.
Keywords: cerebrospinal fluid, shunt infection, quality improvement, protocol, hydrocephalus, pediatric
ABBREVIATIONS : AIC = antibiotic-impregnated catheter, CCC = complex chronic condition, EVD = external ventricular drain, HCRN = Hydrocephalus Clinical Research Network, IV = intravenous, QI = quality improvement
Abstract
OBJECTIVE
Two previous Hydrocephalus Clinical Research Network (HCRN) studies have demonstrated that compliance with a standardized CSF shunt infection protocol reduces shunt infections. In this third iteration, a simplified protocol consisting of 5 steps was implemented. This analysis provides an updated evaluation of protocol compliance and evaluates modifiable shunt infection risk factors.
METHODS
The new simplified protocol was implemented at HCRN centers on November 1, 2016, for all shunt procedures, excluding external ventricular drains, ventricular reservoirs, and subgaleal shunts. Procedures performed through December 31, 2019, were included (38 months). Compliance with the protocol, use of antibiotic-impregnated catheters (AICs), and other variables of interest were collected at the index operation. Outcome events for a minimum of 6 months postoperatively were recorded. The definition of infection was unchanged from the authors’ previous report.
RESULTS
A total of 4913 procedures were performed at 13 HCRN centers. The overall infection rate was 5.1%. Surgeons were compliant with all 5 steps of the protocol in 79.4% of procedures. The infection rate for the protocol alone was 8.1% and dropped to 4.9% when AICs were added. Multivariate analysis identified having ≥ 2 complex chronic conditions (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.26–2.44, p = 0.01) and a history of prior shunt surgery within 12 weeks (OR 1.84, 95% CI 1.37–2.47, p < 0.01) as independent risk factors for shunt infection. The use of AICs (OR 0.70, 95% CI 0.50–0.97, p = 0.05) and vancomycin irrigation (OR 0.36, 95% CI 0.21–0.62, p < 0.01) were identified as independent factors protective against shunt infection.
CONCLUSIONS
The authors report the third iteration of their quality improvement protocol to reduce the risk of shunt infection. Compliance with the protocol was high. These updated data suggest that the incorporation of AICs is an important, modifiable infection prevention measure. Vancomycin irrigation was also identified as a protective factor but requires further study to better understand its role in preventing shunt infection.
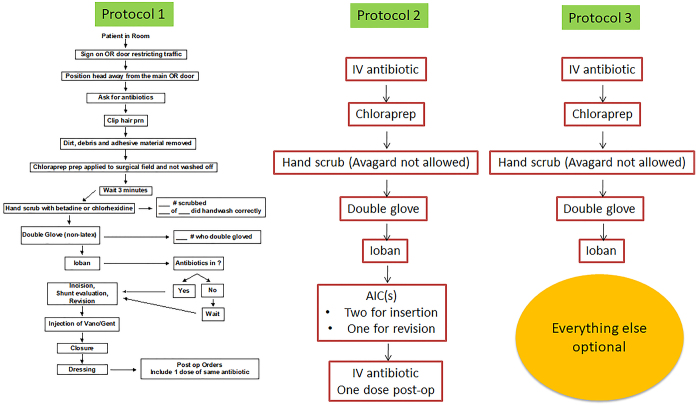
Pediatric CSF shunt infection remains a common complication and carries significant patient morbidity. Additionally, treatment for shunt infections is costly, with estimates as high as $50,000 per occurrence.1 The Hydrocephalus Clinical Research Network (HCRN) has adopted quality improvement (QI) initiatives and implemented standardized surgical protocols to reduce shunt infection. We first reported our 11-step protocol in 2011 (protocol 1; Fig. 1) and successfully reduced network-wide shunt infection from 8.8% to 5.7%.2 Our second report simplified the protocol to 5 core steps plus antibiotic-impregnated catheters (AICs) and postoperative antibiotics (protocol 2; Fig. 1). That protocol resulted in a similar infection rate as the original protocol.3 In this third iteration of the process, we chose to further simplify the protocol, using only the 5 core steps. Other interventions that were intended to reduce infection (including AICs) were recorded but not required. The purpose of the current analysis is to assess the impact of this simplified protocol on shunt infection rates and to identify other potentially modifiable risk factors for shunt infection.
FIG. 1.
HCRN shunt surgery protocols. The results associated with protocols 1 and 2 were described previously.2,3 Gent = gentamycin; OR = operating room; prn = as needed; Vanc = vancomycin. Figure is available in color online only.
Methods
HCRN
The HCRN is a collaborative group of 14 pediatric neurosurgical centers and 22 investigators conducting clinical research in pediatric hydrocephalus. Thirteen centers contributed data to this study. From its inception, the HCRN has used QI methodology in an effort to reduce shunt infection. Data collection at each site was approved by the local IRB. Data analysis was approved by the University of Utah IRB.
