In Brief
The study objective was to define rates of and risk factors for proximal junctional failure (PJF) based on a longer-term follow-up of operatively treated adult symptomatic lumbar scoliosis (ASLS) patients. The overall PJF rate was 28.8% at a mean 4.3-year follow-up. On multivariate analysis, an increased PJF risk was associated with a greater BMI and preoperative thoracic kyphosis and lower preoperative proximal junctional angle. Collectively, this study provides the highest quality data to date on rates of PJF following primary ASLS surgery.
Keywords: adult scoliosis, complications, proximal junctional failure, proximal junctional kyphosis, spinal alignment, spine deformity, spinal instrumentation, spine surgery, lumbar
ABBREVIATIONS : ASD = adult spinal deformity, ASLS = adult symptomatic lumbar scoliosis, BMI = body mass index, LL = lumbar lordosis, MCS = Mental Component Summary, ODI = Oswestry Disability Index, PCO = posterior column osteotomy, PCS = Physical Component Summary, PI = pelvic incidence, PI-LL = mismatch between PI and LL, PJA = proximal junctional angle, PJF = proximal junctional failure, PJK = proximal junctional kyphosis, PROM = patient-reported outcome measure, SRS-22r = Scoliosis Research Society revised 22-item questionnaire, SVA = sagittal vertical axis, TK = thoracic kyphosis, UIV = uppermost instrumented vertebra
Abstract
OBJECTIVE
Proximal junctional failure (PJF) is a severe form of proximal junctional kyphosis. Previous reports on PJF have been limited by heterogeneous cohorts and relatively short follow-ups. The authors’ objectives herein were to identify risk factors for PJF and to assess its long-term incidence and revision rates in a homogeneous cohort.
METHODS
The authors reviewed data from the Adult Symptomatic Lumbar Scoliosis 1 trial (ASLS-1), a National Institutes of Health–sponsored prospective multicenter study. Inclusion criteria were an age ≥ 40 years, ASLS (Cobb angle ≥ 30° and Oswestry Disability Index [ODI] ≥ 20 or Scoliosis Research Society revised 22-item questionnaire [SRS-22r] score ≤ 4.0 in pain, function, or self-image domains), and primary thoracolumbar fusion/fixation to the sacrum/pelvis of ≥ 7 levels. PJF was defined as a postoperative proximal junctional angle (PJA) change > 20°, fracture of the uppermost instrumented vertebra (UIV) or UIV+1 with > 20% vertebral height loss, spondylolisthesis of UIV/UIV+1 > 3 mm, or UIV screw dislodgment.
RESULTS
One hundred sixty patients (141 women) were included in this analysis and had a median age of 62 years and a mean follow-up of 4.3 years (range 0.1–6.1 years). Forty-six patients (28.8%) had PJF at a median of 0.92 years (IQR 0.14, 1.23 years) following surgery. Based on Kaplan-Meier analyses, PJF rates at 1, 2, 3, and 4 years were 14.4%, 21.9%, 25.9%, and 27.4%, respectively. On univariate analysis, PJF was associated with greater age (p = 0.0316), greater body mass index (BMI; p = 0.0319), worse baseline patient-reported outcome measures (PROMs; ODI, SRS-22r, and SF-12 Physical Component Summary [PCS]; all p < 0.04), the use of posterior column osteotomies (PCOs; p = 0.0039), and greater postoperative thoracic kyphosis (TK; p = 0.0031) and PJA (p < 0.001). The use of UIV hooks was protective against PJF (p = 0.0340). On regression analysis (without postoperative measures), PJF was associated with greater BMI (HR 1.077, 95% CI 1.007–1.153, p = 0.0317), lower preoperative PJA (HR 0.607, 95% CI 0.407–0.906, p = 0.0146), and greater preoperative TK (HR 1.362, 95% CI 1.082–1.715, p = 0.0085). Patients with PJF had worse PROMs at the last follow-up (ODI, SRS-22r subscore and self-image, and SF-12 PCS; p < 0.04). Sixteen PJF patients (34.8%) underwent revision, and PJF recurred in 3 (18.8%).
CONCLUSIONS
Among 160 primary ASLS patients with a median age of 62 years and predominant coronal deformity, the PJF rate was 28.8% at a mean 4.3-year follow-up, with a revision rate of 34.8%. On univariate analysis, PJF was associated with a greater age and BMI, worse baseline PROMs, the use of PCOs, and greater postoperative TK and PJA. The use of UIV hooks was protective against PJF. On multivariate analysis (without postoperative measures), a higher risk of PJF was associated with greater BMI and preoperative TK and lower preoperative PJA.
Proximal junctional kyphosis (PJK) is a well-recognized phenomenon following posterior segmental instrumentation for spinal deformity.1–5 The reported incidence ranges from 5.8% to 59%,6 with most reports favoring a 20%–40% range.3,7–9 PJK is a complex, multifactorial issue that requires a multifaceted approach to address.10 However, given the widely used definition of PJK proposed by Glattes and colleagues,6 the majority of patients with PJK do not appear to have symptoms or a need for revision surgery.6,11
The term "proximal junctional failure" (PJF) has since been advanced to reflect more clinically significant junctional pathology.8,12–17 Although the definition of PJF remains unsettled, patients with PJF typically not only meet the PJK Glattes criteria but also exhibit a combination of more significant junctional kyphosis, bony fracture, posterior ligamentous complex disruption, or instrumentation failure. PJF has a reported incidence ranging from 1.4% to 19%1,9,13–16 and is arguably the greatest unsolved problem in adult spinal deformity (ASD) surgery. The development of PJF can be associated with pain, disability, and neurological deficits and frequently requires extensive revision surgery.8,18
Previous studies on PJF have been limited by relatively short follow-ups, limited follow-up rates, single-center or single-surgeon cohorts, and heterogeneous deformity populations.9,13–16,19,20 Further study is needed to better define the long-term incidence of PJF and factors associated with its occurrence. In the present study, we aimed to define the prevalence of PJF and to assess the risk factors for PJF using a longer-term follow-up in a relatively homogeneous patient population from a multicenter, prospective database (from the Adult Symptomatic Lumbar Scoliosis 1 trial [ASLS-1]).21–23
Methods
Study Design and Inclusion Criteria
This is a retrospective review of a prospective, multicenter, consecutive series of patients from a National Institutes of Health–sponsored study (ASLS-1) designed to assess operative versus nonoperative treatment of adults with symptomatic lumbar scoliosis.21,22 Patients were enrolled at 9 sites in North America through a protocol approved by the institutional review boards of each contributing center. Inclusion criteria were as follows: no prior spine fusion or multilevel decompression, age 40–80 years, and ASLS defined on the basis of the presence of a lumbar curve with a coronal Cobb angle ≥ 30° and an Oswestry Disability Index (ODI) ≥ 20 or Scoliosis Research Society revised 22-item questionnaire (SRS-22r) score ≤ 4.0 in the domains of pain, function, or self-image. Patients with neuromuscular scoliosis (e.g., Parkinson’s disease) were excluded from study enrollment. The present study focused on the subset of ASLS-1 patients who had been operatively treated with posterior instrumented fusion/fixation of ≥ 7 vertebral levels that included the sacrum/pelvis.
Data Collection
Patient demographic, clinical, and operative parameters were collected from standardized study forms. Patient-reported outcome measures (PROMs) included the SRS-22r, SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS), ODI, and numeric rating scale for back and legs.
Full-length free-standing posteroanterior and lateral radiographs were obtained at baseline, 3 months postoperatively, and at subsequent clinical visits. Radiographs were measured using Surgimap software (Nemaris Inc.). Sagittal parameters included lumbar lordosis (LL), thoracic kyphosis (TK), sagittal vertical axis (SVA), pelvic tilt, pelvic incidence (PI), mismatch between PI and LL (PI-LL), and T1 pelvic angle, as previously described.24,25 Proximal junctional angle (PJA) was measured as the angle between the inferior endplate of the uppermost instrumented vertebra (UIV) and the superior endplate of the second vertebra above the UIV (UIV+2). Coronal parameters included Cobb angles and global coronal alignment measured as the distance from the midsacral point to the plumbline drawn from the center of C7.25
Radiographic PJK was defined on the basis of the Glattes criteria:6 1) PJA ≥ +10° and 2) PJA at least 10° greater than the preoperative measurement. Based on previous reports,8,9,12,13,16,18,26 PJF was defined as the presence of at least one of the following on postoperative imaging: change in PJA > 20° compared with the preoperative measurement, fracture of UIV and/or UIV+1 with > 20% vertebral height loss, screw dislodgment, or spondylolisthesis of UIV+1 relative to UIV of > 3 mm.