Five-Step Protocol
The 5-step protocol (protocol 3; Fig. 1) was implemented at the HCRN centers from November 1, 2016, through December 31, 2019. The 5 core steps of protocol 3 included intravenous (IV) antibiotic administration before incision, use of chlorhexidine as final preparation, hand scrub by all participants, double gloves by all participants, and application of an iodine-impregnated incision drape (Ioban, 3M) to the surgical field. Patients were monitored for at least 6 months after shunt insertion or revision. During this follow-up, any surgical and/or infectious events were recorded.
Study Sample
The entry and outcome criteria for the study were identical to those used previously.3 All children ≤ 18 years of age who underwent shunt insertion or revision operation at each HCRN center were entered into the study. Operations for ventriculoperitoneal, ventriculoatrial, ventriculopleural, arachnoid cyst, and subdural-peritoneal shunts were included. If a patient first presented with a shunt infection, they were entered into the study cohort at the time of shunt replacement after treatment of their infection. Children who were undergoing external ventricular drain (EVD), Ommaya reservoir, ventricular access device, or subgaleal shunt placement were not eligible for inclusion at the time of those procedures, but these children were eligible if they subsequently underwent a shunt procedure listed above.
Primary Endpoint
Each patient included in the study was evaluated for infection during routine clinical follow-up or if they visited the emergency room or were admitted to the hospital. Evaluation was performed according to the surgeons’ usual clinical practice. As in our previous study,3 the primary endpoint for the study was shunt infection. An identical definition for infection was used: 1) identification of organisms on culture or Gram stain from CSF, wound swab, or pseudocyst fluid; 2) shunt erosion, defined as wound breakdown with visible shunt hardware; 3) abdominal pseudocyst, even in the absence of positive cultures; or 4) positive blood cultures in a child with a ventriculoatrial shunt.3
Shunt Procedures
The shunt procedures were classified based on timing and presence of infection. The four classifications were 1) shunt insertion in a child who had not had one previously (insertion); 2) shunt revision in which a child entered the operating room with all previously implanted shunt equipment and left with all shunt equipment implanted (i.e., they had no externalized components or drains before or after surgery; revision); 3) shunt insertion after external ventricular drainage (not infected); and 4) shunt insertion after infection treatment. All repeat procedures were recorded regardless of type.
Protocol Compliance
Protocol compliance with all steps was assessed in a prospective manner by HCRN coordinators and the surgeons, fellows, and residents. Several surgeon-specific nonprotocol steps that might reduce infection were also recorded if performed. These steps included AICs, preoperative bathing/shampoo (with or without chlorhexidine products), chlorhexidine body wipes, skin preparation, intraoperative guidance (stereotaxis, ultrasound, endoscopy), changing the outer gloves after draping, using a "no-touch" technique in which the shunt equipment is only handled with surgical instruments, injection of antibiotics into the shunt, irrigating wounds before closure with bacitracin solution, and leaving bacitracin powder in the wounds. In addition, an open text field was used to collect "other steps intended to reduce infection."
Complex Chronic Conditions
The following patient complex chronic conditions (CCCs) were recorded: neuromuscular (brain and spinal cord malformations, mental retardation, CNS degeneration and disease, infantile cerebral palsy, muscular dystrophies and myopathies), cardiovascular (heart and great vessel malformations, cardiomyopathies, conduction disorders, dysrhythmias), respiratory (respiratory malformations, chronic respiratory disease, cystic fibrosis), renal (congenital anomalies, chronic renal failure), gastrointestinal (congenital anomalies, chronic liver disease and cirrhosis, inflammatory bowel disease), hematological or immunological (sickle cell disease, hereditary anemias or immunodeficiency, acquired immunodeficiency), metabolic (amino acid, carbohydrate, or lipid metabolism; storage disorders; other metabolic disorders), other congenital or genetic defect (chromosomal, bone and joint, diaphragm and abdominal wall, or other congenital anomalies), and malignancy (malignant neoplasms).4 Data were entered in the HCRN Registry using OpenClinica, a clinical trials management software. A "compliant" procedure was one in which all 5 protocol steps were performed.
At the time of data analysis, we identified one site and one surgeon at another site who consistently used a vancomycin solution as irrigation during the surgery or left it in the wound at the end of surgery. These participants had very low infection rates. We therefore contacted the HCRN principal investigator at each site to ask about the use of vancomycin irrigation at their site. Their responses were added to the data set for analysis.
Statistical Analysis
Infection rates were summarized with frequencies and proportions by shunt procedure type and by center. Protocol and nonprotocol infection risk factors were summarized by infection rate for compliant and noncompliant procedures. Univariate analysis of infection risk factors was conducted with Pearson chi-square tests. Risk factors showing an association of p < 0.2 in univariate analysis were entered into a logistic regression model that adjusted for within-patient correlation (patients often have multiple procedures) using generalized estimating equations. Two multivariate logistic regression models were constructed via backward stepwise selection using all potentially significant variables associated with shunt infection. In one model, the compliance category was simplified into a binary variable based on whether all 5 protocol steps were completed or not and was forced into the model. In a second model, the 5 protocol steps were considered individually for inclusion using a criterion of statistical significance (p ≤ 0.05 in the multivariable model). Statistical analysis was performed using SAS (version 9.4, SAS Institute).