Data and Statistical Analysis
Follow-up started on the date of the index surgical treatment and ended at the first loss to follow-up or death. Kaplan-Meier curves were used to estimate the proportion of patients with PJF across the follow-up period. Demographic, clinical, surgical, and radiographic parameters were compared for patients with and without PJF using the Wilcoxon rank-sum test for continuous variables and the chi-square test and Fisher’s exact test for categorical data according to the sample size. Differences were considered statistically significant if p < 0.05.
Multivariate Cox models were used to determine a subset of risk factors independently associated with PJF. Initial multivariate models included all variables with p < 0.1 from the univariate comparisons. Variables that did not retain a p < 0.1 in the multivariate analysis were then removed, and additional variables known to be potentially important confounders were considered for inclusion before determining the final multivariate model.
Among patients who had PJF, Kaplan-Meier curves were used to estimate the proportion of patients with a revision across the time after PJF. Associations between each parameter used to define PJF and the need for revision surgery were assessed using the chi-square test and Fisher’s exact test for categorical data according to the sample size. Statistical analyses were performed using SAS software (SAS Institute Inc.).
Results
Clinical and Radiographic Characteristics of the Patient Population
A total of 286 patients were enrolled in ASLS-1 between 2010 and 2014, including 182 patients who underwent surgery. Of these 182 patients, 22 were excluded from the present study because of a lack of instrumentation extending to the pelvis (n = 10), < 7 levels fused (n = 11), or a lack of radiographic follow-up (n = 1). The mean total follow-up for the remaining 160 patients included in the study was 4.3 years (range 0.1–6.1 years).
Baseline demographics, radiographic measures, and PROMs are summarized in Table 1. The median age was 62 years (IQR 55.7, 67.9), and 141 of the patients (88.1%) were women. On baseline radiographs, the median PI-LL was 19° (IQR 8, 32), median C7–S1 SVA was 31.5 mm (IQR 13, 66), and median coronal Cobb angle was 51° (IQR 41.5, 67). Baseline PROMs reflected moderate to severe disability and pain.
TABLE 1.
Summary of baseline characteristics of 160 ASLS patients stratified based on the development of PJF
| Characteristic | All Patients | No PJF | PJF | p Value (no vs yes) |
|---|---|---|---|---|
| No. of patients |
160 |
114 |
46 |
|
| Age in yrs |
62 (56, 68) |
60 (56, 66) |
64 (59, 72) |
0.0316
*
|
| Female sex |
141 (88.1) |
100 (87.7) |
41 (89.1) |
0.8028† |
| Race |
|
|
|
0.8121‡ |
| White |
153 (95.6) |
109 (95.6) |
44 (95.7) |
|
| Black |
5 (3.1) |
4 (3.5) |
1 (2.2) |
|
| Other |
2 (1.3) |
1 (0.9) |
1 (2.2) |
|
| Tobacco use |
|
|
|
0.4629‡ |
| Current |
2 (1.3) |
2 (1.8) |
0 (0.0) |
|
| Former |
62 (38.8) |
41 (36.0) |
21 (45.7) |
|
| Never |
96 (60.0) |
71 (62.3) |
25 (54.3) |
|
| BMI |
26.0 (23.4, 29.5) |
25.8 (23.4, 28.2) |
28.0 (24.2, 31.2) |
0.0319
*
|
| Osteopenia/osteoporosis |
|
|
|
0.2914‡ |
| None/NA |
53 (33.1) |
41 (36.0) |
12 (26.1) |
|
| T-score −1 to −1.5 |
46 (28.8) |
28 (24.6) |
18 (39.1) |
|
| T-score −1.6 to −2.4 |
46 (28.8) |
33 (28.9) |
13 (28.3) |
|
| T-score −2.5 or worse§ |
15 (9.4) |
12 (10.5) |
3 (6.5) |
|
| Diabetes |
|
|
|
0.1278‡ |
| No |
150 (93.8) |
107 (93.9) |
43 (93.5) |
|
| Yes, controlled w/ diet |
1 (0.6) |
1 (0.9) |
0 (0.0) |
|
| Yes, controlled w/ oral hypoglycemic |
7 (4.4) |
6 (5.3) |
1 (2.2) |
|
| Yes, insulin dependent |
2 (1.3) |
0 (0.0) |
2 (4.3) |
|
| Radiographic measures |
|
|
|
|
| C7–S1 SVA in mm |
31.5 (13, 66) |
31.5 (13, 60) |
29.5 (16, 87) |
0.4263* |
| TPA in ° |
20.8 (14.4, 28) |
20.8 (14.5, 27.9) |
20.3 (14.4, 29.9) |
0.6477* |
| TK (T4–12) in ° |
30.7 (19.4, 42.5) |
30 (18.1, 41) |
36.5 (27, 44.4) |
0.1* |
| LL (T12–sacrum) in ° |
36.3 (25, 49) |
37 (27, 47) |
32.8 (20, 58) |
0.7672* |
| PI in ° |
54 (48, 63) |
54 (48, 63) |
53 (45.1, 63) |
0.8766* |
| PI-LL in ° |
19 (8, 32) |
18.5 (8.5, 31.1) |
20 (8, 34) |
0.762* |
| PT in ° |
24 (20, 30) |
24 (19, 28) |
24 (21, 31) |
0.3591* |
| Lumbar Cobb angle in ° |
51 (41.5, 67) |
55 (43, 68) |
49 (38, 61) |
0.1497* |
| Coronal alignment absolute value in mm |
21 (9, 38.6) |
21 (10, 39.2) |
20 (8, 34) |
0.6307* |
| PJA in ° |
5.0 (3.0, 8.6) |
5.2 (3, 9) |
4.7 (2, 7.9) |
0.1226* |
| Baseline PROMs |
|
|
|
|
| ODI |
38 (28, 50) |
36 (26, 46) |
47 (36, 54) |
0.0026
*
|
| SRS-22r subscore |
3.0 (2.6, 3.5) |
3.1 (2.7, 3.5) |
2.7 (2.4, 3.4) |
0.0063
*
|
| SRS-22r pain |
2.8 (2.2, 3.4) |
2.8 (2.4, 3.4) |
2.6 (2, 3.2) |
0.0386
*
|
| SRS-22r function |
3.2 (2.5, 3.6) |
3.2 (2.8, 3.8) |
2.7 (2.2, 3.4) |
0.0046
*
|
| SRS-22r self-image |
2.7 (2.1, 3.2) |
2.8 (2.2, 3.2) |
2.5 (2, 3) |
0.0657* |
| SRS-22r mental health |
3.7 (3.0, 4.2) |
3.8 (3.2, 4.4) |
3.4 (2.8, 4) |
0.0283
*
|
| SRS-22r satisfaction |
3.0 (2.0, 3.5) |
3 (2, 3.5) |
2.8 (2, 3.5) |
0.3145* |
| NRS back pain |
7 (5, 8) |
7 (5, 8) |
7 (6, 8) |
0.2615* |
| NRS leg pain |
4 (1, 7) |
4 (1, 7) |
4.5 (2, 7) |
0.0816* |
| SF-12 MCS |
51.7 (41.5, 59.6) |
53.2 (41.5, 59.9) |
47 (40.5, 58.3) |
0.0835* |
| SF-12 PCS | 31.9 (25.8, 39.3) | 33.9 (27.5, 41.7) | 26.7 (22.8, 33.5) | 0.0005 * |
NA = not applicable; NRS = numeric rating scale; PT = pelvic tilt; TPA = T1 pelvic angle.
Values are expressed as the median (Q1, Q3) or number (%), unless indicated otherwise. Boldface type indicates statistical significance.
Wilcoxon rank-sum test.
Chi-square test.
Fisher’s exact test.
Or vertebral compression fracture.
Operative parameters are summarized in Table 2. The mean number of vertebral levels fused posteriorly was 11.5 (IQR 8, 14), with 67 patients (41.9%) having an upper thoracic UIV, 20 (12.5%) having a UIV in the midthoracic spine, and 73 (45.6%) having a UIV in the lower thoracic spine. The most common rod material used was cobalt chromium (84.4%), followed by titanium (10%) and stainless steel (4.4%). Most rods were 5.5 mm in diameter (94.4%). Eighteen patients had supplemental rods.27,28 Posterior column osteotomies (PCOs) were used in the majority of patients, and three-column osteotomies (pedicle subtraction osteotomy or vertebral column resection) were used in 9 patients. PJF prophylaxis measures included hooks at the UIV in 39 patients (24.4%) and cement at the UIV and/or UIV+1 in 7 patients (4.4%). The two most common UIV hook constructs were bilateral transverse process hooks and a pedicle screw on one side with a pedicle hook on the other side. Postoperative radiographic measures, changes in radiographic measurements from baseline, and PROMs obtained at the last follow-up are summarized in Table 3.
TABLE 2.
Index operative parameters for 160 ASLS patients stratified based on the development of PJF
| Parameter | All Patients | No PJF | PJF | p Value (no vs yes) |
|---|---|---|---|---|
| No. of patients |
160 |
114 |
46 |
|
| Estimated blood loss in ml |
1750 (975, 3000) |
1700 (1000, 2600) |
1875 (900, 3500) |
0.6092* |
| Operative time in hrs |
6.5 (5.8, 7.5) |
6.6 (6, 7.7) |
6.5 (5.3, 7) |
0.1415* |
| Surgical approach |
|
|
|
|
| Pst only |
144 (90.0) |
104 (91.2) |
40 (87.0) |
0.4150† |
| Combined ant-pst |
16 (10.0) |
10 (8.8) |
6 (13.0) |
|
| Pst approach details |
|
|
|
|
| Rod material |
|
|
|
0.1301‡ |
| Titanium |
16 (10.0) |
13 (11.4) |
3 (6.5) |
|
| Stainless steel |
7 (4.4) |
6 (5.3) |
1 (2.2) |
|
| Cobalt chromium |
135 (84.4) |
95 (83.3) |
40 (87.0) |
|
| Other |
2 (1.3) |
0 (0.0) |
2 (4.3) |
|
| Rod diameter |
|
|
|
|
| Rod 1 diameter |
|
|
|
0.6905‡ |
| 5.5 mm |
152 (95.0) |
109 (95.6) |
43 (93.5) |
|
| 6.0 mm |
8 (5.0) |
5 (4.4) |
3 (6.5) |
|
| Rod 2 diameter |
|
|
|
0.2096‡ |
| 5.5 mm |
150 (93.8) |
109 (95.6) |
41 (89.1) |
|
| 6.0 mm |
7 (4.4) |
4 (3.5) |
3 (6.5) |
|
| 7.0 mm |
3 (1.9) |
1 (0.9) |
2 (4.3) |
|
| Rod 3 diameter |
|
|
|
>0.99‡ |
| 5.5 mm |
17 (94.4) |
14 (93.3) |
3 (100.0) |
|
| 6.0 mm |
1 (5.6) |
1 (6.7) |
0 (0.0) |
|
| Accessory/satellite rod(s) |
|
|
|
0.2804‡ |
| No |
142 (88.8) |
99 (86.8) |
43 (93.5) |
|
| Yes |
18 (11.3) |
15 (13.2) |
3 (6.5) |
|
| No. of fused levels |
11.5 (8, 14) |
13 (8, 15) |
9 (8, 14) |
0.4354* |
| UIV |
|
|
|
0.4794† |
| Upper thoracic spine (T1–4) |
67 (41.9) |
49 (43.0) |
18 (39.1) |
|
| Mid thoracic spine (T5–8) |
20 (12.5) |
16 (14.0) |
4 (8.7) |
|
| Lower thoracic spine (T9–12) |
73 (45.6) |
49 (43.0) |
24 (52.2) |
|
| Type of pelvic fixation |
|
|
|
0.5964† |
| Iliac screws |
147 (91.9) |
106 (93.0) |
41 (89.1) |
|
| S2-alar-iliac screws |
13 (8.1) |
8 (7.0) |
5 (10.9) |
|
| Osteotomies |
|
|
|
|
| PCO |
101 (63.1) |
64 (56.1) |
37 (80.4) |
0.0039†
|
| Three-column osteotomy |
9 (5.6) |
7 (6.1) |
2 (4.3) |
>0.99‡ |
| PSO |
6 (3.8) |
6 (5.3) |
0 (0.0) |
0.1832‡ |
| VCR |
3 (1.9) |
1 (0.9) |
2 (4.3) |
0.1988‡ |
| PJF prophylaxis measures |
|
|
|
|
| Hooks at UIV |
39 (24.4) |
33 (28.9) |
6 (13.0) |
0.0340†
|
| Cement at UIV &/or UIV+1 |
7 (4.4) |
5 (4.4) |
2 (4.3) |
>0.99‡ |
| Ant/lat approach details |
|
|
|
|
| ALIF |
|
|
|
0.4248‡ |
| 1 |
8 (50.0) |
6 (60.0) |
2 (33.3) |
|
| 2 |
7 (43.8) |
3 (30.0) |
4 (66.7) |
|
| 3 | 1 (6.3) | 1 (10.0) | 0 (0.0) |
ALIF = anterior lumbar interbody fusion; ant = anterior; PSO = pedicle subtraction osteotomy; pst = posterior; VCR = vertebral column resection.
Values are expressed as the median (Q1, Q3) or number (%), unless indicated otherwise. Boldface type indicates statistical significance.
Wilcoxon rank-sum test.
Chi-square test.
Fisher’s exact test.
TABLE 3.
Postoperative radiographic outcome measures and PROMs for 160 ASLS patients stratified based on the development of PJF
| Parameter | All Patients | No PJF | PJF | p Value (no vs yes)* |
|---|---|---|---|---|
| No. of patients |
160 |
114 |
46 |
|
| Postop (3-mo radiographs) |
|
|
|
|
| C7–S1 SVA in mm |
24.7 (12, 39) |
22.4 (11, 35.8) |
29.6 (16.1, 43.1) |
0.0562 |
| TPA in ° |
16 (9, 23) |
16 (10.4, 23) |
17 (8, 25) |
0.8201 |
| TK (T4–12) in ° |
42 (31.3, 48.1) |
40 (31, 46) |
46.8 (36.5, 56.4) |
0.0031
|
| LL (T12–sacrum) in ° |
51 (43.5, 61) |
50.5 (43, 60) |
53 (46, 61) |
0.3836 |
| PI in ° |
54 (47, 61) |
54 (46, 61) |
53.5 (47.4, 61) |
0.8342 |
| PI-LL in ° |
7 (3.6, 13) |
6.6 (3, 13) |
8.5 (5, 13) |
0.2014 |
| PT in ° |
21.9 (15.9, 27) |
21.4 (15.7, 25.2) |
23 (16, 29) |
0.6132 |
| Lumbar Cobb angle in ° |
19.2 (12, 29) |
21 (11.2, 30.2) |
17.3 (12.7, 22) |
0.2449 |
| Coronal alignment absolute value in mm |
15.7 (8, 25.4) |
15.4 (8, 25.4) |
15.8 (8.7, 26.8) |
0.8188 |
| PJA in ° |
11.5 (7.2, 16.9) |
9.5 (6.6, 13.2) |
19.2 (14.3, 26.8) |
<0.0001
|
| Change (postop – baseline) |
|
|
|
|
| C7–S1 SVA in mm |
41 (15.1, 71) |
41 (17.8, 67.2) |
41.6 (12.7, 92.4) |
0.7177 |
| TPA in ° |
8.4 (4.2, 13.1) |
7.6 (4.2, 12.1) |
9.7 (4.3, 14.5) |
0.3947 |
| TK (T4–12) in ° |
13.4 (7.2, 21) |
12.5 (6.9, 21) |
15.9 (8.5, 21.1) |
0.2143 |
| LL (T12–sacrum) in ° |
17 (8, 27) |
16.5 (8, 26.6) |
18 (11, 31.5) |
0.2485 |
| PI in ° |
3 (1.6, 4) |
3 (1, 4) |
3 (2, 4) |
0.6699 |
| PI-LL in ° |
18 (9, 29) |
17.4 (9, 26.9) |
21 (11, 31) |
0.3193 |
| PT in ° |
6 (3, 11.6) |
6 (3, 11.8) |
6 (3.5, 11) |
0.3638 |
| Lumbar Cobb in ° |
34 (24, 41.8) |
34.5 (24.5, 42.7) |
30.6 (19.6, 40) |
0.4993 |
| Coronal alignment absolute value in mm |
25 (10, 46) |
25.7 (13, 46) |
19.9 (7.5, 45.4) |
0.3487 |
| PJA in ° |
6 (3.1, 11.0) |
4.4 (2.2, 7.5) |
14.3 (9.7, 19.9) |
<0.0001
|
| PROMs at last follow-up |
|
|
|
|
| ODI |
22 (8, 38) |
18 (8, 36) |
27 (16, 48) |
0.0091
|
| SRS-22r subscore |
3.9 (3.3, 4.3) |
4 (3.4, 4.4) |
3.6 (3.2, 4.1) |
0.0379
|
| SRS-22r pain |
3.8 (3, 4.4) |
3.8 (3.2, 4.6) |
3.9 (2.5, 4.4) |
0.1378 |
| SRS-22r function |
3.6 (3, 4.2) |
3.8 (3, 4.2) |
3.4 (2.8, 4) |
0.0682 |
| SRS-22r self-image |
4 (3.4, 4.5) |
4.2 (3.5, 4.5) |
3.7 (3.2, 4.3) |
0.0221
|
| SRS-22r mental health |
4 (3.4, 4.6) |
4.2 (3.6, 4.6) |
4 (3.4, 4.6) |
0.4612 |
| SRS-22r satisfaction |
4.5 (4, 5) |
4.5 (4, 5) |
4.5 (3.5, 5) |
0.5686 |
| NRS back pain |
2 (1, 5) |
2 (0, 5) |
3 (1, 5) |
0.1266 |
| NRS leg pain |
1 (0, 4) |
1 (0, 4) |
2 (1, 5) |
0.0554 |
| SF-12 MCS |
52.5 (40.4, 58.9) |
53.7 (41.7, 59.7) |
49.7 (37.6, 57.7) |
0.1350 |
| SF-12 PCS | 43.2 (31.9, 52.1) | 45.3 (33.3, 53.3) | 37.5 (29.3, 47.6) | 0.0238 |
Values are expressed as the median (Q1, Q3), unless indicated otherwise. Boldface type indicates statistical significance.