Results
A total of 4913 shunt procedures were completed at 13 HCRN centers from November 1, 2016, through December 31, 2019. The most common procedure type was shunt revision (n = 2844, 57.9%), followed by shunt insertion (n = 1442, 29.4%), shunt insertion after infection (n = 432, 8.8%), and shunt insertion after EVD (n = 195, 4.0%; Table 1). The overall infection rate was 5.1% (n = 252), and there was no difference in infection rate among procedure types (p = 0.07). HCRN site-specific infection rates ranged from 2.2% to 9.3% (p ≤ 0.01; Table 2).
TABLE 1.
Procedure-specific infection rates
| Shunt Procedure Type | No. of Procedures | No. of Infections (%) |
|---|---|---|
| Insertion |
1442 |
63 (4.4) |
| Revision |
2844 |
145 (5.1) |
| Insertion after EVD (not infection) |
195 |
16 (8.2) |
| Insertion after infection |
432 |
28 (6.5) |
| Total | 4913 | 252 (5.1) |
TABLE 2.
Center-specific case volume and infection rates
| Center No. | Case Volume (n) | Infection Rate (%) |
|---|---|---|
| 1 |
86 |
9.3 |
| 2 |
279 |
3.6 |
| 3 |
315 |
3.8 |
| 4 |
455 |
6.6 |
| 5 |
465 |
7.1 |
| 6 |
308 |
7.8 |
| 7 |
330 |
5.8 |
| 8 |
608 |
4.3 |
| 9 |
116 |
5.2 |
| 10 |
281 |
6.4 |
| 11 |
652 |
4.3 |
| 12 |
561 |
5.0 |
| 13 |
457 |
2.2 |
| Overall | 4913 | 5.1 |
Surgical teams were compliant with all 5 steps of the protocol in 79.4% of procedures (Table 3). Compliance with individual protocol steps was variable. The use of chlorhexidine as final preparation had the lowest compliance rate (83.3%), while Ioban application and surgical scrubbing had the highest (98.8%). Additional nonprotocol factors were also recorded (Table 3). AICs were used in 81.3% of cases and were associated with an infection rate of 4.9%, compared with 6.3% when AICs were not used (p = 0.08). The use of vancomycin irrigation of wounds had a statistically significant effect on infection rate (2.2% compliant vs 5.7% noncompliant, p < 0.01), but it was only used in 15.7% of cases. Conversely, bacitracin irrigation of wounds had a significant and paradoxical increase in infection rate (5.4% compliant vs 3.0% noncompliant, p = 0.01). A similar negative effect was seen when vancomycin and gentamicin were injected into the shunt (7.1% compliant vs 4.9% noncompliant, p = 0.04).
TABLE 3.
Protocol and nonprotocol factor compliance
| Factor | Compliance Rate (%) | Infection Rate (%) |
p Value | |
|---|---|---|---|---|
| Compliant | Noncompliant | |||
| Protocol factors |
|
|
|
|
| IV antibiotics requested before incision |
97.8 |
5.1 |
4.7 |
0.83 |
| Chlorhexidine as final prep |
83.3 |
5.4 |
3.8 |
0.06 |
| All team members performed formal surgical scrub (no antiseptic cream) |
98.8 |
5.1 |
3.5 |
0.58 |
| All team members wore double gloves |
98.5 |
5.1 |
4.2 |
0.71 |
| Ioban applied |
98.8 |
5.2 |
1.7 |
0.23 |
| All 5 steps completed |
79.4 |
5.4 |
4.0 |
0.06 |
| Additional nonprotocol factors |
|
|
|
|
| AIC |
81.3 |
4.9 |
6.3 |
0.08 |
| Vancomycin & gentamicin injected into shunt |
10.0 |
7.1 |
4.9 |
0.04 |
| Outer gloves changed after draping |
87.4 |
5.0 |
6.1 |
0.26 |
| No touch technique |
30.1 |
5.9 |
4.9 |
0.15 |
| Bacitracin irrigation of wounds |
87.1 |
5.4 |
3.0 |
0.01 |
| Vancomycin irrigation of wounds | 15.7 | 2.2 | 5.7 | <0.01 |
Univariate analysis of additional risk factors is shown in Table 4. Compliance category, number of CCCs, and prior shunt surgery were found to be statistically significant. Within compliance category, completion of the 5 steps alone yielded an infection rate of 8.1%. The addition of vancomycin and gentamicin did not significantly change the infection rate (7.8%). The infection rate of 5 steps with the addition of AICs was 4.9%, a relative risk reduction of approximately 40% when compared with the 5 steps alone. The lowest infection rate was observed when the 5 steps were used in combination with AICs and vancomycin wound irrigation (2.0%).
TABLE 4.
Univariate analysis of additional risk factors
| Risk Factor | No. of Procedures | No. of Infections (%) | p Value |
|---|---|---|---|
| Compliance category |
|
|
<0.01 |
| 5 steps alone |
443 |
36 (8.1) |
|
| 5 steps + vancomycin & gentamicin |
218 |
17 (7.8) |
|
| 5 steps + AIC |
2760 |
136 (4.9) |
|
| 5 steps + vancomycin, gentamicin, & AIC |
185 |
17 (9.2) |
|
| 5 steps + AIC & vancomycin irrigation |
297 |
6 (2.0) |
|
| Other combination |
1010 |
40 (4.0) |
|
| CCCs |
|
|
<0.01 |
| 0 |
2693 |
117 (4.3) |
|
| 1 |
1505 |
78 (5.2) |
|
| ≥2 |
715 |
57 (8.0) |
|
| Corrected age at time of procedure |
|
|
0.66 |
| <0 wks |
352 |
19 (5.4) |
|
| 0 to <4 wks |
11 |
0 (0.0) |
|
| 4 to <26 wks |
39 |
4 (10.3) |
|
| 26 to <52 wks |
38 |
2 (5.3) |
|
| 52 to <104 wks |
63 |
2 (3.2) |
|
| ≥104 wks |
4377 |
224 (5.1) |
|
| Unknown |
33 |
1 (3.0) |
|
| Cause of hydrocephalus |
|
|
0.98 |
| Post-infectious |
186 |
10 (5.4) |
|
| Post-IVH secondary to prematurity |
1365 |
76 (5.6) |
|
| Myelomeningocele |
682 |
29 (4.3) |
|
| Aqueductal stenosis |
300 |
15 (5.0) |
|
| Spontaneous ICH/IVH/SAH |
161 |
9 (5.6) |
|
| Posterior fossa tumor |
267 |
12 (4.5) |
|
| Supratentorial tumor |
286 |
15 (5.2) |
|
| Midbrain tumor or other midbrain lesion |
93 |
4 (4.3) |
|
| Post-head injury |
148 |
7 (4.7) |
|
| Encephalocele |
57 |
3 (5.3) |
|
| Posterior fossa cyst, including Dandy-Walker |
171 |
13 (7.6) |
|
| Other intracranial cyst (e.g., arachnoid cyst) |
137 |
6 (4.4) |
|
| Communicating congenital hydrocephalus |
207 |
12 (5.8) |
|
| Other congenital (e.g., schizencephaly) |
126 |
7 (5.6) |
|
| Craniosynostosis |
47 |
4 (8.5) |
|
| Other cause |
255 |
13 (5.1) |
|
| Unknown cause |
46 |
1 (2.2) |
|
| Unknown |
379 |
16 (4.2) |
|
| Prior shunt surgery |
|
|
<0.01 |
| None |
2439 |
98 (4.0) |
|
| <12 wks |
1132 |
90 (8.0) |
|
| 12 to <26 wks |
322 |
22 (6.8) |
|
| 26 to <52 wks |
262 |
9 (3.4) |
|
| >52 wks |
758 |
33 (4.4) |
|
| Prior shunt infection |
|
|
0.36 |
| None |
4584 |
228 (5.0) |
|
| <12 wks |
192 |
14 (7.3) |
|
| 12 to <26 wks |
58 |
3 (5.2) |
|
| 26 to <52 wks |
21 |
2 (9.5) |
|
| >52 wks | 58 | 5 (8.6) |
ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; SAH = subarachnoid hemorrhage.
A multivariate logistic regression model was constructed using all potentially significant variables associated with shunt infection (Table 5). The compliance category was simplified into a binary variable based on whether all 5 protocol steps were completed or not. Significant risk factors that were independently associated with shunt infection included having ≥ 2 CCCs (odds ratio [OR] 1.76, 95% confidence interval [CI] 1.26–2.44, p = 0.01) and history of prior shunt surgery < 12 weeks (OR 1.84, 95% CI 1.37–2.47, p < 0.01). Significant risk factors that were protective against shunt infection were use of AICs (OR 0.70, 95% CI 0.50–0.97, p = 0.05) and use of vancomycin irrigation (OR 0.36, 95% CI 0.21–0.62, p < 0.01). A second model (Table 6) included the same variables, but the 5 protocol steps were considered individually. In this model, none of the individual steps met the criterion for statistical significance (p < 0.05). The same risk factors were identified in both models (Tables 5 and 6).
TABLE 5.
Multivariable analysis
| Variable | OR (95% CI) | p Value |
|---|---|---|
| All 5 protocol steps completed |
1.09 (0.77–1.56) |
0.62 |
| CCCs |
|
0.01 |
| 1 CCC |
1.15 (0.86–1.54) |
|
| ≥2 CCCs |
1.76 (1.26–2.44)
|
|
| History of prior shunt surgery |
|
<0.01 |
| <12 wks |
1.84 (1.37–2.47)
|
|
| 12–26 wks |
1.54 (0.95–2.48) |
|
| 26 to <52 wks |
0.76 (0.38–1.52) |
|
| >52 wks |
0.95 (0.63–1.44) |
|
| AIC |
0.70 (0.50–0.97)
|
0.05
|
| Vancomycin irrigation | 0.36 (0.21–0.62) | <0.01 |
Boldface type indicates statistical significance.