Wilcoxon rank-sum test.
Characteristics of Patients Presenting With PJF
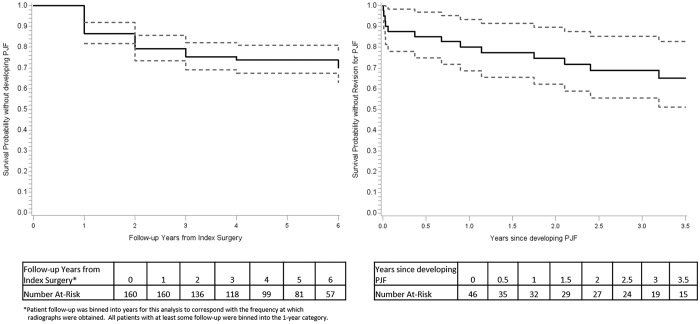
A total of 46 patients (28.8%) had PJF at a median of 335 days (IQR 51, 449 days) from the index surgery (Table 4). Based on Kaplan-Meier analyses, PJF rates at 1, 2, 3, and 4 years were 14.4%, 21.9%, 25.9%, and 27.4%, respectively (Fig. 1 left). Among these 46 patients, the UIV was in the upper thoracic spine in 18 (39.1%), midthoracic spine in 4 (8.7%), and lower thoracic spine in 24 (52.2%). The median PJA at the 3-month follow-up was 19.2° (IQR 14.3°, 26.8°), and 39 patients (84.8%) ultimately had a PJA > 20°. Fracture of the UIV and UIV+1 was found in 18 (39.1%) and 7 (15.2%) patients, respectively. Screw dislodgment and spondylolisthesis of UIV+1 relative to the UIV were seen in 7 (15.2%) and 2 (4.3%) patients, respectively (Table 4).
TABLE 4.
Characteristics and management of ASLS patients with PJF
| Parameter | Value |
|---|---|
| No. of patients |
46/160 (28.8) |
| Time to PJF from index surgery in days |
335 (51, 449) |
| UIV |
|
| T1–4 |
18/46 (39.1) |
| T5–8 |
4/46 (8.7) |
| T9–12 |
24/46 (52.2) |
| Radiographic findings |
|
| PJA >20° |
39/46 (84.8) |
| Fracture of UIV |
18/46 (39.1) |
| Fracture of UIV+1 |
7/46 (15.2) |
| Screw dislodgment |
7/46 (15.2) |
| Spondylolisthesis of UIV+1 relative to UIV |
2/46 (4.3) |
| Treatment for PJF |
|
| None to date |
30/46 (65.2) |
| Revision surgery |
16/46 (34.8) |
| Proximal extension of fusion |
15/16 (93.8) |
| 1–2 vertebral levels |
1/16 (6.3) |
| 3–5 vertebral levels |
6/16 (37.5) |
| 6–10 vertebral levels |
8/16 (50.0) |
| >10 vertebral levels |
0/16 (0) |
| Decompression (laminectomy) at level of PJF |
5/16 (31.3) |
| Complete/partial vertebrectomy at level of PJF |
7/16 (43.8) |
| Partial removal of instrumentation |
1/16 (6.3) |
| Recurrent PJF |
|
| None |
13/16 (81.3) |
| Recurred | 3/16 (18.8) |
Values are expressed as number/total number (%) or median (Q1, Q3).
FIG. 1.
Survival plot for the occurrence of PJF following surgical treatment of ASLS in 160 patients (left). Survival plot for revision surgery following the development of PJF (right). Dashed lines represent the 95% confidence interval.
Assessment of Risk Factors for PJF
On univariate analysis, baseline demographic and radiographic measures were similar between those who did and those who did not have PJF, except for age and body mass index (BMI; Table 1). Patients with PJF were significantly older (median age 64 vs 60 years, p = 0.0316), and had a significantly greater BMI (median 28.0 vs 25.8, p = 0.0319). Patients with PJF also had significantly worse baseline PROM scores for ODI, SRS-22r subscore, SRS-22r pain, SRS-22r function, SRS-22r mental health, and SF-12 PCS (all p < 0.04).
On univariate analysis, operative parameters were similar between those who did and those who did not have PJF, except for the use of PCOs and hooks at the UIV (Table 2). More patients with PJF had been treated with PCOs than those without PJF (80.4% vs 56.1%, p = 0.0039), and fewer patients with hooks at the UIV had PJF (28.9% vs 13.0%, p = 0.0340). Notably, surgical approach, rod material and diameter, number of fused levels, and level of UIV were not associated with PJF.
On univariate analysis, 3-month postoperative radiographic parameters and changes in radiographic alignment (3-month postoperative compared with preoperative) were not different between patients who did and those who did not have PJF, except for postoperative TK, postoperative PJA, and change in PJA (Table 3). Patients with PJF had greater postoperative TK (46.8° vs 40.0°, p = 0.0031), greater postoperative PJA (19.2° vs 9.5°, p < 0.0001), and greater postoperative change in PJA (14.3° vs 4.4°, p < 0.0001). Notably, patients with PJF had significantly worse scores on multiple PROMs at the last follow-up (Table 3).
Multivariate assessment of baseline parameters associated with the development of PJF demonstrated significant associations with BMI, baseline PJA, and baseline TK (Table 5). The occurrence of PJF was associated with greater BMI (HR 1.077, 95% CI 1.007–1.153, p = 0.0317), lower baseline PJA (HR 0.607, 95% CI 0.407–0.906, modeled by 5° increments), and greater baseline TK (HR 1.362, 95% CI 1.082–1.715, p = 0.0085, modeled by 10° increments). Factors that were adjusted for but were not significant in the final model included age, osteopenia/osteoporosis, level of UIV, hooks at the UIV, and baseline C7–S1 SVA (Table 5).
TABLE 5.
Baseline parameters associated with the risk for PJF based on multivariate modeling
| Parameter | HR | 95% CI | p Value |
|---|---|---|---|
| Age at index surgery |
1.438 |
0.921–2.246 |
0.1097 |
| BMI |
1.077 |
1.007–1.153 |
0.0317
|
| Osteopenia/osteoporosis |
1.667 |
0.794–3.499 |
0.1768 |
| UIV |
|
|
|
| T9–12 |
Ref |
Ref |
Ref |
| T5–8 |
0.637 |
0.2–2.032 |
0.4459 |
| T1–4 |
0.632 |
0.288–1.386 |
0.2525 |
| Hooks at UIV |
0.486 |
0.19–1.241 |
0.1315 |
| Baseline PJA* |
0.607 |
0.407–0.906 |
0.0146
|
| Baseline TK (T4–12)† |
1.362 |
1.082–1.715 |
0.0085
|
| Baseline C7–S1 SVA† | 1.043 | 0.971–1.121 | 0.2465 |
Boldface type indicates statistical significance.
Modeled by 5° increments.
Modeled by 10° increments.