TABLE 6.
Stepwise multivariable analysis
| Variable | OR (95% CI) | p Value |
|---|---|---|
| CCCs |
|
0.01 |
| 1 CCC |
1.15 (0.85–1.54) |
|
| ≥2 CCCs |
1.76 (1.26–2.44)
|
|
| History of prior shunt surgery |
|
<0.01 |
| <12 wks |
1.84 (1.37–2.48)
|
|
| 12–26 wks |
1.54 (0.95–2.51) |
|
| 26 to <52 wks |
0.77 (0.38–1.54) |
|
| >52 wks |
0.96 (0.63–1.45) |
|
| AIC |
0.70 (0.50–0.97)
|
0.05
|
| Vancomycin irrigation | 0.35 (0.21–0.60) | <0.01 |
Boldface type indicates statistical significance.
Discussion
The implementation of standardized protocols for infection prevention has become increasingly popular among pediatric neurosurgeons. Adherence to a standardized preoperative preparation protocol reduced surgical site infection risk by 79% for all pediatric neurosurgical procedures at a tertiary care institution.5 Desai et al.6 reported a 50% relative risk reduction in pediatric baclofen pump infections after an infection prevention surgical checklist was implemented. Similarly, Tipper et al. noted a significant reduction in pediatric scoliosis surgery infection rates (from 8.6% to 2.2%) when a standardized perioperative protocol was used.7
This report further reinforces the HCRN’s previous findings that compliance with a standardized protocol reduces shunt infection rate.2,3 Since our last article, the HCRN has expanded from 8 centers to 14 centers, and the total number of procedures has increased more than two-fold compared with the previous study. The overall protocol compliance was similar to that previously reported (79.4% vs 77.0%), and we observed a similar infection rate (5.1% vs 6.0%). Furthermore, the standardization of our shunt infection protocol over multiple HCRN centers enabled us to identify other independently associated risk factors for shunt infection: greater number of CCCs, prior shunt surgery within 12 weeks, use of AICs, and use of vancomycin irrigation. From a QI standpoint, the use of AICs and vancomycin irrigation represent two modifiable risk factors.
Although the data in Table 3 for "chlorhexidine as final prep" and "all 5 steps completed" seem to suggest an increased infection risk, these are univariate comparisons. When evaluated in the multivariable regressions (Tables 5 and 6), none of the 5 steps, individually or as a group, impacted the infection risk. This does not mean that the 5 steps are irrelevant. Compliance with these steps was high (79.4%). In fact, of the noncompliant cases, 90% completed 4 of 5 steps, and in 92% of the 4-step cases, the one missing component was antibiotic compliance. That may mean a different antibiotic or dose, but we believe it is unlikely that no prophylactic antibiotics would have been used in those cases. We therefore believe that any benefit of AICs and vancomycin irrigation is seen when they are used in addition to the 5 protocol steps.
AICs
Several systematic reviews and meta-analyses have indicated that AICs can reduce shunt infection as well as provide a cost savings advantage, but the primary studies have been limited to lower-quality levels of evidence.8–10 Recently, the British Antibiotic and Silver Impregnated Catheters for Ventriculoperitoneal Shunts (BASICS) trial, a multicenter, single-blind randomized controlled trial conducted in the United Kingdom and Ireland, provided high-quality evidence in support of AICs.11 The investigators sought to determine the clinical efficacy and cost-effectiveness of antibiotic- and silver-impregnated catheters at reducing shunt infection. A total of 1605 patients with hydrocephalus (both adult and pediatric) undergoing first-time shunt insertion were randomized to receive a standard shunt catheter, an AIC, or a silver-impregnated catheter. Their primary endpoint was time to shunt failure due to infection. Patients who received AICs had a significantly lower rate of infection compared with both standard and silver-impregnated catheters (2% for AICs vs 6% for both standard and silver-impregnated shunts). Furthermore, the authors expected a cost savings of £135,753 per shunt infection prevented with AICs, despite an inherently higher upfront equipment cost. The authors recommended that AICs should be used for all patients undergoing first-time ventriculoperitoneal shunt insertions.
Our current data and the results of our multivariate analysis support a protective effect of AICs against shunt infection. Since our last report, the use of AICs has become increasingly popular among HCRN surgeons, and they were used in more than 80% of our procedures. In practice, we observed a 40% relative risk reduction in infection when AICs were used in conjunction with our shunt infection protocol. The injection of antibiotics into the shunt reservoir did not convey additional benefit toward infection rate when compared with the shunt infection protocol alone. The comparison between AICs and antibiotic injection could not be made in our last study, but our current data demonstrate an advantage of AICs against shunt infection. Interestingly, when both AIC and antibiotic injection were used, a paradoxical increase in infection rate was observed. Our current study was not designed to address the underlying reason for these findings, which may be subject to future investigations. Overall, our data strongly favor the use of AICs for CSF shunts. We recommend AICs be used for all pediatric shunt procedures. This step has now been included in the HCRN shunt infection protocol.
Vancomycin Irrigation
There is clinical equipoise regarding the use of intraoperative antibiotic wound irrigation, with significant heterogeneity in the literature about its use as an anti-infective measure.12 Nonetheless, all participating HCRN sites included antibiotic wound irrigation before skin closure. Specific details regarding antibiotic irrigation were not included in our protocol and were left to the discretion of the surgeon. Vancomycin irrigation was used in 15% of the procedures, while the remaining 85% used bacitracin irrigation. One site that used vancomycin irrigation consistently had a very low infection rate (2.2%) and one surgeon who used it consistently at another site had no infections. These results prompted us to assess the impact of vancomycin irrigation across the network. Our multivariate analysis demonstrated that vancomycin irrigation had a significant protective effect, whereas bacitracin irrigation was ineffective. We also observed a synergistic effect when vancomycin irrigation was used in combination with the shunt infection protocol and AICs, as this resulted in the lowest shunt infection rate (2.0%) across the network.
A recent study by Goswami et al.13 demonstrated superior antimicrobial activity of vancomycin irrigation over bacitracin irrigation. The authors conducted an in vitro study on the antimicrobial ability of frequently used irrigation solutions, including bacitracin (50,000 U/L) plus polymyxin (500,000 U/L), vancomycin (1 g/L), gentamicin (80 mg/L), 0.9% saline, 0.3% povidone-iodine, 0.05% chlorhexidine, 0.45% Castile soap, and 0.125% sodium hypochlorite, on cultures of Staphylococcus aureus and Escherichia coli. Bacterial colony-forming units were counted after 1 and 3 minutes of exposure to each solution. The authors found that vancomycin irrigation was effective at eradicating S. aureus but had no effect on E. coli, whereas bacitracin-polymyxin solution had no effect on either S. aureus or E. coli. Interestingly, the povidone-iodine solution was found to have the best antimicrobial activity against both S. aureus and E. coli. Nonetheless, these findings may provide a possible explanation of our results because Staphylococcus species are the most common organisms in pediatric shunt infections.14
In January 2020, the US FDA voluntarily withdrew bacitracin for injection from the market because of risks of nephrotoxicity and anaphylactic reactions. As a result, several HCRN sites have transitioned to the use of vancomycin irrigation for their shunt procedures. This will be a significant variable of interest for the next analysis of our shunt infection protocol.
Nonmodifiable Factors: CCCs and Recent Shunt Surgery
The presence of ≥ 2 CCCs as a risk factor associated with shunt infection is also a novel finding. The relationship between increasing medical complexity and shunt infection has often been a spoken tenet in neurosurgery, but our previous work did not demonstrate that having ≥ 2 CCCs was associated with CSF shunt infection.15 One possibility for this difference is that the prior study included only first-time shunts, before CCCs became apparent. Additionally, the current study analyzed significantly more patients, increasing our power to identify risk factors. Although the presence of ≥ 2 CCCs is a nonmodifiable factor, these patients should be counseled that they are at an increased risk of infection with shunt surgery.
We also found that patients who had undergone a recent shunt operation (within 12 weeks) were at risk for infection. This finding aligns with previous HCRN work showing that shunt revisions are a risk factor for shunt infection.16 We observed a nonstatistically significant trend of declining ORs for infection with increasing time from the index shunt operation (Table 5). These data are consistent with the current literature that most shunt infections present within weeks of surgery and the infection risk decreases over time.14,17 Of course, the best way to avoid this risk factor is to minimize the need for shunt revision, but when early revisions are necessary, attention to infection prevention measures are particularly important.
Limitations
Although we have a large sample size and prospectively collected data, there may be residual confounding effects due to variables that could not be recorded. For example, there may be site- and surgeon-specific nuances that have not been documented. Capturing every detail during surgery can be a significant challenge; however, the purpose of our protocol was to create common, standardized steps that all HCRN surgeons could follow to reduce shunt infection. Second, we identified strong associations between AICs and vancomycin wound irrigation against shunt infection, but this does not prove causality and these data must be confirmed in our future QI studies or randomized trials. Finally, our vancomycin data were collected retrospectively and represented a minority of patients from a few HCRN centers; thus, it is possible that our observed effect may be due to type I error and/or center effect. As several HCRN sites have transitioned to vancomycin irrigation exclusively, our sample size for this group will increase in the next iteration of our QI analysis.