Revision Surgery for PJF and PJF Recurrence Rates
Of the 46 patients with PJF, 16 (34.8%) had undergone revision surgery as of the last follow-up (Table 4). The median follow-up after PJF was 902 days (IQR 135, 1363 days; Fig. 1 right). Extension of fusion was performed in all but 1 patient, including 1–2 levels (1 patient), 3–5 levels (6 patients), or 6–10 levels (8 patients). Five (31.3%) of the 16 patients who had undergone revision for PJF had a laminectomy/decompression at the junction and extension of fusion, 7 (43.8%) had a partial or complete vertebrectomy and extension of fusion, 3 (18.8%) had only extension of fusion, and 1 (6.3%) had partial removal of instrumentation (Table 4). A case example of PJF requiring revision surgery is shown in Fig. 2.
FIG. 2.
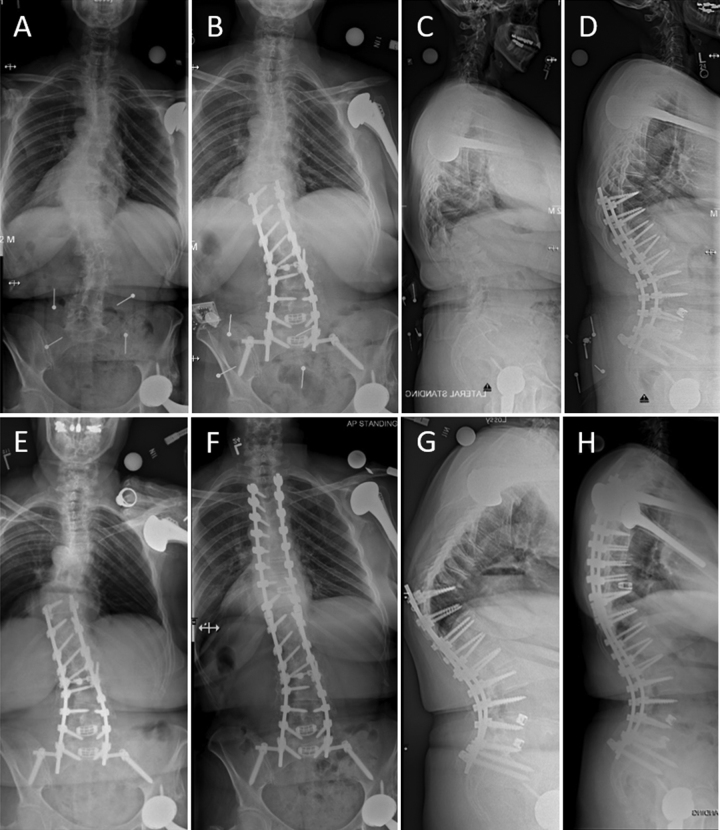
Case example of a 62-year-old woman with ASLS who underwent surgical correction consisting of T10–S1 posterior instrumented arthrodesis with T12–S1 PCOs, L4–S1 transforaminal lumbar interbody fusions, and placement of iliac screws. Full-length free-standing posteroanterior and lateral radiographs at preoperative baseline (A and C, respectively) and at the 3-month postoperative follow-up (B and D, respectively). Following operative treatment, she had marked improvement of her preoperative symptoms; however, she developed a compression fracture at T10 with progressive PJK and positive sagittal malalignment. At approximately 18 months following the index surgery, she underwent revision surgery consisting of partial T9 and T10 vertebral column resections with titanium cage placement and extension of the posterior instrumentation to T3. Full-spine standing posteroanterior and lateral radiographs at approximately 18 months following the index surgery (E and G, respectively) and following the revision surgery (F and H, respectively). Note the T10 compression fracture and markedly increased PJA, which measures 42° (G).
The percentage of patients with PJF who met each of the 4 criteria used to define PJF, with stratification based on whether revision surgery was performed, is summarized in Table 6. Each criterion was significantly associated with the need for revision except for spondylolisthesis of UIV+1, which was only present in 2 patients. A change in PJA > 20° was more common among patients who did not undergo revision for PJF (93.3% vs 68.8%, p = 0.040). In contrast, patients who had undergone revision had a higher rate of fracture of the UIV and/or UIV+1 (93.8% vs 20.0%, p < 0.001) and a higher rate of screw dislodgment (37.5% vs 3.3%, p = 0.005) than those who had not undergone revision surgery. Recurrent PJF developed at the new UIV in 3 (18.8%) of the 16 patients treated for PJF (Table 4).
TABLE 6.
Association between each factor used for the definition of PJF and the need for revision surgery
| Parameter | No Revision | Revision | p Value |
|---|---|---|---|
| No. of patients |
30 |
16 |
|
| Change in PJA >20° |
28 (93.3) |
11 (68.8) |
0.040
|
| Fracture of UIV &/or UIV+1 >20% |
6 (20.0) |
15 (93.8) |
<0.001
|
| Screw dislodgment |
1 (3.3) |
6 (37.5) |
0.005
|
| Spondylolisthesis of UIV+1 by >3 mm | 1 (3.3) | 1 (6.3) | >0.99 |
Values are expressed as number (%), unless indicated otherwise. Boldface type indicates statistical significance.
Rates of PJK and Overlap With PJF
Of the study cohort of 160 patients, 74 (46.3%) had PJK based on Glattes criteria6 at a median of 122 days (IQR 30, 950 days) after surgery. All but 1 of the 46 patients (97.8%) who met the criteria for PJF also met the criteria for PJK. In contrast, there were 29 patients who developed PJK but did not develop PJF, and none of these 29 patients underwent revision surgery. Of the 16 patients who had undergone revision surgery for junctional issues, all met the criteria for PJF and 15 met the criteria for PJK.
Discussion
PJK is most commonly defined on the basis of the criteria established by Glattes and colleagues.6 Among 81 ASD patients, they noted a PJK rate of 26%, but none of these patients underwent revision surgery for PJK, and there was no apparent impact on PROMs. Subsequent studies have reported rates of PJK ranging from 17% to 61.7%,3,8 but significant associations with clinical outcomes have been limited.7 More recently, the term "PJF" was coined to help distinguish the subset of clinically significant PJK patients.18 In the present study, although we report rates of PJK, we focused on PJF. Our definition of PJF is similar to that of Hart et al.,18 with the addition of PJA > 20°.16 We provide assessment of PJF rates, risk factors, and revisions in a multicenter, prospectively collected, relatively homogeneous series of primary ASLS patients with a mean follow-up of more than 4 years. To our knowledge, this study provides the highest quality data to date on rates of PJF following primary ASLS surgery.
In our cohort of 160 patients, 46 (28.7%) had PJF. Based on Kaplan-Meier analyses, PJF rates at 1, 2, 3, and 4 years were 14.4%, 21.9%, 25.9%, and 27.4%, respectively. This suggests that the majority of PJF cases develop within 2 years of surgery. The rate of PJF in this study is higher than those in previous reports, in which the rates have ranged from 1.4% to 19%.1,9,13–16,18 This likely reflects a number of factors, including differences in how PJF was defined across studies, differences in the length of follow-up, variations in surgical technique and correction goals, and marked heterogeneity in patient pathologies. To help ensure the homogeneity of our study population, only patients undergoing primary thoracolumbar fusion/fixation to the sacrum/pelvis of ≥ 7 levels were included. In addition, the spinal pathologies in our study population consisted of coronal deformities, with limited representation of sagittal malalignment.
Although the literature on PJK is extensive, there are few studies that have focused on PJF. Based on a retrospective review of 1668 patients with ASD who were followed for a mean of 4.0 years after surgery, Yagi and colleagues9 reported a PJF rate of 1.4%. They defined PJF as symptomatic PJK requiring surgery, and their cohort was very heterogeneous, including primary and revision cases, with pathologies ranging from adult idiopathic scoliosis to fixed sagittal imbalance, and not all patients received instrumentation to the pelvis. Hart and colleagues18 defined PJF on the basis of meeting the Glattes PJK criteria6 and at least one of the following: fracture of the UIV/UIV+1, disruption of the posterior osseoligamentous complex, or UIV instrumentation pullout. They reported PJF in 57 (4.7%) of 1218 ASD patients whose cases included primary and revision procedures, a mixture of coronal and sagittal plane deformities, no minimum length of instrumentation construct, and variable inclusion of pelvic instrumentation. Hostin and colleagues13 reported a 5.6% rate of acute PJF (within 28 weeks of surgery) among 1218 ASD patients with a broad range of deformities. They defined acute PJF as any one of the following within 28 weeks of surgery: ≥ 15° increase in PJA, fracture of the UIV/UIV+1, failure of UIV fixation, or the need for proximal extension of fusion. Using a definition of PJF as PJK requiring revision surgery, Scheer and colleagues15 reported a 7.3% rate of PJF among 510 ASD patients with a broad range of coronal and sagittal pathologies. Park and colleagues14 retrospectively reviewed 160 ASD patients treated with long instrumented fusion to the sacrum (with or without iliac fixation) and reported a PJF rate of 18.1%. They defined PJF as the presence of PJK (Glattes criteria6) and structural failure, such as UIV/UIV+1 fracture, screw pullout, or implant breakage. Yagi and colleagues16 reported a PJF rate of 19% among 113 surgically treated ASD patients whose cases included primary and revision procedures and a variety of sagittal and coronal deformities. They defined PJF as an increase in the PJA ≥ 20° from baseline and concomitant deterioration of at least one SRS-Schwab29 sagittal modifier grade, or any type of PJK requiring revision. Notably, the PJF rate in the present study is closest to those reported by Yagi et al.16 and Park et al.14 Although the definition of PJF in those studies only partially overlaps with the definition in the present study, both of those studies had high proportions of patients with instrumentation extending to the sacrum/pelvis, which likely adds considerable stiffness to the proximal junction and potentially increases the risk of PJF.