Conclusions
We report the third iteration of our QI protocol to reduce the risk of shunt infection. Compliance with the protocol was high. Patients who have ≥ 2 CCCs and those undergoing recent shunt surgery within 12 weeks are at higher risk for shunt infection. From a QI standpoint, our updated data suggest that use of AICs and vancomycin irrigation of wounds are important infection prevention measures during CSF shunt surgery.
Acknowledgments
We would like to thank our colleagues for their past and ongoing support of HCRN: D. Brockmeyer, M. Walker, R. Bollo, S. Cheshier, J. Blount, J. Johnston, L. Acakpo-Satchivi, W. J. Oakes, P. Dirks, J. Rutka, M. Taylor, P. Dirks, D. Curry, G. Aldave, R. Dauser, A. Jea, S. Lam, H. Weiner, T. Luerssen, R. Ellenbogen, J. Ojemann, A. Lee, A. Avellino, S. Greene, E. Tyler-Kabara, T. Abel, T. S. Park, J. Strahle, S. McEvoy, M. Smyth, N. Tulipan, A. Singhal, P. Steinbok, D. Cochrane, W. Hader, C. Gallagher, M. Benour, E. Kiehna, J. G. McComb, P. Chiarelli, A. Robison, A. Alexander, M. Handler, B. O’Neill, C. Wilkinson, L. Governale, A. Drapeau, J. Leonard, E. Sribnick, A. Shaikhouni, E. Ahn, A. Cohen, M. Groves, S. Robinson, C. M. Bonfield, and C. Shannon. In addition, our work would not be possible without the outstanding support of the dedicated personnel at each clinical site and the data coordinating center. Special thanks go to L. Holman, J. Clawson, P. Martello, N. Tattersall, T. Bach (Salt Lake City); T. Caudill, P. Komarova, A. Arynchyna, A. Bey (Birmingham); H. Ashrafpour, M. Lamberti-Pasculli, L. O’Connor (Toronto); E. Santisbon, E. Sanchez, S. Martinez, S. Ryan (Houston); K. Hall, C. Gangan, J. Klein, A. Anderson, G. Bowen (Seattle); S. Thambireddy, K. Diamond, A. Luther (Pittsburgh); A. Morgan, H. Botteron, D. Morales, M. Gabir, D. Berger, D. Mercer (St. Louis); M. Stone, A. Wiseman, J. Stoll, D. Dawson, S. Gannon (Nashville); A. Cheong, R. Hengel (British Columbia); R. Rashid, S. Ahmed (Calgary); J. Yea, A. Loudermilk (Baltimore); N. Chapman, N. Rea, C. Cook (Los Angeles); S. Staulcup (Colorado); S. Saraswat, A. Sheline (Columbus); and N. Nunn, M. Langley, V. Wall, D. Austin, B. Conley, V. Freimann, L. Herrera, B. Miller (Utah Data Coordinating Center). We thank Kristin Kraus for editorial assistance.
The HCRN is thankful for the following sources of funding: the National Institute of Neurological Disorders and Stroke (grant no. 1RC1NS068943-01 Challenge), private philanthropy, and the Hydrocephalus Association.
Appendix
Hydrocephalus Clinical Research Network Members
The HCRN currently consists of the following clinical centers and investigators: Primary Children’s Hospital, University of Utah (J. Kestle); Children’s Hospital of Alabama, University of Alabama at Birmingham (C. Rozzelle, B. Rocque); Hospital for Sick Children, University of Toronto (J. Drake, A. Kulkarni); Texas Children’s Hospital, Baylor College of Medicine (W. Whitehead); Seattle Children’s Hospital, University of Washington (S. Browd, J. Hauptman); Children’s Hospital of Pittsburgh, University of Pittsburgh (I. Pollack); St. Louis Children’s Hospital, Washington University in St. Louis (D. Limbrick); Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt University Medical Center (J. Wellons, R. Naftel); British Columbia Children’s Hospital, University of British Columbia (M. Tamber); Alberta Children’s Hospital, University of Calgary (J. Riva-Cambrin); The Johns Hopkins Hospital (E. Jackson); Children’s Hospital of Los Angeles (M. Krieger, J. Chu, T. Simon); Children’s Hospital Colorado (T. Hankinson); Nationwide Children’s Hospital (J. Pindrik); HCRN Data Coordinating Center, Department of Pediatrics, University of Utah (R. Holubkov); and no affiliated clinical center (P. McDonald).
Disclosures
Dr. Limbrick reports support of non–study-related clinical or research effort from Microbot Medical Inc. and Medtronic Inc. Dr. Hauptman reports being a consultant for Medtronic and BK Medical.
Author Contributions
Conception and design: all authors. Acquisition of data: Kestle, Chu, Krieger, Kulkarni, Riva-Cambrin, Rozzelle, Limbrick, Wellons, Browd, Whitehead, Pollack, Simon, Tamber, Hauptman, Pindrik, Naftel, McDonald, Hankinson, Jackson, Rocque, Drake. Analysis and interpretation of data: Kestle, Chu, Jensen, Holubkov, Reeder. Drafting the article: Kestle, Chu. Critically revising the article: Kestle, Chu, Jensen, Holubkov, Krieger, Kulkarni, Riva-Cambrin, Rozzelle, Limbrick, Wellons, Browd, Whitehead, Pollack, Simon, Tamber, Hauptman, Pindrik, Naftel, McDonald, Hankinson, Jackson, Reeder, Drake. Reviewed submitted version of manuscript: Kestle, Rocque. Approved the final version of the manuscript on behalf of all authors: Kestle. Statistical analysis: Kestle, Jensen, Holubkov, Reeder. Administrative/technical/material support: Kestle.
Contributor Information
Collaborators: Hydrocephalus Clinical Research Network Members, J. Kestle, C. Rozzelle, B. Rocque, J. Drake, A. Kulkarni, W. Whitehead, S. Browd, J. Hauptman, I. Pollack, D. Limbrick, J. Wellons, R. Naftel, M. Tamber, J. Riva-Cambrin, E. Jackson, M. Krieger, J. Chu, T. Simon, T. Hankinson, J. Pindrik, R. Holubkov, and P. McDonald
References
- 1. Attenello FJ, Garces-Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI. Hospital costs associated with shunt infections in patients receiving antibiotic-impregnated shunt catheters versus standard shunt catheters. Neurosurgery. 2010;66(2):284–289. doi: 10.1227/01.NEU.0000363405.12584.4D. [DOI] [PubMed] [Google Scholar]
- 2. Kestle JR, Riva-Cambrin J, Wellons JC, III, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr. 2011;8(1):22–29. doi: 10.3171/2011.4.PEDS10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kestle JR, Holubkov R, Cochrane D, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2016;17(4):391–396. doi: 10.3171/2015.8.PEDS15253. [DOI] [PubMed] [Google Scholar]
- 4. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980-1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 5. Schaffzin JK, Simon K, Connelly BL, et al. Standardizing preoperative preparation to reduce surgical site infections among pediatric neurosurgical patients. J Neurosurg Pediatr. 2017;19(4):399–406. doi: 10.3171/2016.10.PEDS16287. [DOI] [PubMed] [Google Scholar]
- 6. Desai VR, Raskin JS, Mohan A, et al. A standardized protocol to reduce pediatric baclofen pump infections: a quality improvement initiative. J Neurosurg Pediatr. 2018;21(4):395–400. doi: 10.3171/2017.10.PEDS17248. [DOI] [PubMed] [Google Scholar]
- 7. Tipper GA, Chiwera L, Lucas J. Reducing surgical site infection in pediatric scoliosis surgery: a multidisciplinary improvement program and prospective 4-year audit. Global Spine J. 2020;10(5):633–639. doi: 10.1177/2192568219868200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 2015;122(5):1096–1112. doi: 10.3171/2014.12.JNS14908. [DOI] [PubMed] [Google Scholar]
- 9. Klimo P Jr, Thompson CJ, Ragel BT, Boop FA. Antibiotic-impregnated shunt systems versus standard shunt systems: a meta- and cost-savings analysis. J Neurosurg Pediatr. 2011;8(6):600–612. doi: 10.3171/2011.8.PEDS11346. [DOI] [PubMed] [Google Scholar]
- 10. Arts SH, Boogaarts HD, van Lindert EJ. Route of antibiotic prophylaxis for prevention of cerebrospinal fluid-shunt infection. Cochrane Database Syst Rev. 2019;6:CD012902. doi: 10.1002/14651858.CD012902.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallucci CL, Jenkinson MD, Conroy EJ, et al. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019;394(10208):1530–1539. doi: 10.1016/S0140-6736(19)31603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnes S, Spencer M, Graham D, Johnson HB. Surgical wound irrigation: a call for evidence-based standardization of practice. Am J Infect Control. 2014;42(5):525–529. doi: 10.1016/j.ajic.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 13. Goswami K, Cho J, Foltz C, et al. Polymyxin and bacitracin in the irrigation solution provide no benefit for bacterial killing in vitro. J Bone Joint Surg Am. 2019;101(18):1689–1697. doi: 10.2106/JBJS.18.01362. [DOI] [PubMed] [Google Scholar]
- 14. Test MR, Whitlock KB, Langley M, Riva-Cambrin J, Kestle JRW, Simon TD. Relationship of causative organism and time to infection among children with cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2019;24(1):22–28. doi: 10.3171/2019.2.PEDS18638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon TD, Butler J, Whitlock KB, et al. Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. J Pediatr. 2014;164(6):1462–1468.e2. doi: 10.1016/j.jpeds.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon TD, Whitlock KB, Riva-Cambrin J, et al. Revision surgeries are associated with significant increased risk of subsequent cerebrospinal fluid shunt infection. Pediatr Infect Dis J. 2012;31(6):551–556. doi: 10.1097/INF.0b013e31824da5bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGirt MJ, Leveque JC, Wellons JC, III, et al. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 2002;36(5):248–255. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]