Risk factors for PJF on univariate analysis in the present study included greater age, greater BMI, worse baseline PROMs, the use of PCOs, greater postoperative TK and PJA, and greater change in PJA following surgery. In addition, hooks were used in a greater proportion of patients who did not have PJF. Multivariate analyses to identify risk factors for PJF based only on parameters available preoperatively revealed a greater BMI, lower preoperative PJA, and greater preoperative TK as significant factors. Age has been identified as a risk factor for PJK/PJF in several previous studies3,7,8,14,15 and may reflect an overall greater frailty. BMI has also been reported as a risk factor for PJK/PJF14,15,26,30 and may relate to increased biomechanical stress at the proximal junction. For example, based on the hazard ratio for BMI (1.077) in the present study, the risk of PJF increases by 210% for a patient with a BMI of 35 compared to that of a patient with a BMI of 25. (Hazard ratios are relative measures, so increases in the risk for multiple units are calculated as HRN units; thus, a 10-point increase in BMI corresponds to 1.077.10) Since BMI is a potentially modifiable factor, this finding may offer an opportunity for preoperative optimization to mitigate PJF risk. Previous reports have also noted the potential protective effects of hooks at the UIV for reducing PJK/PJF.19,31 It is possible that hooks may facilitate UIV fixation with less soft tissue disruption than pedicle screws. However, the present study is limited in providing a definitive assessment of hooks since they were used in a relatively small subset of patients (39 of 160). The reasons that worse baseline PROMs and the use of PCOs were associated with PJF occurrence are unclear. It is possible that patients with greater pain and disability at baseline may be less active and more deconditioned, which could contribute to sarcopenia and a greater PJF risk.32
Radiographic alignment measures, including greater global sagittal malalignment, greater correction of global sagittal malalignment, and greater correction of LL, have been suggested as risk factors for PJK/PJF in multiple studies.8,14,15 Similar associations were not observed in the present study, which may be attributable to differences in patient populations. In the present study, spinal deformities were primarily in the coronal plane, and three-column osteotomies were rarely used. This contrasts with previous studies in which the cohorts were heterogeneous and included primary sagittal deformities and a greater use of three-column osteotomies.1,13,16,18 It may seem counterintuitive that the greater use of PCOs, but not greater sagittal correction, would be associated with an increased occurrence of PJF since PCOs are often employed to provide sagittal correction. However, in the present study cohort, since the primary deformity was in the coronal plane, PCOs may have served a greater role in the segmental release of scoliosis than in increasing segmental lordosis.
In the present study, the association between PJF and the development of greater postoperative TK and PJA and a greater change in PJA following surgery may reflect a combination of early junctional kyphosis/failure and greater relaxation of preoperative thoracic compensation.33 Although in the univariate analysis preoperative TK and PJA were not significantly predictive of PJF, both parameters met the threshold for inclusion in the multivariate models and each was significantly associated with PJF occurrence in these models. The increased risk of PJF in patients with greater preoperative TK has been noted in multiple studies8,15 and may result from increased biomechanical stress at the junction and could reflect increased overall frailty and sarcopenia.32 For example, based on the hazard ratio for preoperative TK (1.362, modeled per 10°) in the present study, the risk of PJF increases 2.5-fold (calculated as 1.3623 = 2.53) for a patient with a preoperative TK of 60° compared to that of a patient with a TK of 30°. The association between greater preoperative TK and an increased risk of PJF may have implications for patient counseling and may impact surgical planning with regard to UIV selection.
In the present series, 16 (34.8%) of 46 patients with PJF had undergone revision as of the last follow-up. Thus, even when selecting for patients with more severe proximal junctional pathology (PJF vs Glattes PJK6), only approximately one-third of cases resulted in revision surgery. The decision of whether to pursue revision surgery for PJF is multifactorial and often driven by back and/or radicular pain not relieved by nonoperative treatment, the development of a neurological deficit or myelopathy, increased kyphosis significantly impacting function and/or appearance, and concerns about instability or the progression of junctional failure that may threaten neurological compromise. The mechanism of failure was significantly associated with the need for revision surgery (Table 6), with revision performed in 6 (85.6%) of the 7 patients with screw dislodgment, 15 (71.4%) of the 21 with UIV/UIV+1 fracture, 1 (50.0%) of the 2 with spondylolisthesis of UIV+1, and 11 (28.2%) of the 39 with PJA > 20°. Few previous studies have specifically reported revision rates for PJF, and some studies have defined PJF based on the need for revision surgery.9,15 Hart and colleagues18 reported that 27 (47.4%) of 57 patients with PJF in their cohort underwent revision surgery within 6 months of the index operation. They also identified several factors that seemed to influence the decision to perform revision surgery for PJF, including traumatic etiology of PJF, severity of PJA, higher SVA, and female sex. In the series from Park and colleagues,14 of the 29 patients who had PJF, revision surgery was recommended for 13, only 2 of whom had revision surgery and 11 of whom underwent vertebroplasties at the UIV/UIV+1 as a means of palliative care because the patients had declined revision surgery. In the series from Hostin and colleagues,13 28 (41.2%) of 68 patients with acute PJF underwent revision surgery. In the present series, 3 (18.8%) of 16 patients who had undergone revision for PJF later developed recurrent PJF. This compares with the previously reported rates of recurrent PJK of 31% by Funao and colleagues34 and 44% by Kim and colleagues.35
Strengths of the present study include the prospective multicenter design, relative homogeneity of the patient population, and length of the follow-up. It is also important to recognize the limitations. Although the homogeneity of the cohort is a strength, it is also a limitation since the patients had primarily coronal deformities, which limits the ability to assess the potential impact of sagittal realignment on the risk of PJF. The relatively small number of three-column osteotomies in this cohort limited meaningful analysis of this subgroup with regard to PJF risk. Since the time that patients were enrolled in the ASLS-1 study, there have been advances in our understanding of PJK/PJF prevention that were not employed in and cannot be assessed using the present cohort. For example, the use of proximal junctional tethers19,36–42 and an improved understanding of age-adjusted alignment goals43–45 have been suggested to reduce rates of PJK/PJF. Finally, since there were multiple surgeons from several institutions, there was a potential selection and performance bias that could have influenced the decision for revision surgery.
Conclusions
We used data from the multicenter, prospective ASLS-1 to define rates of PJF based on longer-term follow-up in a relatively homogeneous cohort and to assess risk factors for PJF. In this cohort, the overall PJF rate was 28.8% at a mean 4.3-year follow-up. Based on Kaplan-Meier analyses, PJF rates at 1, 2, 3, and 4 years were 14.4%, 21.9%, 25.9%, and 27.4%, respectively. On univariate analysis, a higher risk of PJF was associated with a greater age and BMI, the use of PCOs, and worse baseline PROMs. The use of hooks at the UIV was protective against PJF. On multivariate analysis, an increased risk of PJF was associated with a greater BMI and preoperative TK and lower preoperative PJA. Sixteen patients (34.8%) underwent revision surgery for PJF, and PJF recurred in 3 of the patients (18.8%). Collectively, this study provides the highest quality data to date on rates of PJF following primary ASLS surgery.
Acknowledgments
This trial was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases division of the National Institutes of Health (5RO1-ARO55176) from 2010 to 2017, and since 2017 it has received funding through the Scoliosis Research Society.
Disclosures
Dr. Smith is a consultant for Zimmer Biomet, Carlsmed, DePuy Synthes, NuVasive, Stryker, SeaSpine, and Cerapedics; receives royalties from Zimmer Biomet, NuVasive, and Thieme; owns stock in Alphatec and NuVasive; receives support from DePuy Synthes/ISSGF, NuVasive, and AO Spine for non–study-related clinical research effort; and receives fellowship support from AO Spine. Dr. Kelly receives honoraria from Wolters Kluwer. Dr. Gupta receives royalties from DePuy, Innomed, and Globus; is a consultant for DePuy, Medtronic, Globus, and Alphatec (2019); owns stock in J&J; receives honoraria from AO Spine, Wright State, LSU, and Malaysia Spine Society; serves on the board of directors for and has received travel funds from the SRS; has received travel funds from DePuy, Medtronic, Alphatec (2019), Medicrea (2019), Mizuho (2019), AO Spine, Globus, and Zimmer Biomet; and volunteers as a scientific advisor to the National Health Spine Foundation. Dr. Yen is a consultant for NuVasive. Dr. Lafage receives honoraria from Stryker, Implanet, and J&J; receives royalties from NuVasive; is a consultant for Globus and Alphatec; has ownership of VFT Solutions and See Sine LLC; and serves on committees for the International Spine Study Group and SRS. Dr. Ames receives royalties from Stryker, Biomet Zimmer Spine, DePuy Synthes, NuVasive, Next Orthosurgical, K2M, and Medicrea; is a consultant for DePuy Synthes, Medtronic, Medicrea, K2M, Carlsmed, and Agada Medical; receives research support from Titan Spine, DePuy Synthes, and International Spine Study Group; serves on editorial board of Operative Neurosurgery; serves on executive committee for International Spine Study Group; serves as director of Global Spinal Analytics; and serves as chair of the SRS Safety and Value Committee. Dr. Bess is a consultant for Atec, Mirus, Cerepedics, and Amgen; holds a patent with Stryker and NuVasive; received support from DePuy Synthes, NuVasive, International Spine Study Group Foundation, and Stryker for the study described; receives support from DePuy Synthes, NuVasive, Stryker, Medtronic, Globus, SI Bone, SeaSpine, and Carlsmed for non–study-related effort; is on the speakers bureau of Atec; and receives royalties from Stryker and NuVasive. Dr. Schwab owns stock in VFT Solutions and SeaSpine; is a consultant for Zimmer Biomet, Medtronic, and Mainstay Medical; receives royalties from Zimmer Biomet and Medtronic; and serves as executive committee member for International Spine Study Group. Dr. Shaffrey is a consultant for NuVasive, Medtronic, SI Bone, and Proprio; owns stock in NuVasive and Proprio; holds patents with NuVasive, Medtronic, and SI Bone; and receives royalties from NuVasive and SI Bone.
Author Contributions
Conception and design: Smith, Kelly, Shaffrey, Bridwell. Acquisition of data: Smith, Kelly, Dial, Hills, Baldus, Lafage, Schwab, Shaffrey, Bridwell. Analysis and interpretation of data: Smith, Lazaro, Sardi, Kelly, Yanik, Gupta, Yen, Ames, Bess, Bridwell. Drafting the article: Smith, Lazaro, Sardi. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Smith. Statistical analysis: Yanik. Administrative/technical/material support: Baldus, Bess, Shaffrey, Bridwell. Study supervision: Bridwell.
Supplemental Information
Previous Presentations
This work was presented at the North American Spine Society Annual Meeting held in Chicago, Illinois, on October 12–15, 2022. Abstract published in The Spine Journal. 2022:22(9):S78.
References
- 1. Buell TJ, Nguyen JH, Mazur MD, et al. Radiographic outcome and complications after single-level lumbar extended pedicle subtraction osteotomy for fixed sagittal malalignment: a retrospective analysis of 55 adult spinal deformity patients with a minimum 2-year follow-up. J Neurosurg Spine. 2018;30(2):242–252. doi: 10.3171/2018.7.SPINE171367. [DOI] [PubMed] [Google Scholar]
- 2. Kim HJ, Yang JH, Chang DG, et al. Incidence and radiological risk factors of proximal junctional kyphosis in adolescent idiopathic scoliosis following pedicle screw instrumentation with rod derotation and direct vertebral rotation: a minimum 5-year follow-up study. J Clin Med. 2021;10(22):5351. doi: 10.3390/jcm10225351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976) 2008;33(20):2179–2184. doi: 10.1097/BRS.0b013e31817c0428. [DOI] [PubMed] [Google Scholar]
- 4. Smith JS, Klineberg E, Lafage V, et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25(1):1–14. doi: 10.3171/2015.11.SPINE151036. [DOI] [PubMed] [Google Scholar]
- 5. Cerpa M, Sardar Z, Lenke L. Revision surgery in proximal junctional kyphosis. Eur Spine J. 2020;29(1)(suppl 1):78–85. doi: 10.1007/s00586-020-06320-y. [DOI] [PubMed] [Google Scholar]
- 6. Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C., II Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005;30(14):1643–1649. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 7. Kim HJ, Bridwell KH, Lenke LG, et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine (Phila Pa 1976) 2013;38(11):896–901. doi: 10.1097/BRS.0b013e3182815b42. [DOI] [PubMed] [Google Scholar]
- 8. Lau D, Clark AJ, Scheer JK, et al. Proximal junctional kyphosis and failure after spinal deformity surgery: a systematic review of the literature as a background to classification development. Spine (Phila Pa 1976) 2014;39(25):2093–2102. doi: 10.1097/BRS.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 9. Yagi M, Rahm M, Gaines R, et al. Characterization and surgical outcomes of proximal junctional failure in surgically treated patients with adult spinal deformity. Spine (Phila Pa 1976) 2014;39(10):E607–E614. doi: 10.1097/BRS.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 10. Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: past, present, and future. J Neurosurg Spine. 2019;30(5):551–567. doi: 10.3171/2019.1.SPINE181494. [DOI] [PubMed] [Google Scholar]
- 11. Maruo K, Ha Y, Inoue S, et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine (Phila Pa 1976) 2013;38(23):E1469–E1476. doi: 10.1097/BRS.0b013e3182a51d43. [DOI] [PubMed] [Google Scholar]
- 12. Hart RA, McCarthy I, Ames CP, Shaffrey CI, Hamilton DK, Hostin R. Proximal junctional kyphosis and proximal junctional failure. Neurosurg Clin N Am. 2013;24(2):213–218. doi: 10.1016/j.nec.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13. Hostin R, McCarthy I, O'Brien M, et al. Incidence, mode, and location of acute proximal junctional failures after surgical treatment of adult spinal deformity. Spine (Phila Pa 1976) 2013;38(12):1008–1015. doi: 10.1097/BRS.0b013e318271319c. [DOI] [PubMed] [Google Scholar]
- 14. Park SJ, Lee CS, Chung SS, Lee JY, Kang SS, Park SH. Different risk factors of proximal junctional kyphosis and proximal junctional failure following long instrumented fusion to the sacrum for adult spinal deformity: survivorship analysis of 160 patients. Neurosurgery. 2017;80(2):279–286. doi: 10.1227/NEU.0000000000001240. [DOI] [PubMed] [Google Scholar]
- 15. Scheer JK, Osorio JA, Smith JS, et al. Development of validated computer-based preoperative predictive model for proximal junction failure (PJF) or clinically significant PJK with 86% accuracy based on 510 ASD patients with 2-year follow-up. Spine (Phila Pa 1976) 2016;41(22):E1328–E1335. doi: 10.1097/BRS.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 16. Yagi M, Fujita N, Tsuji O, et al. Low bone-mineral density is a significant risk for proximal junctional failure after surgical correction of adult spinal deformity: a propensity score-matched analysis. Spine (Phila Pa 1976) 2018;43(7):485–491. doi: 10.1097/BRS.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 17. Hyun SJ, Lee BH, Park JH, Kim KJ, Jahng TA, Kim HJ. Proximal junctional kyphosis and proximal junctional failure following adult spinal deformity surgery. Korean J Spine. 2017;14(4):126–132. doi: 10.14245/kjs.2017.14.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hart R, McCarthy I, O'Brien M, et al. Identification of decision criteria for revision surgery among patients with proximal junctional failure after surgical treatment of spinal deformity. Spine (Phila Pa 1976) 2013;38(19):E1223–E1227. doi: 10.1097/BRS.0b013e31829fedde. [DOI] [PubMed] [Google Scholar]
- 19. Line BG, Bess S, Lafage R, et al. Effective prevention of proximal junctional failure in adult spinal deformity surgery requires a combination of surgical implant prophylaxis and avoidance of sagittal alignment overcorrection. Spine (Phila Pa 1976) 2020;45(4):258–267. doi: 10.1097/BRS.0000000000003249. [DOI] [PubMed] [Google Scholar]
- 20. Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine (Phila Pa 1976) 2010;35(2):138–145. doi: 10.1097/BRS.0b013e3181c8f35d. [DOI] [PubMed] [Google Scholar]
- 21. Kelly MP, Lurie JD, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis. J Bone Joint Surg Am. 2019;101(4):338–352. doi: 10.2106/JBJS.18.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith JS, Kelly MP, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis at 5-year follow-up: durability of outcomes and impact of treatment-related serious adverse events. J Neurosurg Spine. 2021;35(1):67–79. doi: 10.3171/2020.9.SPINE201472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith JS, Shaffrey CI, Kelly MP, et al. Effect of serious adverse events on health-related quality of life measures following surgery for adult symptomatic lumbar scoliosis. Spine (Phila Pa 1976) 2019;44(17):1211–1219. doi: 10.1097/BRS.0000000000003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryan DJ, Protopsaltis TS, Ames CP, et al. T1 pelvic angle (TPA) effectively evaluates sagittal deformity and assesses radiographical surgical outcomes longitudinally. Spine (Phila Pa 1976) 2014;39(15):1203–1210. doi: 10.1097/BRS.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 25. Ames CP, Smith JS, Scheer JK, et al. Impact of spinopelvic alignment on decision making in deformity surgery in adults: a review. J Neurosurg Spine. 2012;16(6):547–564. doi: 10.3171/2012.2.SPINE11320. [DOI] [PubMed] [Google Scholar]
- 26. Bridwell KH, Lenke LG, Cho SK, et al. Proximal junctional kyphosis in primary adult deformity surgery: evaluation of 20 degrees as a critical angle. Neurosurgery. 2013;72(6):899–906. doi: 10.1227/NEU.0b013e31828bacd8. [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Eksi MS, Ames CP, et al. A novel 4-rod technique offers potential to reduce rod breakage and pseudarthrosis in pedicle subtraction osteotomies for adult spinal deformity correction. Oper Neurosurg (Hagerstown) 2018;14(4):449–456. doi: 10.1093/ons/opx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rabinovich EP, Buell TJ, Wang TR, Shaffrey CI, Smith JS. Reduced occurrence of primary rod fracture after adult spinal deformity surgery with accessory supplemental rods: retrospective analysis of 114 patients with minimum 2-year follow-up. J Neurosurg Spine. 2021;35(4):504–515. doi: 10.3171/2020.12.SPINE201527. [DOI] [PubMed] [Google Scholar]
- 29. Schwab F, Ungar B, Blondel B, et al. Scoliosis Research Society—Schwab Adult Spinal Deformity Classification: a validation study. Spine (Phila Pa 1976) 2012;37(12):1077–1082. doi: 10.1097/BRS.0b013e31823e15e2. [DOI] [PubMed] [Google Scholar]
- 30. O’Leary PT, Bridwell KH, Lenke LG, et al. Risk factors and outcomes for catastrophic failures at the top of long pedicle screw constructs: a matched cohort analysis performed at a single center. Spine (Phila Pa 1976) 2009;34(20):2134–2139. doi: 10.1097/BRS.0b013e3181b2e17e. [DOI] [PubMed] [Google Scholar]
- 31. Nicholls FH, Bae J, Theologis AA, et al. Factors associated with the development of and revision for proximal junctional kyphosis in 440 consecutive adult spinal deformity patients. Spine (Phila Pa 1976) 2017;42(22):1693–1698. doi: 10.1097/BRS.0000000000002209. [DOI] [PubMed] [Google Scholar]
- 32. Yuan L, Zeng Y, Chen Z, Li W, Zhang X, Mai S. Degenerative lumbar scoliosis patients with proximal junctional kyphosis have lower muscularity, fatty degeneration at the lumbar area. Eur Spine J. 2021;30(5):1133–1143. doi: 10.1007/s00586-020-06394-8. [DOI] [PubMed] [Google Scholar]
- 33. Protopsaltis TS, Diebo BG, Lafage R, et al. Identifying thoracic compensation and predicting reciprocal thoracic kyphosis and proximal junctional kyphosis in adult spinal deformity surgery. Spine (Phila Pa 1976) 2018;43(21):1479–1486. doi: 10.1097/BRS.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 34. Funao H, Kebaish FN, Skolasky RL, Kebaish KM. Recurrence of proximal junctional kyphosis after revision surgery for symptomatic proximal junctional kyphosis in patients with adult spinal deformity: incidence, risk factors, and outcomes. Eur Spine J. 2021;30(5):1199–1207. doi: 10.1007/s00586-020-06669-0. [DOI] [PubMed] [Google Scholar]
- 35. Kim HJ, Wang SJ, Lafage R, et al. Recurrent proximal junctional kyphosis: incidence, risk factors, revision rates, and outcomes at 2-year minimum follow-up. Spine (Phila Pa 1976) 2020;45(1):E18–E24. doi: 10.1097/BRS.0000000000003202. [DOI] [PubMed] [Google Scholar]
- 36. Buell TJ, Buchholz AL, Quinn JC, et al. A pilot study on posterior polyethylene tethers to prevent proximal junctional kyphosis after multilevel spinal instrumentation for adult spinal deformity. Oper Neurosurg (Hagerstown) 2019;16(2):256–266. doi: 10.1093/ons/opy065. [DOI] [PubMed] [Google Scholar]
- 37. Buell TJ, Bess S, Xu M, et al. Optimal tether configurations and preload tensioning to prevent proximal junctional kyphosis: a finite element analysis. J Neurosurg Spine. 2019;30(5):574–584. doi: 10.3171/2018.10.SPINE18429. [DOI] [PubMed] [Google Scholar]
- 38. Buell TJ, Mullin JP, Nguyen JH, et al. A novel junctional tether weave technique for adult spinal deformity: 2-dimensional operative video. Oper Neurosurg (Hagerstown) 2019;16(2):45–46. doi: 10.1093/ons/opy148. [DOI] [PubMed] [Google Scholar]
- 39. Rabinovich EP, Buell TJ, Sardi JP, Lazaro BCR, Shaffrey CI, Smith JS. A novel weave tether technique for proximal junctional kyphosis prevention in 71 adult spinal deformity patients: a preliminary case series assessing early complications and efficacy. Oper Neurosurg (Hagerstown) 2021;21(6):393–399. doi: 10.1093/ons/opab305. [DOI] [PubMed] [Google Scholar]
- 40. Safaee MM, Deviren V, Dalle Ore C, et al. Ligament augmentation for prevention of proximal junctional kyphosis and proximal junctional failure in adult spinal deformity. J Neurosurg Spine. 2018;28(5):512–519. doi: 10.3171/2017.9.SPINE1710. [DOI] [PubMed] [Google Scholar]
- 41. Safaee MM, Haddad AF, Fury M, et al. Reduced proximal junctional failure with ligament augmentation in adult spinal deformity: a series of 242 cases with a minimum 1-year follow-up. J Neurosurg Spine. 2021;35(6):752–760. doi: 10.3171/2021.2.SPINE201987. [DOI] [PubMed] [Google Scholar]
- 42. Bess S, Harris JE, Turner AW, et al. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: a finite element analysis. J Neurosurg Spine. 2017;26(1):125–133. doi: 10.3171/2016.6.SPINE151477. [DOI] [PubMed] [Google Scholar]
- 43. Lafage R, Passias P, Sheikh Alshabab B, et al. Patterns of lumbar spine malalignment leading to revision surgery for proximal junctional kyphosis: a cluster analysis of over- versus under-correction. Global Spine J. Published online February. 2022;28, doi: 10.1177/21925682211047461. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lafage R, Smith JS, Elysee J, et al. Sagittal age-adjusted score (SAAS) for adult spinal deformity (ASD) more effectively predicts surgical outcomes and proximal junctional kyphosis than previous classifications. Spine Deform. 2022;10(1):121–131. doi: 10.1007/s43390-021-00397-1. [DOI] [PubMed] [Google Scholar]
- 45. Lafage R, Schwab F, Glassman S, et al. Age-adjusted alignment goals have the potential to reduce PJK. Spine (Phila Pa 1976) 2017;42(17):1275–1282. doi: 10.1097/BRS.0000000000002146. [DOI] [PubMed] [Google Scholar]